Abstract
Background
Glioblastoma is an aggressive disease with a defined standard of care offering crucial survival benefits. Disparities in care may influence treatment decisions. This study seeks to evaluate potential patterns in care delivery using the National Cancer Database (NCDB).
Methods
We evaluated the NCDB from 1998 to 2011 for patients diagnosed with glioblastoma older than 20 years of age in order to describe current hospital-based demographics, rates of treatment modality by age, race, gender, likelihood of receiving treatment, and survival probabilities.
Results
From 1998 to 2011, 100672 patients were diagnosed with glioblastoma in the United States. Of these, 54% were younger than 65 years of age, while 20% were 75 years of age or older. The most common type of treatment was surgery (73%), followed by radiation (69%) and chemotherapy (50%). Eleven percent of patients did not receive any form of therapy. Patients receiving no form of treatment were more likely to be older, female, black, or Hispanic. Tumors that did not involve brainstem, ventricles, or the cerebellum were associated with more aggressive treatment and better overall survival. The median survival was 7.5 months. The use of concomitant surgical resection, chemotherapy, and radiation demonstrated greater survival benefit.
Conclusions
Median survival for glioblastoma is significantly less than reported in clinical trials. Sociodemographic factors such as age, gender, race, and socioeconomic status affect treatment decisions for glioblastoma. The elderly are greatly undertreated, as many elderly patients receive no treatment or significantly less than standard of care.
Keywords: Glioblastoma, treatment, survival, patterns of care
Glioblastoma is the most common malignant primary brain tumor and affects over 12000 cases projected annually in the United States.1 It is an infiltrative tumor known to spread along axonal pathways, and cannot be effectively treated by surgery alone.2 Improvements in survival have been demonstrated with receipt of surgery, concurrent chemoradiation, and adjuvant temozolomide; as demonstrated by the European Organization for Research and Treatment of Cancer (EORTC) 22981/26981 and National Cancer Institute of Canada Clinical Trials group (NCIC) CE.3 randomized trial.3 This approach achieved a 14.6-month survival, setting the standard of care.3 Survival is now reported to be greater than 20 months in clinical trials.3–7
Inequalities in treatment delivery have been described in different types of cancer,8–10 due to disparities in receiving optimal and timely medical care. Optimal treatment may not be widely accessible and factors such as race, gender, education level, insurance, and median income may have a higher role than expected in treatment decision making. Evidence from single center experiences have reported inequalities in access to established neuro-oncology centers based on sociodemographic factors.11–13 Our study offers an analysis of the largest clinical cancer registry, the American College of Surgeons National Cancer Database (NCDB), to describe current patterns of care and their influence in survival in patients with glioblastoma.
Methods
We queried the NCDB, from the American College of Surgeons and the American Cancer Society.14 NCDB gathers extensive and retrospective data of newly diagnosed cancer cases in the U.S. from over 1500 accredited hospitals. A total of 100672 glioblastoma patients who were 20 years or older were identified from 1998 to 2011. Glioblastoma was defined with the International Classification of Diseases for Oncology (ICD-O-3) histology/behavior codes 9440/3, 9441/3, and 9442/3. Primary tumor site was limited to C70.0-C72.9, C75.1-C75.3, and was reported as: cerebrum (C71.0) and brain lobes (C71.1, C71.2, C71.3, C71.4), brain stem (C71.7), cerebellum (C71.6) and ventricles (C71.5), and others (71.8–71.9). Data were abstracted according to the Facility Oncology Registry Data Standards (FORDS) manual. Descriptive statistics were reported overall, and stratified arbitrarily by age groups (20–49, 50–64, 65–74, ≥75) using frequencies for categorical variables. Patients were classified in a particular treatment modality group if they received that therapy during their first course of treatment.
The insurance variable was determined at the time of diagnosis and/or treatment. Median household income and education status were derived from the 2000 U.S. Census data based on the patient’s residential zip code at the time of diagnosis.15 The education variable refers to the number of adults who did not graduate from high school and was divided in ≥ 29%, 20–28.9%, 14–19.9%, and ≤14%. Region was defined by comparing the patient’s residential state and county Federal Information Processing Standard (FIPS) code at diagnosis to the 2003 files Rural-Urban Continuum Codes as developed by the U.S. Department of Agriculture Economic Research Service. Counties with a population size larger than 250000 were defined as metropolitan, population size from 2500 to 250000 was designated as urban region, and less than 2500 as rural region.
Logistic regression was performed for factors that influence treatment receipt. Statistically significant variables in univariate models were included in the regression models. Backward model selection was performed to identify critical variables with lower values of Akaike Information Criterion (AIC). Due to missing covariates, 9463 cases were excluded from the treatment combination logistic regression model. Tumor size was removed from the analysis due to the great number of missing values.
