Abstract
Objective
Treating pediatric severe obesity is challenging due to the complex biological, behavioral, and environmental factors that underpin the disease. The multifactorial etiology of obesity combined with the physiologic complexity of the energy regulatory system contributes to treatment variability. The goal of this secondary analysis of pooled data was to describe the degree of individual variation in response to various interventions among adolescents with severe obesity.
Methods
Data from 3 centers across the United States conducting either lifestyle (n=53), pharmacotherapy (n=40), or metabolic and bariatric surgery (MBS; n=78) interventions were pooled. Inclusion criteria were severe obesity at baseline and at least one follow-up visit >30 days after treatment start.
Results
Change in BMI following intervention ranged from −50.2% to +12.9%, with each intervention (lifestyle [range: −25.4% to 5.0%], pharmacotherapy [range: −10.8% to 12.9%], MBS [range: −50.2% to −13.3%]) exhibiting wide individual variation in response. Changes in cardiometabolic risk factors demonstrated similarly high variability.
Conclusions
Adolescents with severe obesity demonstrated a high degree of heterogeneity in terms of BMI reduction and cardiometabolic risk factor response across treatment modalities. Reporting individual response data in trials and identifying factors driving variability in response will be vital for advancing precision medicine approaches to address obesity.
Keywords: Adolescent, Lifestyle, Pharmacotherapy, Bariatric Surgery, Heterogeneity
Introduction
Pediatric severe obesity (Body mass index [BMI] ≥35kg/m2 or 1.2 × 95th BMI-percentile) continues to rise in prevalence and is associated with risk factors for many chronic diseases.(1, 2, 3, 4) Despite the high prevalence and seriousness of pediatric severe obesity, successful treatment remains a challenge.(5) Most studies have evaluated success and failure of treatment based upon mean changes in response to a given intervention.(6, 7, 8, 9) However, this traditional approach fails to capture the individual variability observed in obesity studies regardless of the intervention modalities (e.g., lifestyle, medication, or metabolic and bariatric surgery[MBS]).
While the current paradigm to determine treatment efficacy using group response is likely to remain the standard for randomized controlled trials, it may not by itself be the optimal means by which to interpret results of interventions for the treatment of pediatric severe obesity nor direct individualized treatment efforts. Consistent with the overwhelming evidence supporting the heterogeneous and multifactorial nature of obesity as a disease,(9, 10, 11) treatments that target obesity may be best suited for individualized and precision approaches rather than a “one-size-fits-all” paradigm.(9, 12, 13, 14) Though the degree of heterogeneity in obesity treatment outcomes has been well-described in adults,(10, 15) the pediatric literature, especially among adolescents with severe obesity, is lacking data on the variability in response to different treatment options. Characterization of the range of responses is needed in order to identify individual intervention goals and bridge the gap towards precision medicine approaches.
Therefore, the purpose of this secondary data analysis was to describe the variability in response among adolescents with severe obesity to lifestyle intervention, pharmacotherapy, and MBS. To accomplish this goal, we pooled data from 3 centers across the United States and examined the variability in response to treatment. We a priori hypothesized that each treatment would exhibit considerable heterogeneity in treatment response for weight loss and changes in cardiometabolic risk outcomes.
Methods
The cohorts
Data were pooled from 3 centers (University of Minnesota, Arizona State University, and Cincinnati Children Hospital) across the United States. Inclusion criteria for this secondary analysis were: meeting the definition of severe obesity (>1.2 times the 95th BMI percentile or absolute BMI >35kg/m2)(3, 16) at baseline and a minimum of 30-days of intervention completed with height and weight measurements available at follow-up. We used baseline to last visit within each study for each participant as the period of time to measure change in the outcome variable.
