Abstract
Pain assessments typically depend on self-report of the pain experience. Yet, in individuals with autism spectrum disorders, this can be an unreliable due to communication difficulties. Importantly, observations of behavioral hypo- and hyperresponsivity to pain suggest altered pain sensitivity in autism spectrum disorder. Neuroimaging may provide insight into mechanisms underlying pain behaviors. The neural pain signature reliably responds to painful stimulation and is modulated by other outside regions, affecting the pain experience. In this first functional magnetic resonance imaging study of pain in autism spectrum disorder, we investigated neural responses to pain in 15 adults with autism spectrum disorder relative to a typical comparison group (n = 16). We explored temporal and spatial properties of the neural pain signature and its modulators during sustained heat pain. The two groups had indistinguishable pain ratings and neural pain signature responses during acute pain; yet, we observed strikingly reduced neural pain signature response in autism spectrum disorder during sustained pain and after stimulus offset. The posterior cingulate cortex, a neural pain signature modulating region, mirrored this late signal reduction in autism spectrum disorder. Intact early responses, followed by diminished late responses to sustained pain, may reflect altered pain coping or evaluation in autism spectrum disorder. Evidence of a dichotomous neural response to initial versus protracted pain may clarify the coexistence of both hypo- and hyperresponsiveness to pain in autism spectrum disorder.
Keywords: autism spectrum disorder, functional magnetic resonance imaging, pain, repetitive behaviors, self-injury, sensory
Introduction
Altered sensory responsivity is well documented in autism spectrum disorder (ASD), with evidence of hypo- and hyperresponsivity to sensory stimuli (APA, 2013; Baranek et al., 2006). Yet, very little is known about neural mechanisms of these altered behavioral responses. Recent work suggests that both basic sensory and limbic systems (Green et al., 2013, 2015), as well as systems for higher-order perception and attention (Cascio et al., 2015; Pryweller et al., 2014), are likely involved. Despite a growing literature addressing unusually intense reactions to innocuous sensory stimuli (i.e. hyperreactivity), there is very little known about response to noxious stimulation in ASD. Clinical reports have historically suggested increased pain tolerance (i.e. hyporeactivity) in ASD, possibly as an explanation for self-injurious behavior (SIB). However, this notion is controversial and lacks empirical support (Symons et al., 2009a, 2010). Importantly, an “apparent indifference to pain/temperature” is now specified as a common exemplar of sensory hyporeactivity in Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5), yet, there remains a paucity of study on the topic with no clear consensus on pain responsivity in ASD (Moore, 2015).
Pain is typically measured by verbal self-report, but in ASD, these reports may be unreliable due to different verbal and/or cognitive ability (Duerden et al., 2015; Williamson and Hoggart, 2005). Pain can also be assessed by coding nonverbal responses (such as facial expressions or heart rate) to stimuli, but facial expressions may also be impacted by ASD (Davies et al., 2016). Experimental studies using noxious stimuli have yielded mixed findings in ASD. Individuals with ASD verbally report less pain and discomfort during electrical and thermal pain stimulation (Yasuda et al., 2016). Yet, heart rate and endorphin levels suggest intact or enhanced response to acute pain (Tordjman et al., 2009), as do findings of aberrantly high (Nader et al., 2004) or prolonged (Rattaz et al., 2013) facial responses to pain in ASD. Psychophysical studies report either no differences in pain thresholds (electrical (Bird et al., 2010; Yasuda et al., 2016) and thermal (Yasuda et al., 2016)) or lower thresholds (more sensitive to thermal (Cascio et al., 2008; Duerden et al., 2015) and pressure pain (Fan et al., 2014; Riquelme et al., 2016)) compared to typical comparison (TC) groups. One study reported initial heightened pain sensitivity that did not replicate in a follow-up session with the same sample, suggesting mediation by anxiety or other higher-order factors expected to diminish with repeated exposure (Cascio et al., 2008).
Adding to the importance of characterizing pain perception in ASD, the prevalence of SIBs in the ASD population likely complicates our understanding of pain perception in ASD. SIB is a complex and heterogeneous phenomenon that occurs in approximately half of individuals with ASD (Bodfish et al., 2000), and for which, multiple neurobiological mechanisms have been proposed, including alterations in the somatosensory system (Duerden et al., 2014) and endogenous opioid system (Sandman, 1988) centrally, and nociceptive fiber innervation peripherally (Symons et al., 2008, 2009b). SIBs are also tightly linked to aberrant sensory processing (Duerden et al., 2012). The literature currently lacks consensus on the complex relations between SIB and pain perception and processing in ASD. In addition to SIBs, co-occurring pain-related medical conditions such as gastrointestinal discomfort (Gorrindo et al., 2012) are common in ASD and could reflect altered pain perception. Even without these comorbidities, aberrant pain responsivity can be problematic. Pain hyposensitivity can result inadvertent injury and reduced treatment, while hypersensitivity to innocuous sensory stimuli can result in pain-like behaviors or anxiety (Baranek and Berkson, 1994; Baranek et al., 2006; Liss et al., 2006). Importantly, successful pain management can also impact other issues such as sleep disturbances (Tudor et al., 2015) and mitigate distress for caregivers (Konstantareas and Homatidis, 1989).
With the presence of pain-related comorbidities, uncertainty regarding pain responsivity, and communication deficits that limit the reliability of standard pain assessments, other communication-independent methods are needed to facilitate understanding of pain processing in ASD. Functional neuroimaging during pain may provide a tool for elucidating pain processing in these vulnerable populations. Recent work has characterized a reliable neural pain signature (NPS; Wager et al., 2013; somatosensory cortices, thalamus, insula, striatum, anterior cingulate cortex (ACC), and supplementary motor cortex), for which response to sustained painful stimulation shares a unique temporal structure (Ibinson and Vogt, 2013). Rather than a boxcar-like pattern that directly reflects the onset and offset of stimulation, the NPS exhibits a triphasic response. While the early and intermediate phases have been linked to attentional orienting, late phase blood oxygenation level-dependent (BOLD) signal increase in S1 and S2 is tied to the conscious perception of pain and intensity evaluation (Chen et al., 2002; Moulton et al., 2005), and in the dorsal anterior cingulate cortex (dACC), it is thought to reflect affective modulation and cognitive control associated with pain (Shackman et al., 2011). The level of integration within the NPS (from pain discrimination to cognitive and affective appraisal) suggests understanding NPS response may clarify differences in pain processing that may be difficult to detect with behavioral methods such as pain rating scales. Dynamic interactions between the NPS and other networks such as the default mode network (DMN) may drive individual strategies for coping with pain, for example, by titrating the level of engagement with the external stimulus versus distracting from it by directing attention elsewhere (Kucyi and Davis, 2015; Kucyi et al., 2013).
Given the lack of understanding of pain processing in ASD and the proposed utility of functional magnetic resonance imaging (fMRI) as an objective metric of pain experience in vulnerable populations (Wager et al., 2013), we conducted a functional imaging study of sustained painful heat applied to the calf. The goal of this study was to determine whether the temporal or spatial properties of the NPS differ in individuals with ASD, and if so, what possible modulators outside the NPS contribute to these differences.
