Abstract
Aims
The purpose of this pilot study was to assess the potential usefulness of diastolic stress test (DST) echocardiography in patients with suspected heart failure with preserved ejection fraction (HFpEF).
Methods and results
Patients with suspected HFpEF (left ventricular ejection fraction ≥ 50%, exertional dyspnoea, septal E/e′ at rest 9–14, and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) at rest < 220 pg/mL; n = 13) and a control group constituted from asymptomatic patients with arterial hypertension (n = 19) and healthy subjects (n = 18) were included. All patients were analysed by two‐dimensional and Doppler echocardiography at rest and during exercise (DST) and underwent cardiopulmonary exercise testing and NT‐proBNP analysis during exercise. HFpEF during exercise was defined as exertional dyspnoea and peak VO2 ≤ 20.0 mL/min/kg. In patients with suspected HFpEF at rest, 84.6% of these patients developed HFpEF during exercise, whereas in the group of asymptomatic patients with hypertension and healthy subjects, the rate of developed HFpEF during exercise was 0%. Regarding the diagnostic performance of DST to detect HFpEF during exercise, an E/e′ ratio >15 during exercise was the most accurate parameter to detect HFpEF (accuracy 86%), albeit a low sensitivity (45.5%). Nonetheless, combining E/e′ with tricuspid regurgitation (TR) velocity > 2.8 m/s during exercise provided a significant increase in the sensitivity to detect patients with HFpEF during exercise (sensitivity 72.7%, specificity 79.5%, and accuracy 78%). Consistent with these findings, an increase of E/e′ was significantly linked to worse peak VO2, and the combination of an increase of both E/e′ and TR velocity was associated with elevated NT‐proBNP values during exercise.
Conclusions
The findings of this pilot study suggest that DST using E/e′ ratio and TR velocity could be of potential usefulness to diagnose HFpEF during exercise in patients with suspected HFpEF at rest.
Keywords: Diastolic stress test, Heart failure with preserved ejection fraction, Exercise echocardiography, Exertional dyspnoea
Introduction
Diagnosis of heart failure (HF) with preserved ejection fraction (HFpEF) is still challеnging and has been built on the basis of echocardiographic analyses at rest for many years.1, 2 However, because many patients with HFpEF have symptoms such as dyspnoea only during exercise and because non‐invasive echocardiographic analyses at rest could be insufficiently sensitive to identify these patients,3, 4, 5, 6 the potential usefulness of diastolic stress test (DST) echocardiography in this setting has been suggested.7, 8, 9, 10, 11, 12, 13, 14, 15, 16 In line with this, several studies have shown that left ventricular (LV) diastolic alterations are more manifest during exercise than at rest in HFpEF.17, 18 However, it remains uncertain why some patients with diastolic dysfunction remain asymptomatic during exercise and others not. In addition, many patients with signs and symptoms of HFpEF fall into the “grey zone” of key echocardiographic diagnostic parameters, such as E/e′ 8–15.2 Hence, a technique that may accurately categorize these borderline patients could be of great importance in clinical practice.19 In this respect, we hypothesized that DST could identify with adequate sensitivity and specificity patients with HFpEF who have inconclusive criteria at rest.
The purpose of this study was to assess the potential usefulness of DST in patients with suspected HFpEF by analysing patients with indeterminate criteria for HFpEF at rest and a control group constituted from asymptomatic patients with arterial hypertension (HT) and healthy subjects.
Methods
Study design
This study was a pilot single‐centre study with the aim to assess the potential usefulness of DST in patients with suspected HFpEF. Pre‐screening was obtained within the standard of care work‐up in the Department of Cardiology at the Medical University of Graz. Eligible subjects meeting inclusion/exclusion criteria were considered for the study. Patients were recruited between 2012 and 2014 in the Department of Cardiology at the Medical University of Graz, Graz, Austria. During the screening visit, medical history of the patient as well as demographic data, physical examination to assess congestion and signs of HF, vital signs, New York Heart Association class evaluation, complete laboratory measurement [including N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP)], questionnaires, electrocardiogram, ambulatory blood pressure measurement, cardiopulmonary exercise testing, detailed rest echocardiography, and DST were performed.
