Abstract
Aims
Dyspnoea is common in heart failure (HF) but non‐specific. Lung ultrasound (LUS) could represent a non‐invasive tool to detect subclinical pulmonary congestion in patients with undifferentiated dyspnoea.
Methods and results
We assessed the feasibility of an abbreviated LUS protocol (eight and two zones) in a prospective pilot study of 25 ambulatory patients with undifferentiated dyspnoea undergoing clinically indicated invasive cardiopulmonary exercise testing (iCPET) at rest (LUS 1) and after peak exercise (LUS 2). We also related LUS findings (B‐lines) to invasive haemodynamics stratified by supine pulmonary capillary wedge pressure (PCWP) (Congestion, >15 mmHg; Control, ≤15 mmHg). All enrolled patients (median age 68, 60% women, 32% prior HF, median ejection fraction 59%) had interpretable LUS 1 images in eight zones, and 20 (80%) had adequate LUS 2 images. LUS images were adequate in two posterior zones in 24 patients (96%) for LUS 1 and 18 (72%) for LUS 2. Although B‐line number was numerically higher in the Congestion group at rest and after peak exercise, this difference did not reach statistical significance. In the entire cohort, there was an association between B‐lines and rest systolic pulmonary artery pressure (r = 0.46, P = 0.02) and PCWP (r = 0.54, P = 0.005). There was an inverse relationship between B‐lines and peak VO2 (r = −0.65, P = 0.002).
Conclusions
Among ambulatory patients with undifferentiated dyspnoea, an abbreviated LUS protocol before and after iCPET is feasible in the majority of patients. B‐line number at rest was associated with invasively measured markers of haemodynamic congestion and was inversely related with peak VO2.
Keywords: Dyspnoea, Lung ultrasound, Invasive haemodynamics, Congestion
Introduction
Pulmonary congestion, often manifested by dyspnoea, is the most common sign of heart failure (HF), yet it is non‐specific and can be a symptom of many other conditions. The detection and quantification of pulmonary congestion based on current methods remain challenging owing to poor test sensitivities.1, 2 Lung ultrasound (LUS) is a relatively novel, semi‐quantitative technique to assess pulmonary congestion in patients with known or suspected HF.3 Sonographic B‐lines are hyperechoic, vertical lines on LUS, which provide a graded measure of extravascular lung water and change rapidly with removal of fluid during dialysis or diuresis.4, 5 Data on the diagnostic utility of this method in ambulatory patients with undifferentiated dyspnoea and its association with invasive haemodynamic measures are sparse.
We sought to assess the feasibility of employing an abbreviated eight‐zone lung imaging protocol before and after invasive cardiopulmonary exercise testing (iCPET) with respect to the detection of B‐lines in an outpatient cohort with undifferentiated dyspnoea in order to inform future, larger studies. The secondary, exploratory objectives were to examine the feasibility of two additional posterior zones and the association between eight‐zone B‐line number, invasive haemodynamics, and patient‐reported dyspnoea.
Methods
Study population and protocol
We conducted a prospective observational study in ambulatory patients with unexplained exertional dyspnoea referred to the Brigham and Women's Hospital Dyspnea Center for iCPET. Eligible adults were identified through the exercise laboratory's schedule and review of supine pulmonary capillary wedge pressure (PCWP) readings in the cardiac catheterization laboratory. Main exclusion criteria were ventricular assist device, heart/lung transplantation, pneumothorax, interstitial lung disease, current lung or pleural cancer, chest drain, dialysis, liver failure, and pregnancy. Although patients with a medical history of asthma or chronic obstructive pulmonary disease were included in this study, none of these patients had a drop in SpO2 < 93% (mean SpO2 95%, range 93–98%) during peak exercise. And while seven participants had a history of atrial fibrillation, only one patient had atrial fibrillation on electrocardiography during the iCPET examination. The maximum heart rate in this patient during peak exercise was 106 b.p.m. Patients were divided into two groups on the basis of supine PCWP: Control group (Control) included subjects with supine PCWP ≤ 15 mmHg, and haemodynamic congestion group (Congestion) included those with supine PCWP > 15 mmHg.6 Once on the upright cycle ergometer, LUS (LUS 1) and dyspnoea assessment [numeric ranking scale (NRS) 1; see subsequent details] were performed in sitting position at rest and following peak exercise immediately after transfer to a chair (LUS 2; NRS 2). Patients were excluded from this analysis if invasive haemodynamics were not measured (n = 1) or if no LUS images were acquired at any time point owing to equipment issues (n = 1). This study complies with the Declaration of Helsinki, the local Institutional Review Board approved the research protocol, and informed consent was obtained from all subjects.