Methods and materials
Participants
This study was approved by the internal review board at the University of North Carolina, Chapel Hill. Participants included 24 adults with ASD (clinical diagnosis of either autistic disorder or Asperger’s disorder, Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV); American Psychiatric Association (APA), 2000) and 21 adults without ASD in a TC group recruited for this study. Of those recruited, 16 ASD and 16 TC were able to complete the fMRI protocol, while the other participants did not complete the scan due to an inability to lay still, the uncomfortable nature of the protocol, or equipment issues. One participant in the ASD group was excluded due to head movement during fMRI data acquisition, making the final reported sample 15 adults with ASD and 16 adults in the TC group. Potential participants were excluded for history of neurological disorders or peripheral nerve injury. Inclusion criteria were IQ ≥ 80 and age range of 18–50 years. Participants were group matched on the basis of age, gender, race, and full-scale IQ, measured using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Table 1 summarizes demographic, clinical, and heat pain characteristics of participants. There were no significant differences in any demographic variables; however, participants with ASD tended to have a lower performance IQ (p = 0.08). There were no differences in heat pain thresholds, temperature received in the scanner, or pain ratings. Additionally, there were no differences in the percentage of participants who received a temperature <49°C during the scan (ASD: 20.0%, TC: 25.0%, p = 0.793). Participants with ASD reported SIBs (8 of 12 reporting), with rubbing or scratching (87.5%) and skin picking (75%) as the most highly reported behaviors.
Table 1.
Demographic, clinical, and heat pain characteristics of participants.
| ASD (n= 15) | Control (n=16) | p-value | |
|---|---|---|---|
| % Male | 93.3 | 87.5 | – |
| Age (years), mean (SD) | 27.53 (10.33) | 30.13 (10.87) | 0.5 |
| FSIQ, mean (SD) | 102.27 (20.11) | 110.94 (12.59) | 0.16 |
| PIQ, mean (SD) | 103.47 (18.52) | 113.44 (11.93) | 0.08 |
| VIQ, mean (SD) | 100.27 (20.52) | 106.5 (14.45) | 0.33 |
| ADOS | |||
| Social | 9.93 (2.59) | – | – |
| Communication | 4.6 (1.45) | – | – |
| Stereotyped | 2.6 (2.06) | – | – |
| ADI-R | |||
| Social | 10.38 (5.74) | – | – |
| Verbal communication | 8.46 (5.41) | – | – |
| Repetitive behavior | 6.23 (4.02) | – | – |
| Heat pain threshold (°C), mean ± SD | 41.92 ± 4.81 | 42.92 ± 4.08 | 0.55 |
| Temperature received (°C), mean ± SD | 48.37 ± 1.52 | 48.50 ± 0.97 | 0.77 |
| Temperature percentage of threshold, mean ± SD | 116.92 ± 11.15 | 113.91 ± 9.0 | 0.44 |
| Pain rating, mean ± SD | 7.48 ± 1.47 | 7.53 ± 1.90 | 0.94 |
| Self-injurious behaviors, n (%) | 8 (66.7)a | – | – |
| Mean absolute movement (mm) | 0.55 ± 0.52 | 0.38 ± 0.37 | 0.31 |
ASD: autism spectrum disorders; SD: standard deviation; FSIQ: full-scale IQ; PIQ: performance IQ; VIQ: verbal IQ, ADOS: Autism Diagnostic Observation Schedule; ADI-R: Autism Diagnostic Interview–Revised; RBS-R: Repetitive Behavior Scale–Revised.
Only 12 individuals from the ASD group were evaluated with the RBS-R.
Behavioral measures
The Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) and Autism Diagnostic Interview–Revised (ADI-R; Lord et al., 1994) were administered by research-reliable assessors under supervision of a licensed clinician to confirm ASD diagnosis and estimate symptom severity. Repetitive behavior patterns were assessed with the Repetitive Behavior Scale–Revised (RBS-R; Bodfish et al., 2000) in 12 of the 15 included participants with ASD. The RBS-R uses a 4-point Likert scale and comprises six subscales: stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted behaviors. The instructions were modified for use as an adult self-report. Participants with ASD were categorized by presence (ASD + SIB) or absence (ASD − SIB) of SIBs based on endorsement of any of the eight behaviors comprising the self-injurious subscale of the RBS-R. While a definitive analysis based on this subsetting was not possible due to the size of our sample, an exploratory preliminary analysis of these two groups is presented in the supplementary materials.
Heat pain thresholds
Thermal heat stimuli were applied with a Peltier stimulator (30 × 30 mm thermoconducting surface; TSA II, Medoc, Israel) to the right lateral calf. Heat pain thresholds were measured prior to neuroimaging, using a method of limits procedure. In total, five trials were conducted, each of which comprised a 1°C/s increase in temperature from a baseline of 32°C until the participant indicated pain by button press. The highest and lowest values were discarded, and temperatures for the remaining three trials were averaged as the threshold.
Heat pain fMRI experimental design
In total, two runs of six trials each were conducted using a block design. For each trial, heat was applied to the right lateral calf for 21 s (15 s at target temperature, 3 s ramp up/down) followed by 39 s of no stimulation. Most participants performed two runs; three participants with ASD did not complete the second run due to time constraints (n = 2) or refusal to continue the protocol (n = 1). Participants received 49°C unless they were unable to tolerate it, in which case, the temperature was reduced to an average of 44.8 ± 2.3°C (42°C lower limit, 8.5% heat reduction on average, n = 6). Participants receiving <49°C were split equally across the ASD and TC groups. After each run, participants were asked to verbally rate pain intensity on a scale of 1–10. Pain ratings did not differ for participants who received <49°C (7.4 ± 1.8 compared to 7.3 ± 1.7 for individuals who received 49°C, p = 0.840).
Image acquisition
Images were acquired on a Siemens 3T Allegra magnetic resonance imaging (MRI) scanner. A T1-weighted anatomical image was acquired using MPRAGE sequence (160 axial slices, voxel = 1 × 1 × 1 mm3; repetition time (TR) = 1700 ms, field of view (FOV) = 192 × 256 mm2). Functional images were acquired with T2*-weighted echo planar imaging (EPI) sequence (50 axial slices, voxel size = 3 × 3 × 3 mm, TR = 3 s, FOV = 64 × 64 mm2, interleaved acquisition, flip angle = 90°).
Image processing
Images were processed in FMRI Expert Analysis Tool (FEAT) version 6.00, a part of FMRIB’s Software Library (FSL, www.fmrib.ox.ac.uk/fsl, version 5.0). Functional data were registered to the high-resolution structural image using a boundary-based registration algorithm (Greve and Fischl, 2009), and individual structural images were registered to Montreal Neurological Institute (MNI) 152-T1 1 mm3 template using FMRIB’s linear image registration tool (FLIRT, linear; Jenkinson et al., 2002; Jenkinson and Smith, 2001) and further refined with FMRIB’s nonlinear image registration tool (FNIRT, nonlinear) registration algorithms (Andersson et al., 2007a; 2007b). Preprocessing of functional images included motion correction with MCFLIRT (Jenkinson et al., 2002), brain extraction with brain extraction tool (BET; Smith, 2002), a high-pass filter (180 s), grand-mean intensity normalization of the entire four-dimensional (4D) dataset by a single multiplicative factor and spatial smoothing (full width at half maximum (FWHM) = 5 mm). Time-series statistical analysis was conducted with prewhitening using FMRIB’s improved linear model (FILM) with local autocorrelation correction (Woolrich et al., 2001). Individual runs were rejected based on peak motion of >6 mm (n = 2 for ASD group, n = 1 for TC group). Importantly, groups did not differ in the number of participants with one versus two runs included in the analysis (Fisher’s exact, p = 0.083). Standard motion parameters were included in the general linear model (GLM), with the addition of DVARS (D, temporal derivatives of time courses; VARS, variance of root mean squares of head motion across voxels) and framewise displacement metrics for head motion (Power et al., 2012) to the GLM as confound explanatory variables (EVs) to remove effects of outlier volumes from the parameter estimates of interest. Second-level analyses combined runs for each subject used a fixed-effect model, by forcing random effect variance to zero in FMRIB’s local analysis of mixed effects (FLAME; Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004.)
