Keywords: nerve regeneration, human traumatic brain injuries, long noncoding RNA, messenger RNA, GO analysis, real-time quantitative polymerase chain reaction, biomarkers, microarray analysis, biological processes, medical informatics, neural regeneration
Abstract
Long non-coding RNAs (lncRNAs) play a key role in craniocerebral disease, although their expression profiles in human traumatic brain injury are still unclear. In this regard, in this study, we examined brain injury tissue from three patients of the 101st Hospital of the People’s Liberation Army, China (specifically, a 36-year-old male, a 52-year-old female, and a 49-year-old female), who were diagnosed with traumatic brain injury and underwent brain contusion removal surgery. Tissue surrounding the brain contusion in the three patients was used as control tissue to observe expression characteristics of lncRNAs and mRNAs in human traumatic brain injury tissue. Volcano plot filtering identified 99 lncRNAs and 63 mRNAs differentially expressed in frontotemporal tissue of the two groups (P < 0.05, fold change > 1.2). Microarray analysis showed that 43 lncRNAs were up-regulated and 56 lncRNAs were down-regulated. Meanwhile, 59 mRNAs were up-regulated and 4 mRNAs were down-regulated. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses revealed 27 signaling pathways associated with target genes and, in particular, legionellosis and influenza A signaling pathways. Subsequently, a lncRNA-gene network was generated, which showed an absolute correlation coefficient value > 0.99 for 12 lncRNA-mRNA pairs. Finally, quantitative real-time polymerase chain reaction confirmed different expression of the five most up-regulated mRNAs within the two groups, which was consistent with the microarray results. In summary, our results show that expression profiles of mRNAs and lncRNAs are significantly different between human traumatic brain injury tissue and surrounding tissue, providing novel insight regarding lncRNAs’ involvement in human traumatic brain injury. All participants provided informed consent. This research was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-TCC-13004002) and the protocol version number is 1.0.
Chinese Library Classification No. R446; R604
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability in adults and imposes an enormous economic and health burden on the world every year (Chen and D’Esposito, 2010). Although many resources have been invested in researching TBI, its prognosis is still poor (Holloway and Quill, 2010). Consequently, an increasing number of studies have sought to investigate the specific mechanisms of TBI. Previous studies have shown that primary and secondary injury are two pathological processes of TBI (Ghajar, 2000; Hirschi et al., 2017). Secondary injury is reversible compared with primary injury and is considered to be the main cause of death after TBI. Accordingly, a previous study on the mechanism of secondary injury after brain trauma has received much attention in recent years (Kan et al., 2012). Primary injury is caused by immediate mechanical insult, which leads to focal contusions, further axonal injury, and hematomas (Liu and Wang, 2017). In contrast, secondary injury results from delayed metabolic, biochemical, and cellular changes that are initiated by the primary insult, and it can subsequently trigger microglia activation, astrocyte proliferation, and neuronal cell death (Shi et al., 2011). Despite this understanding, the underlying cellular and molecular mechanisms of TBI remain unclear.
Previous studies have confirmed a number of pathways and genes that are involved in brain injury, including GPR56 (Giera et al., 2015), the Cdk2–p27Kip1 pathway, and Wnt/β-catenin signaling (Fancy et al., 2014; Salmaso et al., 2014). However, increasing evidence shows that despite the presence of protein coding genes, many noncoding RNAs (ncRNAs) that were previously thought to be ‘‘junk RNAs” are also involved in many diseases and biological processes (Chen et al., 2016). Indeed, these RNAs directly impact upon mRNA translation (Twiss et al., 2016). NcRNAs consists of two main classes: long ncRNAs (lncRNAs), which are a new class of non-protein coding transcripts > 200 nucleotides in length; and small ncRNAs, with lengths < 200 nucleotides (e.g., microRNAs) (Jiang et al., 2015). LncRNAs are involved in regulating cell biological behavior. Previous studies have shown that lncRNAs play an important role in various pathophysiological processes of brain diseases. Further, targeting lncRNAs can effectively reverse progression of brain tumors, Alzheimer’s disease, and ischemic stroke (Dharap et al., 2012; Tan et al., 2013). A study also showed that lncRNAs might be involved in methamphetamine-induced neuronal apoptosis by modulating neuronal coding genes (Xiong et al., 2017). In addition, lncRNAs are strongly associated with nervous system development and neurodegenerative diseases (Roberts et al., 2014; D’Haene et al., 2016; Quan et al., 2017). LncRNAs not only have specific expression profiles in brain tissue but also can be used as potential independent prognostic molecular markers (Mercer et al., 2008). Altogether, these studies show that lncRNAs may play an important role in regulating pathological processes of brain diseases. Discovery of lncRNAs and their functional studies in regulatory networks may help us further understand disease pathogenesis. Recently, Zhong et al. (2016) reported that a large amount of lncRNAs are markedly down-regulated or up-regulated in the cortex surrounding the injury region of mice after TBI.
However, as is commonly known, mouse genes and the pathophysiology of brain damage are different compared with humans. Thus, if human brain injury tissue is used to with a lncRNA chip, the findings will be closer to the real situation in human brain injury. Accordingly, to determine the potential role of lncRNAs in human TBI, we performed a comprehensive analysis of mRNA and lncRNA profiles in human TBI tissue and adjacent brain contusion tissue. Moreover, Kyoto Encyclopedia of Genes and Genomes (KEGG) databases and Gene Ontology (GO) were used to analyze differentially expressed mRNAs and determine their biological functions. By constructing a co-expression network, the relationship between differential expression of mRNAs and lncRNAs was analyzed.
This is the first report to investigate the expression profiles of mRNAs and lncRNAs in human TBI by microarray. These differentially expressed lncRNAs may play a partial or key role in progression and/or development of human TBI. In the future, more work is needed to determine if these lncRNAs can be used as novel therapeutic targets for human TBI.
Subjects and Methods
Design
This is an observational study. This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Additional file 1 (153KB, pdf) ).
Patient samples and RNA extraction
Paired brain contusion tissue and adjacent brain contusion tissue were retrospectively randomly collected from three patients with TBI.
Diagnostic criteria
All patients were diagnosed with severe TBI, and underwent surgery at the 101st Hospital of Chinese People’s Liberation Army, China, from January to August 2016. The study protocol was approved by the Medical Ethics Committee of the 101st Hospital of Chinese People’s Liberation Army (approval number: 2016-YXLL-008; Time of Ethics approval: 2016-01-01). Severe TBI mainly refers to: (1) extensive brain contusion, extensive skull fracture, brain stem injury or intracranial hematoma; (2) loss of consciousness for > 6 hours and Glasgow Coma Scale of 3 to 8; and (3) obvious positive signs of nervous system dysfunction, and obvious changes in vital signs (Brain Trauma Foundation, American Association of Neurological Surgeons and Congress of Neurological Surgeons, 2007; Jiang et al., 2014).
