In contrary to the discrete neuronal circuits, astrocytes share their cytoplasm through gap junctional coupling into a syncytium. This distinctive difference in neuronal and astrocytic anatomy recalls the historical debate between Camillo Golgi and Ramón y Cajal on the wiring principle of the nervous system over a century ago (Cimino, 1999). At that neuron-centric era, the Cajal advocated the cell theory that considered the nervous system to be made up of discrete individual cells. Golgi on the other hand favored the reticular theory that viewed the nervous system as a singular continuing network system. Nevertheless, regardless of the difference in their viewpoints, both theories were intended to postulate an anatomical blueprint of neural circuitry, with no attention given to neuroglia. It was until the 1950s, the distinct membrane borders between synapses were revealed by electron microscopy, which means that neurons are indeed discrete individual cells. This declared Ramón y Cajal’s cell theory the victor in the debate, while Golgi’s reticular theory was disregarded. However, the existence of a “low-resistance pathway” between neuroglia was soon identified in the optic nerve in the 1960s (Kuffler et al., 1966), which we now know to be gap junction coupling that connects the cytoplasm of astrocytes into a syncytial network. Ever since, decades of studies clearly demonstrated that syncytial coupling into the network is a most prominent feature of astrocytes and these glial networks are intimately interwoven with the neuronal circuits running across the entire central nervous system (Giaume et al., 2010). Surprisingly, the question of whether a reticular system exists in parallel with the neuronal circuits has received a little research attention to this day. Interestingly, the wiring pattern of astrocyte syncytium is reminiscent of the reticular theory postulated by Golgi. In this perspective, the “revived” use of “reticular theory” is solely dedicated to the brain reticular system established by astrocytes.
Astrocytes vary substantially in cytoarchitecture and spatial organization: Heterogeneity of astrocytes was initially recognized by cellular morphology, which led to the classification of protoplasmic astrocytes in grey matter and fibrous astrocytes in white matter. Now this notion continuously expands to astrocytes’ embryonic origins, gene expression, and physiological functions (Khakh and Sofroniew, 2015). Aided by the latest tissue clearing technology, a striking heterogeneity in astrocyte syncytial spatial organization has been revealed in various brain regions in two recent studies (Huang et al., 2018; Kiyoshi et al., 2018). In these studies, the architecture of astrocyte networks vary substantially from cortical grey matter to white matter, specialized radial shaped Bergmann glia and velate astrocytes in the cerebellum, and grey matter astrocytes in the spinal cord. Even within the visual cortex, astrocyte density is high in layer I, and lower in layers II/III through VI, which is correlated with the neuronal densities in each layer. In the cerebellum, the processes of radial shaped Bergermann glia are aligned with the dendrites of Purkinje neurons. Interestingly, velate astrocytes in the cerebellar granule cell layer exhibit the highest cell density. Therefore, the architecture of astrocyte networks indeed differs across and within brain regions. Yet still little is known how such a heterogeneity is achieved developmentally and maintained throughout adulthood.
How does the astrocyte network operate as a functional system? Aggregation of astrocytes into a syncytium naturally raises a question of whether this evolutionary design endows astrocytes the ability to act as a system in brain function. For decades, electrophysiology has been effectively used to assess neuronal excitability and synaptic transmission. However, lack of a functional readout was a major hurdle to gain insight into the functional state of an astrocyte syncytium (Nimmerjahn and Bergles, 2015). Until recently, an operational mechanism of the astrocyte network has been finally unearthed (Ma et al., 2016). Gap junction coupling is able to lower the electrical resistance among coupled cells. Biophysically, if gap junction coupling is sufficiently strong, it is possible that the electrical barriers between neighboring cells can be nearly eliminated. As a result, the coupled cells can constantly equalize their membrane potentials to comparable levels so that the entire syncytium can electrophysiologically behave as a singular unit. In 2016, this was first demonstrated to be the case in the hippocampal astrocyte network, a phenomenon termed syncytial isopotentiality (Ma et al., 2016) (Figure 1).
Figure 1.
