Abstract
Calcineurin is a phosphatase whose primary targets in T cells are NFAT transcription factors, and inhibition of calcineurin activity by treatment with cyclosporin A (CsA) or FK506 is a cornerstone of immunosuppressive therapies. Here we show that calcineurin is recruited to the T cell receptor signaling complex where it reverses the inhibitory phosphorylation of the tyrosine kinase LCK. on Ser59 (LCKS59). Loss of calcineurin activity impaired phosphorylation of Tyr493 on zeta chain of T cell receptor associated protein kinase 70 (ZAP70Y493), as well as some downstream pathways, in a manner consistent with signaling in cells expressing LCKS59A (a nonphosphorylatable LCK) or LCKS59E (a phosphomimetic mutant). Notably, CsA treatment inhibited activation-induced lymphocyte function-associated antigen (LFA)-1-dependent and NFAT-independent adhesion of T cells to intercellular adhesion molecule 1 (ICAM1), with little effect on cells expressing mutant LCK. These results provide a new understanding of how widely used immunosuppressive drugs interfere with essential processes in the immune response.
Engagement of the T cell receptor (TCR) triggers a complex signaling network that culminates in the activation of effector and differentiation programs. The initial event is the activation of LCK, a SRC family tyrosine kinase that contains a unique N-terminal region, SRC homology 3 (SH3) and SH2 domains that mediate protein–protein interactions, a catalytic domain, and a C-terminal regulatory domain1. LCK that is recruited to the liganded TCR autophosphorylates the activating residue Tyr394 and then phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) in the TCR-ζ chain and in CD3. This recruits the cytosolic tyrosine kinase ZAP70 by binding the latter’s SH2 domains. ZAP70 consists of an N-terminal SH2 domain followed by interdomain-A, a C-terminal SH2 domain, and an interdomain-B that connects to the kinase domain. Interdomain-B exists in an auto-inhibitory conformation that is relieved by LCK-mediated phosphorylation of Tyr315 and Tyr319, a prerequisite for interaction with the cell signaling molecules_CBL (also known as c-Cbl), VAV, CrkII, LCK, and PLC-γ, as well as full activation of ZAP70 (refs. 2–4). The kinase domain of ZAP70 has two other tyrosines (Tyr492 and Tyr493) in the activation loop that are sites of autophosphorylation and/or phosphorylation by LCK5. ZAP70 phosphorylates downstream adaptor molecules like LAT and SLP76, with subsequent recruitment of adaptors and signaling molecules that form a multiprotein complex to promote full cellular activation6.
There is a feedback loop that results in serine phosphorylation of LCK. ERK, one of the prominent serine–threonine kinases that is activated downstream of the TCR, phosphorylates LCK on Ser59 in the unique N-terminal domain7. By using recombinant proteins it has been shown that this phosphorylation diminishes the accessibility or affinity of phosphoproteins to LCK’s SH2 domain7. The functional consequences of LCKS59 phosphorylation in primary mouse T cells is controversial8,9, and its effect on signaling downstream of the TCR has not been studied.
TCR-mediated activation results in elevated intracellular Ca2+ and activation of the Ca2+–calmodulin-dependent serine–threonine phosphatase calcineurin. Calcineurin is composed of a catalytically active A subunit (∼61 kDa) and a small regulatory B subunit (∼19 kDa)10. Among the critical transcription factors activated by TCR signaling are NFATs, in particular NFATC2 (also known as NFAT1) and NFATC1 (also known as NFAT2), which are required for the transcriptional upregulation of critical cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF), and interleukin (IL)-17 (refs. 11,12). NFAT proteins are constitutively phosphorylated on multiple serines and threonines, which causes them to be retained in the cytoplasm. Activated calcineurin dephosphorylates NFATs, leading to their nuclear translocation and the induction of transcription. The widely used immunosuppressive drugs cyclosporin A (CsA) and FK506 prevent the dephosphorylation of NFATs by binding to cytosolic immunophilins (cyclophilin A and FKBP12, respectively), which in turn bind to and inhibit calcineurin and thus NFAT activity13,14. Although NFATs are generally believed to be the primary physiologic target of calcineurin in activated T cells, they have also been shown to positively regulate the transcription factor NF-κB through its interaction with the CBM complex (which is composed of CARD11 (also known as CARMA1), BCL10, and MALT1) and dephosphorylation of BCL10 after stimulation with phorbol ester and a Ca2+ ionophore or via the TCR15. A constitutively active form of calcineurin promotes positive selection and lowers the threshold of antigenic stimulation in mature T cells, although its effects on proximal signaling pathways were not addressed16. Notably, CsA and FK506 treatment prevented the formation of T cell–antigen-presenting cell (APC) conjugates, implying that calcineurin may have non-transcriptionally-related activities downstream of the TCR17. T cell–APC interaction is mediated by LFA-1 (also known as CD11a–CD18 or αLβ2) binding to ICAM1 (ref. 18) after its phosphorylation on β-chain residues Thr758–Thr760, which is crucial for activation19.
CsA and FK506 inhibit activation of p38 MAPK after stimulation with the phorbol ester phorbol 12-myristate 13-acetate (PMA) and the calcium ionophore ionomycin. The decrease in p38, but not ERK, mitogen-activated protein kinase (MAPK) activation was attributed to inhibition of the MAPK kinase MKK6 (refs. 20,21). In contrast, stimulation of T cells via the TCR uses a MAPKK-independent pathway to activate p38 (the alternative pathway), in which ZAP70 phosphorylates p38 on Tyr323 (ref. 22). This results in p38 autophosphorylation on Thr180 and an increase in kinase activity. Because MAPKKs do not contribute to the alternative pathway, we asked whether calcineurin inhibition would also impair p38 activity downstream of the TCR, expecting no effect. However, inhibition of calcineurin did interfere with TCR-mediated activation of p38, which prompted us to examine proximal TCR signaling.
RESULTS
Inhibition of TCR-mediated p38 activation
Calcineurin antagonists diminish PMA- and ionomycin-mediated activation of p38 by inhibiting activation of the upstream kinase MKK6 (refs. 20,21). Because stimulation via the TCR activates p38 by a MAPK-cascade-independent mechanism22, we anticipated that calcineurin inhibition would have no effect on p38 in this setting. Unexpectedly, when Jurkat T cells were activated with cross-linked anti-CD3, we found that treatment with CsA or FK506 reduced phosphorylation of the activation loop of p38 (Fig. 1a). In contrast, the activation of another MAPK, ERK, was unaffected by calcineurin inhibition. This finding was not peculiar to Jurkat cells because primary human T cells behaved in the same manner (Fig. 1b). p38 can also be activated via the classic MAPK cascade in response to stresses such as osmotic shock. Unlike activation via the TCR, shock-induced phosphorylation of p38 (by treatment with sorbitol or UV) was unaffected by inhibition of calcineurin (data not shown). Given that the inhibitors used to inhibit calcineurin act via different immunophilins, these results indicate that calcineurin has a positive role in the alternative activation of p38.
Figure 1.
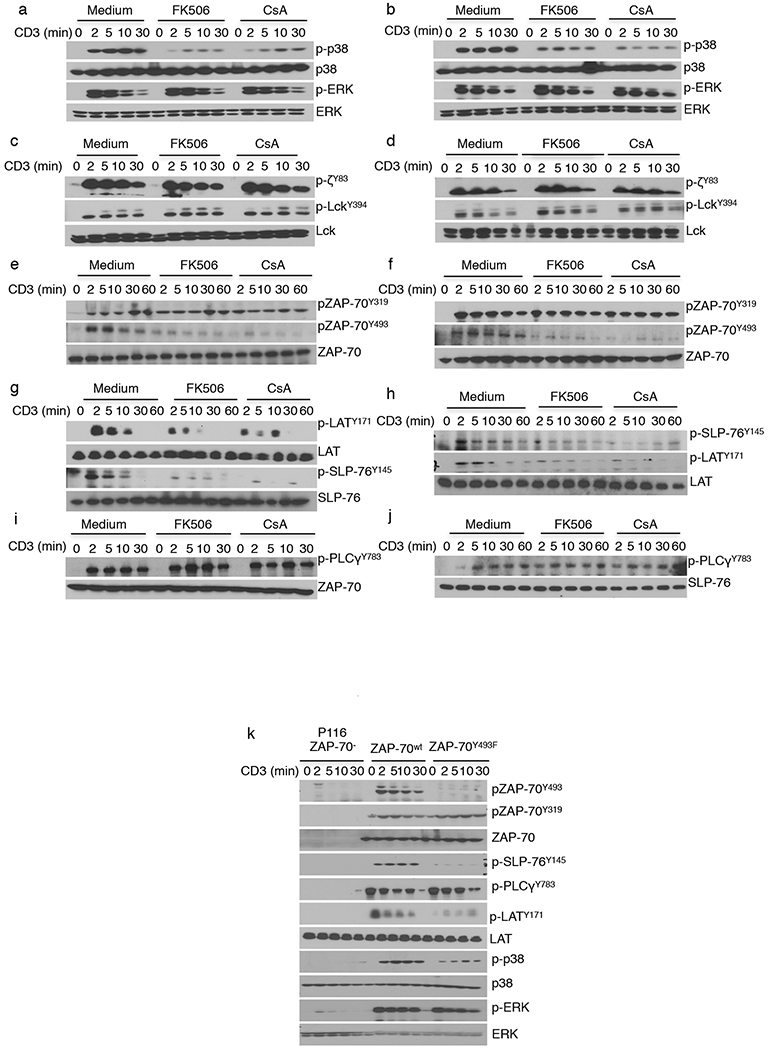
TCR-induced pathways are differentially phosphorylated by CsA and FK506. (a–j) Immunoblot analysis of lysates from Jurkat cells (a,c,e,g,i) and human CD4+ T cells (b,d,f,h,j) that were pretreated with medium, FK506, or CsA at 37 °C and then activated with soluble OKT3 (anti-CD3) and anti-mouse-IgG for the indicated times. After probing for phosphoproteins (designated by the prefix ‘p-’), membranes were stripped and reblotted to visualize the total amounts of the cellular proteins corresponding to the phosphoproteins that were probed. (k) Immunoblot analysis of whole-cell lysates from ZAP70-deficient P116 cells that were retrovirally transduced with ZAP70WT- or ZAP70Y493F-expressing constructs and stimulated for the indicated times. The data in a–k are representative of three independent experiments with similar results.
