Abstract
Pancreatic cancer arises from non-invasive precursor lesions, including pan creatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN), which are curable if detected early enough. Recently, these types of precursor lesions have been extensively characterized at the molecular level, defining the timing of critical genetic alterations in tumorigenesis pathways. The results of these studies deepen our understanding of tumorigenesis in the pancreas, providing novel insights into tumor initiation and progression. Perhaps more importantly, they also provide a rational foundation for early detection approaches that could allow clinical intervention prior to malignant transformation. In this review, we summarize the results of comprehensive molecular characterization of PanINs, IPMNs, and MCNs, and discuss the implications for cancer biology as well as early detection.
Keywords: pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, mucinous cystic neoplasm, pancreatic cancer precursor lesion, somatic mutation, cancer genomics, driver gene
Introduction
Pancreatic cancer is expected to be the second leading cause of cancer-related deaths in the U.S. by 2030, with a current 5-year survival rate of only 8% [1,2]. This dismal prognosis is largely due to a lack of early clinical symptoms, with many patients already presenting with advanced disease at initial diagnosis. Therefore, early detection approaches will be critical to improve outcomes in this disease. A great opportunity for early detection is the treatment of premalignant pancreatic lesions, including pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN). However, prevention of pancreatic cancer must be balanced with potential overtreatment of low-risk lesions. Recent advances in sequencing technologies have deepened our understanding of the genetic changes that characterize these lesions, which offer new opportunities for screening and early detection. In this review, we summarize the current research findings on the molecular genetics of PanIN, IPMN, and MCN, and how these discoveries are being incorporated into cutting-edge early detection approaches.
Pancreatic Intraepithelial Neoplasia (PanIN)
PanINs are the most common precursor to invasive PDAC. Approximately 16% of normal pancreata harbor a PanIN lesion [3]. This prevalence increases in older patients and in the setting of invasive PDAC [3]. PanINs are microscopic (<5 mm in diameter by definition) and arise in small pancreatic ducts [4]. Traditionally, PanIN lesions were grouped by a three-tier classification scheme: PanIN-1A/B, PanIN-2, or PanIN-3, depending on the degree of architectural and nuclear atypia [5,6]. To improve reproducibility and clinical relevance, this classification system has recently been revised and now categorizes these lesions into two groups: low-grade or high-grade PanIN [4] (Figure 1). The cause of these lesions remains unclear, but risk factors such as smoking and excessive alcohol consumption may contribute to their pathogenesis [7]. Due to their microscopic size, PanINs are rarely detected using current imaging modalities [8] and thus require alternative approaches for their identification and management. The molecular features that characterize these lesions are key to understanding PanIN tumorigenesis and early events of pancreatic cancer.
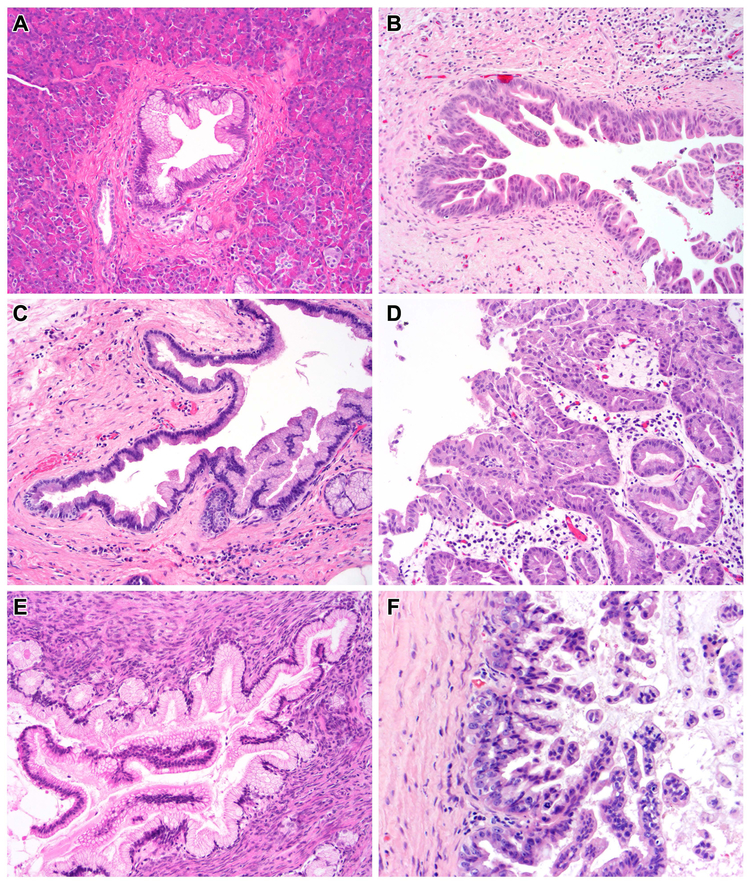
Figure 1. Histologic features of PanIN and IPMN.
(A) This low-grade PanIN has uniform basally-located nuclei and apical mucin. There is minimal cytologic atypia. (B) The cytologic atypia is much more pronounced in this high-grade PanIN, which shows nuclear pleomorphism and marked loss of nuclear polarity. (C) This low-grade IPMN shows gastric-type differentiation, with apical mucin and basal nuclei. Like the low-grade PanIN, there is minimal cytologic atypia. (D) This high-grade IPMN shows pancreatobiliary-type differentiation, with architectural complexity, nuclear pleomorphism and loss of nuclear polarity. (E) The epithelium of this low-grade MCN shows similar cytologic features to low-grade IPMN and PanIN, but the lesion is characterized by its unique ovarian-type stroma. (F) This high-grade MCN shows typical high-grade cytology, including nuclear pleomorphism and loss of nuclear polarity – ovarian-type stroma underlies the neoplastic epithelium.
