Abstract
Although the precise drug mechanism of action of acamprosate remains unclear, its antidipsotropic effect is mediated in part through glutamatergic neurotransmission. We evaluated the effect of 4 weeks of acamprosate treatment in a cohort of 13 subjects with alcohol dependence (confirmed by structured interview DSM-IV-TR) on 1H-MRS glutamate levels in the midline anterior cingulate cortex (MACC). We compared levels of metabolites to a group of 16 healthy controls. The Pennsylvania Alcohol Craving Scale (PACS) was used to assess craving intensity. At baseline, before treatment, the mean cerebrospinal fluid (CSF)-corrected MACC glutamate [Glu] level was significantly elevated in subjects with alcohol dependence compared to controls (P = 0.004). Four weeks of acamprosate treatment reduced glutamate levels (P = 0.025), an effect that was not observed in subjects that did not take acamprosate. At baseline, there was a significant positive correlation between cravings, measured by PACS, and MACC [Glu] levels (P = 0.019). Overall, these data would suggest a normalizing effect of acamprosate on a hyper-glutamatergic state observed in recently withdrawn patients with alcohol dependence and a positive association between MACC glutamate levels and craving intensity in early abstinence. Further research is needed to evaluate utility of these findings for clinical practice, including monitoring of craving intensity and individualized selection of treatment with antidipsotropic medications in subjects with alcohol dependence.
Keywords: acamprosate, glutamate, MACC, MRS, alcohol dependence, craving
INTRODUCTION
Converging evidence indicates that an acute disruption in glutamatergic neurotransmission may be associated with the symptoms of alcohol intoxication and withdrawal (1). Increased and decreased glutamate levels have been reported in key brain regions during acute ethanol withdrawal (1–4) and in early abstinence, respectively (5). Intoxication and withdrawal present significant clinical challenges for acute stabilization of patients with alcohol dependence and generate considerable cost related to emergency room and hospitalization services (6, 7).
Acamprosate is FDA approved for the maintenance of abstinence from alcohol in patients with alcohol dependence. While meta-analyses clearly delineate higher abstinence rates for people treated with acamprosate in comparison to placebo (8–10), treatment response to acamprosate is variable, likely owing to the fact that alcohol dependence is a complex and heterogeneous disorder involving disruption of multiple physiological mechanisms (11). One way to enhance the efficacy of acamprosate treatment is to select the appropriate patients based on biomarkers associated with the drug mechanism of action and predictive of treatment response (12).
While the precise mechanism of action of acamprosate is not known, several lines of preclinical and clinical evidence indicate that it reduces glutamate levels in animal models of excessive ethanol exposure and in alcohol dependent patients (2, 13, 14). Utilization of non-invasive methodology to quantify excitatory/inhibitory tone may provide an opportunity to quantify biological dysregulation of alcohol withdrawal and monitor acamprosate-associated changes in these biological measures.
The anterior cingulate cortex (ACC) is a brain region that is highly activated in functional magnetic resonance spectroscopy studies in response to drug cues and drug-related stimuli in general, which may represent the experience of craving (15). More specifically, an increase in the blood oxygen level–dependent (BOLD) signal in the ACC is evident when patients with alcohol dependence are presented with alcohol-associated stimuli (16, 17). Importantly, BOLD signal activation in the MPFC in response to the taste of alcohol was positively correlated with craving for alcohol (18).
Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive brain imaging technique capable of in vivo monitoring of brain metabolites over time and, thus, allows monitoring of disease progression and effects of pharmacological treatment (3). Given the established work identifying glutamate dysregulation in alcohol intoxication and withdrawal, the theoretical framework for an association between glutamate and relief cravings (19), and the role glutamate may play in the pharmacological effect of acamprosate, the aim of this study was to assess the relationship between glutamate and craving as well as the effect of acamprosate treatment.
MATERIALS AND METHODS
Study Participants
This study was approved by the Institutional Review Board (IRB) of Mayo Clinic Rochester and was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants included in this study provided written informed consent approved by IRB and provided permission to use their information in other research projects.