Lifestyle Modification Therapy (LMT)
Data from two LMT trials were used. The first was a comprehensive, family-based LMT intervention that included weekly nutrition education (60 min-sessions) and moderate to vigorous physical activity (three, 60 min-sessions per week) instruction (12-weeks [mean: 105 days; range 85-129 days on treatment], n=45).(17) The second LMT cohort underwent comprehensive individualized multidisciplinary pediatric weight management (52-weeks [mean: 374 days; range 286-482 days on treatment], n=8).(18)
Pharmacotherapy
Data from three pharmacotherapy clinical trials were included in the analysis. Only data on participants who were randomly assigned to study drug were included (participants assigned to placebo were excluded). Each of the three trials was randomized and controlled and identified change in body mass index (BMI) as the primary outcome variable: one trial evaluated a glucagon-like-peptide receptor agonist (GLP-1RA) (26-weeks [mean: 156 days; range 33-188 days on treatment], n=13),(19) the second trial evaluated a GLP-1RA with a cross-over design (12-weeks [mean: 80 days: range 30-91 days on treatment], n=11),(20) and the third trial evaluated topiramate (28-weeks [mean: 164 days; range 30-216 days on treatment], n=16).(21)
Metabolic and Bariatric Surgery
Data from two MBS cohorts were used: The first cohort included members who underwent Roux-en-Y gastric bypass (52-weeks [mean: 369 days; range 288-441 days on treatment], n=50),(18, 22) while the second cohort included individuals undergoing either Roux-en-Y gastric bypass or vertical sleeve gastrectomy (52-weeks [mean: 373 days; range 202-459 days on treatment], n=28).
Statistical Analysis
Descriptive statistics for each cohort were calculated with mean (s.d.) for continuous variables or N (%) for categorical variables. The proportions of sex were compared between the three intervention types using chi-squared tests. The mean baseline age and BMI were compared between intervention types using one-way ANOVA with Tukey’s HSD post-hoc test to identify significant differences. The heterogeneity of relative and absolute change in BMI between studies was estimated with the I2 statistic assuming a random-effects model.(23) The I2 statistic describes the percentage of variation across studies that is due to heterogeneity rather than chance, with a value of 0% suggesting no variation due to heterogeneity and 100% suggesting all variation is due to heterogeneity between the studies. All data analyses were conducted using the R statistical platform (v3.4.3).(24)
Results
Demographic and clinical characteristics of the cohorts are presented in Table 1. Participants in MBS tended to be older, have a higher BMI, and longer follow-up duration than participants in lifestyle and pharmacotherapy (p<0.001 for all). All three treatment approaches predominantly involved females, with no significant sex distribution differences among the approaches (p=0.109). Risk factor levels were similar across groups.
Table 1.
Baseline descriptive and clinical characteristics of each cohort
| Measurement | Lifestyle | Pharmacotherapy | Metabolic and Bariatric Surgery |
|---|---|---|---|
| (N=53) | (N=40) | (N=78) | |
| Mean Follow-up Duration (baseline to last visit, days) | 144 (102.1) | 138 (61.4) | 370 (47.1) |
| Age (Years) | 15.2 (1.06) | 14.5 (1.88) | 17.3 (1.67) |
| Sex (n [%] Male) | 26 (49.1%) | 13 (32.5%) | 25 (32.1%) |
| BMI (kg/m2) | 39.2 (5.95) | 40.2 (5.99) | 55.8 (11.0) |
| Percent of 95th BMI Percentile | 143 (23.6) | 149 (20.6) | 195 (41.1)6 |
| Total Cholesterol (mg/dL) | 153 (30.4)8 | 154 (22.1) | 167 (32.5)65 |
| Triglycerides (mg/dL) | 141 (72.1)8 | 124 (82.8) | 105 (39.4)65 |
| HDL-Cholesterol (mg/dL) | 39.9 (7.44)8 | 38.6 (7.23) | 40.0 (8.59)65 |
| Non-HDL-Cholesterol (mg/dL) | 113 (30.0)8 | 115 (22.3) | 101 (49.9)65 |
| LDL-Cholesterol (mg/dL) | 84.9 (22.3)8 | 91.6 (18.2)1 | 108 (29.1)65 |
| Fasting Glucose (mg/dL) | 93.3 (7.73)11 | 79.7 (9.71) | 83.0 (14.6)65 |
| Fasting Insulin (U/L) | 31.8 (37.1)12 | 25.0 (17.0)1 | 22.3 (11.2)65 |
| HOMA-IR | 7.47 (9.05)12 | 5.02 (3.6)1 | 4.78 (2.87)65 |
| Systolic BP (mmHg) | 127 (14.7)8 | 122 (11.5) | 125 (13.0)3 |
| Diastolic BP (mmHg) | 73.8 (8.55)8 | 67.9 (9.66) | 73.5 (9.71)3 |
Values expressed are mean (SD) or N (%) where indicated.
Superscripts represent number of observations missing.