Lung ultrasound
All LUS examinations were performed with standard echocardiographic equipment, utilizing 2–5 MHz phased array transducers. LUS data were collected in 10 zones, and the sum of B‐lines in eight predefined zones was used for the primary analyses.7 In secondary analyses, the sum of two non‐standard posterior zones was examined. To assure consistent B‐line analysis blinded to clinical and haemodynamic information and time point (temporal blinding), all de‐identified digital LUS videos were analysed offline by an experienced investigator as previously described.7, 8 Zones in which pleural effusions were present were excluded from the analysis (n = 1 patient).
Invasive cardiopulmonary exercise testing
Patients underwent placement of a Paceport (Edwards Lifesciences Corp.) pulmonary arterial catheter through the internal jugular vein, and a radial artery catheter in the cardiac catheterization laboratory. All subjects completed a single bout of incremental exercise to exhaustion on an upright cycle ergometer (MedGraphics Corival Cycle Ergometer, Medical Graphics Corp., Lode, Groningen, The Netherlands). At least 2 min of rest was followed by 2 min of unloaded cycling. Work was then continuously increased by 5–25 W/min on the basis of the subjects' described exercise tolerance. Ventilation, systemic and pulmonary haemodynamics, and gas exchange were measured as previously described.9, 10 See the Supporting Information for further details.
Patient‐reported dyspnoea, clinical, and demographic data
Subjects were asked to rate their dyspnoea on a NRS from 0 to 10 (NRS; 0: no shortness of breath; 10: severe shortness of breath) at rest (NRS 1) and at completion of the exercise protocol (NRS 2).11 Clinical and demographic data were collected from electronic medical records. Laboratory results were only reported if they were obtained within 60 days of the iCPET, and echocardiographic data within the past 12 months.
Statistical analyses
For the main analysis, we divided patients into two groups on the basis of supine PCWP as described earlier. We examined between‐group comparisons for baseline characteristics using Fisher's exact test for categorical variables and rank sum tests for continuous variables. Continuous variables are presented as medians [inter‐quartile range (IQR)] unless otherwise noted, and categorical variables as counts and percentages. Correlations between LUS and haemodynamic variables were assessed using the Spearman correlation coefficient. A two‐sided significance level of 0.05 was used for all analyses. Data were analysed using STATA SE, Version 14.2 (StataCorp, Texas 2015).
Results
All 25 patients included in this analysis had adequate LUS images in eight zones at rest (LUS 1), and 20 patients (80%) had adequate LUS images after peak exercise (LUS 2). For the two additional posterior zones, 24 patients had adequate LUS 1 images and 18 patients LUS 2 images. The median age of participants was 68 years (range 32–86), 60% were women, 80% were Caucasian, 32% had prior HF, and the median left ventricular ejection fraction (LVEF) was 59% (IQR 50, 60) (Table 1). Baseline characteristics were similar in patients with PCWP ≤ 15 mmHg and in those with PCWP >15 mmHg.
Table 1.
