Abstract
This protocol was developed to assess communication in tumor cells and to provide a dependable and standardized assay for the in vitro determination of gap junction function. The method is noninvasive; in this method, the cell population under study is divided such that 1 fraction is loaded with a lipophilic cell plasma membrane permeable dye, calcein acetoxymethyl ester, that is hydrolyzed upon cellular uptake by cytoplasmic esterases to yield calcein, a fluorescent and membrane-impermeable molecule. The other fraction is loaded with 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ tetramethylindodicarbocyanine perchlorate (DiD)/1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethylindocarbocyanine perchlorate [Dil; DilC18(3)], which is a lipophilic membrane dye that diffuses laterally to stain the entire cell membrane, is impermeable, and attains an orange-red fluorescence upon incorporation into membranes. The 2 fractions are mixed and incubated under coculture conditions. Calcein with MW 890 kD is transferred to the DiD/DiI-stained cells through gap junctions. The assessment of this uptake is made with confocal imaging and quantitated using flow cytometry. Cell lines representing cancer of the breast as well as a nontransformed cell line developed from the buccal mucosa were analyzed for gap junction competency. Confocal imaging with acquisition at specific time points during the in vitro treatment and flow cytometry gave a qualitative and quantitative analysis of the passage of molecules through the gap junctions. Here, the method has been combined to obtain images as well as quantitation and is a simple and effective approach in assessing the functional competency of gap junction in epithelial cells.
Keywords: dye coupling assay; flow cytometry; GJIC function, cell communication, connexins.
INTRODUCTION
In the multicellular organism, one of the ways in which homeostasis is maintained is through modulation of intercellular communication mediated by gap junction channels. Intercellular communication is also important during development, in tissue repair, and in transmitting immune responses. Thus, communication between cells is vital and can be achieved either directly by cell-cell contact or indirectly by the release of signaling factors that can be transmitted via gap junction communication channels. Gap junctions are specialized intercellular membrane channels constituted by the protein connexin (CX) that selectively facilitate the passage of small molecules of <1.5 kD across cells. Typically, they connect cells of the same type and form homo cellular gap junctions; however, they have been found to form between cells of different types and could be hetero cellular in characteristics.1 Gap junctions are dynamic structures with the constituent proteins, CXs, possessing a relatively short half-life of 1–5 h.2, 3 They are tightly regulated by voltage, growth factors, a number of secondary messengers including cAMP, and retinoids, and they are modulated by phosphorylation.4, 5 Several substances, like ions, sugars, nucleotides, amino acids, fatty acids, small peptides, drugs, and carcinogens, are within the permissible size and move between cells through gap junction channels.6 However, proteins, complex lipids, polysaccharides, RNA, and other large molecules cannot traverse through this channel.7 The passage is by passive diffusion, it does not require ATP, and the continuous flux of materials between cells via gap junction channels is known as gap junction intercellular communication (GJIC).8 Studies pertaining to cell-cell communication through gap junctions are carried out either by measuring dye transfer or by measuring electrical conductance and metabolic cooperation.9–12 Our studies on the bystander effect during therapeutic intervention for the treatment of cancer required the assessment of gap junctions. Using cell lines of different tumor types as well as an immortalized normal cell line, this fluorescent dye transfer technique was employed to assay the functional competence of gap junctions. The protocol is described here in detail and the data is obtained from human mammary carcinoma cell lines, a human fetal buccal mucosa cell line, and a reference human cancer cell line, NT8e, with excellent GJIC function. The protocol has been validated with immunofluorescent staining for membrane integrity and CX expression.
MATERIALS AND METHODS
Materials
DMEM, RPMI 1640, fetal bovine serum, and PBS were procured from Thermo Fisher Scientific (Waltham, MA, USA). Calcein (C3099), 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethylindocarbocyanine perchlorate [Dil; DilC18(3)], (V-22885), and 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ tetramethylindodicarbocyanine perchlorate (DiD) (V-22887) were obtained from Thermo Fisher Scientific. Primary antibodies for CX 43 (ab11370; Abcam, Cambridge, MA, USA), CX 26 (ab38584; Abcam), CX 32 (ab11368; Abcam), and E- cadherin (4065; Cell Signaling Technology, Danvers, MA, USA) and a secondary antibody tagged with Alexa 488 were used at appropriate dilutions. All other chemicals were purchased locally and were of analytical grade.
