Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic systemic disorder causing the development of renal and hepatic cysts and decline in renal function. It affects around 1 in 1,000 live births. Early hypertension and progressive renal failure due to massive enlargement of cysts and fibrosis are hallmarks of the disease. This article reviews recent advances in ADPKD and focuses mainly on diagnosis, management, and prediction of the course of the disease.
Keywords: ADPKD, diagnosis, management, treatment, prediction
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic cause of kidney failure, occurring in more than 1 in 400 to 1,000 live births and accordingly representing a major socioeconomic medical problem globally 1. ADPKD arises owing to mutations in the PKD1 gene (about 85% of cases) or the PKD2 gene (about 15% of cases), which encode the proteins polycystin-1 (PC1) and -2 (PC2), respectively 2, 3. Compared with patients who have PKD1 mutations, those with PKD2 mutations have a milder phenotype and reach end-stage renal disease (ESRD) about 20 years later 4– 6. ADPKD is characterized by progressive development and enlargement of cysts in all nephron segments but preferentially in the collecting duct, and ESRD occurs in 50% of patients by the age of 60 years 1. It is associated with cyst formation in other organs, primarily the liver and pancreas, and with cardiovascular anomalies such as intracranial arterial aneurysms and vascular dissections. Huge variation is observed in disease phenotype and progression, even within families; the reason for this is poorly understood, but genetic modifiers, including hypomorphic alleles, probably play a significant role.
Although a definitive curative treatment is not currently available, there is a disease-modifying therapy using a V2 receptor antagonist (tolvaptan) 7, 8. This article guides the reader through recent achievements, and the clinical consequences, within the field of ADPKD.
Genetics and genetic testing
Mutations in other genes, such as GANAB and DNAJB11, have recently been identified in a small subgroup of patients 9, 10, but the phenotypes related to these mutations are not exactly the same as those caused by mutations in PKD1 or PKD2. This may suggest that, instead of using the name “ADPKD”, it would be appropriate to name these diseases after the mutated gene, as has been done, for example, for ADTKD 11. Whereas mutations in GANAB give rise to a mild form of cystic disease that does not progress to ESRD 9, mutations in DNAJB11 cause cystic disease in the context of which ESRD may develop in the absence of marked kidney enlargement 10.
Genetic testing has undergone remarkable progress, as is very evident if one considers the case of ADPKD. When the PKD1 and PKD2 genes were first identified, genetic testing was performed by means of linkage analysis 5. Not only was this time consuming but also, more importantly, several affected and non-affected family members were needed in order to identify the risk haplotype. As soon as the PKD1 gene was identified, it became evident that genetic testing for the gene would be burdensome owing to the large transcript size (>14 kb) and reiteration of the genomic area encoding about 75% of the protein on the same chromosome 12. The group of Harris then developed a technique that used long-range polymerase chain reaction and benefited from the few non-repeated nucleotides 13. Although this allowed diagnosis of a single individual, it remained time consuming and was also expensive. Over the past 3 years, next-generation sequencing (NGS) has changed the scenario 14– 16. The use of NGS for genetic diseases such as ADPKD has reduced costs, improved sensitivity, and reduced turnaround time 17, 18. A further advantage of this relatively new technique is that gene panels or whole exome sequencing can be used, allowing the detection of mutations in ultrarare genes that account for a very small number of cases of renal cystic disease 17. The drawback of testing PKD1 is the huge number of variants of unknown significance (VUSs) that can be identified, around 10 per patient 18. Some of these VUSs have previously been published as mutations, but for many pathogenicity is still to be elucidated 19. This is of the utmost relevance when a patient seeks preimplantation genetic diagnosis (PGD) or when a disease-modifying treatment for ADPKD can be offered but there is uncertainty over the diagnosis. PGD has evolved over recent years to become a valuable technique to offer patients with severe genetic diseases, although there are ethical issues regarding its use for non-severe genetic diseases. ADPKD is halfway between these categories, and genetic counseling is highly recommended to ensure sound understanding of the pros and cons of such a procedure 20. Probably, each case or family should be counseled differently according to the severity of the disease, but patient attitudes to PGD vary considerably depending on how well the family is dealing with the disease, religious aspects, age, disease severity, and so on.
