Abstract
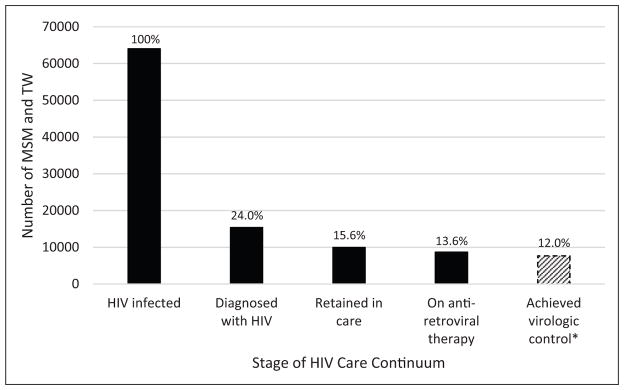
The HIV epidemic in Peru is concentrated in men who have sex with men and transgender women, who have an estimated prevalence > 10%, while the overall population prevalence remains < 1%. Because MSM and TW account for >60% of new infections, it is crucial to understand the full HIV continuum of care for these key populations. We performed a review of the peer-reviewed scientific and grey literature to determine the proportion of HIV-infected MSM and TW in Peru who are diagnosed, linked to and retained in care, taking antiretroviral therapy, and who have attained virologic suppression. Of the estimated 613,080 MSM and TW in Peru in 2015, approximately 63,981 are HIV-infected. Only 24.0% of HIV-infected MSM and TW are aware of their diagnosis, 15.6% are retained in care, 13.6% are on antiretroviral therapy, and 12.0% have achieved adequate virologic control. The largest drop-off in the HIV care continuum occurs at the first step: diagnosis of HIV. Improving HIV serostatus awareness among MSM and TW is crucial to controlling Peru’s HIV epidemic. In the era of ‘treatment as prevention’, understanding the full HIV care continuum may help guide efforts to curb transmission and reduce HIV-related morbidity and mortality.
Keywords: South America, HIV, men, prevention, treatment
Introduction
Recognition of the crucial role of treatment in HIV prevention implies the prioritization of early diagnosis, linkage to care, retention in care, initiation of antiretroviral therapy (ART), and ultimately virologic suppression as components of a comprehensive HIV control strategy.1 This HIV care continuum (HIVCC) was systematically represented in 20112 and has been used widely as a framework for the evaluation of HIV treatment and prevention programs. The HIVCC model has been applied to higher income2–4 and lower/middle income countries,5 regional compilations of country-level estimates,6 and key affected populations such as men who have sex with men (MSM)7 and transgender women (TW).8 MSM and TW are affected by a disproportionate HIV epidemic worldwide, with many regions reporting prevalence >15%.9 An estimation of the worldwide HIVCC among MSM also reports that a substantial proportion has never been tested for HIV, raising the possibility that many are unaware of their diagnosis.7 Within Latin America, an online survey was conducted which estimated the HIVCC among MSM for various countries, including Peru,10 but whether the internet-based findings are generalizable to a national level is unclear.
Peru is an upper-middle-income country with a concentrated HIV epidemic.11 While the overall prevalence in the general population is <1%, several studies have described a prevalence over 10% among MSM and over 20% among TW in Lima, with somewhat lower figures in other cities.12,13 The burden of ongoing HIV transmission is driven mainly by men, as evidenced by the male:female ratio of HIV infection, which has remained around 3:1 since 1998.11 Most women are diagnosed during prenatal care and report being monogamous. HIV prevalence among pregnant women is <0.25%.14 In contrast, MSM and TW account for at least 60% of new HIV diagnoses,15 likely a conservative estimate because many men who may have acquired HIV through sex with other men neither identify as homosexual or bisexual nor report those sexual experiences.16,17 In addition, some women who acquire HIV infection do so from men (and TW) who have sex with both men and women.18 As such, understanding the HIVCC in MSM and TW is crucial to improving treatment and care services for them, controlling HIV transmission in Peru, and shedding light on relevant measures in similar epidemics across the entire Latin American region.
We conducted a literature review to estimate the HIVCC among MSM and TW in Peru. Unfortunately, disaggregated data collection for MSM and TW started only in the last few years, so at this point the scarcity of data makes it unfeasible to conduct separate assessments for those two populations. As uniformly robust statistics were not available for every step in the HIVCC, our goal was to create a snapshot of the HIVCC in these groups and provide a framework for critical improvements in the surveillance and delivery of HIV care in Peru.