Molecular Features of PanIN
Numerous studies have identified somatic mutations in KRAS to be one of the earliest drivers of PanIN progression [9–11] (Table 1). KRAS encodes a small GTPase that regulates signal transduction for many cellular activities such as growth, survival and proliferation [12]. Upon activation, this GTPase stimulates multiple cellular pathways including mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and Ras-like (Ral). Recently, it has been shown that over 90% of all PanINs, regardless of histological grade, contain at least one KRAS alteration [9]. Additionally, several studies have utilized mouse models to demonstrate that endogenous expression of mutant KRAS in murine pancreata is essential for initiating the development of PanIN [13–15]. Altogether, these data suggest that KRAS alterations play an important role early in PanIN development.
Table 1:
Driver gene mutation prevalence in pancreatic precursors
| Lesion | Driver Gene | Mutation Prevalence |
Common alterations | Reference |
|---|---|---|---|---|
| Low-grade PanIN | KRAS | >90% | Missense mutation (codons 12, 13, 61) | [9–11] |
| p16/CDKN2A | 0-12% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [17–24] | |
| TP53 | <5% | Missense mutation/LOH | [11,19,28–30] | |
| SMAD4 | <5% | Inactivating mutation/LOH, homozygous deletion | [11,19,27–30] | |
| High-grade PanIN | KRAS | >95% | Missense mutation (codons 12, 13, 61) | [9–11] |
| p16/CDKN2A | 18% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [17–24] | |
| TP53 | 10-15% | Missense mutation/LOH | [11,19,28–30] | |
| SMAD4 | <5% | Inactivating mutation/LOH, homozygous deletion | [11,19,27–30] | |
| Low-grade IPMN | KRAS | 43-89% | Missense mutation (codons 12, 13, 61) | [47–53] |
| GNAS | 41-77% | Missense mutation (codon 201) | [50,52,54,55] | |
| RNF43 | 10% | Inactivating mutation/LOH | [59] | |
| p16/CDKN2A | <5% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [53,54,59] | |
| TP53 | <5% | Missense mutation/LOH | [53,59] | |
| SMAD4 | <5% | Inactivating mutation/LOH, homozygous deletion | [53,54,59] | |
| High-grade IPMN | KRAS | 34-71% | Missense mutation (codons 12, 13, 61) | [47–53] |
| GNAS | 42-72% | Missense mutation (codon 201) | [50,52,54,55] | |
| RNF43 | 25-75% | Inactivating mutation/LOH | [54,57,59] | |
| p16/CDKN2A | 0-15% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [53,54,59] | |
| TP53 | 18-20% | Missense mutation/LOH | [53,59] | |
| SMAD4 | <5% | Inactivating mutation/LOH, homozygous deletion | [53,54,59] | |
| Low-grade MCN | KRAS | 3-26% | Missense mutation (codons 12, 13, 61) | [91–93] |
| GNAS | 0% | Missense mutation (codon 201) | [50,52,54] | |
| RNF43 | 12% | Inactivating mutation/LOH | [54] | |
| p16/CDKN2A | 0-14% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [93,95,96] | |
| TP53 | 0% | Missense mutation/LOH | [54,93,95] | |
| SMAD4 | 0% | Inactivating mutation/LOH, homozygous deletion | [95,98] | |
| High-grade MCN | KRAS | 50-100% | Missense mutation (codons 12, 13, 61) | [91–93] |
| GNAS | 0% | Missense mutation (codon 201) | [50,52,54] | |
| RNF43 | 25% | Inactivating mutation/LOH | [54] | |
| p16/CDKN2A | 50-59% | Inactivating mutation/LOH, homozygous deletion, promoter hypermethylation | [93,95,96] | |
| TP53 | 25-56% | Missense mutation/LOH | [54,93,95] | |
| SMAD4 | 0% | Inactivating mutation/LOH, homozygous deletion | [95,98] | |
The loss of CDKN2A/p16 expression typically occurs after KRAS mutation and is more prevalent in high grade PanIN (Table 1). During cellular stress (i.e. DNA damage, hyperproliferative signals) the p16 protein acts as an important regulator of cell proliferation by blocking phosphorylation of RB, which inhibits passage through the G1/S cell cycle checkpoint [16]. Loss of p16 expression has been shown in approximately 27%, 55%, and 71% of cancer-associated PanIN-1A/B, PanIN-2, PanIN-3, respectively [17,18]. A more recent study, comprising a larger cohort of lesions, indicated that 32% of low-grade PanIN and 83% of high-grade PanIN show abrogation of p16 expression [19]. This loss can occur by several different mechanisms including homozygous deletion, intragenic mutation coupled with loss of the second allele, and epigenetic silencing by promoter hypermethylation [20–22]. A common occurrence alongside homozygous deletion of CDKN2A is deletion of the MTAP gene, thus producing concordant loss of both p16 and MTAP protein expression [23]. This can allow immunolabelling for both MTAP and p16 proteins to serve as a surrogate marker for homozygous deletion of CDKN2A, which has been found in 8% of PanIN lesions [23]. Hypermethlyation in the promoter region of CDKN2A has been found in 6% and 21% of low-grade and high-grade PanINs, respectively [24]. These findings suggest that loss of p16 increases with grade and is not an initiating event in PanIN tumorigenesis.
