Abstract
Objectives
Care gaps in asthma may be highly prevalent but are poorly characterised. We sought to prospectively measure adherence to key evidence-based adult asthma practices in primary care, and predictors of these behaviours.
Design
One-year prospective cohort study employing an electronic chart audit.
Setting
Three family health teams (two academic, one community-based) in Ontario, Canada.
Participants
884 patients (72.1% female; 46.0±17.5 years old) (4199 total visits; 4.8±4.8 visits/patient) assigned to 23 physicians (65% female; practising for 10.0±8.6 years).
Main outcome measures
The primary outcome was the proportion of visits during which practitioners assessed asthma control according to symptom-based criteria. Secondary outcomes included the proportion of: patients who had asthma control assessed at least once; visits during which a controller medication was initiated or escalated; and patients who received a written asthma action plan. Behavioural predictors were established a priori and tested in a multivariable model.
Results
Primary outcome: Providers assessed asthma control in 4.9% of visits and 15.4% of patients. Factors influencing assessment included clinic site (p=0.019) and presenting symptom, with providers assessing control more often during visits for asthma symptoms (35.0%) or any respiratory symptoms (18.8%) relative to other visits (1.6%) (p<0.01). Secondary outcomes: Providers escalated controller therapy in 3.3% of visits and 15.4% of patients. Factors influencing escalation included clinic site, presenting symptom and prior objective asthma diagnosis. Escalation occurred more frequently during visits for asthma symptoms (21.0%) or any respiratory symptoms (11.9%) relative to other visits (1.5%) (p<0.01) and in patients without a prior objective asthma diagnosis (3.5%) relative to those with (1.3%) (p=0.025). No asthma action plans were delivered.
Conclusions
Major gaps in evidence-based asthma practice exist in primary care. Targeted knowledge translation interventions are required to address these gaps, and can be tailored by leveraging the identified behavioural predictors.
Trial registration number
NCT01070095; Pre-results.
Keywords: asthma, primary care, respiratory medicine (see thoracic medicine), knowledge translation, quality in health care
Strengths and limitations of this study.
This is the largest prospective practice-based audit of primary care adherence to three asthma management practices recommended across international guidelines: assessment of asthma control, initiation/escalation of asthma controller therapy and provision of asthma action plans.
The multivariable modelling in this study allowed for identification of novel behavioural predictors which complement those previously identified through surveys and qualitative studies.
The study was carried out in real-world academic and community primary care settings with broad sociodemographic representation.
Chart review methods are susceptible to underestimation of care due to poor clinician documentation.
None of the study sites included allied health resources for asthma management, whereby findings are limited to settings without such resources.
Introduction
Asthma is one of the most common chronic diseases in the UK, increasing in prevalence and carrying a direct annual healthcare expenditure of more than £1 billion.1 Although effective therapies exist, up to 53% of patients remain poorly controlled.2 3 Poor health outcomes in patients with asthma have been attributed to gaps between evidence-based recommendations and practice, particularly in primary care, where the majority of asthma patients are seen.4 A striking consequence of these gaps was presented in the UK National Review of Asthma Deaths which found that 46% of asthma deaths could have been avoided if appropriate guidelines were followed.5 Although asthma guidelines can be complex6 and sometimes divergent,7 certain recommendations are long-standing and common across guidelines.
First, asthma control should be assessed at each visit.8 9‘ Good asthma control’ is defined by a series of criteria which correlate with improved quality of life and reduced healthcare utilisation. Failure to meet any of these criteria defines the need for initiation or escalation of therapy. These criteria were first articulated in the original (1996) Canadian Asthma Guidelines10 and the 2003 British Asthma Guidelines,11 and have been reiterated in successive guideline updates.
Second, pharmacotherapy should be tailored to asthma control.8 Early initiation of inhaled corticosteroids (ICSs) (a ‘controller’ medication) in poorly controlled asthma improves quality of life and lung function while reducing symptoms, exacerbations and mortality.12–15 This has been recommended consistently since the 1990 British Asthma Guidelines16 and the 1996 Canadian Asthma Guidelines.10 Similarly, addition of a long-acting beta agonist (LABA) in patients with poor control on an ICS improves lung function and reduces rescue bronchodilator use and exacerbations,17 and has been recommended since the 2003 British Asthma Guidelines11 and the 2003 Canadian Asthma Guideline update.18
Finally, a written asthma action plan (AAP) is an individualised self-management plan produced by a healthcare professional for a patient with asthma.19 AAPs reduce hospitalisations, emergency department (ED) visits, unscheduled doctor visits, absenteeism and nocturnal asthma symptoms, and improve quality of life.19 A recommendation that all patients receive a written AAP has also been found in each British Asthma Guideline since 199016 and each Canadian Asthma Guideline since 1996.10 All of these practices are equally recommended in the latest asthma guidance document from the National Institute for Health and Care Excellence.20
Estimates of care gaps across these three fundamental asthma management principles have been limited to patient and provider self-report3 21 and extrapolation from population health databases.22 Furthermore, little is known about factors that predict adherence to these recommendations. Accordingly, our objectives were both to measure adherence to these clinical behaviours and to identify their predictors in Canadian community and academic primary care practices, with a view to targeting future knowledge translation initiatives.