Participants were recruited from a larger clinical trial investigating biomarkers associated with sobriety in alcoholics treated with acamprosate (20). Alcohol-dependent patients ages 18–80 (n = 13) were recruited from patients treated at the residential and outpatient treatment programs affiliated with Mayo Clinic Rochester and the Mayo Clinic Health System sites in Austin, Minnesota, Albert Lea, Minnesota and La Crosse, Wisconsin. In order to be eligible for the study, participants must have had a primary diagnosis of current alcohol dependence based on DSM-IV-TR criteria as determined by the Psychiatric Research Interview for Substance and Mental Disorders (PRISM) and have no active signs of severe alcohol withdrawal. Exclusion criteria included: inability to provide informed consent, any unstable active medical or additional psychiatric condition as determined by the investigator; diagnosis of active substance dependence other than alcohol or nicotine according to DSM-IV-TR criteria as determined by the PRISM, diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, any eating disorder, or obsessive compulsive disorder; active suicidal ideation as determined by responses provided during the PRISM or by the investigator, current treatment with antipsychotic or mood stabilizing medications, history of encephalopathy, hepatic failure, or HIV seropositivity, history of claustrophobia, history of major head trauma with loss of consciousness > 5 minutes or skull fracture, and history of previous neurological event (epilepsy, stroke, transient ischemic attack). Subjects currently taking acamprosate, naltrexone or disulfiram, or those with a history of hypersensitivity or allergic reaction to acamprosate, or women who were pregnant, lactating or planning to become pregnant during the next year were excluded. A healthy control group (n = 16) with no current or lifetime alcohol addiction or current Axis I disorder outside of nicotine dependence was recruited as a comparison group.
There was no random assignment to acamprosate; treatment was based on clinical evaluation and patient choice. All participants also completed other medical and psychiatric assessments to ensure their eligibility for participation in the study.
Clinical Assessments
The presence of alcohol dependence and comorbid psychiatric disorders in cases and controls was determined by Psychiatric Research Interview for Substance and Mental Disorders (PRISM) at the time of recruitment to parent study (20). The Clinical Institute Withdrawal Assessment (CIWA) (21), the Pennsylvania Alcohol Craving Scale (PACS) (22) and the Alcohol Urge Questionnaire (AUQ) (23) were used to measure withdrawal and alcohol craving, respectively. The Timeline Follow Back (TLFB) was used to obtain estimates of daily drinking to create a more complete picture of high and low drinking days over the past 7 (TLFB 7) and 30 days (TLFB 30) (24). The Patient Health Questionnaire (PHQ-9) is a scale used in diagnosing depression based on DSM criteria (25). A percent compliance score was determined by dividing the total number of acamprosate pills that a patient actually took during the course of the study by the number of pills that they should have taken and multiplying by 100.
Magnetic Resonance Imaging and 1H-MRS Techniques
MR imaging and spectroscopy were performed on a GE 3T HDx MRI scanner (GE Medical Systems, Milwaukee Wisconsin) running 16.0 software equipped with an 8-channel head coil. Scans were conducted once for healthy controls and twice for patients; shortly after admission to residential treatment, and upon completion of residential or 4 weeks of acamprosate treatment (mean 31.08 ± 5.71 days).
The axial plane was landmarked in all subjects at the center of the forehead, 1 cm above the eyebrows to standardize head positioning. The forehead was affixed with adhesive tape to the MR bed, and neck support was provided as needed. A neuroradiologist reviewed all structural MRI data for potential exclusionary head and brain pathology.
An MP-RAGE imaging sequence was used to acquire volumetric data for cerebrospinal fluid (CSF) correction (sagittal acquisition; repetition time [TR] = 2300 ms, echo time [TE] = minimum, flip angle = 8°, voxel dimensions = 1.0 × 1.0 × 1.2 mm). A systematic approach to spectroscopy voxel positioning was used in all subjects. Specifically, an axial oblique localizer slice was acquired, positioned on the MP-RAGE images at the level of the genu of the corpus callosum parallel to the average plane of the corpus callosum. On this reference image, a single 8 cm3 1H-MRS voxel (2 × 2 × 2 cm) encompassing the midline anterior cingulate cortex was placed such that: 1) it was centered on the frontal interhemispheric fissure, and 2) the posterior margin of the voxel was placed immediately anterior to the genu of the corpus callosum. This positioning corresponds to the pregenual anterior cingulate cortex (Fig. 1; Brodmann areas 24a, 24b and 32) as described by Vogt (Vogt et al 2003).
