Keywords: nerve regeneration, luteolin, hippocampus, Ts65Dn mice, neurogenesis, ERK, BDNF, nestin, GFAP, NeuN, Down syndrome, neural regeneration
Abstract
Studies have shown that the natural flavonoid luteolin has neurotrophic activity. In this study, we investigated the effect of luteolin in a mouse model of Down syndrome. Ts65Dn mice, which are frequently used as a model of Down syndrome, were intraperitoneally injected with 10 mg/kg luteolin for 4 consecutive weeks starting at 12 weeks of age. The Morris water maze test was used to evaluate learning and memory abilities, and the novel object recognition test was used to assess recognition memory. Immunohistochemistry was performed for the neural stem cell marker nestin, the astrocyte marker glial fibrillary acidic protein, the immature neuron marker DCX, the mature neuron marker NeuN, and the cell proliferation marker Ki67 in the hippocampal dentate gyrus. Nissl staining was used to observe changes in morphology and to quantify cells in the dentate gyrus. Western blot assay was used to analyze the protein levels of brain-derived neurotrophic factor (BDNF) and phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) in the hippocampus. Luteolin improved learning and memory abilities as well as novel object recognition ability, and enhanced the proliferation of neurons in the hippocampal dentate gyrus. Furthermore, luteolin increased expression of nestin and glial fibrillary acidic protein, increased the number of DCX+ neurons in the granular layer and NeuN+ neurons in the subgranular region of the dentate gyrus, and increased the protein levels of BDNF and p-ERK1/2 in the hippocampus. Our findings show that luteolin improves behavioral performance and promotes hippocampal neurogenesis in Ts65Dn mice. Moreover, these effects might be associated with the activation of the BDNF/ERK1/2 pathway.
Chinese Library Classification No. R453; R363; R741
Introduction
Down syndrome, also known as trisomy 21, is one of the most common chromosomal diseases. In 1886, British doctor Lang Down first described the clinical symptoms of the disorder, but it was not until 1959 that this disease was found to be caused by a third copy of chromosome 21. According to epidemiologic studies, the incidence of Down syndrome is about 1/700, with about 200,000 new cases worldwide each year (Heller et al., 2014). In recent years, with the popularity of noninvasive prenatal DNA screening, the number of Down syndrome births has dropped dramatically. Attention has been increasingly focused on the treatment and improvement of the quality of life of the population with Down syndrome. The cognitive deficits in Down syndrome appear to be caused by abnormal neurogenesis, impaired synaptic plasticity and neurochemical changes (Lott and Dierssen, 2010; Dierssen, 2012).
Learning and memory are major functions of the central nervous system and are vital for adaptation and survival. The hippocampal formation is a key brain area involved in learning and memory. A substructure of the hippocampus, the dentate gyrus is a major site of adult neurogenesis and synaptic plasticity, both of which are critical for learning and memory (Snyder et al., 2001; Schmidt-Hieber et al., 2004; Ge et al., 2008).
Over the past few years, an increasing number of studies have explored the exciting possibility of using pharmacological approaches to ameliorate neuronal developmental deficits in Down syndrome mouse models, and a great deal of progress has been made. Studies using the Ts65Dn mouse model have reported that neurogenesis is reduced in the adult dentate gyrus, and that neurogenesis promoting drugs, such as fluoxetine (Clark et al., 2006) and lithium (Contestabile et al., 2013), enhance the proliferation of neural precursor cells in the dentate gyrus. Another study showed that combined environmental enrichment and epigallocatechin gallate therapy in young Ts65Dn mice improved cortico–hippocampal-dependent learning and memory (Catuara-Solarz et al., 2016). Together, these recent studies show that Down syndrome symptoms can be alleviated by appropriate drug treatment.
Natural phytochemicals, including flavones, have been evaluated for their neuroprotective and neurotrophic activities. Flavonoids have various beneficial effects, including scavenging free radicals, alleviating cognitive impairment, and improving learning and memory (Bhullar and Rupasinghe, 2013). For example, baicalein increases the expression levels of phospho-extracellular signal-regulated kinase (p-ERK), phospho-cAMP-response element binding protein and brain-derived neurotrophic factor (BDNF) (Park et al., 2010). Oral administration of the flavonoid chrysin prevents age-associated memory loss, probably by scavenging free radicals and modulating BDNF production (Souza et al., 2015). Curcumin protects against behavioral impairments, neuroinflammation, tau hyperphosphorylation and cell signaling disturbances triggered by amyloid beta in vivo (Hoppe et al., 2013). Furthermore, the BDNF mimetic 7,8-dihydroxyflavone alleviates intellectual disabilities in a Down syndrome model (Jang et al., 2010).
Luteolin (3′,4′, 7-tetrahydroxyflavone), a natural flavonoid, is widely distributed in honeysuckle, chrysanthemum, Dracocephalum ruyschiana and other plants, and has antioxidant, anti-inflammatory and other pharmacological effects. It is clinically used to treat tumors, hepatitis, hypertension, and amyotrophic lateral sclerosis (Lin et al., 2008; Jiang et al., 2013). Luteolin was shown to protect dopaminergic neurons from LPS-induced injury, perhaps by inhibiting microglial activation (Chen et al., 2008). In the PC12 cell line, luteolin induces neurite outgrowth and augments cellular antioxidant defense capacity through the activation of the ERK signaling pathway (Lin et al., 2010).
In the present study, we investigated the effect of luteolin on neurogenesis in the dentate gyrus of Ts65Dn mice, and we examined the underlying mechanisms of action.
Materials and Methods
Animals and luteolin treatment
Three Ts65Dn breeder pairs of mice (Stock No. 001924) were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). Females trisomic for distal chromosome 16 (Ts65Dn) were bred to B6EiC3SnF1/J F1 hybrid male mice and housed in a quiet room at 25°C under a standard 12-hour light/dark cycle. All the females were kept as breeders for colony maintenance, and their offspring, including Ts65Dn and wild-type (WT) euploid littermates, were genotyped according to the protocol provided by Jackson Laboratories.
