Abstract
A hepatic comorbidity of metabolic syndrome, known as nonalcoholic fatty liver disease (NAFLD), is increasing in prevalence in conjunction with the pandemics of obesity and diabetes. The spectrum of NAFLD ranges from simple hepatic fat accumulation to a more severe disease termed nonalcoholic steatohepatitis (NASH), involving inflammation, hepatocyte death, and fibrosis. Importantly, NASH is linked to a much higher risk of cirrhosis, liver failure, and hepatocellular carcinoma, as well as an increased risk for nonhepatic malignancies and cardiovascular disease. Interest in the understanding of the disease processes and search for treatments for the spectrum of NAFLD-NASH has increased exponentially, but there are no approved pharmacologic therapies. In this review, we discuss the existing literature supporting insulin-sensitizing thiazolidinedione compounds as potential drug candidates for the treatment of NASH. In addition, we put these results into new context by summarizing recent studies suggesting these compounds alter mitochondrial metabolism by binding and inhibiting the mitochondrial pyruvate carrier.
Keywords: Nonalcoholic Steatohepatitis, Mitochondria, Pyruvate, Thiazolidinedione
Abbreviations used in this paper: HSC, hepatic stellate cell; HTF-C, high trans-fat, fructose, cholesterol diet; MPC, mitochondrial pyruvate carrier; NAFLD, nonalcoholic fatty liver disease; NAS, nonalcoholic fatty liver disease activity score; NASH, nonalcoholic steatohepatitis; PPARγ, peroxisome proliferator-activated receptor γ; ROS, reactive oxygen species; TCA, tricarboxylic acid; TZD, thiazolidinedione
Summary.
This article reviews the recent studies suggesting a potential role for inhibiting mitochondrial pyruvate metabolism as a strategy for treatment of nonalcoholic steatohepatitis.
Interest in better understanding nonalcoholic fatty liver disease (NAFLD) has increased exponentially as a result of the increasing prevalence of the disease and it now is appreciated how significantly the disease impacts overall health. NAFLD is a spectrum of disease that ranges from simple hepatic steatosis to the more severe nonalcoholic steatohepatitis (NASH), involving hepatocellular injury, inflammation, and hepatic fibrosis. Although most individuals with steatosis do not progress to NASH, because of its prevalence, the number of patients that will develop severe forms of the disease is staggering. In the United States, it is estimated that more than 83.1 million people have NAFLD and as many as 27% of these individuals may have NASH.1 Importantly, NASH increases the risk of developing cirrhosis, liver failure, and hepatocellular carcinoma, as well as an increased predisposition to non–liver-associated diseases such as cardiovascular disease and cancer.2, 3, 4, 5, 6 Despite this high prevalence and the negative health impact of NASH, there are no approved therapeutic agents for treating this spectrum of conditions.
Nutrient oversupply is likely a driving force in developing NAFLD. High levels of dietary fat or sugar, free fatty acids from adipose tissue, and de novo lipogenesis all likely contribute to the overabundance of lipids.7 At some point, and because of factors that are still emerging, lipid accumulation activates inflammatory cascades and tissue injury responses that lead to the development of NASH. Multiple cell types in the liver play a role in this progression. The parenchymal cells of the liver, hepatocytes, are the main site of neutral lipid storage and secrete a number of factors that can communicate with other cell types to drive the progression to NASH. Cells of the immune system also play a role through release of inflammatory cytokines and other factors. Finally, hepatic stellate cells (HSCs) are fibroblastic cells that migrate to the site of injury and secrete extracellular matrix components that make up the fibrotic lesions observed histologically in NASH.8 To be considered successful, phase 3 clinical trials for NASH will need to show significant histologic improvements in fibrosis or NAFLD activity scoring (NAS) (assessment of steatosis, hepatocyte death [ballooning], and inflammation) without worsening other histologic end points.
Obesity-related insulin resistance and type 2 diabetes are tightly linked to the development of NAFLD and progression to NASH.9, 10, 11, 12, 13 Accumulation of fat has been linked to impaired insulin signaling via accumulation of specific species of lipids that activate signaling cascades14, 15 (Figure 1). The cause and effect relationship between hepatic steatosis and insulin resistance is not always clear,16 but abundant evidence suggests that insulin resistance may drive local and systemic alterations in metabolism that promote liver lipid accumulation (Figure 1). For instance, hyperinsulinemia in insulin-resistant states stimulates hepatic de novo lipogenesis,17 and this newly synthesized lipid comprises roughly 25% of the hepatic triglyceride accumulating in NAFLD.18 Insulin-resistant adipose tissue is linked to higher rates of basal lipolysis and thus exposure of the liver to increased plasma free fatty acid concentrations.19, 20 Indeed, roughly 60% of hepatic triglycerides in human NAFLD subjects are derived from plasma fatty acids.18 For some time now, it has been hypothesized that interventions to treat insulin resistance will have utility for concomitant attenuation of NAFLD and NASH.
Figure 1.
Insulin resistance drives hepatic steatosis in NAFLD. In the United States and Europe, the majority of NAFLD is associated with obesity and insulin resistance. For adipose tissue, the inability to respond to insulin results in dysregulated lipolysis and increased free fatty acids, which then are sequestered by the liver. Hyperinsulinemia and hyperglycemia also stimulate de novo lipogenesis, which is the creation of new lipids from carbohydrate or amino acid carbon backbones. Together with enhanced uptake of fats from dietary sources, the liver stores these fatty acids in lipid droplets and becomes steatotic. Although controversial, hepatic steatosis likely further exacerbates insulin resistance.
