Keywords: nerve regeneration, transposition repair, median nerve, functional remodeling, muscle atrophy, surgical intervention, peripheral nerve injury, neural regeneration
Abstract
Our previous studies have confirmed that during nerve transposition repair to injured peripheral nerves, the regenerated nerve fibers of motor neurons in the anterior horn of the spinal cord can effectively repair distal nerve and target muscle tissue and restore muscle motor function. To observe the effect of nerve regeneration and motor function recovery after several types of nerve transposition for median nerve defect (2 mm), 30 Sprague-Dawley rats were randomly divided into sham operation group, epineurial neurorrhaphy group, musculocutaneous nerve transposition group, medial pectoral nerve transposition group, and radial nerve muscular branch transposition group. Three months after nerve repair, the wrist flexion test was used to evaluate the recovery of wrist flexion after regeneration of median nerve in the affected limbs of rats. The number of myelinated nerve fibers, the thickness of myelin sheath, the diameter of axons and the cross-sectional area of axons in the proximal and distal segments of the repaired nerves were measured by osmic acid staining. The ratio of newly produced distal myelinated nerve fibers to the number of proximal myelinated nerve fibers was calculated. Wet weights of the flexor digitorum superficialis muscles were measured. Muscle fiber morphology was detected using hematoxylin-eosin staining. The cross-sectional area of muscle fibers was calculated to assess the recovery of muscles. Results showed that wrist flexion function was restored, and the nerve grew into the distal effector in all three nerve transposition groups and the epineurial neurorrhaphy group. There were differences in the number of myelinated nerve fibers in each group. The magnification of proximal to distal nerves was 1.80, 3.00, 2.50, and 3.12 in epineurial neurorrhaphy group, musculocutaneous nerve transposition group, medial pectoral nerve transposition group, and radial nerve muscular branch transposition group, respectively. Nevertheless, axon diameters of new nerve fibers, cross-sectional areas of axons, thicknesses of myelin sheath, wet weights of flexor digitorum superficialis muscle and cross-sectional areas of muscle fibers of all three groups of donor nerves from different anterior horn motor neurons after nerve transposition were similar to those in the epineurial neurorrhaphy group. Our findings indicate that donor nerve translocation from different anterior horn motor neurons can effectively repair the target organs innervated by the median nerve. The corresponding spinal anterior horn motor neurons obtain functional reinnervation and achieve some degree of motor function in the affected limbs.
Chinese Library Classification No. R459.9; R361; R61
Introduction
In peripheral nerve injury caused by trauma, upper limb nerve injury accounts for approximately 73.5% of cases (Kouyourmdjian, 2006), in which the median nerve is a commonly affected nerve (Noble et al., 1998). Nearly half of patients with median nerve damage lost their ability to work because they do not receive timely and effective treatment (Jaquet et al., 2001). At present, the most effective way to treat peripheral nerve injury is tension-free epineurial neurorrhaphy in situ at the nerve stump (Griffin et al., 2013; Quan et al., 2017). However, trauma often causes too much damage of the peripheral nerve, so it is difficult to achieve epineurial neurorrhaphy (Geuna et al., 2017). Clinical experts suggest that nerve transplantation (with autologous nerve, allogeneic nerve or artificial nerve) and nerve autologous lengthening can be used to repair peripheral nerve injury with such damage. However, when the length of nerve damage is too long or there is root avulsion injury, neither of the above methods is very satisfactory (Kubota et al., 2011; Brooks et al., 2012; Kehoe et al., 2012; Rebowe et al., 2018). A more successful treatment is to use nerve translocation repair. Selecting donor nerves with good connections to the central nervous system to repair and innervate the disabled limbs can not only form a functional reflex arc with the central nervous system, but also reduce the occurrence of complications. Based on this theory, nerve transposition repair has been used in clinical practice.
Doctors performed transposition using small nerves near the affected limb nerve. Ideally, a nerve branch, which does not have an important function, or a proximal nerve that has lost its effector in trauma have been used to repair the innervating nerves of the injured distal nerve and paralyzed limb. When successful, function was restored to the affected limb (Hua et al., 2015). Wang et al. (2007) found that the detrusor muscle, originally innervated by the parasympathetic nerve, could be innervated by a somatic nerve. He successfully established rat models with an artificial somatic-autonomic reflex arc that retained a degree of function of the detrusor. Xiao et al. (2005) confirmed that this method of nerve transposition repair is an effective and safe method for the treatment of bladder self-control and bladder dysfunction in spina bifida patients in clinical trials. Lumbar-sacral nerve re-routing reconstruction was achieved by lumbar and sacral nerve root transposition to control the autonomic nerve of the bladder of lower limb paraplegia patients (Lam Van Ba et al., 2018). Kotoni et al. (1971) used transposition of nerve fibers on the uninjured side to repair avulsion injury of the nerve root on the surface of the spinal cord or of posterior ganglionic injury near the vertebral foramen. Academician Gu Yudong applied cervical 7 nerve root transposition repair on the uninjured side to limb hemiplegia caused by central nervous system diseases (Zheng et al., 2018). Pediatric clinicians used different nerve transposition repair methods to treat birth paralysis, and recovered limb function to varying degrees (Little et al., 2014; Pondaag et al., 2014; Ghanghurde et al., 2016; Murison et al., 2017). Kawabata et al. (2001) used the intercostal nerve to reconstruct the affected brachial plexus to treat birth paralysis caused by fetal weight and breech delivery. These transposition repair methods can restore the function of affected limbs or target organs to a certain extent, suggesting that nerve transposition can be widely used as a clinical tool.