When the mice were at the age of 12 weeks, Ts65Dn and WT euploid littermate males were randomly assigned to the following groups: WT + vehicle group (n = 25), WT + luteolin group (n = 22), Ts65Dn + vehicle group (n = 20), and Ts65Dn + luteolin group (n = 20).
Luteolin was purchased from Sigma-Aldrich (98% purity) and dissolved in saline. According to a previously published method (Yoo et al., 2013), every morning the mice received an intraperitoneal injection of luteolin (or vehicle, 0.9% saline) 10 mg/kg, for 4 weeks. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Jiangsu University Laboratory Animal Management Committee (approval No. UJS-LAER-2017042201) on April 22, 2017.
Morris water maze test
After administration of luteolin for 4 weeks, the water maze test was performed as previously described (Cuadrado-Tejedor et al., 2011). The water maze experiment device (RD1101-MWM-G, Mobiledatum, Shanghai, China) was a stainless-steel sink, with a diameter of 100 cm and a height of 50 cm, containing four quadrants. A circular platform with a diameter of 9 cm and a height of 27 cm was in the center of the platform quadrant, and its position remained unchanged throughout the experiment. Milk was added to the pool to make the water opaque. Water temperature was kept at 23°C. After the mice were adjusted in a quiet test room for 1 hour, the experiment was conducted. On the first day, a visual platform experiment was carried out. The platform was exposed 1 cm above the water surface. Each mouse was put into the pool and allowed to swim for 2 minutes for acclimation.
During the following 2–5 days, the navigation experiment was conducted. The platform was located 1 cm under the surface of the water. The mouse was placed into the water in each of the three quadrants and allowed to swim for 1 minute each time. The mouse either found the platform and remained there for 5 seconds, or the 1-minute test time elapsed. Whether or not the mouse found the platform, it was gently guided to the platform and allowed to remain there for 15 seconds. Then, 1 day after the navigation experiment, the platform was removed for the spatial exploration test. The mouse was placed in the water in the same quadrant. The time to find the platform, the number of entries into the quadrant previously containing the platform, and the time spent in the target quadrant were recorded by an automatic video tracking system above the pool. All experiments were carried out between 9.00 a.m. and 5.00 p.m.
Novel object recognition
Rodents have the natural habit of exploring new objects. The experiment was performed as previously described in Ts65Dn mice (Parrini et al., 2017) and tested in a black empty box, and carried out in a horizontal, quiet environment with a camera mounted 2 m above. Before the test, the mouse was placed into the empty container in the same orientation and position, and allowed to move freely for 10 minutes for acclimation (once a day for 3 consecutive days). During the 4 days of training, two similar objects were placed at the bottom of the empty box (10 cm from each side). The mouse was placed back into the box and returned to the cage after 10 minutes of free activity. After 1 hour, one of the two objects was replaced with a new object (different sizes and materials). The mouse was placed in the box, and activity was observed for a period of 10 minutes. The total time the animal spent exploring each of the objects was recorded. The behaviors recorded included the distance between the nose tip and the object when the animal sniffed the object from less than 2 cm, and the times the nose or front paw directly touched the object. Walking near the object was not considered exploratory behavior. The novel object cognitive discrimination index was defined as: time exploring the novel object/total exploratory time. A solution of 90% alcohol was used to eliminate odors between experiments (to avoid olfactory cues from affecting the exploratory behavior of other animals).
Hippocampal tissue preparation
After behavioral testing, the mice were weighed on an electronic scale (Hengping Scientific Instrument, Shanghai, China). All mice were anesthetized with 4% chloral hydrate. After the thoracic cavity was opened, the needle was carefully inserted through the left ventricle into the aorta, and the right atrium was cut. The mice were then perfused with 50 mL saline followed by 100 mL paraformaldehyde (4%) solution. Subsequently, the brains were gently removed, blotted dry, and quickly weighed with an electronic balance (Sartorius, Gottingen, Germany). The hippocampal tissue was then carefully removed.
Immunohistochemistry
The hippocampal tissue was fixed with 4% paraformaldehyde for 24 hours. The tissue was then dehydrated, paraffin-embedded, dehydrated, and cut into serial coronal sections with a microtome (RM2235 Leica, Wetzlar, Germany). After dewaxing, rehydration and antigen retrieval, each paraffin section was treated with 3% H2O2-methanol solution, blocked at 15–25°C for 10 minutes, and then incubated with 100 μL goat serum at room temperature for 20 minutes.
For immunofluorescence, the following primary antibodies were used: mouse monoclonal anti-nestin (neural stem cell marker; 1:250; Abcam, Cambridge, UK), rabbit polyclonal anti-GFAP (astrocyte marker; 1:500; Abcam), rabbit polyclonal anti-DCX (immature neuron marker; 1:800; Abcam), rabbit monoclonal anti-NeuN (mature neuron marker; 1:250; Abcam) and rabbit polyclonal anti-Ki67 (cell proliferation marker; 1:250; Abcam). Each section was incubated with primary antibody for 2 hours at room temperature. After washing, sections were incubated with FITC or TRITC-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (1:250; Jackson ImmunoResearch; West Grove, PA, USA). Afterwards, the sections were counterstained with DAPI and mounted in antifade medium. The immunoreactivity of protein in cells was observed under a fluorescence microscope (BX43; Olympus, Tokyo, Japan).