Inclusion criteria
(1) The age range was 30–60 years. (2) The Glasgow Coma Scale score at admission was 6–8 points. (3) The cause of TBI was a traffic accident. (4) Craniotomy indications and severe TBI diagnostic criteria refer to TBI treatment guidelines (Brain Trauma Foundation, American Association of Neurological Surgeons and Congress of Neurological Surgeons, 2007).
Exclusion criteria
(1) Brain hernia, shock, or unstable signs of life at hospital admission. (2) Acute brain swelling during surgery. (3) Serious underlying diseases (such as thrombocytopenia and cancer). (4) The family refused to undergo surgery. Clinical and pathological information for the patients is listed in Table 1.
Table 1.
Clinical characteristics of patients with traumatic brain injury
| Patient | Age (year) | Sex | Diagnosis | Brain tissue contusion location | Adjacent brain tissue contusion location | Other illness |
|---|---|---|---|---|---|---|
| 1 | 52 | Female | Severe traumatic brain injury | Right temporal | Right temporal | Hypertension |
| 2 | 36 | Male | Severe traumatic brain injury | Left frontal | Left frontal | / |
| 3 | 49 | Female | Severe traumatic brain injury | Right frontotemporal | Right frontotemporal | / |
Brain contusion tissue and adjacent brain contusion tissue were extracted from each patient. Adjacent brain contusion tissue was labeled as the control group, and brain contusion tissue labeled as the TBI group. Samples were obtained with informed consent. If the patient’s injury was too serious for self-approval, consent was provided by the patient’s guardian or entrusted agent. In the absence of removal of normal brain tissue, adjacent brain contusion tissue was defined as the tissue 1.5 cm from the brain contusion. During the operation, bipolar electrocoagulation was first used to extract contusion tissue from the center of the contusion area, and then adjacent brain contusion tissue was extracted from the non-functional area next to the contusion. Specimens were immediately frozen in liquid nitrogen and stored at −80°C after surgical removal. A total of 100 mg brain tissue was taken, and 1 mL RNA extraction reagent, Trizol (Invitrogen, Carlsbad, CA, USA), was added. Total RNA was exacted from brain contusion tissue and adjacent brain contusion tissue according to the manufacturer’s instructions. RNA was quantified using a NanoDrop 1000. RNA integrity was assessed by standard denaturing agarose gel electrophoresis.
Gene microarray
Arraystar Human lncRNA Microarray version 2.0 (Aksomics, Shanghai, China) was designed for global profiling of human lncRNAs and protein coding transcripts. LncRNAs were collected from authoritative databases including RefSeq, Ensembl, and University of California Santa Cruz (UCSC) Known Genes (Chen et al., 2013; Volders et al., 2013). Differentially expressed lncRNAs and mRNAs showing statistical significance between the two groups were identified by P-value, false discovery rate, and fold change filtering. Hierarchical clustering and combined analysis were performed using homemade scripts.
Gene ontology and KEGG pathway analysis
Gene ontology (GO, http://www.geneontology.org/) is a main bioinformatics tool for describing gene and gene products across all species. It provides an ontology of defined terms representing gene product properties, and covers three domains: biological processes, molecular functions, and cellular components (Gene Ontology, 2006). Two-sided Fisher’s exact test was used to detect overlap between the differentially expressed list and GO annotation list that was greater than expected by chance. False discovery rate was calculated to correct P-values. Enrichment scores were calculated among differentially expressed genes. As the enrichment increases, the corresponding function is more specific. Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) pathways were also used to map differentially expressed genes. Enrichment and statistics calculations were similar to GO analysis.
Co-expression network construction
The lncRNA-mRNA co-expression network was constructed based on correlation between differentially expressed lncRNAs and mRNAs. An algorithm from a previous study was used (Yu et al., 2012). Red color represents up-regulated mRNAs or lncRNAs, and blue color represents down-regulated mRNAs or lncRNAs. Node size represents the degree of change. Solid line indicates a positive correlation, and dashed line indicates a negative correlation.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from brain contusion tissue and adjacent brain contusion tissue using an RNAsimple Total RNA kit (TIANGEN Biotech, Beijing, China). Total RNA was reverse-transcribed using PrimeScript™ RT reagent kit with gDNA Eraser. qRT-PCR was performed using SYBR® Premix Ex Taq™ II and a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) (Wang et al., 2017). Primer sequences are listed in Table 2. Relative quantification of gene expression was calculated using the formula: 2–ΔΔCt, ΔΔCt = (Cttarget gene− Ctβ-actin)injury − (Cttarget gene− Ctβ-actin)injury surrounding. Three independent experiments were performed for each condition.
Table 2.
Primer sequences for quantitative real-time polymerase chain reaction analysis in brain contusion tissue and adjacent brain contusion tissue
| Gene | Sequence (5’–3’) | Product size (bp) |
|---|---|---|
| HSPA1B | Up-stream: CCA TTG AGG AGG TGG ATT AGG G | 100 |
| Down-stream: ATG ACA TAG GAA AAC AGC AGC A | ||
| PPBP | Up-stream: GAA CTC CGC TGC ATG TGT AT | 100 |
| Down-stream: CGA CTT GGT TGC AAT GGG TT | ||
| HSPA1A | Up-stream: TGG AGC TTC AAG ACT TTG CAT | 100 |
| Down-stream: GCA AGT TCA GTA CTT CAC CAA A | ||
| IL-8 | Up-stream: CCA GGA AGA AAC CAC CGG AA | 100 |
| Down-stream: GCA AGT TCA GTA CTT CAC CAA A | ||
| S100A12 | Up-stream: GGG GTT AAC ATT AGG CTG GGA | 100 |
| Down-stream: GCA AGT TCA GTA CTT CAC CAA A | ||
| Actin | Up-stream: AAC AAC GCA TCT CAT ATT TGG AA | 125 |
| Down-stream: AAC AAC GCA TCT CAT ATT TGG AA |
HSPA1B: Heat shock protein family A (Hsp70) member 1B; PPBP: pro-platelet basic protein; HSPA1A: heat shock protein family A (Hsp70) member 1A; IL-8: interleukin 8 (also known as C-X-C motif chemokine ligand 8 [CXCL8]); S100A12: S100 calcium binding protein A12; bp: base pair.