Over 10% of data were missing in the covariates of race, Hispanic heritage, and dwelling region, as well as treatment outcomes of radiation, chemotherapy, and surgery status. We employed 2 methods for handling missing data, including all covariates, and made comparison between both methods to reduce potential bias when fitting the logistic regression and proportional hazard regression models. The methods included: the conventional approach, which is based on treating missing information as an unknown group for each covariate and the multiple imputation approach with fully conditional specification. There was no statistically significant difference in values of the parameter estimates between models and the decision was made to report the results from the conventional approach.
The definition of survival was the interval of time from diagnosis until death due to all causes. Data for survival analysis in this article were available from 1998 to 2006 due to the 5-year NCDB lag in collecting and reporting survival and follow-up data (n = 61346). We analyzed treatment delivery and survival in 2 time periods, from 1998 to 2004 and after 2005, due to the acknowledgment of the standard of care in 2005 by Stupp et al,3 who demonstrated the benefits of temozolomide as concomitant and adjuvant treatment to radiotherapy. Kaplan-Meier estimates of overall survival were calculated. Cox proportional hazards models were employed to assess the risk of mortality according to receiving treatment while adjusting for other potential risk factors. All analyses were performed with SAS software package version 9.4 for Microsoft Windows on x64 (SAS Statistical Institute, Cary, NC).
Results
From 1998 through 2011, a total of 100672 subjects aged 20 years and older diagnosed with glioblastoma were included in the NCDB (Table 1). Almost 54% of patients (n = 54142) were younger than 65 years of age, while 20% were 75 years of age or older (n = 20414). As expected, males were more prevalent than females (57% vs. 43%), and the vast majority of patients were white. Only 5% of cases were noted to be patients of Hispanic heritage. Forty-five percent of patients had private insurance at the time of diagnosis, while 42% of patients had Medicare. Seventy-three percent of patients younger than 65 years of age had private insurance, while 81% of those aged 65 years and older were insured under Medicare. Those with no insurance tended to be younger (6.8% in those under 65 years versus 0.9% in those aged 65 years and older). The majority of patients lived in a county with a median income of $46000 or more and with a low percentage of people who did not graduate from high school. Three-quarters lived in a metropolitan area.
Table 1.
Demographic characteristics of adults diagnosed with glioblastoma in the National Cancer Database, 1998–2011
| 20–49 y/o N = 16336 |
50–64 y/o N = 37806 |
65–74 y/o N = 26116 |
≥ 75 y/o N = 20414 |
Total N = 100672 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Sex | Male | 10268 | 62.9 | 22634 | 59.9 | 14594 | 55.9 | 9779 | 47.9 | 57275 | 56.9 |
| Female | 6068 | 37.1 | 15172 | 40.1 | 11522 | 44.1 | 10635 | 52.1 | 43397 | 43.1 | |
| Race | White | 14282 | 87.4 | 34328 | 90.8 | 24061 | 92.1 | 19132 | 93.7 | 91803 | 91.2 |
| Black | 1259 | 7.7 | 2167 | 5.7 | 1194 | 4.6 | 715 | 3.5 | 5335 | 5.3 | |
| Others | 591 | 3.6 | 879 | 2.3 | 563 | 2.2 | 342 | 1.7 | 2375 | 2.4 | |
| Unknown | 204 | 1.3 | 432 | 1.1 | 298 | 1.1 | 225 | 1.1 | 1159 | 1.2 | |
| Hispanic | Yes | 1209 | 7.4 | 1866 | 4.9 | 1122 | 4.3 | 665 | 3.3 | 4862 | 4.8 |
| No | 13996 | 85.7 | 33019 | 87.3 | 22909 | 87.7 | 18022 | 88.3 | 87946 | 87.4 | |
| Unknown | 1131 | 6.9 | 2921 | 7.7 | 2085 | 8.0 | 1727 | 8.5 | 7864 | 7.8 | |