Baseline characteristics by study group
| All subjects (n = 25) |
Control PCWP ≤ 15 mmHg (n = 12)a |
Congestion PCWP > 15 mmHg (n = 13)a |
P | |
|---|---|---|---|---|
| Age (years) |
68 (60, 74) Range: 32–86 |
62 (59, 70) | 70 (60, 77) | 0.38 |
| Women | 15 (60) | 7 (58) | 8 (62) | 1.00 |
| Race | 0.32 | |||
| White | 20 (80) | 11 (92) | 9 (69) | |
| Other | 5 (20) | 1 (8) | 4 (31) | |
| BMI (kg/m2) | 31 (25, 35) | 29 (25, 34) | 31 (27, 36) | 0.55 |
| Medical history | ||||
| Hypertension | 21 (84) | 11 (92) | 10 (77) | 0.59 |
| Diabetes | 5 (21) | 2 (17) | 3 (25) | 1.00 |
| Myocardial infarction | 4 (16) | 2 (17) | 2 (15) | 1.00 |
| CABG | 2 (8) | 1 (8) | 1 (8) | 1.00 |
| Sleep apnoea | 3 (13) | 2 (17) | 1 (8) | 1.00 |
| Heart failure | 8 (32) | 2 (17) | 6 (46) | 0.20 |
| Prior admission for heart failure | 4 (16) | 0 | 4 (36) | 0.09 |
| Atrial fibrillation/flutter | 7 (29) | 1 (8) | 6 (50) | 0.07 |
| COPD/asthma | 6 (24) | 4 (33) | 2 (15) | 0.38 |
| Ejection fraction (%) | 59 (50, 60) | 60 (55, 65) | 55 (46, 60) | 0.06 |
| Ejection fraction > 40% (n, %) | 20 (91) | 10 (100) | 10 (83) | 0.48 |
| Medications | ||||
| ACE‐inhibitor/ARB | 13 (52) | 9 (75) | 4 (31) | 0.047 |
| Digoxin | 1 (4) | 1 (8) | 0 | 0.48 |
| Spironolactone | 4 (16) | 1 (8) | 3 (23) | 0.59 |
| Diuretic | 12 (48) | 4 (33) | 8 (62) | 0.24 |
| Calcium channel blocker | 9 (36) | 4 (33) | 5 (39) | 1.00 |
| Long acting nitrates | 4 (16) | 2 (17) | 2 (15) | 1.00 |
| Bronchodilator | 9 (36) | 4 (33) | 5 (39) | 1.00 |
| Laboratory results | ||||
| Sodium (mmol/L) (n = 17) | 140 (140, 142) | 142 (141, 142) | 140 (139, 140) | 0.036 |
| Creatinine (mg/dL) (n = 17) | 0.9 (0.9, 1.1) | 0.9 (0.8, 1.0) | 1.1 (0.9, 1.1) | 0.10 |
| Haemoglobin (g/dL) (n = 16) | 14 (12, 14) | 14 (12, 15) | 13 (12, 14) | 0.23 |
| NRS: Dyspnoea (range 0–10; 10 as worst) | ||||
| Dyspnoea at rest | 1 (0, 2.5) | 0.5 (0, 1.5) | 1.5 (0.5, 4) | 0.14 |
Continuous variables: median and inter‐quartile range (IQR) unless otherwise noted. Categorical variables: n, %.
ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BMI, body mass index; CABG, coronary artery bypass surgery; COPD, chronic obstructive pulmonary disease.
PCWP measured supine at rest.
Invasive haemodynamics and lung ultrasound findings
In the entire cohort, B‐line number ranged from 0 to 20 on eight‐zone LUS at rest, and 84% of patients had any B‐lines on eight‐zone LUS (Figure 1). Patients in the Congestion group demonstrated higher supine right‐sided intracardiac pressures and pulmonary vascular resistance than in the Control group (Table 2). In sitting position, both at rest and at peak exercise, pulmonary artery pressures and PCWP were higher in the Congestion group.
Figure 1.