Thiazolidinediones for Treatment of NASH
Given the tight linkage to insulin resistance, the insulin-sensitizing thiazolidinediones (TZDs) rosiglitazone and pioglitazone have been evaluated extensively as NAFLD treatments.21, 22, 23, 24, 25, 26, 27, 28 These antidiabetic and anti-inflammatory compounds are agonists of the nuclear transcription factor peroxisome proliferator-activated receptor γ (PPARγ),29 which activates a transcriptional program driving fatty acid storage and adipocyte differentiation. Unfortunately, PPARγ activation is associated with a number of side effects such as weight gain, edema, and bone mineral density loss.30 Rosiglitazone, the most potent PPARγ agonist, was linked to increased cardiovascular mortality,31, 32, 33 which later was discounted, but caused clinical use of TZDs to plummet.34 In addition to their strong insulin-sensitizing effects in high-fat diet–fed rodents, TZDs markedly reduced inflammation35, 36 and prevented37 or reversed38 hepatic fibrosis, including direct effects on stellate cell activation. Unfortunately, although early studies conducted in human beings produced results that generally were positive for improving steatosis and plasma markers for liver injury such as alanine aminotransferase, they largely did not result in significant histologic improvement with TZD therapy.21, 22, 23, 24, 25, 26, 27, 28, 39 In the majority of the early clinical NASH trials with TZD compounds, drug exposures were quite low and for a limited duration, likely in attempt to avoid these clinical side effects.
Interestingly, a more recent trial using 45 mg/day pioglitazone for 18–36 months resulted in NASH histologic improvement, including fibrosis score improvement, compared with placebo, without significant safety issues.40 Although the response to pioglitazone was quite variable in these subjects, it was shown that the degree of histologic improvements was correlated with blood pioglitazone (as well as active drug metabolite hydroxypioglitazone and ketopioglitazone) concentrations.41 The likelihood of improvement also was linked to genetic variation in the CYP2C8 gene,42 which encodes the cytochrome P450 enzyme isoform that metabolizes pioglitazone.43 In addition, a recent meta-analysis of 8 randomized clinical trials evaluating pioglitazone or rosiglitazone in NASH concluded that NASH resolution and fibrosis improvement is observed with pioglitazone, but not rosiglitazone.44, 45 Because pioglitazone shows significantly weaker affinity for PPARγ compared with rosiglitazone,29 one must consider whether additional molecular targets of TZDs are responsible for the improvements of NASH observed with pioglitazone.
Interestingly, a number of PPARγ-sparing TZD compounds, including Metabolic Solutions Development Company (MSDC)-0160 and MSDC-0602, recently were synthesized and shown to have potent insulin-sensitizing and antifibrotic effects.46, 47, 48 These compounds show PPARγ-binding affinities that are roughly an order of magnitude less than pioglitazone, and 2 orders of magnitude less than rosiglitazone.46 MSDC-0160 and MSDC-0602 also show extremely low PPARγ luciferase reporter activation,46, 49 and treatment with MSDC compounds does not induce PPARγ-target genes in cultured adipocytes46 or cause weight gain in a clinical trial.47 However, the studies conducted in mice show strong anti-inflammatory effects and evidence of adipose tissue browning.46 These PPARγ-sparing TZD compounds also improve insulin sensitivity in both mouse models46 and in clinical trials.47 In addition, the potassium salt of MSDC-0602, MSDC-0602K, reduced liver injury in a mouse model of NASH by reducing the NAS and fibrosis scores, as well as acting directly on isolated stellate cells to suppress their activation. MSDC-0602K is currently in a phase 2b randomized, double-blinded, clinical trial for NASH (Effects of Modulation of the Mitochondrial pyruvate carrier In NASH - Evaluation of a New Chemical Entity [EMMINENCE]; NCT02784444). These findings are consistent with alternative molecular targets for TZDs that may have beneficial pharmacology.
TZDs Target the Mitochondrial Pyruvate Carrier and Inhibit Pyruvate Metabolism
Over the years, a number of studies have suggested that, at higher concentrations, TZDs can exert effects that are independent of PPARγ and transcriptional regulation. Many of the immediate, nongenomic effects of TZDs are on mitochondrial function, including decreased mitochondrial membrane potential, increased permeability, increased reactive oxygen species (ROS) abundance, and increased intracellular calcium.50, 51, 52 TZD infusion into isolated rat liver increased lactate production and decreased glucose output within 10 minutes,53 and similar effects on lactate production in astrocytes were independent of gene transcription or protein translation.54 These effects initially were proposed to be owing to inhibition of mitochondrial complex I activity.55, 56 However, the respiration defects subsequently were reported to be specific to pyruvate-stimulated, and not glutamate-stimulated, respiration, indicating the TZD effect to be upstream of complex I.54, 57 In an attempt to identify a mitochondrial TZD target, a pioglitazone-based 125I-labeled probe was created and shown to bind mitochondrial lysates at an approximately 14- to 17-kilodalton protein. Although initial proteomic studies identified an outer mitochondrial membrane protein called mitoNEET,58 mitoNEET knockdown by small interfering RNA did not affect pioglitazone’s ability to increase lactate production57 and the band cross-linked by the probe at approximately 14 kilodaltons still was present in liver lysates from mitoNEET null mice.59
