Abstract
Aims
Cachexia is a severe consequence of cancer. Although cancer‐induced heart atrophy leads to cardiac dysfunction and heart failure (HF), biomarkers for their diagnosis have not been identified. Neutrophil gelatinase‐associated lipocalin (NGAL) is an aldosterone‐responsive gene increased in HF. We studied NGAL and its association with aldosterone levels in a model of cancer cachexia‐induced cardiomyopathy.
Methods and results
Rats were injected with Yoshida 108 AH‐130 hepatoma cells to induce tumour. Cachectic rats were treated daily, for 16 days, with placebo or with 5 or 50 mg/kg/day of spironolactone. Cardiac function was analysed by echocardiography at baseline and at Day 11. Weight loss and atrophy of lean body and fat mass of cachectic rats were significantly attenuated by spironolactone. Cardiac dysfunction of tumour‐bearing rats was improved by spironolactone. Plasma aldosterone was up‐regulated from 337 ± 7 pg/mL in sham animals to 591 ± 31 pg/mL in the cachectic rats (P < 0.001 vs. sham). Treatment with 50 or 5 mg/kg/day of spironolactone reduced plasma aldosterone to 396 ± 22 and 391 ± 25 pg/mL (P < 0.01 vs. placebo). Plasma levels of NGAL were also increased in cachectic rats (1.462 ± 0.3603 μg/mL) than in controls (0.0936 ± 6 μg/mL, P < 0.001). Spironolactone treatment (50 mg/kg/day) significantly reduced cardiac mRNA and protein NGAL levels (P < 0.05 and P < 0.001 vs. placebo, respectively). NGAL mRNA and protein levels were overexpressed in cachectic animal hearts treated with placebo, compared with control (P < 0.05 and P < 0.01 vs. sham). Spironolactone treatment at 50 mg/kg/day reduced significantly cardiac NGAL (P < 0.05 and P < 0.001 vs. placebo).
Conclusions
Cancer cachexia induced increased levels of aldosterone and NGAL, contributing to worsening cardiac damage in cancer cachexia‐induced cardiomyopathy. Spironolactone treatment may greatly attenuate cardiac dysfunction and lean mass atrophy associated with cancer cachexia.
Keywords: Cancer cachexia, Cardiac wasting, Heart failure, Mineralcorticoid receptor, Neutrophil Gelatinase‐Associated Lipocalin (NGAL), Aldosterone, Spironolactone
Introduction
Cachexia is an important co‐morbidity in cancer patients and an independent factor for impaired survival.1 Cachexia is characterized by an involuntary weight loss due to the atrophy of skeletal muscle with or without loss of adipose tissue2 affecting ~50–80% of patients with cancer and is the direct cause of 30% of cancer deaths.3 Atrophy affects multiple organs including the heart. Although impaired cardiac function in cancer patients is usually attributed to cardiotoxicity of anti‐neoplastic therapies, the effects of cancer cachexia (CC) on cardiac atrophy and function have given rise to the hypothesis that CC itself results in cardiac atrophy and cardiac dysfunction, which lead to heart failure (HF), which is well supported by several pre‐clinical studies.4, 5, 6, 7
Although several inflammatory, hormonal, and oxidative stress molecules have been suggested as markers of prognosis in cachexia,8 there are no universally accepted specific biomarkers for this condition. This scenario becomes even more intriguing and complex considering that CC may lead to the development of HF, which appears as an additional contributing factor that exacerbates wasting in the cancer patients.9, 10 Although the field of HF biomarkers is rich and reflects different mechanisms of HF development and progression,11 none of the available biomarkers are used to assess cardiac impairment in CC.12
Neutrophil gelatinase‐associated lipocalin (NGAL) is a 25 kDa secretory protein belonging to the lipocalins superfamily, which was initially identified as a component of neutrophil granules, associated with matrix metalloproteinase‐913 and later found to be expressed at low levels in several tissues.14 Although the specific cellular role of NGAL is elusive, its expression is enhanced in tissues, plasma, and urine, in many pathological conditions, such as kidney failure and cancer.15, 16 NGAL expression has been documented to be highly up‐regulated at an early stage of kidney injury and rapidly detected in the circulation or in urine.17 Thus, plasma and/or urinary NGAL levels might be diagnostic markers of early prediction of kidney injury.18 Nevertheless, NGAL is not only specific for the kidney; in fact, it has been shown that urinary and blood NGAL levels might be up‐regulated by cardiac dysfunction, in patients with HF,19, 20 following acute myocardial infarction21 and experimental HF.22
Recently, a clear link between NGAL and aldosterone has been reported, suggesting that NGAL is an aldosterone/mineralocorticoid receptor (MR)‐responsive gene. In fact, MR binds directly the promoter of NGAL, upon treatment with aldosterone, and controls its transcription, which is blocked by the MR antagonist spironolactone or by deletion of the hormone response element.23 Moreover, acute or chronic administration of aldosterone induces cardiac NGAL expression and increased plasma NGAL in vivo.23, 24 High plasma level of aldosterone has also been reported in patients suffering from either non‐small cell lung or colorectal cancer, with or without cachexia or in experimental model of CC‐induced cardiomyopathy, resulting in cardiac remodelling due, at least in part, to increased aldosterone production.6
Although increased systemic and myocardial expression of NGAL has been reported in HF,22 NGAL expression and its association with aldosterone levels have never been described in CC‐induced cardiomyopathy.
