Abstract
Aims
Improving quality of life (QoL) in heart failure patients is a key management objective. Validated health‐related QoL (HR‐QoL) measurement tools have been incorporated into clinical trials but not routinely into daily practice. The aims of this study were to investigate the acceptability and feasibility of implementing validated HR‐QoL instruments into heart failure clinics and to examine the impact of patient characteristics on HR‐QoL.
Methods and results
One hundred and sixty‐three patients attending heart failure clinics at a UK tertiary centre were invited to complete three HR‐QoL assessments: the Minnesota Living with Heart Failure Questionnaire (MLHFQ); the EuroQoL 5D‐3L (EQ‐5D‐3L); and the Kansas City Cardiomyopathy Questionnaire (KCCQ) in that order. Data on patient demographics, co‐morbidities, New York Heart Association (NYHA) class, plasma B‐type natriuretic peptide (BNP), renal function, and left ventricular ejection fraction were recorded. 94% of patients attending clinic were willing to participate. The EQ‐5D‐3L had all questions answered by 92% of patients, compared with 86% and 51% for the MLHFQ and KCCQ, respectively. HR‐QoL significantly correlated with NYHA class using each tool (MLHFQ, r = 0.59; KCCQ, r = −0.61; EQ‐5D‐3L, r = −0.44, all P < 0.01). However, within each NYHA class, there was a widespread of HR‐QoL scores. There was no association between patient demographics, left ventricular ejection fraction, plasma B‐type natriuretic peptide, or renal function with HR‐QoL using any tool.
Conclusions
Health‐related QoL assessment by validated questionnaire was acceptable to patients and feasible to perform in routine practice. Although NYHA class correlated significantly with HR‐QoL scores, there was high variability in HR‐QoL within each NYHA class, highlighting its limitation as the sole assessment of HR‐QoL. Clinicians should encourage the assessment of HR‐QoL to facilitate patient‐centred care and make more specific use of HR‐QoL measurement tools.
Keywords: Heart failure, Heath‐related quality of life (HR‐QoL), New York Heart Association (NYHA) class, Minnesota Living with Heart Failure Questionnaire (MLHFQ), EuroQoL 5D‐3L (EQ‐5D‐3L), Kansas City Cardiomyopathy Questionnaire (KCCQ)
Introduction
The negative impact of heart failure on health‐related quality life (HR‐QoL) is well recognised.1 Improving HR‐QoL is acknowledged as a fundamental goal of heart failure management in the North American, European, and UK guidelines.2, 3, 4 The assessment of symptoms and functional status, using the New York Heart Association (NYHA) classification, is a standard practice in the care of heart failure patients. It is recommended in guidelines as a measure of heart failure severity and is integrated into treatment decision algorithms, despite its shortcomings in reproducibilty.4, 5, 6, 7 The use of validated instruments to assess HR‐QoL, however, remains largely limited to clinical trials, with minimal guidance on the practical assessment of HR‐QoL outside of this setting.8, 9
Health‐related quality life is influenced by numerous physical, emotional, and social factors and is uniquely perceived by each individual.10 The structured assessment of HR‐QoL is considered important in promoting patient‐centric care.11 It puts the patient's perspective at the forefront and can identify areas of specific need. This helps to facilitate shared decision‐making and ensure that the preferences of the patient are used to guide management. This is important as patients can place different value on treatment goals, including the trade‐off between quality of life (QoL) and survival.12 It also provides a framework for clinical monitoring. A reduction in HR‐QoL has been shown to be an independent predictor of increased hospitalisation and mortality.13 A number of validated generic and disease‐specific HR‐QoL assessment instruments have been developed.14, 15, 16 However, there remains no consensus on the best tools and methods for assessment of HR‐QoL in heart failure in routine clinical practice.
The aims of this study were to investigate the acceptability and feasibility of implementing validated HR‐QoL instruments into heart failure clinics and to examine the impact of patient characteristics on HR‐QoL.
