Abstract
Background:
Instrumental activities of daily living (IADL) impairment and apathy occur in early-stage Alzheimer’s disease (AD) and are associated with regional atrophy and hypometabolism in vivo and greater tau burden at autopsy.
Objective:
To explore the association between IADL impairment, apathy, and in vivo regional tau in mild cognitive impairment (MCI) and AD dementia.
Methods:
Forty participants (24 MCI, 16 AD dementia) underwent assessments of IADL (Functional Activities Questionnaire, FAQ) and apathy (Apathy Evaluation Scale Informant report, AES-I). Regional tau was assessed using flortaucipir positron emission tomography (PET) and amyloid using Pittsburgh Compound B PET. Regions with unadjusted associations of p≤0.01 were entered into regression models assessing the relationship between tau and FAQ or AES-I, adjusting for age, sex, and cognition, with/without a tau by amyloid interaction.
Results:
Unadjusted IADL impairment but not apathy was associated with greater tau in multiple regions. After adjusting for covariates, for medial orbitofrontal and entorhinal cortex the interaction between tau and amyloid was associated with IADL impairment and for anterior cingulate it was not but independent associations with both tau and amyloid were retained. With whole brain analyses, similar results were seen for IADL, while for apathy tau in small clusters within the right anterior cingulate and dorsolateral prefrontal cortices were seen, which were more pronounced in individuals with greater amyloid.
Conclusions:
This exploratory study suggests that IADL impairment in AD is associated with medial temporal and frontal tau, especially in individuals with elevated amyloid, while apathy may be associated with right frontal tau.
Keywords: Alzheimer’s disease, amyloid, apathy, instrumental activities of daily living, mild cognitive impairment, positron emission tomography, tau
Introduction
Impairment in instrumental activities of daily living (IADL), which consists of difficulties with activities such as handling money and managing finances, driving or using public transportation, shopping for clothes or food, preparing meals, cleaning, managing medications, and keeping track of appointments, is a major contributor to income loss for patients with Alzheimer’s disease and their caregivers and is a source of physical and psychological burden [1]. Apathy consists of a lack of motivation and interest and social withdrawal, and it is one of the most common neuropsychiatric symptoms in AD [2,3]. Additionally, apathy is a major source of frustration and burden for patients and caregivers, and it can lead to the isolation of both patients and caregivers, alienating them from others, and drastically altering their daily routine [4].
IADL impairment and apathy can occur in amnestic mild cognitive impairment (MCI) and worsen in mild AD dementia [2,3,5–9]. Moreover, IADL impairment has been associated with apathy [8,[10]. It is thought that the combination of memory impairment, executive dysfunction, and apathy likely leads to IADL impairment [3,10–12]. Therefore, IADL impairment and apathy are clinically meaningful symptoms that may be inter-related within AD and are critical deficits during disease progression.
IADL impairment and apathy have been associated with regional brain changes in vivo, and to a lesser extent in post-mortem studies. Frontal, parietal, and temporal atrophy, hypometabolism, and hypoperfusion have been associated with IADL impairment at baseline and over time across MCI and AD dementia [13–20]. Atrophy, hypometabolism, hypoperfusion, reduced fractional anisotropy, and reduced functional connectivity most consistently in the anterior cingulate, as well as orbitofrontal, inferior temporal, parietal, striatum, and medial thalamus have been associated with apathy at baseline and over time across the AD spectrum [21–29]. Furthermore, IADL impairment has been associated with lower Aβ1–42 and greater total and phospho-tau in the cerebrospinal fluid (CSF) across the AD spectrum [15,30], while apathy has been associated with greater CSF total and phospho-tau in AD dementia [31]. Using in vivo amyloid imaging with Pittsburgh Compound B (PiB) positron emission tomography (PET), both IADL impairment and apathy have been associated with greater cortical amyloid burden in MCI [32,33]. Finally, in post-mortem studies of individuals with moderate to severe AD dementia, basic ADL impairment has been associated with medial temporal neurofibrillary tangle (tau) burden and more widespread neuritic plaque (amyloid) burden in medial temporal, frontal, and occipital regions, while apathy has been associated with medial frontal neurofibrillary tangle burden but not with amyloid burden [34–36].
Recent studies using flortaucipir (FTP) PET, a.k.a. 18F-AV-1451 or T807, to visualize in vivo cerebral tau burden have shown that FTP binding corresponds with post-mortem Braak staging in AD [37–39]. However, few studies have assessed the relationship between neuropsychiatric symptoms or IADL and in vivo tau imaging in AD. The objective of the current study was to explore the associations among IADL impairment, apathy, and in vivo regional tau, visualized by FTP PET in MCI and AD dementia. We hypothesized that regional tau burden, specifically in the medial frontal cortex, would be associated with apathy and more widespread tau burden would be associated with IADL impairment as seen with other imaging modalities serving as markers of neurodegeneration. We further hypothesized that these associations would be stronger within individuals with greater amyloid burden. Regional tau burden at autopsy tracks better than amyloid with clinical symptoms. Therefore, the current study provides a unique opportunity to localize the neurodegenerative pattern related to two important clinically meaningful symptoms, IADL impairment and apathy.
Methods
Participants
Forty community-dwelling participants (24 MCI, 16 AD dementia) were recruited from the Memory Disorders Units at Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital to participate in an investigator-initiated observational imaging study. As previously described [32,40], participants were healthy or had stable medical conditions, did not have neurological conditions other than cognitive impairment, and did not have active psychiatric disorders. Participants on stable doses of antidepressants were also eligible for the study. Participants met criteria for amnestic MCI single or multiple domain or AD dementia [5,9,41]. They had objective memory impairment and a wide range of global cognitive and IADL impairment.
The study was approved by the Partners Institutional Review Board, and all participants and study partners signed a consent form before any study assessments were completed. Surrogate consent by the study partner was provided when necessary for AD dementia participants.
Clinical Assessments
IADL were assessed with the 10-item informant-based Functional Activities Questionnaire (FAQ) [42]; greater FAQ scores indicate greater functional impairment (range 0–30). The FAQ was administered to study partners for 33 of the 40 participants. Apathy was assessed with the 18-item Apathy Evaluation Scale Informant report (AES-I) [43]; lower AES-I scores indicate greater apathy (range 18–72). The AES-I was administered to study partners for all 40 participants. Depression severity was assessed using two tests: 1) the single depression item from the informant-based Neuropsychiatric Inventory (NPI) [44,45]; higher NPI Depression item scores indicate greater depression severity and frequency (range 0–12); and 2) the self (participant)-based 15-item Geriatric Depression Scale (GDS) [46]; higher GDS scores indicate greater depressive symptoms. Global cognition was assessed with the Mini-Mental State Exam (MMSE) [47]; lower MMSE scores indicate greater cognitive impairment (range 0–30).
