Abstract
Background
In living donor liver transplantation (LDLT), 2 patients undergo surgery, and the advantages and disadvantages for both patients should be considered. This study evaluated the long-term quality of life in living liver donors, and its impact on their activities of daily living focusing on mood and mental health.
Material/Methods
In total, 101 living liver donors (69 female and 32 male patients, median age of 36.8 years) were surveyed at a median time of 61.8 months after liver donation (range 7–169 months). The generic Short Form Health Survey (SF-36), the Patient Health Questionnaire 9 (PHQ-9), and the Questionnaire of Physical Activity (IPAQ) were used. The results of SF-36 were compared to a matched control group (n=72) using the Wilcoxon test; the SF-36, the PHQ-9, and the IPAQ scores were analyzed using Spearman’s rank correlation. Linear regression model was used to check for dependencies between variables of interest. The IPAQ results were compared between the study group and the general Polish population.
Results
There were no significant differences in the SF-36 domains between the study group and control group except body pain, which was higher in the living liver donor group (P<0.05). In 30.6% of patients, the PHQ-9 survey revealed mood disturbances. The PHQ-9 scores were higher in female-donors (P<0.05). Both summary scores of the SF-36 correlated to the PHQ-9 (P<0.001). In 89.1% of patients, physical activity was below the population norm and was lower in female donors than in male donors (P<0.01).
Conclusions
LDLT had no impact on donors’ physical and mental health. Physical activity of living liver donors was lower than that of the general population. The SF-36 and the IPAQ measures seem to be reliable in the care of living liver donors. The PHQ-9 survey results and the inclination to depression of female living liver donors requires further study.
MeSH Keywords: Liver Transplantation, Living Donors, Motor Activity, Quality of Life
Background
Living donor liver transplantation (LDLT) is an option for patients with end-stage liver disease. Due to the scarcity of cadaveric organs, this option is becoming more popular worldwide [1,2]. Until the end of 2014, 1553 cadaveric liver transplantations and 260 LDLT were performed in the Department of General, Transplant and Liver Surgery of Medical University of Warsaw. The number of LDLT has been increasing every year. In Poland, LDLT is performed only from adult to child, and the donor and recipient must be related.
Unlike in cadaveric transplantation, in LDLT, 2 patients undergo surgery. Therefore, we must consider the risks and benefits for both patients. Reduced pre-transplantation waiting time and better match of the organ are obvious benefits for the recipient; reduced cost of treating a patient with liver failure is the benefit for the society [1,3]. On the other hand, a completely healthy donor is subjected to the risk of morbidity. According to the literature, only 10% of donors have physical symptoms for 1 year after the surgery; morbidity rates ranges from 8.8–23.2% and mortality rates from 0.2–0.9% [2–5].
Despite the increasing trend towards LDLT, data regarding quality of life of living liver donors is scant. The aim of this study was to evaluate the long-term quality of life in living liver donors, and the influence of this procedure on their professional, social, and physical activity. Since this is the first study of its kind in Poland, we also focused on their mental health.
Material and Methods
Patients
The analyzed cohort consisted of 101 living liver donors (69 females and 32 males) who underwent the surgery for LDLT between 1999 and 2014 in the Department of General Transplant and Liver Surgery of Medical University of Warsaw. Inclusion criteria were a period of more than 6 months between donation and assessment. A structured donor screening protocol included basic cardio-pulmonary assessment, computed tomography (CT)-based liver volumetry, followed with cholangiography via cystic duct with subsequent cholecystectomy was introduced in every living liver donor. The median age of patients was 36.8 years (range 24 to 59 years). Median time after donation was 61.8 months (range 7 to 169 months). Among all donors, 86 donors (85.1%) were parents of the recipient, while 7 donors (6.9%) were their extended family. Eighty donors (79.2%) of the analyzed individuals were in a relationship, 12 (11.8%) were single, and 2 (1.9%) were widowers. With respect to education, 55.4% (56 donors) had finished their education in college, 35.6% (36 donors) in university and 7.9% (8 donors) in primary school.