Methods
This report adheres to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.23
Study design
This was a prospective cohort study employing an electronic audit of asthma care delivered by prescribers across two academic family health teams (primary healthcare teams including family physicians, nurses and allied health members) in Hamilton, Ontario (population: 536 917)24 and one community-based family health team in Brampton, Ontario (population: 593 638).25 Clinics used the OSCAR electronic medical record (EMR) system (http://oscarcanada.org), were under a capitated funding model, did not have asthma educators or respiratory therapists on site, and were not using any asthma-related decision support tools. Invitations were sent to all physicians and nurse practitioners (NPs). We identified asthma patients through a validated EMR search algorithm including: ‘asthma’ in the cumulative patient profile (a standardised chart component which includes active and past medical history), use of the International Classification of Diseases (ICD)9 diagnostic billing code for asthma/allergic bronchitis (493) within the last 3 years (excluding patients in whom a chronic obstructive pulmonary disease (COPD)-related ICD-9 diagnostic billing code (491, 492, 496) had been used in the last 3 years); and presence of ‘asthma’ in any of the typed chart notes26 (algorithm-generated lists were vetted/modified by clinicians). We included patients with asthma belonging to all consenting clinicians, who were > 16 years old, understood English and had been on asthma medication in the prior 12 months, while excluding patients who had been on a COPD medication in the prior year.26 Patients who were pregnant, or whom the physician deemed to have cognitive limitations or a life expectancy of <1 year were excluded. We reviewed all outpatient visits and asthma-related telephone interactions by staff physicians, residents, NPs, NP students or physician assistants (PAs) between 1 August 2012 and 31 July 2013. We excluded visits exclusively for administration of injection medication(s) (eg, the influenza shot).
Patient involvement
A patient organisation (the Asthma Society of Canada) was involved from project inception and its members helped to guide the choice of research questions and the research design, and will lead efforts to disseminate results to patients.
Data collection
Four trained reviewers entered data in a standardised electronic form. Data elements were agreed on by primary care (GA, DC, AGK, SGo) and respirology experts (SGu, L-PB). The form was refined for clarity and usability through three cycles of testing, each involving 20 visit reviews by each reviewer. Reviewers then independently abstracted data from 40 randomly selected visits to ensure agreement. Abstracted data included visit time/date, presenting symptom, demographics, baseline asthma parameters, baseline and changes to respiratory medications, previous asthma diagnostic testing (spirometry and/or methacholine challenge), previous hospitalisations or ED visits for asthma, previous referrals/visits to respirologists or allergists (and their findings), clinician documentation of asthma control according to symptom-based guideline criteria (table 1),8 9 20 actual asthma control according to symptoms recorded in any place in the chart and provision of a written AAP.
Table 1.
Symptom-based criteria for assessing asthma control8
| Criterion | Controlled | Uncontrolled |
| Daytime symptoms* | <4 days/week | > 4 days/week |
| Night-time symptoms* | <1 night/week | > 1 night/week |
| Physical activity | Normal/no limitations | Restricted due to asthma in previous 3 months |
| Absenteeism | None | Missed work/school/other activities due to asthma in previous 3 months |
| Short-acting bronchodilator use* | <4 doses/week | > 4 doses/week |
*Evaluated as an average of the prior 6 months.
Outcomes
The primary outcome was the proportion of visits during which practitioners assessed asthma control according to symptom-based criteria. Patients were considered to have poor control if they met one or more criteria for uncontrolled asthma (based on review of the current and any prior visits within each corresponding timespan) (table 1). Secondary outcomes included the proportion of: patients who had asthma control assessed at least once; visits during which a controller medication was either initiated or escalated (and the proportion of patients with this); and patients who received a written AAP.
A priori, we identified a set of practically measurable, clinically relevant and plausibly explanatory parameters which might predict these outcomes, through a consensus of study co-investigators and knowledge-users, grounded in existing literature where possible. Parameters included: clinic, practitioner type, objective diagnosis of asthma, asthma control status, documented physician diagnosis of asthma, presenting symptom type, time of visit, billing physician (most responsible physician (MRP)/other) and previous ED visits/hospitalisations for asthma.
We also characterised which control questions were being asked, and analysed current medication use as a function of control status, prior objective diagnosis, and prior ED visit/hospitalisation for asthma.
Analysis
Inter-rater reliability was calculated using percent agreement. We summarised baseline clinician and patient characteristics descriptively, using information from the first visit in patients with multiple visits. We compared patient variables between sites with Fisher’s exact/χ2 tests and analysis of variances, as appropriate, and compared patient subgroups using the Fisher’s exact test. We performed univariate analysis followed by multivariable logistic regression to calculate ORs and p values for predictors of each outcome (covariates tested are listed above). In measuring control assessment, visits occurring within 1 month after a provider assessed control (a standard look-back period for symptom-based control questions) were excluded from the analysis. In measuring controller medication initiation/escalation, visits occurring within 3 months of a controller escalation or initiation were excluded (a standard period during which further medication adjustments are discouraged, in order to allow for the prior medication changes to take effect). In measuring AAP delivery, we eliminated patients who had not been on a controller medication at any time during the study period (controller medication changes in the AAP are only recommended in patients on a baseline controller).27 Analyses were performed using R Statistical Software V.3.2.4. Statistical significance was defined at a two-sided 0.05 level.
Results
Chart review
Agreement between reviewers in chart abstraction was 82.8%–97.3% for control criteria, 97.5% for assessment of medication changes and 100% for AAP delivery.
Model assessment
The goodness of fit of the logistic regression models was assessed with the Hosmer-Lemeshow test, using a range of groupings. All were found to be non-significant, indicating the model was adequately fit. Additionally, we used bootstrap validation to assess the accuracy of the model. Based on the Somers’ Dxy and the slope shrinkage factor, we identified very slight model overfitting.
Population
We recruited 19/42 (45%) physicians and 1/3 (33%) NPs. The NP had patients from an additional four physicians under their care, enabling us to analyse data for 23/42 (55%) physicians. These physicians had been in practice for 10.0±8.6 years (range <1–29) and 15/23 were female (65%). They were the MRP for 884 patients with asthma (table 2). Given that patients could be seen by clinicians other than their MRP, these patients received care from 108 residents (66% female), 46 staff physicians (72% female, in practice for 9.8±10.1 years (range <1–43)), 17 NPs and 2 PAs. Each provider averaged 24.3±39.4 patient visits (range 1–255) over the study period.