PET Acquisition and Analyses
Regional tau burden was assessed using FTP PET and cortical amyloid burden was assessed using PiB PET. PET scans took place at the MGH PET Facility. PET images were acquired using a Siemens HR+ scanner (3D mode; 63 image planes; 15.2cm axial field of view; 5.6mm transaxial resolution and 2.4mm slice interval; 69 frames: 12×15 seconds, 57×60 seconds). FTP was synthesized, prepared and administered according to the MGH Radioactive Drug Research Committee-approved protocols [48]. Following a 10 mCi injection, 18F-FTP data was acquired with a 3D list mode, dynamic protocol using the above PET camera. Static 80–100 minute acquisition was used. PET data were reconstructed and attenuation corrected, and each frame was evaluated to verify adequate count statistics and absence of head motion. Specific binding was expressed in Freesurfer region of interest (ROI) as the standardized uptake value ratio (SUVR) to cerebellar grey as previously described [37,49]. Nine cortical and subcortical ROI’s were assessed: bilateral hippocampus, entorhinal cortex, inferior temporal, lateral temporal, precuneus, supramarginal, anterior cingulate, medial orbitofrontal, and dorsolateral prefrontal cortex. These ROI’s were selected because they either represent regions exhibiting tau burden early on in AD or regions that have been shown to be associated with IADL impairment or apathy as described in the Introduction.
PiB was synthesized and dynamic PiB PET imaging acquisition was performed as previously described [32,50–52]. 15 mCi 11C-PiB was administered, followed immediately by a 70 minute image acquisition. Cortical PiB retention was expressed as distribution volume ratio (DVR), based on Logan plots of time-activity curves (cerebellar grey reference) [50,53]. A value of global PiB retention consisting of an aggregate of cortical regions that typically have elevated PiB retention in AD dementia (frontal, lateral temporal and parietal, and retrosplenial neocortex) was used in the analyses below.
Continuous PiB was used in the primary analyses, which were then repeated using dichotomous PiB. A threshold for PiB-positivity of a DVR of 1.20 has been previously published by our group based on a Gaussian mixture modeling approach [54].
All PET data were also evaluated after magnetic resonance imaging-based atrophy correction for partial volume effect using the symmetric geometric transfer matrix method [53,55,56]. The analyses reported below were performed without atrophy correction initially, and then the main analyses were repeated with atrophy correction.
Statistical Analyses
All analyses in this study were carried out using SPSS version 24.0 (IBM). Unadjusted correlations were performed initially to assess the relationship between FAQ, AES-I, and regional tau burden.
In our primary analyses, regions with associations of p≤0.01 (from the unadjusted correlations) were then entered into separate linear regression models with backward elimination (retention threshold: p<0.1) assessing the relationship between regional tau burden and FAQ or AES-I (dependent variables), adjusting for age, sex, and MMSE (global cognition). Of note, none of the unadjusted associations with the AES-I had p≤0.01, and therefore we did not carry out linear regression models with AES-I as the dependent variable.
In our secondary analyses, models were repeated including an interaction between regional tau and amyloid. Then, the models with FAQ as the dependent variable were repeated adjusting for AES-I. We also ran models further adjusting for depression using either the NPI Depression item or the GDS. All the secondary analyses models were adjusted for age, sex, and MMSE.
The associations among FAQ, AES-I, and regional tau burden were further explored by regressing the clinical variables onto whole brain FTP PET SUVR maps initially unadjusted, and then adjusted for age, sex, and MMSE. Then, analyses were repeated only among participants with greater amyloid burden. PET images were co-registered to the corresponding magnetic resonance imaging T1 image for each participant using six degrees of freedom and rigid body registration. Cerebellar gray was used as reference region. Mean and T-statistics maps were computed using the GLM Flex tools (mrtool.mgh.harvard.edu) implemented in Matlab 9.2 (R2017a; The MathWorks, Inc., Natick, MA). Surface renderings from MNI space were generated using the surfPlot toolbox (mrtool.mgh.harvard.edu). Exploratory vertex-wise maps were computed as an illustration of the ROI results and an initial more liberal and inclusive threshold was set at T>2.65, p<0.01 and then a more stringent threshold for significance was set at T>3.57, p<0.001. No correction for multiple comparisons were applied in these exploratory maps.
Results
Participant demographics and characteristics are presented in Table 1. Greater IADL impairment (higher FAQ score) was strongly associated with greater apathy (lower AES-I score) (r=−0.67, p<0.001). Greater global cognitive impairment (lower MMSE) was more closely associated with greater IADL impairment (r=−0.67, p<0.001) than with greater apathy (r=0.34, p=0.03) though both associations were statistically significant. Unadjusted correlations between FAQ, AES-I, and regional tau burden are shown in Table 2. For 9 out of the 11 regions, associations with FAQ had p≤0.01. For none of the regions did the associations with AES-I yield a p≤0.01. However, there were marginal associations between greater apathy and greater regional tau burden in the bilateral anterior cingulate (r=−0.28, p=0.09) and medial orbitofrontal cortices (r=−0.27, p=0.09).
Table 1.
Participant demographics and characteristics.
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 70.9 | 8.8 | 55-88 |
| Sex (% Male) | 57.5 | ||
| Diagnosis (MCI / AD dementia) | 24 / 16 | ||
| Education (years) | 15.9 | 2.8 | 8-20 |
| AMNART | 116.6 | 9.6 | 94-131 |
| MMSE | 24.7 | 4.1 | 12-30 |
| FAQ | 11.1 | 8.5 | 0-30 |
| AES-I | 55.0 | 10.7 | 30-71 |
| PiB DVR (cortical aggregate) | 1.52 | 0.30 | 1.02-2.07 |
| PiB-positive (%) | 79 (MCI: 70; AD dementia: 93) | ||
AD (Alzheimer’s disease), AES-I (Apathy Evaluation Scale Informant report), AMNART (American National Adult Reading Test), DVR (distribution volume ratio), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), MMSE (Mini-Mental State Exam), PiB (Pittsburgh Compound B), SD (standard deviation).
Table 2.
Unadjusted correlations between FAQ, AES-I, and bilateral regional tau burden.
| FAQ | AES-I | |||
|---|---|---|---|---|
| r | p | r | p | |
| Hippocampal tau | 0.38 | 0.03 | −0.07 | 0.68 |
| Entorhinal cortex tau | 0.55 | 0.001 | −0.15 | 0.35 |
| Inferior temporal tau | 0.58 | <0.001 | −0.19 | 0.25 |
| Lateral temporal tau | 0.50 | 0.003 | −0.15 | 0.35 |
| Precuneus tau | 0.39 | 0.02 | −0.15 | 0.37 |
| Supramarginal tau | 0.45 | 0.009 | −0.17 | 0.29 |
| Anterior cingulate tau | 0.49 | 0.004 | −0.28 | 0.09 |
| Medial orbitofrontal tau | 0.49 | 0.004 | −0.27 | 0.09 |
| Dorsolateral prefrontal cortex tau | 0.53 | 0.001 | −0.23 | 0.15 |
AES-I (Apathy Evaluation Scale Informant report), FAQ (Functional Activities Questionnaire).
Primary IADL regression models:
For most tau regions, tau was no longer significantly or marginally associated with FAQ, while MMSE was significantly associated with FAQ (pr=−0.71, p<0.001), as was male sex (pr=0.37, p=0.04). However, for two regions, tau was retained in the model with marginal associations: Bilateral entorhinal (pr=0.34, p=0.07) and anterior cingulate cortices (pr=0.31, p=0.09).