Human mammary carcinoma cell lines (BT-474 and MDA-MB-468); FBM, an immortalized nontransformed human fetal buccal mucosa cell line characterized for cytokeratin expression;13 and a reference human cancer cell line (NT8e)14 for comparison were selected to develop this protocol.
Methods
Evaluation of gap junctional communication by fluorescent dye transfer
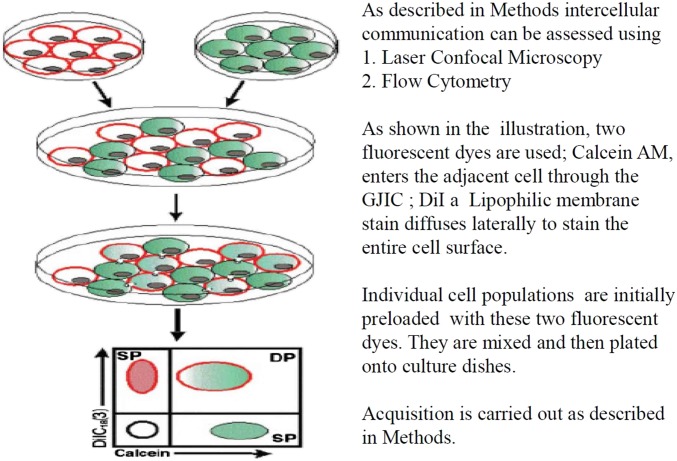
As illustrated in Fig. 1, the dye transfer technique utilizes 2 fluorescent dyes, Calcein and DiI or DiD. Calcein has an MW of ∼900 Da and enters the cell through the gap junctions. It is nonfluorescent and is converted to green fluorescent calcein in live cells after acetoxymethyl (AM) ester hydrolysis by intracellular esterases. DiI/DiD is a lipophilic membrane stain that diffuses laterally to stain the entire cell membrane. It attains an orange-red fluorescence after it is incorporated into membranes. The assay requires “donor” cells that are labeled with 10 μM of calcein-AM for 10 min at 37°C/5% CO2, washed thrice with Dulbecco’s PBS, mixed, and cocultured with “acceptor” cells labeled with 2.5 μM of DiI/DiD, a red fluorescent lipophilic dye that does not diffuse or transfer and stains the cellular membrane. The transfer of calcein from donor to acceptor cells is indicative of a functional and active GJIC. Acceptor cells are converted to dual positive; calcein, and DiI/DiD positive. The assay was analyzed quantitatively as well as qualitatively.
FIGURE 1.
The fluorescent dye transfer assay as depicted in the illustration and described in Methods. This assay was done to assess the function of GJIC. DP, dual positive; SP, single positive.
Laser confocal microscopy
The assay was done for the cell lines as described above. The cells were plated onto a glass bottom 35-mm culture dish. Images at different time points were acquired on a Carl Zeiss LSM 510 Meta Microscope (Carl Zeiss GmbH, Oberkochen, Germany). The controls used were unlabeled cells, only calcein and only DiI, and were maintained for every cell line. A live cell imaging was performed that captured images at specific time points after plating to evaluate junction formations for intercellular communication. Images were captured at ×40 oil and then zoomed at ×2. The filter combinations used were calcein: Excitation 405/488 nm (494), Emission BP 505–530; DiI: Excitation 488/543 nm (549), Emission BP 560–615. The images were analyzed to show transfer of calcein from the donor cells to the DiI-stained acceptor cells. The cut mask function that permits the extraction of the colocalized image pixels has been used to show exclusively the colocalized regions. DiI (V-22885) was used with laser confocal microscopy.
