Abstract
Objective:
Examine if 1 month BMI improvement is predictive of superior 6 and 12 month BMI changes in a national sample of youth in pediatric weight management (PWM) treatment.
Methods:
Participants were 4–18 year olds from the Pediatric Obesity Weight Evaluation Registry (POWER), a prospective study collecting data from 31 PWM programs across the U.S. Response at 1 month was defined as ≥3% BMI reduction; success at 6 and 12 months was defined as ≥5% BMI reduction from baseline. Analyses used linear and logistic regression with robust variance estimation.
Results:
Primary analyses were completed with 687 participants (mean age 12.2 years). One- month responders demonstrated significant improvements in BMI compared to non-responders at 6 months (BMI: −2.05 vs. 0.05; %BMI: −5.81 vs. 0.23; p<0.001 for all) and 12 months (BMI: - 1.87 vs. 0.30; %BMI: −5.04 vs. 1.06; p<0.001 for all). The odds of success for 1 month responders were 9.64 (95% CI: [5.85, 15.87]; p<0.001) times that of non-responders at 6 months and 5.24 ([2.49, 11.02]; p<0.001) times that of non-responders at 12 months.
Conclusions:
In treatment-seeking youth with obesity, early BMI reduction was significantly associated with greater long-term BMI reduction. Non-responders may benefit from early treatment redirection or intensification.
Keywords: pediatrics, obesity, weight management
Pediatric obesity is a significant public health concern that affects approximately 17% of children and adolescents in the United States.1 Current treatment guidelines for youth with obesity advise slow, gradual reductions in weight status using a staged approach, whereby a child may participate in an intervention for 3–6 months prior to changing course, even if unsuccessful.2 At present, there are limited pediatric data that can be used as evidence to support or refute the 3–6 month trial period before redirecting or intensifying interventions. This staged approach to delivery of more intensive therapy may limit the efficacy of anthropometric outcomes of youth in obesity treatment, particularly among those with severe obesity.3,4
In adults with obesity, weight loss early in treatment is associated with greater total weight loss and long-term weight loss maintenance.5 Furthermore, some studies have identified the benefit of providing “rescue” efforts (e.g., treatment change or intensification) to non-responders early in the course of intervention. Delivery of early rescue interventions has resulted in better weight loss outcomes, particularly when non-responders are identified within 3 months of treatment initiation.6 In adults, the timing and amount of weight loss that defines “early response” varies across studies. There is a need to identify the optimal timeline for weight loss, as this would allow clinicians to make appropriate, strategic alterations in treatment that could lead to increased early weight loss, thereby promoting greater and more sustained weight loss long term.6
There is some evidence in the pediatric population to suggest that early BMI reduction and dietary changes predict greater future BMI reduction and maintenance.7–13 However, much of the available data has come from smaller sample sizes or from highly prescriptive treatment regimens, limiting the generalizability of the findings. The goal of the current study was to examine the extent to which early improvement in BMI at 1 month is predictive of superior 6 and 12 month BMI changes in a nationally representative sample of youth participating in multi-component pediatric weight management (PWM) treatment.
Methods
Participants
This project was based on data from the Pediatric Obesity Weight Evaluation Registry (POWER) (ClinicalTrials.gov NCT02121132), a prospective study collecting longitudinal data from a network of 31 multi-component PWM programs across the United States. The purpose of POWER is to identify participant and program characteristics associated with favorable outcomes.14 For inclusion in POWER, programs must provide multicomponent PWM treatment for youth with obesity, and collect the required data elements. These data elements included demographic characteristics, such as race, ethnicity, and age, as well as height and weight from each visit. Sites were responsible for obtaining institution-specific IRB approval as well as informed consent and assent. The inclusion and exclusion criteria for individual participant enrollment were previously described.14 The present study utilized data from POWER participants who were age 4–18 years.
Procedures
Data for this study were not collected systematically at specific time points because intervals of data collection were dependent upon each POWER site’s own clinical protocols. Therefore, measurements at 1, 6, and 12 months (30, 182, and 365 days) were derived algorithmically from the longitudinal records within 14-, 60-, and 90- day windows, respectively, or via interpolation which is described in greater detail below. At 1 month, the closest visit within a 14-day window was used, or, if two visits were equidistant within the window for a given participant (e.g., 26 and 34 days), the average of the two measurements was used. For the 6 and 12 month visits, participant measurements were determined in a two-step algorithm. First, if visits were recorded within 6 months before and after the 182 and 365-day time points, measurements were interpolated for their predicted value at 6 and 12 months (e.g., for the 6 month visit, if there were visits at 95 and 250 days the measurement at 182 was interpolated from these two visits). If a visit did not meet the criteria for interpolation, but the participant had at least one record within the 60- or 90-day window (i.e., 122 to 241 days for 6 months or 275 to 455 days for 12 months) that record was used for the 6 or 12 month visit.