| Insurance | None | 1343 | 8.2 | 2347 | 6.2 | 288 | 1.1 | 137 | 0.7 | 4115 | 4.1 |
| Private | 11455 | 70.1 | 27863 | 73.7 | 4241 | 16.2 | 2167 | 10.6 | 45726 | 45.4 | |
| Medicaid | 1946 | 11.9 | 2660 | 7.0 | 376 | 1.4 | 193 | 0. | 5175 | 5.1 | |
| Medicare | 726 | 4.4 | 3457 | 9.1 | 20399 | 78.1 | 17365 | 85.1 | 41947 | 41.7 | |
| Unknown | 566 | 5.3 | 1479 | 3.9 | 812 | 3.1 | 552 | 2.7 | 3709 | 3.7 | |
| Income | <$30000 | 1808 | 11.1 | 4328 | 11.5 | 3156 | 12.1 | 2456 | 12.0 | 11748 | 11.7 |
| $30000–$34999 | 2597 | 15.9 | 6433 | 17.0 | 4831 | 18.5 | 3855 | 18.9 | 17716 | 17.6 | |
| $35000–$45999 | 4227 | 25.9 | 9807 | 25.9 | 7021 | 26.9 | 5589 | 27.4 | 26644 | 26.5 | |
| ≥ $46000 | 6591 | 40.4 | 14973 | 39.6 | 9610 | 36.8 | 7421 | 36.4 | 38595 | 38.3 | |
| Unknown | 1113 | 6.8 | 2265 | 6.0 | 1498 | 5.7 | 1093 | 5.4 | 5969 | 5.9 | |
| Educationa | ≥29% | 2545 | 15.6 | 5516 | 14.6 | 3844 | 14.7 | 2833 | 13.9 | 14738 | 14.6 |
| 20–28.9% | 3339 | 20.4 | 8023 | 21.2 | 5722 | 21.9 | 4521 | 22.2 | 21605 | 21.5 | |
| 14–19.9% | 3539 | 21.7 | 8381 | 22.2 | 6140 | 23.5 | 4903 | 24.0 | 22963 | 22.8 | |
| ≤14% | 5798 | 35.5 | 13618 | 36.0 | 8906 | 34.1 | 7064 | 34.6 | 35386 | 35.2 | |
| Unknown | 1115 | 6.8 | 2268 | 6.0 | 1504 | 5.8 | 1093 | 5.4 | 5980 | 5.9 | |
| Region | Metropolitan | 12574 | 77.0 | 28413 | 75.2 | 19105 | 73.2 | 15275 | 74.8 | 75367 | 74.9 |
| Urban/rural | 2623 | 16.1 | 7024 | 18.6 | 5347 | 20.5 | 3851 | 18.9 | 18845 | 18.7 | |
| Unknown | 1139 | 7.0 | 2369 | 6.3 | 1664 | 6.4 | 1288 | 6.3 | 6460 | 6.4 | |
| Primary site | Cerebrum/Brain lobes | 12265 | 75.1 | 28381 | 75.1 | 19487 | 74.6 | 14896 | 73 | 75029 | 74.6 |
| Ventricle/cerebellum/ brainstem |
435 | 2.7 | 471 | 1.3 | 252 | 1.0 | 218 | 1.1 | 1376 | 1.4 | |
| Others | 3636 | 22.3 | 8954 | 23.7 | 6377 | 24.4 | 5300 | 26.0 | 24267 | 24.1 | |
| Histology | GBM, NOS | 15716 | 96.2 | 36724 | 97.1 | 25447 | 97.4 | 20025 | 98.1 | 97912 | 97.3 |
| Giant Cell GBM | 233 | 1.4 | 254 | 0.7 | 156 | 0.6 | 76 | 0.4 | 719 | 0.7 | |
| Gliosarcoma | 387 | 2.4 | 828 | 2.2 | 513 | 2.0 | 313 | 1.5 | 2041 | 2.0 | |
| CT | Yes | 10577 | 64.8 | 22698 | 60.0 | 12111 | 46.4 | 5174 | 25.4 | 50560 | 50.2 |
| No | 5277 | 32.3 | 13910 | 36.8 | 13208 | 50.6 | 14721 | 72.1 | 47116 | 46.8 | |
| Unknown | 482 | 3.0 | 1198 | 3.2 | 797 | 3.1 | 519 | 2.5 | 2996 | 3.0 | |
| RT | Yes | 12844 | 78.6 | 28935 | 76.5 | 17892 | 68.5 | 9936 | 48.7 | 69607 | 69.1 |
| No | 3214 | 19.7 | 7878 | 20.8 | 7180 | 27.5 | 8954 | 43.9 | 27226 | 27.0 | |
| Refused | 175 | 1.1 | 743 | 2.0 | 903 | 3.5 | 1431 | 7.0 | 3252 | 3.2 | |
| Unknown | 103 | 0.63 | 250 | 0.66 | 141 | 0.54 | 93 | 0.46 | 587 | 0.6 | |
| Combination | RT+CT+S | 8607 | 52.7 | 17879 | 47.3 | 9096 | 34.8 | 3447 | 16.9 | 39029 | 38.8 |
| RT+CT | 1407 | 8.6 | 3615 | 9.6 | 2267 | 8.7 | 1209 | 5.9 | 8498 | 8.4 | |
| RT+S | 2114 | 12.9 | 5264 | 13.9 | 4299 | 16.5 | 2884 | 14.1 | 14561 | 14.5 | |
| Surgery only | 1867 | 11.4 | 458 | 12.1 | 4219 | 16.2 | 4208 | 20.6 | 14882 | 14.8 | |
| None | 754 | 4.6 | 2280 | 6.0 | 2685 | 10.3 | 5344 | 26.2 | 11063 | 11.0 | |
| Others | 1587 | 9.7 | 4180 | 11.1 | 3550 | 13.6 | 3322 | 16.3 | 12639 | 12.6 | |
| Surgery | Total/Gross/Partial | 13481 | 82.5 | 29742 | 78.7 | 18854 | 72.2 | 11333 | 55.5 | 73410 | 72.9 |
| Biopsy only | 1465 | 9.0 | 4442 | 11.8 | 3537 | 13.5 | 3296 | 16.2 | 12740 | 12.7 | |
| None | 1379 | 8.4 | 3601 | 9.5 | 3698 | 14.2 | 5759 | 28.2 | 14437 | 14.3 | |
| Unknown | 11 | 0.1 | 21 | 0.1 | 27 | 0.1 | 26 | 0.1 | 85 | 0.1 | |
CT, chemotherapy; GBM, glioblastoma; NOS, not otherwise specified; RT, radiotherapy; S, Surgery; y/o: years old
aEducation refers to the percentage of non-High school graduates in the patient’s residential zip code.
