Abstract
Nerve growth factor (NGF) is a powerful trophic factor that provides essential support for the survival and differentiation of sympathetic and sensory neurons during development. However, NGF also activates nociceptors contributing significantly to inflammatory pain and neuropathic pain after tissue injury. As such anti-NGF based therapies represent a promising strategy for pain management. Because of dose-dependent serious side effects such as back pain, injection site hyperalgesia, clinical trials of using NGF to treat various disorders such as diabetic neuropathies, chemotherapy-induced and human immunodeficiency virus-associated peripheral neuropathies were all discontinued. Thus far, worldwide clinical applications of NGF in treating patients are very limited except in China. Hereditary sensory autonomic neuropathy type V (HSAN V) is an extremely rare disease. Genetic analyses have revealed that HSAN V is associated with autosomal recessive mutations in NGF. One of the mutations occurred at the 100th position of mature NGF resulting in a change of residue from arginine to tryptophan (R100W). Although those HSAN V patients associated with the NGFR100W mutation suffer from severe loss of deep pain, bone fractures and joint destruction, interestingly patients with the NGFR100W mutation do not show apparent cognitive deficits, suggesting important trophic support function is preserved. We believe that NGFR100W provides an ideal tool to uncouple the two important functions of NGF: trophic versus nociceptive. Studies from investigators including ourselves have indeed confirmed in animal testing that the NGFR100W no longer induced pain. More importantly, the trophic function seemed to be largely preserved in NGF harboring the R100W mutation. On the mechanistic level, we found that the NGFR100W mutation was capable of binding to and signaling through the tyrosine receptor kinase A receptor. But its ability to bind to and activate the 75 kDa neurotrophic factor was significantly diminished. The significance of these findings is at least two folds: 1) the NGFR100W mutation can be used as an alternative to the wildtype NGF to treat human conditions without eliciting pain; and 2) the 75 kDa neurotrophic factor may serve as a novel target for pain management. We will discuss all the details in this mini-review.
Keywords: hereditary sensory and autonomic neuropathy V, nerve growth factor, NGFR100W mutation, pain, tyrosine receptor kinase A, p75 neurotrophic factor receptor
Nerve growth factor (NGF) is the most thoroughly characterized neurotrophic factor being studied intensely over the past 60 years (Levi-Montalcini, 1964; Aloe et al., 2012). NGF provides support for the survival, development, and differentiation of both basal forebrain cholinergic neurons in the brain and peripheral neurons such as peripheral sensory neurons (Aloe et al., 2012). Because of its robust trophic function, NGF has been extensively investigated for its potential to treat neurodegenerative diseases such as Alzheimer’s disease, peripheral neuropathies such as diabetic neuropathy and human immunodeficiency virus-induced neuropathy, and more (Anand et al., 1996; Aloe et al., 2012). However, all clinical trials using NGF were discontinued largely due to significant side effects including back pain, injection site hyperalgesia, myalgia, and weight loss (Anand et al., 1996; Aloe et al., 2012). Significant pain induced by efficacious doses of NGF is a major obstacle in clinical application of NGF. We have performed a PubMed literature search of articles published in the period January 2009–November 2018 on the subject of hereditary sensory and autonomic neuropathy V involving mutations in NGF.
Recently, a homozygous mutation in NGF (NGFR100W) has been linked to consanguineous families with hereditary, sensory, and autonomic neuropathies type V (HSAN V) (Einarsdottir et al., 2004; Minde et al., 2004). HSAN V is an autosomal recessive genetic disorder characterized by sensory neuropathies accompanied by loss of pain. Clinical symptoms of HSAN V are similar to HSAN IV, which was associated with mutations in the tyrosine receptor kinase A (TrkA), a receptor for NGF. However, unlike HSAN IV patients, patients with HSAN V do not suffer from mental retardation and anhidrosis. This suggests the HSAN V NGF mutation retains important trophic functions, but without pain-eliciting effect. Careful studies on NGFR100W will likely yield important clues to uncoupling the two important biological functions of NGF: nociception versus trophic function.