The main purpose of clinical treatment of peripheral nerve injury is to restore the motor function of the damaged nerves. The common feature of all transposition repair methods is the use of different motor neurons in the anterior horn of the spinal cord to achieve new motor innervation. Therefore, it could be said that the functional reinnervation of motor neurons in the anterior horn of the spinal cord is the most important basis and prerequisite for the repair of nerve transposition. Previously we confirmed that motor neurons in the anterior horn of the spinal cord could effectively form new functional reinnervation when the proximal and the distal ends of common peroneal nerve and tibial nerve was simultaneously repaired (Yu et al., 2016). On the basis of that research, this study repaired damaged median nerve using donor nerve transposition from different motor neurons in the anterior horn of the spinal cord. We then explored the reinnervation after the change in function of the anterior horn cells of the spinal cord.
Materials and Methods
Experimental animals
Thirty specific-pathogen-free male Sprague-Dawley rats aged 6–8 weeks and weighing 200–220 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license no. SCXK(Jing)2016-0006). Animal model production and experimental animal feeding were conducted in the specific-pathogen-free environment of the Animal Center of Peking University People’s Hospital, China, at relative humidity of 40% in the 12-hour light/dark cycle. All protocols were approved by the Animal Ethics Committee of Peking University People’s Hospital (approval No. 2015-50) on December 9, 2015. All rats received gas anesthesia with isoflurane 2.5% 100 mL/min (RWD Life Science, Shenzhen, China) to minimize the post-operative pain.
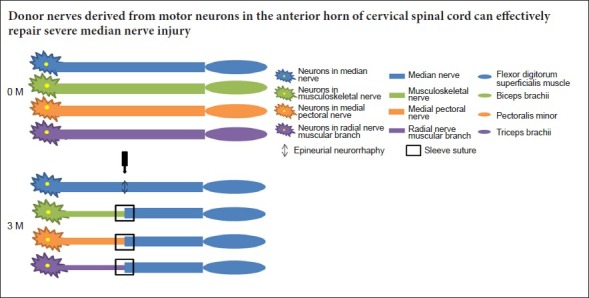
The rats were randomly divided into five groups (n = 6): sham operation group, epineurial neurorrhaphy group (epineurial neurorrhaphy for median nerve in situ), musculocutaneous nerve transposition group (musculocutaneous nerve transposition for repairing median nerve), medial pectoral nerve transposition group (medial pectoral nerve transposition for repairing median nerve), and radial nerve muscular branch transposition group (radial nerve muscular branch transposition for repairing median nerve).
Nerve repair
After anesthesia, the inner skin of the forearm of the left upper limb of the rat was cut and reflected to reveal the muscle. The intermuscular space was bluntly separated to expose the branch of the left brachial plexus. The median nerve and the muscular branches of the musculocutaneous nerve, medial pectoral nerve or radial nerve were bluntly separated according to experimental group. In the sham operation group, after washing with saline, the wound was sutured layer by layer and the wound was closed. In the epineurial neurorrhaphy group, the median nerve was denervated at 5–7 mm from the brachial plexus bifurcation, and then sutured with 10-0 microneedle suture without tension in situ. In the musculocutaneous nerve transposition group, the musculocutaneous nerve and median nerve were denervated at the same level, and the distal end of the musculocutaneous nerve and the proximal end of the median nerve were reflected back on themselves and sutured to an adjacent muscle with 7-0 microneedle suture after ligation. The top of the conical deacetylated chitin sleeve (Patent number: ZL01 136314.2) (Zhang et al., 2008) (inner diameter at the top of 1 mm; the inner diameter at the bottom of 1.5 mm, and the wall thickness of 0.3 mm) was connected to the proximal end of musculocutaneous nerve. Its bottom was connected to the distal end of median nerve, and fixed with 10-0 sutures, leaving a 2 mm gap between the nerves. The procedure and materials used in the medial pectoral nerve transposition and the radial nerve muscular branch transposition groups were the same as those in the musculocutaneous nerve transposition group, only the donor nerve was different (Figure 1). All experimental animals were kept in routine conditions after surgery. General conditions were observed daily and recorded, including activities, diet, surgical incision healing, limb movement, muscle atrophy, and distal forefoot ulceration. Three months later, the wrist flexion function of forelimbs was measured before sacrifice, after which tissue samples were obtained. The local gross morphology of suture sites was observed and histological examinations were conducted.
Figure 1.
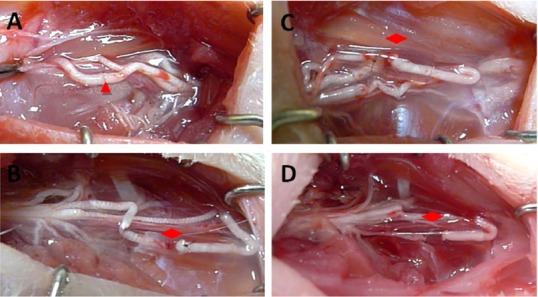
Repair of median nerve injury in rats with several kinds of nerve transposition.
(A) Epineurial neurorrhaphy in situ: The epineurium of median nerve was directly sutured with 10-0 suture under a stereoscope; (B) musculocutaneous nerve transposition for repairing median nerve; (C) medial pectoral nerve transposition for repairing median nerve; (D) radial nerve muscular branch transposition for repairing median nerve. (B–D) The 10-0 sutures and deacetylated chitin sleeve were used for small gap suture. The gap between nerve stumps was approximately 2 mm. Triangle points to the site of epineurial neurorrhaphy; diamonds point to the site of sleeve suture.
Wrist flexion function in rats measured using neuroelectrophysiological test
The rats were fixed on a laboratory-made operating table after anesthesia. The affected limb was held firm with one tack in front of and one behind the elbow joint of the affected limb. An incision of the skin was made in the middle of the upper left arm of the rat to expose and then bluntly separate the nerve. In the sham operation group and epineurial neurorrhaphy group, the median nerves were directly stimulated. In the musculocutaneous nerve transposition, medial pectoral nerve transposition, and radial nerve muscular branch transposition groups, a site 5 mm from the sleeve suture was stimulated with a Synergy electrophysiologic apparatus (Medelec Synergy; Oxford Instrument Inc., United Kingdom) with 0.9 mA continuous square waves at 50 Hz. The position of each left front paw was recorded with a digital camera (Sony, Tokyo, Japan) before and after stimulation. Results were synthesized with Image Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD, USA), and the angle of wrist flexion was measured.
Osmic acid staining
After determining the wrist flexion function, the damaged nerve was exposed along the original surgical incision to harvest 3 mm nerves from both the proximal and distal ends of the sleeve. The nerve tissue was fixed in 4% paraformaldehyde, and stained with 1% osmic acid. After washes with tap water for 6 hours, nerves were dehydrated through a graded alcohol series, permeabilized with xylene, embedded in wax, and sliced into sections. These sections were mounted with neutral resin, and observed under the light microscope (Leica, Solms, Germany). Five fields were randomly selected from each rat at 400× magnification. The number of myelinated nerve fibers, the thickness of myelin sheath, the diameter of axons, and the mean cross-sectional area of axons in the distal and proximal ends of the sleeve were measured with ImageJ image analysis software (National Institutes of Health, Bethesda, MD, USA).
Muscle wet weight
After the rats were executed, the left flexor digitorum superficialis muscle tendon was denervated at the level of the carpal tunnel. The flexor digitorum superficialis muscle was dissociated from the distal end to the proximal end. The flexor digitorum superficialis muscle was weighed with an electronic balance (Tianjin D&T Precision Instrument Co., Ltd., Tianjin, China) after wiping the muscle surface with gauze.
Hematoxylin-eosin staining of muscle fibers
After measuring its muscle wet weight, the left flexor digitorum superficialis muscle belly was fixed in 4% paraformaldehyde at 4°C overnight, dehydrated through a graded alcohol series, permeabilized with xylene, embedded in wax, and serially sliced into 5 μm-thick sections. These sections were stained with hematoxylin and eosin. Under the light microscope (Leica) at 200× magnification, muscle cross section was photographed using a DFC 300FX digital camera (Leica, Heidelberg, Germany). Five fields were randomly selected from each rat at 200× magnification. Muscle cross-sectional area was measured with Image Pro Plus software, and the mean value was calculated.
Statistical analysis
The data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). All data were expressed as the mean ± SD. The difference among groups was compared with one-way analysis of variance. Further paired comparison was conducted using the least significant difference test. Statistical significance was set to α = 0.05.
Results
General status of rats with median nerve injury after repairing with several kinds of nerve transposition
The nutritional status of rats was good after operation in each group. No systemic or local infection or other complications were found, and the hair was glossy. The atrophy to the left upper limb muscles was visibly variable, but no apparent autophagy was seen in any of the nerve transposition groups or the epineurial neurorrhaphy group. There was no obvious inflammatory reaction around the sleeve after exposing the area of operation. Some of the absorbed sleeve tissues had adhered slightly to the surrounding tissues. The nerves in the sleeve had grown in an orderly manner. Careful dissection of the incompletely absorbed sleeve and local soft tissue showed that proximal nerve had connected evenly with the distal nerve. No obvious neurofibroma was found at any operative site from any group.
Wrist flexion function in rats with median nerve injury after repairing with several kinds of nerve transposition
The wrist flexion function of rats was restored but the angle of wrist flexion varied. However, this was similar in each nerve transposition group and the epineurial neurorrhaphy group, but less than in the sham operation group (Figure 2).
Figure 2.

Wrist flexion function in rat forelimbs.
At the proximal end of the suture point, median nerve trunk was stimulated by square wave at a frequency of 50 Hz and a current of 0.9 mA. The wrist flexion angles of rats in each group were observed. 1: Before electrical stimulation; 2: after electrical stimulation; 3: merge images before and after electrical stimulation. A: Sham operation group; B: epineurial neurorrhaphy group; C: musculocutaneous nerve transposition group; D: medial pectoral nerve transposition group; E: radial nerve muscular branch transposition group. The wrist flexion function of rats was restored in each nerve transposition group and the epineurial neurorrhaphy group, and the wrist flexion condition was similar among these groups, but it did not reach the normal median nerve function level.
Several kinds of nerve transposition promotes median nerve regeneration in rats
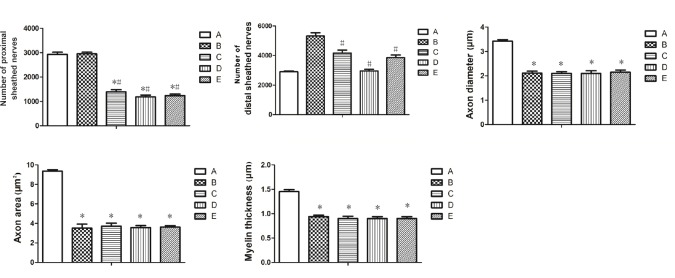
The number of myelinated nerve fibers was significantly less in each nerve transposition group than in the sham operation and epineurial neurorrhaphy groups (P < 0.05). The numbers of myelinated nerve fibers at the proximal end were significantly different among groups (P < 0.05). Histological results verified that the number of original myelinated nerve fibers in musculocutaneous nerve, medial pectoral nerve, and radial nerve muscular branch was less than that in the median nerve. The number of myelinated nerve fibers in the distal end of the sleeve was larger than that in the proximal end of the sleeve in each nerve transposition group and the epineurial neurorrhaphy group. The ratio of the number of distal to proximal myelinated nerve fibers (magnification of the number of regenerated nerve fibers) was 1.80:1, 3.00:1, 2.50:1, and 3.12:1 in the epineurial neurorrhaphy group, musculocutaneous nerve transposition group, medial pectoral nerve transposition group, and radial nerve muscular branch transposition group, respectively. The number of new myelinated nerve fibers was significantly lower in each nerve transposition group than in the epineurial neurorrhaphy group (P < 0.05). However, axon diameter, axon area, and myelin thickness were not significantly different among the epineurial neurorrhaphy group, musculocutaneous nerve transposition group, medial pectoral nerve transposition group, and radial nerve muscular branch transposition group (P > 0.05; Figures 3 and 4).
Figure 3.
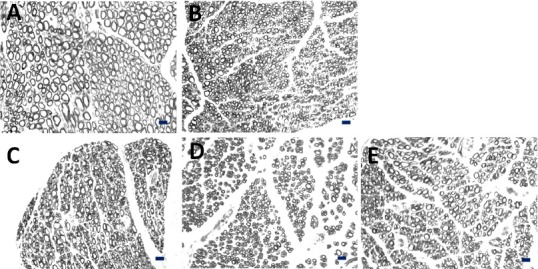
Cross sections of new myelinated nerve fibers at the distal end in rats with median nerve injury, stained with osmic acid.
(A) Sham operation group; (B) epineurial neurorrhaphy group; (C) musculocutaneous nerve transposition group; (D) medial pectoral nerve transposition group; (E) radial nerve muscular branch transposition group. Axon cross-sectional area was smaller in musculocutaneous nerve, medial pectoral nerve, and radial nerve muscular branch than that in median nerve. Axon diameter was less uniform in musculocutaneous nerve, medial pectoral nerve, and radial nerve muscular branch than in normal median nerve. Scale bars: 10 μm.
Figure 4.
Quantitative analysis of regenerative nerve in rats of each group.
A: Sham operation group; B: epineurial neurorrhaphy group; C: musculocutaneous nerve transposition group; D: medial pectoral nerve transposition group; E: radial nerve muscular branch transposition group. There were significant differences in the number of new myelinated nerve fibers on the distal end among groups. Diameter, cross-sectional area of axons and thickness of myelin sheath of newly formed nerve fibers were similar among groups. Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. sham operation group; #P < 0.05, vs. epineurial neurorrhaphy group (one-way analysis of variance and the least significant difference test).
Several kinds of nerve transposition promote muscle recovery in rats with median nerve injury
Hematoxylin-eosin staining results exhibited that the muscle fibers of each group had clear boundaries, uniform dyeing and uniform diameter (Figure 5). Muscle atrophy in varying degrees was observed in each nerve transposition group and the epineurial neurorrhaphy group compared with the sham operation group (Figure 5). Wet weights and cross-sectional areas of muscle fibers were significantly lower in each of the nerve transposition groups and the epineurial neurorrhaphy group than in the sham operation group (P < 0.05; Figure 6). Histological changes of muscles in each nerve transposition group were similar to those in the epineurial neurorrhaphy group (P > 0.05; Figure 6).
Figure 5.
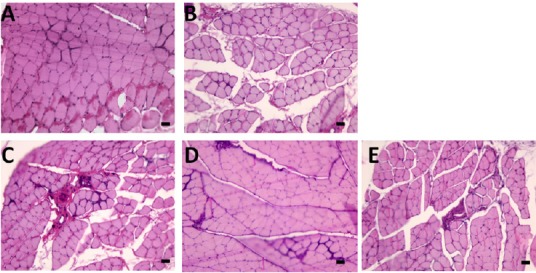
Cross-section of flexor digitorum superficialis muscle of rat left forelimb in each group (hematoxylin-eosin staining).
A: Sham operation group; B: epineurial neurorrhaphy group; C: musculocutaneous nerve transposition group; D: medial pectoral nerve transposition group; E: radial nerve muscular branch transposition group. Muscle atrophy in varying degrees was observed, and fibers between muscle groups increased in each nerve transposition group and the epineurial neurorrhaphy group compared with the sham operation group. Arrows show the site of other fiber ingrowth. Scale bars: 20 μm.
Figure 6.
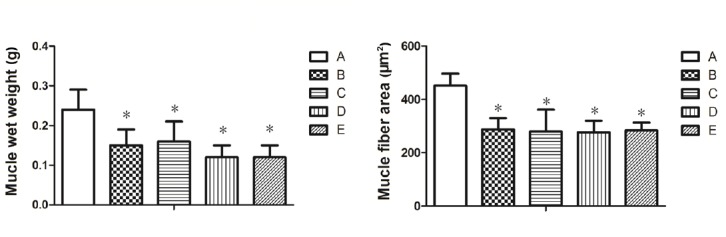
Muscle wet weight and cross-sectional area of muscle fibers of rats in each group.
A: Sham operation group; B: epineurial neurorrhaphy group; C: musculocutaneous nerve transposition group; D: medial pectoral nerve transposition group; E: radial nerve muscular branch transposition group. Muscle histological parameters were similar in each nerve transposition group, but significantly inferior to those of sham operation group. Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. sham operation group (one-way analysis of variance and the least significant difference test).
Discussion
The animal models of median nerve injury were selected in this study, because the median nerve is commonly injured in trauma, mostly caused by sharp cuts and car accidents, and can cause a series of sensorimotor dysfunction such as drop-wrist deformity (Birch et al., 2012). At present, according to the types of median nerve injury, there are two treatment methods. One is no nerve defect, which requires tension-free epineurial suture under a microscope (Pederson, 2014). Another type is median nerve injury with different degrees of defect. When the defect is less than 2 cm, the therapeutic effect of autologous nerve transplantation is similar to that of tension-free epineurial suture (Watchmaker et al., 1991). If the defect is too large a small gap bridging suture with a nerve sleeve made of different materials (Yang et al., 2011) or acellular allogeneic nerve transplantation (Cho et al., 2012; Lin et al., 2013) is performed. For the recovery of effector function, Jiang et al. (2018) suggested that the median nerve trunk could be repaired by transposition of the contralateral cervical 7 plexus instead of a subcutaneous tunnel to repair median nerve endings. This method of transposition repair can restore flexor to some extent, but it did not discuss the recovery of other target organ dysfunction caused by the transposition of a donor nerve and median nerve injury. The therapeutic effects of these methods are not ideal, especially in the face of severe median nerve trauma and other special types of injury, and can result in a series of sequelae and complications (Meek et al., 2005; Thomsen and Schlur, 2013).
In this study, the median nerve regeneration was achieved to some degree in all nerve transposition groups. When the proximal donor nerve is stimulated by electrical stimulation, wrist flexion is induced by the repaired median nerve indicating the reinnervation of motor neurons in different anterior horns of the spinal cord. The histology of the nerve fibers showed that the distal effector of nerve ingrowth was found in all nerve transposition and epineurial neurorrhaphy groups, although the regeneration varied. However, there were no significant differences in the diameter of new axons, cross-sectional area of axons, thickness of myelin sheath, wet weight of flexor digitorum superficialis muscle and cross-sectional area of muscle fibers between groups. These findings suggested that although different motor neurons in the anterior horn of the proximal spinal cord were selected in the different repair groups, there was no significant difference in the axon diameter and the thickness of myelin sheath of the regenerated myelinated nerve fibers at the distal end. Yu et al. (2016) confirmed that in this transposition repair model, the upstream neurons of the target organ innervated by the receptor nerve are motor neurons of the anterior horn of the spinal cord of the donor nerve. Therefore, this study did not use retrograde tracing to demonstrate the dominant neurons in all translocation groups.
The reinnervation of spinal cord anterior horn cells affects not only the regeneration of nerves but also the recovery of muscle function of target organs. This process is associated with the number of effective neuromuscular junctions in muscle (Chao et al., 2013; Pratt et al., 2013). The maintenance of muscle tissue and function depends on effective neuromuscular junctions. The number of motor endplates on muscle fibers is relatively constant but endplates have a short lifetime (Tintignac et al., 2015; Li et al., 2018). Muscle fibers atrophy, fat infiltration, and other types of fiber ingrowth occur when muscle denervation occurs, and the transformation can become irreversible (Bezakova et al., 2001; Sanuki et al., 2015). In this study, the rate of regeneration of new nerve fibers in the different transposition groups was much the same, and the time to reach target organs was relatively similar. We found that the amplification of axonal buds after any of the different donor nerve transposition could meet the needs of motor endplates and form neuromuscular junctions with some muscle functions. There was no significant difference in the time window of complete muscle denervation. This may be the reason for similar muscle morphology and function between different repair groups in this study.
In this study, we used a conical sleeve with different inner diameters at each end for small gap bridging. This suture method can achieve tension-free suture to repair nerves of different diameters. Furthermore, compared with traditional epineurial neurorrhaphy, small gap sleeve technique reserves a clear space for nerve regeneration between nerve stumps, reduces the nerve mismatch rate during nerve regeneration and improves the efficacy of median nerve injury (Broshart et al., 1987; Kou et al., 2013; Zhang et al., 2017). Nevertheless, this study has some limitations. So far there are no good indicators and methods for evaluating motor nerve function of median nerve in rats. We have not yet quantified and evaluated the specific recovery of the median nerve. Remodeling of the corresponding superior neural circuit and the functional area of the brain after transposition repair of the spinal cord anterior horn cells has not been carried out in this study. We will perform related research in primates in the future before a clinical trial for the future human application.
In summary, from functional and histological aspects, this study confirmed that donor nerves derived from motor neurons in the anterior horn of cervical spinal cord can effectively repair severe median nerve injury, and retain the function of target organs of affected limbs. It is indicated that nerve transposition repair can be used to repair severe peripheral nerve injury.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This research was continuously funded by the National Natural Science Foundation of China, No. 31571236, 31571235 (to YHK, PXZ); the National Key Research and Development Program of China, No. 2016YFC1101604 (to DYZ); the National Key Basic Research Program of China (973 Program), No. 2014CB542200 (to BGJ); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to BGJ); the Beijing Science and Technology New Star Cross Program of China, No. 2018019 (to PXZ); the Peking University People’s Hospital Research and Development Funds, No. RDH2017-01 (to HLX). Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: All animal experimental procedures were approved by the Animal Ethics Committee of Peking University People’s Hospital (approval No. 2015-50) on December 9, 2015 and performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Liu XL
Funding: This research was continuously funded by the National Natural Science Foundation of China, No. 31571236, 31571235 (to YHK, PXZ); the National Key Research and Development Program of China, No. 2016YFC1101604 (to DYZ); the National Key Basic Research Program of China (973 Program), No. 2014CB542200 (to BGJ); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to BGJ); the Beijing Science and Technology New Star Cross Program of China, No. 2018019 (to PXZ); the Peking University People’s Hospital Research and Development Funds, No. RDH2017-01 (to HLX).
References
- Bezakova G, Rabben I, Sefland I, Fumagalli G, Lomo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc Natl Acad Sci U S A. 2001;98:9924–9929. doi: 10.1073/pnas.171539698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch R, Misra P, Stewart MP, Eardley WG, Ramasamy A, Brown K, Shenoy R, Anand P, Clasper J, Dunn R, Etherington J. Nerve injuries sustained during warfare: part II: Outcomes. J Bone Joint Surg Br. 2012;94:529–535. doi: 10.1302/0301-620X.94B4.28488. [DOI] [PubMed] [Google Scholar]
- Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE, Wisotsky SM, Cho MS, Wilson C, Cooper EO, Ingari JV, Safa B, Parrett BM, Buncke GM. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. doi: 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- Broshart TM, Seiler WA. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Chao T, Frump D, Lin M, Caiozzo VJ, Mozaffar T, Steward O, Gupta R. Matrix metalloproteinase 3 deletion preserves denervated motor endplates after traumatic nerve injury. Ann Neurol. 2013;73:210–223. doi: 10.1002/ana.23781. [DOI] [PubMed] [Google Scholar]
- Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, Buncke GM. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340–2349. doi: 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Geuna S, Papalia I, Ronchi G, d’Alcontres FS, Natsis K, Papadopulos NA, Colonna MR. The reasons for end-to-side coaptation: how does lateral axon sprouting work. Neural Regen Res. 2017;12:529–533. doi: 10.4103/1673-5374.205081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanghurde BA, Mehta R, Ladkat KM, Raut BB, Thatte MR. Distal transfers as a primary treatment in obstetric brachial plexus palsy: a series of 20 cases. J Hand Surg Eur Vol. 2016;41:875–881. doi: 10.1177/1753193416663887. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- Hua XY, Qiu YQ, Li T, Zheng MX, Shen YD, Jiang S, Xu JG, Gu YD, Xu WD. Contralateral peripheral neurotization for hemiplegic upper extremity after central neurologic injury. Neurosurgery. 2015;76:187–195. doi: 10.1227/NEU.0000000000000590. [DOI] [PubMed] [Google Scholar]
- Jaquet JB, Luijsterburg AJ, Kalmijn S, Kuypers PD, Hofman A, Hovius SE. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51:687–962. doi: 10.1097/00005373-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang L, Lao J, Zhao X. Total brachial plexus injury: contralateral C7 root transfer to the lower trunk versus the median nerve. Neural Regen Res. 2018;13:1968–1973. doi: 10.4103/1673-5374.239444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, Shibata T, Matsui Y, Yasui N. Use of intercostal nerves for neurotization of the musculocutaneous nerve in infants with birth-related brachial plexus palsy. J Neurosurg. 2001;94:386–391. doi: 10.3171/jns.2001.94.3.0386. [DOI] [PubMed] [Google Scholar]
- Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Kotani T, Toshima Y, Matsuda H, Suzuki T, Ishizaki Y. Postoperative results of nerve transposition in brachial plexus injury. Seikei Geka. 1971;22:963–966. [PubMed] [Google Scholar]
- Kou Y, Peng J, Wu Z, Yin X, Zhang P, Zhang Y, Weng X, Qiu G, Jiang B. Small gap sleeve bridging can improve the accuracy of peripheral nerve selective regeneration. Artif Cells Nanomed Biotechnol. 2013;41:402–407. doi: 10.3109/21691401.2012.762007. [DOI] [PubMed] [Google Scholar]
- Kouyourmdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34:785–788. doi: 10.1002/mus.20624. [DOI] [PubMed] [Google Scholar]
- Kubota S, Nishiura Y, Hara Y, Saijilafu, Abe I, Ochiai N. Functional and morphological effects of indirect gradual elongation of peripheral nerve: electrophysiological and morphological changes at different elongation rates. Hand Surg. 2011;16:105–111. doi: 10.1142/S0218810411005199. [DOI] [PubMed] [Google Scholar]
- Lam Van Ba O, Barbe MF, Caremel R, Aharony S, Loutochin O, Jacques L, Wood MW, Tiwari E, Tuite GF, Campeau L, Corcos J, Ruggieri MR Sr. Lumbar to sacral root rerouting to restore bladder function in a feline spinal cord injury model: Urodynamic and retrograde nerve tracing results from a pilot study. Neurourol Urodyn. 2018;37:153–162. doi: 10.1002/nau.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–188. doi: 10.1146/annurev-physiol-022516-034255. [DOI] [PubMed] [Google Scholar]
- Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29:331–348. doi: 10.1016/j.hcl.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Little KJ, Zlotolow DA, Soldado F, Cornwall R, Kozin SH. Early functional recovery of elbow flexion and supination following median and/or ulnar nerve fascicle transfer in upper neonatal brachial plexus palsy. J Bone Joint Surg Am. 2014;96:215–221. doi: 10.2106/JBJS.L.01405. [DOI] [PubMed] [Google Scholar]
- Meek MF, Coert JH, Robinson MD. Poor results after nerve grafting in the upper extremity: quo vadis. Microsurgery. 2005;25:396–402. doi: 10.1002/micr.20137. [DOI] [PubMed] [Google Scholar]
- Murison J, Jehanno P, Fitoussi F. Nerve transfer to biceps to restore elbow flexion and supination in children with obstetrical brachial plexus palsy. J Child Orthop. 2017;11:455–459. doi: 10.1302/1863-2548.11.170125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39:1216–1222. doi: 10.1016/j.jhsa.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Pondaag W, Malessy MJ. Intercostal and pectoral nerve transfers to re-innervate the biceps muscle in obstetric brachial plexus lesions. J Hand Surg Eur Vol. 2014;39:647–652. doi: 10.1177/1753193413501588. [DOI] [PubMed] [Google Scholar]
- Pratt SJP, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 2013;591:559–570. doi: 10.1113/jphysiol.2012.241679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Q, Chang B, Liu RX, Sun S, Wang Y, Lu SB, Peng J, Zhao Q. Peripheral nerve injury and regeneration: application and progress of novel nerve scaffolds. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:962–968. [Google Scholar]
- Rebowe R, Rogers A, Yang X, Kundu SC, Smith TL, Li Z. Nerve repair with nerve conduits: problems, solutions, and future directions. J Hand Microsurg. 2018;10:61–65. doi: 10.1055/s-0038-1626687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanuki T, Yumoto E, Nishimoto K, Kodama N, Kodama H, Minoda R. Laryngeal reinnervation featuring refined nerve-muscle pedicle implantation evaluated via electromyography and use of coronal images. Otolaryngol Head Neck Surg. 2015;152(4):697–705. doi: 10.1177/0194599815568945. [DOI] [PubMed] [Google Scholar]
- Thomsen L, Schlur C. Incidence of painful neuroma after end-to-end nerve suture wrapped into a collagen conduit: a prospective study of 185 cases. Chir Main. 2013;32:335–340. doi: 10.1016/j.main.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015;95:809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Li SR, Wen C, Xiao CG, Su BY. Morphological changes of cholinergic nerve fibers in the urinary bladder after establishment of artificial somatic-autonomic reflex arc in rats. Neurosci Bull. 2007;23:277–281. doi: 10.1007/s12264-007-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchmaker GP, Gumucio CA, Crandall RE, Vannier MA, Weeks PM. Fascicular topography of the median nerve: a computer based study to identify branching patterns. J Hand Surg Am. 1991;16:53–59. doi: 10.1016/s0363-5023(10)80013-9. [DOI] [PubMed] [Google Scholar]
- Xiao CG, Du MX, Li B, Liu Z, Chen M, Chen ZH, Cheng P, Xue XN, Shapiro E, Lepor H. An artificial somatic-autonomic reflex pathway procedure for bladder control in children with spina bifida. J Urol. 2005;173:2112–2116. doi: 10.1097/01.ju.0000158072.31086.af. [DOI] [PubMed] [Google Scholar]
- Yang M, Rawson JL, Zhang EW, Arnold PB, Lineaweaver W, Zhang F. Comparisons of outcomes from repair of median nerve and ulnar nerve defect with nerve graft and tubulization: a meta-analysis. J Reconstr Microsurg. 2011;27:451–460. doi: 10.1055/s-0031-1281526. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang P, Han N, Kou Y, Yin X, Jiang B. Collateral development and spinal motor reorganization after nerve injury and repair. Am J Transl Res. 2016;8:2897–2911. [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Xue F, Kou Y, Fu Z, Zhang D, Zhang H, Jiang B. The experimental study of absorbable chitin conduit for bridging peripheral nerve defect with nerve fasciculu in rats. Artif Cells Blood Substit Immobil Biotechnol 2008. 2008;36:360–371. doi: 10.1080/10731190802239040. [DOI] [PubMed] [Google Scholar]
- Zhang XX, Kou YH, Yin XF, Jiang BG, Zhang PX. Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves. Neural Regen Res. 2017;12:1172–1176. doi: 10.4103/1673-5374.211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng MX, Hua XY, Feng JT, Li T, Lu YC, Shen YD, Cao XH, Zhao NQ, Lyu JY, Xu JG, Gu YD, Xu WD. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378:22–34. doi: 10.1056/NEJMoa1615208. [DOI] [PubMed] [Google Scholar]