IADL regression models including amyloid:
The interaction between regional tau and amyloid was significant for IADL models for two regions, such that in participants with greater amyloid burden, there was an association between greater regional tau burden and greater IADL impairment: Bilateral medial orbitofrontal (tau x amyloid interaction: pr=0.54, p=0.002) and entorhinal cortices (tau x amyloid interaction: pr=0.51, p=0.004) (see Figure 1). For one tau region, the interaction between regional tau and amyloid was not significant, but independent associations with both tau and amyloid were retained: Bilateral anterior cingulate cortex (tau pr=0.37, p=0.05, amyloid pr=0.47, p=0.01), (see Figure 2). For four tau regions, neither the interaction between regional tau and amyloid nor the tau region was retained in the model, and only the amyloid predictor was retained: Bilateral dorsolateral prefrontal (amyloid: pr=0.49, p=0.006), inferior temporal (amyloid: pr=0.49, p=0.006), lateral temporal (amyloid: pr=0.49, p=0.006) and supramarginal cortices (amyloid: pr=0.49, p=0.006). No other predictors were retained in any of these models.
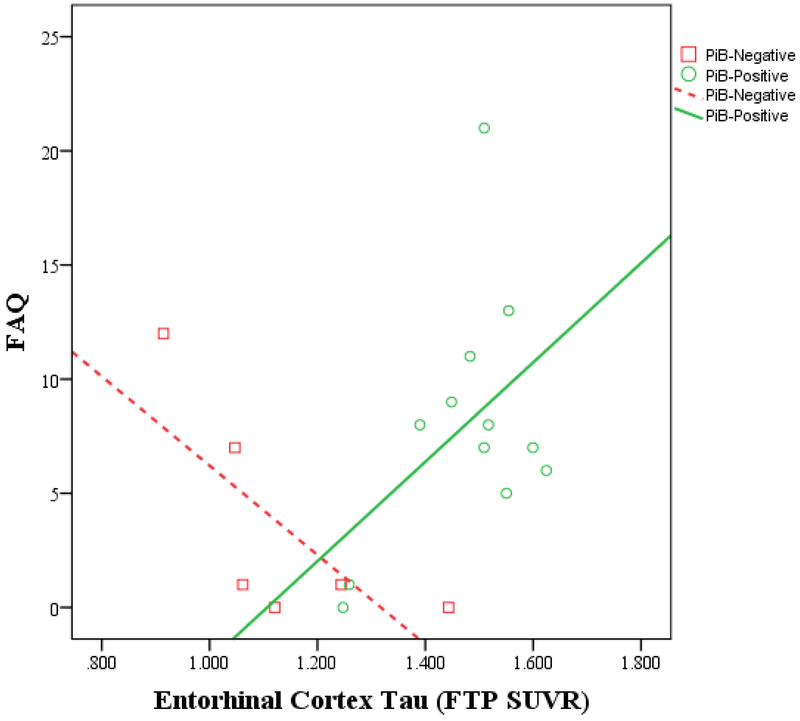
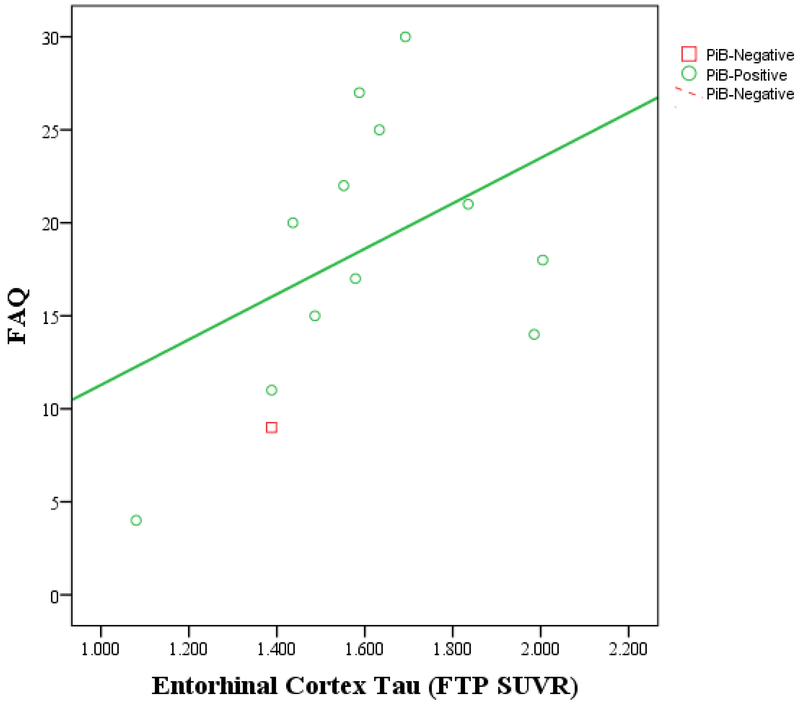
Figure 1.
IADL vs. entorhinal cortex tau burden by amyloid status in MCI participants (A) and in AD dementia participants (B). Greater IADL impairment (higher FAQ score) is associated with greater entorhinal cortex tau burden among PiB-positive participants but not among PiB-negative participants (for both MCI and AD dementia participants). The unadjusted Pearson correlations are shown in the graphs. In the linear regression model adjusted for age, sex, and MMSE, an interaction between tau and amyloid was significant when related to IADL (partial r=0.51, p=0.004). AD (Alzheimer’s disease), FAQ (Functional Activities Questionnaire), FTP (flortaucipir), IADL (instrumental activities of daily living), MCI (mild cognitive impairment), MMSE (Mini-Mental State Exam), PiB (Pittsburgh Compound B), SUVR (standardized uptake value ratio).