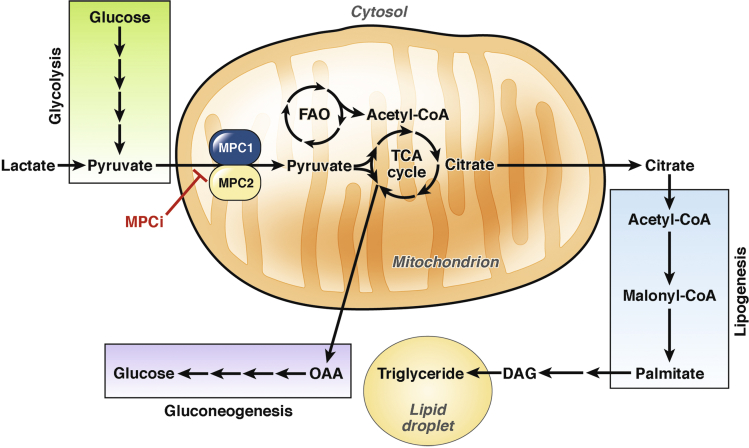
Additional proteomic studies later identified multiple peptides for the mitochondrial pyruvate carrier (MPC).59 Pyruvate generated in the cytosol must enter into the mitochondrial matrix for further metabolism by an inner mitochondrial membrane solute carrier (Figure 2), which only recently has been cloned.60, 61 In the mitochondrial matrix, pyruvate can be oxidized by either pyruvate dehydrogenase to produce acetyl-CoA, or carboxylated by pyruvate carboxylase to form oxaloacetate (Figure 2). Although the composition and structure of the MPC complex is not entirely known, 2 proteins, MPC1 and MPC2, are integral components because deletion of 1 of these subunits causes loss of the other subunit and loss of pyruvate transport activity.60, 61, 62, 63 Consistent with an inhibitory interaction, TZD compounds were shown to reduce pyruvate-stimulated respiration in myocytes, and small interfering RNA–mediated knockdown of the MPC subunits sensitized cells/mitochondria to this inhibitory effect of TZDs.64 The PPARγ-sparing TZD, MSDC-0602, inhibited mitochondrial respiration on pyruvate/malate in a matter of minutes, and this effect was lost in MPC2 knockout liver mitochondria.62 Importantly, because the affinity of MSDC-0160 and MSDC-0602 for mitochondrial binding is nearly identical to the traditional TZDs rosiglitazone and pioglitazone,46, 59 it is likely that the MSDC compounds have a preferential pharmacology toward the mitochondrial target. However, MSDC compounds retain the TZD backbone structure and show minor PPARγ binding and activation. Thus, it is impossible to eliminate the possibility of PPARγ-agonism from their pharmacology.30
Figure 2.
The mitochondrial pyruvate carrier controls hepatic intermediary metabolism. Pyruvate that is generated in the cytosol via glycolysis or from lactate must enter the mitochondrion for further metabolism. The MPC transports pyruvate across the inner mitochondrial membrane into the matrix. Pyruvate then can be converted to acetyl-CoA by pyruvate dehydrogenase to fuel the TCA cycle. Alternatively, a large proportion of pyruvate in hepatic mitochondria is converted to oxaloacetate by pyruvate carboxylase, which can be transported to the cytosol and converted to new glucose via the process of gluconeogenesis. The TCA cycle intermediate citrate can be exported to the cytosol, and becomes the building block for new fatty acids in de novo lipogenesis. These fatty acids ultimately are converted to triglycerides and either stored in lipid droplets or excreted in lipoproteins. Inhibition of the MPC can reduce carbon flow into the TCA cycle and alter the ability for hepatic mitochondria to perform gluconeogenesis and de novo lipogenesis. In addition, reduction of pyruvate oxidation would enhance fatty acid oxidation, potentially also decreasing hepatic lipid storage. DAG, diacylglycerol; FAO, fatty acid oxidation; MPCi, mitochondrial pyruvate carrier inhibitor; OAA, oxaloacetate.
MPC as a Target for Treating NASH
Several recent studies conducted in rodent models have evaluated the effects of modulating mitochondrial pyruvate metabolism on NAFLD and NASH end points. Complete deletion of MPC proteins is lethal at early embryonic stages,65, 66 but mice with liver-specific MPC163 or MPC262 deletion are viable and outwardly normal. Because mitochondrial pyruvate metabolism is essential for the process of gluconeogenesis from pyruvate (Figure 2), both mouse models showed a decreased capacity to convert pyruvate into new glucose.62, 63 The liver knockout mice were protected from hyperglycemia in high-fat diet–induced or genetic (crossed with db/db) models of insulin resistance and diabetes,62, 63 likely owing to diminished hepatic glucose production. However, the MPC-deficient livers were not protected from developing hepatic steatosis in the obese mouse models.62, 63
Most mouse genetic or high-fat diet mouse models do not develop severe liver inflammation or fibrosis. To study the importance of mitochondrial pyruvate metabolism in NASH, we used a diet enriched in trans-fat, fructose, and cholesterol (HTF-C), which previously has been shown to induce NASH-like steatosis, inflammation, and fibrosis in mice.67 Liver-specific MPC2 deletion and/or treatment with the PPARγ-sparing TZD MSDC-0602 did not significantly improve hepatic steatosis in this HTF-C diet model.48 However, accumulation of oxidized lipids in these livers was reduced with MSDC-0602 treatment.48 Histologic scoring and gene expression analyses of these livers showed a reduction in NAS and fibrosis scoring along with reduced expression of markers of HSC activation with either genetic or pharmacologic MPC inhibition.48 In addition, MSDC-0602 could directly limit HSC activation in vitro.48
As indicated earlier, mice with liver-specific MPC2 knockout were protected from stellate cell activation on the HTF-C diet.48 However, both quiescent and activated HSCs from mice with liver-specific MPC2 deletion were shown to normally express Mpc2, indicating that the albumin Cre transgene induced Mpc2 deletion specifically in hepatocytes.48 This led us to believe that hepatocytes could release signals that are received by HSCs to alter their activation. Although the molecular signals have not yet been identified, primary mouse HSCs treated with extracellular vesicles isolated from the plasma of wild-type or MPC2-deficient mice fed the NASH-inducing HTF-C diet showed that extracellular vesicles from the liver-specific MPC2 null mice elicited less HSC activation.48 Elucidating the mediator(s) contained in these vesicles that modulate HSC fibrogenesis is an area of ongoing research.