Seventy-two healthy volunteers, age and gender matched, were enrolled into the study as the control group.
Quality of life assessment
For the assessment of health-related quality of life (HRQoL), we used structured questionnaires about personal life of living liver donors: International Physical Activity Questionnaire (IPAQ), the generic Short Form Health Survey (SF-36), and the Patient Health Questionnaire 9 (PHQ-9). The questionnaires were sent to the donors by mail. The SF-36 is a widely validated generic questionnaire consisting of 36 items that cover questions related to physical health (domains: physical functioning, role limitations due to physical problems, body pain, and general health) and mental health (domains: vitality, social functioning, role limitations due to emotional problems and mental health). There are also 2 summary scores, the Physical Component Score (PCS) and Mental Component Score (MCS), which are scored from 0 to 100 points, with higher scores indicating better HRQoL [6]. The licensed approval certificate (CT132326/OP012559) for the use of the SF-36 questionnaire was obtained for this study.
Physical activity was assessed by the Polish version of the IPAQ. The instrument was established for monitoring levels of physical activity of an adult population and was used in previous published studies with surgical patients [7,8]. It was developed for surveillance activities, and to guide policy development related to health-enhancing physical activity across various domains of life. The full version of the IPAQ investigates 4 physical activity domains (chores, work, leisure, and transport), as well as time spent sitting, as a marker for sedentary behavior. Physical activity is described as a constant score by domain and intensity of physical activity; the following formula was used to calculate its expenditure: number of days spent doing the activity × average duration of the activity per day × energy cost of the activity. The energy cost of an activity was expressed in MET (metabolic equivalent task). The scoring protocol established the following MET values: 3.3 for walking, 4.0 for moderate intensity physical activity, 6.0 for cycling, and 8.0 for vigorous physical activity [9]. The IPAQ questionnaire has been validated in Poland and many other countries [9,10].
The PHQ is a family of short, self-administered screener for common mental health problems found in primary care medical setting, stemming from PRIME-MD (The Primary Care Evaluation of Mental Disorders), and is based on DSM-IV diagnostic system [11]. The Brief-PHQ is a shortened version comprised of PHQ-9, which is a 9-item tool for screening depressive disorders, a panic disorder screening module, and items related to stressors and women‘s health issues. The depression module (PHQ-9) has been validated as a separate tool and as a part of the full PHQ for depression screening and severity assessment [12,13]. Polish validation was published by Tomaszewski et al. [14]. The PHQ-9 consists of 9 items scored 0, 1, 2, and 3, to the response categories from “not at all” to “nearly every day”, with overall scores ranging from 0 to 27. The PHQ-9 allows classic screening based on DSM-IV diagnostic algorithm for depression (the screening is positive if sadness or anhedonia is present, and at least 5 depressive symptoms occur together). Alternatively, total severity scores of 5, 10, 15, and 20 represent cut-offs for positive screening of mild, moderate, moderately severe, and severe depression respectively, although for practical use, positive screening cut-off of >10 is suggested, with sensitivity of 99% and specificity of 94% [13,15]. For intermediate results (6–10) repeating the screening procedure is encouraged.
Ethics
The study protocol was approved by the Local Ethical Committee of Medical University of Warsaw (AKBE/168/16), and the study was performed in accordance with the ethical standards established by the 1975 Declaration of Helsinki (6th revision, 2008).
Statistics
Statistical analyses were performed using MATLAB R2012a (The MathWorks, Inc., Natick, MA, USA). Descriptive statistics (median, range, means, and standard deviation) were calculated for all quantitative variables; percentages and frequencies were generated for qualitative variables. The Wilcoxon signed-rank test was performed to compare the SF-36 results in the analyzed and control groups. The SF-36, the PHQ, and the IPAQ scores were analyzed using Spearman’s rank correlation coefficients. The IPAQ results were compared in the analyzed group with previously reported results for Polish society. A probability (P) value less than 0.05 was considered significant. Additionally, a linear regression model was used to check for dependencies between variables of interest.
