Abstract
Transplantation of human bone marrow mesenchymal stem cells (hMSCs) stands as a potent stroke therapy, but its exact mechanism remains unknown. This study investigated the anti-apoptotic mechanisms by which hMSCs exert neuroprotective effects on cerebral ischemia. Primary mixed cultures of rat neurons and astrocytes were cultured and exposed to oxygen-glucose deprivation. A two-hour period of “reperfusion” in standard medium and normoxic conditions was allowed and immediately followed by hMSCs and/or Bcl-2 antibody treatment. Cell viability of primary rat neurons and astrocytes was determined by 3-(4,5-dimethylthianol-2-yl)-2,5 diphenyl tetrazolium bromide and trypan blue exclusion methods. hMSC survival and differentiation were characterized by immunocytochemistry, while the concentration of Bcl-2 in the supernatant was measured by enzyme-linked immunosorbent assay to reveal the secretory anti-apoptotic function of hMSCs. Cultured hMSCs expressed embryonic-like stem cell phenotypic markers CXCR4, Oct4, SSEA4, and Nanog, as well as immature neural phenotypic marker Nestin. Primary rat neurons and astrocytes were protected from oxygen-glucose deprivation by hMSCs, which was antagonized by the Bcl-2 antibody. However, Bcl-2 levels in the supernatants did not differ between hMSC- and non-treated cells exposed to oxygen-glucose deprivation. Neuroprotective effects of hMSCs against cerebral ischemia were partially mediated by the anti-apoptotic mechanisms. However, further studies are warranted to fully elucidate this pathway.
Keywords: apoptosis, Bcl-2 antibody, human mesenchymal stem cells, ischemia, neuroprotection, oxygen glucose deprivation
Chinese Library Classification No. R459.9; R364; R741
Introduction
Stroke is associated with neuronal dysfunction due to damaged brain parenchyma after the interruption of cerebral blood circulation (Feigin et al., 2009; Kamal et al., 2009). Necrotic cell death in the ischemic core occurs in the early acute phase (hours) (Mehta et al., 2007), while apoptotic cell death takes place in the late stage (days, weeks, and even months) of stroke (Mehta et al., 2007). The most commonly used pharmacological agent in the acute phase of stroke is tissue plasminogen activator (National Institute of Neurological Disorders and Stroke, 1995), but only 2% of acute stroke patients benefit from this treatment (Asahi et al., 2000). Rescuing apoptotic cells in the chronic phase may reduce stroke deficits (Sairanen et al., 2006).
Stem cell transplantation stands as an efficacious stroke therapy. Mesenchymal stem cells (MSCs) can migrate throughout the brain and differentiate into neural-like cells (Dharmasaroja, 2009), expressing markers for astrocytes and neurons, and secreting therapeutic proteins (Chen et al., 2001a; Zhao et al., 2002). Intravenous injection of MSCs at 1 or 7 days after stroke markedly improves functional outcomes compared with non-treated rats (Chen et al., 2001a). Moreover, intracerebral injection of neurotrophic factor modified MSCs, such as the MSC-transfected fibroblast growth factor-2 gene with HSV-1 vector, enhances the cells’ neuroprotective effects against stroke (Ikeda et al., 2005). Translation of these laboratory findings to the clinic appears promising (Borlongan, 2009; Dharmasaroja, 2009).
The mechanisms underlying cell therapy remain not fully understood. MSCs may differentiate into neural cells and endothelial cells both in vitro and in vivo (Nakano et al., 2001; Kim et al., 2002). Additionally, MSCs induce neurogenesis and angiogenesis (Chen et al., 2001a, 2003), upregulate anti-inflammatory while downregulating pro-inflammatory cytokines in the brain (Kim et al., 2009; Liu et al., 2009), and may inhibit cell apoptosis (Chen et al., 2001a, 2003). These represent potential pathways mediating MSC neuroprotection in stroke.
Post-ischemic anti-apoptosis may involve Bcl-2, a member of the Bcl-2 gene family, which acts as a transcription factor in mediating endogenous neuroprotection against stroke (Kitagawa et al., 1998). Upregulation of Bcl-2 and Bcl-xl improves neuroprotection against sublethal forebrain ischemia (Wu et al., 2003). A number of neuroprotective drugs exert their effects by partly mediating Bcl-2 (Cui et al., 2009). Human embryonic neural stem cell transplantation improves neurological function possibly by increasing the number of Bcl-2 positive cells in the penumbra at 7 days post-stroke (Zhang et al., 2009). Injection of Bcl-2 expressing plasmid into the lateral ventricle of the stroke rat brain increases neurogenesis while dampening apoptosis of newborn neurons (Zhang et al., 2006). Similarly, transplantation of embryonic stem cells overexpressing the human anti-apoptotic gene Bcl-2 into the stroke rat cortex promotes functional benefits (Wei et al., 2005).
The purpose of this study was to ascertain whether the anti-apoptotic factor Bcl-2 mediated neuroprotective effects of human bone marrow mesenchymal stem cells (hMSCs) on rat neurons and astrocytes exposed to an in vitro model of stroke.