In the primary analyses, early response at 1 month was defined as ≥3% BMI reduction, which was a cut-off selected by the investigators as a clinically reasonable BMI reduction in the time frame. Success at 6 and 12 months was defined as ≥5% BMI reduction from baseline. This definition of success was based on the Food and Drug Administration suggestions for endpoints of efficacy in clinical trials15. Any participant with a 1 month absolute change in BMI greater than 15% was excluded, as this was considered implausible and could not be verified with the available data.
Statistical Analyses
Summary characteristics were tabulated with respect to 1 month responder status using mean (SD) for continuous variables and frequency (%) for categorical variables. Analyses were based on linear and logistic regression with robust variance estimation, adjusting for age, sex, race (white vs. other), ethnicity, and baseline BMI. An exploratory analysis determined whether increasing or decreasing the definition of response at 1 month affected the prediction of success at 6 and 12 months. Classification criteria, such as sensitivity and specificity, were calculated using the epiR package16 with exact binomial confidence limits to determine the 95% confidence intervals. Similar methods were applied in an additional analysis to determine whether 1 month response influenced rate of return for follow-up since attrition was observed in the cohort. All analyses were conducted in R v3.4.3.17
Results
Participant characteristics
Data from 687 participants were included in the primary analyses. These participants came from an initial sample of 6183 unique participants with at least one visit recorded in the POWER dataset. Of those, 1506 were in the appropriate age range and had data entered at baseline and 1 month (±14 days). From the 1506 participants, 687 had data entered at 6 months, 12 months or both. There were 401 participants with 6 but not 12 month data; 7 participants with 12 but not 6 month data; and 279 participants with both 6 and 12 month data. For the additional analysis to examine the influence of 1 month response on the rate of return, the 1506 participants with baseline and 1 month data were included.
For the study cohort of 687 participants used in the primary analyses, the mean age was 12.2 (SD=3.2) years and 45.1% were male. Please see Table 1 for additional demographic data.
Table 1.
Participant characteristics. Participants were included in the analysis if they met the criteria of having sufficient baseline and follow-up visit data. The table presents the data for the overall cohort, participants who did and did not lose ≥3% BMI at the 1 month window. The values are expressed are mean (SD) or N (%) where indicated.
| Participant Characteristic | Overall Cohort (N=687) |
Lost ≥3% BMI at 1 month Window (N=109) |
Lost <3% BMI at 1 month Window (N=578) |
|---|---|---|---|
| Male | 310 (45.1%) | 50 (45.9%) | 260 (45.0%) |
| Age at Baseline (Years) | 12.2 (3.24) | 12.6 (3.27) | 12.1 (3.23) |
| Age Group at Baseline: | |||
| 4 to 8 | 63 (9.2%) | 7 (6.4%) | 56 (9.7%) |
| 8 to 12 | 287 (41.8%) | 46 (42.2%) | 241 (41.7%) |
| 12 to 16 | 233 (33.9%) | 35 (32.1%) | 198 (34.3%) |
| 16+ | 104 (15.1%) | 21 (19.3%) | 83 (14.4%) |
| Height (cm) | 153 (15.8) | 153 (16.6) | 154 (15.7) |
| Weight (kg) | 81.5 (32.4) | 82.3 (32.2) | 81.4 (32.4) |
| BMI | 33.4 (8.37) | 33.9 (8.01) | 33.3 (8.44) |
| % of the 95th BMI Percentile | 135 (27.1) | 135 (25.6) | 135 (27.4) |
| Race: | |||
| Black/African American | 97 (14.1%) | 9 (8.3%) | 88 (15.2%) |
| White | 406 (59.1%) | 89 (81.7%) | 317 (54.8%) |
| Other/Mixed | 97 (14.1%) | 3 (2.8%) | 94 (16.3%) |
| Unknown/Not Reported | 87 (12.7%) | 8 (7.3%) | 79 (13.7%) |
| Hispanic | 209 (30.4%) | 33 (30.3%) | 176 (30.4%) |
| Missing Hispanic | 71 (10.3%) | 11 (10.1%) | 60 (10.4%) |
| Insurance: | |||
| Unknown | 63 (9.2%) | 9 (8.3%) | 54 (9.3%) |
| Public/Medicaid | 392 (57.1%) | 67 (61.5%) | 325 (56.2%) |
| Private | 226 (32.9%) | 33 (30.3%) | 193 (33.4%) |
| Self-Pay/Other/None | 6 (0.9%) | 0 (0.0%) | 6 (1.0%) |
BMI change at 1 month
At 1 month, 109 (15.9%) achieved an early response to weight management services of ≥3% BMI reduction. Responders had a mean absolute change in BMI of −1.62 (SD=0.73) kg/m2 compared to 0.03 (SD=0.63) among non-responders at 1 month (Table 2).
Table 2.