Calcineurin controls a subset of TCR proximal events
Because p38 is directly activated by ZAP70, a tyrosine kinase involved at the very earliest stage of TCR-mediated signaling, we asked whether TCR-proximal signaling events upstream of p38 were sensitive to calcineurin inhibition. The earliest tyrosine kinase in the signaling pathway is LCK, which phosphorylates TCR-ζ. Activation of LCK, as measured by the phosphorylation of its Tyr394 residue, was unaffected by calcineurin inhibition, as was phosphorylation of TCR-ζ (Fig. 1c,d). In contrast, phosphorylation of the activating ZAP70 kinase domain residue Tyr493 was decreased by CsA and FK506 treatment, whereas phosphorylation of Tyr319 in the interdomain-B region was unaffected (Fig. 1e,f). In addition to p38, phosphorylation of other downstream molecules, such as LAT (on Tyr171) and SLP76 (on Tyr145) was diminished, (Fig. 1g,h). Of note, these drugs had no effect on phosphorylation of ERK or phospholipase C (PLC)-γ1, or on degradation of inhibitor of nuclear factor kappa-B kinase (IκB) (Fig. 1a,b,i,j and Supplementary Fig. 1a,b).
The effect of CsA and FK506 treatment on some but not all of the TCR-induced signaling events led us to ask whether the various pathways differed in their dependence on the most proximal signaling abnormality we detected, phosphorylation of ZAP70Y493. The ZAP70-deficient Jurkat cell variant P116 was stably transduced with vectors encoding either wild-type ZAP70 (ZAP70WT) or a Tyr493Phe mutant (ZAP70Y493F) and stimulated via the TCR (Fig. 1k). As compared to cells expressing the WT protein, those expressing ZAP70Y493F showed diminished phosphorylation of p38, LAT, and SLP76. In contrast, the Tyr493Phe mutation had little, if any, effect on phosphorylation of ERK and PLC-γ1, the latter previously having been shown to be dependent on phosphorylation of ZAP70Y319 (ref. 3). These results recapitulate the observations made with calcineurin inhibitors, strongly suggesting that their effects on more distal signaling events were secondary to those caused by the diminished phosphorylation of ZAP70Y493.
To confirm that the effects of CsA and FK506 were indeed due to calcineurin inhibition, TCR signaling was analyzed in cells in which expression of the gene encoding calcineurin A was knocked down using small interfering RNA (siRNA). Because T cells express both isoforms of the catalytic subunit of calcineurin (the α isoform, encoded by PPP3CA, and the β isoform, encoded by PPP3CB), a cocktail of validated siRNAs was used to achieve efficient knockdown of calcineurin in Jurkat cells and primary CD4+ T cells15. In agreement with previous findings that β-isoform is the predominant isoform, siRNAs targeting the β-isoform (si-β) had a greater effect than that of siRNA targeting the mRNA encoding the α-isoform (si-α), and transfection with siRNAs targeting both resulted in almost complete knockdown. siRNAs targeting PPP3CA and PPP3CB reduced protein levels by ∼80% (Fig. 2a,b). Knockdown resulted in a reduction in anti-TCR-induced phosphorylation of ZAP70Y493 and p38 in Jurkat cells and human CD4+ T cells (Fig. 2c,d), recapitulating the effects observed after treatment with the CsA and FK506. To rule out possible off-target effects of siRNA treatment, we made Jurkat cells that stably expressed codon-optimized calcineurin A, in which the sequences encoding multiple codons were altered in a manner that yielded the same amino acid sequence but conferred resistance to siRNA-mediated knockdown. Treatment with calcineurin-specific siRNAs reduced expression of endogenous calcineurin A in both cells, but the transfected calcineurin A remained unaltered in cells expressing the codon-optimized construct (Fig. 2e). Maintenance of calcineurin expression prevented the decreases in activation-induced phosphorylation of ZAP70Y493, p38, and pSLP76Y145, arguing strongly that the effect of the siRNA was indeed due to its targeting of calcineurin. Taken together, these data demonstrate a role for calcineurin in the positive regulation of TCR signaling.
Figure 2.
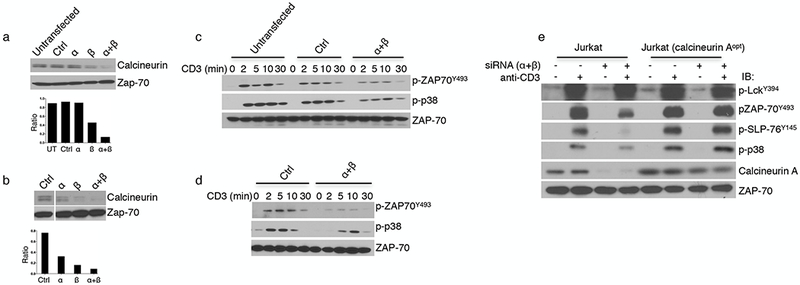
Knockdown of calcineurin expression in T cells recapitulates the effects of the drugs. (a,b) Top, representative immunoblot analysis for the expression of calcineurin in lysates from Jurkat T cells (a) or human CD4+ T cells (b) that were untransfected or electroporated with a control (Ctrl), calcineurin A α-specific, or calcineurin A β-specific siRNA either individually or in combination 72 h after transfection. Bottom, quantification of calcineurin expression relative to ZAP70 levels. In b, the lanes were rearranged for clarity. (c,d) Representative western blot analysis of lysates from siRNA-treated Jurkat cells (c) and human CD4+ T cells (d) after stimulation with OKT3 and anti-mouse-IgG for the indicated times. Stripped membranes were reblotted to ascertain total amounts of the indicated cellular proteins. Data are representative of three independent experiments. (e) Immunoblot analysis of Jurkat cells expressing endogenous or endogenous plus codon-optimized calcineurin A (calcineurin Aopt) after activation with OKT3 for 5 min. The data are representative of three (c,d) or two (e) independent experiments with similar results.
Calcineurin is recruited to the TCR microclusters
Given that manipulation of calcineurin affected the phosphorylation of a number of TCR-proximal signaling molecules, we considered the possibility that calcineurin itself might be in the signaling complex. The subcellular localization of calcineurin was determined by confocal microscopy. Jurkat cells expressing yellow fluorescent protein (YFP)-tagged ZAP70 were suspended at 37 °C and dropped onto coverslips coated with antibodies to CD45 (nonstimulatory control) or CD3 (stimulatory)23. Cells were fixed after 2.5 or 10 min and immunostained with antibodies specific for calcineurin and phosphotyrosine. In the absence of stimulation, ZAP70 was primarily cytosolic, whereas calcineurin was seen in punctate structures, and there was no colocalization of ZAP70 and calcineurin. After TCR ligation, however, there was a clear accumulation of ZAP70–YFP and calcineurin with phosphotyrosine-rich areas at 2.5 min, indicating that both were recruited to the signaling microclusters (Fig. 3a). At 10 min the extent of colocalization was decreased, although clear colocalization was still visible in many microclusters (Fig. 3b). Similar results were seen when activated ZAP70–YFP cells were imaged with anti-calcineurin and anti-pTCR-ζ (Fig. 4a,b).
Figure 3.
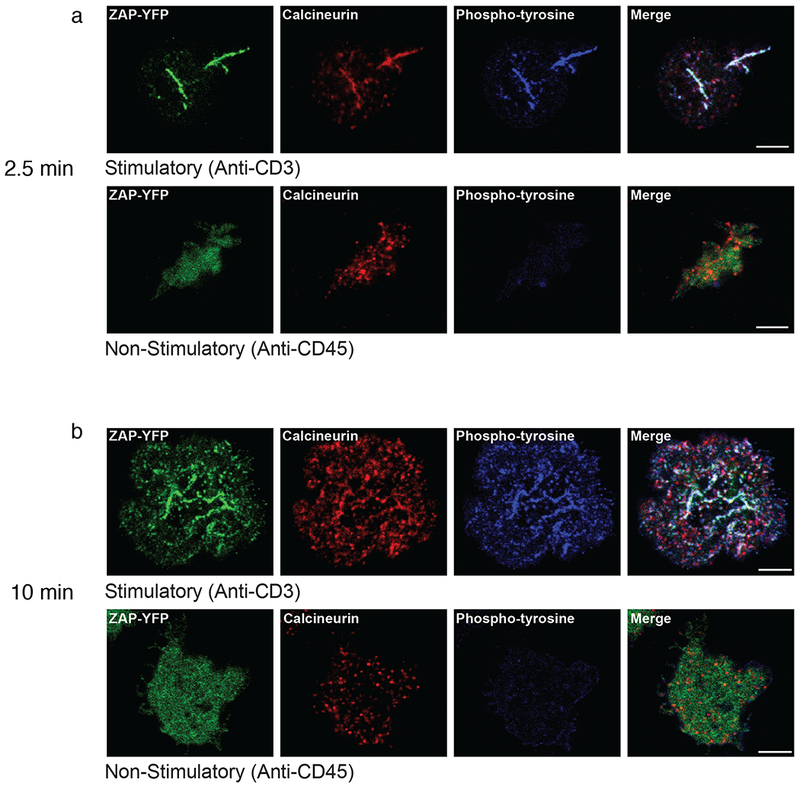
Cytosolic calcineurin is recruited to TCR microclusters. (a,b) Immunostained images of Jurkat cells stably expressing YFP-tagged ZAP70 that were dropped onto coverslips coated with anti-CD3 (stimulatory; top) or anti-CD45 (nonstimulatory; bottom) for 2.5 min (a) or 10 min (b). ZAP–YFP is shown in green, anti-calcineurin-A is shown in red, and phosphotyrosine is shown in blue. The experiment was performed 3 times, and in each case a representative single confocal slice is shown. Scale bars, 5 μm.
Figure 4.
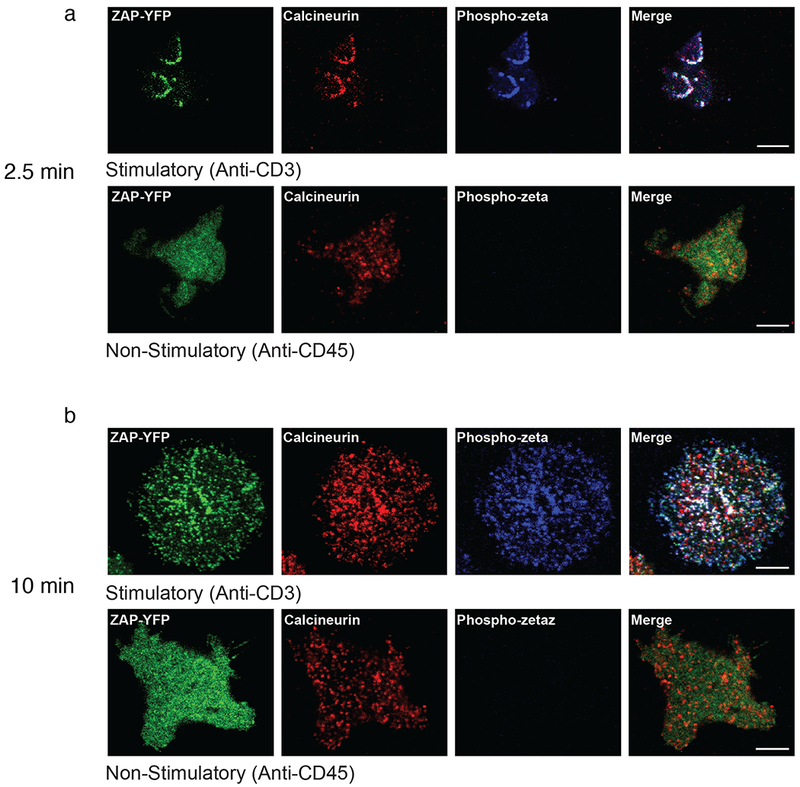
Calcineurin-containing microclusters also contain phosphorylated TCR. Immunostaining of (a,b) Immunostained images of Jurkat cells stably expressing YFP-tagged ZAP70 that were dropped onto anti-CD3-coated (stimulatory) or anti-CD45-coated (nonstimulatory) coverslips for 2.5 min (a) or 10 min (b). ZAP–YFP is shown in green, anti-calcineurin-A is shown in red, and phospho-TCR-ζ is shown in blue. The experiment was performed 3 times, and in each case a representative single confocal slice is shown. Scale bars, 5 μm.
To characterize the molecular nature of the calcineurin interactions in the activation-induced microclusters, TCR-ζ was immunoprecipitated from activated Jurkat cells, and its association with calcineurin was determined by immunoblotting. Consistent with the confocal microscopy data, calcineurin did not co-precipitate with the TCR in unactivated cells but did so soon after activation with anti-CD3 in both Jurkat cells and primary human T cells (Fig. 5a,b). Treatment of Jurkat cells or primary human CD4+ T cells with CsA and FK506 had no effect on TCR-induced calcineurin recruitment, demonstrating that calcineurin’s catalytic activity is dispensable for translocation to the TCR. To determine whether the association of calcineurin with the TCR is the result of signaling, as opposed to a consequence of anti-TCR-induced clustering for example, we used JCam1.6 cells, a Jurkat cell derivative lacking LCK24. Calcineurin was not co-immunoprecipitated with anti-TCR-ζ in the absence of LCK (Supplementary Fig. 1c). LCK could be required because of its role in signal initiation and/or because it acts as an adaptor protein for calcineurin binding. This was addressed by stimulating Jurkat cells in the presence or absence of PP1, a SRC family kinase inhibitor. Inhibition of LCK activity prevented TCR-mediated recruitment of calcineurin to the TCR (Fig. 5c). Consistent with the need for engagement of the TCR-proximal signaling machinery, treatment of Jurkat cells with PMA and ionomycin, which bypasses the TCR to activate T cells, failed to recruit calcineurin (Supplementary Fig. 1d). We conclude that the translocation of calcineurin requires tyrosine-phosphorylation events initiated by LCK.
Figure 5.
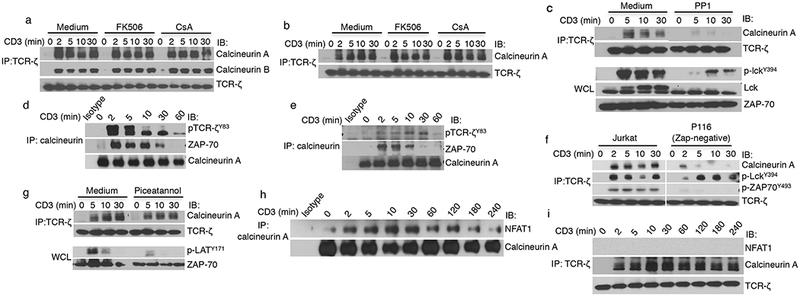
Association of calcineurin with signaling components requires LCK activity and intact ZAP70. (a,b) Immunoblot analysis for co-immunoprecipiation of calcineurin after immunoprecipitation of TCR-ζ from activated Jurkat cells (a) and human CD4+ T cells (b) that were treated as indicated (with or without FK506 or CsA) and stimulated with anti-CD3 for the indicated times. (c) Immunoblot analysis of TCR-ζ immunoprecipitates from Jurkat cells that were incubated with or without the Src kinase family inhibitor PP1 and activated for different amounts of times. Whole-cell lysates (WCL) from these cells were subjected to immunoblot analysis with a phospho-LCKY394-specific antibody to confirm inhibition of kinase activity. (d,e) Immunoblot analysis of anti-calcineurin immunoprecipitates from Jurkat cells (d) and human CD4+ T cells (e) that were activated for the indicated times. Stripped membranes were reprobed with anti-calcineurin to ensure equal amounts of loading of the precipitated complex. (f,g) Immunoblotting of anti-TCR-ζ immunoprecipitates from Jurkat cells and ZAP70-deficient P116 cells (f) or from Jurkat cells that were or were not treated with the ZAP70 kinase inhibitor piceatannol (g) following activation for different amounts of times. Whole-cell lysates were subjected to immunoblot analysis with anti-phospho-LATY171 to confirm inhibition of ZAP70 activity. (h,i) Immunoblot analysis of anti-calcineurin-A (or an isotype control) (h) or anti-TCR-ζ (i) immunoprecipitates from Jurkat cells that were unactivated or activated for the indicated amounts of times. The data in a–i are representative of three independent experiments with similar results.
Calcineurin interacts with TCR-proximal signaling molecules
To determine which TCR-associated proteins interact with calcineurin, Jurkat cells that were activated with anti-CD3 were lysed, and immunoblotting was performed on anti-calcineurin immunoprecipitates. As early as 2 min after activation, ZAP70 and phosphorylated TCR-ζ were found in the calcineurin immunoprecipitates, an association that waned over 10–30 min and was mostly gone by 1 h after activation (Fig. 5d), which was consistent with the very rapid recruitment of calcineurin to, and then attenuation from, the TCR microclusters (Figs. 3 and 4). Similar results were obtained with primary human T cells (Fig. 5e). The requirement for ZAP70 was determined by using ZAP70-deficient P116 cells. Although activation of LCK was normal, as expected, there was little recruitment of calcineurin to the TCR in the absence of ZAP70 (Fig. 5f). The role of ZAP70’s catalytic activity was determined by using the ZAP70 kinase inhibitor piceatannol. Calcineurin was still co-immunoprecipitated with TCR-ζ even when ZAP70 activity was inhibited, as shown by markedly decreased phosphorylation of the ZAP70 substrate LAT (Fig. 5g). These results indicate that calcineurin is recruited to ZAP70 independently of signaling downstream of ZAP70. Because calcineurin is best known for binding to and dephosphorylating NFAT transcription factors, we determined the kinetics of its association with NFAT1. Notably, the kinetics of calcineurin association with NFAT1 was the inverse of its association with the TCR, peaking at 10–30 min and slowly declining thereafter, but it still persisted at 4 h after TCR activation (Fig. 5h). We did not detect NFAT1 in anti-TCR-ζ immunoprecipitates (Fig. 5i), which, along with the appearance of non-TCR-associated calcineurin punctae at 10 min (Figs. 3 and 4), is consistent with the notion that different pools of calcineurin participate in TCR-proximal signaling events versus NFAT1 dephosphorylation.
Calcineurin negatively regulates LCKS59 phosphorylation
TCR-mediated activation of T cells induces a phosphorylation event at LCKS59, which in vitro inhibits LCK activity toward TCR-ζ ITAMs7,25. We observed that activation-induced phosphorylation of LCKS59 was enhanced by inhibition of calcineurin activity, without any effect on LCKY394 phosphorylation, in both Jurkat cells and primary human T cells (Fig. 6a,b). TCR-associated LCK also had increased phospho-LCKS59 after treatment of the cells with CsA or FK506 (Fig. 6c). To examine phospho-LCKS59 induction in a more physiological context, T cells isolated from the lymph nodes of AND TCR-αβ transgenic mice were activated with their cognate antigen, moth cytochrome c (MCC) peptide 88–103 presented on I-Ek. As with TCR cross-linking, we found greater amounts of phospho-LCKS59 after treatment with CsA or FK506 (Supplementary Fig. 2a).
Figure 6.
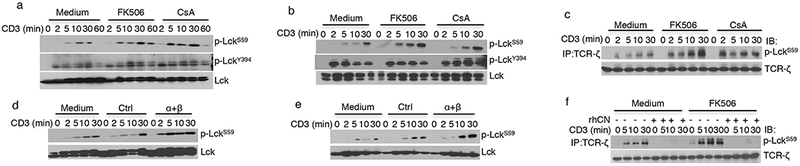
phospho-LCKS59 is a substrate of calcineurin in vivo and in vitro. (a,b) Immunoblot analysis of Jurkat cells (a) and primary human CD4+ T cells (b) that were treated with either medium or the indicated compounds and stimulated with anti-CD3 for the indicated amounts of time. (c) Immunoblot analysis of anti-TCR-ζ immunoprecipitates from Jurkat cells after activation for the indicated amounts of time. (d,e) Immunoblot analysis of proteins from Jurkat cells (d) and human CD4+ T cells (e) that were transfected with a control siRNA or with PPP3CA-specific and PPP3CB-specific siRNAs and that were activated with anti-CD3 for the indicated amounts of time. (f) Immunoblot analysis of TCR-ζ immune-complexes from Jurkat cells that were activated in the absence or presence of FK506 for the indicated times and subjected to an in vitro phosphatase assay using recombinant human calcineurin (rhCN). The data in a–f are representative of three independent experiments.
To complement our findings with the pharmacologic inhibitors, cells in which calcineurin expression was knocked down using siRNA were analyzed. Notably, knockdown of the expression of the calcineurin catalytic subunits enhanced the TCR-mediated phosphorylation of LCKS59 in both Jurkat cells and human CD4+ T cells, recapitulating the effects observed with CsA and FK506 (Fig. 6d,e). These results raised the possibility that phospho-LckS59 is a direct target of calcineurin. To test this hypothesis, TCR-ζ was immunoprecipitated from Jurkat cells that were activated in the absence or presence of CsA or FK506, and an in vitro phosphatase assay was performed with recombinant human calcineurin (Fig. 6f and Supplementary Fig. 2b). Calcineurin completely dephosphorylated LCKS59, confirming in vitro that LCKS59 is a substrate for this serine–threonine phosphatase.
Amino acid substitution at LCKS59 alters downstream signaling
Although in vitro evidence using recombinant proteins indicated that phosphorylation of LCKS59 inhibits kinase activity, the effect of this post-translational modification on downstream signaling at the molecular level has not been addressed. To do this, LCK-deficient JCam1.6 were transduced with a retrovirus encoding the WT (Ser59), Ser59Ala, and Ser59Glu forms of LCK (referred to as LCKWT, LCKS59A, and LCKS59E, respectively), and stable cell lines were established (Supplementary Fig. 2c). After activation with anti-CD3, T cells expressing LckS59A had increased phosphorylation at LckY394, ZAP70Y319 and ZAP70Y493, LATY171, p38T180, and ERKT202,Y204 (Fig. 7a). On the contrary, cells expressing the phosphomimetic mutant LckS59E had markedly blunted phosphorylation of these signaling intermediates (Fig. 7b). In agreement with enhanced TCR signaling, anti-CD3-activated Jurkat cells expressing LCKS59A produced more IL-2 than cells expressing LCKWT, whereas cells expressing LCKS59E produced less IL-2 than the control cells (Supplementary Fig. 2d). This was TCR specific, as the cells responded similarly when stimulated with PMA and ionomycin.
Figure 7.
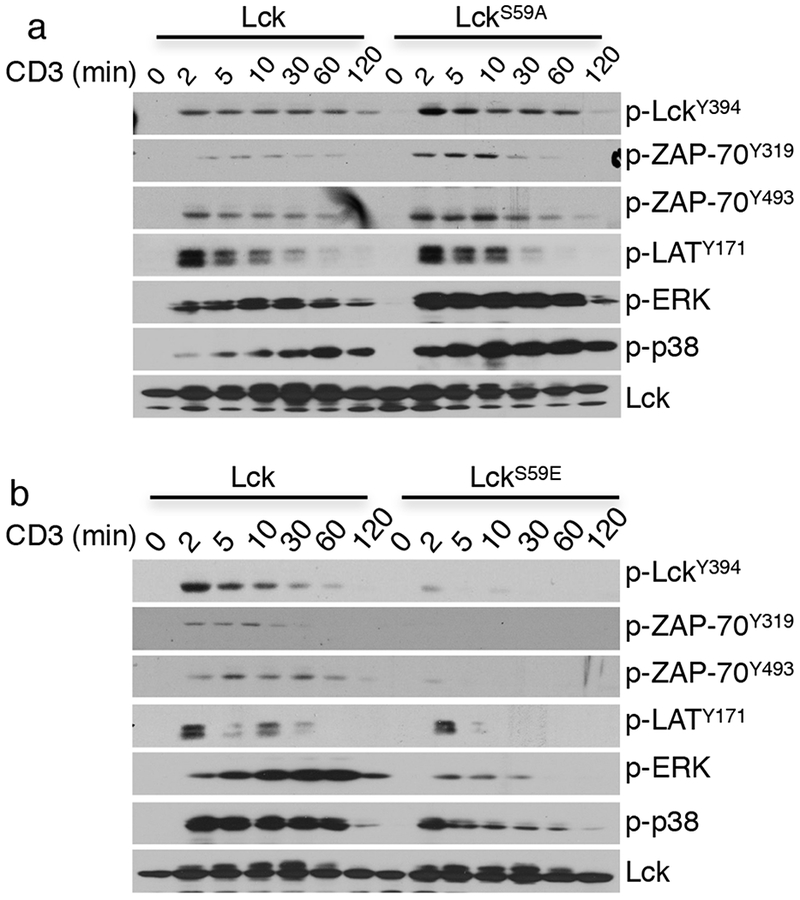
Mutations at LCK Ser59 affect TCR-proximal signaling. (a,b) Immunoblot analysis of signaling proteins from LCK-deficient JCam1.6 cells that were transduced with constructs expressing LCK or LCKS59A (a) or with constructs expressing LCK or LCKS59E and that were stimulated for different amounts of time. The data in a,b are representative of three independent experiments.
T cell adhesion requires dephosphorylation of phopho-LCKS59
The immunosuppressive effects of calcineurin inhibition are largely ascribed to inhibition of NFAT-dependent gene expression. To resolve whether inhibition of calcineurin activity in the TCR signalosome also contributes to immunosuppression, we searched for a calcineurin-dependent and NFAT-independent biological function. One possibility was T cell adhesion, a critical component in the initiation of the adaptive immune response. Activation via the TCR results in ‘inside-out’ signaling, in which the intracellular domain of LFA-1 is phosphorylated on Thr758, rapidly resulting in conformational changes in the extracellular portion of LFA-1 that enhance binding to ICAM1 (refs. 18,19). Given that increased LFA-1-mediated adhesion occurs in minutes, we reasoned that it was unlikely to require transcription and de novo protein synthesis. Indeed, LFA-1-dependent adhesion of anti-CD3-activated Jurkat or human CD4+ T cells to ICAM1-coated plates was unaffected by treatment with the protein synthesis inhibitor cycloheximide (Supplementary Fig. 3a). As expected, activation-induced T cell adhesion was greatly diminished by inhibition of LCK activity with PP1 (Fig. 8a,b). Notably, CsA and FK506 treatment also inhibited LFA-1-mediated adhesion induced by TCR signals; these inhibitors had little effect when adhesion was induced by activation with PMA and ionomycin. To distinguish between NFAT-dependent and NFAT-independent activities we used VIVIT, a peptide reagent that mimics the major calcineurin-docking site on NFATs and prevents calcineurin binding to NFATs without impairing its catalytic activity26,27. Treatment of cells VIVIT or its control peptide, VEET, had no effect on TCR-induced adhesion. All treatments, except those with VEET, inhibited NFAT-dependent IL-2 production (Supplementary Fig. 3b–d). Treatment of cells with CsA or FK506 also had no effect on LFA-1 expression (Supplementary Fig. 4a,b). However, phosphorylation of LFA-1T758 was markedly diminished by CsA and FK506 treatment (Fig. 8c).
Figure 8.
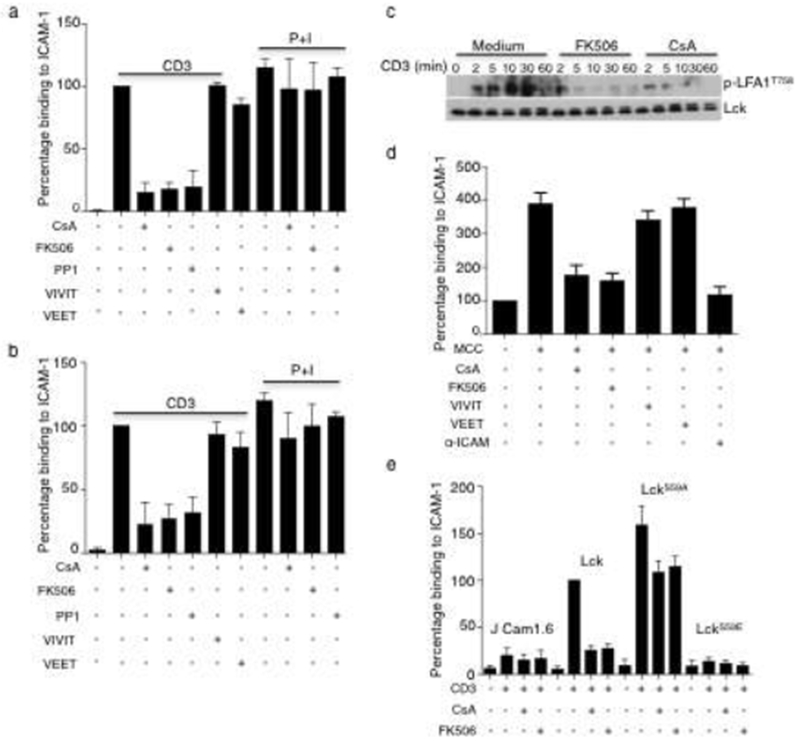
Calcineurin’s effect on inhibitory phospho-LCKS59 promotes TCR-induced LFA-1-mediated cell adhesion. (a,b) Quantification of Jurkat (a) and human CD4+ T (b) cells, as assessed by trypan blue exclusion, that bound to ICAM1-coated plates after treatment with the indicated compounds for 30 min at 37 °C and stimulation with anti-CD3 for an additional 30 min. The percentage binding was calculated for each relative to the activated sample, which was considered to be 100%. (c) Immunoblot analysis of human CD4+ T cells that were treated as indicated and activated with anti-CD3. (d) Numbers OR Quantification of T cells from AND TCR-transgenic mice that bound to ICAM1-coated plates after incubation with MCC-pulsed APCs (I-Ek- and ICAM1-expressing DCEK cells) in the presence of the indicated reagents. (e) Quantification of ICAM1-bound JCam1.6 cells stably expressing LCK, LCKS59A, or LCKS59E that bound to ICAM1-coated plates after activation with anti-CD3 in the presence of FK506 or CsA for 30 min at 37 °C. The data in a,b,e are mean ± s.e.m. and are representative of three (a,b) or four (e) independent experiments. In d, the data are mean ± s.e.m. of four independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001; n.s., not significant; by paired t-test.
To determine whether inhibition of calcineurin affects TCR-induced LFA-1-mediated adhesion in response to a physiologic antigen, purified T cells isolated from the lymph nodes of AND TCR-αβ transgenic mice were activated with their cognate antigen, MCC peptide 88–103 presented by I-Ek, in the presence or absence of the drugs. The mouse fibroblast cell DCEK that has been stably transfected with the genes encoding the relevant MHC-II molecule (I-Ek) and mouse ICAM1 was used to present antigen. Antigen-induced T cell binding to APCs was almost completely inhibited by treatment with CsA and FK506 and, as with anti-CD3-stimulated Jurkat and human T cells, treatment with the NFAT inhibitor VIVIT had no effect (Fig. 8d). Treatment with an ICAM1-specific blocking antibody reduced activation-induced cell adhesion to the baseline level found in the absence of antigen, demonstrating that the enhanced binding was entirely ICAM1 dependent. Thus, calcineurin has an important positive role in TCR-induced adhesion via NFAT-independent modulation of LFA-1 activation.
To determine whether calcineurin’s effects on adhesion were mediated by dephosphorylation of phospho-LCKS59, we examined activation-induced adhesion of JCam1.6 cells expressing the LCKWT, LCKS59A, or LCKS59E. Activated LCKWT-expressing cells bound to ICAM1-coated plates, an event that was prevented by inhibition of calcineurin activity (binding in the presence of CsA or FK506 was reduced to the low levels observed in LCK-negative JCam1.6 cells) (Fig. 8e). LCKS59A-expressing cells reproducibly bound better than LCKWT-expressing cells, consistent with a negative role for phospho-LCKS59 in this process. In fact, activated cells expressing the LckS59E phosphomimetic residue were unable to bind ICAM1-coated plates at all. Notably, although the cells expressed equivalent amounts of LFA-1 on their surface (Supplementary Fig. 4c), treatment with CsA and FK506 had only a small (∼25%) inhibitory effect on the adhesion of LCKS59A-expressing T cells. We conclude that calcineurin is required for activation-induced LFA-1–ICAM1-mediated adhesion largely due to dephosphorylation of phospho-LCKS59.
DISCUSSION
Major targets for calcineurin in activated T cells are members of the NFAT transcription factor family, whose dephosphorylation allows them to migrate to the nucleus with increased DNA-binding and transcriptional activity. Many genes, whose expression is repressed by inhibition of calcineurin (such as those encoding IL-2, IL-2 receptor, IL-4, IFN-γ, TNF, CD40 ligand, and Fas ligand), are critical to T cell proliferation and effector functions, although in not all cases is this known to be due to inhibition of NFAT activity28. However, it is the inhibition of calcineurin-dependent NFAT activity that is thought to underlie the clinical usefulness of immunosuppressive drugs such as CsA and FK506. Yet, there are a few studies that have provided evidence of regulation of TCR-proximal signaling by calcineurin. In one case, treatment with CsA or FK506 increased LAT expression induced by TCR signaling29. In another, inhibition of calcineurin reduced TCR clustering in the immune synapse17. The findings in our report provide a molecular mechanism for, and the biological relevance of, calcineurin’s role in TCR-proximal signaling.
In the resting state calcineurin is a cytosolic protein. In the event of increased levels of intracellular Ca2+, calcineurin binds to and dephosphorylates NFAT family members, allowing both to migrate to the nucleus. It is thought that continued calcineurin phosphatase activity in the nucleus is required to maintain NFAT in a transcriptionally active state while Ca2+ levels remain elevated30,31. The finding that in activated T cells calcineurin first migrates to the plasma membrane was therefore a surprise. We found that maximal association of calcineurin with the TCR occurred within 2 min, slowly waning thereafter, whereas the association of calcineurin with NFAT1 increased over 10–30 min. Whether this represents a single pool of calcineurin that is initially diverted to plasma membrane TCRs and subsequently to intracellular NFAT, or whether these are two distinct pools of calcineurin, remains to be determined. It seems likely that in the TCR signaling complex calcineurin binds phosphorylated ZAP70, as the translocation of calcineurin to the TCR required LCK tyrosine kinase activity and the presence of its substrate, ZAP70. In contrast, ZAP70 kinase activity was dispensable, arguing that further downstream signaling is not required. Furthermore, confocal microscopy studies showed that the calcineurin colocalized with phosphorylated ZAP70 in activated T cells. Techniques that provide higher-resolution characterization of protein–protein interactions, such as fluorescence resonance energy transfer (FRET) or other super-resolution microscopy techniques, may prove useful in further elucidating calcineurin-interacting proteins in the TCR signaling complex.
Phosphorylation of N-terminal residues of LCK was initially reported as a consequence of mitogenic stimulation25, and phopho-LCKS59 was subsequently identified as a target of ERK in phorbol-ester-stimulated and anti-TCR-stimulated T cells7,32. Although one study has suggested that phosphorylation of LCKS59 is a positive event that prevents protein tyrosine phosphatase, non-receptor type 6 (PTPN6; also known as SHP1) recruitment to the TCR9, T cells from antigen-specific TCR-transgenic mice bearing the LCKS59A mutation responded better to antigenic stimulation, with higher numbers of CD69+ cells and enhanced proliferation to peptide–MHC tetramers, than cells from WT mice8. Although in the latter study TCR-proximal signaling was not examined, in vitro kinase assays performed with recombinant Lck showed that its phosphorylation by Erk reduced its activity toward sequences from its natural target, TCR-ζ (ref. 7). In this report, LCK-deficient Jurkat T cells that were reconstituted with LCKWT, LCKS59A (which cannot accept a phosphate at Ala59), or LCKS59E (a phosphomimetic mutant) behaved very differently with regard to activation. Consistent with an inhibitory role of phospho-LCKS59, TCR-proximal signaling was enhanced in cells expressing LCKS59A and markedly inhibited in those expressing LCKS59E. This held true for functional responses, such as activation-induced adhesion to ICAM1-coated surfaces and IL-2 production. It is noteworthy that inhibition of calcineurin activity affected some signaling events but not others. Inhibition of calcineurin activity diminished phosphorylation of the proximal kinase ZAP70 at residue Tyr493 but not at Tyr315 and Tyr319, and downstream signaling bifurcated, with decreases in phosphorylation of LATY171 and SLP76Y145, but not of PLC-γ1Y783, and inhibition of p38 but not ERK. This pattern was recapitulated in cells in which ZAP70Y493 could not be phosphorylated (ZAP70Y493F-expressing cells). These results are consistent with the notion that phospho-LCKS59 may change LCK binding to and/or catalytic activity toward ZAP70, which specifically affects phosphorylation of ZAP70Y493, and may shed light on which downstream pathways are dependent on phospho-ZAP70Y493.
Migration of immune cells into lymph nodes or areas of inflammation is a necessary step in an immune response. A key component in this event is LFA-1 (αLβ2, or CD11a–CD18), a transmembrane integrin expressed on all T cells, as well as on other leukocytes, that binds to ICAM1 (ref. 18). After T cells are activated, LFA-1 undergoes conformational changes from an inactive ‘bent’ to an active ‘extended’ form that exposes ICAM1-binding sites. This is accompanied by phosphorylation of LFA-1 on multiple residues, including αL chain residues Ser1140, which seems to be necessary for the conformational changes, and Thr758, which regulates interactions with intracellular adaptors and enzymes, such as 14–3-3, that are involved in actin cytoskeleton rearrangement33,34. Although it has been shown that Thr758 is a major phosphorylation site in TCR-activated T cells34 and that a number of different protein kinase C (PKC) isoforms can phosphorylate a CD18 peptide fragment containing Thr758 in vitro, the physiologically relevant kinase(s) in vivo is unknown35. Changes in LFA-1-dependent adhesion are among the earliest biological responses to TCR-mediated stimulation, occurring within minutes, and these are dependent on LCK and ZAP70 activity36,37. We found that adhesion is inhibited by CsA treatment but is not affected by inhibition of NFAT activity, consistent with its rapid onset and independence from de novo protein synthesis. Notably, under some conditions inhibition of calcineurin impairs TCR-mediated T cell–APC conjugate formation17. The LFA-1–ICAM1 interaction is critical in an immune response and has been targeted in the treatment of autoimmune disorders, such as plaque psoriasis, although reactivation of John Cunningham (JC) virus has precluded further use38. T cell activation-induced degranulation by cytotoxic T cells is another early event that has been shown to be affected by inhibition of calcineurin39,40, and it is tempting to speculate that this may also be due to its role in TCR-proximal signaling. We conclude that CsA and FK506 inhibit TCR-upregulated LFA-1–ICAM1 binding by negatively regulating TCR-proximal signaling, leading to a defect in cell adhesion. In view of the critical role of cell adhesion in the adaptive immune response, this CsA (or FK506) effect may be an important feature in their clinical usefulness.
METHODS
Methods, including statements of data availability and any associated accession codes and references, are available in the online version of the paper.
ONLINE METHODS
Plasmids and antibodies.
A retroviral plasmid encoding LCK was obtained from Addgene (#20511). Mutation of Ser59 to alanine (S59A) or glutamic acid (S59E) was performed by site-directed mutagenesis following the manufacturer’s protocol (QuikChange XL kit, Agilent Technologies). A plasmid expressing codon-optimized calcineurin A (SFG.wtCNa_opt.IRES.eGFP), in which each of the three siRNA-targeted sequences containing multiple codons were altered from that of the wild-type sequence (while maintaining the same amino acid sequence), was obtained from Addgene (#22489). pSX SRα, encoding human ZAP70 cDNA has been described41. ZAP70 was excised from this vector and cloned into the retroviral vector pMSCV-IRES-GFP (Addgene #52107). The Tyr493Phe mutation was created by site-directed mutagenesis (QuikChange XL kit, Agilent Technologies). All constructs were verified by sequencing. Rabbit antibodies used were as follows: anti-phospho-ZAP70Y319, anti-phospho-PLC-γ1Y783, pan–calcineurin A antibody, anti-phospho-LATY171, anti-phospho-ZAP70Y493, anti-p44/42 MAPK (anti-ERK1/2), anti-phospho-p44/42 MAPK (anti-phospho-Erk1/2) (at Thr202 and Tyr204), anti-ZAP70 (clone 99F2), anti-phospho-SRCY416, anti-phospho-p38T180,Y182, anti-SHP1 (C14H6) and anti-p38 MAPK from Cell Signaling Technologies; anti-CD3-ζ (phospho-Tyr83), anti-phospho-SLP76Y145, anti-CD18 (phospho-Thr758) from Abcam; anti-phospho-LCKY394, anti-IκBα (C-21) from Santa Cruz; anti-phospho-LCKS59 from Sigma; and anti-CD247 (phospho-Tyr142) from BD Pharmingen. Anti-mouse monoclonal antibodies used included anti-phosphotyrosine (clone 4G10, Millipore); anti-Lck (3A5), anti-TCR-ζ (6B10.2) from Santa Cruz; anti-human-CD3 functional grade purified, anti-human-CD28 functional grade purified from Ebioscience, anti-calcineurin α-subunit from Sigma; anti-calcineurin B from R&D Systems; affinity-purified goat anti-mouse IgG (H + L), Jackson Immunoresearch; phycoerythrin (PE)-conjugated anti-human-CD11a and purified anti-mouse-CD54 (ICAM1) from BioLegend; PE-conjugated mouse IgG1, κ isotype control, Pharmingen; Alexa Fluor 564 and Alexa Fluor 647 d, Thermo Fisher; agarose-conjugated CD3-ζ mouse monoclonal IgG1, Santa Cruz.
Cell lines and reagents.