Study patients
The study included 13 ambulatory patients with suspected HFpEF (left ventricular ejection fraction (LVEF) ≥ 50%, exertional dyspnoea, septal E/e′ at rest 9–14, and NT‐proBNP at rest < 220 pg/mL) and a control group constituted from 19 asymptomatic patients with arterial HT and 18 healthy subjects. Asymptomatic patients with HT were determined by the presence or history of arterial HT (arterial blood pressure ≥ 140/90 mmHg) without any evidence of HF. Healthy subjects were defined as individuals with absence of any disease and cardiovascular risk factors such as obesity, diabetes, HT, and hypercholesterolaemia, with no medication and with normal findings in transthoracic echocardiography according to the diagnostic criteria of the European Association of Cardiovascular Imaging (EACVI).20 Exercise HFpEF was defined by dyspnoea during exercise and objective evidence of inadequate functional capacity (i.e. peak VO2 < 20 mL/min/kg).21, 22
Exclusion criteria
In order to avoid causes of LV myocardial dysfunction other than HFpEF, patients with coronary artery disease were excluded (namely, patients with unstable angina or non‐ST‐segment elevation myocardial infarction, patients with ST‐segment elevation acute myocardial infarction, subjects with coronary artery bypass graft, subjects with chronic stable angina, and patients with evidence of myocardial ischaemia). Moreover, with the purpose of excluding other causes of dyspnoea other than HFpEF, patients with the following characteristics were excluded: (i) chronic obstructive pulmonary disease (presence of a post‐bronchodilator first second of forced expiration to the full, forced vital capacity ratio < 0.7023 and concomitant signs and symptoms of chronic obstructive pulmonary disease reference values on spirometry), asthma, or severe pulmonary disease defined as pulmonary pathology with requirement of supplemental oxygen or need of treatment with corticoids; (ii) severe kidney disease defined as estimated glomerular filtration rate < 30 mL/min/1.73m2 for at least 3 months, history of renal transplantation, or severe acute renal failure with dialysis requirement; (iii) severe chronic liver disease or history of liver transplantation; (iv) congenital heart disease; (v) pericardial disease characterized by moderate or severe pericardial effusion (echo‐free space in end‐diastole ≥5 mm) or constrictive pericarditis; (vi) cardiomyopathy; (vii) valvular heart disease defined as mild, moderate, or severe mitral or aortic stenosis; moderate or severe non‐functional mitral or tricuspid regurgitation (TR); and moderate or severe aortic regurgitation (according to the diagnostic criteria of the guidelines for the management of patients with valvular heart disease of the EACVI). Furthermore, to avoid underestimations of myocardial and mitral annular measurements, patients with valvular heart surgery, mitral annular calcification (≥5 mm), cardiac pacing, and poor two‐dimensional quality in ≥2 myocardial segments of the LV were also excluded. In addition, to avoid mistakes or large variations in the measurements of the LV due to variability of R‐R interval, patients with atrial or ventricular arrhythmias were also excluded.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (clinical research project number 24‐318 ex 11/12). All subjects before involvement in the study provided written informed consent.
Conventional echocardiography at rest
Two‐dimensional and Doppler echocardiography at rest were performed in accordance with EACVI recommendations.20 LV end‐systolic and end‐diastolic volume, LVEF, stroke volume, and cardiac output were measured by the modified Simpson's method from the apical four‐chamber view. LV filling pressures were calculated by the ratio of early mitral diastolic inflow velocity to early diastolic mitral septal annular velocity (E/e′) and indirectly by the maximal jet velocity of the TR, using the Bernoulli's equation: 4 × (TR velocity)2 + right atrial pressure for pulmonary artery systolic pressure calculation.
Exercise echocardiography (diastolic stress test) protocol
Patients were analysed during bicycle supine exercise echocardiographic test. The images were obtained from the apical four‐chamber view, and a multistage supine bicycle exercise test was performed. The exercise period began with the subject riding 60 r.p.m. at 25 W and increasing the workload at 25 W each 8 min. When the patient reached the 75 W, the workload was increased 25 W each 5 min. Heart rate, blood pressure (by sphygmomanometer), and echocardiographic parameters were measured at rest, during each stage of exercise, at peak exercise (symptom‐limited), and in the recovery period. The following endpoint parameters were obtained and analysed during DST:
septal E/e′,
LVEF, stroke volume, and cardiac output (apical four‐chamber view),
maximal jet velocity of the TR, and
NT‐proBNP.