Results
Demographic characteristics of the analyzed group are summarized in Table 1. In the study group, there were no fatal or severe complication after liver donation that would have required reoperation or blood transfusion. The total complication rate for the procedure in our department has been established at around 4.5%, with the major complication wound infections.
Table 1.
Demographic characteristic of analyzed and control groups.
| Females | Males | |||
|---|---|---|---|---|
| Study group (n=69) | Control group (n=49) | Study group (n=32) | Control group (n=23) | |
| Age (mean, years) | 35.59 | 36.22 | 39.36 | 37.39 |
| SD | ±5.85 | ±7.09 | ±7.89 | ±8.79 |
| Range (years) | 24–51 | 23–51 | 27–59 | 27–57 |
| Females – study group (n=69) | Males – study group (n=32) | |||
| Role of donor | ||||
| Parents | 58 | 28 | ||
| Extended family | 5 | 2 | ||
| No data | 6 | 2 | ||
| Education | ||||
| University | 26 | 10 | ||
| College | 37 | 19 | ||
| Primary School | 5 | 3 | ||
| No data | 1 | 0 | ||
| Employment | ||||
| Full-time | 20 | 23 | ||
| Half-time | 2 | 2 | ||
| Childcare | 21 | 0 | ||
| Unemployed | 5 | 1 | ||
| Pension | 3 | 0 | ||
| No data | 18 | 6 | ||
| Marital status | ||||
| In relationship | 54 | 26 | ||
| Single | 9 | 3 | ||
| Widower | 1 | 1 | ||
| No data | 5 | 2 | ||
| Months after donation | 61.62 | 62.31 | ||
| SD (±) | 38.32 | 42.65 | ||
| Range | 7–169 | 7–162 | ||
SD – standard deviation.
SF-36
Domains were comparable between female and male liver donors. Results of the SF-36 in the study group and control group are presented in Figure 1, detailed data is presented in Table 2. Body Pain (BP) was lower in the study cohort than in the control group (P<0.05), and it was significantly lower in male patients in the study group compared to healthy male participants (P<0.05).
Figure 1.
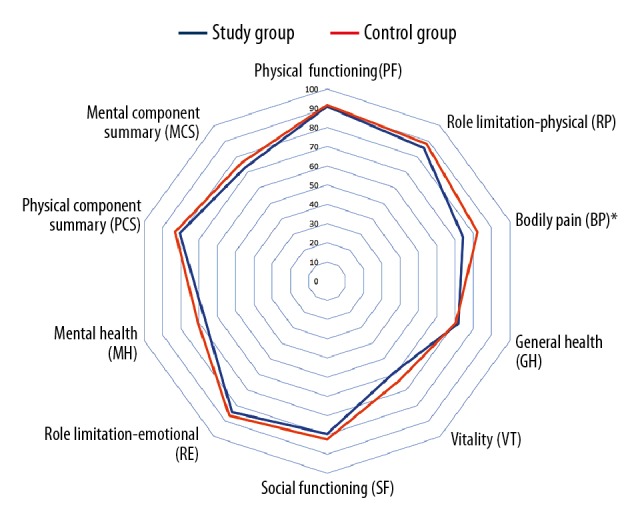
Health-related quality of life estimated by SF-36. Detailed explanations: Results of SF-36 measure in the study and control groups. P value for Wilcoxon test. * P<0.05.
Table 2.
Results of SF-36 measure in study and control groups; P value for Wilcoxon test.
| SF-36 | Study group (n=101) | Control group (n=72) | P value |
|---|---|---|---|
| Physical functioning (PF) | 90.9±15.1 | 91.6±18.3 | 0.613 |
| Role limitation-physical (RP) | 85.5±29.3 | 88.2±26.5 | 0.625 |
| Bodily pain (BP) | 74.3±25.0 | 82.3±23.3 | 0.021 |
| General health (GH) | 71.7±18.4 | 70.4±16.5 | 0.568 |
| Vitality (VT) | 59.0±17.9 | 63.9±17.6 | 0.067 |
| Social functioning (SF) | 79.6±22.4 | 82.5±19.4 | 0.555 |
| Role limitation-emotional (RE) | 84.2±31.5 | 86.6±26.6 | 0.776 |
| Mental health (MH) | 66.5±19.3 | 70.6±18.9 | 0.144 |
| Physical component summary (PCS) | 80.6±17.0 | 83.1±14.5 | 0.427 |
| Mental component summary (MCS) | 72.8±19.2 | 75.9±17.4 | 0.324 |
All data shown as mean ±SD.