Hepatic inflammation is believed to promote the development of fibrosis in NASH. Although MPC2 deletion or MSDC-0602 treatment did not significantly improve inflammation histologically or at the gene expression level,48 another study found that MPC1 deletion decreased hepatic inflammation and fibrosis after a long-term (44 weeks) 60% high-fat diet.68 Moreover, it was shown that MPC inhibition with a specific inhibitor (UK-5099) in isolated hepatocytes acutely decreased inflammatory cytokine expression induced by palmitate and lipopolysaccharide.68 Collectively, these data suggest that MPC inhibition decreases NASH pathology by preventing lipid-induced hepatocellular damage, decreasing inflammation, maintaining HSC in a quiescent state, and by modulating mediators released by hepatocytes that control HSC activation.
How Does Modulating Mitochondrial Pyruvate Metabolism Treat NAFLD?
One of the most significant outstanding questions is how targeting mitochondrial pyruvate metabolism is beneficial in treating NAFLD. There are several possibilities, as summarized in Figure 3. Evidence is now accumulating that mitochondria from NAFLD livers of animal models and human beings show increased tricarboxylic acid (TCA) cycle function,69, 70, 71, 72, 73, 74, 75, 76 but impaired respiratory coupling.70, 77, 78 Anaplerotic/cataplerotic flux (nonoxidative) of metabolites into/out of the TCA cycle also is increased in NAFLD.71 This increase in mitochondrial metabolic flux in conjunction with decreased oxidative capacity can lead to increased ROS formation and hepatocellular damage.71, 73, 79 It also has been postulated that lobular hypoxia secondary to high rates of oxidative metabolism exacerbates ROS formation.77 Increased fatty acid delivery to the liver from insulin-resistant adipose tissue seems to drive this increase in TCA cycle and anaplerosis.71 However, in rodent models some mitochondrial abnormalities can be observed before profound insulin resistance and hepatic steatosis develops.78 Presently, it still is debated whether this increase in TCA cycle flux exists after the transition from steatosis to NASH in human beings. Liver mitochondrial content and oxygen consumption was measured in obese human subjects with and without NAFLD and subjects with NASH.80 Interestingly, both subjects with NAFLD and NASH show increased mitochondrial content, but although NAFLD subjects had increased oxygen consumption rates, liver mitochondria from subjects with NASH showed reduced oxygen consumption, increased ROS production, and oxidative damage.80 Although this human study did not assess TCA cycle flux or anaplerosis/cataplerosis rates, it appears that mice fed a trans-fat diet inducing NASH still show increased TCA cycle activity.73 In addition, pigs fed a NASH-inducing Western diet showed increased citrate synthase activity, which can serve as a proxy for both mitochondrial content and TCA cycle activity.81 With this model of mitochondrial metabolic overload in NAFLD, improvements could be envisioned by either increasing oxidative capacity, as seen with exercise training,79, 82, 83, 84 or by decreasing the metabolite transport into mitochondria, which is driving the increase in TCA flux.68, 71
Figure 3.
The multifaceted benefits of MPC inhibition in NASH. Systemic MPC inhibition and the associated increased insulin sensitivity could improve nearly every aspect of the pathophysiology of NASH. Improved insulin sensitivity would decrease adipose tissue lipolysis of free fatty acids, and reduce the lipid load on the liver. Reduced hyperinsulinemia and increased hepatic insulin sensitivity would decrease de novo lipogenesis and decrease gluconeogenesis. Blocking pyruvate entry into the mitochondrion also decreases TCA cycle flux, which is increased in NAFLD. This normalization of TCA cycle flux to oxidative capacity would result in decreased reactive oxygen species and cell damage signals. This would reduce inflammation. MPC inhibition on inflammatory cells such as Kupffer cells likely also directly reduces inflammation. Reduced inflammation, as well as direct effects, then would reduce the activation and fibrogenesis of hepatic stellate cells. Finally, it also has been observed that MPC inhibition can induce a hepatocyte-derived signal contained within extracellular vesicles that can reduce stellate cell activation. TGF-β, transforming growth factor β.
Inhibiting pyruvate flux into the mitochondrion also would limit metabolic substrates, driving the enhanced TCA cycle flux and anaplerosis in NAFLD/NASH. In many organs, pyruvate is a major metabolite fueling the TCA cycle after pyruvate dehydrogenase converts pyruvate to acetyl-CoA (Figure 2), although in the liver a majority of pyruvate is carboxylated via the pyruvate carboxylase enzyme into oxaloacetate (OAA) under many nutritional conditions (Figure 2). Hepatocytes with MPC1 or MPC2 deletion62, 63, 68 or treated with MSDC-060262 show decreased flux of pyruvate carbons into TCA cycle intermediates. However, more sophisticated isotopomer flux experiments and modeling are required to fully assess the MPCs role in governing TCA cycle and anaplerotic activity. Knockout of MPC proteins likely is well compensated for by increased rates of fatty acid oxidation and amino acid utilization.62, 63 Interestingly, increased glutaminolysis recently was shown to drive HSC activation,85 allowing speculation that MPC inhibition and enhanced glutamine metabolism in hepatocytes could limit the availability of glutamine for HSCs and therefore decrease HSC activation.
