ABSTRACT
Introduction: Hospital mortality among chronic obstructive pulmonary disease (COPD) patients receiving NIV for acute respiratory failure has shown to be significantly higher in clinical settings than in the randomized trials (RCTs) which clinical guidelines are based on. This may be due to the quality of care of NIV or patient selection. In daily clinical practice, we include patients with terminal pulmonary disease with a do-not-intubate (DNI) or a do-not-resuscitate (DNR) order with a high mortality risk compared to highly selected patients in RCTs. The aim of this study was to determine the role of patient selection for in-hospital mortality among patients receiving NIV for acute respiratory failure of COPD.
Methods: We conducted a retrospective study including all patients receiving acute NIV due to acute respiratory failure at the respiratory wards in 2012–2013 at two hospitals in Greater Copenhagen.
Results: Overall in-hospital mortality rate was 30%. In patients with a DNI/DNR order, mortality was 59% and in patients with no limitations in treatment 2%. Patients who fulfilled the exclusion criteria of the RCT by Plant et al. had a mortality of 41% compared to 25% in the remaining population.
Conclusions: High overall in-hospital mortality reflects that patient selection in clinical practice is very different from RCT. Quality of acute NIV treatment seems acceptable in clinical practice for patients with less severe COPD and no limitations in treatment. Higher mortality in patients with DNI/DNR order may be due to inefficient NIV treatment for these patients with more severe COPD.
KEYWORDS: Non-invasive ventilation NIV, acute respiratory failure, chronic obstructive pulmonary disease COPD, mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health concern and is according to the World Health Organization (WHO) the fourth leading cause of death globally [1]. In Denmark, 400,000 people are estimated to have COPD, accountable for 22.000 hospital admissions annually [2,3]. In about 11% of these hospital admissions, patients are treated with non-invasive positive-pressure ventilation (NIV) due to acute hypercapnic respiratory failure (AHRF) secondary to exacerbation of COPD [2].
Treatment with NIV has been implemented globally based on randomized controlled trials (RCT) [4–6] of which the study by Plant et al. [5] published in The Lancet in 2000 is possibly the most renowned. Since NIV has become a primary treatment option as add-on to the usual care for treatment of AHRF due to exacerbation of COPD, there has been a decrease in mortality and need of intubation, more rapid improvement and decrease in hospital stay for this patient group [6].
Acute NIV was implemented in Danish hospitals in 2005–2010 with national recommendations and local guidelines based on the RCTs for indication and practical conduct of treatment. In-hospital mortality among COPD patients receiving NIV for AHRF has shown to be significantly higher in clinical setting than in RCTs [7,8]. Higher mortality can be explained by the quality of care and efficiency of NIV treatment, or by patient selection for treatment. In daily clinical practice, acute NIV treatment is also provided to patients with a terminal condition, with a ‘do-not-intubate’ (DNI) or a ‘do-not-resuscitate’ (DNR) order and a high mortality risk.
In the present study, we aim to (1) conduct a quality review of acute NIV treatment in a standard clinical setting, (2) to describe in-hospital mortality particularly in relation to the role of patient selection in two major respiratory wards in Copenhagen and (3) compare in-hospital mortality in our cohort to the study population of the RCT by Plant et al.
Material and methods
Data extraction
Data on all patients receiving acute NIV for any cause at the respiratory wards at two hospitals in Greater Copenhagen, Bispebjerg Hospital and Gentofte Hospital, from 1 January 2012 to 31 December 2013, were retrospectively retrieved. Records of all patients were reviewed 2 years after discharge from index admission by three of the authors, SHS, DBR and KLA. Data were extracted according to a predefined protocol: baseline characteristics at index admission, data concerning index admission, number of readmissions and mortality.
Guidelines for NIV treatment
All patients were admitted through the acute medical ward, where initial examination was performed with arterial blood gas analysis, blood samples and chest X-ray. Patients with assumed acute exacerbation of COPD were treated with inhaled bronchodilators, systemic corticosteroids and if indicated oxygen therapy and antibiotics.