Table 2.
Patient characteristics
| Characteristic | Overall, n=884 |
Site 1 (academic), n=429 |
Site 2 (academic), n=245 |
Site 3 (non-academic), n=210 |
P value |
| Mean age±SD (years) | 46.0±17.5 | 49.3±17.9 | 43.9±17.4 | 42.7±15.9 | <0.01 |
| Sex, n (%) | 0.604 | ||||
| Female | 638 (72.1) | 307 (71.6) | 174 (71.0) | 157 (74.8) | |
| Male | 246 (27.9) | 123 (28.7) | 71 (29.0) | 53 (25.2) | |
| Smoking status, n (%) | <0.01 | ||||
| Non-smoker | 442 (49.8) | 226 (52.7) | 109 (44.5) | 107 (50.0) | |
| Ex-smoker | 132 (14.9) | 80 (18.6) | 32 (13.1) | 20 (9.5) | |
| Smoker | 168 (19.0) | 75 (17.5) | 47 (19.2) | 46 (21.9) | |
| Not documented | 142 (16.1) | 48 (11.2) | 57 (23.3) | 37 (17.6) | |
| Comorbidities, n (%) | |||||
| Atopy | 359 (40.6) | 192 (44.8) | 104 (42.4) | 63 (30.0) | <0.01 |
| COPD | 68 (7.7) | 46 (10.7) | 13 (5.3) | 9 (4.3) | <0.01 |
| Other resp. diagnosis | 16 (1.8) | 10 (2.3) | 5 (2.0) | 1 (0.5) | 0.243 |
| Previous diagnostic testing, n (%) | |||||
| Spirometry | 342 (38.7) | 198 (46.2) | 97 (39.6) | 47 (22.4) | <0.01 |
| Bronchodilator challenge (% of spirometries) |
237 (69.3) | 137 (69.2) | 64 (66.0) | 36 (76.6) | 0.432 |
| Methacholine challenge | 88 (10.0) | 52 (12.1) | 30 (12.2) | 6 (2.9) | <0.01 |
| Baseline medications, n (%) | |||||
| Short-acting bronchodilator | 564 (63.8) | 281 (65.5) | 149 (60.8) | 57 (27.1) | <0.01 |
| Inhaled corticosteroid alone* | 150 (17.0) | 87 (20.3) | 45 (18.4) | 18 (8.6) | <0.01 |
| Inhaled corticosteroid with long-acting beta-agonist | 132 (14.9) | 67 (15.6) | 30 (12.2) | 35 (16.7) | 0.359 |
| Long-acting beta-agonist alone | 6 (0.7) | 4 (0.9) | 1 (0.4) | 1 (0.5) | 0.669 |
| Leukotriene receptor antagonist | 21 (2.4) | 10 (2.3) | 9 (3.7) | 2 (1.0) | 0.515 |
| Prednisone† | 9 (1.0) | 6 (1.4) | 2 (0.8) | 1 (0.5) | 0.041 |
*Without concurrent use of a long-acting beta-agonist in a combination inhaler or as a separate inhaler.
†Includes only those patients who use prednisone chronically.
COPD, chronic obstructive pulmonary disease; resp., respiratory.
Fifty-five (6%) patients had been seen in the ED or hospitalised for asthma in the prior 10 years. These patients were more likely to be on a controller medication (32/55) (58.1%) than those without an ED visit or admission (243/829) (29.3%) (p<0.01). Ninety (10.2%) patients had an objective diagnosis of asthma (by spirometry or methacholine challenge). Although patients receiving a COPD-specific medication and/or in whom a COPD billing code had been used were excluded through the EMR search algorithm, detailed chart review identified that 7.7% of patients with asthma did have comorbid COPD (table 2).
There were 4199 eligible visits over the study period (4.8±4.8 visits/patient), among which 572 (13.6%) were for respiratory symptoms, including 163 (3.9%) specifically for asthma. During the study period, 331 (37.4%) patients had at least one visit with a respiratory symptom, and 28 (3.2%) were referred to see a respirologist or allergist. A further 159 (18.0%) patients had been seen by a specialist in the prior 10 years. Among these, six (3.8%) had received an AAP from that specialist.
Asthma care
Primary outcome: asthma control assessment
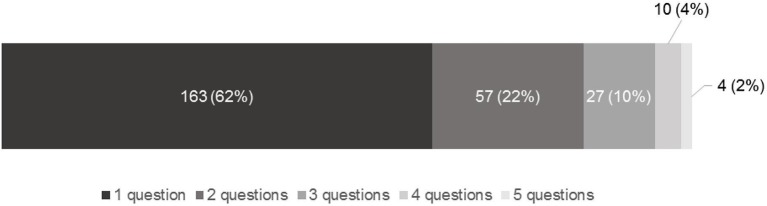
Practitioners determined asthma control in 202/4122 (4.9%) eligible visits. Among 261 (6.2%) visits where any control question was asked, an average of 1.6 questions were asked, as follows: daytime symptoms (60.5%), rescue puffer use (44.8%), night-time symptoms (27.2%), physical activity limitations (23.0%) and school/work absenteeism (4.2%). All five questions were asked in four (1.5%) of these visits (figure 1).
Figure 1.
Proportion of visits* with each number of symptom-based asthma control questions asked. The stacked bar graph provides the number and proportion of visits during which each of one, two, three, four or five symptom-based control questions were asked by the clinician. *Among the 261/4122 visits in which any control question was asked.
In the multivariable model, clinic site (p=0.019) and nature of presenting symptom (p<0.01) were significant predictors of asthma control assessment (table 3).
Table 3.