As noted earlier, intrahepatic triglyceride levels are affected by several metabolic processes, including fatty acid oxidation, de novo lipogenesis, and release of fatty acids from adipose tissues, which are dysregulated in obesity and insulin resistance. Altering mitochondrial pyruvate metabolism could affect each one of these pathways. For example, blocking the ability to oxidize pyruvate from glucose leads to increased oxidation of fatty acids in MPC-deficient hepatocytes62 or hepatocytes treated with MSDC-0602.46 Mitochondrial pyruvate metabolism into citrate is also the initial step in de novo lipogenesis (Figure 2), and thus inhibiting MPC activity may reduce hepatic lipid synthesis. However, reduced MPC expression or MPC inhibition with UK-5099 did not greatly alter lipid synthesis owing to compensation by increased amounts of glutamine carbons into newly synthesized lipids.86, 87 In NAFLD driven by insulin resistance, a large proportion of fatty acids delivered to the liver are released from adipose tissue lipolysis. Because the obese liver-specific MPC-deficient mice still are peripherally insulin-resistant, it is likely that enhanced adipose tissue lipolysis drives the triglyceride accumulation in these livers. On the other hand, improving insulin sensitivity would reduce hyperinsulinemia, which then should reduce hepatic de novo lipogenesis. Indeed, treatment with pioglitazone or MSDC-0602 significantly reversed hepatic triglyceride accumulation in high-fat diet–fed mice,88 potentially by both reducing hepatic de novo lipogenesis and peripheral effects. Taken together, these results suggest that MPC deficiency or inhibition could alter the numerous metabolic pathways regulating hepatic steatosis, but metabolic compensation likely modifies these effects. In our opinion, this further points to the importance of correcting peripheral insulin resistance in the treatment of NASH.
Conclusions
NAFLD and NASH are an increasingly prevalent and costly health burden with no approved therapeutics at this time. The MPC is an attractive target in NASH because of the many metabolic alterations that could occur in both hepatocytes and stellate cells with inhibition of mitochondrial pyruvate metabolism. Intriguingly, this MPC-driven pharmacology could have been an unrecognized benefit of treating NASH with pioglitazone. Indeed, pioglitazone treatment decreases TCA cycle flux and anaplerosis in rodent models of NASH.74 In addition to the liver-centric effects of MPC inhibition, improving peripheral insulin sensitivity with both the traditional and PPARγ-sparing TZDs would both target an underlying driver of NAFLD and reduce fatty acid delivery to the liver (Figure 3).
Footnotes
Author contributions Kyle S. McCommis and Brian N. Finck were both fully involved in the design, drafting, and revision of this review article.
Conflicts of interest Brian N. Finck is a shareholder and member of the Scientific Advisory Board for Cirius Therapeutics. The remaining author discloses no conflicts.
Funding Supported by NIH grants K99 HL136658 and P30 DK052574 (K.S.M.), and R01 DK104735 (B.N.F.).
References
- 1.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harlow K.E., Africa J.A., Wells A., Belt P.H., Behling C.A., Jain A.K., Molleston J.P., Newton K.P., Rosenthal P., Vos M.B., Xanthakos S.A., Lavine J.E., Schwimmer J.B., Nonalcoholic Steatohepatitis Clinical Research Network Clinically actionable hypercholesterolemia and hypertriglyceridemia in children with nonalcoholic fatty liver disease. J Pediatr. 2018;198:76–83 e2. doi: 10.1016/j.jpeds.2018.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon T.G., Bamira D.G., Chung R.T., Weiner R.B., Corey K.E. Nonalcoholic steatohepatitis is associated with cardiac remodeling and dysfunction. Obesity (Silver Spring) 2017;25:1313–1316. doi: 10.1002/oby.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 5.VanWagner L.B., Wilcox J.E., Colangelo L.A., Lloyd-Jones D.M., Carr J.J., Lima J.A., Lewis C.E., Rinella M.E., Shah S.J. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population-based study. Hepatology. 2015;62:773–783. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongjarupong N., Assavapongpaiboon B., Susantitaphong P., Cheungpasitporn W., Treeprasertsuk S., Rerknimitr R., Chaiteerakij R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17:149. doi: 10.1186/s12876-017-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H., Pradere J.P., Schwabe R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim H.W., Bernstein D.E. Risk factors for the development of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including genetics. Clin Liver Dis. 2018;22:39–57. doi: 10.1016/j.cld.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Smits M.M., Ioannou G.N., Boyko E.J., Utzschneider K.M. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol. 2013;28:664–670. doi: 10.1111/jgh.12106. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S., Vanni E., Villanova N., Melchionda N., Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 12.Choudhury J., Sanyal A.J. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575–594. doi: 10.1016/j.cld.2004.04.006. ix. [DOI] [PubMed] [Google Scholar]