Diagnoses of giant cell glioblastoma and gliosarcoma were both rare (0.7% and 2%, respectively) compared to a diagnosis of glioblastoma, not otherwise specified (NOS). Diagnosis was confirmed by pathology in 91.75% of the patients, was based on imaging modalities in 4.81%, and was diagnosed by direct visualization without microscopic confirmation in 0.35% of the patients. Of patients who did not receive treatment, 61.82% underwent biopsy and the rest were diagnosed by clinical and imaging suspicion.
The primary site was mostly cerebrum/brain lobes (74.6%), followed by others (24.1%), and ventricle/cerebellum/brain stem (1.4%). As first course of treatment, only 50% of cases received chemotherapy, 69% received radiation therapy, and 73% underwent surgery. Eleven percent of all patients did not receive any form of therapy. Major differences were found across age groups in treatment frequencies; this was notable among elderly patients. In those aged 75 years and older, only 17% received standard therapy, while 44% did not receive radiation, 72% did not receive chemotherapy, 44% did not receive surgery or biopsy, and 26% did not receive any form of treatment. In those aged 20 to 49 years, 53% received standard care and only 5% received no treatment. The rest of the demographics are summarized in Table 1.
We compared the frequency of combination treatment from 1998 to 2004 and from 2005 to 2011. Before 2005, 12485 patients (26.67%) received radiation, chemotherapy, and surgery and 5506 (11.76%) received no treatment. After 2005, 26544 (49.29%) received radiation, chemotherapy, and surgery, but the no treatment group remained similar at 5557 (10.32%).
Logistic regression was used to determine predictors of treatment by receiving radiation therapy, chemotherapy, and surgery (standard therapy) (Table 2). This analysis was performed overall and in patients diagnosed before 2004 and after 2005; the determinants of standard of care receipt were the same between both time periods. An inverse association was found between age of diagnosis and receiving standard therapy, with increasing receipt of all 3 treatments in progressively younger age groups. After controlling all the other factors in the model, females were about 10% less likely to receive standard therapy than males with glioblastoma. Patients identified as non-white racial groups and those of Hispanic heritage were more than 10% less likely to receive chemotherapy, radiation, and surgery than white and non-Hispanic patients. Patients with any type of insurance were significantly more likely to have received standard of care than those without insurance. Those living in counties with progressively lower median incomes and counties with a progressively lower percentage of patients that graduated high school were increasingly less likely to have received radiation therapy, chemotherapy, and surgery. Compared to patients diagnosed from 1998 to 2004, those diagnosed from 2005 to 2011 were 3 times more likely to have received all 3 treatments in the first course of therapy. Patients with giant cell glioblastoma or gliosarcoma were more likely to have received standard therapy than those with glioblastoma, NOS. Patients receiving standard therapy were almost 3 times more likely to have tumors that did not involve brainstem, ventricles, cerebellum, or the cerebrum compared to primary location in the cerebrum (OR = 2.95, P < .0001).
Table 2.
Multivariate logistic regression of risk factors for receiving standard of care (surgery, chemotherapy, and radiation) of adults diagnosed with glioblastoma in the National Cancer Database, 1998–2011
| Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Age group (years) | 20–49 | 6.15 | 5.77–6.56 | <.0001 |
| 50–64 | 4.36 | 4.12–4.62 | <.0001 | |
| 65–74 | 2.78 | 2.65–2.92 | <.0001 | |
| ≥75 | ref | - | - | |
| Race | Black | 0.78 | 0.73–0.83 | <.0001 |
| Other | 0.87 | 0.79–0.96 | .004 | |
| Unknown | 0.65 | 0.56–0.75 | <.0001 | |
| White | ref | - | - | |
| Sex | Female | 0.90 | 0.88–0.94 | <.0001 |
| Male | ref | - | - | |
| Hispanic | Yes | 0.87 | 0.81–0.94 | .0002 |
| Unknown | 1.05 | 0.99–1.11 | .098 | |
| No | ref | - | - | |
| Insurance | Medicaid | 1.36 | 1.24–1.49 | <.0001 |
| Medicare | 1.65 | 1.51–1.79 | <.0001 | |
| Private | 1.97 | 1.84–2.12 | <.0001 | |
| Not insured | ref | - | - | |
| Income | ≤$30000 | 0.81 | 0.76–0.86 | <.0001 |
| $30000–$34999 | 0.86 | 0.82–0.91 | <.0001 | |
| $35000–$45999 | 0.94 | 0.91–0.98 | .005 | |
| > $46000 | ref | - | - | |
| Educationa | ≥29% | 0.79 | 0.74–0.83 | <.0001 |
| 20–28.9% | 0.87 | 0.83–0.91 | <.0001 | |
| 14–19.9% | 0.94 | 0.90–0.98 | .002 | |
| ≤14% | ref | - | - | |
| Region | Metro | 0.96 | 0.93–1.00 | .077 |
| Unknown | 0.85 | 0.76–0.95 | .005 | |
| Urban/Rural | ref | - | - | |
| Primary tumor site | Others | 1.93 | 1.67–2.22 | <.0001 |
| Cerebrum and brain lobes | 2.95 | 2.56–3.39 | <.0001 | |
| Ventricle/cerebellum/brainstem | ref | - | - | |
| Histology | Gliosarcoma | 1.46 | 1.32–1.61 | <.0001 |
| Giant Cell GBM | 1.33 | 1.12–1.56 | .001 | |
| GBM, NOS | ref | - | - | |
| Years of Diagnosis | 2005–2011 | 3.03 | 2.95–3.13 | <.0001 |
| 1994–2004 | ref | - | - |
GBM, glioblastoma; NOS, not otherwise specified; ref, reference
aEducation refers to the percentage of non-High school graduates in the patient’s residential zip code.
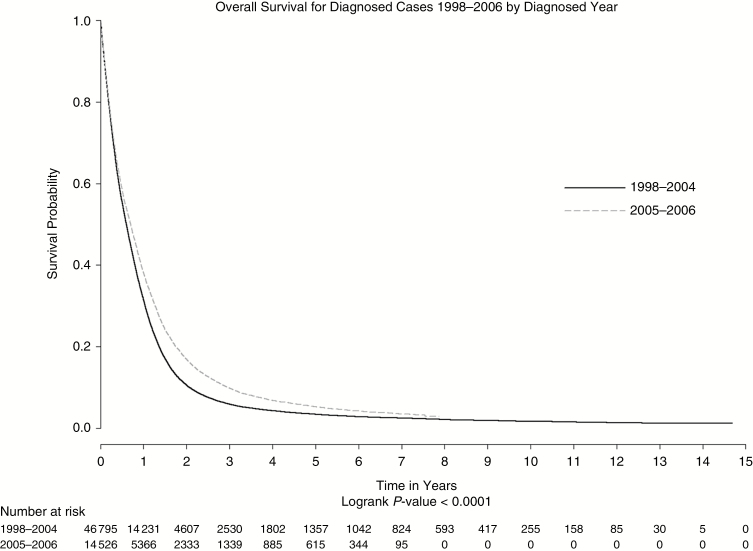
Overall, median survival for glioblastoma patients was 7.5 months, with 2.5% (1560/61346) surviving to 2 years and 2.0% (1234/61346) surviving up to 5 years. Patients diagnosed after 2004 had higher survival estimates compared to patients diagnosed before 2004 (median survival 8.4 months versus 7.2 months, respectively) (Figure 1). The log-rank test comparing survival for these 2 periods was significant (P < .0001)(Figure 1).
Fig. 1.
Overall survival by time period (1998–2004 vs 2005–2006).
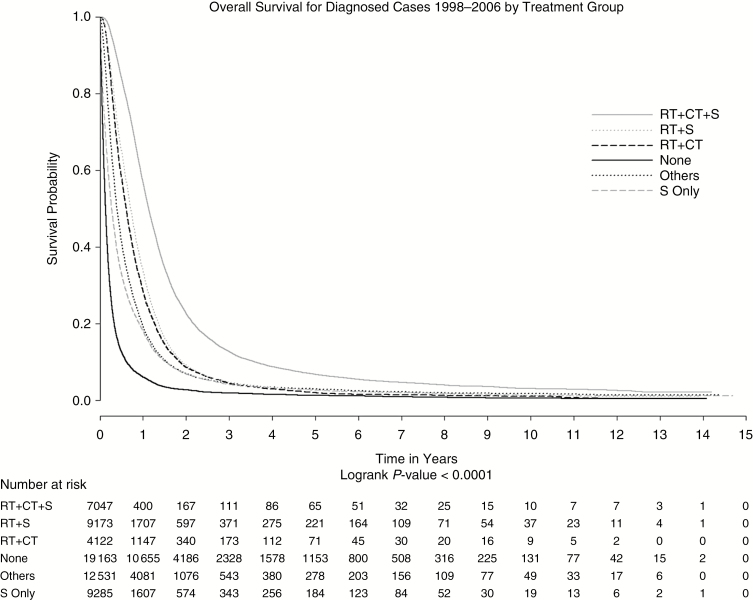
Based on our analysis (Table 1), the majority of patients received some form of treatment. It is important to note, however, that a higher percentage of elderly patients (65 years and older) did not receive (34.67% versus 20.48%) or refused (5.01% versus 1.69%) initial radiation therapy as compared with younger patients (20 to 64 years). Patients who did not receive radiation had lower survival compared to patients who did receive radiation (P < .0001) (Figure 2). The 1-, 3-, and 5-year survival estimates in patients that did not receive radiation were 14%, 3%, and 2% compared with 39%, 7%, and 4% in patients who did receive radiation.