Change in BMI and cardiometabolic risk factors from baseline to last visit are presented in Table 2 (Additional data reporting median and ranges can be found in Table S1). Bariatric surgery resulted in the greatest weight loss (−33.8[9.4]% BMI change), followed by pharmacotherapy (−2.4[4.4]% BMI change) and LMT (−1.8[4.8]% BMI change). Despite overall reductions in BMI in each treatment group, substantial heterogeneity was observed (Figure 1, Panel A; I2=88.5% [95% CI: 72.6%, 97.2%]). The variability of BMI reduction was highest with MBS (range: −50.2% to −13.3%) while both lifestyle and pharmacotherapy had individuals who reduced as well as gained BMI, (range: −25.4% to 5.0% and range: −10.8% to 12.9%, respectively). Similar change and heterogeneity (Figure 1, Panel B) were observed when percent of the 95th BMI-percentile or absolute change in BMI (I2=90.4 [95% CI: 74.8%, 98.5%]) (Figure S1) were used as the primary outcome.
Table 2.
Mean absolute# and relative* change in clinical characteristics from baseline to last visit for each treatment. Values expressed are mean (SD).
| Measurement | Lifestyle Modification Therapy | Pharmacotherapy | Bariatric and Metabolic Surgery |
|---|---|---|---|
| (N=53) | (N=40) | (N=78) | |
| BMI | |||
| - Absolute change (kg/m2) | −0.65 (2.11) | −0.96 (1.71) | −19.1 (7.13) |
| - Relative change (%) | −1.77 (4.79) | −2.35 (4.38) | −33.8 (9.4) |
| Percent of 95th BMI Percentile | |||
| - Absolute change | −3.99 (7.24) | −5.21 (6.33) | −72.0 (25.5)13 |
| Total Cholesterol | |||
| - Absolute change (mg/dL) | −11.2 (18.3)9 | −0.69 (18.3)8 | −4.9 (30.9)68 |
| - Relative change (%) | −6.96 (11.1)9 | −0.25 (11.9)8 | −2.37 (16.5)68 |
| Triglycerides | |||
| - Absolute change (mg/dL) | −17.8 (60.0)9 | −18.5 (43.6)8 | 8.8 (89.9)68 |
| - Relative change (%) | −3.3 (35.8)9 | −4.57 (35.7)8 | 19.0 (77.3)68 |
| HDL-Cholesterol | |||
| - Absolute change (mg/dL) | −2.57 (5.59)9 | 2.36 (5.07)7 | 4.7 (12.4)68 |
| - Relative change (%) | −5.72 (13.8)9 | 7.44 (14.2)7 | 10.8 (27.4)68 |
| Non-HDL-Cholesterol | |||
| - Absolute change (mg/dL) | −7.58 (16.1)10 | −3.0 (17.1)8 | −10.5 (35.7)68 |
| - Relative change (%) | −5.66 (13.3)10 | −2.41 (15.6)8 | −7.6 (26.1)68 |
| LDL-Cholesterol | |||
| - Absolute change (mg/dL) | −3.7 (11.8)10 | −0.03 (14.0)8 | −14.3 (24.3)68 |
| - Relative change (%) | −3.76 (14.0)10 | −0.44 (16.3)8 | −11.8 (22.1)68 |
| Fasting Glucose (mg/dL) | |||
| - Absolute change (mg/dL) | −0.19 (5.66)13 | −2.15 (11.0)7 | −3.51 (12.9)68 |
| - Relative change (%) | 0.01 (6.06)13 | −1.9 (14.6)7 | −2.05 (17.0)68 |
| Fasting Insulin | |||
| - Absolute change (U/L) | −3.84 (10.9)14 | −2.25 (9.48)10 | −8.35 (11.6)69 |
| - Relative change (%) | −2.11 (38.4)14 | −7.24 (47.8)10 | −30.4 (60.1)69 |
| HOMA-IR | |||
| - Absolute change | −0.98 (2.86)14 | −0.55 (2.22)10 | −1.85 (2.78)69 |
| - Relative change (%) | −0.88 (41.4)14 | −7.73 (52.2)10 | −26.6 (70.4)69 |
| Systolic BP | |||
| - Absolute change (mmHg) | −0.75 (13.8)9 | −2.65 (10.5) | −13.5 (13.9)5 |
| - Relative change (%) | 0.3 (10.8)9 | −1.7 (8.38) | −10.1 (10.2)5 |
| Diastolic BP | |||
| - Absolute change (mmHg) | −2.53 (9.4)9 | −1.2 (7.83) | −7.6 (10.4)5 |
| - Relative change (%) | −2.43 (11.0)9 | −0.86 (11.7) | −9.03 (13.7)5 |
Absolute change determined as last visit value minus baseline visit value.
Percent change determined as last visit value minus baseline visit value divided by baseline multiplied by 100%.
Superscripts represent number of observations missing.
Figure 1.
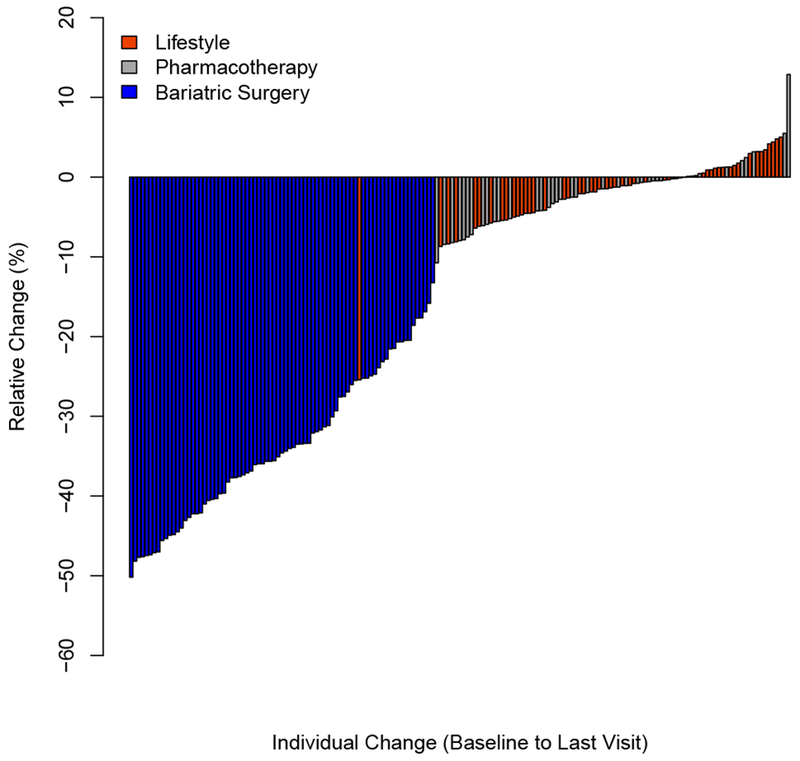
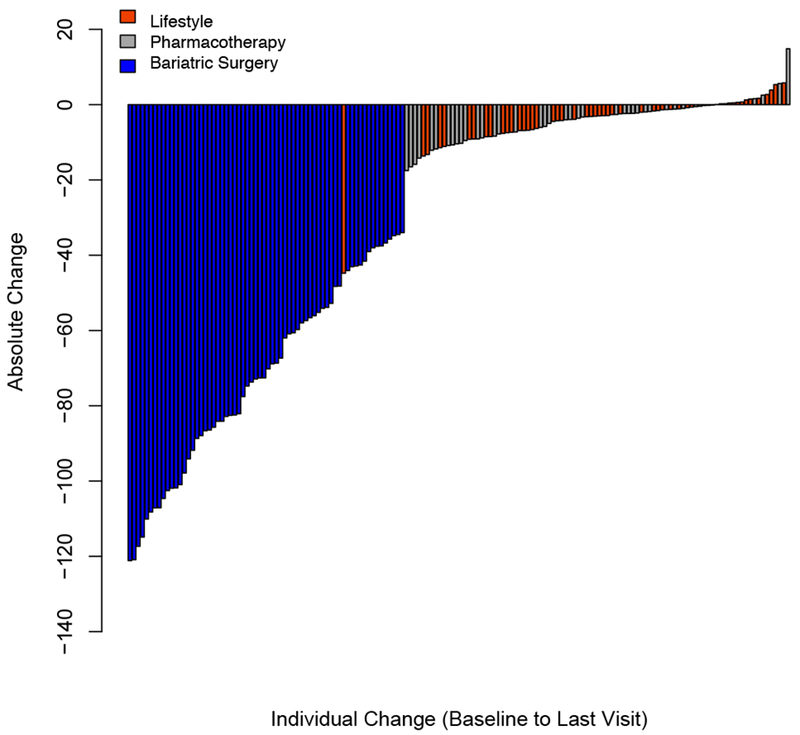
Relative (%) change in BMI (Panel A) and absolute change in percent of the 95th BMI percentile (Panel B) for each individual from baseline to last visit.