Cells were observed under an Olympus CX41 microscope with a 40× objective. Ki67+, DCX+ and NeuN+ cells covering the complete rostro-caudal extension of the dentate gyrus were quantified as previously described (Contestabile et al., 2013). Serial coronal sections with a thickness of 30 μm were obtained, and one section was selected every 200 μm for cell counting. A total of 12 sections were taken from each mouse. The positive cells in 12 sections of the hippocampal granulosa layer and the dentate gyrus were counted. For nestin and GFAP, hippocampal dentate gyrus regions from three sections were selected for each animal, and then three random visual fields were analyzed using IPP6.0 software (Media Cybernetics, Montgomery, MD, USA) at 200× magnification. Results are shown as mean density = (sum of the integrated optical density)/area.
Nissl staining
Paraffin sections were stained with Nissl staining solution (Beyotime, Shanghai, China) for 5 minutes, washed with water and then with 95% ethanol for 5 seconds. The sections were dehydrated through a graded alcohol series and xylene, and mounted with neutral balsam. The morphological and quantitative changes in neural cells in the hippocampal dentate gyrus were observed under an optical microscope (BX43, Olympus).
Western blot assay
Total protein in hippocampal tissue was extracted with a protein extraction kit (Beyotime), and protein concentration was determined using the Bradford protein concentration determination kit (Beyotime). Total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Afterwards, the separated proteins were transferred onto nitrocellulose filter membranes. The blots were blocked with 5% bovine serum albumin for 2 hours at room temperature, and then incubated with the following primary antibodies at 4°C overnight: rabbit monoclonal anti-BDNF (1:1000; Abcam), rabbit polyclonal anti-p-ERK1/2 (phosphoT202+Y204; 1:1500; Abcam), rabbit polyclonal anti-ERK1/2 (1:1000; Abcam) and mouse monoclonal anti-GAPDH (1:1000; Abcam). The next day, the membranes were washed three times with phosphate-buffered saline containing Tween-20 (PBST), and then incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG; 1:1000; Beyotime, Shanghai, China) for 1 hour. The blots were then washed three times with PBST, and then treated with 1–2 mL enhanced chemiluminescence reaction liquid for 1 minute. The membranes were observed and photographed with a Tanon 5200 Luminescence imaging system (Tanon, Shanghai, China). Protein expression was analyzed according to optical density.
Statistical analysis
Data are presented as the mean ± SE. Statistical analysis was performed using SPSS v22.0 software (IBM, Armonk, NY, USA). Data were evaluated using two-way analysis of variance followed by Tukey’s post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Effect of luteolin on behavioral performance in Ts65Dn mice
After administration of luteolin for 4 weeks, Morris water maze results showed that compared with the WT + vehicle group (Figure 1A), the time to find the hidden platform was longer in the Ts65Dn + vehicle group (P < 0.01). In the spatial exploration test (Figure 1B and C), compared with the Ts65Dn + vehicle group, the number of crossings of the target platform/quadrant area was more and the time spent in the platform was longer in the WT + vehicle group (both P < 0.01). These performance deficits indicate defects in learning and memory abilities in Ts65Dn + vehicle mice. After treatment with luteolin, the deficits were partially alleviated, with the time to finding the hidden platform being significantly shortened (P < 0.05). The novel object recognition test (Figure 1D) revealed that the discrimination index was significantly lower in the Ts65Dn + vehicle group than in the WT + vehicle group (P < 0.01). In comparison, after luteolin treatment, novel object recognition ability was significantly improved in the mouse model of Down syndrome (P < 0.05).
Figure 1.
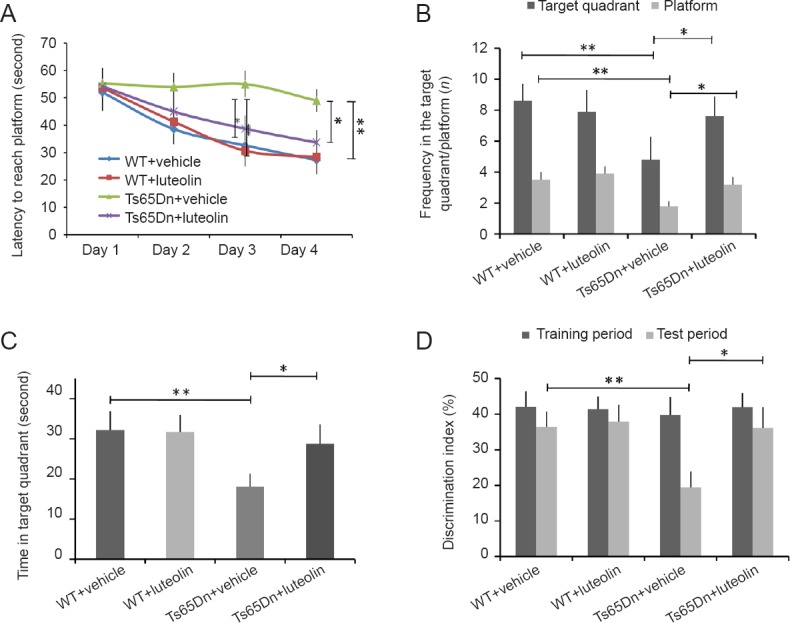
Effect of luteolin on behavioral performance in Ts65Dn mice.
(A) Latency to reach the platform in the Morris water maze test on the last day of testing (genotype (F = 23.5, P < 0.01), treatment (F = 102.4, P < 0.01), genotype × treatment (F = 44, P < 0.01)). (B) Number of entries into the target platform/quadrant area in a period of 60 seconds in the spatial exploration task. Entries into the target quadrant (genotype (F = 27.7, P < 0.01), treatment (F = 7.5, P < 0.01), genotype × treatment (F = 19.6, P < 0.01)). Entries into the platform area (genotype (F = 51.5, P < 0.01), treatment (F = 28.3, P < 0.01), genotype × treatment (F = 9.2, P < 0.01)). (C) Time spent in the target quadrant (genotype (F = 42.9, P < 0.01), treatment (F = 41.4, P < 0.01), genotype × treatment (F = 29.1, P < 0.01)). (D) Discrimination index in the novel object recognition test. Test phase: genotype (F = 51.5, P < 0.01), treatment (F = 28.3, P < 0.01), genotype × treatment (F = 9.2, P < 0.01). Number of animals in each group: WT + vehicle group: n = 12; WT + luteolin group: n = 11; Ts65Dn + vehicle group: n =12; Ts65Dn + luteolin group: n = 12. Data are shown as the mean ± SEM (two-way analysis of variance followed by Tukey’s post hoc test). All experiments were performed more than three times. *P < 0.05, **P < 0.01. WT: Wild-type.