Methods
Heart failure patients attending two specialist cardiology outpatient clinics at the Royal Brompton Hospital London, UK over 24 consecutive months from May 2015 to May 2017 were invited to participate. They were each asked to complete three HR‐QoL assessment tools: the Minnesota Living with Heart Failure Questionnaire (MLHFQ); the EuroQoL 5D‐3L (EQ‐5D‐3L); and the Kansas City Cardiomyopathy Questionnaire (KCCQ) in that order. The MLHFQ and KCCQ are disease‐specific instruments, which have both been validated to assess HR‐QoL in HF patients and widely used in clinical studies.14, 16 For comparison, a generic instrument was chosen, namely, the EQ‐5D‐3L, which is validated to assess HR‐QoL in the general population and heart failure patients.15 The questionnaires were completed prior to the patients' face‐to‐face clinical review with their cardiologist and specialist heart failure nurse. We recorded if a patient requested help with the questionnaires, from either a nurse, carer, or family/friend attending with them. Data were collected from the electronic patient record on demographics, co‐morbidities, NYHA class, plasma B‐type natriuretic peptide (BNP), renal function using the modification of diet in renal disease formula to calculate the estimated glomerular filtration rate (eGFR), and echocardiographic measurements.
The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice standards. All relevant licences were obtained prior to starting data collection.
Questionnaires
Minnesota living with Heart Failure Questionnaire
The MLHFQ consists of 21 questions specific to heart failure and appraises HR‐QoL over the period of the previous month. Questions are answered using a Likert scale of 0–5; 0 indicates that the question has no impact on the patient or is not applicable, and 5 indicates the greatest adverse effect. The questionnaires can be divided into three domains: overall QoL domain (score range 0–105), the physical domain consisting of eight questions (score range 0–40), and the emotional domain made up of five questions (score range 0–25). A higher score represents a poorer HR‐QoL. The authors recommend the overall total score as the best measure as opposed to the other two domains, which were created following factor analysis.
EuroQoL 5D‐3L
The EQ‐5D‐3L is a generic health questionnaire, which is composed of two parts. The first part, the health score, assesses the patient's ability to mobilise, self‐care, and perform their usual activities and scores their pain/discomfort and anxiety/depression levels. The second part is the visual analogue score, which enables the patient to rate their current health state on a scale of 0–100, with 0 being the lowest and 100 being the best possible health state. It provides additional information as a quantitative measure of self‐rated health from the patient's perspective. The health score and the visual analogue scores can be converted into a country‐specific index. The maximum health index value equals 1 and indicates perfect health. For the health index value, it is possible to have a negative score, with a score below 0 indicating ‘a state worse than death’. The visual analogue index score is from 0 to 1 with no negative scores.
Kansas City Cardiomyopathy Questionnaire
The KCCQ is a heart failure disease‐specific questionnaire consisting of 23 questions. It is divided into several domains including physical and social limitations, symptoms, self‐efficacy, and QoL, each transformed to score range between 0 and 100. The clinical summary score combines measures of symptoms and social factors, while the overall summary score brings together all the domains. A higher score is representative of a better health status.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and categorical data as numbers and percentages. Correlation between the HR‐QoL scores of the different questionnaires and between individual variables and the HR‐QoL tools was assessed by Pearson correlation for normally distributed data and Spearman rank correlation for non‐parametric and nominal data. A multivariable linear regression was performed to explore predictive variables of HR‐QoL. Statistical analyses were performed using statistical software SPSS version 24 (IBM Corp., Armonk, NY, USA). P‐values < 0.05 were considered statistically significant.
Results
One hundred and fifty‐two of the 163 patients (93.3%) invited to participate completed the questionnaires. Table 1 shows the baseline characteristics of these patients. The mean age was 68 years, and 73% were male. Left ventricular ejection fraction was preserved (>50%) in 32% of patients. The majority of patients were in NYHA Class II or III (56% and 31%, respectively), and dilated cardiomyopathy was the single most common aetiology (36%). A quarter of the patients (26%) had two or more co‐morbidities identified as either hypertension, diabetes, atrial arrhythmia, or chronic obstructive pulmonary disease, and the median number of co‐morbidities per patient was one.