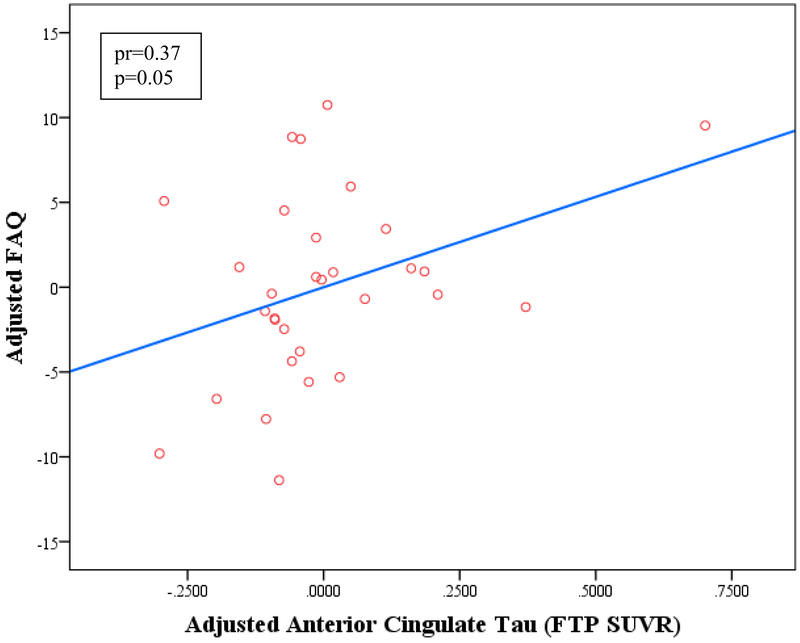
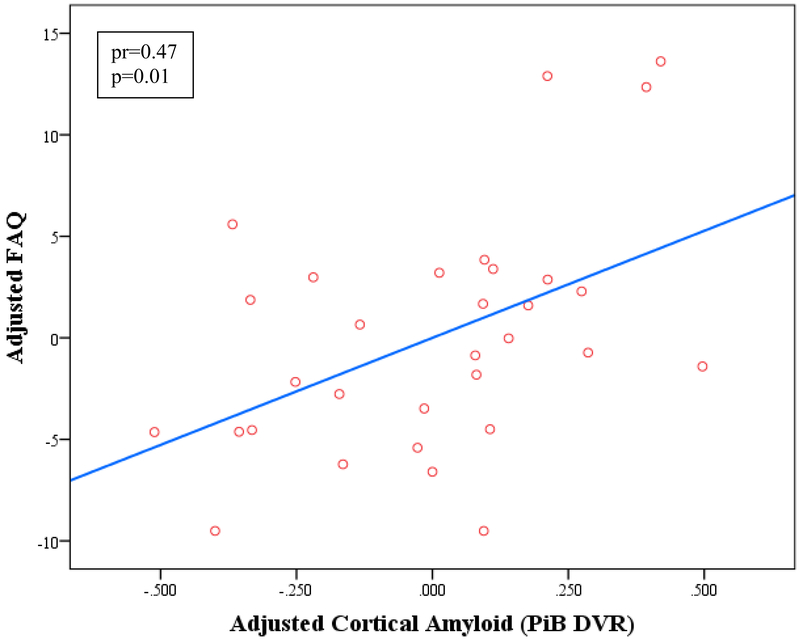
Figure 2.
Linear regression model of IADL vs. anterior cingulate tau burden (A) and cortical amyloid (B) adjusted for age, sex, and MMSE. Greater IADL impairment (higher FAQ score) is associated independently with greater anterior cingulate tau burden and cortical amyloid burden. The interaction between tau and amyloid was not significant when related to IADL. DVR (distribution volume ratio), FAQ (Functional Activities Questionnaire), FTP (flortaucipir), IADL (instrumental activities of daily living), MMSE (Mini-Mental State Exam), PiB (Pittsburgh Compound B), SUVR (standardized uptake value ratio).
When repeating the above models adding another interaction term between MMSE and amyloid, that interaction term was significant (pr=−0.61, p<0.001) and the tau terms (alone or the interaction of tau with amyloid) dropped out from all models except for anterior cingulate cortex tau, which was marginally significant (pr=0.36, p=0.06).
Similar results were obtained when using a dichotomous rather than continuous amyloid variable.
IADL regression models adjusting for AES-I:
The results were not significantly altered after adjusting for AES-I, while AES-I was also significantly associated with FAQ.
IADL analyses separated by diagnostic group:
When repeating the primary analyses within MCI participants separately from AD dementia participants, a similar association between IADL and entorhinal cortex tau burden (pr=0.49, p=0.08) was seen within the AD dementia group to that of the whole sample. However, there were no significant or marginal associations between IADL and regional tau burden within the MCI group.
IADL regression models adjusting for depression:
When using the NPI Depression item or the GDS to adjust for depression, the associations between regional tau and IADL were not significantly altered.
Partial volume correction:
Nearly identical results were obtained when using partial volume corrected PET data in unadjusted and adjusted analyses.
Whole brain voxel-wise analyses:
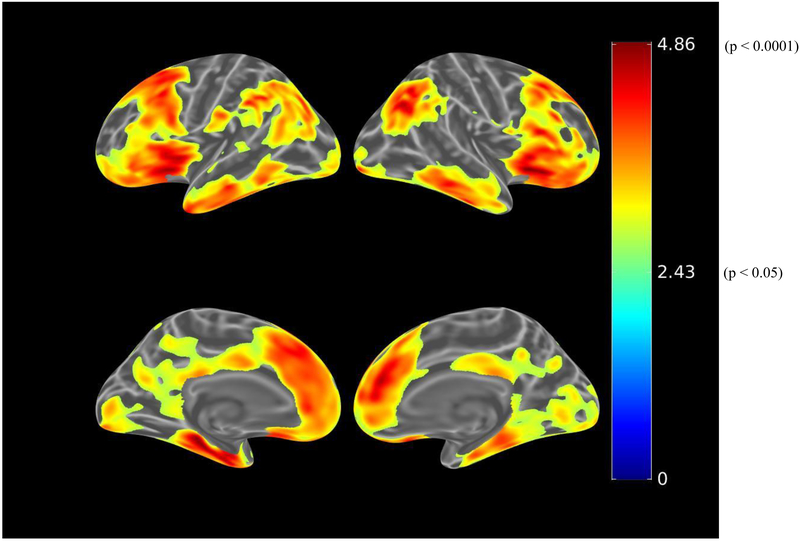
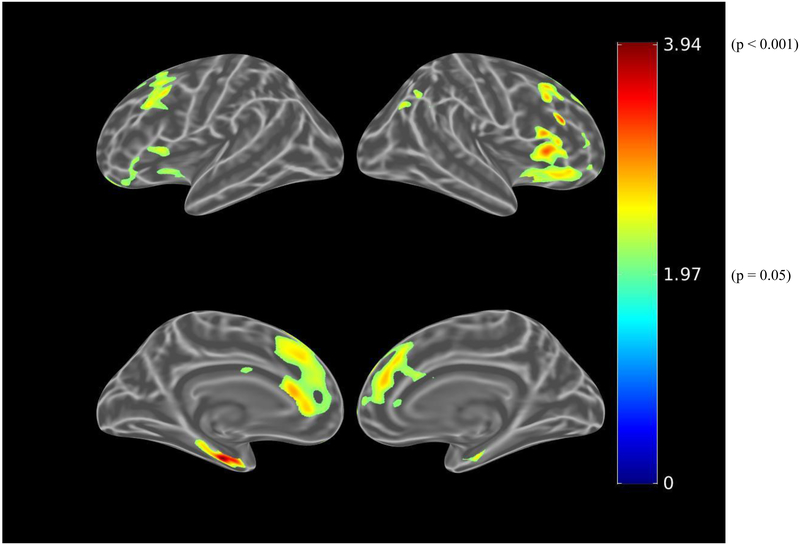