Effect of luteolin on hippocampal cell proliferation in Ts65Dn mice
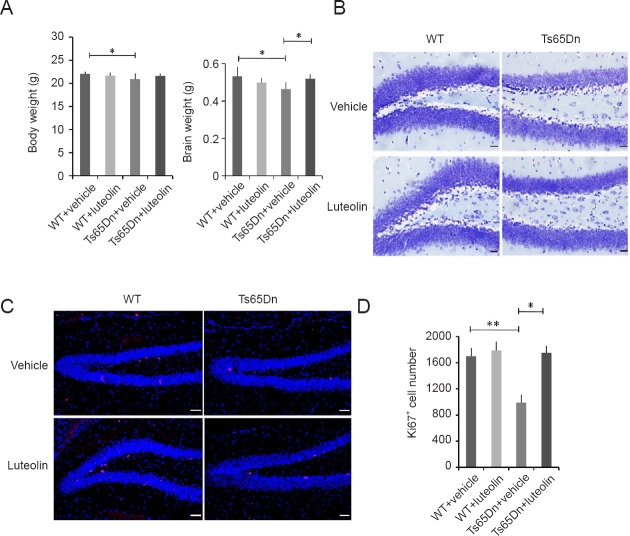
Compared with the WT + vehicle group, body and brain weights were lower in the Ts65Dn + vehicle group (P < 0.05), suggesting developmental retardation. Luteolin treatment restored the body and brain weights in Ts65Dn mice (Figure 2A).
Figure 2.
Effect of luteolin on hippocampal cell proliferation in Ts65Dn mice.
(A) Body and brain weights after 4-week administration of luteolin. Body weight: genotype (F = 7.33, P < 0.01), treatment (F = 0.46, P = 0.49), genotype × treatment (F = 5.86, P < 0.05). Brain weight: genotype (F = 4.1, P < 0.05), treatment (F = 0.83, P = 0.37), genotype × treatment (F = 19.6, P < 0.01). (B) Nissl staining of hippocampal tissue. (C) Immunofluorescence for Ki67 (red, TRITC) in the subgranular zone. Scale bars: 20 μm in B, 50 μm in C. (D) Numbers of Ki67+ cells in each group: genotype (F = 55.4, P < 0.01), treatment (F = 77.4, P < 0.05), genotype × treatment (F = 51.3, P < 0.01). Number of mice in each group: WT + vehicle group: n = 25; WT + luteolin group: n = 22; Ts65Dn + vehicle group: n = 20; Ts65Dn + luteolin group: n = 20. Data are shown as the mean ± SEM (two-way analysis of variance followed by Tukey’s post hoc test). All experiments were performed more than three times. *P < 0.05, **P < 0.01. WT: Wild-type.
The hippocampus plays an important role in cognition and memory (Contestabile et al., 2013). Nissl bodies in hippocampal neurons in the WT + vehicle group were large and abundant, and pyramidal and glial cells were numerous and distributed in an orderly manner. The number of Nissl bodies in hippocampal neurons in the Ts65Dn + vehicle group was decreased, and the staining was faint and not well defined. The number of Nissl bodies seemed to be increased in the Ts65Dn + luteolin group (Figure 2B). As shown in Figure 2C and D, immunostaining revealed that the number of Ki67+ cells in the hippocampal dentate gyrus was significantly smaller in the Ts65Dn + vehicle group than in the WT + vehicle group (P < 0.01). The number of Ki67+ cells was similar in the Ts65Dn + luteolin group and the WT + vehicle group (P > 0.05).
Effects of luteolin on hippocampal neurogenesis in Ts65Dn mice
Neural differentiation impairment is thought to be strongly associated with Down syndrome (Dierssen, 2012), and therefore, we examined the expression of some important differentiation markers in the hippocampal dentate gyrus. The expression of the astrocyte marker GFAP (Nimmerjahn, 2009) and the neural stem cell marker nestin (Eisch et al., 2000) were both decreased in the Ts65Dn + vehicle group (Figure 3A and B). Furthermore, the immature neuronal marker DCX and the mature neuronal marker NeuN were reduced in the Ts65Dn + vehicle group compared with the WT + vehicle group (P < 0.01). However, the expression levels of all of these markers were increased significantly in the Ts65Dn + luteolin group (Figure 3).
Figure 3.
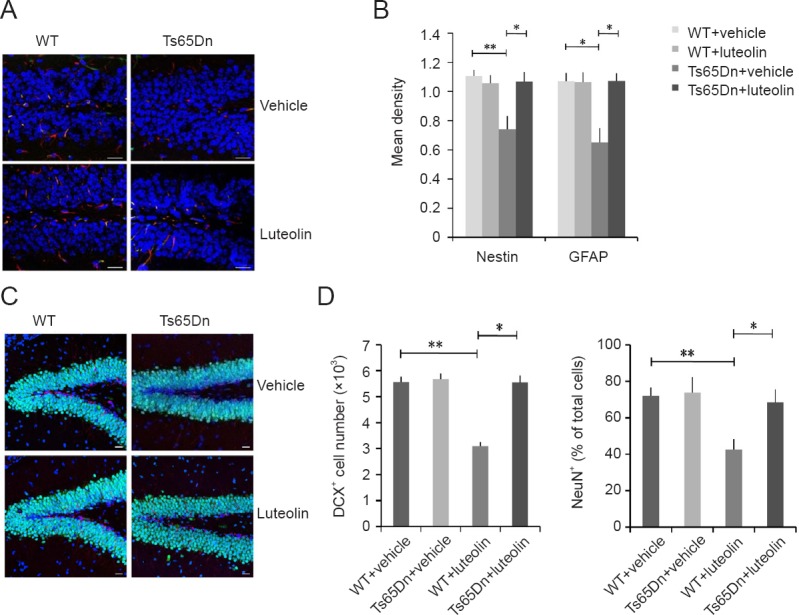
Effects of luteolin on hippocampal neurogenesis in Ts65Dn mice.
