Abstract
Herbaspirillum seropedicae is an endophytic diazotrophic bacterium and a plant growth promoting bacteria. Colletotrichum graminicola causes the anthracnose, one of the most destructive maize diseases worldwide. The main objective of this work was to evaluate the effects of H. seropedicae SmR1 strain on the plant growth and leaf anthracnose of maize plants grown in substrate amended or not amended with humic substances. In the first assay, plants were pre-treated with H. seropedicae and inoculated with C. graminicola at 7, 14 and 21 days after treatment (DAT). In the second assay, plants were treated with H. seropedicae, grown in substrate amended with humic substances and inoculated at 3 and 7 DAT. The anthracnose severity was assessed by measurement of necrotic and chlorotic leaf area, and bacteria were quantified in leaves by quantitative PCR. H. seropedicae did not affect the disease severity in maize leaves, although it efficiently colonized the leaf tissues and it promoted maize leaf growth. Humic substances improved H. seropedicae colonization in maize.
Keywords: Colletotrichum graminicola, Anthracnose, Herbaspirillum seropedicae, Plant growth promoting bacteria, Diazotrophic bacteria, Biocontrol agent
Introduction
The hemibiotrophic fungus Colletotrichum graminicola (Cesati) Wilson causes anthracnose in maize. It combines a transient biotrophic phase, during which the host cells remain alive, with a highly destructive necrotrophic development that kills the cells while spreading itself through the host tissue (Münch et al. 2008; Perfect and Green 2001). Anthracnose is one of the most important diseases affecting maize fields around the world (Bergstrom and Nicholson 1999; Jirak-Peterson and Esker 2011; Nicoli et al. 2016; Palaversic et al. 2009; Sukno et al. 2008), including Brazil (Cota et al. 2012). Although all maize plant tissues can be infected, the fungus attacks more frequently leaves and stalks causing the anthracnose leaf blight and anthracnose stalk rot (Bergstrom and Nicholson 1999), which represent the most economically damaging forms of the disease, resulting in severe yield losses in crops, especially under tropical conditions. The incidence of anthracnose in Brazil has increased in recent years as a result of the successive plantings, adoption of no-tillage without crop rotation and the use of susceptible genotypes. This is explained by the high ability of pathogens to survive in crop residues, leading to accumulation of inoculum in planting areas (Cota et al. 2012; Sukno et al. 2008).
There has been increasing interest in the development and use of new and environmentally safer alternatives for plant nutrition as well as protection against phytopathogens (Guerrero-Molina et al. 2015). In this scenario, the so-called plant growth promoting bacteria (PGPB) exhibit beneficial effects on plant growth and health. PGPB are known to improve plant nutrition and crop yield, while reducing negative impacts of chemical fertilizers on the environment (Babalola 2010; Patil et al. 2016; Perez-Montano et al. 2014; Vejan et al. 2016; Whipps 2001). Some studies have revealed that PGPB can decrease or prevent damage by plant pathogens, through mechanisms such as: (1) antagonism; (2) competition for iron; and (3) induction of systemic resistant against pathogens (Dobbelaere et al. 2003; Glick and Bashan 1997; Perez-Montano et al. 2014). Indeed, PGPB usually inhibit plant pathogens by more than one mechanism (Alabouvette et al. 1993; Patil et al. 2016).
Regarding to the beneficial effects of PGPB as biocontrol agents, several studies can be found using species of Pseudomonas against several pathogens, fungi, bacteria or virus (Cabanas et al. 2014; Elbadry et al. 2006; Liu et al. 1995; Nandakumar et al. 2001; Patil et al. 2016; Yasmin et al. 2016), including Colletotrichum spp. (Bigirimana and Hofte 2002; Gang et al. 1991; Planchamp et al. 2015; Viswanathan and Samiyappan 1999; Viswanathan and Samiyappan 2002), and even C. graminicola in maize (Planchamp et al. 2015). Bacillus has been reported to be effective against C. acutatum (Park et al. 2013) and other pathogens as well (Niu et al. 2011; Raj et al. 2012). Gluconacetobacter diazotrophicus (Arencibia et al. 2006), Brevibacterium iodinum (Son et al. 2014), Azospirillum sp. (Yasuda et al. 2009) and Azospirillum brasilense (Tortora et al. 2011; Tortora et al. 2012) are further examples of bacteria able to control plant pathogens at different extents under controlled conditions. Although all these studies have indicated a high potential for using PGPB as biocontrol agents against different fungal diseases on different hosts, up to now, to the best of our knowledge, there is no information if Herbaspirillum would be able to affect the development of plant diseases.
Herbaspirillum seropedicae is a PGPB able to colonize plant tissues endophytically. It is also diazotrophic (Chubatsu et al. 2012), that is, able to convert atmospheric nitrogen into ammonia, which can be in turn metabolized by the plant (Dobbelaere et al. 2003). H. seropedicae, i.e., the extensively studied SmR1 strain (Monteiro et al. 2012), has its complete genome sequenced (Pedrosa et al. 2011) and is nowadays target for application in agriculure. Herbaspirillim sp. was already suggested as a potential biocontrol agent by Weber et al. (2007), because its presence in plantlets of banana was associated with decrease in the severity of wilt caused by Fusarium oxysporum f. sp. cubense. H. seropedicae has at least 27 genes involved in iron transport and metabolism (Pedrosa et al. 2011). In fact, production of siderophores called serobactins was observed in H. seropedicae Z67 (Rosconi et al. 2013), and these compounds contribute to the competitive performance of H. seropedicae inside the host plant (Rosconi et al. 2016). Besides that, H. seropedicae seems to be able to modulate plant defense responses (Brusamarello-Santos et al. 2012; do Amaral et al. 2014).