Flow cytometry
Cells were acquired on a BD FACSAria (BD Biosciences, San Jose, CA, USA) at different time points: 0, 3, 4, 5, 16, and 24 h. The flow cytometry controls were unlabeled, only calcein and only DiD (V22887; Thermo Fisher Scientific), and labeled cells. The laser lines used are Argon 488 nm for calcein and HeNe 633 for DiD. The BD FACSDiva software (BD Biosciences) was used to calculate the dual positive population. From the quadrant statistics, percent of communication was calculated according to the formula:
where Q2 is the dual population and Q1 is the acceptor cell population marked with the red fluorescent lipophilic dye.15
Cellular localization of CX43, CX32, CX26, and E-cadherin
Cells were grown on coverslips, fixed with ice-cold methanol, blocked with 10% normal goat serum (NGS), and treated with the appropriate primary antibody (1:100 dilution in 3% NGS) at 4°C overnight. Further, coverslips were washed thrice with PBS and treated with the secondary tagged with Alexa 488 at 1:200 dilution in 3% NGS for 60 min. Negative controls (-primary) were treated only with secondary Alexa 488. Finally, coverslips were washed thrice with PBS, stained for nuclei with DAPI, washed, and mounted on slides with 1, 4-diazabicyclo (2.2.2) octane (DABCO), and the images were acquired on laser confocal microscopy.
RESULTS
GJIC competence analyzed using flow cytometry
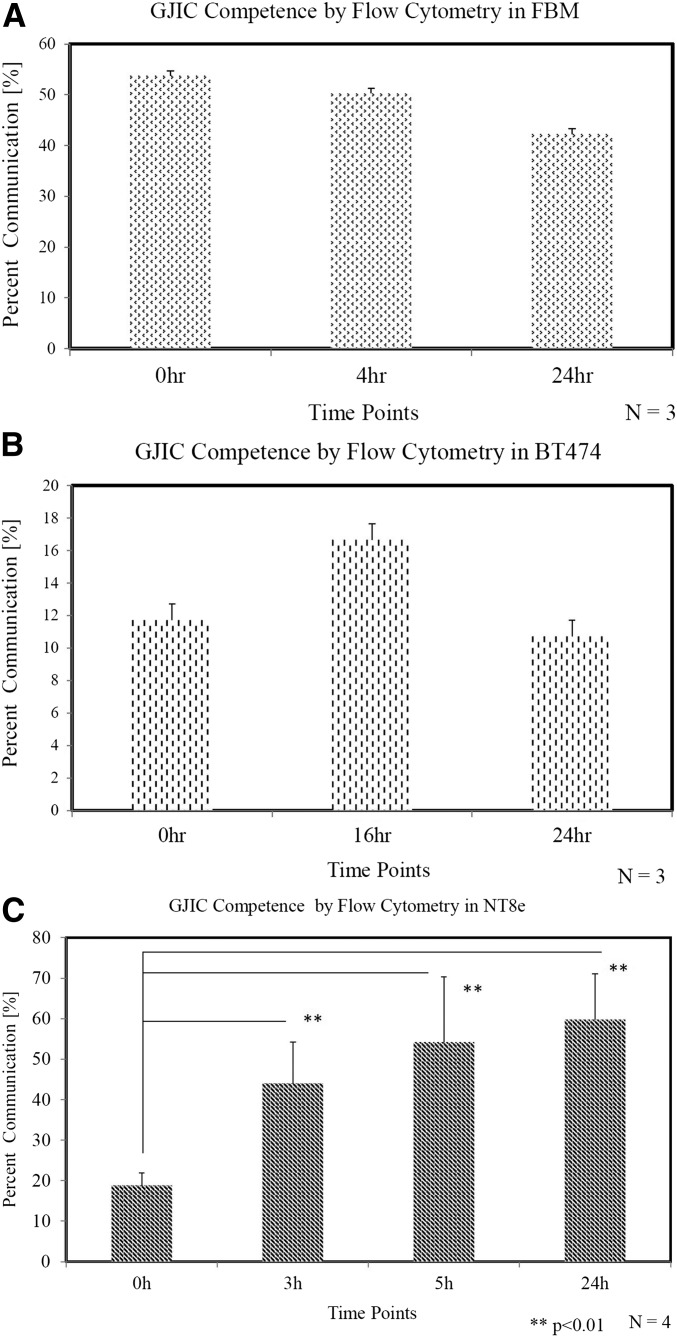
Gap junctions are dynamic entities with a high turnover rate of the CX proteins. The half-life of the molecule is between 1.5 and 5 h. In epithelial cultures, gap junctions form stable communication channels by 5–6 h after plating. For stable communication to form, it is also important that the cell seeding is sufficient. The time points evaluated for complete assessment are the early time points (0, 3 h), stabilized time point (4, 5 h), and the time point of 24 h. Table 1 shows the quadrant percentage of the different populations in the 3 cell lines selected. Q1 is the acceptor population (only DiD), Q2 is the dual population (DiD cells that have accepted calcein AM through gap junctions), Q3 is the negative population, and Q4 is the donor population (only calcein). The results shown are for acquisitions done and repeated. In Fig. 2, the percent communication calculated by the formula