FIGURE 1:
Flow of magnetic resonance spectroscopy data acquisition and quantification. Representative axial brain slice with the midline anterior cingulate cortex (MACC) in plain. Voxel size is 2 × 2 cm in plain and 2 cm thick for a total volume of 8 ml. A representative spectra acquired with the TE80 pulse sequence is present with the LCModel fit of the whole spectra. LCModel fit of glutamate. The residual of the spectra is also presented following LCModel fit.
There are several different spectroscopic sequences that yield reasonably accurate measurements of glutamate (Hancu 2009). As the optimal MRS method for accurately measuring glutamate has yet to be determined, we chose to use a TE-optimized PRESS sequence (PROBE-P PRESS, TR = 2000 ms, TE = 80 ms, 128 water-suppressed and 8 unsuppressed samples; Fig. 1) (26). This sequence provides a reasonable measurement of glutamate while simultaneously preserving the signals from other metabolites such as choline, creatine and N-acetyl-aspartate.
Metabolite Quantification
Spectroscopic imaging data were transferred to a Sun workstation running SAGE-IDL (GE Medical Systems). Data integrity was verified by visual inspection by a trained spectroscopist and subjects whose data was determined to be contaminated by artifact based on the visual inspection were excluded from the study. A quantitative analysis of brain metabolites was performed using the 6.2–1A version of LCModel software (27, 28) using basis sets provided by the scanner vendor. All spectra were water scaled to account for individual coil loading. As a second quality control measure, we discarded glutamate data with a Cramer Rao lower bound (CRLB) estimate > 20%.
The MP-RAGE anatomical data were segmented into gray matter, white matter, and CSF using a technique modified from a previous study (29). Specifically, the FSL package from FMRIB Oxford (30) was used to perform brain segmentation. Briefly, MP-RAGE data were converted into NIFTI format using mri_convert. The T1 volume was skull-stripped using a brain extraction tool with a manually chosen, visually optimal f parameter, then segmented into gray matter, white matter, and CSF using FAST with default parameters. The segmented data were then overlaid with the 1H-MRS voxel location, and the probabilities of each tissue type for each imaging pixel within the spectroscopy voxel were summed. The sums were then divided by the total number of pixels within the voxel to arrive at the fraction of each tissue within the 1H-MRS voxel. CSF–corrected metabolite concentrations, [M]TVC, were then calculated by taking the measured metabolite concentration, [M]M, and applying a correction factor as follows:
where FCSF is fraction of CSF in the spectroscopy voxel. This generated “absolute” (vs relative to creatine) metabolite concentrations in “institutional units” specific to our scanner and technique. These CSF-corrected metabolite concentrations (e.g., [Glu]) were used for all statistical analyses.
Statistical Analysis
Data are presented as mean ± SD (standard deviation). Statistical analyses were performed using unpaired or paired two-tailed Student’s t test paired or unpaired, Wilcoxon signed rank test, Fisher’s exact test or Pearson correlation (Prism v 5.04, GraphPad Software, La Jolla, CA). The primary analysis focused on a glutamate levels between patients with alcohol dependence and controls as well as a correlation between glutamate levels and PACS scores. Because we had glutamate levels and PACS scores in both healthy controls and patients with alcohol dependence, we made a single correlation including all subjects. We adjusted both the difference in glutamate levels between patients with alcohol dependence and controls and the correlation between glutamate levels and PACS scores for potential confounding variables including age, gender, medication (benzodiazepine and/or antidepressant), smoking status, AUQ scores, days since last drink and PHQ-9 scores using JMP 10.0.0 (SAS Institute, Inc., Minneapolis, MN). Results were considered significantly different when P < 0.05.