Jurkat cells were obtained from the American Type Culture Collection. LCK-deficient (J Cam1.6), ZAP70-deficient (P116) cells and stable ZAP–YFP-expressing Jurkat cells were created as described42. Cells were maintained in RPMI 1640 (Gibco) medium supplemented with 10% FBS (Gibco). Stable cell lines were cultured in the same medium with 1.3 mg/ml geneticin sulfate G418 (KSE Scientific, Durham NC). The Phoenix-AMPHO retroviral packaging cell line and L-cells expressing I-Ek (DCEK) were a kind gift from Ron Germain (NIAID). Recombinant human calcineurin (catalog no. 3160-CA, R&D Systems), Lipofectamine 2000 (Life Technologies), calmodulin from bovine testes (catalog no. P1431, Sigma), piceatannol (10009366, Cayman Chemical); 11R-VIVIT (480401) and 11R-VEET (catalog no. 480401, Calbiochem; moth cytochrome c (MCC) peptide (ANERADLIAYLKQATK; Peptide 2.0), recombinant human ICAM1–CD54-Fc chimera (720-IC-050, R&D Systems); human CD4 and mouse T cell recovery column kits (Cedarlane Laboratories Limited), and control siRNA-A (sc-37007), PP2B-Aα siRNA (sc-36304) and PP2B-Aß siRNA (sc-39195) (all from Santa Cruz) were used. FK506 monohydrate and cyclosporin A were from Sigma; the Src kinase inhibitor PP1 was from Calbiochem; and the human IL-2 ELISA kit was from eBioscience. SDS sample buffer was purchased from Quality Biologicals Inc., and Restore PLUS western blot Stripping Buffer was from Thermo Scientific.
Mice.
All experiments followed an approved animal study protocol of the National Institutes of Health. The AND TCR-αβ transgenic mice used for the studies were bred in our facility.
Isolation and activation of T cells and immunoblotting.
T cells were treated with CsA or FK506 (100–200 ng/ml) for 2 h at 37 °C followed by incubation with soluble anti-CD3 (OKT3) on ice. OKT3 was then cross-linked with anti-mouse-IgG for the indicated times, and the cells were lysed in 1% Triton-X 100 lysis buffer supplemented with protease and phosphatase inhibitors (Roche) for 30 min on ice and centrifuged for 20 min at 13,000 r.p.m. The supernatant was boiled in SDS sample buffer for 10 min and subjected to SDS–PAGE, followed by immunoblotting (Trans-Blot Turbo, Bio-Rad). Human CD4+ T cells were isolated from buffy coats of healthy volunteers (NIH Blood Bank) using the human CD4 cell recovery column kit (Cedarlane), according to the manufacturer’s instructions. T cells from lymph nodes of AND TCR-αβ transgenic mice were purified using the mouse T cell recovery column kit (Cedarlane). Inhibition of LCK and ZAP70 activity before stimulation was done by pre-incubation of cells in the presence of 25 µM SRC family kinase inhibitor PP1 or 25 µM SYK family kinase inhibitor piceatannol for 30 min at 37 °C. In some cases, membranes were stripped with stripping buffer for 10 min and reblotted for detection of total protein. Uncropped immunoblots are in Supplementary Data Set 1.
Immunoprecipitation.
T cells were lysed in ice-cold lysis buffer (150 mM NaCl, 20 mM Tris pH 7.5, 1% Triton X-100 supplemented with 1× protease inhibitor and 1× phosphatase inhibitor; Roche). Lysates were cleared by centrifugation at 13,000 r.p.m. for 20 min and subjected to immunoprecipitation using agarose-conjugated TCR-ζ or protein G beads incubated with anti-calcineurin-A or an IgG1 control antibody for 4–6 h. The beads were washed in ice-cold lysis buffer, and the proteins were eluted by boiling in SDS sample buffer and resolved by SDS–PAGE.
Confocal microscopy and image analysis.
Cells were allowed to spread on coverslips as described42. In brief, cells were dropped onto polylysine-treated four-chambered glass coverslips coated with stimulatory anti-CD3 (UCHT-1) or nonstimulatory anti-CD45 (H130) antibodies at 10 µg/ml. Cells were resuspended in warm medium without phenol red, which was supplemented with 25 mM HEPES, pH 7, and dropped at the bottom of the chamber followed by incubation at 37 °C for 2.5 and 10 min. The cells were fixed with 2.4% paraformaldehyde for 30 min and permeabilized with Triton X-100. The slides were blocked in blocking buffer (2% goat serum) for 30 min and incubated with primary antibodies for 60 min at the appropriate dilution (pan–calcineurin A, 1:50; anti-CD247, 1 µg/ml; anti-phosphotyrosine clone 4G10, 1.1 µg/ml). Cells were washed and stained with Alexa-Fluor-568-labeled (1:1,000) or Alexa-Fluor-647-labeled (1:500) secondary antibodies, and images were captured using a Leica SP8 laser-scanning confocal microscope using a 63×, 1.4 numerical aperture (NA) objective (Leica Microsystems Inc, Buffalo Grove IL). 2- to 3-μm z-stacks with a spacing of 0.3 μm were taken of the area contacting the coverslip. Images were processed in Leica AF software and then exported to Adobe Photoshop and Illustrator (Adobe Systems Inc, San Jose CA) to prepare the composite figures. Original images can be found in Supplementary Data Set 2.
Phosphatase assay.
TCR immunoprecipitates from Jurkat cells were washed in assay buffer (20 mM Tris, 10 mM MgCl2, 0.1 mM CaCl2 and 1 mg/ml bovine serum albumin, pH 7.5) and incubated with recombinant human calcineurin (20 ng/ml) for 30 min at 30 °C. The reaction was stopped by adding sample buffer and boiling for 10 min. Dephosphorylation of the substrate was assessed by SDS–PAGE and immunoblotting.
siRNA-mediated knockdown of calcineurin expression.
Five million Jurkat or human CD4+ T cells were resuspended in 500 µl of antibiotic-free RPMI medium with 10 µ1 (100 picomoles) each of the validated siRNAs (PP2B-Aα and PP2B-Aβ) or the control siRNA-A, and they were placed inside a 4-mm cuvette (Bio-Rad). The cuvettes were pulsed using the BTX electroporator (300 V, 10 ms, 960 µF), and the cells were transferred into RPMI medium containing 10% FCS. Cells were analyzed for knockdown of calcineurin expression after 72 h by immunoblot analysis. At the protein level, the extent of calcineurin knockdown was estimated by densitometric analysis using ImageJ software. Jurkat cells expressing codon-optimized calcineurin A were electroporated with calcineurin-A-specific siRNAs (PP2B-Aα and PP2B-Aβ) or a control siRNA as described above.
T cell adhesion assay.
Cells were pretreated with the drugs (CsA, FK506) or inhibitors (PP1, VIVIT, VEET) for 30 min at 37 °C, whereas cycloheximide was added at the time of activation. Jurkat cells and LCK-expressing JCam1.6 cells (1 × 106) or human CD4+ T cells (0.5 × 106) were stimulated with soluble anti-CD3 and cross-linked with anti-mouse-IgG, then dropped into 24-well plates coated with 3 µg/ml recombinant human ICAM1–Fc fragment at 37 °C for 30 min43.Wells were washed three or four times with PBS, and the plate-bound cells were removed with cell-dissociation buffer (0.25% trypsin–EDTA, Gibco). Bound cells were counted by trypan blue exclusion using light microscopy and a hemocytometer. For antigen-induced adhesion, monolayers of fibroblastic DCEK cells that stably expressed I-Ek and mouse ICAM1 (ref. 44) in duplicate 24-well plates were pulsed with medium or MCC (1 μM) for 2 h. Purified T cells from the lymph nodes of AND TCR-transgenic mice were added to the wells in medium alone or with the indicated reagents (CsA and FK506, 100 ng/ml; VIVIT and VEET, 1 µM; anti-ICAM1, 1 μg/ml), centrifuged at 800 r.p.m. for 10 s, and incubated for 1 h at 37 °C. Wells were washed five or six times with PBS, and the adherent cells were then removed with cell-dissociation buffer (0.25% trypsin–EDTA, Gibco). T cells were counted as described above.
Generation of stable Jurkat lines expressing wild-type or mutated LCK, ZAP70, and calcineurin A.
Constructs encoding LCK, LCKS59A, or LCKS59E were transfected in Amphotropic Phoenix packaging cells to produce retroviruses for spin-infection of LCK-negative JCam1.6 cells. Infection of 106 cells was carried out for 2 h at 30 °C in the presence of 8 µg/ml polybrene. The cells were allowed to recover for 48 h, and they were then grown in selection medium (containing 2 mg/ml G418). Expression of the transfected LCK-encoded protein was checked after an additional 48 h by immunoblotting. For ZAP70, constructs encoding ZAP70WT or the ZAP70Y493F mutant were transfected into HEK-293T cells (10 μg of pMSCV containing the gene of interest, 2 μg of gag–pol, and 2 μg of VSV-g) to generate viruses for spin-infection of the ZAP70-negative cell line P116. Infection of 106 cells was carried out as described above, and the cells were subsequently grown in selection medium (containing 0.5 μg/ml puromycin). GFP expression was checked by flow cytometry, and expression of ZAP70 was analyzed by immunoblotting. For calcineurin A expression, a plasmid encoding codon-optimized calcineurin (10 µg) was transfected into HEK 293T cells along with a plasmid (pCMV-VSV-G) encoding envelope and another encoding packaging proteins (pUMVC) to generate viruses for spin-infection. Cells were sorted for GFP expression, and the levels of calcineurin were analyzed by immunoblotting. After sorting, the cells were maintained in 800 μg/ml G418.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism 6 software. Error bars indicate s.e.m. unless otherwise specified. Statistical significance was determined with the paired t-test, and P < 0.05 was considered to be statistically significant.
Data availability
The data that support the findings of this study are available from J.D.A. upon request.
Activation of lymph node T cells.
Lymph nodes were isolated from 4- to 8-week-old AND mice. For analysis of T cell signaling, 2 × 106 cells were added to I-Ek-expressing DCEK cells that had been pulsed with 10 µM MCC (residues 88–103) for 2 h at 37 °C, centrifuged at 800 r.p.m. for 10 s, and incubated at 37 °C for the indicated times. The non-adherent cells were lysed in Triton-X 100 lysis buffer and subjected to SDS–PAGE and immunoblotting.
Enzyme-linked immunosorbent assay (ELISA).
Jurkat cells (105 cells/well) and CD4+ T cells (5 × 105 cells/well) were activated with plate-bound OKT3 (10 µg/ml) and soluble anti-CD28 (1 µg/ml) or with PMA (30 ng/ml) and ionomycin (1 µg/ml), in the presence of CsA (100 ng/ml), FK506 (100 ng/ml), VIVIT (1 µM), VEET (1 µM), PP1 (25 µM), or cycloheximide (10 µg/ml). Culture supernatants were assayed for IL-2 after 8 h (Jurkat) or 24 h (human T cells). The data are the average ± s.d. of two independent experiments except for the cycloheximide experiments, in which the data are the average ± s.e.m. of three independent experiments.
Flow cytometry.
Resting or OKT3-activated Jurkat cells or human CD4+ T cells treated with CsA or FK506 were washed with PBS and incubated with PE-conjugated anti-human-CD11a (1:200 dilution) in PBS with 2% FCS for 1 h on ice. Samples were washed and resuspended in PBS. Flow cytometric data acquisition and analysis were performed with FACS Calibur using the CellQuest software (BD Biosciences).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH and the Overseas Associateship funded by the Indian Department of Biotechnology, Ministry of Science and Technology (L.I.S.). We thank R. Germain (NIAID) for the DCEK cells and Amphotropic Phoenix packaging cell lines, B. Dong for expert technical assistance, and S. Koyasu for suggesting that we ask whether CsA has an effect on the activation of p38 via the alternative pathway.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Boggon TJ & Eck MJ Structure and regulation of Src family kinases. Oncogene 23, 7918–7927 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Di Bartolo V et al. Tyrosine 319, a newly identified phosphorylation site of ZAP70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem 274, 6285–6294 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Williams BL et al. Phosphorylation of Tyr319 in ZAP70 is required for T cell antigen receptor–dependent phospholipase C–γ1 and Ras activation. EMBO J. 18, 1832–1844 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW & Weiss A Intramolecular regulatory switch in ZAP70: analogy with receptor tyrosine kinases. Mol. Cell. Biol 25, 4924–4933 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mège D et al. Mutation of tyrosines 492 and 493 in the kinase domain of ZAP70 affects multiple T cell receptor signaling pathways. J. Biol. Chem 271, 32644–32652 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Wang H et al. ZAP70: an essential kinase in T cell signaling. Cold Spring Harb. Perspect. Biol 2, a002279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts JD, Sanghera JS, Pelech SL & Aebersold R Phosphorylation of serine 59 of p56lck in activated T cells. J. Biol. Chem 268, 23275–23282 (1993). [PubMed] [Google Scholar]
- 8.Paster W et al. A THEMIS–SHP1 complex promotes T cell survival. EMBO J. 34, 393–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefanová I et al. TCR ligand discrimination is enforced by competing ERK -positive and SHP1-negative feedback pathways. Nat. Immunol. 4, 248–254 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Rusnak F & Mertz P Calcineurin: form and function. Physiol. Rev. 80, 1483–1521 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Macian F NFAT proteins: key regulators of T cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Alam MS et al. Counter-regulation of T cell effector function by differentially activated p38. J. Exp. Med. 211, 1257–1270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J et al. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell 66, 807–815 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Liu J et al. Inhibition of T cell signaling by immunophilin–ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry 31, 3896–3901 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Palkowitsch L et al. The Ca2+-dependent phosphatase calcineurin controls the formation of the CARMA1–BCL10–MALT1 complex during T cell receptor–induced NF-κB activation. J. Biol. Chem 286, 7522–7534 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden-Martinez K, Kane LP & Hedrick SM Effects of a constitutively active form of calcineurin on T cell activation and thymic selection. J. Immunol 165, 3713–3721 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Zeyda M et al. Impairment of T cell interactions with antigen-presenting cells by immunosuppressive drugs reveals involvement of calcineurin and NF-κB in immunological synapse formation. J. Leukoc. Biol 81, 319–327 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Smith A et al. The role of the integrin LFA-1 in T lymphocyte migration. Immunol. Rev 218, 135–146 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Nurmi SM, Autero M, Raunio AK, Gahmberg CG & Fagerholm SC Phosphorylation of the LFA-1 integrin β2-chain on Thr758 leads to adhesion, RAC1–CDC42 activation, and stimulation of CD69 expression in human T cells. J. Biol. Chem 282, 968–975 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Matsuda S, Moriguchi T, Koyasu S & Nishida E T lymphocyte activation signals for interleukin-2 production involve activation of MKK6–p38 and MKK7–SAPK (JNK) signaling pathways sensitive to cyclosporin A. J. Biol. Chem 273, 12378–12382 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Matsuda S et al. Two distinct action mechanisms of immunophilin–ligand complexes for the blockade of T cell activation. EMBO Rep. 1, 428–434 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvador JM et al. Alternative p38 activation pathway mediated by T cell receptor–proximal tyrosine kinases. Nat. Immunol 6, 390–395 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Bunnell SC et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol 158, 1263–1275 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straus DB & Weiss A Genetic evidence for the involvement of the LCK tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70, 585–593 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Veillette A, Horak ID, Horak EM, Bookman MA & Bolen JB Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T cell activation. Mol. Cell. Biol 8, 4353–4361 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aramburu J et al. Selective inhibition of NFAT activation by a peptide spanning the calcineurin-targeting site of NFAT. Mol. Cell 1, 627–637 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Aramburu J et al. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285, 2129–2133 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Luo C & Hogan PG Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol 15, 707–747 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Cho CS et al. Rapamycin antagonizes cyclosporin A- and tacrolimus (FK506)-mediated augmentation of linker for activation of T cell expression in T cells. Int. Immunol 15, 1369–1378 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Shibasaki F, Price ER, Milan D & McKeon F Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NFAT4. Nature 382, 370–373 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Al-Daraji WI, Grant KR, Ryan K, Saxton A & Reynolds NJ Localization of calcineurin–NFAT in human skin and psoriasis, and inhibition of calcineurin–NFAT activation in human keratinocytes by cyclosporin A. J. Invest. Dermatol 118, 779–788 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Winkler DG et al. Phosphorylation of Ser42 and Ser59 in the N-terminal region of the tyrosine kinase p56lck. Proc. Natl. Acad. Sci. USA 90, 5176–5180 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez OD et al. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat. Immunol 4, 1083–1092 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Fagerholm SC, Hilden TJ, Nurmi SM & Gahmberg CG Specific integrin α- and β-chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol 171, 705–715 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagerholm S, Morrice N, Gahmberg CG & Cohen P Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem 277, 1728–1738 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Evans R, Lellouch AC, Svensson L, McDowall A & Hogg N The integrin LFA-1 signals through ZAP70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood 117, 3331–3342 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Burbach BJ, Medeiros RB, Mueller KL & Shimizu Y T cell receptor signaling to integrins. Immunol. Rev 218, 65–81 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Brennan M & Cox D in I Domain Integrins 157–178 (Springer, 2014). [DOI] [PubMed] [Google Scholar]
- 39.Grybko MJ, Bartnik JP, Wurth GA, Pores-Fernando AT & Zweifach A Calcineurin activation is only one calcium-dependent step in cytotoxic T lymphocyte granule exocytosis. J. Biol. Chem 282, 18009–18017 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Dutz JP, Fruman DA, Burakoff SJ & Bierer BE A role for calcineurin in degranulation of murine cytotoxic T lymphocytes. J. Immunol 150, 2591–2598 (1993). [PubMed] [Google Scholar]
- 41.Wange RL et al. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP70. J. Biol. Chem 270, 18730–18733 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Bunnell SC, Barr VA, Fuller CL & Samelson LE High-resolution multicolor imaging of dynamic signaling complexes in T cells stimulated by planar substrates. Sci. STKE 2003, PL8 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Au-Yeung BB et al. A genetically selective inhibitor demonstrates a function for the kinase ZAP70 in regulatory T cells independent of its catalytic activity. Nat. Immunol 11, 1085–1092 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahnke K et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II–positive lysosomal compartments. J. Cell Biol 151, 673–684 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from J.D.A. upon request.