described in Methods has been represented for the cell lines used to develop this protocol. In the FBM cell line (Fig. 2A), the percent communication varies from 53 to 42% in the early to the stabilized time points, whereas in the Her2neu overexpressing cell line, BT474 (Fig. 2B), the percent communication is in the range of 10–17%. A reference cancer cell line, NT8e, with remarkably good percent communication was selected to emphasize the variation observed in the GJIC function (Fig. 2C). In this cell line, the percent communication showed a wide range in the mean from 18% immediately after plating the cells (described in Methods) to 44% at the 3-h time point and 54% at the 5-h stabilized time point to 60% at the 24-h time point. Flow cytometry allows a quantitation and gives an estimate of the percent communication observed in different cell lines. It represents the differences observed in junction communication among the different cancer types or subtypes and could also indirectly reflect on the chemotherapeutic response or the differences observed in tumor aggressiveness within these types and subtypes.
TABLE 1.
Quadrant percentages obtained using flow cytometry and calculated percent communication through gap junctions
| Time point | Only DiD (Q1%) | DiD + calcein (Q2%) | Negative (Q3%) | Only calcein (Q4%) | Q1 + Q2 | Q2 ÷ (Q2 + Q1) × 100 | Average | Count | SD |
|---|---|---|---|---|---|---|---|---|---|
| NT8E | |||||||||
| 0 h | 47.5 | 11.4 | 4.6 | 36.5 | 58.9 | 19.35 | 18.81 | 4 | 3.098 |
| 35.8 | 8.7 | 15.8 | 39.7 | 44.5 | 19.55 | ||||
| 31.2 | 5.3 | 21.7 | 41.8 | 36.5 | 14.5 | ||||
| 20 | 5.6 | 0.2 | 74.2 | 25.6 | 21.875 | ||||
| 3 h | 45 | 23.7 | 0.2 | 31.1 | 68.7 | 34.49 | 44.02 | 4 | 10.215 |
| 27.2 | 35.2 | 6.7 | 30.9 | 62.4 | 56.4 | ||||
| 34.1 | 20 | 9.9 | 36 | 54.1 | 36.9 | ||||
| 31 | 29 | 5.5 | 34.5 | 60 | 48.3 | ||||
| 5 h | 39.7 | 31.3 | 0.1 | 28.9 | 71 | 44.08 | 54.22 | 4 | 16.11 |
| 28.2 | 40 | 6.8 | 25 | 68.2 | 58.6 | ||||
| 30.9 | 20 | 3.3 | 45.8 | 50.9 | 39.2 | ||||
| 18.2 | 55.4 | 3.3 | 23.1 | 73.6 | 75 | ||||
| 24 h | 34.5 | 52.9 | 0.4 | 12.2 | 87.4 | 60.52 | 59.75 | 4 | 11.32 |
| 25 | 46 | 0.7 | 28.3 | 71 | 64.7 | ||||
| 32 | 25 | 3.3 | 39.7 | 57 | 43.8 | ||||
| 23.5 | 55.1 | 4.7 | 16.6 | 78.6 | 70 | ||||
| BT474 | |||||||||
| 0 h | 41.4 | 5.2 | 50.5 | 2.9 | 46.6 | 11.15 | 11.71 | 3 | 0.849 |