In the linear regression analysis, both Physical Component Summary (PCS) and Mental Component Summary (MCS) decreased with age (P<0.01 and P<0.05 respectively), and MCS was significantly lower in female in the study group (P<0.01) (Table 3).
Table 3.
Dependencies between results by questioners and sex, age and time from donation by linear regression analysis.
| SF-36: PCS (study) | SF-36: MCS (study) | SF-36: PCS (control) | SF-36: MCS (control) | PHQ-9 (study) | IPAQ (study) | |
|---|---|---|---|---|---|---|
| Const. | 113.183** (p<0.001) | 98.886** (p<0.001) | 91.247** (p<0.001) | 83.153** (p<0.001) | −0.436 n.s. |
451.703** (p<0.01) |
| SEX | −4.775 n.s. |
−9.233** (p<0.01) | −9.428* (p<0.05) | −10.977* (p<0.05) | 2.109* (p<0.05) | −168.195** (p<0.01) |
| AGE | −0.802** (p<0.01) | −0.516* (p<0.05) | −0.030 n.s. |
−0.043 n.s. |
0.101 n.s. |
−1.572 n.s. |
| TIME | 0.012 n.s. |
−0.008 n.s. |
– | – | 0.001 n.s. |
0.145 n.s. |
| R2 | 0.098 | 0.071 | 0.094 | 0.100 | 0.046 | 0.105 |
Indicates significance at 5% confidence level,
at 1% confidence level. n.s. – not significant.
PHQ-9
In 31 patients (30.6%), the PHQ-9 survey revealed mood disturbance (83.9% of this group were parents of the recipient), which required further investigation. Data are shown in Figure 2 and Table 4. The PHQ-9 scores were higher in females in linear regression analysis (P<0.05) (Table 3). Both summary scores of the SF-36 were correlated strongly to the PHQ-9 (P<0.001), but there was no correlation between the PHQ-9 tool and the IPAQ questionnaire results (Table 5).
Figure 2.
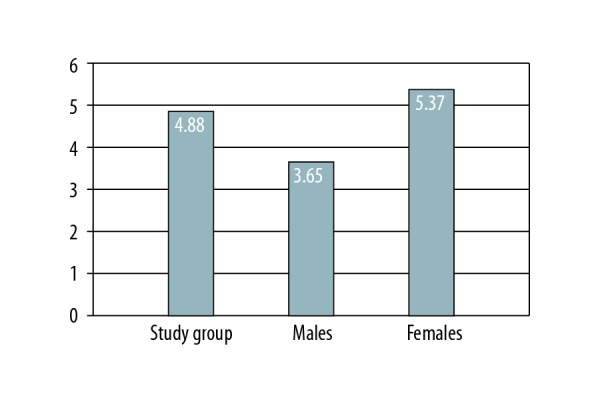
PHQ-9 survey results in analyzed cohort. Detailed explanations: average PHQ-9 scores in the study group. PHQ-9 were higher in females (P<0.05) by linear regression analysis.
Table 4.
Severity of depression among the study group – PHQ-9 results.
| Severity of depression | No signs | Mild | Moderate | Moderately severe | Severe |
|---|---|---|---|---|---|
| Number of patients | 70 | 18 | 7 | 3 | 3 |
| Parents of receipient | 59 | 13 | 7 | 3 | 3 |
Table 5.
Correlation between scores from various questioners; P-value for Spearman correlation.
| SF-36: PCS | SF-36: MCS | PHQ-9 | |
|---|---|---|---|
| SF-36: PCS | – | 0.616 (p<0.001) | −0.516 (p<0.001) |
| SF-36: MCS | 0.616 (p<0.001) | – | −0.746 (p<0.001) |
| PHQ-9 | −0.516 (p<0.001) | −0.746 (p<0.001) | – |
| IPAQ | n.s. | n.s. | n.s. |
n.s. – not significant.