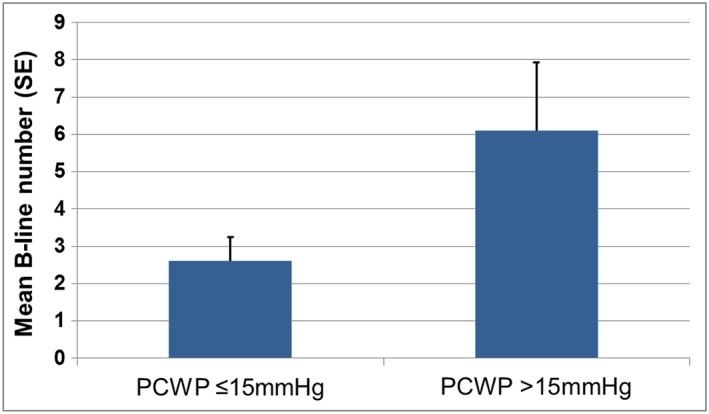
Mean B‐line number at rest by supine PCWP (n = 25). PCWP, mean pulmonary capillary wedge pressure.
Table 2.
Haemodynamics and lung ultrasound findings by study group
| Median (IQR) | All subjects (n = 25) | Control (n = 12) | Congestion (n = 13) | P |
|---|---|---|---|---|
| Supine (at rest) (n = 25) | ||||
| Heart rate (b.p.m.) | 70 (59, 80) | 60 (58, 81) | 74 (65, 78) | 0.34 |
| RA (mean; mmHg) | 7 (4, 10) | 4 (4, 6) | 10 (8, 12) | <0.001 |
| RV (end‐diastolic; mmHg) | 10 (6, 11) | 6 (4, 8) | 11 (11, 14) | <0.001 |
| PCWP (mean; mmHg) | 15 (9, 22) | 9 (7, 12) | 21 (18, 22) | — |
| PASP (mmHg) | 35 (26, 47) | 26 (23, 30) | 47 (41, 49) | <0.001 |
| mPAP (mmHg) | 24 (18, 31) | 18 (15, 21) | 31 (28, 33) | <0.001 |
| CO (L/min) | 5.1 (4.3, 5.7) | 5.6 (5.1, 6.0) | 4.4 (3.9, 4.9) | 0.022 |
| PVR (dyn * s/cm5) | 165 (106, 202) | 108 (89, 154) | 201 (158, 263) | 0.006 |
| Sitting (at rest) (n = 25) | ||||
| Heart rate (b.p.m.) | 66 (62, 81) | 74 (61, 100) | 67 (63, 78) | 0.68 |
| Systolic BP (mmHg) | 150 (138, 170) | 139 (131, 163) | 153 (138, 171) | 0.19 |
| Diastolic BP (mmHg) | 79 (67, 84) | 72 (65, 84) | 82 (68, 88) | 0.17 |
| SaO2 (%) | 96 (95, 97) | 97 (96, 98) | 96 (96, 97) | 0.60 |
| VO2 (mL/min) | 365 (281, 426) | 394 (293, 442) | 299 (281, 392) | 0.08 |
| RA (mean; mmHg) | 5 (3, 7) | 4 (3, 6) | 5 (4, 7) | 0.17 |
| PASP (mmHg) | 25 (21, 32) | 22 (20, 26) | 32 (23, 46) | 0.011 |
| mPAP (mmHg) | 17 (14, 24) | 15 (14, 18) | 23 (16, 33) | 0.019 |
| PCWP (mmHg) | 9 (6, 11) | 7 (6, 9) | 10 (9, 18) | 0.015 |
| CO (L/min) | 5.6 (4.1, 7.1) | 5.7 (4.5, 6.7) | 4.3 (3.5, 6.7) | 0.23 |
| PVR (dyn * s/cm5) | 156 (102, 239) | 116 (99, 175) | 181 (143, 260) | 0.10 |
| TPG (mmHg) | 10 (8, 13) | 8 (7, 11) | 11 (8, 14) | 0.13 |
| 8‐zone LUS 1 (n = 25) | ||||
| B‐lines (mean, SD) | 4.8 (5.6) | 2.6 (2.2) | 6.1 (6.6) | 0.37 |
| 2‐zone post. LUS 1 (n = 24) | ||||
| B‐lines (mean, SD) | 1.4 (2.0) | 0.9 (1.8) | 1.9 (2.2) | 0.13 |
| Sitting (peak exercise) (n = 20) | ||||
| Heart rate (b.p.m.) | 125 (110, 142) | 126 (116, 155) | 122 (86, 131) | 0.31 |
| Systolic BP (mmHg) | 190 (159, 202) | 200 (144, 235) | 185 (140, 204) | 0.24 |
| Diastolic BP (mmHg) | 88 (79, 94) | 85 (79, 91) | 91 (78, 100) | 0.55 |