(A) Immunofluorescence labeling for the neural stem cell marker nestin (green, FITC) and the astrocyte marker GFAP (red, TRITC) in the hippocampal dentate gyrus. (B) Mean density of nestin and GFAP immunofluorescence. Nestin: genotype (F = 7.47, P < 0.05), treatment (F = 6.14, P < 0.05), genotype × treatment (F = 12.9, P < 0.01). GFAP: genotype (F = 14.1, P < 0.01), treatment (F = 12.1, P < 0.01), genotype × treatment (F = 10.6, P < 0.01). (C) Immunofluorescence for DCX (red, TRITC) and NeuN (green, FITC). (D) DCX+ cell number and NeuN+ cell ratios. DCX: genotype (F = 19.1, P < 0.01), treatment (F = 25.4, P < 0.01), genotype × treatment (F = 16.1, P < 0.01). NeuN: genotype (F = 30, P < 0.01), treatment (F = 22.4, P < 0.01), genotype × treatment (F = 15.7, P < 0.01). Scale bars: 20 μm in A and C. Data are shown as the mean ± SEM (two-way analysis of variance followed by Tukey’s post hoc test). All experiments were performed more than three times. *P < 0.05, **P < 0.01. WT: Wild-type; FITC: fluorescein isothiocyanate; GFAP: glial fibrillary acidic protein; DCX: doublecortin.
Luteolin induces BDNF expression and activates the ERK1/2 pathway in Ts65Dn mice
BDNF plays an important role in the survival, differentiation, growth and development of neurons (Lee et al., 2016). Therefore, we examined whether luteolin affects BDNF expression in Ts65Dn mice. Compared with the vehicle group (Figure 4A), BDNF expression was increased in the luteolin group (P < 0.05), consistent with previous studies (Bianchi et al., 2010; Stagni et al., 2015). The BDNF/ERK signaling pathway is associated with neuronal differentiation and survival (Lee et al., 2016; Ohta et al., 2017). p-ERK1/2 levels were increased in the Ts65Dn + luteolin group (P < 0.01; Figure 4A and B), suggesting that the ERK pathway is activated by luteolin. Furthermore, the increased BDNF expression in the hippocampus might improve learning and memory ability by regulating the growth, proliferation and differentiation of neurons (Peng et al., 2018; Sanna et al., 2018).
Figure 4.
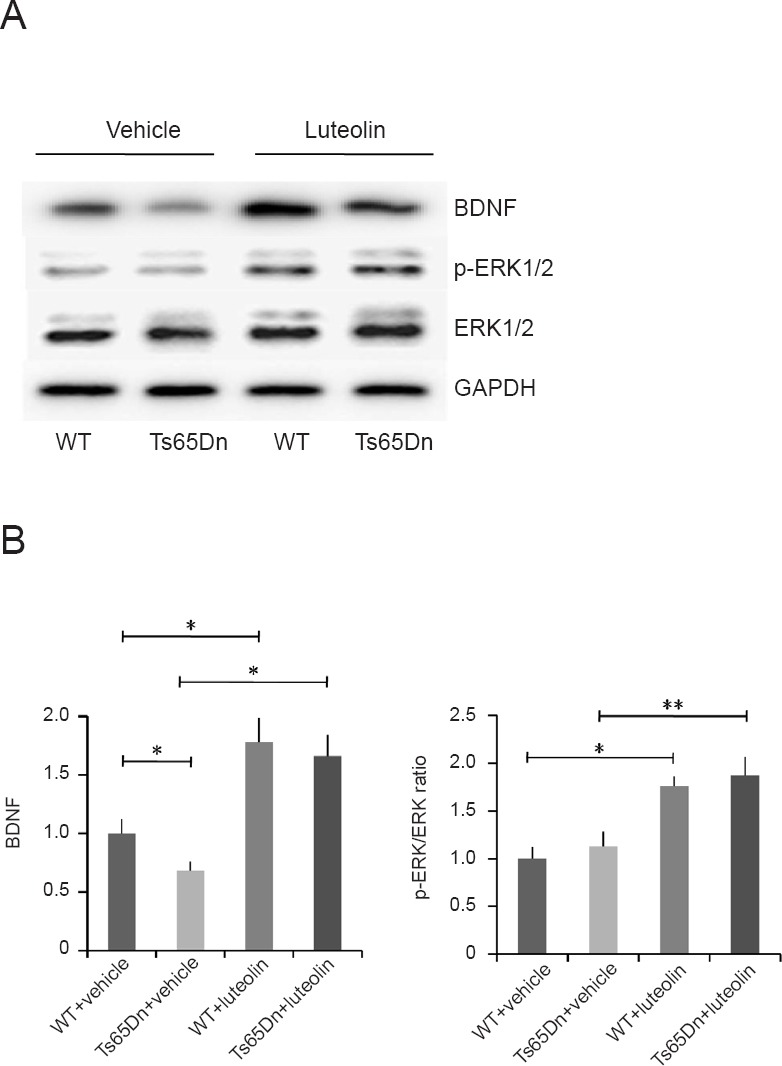
Luteolin increases BDNF expression and activates the ERK1/2 pathway in Ts65Dn mice.