According to local guidelines and national recommendations by the Danish Society of Respiratory Medicine, AHRF with indication for NIV was defined as pH < 7.35 and PaCO2 > 6 kPa after 1–2 h of initial treatment in patients with a recognized or suspected COPD diagnosis, with worsening of dyspnoea and/or respiratory rate of more than 25 per minute and/or PaO2 < 7 kPa without oxygen supply [9]. In contrast to the RCT by Plant et al., recommendations did not include a specific respiratory rate of more than 23 per minute, or a criterion of AHRF within a maximum of 12 h after admission. Patients developing AHRF during admission, regardless of time of debut, were treated with NIV in accordance to clinical guidelines and included in the present study. Arterial blood gasses were taken at debut of respiratory insufficiency and obtained in the data set. Unfortunately as a retrospective study, we were not able to differentiate how many patients who developed AHRF more than 12 h after admission, nor do we have data on their admission previous to the debut of AHRF.
Patients were transferred to the respiratory ward for NIV treatment according to the national recommendations by the Danish Society of Respiratory Medicine. Guidelines were unchanged during the study period. Recommended initial inspiratory positive airway pressure (IPAP) was 10 cmH2O and expiratory positive airway pressure was 4 cmH2O. Medical staff, including physicians and nurses, had all been trained in handling NIV and in guidelines of treatment.
Patients who had been treated with NIV at the intensive care unit (ICU) but had not been intubated were also included in the study as they were moved to the respiratory ward for further NIV treatment.
COPD was defined in accordance to The Global Initiative for Chronic Obstructive Lung Disease guidelines 2012 [10] and diagnosis was made by the treating physician based on clinical history, spirometry and physical examination.
Subgroup analysis
We performed two subgroup analyses:
Patients with a DNI/DNR order were compared to patients with no DNI/DNR order. DNI and DNR orders were always presented combined as DNI/DNR order in the records and never obtained exclusively. DNR/DNI orders were placed after assessment of the patient’s general daily activity level and functional impairment, severity of disease, comorbid conditions and patient’s own wish and with less consideration to the course of current treatment. DNI/DNR orders were placed by a treating physician before, within 2 h after initiation of NIV, or later on during NIV treatment if this had not been done initially. Senior physicians were always consulted and if possible, the patient and their relatives too.
A subgroup analysis was performed for comparison of our study population to the populations of the RCT by Plant et al. [5]. Patients who fulfilled the exclusion criteria of the RCT of Plant et al., pH < 7.25 and Glasgow coma scale (GCS) <8, were compared to the remaining patients in the population. As mentioned above, the exclusion criterion of development of AHRF within 12 h of admission of Plant et al. could not be met since patients in the present study were treated with NIV regardless of time of debut of AHRF. Patients with debut of AHRF more than 12 h after admission could unfortunately not be identified and differentiated from patients with AHRF at time of admission in the present dataset. All patients were therefore included in the subgroup analysis, regardless of time for development of AHRF. DNI/DNR order was not an exclusion criterion.
Statistical analysis
Characteristics were presented as numbers and percentages for categorical variables. Continuous variables were presented as medians and interquartile range.
We compared differences in groups using Pearson’s chi-squared for categorical variables and Wilcoxon rank-sum for continuous variables. Significance was determined at p < 0.05.
Statistical analysis was performed using Microsoft Excel and RStudio Desktop (version 1.1.383).
Ethical considerations
Data and patient information were extracted and handled according to Danish legislation, and with approval from the Scientific Ethics Committee and the Data Protection Board (j.nr: GEH-2014-005, I-Suite nr: 02618).
Results
A total of 304 patients were treated with acute NIV during the 2-year study period. Fifteen patients were excluded due to AHRF not caused by COPD (incorrect COPD diagnosis n = 3, congestive heart failure n = 3, asthma n = 1, pneumonia n = 6, pneumonia and pulmonary oedema n = 2, pneumonia and overdose of opiates n = 1, malignant pleural effusion = 1, idiopathic pulmonary fibrosis and pneumonia n = 1). Accordingly, 286 patients received treatment with NIV for AHRF due to COPD and are referred to as the study population. Among those, 141 (49%) patients had a DNI/DNR order (Figure 1). In 101 patients (72%), the DNI/DNR order was placed before or within 2 h after initiation of NIV treatment, and the remaining 40 patients (28%) received the DNI/DNR order after more than 2 h of NIV treatment. A total of 15 (5.2%) were transferred from the ICU for further treatment in the respiratory ward. Among those, 10 patients needed more NIV treatment while the remaining were stabilized and treated conservative. Further, 21 patients (7.3%) failed NIV in the respiratory ward and were transferred to ICU.
Figure 1.