Statistical analysis
All data are presented as the mean ± SEM. Statistical analyses were performed using SPSS 20 software (IBM, Armonk, NY, USA). Differences between the control group and TBI group were analyzed using Student’s t-test. Spearman correlation analysis was used to analyze co-expression relationships between lncRNAs and mRNAs (Li et al., 2016). A value of P < 0.05 was accepted as statistically significant.
Results
Differentially expressed lncRNAs and mRNAs between control and TBI groups
LncRNA and mRNA expression profiles were detected in three TBI and three control groups. Volcano plot filtering identified 99 differentially expressed lncRNAs (P < 0.05, fold change > 1.2) (Figure 1A and B). Among them, 43 lncRNAs were up-regulated and 56 lncRNAs were down-regulated. In the TBI group, n333955, n332943, n335470, ENST00000384390, and n341115 were the five most significantly up-regulated lncRNAs. While, TCONS_00018733-XLOC_008489, OTTHUMT00000076953, NR_029967, ENST00000433249, and n381234 were the five most significantly down-regulated lncRNAs (Table 3). Next, we compared mRNA expression profiles of the TBI group with the control group.
Figure 1.
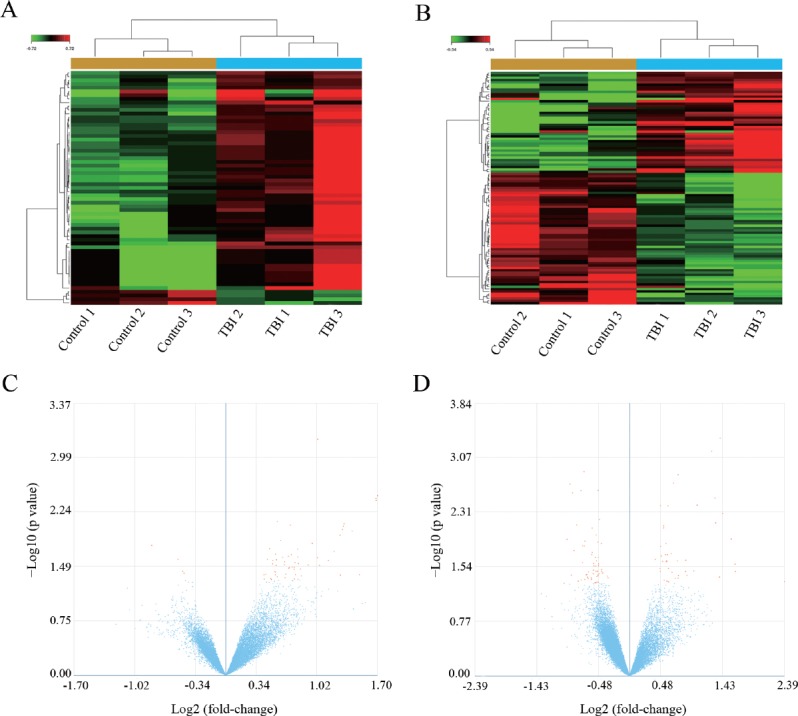
Differential expression profiles of mRNAs and lncRNAs in TBI and control groups.
(A, B) Heat maps comparing expression profiles of mRNAs (A) and lncRNAs (B) in TBI and control groups (> 1.2-fold change; P < 0.05). Green represents down-regulated genes and red represents up-regulated genes. (C, D) Volcano plot of P values as a function of weighted fold change for mRNAs (C) and lncRNAs (D) in frontotemporal tissue of TBI and control groups. The vertical dotted line delimits up- and down-regulation. Red plots represent significant mRNAs and lncRNAs with > 1.2-fold change and corrected P < 0.05. Each sample was analyzed in triplicate. TBI: Traumatic brain injury; mRNAs: messenger RNAs; lncRNAs: long non-coding RNAs.
Table 3.
Top five up-regulated and down-regulated lncRNAs in frontotemporal traumatic brain injury tissue
| LncRNA | log2 (fold change) | P |
|---|---|---|
| Top five up-regulated lncRNAs | ||
| n333955 | 5.240 | 0.047 |
| n332943 | 3.103 | 0.034 |
| n335470 | 3.094 | 0.027 |
| ENST00000384390 | 2.970 | 0.012 |
| n341115 | 2.635 | 0.000 |
| Top five down-regulated lncRNAs | ||
| TCONS_00018733-XLOC_008489 | –2.017 | 0.050 |
| OTTHUMT00000076953 | –1.956 | 0.012 |
| NR_029967 | –1.892 | 0.002 |
| ENST00000433249 | –1.868 | 0.038 |
| n381234 | –1.843 | 0.003 |
Gene symbols for ENST00000384390, OTTHUMT00000076953, and NR_029967 are SNORD14D, HCG24 and MIR329-1, respectively.
In total, we identified 63 differentially expressed mRNAs (P < 0.05, fold change > 1.2) (Figure 1C and D). Among them, 59 mRNAs were up-regulated and 4 mRNAs were down-regulated. In the TBI group, heat shock protein family A (Hsp70) member 1B (HSPA1B), pro-platelet basic protein (PPBP), heat shock protein family A (Hsp70) member 1A (HSPA1A), C-X-C motif chemokine ligand 8 (CXCL8; also known as interleukin 8 (IL-8), and S100 calcium binding protein A12 (S100A12) were the five most significantly up-regulated mRNAs. Olfactory receptor family 7 subfamily A member 5 (OR7A5), retinoic acid receptor responder 3 (RARRES3), meiotic nuclear divisions 1 (MND1), and major histocompatibility complex, class II, and DR Beta 3 (HLA-DRB3) were the four most significantly down-regulated mRNAs (Table 4).
Table 4.
Top five up-regulated and top four down-regulated mRNAs in frontotemporal traumatic brain injury tissue
| Accession number of mRNA | Gene name | log2 (fold change) | P |
|---|---|---|---|
| Top five up-regulated RNAs | |||
| NM_005346 | HSPA1B | 3.260 | 0.003 |
| NM_002704 | PPBP | 2.830 | 0.042 |
| NM_005345 | HSPA1A | 2.513 | 0.008 |
| NM_000584 | IL-8 | 2.443 | 0.042 |
| NM_005621 | S100A12 | 2.223 | 0.027 |
| Top four down-regulated mRNAs | |||
| NM_017506 | OR7A5 | –1.777 | 0.017 |
| NM_004585 | RARRES3 | –1.450 | 0.026 |
| NM_001253861 | MND1 | –1.398 | 0.038 |
| BC001023N | HLA-DRB3 | –1.383 | 0.040 |
HSPA1B: Heat shock protein family A (Hsp70) member 1B; HSPA1A: heat shock protein family A (Hsp70) member 1A; PPBP: pro-platelet basic protein; IL-8: interleukin 8 (also known as C-X-C motif chemokine ligand 8 [CXCL8]); S100A12: S100 calcium binding protein A12; OR7A5: olfactory receptor family 7 subfamily A member 5; RARRES3: retinoic acid receptor responder 3; MND1: meiotic nuclear divisions 1; HLA-DRB3: major histocompatibility complex, class II, DR beta 3.
