Abstract
There is currently much interest in dissecting the mechanisms of tumor immunity. A new study shows that a subset of CD4+ T cells that produce the cytokine interleukin-9 (IL-9) mediate inhibition of melanoma growth in mice and that analogous IL-9-producing T cells are present in human skin (pages 1248–1253). Could such cells be manipulated to develop new therapeutic strategies for melanoma?
Given the myriad and mutable nature of the challenges they face, it seems easy to imagine why T cells would evolve to acquire a diversity of epigenetic states. T helper (TH) cells need to be both specific and plastic, but ascertaining the qualities of these states and their precise roles in immune homeostasis is an enormous challenge for modern-day immunobiologists. Production of IL-9 by TH2-like cells has been known for some time, but the possibility that this cytokine is produced by a distinct TH cell subset—dubbed TH9 cells—has gained substantial currency in recent years1. TH9 cells have been linked to TH2 responses and mast cell biology1,2, and, in this issue of Nature Medicine, Purwar et al.3 ascribe a new role for IL-9 and TH9 cells in the protective antitumor immune response.
TH9 cells join an ever-growing list of what seem to be metastable epigenetic states of TH cell development (Fig. 1). The role ofTH9 cells in tumor immunity had not been previously investigated in mice or humans, but Purwar et al.3 inadvertently tackled this problem. While attempting to examine the role of retinoid-related orphan receptor-γ (ROR-γ), a key transcription factor in the development of the TH17 cell lineage, in antitumor immunity, the authors found increased IL-9 expression in ROR-γ-deficient T cells. Moreover, mice lacking ROR-γ (Rorc−/− mice) showed reduced tumor growth after subcutaneous injection of melanoma cells compared with Rorc+/+ mice. Hypothesizing that TH9 and IL-9 played a part in the protective antitumor immunity they observed in the Rorc−/− mice, the authors employed several complementary approaches to test this possibility3. They found that administration of a monoclonal neutralizing antibody (mAb) against IL-9 promoted melanoma growth in ROR-γ-deficient mice, IL-23 receptor-deficient mice and wild-type mice.
Figure 1.
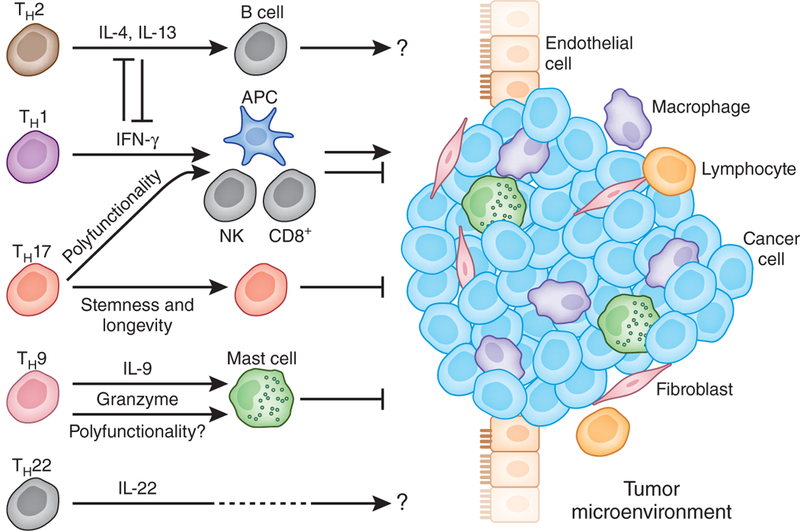
T helper subsets and tumor immunity. Different T helper subsets are found in tumor microenvironments. The roles of these T cell subsets in tumor immunity may be highly context dependent. TH1 cells may mediate antitumor immunity through the interferon-γ(IFN-γ) signaling pathway. TH2 cells may suppress antitumor immunity by inhibiting the TH1 response and inducing alternative macrophages through IL-4 and IL-13. However, it is also possible that TH2 cells may promote tumor immunity through B cell activation. The work of Purwar et al.3 suggests that TH9 cells may mediate antitumor immunity via the direct killing of tumor cells and by mast cells. The polyfunctionality, stemness and longevity of TH17 cells may be associated with their antitumor immunity. The role of TH22 cells in tumor immunity is poorly understood, although they may be associated with poor outcome in patients with gastric cancer and have been linked to hepatocellular carcinoma progression. APC, antigen-presenting cell; NK, natural killer cell.
To examine the role of different TH effector subsets in melanoma immunity (Fig. 1), Purwar et al.3 adoptively transferred in vitro -polarized TH1, TH2, TH9 and TH17 cells specific for ovalbumin peptide-expressing B16 melanoma into syngeneic immunocompetent mice. Consistently with previously published studies, TH17 cells mediated potent antitumor immunity4–9. The effects of TH9 cells were superior to the other effector T cells in this tumor protection model3 although it remains unclear from this study what role, if any, TH9 cells have in the treatment of established vascularized tumors. In support of a protective role of IL-9, TH9 cells or both in tumor immunity, the authors showed that melanoma growth was accelerated in IL-9 receptor-deficient mice compared to heterozygotes for the IL-9 receptor. Furthermore, recombinant IL-9 treatment impaired tumor growth in vaccine and nonvaccine settings. Therefore, IL-9 and/or TH9 cells inhibit tumor growth, at least in the setting of tumor implantation.