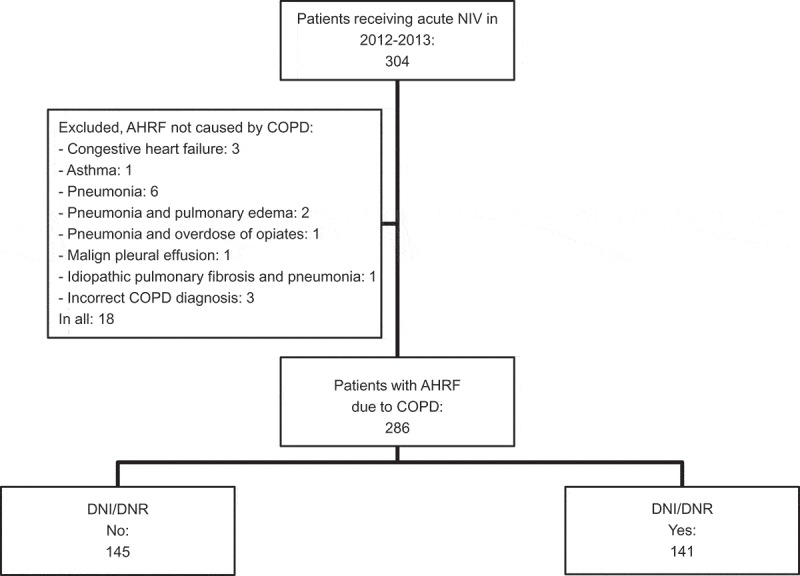
Overview of the study population.
Baseline characteristics
Baseline characteristics are presented in Table 1. Patients with no DNI/DNR order were younger (68 vs. 80 years, p < 0.001), more were male (48% vs. 28%, p = 0.01) and fewer received long-term oxygen therapy (12% vs. 28%, p = 0.004).
Table 1.
Baseline characteristics.
| Total (n = 286) |
DNI/DNR order No (n = 145) |
DNI/DNR order Yes (n = 141) |
p | Fulfilled exclusion criteria, Plant et al. Yes (n = 98) |
Fulfilled exclusion criteria, Plant et al. No (n = 183) |
p | |
|---|---|---|---|---|---|---|---|
| Age (years) Median (IQ range) |
75.5 (66–82) |
68 (61–76) |
80 (75–86) |
<0.001 | 76 (67–82) |
75 (65.5–82) |
0.618 |
| Male n (%) | 109 (38.1%) | 69 (47.6%) | 40 (28.4%) | 0.01 | 37 (37.8%) | 68 (37.3%) | 0.94 |
| FEV1% of predicted (n = 211) Median (IQ range) |
32 (23.3–43) |
34 (25–43.2) |
29 (22–42.3) |
0.221 | 30 (27–42) |
33 (23.3–45.3) |
0.421 |
| MRC score (n = 184) Median (IQ range) |
4 (4–5) |
4 (3–5) |
4 (4–5) |
<0.001 | 4 (4–5) |
4 (4–5) |
0.891 |
| LTOT (n 285) n (%) | 57 (19.9%) | 18 (12.4%) | 39 (27.7%) | 0.004 | 21 (21.6%) | 35 (19.1%) | 0.653 |
| Smoking (n 283) n (%) | |||||||
| – Never | 15 (5.3%) | 2 (1.4%) | 13 (9.4%) | 0.003 | 6 (6.3%) | 9 (4.9%) | 0.656 |
| – Former | 148 (52.3%) | 61 (42.4%) | 87 (62.6%) | 0.019 | 48 (50.0%) | 97 (53.3%) | 0.717 |
| – Current | 120 (42.4%) | 81 (56.3%) | 39 (28.1%) | <0.001 | 42 (43.8%) | 76 (41.8%) | 0.808 |
| Pack years (n 264) Median (IQ range) |
40 (30–55) |
40 (34–50) |
40 (30–60) |
0.590 | 40 (30–51.3) |
45 (33–55.5) |
0.080 |
| Admitted with COPD within past month n (%) | 47 (16.4%) | 21 (14.4%) | 26 (18.4%) | 0.41 | 19 (19.4%) | 27 (14.8%) | 0.360 |
| Admitted with COPD within past year n (%) | 110 (38.5%) | 55 (37.9%) | 55 (39.0%) | 0.88 | 38 (38.8%) | 70 (38.3%) | 0.946 |
| No. of COPD admissions within the last year Median (IQ range) |
0 (0–1) |
0 (0–1) |
0 (0–1) |
0.654 | 0 (0–1) |
0 (0–1) |
0.979 |
We then evaluated whether the patients fulfilled the exclusion criteria of the RCT by Plant et al. (pH < 7.25 and GCS < 8). A total of 98 patients fulfilled the exclusion criteria whereas the remaining 183 patients assumedly would have been eligible for the RCT by Plant et al. (Figure 2). Due to missing data, five patients could not be evaluated for eligibility (missing initial arterial blood gas n = 3, missing GCS n = 2). There was no significant difference in baseline characteristic within the two subgroups (Table 1).