Methods
Since every step of the HIVCC is not routinely evaluated by ongoing HIV surveillance in Peru, we used both the grey literature (i.e. governmental/public health reports) and peer-reviewed publications for our estimates. Public data were obtained from the Peru Ministry of Health (http://www.dge.gob.pe/portal/) and UNAIDS (http://www.unaids.org/en/dataanalysis). International databases (PubMed, Scopus, Web of Science, and EBSCO Academic Search Complete) were searched using the terms ‘Human Immunodeficiency Virus’ or ‘HIV’, ‘Peru’, and ‘men who have sex with men’ or ‘MSM’ or ‘transgender’. In PubMed, MeSH terms ‘HIV’ and ‘Peru’ were also used. Articles in English and Spanish published between 1 January 2000 and 25 June 25 2015 were included if they contained any information on HIV diagnosis or serostatus awareness, linkage to care, retention in care, ART, or virologic suppression. Additional information was obtained through personal communication with experts in HIV epidemiology in Peru who helped identify additional reports and commented on the plausibility of preliminary findings. Because the studies varied greatly in their quality and potential biases, we chose the most recent study that was most representative and most specific to MSM and TW to estimate each HIVCC step. These steps were then combined to create an overall estimate of the HIVCC in 2015. Confidence intervals (95%) were obtained from the cited studies, by contacting the authors, or by calculation using Stata 14.1 (College Station, TX) when estimates were unavailable.
Results
Number of MSM and TW, and HIV prevalence among them
According to the 2013 UNAIDS global report, the estimated number of MSM in Peru in 2010 was 435,426,19 assuming that 6% of Peruvian men ages 15–49 were MSM, which was defined as a person born biologically male (including TW) who reported sex with a man in the past year.15 This estimate was based on a 2002 study of young men (age 18–29) in 20 cities.20 This figure does not account for older men and non-urban areas, where same-sex practices may be lower, but has been used in other epidemiological modelling estimates, 21 as more specific data in these populations are not available. Applying the 6% to 2015 population estimates (n=10,218,000 men),22 we then estimate that there are approximately 613,080 MSM and TW between the ages of 15 and 65 in Peru. We broadened the age range of our estimate because people are living past 49 now that better treatments are available. The prevalence of HIV among MSM and TW was 12.4% (11.2–13.6%) in Lima and Callao and 2.8–5% in the cities of Piura, Iquitos, Ica, and Pucallpa according to the 2011 national HIV surveillance study.13 The study excluded MSM and TW aware of their HIV-positive status, corresponding to 25% of the MSM and TW population according to a surveillance sub-study. Correcting for this and assuming 50% of all Peruvian MSM and TW live in Lima/Callao, we estimate the national HIV prevalence among MSM and TW at 10.4%. Using this prevalence rate and the estimated number of MSM and TW, the approximate number of HIV-infected MSM and TW in Peru is 63,981. While our prevalence estimate includes TW, it is important to note that TW have an overall prevalence that is markedly higher, often double the prevalence among MSM, reaching as high as 29.6% (22.6–38.7).23
HIV-positive MSM and TW aware of their serostatus
Because HIV diagnoses only come to the attention of Peru’s public health authorities after the mandatory reporting of new diagnoses, serostatus awareness estimates must come from research and surveillance studies. Previous estimates came predominantly from two cross-sectional studies based around a prominent STI clinic (Centro de Referencia Alberto Barton in Callao), both of which suggested that >70% were aware of their HIV diagnosis.24,25 However, these likely overestimate the actual serostatus awareness because of the setting of patient recruitment. A more geographically diverse study, Comunidades Positivas, enrolled 718 MSM and TW from 24 neighborhoods in Lima, most of which were lower income neighborhoods. The baseline HIV prevalence was 18%, and only 29.9% (22.1–38.7%) of HIV positives were aware of their infection.26 There was no significant difference found in serostatus awareness rates between MSM and TW. While this study may offer a better geographic sampling of MSM and TW in Lima, where the majority of the HIV-infected population lives,12 it may not be representative of groups with higher educational attainment and income. Nevertheless, these data are concordant with the 2011 epidemiological surveillance study, which compared convenience sampling, time space sampling, and respondent driven sampling (RDS) in Lima and Callao, and found that only 24.0% (16.0–33.6%), 10.0% (3.3–21.8%), and 38.5% (20.2–59.4%) of MSM, respectively were aware of their HIV infection. 13,27 When including the five cities of Lima, Ica, Iquitos, Piura, and Pucallpa, only 10.2% (7.5–13.5%) of the HIV-infected MSM were aware of their infection, 28 though this wider geographic study suffered from selection bias as it excluded those who reported being HIV-positive or having a recent HIV-negative test.13 The 2011 surveillance study’s convenience sample estimate of 24.0% offers the largest, most contemporary estimate of serostatus awareness, and is most consistent with the previous population estimate from Comunidades Positivas.26 Based on these studies, we estimate that only approximately 24% of HIV-infected MSM and TW in Peru are aware of their serostatus.