To further examine these differentially expressed genes, we selected genes that changed > 1.2-fold to build a hierarchical clustering map. As shown in Figure 1A and B, the three TBI groups clustered together in one group, which was mostly distinct from the control group. Overall, changes in the state from control group to TBI group were also found to be separated by differences in expression profiles of mRNAs or lncRNAs (Figure 1). These results show that potential dynamic interactions between coding RNAs and lncRNAs may remold the whole transcriptomic landscape during the pathological process of TBI.
GO and KEGG pathway analyses
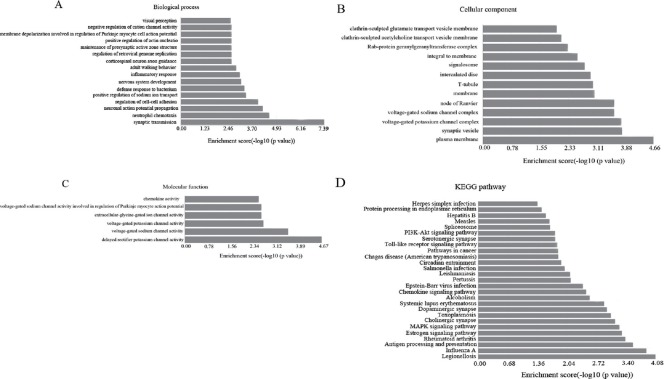
GO analysis of the differentially expressed genes was performed to identify the attributes of gene products in cellular components, biological processes, and molecular functions (Shi et al., 2015). GO analysis showed that the differentially expressed mRNAs were primarily enriched for synaptic transmission, neutrophil chemotaxis, and neuronal action potential propagation (related to biological processes) (Figure 2A), plasma membrane, synaptic vesicle, and voltage-gated potassium channel complex (related to cellular components) (Figure 2B), and voltage-gated potassium channel activity, voltage-gated sodium channel activity, and delayed rectifier potassium channel activity (related to molecular functions) (Figure 2C). Next, we performed pathway analysis using the KEGG database. Dysregulated mRNAs were associated with 27 biological pathways, including legionellosis, influenza A, antigen processing and presentation, rheumatoid arthritis, and the estrogen signaling pathway (Figure 2D). Altogether, these results suggest that the target genes are linked to 27 signaling pathways, especially those involved in legionellosis and influenza A signaling pathways. The top 10 KEGG pathways related to the target genes are listed in Table 5.
Figure 2.
Enrichment analysis of pathways and GO terms for differentially expressed mRNAs in frontotemporal TBI tissue.
(A–C) GO analysis according to biological process, cellular component, and molecular function, respectively. (D) Pathway analysis based on KEGG database. A value of P < 0.05 using two-sided Fisher’s exact test was defined as statistically significant.
Table 5.
Top 10 KEGG pathways related to target genes in frontotemporal traumatic brain injury tissue
| Signaling pathway | Count | P | Gene |
|---|---|---|---|
| Legionellosis | 3 | 0.000 | |HSPA1B|IL-8|HSPA1A| |
| Influenza A | 4 | 0.000 | |IL8|HLA-DRB3|HSPA1B|HSPA1A| |
| Antigen processing and presentation | 3 | 0.000 | |HLA-DRB3|HSPA1B|HSPA1A| |
| Rheumatoid arthritis | 3 | 0.000 | |FOS|IL8|HLA-DRB3| |
| Estrogen signaling pathway | 3 | 0.000 | |HSPA1B|FOS|HSPA1A| |
| MAPK signaling pathway | 4 | 0.001 | |FOS|HSPA1B|FGF12|HSPA1A| |
| Cholinergic synapse | 3 | 0.001 | |GNG3|FOS|KCNQ5| |
| Toxoplasmosis | 3 | 0.001 | |HLA-DRB3|HSPA1B|HSPA1A| |
| Dopaminergic synapse | 3 | 0.001 | |SCN1A|GNG3|FOS| |
| Systemic lupus erythematosus | 3 | 0.001 | |HIST1H2BG|HIST1H2AE|HLA-DRB3| |
HSPA1B: Heat shock protein family A (Hsp70) member 1B; HSPA1A: heat shock protein family A (Hsp70) member 1A; IL-8: interleukin 8 (also known as C-X-C motif chemokine ligand 8 [CXCL8]); HLA-DRB3: major histocompatibility complex, class II, DR beta 3; FOS: Fos proto-oncogene; FGF12: fibroblast growth factor 12; GNG3: G protein subunit gamma 3; KCNQ5: potassium voltage-gated channel subfamily Q member 5; SCN1A: sodium voltage-gated channel alpha subunit 1; HIST1H2BG: histone cluster 1 H2B family member G; HIST1H2AE: histone cluster 1 H2A family member E.
Establishment of a lncRNA-mRNA co-expression network
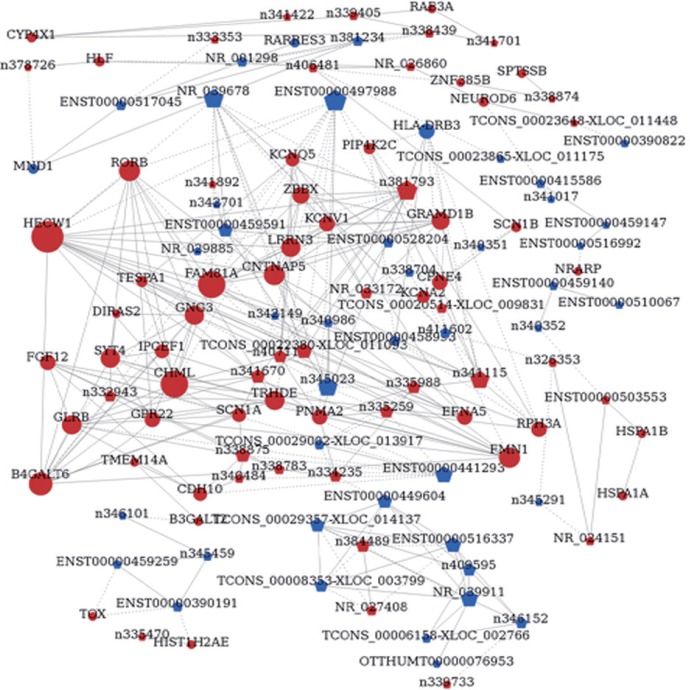
Building lncRNA regulatory networks may help high throughput analysis of interactions between lncRNAs and genes. Using NCBI software, one lncRNA-gene network was established on the basis of interactions between lncRNAs and genes (Figure 3). We found 12 lncRNA-mRNA pairs with absolute correlation coefficient values > 0.99. Most pairs (9/12) were positively correlated, and the remaining (3/12) pairs negatively correlated. The top 8/12 lncRNA-mRNA pairs with correlation coefficients > 0.99 are shown in Table 6. These results show that there is a close relationship between lncRNAs and mRNAs.