In the present study, we hypothesized that cancer cachectic rats would experience greater muscle and cardiac atrophy and greater cardiac dysfunction and be associated with higher cardiac NGAL expression than would sham‐treated and spironolactone‐treated rats. Moreover, we hypothesized a beneficial, dose‐dependent effect of spironolactone (5 vs. 50 mg/kg/day) on preservation of lean tissue and cardiac function in cancer cachectic rats.
Materials and methods
Animal model
Juvenile male Wistar Han rats weighing 186.8 ± 1.1 g of 8 weeks of age were kept under standard laboratory conditions in a specific pathogen‐free animal facility and maintained at 22 ± 2°C with alternating 12 h light–dark cycle and free access to food and water. All the experimental procedures were performed in accordance with the European Commission guidelines for the animals used for scientific purposes.
Study design
Rats were randomized into two groups and i.p. injected with either Yoshida 108 AH‐130 hepatoma cells (n = 42) or saline (n = 9, sham). Tumour‐bearing rats were further divided and treated with placebo (n = 10) or with 5 (n = 16) or 50 mg/kg/day (n = 16) of the aldosterone antagonist spironolactone. Animals were orogastrically gavaged once daily over a period of maximum 16 days. Treatment with spironolactone or placebo started 1 day after tumour inoculation. Body weight and composition, as well as cardiac function, were assessed at baseline, before tumour inoculation. Cardiac function was re‐assessed on Day 11 after tumour inoculation. Body composition and body weight were recorded on Day 16 or the day of the euthanasia if the animals had to be sacrificed for ethical reasons. At the end of the study, the tumour was harvested from the peritoneum and its cell number evaluated using a Neubauer chamber. At the end of the study, plasma was collected, and organs were removed, weighed, and frozen in liquid nitrogen.
Body composition
Total body fat, lean mass, and body fluids were measured using the nuclear magnetic resonance spectroscopy device, EchoMRI‐700 (Echo Medical Systems, Houston, TX, USA), as described before.4
Echocardiography
Echocardiography was performed using the high‐resolution Vevo 770 system (VisualSonics Inc., Toronto, Canada), as described before.4 Briefly, rats were anaesthetized with 1.5% isoflurane and laid in supine position on a heated surface to maintain body temperature and with all legs taped to electrocardiogram electrodes. Fur was removed from the chest using an electrical clipper and a chemical depilatory agent. Recordings were made in B‐mode and M‐mode to assess functional parameters, cardiac function, and dimensions.
Quantitative reverse transcription–PCR
Isolation of total RNA from heart was performed by TRIzol according to the manufacturer's protocol, and quantitative PCR analyses were performed as described.25 Specific primers used for real‐time PCR were as follows:
NGAL: 5′‐TCACCCTGTACGGAAGAACC‐3′ and 5′‐GGTGGGAACAGAGAAAACGA‐3′ (forward and reverse primers);
β‐Actin: 5′‐TTCTACAATGAGCTGCGTGTG‐3′ and 5′‐CAGGTCCAGACGCAGGAT‐3′ (forward and reverse primers).
Protein extraction and sodium dodecyl sulfate polyacrylamide gel electrophoresis western blot
Protein extraction and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) western blot were performed as described before.4 Approximately 50 mg of heart was homogenized in 500 μL ice‐cold lysis buffer [20 mM Tris/HCl pH 7.5, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM EGTA, 1% Triton X‐100, 2.5 mM Na4P2O7, 20 mM NaF, 1 mM dithiothreitol, 1 mM Na3VO4, 1 mM β‐glycerophosphate, and 10 μL/ml freshly added protease and phosphatase inhibitor cocktails] and centrifuged at 18 400 rcf for 20 min at 4°C, and supernatant was collected. A total of 20 μL of the supernatant was used to determine the total protein concentration by Bradford assay (Quick Start Bradford 1× Dye reagent, Biorad #500‐0205, Hercules, CA, USA) using bovine serum albumin (BSA) as a standard (Quick Start bovine serum albumin standard, Biorad #500‐0206, Hercules, California, USA). Proteins were heat denatured for 5 min at 95°C in sample‐loading buffer (500 mM Tris/HCl pH 6.8, 30% glycerol, 10% SDS, 5% β‐mercaptoethanol, and 0.024% bromophenol blue), and 30 μg of protein lysate was resolved by SDS‐PAGE and transferred to nitrocellulose membranes (Amersham Protran 0.2 μm NC 10600001, Little Chalfont, UK). Membranes were blocked with Tris/HCl (pH 7.6) containing 0.1% Tween 20 and 5% BSA for 2 h and incubated overnight at 4°C with shaking with primary antibody against goat anti‐human/mouse/rat lipocalin‐2/NGAL (#AF1757, R&D Systems, Minneapolis, MN, USA). Membranes were then washed in Tris‐buffered saline (pH 7.6) with 0.1% Tween 20 and incubated with horseradish peroxidase‐conjugated anti‐goat IgG secondary antibody (#SC‐2020, Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h at room temperature with shaking. Bound antibody was visualized using the chemiluminescent kit (ECL WB Detection, GE Healthcare RPN210601819, Little Chalfont, UK); immunoblot scanning and analyses were performed using an imaging system (UVITEC Imaging Systems, Cambridge, UK). Quantification of the bands was performed using the ImageJ software (NIH, Bethesda, MD, USA).
Measurement of plasma neutrophil gelatinase‐associated lipocalin and aldosterone levels
Plasma NGAL and aldosterone levels were determined by commercial enzyme‐linked immunosorbent assay kits (BioPorto Diagnostics, Gentofte, Denmark, for NGAL and Asbach Medical Products, Obrigheim, Germany, for aldosterone) according to manufacturers' protocols.