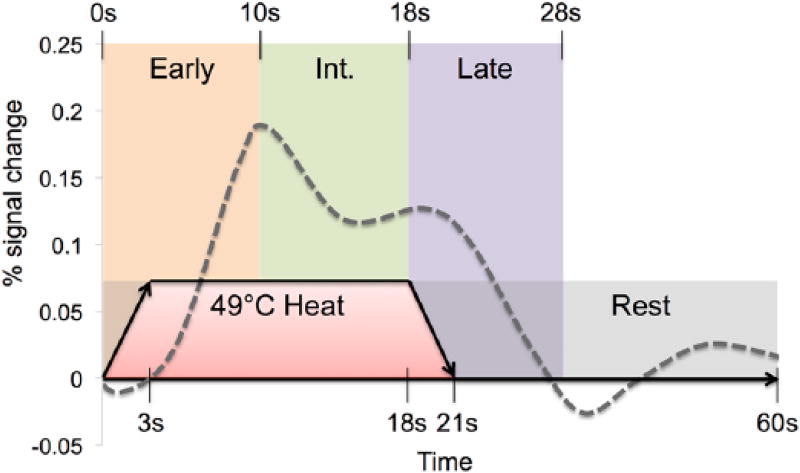
Figure 1 depicts a single trial with the structure of temporal analysis. Based on previous work identifying a three-EV function for a continuous heat pain stimulus (Moulton et al., 2012), we selected three time-based EVs from the beginning of the stimulus: early phase (0–10 s), intermediate phase (10–18 s), and late phase (18–28 s). Stimulus duration was convolved with a double-gamma hemodynamic response function with temporal filtering added to the GLM model. Each EV was modeled as a single contrast (stimulus–baseline). Baseline was defined as the 32 s of the rest period not modeled in the late phase EV.
Figure 1.
Depiction of a single heat pain trial and modeling structure. Time is represented in seconds across the x-axis, with a total of 60 s for one trial. Heat pain is administered for 21 s (15 s at target 49°C, with 3 s ramp up/down) followed by 39 s of no stimulation. Expected triphasic neural response is represented on the y-axis as percent signal change with early (0–10 s), intermediate (10–18 s) and late (18–28 s, as the response lasts longer than physical stimulation) phases shown.
Group-level statistics were calculated using a mixed-effects FLAME 1 model (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). For each diagnostic group, two maps were made: Pain > Rest, using a cluster threshold of z = 2.5 and p-value = 0.01, and Rest > Pain with z = 2.3 and p-value = 0.05 (for both, family-wise error corrected based on clusters defined by Gaussian random field theory; Worsley, 2001). Group contrast maps of (ASD > TC or TC > ASD) were rendered with a cluster threshold of z = 2.3 and p-value = 0.01.
Because this was the first fMRI study of physical pain in ASD, we conducted a whole brain analysis and further interrogated these results using featquery applied to masked regions of interest (ROIs). NPS ROIs were selected based on previous literature (Moulton et al., 2012; Wager et al., 2013) and were defined based on published atlases and previous work that ensured specificity and reproducibility. ROIs included contralateral sensory thalamus (ROI based on connectivity to somatosensory cortex defined by Oxford thalamic connectivity atlas; Behrens et al., 2003) and primary somatosensory cortex (SI, 3A and 3B only; Geyer et al., 2000). Bilateral ROIs included secondary somatosensory cortex (S2, Juelich atlas; Eickhoff et al., 2006a, 2006b), insular cortex (insula; Harvard Cortical Atlas), ACC (Harvard Cortical Atlas), caudate, and putamen (Harvard Subcortical Atlas). NPS modulatory regions were also selected based on previous literature (Kucyi and Davis, 2015): posterior cingulate cortex (PCC; Harvard Cortical Atlas), subgenual prefrontal cortex (sgPFC; Harvard Cortical Atlas) and dorsolateral prefrontal cortex (dlPFC). The dlPFC ROI was taken from a functionally defined atlas (Shirer et al., 2012) to limit overlap with neighboring regions such as ventrolateral prefrontal cortex (PFC). Mean BOLD signal change (%) relative to baseline was extracted from each run for all voxels within the ROI using featquery and then averaged per subject.
Statistical approach
Statistical analysis was performed using Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC) and R (version 3.2.1; https://www.r-project.org/, Vienna, Austria). Descriptive analyses included frequencies, mean, and standard deviation. Student’s t-tests or univariate analysis of variance (ANOVA) were used for group comparisons (Mann–Whitney and Kruskal–Wallis were used where appropriate). Spearman’s ρ was used for nonparametric correlations. Separate mixed models were conducted for each ROI with group and phase as independent variables and percent signal change as the dependent variable. To correct for multiple tests, false discovery rate (FDR) correction was applied to p-values for each model. Significant interactions between group and phase were followed up with post hoc comparisons between the ASD and TC groups by phase. Post hoc tests were Bonferroni corrected for the three post hoc comparisons (one for each phase). Cohen’s d effect sizes were also calculated. All data reported in this article can be accessed by contacting the corresponding author.
Results
Intact NPS in ASD during early pain stimulation
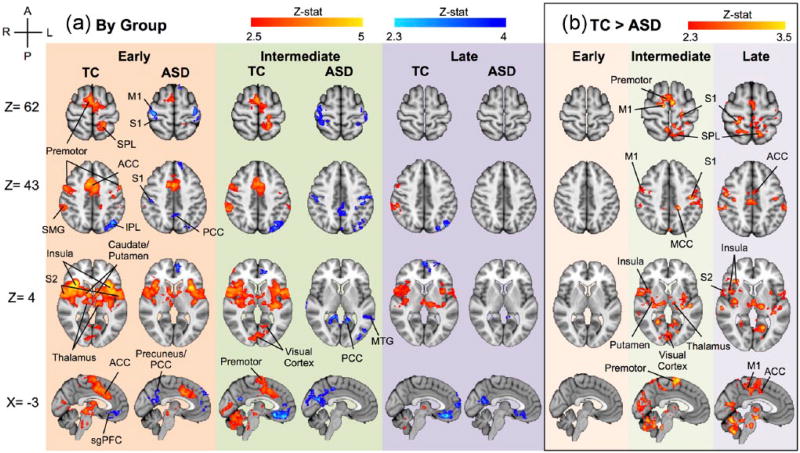
Whole brain analysis during the early phase of pain stimulation showed both groups had increased BOLD response relative to baseline in the NPS (thalamus, S1, S2, premotor cortex, ACC, supramargial gyrus (SMG), insula, caudate, and putamen; Figure 2). Significant clusters with peak voxels and MNI coordinates are reported in Table 2.
Figure 2.
Whole brain analysis. (a) Typical and ASD group maps during early, intermediate, and late phases of pain stimulation. Pain > Rest: z = 2.5, p < 0.01; Rest > Pain: z = 2.3, p < 0.05. While Zmax value for Pain > Rest is z = 5.69, this contrast is rendered from 2.5 < Z < 5 to allow for greater visualization of variation within the group map. (b) Typical > ASD in early, intermediate, and late phases of pain stimulus. Z > 2.3, p < 0.01.
ACC: anterior cingulate cortex; S1: primary somatosensory cortex; S2: secondary somatosensory cortex; SMG: supramargial gyrus; IPL: inferior parietal lobule; SPL: superior parietal lobule; MTG: medial temporal gyrus; dlPFC: dorsolateral prefrontal cortex; PCC: posterior cingulate cortex; M1, primary motor cortex.
Table 2.