Change in BMI values for the study cohort at 1, 6, and 12 months. The table shows the unadjusted data for the overall cohort, participants who did and did not lose ≥3% BMI at 1 month. Data are presented as the changes in absolute BMI, the percent of BMI, the percent of the 95th percentile for BMI, and the BMI z-score.
| Change in BMI | Overall Cohort (N=687) |
Lost >3% BMI at 1 month Window (N=109) |
Lost <3% BMI at 1 month Window (N=578) |
|---|---|---|---|
| Absolute BMI (kg/m2): | |||
| Baseline to 1 month | −0.23 (0.88) | −1.62 (0.73) | 0.03 (0.63) |
| Baseline to 6 months | −0.28 (1.77) a | −2.05 (2.02) | 0.05 (1.51) |
| Baseline to 12 months | −0.06 (2.49) b | −1.87 (3.06) | 0.3 (2.21) |
| Percent of BMI (%): | |||
| Baseline to 1 month | −0.65 (2.62) | −4.78 (1.82) | 0.13 (1.92) |
| Baseline to 6 months | −0.73 (5.18) a | −5.81 (5.17) | 0.23 (4.59) |
| Baseline to 12 months | 0.06 (7.11) b | −5.04 (7.42) | 1.06 (6.61) |
| Percent of 95th BMI Percentile (%): | |||
| Baseline to 1 month | −1.37 (3.55) | −6.92 (2.7) | −0.32 (2.58) |
| Baseline to 6 months | −3.42 (6.97) a | −10.3 (7.81) | −2.11 (5.96) |
| Baseline to 12 months | −4.03 (9.37) b | −11.0 (11.1) | −2.67 (8.37) |
| BMI Z-score Change: | |||
| Baseline to 1 month | −1.0 (2.61) | −5.13 (1.81) | −0.22 (1.92) |
| Baseline to 6 months | −0.07 (0.14) a | -0.18 (0.15) | -0.05 (0.13) |
| Baseline to 12 months | −0.1 (0.21) b | -0.21 (0.22) | −0.08 (0.2) |
N=680 in the overall cohort at 6 months,
N=286 in the overall cohort at 12 months
BMI change at 6 months and 12 months
Figure 1 shows scatterplots of the change in BMI at 6 months based on categories of BMI change at 1 month. In each category of 1 month BMI change, there was a wide distribution of values for 6 month BMI change. Increasing and maintaining BMI at 1 month corresponded to an approximate mean increase and maintenance of BMI at 6 months, respectively. That is, if BMI increased by 1% or more at 1 month, the mean increase at 6 months was 2.7% (SD=4.2); if maintained at 1 month, the mean change at 6 months was +0.1% (SD=4.1). The mean decrease in BMI at 6 months was increasingly more pronounced in groups with increasingly greater BMI decrease at 1 month, with 5+% BMI reduction at 1 month corresponding with 8.0% (SD=5.8) mean reduction at 6 months.
Figure 1. Percent change in BMI at 6 months by groups of percent BMI change at 1 month.
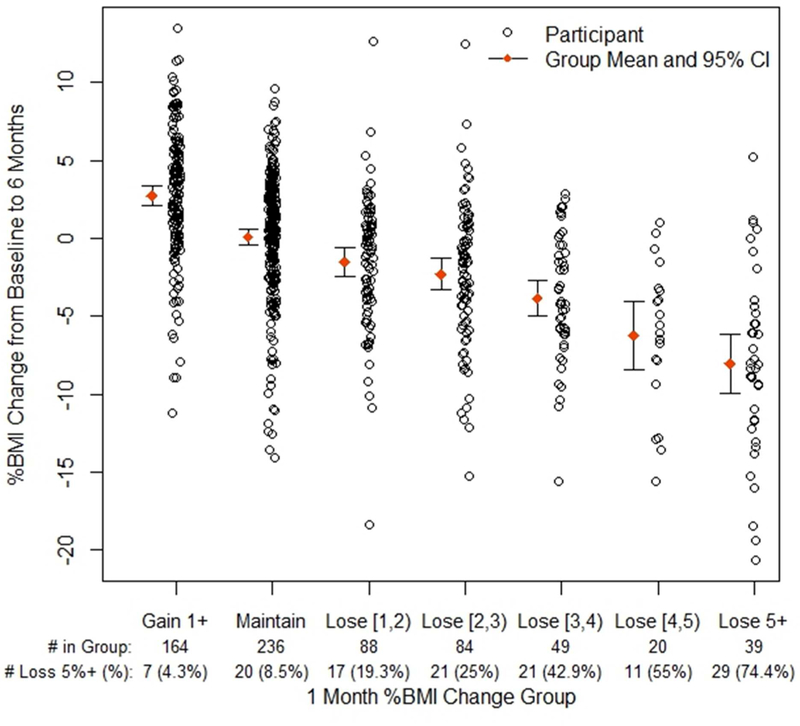
Figure Legend 1. # in Group = 1 month BMI category participants. # Loss 5%+ = number per group who lost ≥5% BMI at 6 months. Maintain indicates %BMI change between −1% and 1%. Loss indicates range, e.g., [1,2) indicates %BMI decrease 1% up to 2% (square bracket is inclusive, parenthesis is not).