Statistics
Data were analysed with GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Results are shown as mean ± SEM. Normality was tested using the D'Agostino–Pearson test. Normally distributed data were analysed by one‐way ANOVA followed by Tukey's test, while data without normal distribution were analysed using Kruskal–Wallis ANOVA and subsequent Dunn's tests. Correlation analyses were assessed using Pearson's correlation coefficient. A P‐value of <0.05 was considered significant.
Results
Spironolactone reduces wasting in cancer cachexia
Baseline weight and lean and fat mass were similar in all the randomized groups before tumour inoculation (data not shown). As expected, tumour‐bearing rats lost a substantial amount of body weight, while sham animals gained weight. The weight loss was significantly attenuated by treatment with spironolactone in tumour‐bearing rats (Figure 1 ). Both lean body mass and fat mass were protected from atrophy by spironolactone (Figure 1 ). This protective effect was also seen on the level of individual muscles and tissues (Table 1).
Figure 1.
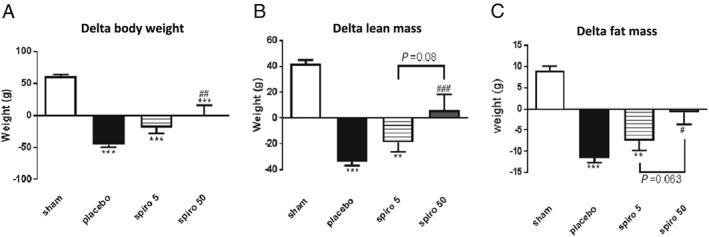
(A–C) Effect of spironolactone treatment on body weight and body composition of the tumour‐bearing rats. The data are presented as mean ± SEM. **P < 0.01, ***P < 0.001 vs. sham; # P < 0.05, ## P < 0.01, ### P < 0.001 vs. placebo. Sham n = 9, placebo n = 10, spironolactone spironolactone 5 mg/kg/day n = 16, spironolactone 50 mg/kg/day n = 16.
Table 1.
Tissue and muscle weight at the end of the study
| Gastrocnemius (mg) | Soleus (mg) | EDL (mg) | Tibialis (mg) | BAT (mg) | WAT (mg) | |
|---|---|---|---|---|---|---|
| Sham | 1209 ± 40.1 | 96.41 ± 3.2 | 104.5 ± 3.3 | 441.11 ± 6 | 271.1 ± 25.1 | 1170 ± 86.8 |
| Placebo | 738.9 ± 27*** | 69.18 ± 1.9*** | 63.8 ± 2.2*** | 280.1 ± 10.2*** | 89.3 ± 6.6*** | 192.1 ± 35.4*** |
| 5 mg/kg/day spiro | 812.3 ± 50.5*** | 70.06 ± 3.1*** | 67.1.4 ± 1*** | 294.9 ± 18*** | 122.5 ± 17.3** | 337.5 ± 110*** |
| 50 mg/kg/day spiro | 939.8 ± 8.1*** , # | 75.31 ± 3.1*** | 80.8 ± 5.4*** , # | 344.3 ± 19*** , # | 131.6 ± 16.8* | 682.1 ± 93.3*** , # |
BAT, brown adipose tissue; EDL, extensor digitalis longus; WAT, white adipose tissue.
P < 0.05.
P < 0.01.
P < 0.001 vs. sham.
P < 0.05 vs. placebo.
Spironolactone prevents tumour‐induced cardiac dysfunction
Baseline echocardiography was similar in all groups before tumour inoculation (P > 0.1, data not shown). Tumour‐bearing animals displayed an overall deterioration of cardiac function: Cachectic animals, treated with placebo, compared with the control group, showed an impaired heart contractility reflected by fractional shortening (%) and ejection fraction (%), which were restored by 50 mg/kg/day of spironolactone (Figure 2 A,B). Also, the left ventricular stroke volume (LVSV), the LV end‐diastolic volume, and the cardiac output (CO) were significantly reduced in cachectic animals treated with placebo, compared with control rats, and significantly restored by spironolactone (Figure 2 C–E). Furthermore, spironolactone significantly protected the heart from the loss of LV diameter in diastole (Figure 2 G). Finally, the loss of LV mass, observed in tumour group treated with placebo, compared with sham rats, was abrogated by 50 mg/kg/day of spironolactone, whereas the 5 mg/kg/day dose was not effective (Figure 2 H).
Figure 2.