Table 1.
Baseline characteristics
| Participants (n = 152) | |
|---|---|
| Male (n, %) | 111 (73) |
| Age (years) | 68.3 ± 12.2 |
| Body mass index (kg/m2) | 28.9 ± 6.2 |
| Systolic BP (mmHg) | 110 ± 25 |
| Heart rate (b.p.m.) | 71 ± 16 |
| LVEDD (cm) | 5.7 ± 1.3 |
| LVESD (cm) | 4.7 ± 1.3 |
| LVEF (%) | 39.3 ± 14.6 |
| Reduced LVEF <40% (n, %) | 81 (53) |
| Mid‐range LVEF 40–49% (n, %) | 23 (15) |
| Preserved EF ≥50% (n, %) | 48 (32) |
| BNP (ng/L) | 224.5 [98–458] |
| eGFR (mL/min/1.73 m2) | 56.4 ± 20.4 |
| NYHA class (n, %) | |
| I | 16 (10) |
| II | 85 (56) |
| III | 47 (31) |
| IV | 4 (3) |
| Dilated cardiomyopathy (n, %) | 54 (36) |
| Ischaemic heart disease (n, %) | 40 (26) |
| ICD/CRT (n, %) | 59 (39) |
| ACE‐I/ARB/ARNI (n, %) (HFrEF/HFpEF) | 72 (89)/32 (67) |
| Beta‐blocker (n, %) (HFrEF/HFpEF) | 68 (84)/36 (75) |
| MRA (n, %) (HFrEF/HFpEF) | 53 (65)/18 (38) |
| ACE‐I/ARB/ARNI + beta‐blocker + MRA (n, %) (HFrEF/HFpEF) | 42 (52)/10 (21) |
| Loop diuretics (n, %) (HFrEF/HFpEF) | 65 (80)/29 (60) |
| Hypertension (n, %) | 32 (21) |
| Diabetes (n, %) | 39 (26) |
| COPD (n, %) | 9 (6) |
| AF/AFl/AT (n, %) | 84 (55) |
| Co‐morbidities (hypertension, diabetes, atrial arrhythmia, COPD) (n, %) | |
| 0 co‐morbidity | 41 (27) |
| 1 co‐morbidity | 71 (47) |
| 2 co‐morbidities | 30 (20) |
| 3 co‐morbidities | 10 (6) |
| MLHFQ total score | 40.9 ± 25.6 |
| EQ‐5D‐3L health score index | 0.64 ± 0.27 |
| EQ‐5D‐3L VAS score index | 0.60 ± 0.19 |
| KCCQ overall summary score | 58.7 ± 24.9 |
Data are presented as mean ± standard deviation, median [interquartile range], or as number (percentage).
ACE‐I, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AFl: atrial flutter; ARB, angiotensin receptor blocker; ARNI, angiotensin II receptor blocker neprilysin inhibitor; AT, atrial tachycardia; BNP, B‐type natriuretic peptide; BP, blood pressure; CRT, cardiac resynchronisation therapy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EQ‐5D‐3L, EuroQoL 5D‐3L; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
The completion rates of questionnaires
The EQ‐5D‐3L questionnaire had the highest completion rate, with all questions being answered by 140 patients (92%) of those who agreed to take part, compared with 130 patients (85%) for the MLHFQ questionnaire and 74 patients (49%) for the KCCQ. The EQ‐5D‐3L had the highest mean response rate per question at 97.6%, while the MLHFQ and KCCQ were lower at 93.4% and 92.5%, respectively. There was no consistent pattern in how well the questionnaires were completed when examined by individual heart failure groups. The heart failure with reduced ejection fraction group had the highest completion on the KCCQ and the lowest completion rate of all on the MLHFQ. Question 23 on the KCCQ, which asked ‘how much does your heart failure affect intimate or sexual relationships?’, was the least answered at 65%. In contrast, the response rate to a similar question on the MLHFQ, ‘did your heart failure prevent you from living as you wanted by making your sexual activities difficult?’, was completed by 94% of patients.