Loss of expression of TP53 and SMAD4 is found almost exclusively in high-grade PanIN and invasive PDAC (Table 1). The proteins encoded by TP53 and SMAD4 play critical roles in mediating cell cycle arrest, cellular senescence and apoptosis in response to cellular stresses [25,26]. Activation of p53 often occurs in response to DNA damage signals, while SMAD4 mediates TGF-β signaling. Several early studies used immunohistochemistry (IHC) to show aberrant p53 and SMAD4 expression in more than 30% of high-grade PanIN lesions, while expression is retained in nearly all low-grade PanINs [19,27,28]. More recently, targeted and whole exome sequencing studies have revealed that only 15% of high-grade PanINs had TP53 mutations and none harbored mutations in SMAD4 [11,29]. Additionally, IHC of these tumor suppressor genes showed rare abnormal expression in high-grade PanIN [29,30]. Discordance in TP53/SMAD4 mutation prevalence between these studies may be explained by a key confounding factor: so-called “cancerization of the ducts,” in which an invasive cancer invades into and spreads within the pancreatic duct system, histologically mimicking high-grade PanIN. This phenomenon has been reported in as many as 70% of resected PDACs [4]. Unlike the aforementioned sequencing studies, the earlier studies sampled PanIN lesions in the setting of invasive PDAC. It is therefore possible that these PanINs were misclassified and actually represented the intraductal spread of invasive carcinoma, which is histologically indistinguishable from high-grade PanIN [4].
In addition to specific gene mutations, there are several other genetic abnormalities that have been implicated in PanIN tumorigenesis. One of the earliest events in PanIN development is telomere shortening [31,32]. Telomeres maintain the integrity of the genome by protecting against chromosomal breakage and interchromosomal fusion [31]. Dysfunctional and shortened telomeres lead to chromosomal instabilities, such as an increased incidence of atypical mitosis and anaphase bridges [33]. Several studies have indicated that approximately 90% of low-grade PanIN lesions have telomere shortening [32,33]. Furthermore, this shortening is exacerbated as PanINs increase in histological grade, with the shortest telomeres belonging to invasive PDAC [33].
A later event in PanIN progression is chromosomal instability and subsequent copy number alterations. These have been widely characterized in PDACs; however, only recently have copy number alterations been described in PanIN. In 2011, Hong et al. analyzed low-grade PanINs from patients with a family history of pancreatic cancer and found that unlike PDAC, these lesions harbor very few chromosomal copy number alterations [34]. Interestingly, a later study of high-grade PanIN found widespread copy number losses in CDKN2A, TP53, and SMDAD4 [35]. There was also strong evidence for bi-allelic inactivation in CDKN2A and TP53, but not SMAD4. This study also identified chromothripsis events in PanIN – a phenomenon characterized by chromosomal shattering and chaotic reassembly. They found that nearly 40% of PanIN lesions were affected by chromothripsis-like events. The vast majority of these chromothripsis events were found in high-grade PanINs; however, it should be noted that these findings are confounded by possible intraductal spread of invasive cancer. Interestingly, Notta et al. identified chromothripsis in 65% of pancreatic cancers, suggesting that these events may promote progression to invasive cancer [36].
Intraductal Papillary Mucinous Neoplasm (IPMN)
IPMN is another common precursor to pancreatic cancer and the most common cyst of the pancreas. Originally thought to be uncommon, improvements and expanded use of imaging modalities have revealed that nearly 14% of the U.S. adult population harbors a pancreatic cyst [37,38]. IPMNs are grossly visible (>1cm in diameter), mucin-producing neoplastic cysts that arise within the main pancreatic duct or branch ducts [6]. Similar to PanINs, IPMNs were formerly grouped by a three-tier classification system based on dysplasia: low, intermediate, or high-grade [6]. This classification was revised to a two-tier system where the former intermediate-grade category is now considered low-grade [4] (Figure 1). IPMNs are also categorized by histologic subtype based on the direction of differentiation of the lining epithelium: gastric, intestinal, pancreatobiliary and oncocytic. Recently, oncocytic-type IPMNs, often referred to as intraductal oncocytic papillary neoplasms (IOPNs), were shown to be unique neoplasms from IPMNs and genetically distinct from the other histologic subtypes [39,40]. Gastric, intestinal and pancreatobiliary-type IPMNs can progress to conventional ductal/tubular carcinomas, while intestinal-type IPMNs can also give rise to colloid carcinomas characterized by extensive stromal mucin accumulation [41,42]. The risk of malignancy associated with an IPMN varies depending on numerous factors (i.e. size, location, grade of dysplasia), with 30–50% of surgically resected IPMNs harboring invasive carcinoma [43–45]. Similar to PanIN, molecular studies of IPMN have demonstrated that the progression from low-grade IPMN to high-grade IPMN is associated with an accumulation of genetic changes that eventually give rise invasive carcinoma [46]. Therefore, an understanding of the molecular drivers that characterize IPMNs is critical for developing effective early detection strategies for pancreatic cancer.
Molecular Features of IPMN
Numerous studies have identified genetic alterations that play a key role in IPMN tumorigenesis. The most common alterations in IPMN are somatic mutations in the oncogenes KRAS and GNAS (Table 1). Mutations in KRAS occur in 50–80% of all IPMNs and are thought to be an early event of IPMN development [47–53]. The identification of GNAS mutations in IPMN was pioneered by Wu et al, which defined a new pathway for pancreatic neoplasia [50]. Since then, GNAS alterations have been found in 40–70% of all IPMNs and remarkably, are not typically found in other pancreatic precursors, or in invasive PDAC not associated with an IPMN [50,52,54–56]. In association with IPMN, GNAS mutations are found in 23–37% of invasive carcinomas [52,55,57]. GNAS encodes the alpha-subunit of a stimulatory guanine nucleotide-binding protein, which activates the cyclic-AMP cascade, leading to cell growth and proliferation [58]. GNAS mutations are most prevalent in intestinal-type IPMNs, found in 70–100% of these neoplasms [50,52,59,60]. Overall, more than 90% of all IPMNs harbor a KRAS and/or GNAS mutation [54], making them important drivers of IPMN development. Moreover, their prevalence in low-grade IPMNs suggests that alterations in these oncogenes may be initiating events in IPMN formation.