Figure 2.
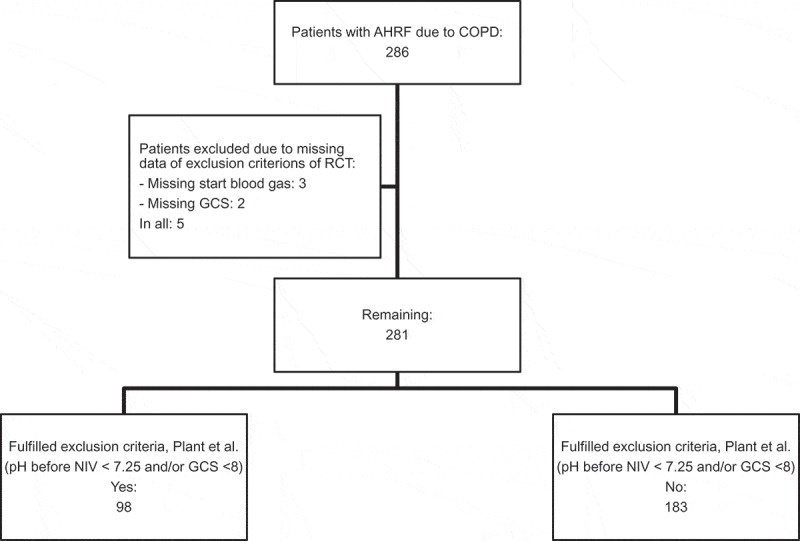
Subgroup analysis for comparison to the RCT by Plant et al.
The index admission
In Table 2, data concerning the index admission with NIV treatment for AHRF due to COPD are listed. Median length of admission was 6 days and median treatment duration of NIV was 20 h. Median delay of initiation of NIV treatment, i.e. time from initial arterial blood gas to initiation of NIV treatment, was 1.73 h with no significant difference between subgroups.
Table 2.
Index admission.
| Total (n = 286) |
DNI/DNR order No (n = 145) |
DNI/DNR order Yes (n = 141) |
p | Fulfilled exclusion criteria, Plant et al. Yes (n = 98) |
Fulfilled exclusion criteria, Plant et al. No (n = 183) |
p | |
|---|---|---|---|---|---|---|---|
| Duration of admission (days) Median (IQ range) |
6 (3–10) |
6 (3–10) |
5 (2–10) |
0.233 | 5 (2–9) |
7 (4–10) |
0.002 |
| Pneumonia (n 284) n (%) | 129 (45.4%) | 64 (44.4%) | 65 (46.4%) | 0.804 | 52 (53.6%) | 75 (41.2%) | 0.144 |
| C-reactive protein max Median (IQ range) |
58 (19–159) |
49 (15–171) |
61 (25–158) |
0.215 | 65 (29–168) |
52 (19.3–152.5) |
0.179 |
| Fever (>38°C) (n 282) n (%) | 27 (9.6%) | 13 (9.1%) | 14 (10.1%) | 0.790 | 11 (11.2%) | 16 (8.8%) | 0.541 |
| Respiratory rate (n 249) Median (IQ range) |
28 (24–32) |
26 (22–30) |
28 (24–32) |
0.016 | 28 (24–32) |
27 (24–30) |
0.169 |
| Antibiotics n (%) | |||||||
| – None – Oral – Iv |
36 (12.6%) 32 (11.2%) 218 (76.2%) |
22 (15.2%) 19 (13.1% 104 (71.7%) |
14 (9.9%) 13 (9.2%) 114 (80.9%) |
0.211 0.326 0.378 |
10 (10.2%) 8 (8.2%) 80 (81.6%) |
24 (13.1%) 23(12.6%) 136 (74.3%) |
0.504 0.289 0.505 |
| Consciousness (n 284) n (%) | |||||||
| – Awake – Drowsy – Awaken by speech – Awaken by stimulation or unconscious |
195 (67.6%) 40 (14.1%) 34 (12.0%) 18 (6.3%) |
109 (76.2%) 19 (13.3%) 11 (7.7%) 4 (2.8%) |
83 (58.9%) 21 (14.9%) 23 (16.3%) 14 (9.9%) |
0.075 0.718 0.036 0.004 |
48 (49.0%) 18 (18.4%) 17 (17.3%) 15 (15.3%) |
142 (77.6%) 22 (12.0%) 32 (17.5%) 3 (1.6%) |
0.005 0.179 0.978 <0.001 |
| pH before NIV (n 283) Median (IQ range) |
7.27 (7.22–7.31) |
7.28 (7.24–7.31) |
7.26 (7.21–7.30) |