The Cattaneo group examined binding/biochemical properties using NGFR100E, a mutation at the same site as NGFR100W (Covaceuszach et al., 2010; Capsoni et al., 2011). Their studies revealed that NGFR100E binding to TrkA is robust. But the binding affinity to p75NTR, the 75 kDa neurotrophic factor receptor for NGF, is substantially reduced. We were able to produce and characterize the naturally occurring mutant, NGFR100W, and have demonstrated that NGFR100W, like NGFR100E, failed to bind to and activate p75NTR-mediated signaling pathways (Sung et al., 2018). Ultimately, we were able to show that NGFR100W was as active as wildtype NGF (NGFWT) in binding and eliciting TrkA-mediated signaling pathways such as phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt and extracellular regulated protein kinase (Erk)1/2. NGFR100W was transported retrograde along the axon of dorsal root ganglion and supported dorsal root ganglion survival and differentiation (Sung et al., 2018), a function likely attributed to TrkA.
We tested the ability of NGFR100W to elicit painful responses both in vitro and in vivo, using NGFWT as a comparison. Our studies demonstrated that NGFWT induced acute potentiation of proton-evoked current in cultured dorsal root ganglion neurons by single cell voltage clamp, but NGFR100W failed to induce potentiation (Sung et al., 2018). Since such fast onset potentiation occurred through post translational change on nociceptive ion channels, failure of those local and acute modification of NGFR100W could be ascribed possibly to absence of p75NTR signaling. Indeed, the p75NTR is implicated in hyperalgesia as shown in many different studies: 1) binding of NGF to p75NTR activates sphingomyelin hydrolysis, which then liberates ceramide (Dobrowsky et al., 1994); 2) Ceramide is known to increase the number of action potentials in sensory neurons (Zhang et al., 2002, 2006); 3) Inhibition of c-Jun N-terminal kinase activity, another p75NTR-downstream signaling effector, was shown to reduce or block thermal hyperalgesia/allodynia (Doya et al., 2005; Obata et al., 2006; Zhuang et al., 2006); 4) Although NGF binds to p75NTR with a lower affinity than to the complex of TrkA/p75NTR, local NGF concentration can be high enough to activate p75NTR after injury or during inflammation (Heumann et al., 1987; Bengzon et al., 1992; Donovan et al., 1995; Widenfalk et al., 2001); 5) Moreover, the majority of small diameter sized nociceptors that express TrkA also co-express p75NTR, which suggests an important role of p75NTR in nociceptive signaling.
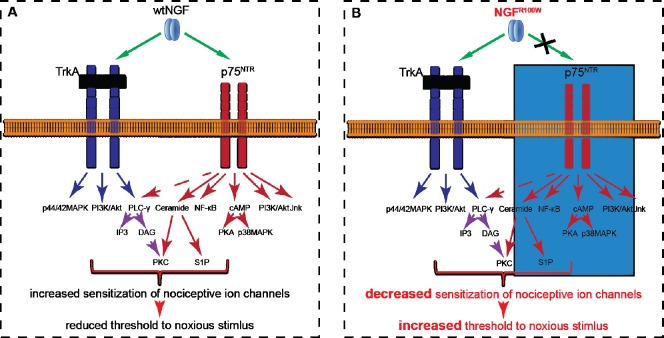
The failure of nociception by NGFR100W was confirmed in our in vivo study (Sung et al., 2018). Intraplantar injection of NGFWT into the hind paws of adult rats induced thermal and mechanical hypersensitization by decreasing the pain threshold significantly. NGFR100W at similar dosages did not induce both thermal and mechanical hyperalgesia (Sung et al., 2018). We also tested hyperaglesic priming, an indicator of the transition from acute to chronic pain: when neurons are unprimed, mild pro-nociceptive molecules cause hyperalgesia which disappears after 1 hour (Ferrari et al., 2015). On the other hand, when primed, this hyperalgesia can be prolonged to more than 4 hours. When tested for chronic pain, NGFR100W was still able to induce priming even 7 days after intraplantar injection, suggesting long-lasting neuroplastic change conceivably involves NGF-TrkA downstream signaling (Sung et al., 2018). Neuroplastic change such as priming involves transcriptional change likely accompanied by retrograde trafficking. This could explain late onset of pain, since the axon can exceed the length of one meter. This provides further evidence that the R100W mutation in NGFR100W does not appear to affect the retrograde TrkA signaling. Token together, our study has suggested that both TrkA and p75NTR contribute to potentiate pain response by wildtype NGF (Figure 1A). In the case NGFR100W, its inability to activate p75NTR signaling may result in an increase in pain threshold (Figure 1B).