(A) Western blot assay for BDNF, p-ERK1/2 and total ERK. (B) Relative optical density of BDNF (normalized to GAPDH expression) and the p-ERK/ERK ratio. BDNF: Genotype (F = 1.04, P = 0.34), treatment (F = 24.7, P < 0.01), genotype × treatment (F = 0.06, P = 0.81). p-ERK/ERK ratio: Genotype (F = 2.64, P = 0.14), treatment (F = 48.6, P < 0.01), genotype × treatment (F = 0.16, P = 0.71). Data are shown as the mean ± SEM (two-way analysis of variance followed by Tukey’s post hoc test). All experiments were performed more than three times. *P < 0.05, **P < 0.01. WT: Wild-type; BDNF: brain-derived neurotrophic factor; ERK: extracellular signal-regulated kinase; p-ERK: phospho-extracellular signal-regulated kinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Flavonoids have been shown to have therapeutic value in neurodegenerative diseases. For example, 7,8-dihydroxyflavone improves hippocampal neurogenesis and hippocampus-dependent memory via the BDNF/TrkB signaling pathway in the Ts65Dn mouse (Lee et al., 2016). Citrus HHMF promotes neurite outgrowth in PC12 cells. Oroxylin A increases BDNF production by activating the MAPK/CREB pathway. Epigallocatechin gallate, a DYRK1A inhibitor, not only rescues cognitive deficits in the Down syndrome mouse model but also in humans (De la Torre et al., 2014).
Although pharmacotherapy has shown promise in the Down syndrome mouse model, safe and effective clinical treatments are still far off. For example, epigallocatechin gallate is still in phase II trials, and its long-term efficacy needs to be assessed (de la Torre et al., 2016). The widely used antidepressant drug fluoxetine was shown to perturb fetal heart development (Reefhuis et al., 2015). Therefore, it is necessary to search for new effective and safe drugs.
Luteolin induces neurite outgrowth, increases expression of GAP-43, a differentiation marker, and activates the ERK1/2 pathway in the PC12 cell line (Lin et al., 2010). In addition, luteolin increases protein kinase A activity in these cells (Lin et al., 2012). Given the beneficial effects of luteolin on neural development, an important feature of this flavonoid is that it can pass through the blood-brain barrier (Dajas et al., 2003). Therefore, in the present study, we treated Ts65Dn mice with luteolin for 4 weeks to assess whether it can improve the hippocampal-dependent behavioral and neurogenetic defects. The Ts65Dn mouse, trisomic for mouse chromosome 16, which contains approximately 92 genes that are orthologous to the Hsa21 genes, is currently the most widely used murine model of Down syndrome, both genetically and phenotypically (Sturgeon and Gardiner, 2011; Antonarakis, 2017). Ts65Dn mice mimic many of the abnormal features of human Down syndrome, including weight loss, craniofacial deformities (Richtsmeier et al., 2000), delay in sensory reaction and motor function (Toso et al., 2008), cognitive and behavioral disorders (Fernandez and Garner, 2008; Belichenko et al., 2009), and a reduction in the number of hippocampal and cerebellar neurons (Moore and Roper, 2007).
Here, we found that behavioral performance in the water maze and novel object recognition test was markedly improved in Ts65Dn mice administrated luteolin. This effect may be associated with changes in hippocampal neurogenesis and neuronal differentiation. The rate of cell proliferation is reduced in the hippocampal subgranular zone of Ts65Dn mice (Contestabile et al., 2013), and a reduced number of Ki67+ cells was found in the subgranular zone. However, after luteolin treatment, the reduction was restored. This suggests that luteolin promotes neuronal proliferation. In the hippocampal dentate gyrus, we detected some markers associated with neurogenesis. The intermediate filament protein nestin is mainly expressed in proliferating cells, especially immature nerve precursor cells, and its expression increases during neurogenesis, and therefore, it is considered an important marker of neural stem cells (Li and Chopp, 1999; Eisch et al., 2000). GFAP, a marker of astrocytes, which are widely distributed in various regions of the brain and maintain normal function of neurons, plays an important role in the development, migration and synaptic connections of neurons (Eroglu, 2009; Nimmerjahn, 2009). In this study, the reduced nestin and GFAP expression in the dentate gyrus of Ts65Dn mice, suggestive of impaired neurogenesis, was substantially restored by luteolin.
In the present study, the number of DCX+ and NeuN+ cells was reduced by 45% and 42%, respectively, in the dentate gyrus of untreated Ts65Dn mice. DCX is a microtubule-related protein that is expressed mainly in immature neurons and plays an important role in cell migration (Manohar et al., 2012). Here, DCX+ cells were mainly distributed in the subgranular zone of the dentate gyrus. As NeuN is a specific marker of mature neurons in the granular layer (Dennie et al., 2016), we speculate that the decrease in immature cells in the subgranular zone leads to reduced migration of mature neurons in the granular layer. Luteolin almost completely ameliorated the defect.
Liu et al. (2009) reported that the protective effects of luteolin against amyloid beta toxicity was associated with the increased expression of BNDF in the cerebral cortex of mice. In the present study, luteolin increased the expression of BDNF in the hippocampus of Ts65Dn and WT mice. It has been suggested that the BDNF/ERK signaling pathway may be associated with cell differentiation and survival of immature progenitor neurons (Lee et al., 2016), and luteolin can activate the ERK1/2 signaling pathway (Lin et al., 2010). Here, we found increased levels of p-ERK1/2 in luteolin-treated mice. This suggests that luteolin enhances hippocampal neurogenesis in Ts65Dn mice by activating the BDNF/ERK pathway.