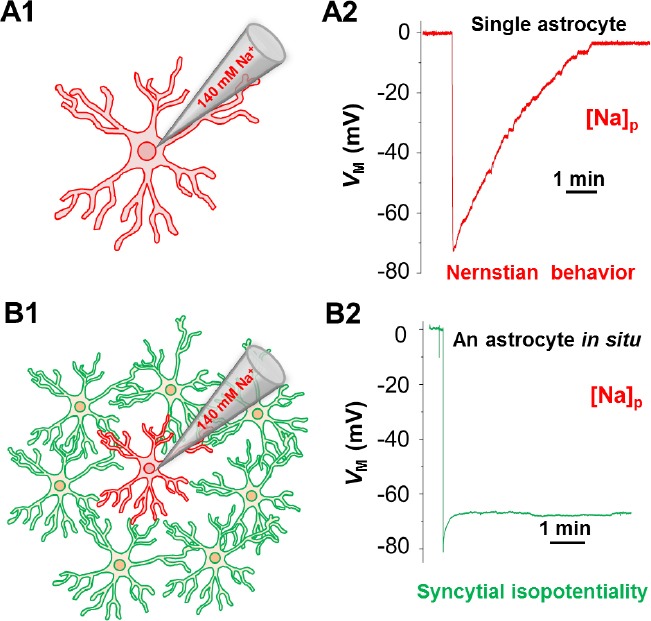
Syncytial isopotentiality is an operational mechanism of astrocyte network.
The drawings illustrate the different electrophysiological behavior of a single freshly isolated (A1, A2), and an astrocyte associated with its network (B1, B2) when the intracellular K+ content was substituted by Na+ during whole-cell recording. In single uncoupled astrocyte, the membrane potential (VM) follows a Nernstian prediction for the change of the K+ gradient across the membrane (A2), or Nernstian behavior. In contrast, the recording from a network coupled astrocyte under the same condition disobeys Nerstian prediction. This results from a strong electrical coupling with its associated network that compensates for the loss the physiological membrane potential. This steady-state quasi-physiological membrane potential, termed syncytial isopotentiality, coordinate astrocytes into a highly efficient system in homeostatic brain function.
Dynamic changes in the functional state of an astrocyte syncytium: The syncytial isopotentiality was discovered with an innovative use of the patch clamp methodology. The rationale behind the methodology is based on the notion that the membrane potential of individual astrocyte obeys the Nernstian prediction for the K+ gradient across the cell membrane. Thus, substitution of the endogenous K+ content by the K+-free/Na+-containing solution dissipates the physiological membrane potential to ~ 0 mV. This “K+-deficient astrocyte”, created through patch clamp recording, will in turn timely report the coupling strength of network through the levels of compensatory quasi-physiological membrane potentials deriving from neighboring astrocytes (Ma et al., 2016). Additionally, a computational modeling has been established for quantitative analysis of the change in the coupling strength of an astrocyte syncytium (Kiyoshi et al., 2018).
Syncytial isopotentiality operates as a universal mechanism in astrocyte networks: The heterogeneity in the cytoarchitecture of individual astrocytes and network organizations patterns discussed above calls into the question of whether syncytial isopotentiality occurs selectively to certain brain regions, such as the hippocampus, or is universal to the entire astrocyte networks in the central nervous system. This question has been answered by two recent studies. This survey showed that syncytial isopotentiality is a shared feature among protoplasmic astrocytes in different cortical regions, in the barrel fields of layer IV somatosensory cortex, and velate astrocytes in the cerebellum. Interestingly, syncytial isopotentiality exists in the syncytium established by Bergmann glia. Although fibrous astrocytes are morphologically distinct from protoplasmic astrocytes and reside in an environment devoid of synapses, syncytial isopotentiality also appears in corpus callosum white matter. Syncytial isopotentiality has also been revealed in grey matter spinalcord astrocytes (Huang et al., 2018). Our results from a large-scale survey favor the notion that syncytial isopotentiality is a general feature of astrocyte networks.
Astrocyte syncytium: a reticular system safeguarding neuronal circuits: A general role of astrocytes is to safeguard neuronal circuits. For example, a K+ channel-mediated homeostatic mechanism, the K+ spatial buffering hypothesis, was formulated over 50 years ago by Kuffler and associates. Should this mechanism be truly operational, one of the speculated requirement is that astrocytes need to be strongly coupled into an isopotential network. Now this crucial mechanism, syncytial isopotentiality, has finally been unearthed. As a demonstrated case, this network mechanism facilitates K uptake with a far greater efficacy. Syncytial isopotentiatlity should also provide a high efficiency for spatial redistribution of K+ ions inside an astrocyte syncytium and with the extracellular space surrounding the very same astrocyte network (Ma et al., 2016).
How does disease conditions affect syncytial isopotentiality? Guided by the discovery of syncytial isopotentiality and the new method developed in our study, a major stroke-induced mechanism underlying astrocyte membrane depolarization was identified. This mechanism was determined to be caused by an energy failure-induced dissipation of K+ content in the entire astrocyte network (Du et al., 2018).