0.009 | 7.21 (7.17–7.23) |
7.29 (7.27–7.31) |
<0.001 |
| pCO2 before NIV (kPa/mmHg) (n 282) Median (IQ range) |
9.4/70.5 (8.1/60.8–11.3/84.8) |
9.0/67.5 (7.8/58.5– 11.5/86.3) |
10.3/77.3 (8.3/62.3–12.3/92.3) |
<0.001 | 11/82.5 (9.1/68.3–12.8/96.0) |
8.8/66.0 (7.8/58.5–10.4/78.0) |
<0.001 |
| Max IPAP (cmH2O) (n 285) Median (IQ range) |
12 (12–14) |
12 (12–14) |
12 (12–14) |
0.958 | 12 (12–16) |
12 (12–14) |
0.037 |
| Max EPAP (cmH2O) Median (IQ range) |
4 (4–5) |
4 (4–5) |
5 (4–5) |
0.060 | 4 (4–5) |
4 (4–5) |
0.579 |
| NIV treatment duration (hours) (n 280) Median (IQ range) |
20 (8.2–47.9) |
17,3 (8–41.9) |
44 (14.8–90.3) |
0.260 | 21 (8.5–51.3) |
19.1 (8.7–49.5) |
0.852 |
| Delay for NIV indication to treatment (hours) (n 284) Median (IQ range) |
1.73 (0.75–3.80) |
1.80 (1.0–4.05) |
1.5 (0.50–3.45) |
0.076 | 1.50 (0.70–3.0) |
1.78 (0.75–4.25) |
0.079 |
When comparing subgroup with no DNI/DNR and with DNI/DNR, significant difference was found in respiratory rate (26 vs. 28 per minute, p = 0.016), level of consciousness – Awaken by speech (8% vs. 16%, p = 0.036), Awaken by stimulation or unconscious (3% vs. 10%, p = 0.004), pH before NIV (7.28 vs. 7.26, p = 0.009) and pCO2 before NIV (9.0 kPa/67.5 mmHg vs. 10.3 kPa/77.3 mmHg, p <0.001).
Comparing patients who fulfilled the exclusion criteria for Plant et al. with the remainder of the population, pH before NIV (7.21 vs. 7.29, p < 0.001), pCO2 before NIV (11 kPa/82.5 mmHg vs. 8.8 kPa/66.0 mmHg, p< 0.001) and level of consciousness – Awaken by stimulation or unconscious (15% vs. 2%, p < 0.001) – were significantly different. Furthermore, maximum IPAP was significantly higher in patients who fulfilled the Plant et al. exclusion criteria compared to remaining patients (12 vs. 12 cmH2O, p = 0.037) (Table 2).
Mortality
Overall in-hospital mortality was 30.1%. In patients with and without DNI/DRI order, in-hospital mortality was 58.9% vs. 2.1% (p < 0.001), mortality 30 days after discharge was 20.7% vs. 9.2% (p = 0.036), 1 year mortality 53.4% vs. 25.6% (p = 0.002) and 2 years mortality 67.2% vs. 33.8% (p = 0.001), respectively (Table 3).
Table 3.