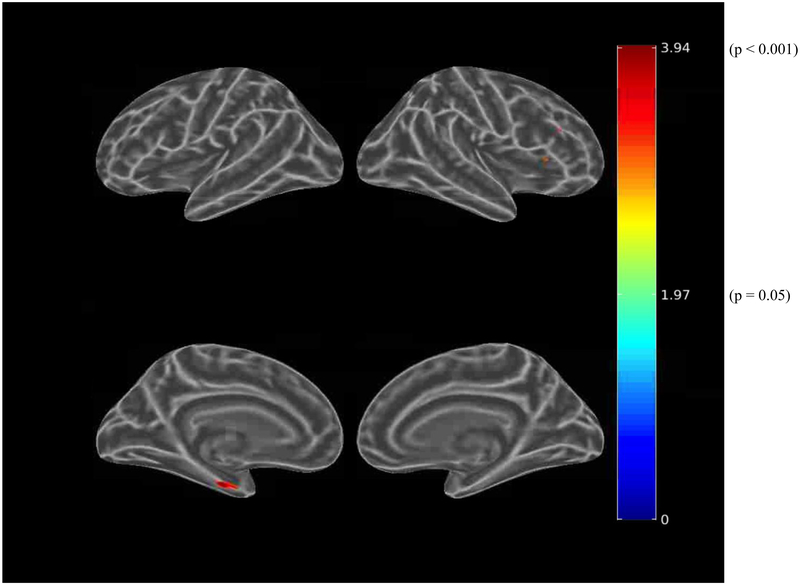
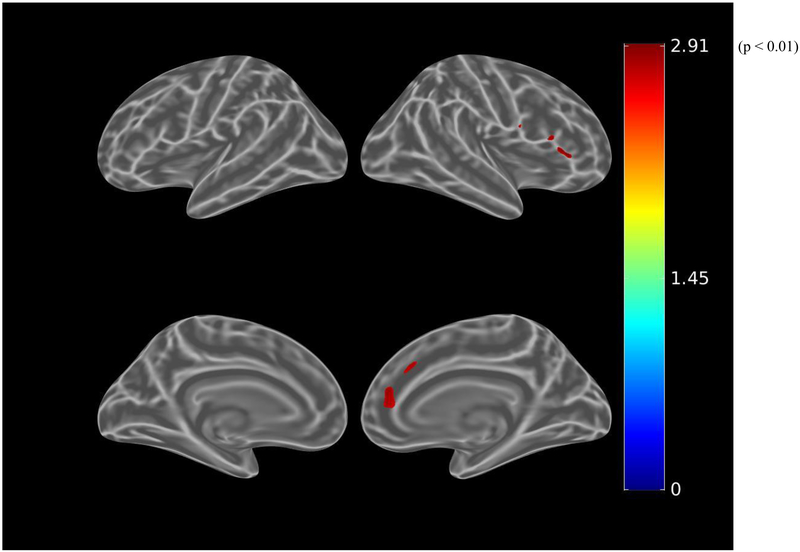
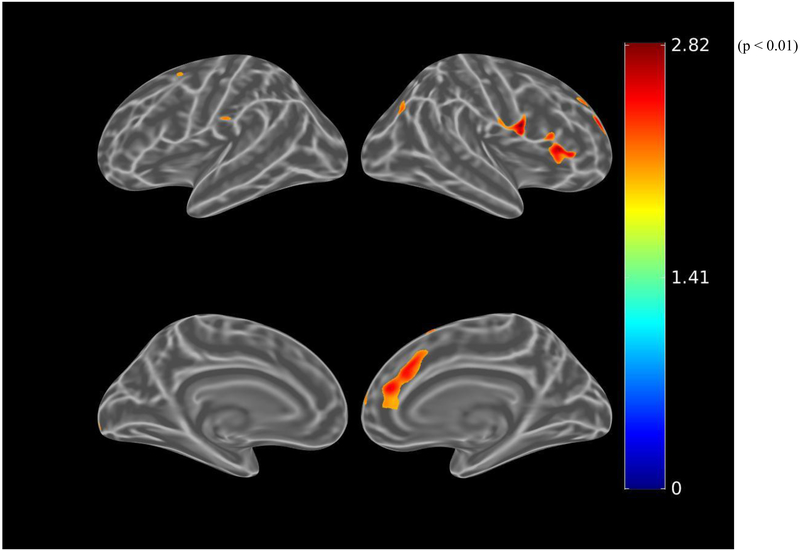
Before adjusting for covariates, greater IADL impairment (greater FAQ score) was associated (using the liberal threshold of p<0.01) with greater tau burden in the bilateral dorsolateral prefrontal, anterior cingulate, medial orbitofrontal, lateral parietal, inferior temporal, entorhinal cortex, and medial occipital regions (see Figure 3.A.). When using the more stringent threshold of p<0.001, all the associations were retained except for the medial occipital region. After adjusting for age, sex, and MMSE (global cognition), there were marginal associations between IADL impairment and bilateral anterior cingulate and left entorhinal cortex tau burden, which were not retrained at the p<0.001 threshold (see Figure 3.B.). Among participants with greater amyloid burden, after adjusting for age, sex, and MMSE, IADL impairment was associated with bilateral dorsolateral prefrontal, bilateral anterior cingulate, right orbitofrontal, and left entorhinal cortex tau burden at a threshold of p<0.01 (see Figure 3.C.). However, at the more stringent threshold of p<0.001, only the association with left entorhinal cortex was retained (see Figure 3.D.).
Figure 3.
Whole brain FTP PET SUVR T-statistics maps showing the unadjusted association between IADL (FAQ) and tau burden (FTP PET SUVR) in all participants (A), the association adjusted for age, sex, and MMSE (global cognition) (B), and in participants with greater amyloid burden (PiB-positive), the association adjusted for age, sex, and MMSE (C). An additional map for PiB-positive participants adjusted for age, sex, and education with a threshold of p<0.001 is shown (D). Threshold on the first 3 maps was set at T>2.65, p<0.01 in order to show a wider range of results. For t=2.0, p=0.05; t=2.65, p=0.01; t=3.0, p=0.005; t=3.57, p=0.001; t=4.0, p=0.0003; t=4.9, p=0.00002. FAQ (Functional Activities Questionnaire), FTP (flortaucipir), IADL (instrumental activities of daily living), MMSE (Mini-Mental State Exam), PET (positron emission tomography), PiB (Pittsburgh Compound B), SUVR (standardized uptake value ratio).