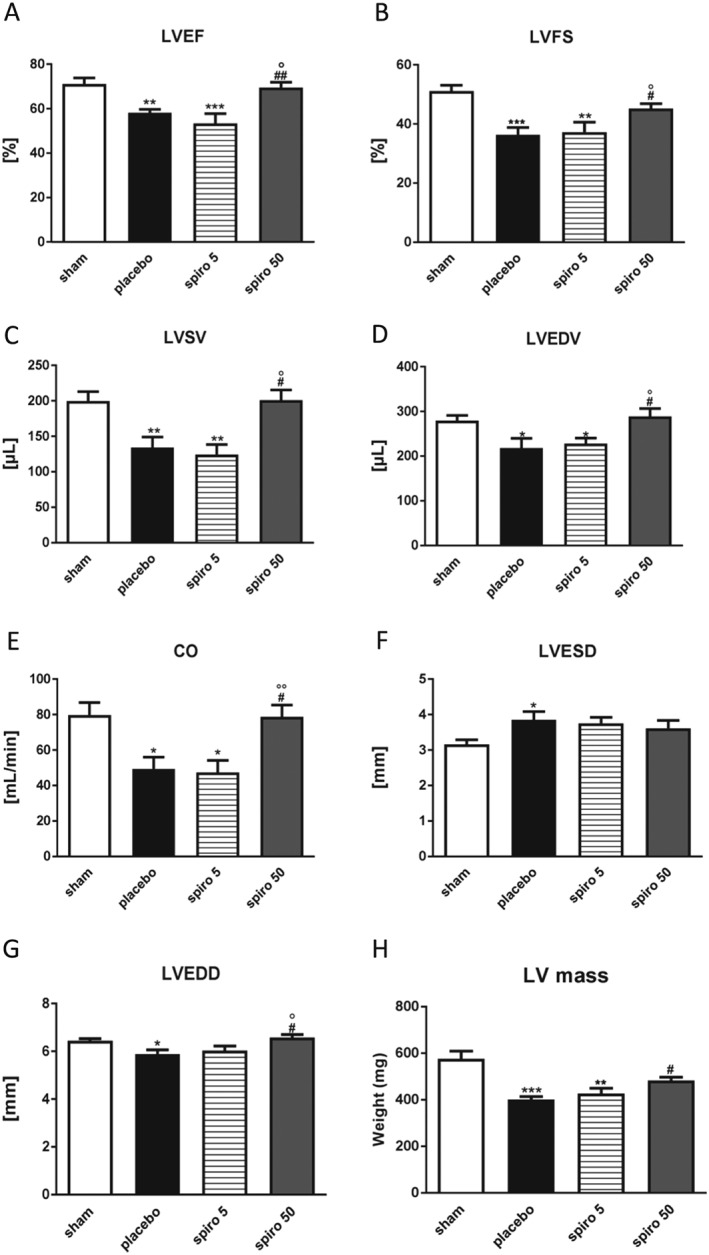
(A–H) Effect of spironolactone treatment in tumour rats on cardiac dimension and function. The data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham; # P < 0.05, ## P < 0.01, vs. placebo; °P < 0.05, °°P < 0.01 vs. spiro 5. Sham n = 9, placebo n = 10, spironolactone 5 mg/kg/day n = 16, spironolactone 50 mg/kg/day n = 16. CO, cardiac output; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction ; LVESD, left ventricular end‐systolic diameter; LVFS, left ventricular fractional shortening; LVSV, left ventricular stroke volume.
Plasma levels of aldosterone and neutrophil gelatinase‐associated lipocalin
Plasma aldosterone was up‐regulated from 337 ± 7 pg/mL in sham animals to 591 ± 31 pg/mL in the placebo group (P < 0.001 vs. sham; Figure 3 ). Treatment with 50 or 5 mg/kg/day of spironolactone reduced aldosterone plasma level to 396 ± 22 and 391 ± 25 pg/mL, respectively (P < 0.01 vs. placebo; Figure 3 ).
Figure 3.
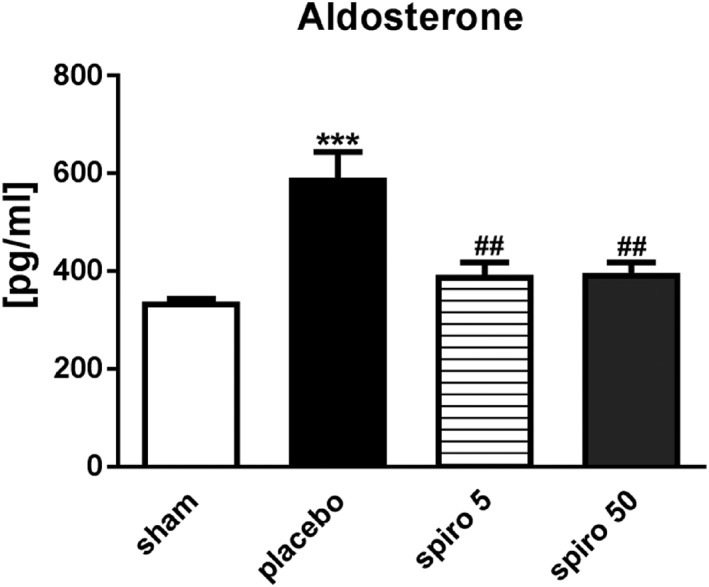
Aldosterone plasma levels were elevated in rats with cancer cachexia and reduced by spironolactone. White bars, sham; black bars, placebo; grid lines bars, spironolactone 5 mg/kg/day; grey bars, spironolactone 50 mg/kg/day. The data are presented as mean ± SEM. ***P < 0.001, ## P < 0.01 vs. placebo. Sham n = 9, placebo n = 10, spironolactone 5 mg/kg/day n = 16, spironolactone 50 mg/kg/day n = 16.
NGAL mRNA expression and protein levels were up‐regulated in the hearts of cachectic animals, treated with placebo, compared with sham rats (P < 0.05 vs. sham for mRNA, Figure 4 A, and P < 0.01 vs. sham for protein level, Figure 5 ). Treatment of the tumour‐bearing rats with 50 mg/kg/day of spironolactone reduced significantly cardiac NGAL mRNA expression (P < 0.05 vs. placebo; Figure 4 A) and NGAL protein levels (P < 0.001 vs. placebo Figure 5 ), while 5 mg/kg/day of spironolactone had no effect on cardiac NGAL expression (Figures 4 A and 5 ). Plasma levels of NGAL were increased in tumour‐bearing rats (1.462 ± 0.3603 μg/mL; Figure 4 B) compared with controls (0.0936 ± 0.006 μg/mL, P < 0.001; Figure 4 B). Spironolactone, 50 mg/kg/day, reduced NGAL levels to 0.5296 ± 0.07 μg/mL (P < 0.05 vs. placebo; Figure 4 B), whereas 5 mg/kg/day had no effect (Figure 4 B).
Figure 4.
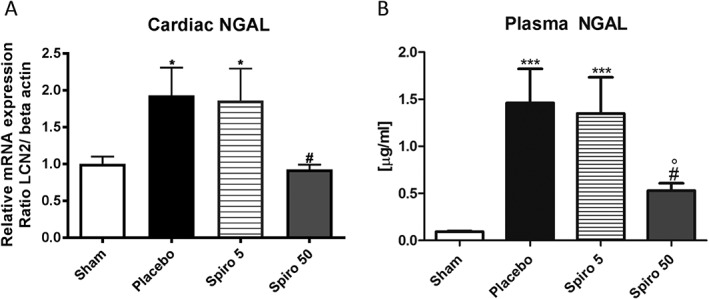
Neutrophil gelatinase‐associated lipocalin (NGAL) mRNA expression in the (A) heart and (B) plasma levels in cachectic rats. White bars, sham; black bars, placebo; grid lines bars, spironolactone 5 mg/kg/day; grey bars, spironolactone 50 mg/kg/day. The data are presented as mean ± SEM. ***P < 0.001, *P < 0.05 vs. sham; # P < 0.05 vs. placebo; °P < 0.05 vs. 5 mg/kg/day. Sham n = 9, placebo n = 10, spironolactone 5 mg/kg/day n = 16, spironolactone 50 mg/kg/day n = 16.
Figure 5.
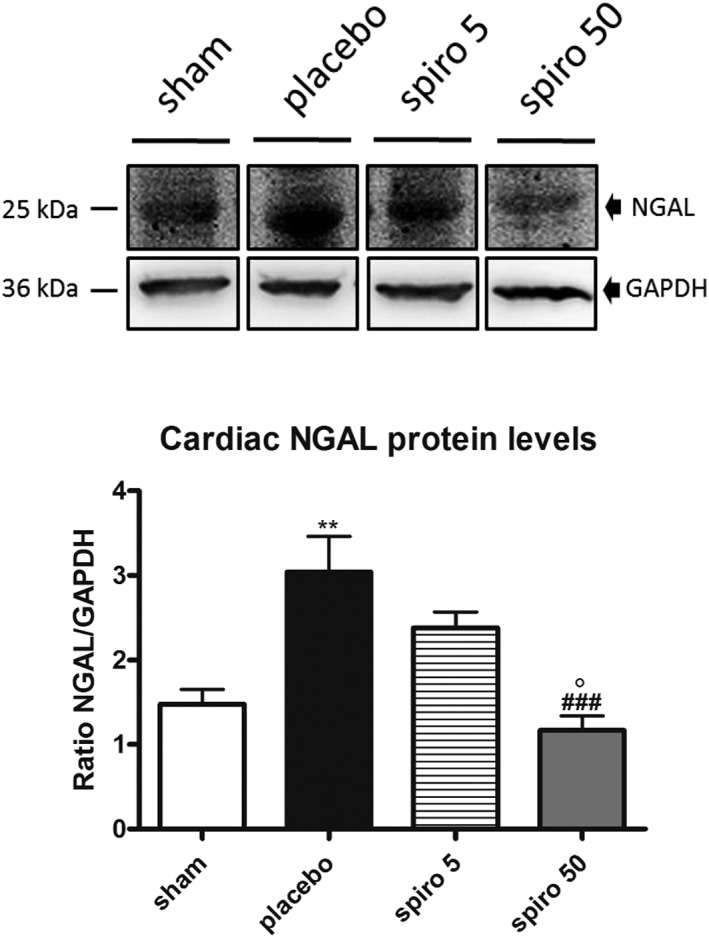
Neutrophil gelatinase‐associated lipocalin (NGAL) protein expression in the heart. White bars, sham; black bars, placebo; grid lines bars, spironolactone 5 mg/kg/day; grey bars, spironolactone 50 mg/kg/day. The data are presented as mean ± SEM. **P < 0.01, vs. sham; ### P < 0.05 vs. placebo; °P < 0.05 vs. 5 mg/kg/day. Sham n = 9, placebo n = 10, spironolactone 5 mg/kg/day n = 16, spironolactone 50 mg/kg/day n = 16.
Increased plasma aldosterone in placebo group correlated positively to plasma NGAL (r = 0.693, P < 0.05; Figure 6 A) and increased cardiac NGAL mRNA expression (r = 0.866, P < 0.01; Figure 6 B) and cardiac protein levels (r = 0.838, P < 0.01; Figure 6 C).
Figure 6.
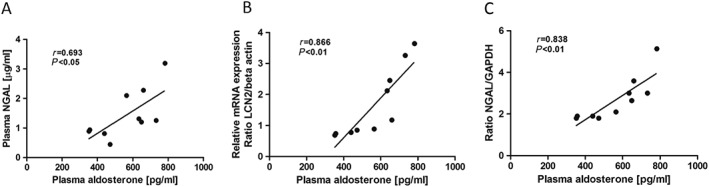
Relationship between plasma aldosterone and plasma NGAL (A), NGAL cardiac mRNA expression (B), and NGAL cardiac protein levels (C) by Pearson‐related analysis. Lines are generated by regression analyses (GraphPad Prism); r = Pearson correlation coefficient. NGAL, neutrophil gelatinase‐associated lipocalin.
Discussion
Here, we have demonstrated that (i) the Yoshida hepatoma model of CC caused wasting and weight loss as well as increased aldosterone levels in plasma and cardiac tissue. (ii) This metabolic alteration was associated with a cardiac dysfunction (reduced ventricular ejection fraction, LVSV, CO), suggesting a close relationship between cardiac and skeletal muscle atrophy and cardiac function.26 (iii) CC caused an increase in aldosterone that was accompanied by an increase in levels of cardiac and plasma NGAL. (iv) Administration of spironolactone reduced wasting and weight loss and prevented further cardiac dysfunction with a decrease in heart and plasma levels of aldosterone and NGAL.
Physiologically, NGAL is involved in the synthesis of prostaglandins, modulation of immune response, and regulation of cell growth and metabolism; on the other hand, its overexpression represents a consequence of malignancies, such as hepatocellular carcinomas,25 and its increased levels in plasma and urine are characteristic in patients suffering from HF. Accordingly, it has been demonstrated that NGAL may be up‐regulated in ‘stressed’ cardiomyocytes in response to pro‐inflammatory cytokines and free radical overproduction.27
Several clinical studies showed that patients with cardiovascular diseases present increased plasma aldosterone levels and that, blocking its receptor, their mortality was reduced.28 Supporting this theory, aldosterone plasma levels are up‐regulated in patients suffering from cancer, with or without cachexia.6 Further evidence shows that excessive MR activation is a hallmark of a number of cardiovascular diseases that may benefit from treatments with MR antagonists.29 We have demonstrated that CC‐dependent cardiac dysfunction was accompanied by an increase in heart and plasma aldosterone levels, which was reverted by spironolactone treatment. Interestingly, blockade of the MR was also able to reduce NGAL. These results suggested that the enhanced levels of both molecules, in either tissue or circulating form, may be involved in the onset and the progression of cardiomyopathy. Our hypothesis is supported and strengthened by recent studies providing evidence that NGAL represents a mineralocorticoid‐target gene in the cardiovascular system. Indeed, an excess of mineralocorticoids caused NGAL overexpression in the heart, aorta, and plasma through the binding of activated MR to the NGAL promoter.23
Although spironolactone was able to inhibit aldosterone levels dose dependently, we observed that it did not affect NGAL expression in the same manner, suggesting that the protective down‐regulation of NGAL against cardiac dysfunction, at higher doses of spironolactone, could be exerted through an additional protective mechanism, independent of aldosterone. Accordingly, recent evidence highlights a direct role for spironolactone in preventing the development of inflammatory cardiovascular disease, differently by MR, which involves the inhibition of inflammation. Several factors might contribute to determine NGAL expression levels, either in physiological situations or in pathology. It has been shown that nuclear factor (NF)‐κB can directly enhance NGAL expression30, 31 and that the direct suppression of NF‐κB, by high dose of spironolactone, independent of the MR, might justify the protective down‐regulation of NGAL against cardiac dysfunction. It has been shown that MR binding is not involved in spironolactone‐induced suppression of NF‐κB activity29 and that this effect could be mediated by the degradation of the ATP‐dependent DNA helicase XPB, able to suppress both NF‐κB and AP‐1 signalling.32 Thus, data from humans,33 animal models,34 and in vitro studies35 show a clear role of aldosterone‐induced oxidative stress28 that, together with the overexpression of NGAL, might explain the cardiac damage induced by the enhanced aldosterone levels caused by hepatocellular carcinoma.36, 37 Moreover, it has been hypothesized that NGAL overexpression is associated with advanced cardiac dysfunction and might affect Nrf2 regulation, causing the failure to maintain the redox homeostasis by antioxidant enzymes. In turn, the persistently oxidative stress results in cardiac remodelling and finally HF.38 Recent evidence revealed a direct correlation between NGAL levels and cardiac tissue damage, suggesting that NGAL levels can reflect several cardiovascular diseases including hypertensive cardiac hypertrophy,39 coronary artery disease,40 and acute HF.41 Human studies revealed a central role for NGAL in the onset and development of cardiac hypertrophy and HF, which was independent of renal function.39 CC, induced by the Yoshida hepatoma, drives to a ‘non‐canonical’ form of cardiomyopathy, where cardiac wasting is the hallmark.6 Thus, here, we present an animal model of cancer cachectic cardiomyopathy without LV hypertrophy and dilatation, with elevated NGAL levels, that may be connected to cardiac damage and dysfunction.
Although plasma NGAL has achieved a clinical significance only as a biomarker of cardiovascular disease in patients with chronic kidney disease,40 latter discoveries, together with our study, suggest that NGAL may be considered as a potential biomarker able to detect the development of heart dysfunction and HF.
Symptoms of CC are shortness of breath, fatigue, and impaired exercise capacity, representing typical signs of HF.6 Growing evidence shows that aldosterone and MR contribute to the endocrine basis of HF, regulating the expression of several genes implicated in pathologic cardiac remodelling, which can be inhibited by pharmacologic blockers of translation and transcription and/or by MR antagonist drugs,42 although the beneficial properties of high doses of spironolactone on skeletal muscle mass depend on its ability to inhibit aldosterone effects. In fact, evidence exists that in patients suffering from congestive HF, the treatment with angiotensin‐converting enzyme inhibitors and AT‐1 receptor antagonists induces an amelioration in exercise capacity mediated by a change in myosin heavy chain (MHC) composition. In particular, slow MHC1 increased as compared with fast oxidative MHC2a and fast glycolytic MHC2b isoforms, and this enhancement correlated with the net peak V(O2) gain.43
Thus, our results suggest that, although under CC atrophy seems to affect predominantly glycolytic fibres,44, 45 the favourable shift in contractile proteins towards fatigue‐resistant oxidative fibres, occurring in the skeletal muscle after spironolactone administration, might imply an improved exercise capacity, thus ameliorating the quality of life of cancer patients with HF.
Conclusions
In summary, we demonstrate that CC‐induced increases in aldosterone levels may contribute to enhanced expression of plasma and cardiac expression of NGAL, which are also associated with worse cardiac function. Therefore, spironolactone treatment may greatly attenuate cardiac dysfunction and lean mass atrophy associated with CC.
Conflict of interest
None declared.
Funding
This work was supported by Ministero dell'Istruzione, dell'Universitá e della Ricerca (MIUR): Programma Operativo Nazionale (PON03PE_00078_1 and PON03PE_00078_2).
Musolino, V. , Palus, S. , Latouche, C. , Gliozzi, M. , Bosco, F. , Scarano, F. , Nucera, S. , Carresi, C. , Scicchitano, M. , von Haehling, S. , Jaisser, F. , Hasenfuss, G. , Anker, S. D. , Mollace, V. , and Springer, J. (2019) Cardiac expression of neutrophil gelatinase‐associated lipocalin in a model of cancer cachexia‐induced cardiomyopathy. ESC Heart Failure, 6: 89–97. 10.1002/ehf2.12372.
References
- 1. Muscaritoli M, Molfino A, Lucia S, Rossi FF. Cachexia: a preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol 2015; 94: 2519. [DOI] [PubMed] [Google Scholar]
- 2. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 3. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update. J Cachexia Sarcopenia Muscle 2014; 5: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Musolino V, Palus S, Tschirner A, Drescher C, Gliozzi M, Carresi C, Vitale C, Muscoli C, Doehner W, von Haehling S, Anker SD, Mollace V, Springer J. Megestrol acetate improves cardiac function in a model of cancer cachexia‐induced cardiomyopathy by autophagic modulation. J Cachexia Sarcopenia Muscle 2016; 7: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res 2011; 71: 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Potsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argilés JM, Thum T, Foldes G, Doehner W, Hilfiker‐Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014; 35: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer‐induced cachexia in mice. Int J Oncol 2010; 37: 347–353. [DOI] [PubMed] [Google Scholar]
- 8. Drescher C, Konishi M, Ebner N, Springer J. Loss of muscle mass: current developments in cachexia and sarcopenia focused on biomarkers and treatment. J Cachexia Sarcopenia Muscle 2015; 6: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mamidanna R, Nachiappan S, Bottle A, Aylin P, Faiz O. Defining the timing and causes of death amongst patients undergoing colorectal resection in England. Colorectal Dis 2016; 18: 586–593. [DOI] [PubMed] [Google Scholar]
- 10. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 2014; 64: 1310–1319. [DOI] [PubMed] [Google Scholar]
- 11. Mueller C. Clinical utility of biomarkers in heart failure. Eur J Heart Fail 2017; 19: 1176–1178. [DOI] [PubMed] [Google Scholar]
- 12. Loncar G, Omersa D, Cvetinovic N, Arandjelovic A, Lainscak M. Emerging biomarkers in heart failure and cardiac cachexia. Int J Mol Sci 2014; 22: 23878–23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993; 15: 10425–10432. [PubMed] [Google Scholar]
- 14. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase‐associated lipocalin from humans. Genomics 1997; 45: 17–23. [DOI] [PubMed] [Google Scholar]
- 15. Mårtensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif 2014; 37: 304–310. [DOI] [PubMed] [Google Scholar]
- 16. Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, McCubrey JA, Libra M. Roles of neutrophil gelatinase‐associated lipocalin (NGAL) in human cancer. Oncotarget 2014; 5: 1576–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt‐Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin–siderophore–iron complex rescues the kidney from ischemia–reperfusion injury. J Clin Invest 2005; 115: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zarbock A, Kellum JA, Schmidt C, van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016; 315: 2190–2199. [DOI] [PubMed] [Google Scholar]
- 19. Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail 2008; 10: 997–1000. [DOI] [PubMed] [Google Scholar]
- 20. van Deursen VM, Damman K, Voors AA, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL. Prognostic value of plasma neutrophil gelatinase‐associated lipocalin for mortality in patients with heart failure. Circ Heart Fail 2014; 7: 35–42. [DOI] [PubMed] [Google Scholar]
- 21. Sahinarslan A, Kocaman S, Bas D, Akyel A, Ercin U, Zengin O, Timurkaynak T. Plasma neutrophil gelatinase‐associated lipocalin levels in acute myocardial infarction and stable coronary artery disease. Coron Artery Dis 2011; 22: 333–338. [DOI] [PubMed] [Google Scholar]
- 22. Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Frøland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Øie E, Gullestad L, Aukrust P. Increased systemic and myocardial expression of neutrophil gelatinase‐associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009; 30: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 23. Latouche C, El Moghrabi S, Messaoudi S, Nguyen Dinh Cat A, Hernandez‐Diaz I, Alvarez de la Rosa D, Perret C, López Andrés N, Rossignol P, Zannad F, Farman N, Jaisser F. Neutrophil gelatinase‐associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012; 59: 966–972. [DOI] [PubMed] [Google Scholar]