Fig. 2.
Overall survival by combination therapy from 1998–2006.
Chemotherapy was associated with an increase in survival. The 1-, 3-, and 5-year survival estimates in patients who did not receive chemotherapy was 19%, 3%, and 2% compared with 49%, 10%, and 5% in patients who did receive chemotherapy. Surgery was also related to an increase in survival (P < .0001). The 1-, 3-, and 5-year survival estimates in patients who did not receive surgery as initial treatment was 12%, 25%, and 1% compared with 39%, 8%, and 4% in patients who did receive surgery as initial treatment.
Using Cox proportional hazards modeling, average effects of predictors on survival were examined (Table 3). As expected, improved survival was inversely associated with age at diagnosis, with those aged 20 to 49 years having 65% longer survival than those aged 75 years or more. Glioblastoma patients who were of non-white race and/or that were of Hispanic heritage had better survival than those who reported white race and non-Hispanic heritage. Females had 5% better survival than male patients.
Table 3.
Factors associated with mortality in adults diagnosed with GBM from the National Cancer Data Base, 1998–2006 from a multivariate Cox proportional hazards model
| Hazard ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Age group (years) | 20–49 | 0.35 | 0.34–0.37 | <.0001 |
| 50–64 | 0.54 | 0.52–0.56 | <.0001 | |
| 65–74 | 0.73 | 0.71–0.75 | <.0001 | |
| ≥75 | ref | - | - | |
| Race | Black | 0.88 | 0.85–0.92 | <.0001 |
| Other | 0.72 | 0.67–0.76 | .007 | |
| Unknown | 0.82 | 0.75–0.89 | <.0001 | |
| White | ref | - | - | |
| Sex | Female | 0.95 | 0.94–0.97 | <.0001 |
| Male | ref | - | - | |
| Hispanic | Yes | 0.80 | 0.77–0.84 | .0002 |
| Unknown | 1.05 | 1.01–1.08 | .005 | |
| No | ref | - | - | |
| Insurance | Medicaid | 1.11 | 1.05–1.18 | <.0001 |
| Medicare | 1.12 | 1.07–1.18 | <.0001 | |
| Private | 0.98 | 0.94–1.03 | <.0001 | |
| Not insured | ref | - | - | |
| Income | ≤$30000 | 1.08 | 1.04–1.12 | <.0001 |
| $30000–$34999 | 1.10 | 1.07–1.14 | <.0001 | |
| $35000–$45999 | 1.07 | 1.05–1.10 | <.0001 | |
| > $46000 | ref | - | - | |
| Educationa | ≥29% | 0.96 | 0.93–0.99 | .002 |
| 20–28.9% | 0.98 | 0.95–1.01 | <.0001 | |
| 14–19.9% | 1.01 | 0.99–1.04 | <.0001 | |
| ≤14% | ref | - | - | |
| Primary site | Others | 1.03 | 0.96–1.11 | .443 |
| Cerebrum and brain lobes | 0.89 | 0.82–0.96 | .002 | |
| Ventricle/cerebellum/brainstem | ref | - | - | |
| Histology | Gliosarcoma | 1.03 | 0.97–1.10 | .32 |
| Giant Cell GBM | 0.74 | 0.66–0.82 | <.0001 | |
| GBM, NOS | ref | - | - | |
| Years of Diagnosis | 2005–2006 | 0.90 | 0.88–0.92 | <.0001 |
| 1998–2004 | ref | - | - | |
| Treatment | Radiation + Chemotherapy+Surgery | 0.25 | 0.25–0.26 | <.0001 |
| Radiation+Surgery | 0.34 | 0.33–0.36 | <.0001 | |
| Radiation+Chemotherapy | 0.40 | 0.38–0.41 | <.0001 | |
| Surgery | 0.57 | 0.55–0.59 | <.0001 | |
| Other Combinations | 0.47 | 0.46–0.49 | <.0001 | |
| None | ref | - | - |
GBM, glioblastoma; NOS, not otherwise specified
aEducation refers to the percentage of non-High school graduates in the patient’s residential zip code.
Patients on Medicaid and Medicare had an increased risk of mortality compared to those with no insurance (11% and 12%, respectively). Those with private insurance had similar survival outcomes to those with no insurance. Income levels below $46000 were associated with a 7% to 10% increased risk of mortality. A diagnosis of giant cell glioblastoma was associated with 25% better survival than a diagnosis of glioblastoma, NOS, while those with a glioblastoma in the cerebrum had the poorest survival. Those receiving any treatment had statistically significantly better survival than those receiving no treatment. However, comparing the various treatment combinations revealed that patients receiving radiation, chemotherapy, and surgery had significantly better survival than any other treatment combination (Figure 2).
Discussion
Using the NCDB we found 100672 patients with a diagnosis of glioblastoma. This group had a median survival of 7.5 months, which is significantly less than in published clinical trials, but similar to population based analysis16; this likely reflects a real-world survival estimation of this disease. Our study includes an overview of current hospital-based patterns of care in glioblastoma, representing over 70% of the population.
The frequency of glioblastoma was higher in males, whites, and non-Hispanics. These findings match the demographics described in other U.S. population studies.1,11 The strongest determinant of treatment was age; however, other factors such as race, gender, histology, primary site location, education, and insurance status also influenced the likelihood and modality of treatment receipt. We found females and Hispanics were 10% less likely to receive standard therapy, but female gender was found to be a protective factor for mortality compared to males. The survival benefits of the female gender have also been reported in smaller cohorts.17 This may be associated with less comorbidities compared to males,18 and the overall younger age of females in our cohort; over 75% of women were less than 65 years of age. Females are also reported to be more likely to have IDH-mutant glioblastoma,19 which is associated with a better prognosis.
We found the use of standard combination therapy—tumor resective surgery, radiation, and chemotherapy—was low and was more common in younger patients, among patients with higher socioeconomic status, and among those living in a region with higher educational level. Only 53% of patients from 20 to 49 years old received standard therapy. These findings demonstrate the uneven distribution of care even in younger patients.
Overall, the most commonly used method of treatment was surgery (73%), followed by radiotherapy and chemotherapy (69% and 50%, respectively). Elderly patients were commonly undertreated, presenting lower frequencies for any form of treatment. We found a 2-fold decrease in treatment delivery with increasing age group. The cause of the large variation in treatment receipt could be related to both difficulties in access to neuro-oncology services and physicians’ beliefs on the usefulness of surgery and radiation therapy in nonoptimal patients.12,13,20–22
Following the EORTC/NCIC trial published in 2005, there has been a significant change in the overall survival of glioblastoma patients.23 Our analysis demonstrated an increased frequency of the standard treatment of radiation, chemotherapy, and surgery receipt after 2005, and also a decreased frequency of receiving surgery only or no treatment. Population-based analysis has also demonstrated a significant improved survival after temozolomide adoption irrespective of age.16
Treatment delivery regardless of modality was associated with better survival. Similar effects are found in other cohorts, including in elderly patients, particularly for gross total resection and adjuvant therapies20,24; even if higher complication rates are expected.12,24 No large study has shown significant side effects or decreased quality of life in elderly patients that receive chemotherapy or radiation alone,25,26 and the survival benefits were comparable to younger adults.27,28 However, elderly patients are more likely to have greater comorbidities and are at higher risk of mortality. Standard treatment, short course radiotherapy schemes, and temozolomide monotherapy are alternative therapies in elderly patients that have demonstrated survival benefits.29–32 Nevertheless, even after adjusting for comorbidities and treatment type, elderly patients tend to have the worst outcomes. This observation may be partially explained by a more aggressive tumor biology, driven by the higher number of mutations and a less favorable molecular profiling.33
The majority of glioblastoma patients in our analysis lived in metropolitan areas and had a high socioeconomic status. The median survival of 7.5 months demonstrates the working state of care for glioblastoma in the U.S. and lack of optimal receipt of standard treatment. Patients with private insurance were more likely to be treated; still, survival was similar when comparing patients with private and no insurance. Similar results regarding socioeconomic factors have been previously described in the literature.34
Survival analysis of patients on Medicaid and Medicare demonstrated an increased risk of mortality (11% and 12%, respectively), compared to patients without insurance. Other studies have also highlighted the increased mortality risk of patients in Medicaid.35 Several factors related to patients’ age and lifestyle differences between payer groups maybe more important than insurance status. Previous investigation of the NCDB in patients with glioblastoma demonstrated over 80% of elderly patients had either Medicare or Medicaid.36 This large elderly population could result in a higher number of Medicare and Medicaid patients being frail and having increased risk of mortality. Other possible explanations could be related to longer care wait-time, economic difficulties in treatment distribution, and a higher likelihood of receiving palliative care due to its coverage under Medicare. Health care provider bias and institutional type may also play a role impacting outcomes for Medicaid and Medicare patients; private insurance may result in more specialized care.