Another commonly mutated gene in IPMN is RNF43, found in 10–75% of IPMNs [54,57,59] (Table 1). Alterations in RNF43 are typically inactivating (i.e. nonsense, frameshift) and accompanied by loss of heterozygosity, implicating RNF43 as a tumor suppressor gene in IPMN tumorigenesis. The RNF43 protein is a transmembrane E3 ubiquitin ligase that serves as a negative regulator of Wnt signaling, thereby inhibiting cell proliferation [61]. Whole-exome and targeted sequencing studies have identified mutations in RNF43 in 6–11% of all invasive PDACs, not just those associated with an IPMN [56,62,63]. Recently, loss of function RNF43 mutations have been reported in other cystic precursors and less frequently in PanIN [29,54].
Inactivation of CDKN2A/p16 has been shown to play a role in IPMN progression, particularly during late-stage development (Table 1). Many studies performing IHC have found p16 loss in IPMNs with high-grade dysplasia (50–100%), and less frequently in low-grade IPMNs (10–51%) [19,53,64,65]. However, several next-generation sequencing (NGS) studies have described CDKN2A mutations at a much lower prevalence: 0–2% of low-grade IPMNs and 0–15% of high-grade IPMNs [53,54,59]. This discrepancy may be explained by alternative mechanisms of CDKN2A gene silencing. For example, epigenetic silencing by promoter hypermethlyation has been described in 21% of high-grade IPMNs [66]. Additionally, allelic loss of chromosome 9p was found in 18–62% of IPMNs [67,68]. Altogether, these studies indicate that loss of CDKN2A/p16 is mediated by the same three mechanisms described in PanIN and is a later event in IPMN tumorigenesis.
Mutations in TP53 are extremely rare in low-grade IPMNs but appear much more frequently in high-grade IPMNs (Table 1). Prior to the widespread use of NGS technologies, the literature described variable p53 expression in IPMNs [69]. Several studies have found diffuse nuclear p53 labeling in invasive carcinomas but were unable to detect p53 in IPMN [70,71]. Others showed variable p53 staining in 40–50% of high-grade IPMNs [19,48]. Targeted, massively-parallel sequencing studies have identified TP53 mutations in 15–20% of high-grade IPMNs and 0–5% of low-grade IPMNs [53,59]. These data suggest that TP53 mutations are late-occurring alterations and may play a role in the malignant progression of IPMN.
Unlike the previously mentioned tumor suppressor genes, loss of SMAD4 is mainly confined to invasive carcinomas (Table 1). Several studies have analyzed the immunohistochemical expression of SMAD4 in IPMNs and invasive cancers [53,64,72,73]. They all found retained expression of SMAD4 in the vast majority of IPMNs, while typically half of invasive carcinomas show loss of SMAD4. In concordance with the IHC findings, targeted and whole exome sequencing studies also found SMAD4 mutations to be very rare in IPMN [53,54,59]. However, loss of heterozygosity (LOH) studies using polymerase-chain reaction (PCR)-based microsatellite analysis found allelic loss of 18q in 22–38% of IPMNs [67,68]. Overall, it seems SMAD4 inactivation is not involved in early-stages of IPMN development but is important for its transition to invasive carcinoma.
Mutations in several other cancer-related genes have been reported in IPMNs at low prevalence, such as PIK3CA, BRAF, PTEN and STK11 [49,74–77]. Mutations in the oncogenes PIK3CA and BRAF have also been reported to be drivers in invasive PDAC and many other cancer types [49,56,74]. Early studies reported loss-of-function mutations in PTEN at relatively low frequencies in invasive PDAC; however, more recent studies have found loss of at least one copy of the PTEN gene can help drive malignant progression of both human and mouse PDACs [78,79]. Peutz-Jeghers syndrome patients have an elevated risk of pancreatic malignancy, and commonly harbor germline mutations in the tumor suppressor gene STK11 [80,81]. Several studies have demonstrated LOH at the STK11 locus in sporadic PDACs and other cancers of the gastrointestinal tract, breast, and ovaries [82–85].
Mucinous Cystic Neoplasm (MCN)
MCN is the third most common precursor to PDAC and make up approximately 10% of pancreatic cysts [54,86]. MCNs are mucin-producing lesions and are distinguished from IPMNs by multiple gross and microscopic features, such as lack of involvement with the pancreatic ductal system and characteristic ovarian-type stroma [86,87]. Akin to PanINs and IPMNs, MCNs were previously categorized into three groups based on dysplasia, but this has since been revised into a two-tiered classification system [4]. Approximately 11–16% of surgically resected MCNs have been reported to harbor invasive carcinoma, demonstrating the importance of understanding the common molecular features that characterize these cysts [86,88,89].
Molecular Features of MCN
Like PanIN and IPMN, MCNs harbor genetic changes that lead to progression of these tumors and pancreatic carcinogenesis. KRAS mutations are reported most frequently, found in 3–100% of MCNs [50,52,90–93]. The frequency of KRAS alterations also seems to increase with grade of dysplasia [92–94]. Unlike IPMNs, mutations in GNAS have not been found in MCNs [50,52,54]. While alterations in RNF43 have only been interrogated in a small number of MCNs, Wu et al found RNF43 mutations in 12% of low-grade MCNs and 25% of high-grade MCNs in their cohort [54]. Loss of CDKN2A/p16 may play a role in later stages of MCN progression. One study found loss of CDKN2A in 5/9 high-grade MCNs, but not in low-grade [93]. Of note, only 2 of these high-grade cysts had biallelic deletion of CDKN2A and complete loss of p16 expression. Promoter hypermethylation of CDKN2A also seems to be more prevalent in high-grade MCNs, reported in 14% of low-grade and 50% of high-grade lesions [95,96]. Aberrant expression of TP53 also appears to occur late in MCN development. Early studies have shown a lack of p53 overexpression in low-grade MCNs, yet found strong positive staining concentrated in areas of high-grade dysplasia and invasion [92,97]. More recently, sequencing approaches have found 25–56% of high-grade/invasive MCNs harbor mutations in TP53, but not in low-grade MCNs [54,93,95]. Similar to IPMNs, loss of SMAD4 expression appears predominantly in invasive MCN and PDAC. In one study, which examined 36 MCNs, SMAD4 expression was retained in both low and high-grade MCNs, while 86% of invasive carcinomas arising from MCNs showed loss of SMAD4 expression [98]. Another study used both a sequencing-based approach and IHC to identify mutations in exons 1–11 of SMAD4 [95]. These researchers found 71% of invasive MCNs harbored somatic mutations in SMAD4. Several other less frequently altered genes have been reported in MCN, including PIK3CA and PTEN [99,100].