- 24. Messaoudi S, Gravez B, Tarjus A, Pelloux V, Ouvrard‐Pascaud A, Delcayre C, Samuel J, Launay JM, Sierra‐Ramos C, Alvarez de la Rosa D, Clément K, Farman N, Jaisser F. Aldosterone‐specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension 2013; 61: 361–367. [DOI] [PubMed] [Google Scholar]
- 25. Song B, Zhang H, Jiang L, Chi Y, Tian J, Du W, Yu B, Han Z. Down‐regulation of lipocalin 2 suppresses the growth of human lung adenocarcinoma through oxidative stress involving Nrf2/HO‐1 signaling. Acta Biochim Biophys Sin (Shanghai) 2015; 47: 805–814. [DOI] [PubMed] [Google Scholar]
- 26. Ishida J, Saitoh M, Doehner W, von Haehling S, Anker M, Anker SD, Springer J. Animal models of cachexia and sarcopenia in chronic illness: cardiac function, body composition changes and therapeutic results. Int J Cardiol 2017; 238: 12–18. [DOI] [PubMed] [Google Scholar]
- 27. Tawfeek MS, Raafat DM, Saad K, Idriss NK, Sayed S, Fouad DA, El‐Houfey AA. Plasma levels of neutrophil gelatinase‐associated lipocalin in children with heart failure. Ther Adv Cardiovasc Dis 2016; 10: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Queisser N, Schupp N. Aldosterone, oxidative stress, and NF‐κB activation in hypertension‐related cardiovascular and renal diseases. Free Radic Biol Med 2012; 53: 314–327. [DOI] [PubMed] [Google Scholar]
- 29. Kolkhof P, Bärfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. Endocrinol 2017; 234: T125–T140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase‐associated lipocalin in vascular injury via activation of nuclear factor‐κB. Am J Pathol 2006; 169: 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase‐associated lipocalin expression by co‐stimulation with interleukin‐17 and tumor necrosis factor‐α is controlled by Iκb‐zeta but neither by c/EBP‐β nor c/EBP‐δ. J Biol Chem 2010; 285: 14088–14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sønder SU, Woetmann A, Odum N, Bendtzen K. Spironolactone induces apoptosis and inhibits NF‐kappaB independent of the mineralocorticoid receptor. Apoptosis 2006; 11: 2159–2165. [DOI] [PubMed] [Google Scholar]
- 33. Elinoff JM, Chen LY, Dougherty EJ, Awad KS, Wang S, Biancotto A, Siddiqui AH, Weir NA, Cai R, Sun J, Preston IR, Solomon MA, Danner RL. Spironolactone‐induced degradation of the TFIIH core complex XPB subunit suppresses NF‐kB and AP‐1 signaling. Cardiovasc Res 2018; 114: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF Jr, Sawyer DB, Cupples LA, Colucci WS, Sam F. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail 2006; 12: 122–127. [DOI] [PubMed] [Google Scholar]
- 35. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone‐induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002; 161: 1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayashi H, Kobara M, Abe M, Tanaka N, Gouda E, Toba H, Yamada H, Tatsumi T, Nakata T, Matsubara H. Aldosterone nongenomically produces NADPH oxidase‐dependent reactive oxygen species and induces myocyte apoptosis. Hypertens Res 2008; 3: 363–375. [DOI] [PubMed] [Google Scholar]
- 37. Wong CC, Wong CM, Ng IO. Hormonal control of the metabolic machinery of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016; 5: 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 1826; 2012: 129–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou S, Sun W, Zhang Z, Zheng Y. The role of Nrf2‐mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev 2014; 2014: 260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marques FZ, Prestes PR, Byars SG, Ritchie SC, Würtz P, Patel SK, Booth SA, Rana I, Minoda Y, Berzins SP, Curl CL, Bell JR, Wai B, Srivastava PM, Kangas AJ, Soininen P, Ruohonen S, Kähönen M, Lehtimäki T, Raitoharju E, Havulinna A, Perola M, Raitakari O, Salomaa V, Ala‐Korpela M, Kettunen J, McGlynn M, Kelly J, Wlodek ME, Lewandowski PA, Delbridge LM, Burrell LM, Inouye M, Harrap SB, Charchar FJ. Experimental and human evidence for lipocalin‐2 (neutrophil gelatinase‐associated lipocalin [NGAL]) in the development of cardiac hypertrophy and heart failure. J Am Heart Assoc 2017; 6: e005971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasegawa M, Ishii J, Kitagawa F, Takahashi H, Sugiyama K, Tada M, Kanayama K, Takahashi K, Hayashi H, Koide S, Nakai S, Ozaki Y, Yuzawa Y. Plasma neutrophil gelatinase‐associated lipocalin as a predictor of cardiovascular events in patients with chronic kidney disease. Biomed Res Int 2016; 2016: 8761475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Julie He B, Anderson ME. Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab 2013; 24: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vescovo G, Dalla Libera L, Serafini F, Leprotti C, Facchin L, Volterrani M, Ceconi C, Ambrosio GB. Improved exercise tolerance after losartan and enalapril in heart failure—correlation with changes in skeletal muscle myosin heavy chain composition. Circulation 1998; 98: 1742–1749. [DOI] [PubMed] [Google Scholar]
- 44. Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael‐Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 2005; 8: 421–432. [DOI] [PubMed] [Google Scholar]
- 45. Marin‐Corral J, Fontes CC, Pascual‐Guardia S, Sanchez F, Olivan M, Argiles JM, Busquets S, López‐Soriano FJ, Barreiro E. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal 2010; 12: 365–380. [DOI] [PubMed] [Google Scholar]