IPAQ
In 90 patients (89.1%), physical activity was below 600 METs, which was lower than the population norm. There was no correlation between the IPAQ questionnaire and both domains of the SF-36 measure and the PHQ-9 tool (Table 5). Linear regression analysis showed that in the study group, physical activity was lower in female donors (P<0.01) than in male donors (Table 3).
Discussion
This study is the first attempt to evaluate wider aspects of health-related quality of life (HRQoL) in general, with special attention to mental health and mood disturbances, as well as physical activity of living liver donors. In this single-center study, we used the SF-36, the IPAQ and the PHQ-9 questionnaires, validated in the Polish population, to evaluate the impact of donation of a part of the liver on some aspects of a donor’s life.
Medical complications affecting donors have been well characterized in some studies [16]. In a review of 19 studies, Parikh et al. concluded that there was lack of long-term data on donor quality of life after LDLT [17]. Since then, numerous papers have been published concerning quality of life [2–5,18–20]. However, in most of these studies, the SF-36 questionnaire was used and the median time after LDLT was 1–3 years. Fukada et al. suggested that very little is known about the role of psychosocial aspects of this procedure on the donors’ well-being [18]. Takeda et al. stated that for living donors, the potential endpoint of donation is primarily psychological, because no medical benefit is gained for donors after LDLT [20]. Using not only the SF-36, but also the PHQ-9 and the IPAQ, we checked wider aspects of the impact of donation on donors’ daily activities and their mental health after the procedure. In this study, we found no significant differences in the social and mental health between donors and matched healthy volunteers. However, we noticed lower body pain in the surveyed group than in the control group, at a much longer median time after the procedure, than previously reported. Some papers revealed that body pain and others physical symptoms decrease after LDLT but may recur in 1–2 years [21]. The Physical Component Score (the PCS section of the SF-36) decreased immediately after the liver donation, and then returned to the baseline within 6 to 12 months, while the Mental Component Summary (the MCS section of the SF-36) remained comparable to that of normative population throughout the procedure [22]. Most living donors maintained above average HRQoL up to 11 years post-operatively, although predictors of poor PCS and MCS scores included recipient’s death within 2 years preceding the survey and an education level less than a bachelor’s degree [19]. Living liver donors experienced increased self-esteem, empowerment, and community awareness, but also complained of postoperative pain and a lack of emotional support in a meta-analysis of 23 studies by Thys et al. [23].
Our study revealed that 30.6% of donors had signs of depression and 12.8% had symptoms of moderate to severe depression. Our findings could be explained by the fact that in Poland, LDLT is possible only from an adult to a child and 85% of donors were parents of the recipients. Fukada et al. showed that there is a correlation between donors’ daily activity and relationship with recipient (as parents) [18]. There are no clear data regarding the prevalence of depression in living liver donor population and the impact of the long-term results of the procedure on recipients. However, Kimura et al. found some psychiatric issues in 4.2% of living liver donors [24]. Neither mental health quality of life nor depression showed significant changes across time after the liver donation, while anxiety was reported to decrease in a study by Kroencke et al. Adult to pediatric donors experienced more preoperative psychological strain, which improved after donation; and 1 to 2 years after donation, quality of life was not substantially impaired in a majority of the donors [25]. Studies have shown that a successful surgery can stabilize donor mentality, and 3 months after surgery, donors demonstrated better mental health quality of life, with a reduction in anxiety [26–28]. These aspects of HRQoL, and the impact of recipient outcome on donor HRQoL were not studied in detail in our report, which is its limitations. However, further studies are required to determine the sensitivity and specificity of the screener PHQ-9 in detecting depression according to DSM-IV diagnostic algorithm for depression in liver donors, as well as the impact of recipient condition on the PHQ-9 results. Interestingly, the PHQ-9 scoring in our study was higher in female liver donors.