Genetic Heterogeneity and Multifocal Neoplasia
Genetic heterogeneity of driver gene alterations in PanIN and IPMN have recently been described due to increased sensitivity of NGS approaches. Several investigators have identified multiple KRAS mutations within a single PanIN lesion [11,29,30]. Remarkably, more than one KRAS mutation occurs in as many as 30% of PanINs, suggesting apparent genetic heterogeneity with respect to driver genes in these pancreatic precursors. In IPMN, Wu et al. used targeted sequencing to analyze cyst fluid from 19 patients [50]. They found 11% of IPMNs contained two different KRAS mutations, 2% contained three different KRAS mutations and 4% contained two different GNAS mutations. Additionally, Felsenstein et al. microdissected epithelium from two distinct regions of IPMN and subsequently performed deep targeted sequencing of pancreatic driver genes [57]. These studies reported that 23% of IPMNs had multiple KRAS and/or GNAS mutations, and remarkably one of these IPMNs contained four unique KRAS mutations. The finding of multiple KRAS and GNAS mutations within a single PanIN/IPMN suggest a more complex pattern of clonal evolution in these precursor lesions than previously appreciated.
In addition to the description of genetic heterogeneity within single precursor lesions, the results of several studies have provided evidence for multifocal neoplasia in the pancreas. First, Hosoda et al. analyzed high-grade PanIN and adjacent low-grade PanIN using targeted NGS in ten patients [29]. Surprisingly, they found only one pair of low-grade/high-grade PanIN that were ‘likely related,’ while the vast majority of pairs were genetically independent. This suggests the possibility of an alternative mechanism for PanIN progression – de novo development of high-grade PanIN adjacent to an independent, unrelated low-grade PanIN [7]. Next, Pea et al. performed targeted sequencing on IPMN and PDAC lesions from 13 patients who developed disease progression in their remnant pancreas following resection of IPMN [101]. Analysis of both the primary and recurrent IPMN revealed that more than half of the cases had genetic alterations not shared between these neoplasms and were classified as ‘likely independent.’ Finally, Felsenstein et al. described the genetic relationship between invasive carcinoma and co-occurring IPMN [57]. Interestingly, 18% of PDACs and co-occurring IPMNs did not share any driver gene mutation and were considered ‘likely independent,’ despite their close anatomic proximity. While this has not been investigated in detail with respect to MCN, clinical data suggests these lesions tend to be unifocal [89]. Altogether, these studies support the concept of a field defect in a subset of patients, which leads to an increased risk of neoplasia throughout the pancreas.
Early detection of pancreatic precursors
As mentioned previously, PanIN, IPMN, and MCN are common precursors of PDAC and therefore represent key targets for early detection approaches. Imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) are commonly used to detect lesions in the pancreas. In a multi-institution study conducted by Canto et al., these imaging methods were used to screen 225 asymptomatic, high-risk individuals (HRI) [102]. They found that 42% of HRIs had at least one pancreatic mass or a dilated pancreatic duct. Among these, proven or suspected lesions were later identified in 92% of patients. These imaging modalities can also be augmented to target specific structures and molecules. For example, Neesse et al. designed a fluorochrome that specifically targets claudin-4 [103], a protein known to be upregulated in pancreatic neoplasia [104,105].
While these imaging-based approaches are useful to detect pancreatic cysts, they may not reliably differentiate cyst type or important histological features (i.e. grade of dysplasia), which can better predict likelihood of progression. This distinction is clinically important because pancreatic cysts represent a diverse group of lesions, some of which are low-risk while others progress to invasive carcinoma. As a result, a more reliable determination of precursors with a higher malignant potential will be critical. Many studies have demonstrated the value of collecting cyst fluid by EUS-fine needle aspiration from patients diagnosed with pancreatic cysts. Several reports highlight the importance of cytological evaluation of cyst fluid for atypical epithelial cells, which can serve as a predictor of malignancy [106–108]. Others have reported on the diagnostic value of biochemical markers in cyst-fluid for differentiating likely benign, serous cysts from mucinous cysts which have greater risk of malignancy [109–111]. Furthermore, two independent studies used targeted NGS to analyze cyst-fluid [100,112]. The investigators used a combination of molecular markers to categorize a cyst as IPMN with 76–100% sensitivity and 84–96% specificity or as MCN with 100% sensitivity and 75% sensitivity. This approach identified IPMNs with high-grade dysplasia or invasive carcinoma with 88% sensitivity and 69–97% specificity. These results highlight the ability of cyst fluid sequencing to preoperatively determine cyst type as well as predict grade of dysplasia in premalignant cysts. Another marker that could be used to differentiate grade of dysplasia in IPMN is telomere fusion, which frequently occurs in critically short telomeres. Hata et al. developed a real-time quantitative PCR method to detect telomere fusion in cyst-fluid [113]. They detected telomere fusions in 27% of high-grade IPMNs, but not in low-grade IPMNs. Additionally, several studies have used cyst fluid to identify other molecular changes such as telomerase activity and microRNA levels [114,115]. As a result from these findings and others, several institutions are implementing NGS-based molecular tests using cyst fluid to aid in the clinical evaluation and diagnosis of pancreatic cysts.