Cardiopulmonary exercise testing
Ventilatory exchange, oxygen uptake (peak VO2), and other cardiopulmonary variables were acquired during the CPET by averaging breath‐by‐breath measurements over 10 s intervals. A standard 12 lead electrocardiogram was monitored continuously for heart rate, ST‐segment changes, and arrhythmias. Blood pressure was recorded at rest and then every 2 min.
Blood sample measurements
An intravenous polyethylene cannula was inserted prior to resting echocardiography. After 10 min of supine rest, venous blood was collected in an edetic acid tube; a second sample was obtained within 1 min following the termination of exercise, while stress images were being acquired. Samples were centrifuged, frozen at the Biobank Graz, and analysed after recruitment of the last patient. Plasma NT‐proBNP levels were measured on the Elecsys proBNP platform (Roche Diagnostics, Mannheim, Germany) with chemiluminescence technology. The detection limit of the NT‐proBNP assay was 5 pg/mL. All other parameters were determined by routine laboratory procedures.
Statistical analysis
Continuous data were presented as mean ± standard deviation and dichotomous data in percentage. Differences in continuous variables between groups were analysed using Student's t‐test. Categorical variables were compared by χ2 test and Fisher's exact test as appropriate. Comparisons between three or more groups were analysed using a one‐way analysis of variance. All statistical analyses were performed with Statview 5.0 (SAS Institute) and SPSS 22.0 (IBM). Differences were considered statistically significant when P < 0.05.
Results
Clinical and echocardiographic characteristics of the study population at rest
A total of 50 subjects were included in the study (13 with suspected HFpEF, 19 asymptomatic patients with HT, and 18 healthy subjects). Clinical characteristics of these subjects are shown in Table 1. There were no significant differences in conventional and haemodynamic parameters such as LVEF, TR velocity, left atrial volume index, heart rate between patients with suspected HFpEF, asymptomatic patients with HT, and healthy subjects at rest (see Table 1). Nonetheless, patients with suspected HFpEF were older and had a higher BMI, higher NT‐proBNP levels, LV mass index, and septal E/e′ at rest.
Table 1.
Clinical and echocardiographic characteristics of the study population
| Healthy subjects (n = 18) | Asymptomatic HT (n = 19) | Suspected HFpEF (n = 13) | P‐value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 53.3 ± 6.2 | 59.2 ± 7.4 | 67.0 ± 7.4 | <0.01 |
| Men | 50.0% | 63.2% | 30.8% | 0.20 |
| Body mass index, kg/m2 | 24.1 ± 2.6 | 27.9 ± 3.1 | 28.6 ± 4.1 | <0.01 |
| Systolic blood pressure, mmHg | 106 ± 10 | 115 ± 12 | 117 ± 15 | 0.04 |
| Diastolic blood pressure, mmHg | 72 ± 9 | 81 ± 9 | 79 ± 9 | 0.01 |
| Heart rate, per min | 61 ± 7 | 62 ± 9 | 67 ± 11 | 0.23 |
| Peak VO2, mL/min/kg | 27.0 ± 7.8 | 21.5 ± 4.9 | 15.9 ± 3.2 | <0.01 |
| NT‐proBNP at rest, pg/mL | 66.2 ± 43.7 | 68.6 ± 45.7 | 105.7 ± 71 | 0.09 |
| Hypertension | 0% | 100% | 100% | <0.01 |
| Diabetes | 0% | 15.8% | 23.1% | 0.12 |
| Coronary artery disease | 0% | 0% | 0% | n/a |
| Atrial fibrillation | 0% | 0% | 0% | n/a |
| Echocardiographic measurements at rest | ||||
| LV ejection fraction, % | 60.3 ± 3.6 | 61.3 ± 3.9 | 61.8 ± 6.7 | 0.68 |
| LV mass index, g/m2 | 67.7 ± 31.4 | 93.0 ± 19.7 | 93.6 ± 19.5 | <0.01 |
| Septal e′ mitral annular velocity by TDI, cm/s | 10.7 ± 2.5 | 7.4 ± 1.0 | 6.8 ± 1.6 | <0.01 |
| Mitral early diastolic inflow velocity (E), cm/s | 74.5 ± 12.7 | 70.2 ± 10.2 | 68.2 ± 12.9 | 0.31 |
| Mitral E/e′ septal ratio | 7.5 ± 1.8 | 9.5 ± 1.6 | 10.3 ± 2.2 | <0.01 |
| Tricuspid regurgitation velocity, m/s | 2.02 ± 0.3 | 1.97 ± 0.3 | 2.1 ± 0.2 | 0.30 |
| LAVI, mL/m2 | 27.0 ± 6.7 | 29.1 ± 12.6 | 31 ± 5.9 | 0.50 |
e′, early diastolic peak velocity by pulsed tissue Doppler imaging; HFpEF, heart failure with preserved ejection fraction; HT, hypertension; LAVI, left atrial volume index; LV, left ventricular; n/a, not applicable; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; TDI, pulsed tissue Doppler imaging.