Linked to and retained in care
There are currently no routine surveillance measures of timely linkage to care after HIV diagnosis in Peru. However, a few studies do provide indicators of the proportion of people living with HIV (PLWH) who are accessing care and retained in care, though their definitions of retention are not uniform. A 2008 study recruited 863 PLWH using RDS and found that 96.4% (94.9–97.5%) reported currently receiving any HIV-related health service.29 However, this figure is not specific to MSM and TW and the study used RDS to recruit from HIV-related organizations, which tend to serve those who are well-integrated in HIV care. The largest public HIV clinic in Lima, at the Alexander von Humboldt Tropical Medicine Institute and the Hospital Nacional Cayetano Heredia (HNCH), identified 736 out-of-care participants (≥1 provider visit but none within the last 12 months) out of approximately 2240 patients who initiated HIV care between May 2004 and March 2010. Thus, approximately 67.1% were retained in care over this period of time.30 After contacting the authors, we found that the time periods for those out-of-care and the total number of patients who initiated HIV care do not match perfectly; nevertheless, this provides a rough estimate of retention in care. A similar study conducted by Peru’s National Institute of Health found that 60.5% (58.9–62.0%) of the 3981 PLWH who initiated care in 11 public hospitals and 4 private clinics from May 2004 to December 2006 maintained good retention (≤9 months between visits) when followed through 2012. Among the men studied, the retention rate was similar (62%) (personal communication, Caballero). Finally, the only study that was specific to MSM was an internet-based study which found similar statistics: 64.9% (53.2–75.5%) of MSM who reported being HIV-positive also reported currently receiving medical care for HIV, though there was no strict definition of what constituted current medical care for HIV, and participants were mostly educated and upper middle class.10 Nevertheless, the data are consistent with the aforementioned studies done among the general population, and we estimate that 64.9% of MSM and TW diagnosed with HIV (15.6% of all HIV-infected MSM and TW) are retained in care.
On antiretroviral therapy
In Peru, there have been many changes in the ART eligibility criteria in recent years, following the evolving WHO guidance. Until 2012, the cutoff for ART eligibility was a CD4 count of 200 cells/μL. The cutoff then was raised to 350 cells/μL, until December 2014 when the threshold was again raised to 500 cells/μL.31 With such dynamic criteria, it is difficult to estimate the overall coverage of ART. As a result, studies generally report ART coverage as a proportion of those in care at the time of study completion. A study of 578 men and women in Peru (27.5% women, 7.8% transgender) who were receiving HIV care through the national program in five cities revealed that 85.3% (82.1–88.0%) of participants receiving care were on ART in 2007.32 Another study of 863 PLWH (men and women) in 2009 found that 84.7% (82.1–87.1%) of those reporting access to HIV care also reported being on ART.29 A 2012 study of 302 MSM and TW recruited from HIV clinics in Lima suggests that a similar proportion of MSM and TW is also receiving ART (87.1% (82.8–90.7%)).33 Finally, in the internet-based survey of MSM from social and sexual networking sites, 96% of Peruvian MSM reporting being in HIV care were also on ART,10 though the generalizability and accuracy of online surveys are unclear and the number of HIV-infected MSM was small (n=77). Based on these data, we believe the 2012 study33 provides the best estimate of ART usage (87.1%) among MSM and TW in Peru. Thus, approximately 13.6% of all HIV-infected MSM and TW are also on ART.
Achieved adequate virologic control
There is evidence that there is good maintenance of ART therapy in Peru once it is initiated: 88% (86.0–89.9%) of men who initiated ART in the principal hospitals of Lima in 2012 remained on ART 12 months later.12 In addition, a study completed at the HNCH, the largest public HIV treatment clinic in Lima, showed that 75.7% (73.4–77.9%) of the 1478 patients on treatment during 2004–2009 did not have virologic failure, defined as >1000 copies/mL after 24 weeks of ART.34 Another study by the National Institute of Health in Peru showed that 88.4% (87.3–89.5%) of the 3370 men who initiated ART between 2004 and 2006 in the Peruvian National Program had attained virologic suppression when followed through 2012.35 Although this study did not report a figure specifically for MSM and TW, this is the closest gender-specific estimate that is available. Based on this figure, we estimate that 12.0% of all HIV-infected MSM and TW have adequate virologic control.
The studies that were used to create these estimates are summarized in Table 1, and these steps of the HIVCC in Peruvian MSM and TW can be visualized graphically in Figure 1.
Table 1.