Similar to the 6 month data, increasing and maintaining BMI at 1 month appeared to correspond to an analogous mean increase and maintenance of BMI, respectively, at 12 months (Figure 2). Reducing 2% to 4% of BMI at 1 month resulted in BMI reduction at 12 months, while losing 4% or more resulted in an apparently greater 12 month BMI reduction. (For additional information about changes in BMI from baseline to 6 months or 12 months see Table 2).
Figure 2. Percent change in BMI at 12 months by groups of percent BMI change at 1 month.
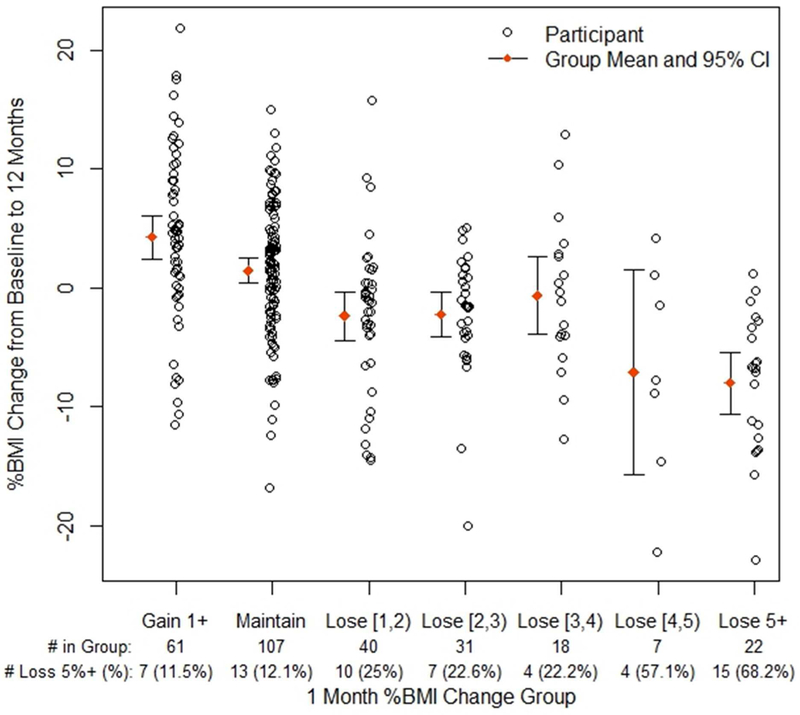
Figure Legend 2. # in Group = 1 month BMI category participants. # Loss 5%+ = number per group who lost ≥5% BMI at 12 months. Maintain indicates %BMI change between −1% and 1%. Loss indicates range, e.g., [1,2) indicates %BMI decrease 1% up to 2% (square bracket is inclusive, parenthesis is not).
The mean change in BMI at 6 and 12 months for responders (> 3% BMI reduction at 1 month) was higher than that of non-responders (< 3% BMI reduction at one month) (Table 3). Results were significant with and without adjustment for age, sex, race (white vs. other), ethnicity, and baseline BMI. The change in BMI was evaluated as change in absolute mean BMI (BMI), percent BMI (%BMI), percent of the BMI 95th percentile (%BMIp95), and BMI z-score (zBMI). Responders at 1 month demonstrated significant improvements in BMI compared to non-responders at 6 months (BMI: −2.05 vs. 0.05; %BMI: −5.81 vs. 0.23; %BMIp95: −10.34 vs. - 2.11; zBMI −0.18 vs. −0.05; p<0.001 for all) and 12 months (BMI: −1.87 vs. 0.30; %BMI: −5.04 vs. 1.06; %BMIp95: −10.97 vs. −2.67; zBMI: −0.21 vs. −0.08; p<0.001 for all).
Table 3.
BMI-related outcomes at 6 and 12 months for responders at 1 month (at least 3% BMI reduction) vs. non-responders at 1 month (less than a 3% BMI reduction) adjusted for age, sex, race (Caucasian vs. other), ethnicity, and baseline BMI with robust variance estimation. At 6 months: responder n=97, non-responder n=512 (total n=609); at 12 months: responder n=44, non-responder n=218, total n=262). Results are represented as the absolute mean (standard deviation, SD) BMI change (kg/m2), the percent BMI change (%), 95th BMI percentile change (%), and the BMI z-score change. R=responder, NonR=non-responder, CI=confidence interval.