Prognostic data.
| Total (n = 286) |
DNI/DNR order No (n = 145) |
DNI/DNR order Yes (n = 141) |
p | Fulfilled exclusion criteria, Plant et al. Yes (n = 98) |
Fulfilled exclusion criteria, Plant et al. No (n = 183) |
p | |
|---|---|---|---|---|---|---|---|
| Mortality | |||||||
| – In-hospital | 86 (30.1%) | 3 (2.1%) | 83 (58.9%) | <0.001 | 40 (40.8%) | 46 (25.1%) | 0.034 |
| Mortality after discharge | |||||||
| – 30 days | 25 (12.5%) | 13 (9.2%) | 12 (20.7%) | 0.036 | 8 (13.8%) | 16 (11.7%) | 0.700 |
| – 1 year | 67 (33.3%) | 36 (25.6%) | 31 (53.4%) | 0.002 | 22 (37.9%) | 43 (31.4%) | 0.469 |
| – 2 years | 87 (43.3%) | 48 (33.8%) | 39 (67.2%) | 0.001 | 28 (47.5%) | 57 (41.7%) | 0.519 |
Comparing patients who fulfilled Plant et al. exclusion criteria and the remaining patients, in-hospital mortality was higher in those fulfilling the criteria (40.8% vs. 25.1%, p = 0.034) and the median length of hospital stay was shorter (5 vs. 7 days, p = 0.002).
Discussion
In this large retrospective study of 286 patients treated with NIV for AHRF due to exacerbation of COPD in a regular clinical setting, we found an overall high in-hospital mortality of 30%. Patients with a DNI/DNR order had a high in-hospital mortality of 59%. In a subgroup analysis of patients with severe respiratory acidosis (pH < 7.25) who were treated outside the guidelines based on RCTs, we found a significantly higher in-hospital mortality and 1-year mortality compared to the rest of the study population.
The early RCTs on acute NIV by Plant et al. and Brochard et al. found an in-hospital mortality of 9–10% in patients treated with NIV [4,5]. In a recent Cochrane review, evaluating 17 RCTs, similar in-hospital mortality was found: 9.9% in patients treated with NIV vs. 18.3% for patients receiving standard care [6]. When comparing the result of clinical trials with the mortality found in retrospective studies of NIV treatment in clinical practice, outcomes are less encouraging. A national audit of 1077 patients treated with NIV at 232 hospitals in the United Kingdom in 2008 found an in-hospital mortality of 25% [7]. Likewise, a smaller Italian study performed in 2008–2012 by Sainaghi et al. demonstrated a high in-hospital mortality of 27% [11].
Patients with severe disease are rarely eligible for RCTs although they represent a major part of patients treated in everyday clinical practice. In the present study, the study population was a rather heterogeneous group of COPD patients including the most chronically disabled, whereas a RCT includes a more homogeneous, selected population according to strict predefined criteria. Looking at the baseline characteristics of the RCT by Plant et al., there are considerable differences compared to the study population in the present study. The population of the RCT was younger than in the present study, average 69 years compared to median 75.5 years in the present population. Likewise, pH before NIV was 7.32 versus 7.27 in the present study. Plant et al. excluded patients with pH < 7.25, arguing that prognosis of these patients is poor without ventilator support and therefore it would be unethical to randomize these patients. In the present study, patients with low GCS, pH < 7.25 and high pCO2 were also treated with NIV as this is considered as the last chance of survival. These factors result in the poorer survival of patients receiving NIV treatment in the present study.
The in-hospital mortality of the present study of 2% in patients with no DNI/DNR order, 59% in patients with a DNI/DNR order and 41% in patients who would have been excluded in the Plant study reflects the clinical reality and heterogeneity compared to the RCTs on which our guidelines are based. Still, a considerable proportion (41%) of the most ill COPD patients, patients with a DNI/DNR order, survived an acute exacerbation with need of NIV treatment in the general respiratory ward. Nevertheless, the overall mortality in the present study was high and may reflect the fairly conservative national NIV guidelines at the time of the study, recommending rather and modest peak IPAP. Especially for patients with lack of initial response to NIV, better outcome can be assumed if IPAP is increased to 18–20 cmH2O within the first hour and rapidly increased further if needed. Unfortunately as a retrospective study, we were not able to collect sufficient data on pressure settings and the increase of those in accordance to blood gas pH at hours 1 and 4 of NIV treatment. This is essential for further analysis and a limitation of the study.
Studies of long-term NIV at home in COPD with chronic hypercapnia have shown that high IPAP of up to 30 cmH2O is possible and well tolerated by patients and increases survival [12]. In daily practice, we find that high IPAP is tolerated by many patients in the acute setting too. Studies are needed to demonstrate whether use of higher IPAP when needed improves survival.