Summary of studies used to construct the HIV care continuum for MSM and TW in Peru.
| Stage of HIVCC | Reference # | Year | Population | # of participants | Setting | Fraction | Crude % | Crude 95% confidence interval | Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Serostatus awareness | 24, 25 | 2007 | MSM and TW | 560 | STI clinic (n = 438) + community outreach (n = 122) in Callao | 91/125 | 72.8% | 64.1–80.4% | Sample mostly from STI clinic |
| 26 | 2008–2009 | MSM and TW | 713 | 24 Lima neighborhoods, RDS | 38/127 | 29.9% | 22.1–38.7% | Focused on low income population, limited to Lima | |
| 13, 27 | 2011 | MSM and TW | 748 | Lima and Callao – community outreach efforts, referred to STI clinic | 24/100 | 24.0%a | 16.0–33.6% | Convenience sample, required follow-up at STI clinic | |
| 13, 27 | 2011 | MSM and TW | 233 | Lima and Callao – time space sampling | 5/50 | 10.0% | 3.3–21.8% | Focused only on popular MSM/TW venues | |
| 13, 27 | 2011 | MSM and TW | 127 | Lima and Callao — RDS | 10/26 | 38.5% | 20.2–59.4% | Small sample size | |
| 28 | 2011 | MSM and TW | 5148 | 5 Peruvian cities | 43/420 | 10.2% | 7.5–13.5% | Excluded those who reported being HIV + or having a recent HIV-negative test | |
| Retained in care | 29 | 2008–2009 | Men and women | 863 | Organizations for PLWH in 4 cities in Peru, RDS | 832/863 | 96.4% (95.6% RDS adjusted) | 94.9–97.5% | Not specific to MSM and TW; measured access to HIV care and not retention |
| 30 | 2011 | Men and women | ~2240 | Large public HIV clinic in Lima | 736/~2240 | ~67.1% | Unable to calculate | Time periods for those out-of-care and the total number of HIV patients do not match perfectly; exact denominator not given | |
| Personal communication, Caballero | 2012 | Men and women | 3981 | Peru National Institute of Health data from 11 public hospitals and 4 private clinics | 2408/3981 | 60.5% (62% among men) | 58.9–62.0% | No specific data for MSM and TW | |
| 10 | 2012 | MSM | 869 | Online survey from social and sexual networking site | 50/77 | 64.9%a | 53.2–75.5% | Online survey likely not representative of population; rigorous definition of retention in care not used | |
| On antiretroviral therapy | 32 | 2007 | Men and Women | 578 | 7 hospitals in 5 cities in Peru | 493/578 | 85.3% | 82.1–88.0% | Convenience sample; not specific to MSM and TW, though TW were included |
| 29 | 2008–2009 | Men and women | 863 | Organizations for PLWH in 4 cities in Peru, RDS | 703/830 | 84.7% (76.8% RDS adjusted) | 82.1–87.1% | Not specific to MSM and TW | |
| 33 | 2012 | MSM and TW | 302 | 3 HIV clinics in Lima | 263/302 | 87.1%a | 82.8–90.7% | Convenience sample; limited to Lima | |
| 10 | 2012 | MSM | 869 | Online survey from social and sexual networking site | 48/50 | 96.0% | 86.3–99.5% | Online survey likely not representative of population; small number of HIV-positives; based on self-report | |
| Achieved adequate virologic control | 12 | 2012 | Men | 1079 | Principal public hospitals of Lima | 950/1079 | 88.0% | 86.0–89.9% | Not specific to MSM and TW; only measured the proportion who remained on ART 12 months after initiating it, not virologic suppression |
| 34 | 2004–2009 | Men and women | 1478 | Largest public HIV treatment clinic in Lima | 1119/1478 | 75.7% | 73.4–77.9% | Not specific to MSM and TW; Used a high cutoff (1000 copies/mL) to define virologic failure | |
| 35 | 2012 | Men | 3370 | Peru Instituto Nacional de Salud data for those who initiated ART from 2004–06, followed through 2012 | 2979/3370 | 88.4%a | 87.3–89.5% | Not specific to MSM and TW |
MSM: men who have sex with men; TW: transgender women; RDS: respondent driven sampling; ART: antiretroviral therapy.
The study used to represent each stage of the HIVCC.
Figure 1.
The HIV care continuum for MSM and TW in Peru.
*No data specific to MSM and TW are available; based on national data among men.
MSM: men who have sex with men, TW: transgender women.