Vermicompost, a renewable organic product containing humic substances (humics) with high biological activity (Martinez-Balmori et al. 2014), has been demonstrated to exert a synergistic interaction with PGPB. In this sense, vermicompost enhances the beneficial effects of PGPB agents, such as soil nutrients availability, microbial biomass and crop yield and quality. Although such effects strongly depend on the dose of vermicompost and crop species, this integrative practice has been suggested to substitute regular chemical fertilization (Song et al. 2015). Indeed, it has been shown that maize inoculation with H. seropedicae and humics can synergistically improve bacterial colonization in roots (Canellas et al. 2013). Combining vermicompost and H. seropedicae has proven to increase growth and development of tomato seedlings (Olivares et al. 2015).
Taking into account the potential use of H. seropedicae in maize crop, this work was the first aimed at evaluating its effect when associated or not associated with humics to control the leaf anthracnose.
Materials and methods
Microrganisms growth conditions
H. seropedicae SmR1, a spontaneous streptomycin resistant mutant of strain Z78 (ATCC 35893), was grown in an orbital shaker (120 rpm) at 30 °C in 30 mL NFbHPN medium supplemented with 5 mg/L malic acid (Klassen et al. 1997).
C. graminicola isolate MANE53 is deposited in the mycological collection (MANE) of the Laboratory of Plant Pathology, Federal University of Santa Catarina, Brazil. For fungal cultivation, pure cultures of the isolate were grown on potato dextrose agar medium (PDA) and incubated for 21 days under fluorescent light, with 12 h photoperiod at ± 25 °C.
H. seropedicae pre-treatment and growth conditions of maize plants
Maize seeds (cv. Pioneer 30F53) were surface-sterilized by immersion in 70% ethanol for 5 min, and in 2% sodium hypochlorite plus 2.5% Tween-20 solution for 30 min. Seeds were then rinsed 3 times with sterile distilled water, germinated in water moist filter paper and incubated at ± 25 °C in the dark for 3 days. In the pre-treatment, the 3-days-old seedlings remained for 30 min under constant agitation (80 rpm) (Balsanelli et al. 2010; do Amaral et al. 2014) in contact with a culture of 107 cells of H. seropedicae SmR1 mL−1 diluted in sterile NFb malate broth without nitrogen source (the Optical Density of bacterial cell cultures was measured at 600 nm using Hitachi U2910 Spectrophotometer, Tokyo, Japan). Sterile broth served as control. Seedlings were then transferred to 2 L pots containing soil.
Effect of H. seropedicae on leaf anthracnose
The distrophic yellowish-red latosol, soil was collected from layer 0–20 cm in Cerro Negro, Santa Catarina, Brazil. Each kg of soil was previously supplemented with 50 mL nutrient solution (Rodriguez-Salazar et al. 2009) with reduced nitrogen (0.5 mM KNO3). The physical composition of each kg of soil consisted of 600 g of clay, 350 g of silt and 50 g of sand. Plants were grown in greenhouse under natural light, without temperature control (average temperature about 28 °C), watered daily and weekly were added with 100 mL of nutrient solution per pot.
C. graminicola inoculation was performed at 7, 14 and 21 days after treatment (DAT) with H. seropedicae. First, the conidia suspension was filtered through sterile double layer cheesecloth and washed twice by centrifugation at 25 °C and 1098×g for 8 min. Whole plants were sprayed with a suspension of 5 × 105 conidia mL−1 plus Tween 20 (1 μL per 10 mL inoculum) using an atomizer connected to an air compressor (58 psi), delivering about 5 mL plant−1. Plants were placed in a moist chamber for 24 h (100% humidity), in the dark. Then, they were transferred to the greenhouse again. The samples were harvested at 5 days after the pathogen inoculation, when plants were 15 (7 DAT), 22 (14 DAT) and 29-day-old (21 DAT). Two leaves per plant were sampled (F1 and F2), digitally scanned and used to determine the percentages of necrotic and chlorotic leaf area, as well as the total leaf area by means of Quant® software (v.1.02, Federal University of Viçosa, 2003).
Effect of H. seropedicae on anthracnose of plants grown in humics-amended substrate
The loamy sandy fulvic neosol soil was collected in Florianopolis-SC from layer of 0–20 cm and it was composed of clay 100 kg−1, silt 100 kg−1 and sand 800 kg−1. By the time the maize plantlets were sown, soil was treated with humics (or water). Humics were added (50 mg CL−1), in a proportion of 10% v/m (or water). Humics were originated from vermicompost prepared using sugarcane filter cake as described early (Jindo et al. 2016). Plants were grown in greenhouse under natural light without temperature control (average temperature about 26.5 °C) and watered daily.
C. graminicola inoculation, now using a spore suspension of 1 × 105 conidia mL−1, was performed after 3 and 7 DAT with H. seropedicae, as described above. Plants were placed in a moist chamber for 24 h in the dark. Then, they were transferred to the greenhouse again. Five days after pathogen inoculation, two leaves per plant per time of treatment were detached. Harvested plants were 11 (3 DAT) and 15-days-old (7 DAT). The oldest leaf collected was digitally scanned to determine the percentage of necrotic and chlorotic leaf area and to access the leaf area using the software Quant®, while the youngest leaf was immediately frozen in liquid nitrogen and stored − 80 °C for further analysis.
H. seropedicae quantification
H. seropedicae SmR1 was quantified by qPCR (expressed as DNA copy number and CFU) using already designed pair of primers (Pereira et al. 2014) and TaqMan probe (Dall’Asta et al. 2017). We estimated H. seropedicae DNA copy number/g leaf and H. seropedicae CFU/g leaf. Therefore, total DNA was isolated from maize tissue using DNeasy Plant Mini Kit (Qiagen) according to manufacturer instructions, and each extract was pooled of three leaves. DNA isolation from H. seropedicae pure culture was performed using Wizard® Genomic DNA purification kit (Promega™, Madison, WI, USA) with modifications. DNA concentrations were assessed on a Thermo Scientific NanoDrop 2000 spectrophotometer (Willmington, DE, USA).
H. seropedicae DNA copy number/g leaf was established as already described, using a standard curve constructed from DNA isolated from H. seropedicae pure culture by plotting Cq value versus log H. seropedicae DNA copy number (Dall’Asta et al. 2017; Pereira et al. 2014). Furthermore, H. seropedicae CFU/g leaf was estimated using a standard curve constructed with DNA isolated from a serial dilution of H. seropedicae culture mixed with crushed plant-leaf tissues as described by Stets et al. (2015), with modifications. Maize seeds (P30F53) were surface-sterilized as described above and plants were grown under axenic conditions as in vitro experiment described previously (Pereira et al. 2014). On the 11th day, leaves were harvested and ground in liquid nitrogen using a mortar and pestle. H. seropedicae culture (OD600 = 0.8; 108 cells of H. seropedicae SmR1 mL−1), was serially diluted in autoclaved 0.9% saline buffer ranging from 108 to 102. A volume of 100 μL of each culture dilution was added to 100 mg of crushed leaves, and this mixture was incubated for 1 h in room temperature and stored in ultra-freezer until DNA isolation. DNA was isolated from this whole mixture using DNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions and DNA concentrations were assessed by measurement of the optical density at 260 and 280 nm on a Thermo Scientific NanoDrop 2000 spectrophotometer (Willmington, DE, USA). A standard curve was generated by plotting Cq versus number of H. seropedicae CFU added to each reaction tube. For both standard curves generated, amplification efficiencies were determined as described previously (Dall’Asta et al. 2017). qPCR was performed in ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA, USA) as described previously (Dall’Asta et al. 2017).
Experimental design and data analysis
Both experiments were performed in completely randomized blocks, with 4 biological replicates. In the first experiment, realized to observe the effect of H. seropedicae leaf anthracnose, T test was used to compare anthracnose extension in control and treated groups and Wilcoxon-Mann–Whitney was used to compare leaf area assessments. The experiment was repeated twice with similar results. In the second experiment, used to observe the effect of H. seropedicae on anthracnose of plants grown in humics-amended substrate, statistical analysis consisted of Kruskal–Wallis test to compare the groups in measurement of chlorotic and necrotic maize leaf area and also to H. seropedicae DNA quantification. All tests were conducted under 95% of significance using Assistat (v. 7.7 beta, Federal University of Campina Grande, Brazil, 2016).
Results
Effect of H. seropedicae in leaf anthracnose and maize plant growth
Two days after C. graminicola inoculation, leaves of control plants presented clarified areas in the site of pathogen entrance. In the third day, these sites evolved and originated oval lesions with tan centers and reddish-brown borders. At this point, some leaves already showed elongated brown lesions, which progressed through necrosis enlargement in the next 2 days, until the leaves harvesting. H. seropedicae pre-treatment did not affect the anthracnose disease in leaves of maize plants in this situation (Table 1). It was assessed with two leaves per plant at each analyzed period of time and no relevant differences were observed even at 7, 14 or 21 DAT. Difference was observed just at 14 DAT in one analyzed leaf in chlorotic area. The experiment was repeated twice with similar results. H. seropedicae promoted maize leaf growth at 21 DAT, demonstrated through the increment in maize leaf area, however, no differences were observed at 7 and 14 DAT (Table 2).
Table 1.
Chlorotic and necrotic area of maize leaves pre-treated with H. seropedicae and inoculated with C. graminicola at 7, 14 and 21 days after treatment (DAT)
|
C. graminicola inoculation DAT |
Leafa | Chlorotic (% area) | Necrotic (% area) | ||
|---|---|---|---|---|---|
| Control | H.s | Control | H.s | ||
| 7 | L1 | 5.0 ± 5.8 | 9.3 ± 7.7 | 91.9 ± 5.9 | 85.8 ± 11.2 |
| L2 | 0.4 ± 0.6 | 0.9 ± 1.4 | 33.5 ± 8.6 | 30.1 ± 3.2 | |
| 14 | L1 | 8.0 ± 5.1 | 12.7 ± 6.7 | 70.2 ± 12.1 | 62.9 ± 12.2 |
| L2 | 8.2b ± 1.5 | 5.1 ± 1.4 | 13.7 ± 8.3 | 6.5 ± 4.4 | |
| 21 | L1 | 2.4 ± 1.7 | 0.8 ± 1.0 | 84.8 ± 7.3 | 89.4 ± 10.0 |
| L2 | 1.7 ± 1.2 | 1.5 ± 1.2 | 47.4 ± 14.2 | 64.3 ± 12.9 | |
Mean values correspond to the average of four replicates, each one being comprised of three leaves (n = 12). Data are presented as mean ± SD. The groups represent: Control (non treated with H. seropedicae) and H.s (treated with H. seropedicae)
aLeaves were assessed at 5 days after inoculation with C. graminicola
bSignificant difference comparing H. seropedicae treated and untreated leaves using Student’s t test with 95% significance level
Table 2.
Maize leaves area (cm2) pre-treated with H. seropedicae and inoculated with C. graminicola at 7, 14 and 21 days after treatment (DAT)
|
C. graminicola inoculation DAT |
Leafa | Leaf area (cm2) | |
|---|---|---|---|
| Control | H.s | ||
| 7 | L1 | 28.4 ± 3.2 | 25.8 ± 6.5 |
| L2 | 80 ± 10.2 | 81.1 ± 6.6 | |
| 14 | L1 | 67 ± 22.3 | 67.9 ± 20.3 |
| L2 | 204.3 ± 26.6 | 187.7 ± 26 | |
| 21 | L1 | 38.1 ± 27.9 | 118.3 ± 38.4b |
| L2 | 58 ± 9.1 | 176.6 ± 37.4b | |
Mean values correspond to the average of four replicates, each one being comprised of three leaves (n = 12). Data are presented as mean ± SD. The groups represent: control (non treated with H. seropedicae) and H.s (treated with H. seropedicae)
aLeaves were assessed at 5 days after inoculation with C. graminicola
bSignificant difference using Wilcoxon-Mann–Whitney with 95% significance level
Effect of H. seropedicae and humics in anthracnose and H. seropedicae maize colonization
H. seropedicae combined with humics did not affect the maize leaf anthracnose, once disease severity assessment evaluation showed no difference in chlorotic or necrotic leaf area between the groups at 3 or 7 DAT with H. seropedicae (Table 3).
Table 3.
Chlorotic and necrotic area of maize leaves pre-treated with H. seropedicae with or without humic substances and inoculated with C. graminicola at 3 and 7 days after treatment (DAT)
|
C. graminicola inoculation DAT |
Chlorotic (% area) | Necrotic (% area) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | −H.s +Humics |
+H.s −Humics |
+H.s +Humics |
Control | −H.s +Humics |
+H.s −Humics |
+H.s +Humics |
|
| 3a | 1.9 ± 3.7 | 1.3 ± 2.7 | 3.9 ± 2.9 | 1.4 ± 2.8 | 18.8 ± 6.9 | 15.5 ± 3.5 | 14.2 ± 7.8 | 15.4 ± 2.3 |
| 7 | 7.6 ± 2.8 | 11.5 ± 1.3 | 7.0 ± 3.8 | 7.8 ± 3.0 | 56.9 ± 11.1 | 50.5 ± 13.9 | 54.3 ± 11.6 | 63.9 ± 10.2 |
Mean values correspond to the average of four replicates, each one being comprised of three leaves (n = 12). Data are presented as mean ± SD. The groups represent: −H − HS (non-treated, without humic substances), −H + HS (non-treated, with humic substances), +H − HS (treated, without humic substances) and +H+HS (treated, with humic substances). No significant differences were obtained using Kruskal–Wallis tests (α = 0.05)
aAssessments were realized at 5 days after inoculation with C. graminicola
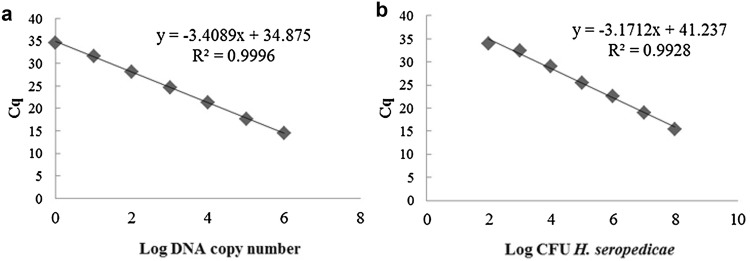
In order to confirm H. seropedicae colonization in leaves, H. seropedicae DNA and CFU were quantified by qPCR using standard curves. The standard curve constructed by plotting Cq x Log H. seropedicae DNA copy number (Fig. 1A) presented R2 = 0.99 and s = − 3.4, which correspond to an amplification E of 96%. The standard curve corresponding to Cq x Log CFU H. seropedicae (Fig. 1B), showed R2 = 0.99, s = − 3.2 and E = 107%.
Fig. 1.
qPCR standard curves for H. seropedicae quantification. a Cq versus log DNA copy number. Curve generated using DNA isolated from pure culture of H. seropedicae SmR1 as template. b Cq versus log CFU. Curve generated using DNA isolated from 100 µL of each serial dilution of H. seropedicae culture mixed with 100 mg of crushed in vitro plant-leaf tissues as template
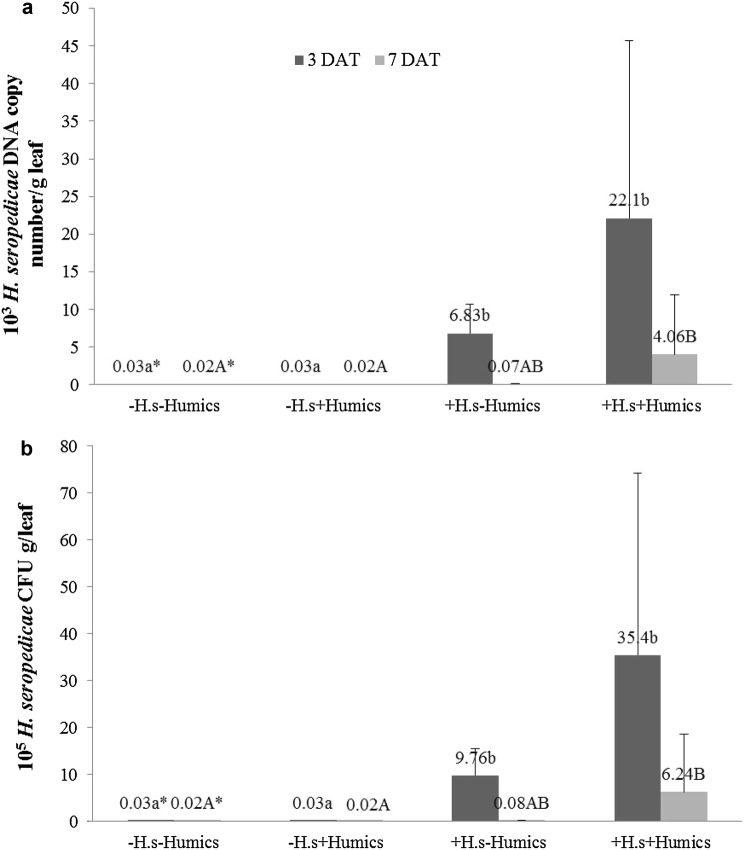
H. seropedicae DNA and CFU quantification presented similar results at 3 and 7 DAT (Fig. 2a, b). In view of H. seropedicae treated groups (+H.s − Humics and +H.s + Humics) at 3 DAT, the combined group (+H.s + Humics) tend to improve bacterial colonization in leaves. Despite that, bacterial presence was statistically higher in H. seropedicae treated groups (+H.s − Humics and +H.s + Humics) in comparison with H. seropedicae untreated groups (−H.s − Humics and –H.s + Humics) at 3 DAT. Regarding bacterial quantification at 7 DAT, H. seropedicae DNA and CFU found in H. seropedicae untreated groups (−H.s − Humics and –H.s + Humics) were statistically lower than in group with H. seropedicae and humics association (+H.s + Humics), suggesting that this consortium favors bacteria colonization.
Fig. 2.
H. seropedicae DNA quantification in leaves of P30F53 Zea mays, after inoculation with C. graminicola 3 and 7 days after treatment (DAT) with H. seropedicae and with or without humic substances. The groups represent: −H.s − Humics (non treated with H. seropedicae, without humics), −H.s + Humics (non treated with H. seropedicae, with humics), +H.s − Humics (treated with H. seropedicae, without humics) and +H.s + Humics (treated with H. seropedicae, with humics): aH. seropedicae DNA copy number/g leaf (fresh weight) using standard curve generated with DNA isolated from H. seropedicae pure culture. bH. seropedicae CFU/g leaf (fresh weight) using standard curve generated with DNA isolated from serial dilution of H. seropedicae culture mixed with crushed plant-leaf tissues. Values presented as mean ± standard deviation (n = 8). Lowercase and capital letters indicate statistical difference using Kruskal–Wallis test (a = 0.05) at 3 and 7 DAT, respectively
Discussion
The capacity of H. seropedicae SmR1 associated or not to humics to control leaf anthracnose caused by C. graminicola was tested and colonization of H. seropedicae in maize leaves was assessed. Although no differences were detected in anthracnose severity in treated plants, H. seropedicae presence was confirmed, showing that the absence of response is not due to the failure of colonization by H. seropedicae.
H. seropedicae colonization in leaves was assessed through H. seropedicae DNA and CFU quantification based on standard curves. The reliability of these standard curves was analyzed according to previously established patterns. According to that, reliable standard curves should have R2 values higher than 0.95 and slope between − 3.0 and − 3.9, corresponding to PCR efficiencies of 80–115% (Zhang and Fang 2006). All qPCR parameters found in this study (Fig. 1) are in accordance to the parameters established by Zhang and Fang (2006). Moreover, the present study’s values represent reliable standard curves even if using the more restricted parameters (ENGL 2008), which settles minimum performance requirements for qPCR analytical methods (R2 ≥ 0.98, slope between − 3.1 and − 3.6, and E values from 90 to 110%). The method of using a mix of H. seropedicae pure culture plus maize tissue to establish the CFU/g plant tissue was used to quantify H. seropedicae presence in maize. This method, that combines the bacterial and plant tissue in a single extract to perform the standard curve was already used to determine Azospirillum sp. quantity in presence of matrix as DNA template to qPCR reactions (Couillerot et al. 2010a, b; Stets et al. 2015). However, in our study, the method was used for H. seropedicae CFU quantification. To the best of our knowledge, it was the first time that this method was used to estimate H. seropedicae CFU/g plant tissue.
Regarding maize leaf colonization (Fig. 2), results showed a tendency of increment in H. seropedicae DNA copy number and CFU when H. seropedicae was associated with humics, if compared to H. seropedicae treatment isolated at 3 DAT, despite not statistically significant. At 7 DAT, it was shown that bacteria associated with humics (+H.s + Humics) presented highest bacteria presence in comparison to H. seropedicae non-treated groups (−H.s − Humics and –H.s + Humics). It was already shown that humics helped the maintenance of H. seropedicae population in maize roots (Canellas et al. 2013), mainly using vermicompost extracted from sugarcane cake residue (Martinez-Balmori et al. 2013). In addition, it was shown that coating maize seeds with H. seropedicae Z67 and humics increased the number of beneficial bacteria viable cells (da Conceicao et al. 2008). Recently it was shown that humics enhance maize root border cells survival at the same time that increase the fitness of the plant/microbial interaction. In this sense, humics are able to improve colonization of the root cap zone and influence the number and shape of border cells in maize root tips (Canellas and Olivares 2017). Despite that, our colonization results were effective, showing the accuracy of HERBAS1 pair of primers in detecting H. seropedicae in planta (Pereira et al. 2014), even in maize leaves inoculated with a phytopathogen and in variable experimental conditions.
At 21 DAT, H. seropedicae treated leaves presented an increment in its area in comparison with untreated ones, what can indicate that H. seropedicae promoted leaf growth. H. seropedicae inoculation has already shown to improve maize yield up to 34%, depending on the plant genotype (Alves et al. 2015) as well as increase maize dry weight and shoot and root lengths (Ribaudo et al. 2006). Increase in dry and fresh weight of rice plants inoculated with H. seropedicae was also observed at 28 (James et al. 2002) and at 30 days after inoculation (Baldani et al. 2000; Gyaneshwar et al. 2002). In fact, in view of H. seropedicae SmR1 genome, some genes related to plant growth promoting characteristics were identified, such as nitrogen fixation, siderophores production (and their receptors) and auxin synthesis genes, as well as a 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase gene (Monteiro et al. 2012; Pedrosa et al. 2011).
Maize plants were effectively colonized by bacteria H. seropedicae in this study, and the increased leaf area in treated plants observed can serve as an indicative of plant growth promotion by H. seropedicae. However, concerning the assessment of H. seropedicae control of anthracnose disease in maize plants, H. seropedicae SmR1 plants treatment do not exert biocontrol activity against anthracnose in maize. The association between this PGPB and humics was tested. The use of integrated disease management strategies to effectively manage diseases has been suggested as an alternative, because in general only a biocontrol agent may not be able to play this role by itself (Sahni et al. 2008). However, in our study, even the use of H. seropedicae SmR1 combined with humics management was not able to control the disease caused by C. graminicola in maize plants. In this work we tested only the SmR1 H. seropedicae strain and taking in account the existence of other available strains and beneficial bacteria species, there is the possibility that other strains (da Conceicao et al. 2008; Marques et al. 2008), the consortium of H. seropedicae strains or even with other bacteria species (da Silva et al. 2017) may be able to exert any biological activity against disease or other beneficial effect. Additionally, C. graminicola is considered a highly aggressive phytopathogen (Bergstrom and Nicholson 1999) and the genotype of maize contributes to the severity of symptoms resulting from infection with C. graminicola (Weihmann et al. 2016), it would be interesting to test its effect against other isolates and on maize plants exhibiting different levels of resistance.
Conclusion
Our data show that HERBAS1 qPCR assay efficiently quantify H. seropedicae plant growth promoting bacteria in maize leaves even in the presence of C. graminicola pathogen. Treatment with H. seropedicae and humics-amended substrates were not able to control anthracnose in P30F53 maize, although humics presence augmented H. seropedicae colonization in plants. Leaf area was increased in H. seropedicae colonized plants compared to control plants, H. seropedicae inoculation of maize leads to leaf growth promotion.
Acknowledgements
This work was financially supported by the National Institute of Science and Technology-Biological Nitrogen Fixation (INCT-FBN), National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil. The author would like to thank Professor Fábio Lopes Olivares, from University of North Fluminense, Brazil, who kindly provided the humic substances.
Author contributions
This research is part of the doctoral thesis of Pâmela Dall’Asta. Aline C. Velho and Tomás P. Pereira contributed in the Colletotrichum graminicola and Herbaspirillum seropedicae practical procedures, respectively. Professors Ana Carolina M. Arisi and Marciel Stadnik are the supervisors. Pâmela Dall´Asta wrote the basic text and all the authors contributed by reading, revising and improving it, especially the professors Ana Carolina M. Arisi and Marciel Stadnik.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alabouvette C, Lemanceau P, Steinberg C. Recent advances in the biological-control of Fusarium wilts. Pestic Sci. 1993;37:365–373. [Google Scholar]
- Alves GC, Videira SS, Urquiaga S, Reis VM. Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil. 2015;387:307–321. [Google Scholar]
- Arencibia AD, et al. Gluconoacetobacter diazotrophicus Elicitate a Sugarcane Defense Response Against a Pathogenic Bacteria Xanthomonas albilineans Plant Signaling & Behavior. 2006;1:265–273. doi: 10.4161/psb.1.5.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalola OO. Beneficial bacteria of agricultural importance. Biotechnol Lett. 2010;32:1559–1570. doi: 10.1007/s10529-010-0347-0. [DOI] [PubMed] [Google Scholar]
- Baldani VLD, Baldani JI, Dobereiner J. Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils. 2000;30:485–491. [Google Scholar]
- Balsanelli E, et al. Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization. Environ Microbiol. 2010;12:2233–2244. doi: 10.1111/j.1462-2920.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom GC, Nicholson RL. The biology of corn anthracnose—knowledge to exploit for improved management. Plant Dis. 1999;83:596–608. doi: 10.1094/PDIS.1999.83.7.596. [DOI] [PubMed] [Google Scholar]
- Bigirimana J, Hofte M. Induction of systemic resistance to Colletotrichum lindemuthianum in bean by a benzothiadiazole derivative and rhizobacteria. Phytoparasitica. 2002;30:159–168. [Google Scholar]
- Brusamarello-Santos LCC, et al. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil. 2012;356:113–125. [Google Scholar]
- Cabanas CGL, Schiliro E, Valverde-Corredor A, Mercado-Blanco J. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol. 2014;5:14. doi: 10.3389/fmicb.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellas LP, Olivares FL. Production of border cells and colonization of maize root tips by Herbaspirillum seropedicae are modulated by humic acid. Plant Soil. 2017;417:403–413. [Google Scholar]
- Canellas LP, et al. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.) Plant Soil. 2013;366:119–132. [Google Scholar]
- Chubatsu LS, et al. Nitrogen fixation control in Herbaspirillum seropedicae. Plant Soil. 2012;356:197–207. [Google Scholar]
- Cota LV, da Costa RV, Silva DD, Casela CR, Parreira DF. Quantification of yield losses due to anthracnose stalk rot on corn in Brazilian conditions. J Phytopathol. 2012;160:680–684. [Google Scholar]
- Couillerot O, Bouffaud ML, Baudoin E, Muller D, Caballero-Mellado J, Moenne-Loccoz Y. Development of a real-time PCR method to quantify the PGPR strain Azospirillum lipoferum CRT1 on maize seedlings. Soil Biol Biochem. 2010;42:2298–2305. [Google Scholar]
- Couillerot O, Poirier MA, Prigent-Combaret C, Mavingui P, Caballero-Mellado J, Moenne-Loccoz Y. Assessment of SCAR markers to design real-time PCR primers for rhizosphere quantification of Azospirillum brasilense phytostimulatory inoculants of maize. J Appl Microbiol. 2010;109:528–538. doi: 10.1111/j.1365-2672.2010.04673.x. [DOI] [PubMed] [Google Scholar]
- da Conceicao PM, Vieira HD, Canellas LP, Marques RB, Olivares FL. Corn seed coating with humic acids and endophytic diazotrophic bacteria. Pesquisa Agropecuaria Brasileira. 2008;43:545–548. [Google Scholar]
- da Silva SF, Olivares FL, Canellas LP. The biostimulant manufactured using diazotrophic endophytic bacteria and humates is effective to increase sugarcane yield. Chem Biol Technol Agric. 2017;4:24. [Google Scholar]
- Dall’Asta P, Pereira TP, do Amaral FP, Arisi ACM. Tools to evaluate Herbaspirillum seropedicae abundance and nifH and rpoC expression in inoculated maize seedlings grown in vitro and in soil. Plant Growth Regul. 2017;83:397–408. [Google Scholar]
- do Amaral FP, Ferreira Bueno JC, Hermes VS, Arisi ACM. Gene expression analysis of maize seedlings (DKB240 variety) inoculated with plant growth promoting bacterium Herbaspirillum seropedicae. Symbiosis. 2014;62:41–50. [Google Scholar]
- Dobbelaere S, Vanderleyden J, Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci. 2003;22:107–149. [Google Scholar]
- Elbadry M, Taha RM, Eldougdoug KA, Gamal-Eldin H. Induction of systemic resistance in faba bean (Vicia faba L.) to bean yellow mosaic potyvirus (BYMV) via seed bacterization with plant growth promoting rhizobacteria. J Plant Dis Prot. 2006;113:247–251. [Google Scholar]
- ENGL (2015) Definition of minimum performance requirements for analytical methods of GMO testing. European Network of GMO Laboratories. http://gmo-crl.jrc.ec.europa.eu/doc/MPR%20Report%20Application%2020_10_2015.pdf. Accessed Dec 2016
- Gang W, Kloepper JW, Tuzun S. Induction of systemic resistance of cucumber to Colletotrichum-Orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology. 1991;81:1508–1512. [Google Scholar]
- Glick BR, Bashan Y. Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv. 1997;15:353–378. doi: 10.1016/s0734-9750(97)00004-9. [DOI] [PubMed] [Google Scholar]
- Guerrero-Molina MF, Lovaisa NC, Salazar SM, Martinez-Zamora MG, Diaz-Ricci JC, Pedraza RO. Physiological, structural and molecular traits activated in strawberry plants after inoculation with the plant growth-promoting bacterium Azospirillum brasilense REC3. Plant Biol. 2015;17:766–773. doi: 10.1111/plb.12270. [DOI] [PubMed] [Google Scholar]
- Gyaneshwar P, James EK, Reddy PM, Ladha JK. Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Phytol. 2002;154:131–145. [Google Scholar]
- James EK, et al. Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant-Microbe Interact. 2002;15:894–906. doi: 10.1094/MPMI.2002.15.9.894. [DOI] [PubMed] [Google Scholar]
- Jindo K, et al. Phosphorus speciation and high-affinity transporters are influenced by humic substances. J Plant Nutr Soil Sci. 2016;179:206–214. [Google Scholar]
- Jirak-Peterson JC, Esker PD. Tillage, crop rotation, and hybrid effects on residue and corn anthracnose occurrence in Wisconsin. Plant Dis. 2011;95:601–610. doi: 10.1094/PDIS-11-10-0837. [DOI] [PubMed] [Google Scholar]
- Klassen G, Pedrosa FO, Souza EM, Funayama S, Rigo LU. Effect of nitrogen compounds on nitrogenase activity in SMR1. Can J Microbiol. 1997;43(9):887–891. [Google Scholar]
- Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber against Fusarium-wilt by plant growth-promoting rhizobacteria. Phytopathology. 1995;85:695–698. [Google Scholar]
- Marques RB, Jr, Canellas LP, da Silva LG, Olivares FL. Rooting of micro seed pieces by combined use of humic substances and endopitytic diazotrophic bacteria in sugarcane. Revista Brasileira De Ciencia Do Solo. 2008;32:1121–1128. [Google Scholar]
- Martinez-Balmori D, et al. Molecular characteristics of vermicompost and their relationship to preservation of inoculated nitrogen-fixing bacteria. J Anal Appl Pyrol. 2013;104:540–550. [Google Scholar]
- Martinez-Balmori D, Spaccini R, Aguiar NO, Novotny EH, Olivares FL, Canellasa LP. Molecular characteristics of humic acids isolated from vermicomposts and their relationship to bioactivity. J Agric Food Chem. 2014;62:11412–11419. doi: 10.1021/jf504629c. [DOI] [PubMed] [Google Scholar]
- Monteiro RA, et al. Herbaspirillum–plant interactions: microscopical, histological and molecular aspects. Plant Soil. 2012;356:175–196. [Google Scholar]
- Münch S, Lingner U, Floss DS, Ludwig N, Sauer N, Deising HB. The hemibiotrophic lifestyle of Colletotrichum species. J Plant Physiol. 2008;165:41–51. doi: 10.1016/j.jplph.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Nandakumar R, Babu S, Viswanathan R, Raguchander T, Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol Biochem. 2001;33:603–612. [Google Scholar]
- Nicoli A, Zambolim L, da Costa RV, Cota L, da Silva DD. Colletotrichum graminicola from leaves or stalks are similarly aggressive in cross-tissue inoculation of five maize hybrids. Trop Plant Pathol. 2016;41:57–61. [Google Scholar]
- Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY, Jin HL, Guo JH. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol Plant-Microbe Interact. 2011;24:533–542. doi: 10.1094/MPMI-09-10-0213. [DOI] [PubMed] [Google Scholar]
- Olivares FL, Aguiar NO, Rosa RCC, Canellas LP. Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Sci Hortic. 2015;183:100–108. [Google Scholar]
- Palaversic B, Jukic M, Buhinicek I, Vragolovic A, Kozic Z. Breeding maize for resistance to stalk anthracnose. Maydica. 2009;54:229–232. [Google Scholar]
- Park JW, Balaraju K, Kim JW, Lee SW, Park K. Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol Control. 2013;65:246–257. [Google Scholar]
- Patil SV, Jayamohan NS, Kumudini BS. Strategic assessment of multiple plant growth promotion traits for shortlisting of fluorescent Pseudomonas spp. and seed priming against ragi blast disease. Plant Growth Regul. 2016;80:47–58. [Google Scholar]
- Pedrosa FO, et al. Genome of Herbaspirillum seropedicae Strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLOS Genet. 2011;7:10. doi: 10.1371/journal.pgen.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira T, do Amaral F, Dall’Asta P, Brod F, Arisi A. Real-time PCR quantification of the plant growth promoting bacteria Herbaspirillum seropedicae Strain SmR1 in maize. Roots Mol Biotechnol. 2014;56:660–670. doi: 10.1007/s12033-014-9742-4. [DOI] [PubMed] [Google Scholar]
- Perez-Montano F, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Perfect SE, Green JR. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol. 2001;2:101–108. doi: 10.1046/j.1364-3703.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- Planchamp C, Glauser G, Mauch-Mani B. Root inoculation with Pseudomonas putida KT2440 induces transcriptional and metabolic changes and systemic resistance in maize plants. Front Plant Sci. 2015;5:10. doi: 10.3389/fpls.2014.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SN, Lavanya SN, Amruthesh KN, Niranjana SR, Reddy MS, Shetty HS. Histo-chemical changes induced by PGPR during induction of resistance in pearl millet against downy mildew disease. Biol Control. 2012;60:90–102. [Google Scholar]
- Ribaudo CM, Rondanini DP, Trinchero GD, Cura JA. Effect of Herbaspirillum seropedicae inoculation on maize nitrogen metabolism. Maydica. 2006;51:481–485. [Google Scholar]
- Rodriguez-Salazar J, Suarez R, Caballero-Mellado J, Iturriaga G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. Fems Microbiology Letters. 2009;296:52–59. doi: 10.1111/j.1574-6968.2009.01614.x. [DOI] [PubMed] [Google Scholar]
- Rosconi F, et al. Identification and structural characterization of serobactins, a suite of lipopeptide siderophores produced by the grass endophyte Herbaspirillum seropedicae. Environmental Microbiology. 2013;15:916–927. doi: 10.1111/1462-2920.12075. [DOI] [PubMed] [Google Scholar]
- Rosconi F, Trovero MF, de Souza EM, Fabiano E. Serobactins-mediated iron acquisition systems optimize competitive fitness of Herbaspirillum seropedicae inside rice plants. Environ Microbiol. 2016;18:2523–2533. doi: 10.1111/1462-2920.13202. [DOI] [PubMed] [Google Scholar]
- Sahni S, Sarma BK, Singh DP, Singh HB, Singh KP. Vermicompost enhances performance of plant growth-promoting rhizobacteria in Cicer arietinum rhizosphere against Sclerotium rolfsii. Crop Prot. 2008;27:369–376. [Google Scholar]
- Son JS, Sumayo M, Hwang YJ, Kim BS, Ghim SY. Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Appl Soil Ecol. 2014;73:1–8. [Google Scholar]
- Song XC, Liu MQ, Wu D, Griffiths BS, Jiao JG, Li HX, Hu F. Interaction matters: synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl Soil Ecol. 2015;89:25–34. [Google Scholar]
- Stets MI, Alqueres S, Souza EM, Pedrosa FO, Schmid M, Hartmann A, Cruz LM (2015) Quantification of Azospirillum brasilense FP2 in wheat roots by strain-specific qPCR. Appl Environ Microbiol. 10.1128/aem.01351-15 [DOI] [PMC free article] [PubMed]
- Sukno SA, Garcia VM, Shaw BD, Thon MR. Root infection and systemic colonization of maize by Colletotrichum graminicola. Appl Environ Microbiol. 2008;74:823–832. doi: 10.1128/AEM.01165-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora ML, Diaz-Ricci JC, Pedraza RO. Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch Microbiol. 2011;193:275–286. doi: 10.1007/s00203-010-0672-7. [DOI] [PubMed] [Google Scholar]
- Tortora ML, Diaz-Ricci JC, Pedraza RO. Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil. 2012;356:279–290. [Google Scholar]
- Vejan P, Abdullah R, Khadiran T, Ismail S, Boyce AN. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules. 2016;21:17. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan R, Samiyappan R. Induction of systemic resistance by plant growth promoting rhizobacteria against red rot disease in sugarcane. Sugar Tech. 1999;1:67. [Google Scholar]
- Viswanathan R, Samiyappan R. Induced systemic resistance by fluorescent pseudomonads against red rot disease of sugarcane caused by Colletotrichum falcatum. Crop Prot. 2002;21:1–10. [Google Scholar]
- Weber OB, Muniz CR, Vitor AO, Freire FCO, Oliveira VM. Interaction of endophytic diazotrophic bacteria and Fusarium oxysporum f. sp cubense on plantlets of banana ‘Maca’. Plant Soil. 2007;298:47–56. [Google Scholar]
- Weihmann F, Eisermann I, Becher R, Krijger JJ, Hubner K, Deising HB, Wirsel SGR. Correspondence between symptom development of Colletotrichum graminicola and fungal biomass, quantified by a newly developed qPCR assay, depends on the maize variety. BMC Microbiol. 2016;16:14. doi: 10.1186/s12866-016-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52:487–511. doi: 10.1093/jexbot/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- Yasmin S, et al. Plant growth promotion and suppression of bacterial leaf blight in rice by inoculated bacteria. PLoS ONE. 2016;11:19. doi: 10.1371/journal.pone.0160688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Isawa T, Shinozaki S, Minamisawa K, Nakashita H. Effects of colonization of a bacterial endophyte, Azospirillum sp B510, on disease resistance in rice. Biosci Biotechnol Biochem. 2009;73:2595–2599. doi: 10.1271/bbb.90402. [DOI] [PubMed] [Google Scholar]
- Zhang T, Fang HHP. Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol. 2006;70:281–289. doi: 10.1007/s00253-006-0333-6. [DOI] [PubMed] [Google Scholar]