A recent Cochrane review showed significantly greater benefits of NIV in patients with severe acidosis (pH < 7.30) than in patients with mild acidosis (pH 7.30–7.35) [6] but these patients must be monitored closely due to high risk of treatment failure and need of ICU treatment. Accordingly the latest ERS/ATS guidelines suggest no lower limit of pH for acute NIV treatment of patients with COPD and AHRF, though low pH is associated with a high risk of treatment failure [13]. Treatment of severe acidosis with NIV in ICU settings has been found efficient and safe. In a RCT, Khilnani et al. investigated NIV for treatment of severe acidosis (pH < 7.25) in COPD patients and concluded that the treatment was safe with rapid improvement of blood gases, less need for intubation and reduction in length of hospital stay compared to patients treated with conventional therapy [14]. In a prospective observational study, Diaz et al. studied treatment of patients with AHRF and coma (GCS < 8) with NIV, where COPD patients represent the largest subgroup. In-hospital mortality rates were generally high, with no difference in patients in coma or awake. Response rate was high in patients with COPD, with rapid improvement of GCS, pH and pCO2 within the first hour of NIV treatment [15].
In the present study, pH < 7.25 and low level of consciousness were associated with a higher degree of DNI/DNR orders and accordingly associated with even higher mortality than need of ICU treatment. This means that mortality may as well reflect the willingness to try NIV in the most severely (acute and chronic) ill COPD patients where NIV may offer the last chance of survival. It is crucial with further prospective studies on acute NIV treatment, performed on patients with more severe disease and with higher risk of treatment failure to investigate possible association with more aggressive NIV treatment and higher IPAP on successful treatment and survival. New danish national recommendations and local NIV guidelines implemented in 2017 recommend accelerated rise in IPAP if there is no significant effect of initial NIV treatment.
As for all retrospective studies, we have the issue of missing data in reviewed records, reducing the size and power of a study. For all parameters though, missing data were randomly distributed in subgroups, minimizing potential confounding factors. There is always the concern of selection bias, detection bias, reporting bias and confounding and results are therefore not directly comparable to RCTs. To minimize risk of bias, all records were reviewed according to a predefined study protocol. As the present study is a descriptive study of clinical practice, it is most likely presenting a more accurate description of challenges in treating patients in everyday ward than the RCTs.
As a retrospective study in a clinical setting, the data available was collected for clinical purposes of treatment without considering precise time or protocols. Clinical guidelines and criteria for AHRF and NIV treatment and physicians assessment do not fully meet the inclusion and exclusion criteria of RCT and data are therefore difficult to compare fairly. As previously mentioned, the national guidelines of the Danish Society of Respiratory Medicine differed from the inclusion and exclusion criteria of Plant et al. with no strict limit of respiratory rate and not including ‘AHRF within 12 h of admission’. In the clinical setting, patients were treated with NIV regardless of onset of AHRF on admission or later during admission. Unfortunately, the dataset does not allow us to identify the time for debut of AHRF. Attempting to make a true comparison with the population of the RCT by Plant et al. and the study population is therefore limited as exclusion criteria are not completely met. Nevertheless, we find the comparison important from a clinician’s perspective with less strict inclusion and exclusion criteria in daily practice. The British NCEPOD report of 2017 indicates higher mortality if NIV treatment was initiated at a later stage of admission [16]. This may also be reflected in the results of the present study.
Conclusion
This large retrospective study showed that patients receiving NIV at respiratory wards in Denmark are often more severely ill compared to the study populations in randomized controlled trials of NIV, resulting in a poorer prognosis. High-quality prospective studies are required to investigate the appropriate application of NIV to patient groups with more severe COPD and including patients with severe acidosis, reflecting patients treated in everyday clinical practice.
Biographies
Caroline Hedsund, MD, is a resident in specialist training in pulmonary diseases at the Department of Pulmonary medicine at Herlev and Gentofte Hospital. Research interests include COPD and NIV.
Kasper Linde Ankjærgaard, MD, PhD, Department of Clinical Medicine, University of Copenhagen. Main research focus are COPD and NIV.
Daniel Bech Rasmussen, MD, is a specialist in training in pulmonary diseases and a PhD student at Respiratory Research Unit Zealand, Department of Respiratory Medicine, Naestved Hospital and the Department of Regional Health Research, University of Southern Denmark. Main research topics are COPD and beta blockers.