Summary of significant clusters with peak voxels and MNI coordinates for each group in each phase.
| Phase | Typical comparison group | Autism spectrum disorders group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Region of activation | Cluster size |
Coordinates | Z-score peak |
p-value | Region of activation | Cluster size |
Coordinates | Z-score peak |
p-value | |||||
|
|
|
|||||||||||||
| x | y | z | x | y | z | |||||||||
| Early phase | ||||||||||||||
| Pain > Rest | Right insula (also bilateral insula, S2, caudate, putamen, thalamus, brainstem) | 149,684 | 35 | 26 | 4 | 5.69 | <1E–28 | Left insula (also bilateral insula, caudate, putamen, thalamus, brainstem) | 53,732 | −35 | 13 | 4 | 5.14 | 1.51E–28 |
| Right premotor (also bilateral premotor, ACC, left S1, left SPL) | 42,154 | 7 | 10 | 46 | 4.86 | 4.19E–24 | Right ACC (also right premotor) | 17,517 | 1 | 7 | 44 | 4.63 | 6.82E–13 | |
| Left cerebellum | 5773 | −30 | −56 | −28 | 3.99 | 1.59E–05 | Right S2 | 3092 | 65 | −24 | 26 | 3.4 | 0.00308 | |
| Right cerebellum | 4357 | 32 | −35 | −34 | 4.18 | 0.000224 | Left SPL (also left S1) | 2959 | −21 | −46 | 74 | 4.51 | 0.00413 | |
| Right IFG (also right dlPFC) | 4104 | 36 | 36 | 16 | 4 | 0.00037 | ||||||||
| Left V1 (also right V1) | 3325 | −7 | −67 | 8 | 3.49 | 0.00186 | ||||||||
| Left premotor | 2834 | −53 | 3 | 42 | 3.96 | 0.00547 | ||||||||
| Rest > Pain | Left IPL | 6463 | −27 | −77 | 47 | 3.8 | 9.61E–05 | Left lateral occipital cortex (also left IPL) | 5472 | −50 | −64 | 31 | 3.55 | 0.000416 |
| Right sgPFC (also left sgPFC) | 3675 | 5 | 24 | −12 | 3.41 | 0.00762 | Left precuneus (also left PCC) | 4603 | −8 | −67 | 20 | 3.86 | 0.00162 | |
| Left S1 (also left M1) | 4212 | −52 | −19 | 56 | 3.77 | 0.00307 | ||||||||
| Right S1 (also right M1) | 4206 | 39 | −25 | 59 | 4.05 | 0.0031 | ||||||||
| Right MFG (also left MFG) | 3673 | 7 | 55 | 8 | 3.78 | 0.00764 | ||||||||
| Left dlPFC | 3390 | −10 | 63 | 28 | 3.46 | 0.0125 | ||||||||
| Intermediate phase | ||||||||||||||
| Pain > Rest | Left thalamus (also bilateral insula, S2, caudate, putamen, thalamus, brainstem) | 181,309 | −26 | −25 | 13 | 4.92 | <1E–28 | None | ||||||
| Right premotor (also bilateral ACC) | 25,241 | 2 | −1 | 65 | 4.9 | 1.96E–13 | ||||||||
| Left SPL (also left S1, left M1) | 7237 | −18 | −42 | 64 | 4.54 | 3.03E–05 | ||||||||
| Right V1 (also left V1) | 5874 | 18 | −89 | 2 | 3.76 | 0.000199 | ||||||||
| Right dlPFC | 4699 | 50 | 45 | 7 | 3.87 | 0.00114 | ||||||||
| Rest > Pain | Left sgPFC (also right sgPFC, left vmPFC) | 16,089 | −2 | 22 | −14 | 4.25 | 5.96E–08 | Left PCC (also bilateral PCC, precuneus, and V1; left hippocampus; left parahippocampal gyrus; left amygdala) | 40,054 | −11 | −52 | 8 | 4.09 | 2.62E–15 |
| Right STG | 6045 | 49 | 13 | −30 | 3.93 | 0.0017 | Left S1 (also left M1, left SPL) | 9342 | −36 | −40 | 56 | 3.58 | 4.53E–05 | |
| Left precuneus (also left lateral occipital cortex) | 5249 | −27 | −81 | 49 | 3.7 | 0.00445 | Right S1 (also right M1, right SPL) | 8698 | 44 | −31 | 57 | 3.88 | 8.85E–05 | |
| Right PCC (also left PCC, right precuneus) | 4702 | 1 | −57 | 24 | 4.05 | 0.00888 | Right MTG (also right STG) | 6499 | 65 | −10 | −11 | 3.63 | 0.000996 | |
| Right parahippocampal gyrus (also right hippocampus) | 5990 | 22 | −28 | −20 | 3.96 | 0.00181 | ||||||||
| Left MTG (also left STG) | 3617 | −44 | −31 | −1 | 3.37 | 0.0377 | ||||||||
| Late phase | ||||||||||||||
| Pain > Rest | Right S2 (also right insula, putamen, SMG, IPL, premotor) | 32,655 | 48 | −7 | 9 | 4.36 | 3.91E–15 | None | ||||||
| Left insula (also left S2, putamen, SMG, IPL, bilateral thalamus) | 23,698 | −33 | −20 | 21 | 4.54 | 5.01E–12 | ||||||||
| Left cerebellum | 4088 | −21 | −72 | −51 | 4.04 | 0.00499 | ||||||||
| Rest > Pain | Left sgPFC (also bilateral sgPFC, OFC, vmPFC) | 9859 | −4 | 38 | −16 | 4.37 | 6.08E–05 | Right MTG (also right fusiform, parahippocampal, amygdala) | 6772 | 42 | −6 | −37 | 3.64 | 0.00135 |
| Left vmPFC (also left dlPFC) | 5445 | −26 | 64 | 9 | 3.66 | 0.00588 | Left fusiform (also left cerebellum, parahippocampal gyrus) | 5127 | −30 | −39 | −22 | 3.62 | 0.0085 | |
| Left IPL (also left lateral occipital cortex) | 4279 | −42 | −74 | 34 | 3.85 | 0.0236 | Left lateral occipital cortex | 5018 | −35 | −84 | 24 | 3.85 | 0.00966 | |
| Left PCC (also right PCC, right V1) | 4966 | −6 | −55 | 16 | 3.96 | 0.0103 | ||||||||
| Right parahippocampal gyrus (also right fusiform) | 4727 | 28 | −45 | −12 | 3.6 | 0.0137 | ||||||||
| Left MTG | 4017 | −50 | 4 | −20 | 3.88 | 0.0327 | ||||||||
| Left sgPFC (also bilateral sgPFC, OFC, caudate) | 3786 | 5 | 25 | −17 | 3.21 | 0.0437 | ||||||||
MNI: Montreal Neurological Institute; STG: superior temporal gyrus; SPL: superior parietal lobule; S1: primary somatosensory cortex; M1: primary motor cortex; V1: primary visual cortex; S2: secondary somatosensory cortex; ACC: anterior cingulate cortex; sgPFC: subgenual prefrontal cortex; vmPFC: ventromedial prefrontal cortex; MFG: middle frontal gyrus; PCC: posterior cingulate cortex; MTG: middle temporal gyrus; dlPFC: dorsolateral prefrontal cortex; OFC: orbiofrontal cortex; IPL: Inferior parietal lobule.
Altered NPS in ASD during sustained pain stimulation
With whole brain analysis during the intermediate phase of pain stimulation, the TC group showed significant increased BOLD signal relative to baseline throughout the NPS, while this pattern was absent in the ASD group. During the late phase of pain stimulation, the TC group showed a spatially reduced NPS with increases in premotor, SMG, insula, S2, and thalamus, while this pattern was absent in the ASD group (Figure 2(a)). Significant clusters with peak voxels and MNI coordinates are reported in Table 2.
Group comparisons of whole brain BOLD signal changes during the three phases of pain stimulation are shown in Figure 2(b), illustrating increased BOLD signal in the TC group compared to the ASD group (shown at Z > 2.3, p < 0.01, corrected). There were no significant differences in BOLD signal between the two groups during the early phase of pain stimulation. During intermediate and late phases, the TC group had an increased BOLD signal relative to the ASD group in the NPS. No regions showed increased signal in ASD relative to TC, in any phase.