Forty‐eight (32%) patients required assistance completing the questionnaires. There was an equal division between patients who requested help from a nurse and those seeking help from family members, friends, or carers. If advice was sought from a nurse, the recommended approach in the questionnaire manuals was used, helping to limit bias.
Health‐related quality of life scores
There was good correlation between all three HR‐QoL assessment tools. The strongest correlation was observed between the two disease‐specific questionnaires, the MLHFQ total score and KCCQ overall summary score (r = −0.88, P = < 0.01) (Table 2).
Table 2.
Correlation between the mean QoL scores
| MLHFQ total score HFQ | EQ‐5D‐3L health score | VAS health score | KCCQ overall summary score | |
|---|---|---|---|---|
| MLHFQ total score | 1.00 | −0.68** | −0.65** | −0.88** |
| EQ‐5D‐3L health score | −0.68** | 1.00 | 0.61** | 0.74 |
| VAS health score | −0.65** | 0.61** | 1.00 | 0.69** |
| KCCQ overall summary score | −0.88** | 0.74 | 0.69** | 1.00 |
EQ‐5D‐3L, EuroQoL 5D‐3L; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; QoL, quality of life; VAS, visual analogue scale.
Correlation is significant at the 0.01 level (two‐tailed).
Correlation of patient characteristics with health‐related quality of life
On univariable analysis, NYHA class was the only clinical and demographic variable to correlate significantly with each of the HR‐QoL assessment tools (Table 3). The correlation was strongest with the disease‐specific questionnaires, KCCQ (r = −0.61, P = < 0.01) and MLHFQ (r = 0.59, P = < 0.01). However, within each NYHA class, there was a wide distribution of HR‐QoL summary scores (Figures 1 and 2 ). There was a significant correlation between BNP and NYHA class (r = 0.325, P = < 0.01) but not between BNP and HR‐QoL.
Table 3.
Correlation between the mean QoL scores and clinical variables
| MLHFQ total score | EQ‐5D‐3L health score | KCCQ overall score | |
|---|---|---|---|
| Age | −0.164 | 0.110 | 0.095 |
| Gender | 0.078 | 0.025 | −0.069 |
| NYHA class | 0.588** | −0.436** | −0.613** |
| BMI | −0.019 | −0.110 | −0.008 |
| LVEF | −0.172* | −0.095 | 0.074 |
| Systolic BP | −0.065 | −0.001 | 0.079 |
| Heart rate | 0.111 | −0.138 | −0.174* |
| eGFR | 0.058 | −0.083 | −0.030 |
| BNP | 0.069 | 0.083 | −0.071 |
| Co‐morbidities | 0.036 | −0.040 | −0.088 |
BMI, body mass index (kg/m2); BNP, B‐type natriuretic peptide; BP, blood pressure; eGFR, estimated glomerular filtration rate; EQ‐5D‐3L, EuroQoL 5D‐3L; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; QoL, quality of life.
Correlation is significant at the 0.05 level (two‐tailed).
Correlation is significant at the 0.01 level (two‐tailed).
Figure 1.
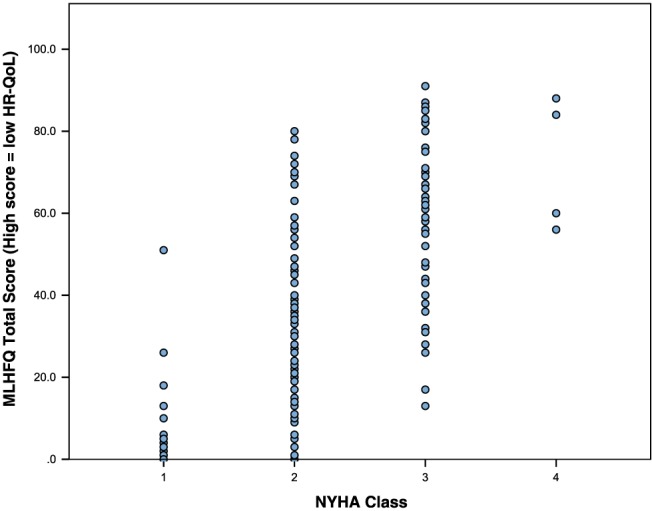
Distribution of Minnesota Living with Heart Failure Questionnaire (MLHFQ) total scores by New York Heart Association (NYHA) class.