Predictors of asthma control assessment*
| Control not assessed (n=3920 visits) | Control assessed (n=202 visits) | P value† | OR* (95% CI) | |
| Primary care clinic, n (%) | 0.019‡ | |||
| Site 1 | 1727 (95.0) | 90 (5.0) | ||
| Site 2 | 801 (92.7) | 63 (7.3) | 1.37 (0.93 to 2.02) | |
| Site 3 | 1392 (96.6) | 49 (3.4) | 0.72 (0.45 to 1.14) | |
| Appointment provider type, n (%) | 0.11§ | |||
| Physician | 1847 (97.1) | 55 (2.9) | ||
| Nurse practitioner | 414 (95.4) | 20 (4.6) | 1.17 (0.60 to 2.29) | |
| Resident | 1417 (92.6) | 114 (7.4) | 1.79 (1.07 to 3.00) | |
| Physician assistant | 242 (94.9) | 13 (5.1) | 1.26 (0.57 to 2.77) | |
| Clinical diagnosis of asthma, n (%) | 0.074 | |||
| Yes | 2296 (94.1) | 145 (5.9) | ||
| No | 1624 (96.6) | 57 (3.4) | 0.73 (0.51 to 1.03) | |
| Objective diagnosis of asthma, n (%) | 0.79 | |||
| Yes | 357 (93.5) | 25 (6.5) | ||
| No | 3563 (95.3) | 177 (4.7) | 0.93 (0.58 to 1.52) | |
| Presenting symptom, n (%) | <0.001¶ | |||
| Non-respiratory symptom | 3461 (98.3) | 59 (1.7) | ||
| Asthma | 101 (63.9) | 57 (36.1) | 29.8 (19.3 to 45.7) | |
| Other respiratory symptom | 358 (80.6) | 86 (19.4) | 14.5 (10.1 to 20.8) | |
| Time of visit, n (%) | 0.11 | |||
| On hours | 3478 (94.8) | 191 (5.2) | ||
| Weekend/after hours | 442 (97.6) | 11 (2.4) | 0.57 (0.29 to 1.13) | |
| Previous ED visit/hospitalisation for asthma, n (%) | NA** | |||
| Yes | 63 (100) | 0 (0) | ||
| No | 3857 (95.0) | 202 (5.0) | ||
| Patient seen by own MRP, n (%)** | 0.33 | |||
| Yes | 1269 (97.5) | 33 (2.5) | ||
| No | 2707 (94.1) | 169 (5.9) | 1.33 (0.75 to 2.44) |
*In measuring asthma control assessment, we eliminated visits in which asthma control had been assessed within the prior 1 month (a standard look-back period for symptom-based asthma control assessment).28
†P values and ORs for each variable shown are from the multivariable model.
‡Although significant across all sites, differences were not significant in pairwise comparisons.
§Although not significant across all provider types, in pairwise comparisons, residents were more likely to assess control compared with staff physicians (OR 1.8, 95% CI 1.1 to 3.0).
¶In pairwise comparisons, control was assessed more often in asthma-related visits than in non-respiratory visits (OR 29.8, 95% CI 19.3 to 45.9) and in any respiratory-related visits than in non-respiratory visits (OR 14.5, 95% CI 10.1 to 20.8).
**This covariate was removed from the multivariable model due to no subjects having this variable among those who had their control assessed; the univariate p value was 0.074.
ED, emergency department; MRP, most responsible physician; NA, not applicable.
Of 884 patients, 136 (15.4%) had their control status determined at least once in the study year, with 135 (15.3%) having poor control and one (0.01%) having good control. Among the patients with poor control, 61/135 (45.2%) were on a controller medication (31/61 (50.8%) ICS alone; 27/61 (44.3%) ICS/LABA; 3/61 (5.0%) ICS +leukotriene receptor antagonist (LTRA)), compared with 221/749 (29.5%) of the patients with unknown or good control (p<0.01) (104/221 (47.0%) ICS alone; 110/221 (49.8%) ICS/LABA; 7/221 (3.2%) ICS +LTRA)).
Secondary outcomes
Controller medication initiation or escalation
Controller medications were initiated or escalated by prescribers in 138/4159 (3.3%) eligible visits. Of 884 study patients, 136 (15.4%) had a controller medication initiated or escalated at least once in the study year. There was only one eligible visit (0.02%) in which a medication de-escalation was made.
In the multivariable model, clinic site (p<0.01) and nature of presenting symptom (p<0.01) were significant predictors of initiation or escalation (table 4), as was the absence of a prior objective diagnosis of asthma (p=0.025). However, patients lacking a prior objective diagnosis of asthma were less likely to already be on a controller medication (380/794) (47.9%) than those with an objective diagnosis (61/90) (67.8%) (OR 0.44, 95% CI 0.27 to 0.69) (p<0.01). Uncontrolled asthma predicted initiation or escalation in a univariate analysis, but could not be added to the multivariable model (table 4).
Table 4.