Changes in cardiometabolic risk factors exhibited even greater heterogeneity within and between interventions (Figure 2). Insulin resistance estimated by the homeostatic model assessment of insulin resistance (HOMA-IR) demonstrated a range of −85.7% to 159.1% (Figure 2, Panel A). The components which were used to calculate HOMA-IR, insulin (Figure S6) and glucose (Figure S7), displayed similar distributions and heterogeneity, with nearly equal distributions of individuals who experienced increases a decrease in concentrations of these analytes. Similar distributions were found for lipids including the atherogenic non-high-density lipoprotein (non-HDL) cholesterol (Figure 2, Panel B), HDL-cholesterol (Figure S2), LDL-cholesterol (Figure S3), total cholesterol (Figure S4), and triglycerides (Figure S5). Systolic blood pressure (SBP) represented the greatest relative reduction in the MBS group (−10.1% [10.2]). However, despite many individuals having significant reductions in SBP, several MBS participants (11 out of 73 increased) also had increases in SBP (Figure 2, Panel C). This contrast in outcomes is also well illustrated by individuals who underwent LMT (17 out of 44 increased) and pharmacotherapy (16 out of 40 increased) showing similar heterogeneity within groups for individuals with reductions and gains in SBP.
Figure 2.


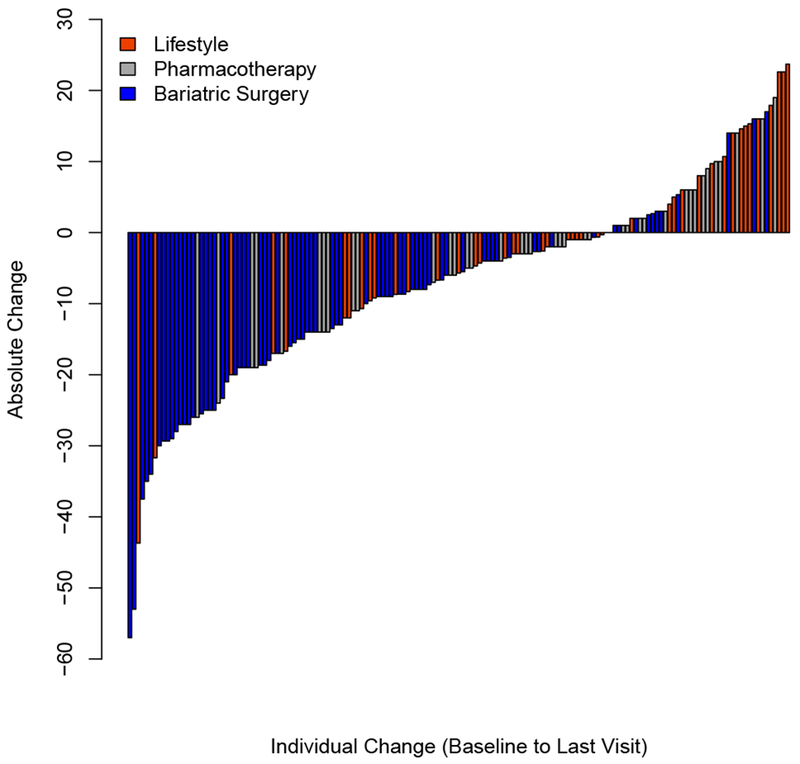
Absolute change in HOMA-IR (Panel A), non-HDL cholesterol (Panel B), and systolic blood pressure (Panel C) for each individual from baseline to last visit.
Discussion
The present study highlights wide variation in treatment responses among adolescents with severe obesity using the three most common approaches. Within each treatment (LMT, pharmacotherapy, and MBS) heterogeneous responses in weight loss and clinical variables were noted. Despite weight loss in every participant who underwent MBS, and despite the fact that the greatest mean reduction in BMI was seen following MBS, a high degree of heterogeneity in cardiometabolic risk factor changes was observed. By contrast, LMT and pharmacotherapy demonstrated significantly less mean weight loss compared to MBS yet a similar degree of variability in response with some participants gaining weight. Similar to MBS, LMT and pharmacotherapy demonstrated a high degree of heterogeneity in terms of cardiometabolic risk factor changes
Adult recommendations for weight loss suggest that 3–5% weight reduction may lead to clinically meaningful improvements in cardiometabolic risk factors and practice guidelines suggest an initial goal of 5–10% reduction from baseline weight over 6-months.(25) As such, results of clinical trials have traditionally reported mean reductions in body weight without much attention paid to variability in response. However, it is now becoming more common that in addition to mean changes, the proportion of persons achieving specified weight loss milestones (e.g. >5% and >10% weight-loss) are being reported as key outcomes. This way of reporting the outcome tends to capture the wide variability better than traditional descriptions of central tendency such as mean or median and is consistent with FDA decision-making regarding drug approval. For example, in a 52-week randomized control trial of liraglutide, a glucagon-like peptide-1 analogue, Pi-Sunyer and colleagues reported a mean weight loss of 8.4±7.3kg with 63.2% achieving >5% weight loss and 33.1% achieving 10% weight loss.(26) Thus, while heterogeneity was clearly present, the likelihood of achieving target goals of >5% and >10% was reported as a metric by which healthcare providers can categorically assess treatment response in individual patients against a published reference group for a particular treatment approach. However, a proportion of individuals fail to achieve clinically meaningful weight-loss and this means of outcome reporting does not fully capture the degree of heterogeneity observed. We strongly encourage future clinical trials, interventions and longitudinal studies to report individual data (e.g., individual trajectories and/or histogram figures), along with means, to help researchers and clinicians better understand the degree of variability in a given outcome variable.
In studies with large sample sizes, the variability in outcome might be as important as the overall effect observed. For instance, the HERITAGE family study demonstrated that for individuals who participated in a 20-week endurance exercise training program, changes in insulin sensitivity, exercise capacity, lipids, and blood pressure responses varied widely.(27, 28, 29, 30, 31) The power of this study was how their data were displayed. Rather than only reporting means, ranges, or target metrics, the authors displayed individual response on the abscissa to a given metric (health outcome) on the ordinate. When reporting on individual changes, this simple, yet powerful tool provides a graphic representation of the proportion of individuals who improve, get worse, or remain stable in response to treatment. We utilized a similar approach to present our findings. Our reported outcomes of HOMA-IR produce a similar S-shaped pattern of response that was observed in the HERITAGE family study using more robust measures of insulin sensitivity (intravenous glucose tolerance test).(27) The same pattern is observed in many other outcomes including lipids and blood pressure. Collectively, these findings support the notion that that not all individuals improve following intervention and that a proportion of individuals will decompensate over the course of the trial.
Data depicting heterogeneity in weight loss and BMI response in pediatric obesity trials are sparse, thus the impetus for this secondary analysis. Indeed, only reporting mean changes unnecessarily limits the interpretation of findings and can lend to misinterpreting the data and potentially erroneous conclusions being drawn. Data from Chanoine et al. in the largest trial evaluating orlistat in an adolescent population with obesity concluded that the addition of orlistat to lifestyle modification significantly improved 1 year weight loss as compared to placebo. While the mean BMI response compared to placebo supported their conclusion (mean BMI change = −0.55kg/m2 orlistat vs +0.31kg/m2 placebo, p=0.001), the mean weight loss responses (mean weight loss = +0.53kg orlistat vs +3.14kg, p<0.001) and percent achieving clinically meaningful weight loss (19% with >5% weight loss and 10% with >10% weight loss) support the conclusion that neither treatment is effective for supporting clinically meaningful weight loss in the majority (81%) of individuals. Moreover, only means without standard deviation were reported, creating further bias and limitation in utility for outcomes reporting.
The importance of reporting heterogeneity in clinical trials is not confined to obesity interventions. A proposal by Kent and colleagues offers a framework for assessing and reporting multivariate risk-based heterogeneity in treatment effects that can be applied to a wide variety of conditions.(32) Although this framework has yet to be applied widely,(33) there is increasing support for updating the conventional methods for evaluating treatment efficacy in order to appreciate the known heterogeneity in clinical trials.(34) The manner in which data are presented and interpreted are vital to understanding variability in response and may help differentiate statistically significant findings from clinically meaningful outcomes. Standard approaches to summarizing data, such as presenting only the mean and standard deviation, may obfuscate important clinical heterogeneity in individual response to a given treatment. There is increasing recognition that heterogeneity may be a biologically derived phenomenon which necessitates characterization of biological factors (e.g. genomics, metabolomics, microbiome) which is still in its infancy for evaluation in obesity trials.(13) Furthermore, in order to help expedite the process of transitioning from a “one-size-fits all” treatment approach towards a precision medicine based approach for obesity treatment and management, reporting of individual variation in treatment response using various biological factors will be essential.