Data are expressed as mean ± standard deviation or percentages.
Diastolic and haemodynamic changes during exercise in patients with suspected heart failure with preserved ejection fraction
Patients with suspected HFpEF had significantly lower values of septal e′, stroke volume, and cardiac output as well as higher values of septal E/e′, TR velocity, and NT‐proBNP during exercise than asymptomatic patients with HT and healthy subjects (see Tables 2 and 3). Consistent with these findings, 84.6% of patients with suspected HFpEF developed HFpEF during exercise, whereas in the group of asymptomatic patients with HT and healthy subjects, the rate of HFpEF during exercise was 0%.
Table 2.
Diastolic changes during exercise in the study population
| Healthy subjects (n = 18) | Asymptomatic HT (n = 19) | Suspected HFpEF (n = 13) | P‐ANOVA value | |
|---|---|---|---|---|
| Mitral Septal E/e′ | ||||
| At rest | 7.5 ± 1.8 | 9.5 ± 1.6 | 10.3 ± 2.2 | |
| During exercise (at maximal workload) | 8.1 ± 1.5 | 9.3 ± 1.7 | 14.1 ± 3.1 | <0.01 |
| P‐value | 0.16 | 0.61 | <0.01 | |
| NT‐proBNP, pg/mL | ||||
| At rest | 66.2 ± 43.7 | 68.6 ± 45.7 | 105.7 ± 71.0 | |
| During exercise (at maximal workload) | 74.6 ± 42.2 | 71.7 ± 49.3 | 125.7 ± 92.4 | 0.04 |
| P‐value | 0.27 | <0.01 | 0.02 | |
| TR, m/s | ||||
| At rest | 2.03 ± 0.2 | 1.97 ± 0.3 | 2.1 ± 0.2 | |
| During exercise (at maximal workload) | 2.00 ± 0.2 | 2.12 ± 0.6 | 2.52 ± 0.6 | 0.02 |
| P‐value | 0.92 | 0.05 | 0.01 | |
| Mitral septal e′, cm/s | ||||
| At rest | 10.7 ± 2.5 | 7.4 ± 1.0 | 6.8 ± 1.6 | |
| During exercise (at maximal workload) | 14.8 ± 2.6 | 11.3 ± 1.4 | 9.2 ± 1.7 | <0.01 |
| P‐value | <0.01 | <0.01 | <0.01 | |
HFpEF, heart failure with preserved ejection fraction; HT, hypertension; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; TR, tricuspid regurgitation.
Table 3.
Haemodynamic changes during exercise in the study population
| Healthy subjects (n = 18) | Asymptomatic HT (n = 19) | Suspected HFpEF (n = 13) | P‐ANOVA value | |
|---|---|---|---|---|
| Stroke volume, mL | ||||
| At rest | 50.9 ± 12.8 | 52.8 ± 12.1 | 45.4 ± 11.3 | |
| During exercise (at maximal workload) | 70.0 ± 16.2 | 61.5 ± 13.2 | 52.4 ± 12.0 | 0.05 |
| P‐value | <0.01 | <0.01 | <0.01 | |
| Cardiac output, L/min | ||||
| At rest | 3.1 ± 0.6 | 3.2 ± 0.7 | 3.0 ± 0.6 | |
| During exercise (at maximal workload) | 7.8 ± 2.0 | 6.6 ± 1.5 | 5.4 ± 0.9 | <0.01 |
| P‐value | <0.01 | <0.01 | <0.01 | |
HFpEF, heart failure with preserved ejection fraction; HT, hypertension.