Figure 2.
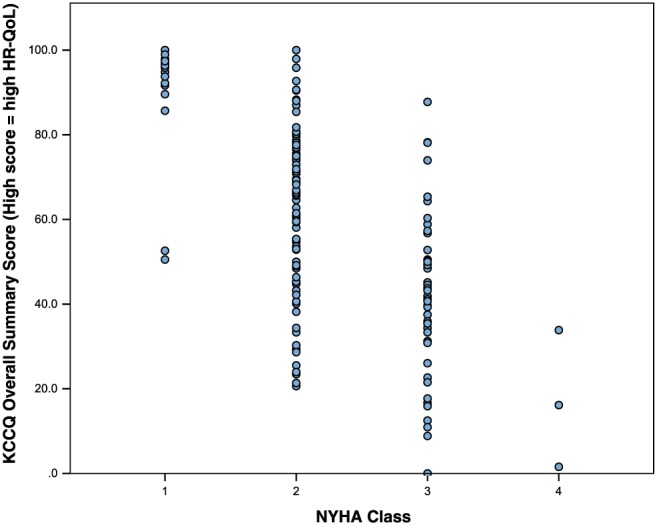
Distribution of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary scores by New York Heart Association (NYHA) class.
On multivariable linear regression analysis, NYHA class remained significantly associated with the HR‐QoL outcomes scores of each of the three questionnaires (all P < 0.005). (Table 4).
Table 4.
Multivariable linear regression
| MLHFQ | EQ‐5D‐3L | KCCQ | ||||
|---|---|---|---|---|---|---|
| Β coefficient | P‐value | Β coefficient | P‐value | Β coefficient | P‐value | |
| Age | −0.054 | 0.789 | −0.001 | 0.602 | −0.025 | 0.895 |
| Gender | 8.091 | 0.119 | 0.033 | 0.545 | −5.626 | 0.225 |
| BMI | 0.301 | 0.418 | −0.007 | 0.093 | −0.450 | 0.183 |
| Systolic BP | 0.095 | 0.322 | 0.000 | 0.656 | −0.042 | 0.604 |
| Heart rate | 0.168 | 0.256 | −0.002 | 0.283 | −0.170 | 0.211 |
| LVEF | −0.316 | 0.100 | −0.004 | 0.082 | 0.142 | 0.393 |
| NYHA class | 18.115 | 0.000 | −0.206 | 0.000 | −23.890 | 0.000 |
| eGFR, mL/min | 0.191 | 0.095 | −0.002 | 0.096 | −0.178 | 0.090 |
| BNP | −0.005 | 0.467 | 2.451E‐5 | 0.735 | 0.009 | 0.163 |
| No. of co‐morbidities | 0.958 | 0.758 | 0.035 | 0.284 | −0.253 | 0.928 |
BMI, body mass index (kg/m2); BNP, B‐type natriuretic peptide; BP, blood pressure; eGFR, estimated glomerular filtration rate; EQ‐5D‐3L, EuroQoL 5D‐3L; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association.