| 35 | 4.5 | 58.3 | 2.2 | 39.5 | 11.3 | ||||
| 33 | 4.8 | 56.7 | 5.5 | 37.8 | 12.69 | ||||
| 16 h | 12.9 | 2.6 | 82.9 | 1.6 | 15.5 | 16.77 | 16.6 | 3 | 2.70 |
| 42 | 6.8 | 49.2 | 2 | 48.8 | 13.9 | ||||
| 33 | 7.9 | 55.9 | 3.2 | 40.9 | 19.3 | ||||
| 24 h | 61.2 | 6.9 | 31.8 | 0.1 | 68.1 | 10.1 | 10.71 | 3 | 1.66 |
| 45 | 4.7 | 48.2 | 2.1 | 49.7 | 9.45 | ||||
| 38 | 5.5 | 54.7 | 1.8 | 43.5 | 12.6 | ||||
| FBM | |||||||||
| 0 h | 38.2 | 44.3 | 0.3 | 17.2 | 82.5 | 53.6 | 53.753 | 3 | 1.33 |
| 32.1 | 39.5 | 0.4 | 28 | 71.6 | 55.16 | ||||
| 28.5 | 31.5 | 2.5 | 37.5 | 60 | 52.5 | ||||
| 4 h | 30.8 | 30.5 | 0.1 | 38.6 | 61.3 | 49.75 | 50.28 | 3 | 4.97 |
| 30 | 25.2 | 2.8 | 42 | 55.2 | 45.6 | ||||
| 32 | 40 | 0.6 | 27.4 | 72 | 55.5 | ||||
| 24 h | 44.4 | 31.7 | 0.9 | 23 | 76.1 | 41.65 | 42.25 | 3 | 4.83 |
| 38.6 | 23.4 | 1.5 | 36.5 | 62 | 37.74 | ||||
| 40 | 36 | 2.2 | 21.8 | 76 | 47.36 |
FIGURE 2.
GJIC competences by flow cytometry. Percent communication calculated by the formula described in Methods for different time points has been represented for the cell lines used to develop this protocol. FBM (A), BT474 (B), and reference human cancer cell line, NT8e (C). **P < 0.01.
GJIC competence analyzed using laser confocal microscopy
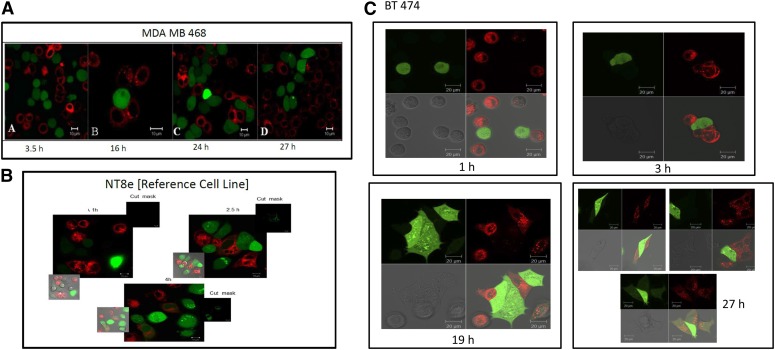
Microscopy ensures visualization of the cells and a validation of the gap junction functional competence. The assay was done as described in Methods, and live cell images of cells in culture were captured at different time points. Figure 3 is an evaluation of gap junction communication using confocal microscopy with 3 different cell lines of varied GJIC competence. The triple negative breast cancer cell line, MDA MB 468, does not show any evidence of transfer of calcein even after 27 h of plating and coculture, which is indicative of negligible GJIC function (Fig. 3A). However, the reference cancer cell line (NT8e) shows a gap junction communication with initial transfer of calcein at 2.5 h after plating and a considerable transfer at 4 h after plating under coculture conditions (Fig. 3B). The Her2 overexpressing cell line BT474 does not show transfer of calcein till the 19-h time point. At the 27-h time point, there are a few fields that show calcein transfer, which are represented in Fig. 3C.
FIGURE 3.