- 13.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F., Roverato A., Guaraldi G., Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 14.Jelenik T., Kaul K., Sequaris G., Flogel U., Phielix E., Kotzka J., Knebel B., Fahlbusch P., Horbelt T., Lehr S., Reinbeck A.L., Muller-Wieland D., Esposito I., Shulman G.I., Szendroedi J., Roden M. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. 2017;66:2241–2253. doi: 10.2337/db16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaurasia B., Summers S.A. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Farese R.V., Jr., Zechner R., Newgard C.B., Walther T.C. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15:570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferre P., Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt H.B., Wild S.H., Wood P.J., Zhang J., Darekar A.A., Dewbury K., Poole R.B., Holt R.I., Phillips D.I., Byrne C.D. Non-esterified fatty acid concentrations are independently associated with hepatic steatosis in obese subjects. Diabetologia. 2006;49:141–148. doi: 10.1007/s00125-005-0070-x. [DOI] [PubMed] [Google Scholar]
- 20.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Oliver D., Bacon B.R. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 22.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R., Nash C.R.N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldwell S.H., Hespenheide E.E., Redick J.A., Iezzoni J.C., Battle E.H., Sheppard B.L. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Sponseller C.A., Hampton K., Bacon B.R. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol. 2003;38:434–440. doi: 10.1016/s0168-8278(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 25.Promrat K., Lutchman G., Uwaifo G.I., Freedman R.J., Soza A., Heller T., Doo E., Ghany M., Premkumar A., Park Y., Liang T.J., Yanovski J.A., Kleiner D.E., Hoofnagle J.H. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 26.Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., Podevin P., Lacorte J.M., Bernhardt C., Bruckert E., Grimaldi A., Poynard T., Group L.S. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 27.Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., Austin A.S., Freeman J.G., Morgan L., Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G., Hartmann-Heurtier A., Bruckert E., Poynard T., Group L.S. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 30.Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dormandy J.A., Charbonnel B., Eckland D.J., Erdmann E., Massi-Benedetti M., Moules I.K., Skene A.M., Tan M.H., Lefebvre P.J., Murray G.D., Standl E., Wilcox R.G., Wilhelmsen L., Betteridge J., Birkeland K., Golay A., Heine R.J., Koranyi L., Laakso M., Mokan M., Norkus A., Pirags V., Podar T., Scheen A., Scherbaum W., Schernthaner G., Schmitz O., Skrha J., Smith U., Taton J., Investigators PROactive. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 32.Kernan W.N., Viscoli C.M., Furie K.L., Young L.H., Inzucchi S.E., Gorman M., Guarino P.D., Lovejoy A.M., Peduzzi P.N., Conwit R., Brass L.M., Schwartz G.G., Adams H.P., Jr., Berger L., Carolei A., Clark W., Coull B., Ford G.A., Kleindorfer D., O'Leary J.R., Parsons M.W., Ringleb P., Sen S., Spence J.D., Tanne D., Wang D., Winder T.R. IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA Drug Safety Communication. FDA eliminates the Risk Evaluation and Mitigation Strategy (REMS) for rosiglitazone-containing diabetes medicines. December 16, Available from: https://www.fda.gov/Drugs/DrugSafety/ucm476466.htm. Accessed: July 18, 2018.
- 34.Sharma M., Nazareth I., Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6:e010210. doi: 10.1136/bmjopen-2015-010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nan Y.M., Han F., Kong L.B., Zhao S.X., Wang R.Q., Wu W.J., Yu J. Adenovirus-mediated peroxisome proliferator activated receptor gamma overexpression prevents nutritional fibrotic steatohepatitis in mice. Scand J Gastroenterol. 2011;46:358–369. doi: 10.3109/00365521.2010.525717. [DOI] [PubMed] [Google Scholar]
- 36.Yu J., Zhang S., Chu E.S., Go M.Y., Lau R.H., Zhao J., Wu C.W., Tong L., Zhao J., Poon T.C., Sung J.J. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42:948–957. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi K., Sakaida I., Tsuchiya M., Omori K., Takami T., Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315:187–195. doi: 10.1016/j.bbrc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Uto H., Nakanishi C., Ido A., Hasuike S., Kusumoto K., Abe H., Numata M., Nagata K., Hayashi K., Tsubouchi H. The peroxisome proliferator-activated receptor-gamma agonist, pioglitazone, inhibits fat accumulation and fibrosis in the livers of rats fed a choline-deficient, l-amino acid-defined diet. Hepatol Res. 2005;32:235–242. doi: 10.1016/j.hepres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 39.He L., Liu X., Wang L., Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: a meta-analysis of randomized clinical trials. Medicine (Baltimore) 2016;95:e4947. doi: 10.1097/MD.0000000000004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland C., Musi N., Webb A., Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi-Suzuki M., Bril F., Kalavalapalli S., Cusi K., Frye R.F. Concentration-dependent response to pioglitazone in nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;46:56–61. doi: 10.1111/apt.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi-Suzuki M., Cusi K., Bril F., Gong Y., Langaee T., Frye R.F. A genetic score associates with pioglitazone response in patients with non-alcoholic steatohepatitis. Front Pharmacol. 2018;9:752. doi: 10.3389/fphar.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaakkola T., Laitila J., Neuvonen P.J., Backman J.T. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006;99:44–51. doi: 10.1111/j.1742-7843.2006.pto_437.x. [DOI] [PubMed] [Google Scholar]