Figure 3.
LncRNA-mRNA network analysis of frontotemporal traumatic brain injury tissue.
Dots represent mRNAs, and pentagons represent lncRNAs. Red color represents up-regulated mRNA or lncRNA, and blue color represents down-regulated mRNA or lncRNA. Node size represents the degree of change. Solid line indicates a positive correlation, and dashed line indicates a negative correlation.
Table 6.
Top 8 lncRNA-mRNA pairs with correlation coefficients > 0.99 in frontotemporal traumatic brain injury tissue
| mRNA | LncRNA | COR |
|---|---|---|
| Positive correlation | ||
| KCNV1 | NR_0331 | 0.996 |
| FGF12 | n338875 | 0.995 |
| CDH10 | n340484 | 0.995 |
| HLA-DRB3 | ENST00000458993 | 0.995 |
| CDH10 | n338875 | 0.994 |
| Negative correlation | ||
| TOX | ENST00000459259 | –0.991 |
| CDH10 | ENST00000441293 | –0.994 |
| PIP4K2C | ENST00000458993 | –0.996 |
COR: Correlation coefficients; KCNV1: potassium voltage-gated channel modifier subfamily V member 1; FGF12: fibroblast growth factor 12; CDH10: cadherin 10; HLA-DRB3: major histocompatibility complex, class II, DR beta 3; TOX: thymocyte selection associated high mobility group box; PIP4K2C: phosphatidylinositol-5-phosphate 4-kinase type 2 gamma.
Expression levels of mRNAs measured by qRT-PCR
To confirm our microarray data, five up-regulated mRNAs (HSPA1B, PPBP, HSPA1A, IL8, and S100A12) were chosen, and their expression levels were further examined by qRT-PCR. CT values were normalized to actin. The chosen mRNAs show differential expression in the TBI group compared with the control group (Figure 4), confirming our microarray results.
Figure 4.
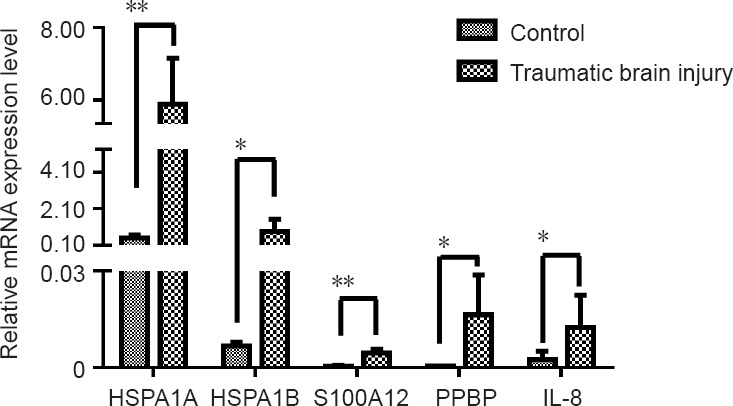
Quantitative real-time polymerase chain reaction of mRNA expression in frontotemporal traumatic brain injury tissue.
Expression levels of the five mRNAs shown are consistent with microarray data. All results are expressed as the mean ± SD of three independent experiments (*P < 0.05, **P < 0.01; Student’s t-test). HSPA1A: Heat shock protein family A (Hsp70) member 1A; HSPA1B: heat shock protein family A (Hsp70) member 1B; S100A12: S100 calcium binding protein A12; PPBP: pro-platelet basic protein; IL-8: interleukin 8 (also known as C-X-C motif chemokine ligand 8 [CXCL8]).
Discussion
TBI is a problem of epidemic magnitude involving both civilian, military service members, and professional athletes. In the United States, more than 1.3 million emergency department visits account for TBI, while TBI accounts for a third of almost all injury-related deaths. It is estimated that the annual financial burden of TBI in the United States is $76.5 billion, including lifetime direct medical expenses and productivity losses (Rockhill et al., 2012). TBI can also result in a variety of sensory and motor deficits, emotional impairments, and sleep disturbances (Qu et al., 2016). Neurological effects of TBI include primary injury events occurring at the time of trauma, as well as subsequent cell death and neuronal tissue damage caused by secondary injury events (Loane and Faden, 2010; Algattas and Huang, 2013; Simon et al., 2017). These sequelae and injuries cause many difficulties for the daily lifes of patients. In recent years, mechanistic research on TBI has become a focus, as the findings can help alleviate tissue damage caused by secondary injury and improve prognosis of TBI. For example, in an in vitro TBI model, neuronal apoptosis was reduced by up-regulation of miR-21 with miR-21 agomir, which was mediated via the PTEN-AKT signaling pathway (Han et al., 2014). Inflammatory factors (such as tumor necrosis factor and IL-1α/β) are thought to be targets for reducing inflammation or apoptosis in TBI (Shojo et al., 2010). Many lncRNAs in the cortex of injury sites are markedly up-regulated or down-regulated in mice after TBI, which may be involved in various pathological processes (Zhong et al., 2016).