| SaO2 (%) | 96 (95, 97) | 96 (96, 97) | 96 (94, 97) | 0.38 |
| VO2 (mL/min) | 1443 (985, 1611) | 1552 (1240, 1739) | 1285 (747, 1611) | 0.33 |
| VO2/kg | 15.4 (11.9, 18.3) | 16.9 (14.1, 18.3) | 15.8 (9.2, 19.7) | 0.65 |
| RA (mean; mmHg) | 8 (6, 10) | 6 (5, 8) | 10 (7, 13) | 0.05 |
| PASP (mmHg) | 51 (42, 59) | 45 (41, 50) | 59 (55, 69) | 0.003 |
| mPAP (mmHg) | 35 (27, 41) | 30 (27, 32) | 36 (35, 47) | 0.019 |
| PCWP (mmHg) | 17 (11, 26) | 14 (10, 16) | 24 (19, 28) | 0.005 |
| CO (L/min) | 10.8 (8.1, 13.3) | 11.9 (10.8, 13.3) | 9.5 (7.9, 13.5) | 0.20 |
| PVR (dyn * s/cm5) | 121 (89, 190) | 105 (89, 127) | 146 (98, 241) | 0.08 |
| TPG (mmHg) | 16 (14, 21) | 17 (14, 18) | 18 (15, 25) | 0.50 |
| 8‐zone LUS 2 (n = 20) | ||||
| B‐lines (mean, SD) | 3.8 (±6.0) | 2.1 (±1.5) | 5.4 (±8.3) | 0.94 |
| 2‐zone post. LUS 2 (n = 18) | ||||
| B‐lines (mean, SD) | 5.6 (±8.2) | 3.4 (±2.7) | 7.7 (±11.1) | 0.65 |
| Additional iCPET parameters (n = 25) | ||||
| Peak O2 pulse | 10 (8, 13) | 12 (8, 15) | 9 (8, 12) | 0.29 |
| Peak VE | 52 (35, 62) | 54 (46, 65) | 37 (34, 62) | 0.19 |
| VE/VCO2 slope | 34 (32, 36) | 34 (33, 35) | 34 (31, 36) | 0.89 |
CO, cardiac output; mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PCWP, mean pulmonary capillary wedge pressure; peak O2 pulse, amount of oxygen consumed per heart beat; PVR, pulmonary vascular resistance; RA, right atrial pressure; RV, right ventricular pressure; SaO2, arterial oxygen saturation; TPG, transpulmonary gradient; VE/VCO2 slope, minute ventilation/carbon dioxide production relationship; VE, minute ventilation; VO2, peak oxygen uptake.
Although B‐line number was numerically higher in the Congestion group, both at rest and after peak exercise, this difference did not reach statistical significance (P = 0.37 for eight‐zone LUS at rest; P = 0.94 after exercise). In the entire cohort, there was an association between B‐lines on LUS and supine and sitting (rest) pulmonary artery and PCWP [Figures 2 A and 2 B]. For peak exercise haemodynamics, there was an inverse relationship between B‐lines and peak VO2 and cardiac output. By contrast, patient‐reported dyspnoea was only associated with sitting PCWP at rest (r = 0.48, P = 0.017) but not with other haemodynamic measures (Table 3).
Figure 2.