Haemodynamic and myocardial characteristics of patients with heart failure with preserved ejection fraction during exercise
Patients with HFpEF during exercise had principally significantly higher values of septal E/e′ and TR velocity than those without HFpEF (see Table 4). In line with these findings, patients with developed HFpEF during exercise had significantly lower functional exercise capacity (peak VO2) and higher NT‐proBNP values than those without HFpEF (see Table 4).
Table 4.
Diastolic and haemodynamic differences in patients with developed HFpEF during exercise
| Non‐HF (n = 37) | HFpEF (n = 13) | P‐value | |
|---|---|---|---|
| E/e′ septal > 15 during exercise | 3.7% | 45.5% | <0.01 |
| TR > 2.8 m/s during exercise | 20.5% | 36.4% | 0.28 |
| No increase of SV during exercise | 2.6% | 9.1% | 0.27 |
| No increase of CO during exercise | 0% | 0% | n/a |
| NT‐proBNP during exercise > 125 pg/mL | 10.3% | 36.4% | 0.03 |
| NT‐proBNP during exercise > 220 pg/mL | 2.6% | 18.2% | 0.05 |
| Peak VO2, mL/min/kg | 24.2 ± 6.9 | 15.0 ± 2.4 | <0.01 |
CO, cardiac output; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; SV, stroke volume; TR, tricuspid regurgitation.
Diagnostic performance of diastolic stress test to determine heart failure with preserved ejection fraction during exercise
Regarding the diagnostic performance of DST to detect HFpEF during exercise, an E/e′ ratio > 15 during exercise was the most accurate parameter to detect HFpEF (accuracy 86%), albeit has a low sensitivity (45.5%) (see Table 5). Nonetheless, combining E/e′ with TR velocity during exercise provided a significant increase in the sensitivity to detect patients with HFpEF during exercise (sensitivity 72.7%, specificity 79.5%, and accuracy 78%) (see Table 5). Consistent with these findings, an increase in E/e′ was significantly linked to worse peak VO2, and the combination of an increase of both E/e′ and TR velocity was associated with elevated NT‐proBNP values during exercise (see Figures 1 and 2 ).
Table 5.
Diagnostic performance of diastolic stress test to detect HFpEF during exercise
| Variable | Sensitivity (%) | Specificity (%) | VPP (%) | VPN (%) | Accuracy (%) |
|---|---|---|---|---|---|
| E/e′ septal > 15 or TR > 2.8 m/s during exercise | 72.7 | 79.5 | 50 | 91.2 | 78 |
| E/e′ septal > 15 during exercise alone | 45.5 | 97.4 | 83.3 | 86.3 | 86 |
| TR > 2.8 m/s during exercise alone | 36.4 | 79.5 | 33.3 | 81.6 | 70 |
| No increase of SV during exercise | 9.1 | 97.4 | 50 | 79.1 | 78 |
| NT‐proBNP during exercise > 125 pg/mL | 36.4 | 89.7 | 50 | 83.3 | 78 |
| NT‐proBNP during exercise > 220 pg/mL | 18.2 | 97.4 | 66.7 | 80.8 | 80 |
HFpEF, heart failure with preserved ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SV, stroke volume; TR, tricuspid regurgitation; VPN, negative predictive value; VPP, positive predictive value.
Figure 1.
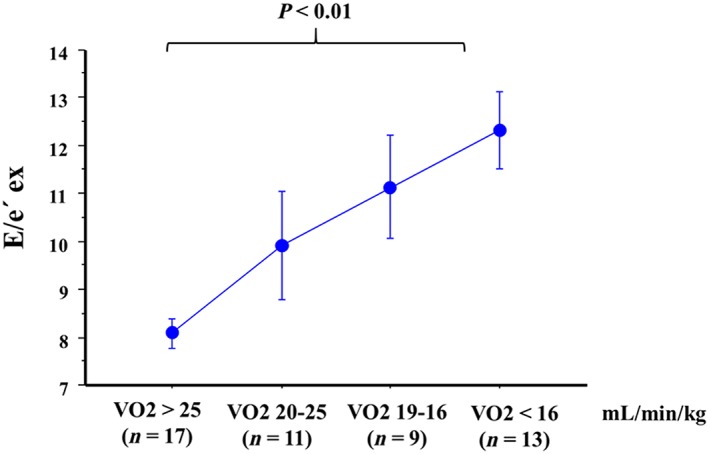
Association of an increase of E/e′ septal with worse functional capacity (peak VO2).