Materials and Methods
Cell culture
Primary mixed cultures of neurons and astrocytes derived from a rat striatum were obtained from BrainBits (E18 Sprague-Dawley (SD) rat striatum; BrainBits LLC, Springfield, IL, USA) and maintained in culture following the supplier’s protocol and similar to our previous study (Kaneko et al., 2014). Immediately after thawing, cells (4 × 104 cells/well) were routinely seeded and grown in a 96-well plate coated with poly-lysine in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA, USA) containing 4.5 g/L D-glucose, L-glutamine, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 10% fetal bovine serum (Sigma, St. Louis, MO, USA) for 5 days in a humidified atmosphere containing 5% CO2 in air at 37°C. Moreover, we confirmed that these cells were appropriate for the oxygen glucose deprivation (OGD) injury model and the ratio of neurons to astrocytes was ~1:1, as revealed by the expression of glutamate receptors (determined immunocytochemically by using vesicular glutamate transporter-1) in 50% of the neuronal and astrocytic cell population (Kaneko et al., 2014).
Oxygen-glucose deprivation
Mixed cultures of neurons and astrocytes were exposed to the OGD injury model as described previously (Matsukawa et al., 2009) with few modifications. Briefly, the culture medium was replaced by a glucose-free Dulbecco’s phosphate buffered saline (DPBS/Modified, Hyclone, Logan, UT, USA) with calcium and magnesium. Cultured cells were placed in a humidified chamber, and then equilibrated with a continuous flow of 92% N2 and 8% O2 gas for 15 minutes. After equilibrium was achieved, the chamber was sealed and placed into the incubator at 37°C for 90 minutes. After this period, OGD was terminated by replacing the high glucose DMEM with the standard 95% O2 and 5% CO2 incubator (Thermo Fisher, Waltham, MA, USA). A two-hour period of “reperfusion” in standard medium and normoxic conditions was allowed, then hMSCs and/or Bcl-2 antibody (Bcl-2 (C-2), mouse monoclonal IgG1, Santa Cruz Biotechnology, Santa Cruz, CA, USA) treatment was initiated. The dose of hMSCs was 4 × 104 cells/well. The dose of Bcl-2 antibody was 58, 117, or 235 ng/mL. Both hMSC and Bcl-2 doses were based on pilot studies demonstrating their potencies. Cryopreserved human bone marrow CD34+ cells (hBM34+) were purchased from AllCells (Alameda, CA, USA). The exposure time of hMSCs and/or Bcl-2 antibody with the neuronal-glial culture lasted for either 2 or 3 hours. The supernatants and the hMSCs were separated from the mixed culture at the end of the 2- or 3-hour exposure. Overall, a 90-minute OGD injury, 2-hour reperfusion, and 2- or 3-hour exposure time of the neuronal-glial culture to hMSCs and Bcl-2 antibodies were performed. Thereafter, cell viability was assessed by 3-(4,5-dimethylthianol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay and trypan blue exclusion methods. Control cell cultures (those not deprived of oxygen and glucose) were incorporated into the experiment.
MTT assay
The colorimetric MTT reduction assay (Borlongan et al., 2010) was conducted by following the instructions for use of Promega Corporation products (Cell Titer 96, Non-Radioactive Cell Proliferation Assay, Promega Corporation, Madison, WI, USA). This method assessed mitochondrial activity and thus cell viability by measuring the ability of cultured cells to convert yellow MTT to purple formazan dye. The supernatant and the hMSCs were separated from the mixed culture at the end of the 3-hour exposure time. Approximately 100 μL DMEM without phenol red was added, then 20 μL of the dye solution was added to each well, and the mixture was incubated on the plate at 37°C for 3 hours in a humidified, 5% CO2 atmosphere. After incubation, 100 μL of the solubilization solution/stop mix was added to each well, and the plate was allowed to stand overnight in the humidified, 5% CO2 incubator at 37°C. The absorbance was quantified spectrophotometrically at a wavelength of 570 nm and with a reference wavelength of 900 nm in the BioTek Synergy HT 96-well microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Data were expressed as the percentage of viable cells in OGD-exposed plates compared with control normoxic plates.
Trypan blue exclusion method
Cell death was analyzed by the trypan blue exclusion method (Kaneko et al., 2014). Four wells were used for each condition, and each experiment was repeated at least three times. Briefly, 0.4% Trypan blue (Gibco) was added to the cells for 30 seconds and an estimation of cell viability was conducted using randomly selected and photographically captured visual fields (120,000 μm2). In addition, we randomly selected two fields in each photograph (n = 8 in each group).