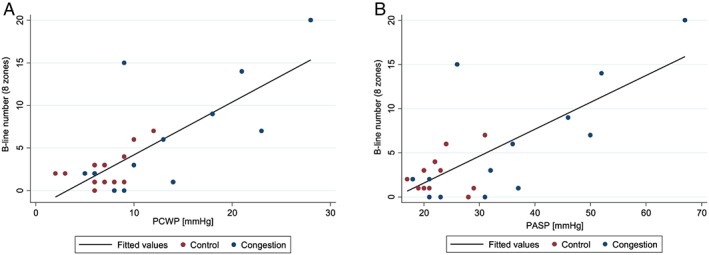
(A) Scatterplot of B‐line number by PCWP at rest. PCWP, mean pulmonary capillary wedge pressure. (B) Scatterplot of B‐line number by PASP at rest. PASP, pulmonary artery systolic pressure.
Table 3.
Spearman correlation between B‐lines and haemodynamics
| Rho (P value)a | |||
|---|---|---|---|
| LUS 1 and supine haemodynamics (at rest; n = 25) | LUS 1 and sitting haemodynamics (at rest; n = 25) | LUS 2 and sitting haemodynamics (peak exercise; n = 20) | |
| SaO2 | — | −0.16 | −0.15 |
| VO2 | — | 0.14 | −0.65 (0.002) |
| VO2/kg | — | — | −0.64 (0.002) |
| RA | 0.16 | 0.06 | −0.11 |
| PASP | 0.41 (0.043) | 0.46 (0.020) | 0.09 |
| mPAP | 0.40 (0.049) | 0.42 (0.034) | 0.08 |
| PCWP | 0.42 (0.039) | 0.54 (0.005) | 0.01 |
| CO | 0.04 | −0.06 | −0.58 (0.007) |
| PVR | 0.26 | 0.28 | 0.39 |
| TPG | — | 0.36 | 0.21 |
CO, cardiac output; mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PCWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial pressure; SaO2, arterial oxygen saturation; TPG, transpulmonary gradient; VO2, peak oxygen uptake.
Only P values < 0.05 are shown. All other P values are non‐significant.
Discussion
The main findings of this feasibility study are as follows: (i) Among ambulatory patients with undifferentiated dyspnoea, B‐lines can be detected at rest with an eight‐zone protocol in the majority of patients. (ii) Our data suggest that at rest, B‐lines are associated with supine and upright pulmonary artery pressures and PCWP. (iii) There appears to be an inverse relationship between B‐lines after peak exercise and peak VO2.
Few studies have investigated the association between invasive haemodynamics and LUS findings in patients with undifferentiated dyspnoea or HF. Two small studies in patients admitted to intensive care units with a variety of diagnoses, including patients on mechanical ventilation, found associations between B‐lines and extravascular lung water, but associations with PCWP were inconsistent.5, 12 A prior study of non‐intubated patients undergoing right heart catheterization, which included 44% with prior HF, showed no association between B‐lines and PCWP but rather with pulmonary artery pressures.8 By contrast, in our study of ambulatory patients with undifferentiated dyspnoea, B‐lines were associated with both PCWP and pulmonary artery pressures. Although our results should be considered hypothesis generating given the small sample size, it is conceivable that patients with left‐sided HF leading to right‐sided HF may demonstrate a higher degree of pulmonary congestion on LUS than those with isolated left HF. Prior studies may have led to inconsistent results due to heterogeneous study cohorts.
Data on LUS findings following exercise are sparse. One prior study investigated LUS findings in a heterogeneous cohort referred for stress echocardiography found an association between B‐lines and echocardiographically estimated pulmonary artery systolic pressure and PCWP.13 Another study examined supine LUS findings before and after non‐invasive CPET in ambulatory and hospitalized HF with reduced ejection fraction patients and demonstrated an association with post‐exercise B‐line number and log‐BNP and an inverse relationship of B‐lines with peak VO2.14 Similarly, we found an association between resting B‐lines and PCWP, which was invasively measured in our study, and post‐exercise B‐lines and peak VO2. In contrast to our study, both prior investigations detected an increase in B‐line number following exercise. These differences could be due to differences in patient cohorts, patient positioning during LUS (sitting in our study vs. supine during prior investigations), and timing of post‐exercise LUS images.15 Technical challenges in obtaining post‐exercise LUS images could contribute to suboptimal or missing post‐exercise images. In addition, temporal blinding and offline B‐line quantification in our study may have resulted in a smaller observed change in B‐line number than previously reported, as prior studies did not employ temporal blinding. Finally, differences in the number of LUS zones evaluated (28 vs. eight zones) could have resulted in a smaller effect size, although 84% of patients in our study had a detectable number of B‐lines at rest, suggesting that an eight‐zone protocol may be sensitive enough to detect low levels of pulmonary congestion.