Discussion
Improving QoL is an accepted goal in shared decision‐making for patients with heart failure. Validated HR‐QoL tools are widely used in clinical trials but are not yet used routinely in clinical practice. This study set out to explore the feasibility of implementing validated HR‐QoL instruments in the routine heart failure clinic setting. The vast majority of our patients were willing to complete the HR‐QoL assessments. The questionnaires were self‐administered in the clinic waiting area prior to the medical consultation, for time efficiency and to reduce potential bias of opinion generated by interaction with clinical staff. However, nearly a third of the patients requested assistance with the task. Whether or not the help sought from relatives and friends, as opposed to more impartial support from nurses, influenced an individual's response was not assessed. Administration instructions given for the MLHFQ state that patients should not receive help from family members. The use of proxy respondents for patient‐reported outcomes, in stroke patients, however, has been shown to have moderate to substantial reliability for QoL indices.17
The generic EQ‐5D‐3L questionnaire had the highest total completion rate of all the instruments. With respect to the disease‐specific questionnaires, over 40% more patients completed the MLHFQ without omissions compared with the KCCQ. However, the overall mean response rates per question for the MLHFQ and KCCQ was similar, suggesting only minor omissions in the non‐completed KCCQ. The explanation for these findings may relate to response burden. Response burden is the effort needed by a patient to answer a questionnaire. It can be influenced by a number of factors including the questionnaire length and format, complexity of the task, and repetition of sampling.18 These in turn can contribute to lower response rates. In this study, the three questionnaires were administered in a set order, with the KCCQ being last. As such, it is plausible that the lower KCCQ completion rates were a reflection of higher resource burden encountered by that stage. In contrast, the more simplified and shorter style of the EQ‐5D‐3L may have accounted for its higher completion rate without omissions. It is also likely that it contributed to the discrepancy in the answering of questions related to intimacy and sexual activity by the same patients. On the MLHFQ, the question was answered by 94% of patients, indicating that they were not evading answering any questions on this topic. The contrasting low response rate on the KCCQ therefore may reflect its position as the final question of all three questionnaires and the impact of response burden or the phraseology used. The length and time to complete questionnaires can be a barrier to implement validated HR‐QoL instruments into routine practice. In 2015, Spertus and Jones validated a shorter, 12‐item version of the KCCQ with the aim to reduce response burden.19 Additionally, the validation of single‐item questions on self‐reported health has been performed.20, 21
Besides NYHA class, our results demonstrated no biological factors that correlated with HR‐QoL scores. As previously reported, left ventricular ejection fraction was not a predictor of HR‐QoL.22 NYHA class is recommended in all guidelines as a useful tool to assess the functional limitations imposed on a patient by their heart failure. NYHA class is typically rated from the clinician's perspective rather than from the patients. A comparison between physician and patient‐reported NYHA class demonstrated agreement in only half of the cases.23 The association between more impaired HR‐QoL scores and worsening NYHA has previously been shown.22, 24 However, in our study, although NYHA class was strongly correlated with HR‐QoL scores, particularly measured with the disease‐specific tools, within each NYHA class, there was a very wide variability in HR‐QoL. This suggests that NYHA class does not capture all elements of HR‐QOL at an individual level. The limitations of NYHA include its subjective assessment and high inter‐observer variability, previously reported at 56%.4, 5, 6 This is particularly important to note in outpatient clinics where the patient may be reviewed by different clinicians at each clinic visit.
The formal assessment of HR‐QoL has yet to be incorporated into routine clinical practice. The use of validated HR‐QoL tools can readily highlight areas in which patients need the greatest support and open up discussion with healthcare professionals. Without a standardised approach to the evaluation of HR‐QoL, incorrect assumptions may be made with a potentially negative impact on shared decision‐making around appropriate care options. Debate, however, remains on the best instrument to use.
This study focuses on the pragmatic approach of using validated HR‐QoL instruments in everyday clinical practice in an unselected sample of heart failure patients, showing high patient acceptance, and provides insight into completion rates in routine practice. Consistent with previous studies, we found NYHA class to be an independent predictor of HR‐QoL.25 Additionally, we have demonstrated the drawbacks of NYHA class as a surrogate marker of HR‐QoL at an individual level. Further research is required to assess the impact of routine assessment of HR‐QoL in clinical practice on decision‐making, outcomes, and patient satisfaction with healthcare interactions.
Limitations
This study was conducted in a convenience sample within an urban population based at a single tertiary centre. This has potential limitations on being able to generalise the results. However, the sample was an unselected cohort of patients, with both reduced and preserved systolic function, attending a routine heart failure clinic and reflective of daily clinical practice. The impact of social demographics on HR‐QoL and completion rates was not assessed as part of this study. The non‐omission completion rates of the validated instruments are likely to have been confounded by the set order in which they were administered, which should be taking into account particularly when interpreting the results of the KCCQ. The shorter 12‐item KCCQ was not included as it was introduced after the commencement of this study. Further studies into the real‐life completion rates of validated HR‐QoL instruments would be beneficial.