A recent study suggested that lncRNAs play a key role in gene regulation and expression and actively participate in various pathological processes of brain diseases (Yan et al., 2015). However, comprehensive research regarding lncRNAs in TBI is still lacking, and understanding of their role in TBI is limited. Accordingly, in this study, we obtained for the first time complete mRNA and lncRNA expression profiles of three human TBI and three control samples by mRNA and lncRNA gene expression microarray. Differentially expressed mRNAs and lncRNAs were screened by the volcano plot method. A total of 99 lncRNAs (43 up-regulated and 56 down-regulated) and 63 mRNAs (59 up-regulated and 4 down-regulated) were found to be differentially expressed between TBI and control samples. Expression of these genes could be clearly distinguished by hierarchical clustering. Moreover, five mRNAs were chosen and their expression determined by qRT-PCR. Encouragingly, the qRT-PCR results were in good agreement with the microarray results, demonstrating reliability and accuracy of our microarray data. In the present study, HSPA1B, PPBP, HSPA1A, IL8, and S100A12 were the five most significantly up-regulated mRNAs, while OR7A5, RARRES3, MND1, and HLA-DRB3 were the four most significantly down-regulated mRNAs in the TBI group. Heat shock proteins are encoded by gene families and play key roles in tumor progression, cell survival, and tumorigenesis (Wang et al., 2018). Hsp70-1a and hsp70-1b are the two major hsp70 proteins, sharing more than 99% of their amino acids. Hsp70-1a and hsp70-1b are encoded by the HSPA1A and HSPA1B genes, respectively. They play key roles in maintaining protein synthesis and integrity. HSP70 may play an important role in neuronal survival after TBI. HSP70 loss increases brain damage after TBI in mice (Mayer and Bukau, 2005; Daugaard et al., 2007; Eroglu et al., 2014). PPBP-encoded protein is a platelet-derived growth factor belonging to the CXC chemokine family and an effective chemokine and activator of neutrophils. PPBP has been shown to stimulate a variety of cellular processes, including intracellular cAMP accumulation, prostaglandin E2 secretion, mitosis, and glycolysis. Further, PPBP may prevent excitotoxic brain injury of newborns by reducing microglial activation in vivo and maintaining mitochondrial membrane potential in vitro (Wegleiter et al., 2014; Guo et al., 2017; Kinouchi et al., 2017). IL-8-encoded protein is a primary mediator of inflammatory responses and belongs to the CXC chemokine family. IL-8 is thought to be involved in blood-brain barrier dysfunction, causinxg microvascular injury that is neutrophil-dependent (Jiang et al., 2017; Kim et al., 2017). S100A12 plays an important role in immune responses and inflammatory processes and is a copper-, zinc- and calcium- binding protein. Its pro-inflammatory activity includes chemokine production, promotion of cytokines, and recruitment of leukocytes. Determination of serum S100A12 concentration might contribute to early prognosis in severe TBI patients, and may be associated with brain inflammation (Berrocal-Almanza et al., 2016; Zhao et al., 2017; Feng et al., 2018). In this study, these genes were differentially expressed. Considering the above studies, we believe that these genes may play a key role in human TBI and contribute to diagnosis and treatment of TBI in the future. Moreover, OR7A5, RARRES3, MND1, and HLA-DRB3 were the four most significantly down-regulated mRNAs in the TBI group. A previous study found that OR7A5 is a protein coding gene that interacts with odor molecules in the nose to trigger a neuronal response that results in olfactory sense. Among its related pathways are GPCR signaling and the olfactory signaling pathway (Fuchs et al., 2002). RARRES3 is a growth regulator or tumor suppressor whose expression is up-regulated by the synthetic retinoic acid, tazarotene (Suarez-Calvet et al., 2017). The product of the MND1 gene can stimulate recombinase activity of RAD51 and DMC1, which are required for resolution of meiotic double-strand breaks (Chi et al., 2007; Dorosh et al., 2013). HLA–DRB3 plays a central role in the immune system by presenting peptides derived from extracellular proteins, which belong to HLA class II beta chain paralogues (Chowdhary et al., 2015). We have not found any previous study examining these genes in TBI models of animals or humans. Considering that our experimental results show marked down-regulation, their specific biological functions in human TBI deserve to be further investigated. In this study, we also found that the five most significantly up-regulated lncRNAs were n333955, n332943, n335470, ENST00000384390, and n341115. Furthermore, TCONS_00018733-XLOC_008489, OTTHUMT00000076953, NR_029967, ENST00000433249, and n381234 were the five most significantly down-regulated lncRNAs. LncRNAs are recognized as transcriptional regulators (Yoon et al., 2013; Long et al., 2017) that regulate protein coding gene expression by trans and cis-action mechanisms. However, no studies have so far reported association of these differentially expressed lncRNAs with TBI. Nevertheless, regarding expression of mRNAs, we believe that these up-regulated lncRNAs are also involved in the pathological process of TBI. Therefore, further experiments will be performed to investigate the function of these differentially expressed lncRNAs in TBI.
For comprehensive understanding of these differentially expressed mRNA genes, their possible biological functions were analyzed using GO and KEGG databases. Our GO analysis revealed that the largest portion of mRNAs are located in the plasma membrane and involved in synaptic transmission and delayed rectifier potassium channel activity. According to KEGG pathway analysis, the most enriched pathways for the differentially expressed coding genes were legionellosis, influenza A, antigen processing and presentation, rheumatoid arthritis, estrogen signaling pathway, and MAPK signaling pathway, among others.
Many protein coding genes require lncRNAs for transcriptional regulation. Thus, the differentially expressed mRNAs with differentially expressed lncRNAs were combined to build a co-expression network and gain new understanding on the function of lncRNAs. The results identified a total of 119 network nodes and 299 connections between 46 coding genes and 73 lncRNAs. In the co-expression network, 213 pairs showed positive and 86 pairs showed negative connections. However, this co-expression network analysis is not very reliable and can only provide a preliminary prediction.
The results of our study are somewhat different from a previous study (Zhong et al., 2016). We believe there are several reasons for this. First, humans and mice are different species, with natural differences between them. Second, in the study of Zhong et al., experiments were performed between experimental and control groups in different mice. In contrast, the experiments in our study were performed in the same patient; therefore we minimized errors as much as possible. Third, in this study, brain tissue in the control group was not normal and instead was obtained from tissue surrounding the brain injury. Consequently, edema, exudation, or other inflammatory reactions may be present, which may affect the experimental results. Last, our sample size is relatively small. Therefore, although our results provide a clue for further study of potential mechanisms of gene regulation in human TBI patients, we must verify and investigate the related genes.
TBI is one of the main causes of injury-related death at present, with a serious social burden worldwide. The potential molecular mechanisms and pathophysiology of TBI are unknown, and more functional studies of candidate lncRNAs are needed to comprehensively understand the roles of these molecules in TBI. The expression profile of differentially expressed mRNAs and lncRNAs in human TBI tissue has been first shown in this study. Altogether, our results show that expression levels of lncRNAs in TBI tissue are markedly different from control tissue, indicating that these lncRNAs might be involved in the pathophysiological process of human TBI. It is necessary to further study the molecular regulatory mechanism of lncRNAs in TBI to further understand the pathogenesis of TBI, which might indicate underlying prognostic indicators and biological targets of TBI in the future.
Additional files: Open peer review report 1 (123KB, pdf) and STROBE checklist.