| Outcome | Responder Mean (SD) | NonResponder Mean (SD) | Adjusted Mean Difference [R-NonR] (95% CI) | p-value |
|---|---|---|---|---|
| BMI from Baseline to 6 months | −2.05 (2.02) | 0.05 (1.51) | −2.03 (−2.44,−1.62) | <0.001 |
| BMI from Baseline to 12 months | −1.87 (3.06) | 0.30 (2.21) | −2.09 (−2.99,−1.19) | <0.001 |
| Percent BMI from Baseline to 6 months | −5.81 (5.17) | 0.23 (4.59) | −5.84 (−6.94,−4.74) | <0.001 |
| Percent BMI from Baseline to 12 months | −5.04 (7.42) | 1.06 (6.61) | −5.97 (−8.32,−3.62) | <0.001 |
| 95th BMI Percentile from Baseline to 6 months | −10.34 (7.81) | −2.11 (5.96) | −8.08 (−9.67,−6.50) | <0.001 |
| 95th BMI Percentile from Baseline to 12 months | −10.97 (11.08) | −2.67 (8.37) | −8.26 (−11.58,−4.94) | <0.001 |
| BMI Z-score from Baseline to 6 months | −0.18 (0.15) | −0.05 (0.13) | −0.13 (−0.16,−0.10) | <0.001 |
| BMI Z-score from Baseline to 12 months | −0.21 (0.22) | −0.08 (0.20) | −0.14 (−0.21,−0.07) | <0.001 |
Likelihood of success at 6 and 12 months based on response of ≥3% BMI reduction
Odds ratios were calculated to represent likelihood of success at 6 and 12 months, in responders vs non-responders, with adjustment for age, sex, race (white vs. other), ethnicity, and baseline BMI. At 6 months, the odds of success for responders were 9.64 times that of nonresponders while holding all other variables in the model constant (Table 4; 95% CI: 5.85, 15.87; p<0.001). At 12 months, the odds of success for responders at 1 month were 5.24 times (95% CI 2.49, 11.02; p<0.001) that of non-responders while holding all other variables in the model constant.
Table 4.
Odds ratios of success at 6 and 12 months for responders at 1 month in reference to nonresponders at 1 month (based on 3% BMI reduction) adjusted for age, sex, race (Caucasian vs. other), ethnicity, and baseline BMI with robust variance estimation.
| Outcome | Responder at 1 month % |
Nonresponder at 1 month % |
Odds Ratio [Responders=1] (95% CI) |
p- value |
|---|---|---|---|---|
| Successful at 6 months | 56.5% | 11.4% | 9.64 (5.85,15.87) | <0.001 |
| Successful at 12 months | 48.9% | 15.5% | 5.24 (2.49,11.02) | <0.001 |
Prediction models
The results outlined above are based on using a cut-off of ≥3% reduction in BMI at 1 month, which was chosen as a clinically reasonable BMI reduction. However, as observed in Figure 1 and 2, there were participants who were successful at 6 and 12 months despite being non-responders based on a ≥3% cut-off. Therefore, we explored how cut-off values above or below 3% may affect the prediction of success (Table 5). Prevalence of success was defined as the percentage of individuals with ≥5% reduction in BMI from baseline at the 6 and 12 month time points. Sensitivity and specificity, as well as positive and negative predictive values, were calculated. Sensitivity and specificity provides the probability of a test (e.g., 3% loss at 1 month) being positive or negative when the condition (i.e., ≥5% later weight reduction) is present or absent, respectively. Positive and negative predictive values indicate the probability of achieving ≥5% BMI reduction when there is at least X% (i.e., 2, 3, 4 or 5) reduction at 1 month or not, respectively. Overall accuracy is the proportion of all tests that give a “correct” result.
Table 5.
Selected 1 month % BMI reduction cut-offs for early weight loss as predictors of ≥5% BMI reduction at 6 month visit and at 12 -month visits. Data are presented with the 95% CI provided in parentheses.