Progression of autosomal dominant polycystic kidney disease
A large number of factors have been identified as influencing disease progression 21, and whereas some are modifiable, many are not 22. Among the latter are male sex, PKD1 mutations (worse if protein-truncating (PT)), early development of renal symptoms, early detection of high blood pressure 23, and large kidney volumes in relation to age and height 24, 25. Modifiable factors that should be highlighted in patients with ADPKD include smoking; blood pressure; lipid levels; water, protein, and calorie intake; and body mass index (BMI) 26– 31. Also, some biomarkers, such as MCP-1, FGF23, and copeptin, have been found to be predictive factors of ADPKD progression 32– 34.
The prediction of which patients will have a rapid progression is crucial not only for the decision of whether to use a disease-modifying drug but also for the recruitment of patients to clinical trials 35. Although in the past few years many attempts have been made to provide tools predictive of rapid disease progression, to date there is no gold standard. Some of these predictive tools employ imaging; an example is the Mayo Clinic ADPKD calculator, which uses the height-adapted total kidney volume (htTKV) to classify patients into one of five classes (A–E) on the basis of growth rates: less than 1.5%, 1.5–3%, 3–4.5%, 4.5–6%, or more than 6% per year 36. Measuring TKV for use as a prognostic biomarker does not require high precision. Measurement by the ellipsoid equation as well as by means of various imaging modalities is possible 27, 37. Another imaging approach that can be used to predict PKD progression involves the measurement of renal length. Bhutani et al. demonstrated that a renal diameter of 16.8 cm is predictive of chronic kidney disease (CKD) stage 3 within 8 years 38. An alternative to imaging-based prognostic strategies is the Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) scoring system, as shown in a large cohort from Brittany, France 23. This scoring system uses both clinical and genetic data to stratify risk of disease progression. Patients may reach a score suggestive of rapid progression if they have hypertension or early urinary symptoms before the age of 35 years plus a PT mutation. Male sex is also included in the scoring system. Obvious drawbacks of this predictive tool are the need to perform genetic testing, which may not be available in many centers, and the impossibility of employing it in patients younger than 35 who are asymptomatic. In addition, it is subject to the need to establish a relationship between early symptoms and ADPKD and the limitation that many rapid progressors with PT mutations do not develop early symptoms.
The European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Working Groups on Inherited Kidney Disorders and European Renal Best Practice (WGIKD/ERBP) developed a hierarchical decision-making algorithm that may offer guidance on the identification and prediction of rapid disease progression in patients with ADPKD and subsequently identify candidates for tolvaptan treatment 39. When this algorithm was tested in a cohort of 305 patients, it was found that 15.7% fulfilled the ERA-EDTA criteria and that the overall proportion of patients with rapid progression rose to 27% upon incorporation of expanded criteria based on data from the REPRISE (Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD) trial 40 (that is, age of less than 56 years and estimated glomerular filtration rate [eGFR] of more than 30 mL/min/m 2) 41. This study, together with a recent assessment of an unpublished large series of patients, calls into question the suitability of the algorithm, especially in its first steps when limiting for age/eGFR and using retrospective eGFR. Each country, even region, in Europe has either approved the use of the drug on these terms or proposed some changes. For example, the National Institute for Health and Care Excellence (NICE) decision in the UK was to treat on the basis of these criteria but only patients with CKD stage 2 to 3 without limiting age, whereas in Scotland they approved its use in stage 1. In the US, the preferred approach for definition of a rapid progressor is based on htTKV, age, and eGFR, basically using the Mayo ADPKD calculator 42. Risk assessment in ADPKD is an evolving process that will undergo further refinement as new clinical data are obtained and prediction tools are developed. The performance of risk assessment guidelines needs to be evaluated and validated by real-life clinical data, but it is probable that the use of a composite tool including age, renal function, imaging, family history and possibly genetic testing will be able to define rapid progression very accurately.
Basic renal protective measures
Based on the HALT-PKD (Halt Progression of Polycystic Kidney Disease) trial, the recommendations regarding blood pressure control for ADPKD were recently lowered 26. The target should now be 95–110/60–75 mmHg in 15- to 49-year-old patients with an eGFR of more than 60 mL/min/1.73 m 2. First-line management of hypertension should include a blocker of the renin–angiotensin–aldosterone system (angiotensin-converting enzyme inhibitors and angiotensin-II receptor blockers) 27. Second- and third-line treatments should be diuretics and beta-blockers. Calcium channel blockers (particularly the dihydropyridine class) should be considered if blood pressure is not controlled by the other agents 27. The CRISP (Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease) and HALT studies suggest that moderate dietary sodium restriction (2.3–3 g) be recommended to patients with ADPKD 22.
In patients with cyst infection, a positron emission tomography/computed tomography scan is recommended to enable determination of the location of cysts for the purpose of diagnosis or drainage 43. Fluoroquinolones remain the first-line antibiotic treatment, but their side effects and the increasing prevalence of fluoroquinolone-resistant Gram-negative bacilli mean that cyst infections frequently represent a very large burden.
Until more information becomes available 44, 45, moderate enhancement of hydration, spread out over the course of 24 hours, is recommended with the goal of maintaining an average urine osmolality of not more than 280 mOsm/L 27, 46.
Based on experimental studies and lessons learned from CKD in general, a daily protein intake of 0.8–1.0 g/kg ideal body weight seems appropriate in ADPKD 27. Although protein-restricted diets are usually already low in phosphorus, patient education on the need to avoid processed foods that contain readily absorbed inorganic phosphates is appropriate. Sodium bicarbonate supplementation is recommended in order to treat metabolic acidosis, and the aim is to maintain the plasma bicarbonate level at a minimum of 22 mmol/L 27.
Mild to moderate food restriction has been found to markedly reduce cystogenesis in animal models of PKD 47, 48. This fact, together with evidence that excess food intake has a detrimental effect both in PKD trials and in CKD in general, means that moderation of caloric intake and avoidance of an above-average BMI should be recommended to patients with ADPKD 29.
A small trial with statins in children has yielded positive results 49, but a post-hoc analysis of the HALT-PKD trials failed to demonstrate a benefit of statin therapy on renal outcomes 50. However, evidence in ADPKD 22, 31, and in CKD in general, indicates that it is appropriate to keep the low-density lipoprotein (LDL) cholesterol level at not more than 100 mg/dL.
Disease-modifying treatments
In the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3:4 trial, the vasopressin receptor antagonist tolvaptan was shown to slow the growth of cystic kidneys and the deterioration of renal function 7. Based on these results, the European Medicines Agency in 2015 approved tolvaptan to slow the progression of cyst development and renal insufficiency in ADPKD in adults with CKD stages 1–3 at initiation of treatment and evidence of rapidly progressing disease 51. TEMPO 4:4 showed that benefit is sustained over time 52.
In 2018, tolvaptan was approved by the US Food and Drug Administration for the purpose of slowing decline in kidney function in adults at risk of rapidly progressing ADPKD. This decision was based on additional information provided by the REPRISE trial, which demonstrated slowing in the deterioration of renal function, even in the later stages of renal failure 8.
Currently, more than 6,000 patients with ADPKD are being treated with tolvaptan around the world. However, tolvaptan does have side effects. It markedly impairs urinary concentrating ability and therefore patients experience polyuria, nocturia, and polydipsia 7, 8. In addition, a small proportion of patients develop liver function abnormalities; it appears that whereas some of these abnormalities can be significant, all resolve upon drug discontinuation 53. This rare drug-induced liver injury led to the institution of a risk evaluation and mitigation strategy with monthly liver enzyme tests for the first 18 months and then at 3-month intervals.
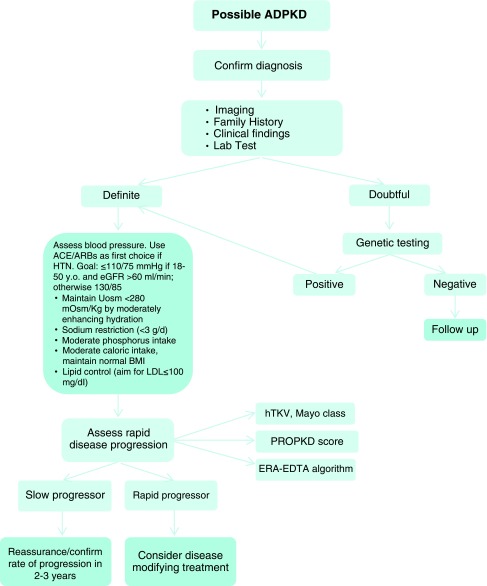
A few other drugs, such as mammalian target of rapamycin (mTOR) inhibitors, somatostatin analogues, and bosutinib, also underwent clinical trials but failed to show any positive effect on disease progression 54– 57. Recommendations for the management of ADPKD are shown in Figure 1.
Figure 1. Diagnosis and management of autosomal dominant polycystic kidney disease.
ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; ERA-EDTA, European Renal Association-European Dialysis and Transplant Association; hTKV, height-adapted total kidney volume; HTN, hypertension; LDL, low-density lipoprotein; PROPKD, Predicting Renal Outcome in Polycystic Kidney Disease; Uosm, urine osmolality. Modified from Chebib et al. 27 and Chebib et al. 42.
Many new drugs are being tested, and it is probable that more than one drug will be needed to target the different abnormal pathways found in PKD cells.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
John Sayer, Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, NE1 3BZ, UK
Chang-Lin Mei, Kidney Institute, Department of Nephrology, Shanghai Changzheng Hospital, Second Military Medical University, Shanghai, China
Funding Statement
This work was funded by the ISCIII: RETIC REDINREN RD16/0009 FIS FEDER FUNDS (PI15/01824 and PI16/01998) and the Catalan Government (AGAUR 2014/SGR-1441).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–85. 10.1056/NEJMcp0804458 [DOI] [PubMed] [Google Scholar]
- 2. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell. 1994;78(4):725. [PubMed] [Google Scholar]
- 3. Mochizuki T, Wu G, Hayashi T, et al. : PKD2, a Gene for Polycystic Kidney Disease That Encodes an Integral Membrane Protein. Science. 1996;272(5266):1339–42. 10.1126/science.272.5266.1339 [DOI] [PubMed] [Google Scholar]
- 4. Audrézet MP, Cornec-Le Gall E, Chen JM, et al. : Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat. 2012;33(8):1239–50. 10.1002/humu.22103 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Torra R, Badenas C, Darnell A, et al. : Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142–51. [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Cornec-Le Gall E, Audrézet MP, Le Meur Y, et al. : Genetics and Pathogenesis of Autosomal Dominant Polycystic Kidney Disease: 20 Years On. Hum Mutat. 2014;35(12):1393–406. 10.1002/humu.22708 [DOI] [PubMed] [Google Scholar]
- 7. Torres VE, Chapman AB, Devuyst O, et al. : Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2012;367(25):2407–18. 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Torres VE, Chapman AB, Devuyst O, et al. : Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017;377(20):1930–1942. 10.1056/NEJMoa1710030 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Porath B, Gainullin VG, Cornec-Le Gall E, et al. : Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98(6):1193–1207. 10.1016/j.ajhg.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Cornec-Le Gall E, Olson RJ, Besse W, et al. : Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am J Hum Genet. 2018;102(5):832–844. 10.1016/j.ajhg.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Eckardt KU, Alper SL, Antignac C, et al. : Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015;88(4):676–83. 10.1038/ki.2015.28 [DOI] [PubMed] [Google Scholar]
- 12. Perales MA, Schwartz DH, Fabry JA, et al. : A vaccinia-gp160-based vaccine but not a gp160 protein vaccine elicits anti-gp160 cytotoxic T lymphocytes in some HIV-1 seronegative vaccinees. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(1):27–35. 10.1097/00042560-199509000-00004 [DOI] [PubMed] [Google Scholar]
- 13. Peral B, Gamble V, Strong C, et al. : Identification of mutations in the duplicated region of the polycystic kidney disease 1 gene ( PKD1) by a novel approach. Am J Hum Genet. 1997;60(6):1399–410. 10.1086/515467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carrera E, Maeder-Ingvar M, Rossetti AO, et al. : Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007;24(1):97–103. 10.1159/000103123 [DOI] [PubMed] [Google Scholar]
- 15. Cornec-Le Gall E, Chebib FT, Madsen CD, et al. : The Value of Genetic Testing in Polycystic Kidney Diseases Illustrated by a Family With PKD2 and COL4A1 Mutations. Am J Kidney Dis. 2018;72(2):302–8. 10.1053/j.ajkd.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Rossetti S, Hopp K, Sikkink RA, et al. : Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23(5):915–33. 10.1681/ASN.2011101032 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Bullich G, Domingo-Gallego A, Vargas I, et al. : A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int. 2018;94(2):363–71. 10.1016/j.kint.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 18. Bergmann C: Recent advances in the molecular diagnosis of polycystic kidney disease. Expert Rev Mol Diagn. 2017;17(12):1037–54. 10.1080/14737159.2017.1386099 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Cornec-Le Gall E, Torres VE, Harris PC: Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J Am Soc Nephrol. 2018;29(1):13–23. 10.1681/ASN.2017050483 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Swift O, Vilar E, Rahman B, et al. : Attitudes in Patients with Autosomal Dominant Polycystic Kidney Disease Toward Prenatal Diagnosis and Preimplantation Genetic Diagnosis. Genet Test Mol Biomarkers. 2016;20(12):741–6. 10.1089/gtmb.2016.0050 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Schrier RW, Brosnahan G, Cadnapaphornchai MA, et al. : Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25(11):2399–418. 10.1681/ASN.2013111184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torres VE, Grantham JJ, Chapman AB, et al. : Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(3):640–7. 10.2215/CJN.03250410 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Cornec-Le Gall E, Audrézet MP, Rousseau A, et al. : The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2016;27(3):942–51. 10.1681/ASN.2015010016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Grantham JJ, Torres VE: The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol. 2016;12(11):667–77. 10.1038/nrneph.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Yu ASL, Shen C, Landsittel DP, et al. : Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2018;93(3):691–9. 10.1016/j.kint.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Schrier RW, Abebe KZ, Perrone RD, et al. : Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371(24):2255–66. 10.1056/NEJMoa1402685 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Chebib FT, Torres VE: Recent Advances in the Management of Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol. 2018;13(11):1765–76. 10.2215/CJN.03960318 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Torres VE, Abebe KZ, Chapman AB, et al. : Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371(24):2267–76. 10.1056/NEJMoa1402686 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Nowak KL, You Z, Gitomer B, et al. : Overweight and Obesity Are Predictors of Progression in Early Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2018;29(2):571–8. 10.1681/ASN.2017070819 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Torres VE, Bankir L, Grantham JJ: A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1140–50. 10.2215/CJN.00790209 [DOI] [PubMed] [Google Scholar]
- 31. Klahr S, Breyer JA, Beck GJ, et al. : Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol. 1995;5(12):2037–47. [DOI] [PubMed] [Google Scholar]
- 32. Messchendorp AL, Meijer E, Boertien WE, et al. : Urinary Biomarkers to Identify Autosomal Dominant Polycystic Kidney Disease Patients With a High Likelihood of Disease Progression. Kidney Int Rep. 2018;3(2):291–301. 10.1016/j.ekir.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Pavik I, Jaeger P, Ebner L, et al. : Soluble klotho and autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(2):248–57. 10.2215/CJN.09020911 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Meijer E, Bakker SJL, van der Jagt EJ, et al. : Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):361–8. 10.2215/CJN.04560510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornec-Le Gall E, Blais JD, Irazabal MV, et al. : Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial. Nephrol Dial Transplant. 2017. 10.1093/ndt/gfx188 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Irazabal MV, Rangel LJ, Bergstralh EJ, et al. : Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–72. 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Magistroni R, Corsi C, Martí T, et al. : A Review of the Imaging Techniques for Measuring Kidney and Cyst Volume in Establishing Autosomal Dominant Polycystic Kidney Disease Progression. Am J Nephrol. 2018;48(1):67–78. 10.1159/000491022 [DOI] [PubMed] [Google Scholar]
- 38. Bhutani H, Smith V, Rahbari-Oskoui F, et al. : A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88(1):146–51. 10.1038/ki.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Gansevoort RT, Arici M, Benzing T, et al. : Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–48. 10.1093/ndt/gfv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wyatt CM, Le Meur Y: REPRISE: tolvaptan in advanced polycystic kidney disease. Kidney Int. 2018;93(2):292–5. 10.1016/j.kint.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 41. Furlano M, Loscos I, Martí T, et al. : Autosomal Dominant Polycystic Kidney Disease: Clinical Assessment of Rapid Progression. Am J Nephrol. 2018;48(4):308–17. 10.1159/000493325 [DOI] [PubMed] [Google Scholar]
- 42. Chebib FT, Perrone RD, Chapman AB, et al. : A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J Am Soc Nephrol. 2018;29(10):2458–70. 10.1681/ASN.2018060590 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Lantinga MA, Casteleijn NF, Geudens A, et al. : Management of renal cyst infection in patients with autosomal dominant polycystic kidney disease: a systematic review. Nephrol Dial Transplant. 2017;32(1):144–50. 10.1093/ndt/gfv452 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. El-Damanawi R, Harris T, Sandford RN, et al. : Patient Survey of current water Intake practices in autosomal dominant Polycystic kidney disease: the SIPs survey. Clin Kidney J. 2017;10(3):305–9. 10.1093/ckj/sfw153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong ATY, Mannix C, Grantham JJ, et al. : Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ Open. 2018;8(1):e018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark WF, Sontrop JM, Huang SH, et al. : Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am J Nephrol. 2016;43(4):281–92. 10.1159/000445959 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Warner G, Hein KZ, Nin V, et al. : Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J Am Soc Nephrol. 2016;27(5):1437–47. 10.1681/ASN.2015020132 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Kipp KR, Rezaei M, Lin L, et al. : A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol. 2016;310(8):F726–F731. 10.1152/ajprenal.00551.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Cadnapaphornchai MA, George DM, McFann K, et al. : Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9(5):889–96. 10.2215/CJN.08350813 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Brosnahan GM, Abebe KZ, Rahbari-Oskoui FF, et al. : Effect of Statin Therapy on the Progression of Autosomal Dominant Polycystic Kidney Disease. A Secondary Analysis of the HALT PKD Trials. Curr Hypertens Rev. 2017;13(2):109–20. 10.2174/1573402113666170427142815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. European Medicines Agency: Assessment report: Jinarc. Procedure No. EMEA/H/C/002788/0000. EMA/154879/2015. London, England: European Medicines Agency:2015. Reference Source [Google Scholar]
- 52. Torres VE, Chapman AB, Devuyst O, et al. : Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2018;33(3):477–489. 10.1093/ndt/gfx043 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Watkins PB, Lewis JH, Kaplowitz N, et al. : Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug Saf. 2015;38(1):1103–13. 10.1007/s40264-015-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Serra AL, Poster D, Kistler AD, et al. : Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):820–9. 10.1056/NEJMoa0907419 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Tesar V, Ciechanowski K, Pei Y, et al. : Bosutinib versus Placebo for Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2017;28(11):3404–13. 10.1681/ASN.2016111232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walz G, Budde K, Mannaa M, et al. : Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–40. 10.1056/NEJMoa1003491 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Meijer E, Visser FW, van Aerts RMM, et al. : Effect of Lanreotide on Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease: The DIPAK 1 Randomized Clinical Trial. JAMA. 2018;320(19):2010–9. 10.1001/jama.2018.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation