Abstract
Expending a considerable amount of physical energy inevitably leads to fatigue during both training and competition in football. An increasing number of experimental findings have confirmed the relationship between the generation and clearance of free radicals, fatigue, and exercise injury. Recently, hydrogen was identified as a new selective antioxidant with potential beneficial applications in sports. The present study evaluated the effect of 2-month consumption of hydrogen-rich water on the gut flora in juvenile female soccer players from Suzhou. As demonstrated by enzyme linked immunosorbent assay and 16S rDNA sequence analysis of stool samples, the consumption of hydrogen-rich water for two months significantly reduced serum malondialdehyde, interleukin-1, interleukin-6, tumour necrosis factor-α levels; then significantly increased serum superoxide dismutase, total antioxidant capacity levels and haemoglobin levels of whole blood. Furthermore, the consumption of hydrogen-rich water improved the diversity and abundance of the gut flora in athletes. All examined indices, including the shannon, sobs, ace, and chao indices, were higher in the control group than those proposed to result from hydrogen-rich water consumption prior to the trial, but these indices were all reversed and were higher than those in the controls after the 2-month intervention. Nevertheless, there were some differences in the gut flora components of these two groups before the trial, whereas there were no significant changes in the gut flora composition during the trial period. Thus, the consumption of hydrogen-rich water for two months might play a role modulating in the gut flora of athletes based on its selective antioxidant and anti-inflammatory activities. The study protocol was approved by the ethics committee of the Suzhou Sports School (approved number: SSS-EC150903).
Keywords: hydrogen-rich water, anti-oxidant, anti-inflammatory, gut flora, diversity, soccer player, Suzhou
INTRODUCTION
A number of studies have confirmed that the occurrence of exercise-induced fatigue is closely related to the level of oxidative stress in the body.1,2 The lipid peroxidative damage caused by the accumulation of free radicals in the body and the corresponding chain reaction are considered important factors responsible for decreased function of the body.3,4,5
The antioxidant capacity of professional athletes is much higher than that of ordinary people, and athletes develop a greater ability to withstand the accumulation of free radicals and oxidative damage generated in sports.6 However, there are still many problems regarding protection against and alleviation and removal of the oxidative stress reaction induced by free radical accumulation in the aftermath of exercise and sports. Currently, the effects of antioxidants used in exercise practice vary, and studies have indicated that some of these substances may induce more significant skeletal muscle injury in athletes.7,8,9 Therefore, the search for safe and effective selective antioxidants has become an important research endeavour.
The selective antioxidant activity of hydrogen was first reported in 2007 by Ohsawa et al.10 Thereafter, a significant number of studies confirmed that hydrogen-rich water, prepared by dissolving hydrogen in water, shows selective antioxidant activity. Currently, sports science researchers are paying increasing attention to the selective antioxidant, anti-inflammatory, and anti-apoptotic effects of hydrogen and its regulation of the alkalinizing environment of the body.11,12 The beneficial protective effect of hydrogen-rich water has gradually been confirmed in both animal and human experiments.
The human symbiotic gut flora, considered the body’s “second genome”, has significant effects on human health.11,12 In recent years, studies have confirmed that imbalance of the intestinal flora is directly related to oxidative stress.13,14 The results of human experiments on athletes have shown that a greater exercise intensity results in increased oxidative stress in the body and, thus, a higher incidence of gastrointestinal stress symptoms. Therefore, in the training process, athletes should drink a sufficient amount of selective antioxidant hydrogen-rich water to regulate their gut flora, which might have a protective effect on the gastrointestinal tract and reduce stress reactions.
PARTICIPANTS AND METHODS
Participants and grouping
Thirty-eight juvenile female football players from the Suzhou Sports School showing a healthy status and absence of sports injury, without any obvious food preference, and with no significant reported intake of nutritional supplements and antibiotics for 3 months were randomly divided into two groups: the control group (n = 10) and the hydrogen-rich water treatment group (n = 28) (Figure 1). Written informed consent was obtained from each participant prior to admission to the protocol, and the study protocol was approved by the ethics committee of the Suzhou Sports School (approved number: SSS-EC150903). This study follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines. During the experiment, the athletes in the hydrogen-rich water treatment group drank hydrogen-rich water in an amount equivalent to the amount of normal water they had previously consumed daily, while athletes in the control group continued to drink standard water in amounts consistent with their previous habits. The experiment lasted for 2 months. The basic information of the subjects is shown in Table 1.
Figure 1.
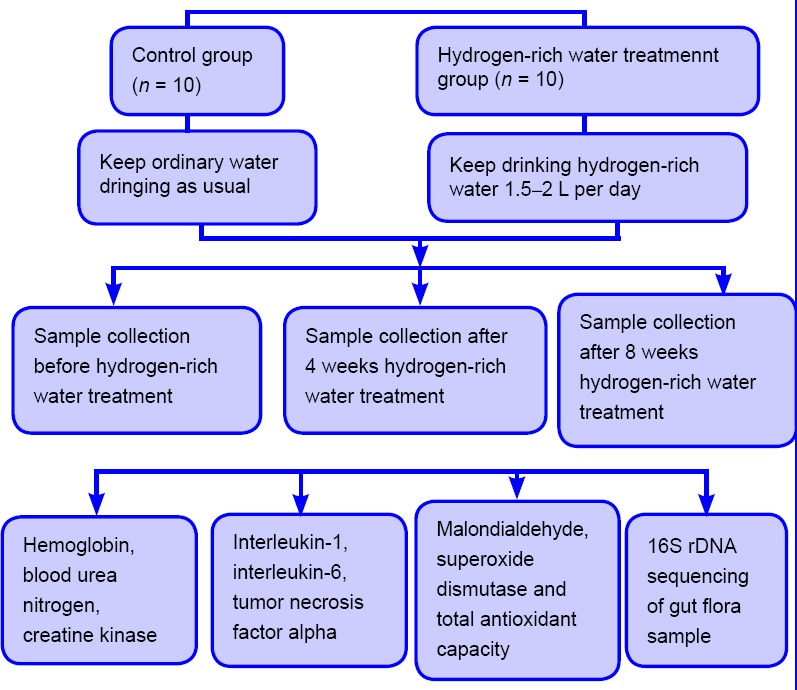
Trial flow chart.
Table 1.
Characteristics of all subjects
| Characteristics | Control group (n = 10) | Hydrogen-rich water treatment group (n = 28) |
|---|---|---|
| Age (year) | 13.7±1.06 | 12.18±0.86 |
| Height (cm) | 159.1±5.51 | 149.32±8.69 |
| Body weight (kg) | 48.97±4.56 | 40.15±7.56 |
| Training period (year) | 3.4±1.51 | 1.21±0.6 |
Note: Data as expressed as the mean ± SD.
Sample collection
During the experiment, the athletes followed their previous dietary and resting regimes and other aspects of their normal daily routine. The training content, exercise intensity, frequency of exercise, and other parameters were consistent with the routine training regimen of the athletes.
Blood sample test
We collected 5 mL samples of venous blood (fasting) from all 38 athletes at a predetermined time in the morning, and 100 μL of whole blood was sampled for the measurement of haematological parameters in a blood cell analyser. The remaining blood samples were centrifuged at 3000 × g for 5 minutes. The serum samples were then collected and analysed with an automatic biochemical analysis apparatus to determine hemoglobin (HGB), blood urea nitrogen (BUN) and creatine kinase (CK). Then, the serum samples were analysed for oxidative response indices (malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC)) and inflammatory indices (interleukin-1 (IL-1), interleukin-6 (IL-6), and tumour necrosis factor-alpha (TNF-α)) using enzyme linked immunosorbent assay.
16S rDNA sequencing analysis of gut flora samples
Faecal flora samples were collected from all 38 athletes according to the specifications for stool sampling and stored at –80°C. The subsequent DNA sample extraction and 16S rDNA sequencing analysis were performed with the assistance of the Novagene Genomics Institute.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The results were expressed as the mean ± SD. Significant differences between the two groups were analysed with repeated measured one-way analysis of variance, and the significance level was set at P < 0.05.
RESULTS
Effects of long-term consumption of hydrogen-rich water on routine indices of juvenile female football players
Hemoglobin
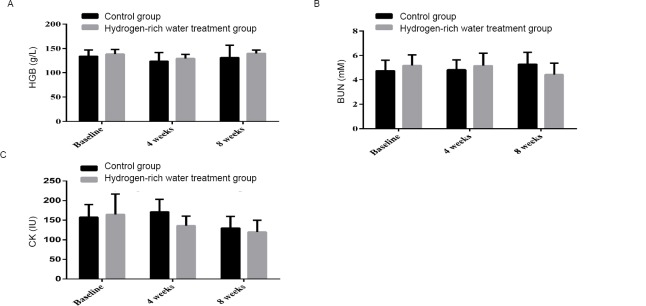
After 4 weeks, HGB decreased from 134.3 ± 12.95 g/L to 124.00 ± 17.75 g/L in the control group, while that in the hydrogen-rich water treatment group decreased from 138.74 ± 9.38 g/L to 129.59 ± 8.57 g/L. After 8 weeks, HGB increased from 124.00 ± 17.75 g/L to 131.6 ± 25.31 g/L in the control group, while that in the hydrogen-rich water treatment group increased from 129.59 ± 8.57 g/L to 139.89 ± 7.02 g/L (Figure 2A). The increasing trend and amplitude of HGB were more significant in the hydrogen-rich water treatment group (P = 0.032).
Figure 2.
Changes in HGB, BUN and CK before and after hydrogen-rich water consumption.
Note: (A) The shift of HGB before and after hydrogen-rich water consumption; (B) The shift of BUN before and after hydrogen-rich water consumption; (C) The shift of CK before and after hydrogen-rich water consumption. HGB: Hemoglobin; BUN: blood urea nitrogen; CK: creatine kinase.
Blood urea nitrogen
After 4 weeks, the level of BUN increased from 4.73 ± 0.88 to 4.83 ± 0.81 mM in the control group, while that in the hydrogen-rich water treatment group changed from 5.19 ± 0.85 to 5.17 ± 1.03 mM. After 8 weeks, the level of BUN in the control group continued to increase, from 4.83 ± 0.81 to 5.29 ± 0.97 mM, while that in the hydrogen-rich water treatment group decreased from 5.17 ± 1.03 to 4.42 ± 0.95 mM (Figure 2B). There was a more distinct difference between the two groups (P = 0.887).
Creatine kinase
After 4 weeks, CK in the control group increased from 157.3 ± 17.37 to 171.3 ± 31.96 IU, while that in the hydrogen-rich water treatment group decreased from 149.3 ± 30.43 to 135.85 ± 24.44 IU (Figure 2C). After 8 weeks, CK decreased from 171.3 ± 31.96 to 129.7 ± 30.05 IU in the control group and from 135.85 ± 24.44 to 119.85 ± 29.93 IU in the hydrogen-rich water treatment group (P = 0.061).
Compared with HGB and BUN, CK was more sensitive to changes in the exercise load. These results suggest that the hydrogen-rich water treatment exerted a somewhat effect to enhance the whole blood HGB level of the athletes.
Effects of long-term consumption of hydrogen-rich water on oxidative response indices of juvenile female football players
Malondialdehyde
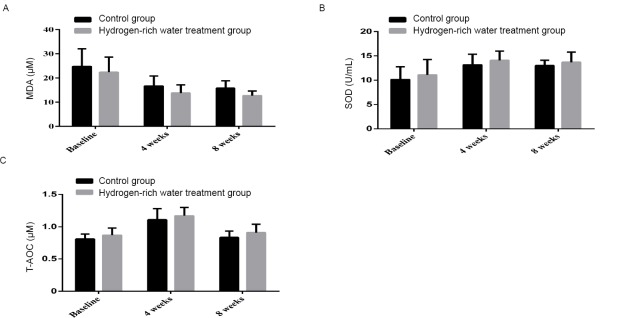
After 4 weeks, serum MDA decreased from 24.77 ± 7.32 to 16.67 ± 4.19 μM in the control group, while that decreased from 22.39 ± 6.20 to 13.80 ± 3.33 μM in the hydrogen-rich water treatment group. After 8 weeks, serum MDA changed from 16.67 ± 4.19 to 15.79 ± 3.07 μM in the control group and from13.80 ± 3.33 to 12.69 ± 1.94 μM in the hydrogen-rich water treatment group, with significant differences being observed between the two groups (P = 0.000; Figure 3A).
Figure 3.
Changes in MDA, SOD and T-AOC before and after hydrogen-rich water consumption.
Note: (A) The shift of MDA before and after hydrogen-rich water consumption; (B) The shift of SOD before and after hydrogen-rich water consumption; (C) The shift of T-AOC before and after hydrogen-rich water consumption. MDA: Malondialdehyde; SOD: superoxide dismutase; T-AOC: total antioxidant capacity.
Superoxide dismutase
After 4 weeks, the serum SOD level increased from 10.14 ± 2.60 to 13.14 ± 2.18 U/mL in the control group and from 11.09 ± 3.17 to 14.07 ± 1.91 U/mL in the hydrogen-rich water treatment group. After 8 weeks, the serum SOD level in the control group decreased from 13.14 ± 2.18 to 13.01 ± 1.08 U/mL, while that in the hydrogen-rich water treatment group decreased from 14.07 ± 1.91 to 13.69 ± 2.10 U/mL, with significant differences between the two groups (P = 0.027; Figure 3B).
Total antioxidant capacity
After 4 weeks, serum T-AOC increased from 0.8 ± 0.08 to 1.11 ± 0.17 μM in the control group, while serum T-AOC in the hydrogen-rich water treatment group changed from 0.87 ± 0.11 to 1.17 ± 0.13 μM. After 8 weeks, T-AOC changed from 1.17 ± 0.13 to 0.84 ± 0.09 μM in the control group and from 1.17 ± 0.13 to 0.9 ± 0.13 μM in the hydrogen-rich water treatment group, with significant differences between the two groups (P = 0.004, Figure 3C).
These results suggest that the hydrogen-rich water treatment exerted an anti-oxidative effect.