Acknowledgments
We would like to appreciate Lang Ma who serves in Gene-Cloud of Biotechnology Information (GCBI) Co., to give us the technical support.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81571939 (to KX), 81601719 (to JZ) and 81772134 (to KX); the Key Research and Development Program of Hunan Province of China, No. 2018SK2091 (to KX); the Wu Jie-Ping Medical Foundation of the Minister of Health of China, No. 320.6750.14118 (to KX); and the Teacher Research Foundation of Central South University of China, No. 2014JSJJ026 (to KX). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study protocol was approved by the Medical Ethics Committee of the 101st Hospital of Chinese People’s Liberation Army (approval number: 2016-YXLL-008; time of ethics approval: 2016-01-01). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patients or their legal guardians have given their consent for patients’ images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the 101st Hospital of People’s Liberation Army, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jiang Li, University of Qingdao, China.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81571939 (to KX), 81601719 (to JZ) and 81772134 (to KX); the Key Research and Development Program of Hunan Province of China, No. 2018SK2091 (to KX); the Wu Jie-Ping Medical Foundation of the Minister of Health of China, No. 320.6750.14118 (to KX); and the Teacher Research Foundation of Central South University of China, No. 2014JSJJ026 (to KX).
P-Reviewer: Li J; C-Editor: Zhao M; S-Editors: Yu J, Wang J, Li CH; L-Editors: James R, Pack M, Qiu Y, Song LP; T-Editor: Liu XL
References
- Algattas H, Huang JH. Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci. 2013;15:309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Almanza LC, Goyal S, Hussain A, Klassert TE, Driesch D, Grozdanovic Z, Sumanlatha G, Ahmed N, Valluri V, Conrad ML, Dittrich N, Schumann RR, Lala B, Slevogt H. S100A12 is up-regulated in pulmonary tuberculosis and predicts the extent of alveolar infiltration on chest radiography: an observational study. Sci Rep. 2016;6:31798. doi: 10.1038/srep31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Trauma Foundation, American Association of Neurological Surgeons; Congress of Neurological Surgeons (2007) Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 24(Suppl 1):S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Chen AJ, D’Esposito M. Traumatic brain injury: from bench to bedside [corrected] to society. Neuron. 2010;66:11–14. doi: 10.1016/j.neuron.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, Zhang Q, Yan G, Cui Q. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:983–986. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Liu L, Xiao M, Wang F, Lin X. Microarray expression profile analysis of long noncoding RNAs in premature brain injury: a novel point of view. Neurosci. 2016;319:123–133. doi: 10.1016/j.neuroscience.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Chi P, San Filippo J, Sehorn MG, Petukhova GV, Sung P. Bipartite stimulatory action of the Hop2-Mnd1 complex on the Rad51 recombinase. Gene Dev. 2007;21:1747–1757. doi: 10.1101/gad.1563007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary VR, Dai C, Tilahun AY, Hanson JA, Smart MK, Grande JP, Rajagopalan G, Fu SM, David CS. A central role for HLA-DR3 in anti-smith antibody responses and glomerulonephritis in a transgenic mouse model of spontaneous lupus. J Immunol. 2015;195:4660–4667. doi: 10.4049/jimmunol.1501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haene E, Jacobs EZ, Volders PJ, De Meyer T, Menten B, Vergult S. Identification of long non-coding RNAs involved in neuronal development and intellectual disability. Sci Rep. 2016;6:28396. doi: 10.1038/srep28396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosh A, Tepla O, Zatecka E, Ded L, Koci K, Peknicova J. Expression analysis of MND1/GAJ, SPATA22, GAPDHS and ACR genes in testicular biopsies from non-obstructive azoospermia (NOA) patients. Reprod Biol Endocrinol. 2013;11:42. doi: 10.1186/1477-7827-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu B, Kimbler DE, Pang J, Choi J, Moskophidis D, Yanasak N, Dhandapani KM, Mivechi NF. Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J Neurochem. 2014;130:626–641. doi: 10.1111/jnc.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Baranzini SE, Silbereis JC, Shiow LR, Yuen TJ, Huang EJ, Lomvardas S, Rowitch DH. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci. 2014;17:506–512. doi: 10.1038/nn.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng MJ, Ning WB, Wang W, Lv ZH, Liu XB, Zhu Y, Gao W, Jin HZ, Gao SS. Serum S100A12 as a prognostic biomarker of severe traumatic brain injury. Clin Chim Acta. 2018;480:84–91. doi: 10.1016/j.cca.2018.01.044. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Malecova B, Linhart C, Sharan R, Khen M, Herwig R, Shmulevich D, Elkon R, Steinfath M, O’Brien JK, Radelof U, Lehrach H, Lancet D, Shamir R. DEFOG: a practical scheme for deciphering families of genes. Genomics. 2002;80:295–302. doi: 10.1006/geno.2002.6830. [DOI] [PubMed] [Google Scholar]
- Gene Ontology C. The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322–D326. doi: 10.1093/nar/gkj021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- Giera S, Deng Y, Luo R, Ackerman SD, Mogha A, Monk KR, Ying Y, Jeong SJ, Makinodan M, Bialas AR, Chang BS, Stevens B, Corfas G, Piao X. The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat Commun. 2015;6:6121. doi: 10.1038/ncomms7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Jian Z, Jia B, Chang L. CXCL7 promotes proliferation and invasion of cholangiocarcinoma cells. Oncol Rep. 2017;37:1114–1122. doi: 10.3892/or.2016.5312. [DOI] [PubMed] [Google Scholar]
- Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014;1582:12–20. doi: 10.1016/j.brainres.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Hirschi R, Rommel C, Hawryluk GWJ. Should we have a guard against therapeutic nihilism for patients with severe traumatic brain injury. Neural Regen Res. 2017;12:1801–1803. doi: 10.4103/1673-5374.219037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RG, Quill TE. Treatment decisions after brain injury-tensions among quality, preference, and cost. N Engl J Med. 2010;362:1757–1759. doi: 10.1056/NEJMp0907808. [DOI] [PubMed] [Google Scholar]
- Jiang BC, Sun WX, He LN, Cao DL, Zhang ZJ, Gao YJ. Identification of lncRNA expression profile in the spinal cord of mice following spinal nerve ligation-induced neuropathic pain. Mol Pain. 2015;11:43. doi: 10.1186/s12990-015-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang X, Miao W, Wang B, Qiu Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. APMIS. 2017;125:773–780. doi: 10.1111/apm.12721. [DOI] [PubMed] [Google Scholar]