While these studies provide important data for the early detection of IPMN, they are limited by sampling only cyst-fluid. As previously mentioned, Felsenstein et al found that a substantial portion of PDACs with co-occurring IPMNs are unrelated [57]; therefore, analysis of cyst fluid may not detect the true precursor of the cancer. Furthermore, traditional imaging tests are unable to detect PanIN lesions altogether. A promising approach is genetic analysis of secretin-stimulated pancreatic juice collected from the duodenum. Kanda et al. determined the prevalence of TP53 mutations in pancreatic juice from individuals undergoing pancreatic evaluation [116]. The prevalence of mutant TP53 in pancreatic juice samples from patients whose highest grade lesion was PanIN-3 (40%) is very similar to its prevalence in primary resected PanIN-3 lesions (48%). More recently, Yu et al. developed a digital NGS approach to detect low-abundance mutations in pancreatic juice samples [117]. In two cases of high-risk individuals, digital NGS was able to detect SMAD4 or TP53 mutations more than one year before their pancreatic cancer diagnosis. Finally, Suenaga et al. performed digital NGS using a targeted 12-gene panel to evaluate mutation concentrations in pancreatic juice samples [118]. Mutation concentrations in genes other than KRAS/GNAS were higher in patients with PDAC or high-grade precursors relative to all other subjects. Consistent with previous studies and the molecular progression of PanIN and IPMN, this pancreatic juice analysis found several predictors of pancreatic cancer or a high-grade precursor: presence of SMAD4 mutations, high SMAD4/TP53 mutation concentrations and high overall mutation concentrations. While the lack of these mutations does not indicate absence of disease, these results highlight the potential value of a pancreatic juice NGS screening test for patients undergoing pancreatic evaluation. Finally, analysis of molecules and cells in the blood also does not require sampling of a specific lesion in the pancreas and thus is an alternative approach to early detection. For example, some groups have reported analysis of circulating epithelial cells (CECs) in the bloodstream in patients with pancreatic cancer as well as IPMN [119,120]. This method has been used to detect CECs in patients with cystic lesions prior to the clinical diagnosis of invasive PDAC; however, the sensitivity of this method to detect PanIN is not known but is likely to be low considering the lack of access of these precursor lesions to the bloodstream. Numerous serum markers have also been suggested as potential biomarkers for early detection of pancreatic neoplasia, but these remain to be systematically evaluated [121].
In the future, more comprehensive analyses of the genetic heterogeneity in pancreatic cancer precursor lesions can further elucidate their clonal evolution and neoplastic progression. Additionally, the identification and validation of molecular markers that can reliably distinguish low-risk lesions from lesions with a high risk of malignant progression will be fundamental for effective early detection approaches. These promising approaches require a deep understanding of the molecular alterations that occur during pancreatic tumorigenesis via the PanIN, IPMN and MCN pathways. Pathologists will play a key role in the implementation of these approaches for the better management of these precursor lesions and ultimately the prevention of pancreatic cancer.
Acknowledgements
The authors acknowledge the following sources of funding: NIH/NCI P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bernard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees)
Footnotes
Conflict of interest: LDW is a paid consultant for Personal Genome Diagnostics. The other authors report no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: A comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003; 16: 996–1006. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O, Hong S-M, Wood LD, et al. A revised classification system and recommendations from the Baltimore Consensus for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015; 39: 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruban RH, Adsay NV, Albores–Saavedra J, et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 6.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004; 28: 977–987. [DOI] [PubMed] [Google Scholar]
- 7.Sipos B, Frank S, Gress T, et al. Pancreatic intraepithelial neoplasia revisited and updated. Pancreatology 2009; 9: 45–54. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008; 1: 306–316. [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012; 142: 730–733.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Löhr M, Klöppel G, Maisonneuve P, et al. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-Analysis. Neoplasia 2005; 7: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology 2013; 145: 1098–1109.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takashima A, Faller DV. Targeting the RAS oncogene. Expert Opin Ther Targets 2013; 17: 507–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4: 437–450. [DOI] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7: 469–483. [DOI] [PubMed] [Google Scholar]
- 15.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: Consensus report and recommendations. Cancer Res 2006; 66: 95–106. [DOI] [PubMed] [Google Scholar]
- 16.LaPak KM, Burd CE. The molecular balancing act of p16INK4a in cancer and aging. Mol Cancer Res 2014; 12: 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosty C, Geradts J, Sato N, et al. p16 inactivation in pancreatic intraepithelial neoplasias (PanINs) arising in patients with chronic pancreatitis. Am J Surg Pathol 2003; 27: 1495–1501. [DOI] [PubMed] [Google Scholar]
- 18.Wilentz RE, Geradts J, Maynard R, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: Loss of intranuclear expression. Cancer Res 1998; 58: 4740–4744. [PubMed] [Google Scholar]
- 19.Furukawa T, Fujisaki R, Yoshida Y, et al. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol 2005; 18: 1034–1042. [DOI] [PubMed] [Google Scholar]
- 20.Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 1994; 8: 27–32. [DOI] [PubMed] [Google Scholar]
- 21.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997; 57: 3126–3130. [PubMed] [Google Scholar]
- 22.Geradts J, Hruban RH, Schutte M, et al. Immunohistochemical p16INK4a analysis of archival tumors with deletion, hypermethylation, or mutation of the CDKN2/MTS1 gene. A comparison of four commercial antibodies. Appl Immunohistochem Mol Morphol 2000; 8: 71–79. [DOI] [PubMed] [Google Scholar]
- 23.Hustinx SR, Leoni LM, Yeo CJ, et al. Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion. Mod Pathol 2005; 18: 959–963. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima N, Sato N, Ueki T, et al. Aberrant methylation of preproenkephalin and p16 genes in pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma. Am J Pathol 2002; 160: 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 2014; 14: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia X, Wu W, Huang C, et al. SMAD4 and its role in pancreatic cancer. Tumour Biol 2015; 36: 111–119. [DOI] [PubMed] [Google Scholar]