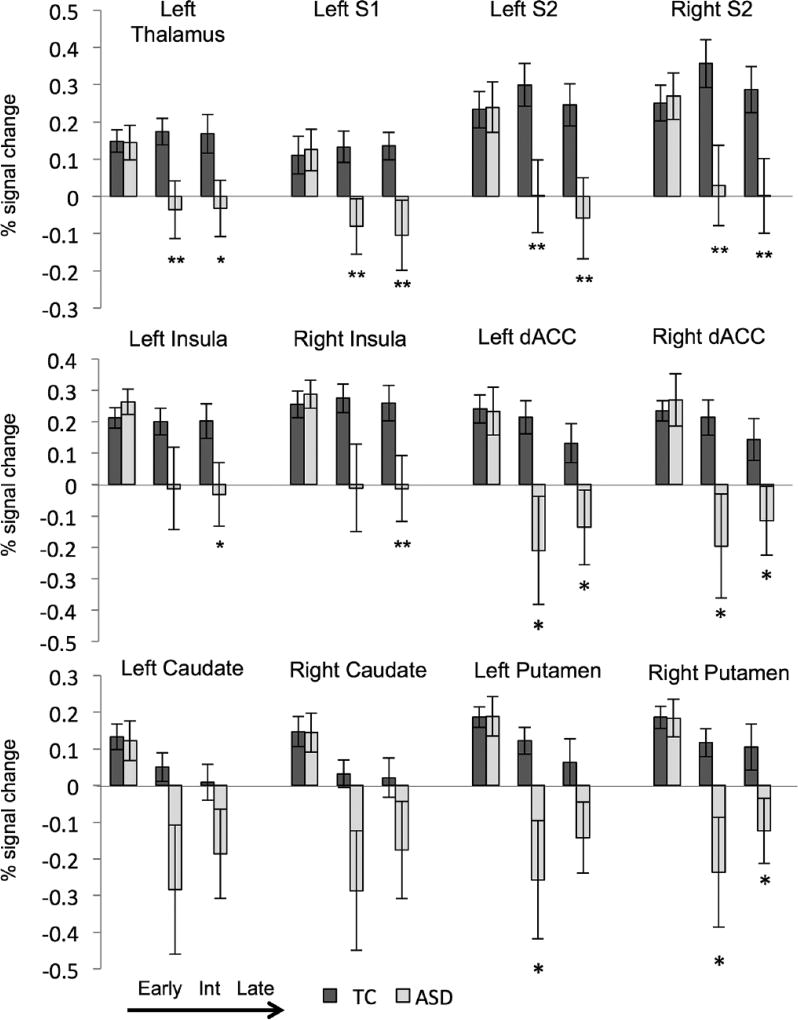
To explore group differences, mean percent signal change was calculated for NPS ROIs: contralateral thalamus and S1, bilateral S2, insula, dACC, caudate, and putamen (Figure 3). Mixed models were run for each ROI (Table 3): all regions had a significant interaction of ASD*phase (p < 0.05, uncorrected) except for the left and right caudate which had significant effects of ASD and phase, but no significant interaction (L: p = 0.127, R: p = 0.117). Interactions that survive FDR correction (p < 0.05) are denoted in Table 3. In all regions explored, there were no group differences in the early phase. In fact, early NPS signal change (compared to baseline) was robust in both groups, with effect sizes that range from Cohen’s d of 0.5–0.9. However, during intermediate and late phases, the ASD group had significantly less BOLD signal in left sensory thalamus, left S1, bilateral S2, and bilateral dACC (p < 0.05 for all comparisons, FDR correction survival is denoted in Table 3). The ASD group also had less signal change in the bilateral putamen during the intermediate phase and bilateral insula during the late phase (p < 0.05 for all comparisons, FDR correction survival is denoted in Table 3). For all significant group differences reported, Cohen’s d ranged from 0.5 to 0.7 for significant regions within the NPS.
Figure 3.
Region of interest analysis: percent signal change in typical (TC, dark gray) and individuals with autism spectrum disorder (ASD, light gray) within targeted regions of interest during early, intermediate, and late phases of painful stimulus presentation.
S1: primary somatosensory cortex; S2: secondary somatosensory cortex; dACC: dorsal anterior cingulate cortex; Int: intermediate.
ASD compared to typical: **p < 0.01, *p < 0.05, #p < 0.10.
Table 3.
Mixed models for each region of interest within the pain network.
| Variable | Fa | p-value | Post hoc | t | p-value | Cohen’s d | r | |
|---|---|---|---|---|---|---|---|---|
| Left thalamus | Group | 7.12 | 0.012 | Early | 0.07 | 0.944 | ||
| Phase | 1.93 | 0.154 | Intermediate | 2.51 | 0.015b | 0.66 | 0.31 | |
| Group*phase | 3.24 | 0.046 | Late | 2.34 | 0.023 | 0.61 | 0.29 | |
| Left S1 | Group | 5.48 | 0.026 | Early | −0.18 | 0.861 | ||
| Phase | 2.31 | 0.108 | Intermediate | 2.55 | 0.014b | 0.67 | 0.32 | |
| Group*phase | 3.56 | 0.035 | Late | 2.55 | 0.013b | 0.67 | 0.32 | |
| Left S2 | Group | 6.05 | 0.020 | Early | −0.07 | 0.944 | ||
| Phase | 2.61 | 0.082 | Intermediate | 2.67 | 0.010b | 0.70 | 0.33 | |
| Group*phase | 4.65 | 0.013b | Late | 2.72 | 0.009b | 0.71 | 0.34 | |
| Right S2 | Group | 5.46 | 0.027 | Early | −0.21 | 0.833 | ||
| Phase | 2.01 | 0.144 | Intermediate | 2.64 | 0.011b | 0.69 | 0.33 | |
| Group*phase | 5.80 | 0.005b | Late | 2.69 | 0.009b | 0.71 | 0.33 | |
| Left insula | Group | 3.08 | 0.090 | Early | −0.89 | 0.378 | ||
| Phase | 4.89 | 0.011 | Intermediate | 1.59 | 0.118 | |||
| Group*phase | 4.24 | 0.019b | Late | 2.28 | 0.026 | 0.60 | 0.29 | |
| Right insula | Group | 4.25 | 0.048 | Early | −0.46 | 0.647 | ||
| Phase | 4.33 | 0.018 | Intermediate | 1.99 | 0.052 | |||
| Group*phase | 4.96 | 0.010b | Late | 2.58 | 0.012b | 0.68 | 0.32 | |
| Left dACC | Group | 5.10 | 0.031 | Early | 0.07 | 0.943 | ||
| Phase | 7.67 | 0.001 | Intermediate | 2.42 | 0.019 | 0.64 | 0.30 | |
| Group*phase | 4.03 | 0.023b | Late | 2.20 | 0.032 | 0.58 | 0.28 | |
| Right dACC | Group | 4.58 | 0.041 | Early | −0.36 | 0.718 | ||
| Phase | 7.73 | 0.001 | Intermediate | 2.39 | 0.020 | 0.63 | 0.30 | |
| Group*phase | 4.64 | 0.014b | Late | 2.21 | 0.031 | 0.58 | 0.28 | |
| Left caudate | Group | 4.49 | 0.043 | Early | 0.16 | 0.876 | ||
| Phase | 7.12 | 0.002 | Intermediate | 1.91 | 0.062 | |||
| Group*phase | 2.14 | 0.127 | Late | 1.62 | 0.116 | |||
| Right caudate | Group | 4.21 | 0.049 | Early | 0.04 | 0.971 | ||
| Phase | 8.01 | 0.001 | Intermediate | 1.97 | 0.054 | |||
| Group*phase | 2.23 | 0.117 | Late | 1.5 | 0.140 | |||
| Left putamen | Group | 6.71 | 0.015 | Early | −0.04 | 0.964 | ||
| Phase | 9.3 | ≤0.001 | Intermediate | 2.37 | 0.021 | 0.62 | 0.30 | |
| Group*phase | 3.4 | 0.040 | Late | 1.86 | 0.068 | |||
| Right putamen | Group | 7.29 | 0.011 | Early | 0.04 | 0.970 | ||
| Phase | 8.38 | 0.001 | Intermediate | 2.35 | 0.022 | 0.62 | 0.29 | |
| Group*phase | 3.56 | 0.035 | Late | 2.21 | 0.031 | 0.58 | 0.28 |
S1: primary somatosensory; S2: secondary somatosensory; dACC: dorsal anterior cingulate cortex.