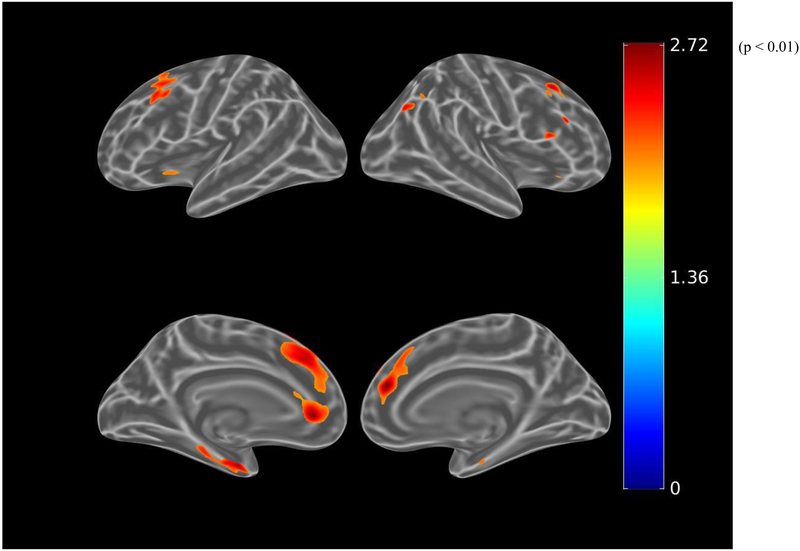
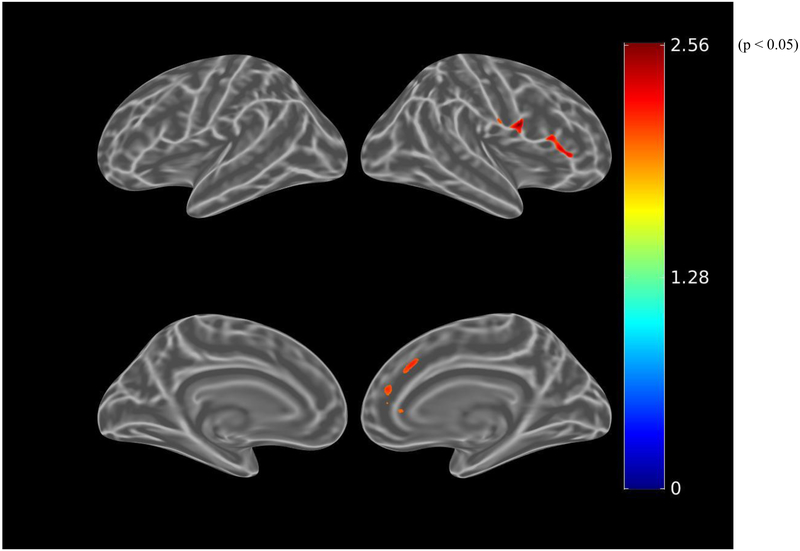
Before adjusting for covariates, greater apathy (lower AES-I) was associated (using the liberal threshold of p<0.01) with greater tau burden in small clusters within the right anterior cingulate and dorsolateral prefrontal cortical regions (see Figure 4.A.). When using the more stringent threshold of p<0.001, these associations were not retained. After adjusting for age, sex, and MMSE, there were marginal associations with greater tau burden in small clusters within the right anterior cingulate and dorsolateral prefrontal cortical regions, again not retained at the p<0.001 threshold (see Figure 4.B.). Among participants with greater amyloid burden, after adjusting for age, sex, and MMSE, this association was more pronounced, but again not retained at the p<0.001 threshold (see Figure 4.C.).
Figure 4.
Whole brain FTP PET SUVR T-statistics maps showing the unadjusted association between apathy (AES-I) and tau burden (FTP PET SUVR) in all participants (A), the association adjusted for age, sex, and MMSE (global cognition) (B), and in participants with greater amyloid burden (PiB-positive), the association adjusted for age, sex, and MMSE (C). Of note, the associations shown in these maps do not meet the p<0.001 threshold. Threshold was set at T>2.65, p<0.01 in order to show a wider range of results. For t=2.0, p=0.05; t=2.65, p=0.01; t=3.0, p=0.005. AES-I (Apathy Evaluation Scale Informant report), FTP (flortaucipir), MMSE (Mini-Mental State Exam), PET (positron emission tomography), PiB (Pittsburgh Compound B), SUVR (standardized uptake value ratio).
Discussion
In this exploratory study, we found that IADL impairment in cognitively impaired individuals with AD may be associated with medial temporal and medial frontal tau burden, while apathy may be associated with right frontal tau burden. For IADL impairment, some of these associations with tau burden (in the bilateral medial orbitofrontal and entorhinal cortices) were modified by amyloid burden such that they were seen in individuals with greater amyloid burden, while for another region (bilateral anterior cingulate cortices) tau and amyloid were related independently to IADL impairment. After adjusting for global cognition, the association between tau and IADL impairment was no longer significant for most regions and marginal for only two regions (bilateral entorhinal and anterior cingulate cortices) suggesting that cognition may be driving that association with IADL impairment merely serving as a proxy of global cognitive impairment. However, this association was significant in individuals with greater amyloid burden.
IADL impairment in AD has been associated with markers of neurodegeneration, including atrophy, hypoperfusion, and hypometabolism across many brain regions [14–16,18]. In the current study, we used FTP PET to visualize in vivo tau burden, an arguably more direct marker of AD neurodegeneration, and showed an unadjusted association in MCI and AD dementia between IADL impairment and bilateral medial frontal, lateral frontal, lateral parietal, inferior temporal, lateral temporal, and medial temporal tau burden, which was reduced to marginal associations with medial temporal and medial frontal after adjusting for global cognition. Prior autopsy studies in AD dementia have shown widespread associations of amyloid burden and basic ADL impairment in more impaired individuals, but more circumscribed associations with tau burden primarily in the medial temporal region [34,36]. In the current study, we found that amyloid burden in an aggregate of cortical regions modifies the association between IADL impairment and tau for some regions of tau (bilateral medial orbitofrontal and entorhinal cortices) such that the association is stronger in individuals with greater amyloid burden. In another tau regions (bilateral anterior cingulate cortex), amyloid was associated independently of tau with IADL impairment. It is unclear why amyloid modified this association for some tau regions but not others. This needs to be further explored in other samples in order to either replicate or refute these results.
Similar to IADL impairment, apathy in AD has been associated with markers of neurodegeneration, including atrophy, hypoperfusion, and hypometabolism across many brain regions, but in particular in the medial frontal regions [21,22,25,26,28]. In the current study, in our ROI-based unadjusted analyses, we found marginal associations between apathy and bilateral anterior cingulate and medial orbitofrontal tau burden. This was replicated in the whole brain analyses, where we found marginal associations with small clusters of tau burden within the right anterior cingulate and dorsolateral prefrontal cortex, which were more pronounced among participants with greater amyloid burden. A prior autopsy study in AD dementia demonstrated an association between apathy and anterior cingulate tau burden but not with amyloid burden [35], partly in line with the finding of our in vivo study. Therefore, future studies in larger samples focused on these particular regions may help further determine the regionally specific pathology apathy relates to in AD.
Few prior studies have investigated the association between in vivo cerebral tau deposition and neuropsychiatric symptoms. These studies have focused on affective symptoms in clinically normal older adults at risk for AD. Recently, we showed a modest association between minimal depressive symptoms and inferior temporal and entorhinal cortex tau burden in a cohort of clinically normal elderly [57]. Delving further into depression constructs, which could consist of apathy, anhedonia, or anxiety, as the possible drivers of this relationship is required. Another pilot study attempted to do that in a cohort of non-demented individuals with late life depression where they showed an association between apathy and anterior cingulate pathologic burden with a non-specific PET tracer thought to bind to both tau and amyloid [58].