No association was found between the PHQ-9 and the IPAQ measure results. The positive association between physical activity and better HRQoL was previously described in different populations. Physical activity was associated with a lower prevalence of depressive symptoms in cardiac patients and up to 40% of patients, who reported low physical activity, were depressed after cardiac surgery in a study by Horne et al. [29]. Surprisingly, the IPAQ results showed that the group of liver donors was much less physically active, compared to the Polish population. These results were contrary to the donors’ attentiveness towards their health. Efforts should be made to educate donors, and special attention should be paid to their pro-health behaviors.
In conclusion, our research clearly indicated that LDLT donors can endure the donation procedure and return to their normal daily life without major problems. There was no significant effect on the mental status and the procedure had only a slight impact on social activity; these results were in line with other papers that have reported that HRQoL of LDLT donor groups was comparable to healthy control groups [30–35]. However, donors were far less physically active, and physical domains of the SF-36 tool were mostly decreased. The SF-36 and the IPAQ were found to be very helpful and reliable in the holistic care of living liver donors; however, the role of the PHQ9, which meets the criteria for screening of depression, requires further study in this population.
Conclusions
Liver donation had no impact on Polish living liver donors’ physical and mental aspects of quality of life as compared to healthy individuals. Interestingly, donors are much less physically active than the general population. The SF-36 generic questionnaire and the IPAQ measure seem to be helpful and reliable in the holistic care of living donors. However, the role of the PHQ-9 survey, and female donors’ tendency to depression, require further study.
Footnotes
Source of support: Departmental sources
References
- 1.Parolin MB, Lazzaretti CT, Lima JHF, et al. Donor quality of life after living donor liver transplantation. Transplant Proc. 2004;36:912–13. doi: 10.1016/j.transproceed.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 2.Toyoki Y, Ishido K, Kudo D, et al. Donor quality of life after living donor liver transplantation: Single-institute experience. Transplant Proc. 2012;44:341–43. doi: 10.1016/j.transproceed.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Kousoulas L, Emmanouilidis N, Klempnauer J, Lehner F. Living-donor liver transplantation: impact on donor’s health-related quality of life. Transplant Proc. 2011;43:3584–87. doi: 10.1016/j.transproceed.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaki M, Kaibori M, Matsui K, Kwon A. Change in donor quality of life after living donor liver transplantation surgery: A single-institution experience. Transplant Proc. 2012;44:344–46. doi: 10.1016/j.transproceed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh CB, Tsai CS, Chen TW, et al. Correlation between SF-36 and six-minute walk distance in liver donors. Transplant Proc. 2010;42:3597–99. doi: 10.1016/j.transproceed.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 7.Bond DS, Phelan S, Wolfe LG, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health related quality of life. Obesity. 2009;17:78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- 8.Kotarska K, Wunsch E, Kempińska-Podhorodecka A, et al. Factors affecting health-related quality of life and physical activity after liver transplantation for autoimmune and nonautoimmune liver diseases: a prospective, single centre study. J Immunol Res. 2014;2014 doi: 10.1155/2014/738297. 738297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 10.Biernat E, Stupnicki R, Gajewski AK. International physical activity questionnaire (IPAQ) – Polish version. Physical Education and Sport. 2007;5(1):47–54. [Google Scholar]
- 11.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:1–7. [Google Scholar]
- 14.Tomaszewski K, Zarychta M, Bieńkowska A, et al. Validation of the Patient Health Questionnaire-9 Polish version in the hospitalised elderly population. Psychiatr Pol. 2011;45:223–33. [PubMed] [Google Scholar]
- 15.Jowsey SG, Jacobs C, Gross CR, et al. Emotional well-being of living kidney donors: Findings from the RELIVE Study. Am J Transplant. 2014;14:2535–44. doi: 10.1111/ajt.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashikura Y, Ichida T, Umeshita K, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation. 2009;88:110–14. doi: 10.1097/TP.0b013e3181aaccb0. [DOI] [PubMed] [Google Scholar]