Predictors of controller medication initiation or escalation*
| Controller not initiated or escalated (n=4021 visits) |
Controller initiated or escalated (n=138 visits) |
P value† | OR† (95% CI) | |
| Primary care clinic, n (%) | <0.01 | |||
| Site 1 | 1781 (97.8) | 40 (2.2) | ||
| Site 2 | 869 (98.3) | 15 (1.7) | 0.68 (0.41 to 1.14) | |
| Site 3 | 1371 (94.3) | 83 (5.7) | 1.61 (1.05 to 2.48) | |
| Appointment provider type, n (%) | 0.72 | |||
| Physician | 1845 (96.6) | 65 (3.4) | ||
| Nurse practitioner | 419 (95.0) | 22 (5.0) | 0.92 (0.51 to 1.65) | |
| Resident | 1512 (97.5) | 39 (2.5) | 0.81 (0.49 to 1.35) | |
| Physician assistant | 245 (95.3) | 12 (4.7) | 0.68 (0.33 to 1.42) | |
| Clinical diagnosis of asthma, n (%) | 0.47 | |||
| Yes | 2369 (96.3) | 92 (3.7) | ||
| No | 1652 (97.3) | 46 (2.7) | 0.88 (0.62,1.25) | |
| Objective diagnosis of asthma, n (%) | 0.025 | |||
| Yes | 383 (98.7) | 5 (1.3) | ||
| No | 3638 (96.5) | 133 (3.5) | 2.44 (1.12 to 5.26) | |
| Presenting symptom, n (%) | <0.01‡ | |||
| Non-respiratory symptom | 3503 (98.5) | 52 (1.5) | ||
| Asthma | 124 (79.0) | 33 (21.0) | 17.8 (11.3 to 28.0) | |
| Other respiratory symptom | 394 (88.1) | 53 (11.9) | 7.67 (5.73 to 11.2) | |
| Time of visit, n (%) | 0.66 | |||
| On hours | 3586 (97.0) | 112 (3.0) | ||
| Weekend/after-hours | 435 (94.4) | 26 (5.6) | 1.11 (0.69 to 1.80) | |
| Previous ED visit/hospitalisation for asthma, n (%) | 0.86 | |||
| Yes | 60 (95.2) | 3 (4.8) | ||
| No | 3961 (96.7) | 135 (3.3) | 1.11 (0.37 to 3.33) | |
| Patient seen by MRP, n (%) | 0.17 | |||
| Yes | 1273 (96.8) | 42 (3.2) | ||
| No | 2748 (96.6) | 96 (3.4) | 1.43 (0.86 to 2.38) | |
| Asthma control level, n (%) | N/A§ | |||
| Uncontrolled | 636 (93.9) | 41 (6.1) | ||
| Unknown or controlled | 3385 (97.2) | 97 (2.8) |
*In measuring controller escalation/initiation, we eliminated visits in which patients had a controller medication escalated within the last 3 months (the typical duration of a therapeutic trial).56 Initiation included starting of any of the following medications: ICS alone, ICS with LABA, LABA alone, LTRA, LAAC. Escalation included an increase in the dose of an ICS or a combination ICS/LABA, addition of a LABA to an ICS, addition of an LTRA to an ICS or ICS/LABA, or addition of a LAAC to an ICS, ICS/LABA or LTRA.
†P values and ORs for each variable shown are from the multivariable model.
‡In pairwise comparisons, controller medications were initiated/escalated more often in asthma-related visits than in non-respiratory visits (OR 17.8, 95% CI 11.3 to 27.956) and in any respiratory-related visits than in non-respiratory visits (OR 7.7, 95% CI 5.7 to 11.159).
§This covariate was removed from the multivariable model since there were no subjects that had controlled asthma who had a controller initiated or escalated; the univariate p value was <0.01.
ED, emergency department; ICS, inhaled corticosteroid; LAAC, long-acting anticholinergic; LABA, long-acting beta-agonist; LTRA, leukotriene receptor antagonist; MRP, most responsible physician.
AAP delivery
There were no AAPs delivered by any prescriber over the 1-year study period to patients on a controller medication.
Discussion
We reviewed Canadian primary care asthma management and identified large gaps across three fundamental evidence-based practices.28 To our knowledge, this is the largest report to objectively characterise these gaps and to measure their predictors in a primary care asthma population.
Asthma control assessment was seldom performed, but was more common at academic sites (table 3). This may be due to practice variation, as seen in other care practices (table 2) and/or to population differences (table 1). Control was more often assessed if the presenting symptom was asthma or respiratory related. This may reflect formal control assessment or the effect of expected targeted questioning and/or patient symptom report. Although it might not be reasonable to expect clinicians to ascertain control at each visit, 85% of patients did not have control assessed despite an average of approximately five visits over the year and with 37% of patients having had at least one visit with a respiratory symptom. A lack of familiarity with control criteria may be a cause of this gap.29 In a Canadian study, primary care physicians identified an average of 2.2 out of 8 control criteria.30 Similarly, 26% of US primary care physicians were confident that they could assess asthma control.31 This problem is compounded by the fact that patients also underperceive their poor control and thus seldom volunteer poor control to their providers21. Additional barriers include lack of time, forgetting to assess control and patient preferences for consultation content.30 At least some of these barriers could be addressed by a periodic physician prompt with embedded questions30 (paper or electronic), and/or a self-directed patient asthma control questionnaire which could be completed before the clinical visit. Certain control criteria, such as absenteeism, were rarely ascertained and their importance should be emphasised in future behavioural interventions.
Although we did not find a report of this gap in a Canadian setting, a US review of 430 primary care charts noted that all control criteria were assessed in 1% (0.1% of visits in our cohort), and at least one criterion was assessed in 59% of visits (6% of visits in our cohort).31 In a 2014 UK review, among 135 patients who died of asthma and whose last asthma care visit had been in primary care, only 37 (27%) had asthma control assessed at that visit.5 Considering that a majority of primary care patients are found to be uncontrolled when asked all five symptom-based criteria, and that our data and others’2 suggest that practitioners are more likely to alter therapy in uncontrolled patients, our findings support a hypothesis that failure to recognise poor asthma control is a contributor to undermedication.