RESULTS
Demographic and Clinical Assessment
Demographic and clinical information of the study participants is presented in Table 1. When comparing the group of patients with alcohol dependence to healthy controls, the groups did not differ by gender, but the alcohol dependence group was significantly older and reported higher rates of depressive symptoms (PHQ-9), antidepressant use, smoking, alcohol craving (PACS and AUQ) and alcohol use (TLFB; all P < 0.05 by Student’s two-tailed t test). The mean number of days since the last drink of patients with alcohol dependence was 6.00 ± 4.29. The mean number of days since last drink and TLFB7 drinking days, prior to study enrollment and scan, represents entering addiction programming with recent (within last week) cessation of alcohol ingestion. Patients were separated into a group of those that chose to go on acamprosate and those that did not. Patients that decided to go on acamprosate were on average 88% compliant during the 4 weeks of treatment based on pill counting. On average, patients were on acamprosate for mean 31.08 ± 5.71 days. As shown in Table 1, there were no group differences in clinical demographics between patients that chose to go on acamprosate and those that did not.
Table 1.
Demographical information in patients treated with acamprosate
| Healthy Control | Alcohol Dependence | P value | No Acamprosate | Acamprosate | P value | |
|---|---|---|---|---|---|---|
| Total N | 16 | 13 | 4 | 9 | ||
| Gender male, N (%) | 4 (25.00) | 6 (41.18) | 0.27 | 2 (50.00) | 4 (44.44) | 1.00 |
| Age, mean (SD) | 26.56 (5.90) | 43.62 (13.36) | < 0.01 | 37.50 (17.46) | 46.33 (11.25) | 0.29 |
| Benzodiazepine (Lorazepam eq. mg), mean (SD) | 0.00 (0.00) | 0.63 (0.58) | n.d. | 0.76 (0.71) | 0.50 (0.00) | 0.50 |
| On benzodiazepine, N (%) | 0 (0.00) | 3 (23.08) | 0.10 | 2 (50.00) | 1 (11.11) | 0.20 |
| With depression, N (%) | 0 (0.00) | 10 (62.50) | 0.08 | 1 (25.00) | 9 (75.00) | 0.18 |
| On antidepressent, N (%) | 0 (0.00) | 6 (46.15) | < 0.01 | 0 (0.00) | 6 (66.67) | 0.07 |
| Smoke. N (%) | 1 (8.33) | 6 (46.15) | 0.03 | 3 (75.00) | 3 (33.33) | 0.27 |
| CIWA, mean (SD) | n.d. | 1.46 (3.83) | n.d. | 0.00 (0.00) | 2.11 (3.95) | 0.25 |
| AUQ, mean (SD) | 9.13 (2.03) | 21.46 (8.68) | < 0.01 | 21.50 (9.15) | 21.44 (9.03) | 0.99 |
| PACS, mean (SD) | 0.50 (1.10) | 15.15 (7.90) | < 0.01 | 14.00 (5.71) | 15.67 (8.97) | 0.74 |
| Days since last drink, mean (SD) | 31.75 (36.32) | 6.00 (4.29) | 0.02 | 6.00 (1.83) | 6.00 (5.24) | 1.00 |
| PHQ-9, mean (SD) | 0.75 (0.93) | 10.31 (4.15) | < 0.01 | 8.00 (3.37) | 11.33 (4.21) | 0.19 |
| TLFB 7 | ||||||
| Drinking days, mean (SD) | 0.50 (0.97) | 2.39 (1.98) | < 0.01 | 1.75 (1.71) | 2.67 (2.12) | 0.47 |
| Drinks/drinking day, mean (SD) | 0.34 (0.65) | 7.77 (5.89) | < 0.01 | 6.63 (4.72) | 8.28 (6.53) | 0.66 |
| Total drinks, mean (SD) | 0.63 (1.15) | 18.46 (15.57) | < 0.01 | 14.00 (11.43) | 20.44 (17.32) | 0.51 |
| TLFB 30 | ||||||
| Drinking days, mean (SD) | 1.75 (1.57) | 18.92 (7.23) | < 0.01 | 21.25 (3.50) | 17.89 (8.34) | 0.46 |
| Drinks/drinking day, mean (SD) | 0.90 (0.70) | 9.45 (6.54) | < 0.01 | 8.18 (0.78) | 10.01 (7.93) | 0.66 |
| Total drinks, mean (SD) | 2.38 (2.42) | 173.20 (124.80) | < 0.01 | 156.00 (41.32) | 180.80 (150.00) | 0.76 |
SD: standard deviation, n.d.: not determined, CIWA: clinical institute withdrawal assessment, AUQ: alcohol urge questionnaire, PACS: Pennsylvania alcohol craving scale, PHQ-9: 9 item patient health questionnaire, TLFB 7: 7 day time line follow back, TLFB 30: 30 day time line follow back. Statistics by two-tailed unpaired Student’s t test for continuous variables or Fisher’s exact test for categorical variables. The Wilcoxon signed rank test was used when standard deviations were zero for continuous variables.