Degrees of freedom for each model, group (1, 29), phase (2, 58), and group*phase (2, 58).
p-values that survive false discovery rate correction across all regions investigated.
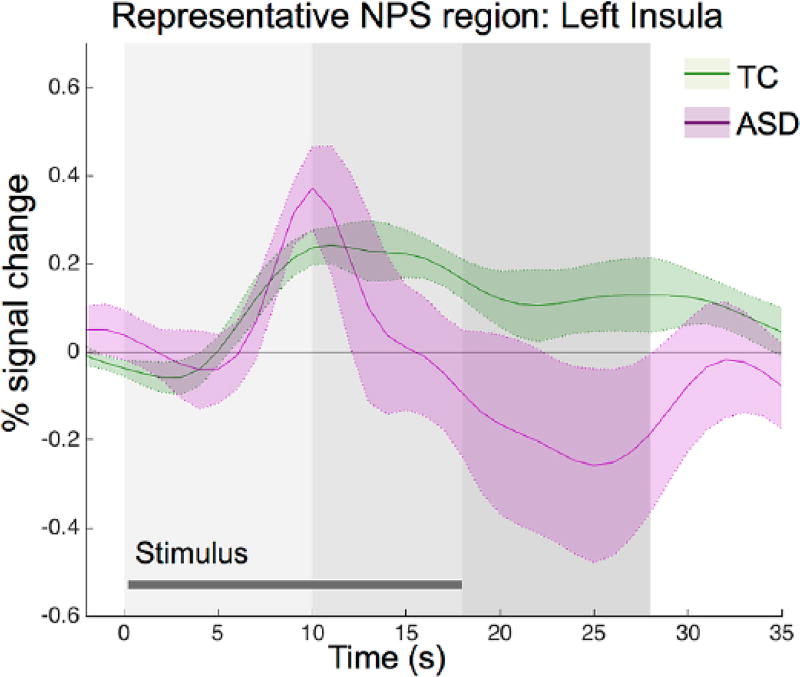
Time course of NPS response in ASD during pain stimulation
As the BOLD signal response was similar across the NPS, Figure 4 illustrates the pattern we observed across the NPS, using a representative NPS region (left insula) for illustration. In the NPS, the ASD group shows early phase increases and sustained intermediate/late phase decreases in BOLD signal compared to the TC group.
Figure 4.
Region of interest analysis: time course of the hemodynamic response within the typical comparison (TC) group and ASD group in a representative region from the neural pain signature (left insula). Similar response patterns exist across regions of the NPS. Gray shading indicates phase of pain stimulation (early, light to late, and dark).
To explore the range of response observed in the intermediate and late phases within the ASD group, we conducted a preliminary analysis of subgroup differences by the presence or absence of SIBs. Subgroup differences are especially important to consider given that 66% of individuals in the ASD group endorsed SIB. Importantly, there were no differences in IQ, pain rating, or heat pain thresholds between SIB subgroups. As seen in Supplementary Figure 1, individuals in the ASD + SIB group (n = 8) have diminished NPS response in the intermediate and late phases, while late phase decreases are absent in the ASD − SIB (n = 4) group. While these exploratory results hint that those individuals with ASD and SIB may drive the observed pattern seen in the ASD group, the small number of participants in the two subgroups precludes a definitive conclusion.
Putative cortical modulators of pain experience
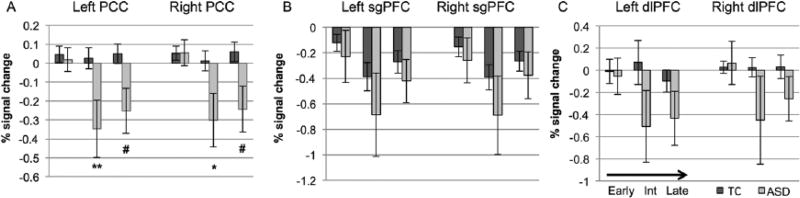
Figure 2 and Table 2 also illustrate differences in cortical regions outside the NPS, specifically in regions such as sgPFC, PCC, and dlPFC, all known to modulate the pain experience. During the early phase, the TC group shows decreased relative BOLD signal (compared to baseline) in the sgPFC, a cortical region that projects to the descending antinociceptive network (Cheng et al., 2015) and may be important for emotional strategies for coping with pain (Rainville, 2002). However, during the early phase, the ASD group showed decreased relative BOLD signal (compared to baseline) in the PCC, a hub of the DMN that may be engaged when mind wandering is used as a coping strategy (Kucyi et al., 2013). The TC group also showed increased signal in dlPFC, which may represent active cognitive coping strategies, while the ASD had decreased signal in the dlPFC. In the intermediate phase, the TC group had increased signal in dlPFC but decreased BOLD signal in the PCC and sgPFC. The ASD group had a more widespread pattern of deactivation that included the PCC. In the late phase, subgenual deactivation persists for both groups, while the TC group showed decreased BOLD signal in dlPFC, and the ASD had decreased signal in the PCC. Direct group contrasts revealed greater response in the TC than the ASD group in the PCC in the intermediate and late phases. These individual group maps and the group contrasts suggest different temporal responses in these putative modulators of the pain experience.
Figure 5 demonstrates mean percent signal change in the bilateral PCC, sgPFC, and dlPFC ROIs during each phase of pain stimulation. The ASD group showed significantly reduced percent signal change in the PCC during the intermediate phase (left, p = 0.002, uncorrected; right, p = 0.014, uncorrected). There were no significant group differences during any phase in sgPFC or dlPFC.
Figure 5.
Region of interest analysis: percent signal change in typical (TC, dark gray) and individuals with autism spectrum disorder (ASD, light gray) within regions that may modulate the pain experience during early, intermediate, and late phases of painful stimulus presentation.
PCC: posterior cingulate cortex; sgPFC: subgenual prefrontal cortex; dlPFC: dorsolateral prefrontal cortex: Int: intermediate.
ASD compared to typical: **p < 0.01, *p < 0.05, #p < 0.10.
Phase-specific associations with pain ratings
Previous studies demonstrate that NPS BOLD signal during pain stimulation reflects subjective pain intensity (Wager et al., 2013) and that this association is strongest in the intermediate and late phases of pain stimulation (Chen et al., 2002; Moulton et al., 2012; Wager et al., 2004). Nonparametric correlations between subjective pain rating and mean percent signal change in NPS and potential modulatory regions during intermediate and late phases revealed no significant associations with pain rating in the TC group. In the ASD group, both left sgPFC (intermediate phase, ρ = −0.714, p = 0.004; late phase, ρ = −0.792, p < 0.0001, uncorrected) and right sgPFC (intermediate phase, ρ = −0.892, p < 0.0001; late phase, ρ = −0.700, p = 0.005, uncorrected) were correlated with pain ratings (p < 0.05 following FDR correction).
Discussion
This study is the first to use fMRI to assess response to painful stimuli in adults with ASD. Contrary to reports that individuals with ASD may not register pain (APA, 2013), we noted intact early responses throughout the NPS in the early phase of sustained painful heat. However, following this early response, the time course of the ASD group differed radically from the TC group, with an exaggerated drop in signal during intermediate and late phases of the response. As this group difference was noted across all regions of the NPS included in the ROI analysis, it is difficult to attribute a particular aspect of pain processing (e.g. sensory vs cognitive) that distinguishes the ASD response, although it is reasonable to assume that differences in lower level ROIs such as thalamus, SI, and SII are propagating to the other NPS regions. Future studies with more nuanced temporal analyses and connectivity approaches may be able to better address this possibility. The widespread group difference in neural response was not reflected in pain ratings, which were indistinguishable between the two groups.