Figure 1.
A proposed model underlying the loss of deep pain perception in HSAN V patients.
(A) NGF acts through both TrkA and p75NTR to induce intracellular signaling cascades such as PLC-γ to potentiate sensitization. (B) Although still capable of inducing TrkA signaling, NGFR100W no longer activates p75NTR downstream signaling effectors, which results an increase in pain threshold. HSAN V: Hereditary sensory autonomic neuropathy type V; NGF: nerve growth factor; PLC-γ: phospholipase C gamma; TrkA: tyrosine receptor kinase A; wt: wildtype; NF-κB: nuclear factor kappa B; cAMP: cyclic adenosine monophosphate; IP3: inositol 1,4,5-trisphosphate; DAG: diacylglycerol; PKA: protein kinase A; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; Jnk: c-Jun N-terminal kinase; PKC: protein kinase C; S1P: sphingosine-1-phosphate; MAPK: mitogen-activated protein kinase; R100W: a change of residue from arginine to tryptophan.
However, the question remains why HSAN IV patients lose pain sensation despite normal TrkA activation. Many studies support the notion that TrkA mediates nociceptive signaling. For example, intrathecal injection of antisense oligodeoxynucleotide for TrkA reduces NGF-induced hyperalgsia (Malik-Hall et al., 2005). Moreover, HSAN IV patients with CIPA (congenital insensitivity to pain with anhidrosis), which is associated with numerous mutations in TrkA, suggests that TrkA signaling does mediate hypersensitivity (Indo, 2001). Furthermore, many effectors downstream of TrkA have been implicated in transmitting NGF-induced hyperalgesia. Administration of Erk inhibitors, such as U0126, attenuates thermal hyperalgesia induced in a capsaicin dose dependent manner (Aley et al., 2001). Given that NGFR100W activated Erk1/2 is similar to NGFWT, it is unclear why NGFR100W failed to induce acute sensitization. One possible explanation is that TrkA downstream pathways involved in sensing pain could be negatively affected by intertwined communication between p75NTR and TrkA. When we inhibited both TrkA and p75NTR in our in vivo study, we observed a synergistic effect in inhibiting both acute and chronic pain when compared to the use of single inhibitor. These studies suggest the crosstalk between p75NTR and TrkA helps to mediate and regulate the pain threshold. Based on these observations, we speculate that both NGF receptors, TrkA and p75NTR, mediate pain signaling. But, their relative contribution to peripheral sensitization is most likely dependent on many variable conditions such as the ratio between TrkA and p75NTR at the cell surface.
Clinical studies of HSAN V patients showed orthopedic complications, such as bone fracture, due to loss of pain mainly in distal legs and arms (Einarsdottir et al., 2004; Minde et al., 2004). Their sural nerve biopsy also revealed a reduced number of C fibers and moderate loss of A delta neurons. Nerve fibers positive transient receptor potential cation channel (TRPV)1, TRPV2, TRPV8, calcitonin gene-related peptide and substance P were substantially reduced in both homozygous and heterozygous patients (Axelsson et al., 2009). Considering that production and secretion of mature NGFR100W was educed in many sources (Axelsson et al., 2009; Covaceuszach et al., 2010; Capsoni et al., 2011; Sung et al., 2018), atrophy, or developmental deficit of peripheral sensory fibers, appears due to insufficient NGF availability.