Baseline MACC CSF-Corrected Glutamate ([Glu]) Levels are Elevated in Patients with alcohol dependence
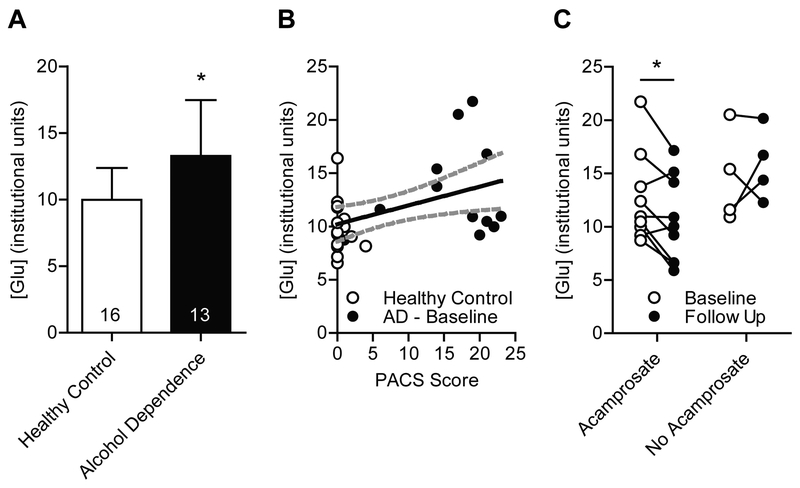
The mean MACC [Glu] level was significantly higher in patients with alcohol dependence (n = 13) compared to healthy controls (n = 16; t(27) = 2.65; P = 0.013; Fig. 2A). Importantly, when the difference in glutamate levels between patients with alcohol dependence and controls was adjusted for potential confounding variables including age, gender, medication (benzodiazepine and/or antidepressant), smoking status, AUQ scores, days since last drink and PHQ-9 scores, the effect was strengthened (P = 0.004).
FIGURE 2:
Acamprosate reduces CSF-corrected glutmate levels in the MACC. (A) Baseline glutamate levels are significantly higher in patients with alcohol dependence (n = 13) compared to healthy control subjects (n = 16; t(27) = 2.65; P = 0.013). Data are presented as mean ± SD. (B) Baseline CSF-corrected glutamate levels in the MACC are positively correlated with alcohol craving assessed using the Pennsylvania Alcohol Craving Scale (PACS; grey dotted line indicates the 95% confidence interval; n = 29 (16 control and 13 alcohol dependence (AD)); Pearson r = 0.44, P = 0.018). (C) Acamprosate treatment (n = 9) reduces this baseline (n = 9) hyperglutamatergic state (paired t test; t(8) = 2.75; P = 0.025). This effect was not observed in subjects that did not take acamprosate. Follow up levels of glutamate (n = 4) were similar to levels at baseline (n = 4) in subjects that did not take acamprosate (paired t test; t(3) = 0.66; P = 0.56).
Baseline MACC CSF-Corrected Glutamate ([Glu]) Levels are Correlated with Alcohol Craving
There was a significant positive correlation between baseline [Glu] levels and alcohol craving measured by PACS [n = 28 (16 control and 12 alcohol dependence); Pearson r = 0.44, P = 0.018; Fig. 2B]. Because healthy controls and patients with alcohol dependence differed on several clinical and demographical variables, we included glutamate and PACS in a model with age, gender, medication (benzodiazepine and/or antidepressant), smoking status, AUQ scores, days since last drink and PHQ-9 scores to adjust for these potential confounding variables. After adjustment for these variables, glutamate levels remained significantly correlated with PACS scores (P = 0.019). No correlation was found between glutamate levels and AUQ scores [n = 29 (16 control and 12 alcohol dependence); Pearson r = 0.27, P = 0.16].