| A. Prediction of BMI reduction at 6 months | ||||||
| Cut-off values for losing X% BMI at 1 month | Prevalence (%)of 5% reduction | Sensitivity (%) for 5% reduction | Specificity (%) for 5% reduction | Positive predictive value (%) for X% reduction at 1 month | Negative predictive value (%) for X% reduction at 1 month | Overall accuracy of the test % |
| ≥2% | 65.1 (56.1,73.4) | 80.1 (76.6,83.4) | 42.7 (35.6,50.0) | 91.0 (88.1,93.4) | 77.4 (74.0,80.4) | |
| ≥3% | 18.5 | 48.4 (39.4,57.5) | 91.5 (88.9,93.7) | 56.5 (46.6,66.0) | 88.6 (85.7,91.1) | 83.5 (80.5,86.2) |
| ≥4% | (15.7,21.7) | 31.7 (23.7,40.6) | 96.6 (94.7,97.9) | 67.8 (54.4,79.4) | 86.2 (83.2,88.8) | 84.6 (81.6,87.2) |
| ≥5% | 23.0 (16.0,31.4) | 98.2 (96.7,99.1) | 74.4 (57.9,87.0) | 84.9 (81.9,87.6) | 84.3 (81.3,86.9) | |
| B. Prediction of BMI reduction at 12 months | ||||||
| Cut-off values for losing X% BMI at 1 month | Prevalence (%) of 5% reduction | Sensitivity (%) for 5% reduction | Specificity (%) for 5% reduction | Positive predictive value (%) for X% reduction at 1 month | Negative predictive value (%) for X% reduction at 1 month | Overall accuracy of the test % |
| ≥2% | 50.0 (36.8,63.2) | 78.8 (72.8,83.9) | 38.5 (27.7,50.2) | 85.6 (80.1,90.1) | 72.7 (67.2,77.8) | |
| ≥3% | 21.0 | 38.3 (26.1,51.8) | 89.4 (84.6,93.1) | 48.9 (34.1,63.9) | 84.5 (79.3,88.9) | 78.7 (73.5,83.3) |
| ≥4% | (16.4,26.2) | 31.7 (20.3,45.0) | 95.6 (92.0,97.9) | 65.5 (45.7,82.1) | 84.0 (79.0,88.3) | 82.2 (77.2,86.4) |
| ≥5% | 25.0 (14.7,37.9) | 96.9 (93.7,98.7) | 68.2 (45.1,86.1) | 83.0 (77.9,87.3) | 81.8 (76.9,86.1) | |
For 6 month data, as the cut-off criteria increased from ≥2% to ≥5%, sensitivity of response at 1 month decreased and specificity increased. The maximum overall accuracy observed was 84.6% with ≥4% BMI decrease at 1 month.
Similarly, for 12 month data, sensitivity of response at 1 month decreased and specificity increased as the cut-off criteria involved greater % BMI decrease. The maximum overall accuracy was observed at 82.2% with ≥4% BMI decrease at 1 month.
Return rates for visits after 1 month in responders and non-responders
We also explored whether response at 1 month might influence the rate of return for future visits. Table 6 shows the rates of return any time after a 1 month visit for responders (73%, n=211) and non-responders (67.3%, n=1295). The odds ratio based on response at 1 month for returning for at least 1 visit after that time was determined and adjusted for age, sex, race (white vs. other), ethnicity, and baseline BMI. There was no significant difference in return rates between responders and non-responders.
Table 6.
Odds ratios for returning after 1 month visit for at least one visit based on responder status at 1 month with 3% BMI reduction cut-off. Models for “unadjusted” and adjusted for age, sex, race (Caucasian vs. other), ethnicity, and baseline BMI analyses are presented with robust variance estimation. Responders n=211, non-responders n=1295 (total n=1506). There was no significant difference in return rates between responders and non-responders.
| Model | Outcome | 1 month Responder Return % |
1 month Nonresponder Return % |
Odds Ratio [Responders=1] (95% CI) |
p- value |
|---|---|---|---|---|---|
| Unadjusted | Return after 1 month visit | 73.0% | 67.3% | 1.31 (0.95,1.81) | 0.103 |
| Adjusted | Return after 1 month visit | 73.0% | 67.3% | 1.28 (0.90,1.83) | 0.167 |
Discussion
The findings from this study support that early (1 month) treatment response is significantly and strongly associated with greater long-term (6 and 12 month) BMI improvement among a large national sample of youth treated in PWM. In particular, participants who achieved ≥3% BMI reduction at 1 month, compared to those who did not achieve this benchmark, had over 9 times greater odds of achieving ≥5% BMI reduction at 6 months, and over 5 times greater odds of achieving ≥5% BMI reduction at 12 months. These data are consistent with findings from adult samples, and raise questions about current pediatric obesity guidelines that recommend waiting 3–6 months to assess response to initial interventions before considering redirecting or intensifying treatment.
In the current study, only 16% of the sample met the responder status of ≥3% BMI reduction at 1 month. When compared to several other studies examining outcomes of lifestyle modification therapy for youth with severe obesity, mean BMI reduction in this group was similar.3,18 It is unclear what combination of individual (e.g., bio-psycho-social) or treatment factors contributed to the early success of the small subset of early responders. Given the retrospective nature of this analysis and the limited availability of individual-level information in this registry, in-depth analyses regarding these factors was not possible. Future studies of contributors to early response are indicated, as these may elucidate individual or treatment factors that are important for early response, and could promote longer-term BMI reduction.