- Jiang YZ, Lan Q, Wang QH, Song DL, Lu H, Wu WJ. Gradual and controlled decompression for brain swelling due to severe head injury. Cell Biochem Biophys. 2014;69:461–466. doi: 10.1007/s12013-014-9818-6. [DOI] [PubMed] [Google Scholar]
- Kan EM, Ling EA, Lu J. Microenvironment changes in mild traumatic brain injury. Brain Res Bull. 2012;87:359–372. doi: 10.1016/j.brainresbull.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kim AR, Ahn KB, Kim HY, Seo HS, Kum KY, Yun CH, Han SH. Streptococcus gordonii lipoproteins induce IL-8 in human periodontal ligament cells. Mol Immunol. 2017;91:218–224. doi: 10.1016/j.molimm.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Kinouchi T, Uemura M, Wang C, Ishizuya Y, Yamamoto Y, Hayashi T, Matsuzaki K, Nakata W, Yoshida T, Jingushi K, Kawashima A, Ujike T, Nagahara A, Fujita K, Imamura R, Ueda Y, Kitae K, Tsujikawa K, Nonomura N. Expression level of CXCL7 in peripheral blood cells is a potential biomarker for the diagnosis of renal cell carcinoma. Cancer Sci. 2017;108:2495–2502. doi: 10.1111/cas.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shi X, Yang W, Lu Z, Wang P, Chen Z, He J. Transcriptome profiling of lncRNA and co-expression networks in esophageal squamous cell carcinoma by RNA sequencing. Tumor Biol. 2016;37:13091–13100. doi: 10.1007/s13277-016-5227-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang H. Effect of Danshen injection with neural stem cells transplantation in rats with craniocerebral injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:4709–4715. [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Wang X, Youmans DT, Cech TR. How do lncRNAs regulate transcription? Sci Adv. 2017;3:eaao2110. doi: 10.1126/sciadv.aao2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci USA. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Liu NK, Xie XM, Li R, Xu XM. Automated monitoring of early neurobehavioral changes in mice following traumatic brain injury. Neural Regen Res. 2016;11:248–256. doi: 10.4103/1673-5374.177732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Z, Zheng D, Qing H. Regulatory roles of long non-coding RNAs in the central nervous system and associated neurodegenerative diseases. Front Cell Neurosci. 2017;11:175. doi: 10.3389/fncel.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TC, Morris KV, Wood MJ. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130507. doi: 10.1098/rstb.2013.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill CM, Jaffe K, Zhou C, Fan MY, Katon W, Fann JR. Health care costs associated with traumatic brain injury and psychiatric illness in adults. J Neurotrauma. 2012;29:1038–1046. doi: 10.1089/neu.2010.1562. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zhao W, Shen A, Shao B, Wu X, Yang J, Ni L, Wu Q, Chen J. Traumatic brain injury induces an up-regulation of Hs1-associated protein X-1 (Hax-1) in rat brain cortex. Neurochem Res. 2011;36:375–382. doi: 10.1007/s11064-010-0332-y. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zhao C, Long W, Ding H, Shen R. Microarray expression profile analysis of long non-coding RNAs in umbilical cord plasma reveals their potential role in gestational diabetes-induced macrosomia. Cell Physiol Biochem. 2015;36:542–554. doi: 10.1159/000430119. [DOI] [PubMed] [Google Scholar]
- Shojo H, Kaneko Y, Mabuchi T, Kibayashi K, Adachi N, Borlongan CV. Genetic and histologic evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171:1273–1282. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet X, Gallardo E, Pinal-Fernandez I, De Luna N, Lleixa C, Diaz-Manera J, Rojas-Garcia R, Castellvi I, Martinez MA, Grau JM, Selva-O’Callaghan A, Illa I. RIG-I expression in perifascicular myofibers is a reliable biomarker of dermatomyositis. Arthritis Res Ther. 2017;19:174. doi: 10.1186/s13075-017-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Yu JT, Hu N, Tan L. Non-coding RNAs in Alzheimer’s disease. Mol Neurobiol. 2013;47:382–393. doi: 10.1007/s12035-012-8359-5. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Kalinski AL, Sachdeva R, Houle JD. Intra-axonal protein synthesis - a new target for neural repair. Neural Regen Res. 2016;11:1365–1367. doi: 10.4103/1673-5374.191193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PJ, Helsens K, Wang X, Menten B, Martens L, Gevaert K, Vandesompele J, Mestdagh P. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:246–251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liao L, Wang M, Zhou H, Huang Y, Wang Z, Chen D, Ji D, Xia X, Wang Y, Liu F, Huang J, Xiong K. Pin1 promotes regulated necrosis induced by glutamate in rat retinal neurons via CAST/Calpain2 pathway. Front Cell Neurosci. 2017;11:425. doi: 10.3389/fncel.2017.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo LM, Wang Y, Zhou HK, Wang SC, Chen D, Huang JF, Xiong K. Inhibition of HSP90alpha protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J Cell Physiol. 2018;233:4864–4884. doi: 10.1002/jcp.26294. [DOI] [PubMed] [Google Scholar]
- Wegleiter K, Hermann M, Posod A, Wechselberger K, Stanika RI, Obermair GJ, Kiechl-Kohlendorfer U, Urbanek M, Griesmaier E. The sigma-1 receptor agonist 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) protects against newborn excitotoxic brain injury by stabilizing the mitochondrial membrane potential in vitro and inhibiting microglial activation in vivo. Exp Neurol. 2014;261:501–509. doi: 10.1016/j.expneurol.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Xiong K, Long L, Zhang X, Qu H, Deng H, Ding Y, Cai J, Wang S, Wang M, Liao L, Huang J, Yi CX, Yan J. Overview of long non-coding RNA and mRNA expression in response to methamphetamine treatment in vitro. Toxicol In Vitro. 2017;44:1–10. doi: 10.1016/j.tiv.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Yan Y, Zhang L, Jiang Y, Xu T, Mei Q, Wang H, Qin R, Zou Y, Hu G, Chen J, Lu Y. LncRNA and mRNA interaction study based on transcriptome profiles reveals potential core genes in the pathogenesis of human glioblastoma multiforme. J Cancer Res Clin. 2015;141:827–838. doi: 10.1007/s00432-014-1861-6. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Yao W, Wang J, Ma X, Xiao W, Li H, Xia D, Yang Y, Deng K, Xiao H, Wang B, Guo X, Guan W, Hu Z, Bai Y, Xu H, Liu J, Zhang X, Ye Z. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377. doi: 10.1371/journal.pone.0042377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhong A, Friedrich EE, Jia S, Xie P, Galiano RD, Mustoe TA, Hong SJ. S100A12 induced in the epidermis by reduced hydration activates dermal fibroblasts and causes dermal fibrosis. J Invest Dermatol. 2017;137:650–659. doi: 10.1016/j.jid.2016.10.040. [DOI] [PubMed] [Google Scholar]
- Zhong J, Jiang L, Cheng C, Huang Z, Zhang H, Liu H, He J, Cao F, Peng J, Jiang Y, Sun X. Altered expression of long non-coding RNA and mRNA in mouse cortex after traumatic brain injury. Brain Res. 2016;1646:589–600. doi: 10.1016/j.brainres.2016.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.