- 44.Musso G., Cassader M., Paschetta E., Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bril F., Kalavalapalli S., Clark V.C., Lomonaco R., Soldevila-Pico C., Liu I.C., Orsak B., Tio F., Cusi K. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol. 2018;16:558–566 e2. doi: 10.1016/j.cgh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z., Vigueira P.A., Chambers K.T., Hall A.M., Mitra M.S., Qi N., McDonald W.G., Colca J.R., Kletzien R.F., Finck B.N. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. J Biol Chem. 2012;287:23537–23548. doi: 10.1074/jbc.M112.363960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colca J.R., VanderLugt J.T., Adams W.J., Shashlo A., McDonald W.G., Liang J., Zhou R., Orloff D.G. Clinical proof-of-concept study with MSDC-0160, a prototype mTOT-modulating insulin sensitizer. Clin Pharmacol Ther. 2013;93:352–359. doi: 10.1038/clpt.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCommis K.S., Hodges W.T., Brunt E.M., Nalbantoglu I., McDonald W.G., Holley C., Fujiwara H., Schaffer J.E., Colca J.R., Finck B.N. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology. 2017;65:1543–1556. doi: 10.1002/hep.29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolten C.W., Blanner P.M., McDonald W.G., Staten N.R., Mazzarella R.A., Arhancet G.B., Meier M.F., Weiss D.J., Sullivan P.M., Hromockyj A.E., Kletzien R.F., Colca J.R. Insulin sensitizing pharmacology of thiazolidinediones correlates with mitochondrial gene expression rather than activation of PPAR gamma. Gene Regul Syst Bio. 2007;1:73–82. [PMC free article] [PubMed] [Google Scholar]
- 50.Haskins J.R., Rowse P., Rahbari R., de la Iglesia F.A. Thiazolidinedione toxicity to isolated hepatocytes revealed by coherent multiprobe fluorescence microscopy and correlated with multiparameter flow cytometry of peripheral leukocytes. Arch Toxicol. 2001;75:425–438. doi: 10.1007/s002040100251. [DOI] [PubMed] [Google Scholar]
- 51.Bova M.P., Tam D., McMahon G., Mattson M.N. Troglitazone induces a rapid drop of mitochondrial membrane potential in liver HepG2 cells. Toxicol Lett. 2005;155:41–50. doi: 10.1016/j.toxlet.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Ortiz J.M., Tranque P., Vaquero C.F., Domingo B., Molina F., Calvo S., Jordan J., Cena V., Llopis J. Glitazones differentially regulate primary astrocyte and glioma cell survival. Involvement of reactive oxygen species and peroxisome proliferator-activated receptor-gamma. J Biol Chem. 2004;279:8976–8985. doi: 10.1074/jbc.M308518200. [DOI] [PubMed] [Google Scholar]
- 53.Preininger K., Stingl H., Englisch R., Furnsinn C., Graf J., Waldhausl W., Roden M. Acute troglitazone action in isolated perfused rat liver. Br J Pharmacol. 1999;126:372–378. doi: 10.1038/sj.bjp.0702318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dello Russo C., Gavrilyuk V., Weinberg G., Almeida A., Bolanos J.P., Palmer J., Pelligrino D., Galea E., Feinstein D.L. Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem. 2003;278:5828–5836. doi: 10.1074/jbc.M208132200. [DOI] [PubMed] [Google Scholar]
- 55.Brunmair B., Staniek K., Gras F., Scharf N., Althaym A., Clara R., Roden M., Gnaiger E., Nohl H., Waldhausl W., Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 56.Scatena R., Bottoni P., Martorana G.E., Ferrari F., De Sole P., Rossi C., Giardina B. Mitochondrial respiratory chain dysfunction, a non-receptor-mediated effect of synthetic PPAR-ligands: biochemical and pharmacological implications. Biochem Biophys Res Commun. 2004;319:967–973. doi: 10.1016/j.bbrc.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 57.Feinstein D.L., Spagnolo A., Akar C., Weinberg G., Murphy P., Gavrilyuk V., Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 58.Colca J.R., McDonald W.G., Waldon D.J., Leone J.W., Lull J.M., Bannow C.A., Lund E.T., Mathews W.R. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 59.Colca J.R., McDonald W.G., Cavey G.S., Cole S.L., Holewa D.D., Brightwell-Conrad A.S., Wolfe C.L., Wheeler J.S., Coulter K.R., Kilkuskie P.M., Gracheva E., Korshunova Y., Trusgnich M., Karr R., Wiley S.E., Divakaruni A.S., Murphy A.N., Vigueira P.A., Finck B.N., Kletzien R.F. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., Kunji E.R., Martinou J.C. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 61.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C., Cox J.E., Cardon C.M., Van Vranken J.G., Dephoure N., Redin C., Boudina S., Gygi S.P., Brivet M., Thummel C.S., Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCommis K.S., Chen Z., Fu X., McDonald W.G., Colca J.R., Kletzien R.F., Burgess S.C., Finck B.N. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 2015;22:682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray L.R., Sultana M.R., Rauckhorst A.J., Oonthonpan L., Tompkins S.C., Sharma A., Fu X., Miao R., Pewa A.D., Brown K.S., Lane E.E., Dohlman A., Zepeda-Orozco D., Xie J., Rutter J., Norris A.W., Cox J.E., Burgess S.C., Potthoff M.J., Taylor E.B. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 2015;22:669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divakaruni A.S., Wiley S.E., Rogers G.W., Andreyev A.Y., Petrosyan S., Loviscach M., Wall E.A., Yadava N., Heuck A.P., Ferrick D.A., Henry R.R., McDonald W.G., Colca J.R., Simon M.I., Ciaraldi T.P., Murphy A.N. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vigueira P.A., McCommis K.S., Schweitzer G.G., Remedi M.S., Chambers K.T., Fu X., McDonald W.G., Cole S.L., Colca J.R., Kletzien R.F., Burgess S.C., Finck B.N. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014;7:2042–2053. doi: 10.1016/j.celrep.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowman C.E., Zhao L., Hartung T., Wolfgang M.J. Requirement for the mitochondrial pyruvate carrier in mammalian development revealed by a hypomorphic allelic series. Mol Cell Biol. 2016;36:2089–2104. doi: 10.1128/MCB.00166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clapper J.R., Hendricks M.D., Gu G., Wittmer C., Dolman C.S., Herich J., Athanacio J., Villescaz C., Ghosh S.S., Heilig J.S., Lowe C., Roth J.D. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol. 2013;305:G483–G495. doi: 10.1152/ajpgi.00079.2013. [DOI] [PubMed] [Google Scholar]