Evaluation of gap junction communication by confocal microscopy. The assay was carried out as described in Methods. Live cell images were captured at different time points. MDA MB 468 (A). Scale bar, 10 μm. NT8e (B). Scale bar, 10 μm. BT474 (C). Scale bar, 20 μm.
Cellular localization of CXs and E-cadherin
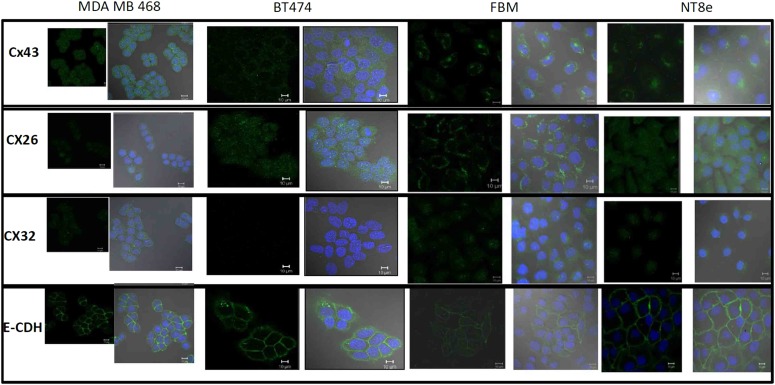
CXs are membrane proteins, and functional competence is determined by their appropriate localization on the membrane and is a primary requirement for the ability to form gap junctions.16 Figure 4 shows a comparison of the staining patterns of CX 43, 32, and 26 in the selected cell lines. In both the breast cancer cell lines, CX 43 localization is shifted from the membrane to the cytoplasm, whereas in the NT8e and FBM, CX43 is present on the plasma membrane. Similarly, CX32 and CX26 show a cytoplasmic presence in NT8e and the breast cancer cell lines. In FBM, CX26 localizes to the membrane and CX32 shows a shift to cytoplasmic localization. The integrity of the plasma membrane is established with immunostaining for E-cadherin, which is a membrane protein and is present in all the cell lines.17
FIGURE 4.
Cellular localization of CXs and E-cadherin. Comparison of the immunofluorescence staining pattern of CXs (43, 26, and 32) and E-cadherin in MDA MB 468, BT 474, FBM, and NT8e. The cells were fixed and stained with primary and secondary antibodies as described in Methods. The nuclei were stained with DAPI and coverslips were mounted on slides and acquired on the Zeiss LSM 510 Meta Microscope. Scale bars, 10 μm.
DISCUSSION
The developed assay could be selected to determine communication in cells and used to assess the transfer of size permissible therapeutics to diseased tissues and cells. Tumors have altered expression of the CX proteins, a main requisite for the formation of CX and gap junctions. Our observation with the breast cancer cell line, BT474, with the flow cytometry protocol showed a range of 10–17% communication and was commensurate with the total absence of CX 43 and CX 26 from the membrane, and it altered localization to the cytoplasm where the functional capability would be completely compromised.16 Further, CX 32 was wholly absent; however, E-cadherin localization was on the membrane, indicating a well-formed and organized plasma membrane (Fig. 4). The percent communication for the FBM cell line and the reference human cancer cell line, NT8e, was over 50%, as shown in Fig. 2A, C. In these cell lines, the membrane integrity was confirmed with the presence of E-cadherin, and CX 43 showed membrane localization in both the cell lines. Additionally, FBM also showed CX 26 presence on the membrane. Both cell lines showed a cytoplasmic localization for CX 32 (Fig. 4), which is indicative of no contribution to the GJIC function.
Furthermore, confocal images (Fig. 3) also confirmed the percent communication data obtained for the NT8e as the images showed colocalization at the early and stabilized time points. The images obtained from the breast cancer cell line indicated absence or lowered intercellular communication that was confirmed with the immunostaining (Fig. 4) for CX43, CX26, and CX32.