Acamprosate Reduces CSF-Corrected Glutamate Levels in the MACC
Nine out of the 13 subjects enrolled into the study went on to take acamprosate for 4 weeks. Acamprosate treatment (n = 9) significantly reduced glutamate levels in the MACC compared to baseline levels (n = 9; paired t test; t(8) = 2.75; P = 0.025; Fig. 2C). Glutamate levels in subjects that did not go on to take acamprosate (n = 4) remained similar to levels at baseline (n = 4; paired t test; t(3) = 0.66; P = 0.56; Fig. 2C). Next, because the length of abstinence can affect glutamate levels, we adjusted these baseline to endpoint changes for days since last drink. Following adjustment for days since last drink, glutamate levels remained significantly decreased following acamprosate treatment compared to baseline (P = 0.013), while there was no change in glutamate levels in the group not treated with acamprosate (P = 0.687).
DISCUSSION
Our findings indicate that glutamate levels in the midline anterior cingulate cortex (MACC) of alcohol dependent subjects in early abstinence are higher compared to healthy controls. These data are consistent with increased glutamate levels in striatum and medial prefrontal cortex of rodents and the anterior cingulate cortex of humans during acute ethanol withdrawal (1–4). However, lower glutamate levels in the ACC have been found during early abstinence (~7 days since last drink) with normalization by 5 weeks, while no differences were found in the dorsolateral prefrontal cortex (5). While the overall impression of these studies in total is limited by preclinical vs clinical comparison, variable design of first scan, timing of alcohol use vs withdrawal, and glutamate spectroscopic quantification methodology, it is possible that glutamate levels are dynamic during the course of early withdrawal (increased) and early abstinence (decreased).
We further demonstrated that acute treatment with acamprosate reduced glutamate levels in patients with alcohol dependence. However, our data are consistent with a previously published study that found that acamprosate treatment for ~21 days significantly reduced Glx/Cr levels in the ACC of patients with alcohol dependence compared to placebo control (13). Importantly, our study found that acamprosate not only reduced CSF-corrected glutamate levels but also normalized them to a level similar to healthy control levels. It has been shown that acamprosate treatment prior to ethanol withdrawal prevents withdrawal elevation of glutamate levels (13, 31) while treatment of acamprosate during ethanol withdrawal reduces withdrawal elevated glutamate levels in the nucleus accumbens (2). It has been speculated that acamprosate may act as a glutamate stabilizer, which is capable of preventing acute withdrawal-elevated glutamate levels and restoring withdrawal-reduced glutamate levels (32). It is interesting that levels of glutamate remained elevated in subjects that did not take acamprosate treatment; it may reflect an ongoing protracted withdrawal-associated biological dysregulation that may take a longer time to normalize without glutamate stabilizing treatment. However, since our study is limited by non-random assignment and small sample size, we were unable to evaluate relationships between early MRS changes and longer-term abstinence rates.
The search for clinical correlates that are associated with acamprosate response have been inconclusive. Although initial European publications underscored importance of physiological dependence in responders to acamprosate (33), it was not supported by more resent pooled analysis of the European studies (34). However, as discussed by other investigators, a small number of patients in the subgroups with the highest severity of physiological dependence made interpretation difficult (34). The presence or absence of the psychiatric comorbidity has been considered an important predictor of the response to acamprosate (35, 36), suggesting that a more classical, primary type of patient with alcohol dependence, rather than the alcohol dependent patient with other psychiatric or organic disorder or many social problems, is more likely to benefit from acamprosate (37). Pooled analysis of the European studies (34) supported importance of anxiety in treatment but found no association with response to acamprosate. This may be associated with a lack of standardized methodology in the assessment of study participants as an important problem (34).