- 27.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000; 60: 2002–2006. [PubMed] [Google Scholar]
- 28.Lüttges J, Galehdari H, Bröcker V, et al. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol 2001; 158: 1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol 2017; 242: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokode M, Akita M, Fujikura K, et al. High‐grade PanIN presenting with localized stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology 2018; 73: 247–258. [DOI] [PubMed] [Google Scholar]
- 31.Hong S-M, Heaphy CM, Shi C, et al. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod Pathol 2011; 24: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 2002; 161: 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda Y, Ishiwata T, Izumiyama-Shimomura N, et al. Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS One 2015; 10: e0117575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S-M, Vincent A, Kanda M, et al. Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin Cancer Res 2012; 18: 4303–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hata T, Suenaga M, Marchionni L, et al. Genome-wide somatic copy number alterations and mutations in high-grade pancreatic intraepithelial neoplasia. Am J Pathol 188: 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016; 538: 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010; 105: 2079–2084. [DOI] [PubMed] [Google Scholar]
- 38.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008; 191: 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basturk O, Chung SM, Hruban RH, et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch 2016; 469: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basturk O, Tan M, Bhanot U, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod Pathol 2016; 29: 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 2001; 25: 26–42. [DOI] [PubMed] [Google Scholar]
- 42.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J Surg Pathol 2004; 28: 839–848. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- 44.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2004; 239: 788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grützmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: Biology, diagnosis, and treatment. Oncologist 2010; 15: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid MD, Saka B, Balci S, et al. Molecular genetics of pancreatic neoplasms and their morphologic correlates: An update on recent advances and potential diagnostic applications. Am J Clin Pathol 2014; 141: 168–180. [DOI] [PubMed] [Google Scholar]
- 47.Schönleben F, Allendorf JD, Qiu W, et al. Mutational analyses of multiple oncogenic pathways in intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2008; 36: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satoh K, Shimosegawa T, Moriizumi S, et al. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas 1996; 12: 362–368. [DOI] [PubMed] [Google Scholar]
- 49.Schönleben F, Qiu W, Bruckman KC, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett 2007; 249: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3: 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuboki Y, Shimizu K, Hatori T, et al. Molecular biomarkers for progression of intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2015; 44: 227–235. [DOI] [PubMed] [Google Scholar]
- 52.Hosoda W, Sasaki E, Murakami Y, et al. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch 2015; 466: 665–674. [DOI] [PubMed] [Google Scholar]
- 53.Amato E, Molin M dal, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014; 233: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Jiao Y, Molin MD, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011; 108: 21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011; 1: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015; 6: 6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felsenstein M, Noë M, Masica DL, et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut 2018; [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lania AG, Mantovani G, Spada A. Mechanisms of Disease: mutations of G proteins and G-protein-coupled receptors in endocrine diseases. Nat Rev Endocrinol 2006; 2: 681–693. [DOI] [PubMed] [Google Scholar]
- 59.Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg 2015; 220: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molin MD, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 2013; 20: 3802–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koo B-K, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012; 488: 665–669. [DOI] [PubMed] [Google Scholar]
- 62.Waddell N, Pajic M, Patch A-M, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molin MD, Zhang M, de Wilde RF, et al. Very long-term survival following resection for pancreatic cancer is not explained by commonly mutated genes: results of whole-exome sequencing analysis. Clin Cancer Res 2015; 21: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biankin AV, Biankin SA, Kench JG, et al. Aberrant p16INK4A and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut 2002; 50: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohri D, Asaoka Y, Ijichi H, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol 2012; 47: 203–213. [DOI] [PubMed] [Google Scholar]
- 66.Sato N, Ueki T, Fukushima N, et al. Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology 2002; 123: 365–372. [DOI] [PubMed] [Google Scholar]
- 67.Abe T, Fukushima N, Brune K, et al. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res 2007; 13: 6019–6025. [DOI] [PubMed] [Google Scholar]
- 68.Fujii H, Inagaki M, Kasai S, et al. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 1997; 151: 1447–1454. [PMC free article] [PubMed] [Google Scholar]
- 69.Chadwick B, Willmore-Payne C, Tripp S, et al. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl Immunohistochem Mol Morphol 2009; 17: 31–39. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki S, Yamamoto H, Kaneto H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep 2003; 10: 21–25. [PubMed] [Google Scholar]
- 71.Sakai Y, Yanagisawa A, Shimada M, et al. K-ras gene mutations and loss of heterozygosity at the p53 gene locus relative to histological characteristics of mucin-producing tumors of the pancreas. Hum Pathol 2000; 31: 795–803. [DOI] [PubMed] [Google Scholar]
- 72.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: Comparison with conventional ductal denocarcinomas. Am J Pathol 2000; 157: 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furukawa T, Fujisaki R, Yoshida Y, et al. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol 2005; 18: 1034–1042. [DOI] [PubMed] [Google Scholar]