Figure 2.
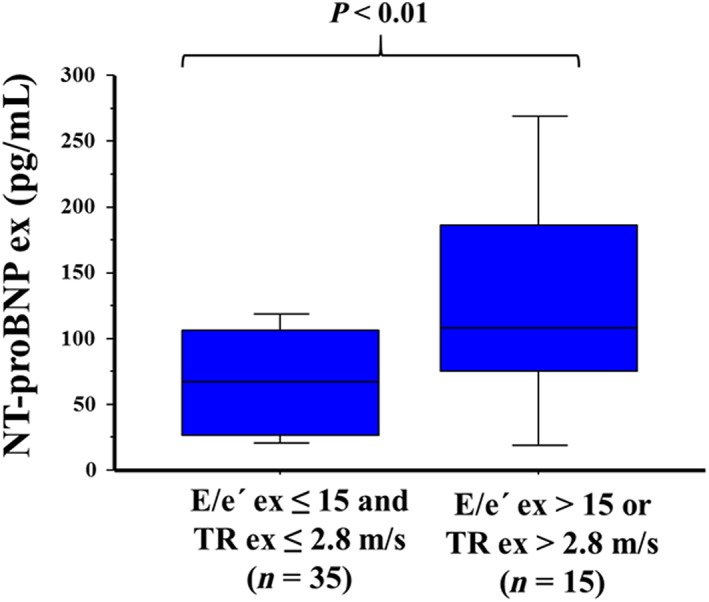
Association of an increase of E/e′ septal and tricuspid regurgitation (TR) velocity with an increase of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) values during exercise. TR and NT‐proBNP values were not available in five patients during exercise.
Discussion
In the present study, analysing patients with suspected HFpEF and a control group constituted from asymptomatic patients with HT and healthy subjects, we found that in patients with suspected HFpEF at rest, 86.4% of these patients developed HFpEF during exercise, whereas in the group of asymptomatic patients with HT and healthy subjects, the rate of developed HFpEF during exercise was 0%. Moreover, DST had a diagnostic accuracy of 78% to detect HFpEF during exercise when elevations of either E/e′ or TR velocity were combined as rule‐in parameters.
Rest echocardiography remains an important method to characterize the underlying functional and structural changes in HFpEF, and E/e′ ratio constitutes a key parameter in the evaluation of these patients.2, 12 The E/e′ rаtio ⩾15 if using e′ of septаl site of the mitrаl annulus or ⩾13 if using averаge values of septal and laterаl sites indicates accurately increased LV end‐diastolic pressure, whereas an E/e′ value <8 indicаtes normal filling pressures.24 Several studies have shown that E/e′ > 15 may be аble to provide stand‐alone evidence of diаstolic LV dysfunction without further need of seriаl non‐invаsive tests in HFpEF patients.2, 25 However, many patients with signs and symptoms of HFpEF fall into the “grey zone” of key echocardiographic diagnostic parameters, such as E/e′ 8–15, and thus, other echocardiographic indices should be used.2 Hence, a technique that may accurately categorize these borderline patients with E/e′ 8–15 as truth HFpEF could be of great importance in the clinical practice.19
Several studies have shown that LV diastolic alterations are more manifest during exercise than at rest in HFpEF.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Nonetheless, in patients with suspected HFpEF (i.e. exertional dyspnoea and borderline values of E/e′ and NT‐proBNP), an optimal diagnostic approach to diagnose HFpEF remains uncertain.29 In the present study an E/e′ ratio > 15 during exercise was the most accurate parameter to detect HFpEF during exercise (accuracy 86%) in patients with suspected HFpEF in the “grey zone,” albeit has a low sensitivity (45.5%). Nonetheless, combining E/e′ with TR velocity provided a significant increase in the sensitivity to detect patients with HFpEF during exercise (sensitivity 72.7%, specificity 79.5%, and accuracy 78%). In addition, both an increase of E/e′ and TR velocity during exercise were significantly linked to worse peak VO2 and elevated NT‐proBNP values during exercise. These findings are in agreement with recent studies.30 In this regard, Obokata et al.1 using invasive LV filling pressure measurements found that the combination of E/e′ and TR velocity during exercise had an adequate sensitivity and specificity to diagnose HFpEF. In addition, Donal et al.31 showed that both E/e′ and TR velocity during exercise were the main echocardiographic parameters linked to worse cardiovascular prognosis in patients with HFpEF. Furthermore, Kosmala et al.32 demonstrated that E/e′ during exercise was significantly linked to cardiovascular outcomes in patients with suspected HFpEF. Therefore, we consider that the combination of E/e′ and TR velocity during exercise could be of clinical usefulness in patients in the “grey zone” with suspected HFpEF.