Limitations
This was a small study conducted at a single centre with the goal to assess the feasibility of a novel LUS protocol before and after iCPET. Laboratory values, including NT‐proBNP, were not consistently available in all study patients. As this was a pilot study, we did not collect long‐term outcome data.
Conclusions
Our data suggest that LUS in eight zones before and after iCPET is feasible. The number of B‐lines at rest appears to be associated with invasively measured markers of haemodynamic congestion, and following peak exercise, there may be an inverse relationship with peak VO2.
Conflict of interest
None declared.
Funding
This work was supported by a grant from the William F. Milton Fund (Harvard University) (E.P.). The writing of this manuscript was supported by a grant from the National Heart, Lung, and Blood Institute (grant number K23HL123533) (E.P.). The sponsors had no input or contribution in the development of the research and manuscript.
Disclosures
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Supporting information
Data S1. Supporting information
Platz, E. , Merz, A. , Silverman, M. , Lewis, E. , Groarke, J. D. , Waxman, A. , and Systrom, D. (2019) Association between lung ultrasound findings and invasive exercise haemodynamics in patients with undifferentiated dyspnoea. ESC Heart Failure, 6: 202–207. 10.1002/ehf2.12381.
References
- 1. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294: 1944–1956. [DOI] [PubMed] [Google Scholar]
- 2. Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail 2015; 17: 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, Volpicelli G, Balzaretti P, Banderali A, Iacobucci A, Locatelli S, Casoli G, Stone MB, Maule MM, Baldi I, Merletti F, Cibinel GA. Lung ultrasound‐implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest 2015; 148: 202–210. [DOI] [PubMed] [Google Scholar]
- 4. Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, Picano E, Zoccali C. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 2010; 3: 586–594. [DOI] [PubMed] [Google Scholar]
- 5. Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. “Ultrasound comet‐tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest 2005; 127: 1690–1695. [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 8. Platz E, Lattanzi A, Agbo C, Takeuchi M, Resnic FS, Solomon SD, Desai AS. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail 2012; 14: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 9. Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation 2013; 127: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 10. Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail 2015; 8: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson MJ, Oxberry SG, Cleland JG, Clark AL. Measurement of breathlessness in clinical trials in patients with chronic heart failure: the need for a standardized approach: a systematic review. Eur J Heart Fail 2010; 12: 137–147. [DOI] [PubMed] [Google Scholar]
- 12. Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, Luberto L, Anile A, Cerutti E, Radeschi G, Frascisco MF. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014; 121: 320–327. [DOI] [PubMed] [Google Scholar]
- 13. Agricola E, Picano E, Oppizzi M, Pisani M, Meris A, Fragasso G, Margonato A. Assessment of stress‐induced pulmonary interstitial edema by chest ultrasound during exercise echocardiography and its correlation with left ventricular function. J Am Soc Echocardiogr 2006; 19: 457–463. [DOI] [PubMed] [Google Scholar]
- 14. Scali MC, Cortigiani L, Simionuc A, Gregori D, Marzilli M, Picano E. Exercise‐induced B‐lines identify worse functional and prognostic stage in heart failure patients with depressed left ventricular ejection fraction. Eur J Heart Fail 2017; 19: 1468–1478. [DOI] [PubMed] [Google Scholar]
- 15. Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care 2015; 4: 326–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