Signe Høyer Schwaner, MD, is a specialist in respiratory medicine and working as a consultant at the Department of Respiratory Medicine at Bispebjerg Hospital, Copenhagen University. Main research interests are COPD and NIV.
Helle Frost Andreassen, MD, PhD, is a specialist in respiratory medicine and head of the Department of Respiratory Medicine at Bispebjerg Hospital, Copenhagen University.
Ejvind Farusing Hansen, MD, PhD, is a specialist in respiratory medicine and consultant at the Department of Pulmonary Medicine, Hospital Hvidovre, Copenhagen University. Main research topics are COPD, NIV, Telemedicine and titrated oxygen treatment.
Torgny Wilcke, MD, PhD, is a specialist in respiratory medicine and consultant at the Department of Pulmonary Medicine at Herlev and Gentofte Hospital. He is associate Professor at clinical section of University of Copenhagen and main research focus is COPD, rehabilitation, acute and long term NIV and palliation. Torgny is a member of the Danish Respiratory Society.
Funding Statement
The authors did not receive any grants for this specific study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Geolocation information
The study was performed in greater Copenhagen, Denmark.
References
- [1].World Health Organization (WHO) Top 10 causes of death world wide. 2017January [cited 2017 Okt 11]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- [2].DrKOL: Dansk register for Kronisk Obstruktiv Lungesygdom National Årsrapport 2013. January 1, 2013-December 31, 2013 [Danish Register of Chronic Obstructive Pulmonary Disease. National Annual Report 2013. Jan 1, 2013-December 31, 2013]. 2014June [cited 2017 Okt 11]. Available from: https://www.sundhed.dk/content/cms/90/4690_drkol-%C3%A5rsrapport-2013_kommenteret_v5_20062014.pdf
- [3].Lokke A, Fabricius PG, Vestbo J, et al. Prevalence of chronic obstructive pulmonary disease in Copenhagen. Results from the Copenhagen City heart study. Ugeskr Laeger. 2007November12;169(46):3956–8. [PubMed] [Google Scholar]
- [4].Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995September28;333(13):817–822. [DOI] [PubMed] [Google Scholar]
- [5].Plant PK, Owen JL, Elliott MW.. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000June3;355(9219):1931–1935. [DOI] [PubMed] [Google Scholar]
- [6].Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017July;13(7):CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roberts CM, Stone RA, Buckingham RJ, et al. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011January;66(1):43–48. [DOI] [PubMed] [Google Scholar]
- [8].Titlestad IL, Lassen AT, Vestbo J. Long-term survival for COPD patients receiving noninvasive ventilation for acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2013;8:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Løkke A, Dahl R, Lange P, et al. Danish COPD guidlines 2012 - Danish lung disease society Danske KOL-guidelines DLS-2012. 2012. [Cited 2017 Okt 11]. Available from: https://www.lungemedicin.dk/fagligt/101-dansk-kol-retningslinje-2012/file.html
- [10].Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013February15;187(4):347–365. [DOI] [PubMed] [Google Scholar]
- [11].Sainaghi PP, Colombo D, Re A, et al. Natural history and risk stratification of patients undergoing non-invasive ventilation in a non-ICU setting for severe COPD exacerbations. Intern Emerg Med. 2016October;11(7):969–975. [DOI] [PubMed] [Google Scholar]
- [12].Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014September;2(9):698–705. [DOI] [PubMed] [Google Scholar]
- [13].Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017August;50: 1602426. [DOI] [PubMed] [Google Scholar]
- [14].Khilnani GC, Saikia N, Banga A, et al. Non-invasive ventilation for acute exacerbation of COPD with very high PaCO2: a randomized controlled trial. Lung India. 2010Jul-Sep;27(3):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diaz GG, Alcaraz AC, Talavera JCP, et al. Noninvasive positive-pressure ventilation to treat hypercapnic coma secondary to respiratory failure. Chest. 2005March;127(3):952–960. [DOI] [PubMed] [Google Scholar]
- [16].Juniper M, Ellis G, Smith NCE, et al. The national confidential enquiry into patient outcome and death. Inspiring change: a review of the quality of care provided to patients receiving non-invasive ventilation. London. NCEPOD. 2017July [cited 2018 Dec 18]. Available from: http://www.ncepod.org.uk/2017niv.html