To gain mechanistic insights into how IL-9-producing T cells might inhibit the implantation of experimentally transplanted melanoma, the authors studied TH9 cells in vitro, finding that TH9 cells were capable of direct tumor lysis, which was granzyme B dependent3. Furthermore, IL-9 administration reduced tumor growth in Rag1-deficient mice, which lack B and T cells, but had no effects on tumor growth in mast cell-deficient mice. These data suggest that tumor growth inhibition mediated by IL-9 depends on the presence of mast cells but not T or B cells. However, the relative impact of mast cells and direct cytotoxicity, or a possible involvement of natural killer cells, in mediating the antitumor effects of IL-9 still remain to be addressed in future studies (Fig. 1).
In correlative studies, the authors identified TH9 cells in healthy skin in humans as well as in tumor tissues from individuals with melanoma3. They also found that T cells from metastatic melanoma produced less IL-9 than that extracted from healthy donors. These early data may stimulate additional work designed to assess a role for TH9 cells in tumor progression.
Whether there is a causal relationship between ROR-γ deficiency and IL-9 production and TH9 development remains unexplored. However, given that the transcription factor PU.1 is required for the development of TH9 cells10, it may be interesting to investigate whether ROR-γ deficiency biochemically and functionally affects transcription events downstream of PU.1.
Many questions remain to be answered. It is known that the development of both TH9 and TH17 cells may be heavily influenced by the transforming growth factor-γ (TGF-P) signaling pathway, but to what extent are TH9 molecular and functional programming similar to or different from the unique features of TH17 cells? TH17 cells can induce potent tumor immunity, and this may be due to the polyfunctionality and plasticity of these cells7,8. Perhaps more importantly, TH17 cells have the elusive qualities of ‘sternness’ and longevity8,9. The hypoxia-inducible factor-1α, Notch and Bcl-2 signaling pathways are important for maintaining the TH17 cell pool8,11,12, but to what extent do TH9 cells possess these evolutionarily conserved ‘stem cell-like’ pathways?
In human epithelial cancers, TH17 cells constitute a small population compared with other effector T cells5,11. Intratumoral TH17 number and/or IL-17 abundance correlate with improved survival in multiple human cancers4. High amounts of TGF-β, IL-6 and IL-1, which should promote TH17 cell development, have been detected in the tumor microenvironment13. However, TH17 cells are tightly regulated and suppressed by regulatory T cells in the tumor microenvironment4,7,14. Given that Purwar et al.3 observed that the numbers of TH9 cells were lower in advanced melanoma, similar to what has been observed for TH17 cells, are TH9 cells also inhibited by regulatory T cells? In addition to melanoma, are TH9 cells capable of invading the microenvironments of other human cancers?
Adoptive T cell therapy can induce tumor regression and result in objective clinical responses in patients with cancer15,16. The work of Purwar et al.3 may imply that IL-9 and TH9 cells have a role in immune-based anticancer therapy, but the uses of TH9 cells in tumor treatment remain unresolved in the present study, where treatment of tumors is performed at the time of their implantation. Future work will be required to determine whether TH9 cells have the ‘staying power’ to eradicate established tumors. As we approach a new set of potent new T cell-based immunotherapies, translational immunologists will need to ascertain if TH9 cells have the qualities to eradicate swiftly growing tumor cells in an immunosuppressive and hypoxic tumor microenvironment, qualities that are not assessed by ‘day 0’ treatment models. Could TH9 cells have the feline qualities of ‘nine lives’?
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Weiping Zou, Department of Surgery, Graduate Programs in Immunology and Tumor Biology, and University of Michigan Comprehensive Cancer Center, University of Michigan, Ann Arbor, Michigan, USA..
Nicholas P Restifo, National Cancer Institute, Bethesda, Maryland, USA..
References
- 1.Veldhoen M et al. Nat. Immunol. 9, 1341–1346 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Dardalhon V et al. Nat. Immunol. 9, 1347–1355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purwar R et al. Nat. Med. 18, 1248–1253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W & Restifo NPT Nat. Rev. Immunol. 10, 248–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muranski P et al. Blood 112, 362–373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Orozco N et al. Immunity 31, 787–798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryczek I et al. Blood 114, 1141–1149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kryczek I et al. Sci. Transl. Med. 3, 104–100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muranski P et al. Immunity 35, 972–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HC et al. Nat. Immunol. 11, 527–534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi LZ et al. J. Exp. Med. 208, 1367–1376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang EV et al. Cell 146, 772–784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W Nat. Rev. Cancer 5, 263–274 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Kryczek I et al. J. Immunol. 179, 1423–1426 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Dudley ME et al. Science 298, 850–854 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Powell DJ Jr., Rosenberg SA & Restifo NP Nat. Rev. Immunol. 6, 383–393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]