Discussion
Overall, there are several important trends in the HIVCC for MSM and TW in Peru. First, the largest drop-off occurs at the first step; only 24% of HIV-infected MSM and TW in Peru are aware of their infection. While this proportion seems low in comparison to nation-wide estimates (53.7%),6 it comes from a large surveillance study that confirms low serostatus awareness using three different sampling methodologies.13,27 Nevertheless, a better assessment of serostatus awareness is warranted in smaller cities that may have lower HIV prevalence and different sexual networks. The marked drop-off in serostatus awareness is likely driven by inadequate diffusion and uptake of HIV testing. Several studies have found that 20–60% of Peruvian MSM and TW have never had an HIV test.27,36–42 In a Peru-based internet study, the main reasons for not testing among high risk MSM were ‘Fear of the consequences of a positive result’, ‘I don’t know where I can get tested’, and ‘I can’t pay for the HIV test’.39 Low self-perceived risk is also a large barrier.36 Much still needs to be done to decrease stigma surrounding HIV and to increase accessibility of HIV testing. Historically, extensive counseling and a signed consent form has been required for HIV testing in Peru,43 which while well-intentioned, may perpetuate stigma surrounding testing. There is now recognition of the need to adapt these rigid requirements to decrease testing-related stigma.44 In addition, new methods of reaching MSM and TW, such as internet-based testing, are being used to increase testing.41 The United States (US) Centers for Disease Control recommend universal screening for HIV45; adopting similar measures in Peru may decrease stigma and improve rates of testing. A US-based study that modeled the transmission risk at each step of the HIVCC revealed that the highest transmission rates occur in those who are undiagnosed,46 while another US and Peru-based study suggested that similar proportions of infections stem from partners whose infection is undiagnosed, diagnosed but untreated, and currently being treated.47 Regardless, the magnitude of the drop-off in diagnosis among MSM and TW combined with this high risk of transmission make this step a significant driver of incident HIV infection in Peru.
Secondly, there is a significant drop-off that occurs at the steps of linkage to and retention in care. Admittedly, there are limitations to our estimation. Linkage to care, the process of establishing timely HIV care after diagnosis,2 has been measured in other studies directly through surveys7,8 and time-to-completion of CD4 counts or viral loads.48 Information systems in Peru that track HIV diagnoses and clinical care for those diagnosed with HIV are currently unlinked, so it is not possible to know on a population level the proportion of those diagnosed who went to access care in a timely manner. Though there is evidence that access to HIV care in Peru is generally good,29 transgender identity and younger age (<35) were associated with lower access to HIV services,29 highlighting the need to improve accessibility of services to these groups. Also, there are no studies that study linkage and retention in a representative sample of MSM or TW; thus, the true proportion may be lower than our estimate. Furthermore, while stigma has been found to be a barrier to retention in care,30 other patient-level factors need to be identified and studied. Ultimately, linking information systems and strengthening patient referral systems post-diagnosis are needed, as poor linkage to and retention in care likely account for a substantial proportion of HIV transmission in Peru, as in the US.46,47
Next, while our estimate suggests that the majority of MSM and TW (87.1%) in care have initiated ART, it is important to note that it is based on data collected only in Lima. Disaggregating country-wide ART data by MSM and TW when reported to public health authorities will help to better inform the true national estimate. There is also evidence that about a third of PLWH in Peru in 2007 reported not being on ART despite having received an indication for ART. The authors cited difficulty with completing all the required laboratory tests as the most common reason.32 As the WHO treatment guidelines have evolved, an increasingly greater proportion of PLHA has initiated ART. Peru has expanded access to ART to those with <500 CD4 cells/μL, according to the 2013 WHO guidelines.49 There is strong evidence for the strategy of ‘treatment as prevention’50 since early initiation of therapy drastically decreases HIV transmission.1 Additionally, results from the Strategic Timing of AntiRetroviral Treatment (START) study, which confirmed that initiating ART at >500 CD4 cells/μl was associated with decreased morbidity and mortality,51 have led the WHO to release an early update to their guidelines recommending ART initiation regardless of CD4 counts.52 According to Peru’s 2014 UNAIDS report, there were 18,386 men in Peru receiving ART in 2013,12 though it is unknown how many of those were MSM or TW. Based on our estimate of the total number of HIV-infected MSM and TW (n=63,981), it is clear that many HIV-infected MSM and TW still are not on ART, though the changing eligibility criteria will likely improve this. As we move toward increasing access to ART, policy makers, public health experts, and clinicians all have important roles in closing this gap by ensuring funding for, availability of, and successful initiation of and adherence to ART.
Finally, we found that the majority of patients on ART also achieved virologic suppression, though data specific to MSM and TW are currently not available. Nevertheless, there is still room for improvement. The HNCH study identified risk factors for virologic failure which included clinical factors (i.e. previous ART use, medication toxicity, opportunistic infections, CD4 count <100 cells/μl), and adherence. Of note, 47.2% of participants initiated ART when their CD4 count was <100 cells/μl, indicating very late initiation,34 which could reflect poor care engagement or late diagnosis. Young age (18–29 or 30–39 years at care initiation) has also been found to be a risk factor for interruption of virologic suppression.35 An online survey specific to MSM found that only 29.3% of MSM on ART reported 100% adherence to ART.10 Finally, studies among the general population of PLWH in Peru have shown that ART drug shortages have also been a problem for those taking ART.32 Such compromises in drug supply could increase the risk of virologic failure. These findings suggest that closing this gap will require a consistent supply of ART and better treatment practices such as earlier initiation of ART, improved ART regimens with less toxicity, and supporting better adherence.