Immunocytochemical detection of hMSCs phenotype
hMSCs were cultured using commercially available media systems. The cells were thawed, and the freeze-thaw process demonstrated a high level of viability, close to 100%. Cells were thawed in a 37°C water bath, transferred to DMEM with FBS, centrifuged at 120 × g for 10 minutes, and re-suspended in the media. Thereafter, they were seeded in culture slides (CultureSlides, BD FalconTM, BD BioCoatTM, Bedford, MA, USA) at 1 × 104 cells/well under the temperature of 37°C in a humidified atmosphere containing 5% CO2. The medium was changed every 4 days. After cells attained 70–80% confluence, they were analyzed by immunocytochemistry assays in order to detect phenotypic characteristics. The cells were rinsed three times with phosphate buffered saline (PBS) and then fixed in 4% paraformaldehyde (PFA) for 30 minutes at room temperature. In order to understand the status of hMSCs, neural stem cell-specific (Nestin, mouse monoclonal IgG1, 1:200; Millipore Corporation, Temecula, CA, USA), putative stem cell (CXCR4, rabbit polyclonal IgG, 1:200, Abcam, Cambridge, MA, USA), differentiated, and embryonic stem cell markers (Oct4, rabbit polyclonal IgG, 1:200, Abcam; SSEA4, mouse monoclonal IgG3, 1:150, Abcam; Nanog, mouse monoclonal IgG1,1:200, Abcam) were employed. After blocking the reaction with 10% normal goat serum (Vector, CA, USA), cells were incubated overnight (not less than 18 hours) at 4°C with each primary antibody, separately. After several rinses in PBS, cells were incubated for 90 minutes at room temperature in goat anti-mouse IgG Alexa Fluor 594 conjugate (1:500; Invitrogen, Carlsbad, CA, USA) or goat anti-rabbit IgG Alexa Fluor 594 conjugate (1:500; Invitrogen). Then, cells were processed for DAPI (1:1000; Sigma, St. Louis, MO, USA) staining. The cells were then washed three times in PBS and embedded with mounting medium (Biomeda, Foster City, CA, USA). Immunofluorescent images were visualized using Zeiss AxioImager (Thornwood, NY, USA). In addition, control studies have included the exclusion of the primary antibody and substituted with a 10% normal goat serum condition. No immunoreactivity was observed in these controls. Assessment was performed blindly by an independent investigator.
Enzyme-linked immunosorbent assay for Bcl-2
The levels of Bcl-2 were assessed in triplicates from the collected supernatants using a standard sandwich antibody ELISA system (Abcam) according to the manufacturer’s protocol. For the measurement of Bcl-2, mouse anti-human Bcl-2 antibody (R & D Systems, Inc., Minneapolis, MN, USA) was used as a capture antibody and biotinylated mouse anti-human Bcl-2 antibody (R & D Systems) was used as a detection antibody. Bcl-2 levels were quantified spectrophotometrically at a wavelength of 450 nm with 900 nm used as the reference wavelength. The BioTek Synergy HT 96-well microplate reader (BioTek Instruments, Inc.) was used to measure optical densities.
Statistical analysis
All data were expressed as means ± standard deviation (SD). Statistical differences were analyzed using one-way analysis of variance, followed by post hoc testing using Fisher’s protected least significant difference using SPSS 13.0 software (SPSS, Chicago, IL, USA). A P-value of 0.05 or less was used to indicate statistical significance.
Results
hMSCs display typical embryonic stem cell-like features
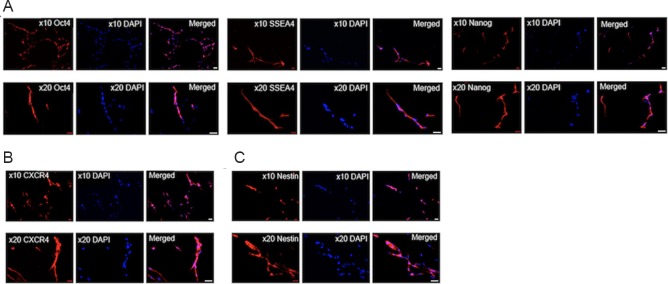
Immunocytochemical assays of hMSCs revealed that they expressed embryonic-like stem cell phenotypic markers (Oct4, SSEA4, and Nanog) (Figure 1A). More than 90% of the cells were positive for the markers of Oct4 and SSEA4. The images showed that the expressed hMSCs were characterized by specific nuclear staining for Oct4 and Nanog, and the cytoplasmic/axonal labeling for SSEA4. Furthermore, these hMSCs were also CXCR4 positive (Figure 1B), a stem cell chemotaxis marker, and approximately 40% were Nestin-positive (Figure 1C), an immature neuronal phenotypic marker.
Figure 1.
Immunocytochemical assays revealing human bone marrow mesenchymal stem cells (hMSCs) display typical embryonic stem cell-like features.
(A) hMSCs express embryonic-like stem cell phenotypic markers (Oct4, SSEA4, and Nanog). (B) hMSCs were positive for CXCR4, a stem cell chemotaxis marker. (C) hMSCs expressed Nestin, an immature neuronal phenotypic marker. All studies were conducted in triplicates. Red: Nestin, CXCR4, Oct4, SSEA4, Nanog; Blue: DAPI. The nuclei of the cells were stained with DAPI at 10× and 20× magnification in A–C. Scale bars: 50 μm.
hMSC protection on injured rat cells is blocked by Bcl-2 antibody
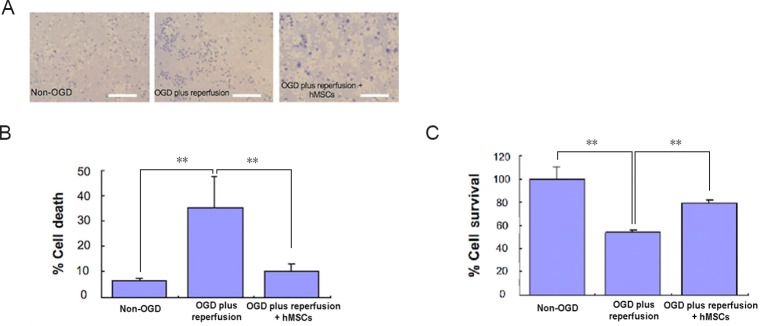
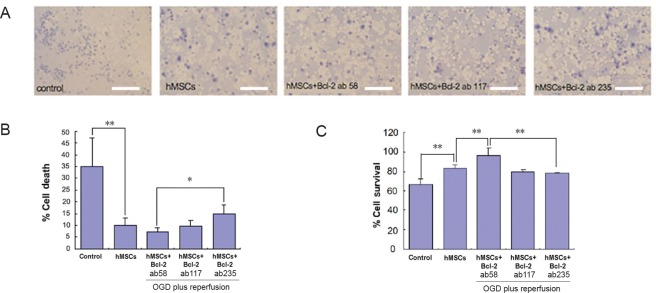
Following a 90-minute OGD injury, 2-hour reperfusion, and 2- or 3-hour exposure time of the neuronal-glial culture to hMSCs and Bcl-2 antibodies, the colorimetric trypan blue and MTT assays showed a significant loss of rat cells, which was partially hindered by the addition of hMSCs (Figure 2). We found that in the vehicle rat cells treated only with Bcl-2 antibody (high dose, 235 ng/mL), cell viability was not different from the OGD group (data not shown), which showed that the Bcl-2 antibody alone did not affect the rat cell viability. When cell survival rates were compared between the hMSCs alone and the co-treatment with Bcl-2 antibody, the results showed that hMSCs (4 × 104 cells/well, 1:1 with the number of the rat cells per well) provided neuroprotection in the rat cells after OGD and reperfusion, but with increasing doses of the Bcl-2 antibody, this hMSC-mediated neuroprotection was blocked (Figure 3). Interestingly, low dose Bcl-2 antibody (58 ng/mL) enhanced hMSC neuroprotection against OGD and reperfusion (Figure 3).
Figure 2.
Cell death (Trypan Blue assay) and cell survival (MTT assay) in OGD model for cerebral ischemia involving primary mixed cultures of rat neurons and astrocytes.
(A) Images of cultures expressing dead cells (blue) in media. Scale bars: 50 µm. (B) Cultures without OGD treatment showed ~7% of cells died. Cell death increased significantly (P < 0.01) in a 90-minute OGD treatment followed by a 2-hour reperfusion (standard medium and normoxic conditions) period. When treated with hMSCs, OGD model plus reperfusion showed a decrease in cell death (P < 0.01). All experiments were conducted in triplicates. (C) Cells that did not receive OGD showed ~100% survival rate. However, cells showed a significant decrease after receiving a 90-minute OGD treatment followed by a 2-hour reperfusion (standard medium and normoxic conditions) period (P < 0.01). When treated with hMSCs, OGD model plus reperfusion showed an increased survival rate (P < 0.01). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by post hoc Fisher’s protected least significant difference). All experiments were conducted in triplicate. hMSCs: Human bone marrow mesenchymal stem cells; OGD: oxygen glucose deprivation; MTT: 3-(4,5-dimethylthianol-2-yl)-2,5 diphenyl tetrazolium bromide.
Figure 3.
Cell death (Trypan Blue assay) and cell survival (MTT assay) in OGD model for cerebral ischemia involving primary mixed cultures of rat neurons and astrocytes.
(A) Images of rat neuron and astrocyte cultures expressing dead cells (blue) in media with different treatment regimens. Scale bars = 50 µm. (B) There was a significant (P < 0.01) decrease in cell death between the control and hMSCs groups. In contrast, the hMSCs + Bcl-2 antibody (235 ng/mL) group showed a significant (P < 0.05) increase in cell death compared to the hMSCs + Bcl-2 antibody (58 ng/mL) group. The dose of Bcl-2 antibody was 58, 117, or 235 ng/mL. All experiments were conducted in triplicates. (C) A graphical representation showing the significance between different treatment regimens. Cell survival significantly (P < 0.01) increased when treated with hMSCs and hMSCs + Bcl-2 antibody (58 ng/mL). However, when increased amounts of Bcl-2 antibody was administered, a significant (P < 0.01) decrease can be seen in the 117 ng/mL and 235 ng/mL groups. Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by post hoc Fisher’s protected least significant difference. All experiments were conducted in triplicate. hMSCs: Human bone marrow mesenchymal stem cells; OGD: oxygen glucose deprivation; MTT: 3-(4,5-dimethylthianol-2-yl)-2,5 diphenyl tetrazolium bromide.
Co-treatment with Bcl-2 antibody does not change the effect of hMSCs on Bcl-2 level
The levels of the Bcl-2 protein in culture medium were measured using a standard sandwich antibody ELISA system. Bcl-2 protein levels substantially increased with OGD treatment, which suggested the cells’ self-protective properties after injury. Under the 450 nm wavelength, the optical density of the sample from the OGD treatment was 0.382 ± 0.036. The level of secreted Bcl-2 protein increased from 11.81 pg/mL to 83.65 pg/mL in cells treated with OGD. Furthermore, Bcl-2 secretion in OGD + hMSCs-treated cells for 3 hours was less than in the group not treated with hMSCs. This discrepancy from other reports may be due to varying time points of hMSC treatment. We found that Bcl-2 secretion in OGD + hMSCs-treated for 2 hours did not differ from OGD treatment alone (Figure 4A). Moreover, co-treatment with the Bcl-2 antibody did not alter Bcl-2 protein expression levels in the supernatants (Figure 4B).
Figure 4.
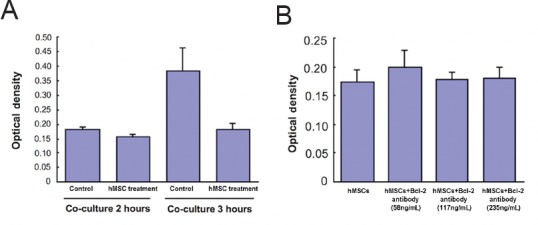
The effect of varying treatment durations on hMSCs and Bcl-2 levels in culture medium.
(A) Each group underwent OGD + hMSCs treatment with different treatment durations. (B) Bcl-2 levels were measured with a standard sandwich antibody ELISA kit. Co-cultured treatment of the primary neuronal-glial culture with hMSCs or Bcl-2 antibodies did not seem to change the Bcl-2 protein levels. Data are expressed as the mean ± SD. All experiments were conducted in triplicate. hMSCs: Human bone marrow mesenchymal stem cells; OGD: oxygen glucose deprivation; ELISA: enzyme-linked immuno sorbent assay.
Discussion
The present study demonstrated that hMSCs, which expressed both embryonic like-stem cell and neuronal phenotypic markers, increased neuroprotection in rat cells following a 90-minute OGD with a 2-hour “reperfusion”. Additionally, the Bcl-2 antibody, depending on the dose, impeded hMSC-associated neuroprotection.
Characterizing the phenotype of the stem cells is crucial for quality control and assurance that the cell population is well-defined. The array of phenotypic markers implemented in the present study can track the survival, migration, and differentiation of the cells after transplantation (Borlongan, 2009). Phenotypic expression assays revealed that the hMSCs expressed CXCR4, Oct4, SSEA4, Nanog, and Nestin, indicating that the population of hMSCs employed embryonic as well as neuronal characteristics. The phenotype of all derived stem cells should be checked in both in vivo and in vitro experiments (Bużańska et al., 2002; Zigova et al., 2002). Our previous findings showed that menstrual blood may be an important source of autologous stem cells (Borlongan et al., 2010). These menstrual blood derived cells express embryonic like-stem cell phenotypic markers (Oct4, SSEA, Nanog) and neuronal phenotypic markers (Nestin, MAP2) (Borlongan et al., 2010). In contrast, identification of a similar cell population reveals the expression of embryonic and other intracellular cell surface markers such as Oct-4, SSEA-4, and c-kit, along with CD90, CD105, and CD166 (Patel and Silva, 2008).
Stem cells protect brain tissue from ischemic damage in vivo and in vitro (Chen et al., 2001a; Zhao et al., 2002; Ikeda et al., 2005; Borlongan, 2009; Dharmasaroja et al., 2009). The present study used the OGD model in vitro to assess the neuroprotective capabilities of hMSCs. Specifically, re-oxygenation of the cultured rat cells after OGD was used to mimic the ischemic conditions in vivo (Stasser and Fisher, 1995; Sáez-Valero et al., 2003). The OGD plus “reperfusion” in vitro model of ischemia is effective mainly through the apoptotic mechanism used to induce brain cell death (Wang et al., 2010). Using this model, the present findings support the protective effects of hMSCs on primary mixed rat cells of neurons and astrocytes injured by a 90-minute OGD followed by a 2-hour re-oxygenation.
Apoptosis, a prominent pathway of neuronal death in ischemic stroke, represents an important therapeutic target in the field of stroke therapy (Smrcka et al., 2003; Kawaguchi et al., 2004; Culmsee et al., 2005). Apoptotic processes occur differently in various regions of the ischemic zone (Didenko et al., 2002). In conditions of severe energy depletion, the reactions of cellular disassembly and packaging into apoptotic bodies are accomplished without either caspase-3 expression or the activation of caspase-3-dependent deoxyribonuclease. Apoptosis occurs in the surrounding penumbra region over a period of days to weeks. The possibility of rescuing these dying cells thus offers an attractive window for therapeutic intervention. Many neuroprotective strategies lie in the prevention of apoptosis for neurodegenerative diseases including stroke (Rau et al., 2003; Smrcka et al., 2003; Lovekamp-Swan et al., 2007). Transplantation of human embryonic neural stem cells into the cortical penumbra 24 hours after focal cerebral ischemia significantly reduces the number of TUNEL- and Bax-positive cells at 7 days post-grafting (Zhang et al., 2009). Moreover, MSC transplantation reduces the number of TUNEL+ cells and shows anti-apoptotic effects (Kim et al., 2008). Intravenous delivery of MSCs reduces infarct size and ameliorates functional deficits in rat cerebral ischemia models (Nomura et al., 2005). Animals treated with hMSCs in the ischemic boundary zone exhibit significantly fewer TUNEL-positive cells 7 days after ischemia (Liu et al., 2006), suggesting one of the mechanisms underlying the therapeutic effects of hMSCs (Chen et al., 2003). Bcl-2 has been reported as a transcription factor mediating the endogenous neuroprotective and anti-apoptotic mechanisms against stroke (Kitagawa et al., 1998). Our results showed that the Bcl-2 antibody, at a specific dose, blocked the hMSC-induced neuroprotection, which advances these previous findings by highlighting that the reduction of apoptosis elicited by stem cell transplantation involves the Bcl-2 pathway, and emphasizing this pathway as a therapeutic target for stroke and other neurodegenerative maladies.
Ischemic preconditioning, the process of self-protection initiated during OGD, has neuroprotective benefits for the injured tissues or cells. The present study found that the low dose of the Bcl-2 antibody enhanced the protective effect, even though it diminished the neuroprotective abilities of hMSCs at the medium to high doses. The possible relevance of these findings to the preconditioning mechanism represents a future direction that should be explored. To this end, multiple possible therapeutic pathways exist for cell therapy treatments of ischemic brain injury, including the possibility that the therapeutic cells may release factors that support the induction of brain remodeling and survival of at-risk cells (The STEPS Participants, 2009). This compelled us to explore whether the neuroprotective effect of hMSCs was related to the change in Bcl-2 protein levels in the supernatant. MSCs secrete growth factors, which are considered key to the benefits provided by transplanted MSCs in the ischemic brain (Chen et al., 2002; Ikeda et al., 2005). We hypothesized that increments of Bcl-2 protein secretion might exert protective effects against OGD, especially during the early phase in which there was likely a short increment of Bcl-2 secretion after OGD. However, we did not find any changes in Bcl-2 secretion in the supernatants despite adding various doses of the Bcl-2 antibody to the co-cultured rat cells, revealing that co-treatment with the Bcl-2 antibody did not alter the effect of hMSCs on Bcl-2 protein levels. Additional studies are warranted to elucidate the precise anti-apoptotic mechanism surrounding the observed neuroprotection.
The anti-apoptotic effect of stem cells serves as a major neuroprotective process for stroke therapy (Park et al., 2017; Liu et al., 2018; Yang et al., 2018). Such anti-apoptotic effects of hMSCs after acute ischemic stroke (Calio et al., 2014; Park et al., 2017; Zhang et al., 2018) entail multiple mechanisms such as secretion of growth factors and exosomes (Ghazavi et al., 2017; Cunningham et al., 2018), prevention of dysfunctional matrix metalloproteinases (MMP) (Chelluboina et al., 2017), stimulation of cell survival signaling pathways including phosphorylation of STAT3 and Akt (Schiebe et al., 2012), deactivation of cell death signals like caspase-3 (Stonesifer et al., 2017), and release of extracellular vesicles to increase blood flow (Bian et al., 2013). Probing the established PI3K/Akt apoptosis pathway supports its important role in the stroke neuroprotection afforded by stem cells (Schiebe et al., 2012; Huang et al., 2014; Liu et al., 2018). Moreover, inhibition of the Bcl-2 gene blocks the anti-apoptotic effects of stem cells (Calio et al., 2014; Yang et al., 2018). The present study further revealed, via pharmacological modulation, that the Bcl-2 signaling pathway, likely in tandem with the PI3K/Akt, closely participates in the anti-apoptotic action of stem cells against stroke.
In summary, hMSCs exert neuroprotection against stroke in vitro at least in part via an anti-apoptotic mechanism (Figure 5). Because stem cell therapy targets the secondary cell death that occurs several hours to days after stroke, a large number of patients may benefit from this therapy. Further studies are needed to assess the long-term safety and efficacy of hMSC transplant therapy in animal models of stroke.
Figure 5.
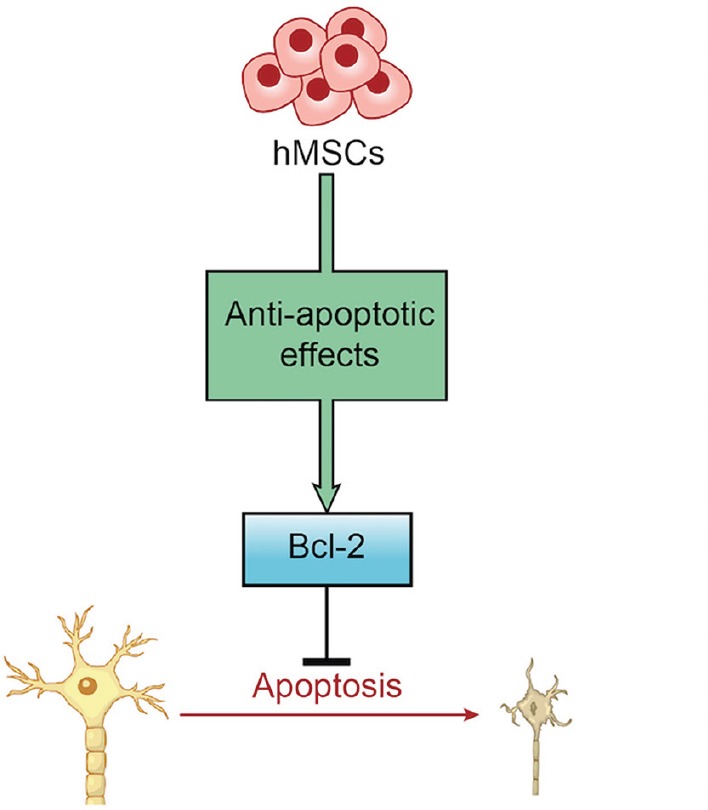
Diagram illustrating how hMSCs provide neuroprotective effects against ischemic stroke.
hMSCs exert anti-apoptotic effects via apoptosis-regulating pathways involving Bcl-2 that mitigates cerebral ischemia-induced apoptosis and provides neuroprotection against ischemic stroke. hMSCs: Human bone marrow mesenchymal stem cells.
Acknowledgments
We thank all the students and staff of the Borlongan Lab at USF Center of Excellence for Aging and Brain Repair for critical discussion of this study.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: Cesar V. Borlongan was funded by NIH R01NS071956, NIH R01 NS090962, NIH R21NS089851, NIH R21 NS094087, and VA Merit Review I01 BX001407. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Min Ye, Nanjing BenQ Hospital, China.
Funding: Cesar V. Borlongan was funded by NIH R01NS071956, NIH R01 NS090962, NIH R21NS089851, NIH R21 NS094087, and VA Merit Review I01 BX001407.
P-Reviewer: Ye M; C-Editor: Zhao M; S-Editor: Li CH; T-Editor: Liu XL
References
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2013;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40(3 Suppl):S146–148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bużańska L, Machaj EK, Zabłocka B, Pojda Z, Domańska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115(Pt 10):2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- Calio M, Marinho D, Ko G, Ribeiro R, Carbonel A, Oyama L, Ormanji M, Guirao T, Calio P, Reis L, Simones M, Nascimento T, Ferreira A, Bertoncini C. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med. 2014;70:141–154. doi: 10.1016/j.freeradbiomed.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Chelluboina B, Nalamolu K, Mendez G, Klopfenstein J, Pinson D, Wang D, Veeravalli K. Mesenchymal stem cell treatment prevents post-stroke dysregulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Cell Physiol Biochem. 2017;44:1360–1369. doi: 10.1159/000485533. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Cui HS, Matsumoto K, Murakami Y, Hori H, Zhao Q, Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B-cell lymphoma 2 phosphorylation suppression. Biol Pharm Bull. 2009;32:79–85. doi: 10.1248/bpb.32.79. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Redondo-Castro E, Allan S. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Didenko VV, Ngo H, Minchew CL, Boudreaux DJ, Widmayer MA, Baskin DS. Caspase-3-dependent and -independent apoptosis in focal brain ischemia. Mol Med. 2002;8:347–352. [PMC free article] [PubMed] [Google Scholar]
- Ghazavi H, Hoseini S, Bideskan A, Mashkani B, Mehri S, Ghorbani A, Sadri K, Mahdipour E, Ghasemi F, Forouzanfar F, Hoseini A, Pasdar A, Sadeghnia H, Mobarhan M. Fibroblast growth factor type 1 (FGF1)-overexpressed adipose-derived mesenchaymal stem cells (Ad-MSCFGF1) induce neuroprotection and functional recovery in a rat stroke model. Stem Cell Rev. 2017;13:670–685. doi: 10.1007/s12015-017-9755-z. [DOI] [PubMed] [Google Scholar]
- Huang P, Gebhart N, Richelson E, Brott T, Meschia J, Zubair A. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy. 2014;16:1336–1344. doi: 10.1016/j.jcyt.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake S. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- Kamal AK, Itrat A, Murtaza M, Khan M, Rasheed A, Ali A, Akber A, Akber Z, Iqbal N, Shoukat S, Majeed F, Saleheen D. The burden of stroke and transient ischemic attack in Pakistan: a community-based prevalence study. BMC Neurol. 2009;9:58. doi: 10.1186/1471-2377-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Tajiri N, Shojo H, Borlongan CV. Oxygen-glucose-deprived rat primary neural cells exhibit DJ-1 translocation into healthy mitochondria: a potent stroke therapeutic target. CNS Neurosci Ther. 2014;20:275–281. doi: 10.1111/cns.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Drummond JC, Cole DJ, Kelly PJ, Spurlock MP, Patel PM. Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth Analg. 2004;98:798–805. doi: 10.1213/01.ane.0000105872.76747.f6. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Seo JH, Bubien JK, Oh YS. Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuro report. 2002;13:1185–1188. doi: 10.1097/00001756-200207020-00023. [DOI] [PubMed] [Google Scholar]
- Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cho HS, Yang SH, Shin JY, Kim JS, Lee ST, Chu K, Roh JK, Kim SU, Park CG. Soluble mediators from human neural stem cells play a critical role in suppression of T-cell activation and proliferation. J Neurosci Res. 2009;87:2264–2272. doi: 10.1002/jnr.22050. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC, Hori M, Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischemia. Brain. 2006;129(Pt 10):2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li Y, Zhou L, Li Y, Xu P, Liu X, Lv Q, Li J, Guo H, Cai H, Sun R, Liu X. GRP78 promotes neural stem cell antiapoptosis and survival in response to oxygen-glucose deprivation (OGD)/reoxygenation through PI3K/Akt, ERK1/2, and NF-κB/p65 pathways. Oxid Med Cell Longev. 2018;2018:3541807. doi: 10.1155/2018/3541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Glendenning M, Schreihofer DA. A high soy diet reduces programmed cell death and enhances bcl-xL expression in experimental stroke. Neuroscience. 2007;148:644–652. doi: 10.1016/j.neuroscience.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K, Hess DC, Borlongan CV. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Allouard Q, Pettengell R. Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp Cell Res. 2004;295:350–359. doi: 10.1016/j.yexcr.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Nakano K, Migita M, Mochizuki H, Shimada T. Differentiation of transplanted bone marrow cells in the adult mouse brain. Transplantation. 2001;71:1735–1740. doi: 10.1097/00007890-200106270-00006. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Eng J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis J. I.V. Infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kim Y, Chang J, Yang Y, Oh W, Lee J, Park H, Kim D, Paek S. Effect of single and double administration of human umbilical cord blood-derived mesenchymal stem cells following focal cerebral ischemia in rats. Exp Neurobiol. 2017;26:55–65. doi: 10.5607/en.2017.26.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AN, Silva F. Menstrual blood stromal cells: the potential for regenerative medicine. Regen Med. 2008;3:443–444. doi: 10.2217/17460751.3.4.443. [DOI] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Böttner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Valero J, González-García C, Ceña V. Acetylcholinesterase activation in organotypic rat hippocampal slice cultures deprived of oxygen and glucose. Neurosci Lett. 2003;348:123–125. doi: 10.1016/s0304-3940(03)00790-0. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Karjalainen-Lindsberg ML, Paetau A, Ijäs P, Lindsberg PJ. Apoptosis dominant in the periinfarct area of human ischaemic stroke - a possible target of antiapoptotic treatments. Brain. 2006;129(Pt 1):189–199. doi: 10.1093/brain/awh645. [DOI] [PubMed] [Google Scholar]
- Schiebe F, Klein O, Lose J, Priller J. Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol. 2012;32:567–576. doi: 10.1007/s10571-012-9798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka M, Horký M, Otevrel F, Kuchtícková S, Kotala V, Muzík J. The onset of apoptosis of neurons induced by ischemia-reperfusion injury is delayed by transient period of hypertension in rats. Physiol Res. 2003;52:117–122. [PubMed] [Google Scholar]
- Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta S, Borlongan C. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser U, Fischer G. Protection from neuronal damage induced by combined oxygen and glucose deprivation in organotypic hippocampal cultures by glutamate receptor antagonists. Brain Res. 1995;687:167–174. doi: 10.1016/0006-8993(95)00519-v. [DOI] [PubMed] [Google Scholar]
- The STEPS Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Valery LF, Carlene MML, Derrick AB, Suzanne LB, Varsha P. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Gong Q, Wu Q, Shi J. Neuroprotective effects of Dendrobium alkaloids on rat cortical neurons injured by oxygen-glucose deprivation and reperfusion. Phytomedicine. 2010;17:108–115. doi: 10.1016/j.phymed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM Jr, Yu SP, Choi DW. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Wu C, Fujihara H, Yao J, Qi S, Li H, Shimoji K, Baba H. Different expression patterns of Bcl-2, Bcl-xl, and Bax proteins after sublethal forebrain ischemia in C57Black/Crj6 mouse striatum. Stroke. 2003;34:1803–1808. doi: 10.1161/01.STR.0000077255.15597.69. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang C, Chen H, Li L, Ma S, Wang H, Fu Y, Qu T. Neural stem cell-conditioned medium ameliorated cerebral ischemia-reperfusion injury in rats. Stem Cells Int 2018. 2018:4659159. doi: 10.1155/2018/4659159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Kang Q, Zhano J, Li W. Human neural stem cell transplantation attenuates apoptosis and improves neurological functions after cerebral ischemia in rats. Acta Anaesthesiol Scand. 2009;53:1184–1191. doi: 10.1111/j.1399-6576.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiol Dis. 2006;24:345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan C, Zhu J, Bao X, Tong Q, Wu X, Tang X, Xue T, Liu J, Zheng G, Wang Y. Additive neuroprotective effect of borneol with mesenchymal stem cells on ischemic stroke in mice. Front Physiol. 2018;8:1133. doi: 10.3389/fphys.2017.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- Zigova T, Song S, Willing AE, Hudson JE, Newman MB, Saporta S, Sanchez-Ramos J, Sanberg PR. Human umbilical cord blood cells express neural antigens after transplantation into the developing rat brain. Cell Transplant. 2002;11:265–274. [PubMed] [Google Scholar]