Correspondingly, therapy was infrequently augmented. Augmentation was more common in the non-academic site and during visits with asthma-related or respiratory-related symptoms (table 4). A lack of objective diagnosis also predicted augmentation, likely because these patients were less commonly on a controller medication. Augmentation was more common during visits with poor asthma control, but occurred in only 6% of such visits, suggesting that other barriers play a role. Although clinicians seem to be aware of the importance of systematic therapeutic escalation and recognise its expected favourable impact on outcomes,32 barriers include a lack of knowledge of specific guideline-recommended thresholds for initiating/escalating therapy,33–35 poor implementability of guidelines3 6 and patient factors such as medication affordability and ICS aversion.3 36 Overall, only 15% of patients received augmentation, compared with an estimated poor asthma control prevalence of 59% in prior studies.2 3 22 37 Whereas the British Asthma Guideline suggests reducing therapy after achieving control to minimise side-effects and cost,9 medication de-escalation occurred only once during the study period. Accordingly, our data may also suggest an ‘overtreatment’ care gap among the ~35% of patients who were on controller medications. To address this gap, future behaviour change interventions could use methods to elicit respiratory symptoms from patients and alert physicians to these, and/or could exclusively target visits with respiratory symptoms which appear to be an enabler of medication optimisation.
In a Canadian administrative database review, 37% of patients with poor control (defined based on short-acting bronchodilator prescriptions, ER/hospital visits or asthma deaths) were not prescribed an ICS, compared with 54.8% in our study. The study also found that 74% of those with poor control on a high dose ICS were not prescribed an add-on LABA,22 compared with 55.7% of patients with poor control on any ICS dose in our study. A similar administrative database review found that 47% of poorly controlled patients were not prescribed an ICS.38 A practice audit of 15 Scottish primary care practices also suggested underuse of LABA therapy, with 180/547 (32.9%) patients on high dose ICS not on add-on LABAs.32
We did not record any AAP delivery over the study period. Previously, 12.8% of surveyed Scottish GPs reported providing their patients with AAPs,32 and 11% of surveyed Canadian patients reported receiving an AAP.3 However, as is the case with other surveys,2 these data were likely affected by both reporting bias and selection bias. In contrast, both Canadian39 and US31 chart audits found results much closer to ours, with only 2% of patients having received an AAP. In a survey of Scottish patients who had an acute asthma attack requiring steroids or hospitalisation in the previous 6 months, 58/254 (22.8%) reported possession of a written AAP, however only 11 (3.9%) had received it from their GP.32 Similarly, the UK National Review of Asthma Deaths revealed that only 23% of patients who died of asthma had ever received an AAP (from primary or secondary care).5 Surveys and qualitative studies indicate that a majority of physicians are aware of guideline recommendations for AAPs and consider AAPs to be important,32 but fail to provide them due to a lack of time,3 40 41 experience and confidence,32 42 and lacking availability at the point of care.3 32 40 41 43–46 In a Canadian study, 30% of physicians attending an asthma skills workshop were unable to prepare an adequate AAP,45 while in a Scottish survey, an identical 30% of respondents indicated that they were ‘not at all’ confident in preparing AAPs for their patients.32 In the same survey, 47.7% of respondents indicated that AAP templates were not available in their practice.32 Practices with access to allied healthcare team members with specific asthma management skills and knowledge and effective communication for delegation of tasks have been shown to have higher asthma guideline adherence.47 Correspondingly, 46% of Scottish GPs indicated that a reorganisation of care would enable them to improve implementation,32 Accordingly, for this particular care gap, an organisational change may be required for increased uptake. Other complex interventions, such as a point-of-care computerised clinical decision support system which autogenerates an AAP might also be considered.48 Of interest, our data suggest that this problem is not limited to the primary care setting, given that less than 4% of patients seen by specialists received an AAP.
We believe that our sample may be reasonably representative of primary care academic and non-academic environments. We measured the behaviour of 46 staff physicians, 108 residents, 17 NPs and 2 PAs spanning a wide range of practice experience. No sites had access to allied health resources for asthma management. Accordingly, clinicians managed asthma individually, as would occur in smaller group or solo practices. The divergent sociodemographic compositions of the two involved cities (Hamilton and Brampton) also support generalisability. Hamilton is a large urban centre with an average age of 41.3 years24, median income of $87 59049 14.3% visible minorities50 and 6.3% unemployment.51 In contrast, Brampton is a suburb within the Greater Toronto Area, has an average age of 36.5 years,52 median income of $68 78253 66.4% visible minorities54 and 9.5% unemployment.55
Our study has several limitations. Our approach may have underestimated asthma control assessment and AAP delivery due to poor chart documentation. However, we believe that clinicians would be very likely to document poor asthma control if ascertained, given its clinical relevance and influence on treatment decisions. Furthermore, only 15.3% of patients had poor control documented, compared with the expected 59% prevalence of poor control,2 3 22 37 supporting the presence of an assessment care gap. Although chart reviews were performed remotely and contact with clinicians was minimal, clinicians may have improved care as a result of observation. Participation bias may have favoured those with an interest in asthma. Our sample may not be representative of jurisdictions with vastly different sociodemographic compositions and/or practice models than those studied. Additionally, although we used a validated algorithm to identify patients with asthma26 and physicians vetted algorithm-generated lists, some diagnostic misclassification likely occurred. Finally, our analysis was unable to account for repeated measures within subjects.
Conclusions
Large care gaps exist in primary care settings, across basic asthma care recommendations that have been found across international guidelines for over 15 years, and that are widely considered to be the standard of care. These care gaps are larger than previously found in self-report and survey-based studies. Complex implementation strategies will be required to overcome these gaps. Behavioural predictors identified quantitatively in this study complement those identified previously through surveys and qualitative studies. These factors should now be used to tailor and then test specific implementation strategies to effect behaviour change for each key care gap.
Supplementary Material
Footnotes
Contributors: CP contributed to the data analysis, interpretation and manuscript preparation; GA, DC and SGo contributed to study design, recruitment and manuscript revisions; SES, AGK and L-PB contributed to study design and manuscript revisions; MMM and GL contributed to study design, statistical analysis and interpretation, and manuscript revisions; SGu conceived of the study and oversaw all aspects of the study, including manuscript review.