We found that baseline CSF-corrected glutamate levels in the MACC were reduced by acamprosate treatment and that baseline CSF-corrected glutamate levels in the MACC were positively correlated with baseline alcohol craving scores. Our data are consistent with the observation that acamprosate may be most effective for maintenance of abstinence rather than for reducing alcohol consumption. The positive correlation between baseline CSF-corrected glutamate levels in the MACC is consistent with a previous report that found that Glx levels in the anterior cingulate are positively correlated with the obsessive compulsive drinking scale (OCDS), which is a measure of alcohol craving (38). Taken together, these data support the concept that acamprosate may help to alleviate craving for alcohol by reducing glutamate levels.
It is important to note that we did not find a significant correlation between CSF-corrected glutamate levels measured using MRS and AUQ scores. PACS and AUQ are both questionnaires used to evaluate craving for alcohol, AUQ evaluates immediate desire to drink (39) while PACS examines overall craving during last week (22). Thus, the AUQ may be more suitable for techniques such as fMRI that present images or tastes while a subject is in the scanner to induce cravings. Indeed, BOLD signal activation in the MPFC in response to the taste of alcohol was positively correlated with craving for alcohol (18). On the other hand, PACS assesses craving over the past week, which may be better representative of the MRS signal that was measured in the present study as MRS measures less transient changes in metabolite levels. Glutamate levels in the ACC have been found to be elevated 1 day after ceasing alcohol consumption (3). In the present study, we found that glutamate levels were increased in patients with alcohol dependence 6 days after last drink. Hermann et al. (2012) showed that glutamate levels normalized within 14 days from last drink but we found that glutamate levels are still increased compared to healthy control levels 6 days after last drink. Furthermore, we found that glutamate levels in subjects that did not take acamprosate but remained abstinent for the next 4 weeks after scan remained elevated compared to healthy controls while acamprosate reduced this hyperglutamatergic state.
Our findings should be considered in the context of the following limitations, most notably a non-randomized small sample size. The number of subjects per group is less than 20 and in the no acamprosate group we only had 4 subjects. Thus, these data should be considered preliminary in nature. As acamprosate was prescribed by non-random assignment and there was no placebo control group, it is possible that our treatment group is biased towards people more motivated to achieve sobriety. However, while non-random, the acamprosate vs no acamprosate groups did not differ in any demographic variable including alcohol use, craving scores, antidepressant use, or depressive symptoms. Furthermore, although we targeted 4 weeks of treatment with acamprosate, based on scanner or patient scheduling, the length of treatment at time of 2nd scan varied.
In conclusion, our findings indicate that the use of MRS-based methodology allowed detection of elevated glutamate levels, reported as CSF corrected glutamate, in the MACC of alcoholics during early recovery and their normalization following acute acamprosate treatment to levels observed in non-alcoholic control subjects. These findings support theoretical considerations and experimental findings related to the effect of acamprosate on alcohol-related brain metabolite changes (13, 31). These data underscore that spectroscopy has the potential to provide in vivo quantification of alcohol and acamprosate-related brain metabolite changes in humans but larger future studies will need to standardize timing of scans in the context of withdrawal commencement and initiation of acamprosate and have longer term follow up to assess conventional alcohol related outcome measures. The goal of future studies should be to not only confirm these early withdrawal and acamprosate associated changes in glutamate, but to further investigate if these brain changes are associated with longer term clinical outcomes of craving reduction and maintenance of sobriety.
ACKNOWLEDGEMENTS
We thank Mandie Maroney-Smith for her expertise performing the MR scans. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic (V.M.K, D.S.C.) and by grants from the National Institutes of Health (NIH) to M.A.F, V.M.K, D.A.M, D.S.C (P20 AA017830).
Acknowledgements and Funding Disclosure: We thank Mandie Maroney-Smith for her expertise performing the MR scans and Lori Solmonson for manuscript preparation. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic (V.M.K, D.S.C.) and by grants from the National Institutes of Health (NIH) to M.A.F, V.M.K, D.A.M, D.S.C (P20 AA017830).
Footnotes
Declaration of Interests: M.A.F. has had grant support from Assurex, Myriad and Pfizer, and has served as an unpaid consultant for Janssen Global Services, LLC, Mitsubishi Tanabe Pharma Corporation, Myriad, Sunovion, Supernus Pharmaceuticals, Teva Pharmaceuticals. D.J.H, V.M.K., J.M.B, L.J.G., S.E.F., D.S.C., J.D.P. report no disclosures.