With the reliance on self-report for pain assessment, the need for more objective measures is imperative to understanding pain processing in ASD. While this study is limited by its sample size, it represents a unique and crucial dataset. This protocol can be challenging for participants to tolerate, with 21 s of sustained heat pain administered repeatedly in an MRI scanner, contributing to attrition or refusal to participate. The tradeoff is that pain stimuli are highly salient and produce a robust BOLD response. In fact, in typical populations, this robust response continues even after the noxious stimulus is removed (Buhle et al., 2012; Wager et al., 2004). This residual response is thought to reflect emotional evaluation or coping post-stimulus (Becerra et al., 2001; Wager et al., 2004). Thus, our data suggest individuals with ASD show reduced neural responses specifically during the evaluation or coping phase of processing painful stimuli. If this reduced neural response is reflective of diminished emotional valuation of pain, this could greatly impact empathic ability or processing of vicarious pain (Lamm et al., 2011), although this work suggests intact vicarious responses to physical pain in ASD (Krach et al., 2015).
In order to explore the reduction of the NPS response during sustained pain in ASD, we interrogated cortical hubs for three candidate networks associated with modulation of pain: (1) the sgPFC (descending antinociceptive network), (2) the dlPFC (cognitive control network), and (3) PCC (DMN). We found the same pattern of intermediate and late deactivation seen in the pain network in the PCC of the ASD group but no significant group differences in the other two regions. It is not immediately clear what this parallel reduction in the PCC (with possibly the rest of the DMN) and the NPS means with regard to coping strategies in ASD. Recent findings report that the DMN is engaged when individuals’ minds wander away from pain (Kucyi et al., 2013). However, the deactivation of the PCC in individuals with ASD suggests that they are likely not employing this kind of strategy during sustained painful stimulation and may be instead attending to the painful stimuli. Overall, our findings in modulatory regions suggest that the TC group may have a wider variety of strategies available to cope with pain. Our findings with cortical modulating regions are not conclusive; thus, it is possible that reduced response of the pain network in ASD is not the result of suppression from an external modulator region but an intrinsic inhibition within the NPS itself.
We noted a lack of correspondence between NPS response and subjective pain ratings in both groups. While some previous studies demonstrate within-subject correlations of NPS response to pain intensity ratings with parametrically increasing temperatures, our work is consistent with other reports of tenuous associations between cortical response to pain and subjective pain ratings (Ibinson and Vogt, 2013; Kramer et al., 2015). However, our pain stimulus did not change in intensity; thus, our paradigm may have been too coarse to detect these relationships. In future studies, it will be important to understand how both pain and heat intensity can modulate neural responses. Previous work suggests that verbal self-reports of pain are robustly associated with peak pain intensity and immediate recall (Kahneman et al., 1993; Redelmeier and Kahneman, 1996). Thus, in our work, these ratings likely reflect pain at the end of a run, leading to some variation due to possible habituation across the run (at the end of six trials). Given this limitation, we are likely at a disadvantage to identify correlations between pain ratings and BOLD response in the NPS. Collecting pain ratings across different phases of the stimulus will help parse out differences in acute versus sustained pain ratings in the future. As this is a sustained pain stimulus, future studies should also examine pain tolerance as a behavioral metric that may better reflect neural response over time. Similarly, we collected ordinal pain ratings; future studies using visual analog scales along with varying intensities of pain stimuli are needed to provide insight into NPS-intensity relationships in ASD. Yet, we observed robust associations between higher pain ratings and intermediate/late decreased sgPFC signal in ASD. As sgPFC feeds into the descending antinociceptive system (Cheng et al., 2015), this decreased sgPFC activity may indicate inhibition of descending antinociception, resulting in increased perceived pain.
This work will be highly important to consider in the context of several other features and comorbidities related to ASD. SIBs are important to consider especially given the lack of consensus on the role of pain in SIB associated with developmental disabilities. Because our sample was small and composed of high-functioning adults with a limited range of SIB assessed with a parent-report instrument adapted for self-report (Bodfish et al., 2000), we could not definitively quantify differences based on SIBs. However, our supplementary analysis does suggest variation by SIB in NPS response within the late phase of pain stimulation. Future studies using discrete or acute painful stimuli (as opposed to sustained pain stimuli) may be needed as these are likely a more ecologically valid representations of the repetitive bouts of painful self-stimulation characteristic of SIB in ASD. Additionally, sensory responsiveness will need to be considered given hyperresponsiveness to innocuous stimuli that resembles pain-like behaviors (Baranek and Berkson, 1994; Baranek et al., 2006; Liss et al., 2006) is likely to influence pain responsivity. Heightened anxiety is also very common in ASD (Gotham et al., 2015; Mayes et al., 2011) and may impact pain responsivity. It may also be informative to examine common comorbid pain conditions in ASD (e.g. gastrointestinal discomfort; Gorrindo et al., 2012) to fully understand heterogeneity in pain responsivity in ASD. Larger studies will be needed to specifically address these possible sources of heterogeneity within ASD. Even with this possible source of variation, our data show significant reductions in intermediate/late phase NPS response across the ASD group.
In summary, we report in this first fMRI study of pain processing in adults with ASD an intact early response to pain throughout the NPS, refuting some clinical reports that individuals with ASD do not register pain. Our work corroborates considerable behavioral evidence in this regard (Symons et al., 2009a). However, we also saw a striking reduction of response throughout the pain network and a hub of the DMN as the stimulation continued. These findings are consistent with altered coping strategies or top-down modulation of sustained painful experiences in individuals with ASD and could partially account for the ability to repeatedly self-injure in these individuals. However, it should be reiterated that our small sample consisted of high-functioning adults with ASD, due to the difficulty in tolerating pain protocols; thus, our results may not be generalizable to the entire autism spectrum.
Our findings of reduced NPS response along with high levels of reported pain in ASD may call into question the utility of the NPS as an objective measure of pain experience in individuals with communication challenges (Wager et al., 2013). Alternatively, it is possible that communication deficits in the ASD group, in addition to limitations of our study design, masked true differences in subjective pain experience that would be suggested by the dramatically altered neural response in ASD. Future studies will be necessary to distinguish between these alternative interpretations and should explore alternative pain rating scales and nonverbal measures of pain such as heart rate and facial expressions. More systematic investigation of the relationship of altered pain processing to SIBs in individuals with ASD, across a range of ages and functioning levels is needed. Our findings in adults may not account for developmental trajectories of sensory responsivity in ASD (Baranek et al., 2013; Freuler et al., 2012); thus, future work will be needed to examine pain responsivity across the lifespan. Evidence of consistent NPS response across the lifespan from infancy to adulthood in typical populations (Goksan et al., 2015) hints at the possibility of the NPS as a useful avenue for early biomarkers of ASD, adding clinical significance to the phenomenon of altered neural response to pain in ASD.