Although luteolin has neuroprotective effects, the underlying mechanisms remain unclear. It was reported that increased levels of free redox-active iron, associated with enhanced lipid peroxidation, may be involved in neurodegeneration and cognitive decline in Down syndrome (Manna et al., 2016). We speculate that the neuroprotective effect of luteolin might be associated with its strong antioxidant capacity, inhibition of neuroinflammation and ferrous ion chelating activity. In addition, the ability of luteolin to increase the expression of neurotrophins might involve estrogen signaling pathways, as the phosphorylated estrogen receptor dimer was previously demonstrated to elevate BDNF mRNA levels (Sohrabji et al., 1995; Moosavi et al., 2016). Furthermore, given its structural similarity to epigallocatechin gallate, luteolin might also activate the hypoxia signal transduction pathway, leading to increased transcription of neurotrophic genes mediating compensatory neuronal survival and differentiation (Weinreb et al., 2009).
A great deal of further study is required to elucidate the mechanisms underlying the neuroprotective effect of luteolin in the Down syndrome mouse model, including its effects on other regions of the brain and hippocampus, and the changes elicited in synaptic plasticity, electrophysiology, and apoptosis. Different luteolin concentrations and treatment durations need to be tested to more thoroughly assess efficacy and safety.
Taken together, our findings show that luteolin protects against behavioral deficits and enhances hippocampal neurogenesis in the mouse model of Down syndrome. Therefore, luteolin may have therapeutic potential for treating neurodegenerative diseases.
Additional file: Open peer review report 1 (108.2KB, pdf) .
Acknowledgments
We thank all of the project participants for their contributions.
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This study was supported by the Project Funding for the Training of High Level Health Professionals in Changzhou of China, No. 2016CZLJ013 (to BY); the Science and Technology Support Project of Changzhou of China, No. Social Development CE20175021 (to BY); the Application Basic Research Project of Changzhou of China, No. CJ20160036 (to WBZ). The funding sources had no role in study design, conception, analysis or interpretation of data, writing and deciding to submit this paper for publication.
Institutional review board statement: The experimental protocol was approved by the Jiangsu University Laboratory Animal Management Committee, China (approval No. UJS-LAER-2017042201) on April 22, 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Sijie Tan, University of South China, China.
Funding: This study was supported by the Project Funding for the Training of High Level Health Professionals in Changzhou of China, No. 2016CZLJ013 (to BY); the Science and Technology Support Project of Changzhou of China, No. Social Development CE20175021 (to BY); the Application Basic Research Project of Changzhou of China, No. CJ20160036 (to WBZ).
P-Reviewer: Tan SJ; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Stow A, Qiu Y, Song LP; T-Editor: Liu XL
References
- Antonarakis SE. Down syndrome and the complexity of genome dosage imbalance. Nat Rev Genet. 2017;18:147–163. doi: 10.1038/nrg.2016.154. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29:5938–5948. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar KS, Rupasinghe HP. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Guidi S, Trazzi S, Felice D, Grossi G, Fernandez M, Giuliani A, Calza L, Bartesaghi R. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catuara-Solarz S, Espinosa-Carrasco J, Erb I, Langohr K, Gonzalez JR, Notredame C, Dierssen M. Combined treatment with environmental enrichment and (-)-epigallocatechin-3-gallate ameliorates learning deficits and hippocampal alterations in a mouse model of Down syndrome. eNeuro. 2016;3:ENEURO.0103-0116. doi: 10.1523/ENEURO.0103-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett. 2008;448:175–179. doi: 10.1016/j.neulet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Clark S, Schwalbe J, Stasko MR, Yarowsky PJ, Costa AC. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp Neurol. 2006;200:256–261. doi: 10.1016/j.expneurol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 2013;123:348–361. doi: 10.1172/JCI64650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Ricobaraza A, Del Rio J, Frechilla D, Franco R, Perez-Mediavilla A, Garcia-Osta A. Chronic mild stress in mice promotes cognitive impairment and CDK5-dependent tau hyperphosphorylation. Behav Brain Res. 2011;220:338–343. doi: 10.1016/j.bbr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, Echeverry C, Lafon L, Heizen H, Ferreira M, Morquio A. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003;36:1613–1620. doi: 10.1590/s0100-879x2003001200002. [DOI] [PubMed] [Google Scholar]
- De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, Fitó M, Benejam B, Langohr K, Rodriguez J, Pujadas M, Bizot JC, Cuenca A, Janel N, Catuara S, Covas MI, Blehaut H, Herault Y, Delabar JM, Dierssen M. Epigallocatechin-3-gallate, a DYRK1A, inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58:278–288. doi: 10.1002/mnfr.201300325. [DOI] [PubMed] [Google Scholar]
- de la Torre R, de Sola S, Hernandez G, Farré M, Pujol J, Rodriguez J, Espadaler JM, Langohr K, Cuenca-Royo A, Principe A, Xicota L, Janel N, Catuara-Solarz S, Sanchez-Benavides G, Bléhaut H, Dueñas-Espín I, Del Hoyo L, Benejam B, Blanco-Hinojo L, Videla S, et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:801–810. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- Dennie D, Louboutin JP, Strayer DS. Migration of bone marrow progenitor cells in the adult brain of rats and rabbits. World J Stem Cells. 2016;8:136–157. doi: 10.4252/wjsc.v8.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierssen M. Down syndrome: the brain in trisomic mode. Nat Rev Neurosci. 2012;13:844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. Journal of cell communication and signaling. 2009;3:167–176. doi: 10.1007/s12079-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Garner CC. Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav Brain Res. 2008;188:233–237. doi: 10.1016/j.bbr.