Funding: CP has been supported by the The Keenan Research Centre in the Li Ka Shing Knowledge Institute Summer Student Program; SGu is supported by the Michael Locke Term Chair in Knowledge Translation and Rare Lung Disease Research. SES is supported by a Tier 1 Canada Research Chair in Knowledge Translation, and the Mary Trimmer Chair in Geriatric Medicine. This work was supported by the Canadian Institutes of Health Research (236225, 322013).
Disclaimer: Funders had no input into the design, execution, analysis or reporting of this work.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: St. Michael’s Hospital Research Ethics Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All study data can be made available upon request to the corresponding author.
References
- 1. Mukherjee M, Gupta R, Farr A, et al. Estimating the incidence, prevalence and true cost of asthma in the UK: secondary analysis of national stand-alone and linked databases in England, Northern Ireland, Scotland and Wales-a study protocol. BMJ Open 2014;4:e006647 10.1136/bmjopen-2014-006647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapman KR, Boulet LP, Rea RM, et al. Suboptimal asthma control: prevalence, detection and consequences in general practice. Eur Respir J 2008;31:320–5. 10.1183/09031936.00039707 [DOI] [PubMed] [Google Scholar]
- 3. FitzGerald JM, Boulet LP, McIvor RA, et al. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006;13:253–9. 10.1155/2006/753083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Asthma Physician and Patient Survey. 2005. http://www.gappsurvey.org/media-key-findings.html (Accessed 1 Feb 2018).
- 5. Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) confidential enquiry report. London: Royal College of Physicians, 2014. [Google Scholar]
- 6. Gupta S, Rai N, Bhattacharrya O, et al. Optimizing the language and format of guidelines to improve guideline uptake. CMAJ 2016;188:E362–68. 10.1503/cmaj.151102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, Paolucci E, Kaplan A, et al. Contemporaneous international asthma guidelines present differing recommendations: an analysis. Can Respir J 2015:30. [PubMed] [Google Scholar]
- 8. Lougheed MD, Lemière C, Dell SD, et al. Canadian Thoracic Society Asthma Management Continuum--2010 Consensus Summary for children six years of age and over, and adults. Can Respir J 2010;17:15–24. 10.1155/2010/827281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. 2016. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/ (Cited Feb 2018).
- 10. Ernst P, Fitzgerald JM, Spier S. Canadian asthma consensus conference summary of recommendations. Can Respir J 1996;3:89–101. 10.1155/1996/314657 [DOI] [Google Scholar]
- 11. British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2003;58(Suppl 1):i1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams NP, Bestall JC, Jones P. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 2008:CD003274. [Google Scholar]
- 13. Adams NP, Bestall JC, Lasserson TJ, et al. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev 2008:CD003135 10.1002/14651858.CD003135.pub4 [DOI] [PubMed] [Google Scholar]
- 14. Adams NP, Bestall JB, Malouf R, et al. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev 2005:CD002738 10.1002/14651858.CD002738.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev 2008:CD006217 10.1002/14651858.CD006217.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. British Thoracic Society, Research Unit of the Royal College of Physicians of London, King’s Fund Centre, National Asthma Campaign. Guidelines for management of asthma in adults: I--Chronic persistent asthma. Statement by the British Thoracic Society, Research Unit of the Royal College of Physicians of London, King’ s Fund Centre, National Asthma Campaign. BMJ 1990;301:651–3. 10.1136/bmj.301.6753.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev 2010:CD005535 10.1002/14651858.CD005535.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lemière C, Bai T, Balter M, et al. Adult asthma consensus guidelines update 2003. Can Respir J 2004;11:9A–18. 10.1155/2004/271362 [DOI] [PubMed] [Google Scholar]
- 19. Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003:CD001117 10.1002/14651858.CD001117 [DOI] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence (NICE). Asthma: diagnosis, monitoring and chronic asthma management. London: NICE, 2017. [PubMed] [Google Scholar]
- 21. Chapman KR, Ernst P, Grenville A, et al. Control of asthma in Canada: failure to achieve guideline targets. Can Respir J 2001;8:35A–40. 10.1155/2001/245261 [DOI] [PubMed] [Google Scholar]
- 22. Klomp H, Lawson JA, Cockcroft DW, et al. Examining asthma quality of care using a population-based approach. CMAJ 2008;178:1013–21. 10.1503/cmaj.070426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Census Profile: Hamilton Ontario. 2016. http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMACA&Code1=537&Geo2=PR&Code2=35&Data=Count&SearchText=Hamilton&SearchType=Begins&SearchPR=01&B1=All (Accessed 25 Jul 2017).
- 25. Census Profile: Brampton Ontario. 2016. http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CSD&Code1=3521010&Geo2=PR&Code2=35&Data=Count&SearchText=Peel&SearchType=Begins&SearchPR=01&B1=All (Accessed 25 Jul 2017).