In various kind of neurological injuries and diseases, it has been increasingly recognized that astrocytes become reactive (Sofroniew, 2014). Changes in astrocyte morphology, gene expression, and the organization patterning could altogether occur under disease conditions. This brings into question of how such multifaceted changes could alter the strength of network coupling and function. This is a new research area that is wide open for the future. It remains completely unknown the extent to which an impaired syncytial isopotentiality contributes either as a cause or an intermediate step in neurological diseases. For instance, the syncytial architecture as a whole can be altered in certain disease conditions, such as epilepsy. Also, the Cx43 expression is known to be altered in disease conditions (Giaume et al., 2010). Therefore, a potential loss or gain of syncytial network connectivity may both occur in a context-dependent manner (Sofroniew, 2014). However, what happens to the astrocyte network communication remains to be determined. Now the availability of a new and powerful methodology should facilitate the future establishment of a causal relationship between an impaired syncytial isopotentiality and a specific type of neurological disorder.
Future research perspectives: Evidence from others and our studies indicate that establishment of syncytial coupling is a ubiquitous feature of astrocytes across the brain. Now, syncytial isopotentiality has been revealed as an operational mechanism of the astrocyte network and a methodology has also been established for further exploration of this mysterious reticular system in the brain. Nowadays, the “connectome” is used to refer to the structural and functional mapping of neural connectivity in the brain. Although astrocytes are wired together into a syncytium, much of the current effort is concentrated on creating wiring diagrams of neurons in different brain areas. It is time to put equal amounts of effort to establish the architectural wiring principle and function of this reticular system.
It should also be noted that the astrocyte syncytium is an electrically low-resistance reticular system. Although a better understanding of this “low-resistance pathway” has been gained at the functional level (Ma et al., 2016), the ultrastructural underpinning and biophysical rationale remain to be established. The low-resistance reticular system also means a low membrane resistance that is mostly caused by a high permeability to K+ ions (Du et al., 2015). In this front, what remains elusive is the identity of the entire repertoire of the channels that create the astrocyte membrane to be so “leaky”. Although such a low-resistance system was first brought to light by Stephen Kuffler over 50 years (Kuffler et al., 1966), the role of this reticular system in normal brain function and the diseased brain continues to be a mystery.
Interestingly, newborn astrocytes are solitary individual cells (Zhong et al., 2016). Curiously, how newly generated astrocytes converge into a shared astrocyte network and when a functional astrocyte network emerges in the developing brain are basic neurobiological questions to be determined. Additionally, a functional readout for defining an astrocyte syncytium that is developmentally mature is yet to be established. This functional readout would be highly useful to determine whether a delay or reversal of this developmental process could be causative to a disease under investigation.
This work was sponsored by grants from the National Institute of Neurological Disorders and Stroke RO1NS062784, R56NS097972.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- Cimino G. Reticular theory versus neuron theory in the work of Camillo Golgi. Physis Riv Int Stor Sci. 1999;36:431–472. [PubMed] [Google Scholar]
- Du Y, Ma B, Kiyoshi CM, Alford CC, Wang W, Zhou M. Freshly dissociated mature hippocampal astrocytes exhibit similar passive membrane conductance and low membrane resistance as syncytial coupled astrocytes. J Neurophysiol. 2015;113:3744–3750. doi: 10.1152/jn.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Wang W, Lutton AD, Kiyoshi CM, Ma B, Taylor AT, Olesik JW, McTigue DM, Askwith CC, Zhou M. Dissipation of transmembrane potassium gradient is the main cause of cerebral ischemia-induced depolarization in astrocytes and neurons. Exp Neurol. 2018;303:1–11. doi: 10.1016/j.expneurol.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Huang M, Du Y, Kiyoshi CM, Wu X, Askwith CC, McTigue DM, Zhou M. Syncytial isopotentiality: an electrical feature of spinal cord astrocyte networks. Neuroglia. 2018;1:271–279. [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyoshi CM, Du Y, Zhong S, Wang W, Taylor AT, Xiong B, Ma B, Terman D, Zhou M. Syncytial isopotentiality: a system-wide electrical feature of astrocytic networks in the brain. Glia. 2018 doi: 10.1002/glia.23525. doi:10.1002/glia.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W, McTigue DM, Enyeart JJ, Terman D, Zhou M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia. 2016;64:214–226. doi: 10.1002/glia.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Bergles DE. Large-scale recording of astrocyte activity. Curr Opin Neurobiol. 2015;32:95–106. doi: 10.1016/j.conb.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Du Y, Kiyoshi CM, Ma B, Alford CC, Wang Q, Yang Y, Liu X, Zhou M. Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol Brain. 2016;9:34. doi: 10.1186/s13041-016-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