Supplementary Material
Acknowledgments
We thank Rachel Wachter, Abby Carroll, and Jenna Barnwell for assistance with data collection and Dr Jim Bodfish and Dr Todd Monroe for helpful comments on this article. Requests for research materials and data can be made to the corresponding author.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Autism Speaks 2082 and NIMH 1R01MH102272.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: APA; 2013. [Google Scholar]
- Andersson JL, Jenkinson M, Smith S. FMRIB technical report TR07JA1. FMRIB Centre, University of Oxford; Oxford: 2007a. [accessed 15 June 2016]. Non-linear optimisation. Available at: http://fsl.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf. [Google Scholar]
- Andersson JL, Jenkinson M, Smith S, et al. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; Oxford: 2007b. [accessed 15 June 2016]. Non-linear registration, aka spatial normalisation. Available at: https://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf. [Google Scholar]
- Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: responsiveness and habituation. Journal of Autism and Developmental Disorders. 1994;24(4):457–471. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, et al. Sensory experiences questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Boyd BA, et al. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology. 2013;25(2):307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, et al. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, et al. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133(Pt. 5):1515–1525. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, et al. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Kober H, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss038. Epub ahead of print 24 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Developmental Disorders. 2008;38:127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Gu C, Schauder KB, et al. Somatosensory event-related potentials and association with tactile behavioral responsiveness patterns in children with ASD. Brain Topography. 2015;28:895–903. doi: 10.1007/s10548-015-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-I, Ha B, Bushnell MC, et al. Differentiating noxious- and innocuous-related activation of human somatosensory cortices using temporal analysis of fMRI. Journal of Neurophysiology. 2002;88(1):464–474. doi: 10.1152/jn.2002.88.1.464. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Erpelding N, Kucyi A, et al. Individual differences in temporal summation of pain reflect pronociceptive and antinociceptive brain structure and function. Journal of Neuroscience. 2015;35(26):9689–9700. doi: 10.1523/JNEUROSCI.5039-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Wolz I, Leppanen J, et al. Facial expression to emotional stimuli in non-psychotic disorders: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2016;64:252–271. doi: 10.1016/j.neubiorev.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Card D, Roberts SW, et al. Self-injurious behaviours are associated with alterations in the somatosensory system in children with autism spectrum disorder. Brain Structure & Function. 2014;219(4):1251–1261. doi: 10.1007/s00429-013-0562-2. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Oatley HK, Mak-Fan KM, et al. Risk factors associated with self-injurious behaviors in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(11):2460–2470. doi: 10.1007/s10803-012-1497-9. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Taylor M, Lee M, et al. Decreased sensitivity to thermal stimuli in adolescents with autism spectrum disorder: relation to symptomatology and cognitive ability. Journal of Pain. 2015;16(5):463–471. doi: 10.1016/j.jpain.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, et al. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cerebral Cortex. 2006a;16(2):268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, et al. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cerebral Cortex. 2006b;16(2):254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Fan Y-T, Chen C, Chen S-C, et al. Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience. 2014;9(8):1203–1213. doi: 10.1093/scan/nst101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuler A, Baranek GT, Watson LR, et al. Precursors and trajectories of sensory features: qualitative analysis of infant home videos. American Journal of Occupational Therapy. 2012;66(5):e81–e84. doi: 10.5014/ajot.2012.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, et al. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. NeuroImage. 2000;11(6 Pt. 1):684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Goksan S, Hartley C, Emery F, et al. fMRI reveals neural activity overlap between adult and infant pain. eLife. 2015;4:e06356. doi: 10.7554/eLife.06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, et al. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Research. 2012;5(2):101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Brunwasser SM, Lord C. Depressive and anxiety symptom trajectories from school age through young adulthood in samples with autism spectrum disorder and developmental delay. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(5):369.e3–376.e3. doi: 10.1016/j.jaac.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, et al. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72:778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, et al. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibinson JW, Vogt KM. Pain does not follow the boxcar model: temporal dynamics of the BOLD fMRI signal during constant current painful electric nerve stimulation. Journal of Pain. 2013;14(12):1611–1619. doi: 10.1016/j.jpain.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Fredrickson BL, Schreiber CA, et al. When more pain is preferred to less: adding a better end. Psychological Science. 1993;4(6):401–405. [Google Scholar]
- Konstantareas MM, Homatidis S. Assessing child symptom severity and stress in parents of autistic children. Journal of Child Psychology and Psychiatry. 1989;30(3):459–470. doi: 10.1111/j.1469-7610.1989.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Krach S, Kamp-Becker I, Einhäuser W, et al. Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Human Brain Mapping. 2015;36:4730–4744. doi: 10.1002/hbm.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JLK, Jutzeler CR, Haefeli J, et al. Discrepancy between perceived pain and cortical processing: a voxel-based morphometry and contact heat evoked potential study. Clinical Neurophysiology. 2015;127:762–768. doi: 10.1016/j.clinph.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The dynamic pain connectome. Trends in Neurosciences. 2015;38(2):86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, et al. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, et al. The Autism Diagnostic Observation Schedule (ADOS) Torrance, CA: Western Psychological Services; 1999. [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, et al. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders. 2011;5(1):474–485. [Google Scholar]
- Moore DJ. Acute pain experience in individuals with autism spectrum disorders: a review. Autism. 2015;19(4):387–399. doi: 10.1177/1362361314527839. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, et al. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. Journal of Neurophysiology. 2005;93(4):2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Pendse G, Becerra LR, et al. BOLD responses in somatosensory cortices better reflect heat sensation than pain. Journal of Neuroscience. 2012;32(17):6024–6031. doi: 10.1523/JNEUROSCI.0006-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader R, Oberlander TF, Chambers CT, et al. Expression of pain in children with autism. The Clinical Journal of Pain. 2004;20(2):88–97. doi: 10.1097/00002508-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryweller JR, Schauder KB, Anderson AW, et al. White matter correlates of sensory processing in autism spectrum disorders. NeuroImage. 2014;6:379–387. doi: 10.1016/j.nicl.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology. 2002;12(2):195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rattaz C, Dubois A, Michelon C, et al. How do children with autism spectrum disorders express pain? A comparison with developmentally delayed and typically developing children. Pain. 2013;154(10):2007–2013. doi: 10.1016/j.pain.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66(1):3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- Riquelme I, Hatem SM, Montoya P. Abnormal pressure pain, touch sensitivity, proprioception, and manual dexterity in children with autism spectrum disorders. Neural Plasticity. 2016;2016:e1723401. doi: 10.1155/2016/1723401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA. Beta-endorphin dysregulation in autistic and self-injurious behavior: a neurodevelopmental hypothesis. Synapse. 1988;2(3):193–199. doi: 10.1002/syn.890020304. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain, and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Harper VN, McGrath PJ, et al. Evidence of increased non-verbal behavioral signs of pain in adults with neurodevelopmental disorders and chronic self-injury. Research in Developmental Disabilities. 2009a;30(3):521–528. doi: 10.1016/j.ridd.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Harper V, Shinde SK, et al. Evaluating a sham-controlled sensory-testing protocol for nonverbal adults with neurodevelopmental disorders: self-injury and gender effects. Journal of Pain. 2010;11(8):773–781. doi: 10.1016/j.jpain.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Wendelschafer-Crabb G, Kennedy W, et al. Evidence of altered epidermal nerve fiber morphology in adults with self-injurious behavior and neurodevelopmental disorders. Pain. 2008;134(1–2):232–237. doi: 10.1016/j.pain.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Wendelschafer-Crabb G, Kennedy W, et al. Degranulated mast cells in the skin of adults with self-injurious behavior and neurodevelopmental disorders. Brain Behavior and Immunity. 2009b;23(3):365–370. doi: 10.1016/j.bbi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, et al. Pain reactivity and plasma β-endorphin in children and adolescents with autistic disorder. PLoS ONE. 2009;4(8):e5289. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor ME, Walsh CE, Mulder EC, et al. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism. 2015;19(3):292–300. doi: 10.1177/1362361313518994. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, et al. An fMRI-based neurologic signature of physical pain. New England Journal of Medicine. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. Journal of Clinical Nursing. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, et al. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Chapter 14. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- Yasuda Y, Hashimoto R, Nakae A, et al. Sensory cognitive abnormalities of pain in autism spectrum disorder: a case–control study. Annals of General Psychiatry. 2016;15:8. doi: 10.1186/s12991-016-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.