Our study has many strengths including standardized collection of anthropometrics and cardiometabolic risk factors within each study pooled, use of random allocation to lifestyle and pharmacotherapy, and a large sample size focused exclusively of adolescent severe obesity. Limitations in the fact that the interventional time period was different for each cohort and we were unable to match for pubertal maturation; thus, this may have contributed to differences in changes in outcomes, particularly HOMA-IR. However, it should be noted that the purpose of this secondary analysis was simply to describe the treatment heterogeneity observed rather than conduct a comparative effectiveness evaluation. Since studies were conducted across different sites, possible differences in measurement techniques may have contributed to some of the heterogeneity observed. However, the fact that heterogeneity was observed for BMI measures, which are likely uniformly captured across studies supports the notion that treatment heterogeneity is operationally important. The retrospective nature of the study design does not allow us to infer the causality of the heterogeneity observed. Formal statistical testing for heterogeneity of all outcomes, both at baseline and change over treatment, were considered using Cochran’s Q and I2 statistics based on meta-analyses for pooled estimates. However, the small number of studies and available outcome data for each study makes estimation of these parameters challenging, so only the changes in BMI, which had no missing observations, were determined. Future summaries comparing more studies will enable formal comparisons of heterogeneity. Lastly, our study was limited to more traditional clinical measures across all studies; future studies should explore novel or nontraditional measures and body composition.
Conclusion.
Treatment of adolescent severe obesity results in significant heterogeneity in response. This individual variation in response is observed within and across lifestyle, pharmacotherapy, and metabolic and bariatric surgery but is often not captured in most studies that only report mean changes as the primary analysis of outcomes. In order for the field of pediatric obesity medicine to improve treatment outcomes, it will be essential to begin characterizing and understanding factors responsible for this heterogeneity to pave the way for precision medicine approaches to treatment. It is therefore our recommendation that future studies report individual data, along with means, in order for the field to better understand the degree of individual variation and heterogeneity in response to a given outcome of interest. We further recommend that, when possible, authors attempt to identify predictors of treatment response in order to advance work in the area of precision obesity medicine.
Supplementary Material
What is already known about this subject?
Treatment of adolescent severe obesity is challenging
Heterogeneity in treatment response is often present in terms of both weight/BMI and risk factor response
What does this study add?
We describe and present the degree of heterogeneity in treatment response among the primary interventions for pediatric severe obesity
Variation in treatment response is significant within and between differing treatment modalities
Understanding the heterogeneity in treatment response will be important for future studies to improve outcomes
Reporting individual responses, along with mean data, will be important in advancing our knowledge of treatment response
Acknowledgements
We would like to thank all of the children who participated in these studies. Funding for the studies were provided by the following: Minnesota Obesity Center (NIH grant P30DK050456 NORC) and GCRC (M01-RR00400, General Clinical Research Center Program, NCRR/NIH); Community Health Collaborative grant from the University of Minnesota Clinical and Translational Science Institute, by award 1UL1RR033183 from the National Center for Research Resources, and by grant 8UL1TR000114-02 from the National Center for Advancing Translational Sciences of the National Institutes of Health; Vikings Children’s Fund; the National Center for Advancing Translational Sciences (Award Number UL1TR000114); an individual training grant from the NIH/NHLBI (F32HL127851); National Institute of Diabetes and Digestive and Kidney Diseases (R01DK105953); National Institutes of Health/National Institute on Minority Health and Health Disparities (P20MD002316 and U54MD002316). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Disclosures: Dr. Ryder receives support from Boehringer Ingelheim Pharmaceuticals in the form of drug/placebo. Dr. Inge has received bariatric research grant funding from Ethicon Endosurgery, and has served as consultant for Sanofi Corporation, NPS Pharma, and Up To Date, and Independent Medical Expert Consulting Services, all unrelated to this project. Dr. Kelly receives research support from Astra Zeneca Pharmaceuticals in the form of drug/placebo and serves as a consultant for Takeda Pharmaceuticals, Orexigen Pharmaceutical, and Novo Nordisk Pharmaceuticals but does not accept personal or professional income for these activities.
Bibliography
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999-2016. Pediatrics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. New England Journal of Medicine 2015;373: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 3.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J , et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 4.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. Jama 2018;319: 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryder JR, Fox CK, Kelly AS. Treatment Options for Severe Obesity in the Pediatric Population: Current Limitations and Future Opportunities. Obesity (Silver Spring) 2018;26: 951–960. [DOI] [PubMed] [Google Scholar]
- 6.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. Journal of the American Dietetic Association 2007;107: 1755–1767. [DOI] [PubMed] [Google Scholar]
- 7.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: Current state of the field, review of the literature, and clinical trial considerations. Int J Obes (Lond) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Treatment of Pediatric Obesity: A Systematic Review and Meta-Analysis of Randomized Trials. The Journal of Clinical Endocrinology & Metabolism 2008;93: 4600–4605. [DOI] [PubMed] [Google Scholar]
- 9.Brownell KD, Wadden TA. The heterogeneity of obesity: fitting treatments to individuals. Behavior Therapy 1991;22: 153–177. [DOI] [PubMed] [Google Scholar]
- 10.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. American journal of physiology Regulatory, integrative and comparative physiology 2011;301: R581–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond) 2015;39: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. New England Journal of Medicine 2017;376: 254–266. [DOI] [PubMed] [Google Scholar]
- 13.Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA 2018;319: 223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly AS, Marcus MD, Yanovski JA, Yanovski SZ, Osganian SK. Working toward precision medicine approaches to treat severe obesity in adolescents: report of an NIH workshop. Int J Obes (Lond) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum M, Agurs-Collins T, Bray MS, Hall KD, Hopkins M, Laughlin M, et al. Accumulating Data to Optimally Predict Obesity Treatment (ADOPT): Recommendations from the Biological Domain. Obesity (Silver Spring) 2018;26 Suppl 2: S25–s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics 2012;130: 1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soltero EGOM, Williams AN, Konopken YP, Castro FG, Arcoleo KJ, Keller CS, Patrick DL, Ayers SL, Barraza E, Shaibi GQ. Effects of a Community-Based Diabetes Prevention Program for Latino Youth with Obesity: A randomized controlled trial. . Obesity 2018;in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryder JR, Gross AC, Fox CK, Kaizer AM, Rudser KD, Jenkins TM, et al. Factors associated with long-term weight-loss maintenance following bariatric surgery in adolescents with severe obesity. Int J Obes (Lond) 2018;42: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA pediatrics 2013;167: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012;20: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox CK, Kaizer AM, Rudser KD, Nathan BM, Gross AC, Sunni M, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity (Silver Spring) 2016;24: 2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. The lancet Diabetes & endocrinology 2017;5: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing,. 2015. [Google Scholar]
- 25.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society 2014;63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 26.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New England Journal of Medicine 2015;373: 11–22. [DOI] [PubMed] [Google Scholar]
- 27.Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, et al. Effects of Exercise Training on Glucose Homeostasis. The HERITAGE Family Study 2005;28: 108–114. [DOI] [PubMed] [Google Scholar]
- 28.Skinner JS, Jaskolski A, Jaskolska A, Krasnoff J, Gagnon J, Leon AS, et al. Age, sex, race, initial fitness, and response to training: the HERITAGE Family Study. Journal of applied physiology (Bethesda, Md : 1985) 2001;90: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 29.Rice T, An P, Gagnon J, Leon AS, Skinner JS, Wilmore JH, et al. Heritability of HR and BP response to exercise training in the HERITAGE Family Study. Med Sci Sports Exerc 2002;34: 972–979. [DOI] [PubMed] [Google Scholar]
- 30.Green JS, Stanforth PR, Rankinen T, Leon AS, Rao Dc D, Skinner JS, et al. The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: the HERITAGE family study. Metabolism 2004;53: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 31.An P, Borecki IB, Rankinen T, Perusse L, Leon AS, Skinner JS, et al. Evidence of major genes for exercise heart rate and blood pressure at baseline and in response to 20 weeks of endurance training: the HERITAGE family study. Int J Sports Med 2003;24: 492–498. [DOI] [PubMed] [Google Scholar]
- 32.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabler NB, Duan N, Raneses E, Suttner L, Ciarametaro M, Cooney E, et al. No improvement in the reporting of clinical trial subgroup effects in high-impact general medical journals. Trials 2016;17: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Embracing patient heterogeneity. Nature Medicine 2014;20: 689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