- 17.Parikh ND, Ladner D, Abecassis M, Butt Z. Quality of life for donors after living donor liver transplantation: A review of the literature. Liver Transpl. 2010;16:1352–58. doi: 10.1002/lt.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda A, Sakamoto S, Shigeta T, et al. Clinical outcomes and evaluation of the quality of life of living donors for pediatric liver transplantation: A single-center analysis of 100 donors. Transplant Proc. 2014;46:1371–76. doi: 10.1016/j.transproceed.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 19.Ladner DP, Dew MA, Forney S, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL) J Hepatol. 2015;62:346–53. doi: 10.1016/j.jhep.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takada Y, Suzukamo Y, Oike F, et al. Long-term quality of life of donors after living donor liver transplantation. Liver Transpl. 2012;18:1343–52. doi: 10.1002/lt.23509. [DOI] [PubMed] [Google Scholar]
- 21.Togashi J, Sugawara Y, Tamura S, et al. Donor quality of life after living donor liver transplantation: A prospective study. J Hepatobiliary Pancreat Sci. 2011;18(2):263–67. doi: 10.1007/s00534-010-0340-y. [DOI] [PubMed] [Google Scholar]
- 22.Xu DW, Long XD, Xia Q. A review of life quality in living donors after transplantation. Int J Clin Exp Med. 2015;8(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Thys K, Schwering KL, Siebelink M, et al. Psychosocial impact of pediatric living-donor kidney and liver transplantation of recipients, donors, and the family: A systemic review. Transpl Int. 2015;28(3):270–80. doi: 10.1111/tri.12481. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H, Onoshi Y, Sunada S, et al. Postoperative psychiatric complications in living liver donors. Transplant Proc. 2015;47(6):1860–65. doi: 10.1016/j.transproceed.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Kroencke S, Nashan B, Fischer L, et al. Donor quality of life up to two years after living donor liver transplantation: A prospective study. Transplantation. 2014;97(5):582–89. doi: 10.1097/01.TP.0000438206.04348.b2. [DOI] [PubMed] [Google Scholar]
- 26.Shibata N, Shimazaki H, Sano N, et al. Psychiatric and psychological outcomes of Japanese living donors following liver transplantation. Psychiatry Clin Neurosci. 2009;63(4):583–85. doi: 10.1111/j.1440-1819.2009.01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Schulz KH, Kroencke S, Beckmann M, et al. Mental and physical quality of life in actual living liver donors versus potential living donors: A prospective, controlled multicenter study. Liver Transpl. 2009;15(12):1676–87. doi: 10.1002/lt.21917. [DOI] [PubMed] [Google Scholar]
- 28.Dew MA, DiMartini AF, DeVito Dabbs AJ. Preventive intervention for living donor psychosocial outcomes: Feasibility and efficacy in a randomized controlled trial. Am J Transplant. 2013;13(10):2672–84. doi: 10.1111/ajt.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horne D, Kehler DS, Kaoukis G, et al. Impact of physical activity on depression after cardiac surgery. Can J Cardiol. 2013;29(12):1649–56. doi: 10.1016/j.cjca.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Trotter JF, Talamantes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transpl. 2001;7:485–93. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 31.Pascher A, Sauer IM, Walter M, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult to-adult living donor liver transplantation. Liver Transpl. 2002;8:829–37. doi: 10.1053/jlts.2002.34896. [DOI] [PubMed] [Google Scholar]
- 32.Walter M, Dammann G, Papachristou C, et al. Quality of life of living donors before and after living donor liver transplantation. Transplant Proc. 2003;35:2961–63. doi: 10.1016/j.transproceed.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Kim-Schluger L, Florman SS, Schiano T, et al. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002;73:1593–97. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 34.Basaran O, Karakayali H, Emiroglu R, et al. Donor safety and quality of life after left hepatic lobe donation in living-donor liver transplantation. Transplant Proc. 2003;35:2768–69. doi: 10.1016/j.transproceed.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 35.Beavers KL, Sandler RS, Fair JH, et al. The living donor experience: Donor health assessment and outcomes after living donor liver transplantation. Liver Transpl. 2001;7:943–47. doi: 10.1053/jlts.2001.28443. [DOI] [PubMed] [Google Scholar]