Unfortunately, there is no consensus on a standard time frame or amount of BMI reduction that clinicians should use to make treatment-altering decisions. In the current study, an a priori decision was made to define early response as ≥3% BMI reduction at 1 month. Ideally, pediatric obesity providers would have clearer guidance on when to make intervention changes, and with whom. As a step toward identifying these standards for the treatment of pediatric obesity, predictive assessments using various 1 month weight loss cut-points were evaluated with the current data. From these results, specificity and negative prediction were higher than sensitivity or positive prediction, suggesting that non-response at 1 month was highly predictive of later non-response. This negative predictive value may be due to the high prevalence of nonresponders. Practically, this indicates that non-responders generally do not change course, and instead, continue to struggle with BMI reduction. This suggests that the non-responders may require either an increase in treatment intensity or a change in modality (e.g., pharmacotherapy, alteration of macronutrient content of dietary regimen, adjunctive behavioral health intervention) to achieve meaningful BMI reduction. Thus, waiting 3–6 months as recommended by current pediatric obesity guidelines, may contribute to sub-optimal outcomes, particularly among youth with severe obesity.
Attrition is often a concern with PWM and it is reasonable to consider treatment response as a possible factor contributing to PWM adherence. However, the data from the present study suggests that early response to intervention is not associated with attrition from weight management intervention. Over two thirds of each group, responders and non-responders, returned at least once following the 1 month time point.
This study has many strengths, including a large sample size that was diverse with respect to gender, race, and ethnicity. Furthermore, there was participation from numerous PWM centers across the United States, enhancing the generalizability of the findings. However, the lack of standardized data collection methods in POWER, as well as of the PWM interventions themselves, limits the reproducibility of this study’s outcomes. Furthermore, it is unclear if the methods by which some participants lost significant amounts of weight are a result of intervention guidelines or unhealthy weight loss behaviors. Additional limitations include the challenge in defining success in weight management. This study focused on the outcome of BMI reduction, which is just one aspect of change. Individuals may experience metabolic benefits without attaining the definitions of response and success used in this paper, and therefore, further studies are needed to define criteria for early response and longer-term success. Finally, there is no consensus on how to report BMI metrics in children19. This study displayed results using multiple BMI metrics to increase interpretability and ability to compare outcomes with other studies.
Conclusion
The current study shows that early (1 month) BMI reduction of ≥3% within the context of PWM programs is significantly associated with greater long-term (6 and 12 month) BMI reduction of ≥5%. Early non-responders may be ideal candidates for early redirection or intensifications of treatment, contrary to current pediatric obesity treatment guidelines.
What’s Known on This Subject:
Approximately 17% of youth in the United States have obesity. In adults with obesity, weight reduction early in treatment is associated with better longterm maintenance. Pediatric treatment guidelines recommend slow, gradual weight loss despite limited evidence to support this recommendation.
What This Study Adds:
Early BMI treatment response was significantly associated with later BMI treatment success in a national sample of youth receiving treatment in multi-component pediatric weight management programs. One-month responders had significantly greater BMI reduction at 6- and 12-months vs. non-responders.
Acknowledgements
We thank Shelley Kirk, PhD, RD, LD - Principal Investigator for the POWER study, Eileen King, PhD - Director of the POWER Data Coordinating Center, and the POWER Governance Board for their support. We deeply appreciate the participation of the POWER patients and their families. It is our privilege to care for you.
Funding: This work was supported in part by this NCATS award UL1TR002494. The Be Forever Fit Program at Harbor-UCLA Medical Center and the EMPOWER (Energy Management for Personalized Weight Reduction) clinic at CHLA receive support from UniHealth Foundation to support their participation in POWER.
Footnotes
*POWER Work Group - Site Leads/Co-leads and Affiliations: Abraham-Pratt I, Florida Hospital for Children, Orlando, FL; Ali L, OU Physicians - Early Lifestyle Interventions Clinic, Tulsa, OK; Armstrong S, Duke Healthy Lifestyles, Durham, NC; Binns H and Ariza A, Ann & Robert H. Lurie Children’s Hospital, Chicago, IL; Borzutzky B, Children’s Hospital Los Angeles, Los Angeles, CA; Brubaker J, Cleveland Clinic Children’s, Avon, OH; Cristison A, Universtiy of Illinois College of Medicine at Peoria, Healthy Kids U, Peoria, IL; Cuda S, Children’s Hospital of San Antonio, San Antonio, TX; Fox C, University of Minnesota Masonic Children’s Hospital, Minneapolis, MN; Gordon C, Barbara Bush Children’s Hospital at Maine Medical Center, Portland, ME; Hendrix S, Arkansas Children’s Hospital, Little Rock, AR; Hes D, Gramercy Pediatrics, New York, NY; Jenkins L, Dell Children’s Hospital Medical Center, Austin, TX; Joseph M, UF Health Pediatric Weight Management Center - Wolfson Children’s Hospital, Jacksonville, FL; Kirk S, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Kumar S and Heyrman M, Mayo Clinic, Rochester, MN; Liu L, Seattle Children’s Hospital, Seattle, WA; McClure A and Hofley M, Children’s Hospital at Dartmouth Pediatric Lipid & Weight Management Center, Lebanon, NH; Negrete S, University of New Mexico Children’s Hospital, Albuquerque, NM; Novick M, Penn State Hershey Children’s Hospital, Hershey, PA; O’Hara V, Eastern Maine Medical Center, Bangor, ME; Rodrue J, Arnold Palmer Hospital Center for Digestive Health & Nutrition, Orlando, FL; Santos M, Connecticut Children’s Medical Center, Hartford, CT; Stoll J, St. Louis Children’s Hospital, St. Louis, MO; Stratbucker W, Helen DeVos Children’s Hospital-Spectrum Health, Grand Rapids, MI; Sweeney B, Children’s Mercy Hospital, Kansas City, MO; Tester J, UCSF Benioff Children’s Hospital Oakland, Oakland, CA; Walka S and deHeer H, Fit Kids of Arizona at Northern Arizona Healthcare, Flagstaff, AZ; Wallace S, UAB Pediatrics/Children’s of Alabama, Birmingham, AL; Walsh S, Children’s Healthcare of Atlanta (Strong4Life Clinic), Atlanta, GA; Wittcopp C, Baystate Children’s Hospital, Springfield, MA; Weedn A, University of Oklahoma Health Sciences Center, Oklahoma City, OK; and Yee J and Grace B, Harbor-UCLA Medical Center, Torrance, CA.