An important caveat to keep in mind is that both IADL impairment and apathy may be serving as a proxies of global cognition as illustrated by the strong unadjusted correlations between these measures, especially IADL impairment and global cognition, in our sample. Alternatively, IADL impairment and apathy may be highly related to and dependent upon certain aspects of cognitive function. To put this to the test, we adjusted our models for global cognition (using the MMSE). When doing so, the association between regional tau burden and IADL impairment was no longer significant for any region but there was a marginal association with bilateral entorhinal and anterior cingulate cortices. These associations became significant again when focusing on individuals with greater amyloid burden. Since the unadjusted associations between apathy and tau ROI’s were only at a trend level, we did not include apathy in these regression models adjusting for global cognition. This suggests that the association between IADL and tau in MCI and AD dementia may be mediated by global cognition. The unadjusted correlation between global cognition and IADL was strong, and therefore these results are not surprising. Moreover, before adjusting for covariates, IADL impairment was associated with widespread cerebral tau burden, suggesting a global process, as is the case with global cognition. That said, in the whole brain analyses, using a liberal threshold, among participants with greater amyloid burden, after adjusting for global cognition, IADL impairment was associated with left entorhinal cortex, right dorsolateral prefrontal cortex, and bilateral anterior cingulate tau burden, and apathy was associated with greater tau burden in small clusters within the right anterior cingulate and dorsolateral prefrontal cortical regions. When using a stringent threshold, only the association between IADL and left entorhinal cortex tau was retained. Therefore, it is possible that while cognition drives the association with most regional tau, more circumspect medial temporal and frontal regions may be associated with IADL impairment and apathy. Additionally, it is likely that both cognitive impairment and apathy are manifestations of cerebral tau burden that then contribute to IADL impairment. In order to better understand the nature of these complex and inter-related clinical assessments, future studies with larger samples, longitudinal follow-up, and more sensitive cognitive, IADL, and affective assessment measures will be necessary.
The current study had several limitations. First, the sample size was small, which has been the case for studies of cognitively symptomatic individuals with tau PET tracers. As such, a few participants may be driving the results observed, but we did not have statistically significant outliers in our sample. We did, however, note that when looking at diagnostic groups separately, the AD dementia group appeared to drive the associations between IADL and regional tau burden. That said, larger samples at our center and other centers are being collected in order to further address these issues address these issues and test our hypotheses. Second, this sample did not include clinically normal elderly, who may be at risk for AD. It could be argued that apathy and IADL impairment are rare in such samples of clinically normal elderly. However, future longitudinal studies using more sensitive assessments of apathy and IADL will attempt to address this issue. Third, many models were employed in the above analyses, which were exploratory in nature. In order to partly mitigate this, the initial unadjusted correlations between the clinical variables (IADL and apathy) and tau burden had a stricter significance threshold of 0.01, and the threshold for the whole brain analyses was 0.001. Finally, the participants in the current study were highly educated and intelligent. This is a convenience sample similar to that of other observational imaging studies. As such, these findings will need to be replicated in population-based cohorts.
In conclusion, in this exploratory small study of MCI and AD dementia participants, we showed a cross-sectional association between IADL impairment and in vivo bilateral medial frontal and medial temporal tau burden and a possible association between apathy and right frontal tau burden. For IADL impairment, these associations persisted after adjusting for amyloid burden, depressive symptoms, and apathy. After adjusting for global cognition the association weakened and was limited to small frontal regions, but became significant again in individuals with greater amyloid burden. These findings need to be extended in larger scale longitudinal analyses to explore the interplay of these clinically meaningful symptoms—apathy, IADL impairment, and cognition—, which were strongly associated in our sample. Moreover, exploring the sub-domains of apathy (lack of initiative, lack of interest, and emotional blunting) will be important in further teasing these apart.
Acknowledgments
This study was supported by the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s disease, NIH/NIA K23 AG033634, K24 AG035007, R01 AG027435, and R01 AG046396.
Footnotes
Conflicts of Interest/Disclosure Statement
The authors have received research salary support from Eisai Inc. (GAM, NJD), Eli Lilly and Company (GAM, KAJ, NJD, RAS), Janssen Alzheimer Immunotherapy (GAM, RAS), Novartis (GAM), and Avid Radiopharmaceuticals (KAJ). Additionally, DMR has served as a consultant for Eli Lilly, Neurotrack, and Lundbeck, GAM has served as a consultant for Grifols Shared Services North America, Inc. and Pfizer, KAJ has served as a consultant for Eli Lilly, Novartis, Janssen, Roche, Piramal, GE Healthcare, Siemens, ISIS Pharma, AZTherapy, and Biogen, NJD has served as a consultant for Avanir, and RAS has served as a consultant for AbbVie, Biogen, Bracket, Genentech, Lundbeck, Merck, Pfizer, Roche, and Sanofi. NJD’s spouse is employed by Alkermes.
References
- [1].Marshall GA, Amariglio RE, Sperling RA, Rentz DM (2012). Activities of daily living: Where do they fit in the diagnosis of Alzheimer’s disease. Neurodegener Dis Manag. 2(5), 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002). Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 288(12), 1475–1483. [DOI] [PubMed] [Google Scholar]
- [3].Wadsworth LP, Lorius N, Donovan NJ, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA, Alzheimer’s Disease Neuroimaging Initiative (2012). Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement Geriatr Cogn Disord. 34(2), 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, Evans J, Lee M, Porsteinsson A, Lanctot KL, Rosenberg PB, Sultzer DL, Francis PT, Brodaty H, Padala PP, Onyike CU, Ortiz LA, Ancoli-Israel S, Bliwise DL, Martin JL, Vitiello MV, Yaffe K, Zee PC, Herrmann N, Sweet RA, Ballard C, Khin NA, Alfaro C, Murray PS, Schultz S, Lyketsos CG, Neuropsychiatric Syndromes Professional Interest Area of I (2013). Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement. 9(5), 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3), 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Apostolova LG, Cummings JL (2008). Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 25(2), 115–126. [DOI] [PubMed] [Google Scholar]
- [7].Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM (2014). Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer’s disease. Am J Geriatr Psychiatry. 22(12), 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA, Alzheimer’s Disease Neuroimaging Initiative (2011). Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 7(3), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL (2003). Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 11(2), 214–221. [PubMed] [Google Scholar]
- [11].Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G (2010). Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 20(1), 175–183. [DOI] [PubMed] [Google Scholar]
- [12].Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ, Committee on Research of the American Neuropsychiatric A (2007). The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 19(3), 249–265. [DOI] [PubMed] [Google Scholar]