- 74.Schönleben F, Qiu W, Ciau NT, et al. PIK3CA Mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res 2006; 12: 3851–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 2001; 159: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Carracedo D, Turk AT, Fine SA, et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2013; 19: 6830–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durante S, Vecchiarelli S, Astolfi A, et al. Copy number gain of chromosome 3q is a recurrent event in patients with intraductal papillary mucinous neoplasm (IPMN) associated with disease progression. Oncotarget 2016; 7: 74797–74806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ying H, Elpek KG, Vinjamoori A, et al. Pten is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov 2011; 1: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kopp JL, Dubois CL, Schaeffer DF, et al. Loss of Pten and activation of Kras synergistically induce formation of intraductal papillary mucinous neoplasia from pancreatic ductal cells in mice. Gastroenterology 2018; 154: 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 1998; 391: 184–187. [DOI] [PubMed] [Google Scholar]
- 81.Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Int Med 1998; 128: 896–899. [DOI] [PubMed] [Google Scholar]
- 82.Resta N, Simone C, Mareni C, et al. STK11 mutations in Peutz-Jeghers syndrome and sporadic colon cancer. Cancer Res 1998; 58: 4799–4801. [PubMed] [Google Scholar]
- 83.Forster LF, Defres S, Goudie DR, et al. An investigation of the Peutz-Jeghers gene (LKB1) in sporadic breast and colon cancers. J Clin Pathol 2000; 53: 791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang ZJ, Churchman M, Campbell IG, et al. Allele loss and mutation screen at the Peutz-Jeghers (LKB1) locus (19p13.3) in sporadic ovarian tumours. Br J Cancer 1999; 80: 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol 1999; 154: 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jang K-T, Park SM, Basturk O, et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma. Am J Surg Pathol 2015; 39: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: Demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol 2004; 2: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 88.Baker ML, Seeley ES, Pai R, et al. Invasive mucinous cystic neoplasms of the pancreas. Exp Mol Pathol 2012; 93: 345–349. [DOI] [PubMed] [Google Scholar]
- 89.Crippa S, Castillo CF, Salvia R, et al. Mucin-producing neoplasms of the pancreas: An analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol 2010; 8: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011; 108: 21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuboki Y, Shiratori K, Hatori T, et al. Association of epidermal growth factor receptor and mitogen-activated protein kinase with cystic neoplasms of the pancreas. Mod Pathol 2010; 23: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 92.Jimenez RE, Warshaw AL, Z’graggen K, et al. Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann Surg 1999; 230: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conner JR, Mariño-Enríquez A, Mino-Kenudson M, et al. Genomic characterization of low- and high-grade pancreatic mucinous cystic neoplasms reveals recurrent KRAS alterations in ‘high-risk’ lesions. Pancreas 2017; 46: 665–671. [DOI] [PubMed] [Google Scholar]
- 94.Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014; 20: 4381–4389. [DOI] [PubMed] [Google Scholar]
- 95.Gerdes B, Wild A, Wittenberg J, et al. Tumor-suppressing pathways in cystic pancreatic tumors. Pancreas 2003; 26: 42–48. [DOI] [PubMed] [Google Scholar]
- 96.Kim SG, Wu T-T, Lee JH, et al. Comparison of epigenetic and genetic alterations in mucinous cystic neoplasm and serous microcystic adenoma of pancreas. Mod Pathol 2003; 16: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 97.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: Clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999; 23: 410–422. [DOI] [PubMed] [Google Scholar]
- 98.Iacobuzio-Donahue CA, Wilentz RE, Argani P, et al. Dpc4 protein in mucinous cystic neoplasms of the pancreas: Frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol 2000; 24: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Carracedo D, Chen Z-M, Qiu W, et al. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas 2014; 43: 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut September 2017: gutjnl - 2016–313586 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pea A, Yu J, Rezaee N, et al. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg 2017; 266: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neesse A, Hahnenkamp A, Griesmann H, et al. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut 2013; 62: 1034–1043. [DOI] [PubMed] [Google Scholar]
- 104.Gress TM, Müller-Pillasch F, Geng M, et al. A pancreatic cancer-specific expression profile. Oncogene 1996; 13: 1819–1830. [PubMed] [Google Scholar]
- 105.Geng MM, Ellenrieder V, Wallrapp C, et al. Use of representational difference analysis to study the effect of TGFB on the expression profile of a pancreatic cancer cell line. Genes Chromosomes Cancer 1999; 26: 70–79. [DOI] [PubMed] [Google Scholar]
- 106.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg 2011; 254: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: Predictors of malignant and invasive pathology. Ann Surg 2007; 246: 644–651. [DOI] [PubMed] [Google Scholar]
- 108.Wiesenauer CA, Schmidt CM, Cummings OW, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg 2003; 138: 610–618. [DOI] [PubMed] [Google Scholar]
- 109.Allen PJ, Qin L-X, Tang L, et al. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg 2009; 250: 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewandrowski KB, Southern JF, Pins MR, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg 1993; 217: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330–1336. [DOI] [PubMed] [Google Scholar]
- 112.Springer S, Wang Y, Molin MD, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015; 149: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hata T, Dal Molin M, McGregor-Das A, et al. Simple detection of telomere fusions in pancreatic cancer, intraductal papillary mucinous neoplasm, and pancreatic cyst fluid. J Mol Diagn 2018; 20: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hata T, Molin MD, Suenaga M, et al. Cyst fluid telomerase activity predicts the histologic grade of cystic neoplasms of the pancreas. Clin Cancer Res 2016; 22: 5141–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ryu JK, Matthaei H, dal Molin M, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology 2011; 11: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013; 11: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu J, Sadakari Y, Shindo K, et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 2017; 66: 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suenaga M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res 2018; 24: 2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poruk KE, Valero V, He J, et al. Circulating epithelial cells in intraductal papillary mucinous neoplasms and cystic pancreatic lesions. Pancreas 2017; 46: 943–947. [DOI] [PubMed] [Google Scholar]
- 120.Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014; 146: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Young MR, Wagner PD, Ghosh S, et al. Validation of biomarkers for early detection of pancreatic cancer: Summary of The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas 2018; 47: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]