Conflict of interest
None declared.
Funding
The salary of Rebecca Lucas was supported by an unrestricted educational grant from Bayer.
Gallagher, A. M. , Lucas, R. , and Cowie, M. R. (2019) Assessing health‐related quality of life in heart failure patients attending an outpatient clinic: a pragmatic approach. ESC Heart Failure, 6: 3–9. 10.1002/ehf2.12363.
References
- 1. Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002; 87: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 4. National Institute for Health and Clinical Excellence . Chronic heart failure in adults: management. (Clinical Guideline CG108). 2010. www.nice.org.uk/guidance/Cg108 (2 April 2018).
- 5. Williams BA, Doddamani S, Troup MA, Mowery AL, Kline CM, Gerringer JA, Faillace RT. Agreement between heart failure patients and providers in assessing New York Heart Association functional class. Heart Lung 2017; 46: 293–299. [DOI] [PubMed] [Google Scholar]
- 6. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart 2007; 93: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981; 64: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 8. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ, American Heart Association Council on Quality of Care and Outcomes Research CoCaSN , Council on Epidemiology and Prevention , Council on Peripheral Vascular Disease , Stroke Council . Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation 2013; 127: 2233–2249. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I, Kirchhof P. The importance of patient‐reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014; 35: 2001–2009. [DOI] [PubMed] [Google Scholar]
- 10. Testa MA, Simonson DC. Assessment of quality‐of‐life outcomes. N Engl J Med 1996; 334: 835–840. [DOI] [PubMed] [Google Scholar]
- 11. Rumsfeld JS. Health status and clinical practice: when will they meet? Circulation 2002; 106: 5–7. [DOI] [PubMed] [Google Scholar]
- 12. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001; 20: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 13. Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near‐term cardiovascular events with serial health status assessments. Circulation 2007; 115: 1975–1981. [DOI] [PubMed] [Google Scholar]
- 14. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure Questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993; 71: 1106–1107. [DOI] [PubMed] [Google Scholar]
- 15. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ‐5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 17. Oczkowski C, O'Donnell M. Reliability of proxy respondents for patients with stroke: a systematic review. J Stroke Cerebrovasc Dis 2010; 19: 410–416. [DOI] [PubMed] [Google Scholar]
- 18. Rolstad S, Adler J, Ryden A. Response burden and questionnaire length: is shorter better? A review and meta‐analysis. Value Health 2011; 14: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 19. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015; 8: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inkrot S, Lainscak M, Edelmann F, Loncar G, Stankovic I, Celic V, Apostolovic S, Tahirovic E, Trippel T, Herrmann‐Lingen C, Gelbrich G, Dungen HD. Poor self‐rated health predicts mortality in patients with stable chronic heart failure. Eur J Cardiovasc Nurs 2016; 15: 504–512. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Hobkirk J, Carroll S, Pellicori P, Clark AL, Cleland JG. Exploring quality of life in patients with and without heart failure. Int J Cardiol 2016; 202: 676–684. [DOI] [PubMed] [Google Scholar]
- 22. Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA, Investigators C. Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9: 83–91. [DOI] [PubMed] [Google Scholar]
- 23. Goode KM, Nabb S, Cleland JG, Clark AL. A comparison of patient and physician‐rated New York Heart Association class in a community‐based heart failure clinic. J Card Fail 2008; 14: 379–387. [DOI] [PubMed] [Google Scholar]
- 24. Nesbitt T, Doctorvaladan S, Southard JA, Singh S, Fekete A, Marie K, Moser DK, Pelter MM, Robinson S, Wilson MD, Cooper L, Dracup K. Correlates of quality of life in rural patients with heart failure. Circ Heart Fail 2014; 7: 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3‐year follow‐up study assessing hospitalization and mortality. Eur J Heart Fail 2010; 12: 1002–1008. [DOI] [PubMed] [Google Scholar]