Disclosure: Dr. Kelly receives research support (drug/placebo) from Astra Zeneca Pharmaceuticals and serves as a consultant for Novo Nordisk, Orexigen, and Vivus Pharmaceuticals but does not accept personal or professional income for these activities. Dr. Ryder receives research support in the form of drug/placebo from Boehringer Ingelheim. Dr. Fox receives research support from Novo Nordisk. Drs. Gross, Kaizer, Borzutsky, Santos, Tucker and Yee have no conflicts of interest to disclose.
References
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–192. [DOI] [PubMed] [Google Scholar]
- 3.Danielsson P, Kowalski J, Ekblom Ö, Marcus C. Response of Severely Obese Children and Adolescents to Behavioral Treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108. [DOI] [PubMed] [Google Scholar]
- 4.Knop C, Singer V, Uysal Y, Schaefer A, Wolters B, Reinehr T. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes. 2015;10(1):7–14. [DOI] [PubMed] [Google Scholar]
- 5.Unick JL, Neiberg RH, Hogan PE, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity. 2015;23(7):1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unick J, Pellegrini C, Demos K, Dorfman L. Initial Weight Loss Response as an Indicator for Providing Early Rescue Efforts to Improve Long-term Treatment Outcomes. Curr Diab Rep. 2017;17(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braet C Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity (Silver Spring). 2006;14(1):148. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LH, Wing RR, Koeske R, Valoski A. A comparison of lifestyle exercise, aerobic exercise, and calisthenics on weight loss in obese children. Behav Ther. 1985;16(4):345–356. [Google Scholar]
- 9.Goldschmidt AB, Stein RI, Saelens BE, Theim KR, Epstein LH, Wilfley DE. Importance of early weight change in a pediatric weight management trial.(Clinical report). Pediatrics. 2011;128(1):E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CN, Jelalian E, Raynor HA, et al. Early patterns of food intake in an adolescent weight loss trial as predictors of BMI change. Eat Behav. 2010;11(4):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jelalian E, Hart CN, Mehlenbeck RS, et al. Predictors of attrition and weight loss in an adolescent weight control program. Obesity (Silver Spring). 2008;16(6):1318. [DOI] [PubMed] [Google Scholar]
- 12.Gow ML, Baur LA, Ho M, et al. Can early weight loss, eating behaviors and socioeconomic factors predict successful weight loss at 12- and 24-months in adolescents with obesity and insulin resistance participating in a randomised controlled trial?(Report). Int J Behav Nutr Phys Act. 2016;13(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegand S, Keller K-M, Lob-Corzilius T, et al. Predicting Weight Loss and Maintenance in Overweight/Obese Pediatric Patients. Hormone Research in Paediatrics. 2015;82(6):380–387. [DOI] [PubMed] [Google Scholar]
- 14.Kirk S, Armstrong S, King E, et al. Establishment of the Pediatric Obesity Weight Evaluation Registry: A National Research Collaborative for Identifying the Optimal Assessment and Treatment of Pediatric Obesity. Child Obes. 2017;13(1):9–17. [DOI] [PubMed] [Google Scholar]
- 15.Food Colman E. and Drug Administration’s Obesity Drug Guidance Document: a short history. Circulation. 2012;125(17):2156. [DOI] [PubMed] [Google Scholar]
- 16.epiR: Tools for the Analysis of Epidemiological Data. R package version 0.9–93. [computer program]. 2017.
- 17.A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 18.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller Weight Changes in Standardized Body Mass Index in Response to Treatment as Weight Classification Increases. J Pediatr. 2011;158(4):624–627. [DOI] [PubMed] [Google Scholar]
- 19.Kelly A, Fox C, Rudser K, Gross A, Ryder Jr. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. In. Int. J. Obes. Vol 402016:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]