- 68.Rauckhorst A.J., Gray L.R., Sheldon R.D., Fu X., Pewa A.D., Feddersen C.R., Dupuy A.J., Gibson-Corley K.N., Cox J.E., Burgess S.C., Taylor E.B. The mitochondrial pyruvate carrier mediates high fat diet-induced increases in hepatic TCA cycle capacity. Mol Metab. 2017;6:1468–1479. doi: 10.1016/j.molmet.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satapati S., Sunny N.E., Kucejova B., Fu X., He T.T., Mendez-Lucas A., Shelton J.M., Perales J.C., Browning J.D., Burgess S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satapati S., Kucejova B., Duarte J.A., Fletcher J.A., Reynolds L., Sunny N.E., He T., Nair L.A., Livingston K.A., Fu X., Merritt M.E., Sherry A.D., Malloy C.R., Shelton J.M., Lambert J., Parks E.J., Corbin I., Magnuson M.A., Browning J.D., Burgess S.C. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125:4447–4462. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satapati S., Kucejova B., Duarte J.A., Fletcher J.A., Reynolds L., Sunny N.E., He T., Nair L.A., Livingston K.A., Fu X., Merritt M.E., Sherry A.D., Malloy C.R., Shelton J.M., Lambert J., Parks E.J., Corbin I., Magnuson M.A., Browning J.D., Burgess S.C. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2016;126:1605. doi: 10.1172/JCI86695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patterson R.E., Kalavalapalli S., Williams C.M., Nautiyal M., Mathew J.T., Martinez J., Reinhard M.K., McDougall D.J., Rocca J.R., Yost R.A., Cusi K., Garrett T.J., Sunny N.E. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310:E484–E494. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalavalapalli S., Bril F., Koelmel J.P., Abdo K., Guingab J., Andrews P., Li W.Y., Jose D., Yost R.A., Frye R.F., Garrett T.J., Cusi K., Sunny N.E. Pioglitazone improves hepatic mitochondrial function in a mouse model of nonalcoholic steatohepatitis. Am J Physiol Endocrinol Metab. 2018;315:E163–E173. doi: 10.1152/ajpendo.00023.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sunny N.E., Bril F., Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab. 2017;28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Hyotylainen T., Jerby L., Petaja E.M., Mattila I., Jantti S., Auvinen P., Gastaldelli A., Yki-Jarvinen H., Ruppin E., Oresic M. Genome-scale study reveals reduced metabolic adaptability in patients with non-alcoholic fatty liver disease. Nat Commun. 2016;7:8994. doi: 10.1038/ncomms9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L., Abrams G.A., Doeller J.E., Kraus D.W., Darley-Usmar V.M., Bailey S.M. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rector R.S., Thyfault J.P., Uptergrove G.M., Morris E.M., Naples S.P., Borengasser S.J., Mikus C.R., Laye M.J., Laughlin M.H., Booth F.W., Ibdah J.A. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris E.M., McCoin C.S., Allen J.A., Gastecki M.L., Koch L.G., Britton S.L., Fletcher J.A., Fu X., Ding W.X., Burgess S.C., Rector R.S., Thyfault J.P. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J Physiol. 2017;595:4909–4926. doi: 10.1113/JP274281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., Herder C., Carstensen M., Krausch M., Knoefel W.T., Schlensak M., Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Panasevich M.R., Meers G.M., Linden M.A., Booth F.W., Perfield J.W., 2nd, Fritsche K.L., Wankhade U.D., Chintapalli S.V., Shankar K., Ibdah J.A., Rector R.S. High-fat, high-fructose, high-cholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am J Physiol Endocrinol Metab. 2018;314:E78–E92. doi: 10.1152/ajpendo.00015.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris E.M., Meers G.M., Koch L.G., Britton S.L., Fletcher J.A., Fu X., Shankar K., Burgess S.C., Ibdah J.A., Rector R.S., Thyfault J.P. Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2016;311:E749–E760. doi: 10.1152/ajpendo.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Linden M.A., Sheldon R.D., Meers G.M., Ortinau L.C., Morris E.M., Booth F.W., Kanaley J.A., Vieira-Potter V.J., Sowers J.R., Ibdah J.A., Thyfault J.P., Laughlin M.H., Rector R.S. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. J Physiol. 2016;594:5271–5284. doi: 10.1113/JP272235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linden M.A., Fletcher J.A., Morris E.M., Meers G.M., Laughlin M.H., Booth F.W., Sowers J.R., Ibdah J.A., Thyfault J.P., Rector R.S. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc. 2015;47:556–567. doi: 10.1249/MSS.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du K., Hyun J., Premont R.T., Choi S.S., Michelotti G.A., Swiderska-Syn M., Dalton G.D., Thelen E., Rizi B.S., Jung Y., Diehl A.M. Hedgehog-yap signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154:1465–1479 e13. doi: 10.1053/j.gastro.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vacanti N.M., Divakaruni A.S., Green C.R., Parker S.J., Henry R.R., Ciaraldi T.P., Murphy A.N., Metallo C.M. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang C., Ko B., Hensley C.T., Jiang L., Wasti A.T., Kim J., Sudderth J., Calvaruso M.A., Lumata L., Mitsche M., Rutter J., Merritt M.E., DeBerardinis R.J. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vigueira P.A., McCommis K.S., Hodges W.T., Schweitzer G.G., Cole S.L., Oonthonpan L., Taylor E.B., McDonald W.G., Kletzien R.F., Colca J.R., Finck B.N. The beneficial metabolic effects of insulin sensitizers are not attenuated by mitochondrial pyruvate carrier 2 hypomorphism. Exp Physiol. 2017;102:985–999. doi: 10.1113/EP086380. [DOI] [PMC free article] [PubMed] [Google Scholar]