The main limitations of our analysis are based on its retrospective nature; we performed multivariate analysis and Akaike Information Criterion to mitigate bias and ensure a correct interpretation of the variables. Due to a great number of missing values and the inherent limitations of our model we were unable to adjust for secondary glioblastoma subtype, which are well-known prognostic factors (eg, IDH1/2 mutations and MGMT promoter methylation status). In addition, the lack of specific chemotherapy received, single vs multiple chemotherapy agent, radiation dosing, disease progression, and treatment failure information may pose limitations in analyzing our findings. As NCDB only gathers cancer-accredited centers, it is possible that patients with fewer options for treatment were not captured. Our data, however, show important differences in treatment distribution related to age, race, gender, and socioeconomic status, even in settings where clinical trial enrollment and maximal therapy is expected.
In summary, despite the acknowledged benefits of the EORTC regimen, age, race, gender, and socioeconomic status are important determinants that influence treatment delivery in glioblastoma. Moreover, overall survival in patients with glioblastoma is dramatically less than the survival reported in published clinical trials.
Funding
Dr. Dressler, Dr. Dolecek, and Dr. Villano were supported by the National Cancer Institute (R03CA156561) and Dr. Dressler and Dr. Liu were members of the Biostatistics and Bioinformatics Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558).
Conflict of interest statement: All authors declare no competing interests.
Acknowledgements
We would like to thank Bridget J. McCarthy, Ph.D. for being a resource for ideas and inspiration.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin JY, Yoon JK, Shin AK, et al. Association of insurance and community-level socioeconomic status with treatment and outcome of squamous cell carcinoma of the pharynx. JAMA Otolaryngol Head Neck Surg. 2017;143(9):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller BJ, Gao Y, Duchman KR. Socioeconomic measures influence survival in osteosarcoma: an analysis of the National Cancer Data Base. Cancer Epidemiol. 2017;49:112–117. [DOI] [PubMed] [Google Scholar]
- 10. Celie KB, Jackson C, Agrawal S, et al. Socioeconomic and gender disparities in anal cancer diagnosis and treatment. Surg Oncol. 2017;26(2):212–217. [DOI] [PubMed] [Google Scholar]
- 11. Chang SM, Parney IF, Huang W, et al. ; Glioma Outcomes Project Investigators Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 12. Iwamoto FM, Reiner AS, Panageas KS, et al. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 13. Yabroff KR, Harlan L, Zeruto C, et al. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amercian College of Surgeons. National Cancer Data Base On-Line Data Dictionary http://ncdbpuf.facs.org/?q=node/259. Published 2014. AccessedMay 08, 2016.
- 16. Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shinojima N, Kochi M, Hamada J, et al. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg. 2004;101(2):219–226. [DOI] [PubMed] [Google Scholar]
- 18. Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012;13(7):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oszvald A, Güresir E, Setzer M, et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J Neurosurg. 2012;116(2):357–364. [DOI] [PubMed] [Google Scholar]
- 21. Graus F, Bruna J, Pardo J, et al. Patterns of care and outcome for patients with glioblastoma diagnosed during 2008-2010 in Spain. Neuro Oncol. 2013;15(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka S, Meyer FB, Buckner JC, et al. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118(4):786–798. [DOI] [PubMed] [Google Scholar]
- 23. Dubrow R, Darefsky AS, Jacobs DI, et al. Time trends in glioblastoma multiforme survival: the role of temozolomide. Neuro Oncol. 2013;15(12):1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babu R, Komisarow JM, Agarwal VJ, et al. Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg. 2016;124(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 25. Keime-Guibert F, Chinot O, Taillandier L, et al. ; Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 26. Zarnett OJ, Sahgal A, Gosio J, et al. Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015;72(5):589–596. [DOI] [PubMed] [Google Scholar]
- 27. Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85(2):171–180. [DOI] [PubMed] [Google Scholar]
- 28. Chiocca EA. Being old is no fun: treatment of glioblastoma multiforme in the elderly. J Neurosurg. 2008;108(4):639–640. [DOI] [PubMed] [Google Scholar]
- 29. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 30. Baker H. Radiotherapy in the elderly and frail with glioblastoma. Lancet Oncol. 2015;16(15):e530. [DOI] [PubMed] [Google Scholar]
- 31. Mak KS, Agarwal A, Qureshi MM, et al. Hypofractionated short-course radiotherapy in elderly patients with glioblastoma multiforme: an analysis of the National Cancer Database. Cancer Med. 2017;6(6):1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guedes de Castro D, Matiello J, Roa W, et al. Survival outcomes with short-course radiation therapy in elderly patients with glioblastoma: data from a randomized phase 3 trial. Int J Radiat Oncol Biol Phys. 2017;98(4):931–938. [DOI] [PubMed] [Google Scholar]
- 33. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krex D, Klink B, Hartmann C, et al. ; German Glioma Network Long-term survival with glioblastoma multiforme. Brain. 2007;130(Pt 10):2596–2606. [DOI] [PubMed] [Google Scholar]
- 35. LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252(3):544–50; discussion 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glaser SM, Dohopolski MJ, Balasubramani GK, et al. Glioblastoma multiforme (GBM) in the elderly: initial treatment strategy and overall survival. J Neurooncol. 2017;134(1):107–118. [DOI] [PubMed] [Google Scholar]