To elaborate further, nonfluorescent calcein-AM is transported through the plasma membrane via the gap junctions to the cytosol where it is hydrolyzed by endogenous esterases to calcein, which is fluorescent. It is quite likely that MDR1, the multidrug resistant protein depending on the level of expression, may intercept calcein-AM during its passage and actively extrude it.18 This may affect the net influx of calcein-AM and the accumulation of fluorescent calcein in the cytosol. So, the end result would be that the fluorescence signal would build up more slowly in the presence of MDR1 than its absence, and hence will not result in a complete absence of the fluorescence signal or compromise the evaluation of gap junction function. The FBM and NT8E cell lines have not been examined for MDR1, whereas BT474 has been reported to have negligible basal expression19 as opposed to MDAMB 468, which has a higher basal expression.20 The strength of this assay is that gap junction communication is determined at different time points and allows assessment and comparisons of GJIC competence in different cell types.
In summary, by combining 2 techniques and validating the integrity of the membrane, the protocol developed can provide a qualitative and quantitative assessment of intercellular gap junction function.
ACKNOWLEDGMENTS
The author thanks the Council of Scientific and Industrial Research, New Delhi, India, for the grant support (27/167/07 EMR II) toward research funding. The author declares no conflicts of interest.
REFERENCES
- 1.Solan JL, Lampe PD. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim Biophys Acta Biomembr 2018;1860:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 1990;116:163–175. [DOI] [PubMed] [Google Scholar]
- 3.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 1991;273:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 2003;83:1359–1400. [DOI] [PubMed] [Google Scholar]
- 5.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 2007;94:120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedner P, Steinhäuser C, Theis M. Functional redundancy and compensation among members of gap junction protein families? Biochim Biophys Acta 2012;1818:1971–1984. [DOI] [PubMed] [Google Scholar]
- 7.Skerrett IM, Williams JB. A structural and functional comparison of gap junction channels composed of connexins and innexins. Dev Neurobiol 2017;77:522–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araya-Secchi R, Perez-Acle T, Kang SG, et al. Characterization of a novel water pocket inside the human Cx26 hemichannel structure. Biophys J 2014;107:599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Siragam V, Chen J, Fridman MD, Hamilton RM, Sun Y. High-throughput measurement of gap junctional intercellular communication. Am J Physiol Heart Circ Physiol 2014;306:H1708–H1713. [DOI] [PubMed] [Google Scholar]
- 10.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 2008;131:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche JM, Chang HC, Weber PA, Nicholson BJ. A transient diffusion model yields unitary gap junctional permeabilities from images of cell-to-cell fluorescent dye transfer between Xenopus oocytes. Biophys J 2004;86:2058–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang T, Shao Q, MacDonald A, et al. Autosomal recessive GJA1 (Cx43) gene mutations cause oculodentodigital dysplasia by distinct mechanisms. J Cell Sci 2013;126:2857–2866. [DOI] [PubMed] [Google Scholar]
- 13.Raul U, Sawant S, Dange P, Kalraiya R, Ingle A, Vaidya M. Implications of cytokeratin 8/18 filament formation in stratified epithelial cells: induction of transformed phenotype. Int J Cancer 2004;111:662–668. [DOI] [PubMed] [Google Scholar]
- 14.Mulherkar R, Goud AP, Wagle AS, et al. Establishment of a human squamous cell carcinoma cell line of the upper aero-digestive tract. Cancer Lett 1997;118:115–121. [DOI] [PubMed] [Google Scholar]
- 15.Tomasetto C, Neveu MJ, Daley J, Horan PK, Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol 1993;122:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips SL, Williams CB, Zambrano JN, Williams CJ, Yeh ES. Connexin 43 in the development and progression of breast cancer: what’s the connection? (Review). Int J Oncol 2017;51:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 2008;36:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 2018;18:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Wang Y, Deosarkar S, Soroush F, Kiani MF, Wang B. Fast, stable induction of P-glycoprotein-mediated drug resistance in BT-474 breast cancer cells by stable transfection of ABCB1 gene. Anticancer Res 2015;35:2531–2538. [PubMed] [Google Scholar]
- 20.Thomas NK, Brown TJ. ABC transporters do not contribute to extracellular translocation of hyaluronan in human breast cancer in vitro. Exp Cell Res 2010;316:1241–1253. [DOI] [PubMed] [Google Scholar]