Limitations
Some considerations should be taken into account on this study. One main limitation is the low sample size of this pilot study. Hence, the findings of this study regarding the potential usefulness of DST in the setting of patients with suspected HFpEF should be validated in further studies with larger sample size. In addition, it is important to note that the diagnosis of HFpEF during exercise was not validated by using invasive gold standard measurements such as LV filling pressures or pulmonary capillary wedge pressure. Therefore, the results of this study regarding the diagnostic performance of DST in the setting of patients with suspected HFpEF should be confirmed in further studies with invasive measurements. In addition, it is worth highlighting that the findings of this study are lacking prognostic significance because in this study, no outcomes analyses were performed. However, recent studies have suggested that an elevated E/e′ and TR during exercise in patients with HFpEF are markers of poor cardiovascular outcomes in these patients.30, 32
Conclusions
The findings of this pilot study suggest that DST using E/e′ ratio and TR velocity could be of potential usefulness to diagnose HFpEF during exercise in patients with suspected HFpEF at rest. Further larger studies analysing the diagnostic performance of DST in patients with suspected HFpEF are warranted, because the findings of this pilot suggest a clinical usefulness of DST in this setting.
Conflict of interest
None declared.
Funding
We thank the Heart Failure Association of the European Society of Cardiology for the financial support. E.B. received the HFA/ESC Grant.
Acknowledgements
We thank all patients and staff of the Department of Cardiology, Laboratory of the Division of Endocrinology and Metabolism of Medical University Graz, Biobank Graz (COO – v.h.Prof, Karine Sargsyan) for their participation in this study.
Belyavskiy, E. , Morris, D. A. , Url‐Michitsch, M. , Verheyen, N. , Meinitzer, A. , Radhakrishnan, A.‐K. , Kropf, M. , Frydas, A. , Ovchinnikov, A. G. , Schmidt, A. , Tadic, M. , Genger, M. , Lindhorst, R. , Bobenko, A. , Tschöpe, C. , Edelmann, F. , Pieske‐Kraigher, E. , and Pieske, B. (2019) Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study. ESC Heart Failure, 6: 146–153. 10.1002/ehf2.12375.