REFERENCES
- 1.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med 1998;49:173–184. [DOI] [PubMed] [Google Scholar]
- 2.Hinton DJ, Lee MR, Jacobson TL, et al. Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology 2012;62(8):2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermann D, Weber-Fahr W, Sartorius A, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 2012;71(11):1015–1021. [DOI] [PubMed] [Google Scholar]
- 4.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 1995;283(1–3):177–183. [DOI] [PubMed] [Google Scholar]
- 5.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 2012;125(1–2):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathlev NK, Ulrich A, Shieh TC, Callum MG, Bernstein E, D’Onofrio G. Etiology and weekly occurrence of alcohol-related seizures. Acad Emerg Med 2002;9(8):824–828. [DOI] [PubMed] [Google Scholar]
- 7.McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry 2008;79:854–862. [DOI] [PubMed] [Google Scholar]
- 8.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res 2004;28(1):51–63. [DOI] [PubMed] [Google Scholar]
- 9.Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311(18):1889–1900. [DOI] [PubMed] [Google Scholar]
- 10.Rosner S, Leucht S, Lehert P, Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol 2008;22(1):11–23. [DOI] [PubMed] [Google Scholar]
- 11.Mann K, Lemenager T, Hoffmann S, et al. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol 2013;18(6):937–946. [DOI] [PubMed] [Google Scholar]
- 12.Nam HW, Karpyak VM, Hinton DJ, et al. Elevated baseline serum glutamate as a pharmacometabolomic biomarker for acamprosate treatment outcome in alcohol-dependent subjects. Transl Psychiatry 2015;5:e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umhau JC, Momenan R, Schwandt ML, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 2010;67(10):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Witte P Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addictive behaviors 2004;29(7):1325–1339. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002;159(10):1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grusser SM, Wrase J, Klein S, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. [DOI] [PubMed] [Google Scholar]
- 17.Heinz A, Wrase J, Kahnt T, et al. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res 2007;31(7):1138–1147. [DOI] [PubMed] [Google Scholar]
- 18.Filbey FM, Claus E, Audette AR, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 2008;33(6):1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol 1999;34(2):197–222. [DOI] [PubMed] [Google Scholar]
- 20.Karpyak VM, Biernacka JM, Geske JR, et al. Genetic markers associated with abstinence length in alcohol-dependent subjects treated with acamprosate. Transl Psychiatry 2014;4:e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84(11):1353–1357. [DOI] [PubMed] [Google Scholar]
- 22.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- 23.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 1995;19(3):600–606. [DOI] [PubMed] [Google Scholar]
- 24.Sobell LC, Sobell MB. Timeline Follow-back: A Technique for Assessing Self-report Alcohol Consumption In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption: Psychosocial and Biomechanical Methods. Totowa: Humana Press; 1992: 41–72. [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 1987;508:333–348. [DOI] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 28.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14(4):260–264. [DOI] [PubMed] [Google Scholar]
- 29.Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Research 2008;162(2):113–121. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 31.Dahchour A, De Witte P. Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res 2003;27(3):465–470. [DOI] [PubMed] [Google Scholar]
- 32.Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci 2008;29(3):109–115. [DOI] [PubMed] [Google Scholar]
- 33.Lesch KP. Variation of serotonergic gene expression: neurodevelopment and the complexity of response to psychopharmacologic drugs. Eur Neuropsychopharmacology 2001;11(6):457–474. [DOI] [PubMed] [Google Scholar]
- 34.Verheul R, Lehert P, Geerlings PJ, Koeter MWJ, van den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology (Berl) 2005;178(2–3):167–173. [DOI] [PubMed] [Google Scholar]
- 35.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274(5292):1527–1531. [DOI] [PubMed] [Google Scholar]
- 36.Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry 1997;171:73–77. [DOI] [PubMed] [Google Scholar]
- 37.Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol 2000;35(2):176–187. [DOI] [PubMed] [Google Scholar]
- 38.Bauer J, Pedersen A, Scherbaum N, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 2013;38(8):1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health 1999;23(3):179–186. [PMC free article] [PubMed] [Google Scholar]