- 26. Xi N, Wallace R, Agarwal G, et al. Identifying patients with asthma in primary care electronic medical record systems Chart analysis-based electronic algorithm validation study. Can Fam Physician 2015;61:e474-83. [PMC free article] [PubMed] [Google Scholar]
- 27. Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J 2012;19:127–64. 10.1155/2012/635624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. To T, Guttmann A, Lougheed MD, et al. Evidence-based performance indicators of primary care for asthma: a modified RAND Appropriateness Method. Int J Qual Health Care 2010;22:476–85. 10.1093/intqhc/mzq061 [DOI] [PubMed] [Google Scholar]
- 29. Boulet LP, Bourbeau J, Skomro R, et al. Major care gaps in asthma, sleep and chronic obstructive pulmonary disease: a road map for knowledge translation. Can Respir J 2013;20:265–9. 10.1155/2013/496923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Renzi PM, Ghezzo H, Goulet S, et al. Paper stamp checklist tool enhances asthma guidelines knowledge and implementation by primary care physicians. Can Respir J 2006;13:193–7. 10.1155/2006/825281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cicutto L, Dingae MB, Langmack EL. Improving asthma care in rural primary care practices: a performance improvement project. J Contin Educ Health Prof 2014;34:205–14. 10.1002/chp.21254 [DOI] [PubMed] [Google Scholar]
- 32. Wiener-Ogilvie S, Pinnock H, Huby G, et al. Do practices comply with key recommendations of the British Asthma Guideline? If not, why not? Prim Care Respir J 2007;16:369–77. 10.3132/pcrj.2007.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goeman DP, Hogan CD, Aroni RA, et al. Barriers to delivering asthma care: a qualitative study of general practitioners. Med J Aust 2005;183:457–60. [DOI] [PubMed] [Google Scholar]
- 34. Doerschug KC, Peterson MW, Dayton CS, et al. Asthma guidelines: an assessment of physician understanding and practice. Am J Respir Crit Care Med 1999;159:1735–41. 10.1164/ajrccm.159.6.9809051 [DOI] [PubMed] [Google Scholar]
- 35. Pinnock H, Holmes S, Levy ML, et al. Knowledge of asthma guidelines: results of a UK General Practice Airways Group (GPIAG) web-based ‘Test your Knowledge’ quiz. Prim Care Respir J 2010;19:180–4. 10.4104/pcrj.2009.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cooper V, Metcalf L, Versnel J, et al. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med 2015;25:15026 10.1038/npjpcrm.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McIvor RA, Boulet LP, FitzGerald JM, et al. Asthma control in Canada: no improvement since we last looked in 1999. Can Fam Physician 2007;53:672–7. [PMC free article] [PubMed] [Google Scholar]
- 38. Ahmed S, Tamblyn R, Winslade N. Using decision support for population tracking of adherence to recommended asthma guidelines. BMJ Open 2014;4:e003759 10.1136/bmjopen-2013-003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsuyuki RT, Sin DD, Sharpe HM, et al. Management of asthma among community-based primary care physicians. J Asthma 2005;42:163–7. 10.1081/JAS-54615 [DOI] [PubMed] [Google Scholar]
- 40. Moffat M, Cleland J, van der Molen T, et al. Poor communication may impair optimal asthma care: a qualitative study. Fam Pract 2007;24:65–70. 10.1093/fampra/cml062 [DOI] [PubMed] [Google Scholar]
- 41. To T, McLimont S, Wang C, et al. How much do health care providers value a community-based asthma care program? A survey to collect their opinions on the utilities of and barriers to its uptake. BMC Health Serv Res 2009;9:77 10.1186/1472-6963-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ring N, Jepson R, Hoskins G, et al. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Patient Educ Couns 2011;85:e131–43. 10.1016/j.pec.2011.01.025 [DOI] [PubMed] [Google Scholar]
- 43. Labelle M, Beaulieu M, Renzi P, et al. Integrating clinical practice guidelines into daily practice: impact of an interactive workshop on drafting of a written action plan for asthma patients. J Contin Educ Health Prof 2004;24:39–49. 10.1002/chp.1340240107 [DOI] [PubMed] [Google Scholar]
- 44. Partridge MR. Written asthma action plans. Thorax 2004;59:87–8. 10.1136/thx.2003.016451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lougheed MD, Moosa D, Finlayson S, et al. Impacts of a provincial asthma guidelines continuing medical education project: the Ontario asthma plan of action’s provider education in asthma care project. Can Respir J 2007;14:111–7. 10.1155/2007/768203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamontagne AJ, Pelàez S, Grad R, et al. Facilitators and solutions for practicing optimal guided asthma self-management: the physician perspective. Can Respir J 2013;20:285–93. 10.1155/2013/146839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiener-Ogilvie S, Huby G, Pinnock H, et al. Practice organisational characteristics can impact on compliance with the BTS/SIGN asthma guideline: qualitative comparative case study in primary care. BMC Fam Pract 2008;9:9 10.1186/1471-2296-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bousquet J, Chavannes NH, Guldemond N, et al. Realising the potential of mHealth to improve asthma and allergy care: how to shape the future. Eur Respir J 2017;49:17 10.1183/13993003.00447-2017 [DOI] [PubMed] [Google Scholar]
- 49. Median total income, by family type, by census metropolitan area. 2017. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/famil107a-eng.htm (Accessed 25 Jul 2017).
- 50. Statistics Canada. Visible minority population and top three visible minority groups, selected census metropolitan areas, Canada. 2011. http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/2011001/tbl/tbl2-eng.cfm (Accessed 25 Jul 2017).
- 51. Statistics Canada. Labour force characteristics by census metropolitan area, three-month moving average, seasonally adjusted and unadjusted, last 5 months. 2017. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/lfss04f-eng.htm (Accessed 10 Aug 2017).
- 52. Statistics Canada. Census subdivision of Brampton, CY-Onario. 2011. http://www12.statcan.gc.ca/census-recensement/2011/as-sa/fogs-spg/Facts-csd-eng.cfm?LANG=Eng&GK=CSD&GC=3521010 (Accessed 25 Jul 2017).
- 53. General Facts. 2011. https://www.peelregion.ca/planning/pdc/data/quickfacts.htm (Accessed 25 Jul 2017).
- 54. Immigration and ethnocultural diveristy in Canada: visible minority population. 2011. http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm#a4 (Accessed 25 Jul 2017).
- 55. Statistics Canada. NHS Profile, Brampton, CY, Ontario, 2011. 2011. http://www12.statcan.gc.ca/nhs-enm/2011/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=CSD&Code1=3521010&Data=Count&SearchText=Peel&SearchType=Begins&SearchPR=01&A1=All&B1=All&Custom= (Accessed 25 Jul 2017).
- 56. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org (Cited Feb 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.