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HC, Salehi A, Chuluun B, Das D, Lin B, Moghadam S, Garner CC, Colas D. Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors. Neurobiol Learn Mem. 2014;116:162–171. doi: 10.1016/j.nlm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Hoppe JB, Coradini K, Frozza RL, Oliveira CM, Meneghetti AB, Bernardi A, Pires ES, Beck RC, Salbego CG. Free and nanoencapsulated curcumin suppress beta-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3beta signaling pathway. Neurobiol Learn Mem. 2013;106:134–144. doi: 10.1016/j.nlm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7, 8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Li D, Wu W. Inhibitory effects and mechanisms of luteolin on proliferation and migration of vascular smooth muscle cells. Nutrients. 2013;5:1648–1659. doi: 10.3390/nu5051648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yang M, Kim J, Son Y, Kim J, Kang S, Ahn W, Kim SH, Kim JC, Shin T, Wang H, Moon C. Involvement of BDNF/ERK signaling in spontaneous recovery from trimethyltin-induced hippocampal neurotoxicity in mice. Brain Res Bull. 2016;121:48–58. doi: 10.1016/j.brainresbull.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem. 2010;58:4477–4486. doi: 10.1021/jf904061x. [DOI] [PubMed] [Google Scholar]
- Lin LF, Chiu SP, Wu MJ, Chen PY, Yen JH. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS One. 2012;7:e43304. doi: 10.1371/journal.pone.0043304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Gao M, Qiang GF, Zhang TT, Lan X, Ying J, Du GH. The anti-amnesic effects of luteolin against amyloid beta(25-35) peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience. 2009;162:1232–1243. doi: 10.1016/j.neuroscience.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9:623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- Manna C, Officioso A, Trojsi F, Tedeschi G, Leoncini S, Signorini C, Ciccoli L, De Felice C. Increased non-protein bound iron in Down syndrome: contribution to lipid peroxidation and cognitive decline. Free Radic Res. 2016;50:1422–1431. doi: 10.1080/10715762.2016.1253833. [DOI] [PubMed] [Google Scholar]
- Manohar S, Paolone NA, Bleichfeld M, Hayes SH, Salvi RJ, Baizer JS. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2012;202:169–183. doi: 10.1016/j.neuroscience.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Roper RJ. The power of comparative and developmental studies for mouse models of Down syndrome. Mamm Genome. 2007;18:431–443. doi: 10.1007/s00335-007-9030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther. 2016;10:23–42. doi: 10.2147/DDDT.S96936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A. Astrocytes going live: advances and challenges. J Physiol. 2009;587:1639–1647. doi: 10.1113/jphysiol.2008.167171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta KI, Suzuki S, Warita K, Kaji T, Kusaka T, Miki T. Prolonged maternal separation attenuates BDNF-ERK signaling correlated with spine formation in the hippocampus during early brain development. J Neurochem. 2017;141:179–194. doi: 10.1111/jnc.13977. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim DH, Kim JM, Shin CY, Cheong JH, Ko KH, Ryu JH. Mismatch between changes in baicalein-induced memory-related biochemical parameters and behavioral consequences in mouse. Brain Res. 2010;1355:141–150. doi: 10.1016/j.brainres.2010.07.098. [DOI] [PubMed] [Google Scholar]
- Parrini M, Ghezzi D, Deidda G, Medrihan L, Castroflorio E, Alberti M, Baldelli P, Cancedda L, Contestabile A. Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci Rep. 2017;7:16825. doi: 10.1038/s41598-017-17201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Yan HZ, Liu PR, Shi XW, Liu CL, Liu Q, Zhang Y. Phosphodiesterase 4 inhibitor roflumilast protects rat hippocampal neurons from sevoflurane induced injury via modulation of MEK/ERK signaling pathway. Cell Physiol Biochem. 2018;45:2329–2337. doi: 10.1159/000488180. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Devine O, Friedman JM, Louik C, Honein MA National Birth Defects Prevention Study. Specific SSRIs and birth defects: Bayesian analysis to interpret new data in the context of previous reports. BMJ. 2015;351:h3190. doi: 10.1136/bmj.h3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtsmeier JT, Baxter LL, Reeves RH. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn. 2000;217:137–145. doi: 10.1002/(SICI)1097-0177(200002)217:2<137::AID-DVDY1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sanna MD, Mello T, Masini E, Galeotti N. Activation of ERK/CREB pathway in noradrenergic neurons contributes to hypernociceptive phenotype in H4 receptor knockout mice after nerve injury. Neuropharmacology. 2018;128:340–350. doi: 10.1016/j.neuropharm.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza LC, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT, Donato F, Prigol M, Boeira SP, Jesse CR. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav. 2015;134:22–30. doi: 10.1016/j.pbb.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Stagni F, Giacomini A, Guidi S, Ciani E, Ragazzi E, Filonzi M, De Iasio R, Rimondini R, Bartesaghi R. Long-term effects of neonatal treatment with fluoxetine on cognitive performance in Ts65Dn mice. Neurobiol Dis. 2015;74:204–218. doi: 10.1016/j.nbd.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Sturgeon X, Gardiner KJ. Transcript catalogs of human chromosome 21 and orthologous chimpanzee and mouse regions. Mamm Genome. 2011;22:261–271. doi: 10.1007/s00335-011-9321-y. [DOI] [PubMed] [Google Scholar]
- Toso L, Cameroni I, Roberson R, Abebe D, Bissell S, Spong CY. Prevention of developmental delays in a Down syndrome mouse model. Obstet Gynecol. 2008;112:1242–1251. doi: 10.1097/AOG.0b013e31818c91dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Mandel S, Youdim MB. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes & nutrition. 2009;4:283–296. doi: 10.1007/s12263-009-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo DY, Choi JH, Kim W, Nam SM, Jung HY, Kim JH, Won MH, Yoon YS, Hwang IK. Effects of luteolin on spatial memory, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia model. Neurol Res. 2013;35:813–820. doi: 10.1179/1743132813Y.0000000217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.