- [13].de Gois Vasconcelos L, Jackowski AP, Oliveira MO, Flor YM, Bueno OF, Brucki SM (2011). Voxel-based morphometry findings in Alzheimer’s disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (Sao Paulo). 66(6), 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ (2011). Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 32(7), 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marshall GA, Lorius N, Locascio JJ, Hyman BT, Rentz DM, Johnson KA, Sperling RA, Alzheimer’s Disease Neuroimaging Initiative (2014). Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer disease spectrum. J Alzheimers Dis. 41(3), 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nadkarni NK, Levy-Cooperman N, Black SE (2012). Functional correlates of instrumental activities of daily living in mild Alzheimer’s disease. Neurobiol Aging. 33(1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Okonkwo OC, Alosco ML, Jerskey BA, Sweet LH, Ott BR, Tremont G (2010). Cerebral atrophy, apolipoprotein E varepsilon4, and rate of decline in everyday function among patients with amnestic mild cognitive impairment. Alzheimers Dement. 6(5), 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roy K, Pepin LC, Philiossaint M, Lorius N, Becker JA, Locascio JJ, Rentz DM, Sperling RA, Johnson KA, Marshall GA, Alzheimer’s Disease Neuroimaging Initiative (2014). Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis. 42(1), 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Salmon E, Lespagnard S, Marique P, Peeters F, Herholz K, Perani D, Holthoff V, Kalbe E, Anchisi D, Adam S, Collette F, Garraux G (2005). Cerebral metabolic correlates of four dementia scales in Alzheimer’s disease.[erratum appears in J Neurol. 2005 Sep;252(9):1138]. J Neurol. 252(3), 283–290. [DOI] [PubMed] [Google Scholar]
- [20].Vidoni ED, Honea RA, Burns JM (2010). Neural correlates of impaired functional independence in early Alzheimer’s disease. J Alzheimers Dis. 19(2), 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM (2007). Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 24(2), 91–97. [DOI] [PubMed] [Google Scholar]
- [22].Donovan NJ, Wadsworth LP, Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2014). Regional Cortical Thinning Predicts Worsening Apathy and Hallucinations Across the Alzheimer Disease Spectrum. Am J Geriatr Psychiatry. 22(11), 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guercio B, Donovan NJ, Ward A, Schultz A, Lorius N, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2015). Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly. J Neuropsychiatry Clin Neurosci. 27(1), e22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim JW, Lee DY, Choo IH, Seo EH, Kim SG, Park SY, Woo JI (2011). Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer disease. Am J Geriatr Psychiatry. 19(7), 644–653. [DOI] [PubMed] [Google Scholar]
- [25].Lanctot KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, Black SE (2007). A SPECT study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 24(1), 65–72. [DOI] [PubMed] [Google Scholar]
- [26].Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL (2007). Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 64(7), 1015–1020. [DOI] [PubMed] [Google Scholar]
- [27].Munro CE, Donovan NJ, Guercio BJ, Wigman SE, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2015). Neuropsychiatric symptoms and functional connectivity in mild cognitive impairment. J Alzheimers Dis. 46(3), 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robert PH, Darcourt G, Koulibaly MP, Clairet S, Benoit M, Garcia R, Dechaux O, Darcourt J (2006). Lack of initiative and interest in Alzheimer’s disease: a single photon emission computed tomography study. Eur J Neurol. 13(7), 729–735. [DOI] [PubMed] [Google Scholar]
- [29].Gatchel JR, Donovan NJ, Locascio JJ, Becker JA, Rentz DM, Sperling RA, Johnson KA, Marshall GA, Alzheimer’s Disease Neuroimaging Initiative (2017). Regional 18F-fluorodeoxyglucose hypometabolism is associated with higher apathy scores over time in early Alzheimer’s Disease. Am J Geriatr Psychiatry. 25(7), 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, Tremont G (2010). Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 67(6), 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Skogseth R, Mulugeta E, Jones E, Ballard C, Rongve A, Nore S, Alves G, Aarsland D (2008). Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 25(6), 559–563. [DOI] [PubMed] [Google Scholar]
- [32].Marshall GA, Donovan NJ, Lorius N, Gidicsin CM, Maye J, Pepin LC, Becker JA, Amariglio RE, Rentz DM, Sperling RA, Johnson KA (2013). Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 25(4), 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marshall GA, Olson LE, Frey MT, Maye J, Becker JA, Rentz DM, Sperling RA, Johnson KA, Alzheimer’s Disease Neuroimaging Initiative (2011). Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord. 31(6), 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2006). Neuropathologic correlates of activities of daily living in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 20(1), 56–59. [DOI] [PubMed] [Google Scholar]
- [35].Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2006). Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 21(3), 144–147. [DOI] [PubMed] [Google Scholar]
- [36].Roth M, Tomlinson BE, Blessed G (1966). Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 209(18), 109–110. [DOI] [PubMed] [Google Scholar]
- [37].Johnson K, Schultz A, Betensky R, Becker J, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R (2016). Tau PET imaging in aging and early Alzheimer’s disease. Ann Neurol. 79(1), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Vasdev N, Dickerson BC, Gomperts SN, Growdon JH, Johnson KA, Frosch MP, Hyman BT, Gomez-Isla T (2015). Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 78(5), 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pontecorvo MJ, Devous MD Sr., Navitsky M, Lu M, Salloway S, Schaerf FW, Jennings D, Arora AK, McGeehan A, Lim NC, Xiong H, Joshi AD, Siderowf A, Mintun MA, investigators FA-A (2017). Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 140(3), 748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guercio B, Donovan NJ, Munro CE, Aghjayan SL, Wigman SE, Locascio JJ, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2015). The Apathy Evaluation Scale: A Comparison of Subject, Informant, and Clinician Report in Cognitively Normal Elderly and Mild Cognitive Impairment. J Alzheimers Dis. 47(2), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petersen R (2004). Mild cognitive impairment as a diagnostic entity. J Intern Med. 256(3), 11. [DOI] [PubMed] [Google Scholar]
- [42].Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S (1982). Measurement of functional activities in older adults in the community. J Gerontol. 37(3), 323–329. [DOI] [PubMed] [Google Scholar]
- [43].Marin RS, Biedrzycki RC, Firinciogullari S (1991). Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 38(2), 143–162. [DOI] [PubMed] [Google Scholar]
- [44].Cummings JL (1997). The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 48(5 Suppl 6), S10–16. [DOI] [PubMed] [Google Scholar]
- [45].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994). The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 44(12), 2308–2314. [DOI] [PubMed] [Google Scholar]
- [46].Sheikh JI, Yesavage JA (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version Clinical Gerontology : A Guide to Assessment and Intervention. New York: The Haworth Press; 165–173. [Google Scholar]
- [47].Folstein MF, Folstein SE, McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- [48].Shoup TM, Yokell DL, Rice PA, Jackson RN, Livni E, Johnson KA, Brady TJ, Vasdev N (2013). A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J Labelled Comp Radiopharm. 56(14), 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Shankle WR, Elizarov A, Kolb HC (2013). Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 34(2), 457–468. [DOI] [PubMed] [Google Scholar]
- [50].Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve D, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA (2011). Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 69(6), 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, DeKosky ST, Fischman AJ, Greenberg SM (2007). Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 62(3), 229–234. [DOI] [PubMed] [Google Scholar]
- [52].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 55(3), 306–319. [DOI] [PubMed] [Google Scholar]
- [53].Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA (2005). Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 25(11), 1528–1547. [DOI] [PubMed] [Google Scholar]
- [54].Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA, Alzheimer’s Disease Neuroimaging I, Australian Imaging B, Lifestyle Flagship Study of A, Harvard Aging Brain S (2014). Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 82(20), 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, Becker JA, Svarer C, Knudsen G, Sperling RA, Johnson KA (2016). Different partial volume correction methods lead to different conclusions: An F-FDG PET Study of aging. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Lin MP, Price JC (1999). Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 40(12), 2053–2065. [PubMed] [Google Scholar]
- [57].Gatchel JR, Donovan NJ, Locascio JJ, Schultz AP, Becker JA, Chhatwal J, Papp KV, Amariglio RE, Rentz DM, Blacker D, Sperling RA, Johnson KA, Marshall GA (2017). Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. . J Alzheimers Dis. 59(3), 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eyre HA, Siddarth P, van Dyk K, St Cyr N, Baune BT, Barrio JR, Small GW, Lavretsky H (2017). Neural correlates of apathy in late-life depression: a pilot [18 F]FDDNP positron emission tomography study. Psychogeriatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]