References
- 1. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation 2017; 135: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 3. Park JH, Marwick TH. Use and limitations of E/e′ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound 2011; 19: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tschöpe C, Lam CS. Diastolic heart failure: what we still don't know. Looking for new concepts, diagnostic approaches, and the role of comorbidities. Herz 2012; 37: 875–879. [DOI] [PubMed] [Google Scholar]
- 5. Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. E/e′ ratio in patients with unexplained dyspnea: lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail 2015; 8: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams‐Huet B, Thomas JD, Grayburn PA. Levine BD echocardiographic indices do not reliably track changes in left‐sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging 2011; 4: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 2005; 18: 63–68. [DOI] [PubMed] [Google Scholar]
- 8. Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of left ventricular filling pressure with exercise. J Am Coll Cardiol 2006; 47: 1891–1900. [DOI] [PubMed] [Google Scholar]
- 9. Holland DJ, Prasad SB, Marwick TH. Contribution of exercise echocardiography to the diagnosis of heart failure with preserved ejection fraction (HFPEF). Heart 2010; 96: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 10. Sanderson JE. Exercise echocardiography and the diagnosis of heart failure with a normal ejection fraction. Heart 2010; 96: 997–998. [DOI] [PubMed] [Google Scholar]
- 11. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 13. Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, Edvardsen T, Garbi M, Ha JW, Kane GC, Kreeger J, Mertens L, Pibarot P, Picano E, Ryan T, Tsutsui JM, Varga A. The clinical use of stress echocardiography in non‐ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2016; 17: 1191–1229. [DOI] [PubMed] [Google Scholar]
- 14. Belyavskiy E, Morris DA, Kraigher‐Krainer E, Kropf M, Radha Krishnan AK, Schmidt A, Pieske B. Clinical perspectives and evidence of diastolic stress test in heart failure with preserved ejection fraction. Egypt Heart J 2015; 67: 279–288. [Google Scholar]
- 15. Erdei T, Smiseth OA, Marino P, Fraser AG. A systematic review of diastolic stress test in heart failure with preserved ejection fraction, with proposals from EU‐FP7 MEDIA study group. Eur J Heart Fail 2014; 16: 1345–1361. [DOI] [PubMed] [Google Scholar]
- 16. Takagi TJ. Diastolic stress echocardiography. Echocardiography 2017; 15: 99–109. [DOI] [PubMed] [Google Scholar]
- 17. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meluzín J, Sitar J, Krístek J, Prosecky R, Pesl M, Podrouzková H, Soska V, Panovsky R, Dusek L. The role of exercise echocardiography in the diagnostics of heart failure with normal left ventricular ejection fraction. Eur J Echocardiogr 2011; 12: 591–602. [DOI] [PubMed] [Google Scholar]
- 19. Jang JY, Lee S, Kim DH, Song JM, Kang DH, Song JK. Variable hemodynamic responses during diastolic stress echocardiography in patients who have relaxation abnormality with possible elevated filling pressure. Korean Circ J 2018; 48: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39. [DOI] [PubMed] [Google Scholar]
- 21. Guazzi M, Adams V, Conraads V, Halle M, Mezzeni A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012; 126: 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133: e694–e711. [DOI] [PubMed] [Google Scholar]
- 23. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez‐Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 24. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 25. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures. A comparative simultaneous Doppler‐catheterization study. Circulation 2000; 102: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 26. Kasner M, Sinning D, Lober J, Post H, Fraser AG, Pieske B, Burkhoff D, Tschöpe C. Heterogeneous responses of systolic and diastolic left ventricular function to exercise in patients with heart failure and preserved ejection fraction. ESC Heart Failure 2015; 2: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction. J Am Coll Cardiol 2009; 54: 36–46. [DOI] [PubMed] [Google Scholar]
- 28. Donal E, Thebault C, Lund LH, Kervio G, Reynaud A, Simon T, Drouet E, Nonotte E, Linde C, Daubert JC. Heart failure with a preserved ejection fraction additive value of an exercise stress echocardiography. Eur Heart J Cardiovasc Imaging 2012; 13: 656–665. [DOI] [PubMed] [Google Scholar]
- 29. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nedeljkovic I, Banovic M, Stepanovic J, Giga V, Djordjevic‐Dikic A, Trifunovic D, Nedeljkovic M, Petrovic M, Dobric M, Dikic N, Zlatar M, Beleslin B. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur J Prev Cardiol 2016; 23: 71–77. [DOI] [PubMed] [Google Scholar]
- 31. Donal E, Lund LH, Oger E, Reynaud A, Schnell F, Persson H, Drouet E, Linde C, Daubert JC, KaRen investigators . Value of exercise echocardiography in heart failure with preserved ejection fraction: a substudy from the KaRen study. Eur Heart J Cardiovasc Imaging 2016; 17: 106–113. [DOI] [PubMed] [Google Scholar]
- 32. Kosmala W, Przewlocka‐Kosmala M, Rojek A, Marwick TH. Comparison of the diastolic stress test with a combined resting echocardiography and biomarker approach to patients with exertional dyspnea: diagnostic and prognostic implications. JACC Cardiovasc Imaging 2018. 10.1016/j.jcmg.2017.10.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