Effects of long-term consumption of hydrogen-rich water on inflammatory indices of juvenile female football players
Interleukin-1
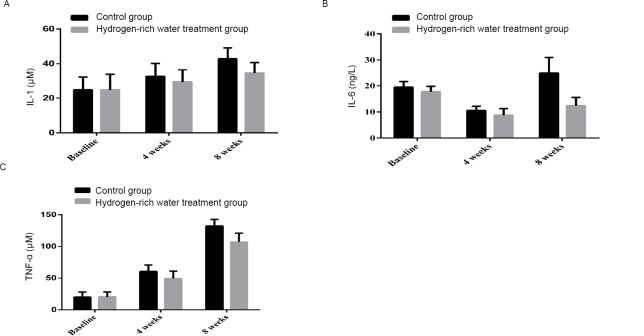
After 4 weeks, the level of serum IL-1 in the control group increased from 24.77 ± 7.32 to 32.56 ± 7.61 μM, and that in the hydrogen-rich water treatment group increased from 24.79 ± 8.94 to 29.32 ± 7.09 μM. After 8 weeks, the IL-1 level increased from 32.56 ± 7.61 to 42.94 ± 6.24 μM in the control group and from 29.32 ± 7.09 μM to 34.47 ± 6.22 μM in the hydrogen-rich water treatment group, with significant differences between the two groups (P = 0.002, Figure 4A).
Figure 4.
Changes in IL-1, IL-6 and TNF-α before and after hydrogen-rich water consumption.
Note: (A) The shift of IL-1 before and after hydrogen-rich water consumption; (B) The shift of IL-6 before and after hydrogen-rich water consumption; (C) The shift of TNF-α before and after hydrogen-rich water consumption. IL: Interleukin; TNF-α: tumour necrosis factor alpha.
Interleukin-6
After 4 weeks, the level of serum IL-6 decreased from 19.48 ± 2.16 to 10.53 ± 1.62 ng/L in the control group and from 17.72 ± 2.1 to 8.74 ± 2.57 ng/L in the hydrogen-rich water treatment group. After 8 weeks, the level of serum IL-6 in the control group increased from 10.53 ± 1.62 ng/L to 24.88 ± 6.11 ng/L, while that in the hydrogen-rich water treatment group increased from 8.74 ± 2.57 to 12.37 ± 3.2 ng/L, with significant differences between the two groups (P = 0.000, Figure 4B).
Tumour necrosis factor-α
After 4 weeks, serum TNF-α increased from 20.04 ± 7.99 to 60.57 ± 10.09 μM in the control group and increased from 20.44 ± 7.75 to 49.46 ± 11.59 μM in the hydrogen-rich water treatment group. After 8 weeks, serum TNF-α increased from 60.57 ± 10.09 to 132.24 ± 10.46 μM in the control group and from 49.46 ± 11.59 to 107.00 ± 13.89 μM in the hydrogen-rich water treatment group, with significant differences between the two groups (P = 0.000, Figure 4C).
These results suggest that the hydrogen-rich water treatment exerted an anti-inflammatory effect.
Effects of long-term consumption of hydrogen-rich water on gut flora components of juvenile female football players
Classification by phylum
In the samples collected from the athletes after pre-treatment with hydrogen-rich water, the number of Actinobacteria in the control group was higher than that in the treatment group, and the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group. Moreover, the number of Clostridia in the control group was slightly higher than that in the hydrogen-rich water treatment group. However, there were no significant differences in the numbers of these bacterial groups after 2 months of hydrogen-rich water treatment.
Classification by class
In samples collected from the athletes after pre-treatment with hydrogen-rich water, the number of Actinobacteria in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group, and the numbers of Clostridia, Coriobacteria, and Erysipelotrichia in the control group were higher than those in the hydrogen-rich water treatment group. However, there was no significant difference in the numbers of these bacterial groups after 2 months of hydrogen-rich water treatment.
Classification by order
In samples collected from the athletes after pre-treatment with hydrogen-rich water, the number of Actinobacteria in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group, and the numbers of Clostridia and Coriobacteria in the control group were higher than those in the hydrogen-rich water treatment group. The number of Erysipelotrichia in the control group was higher than that in the hydrogen-rich water treatment group, although this difference was not significant. Nevertheless, there were no significant differences in the numbers of related bacteria after 2 months of hydrogen-rich water treatment.
Classification by family
In samples collected from the athletes after pre-treatment with hydrogen-rich water, the numbers of Acidaminococcaceae, Bacteriodaceae, Bifidobacteriaceae, Coriobacteriaceae, Desulforibrionaceae, Erysipelotrichaceae and Ruminococcaceae were higher than those in the hydrogen-rich water treatment group, with differences being observed in the number of Bifidobacteriaceae, Ruminococcaceae, Coriobacteriaceae and Erysipelotrichaceae. There was no difference in the number of Lachnospiraceae between the two groups. The number of Prevotellaceae in the hydrogen-rich water treatment group was higher than that in the control group. However, there were no significant differences in the number of these bacterial groups after 2 months of hydrogen-rich water treatment.
Classification by genus
In samples collected from the athletes after pre-treatment with hydrogen-rich water, the numbers of Bifidobacterium and Oscillibacter in the control group were higher than those in the hydrogen-rich water treatment group, with a difference being observed in the number of Bifidobacteriaceae. The number of Prevotella in the hydrogen-rich water treatment group was higher than that in the control group, although this difference was not significant. There were no significant differences in the number of these bacterial groups after 2 months of hydrogen-rich water treatment.
Effects of long-term consumption of hydrogen-rich water on gut flora diversity and abundance in juvenile female football players
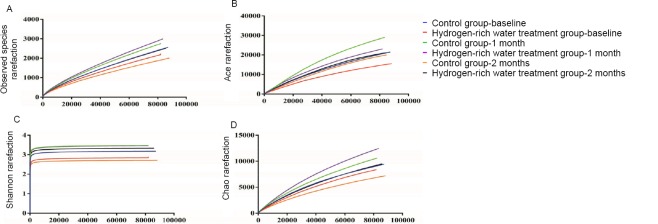
The actual number of operational taxonomic units (sobs) and the ace, chao and shannon indices were determined, and then a dilution curve was drawn. The recorded changes indicated that the sobs, ace, chao and shannon indices of the control group were all higher than those of the hydrogen-rich water treatment group, suggesting that the abundance and diversity of the gut flora in the control group were higher than those in the hydrogen-rich water treatment group.
After 1 month of hydrogen-rich water treatment, the sobs, ace, and chao indices were higher in the hydrogen-rich water treatment group than those in the control group. The trend was slightly reversed, indicating that the abundance of gut flora was higher in the hydrogen-rich water treatment group than in the control group. The shannon index of the treatment group at that time was essentially the same as that in the control group, indicating that treatment with hydrogen-rich water could also enhance the diversity of the gut flora. After 2 months of hydrogen-rich water treatment, the sobs, ace, chao and shannon indices were much higher than those in the control group (P = 0.479, P = 0.710, P = 0.369, P = 0.369). Indicating that treatment with hydrogen-rich water can enhance the gut flora abundance and diversity of the gut flora (Figure 5).
Figure 5.
Changes in gut flora diversity and abundance before and after hydrogen-rich water consumption.
Note: (A) The shift of sobs before and after hydrogenrich water consumption; (B) The shift of ace index before and after hydrogen-rich water consumption; (C) The shift of chao index before and after hydrogen-rich water consumption; (D) The shift of shannon index before and after hydrogen-rich water consumption.
DISCUSSION
The existing experimental and clinical studies have shown that animals or humans need only to breathe hydrogen or drink or inject hydrogen-rich water to protect the heart, brain, liver, kidney, lung, and small intestine from ischaemia/reperfusion oxidative injury or inflammatory injury after cardiac organ transplantation.15,16
The potential biological effects of hydrogen in sports have drawn much attention from researchers in sports science. The beneficial protective effects of hydrogen-rich water on the body have been gradually confirmed in both animal and human experiments. Ostojic summarized the current applications of hydrogen in the field of sports, emphasizing that hydrogen 1) can effectively remove a large number of harmful free radicals generated through movement, thus enhancing the antioxidant capacity; 2) is an effective alkalizing agent in the internal environment that can effectively inhibit blood acidification induced by lactic acid accumulation in sports; and 3) is an important gas signalling molecule that can participate in physiological regulatory processes such as anti-inflammatory, anti-apoptotic, and anti-autophagy processes.17,18 This regulation does not involve the same signalling pathway as antioxidative stress.
Analysis of the effect of long-term consumption of hydrogen-rich water on routine indices of juvenile female football players
HGB is one of the classic indicators reflecting the level of endurance exercise. The shift of HGB after 4 weeks was caused by increases in the amount or intensity of exercise and seasonal factors during winter training. The HGB level began to gradually increase, suggesting that the athletes had adapted well to the winter training load. The increase in the HGB level was higher overall in the hydrogen-rich water treatment group suggested that long-term hydrogen-rich water treatment could help increase the HGB level.
Urea nitrogen is the final product of protein metabolism. The participation of protein catabolism in the energy supply is enhanced during long-term and high-intensity exercise, thus increasing the amount of urea nitrogen in the blood and urine with increased decomposition of proteins and amino acids. The shift of the BUN level of all 38 athletes increased slightly due to winter training and seasonal factors. After 8 weeks, the decrease in the serum urea nitrogen level and the increase in the HGB level indicated that long-term hydrogen-rich water treatment has beneficial effects on the physiological functions of athletes.
CK is the key enzyme in energy metabolism in skeletal muscle cells, whose activity directly affects the short-term maximum intensity of the exercise capacity. After a high-intensity muscle load, muscle soreness and serum CK levels are highly and positively correlated. Clarke et al.37 found that the level of CK in the serum of professional rugby athletes is markedly high. CK is an important index reflecting the exercise load, particularly that suffered by the skeletal muscle. Thus, CK could indirectly reflect the levels of injury and active repair of the skeletal muscle ultrastructure.
After 8 weeks, the level of serum CK in both the control and hydrogen-rich water treatment groups continued to decrease.
Analysis of the effect of long-term consumption of hydrogen-rich water on the serum oxidative response of juvenile female football players
Tsubone et al.19 compared the effects of drinking hydrogen-rich water on the levels of oxidative stress and antioxidant metabolites in the serum of British thoroughbred horses and found that hydrogen-rich water treatment had a beneficial antioxidant effect. Aoki et al.20 conducted studies on football players and showed that drinking hydrogen-rich water for 1 week could reduce exercise fatigue and lactic acid accumulation after exercise but had no significant effect on the oxidative response index.
Li et al.21 showed that hydrogen-rich water could significantly prolong the duration of exercise before exhaustion in rats and improve their exercise capacity, indicating a significant anti-fatigue effect. Zhao and Zhang22 showed that supplementation of hydrogen-rich water at different times before, during, and after exercise exerted significant protective effects against oxidative stress injury in swimming athletes during high-intensity exercise. This supplementation of hydrogen-rich water can reduce the production of excessive free radicals and enhance the activity of antioxidant enzymes and the antioxidant capacity of the body, thereby promoting physical recovery after high-intensity exercise. Hu and Zhang23 showed that high-intensity intermittent training increases the concentration of O2 –, •OH and H2O2. Hydrogen-rich water can significantly enhance the body’s inhibition of O2 – and •OH, showing a higher rate of •OH inhibition, fully reflecting its selective antioxidant effect. Li et al.24 found that hydrogen-rich water treatment could effectively reduce oxidative stress injury induced in skeletal muscle by severe exercise while improving the muscle ultrastructure. Wang et al.25 reported that hydrogen-rich water treatment could up-regulate the expression of SIRT3, enhance the activity of antioxidant enzymes, and reduce the inflammatory response after centrifugal exercise.
MDA is one of the classic indicators reflecting the level of lipid peroxidation. After 8 weeks, the difference of serum MDA between the two groups was significant, it suggested that long-term hydrogen-rich water treatment exerts an antioxidant effect.
SOD is one of the classic indicators reflecting the free radical-scavenging antioxidant capacity. The SOD levels of both the control and hydrogen-rich water treatment groups slightly increased after 4 weeks. And the mean serum SOD level of the hydrogen-rich water treatment group was consistent higher than the control group after 8 weeks.
Serum antioxidant substances can be divided into the enzymatic antioxidant system and the non-enzymatic antioxidant system. The enzymatic antioxidant system mainly involves substances such as SOD, glutathione peroxidase, glutathione reductase, and catalase. The non-enzymatic antioxidant system mainly involves water-soluble substances, such as vitamin C, bilirubin, fat-soluble vitamin E, coenzyme Q, carotenoids, and flavonoid antioxidants. In terms of their function, serum antioxidant substances can be divided into three types: preventive antioxidants; capture-type antioxidants; and repair and regeneration antioxidants. The total antioxidant capacity represents the sum of the above substances and functions.
The observed changes in serum T-AOC suggested that 4 weeks of hydrogen-rich water treatment group indeed improved the free radical-scavenging ability of antioxidants. These results suggest that long-term hydrogen-rich water treatment exerts an antioxidant effect.
Analysis of the effect of long-term consumption of hydrogen-rich water on serum inflammatory indices of juvenile female football players
Inflammatory factors will increase, and inflammation will intensify during exercise due to increases in energy consumption, free radicals, and intensification of oxidative stress. However, there are three anti-inflammatory mechanisms that may be deployed in the course of exercise. 1) Exercise can increase energy consumption, thereby reducing visceral fat volume and alleviating the infiltration of fat into inflammatory lymphocytes. 2) Exercise can effectively increase the production and release of muscle-derived anti-inflammatory cytokines during skeletal muscle contraction; skeletal muscle accounts for 35–45% of the total body weight, and the regulatory effects of this major endocrine organ on human homeostasis cannot be ignored. 3) Exercise can effectively reduce toll-like receptor expression on the membrane surface of monocytes and macrophages, which may lead to a decreased downstream response, including reduced secretion of inflammatory agents, decreased expression of compatibility complexes in major organs, and decreases in co-stimulatory Mecules.26,27 These three effects can ensure that the levels of inflammatory agent factors in athletes participating in strenuous exercise will not increase and may even decrease. However, the effect of oxidative stress on the body will not be weakened. After 8 weeks of hydrogen-rich water treatment, the levels of IL-1, IL-6 and TNF-α in the hydrogen-rich water treatment group were lower than those in the control group and with significant differences between the two groups. Compared with the abovementioned changes in the oxidative stress indices, long-term hydrogen-rich water treatment showed a stronger anti-inflammatory effect in addition to an antioxidant effect.
Analysis of the effect of long-term consumption of hydrogen-rich water on gut flora components of juvenile female football players
Analysis of the structural components of the gut flora at different levels of classification in the two groups showed some differences between the two groups at different stages of the experiment. However, there were no significant changes in the structural components of flora between the two groups in terms of the oxidative response and the anti-inflammatory effect. These results suggest that two months of hydrogen-rich water treatment did not significantly change the structural components of the gut flora of the juvenile female football players. Differences in the composition of the flora between the two groups are an expected result of differences in age, particularly regarding the number of training years.
In 2007, Ohsawa et al.10 suggested that the selective antioxidant activity of hydrogen-rich water, and particularly its selective elimination of •OH, is superior to that of traditional antioxidants, while its overall antioxidant capacity is much lower than that of traditional antioxidants. Therefore, the effect of 2 months of hydrogen-rich water treatment on the regulation of gut flora was also much lower than that of established supplements such as resveratrol, grape antioxidant dietary fibre, selenium supplements, anthocyanin, and pomegranate peel polyphenols.28,29,30,31,32,33
Analysis of the effect of long-term consumption of hydrogen-rich water on gut flora diversity and abundance in juvenile female football players
As a complex and variable micro-ecological system, the gut flora is constantly undergoing changes in its dynamic equilibrium. The richness and diversity of its components are important indicators of the health of this ecological system.34 The richness of the gut flora in patients with inflammatory bowel disorder is decreased in elderly and obese individuals.35 Le Chatelier et al.36 compared the composition of the gut flora of 123 non-obese and 169 obese Danes and found that the gut flora richness of these two groups differed, as did the number of genes in their gut flora. Individuals with lower gut flora richness were found to exhibit more significant obesity characteristics, insulin resistance, and lipid metabolic disorders as well as more severe inflammatory phenotypes.35,36
As a strong stressor, long-term and high-intensity professional sports training eventually has a corresponding impact on the gut flora. Clarke et al.37 found that professional rugby athletes exhibited a more abundant gut flora in their intestines compared with control groups of individuals with a body mass index (BMI) < 25 or BMI > 28. In samples from the professional rugby athletes, the total microorganisms identified came from 22 phyla, 68 families, and 113 genera. In the control group with a BMI < 25, a total of 11 phyla, 33 families, and 65 genera of microorganisms were detected, whereas the microorganisms in the control group with a BMI > 28 came from 9 phyla, 33 families, and 61 genera. The richness and diversity of the gut flora were lowest in obese individuals, while the professional athletes exhibited the highest richness and diversity levels.
Before treatment with hydrogen-rich water, the richness and diversity of the gut flora were higher in the control group (3.4 ± 1.51 years of training) than in the treatment group (1.21 ± 0.6 years of training), and the training period was the main factor leading to this difference. Individuals who had a longer training period exhibited a higher richness and diversity in their gut flora; this trend is consistent with the results of Clarke et al.37
After 4 weeks of treatment with hydrogen-rich water, the trend was slightly reversed. The richness and diversity of the gut flora were higher in athletes who had a shorter training period than those who had a longer training period. This finding indicated that drinking hydrogen-rich water for a long period of time may plays an important role in enhancing the richness and diversity of the gut flora. At the same time, the levels of serum MDA, IL-1, IL-6 and TNF-α decreased in the treatment group, and the SOD, T-AOC level increased. Such changes are closely related to changes in the richness and diversity of the gut flora.
After 8 weeks of treatment with hydrogen-rich water, the richness and diversity of the gut flora were still higher in athletes who had a shorter training period than in control individuals who had a longer training. Additionally, the serum levels of MDA, IL-1, IL-6 and TNF-α decreased, and the levels of HGB SOD, T-AOC level increased to various degrees in the hydrogen-rich water treatment group. The trend of favourable changes of motor function indices, the oxidative response index, and inflammatory factor indices were almost consistent with the changes in the richness and diversity of the gut flora.
The above results showed that long-term consumption of hydrogen-rich water not only exerts certain antioxidant and anti-inflammatory effects but also enhances the diversity and abundance of the gut flora of the subjects.
Footnotes
Funding: The study was supported by the National Basic Research Project of China (973 Program), No. 2012CB518200 (to ZCG), the General Program of the Natural Science Foundation of China, No. 81371232, 81573251 (to ZCG), and the Special Key Programs for Science and Technology of China, No. 2012ZX09102301-016 and 2014ZX09J14107-05B (to ZCG).
Conflicts of interest
There is no conflict of interest.
Financial support
The study was supported by The National Basic Research Project of China (973 Program), No. 2012CB518200, the General Program of the Natural Science Foundation of China, No. 81371232, 81573251, and the Special Key Programs for Science and Technology of China, No. 2012ZX09102301-016, 2014ZX09J14107-05B.
Institutional review board statement
The institutional review board approval of Suzhou Sports School was obtained for this study.
Declaration of participant consent
The authors certify that they have obtained participant consent forms. In the form, participants have given their consent for their images andother clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement
This study follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of the State Key Laboratory of Proteomics, Cognitive and Mental Health Research Center, Beijing, China.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 2.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Cobley JN, McHardy H, Morton JP, Nikolaidis MG, Close GL. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic Biol Med. 2015;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Pingitore A, Pereira Lima GP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–922. doi: 10.1016/j.nut.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Slattery K, Bentley D, Coutts AJ. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015;45:453–471. doi: 10.1007/s40279-014-0282-7. [DOI] [PubMed] [Google Scholar]
- 7.Mankowski RT, Anton SD, Buford TW, Leeuwenburgh C. Dietary antioxidants as modifiers of physiologic adaptations to exercise. Med Sci Sports Exerc. 2015;47:1857–1868. doi: 10.1249/MSS.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAnulty LS, Miller LE, Hosick PA, Utter AC, Quindry JC, McAnulty SR. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl Physiol Nutr Metab. 2013;38:760–765. doi: 10.1139/apnm-2012-0455. [DOI] [PubMed] [Google Scholar]
- 9.Carrera-Quintanar L, Funes L, Vicente-Salar N, et al. Effect of polyphenol supplements on redox status of blood cells: a randomized controlled exercise training trial. Eur J Nutr. 2015;54:1081–1093. doi: 10.1007/s00394-014-0785-x. [DOI] [PubMed] [Google Scholar]
- 10.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L. Genomics: The tale of our other genome. Nature. 2010;465:879–880. doi: 10.1038/465879a. [DOI] [PubMed] [Google Scholar]
- 12.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 13.Espley RV, Butts CA, Laing WA, et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144:146–154. doi: 10.3945/jn.113.182659. [DOI] [PubMed] [Google Scholar]
- 14.Placha I, Chrastinova L, Laukova A, et al. Effect of thyme oil on small intestine integrity and antioxidant status, phagocytic activity and gastrointestininal microbita in rabbits. Acta Vet Hung. 2013;61:197–208. doi: 10.1556/AVet.2013.012. [DOI] [PubMed] [Google Scholar]
- 15.Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 16.Noda M, Fujita K, Ohsawa I, Ito M, Ohno K. Multiple effects of Mecular hydrogen and its distinct mechanism. J Neurol Disord. 2014;2:1–8. [Google Scholar]
- 17.Ostojic SM, Stojanovic MD. Hydrogen-rich water affected blood alkalinity in physically active men. Res Sports Med. 2014;22:49–60. doi: 10.1080/15438627.2013.852092. [DOI] [PubMed] [Google Scholar]
- 18.Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–279. doi: 10.1055/s-0034-1395509. [DOI] [PubMed] [Google Scholar]
- 19.Tsubone H, Hanafusa M, Endo M, et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J Equine Sci. 2013;24:1–8. doi: 10.1294/jes.24.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. doi: 10.1186/2045-9912-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li A, Zhang L, Zhou J, Sun X. Effects of supplementation of hydrogen-rich water on the oxidative stress-induced damage in skeletal muscle after acute exhaustive exercise. Zhongguo Yundong Yixue. 2011;30:452–455. [Google Scholar]
- 22.Zhao YY, Zhang L. Master Dissertation. Suzhou: Schoow University; 2014. The influence of drinking hydrogen-rich water at different phase and high-intensity exercise to swimming athletes in free radical metabolism. [Google Scholar]
- 23.Hu J, Zhang L. Master Dissertation. Suzhou: Schoow University; 2014. A comparative research of the influence of different antioxidants on antioxidant system about the short distance swimmer. [Google Scholar]
- 24.Li C, Li CX, Pang L, Wu L. Hydrogen-rich water on oxidative stress injury in rat skeletal muscle after exhaustive exercise was repeated. Taishan Yixueyuan Xuebao. 2015;36:371–375. [Google Scholar]
- 25.Wang L, Liu ZQ, Hou YL, Ge YJ. Hydrogen-rich water inhibits mitochondrial oxidative stress and inflammation in the skeletal muscle after eccentric exercise. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:4682–4687. [Google Scholar]
- 26.Ji LL, Zhang Y. Antioxidant and anti-inflammatory effects of exercise: role of redox signaling. Free Radic Res. 2014;48:3–11. doi: 10.3109/10715762.2013.844341. [DOI] [PubMed] [Google Scholar]
- 27.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 28.Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Sun J, Li X, et al. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr Res. 2013;33:971–981. doi: 10.1016/j.nutres.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Jose Pozuelo M, Agis-Torres A, Hervert-Hernandez D, et al. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. J Food Sci. 2012;77:H59–H62. doi: 10.1111/j.1750-3841.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 31.Kasaikina MV, Kravtsova MA, Lee BC, et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25:2492–2499. doi: 10.1096/fj.11-181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakobsdottir G, Blanco N, Xu J, et al. Formation of short-chain Fatty acids, excretion of anthocyanins, and microbial diversity in rats fed blackcurrants, blackberries, and raspberries. J Nutr Metab. 2013;2013:202534. doi: 10.1155/2013/202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neyrinck AM, Van Hee VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr. 2013;109:802–809. doi: 10.1017/S0007114512002206. [DOI] [PubMed] [Google Scholar]
- 34.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 35.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 36.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 37.Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]