Interestingly, the HSAN V patients still reported visceral pain, such as abdominal pain, despite of lack of pain in arms and legs (Einarsdottir et al., 2004). Visceral pains differ from somatic pain (inflammatory lesion of skin, muscle, joints, or peripheral nerve injury) in several points including: 1) pain could be induced by a psychophysical condition; 2) visceral pain is generally dull and its location hard to pinpoint. Taking those major differences into consideration, the mechanism underlying visceral and somatic pains are likely different. Therefore, the impact of NGFR100W in HSAN V patients is expected to be mainly associated with loss of somatic pain, but not much visceral pain.
In summary, the well-established pain-inducing effect of NGF is the predominant hurdle in using NGF to treat human diseases and conditions. Uncoupling pain from trophic function of NGF will thus allow various clinical trials to fully utilize the benefits of NGF as a therapeutic drug without unwanted side effects. Detailed characterization of NGFR100W will undoubtedly help to uncover novel target(s) and means for accomplishing our eventual goal.
Additional file: Open peer review report 1 (91.3KB, pdf) .
Acknowledgments
We thank Ms Jesse Kim (University of California San Diego, USA) for copyediting of the manuscript.
Footnotes
Conflicts of interest: The authors have no actual or potential conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Armin Blesch, Indiana University School of Medicine, USA.
P-Reviewer: Blesch A; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- Axelsson HE, Minde JK, Sonesson A, Toolanen G, Hogestatt ED, Zygmunt PM. Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience. 2009;162:1322–1332. doi: 10.1016/j.neuroscience.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Soderstrom S, Kokaia Z, Kokaia M, Ernfors P, Persson H, Ebendal T, Lindvall O. Widespread increase of nerve growth factor protein in the rat forebrain after kindling-induced seizures. Brain Res. 1992;587:338–342. doi: 10.1016/0006-8993(92)91016-8. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Covaceuszach S, Marinelli S, Ceci M, Bernardo A, Minghetti L, Ugolini G, Pavone F, Cattaneo A. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One. 2011;6:e17321. doi: 10.1371/journal.pone.0017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaceuszach S, Capsoni S, Marinelli S, Pavone F, Ceci M, Ugolini G, Vignone D, Amato G, Paoletti F, Lamba D, Cattaneo A. In vitro receptor binding properties of a “painless” NGF mutein, linked to hereditary sensory autonomic neuropathy type V. Biochem Biophys Res Commun. 2010;391:824–829. doi: 10.1016/j.bbrc.2009.11.146. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- Doya H, Ohtori S, Fujitani M, Saito T, Hata K, Ino H, Takahashi K, Moriya H, Yamashita T. c-Jun N-terminal kinase activation in dorsal root ganglion contributes to pain hypersensitivity. Biochem Biophys Res Commun. 2005;335:132–138. doi: 10.1016/j.bbrc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13:799–805. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Reichling DB, Levine JD. Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci. 2015;35:495–507. doi: 10.1523/JNEUROSCI.5147-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo Y. Molecular basis of congenital insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum Mutat. 2001;18:462–471. doi: 10.1002/humu.1224. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor. Ann N Y Acad Sci. 1964;118:149–170. doi: 10.1111/j.1749-6632.1964.tb33978.x. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Minde J, Toolanen G, Andersson T, Nennesmo I, Remahl IN, Svensson O, Solders G. Familial insensitivity to pain (HSAN V) and a mutation in the NGFB gene. A neurophysiological and pathological study. Muscle Nerve. 2004;30:752–760. doi: 10.1002/mus.20172. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Noguchi K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J Neurosci. 2006;26:11974–11986. doi: 10.1523/JNEUROSCI.3188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K, Ferrari LF, Yang W, Chung C, Zhao X, Gu Y, Lin S, Zhang K, Cui B, Pearn ML, Maloney MT, Mobley WC, Levine JD, Wu C. Swedish nerve growth factor mutation (NGF(R100W)) defines a role for TrkA and p75(NTR) in nociception. J Neurosci. 2018;38:3394–3413. doi: 10.1523/JNEUROSCI.1686-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006;575:101–113. doi: 10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.