Our HIVCC model is not without limitations, although those are not unique to this study, let alone to Peru. First, we made some assumptions around geographical and demographic variables to calculate the actual number of MSM and their HIV prevalence. Nevertheless, calculations were provided to impart a rough sense of the magnitude of the HIV epidemic among MSM and TW in Peru using available data. Also, the data used to construct this model come from a wide variety of sources of varying quality. Ideally, monitoring of each step in the HIVCC would be centralized, name-based, and include disaggregated data for MSM and TW. This kind of monitoring will be invaluable in helping to determine the needs and priorities of Peru’s HIV programs. Achieving this will require a concerted effort among public health stakeholders. In the meantime, this model provides a rough estimate of the HIVCC among MSM and TW in Peru and provides guidance on how better to monitor and intervene at the various steps in the HIVCC. Similar models in key populations in other Latin American countries are also needed as HIVCCs are highly context-dependent. Finally, further research and information systems that disaggregate the population by sexual orientation and gender identity are needed to elucidate the impact of these factors on the HIVCC in these populations.
Conclusions
Our review showed that the largest drop-off in the HIVCC in Peruvian MSM and TW occurs at the level of diagnosis. It also revealed that information about timely linkage to care is not systematically collected, though there are indicators that this step has not been optimized. In order to progress with the strategy of treatment as prevention, much work needs to be done to increase HIV serostatus awareness and remove the barriers that hinder HIV testing in MSM and TW, as well as to improve linkage to care among those found to be HIV-infected. Additional efforts need to be made to provide sufficient information on the stages of the HIVCC for MSM and TW as separate groups as well. The lack of this information hinders improved prevention efforts and only serves to hide TW. Efforts to improve these and the other steps, i.e. retention in care, early initiation of ART, optimal adherence and virologic suppression, are warranted to improve the country’s overall ability to control the transmission of HIV.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Mental Health [5T32MH080634-09 to JC and 1R21MH102135-01 to CC], the National Institute of Allergy and Infectious Diseases [5R01AI099727-03 to CC and JK], and the National Institute on Drug Abuse [US-Mexico Drug Abuse Prevention Research Fellowship to AB] at the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test- and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV – United States, 2011. MMWR Morbid Mortal Week Rep. 2014;63:1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 4.Nosyk B, Montaner JS, Colley G, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14:40–49. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genberg BL, Naanyu V, Wachira J, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counseling and testing. Lancet HIV. 2015;2:e20–e26. doi: 10.1016/S2352-3018(14)00034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineirua A, Sierra-Madero J, Cahn P, et al. The HIV care continuum in Latin America: challenges and opportunities. Lancet Infect Dis. 2015;15:833–839. doi: 10.1016/S1473-3099(15)00108-5. [DOI] [PubMed] [Google Scholar]
- 7.Ayala G, Makofane K, Santos G-M, et al. HIV Treatment cascades that leak: correlates of drop-off from the HIV Care continuum among men who have sex with men worldwide. J AIDS Clin Res. 2014;5:2. [Google Scholar]
- 8.Santos GM, Wilson EC, Rapues J, et al. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sex Transm Infect. 2014;90:430–433. doi: 10.1136/sextrans-2013-051342. [DOI] [PubMed] [Google Scholar]
- 9.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magidson JF, Biello KB, Safren SA, et al. Engagement in HIV care and sexual transmission risk behavior among men who have sex with men using online social/sexual networking in Latin America. AIDS Care. 2015;27:1055–1062. doi: 10.1080/09540121.2015.1017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Análisis de la situación epidemiológica del VIH/SIDA en el Perú. Lima, Peru: Ministerio de Salud, Dirección General de Epidemiología; 2013. [accessed 3 March 2015]. http://www.dge.gob.pe/portal/docs/ASISVIH2013.pdf. [Google Scholar]
- 12.Informe Nacional Sobre Los Progresos Realizados en el País: Peru. 2014. Lima, Peru: United Nations General Assembly Special Session (UNGASS) on HIV/AIDS; 2014. [accessed 3 March 2015]. p. 175. 2012-December 2013. http://www.unaids.org/sites/default/files/en/dataanalysis/knowyourresponse/countryprogressreports/2014countries/PER_narrative_report_2014.pdf. [Google Scholar]
- 13.Estudio de Vigilancia Epidemiológica de ITS y VIH en Hombres que Tienen Sexo con Hombres Comparando las Metodologías de Reclutamiento: Muestreo por Conveniencia, Muestreo por Tiempo y Espacio y el Muestreo Dirigido por Participantes. Lima, Peru: Coordinadora Nacional Multisectorial en Salud, Fondo Mundial de Lucha Contra el Sida, la Tuberculosis y la Malaria, CARE-Peru; Nov 11, 2011. [Google Scholar]
- 14.Alarcon JO, Johnson KM, Courtois B, et al. Determinants and prevalence of HIV infection in pregnant Peruvian women. AIDS. 2003;17:613–618. doi: 10.1097/00002030-200303070-00017. [DOI] [PubMed] [Google Scholar]
- 15.Modos de Transmisión del VIH en América Latina: Resultados de la aplicación del modelo. Lima, Peru: Organización Panamericana de la Salud; 2009. [accessed 3 March 2015]. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/countryreport/2009/20090810_MOT_Peru_es.pdf. [Google Scholar]
- 16.Clark J, Salvatierra J, Segura E, et al. Moderno love: sexual role-based identities and HIV/STI prevention among men who have sex with men in Lima, Peru. AIDS Behav. 2013;17:1313–1328. doi: 10.1007/s10461-012-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabet S, Sanchez J, Lama J, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16:1271–1277. doi: 10.1097/00002030-200206140-00010. [DOI] [PubMed] [Google Scholar]
- 18.Clark JL, Konda KA, Munayco CV, et al. Prevalence of HIV, herpes simplex virus-2, and syphilis in male sex partners of pregnant women in Peru. BMC Public Health. 2008;8:65. doi: 10.1186/1471-2458-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Report: UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [accessed 3 March 2015]. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 20.Garcia PJ, Holmes KK, Carcamo CP, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet. 2012;379:1120–1128. doi: 10.1016/S0140-6736(11)61846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cáceres Carlos FWM, Konda Kelika, Lescano Andrés. Nuevas Evidencias para las Políticas y Programas de Salud en VIH/SIDA e Infecciones de Transmisión Sexual en el Perú – Información disponible hasta febrero – 2007. Lima, Peru: Universidad Peruana Cayetano Heredia; Organización Panamericana de la Salud – Representación en Perú; 2007. p. 41. [Google Scholar]
- 22.World Population Prospects, the 2015 Revision. United Nations Department of Economic and Social Affairs, Population Division; 2015. [access 17 August 2015]. http://esa.un.org/unpd/wpp/DVD/ [Google Scholar]
- 23.Silva-Santisteban A, Raymond HF, Salazar X, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav. 2012;16:872–881. doi: 10.1007/s10461-011-0053-5. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Brumer AG, Konda KA, Salvatierra HJ, et al. Prevalence of HIV, STIs, and risk behaviors in a cross-sectional community- and clinic-based sample of men who have sex with men (MSM) in Lima, Peru. PLoS One. 2013;8:e59072. doi: 10.1371/journal.pone.0059072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark JL, Konda KA, Segura ER, et al. Risk factors for the spread of HIV and other sexually transmitted infections among men who have sex with men infected with HIV in Lima, Peru. Sex Transm Infect. 2008;84:449–454. doi: 10.1136/sti.2008.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo R, Konda KA, Leon SR, et al. Human Immunodeficiency Virus (HIV) and Sexually Transmitted Infection (STI) incidence and associated risk factors among high-risk MSM and male-to-female transgender women in Lima, Peru. J Acquir Immune Defic Syndr. 2015;69:567–575. doi: 10.1097/QAI.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark JL, Konda KA, Silva-Santisteban A, et al. Sampling methodologies for epidemiologic surveillance of men who have sex with men and transgender women in Latin America: an empiric comparison of convenience sampling, time space sampling, and respondent driven sampling. AIDS Behav. 2014;18:2338–2348. doi: 10.1007/s10461-013-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vagenas P, Ludford KT, Gonzales P, et al. Being unaware of being HIV-infected is associated with alcohol use disorders and high-risk sexual behaviors among men who have sex with men in Peru. AIDS Behav. 2014;18:120–127. doi: 10.1007/s10461-013-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva-Santisteban A, Segura ER, Sandoval C, et al. Determinants of unequal HIV care access among people living with HIV in Peru. Global Health. 2013;9:22. doi: 10.1186/1744-8603-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valenzuela C, Ugarte-Gil C, Paz J, et al. HIV stigma as a barrier to retention in HIV care at a general hospital in Lima, Peru: A case-control study. AIDS Behav. 2014;19:235–245. doi: 10.1007/s10461-014-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norma técnica de salud de atención integral del adulto con infección por el Virus de la Inmunodeficiencia Humana (VIH) Lima, Peru: Ministerio de Salud, Republica del Peru; Dec 11, 2014. NTS No 097 – MINSA/DGSP-v.02. Resolución Ministerial No. 962-2014/MINSA. [Google Scholar]
- 32.Girón VJM, Segura ER, CVS, ARV, LXS, Cáceres CF. Percepciones de las personas viviendo con VIH/SIDA sobre los servicios de salud y el tratamiento antirretroviral de gran actividad: un estudio transversal en cinco ciudades del Perú (Spanish) Rev Peru Med Exp Salud Publica. 2007;24:211–217. [Google Scholar]
- 33.Ferro EG, Weikum D, Vagenas P, et al. Alcohol use disorders negatively influence antiretroviral medication adherence among men who have sex with men in Peru. AIDS Care. 2015;27:93–104. doi: 10.1080/09540121.2014.963013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alave J, Paz J, Gonzalez E, et al. Risk factors associated with virologic failure in HIV-infected patients receiving antiretroviral therapy at a public hospital in Peru. Rev Chilena Infectol. 2013;30:42–48. doi: 10.4067/S0716-10182013000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caballero Ñopo ZP. Efecto del tratamiento antirretroviral del Programa del Ministerio de Salud del Perú en la supresión de la carga viral de pacientes con VIH/SIDA: estudio longitudinal retrospectivo/Effect of antiretroviral treatment of the Ministry of Health Peru Program in suppressing viral load in patients with HIV/AIDS: a longitudinal retrospective study. Escola Nacional de Sauúde Pública Sergio Arouca. Rio de Janeiro, Brazil: Escola Nacional de Saúde Pública Sergio Arouca, Fiocruz; 2014. p. 64. [Google Scholar]
- 36.Lee SW, Deiss RG, Segura ER, et al. A cross-sectional study of low HIV testing frequency and high-risk behaviour among men who have sex with men and transgender women in Lima, Peru. BMC Public Health. 2015;15:408. doi: 10.1186/s12889-015-1730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsitz MC, Segura ER, Castro JL, et al. Bringing testing to the people – benefits of mobile unit HIV/syphilis testing in Lima, Peru, 2007–2009. Int J STD AIDS. 2014;25:325–331. doi: 10.1177/0956462413507443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludford KT, Vagenas P, Lama JR, et al. Screening for drug and alcohol use disorders and their association with HIV-related sexual risk behaviors among men who have sex with men in Peru. PLoS One. 2013;8:e69966. doi: 10.1371/journal.pone.0069966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blas MM, Alva IE, Cabello R, et al. Risk behaviors and reasons for not getting tested for HIV among men who have sex with men: an online survey in Peru. PLoS One. 2011;6:e27334. doi: 10.1371/journal.pone.0027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caceres CF, Konda KA, Salazar X, et al. New populations at high risk of HIV/STIs in low-income, urban coastal Peru. AIDS Behav. 2008;12:544–551. doi: 10.1007/s10461-007-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blas MM, Alva IE, Cabello R, et al. Internet as a tool to access high-risk men who have sex with men from a resource-constrained setting: a study from Peru. Sex Transm Infect. 2007;83:567–570. doi: 10.1136/sti.2007.027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark JL, Caceres CF, Lescano AG, et al. Prevalence of same-sex sexual behavior and associated characteristics among low-income urban males in Peru. PLoS One. 2007;2:e778. doi: 10.1371/journal.pone.0000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guía Nacional de Consejería en ITS/VIH y el SIDA. Lima, Peru: Ministerio de Salud, República del Perú, Dirección General de Salud de las Personas – Estrategia Sanitaria Nacional Prevención y Control de Infecciones de Transmisión Sexual y VIH-SIDA y el Proyecto Vigía; 2006. [accessed 22 April 2015]. ftp://ftp2.minsa.gob.pe/docconsulta/documentos/dgsp/vihsida/GuiaNacionalConsejeriaITS_VIH.pdf. [Google Scholar]
- 44.Evaluación de la Estrategia Sanitaria Nacional de ITS, VIH, y Hepatitis B en el Marco de la Reforma del Sector Salud. Lima, Perú: Ministerio de Salud, República del Perú; Dec 18, 2014. Resolución Ministerial No. 872-2014/MINSA. [Google Scholar]
- 45.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recommend Rep. 2006;55:1–17. (quiz CE1–4) [PubMed] [Google Scholar]
- 46.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. JAMA Int Med. 2015;175:588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 47.Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS One. 2012;7:e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Int Med. 2013;173:1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. [accessed 12 August 2015];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, June 2013. 2013 http://apps.who.int/iris/bit-stream/10665/179870/1/9789241508926_eng.pdf?ua=1. [PubMed]
- 50.Montaner JS. Treatment as prevention: toward an AIDS-free generation. Topics Antiviral Med. 2013;21:110–114. [PMC free article] [PubMed] [Google Scholar]
- 51.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. [accessed 1 October 2015];Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV- September 2015. 2015 http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed]