Abstract
Purpose of Review:
Due to the organ shortage, which prevents over 90,000 individuals in the U.S. from receiving life-saving transplants, the transplant community has begun to critically reevaluate whether organ sources that were previously considered too risky provide a survival benefit to waitlist candidates.
Recent Findings:
Organs that many providers were previously unwilling to use for transplantation, including kidneys with a high Kidney Donor Profile Index (KDPI) or from increased risk donors (IRDs) who have risk factors for window period hepatitis C (HCV) and human immunodeficiency virus (HIV) infection, have been shown to provide a survival benefit to transplant waitlist candidates compared to remaining on dialysis. The development of direct-acting antivirals (DAAs) to cure HCV infection has enabled prospective trials on the transplantation of organs from HCV-infected donors into HCV-negative recipients, with promising preliminary results. Changes in legislation through the HOPE Act have legalized transplantations from HIV-positive deceased donors to HIV-positive recipients for the first time in the U.S.
Summary:
Critical reexamination of deceased donor organs that were previously discarded has resulted in greater utilization of these organs, an increased number of deceased donor transplants, and the provision of life-saving treatment to more transplant waitlist candidates.
Keywords: organ transplantation, deceased donors, kidney transplantation, HIV, HCV
INTRODUCTION
There are over 90,000 individuals in the United States waiting for a kidney transplant (1). The organ transplant waitlist has grown substantially over the past several decades, with an annual growth rate of 2.9% from 2005-2014, due to an increasing demand for transplants and an insufficient supply of kidneys (2). There have been important advances in living donor kidney transplantation, including kidney exchanges (3-7), incompatible transplants (8), donor champion (9) and social media programs (10, 11), and improved donor selection through data-driven live donor risk prediction (12-15), but not all patients are lucky enough to identify living donors. As a result, transplant providers have had to critically reevaluate deceased donor kidneys that were previously discarded, recognizing that transplantation with suboptimal organs might still confer substantial survival benefit over waiting for a “better” kidney. This reevaluation of previously underutilized deceased donor organs, which has coincided with an increase in donors due to the opioid epidemic (16), caused the size of the kidney transplant waitlist to decrease in 2016 for the first time in more than a decade (2). In this review, we describe several types of deceased donor kidneys that are now recognized as underutilized for transplantation despite providing a survival benefit for transplant candidates.
HIGH KDPI KIDNEYS
Historically, deceased donor kidneys were dichotomously classified as coming from standard criteria donors (SCDs) or from expanded criteria donors (ECDs) based on age, creatinine, history of hypertension, and cause of death. ECD kidneys were associated with a higher risk of graft failure and were more likely to be discarded (17). However, ECD organs were found to confer a survival benefit to many transplant candidates, particularly those who were older, diabetic, unsensitized to donor antigens, and facing longer transplant wait times (18). In organ procurement organizations (OPOs, the local unit of the organ allocation system) with long median waitlist times for kidney transplant candidates, acceptance of ECD kidneys was associated with a 27% lower risk of death than waiting for an SCD kidney offer (18). Additionally, among patients predicted to benefit from ECD transplants (older adults, diabetics, unsensitized, and registrants at centers with long wait times) (18), willingness to accept an ECD kidney was associated with 12% lower risk of death (p<0.001) (19).
Today, kidney allocation in the United States has transitioned from SCD/ECD to a more granular Kidney Donor Profile Index (KDPI). The KDPI assigns a continuous risk score to deceased donor kidneys based on 10 donor characteristics (e.g. age, race, and comorbidities). The KDPI is normalized such that a donor’s score represents their percentile of donor quality; that is, a kidney from a donor with a KDPI of 60 is predicted to be of lower quality than 60% of the organs offered in the prior year. Kidneys with a high KDPI (>85%) have 1.46-times higher odds of being discarded than kidneys with a lower KDPI, as they are viewed as low-quality organs (20). Deceased-donor kidneys with a KDPI of 0-20% are expected to function an average of 11.5 years after transplant, compared to an average of 9 years for kidneys with a KDPI of 21-85% and 5.5 years for kidneys with a KDPI greater than 85% (21).
However, as with ECD kidneys, transplant providers have increasingly recognized that high-KDPI organs can still provide a survival benefit for certain patients on the transplant waitlist. For example, transplants with high-KDPI kidneys are associated with increased short-term risk of mortality but decreased long-term risk of mortality compared to waiting for a lower-KDPI organ offer (22). At five years post-transplant, recipients of transplants with kidneys with KDPIs of 71-90, 81-90, and 91-100 all had higher cumulative survival than candidates who chose to wait for a lower-KDPI offer (22). In general, transplant candidates who waited for a “better” kidney were more likely to die than patients who accepted the high-KDPI kidney offer, underscoring the risks of remaining on dialysis; however, this is not true for all patients, and decision trees can help patients and their clinicians understand risk on a more individual level (Figures 1 and 2).
Figure 1. Survival benefit of high-KDPI kidneys.
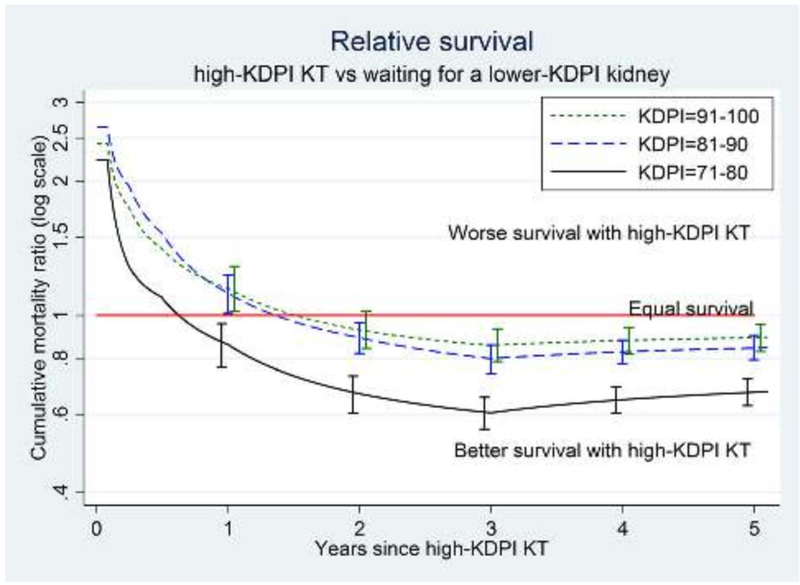
This figure quantifies the relative risk of mortality associated with receiving a transplant with a high-KDPI kidney versus remaining on the transplant waitlist in search of a lower-KDPI (better) offer. Patients receiving higher-KDPI kidneys (KDPI 71-80, 81-90, or 91-100) have a higher risk of dying in the first weeks after transplant surgery than patients who remain on the waitlist or receive a lower-KDPI kidney (KDPI 0-70), largely due to the risks of undergoing transplant surgery. After 1-2 months, patients who accepted these higher-KDPI kidneys are more likely to still be alive (i.e. they have a lower risk of dying) than patients who remain on the waitlist or receive a lower-KDPI kidney (KDPI 0-70). This “survival benefit” from accepting a higher-KDPI organ highlights the benefits of high KDPI kidneys and the risks of remaining on dialysis.
Figure 2. Decision trees for acceptance of high-KDPI organ offers by participant and center characteristics.
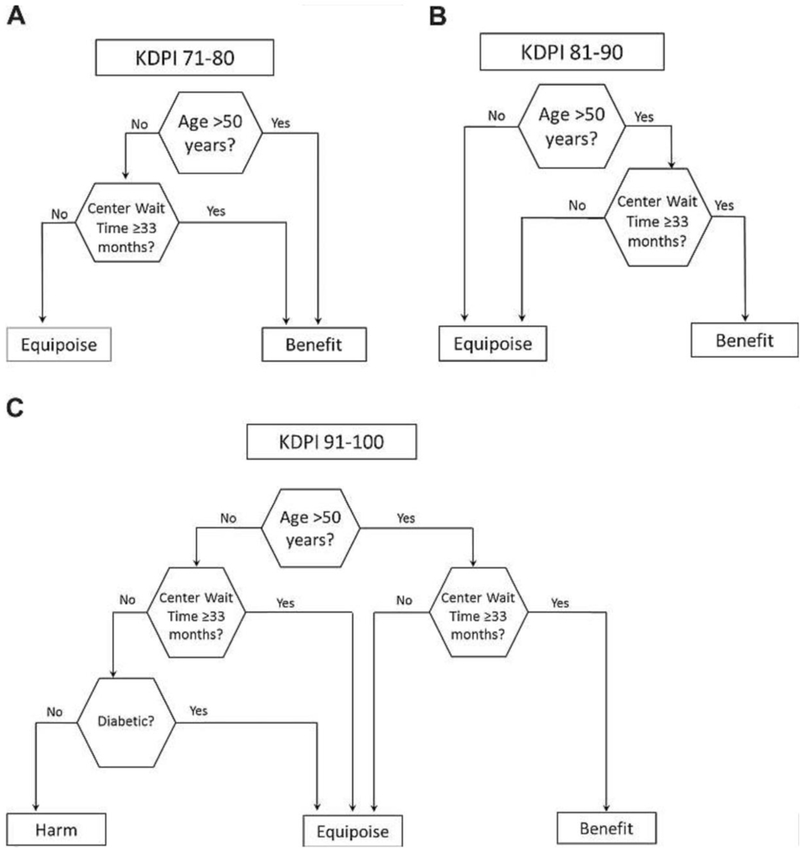
Reproduced with permission.
Despite the demonstrated survival benefit of transplants with high-KDPI kidneys, this pool of organs remains underutilized. Kidneys that would have been classified as SCD that are assigned a high KDPI are now at increased risk of discard in the KDPI era, a sort of “labeling effect” (23). In addition, fear of regulatory action by the OPTN or CMS has also impacted center comfort with using high-KDPI kidneys (24-27). From 2012-2014, 50.6% of kidneys with a KDPI of 61-80 and 71.6% of kidneys with a KDPI of 81-100 were discarded (23). Therefore, although strides have been made to better assess donor quality, understand the survival benefit associated with high-KDPI organs, and identify groups most likely to benefit from these transplants, high-KDPI organs remain an underutilized source of organs for transplantation.
IRD KIDNEYS
The United States Public Health Service (PHS) has provided guidelines to reduce the risk of transmitting infectious diseases through organ transplantation. Guidelines published in 1994 classified certain donors as “high risk” (colloquially referred to as “CDC high risk”) based on their above-average risk for acquiring human immunodeficiency virus (HIV) during the window period of serologic detectability (28). While the risk is elevated, there is nothing “high” about the risk of window period infections in these patients, which range from 0.04-4.9 per 10,000 donors for HIV and 0.027-32.4 per 10,000 donors for HCV (Table 1) (29, 30).
Table 1. Risk per 10,000 donors of a hepatitis C (HCV) or human immunodeficiency virus (HIV) infection occurring during the window period, by nucleic acid testing (NAT).
| Risk (95% CI) per 10,000 donors, by NAT | ||
|---|---|---|
| Population | HCV | HIV |
| Men who have sex with men | 3.5 (3.3-3.8) | 4.2 (3.9-4.5) |
| Injection drug users | 32.4 (29.7-35.3) | 4.9 (4.3-5.6) |
| Hemophiliacs | 0.027 (0.023-0.034) | 0.035 (0.027-0.043) |
| Commercial sex workers | 12.3 (11.3-13.4) | 2.7 (2.2-3.0) |
| Sex with a partner in categories 1-4 | 12.3 (11.1-13.2) | 0.3 (0.2-0.4) |
| HIV exposure through blood | 0.4 (0.09-1.2) | 0.6 (0.4-1.0) |
| Incarcerated* | 0.8 (0.08-2.5) | 0.9 (0.5-1.7) |
Based on only one study of intraprison incidence of HCV infection in incarcerated individuals
In 2013, the PHS provided updated criteria based on donor risk factors for HIV, hepatitis B, and hepatitis C (HCV) infections and termed this group “increased risk” donors (IRDs). We prefer the term “infectious risk donors” to clarify that the risk is strictly infectious, and that, for most other measures of organ risk, most IRD kidneys are among the best kidneys available. IRDs account for almost 20% of the deceased donor pool today, and this proportion will likely increase even further in the context of the modern opioid epidemic (31). Donors who die of drug overdoses, a population that overlaps with the IRD population, accounted for only 1.1% of organ donors in 2000 and rose dramatically to 13.4% of donors in 2017; kidneys from overdose donors, despite excellent outcomes, were more likely to be discarded than those from donors who died of trauma (5.2% vs. 1.5%) (32).
The potential for infection of a recipient with HIV or HCV makes some transplant candidates and their providers uncomfortable accepting these organs. In 2007, in response to the first reported case of HIV infection from an IRD transplant in the 20 years of US transplant data collection, 32% of transplant surgeons reported changing their practice (33). Additionally, providers have reported obstacles to use of IRD organs including lack of comfort obtaining IRD-specific consent, lack of guidelines for IRD-specific consent, and failure to discuss the use of IRDs with candidates at the time of listing (34-36). Transplant candidates also view IRD organs as a less desirable option; focus groups perceived that IRD organs were most appropriate for patients at high risk of death or who have poor quality of life on dialysis (37). In fact, one study found that 42% of kidney transplant candidates would reject IRD kidneys under all circumstances (38). The reluctance to use IRD organs is perhaps even greater when treating pediatric transplant candidates, despite the fact that IRD kidneys are associated with similar allograft and patient survival and that only one unintended bloodborne pathogen transmission occurred in 8,000 unique pediatric transplants from 2008-2015 (39).
The reluctance to use IRD kidneys is harmful to patients, as studies have demonstrated that IRD transplants provide substantial benefits for recipients. Simulation studies have found that increased use of IRDs would increase the number of transplants, increase quality-adjusted life years, lower the cost of care, and decrease the number of viral infections because of reduced time on hemodialysis (during which patients incur risk of viral transmission) (40). Additionally, a calculator designed to help an individual patient decide between accepting an IRD offer or waiting for a non-IRD offer (www.transplantmodels.com/ird; Figure 3) showed that accepting an IRD kidney offer would provide a 5-year survival benefit for most patients, and that patients most likely to benefit from these transplants could be identified (31). Subsequent analysis of national registry data has confirmed these findings: among transplant candidates who declined an IRD, only 31% later received a non-IRD deceased donor kidney transplant, and the non-IRD allografts accepted were of substantially lower quality (higher KDPI, 52 vs. 21) than the declined IRD kidneys (41). By 6 months post-transplant, accepting an IRD kidney was associated with a 48% lower risk of death than continuing to wait for a non-IRD kidney (41).
Figure 3. Increased Risk Donor (IRD) kidney transplant calculator.
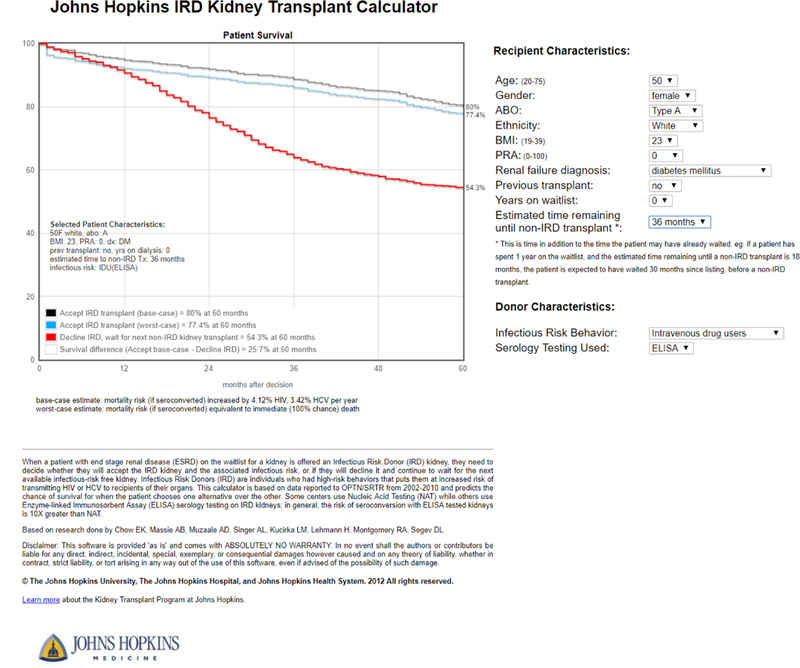
This calculator was designed to assist clinicians and patients in decision-making related to IRD kidney offers. The user enters the recipient and donor information, and a Markov decision process model estimates a personalized 5-year survival curve if the recipient accepts versus declines the IRD offer. The calculator is available at http://transplantmodels.com/ird/. The methodology and decision process model development used to produce this calculated was described by Chow et al (31).
In summary, IRD kidneys remain an underutilized source of organs for transplantation, presumably due to stigma of HIV causing both provider and transplant candidate discomfort. Further studies are necessary to evaluate the effect of improved education and resources on willingness to consider IRD organ offers. Additionally, improvements in infectious disease detection, such as the reduction in the window period of detectability (42), continue to reduce the risk of disease transmission from IRD kidneys and might affect willingness to accept IRD organs.
HIV+ DONOR KIDNEYS AND HOPE
While IRD organs are available to all transplant candidates, organs from donors with known human immunodeficiency virus (HIV) infections were historically banned from use in organ transplantation. However, as methods for controlling HIV infection have turned a fatal diagnosis into a chronic disease that is relatively easily controlled, an increasing number of HIV-positive (HIV+) patients have survived with HIV, developed end-stage renal disease, and been placed on the kidney transplant waitlist (43). For two decades, these HIV+ transplant candidates have received HIV-negative (HIV-) organs with good outcomes and well-controlled HIV following transplantation (44). In fact, HIV-monoinfected recipients (i.e. those who are HIV+ and are not coinfected with hepatitis C) can have similar 5- and 10-year graft and patient survival to their HIV-negative counterparts (45). Induction immunosuppression in HIV+ recipients is associated with lower risk of delayed graft function and graft loss and does not increase risk of infection (46). These findings suggest that kidney transplantation is a safe and effective treatment of end-stage renal disease in HIV+ patients.
The promising transplant outcomes of HIV+ recipients, including continued control of their HIV infections, suggested that the use of HIV+ donor organs should be reevaluated (Figure 4). In 2010, Muller et. al published the results of the first four kidney transplants from HIV+ donors to HIV+ recipients (HIV-to-HIV transplantation) in South Africa, all of which were successful (47). Results at 3 and 5 years for the first 27 HIV-to-HIV kidney transplants were similarly encouraging, with graft survival of 93% at 1 year, 84% at 3 years, and 84% at 5 years. In all patients, HIV infection remained well-controlled, with undetectable virus in blood (48) and no evidence of HIV superinfection (49). HIV-to-HIV transplants are advantageous to both HIV positive and negative candidates by increasing the overall donor pool (50).
Figure 4. Risk of HIV superinfection and drug resistance associated with HIV-positive organ donors.
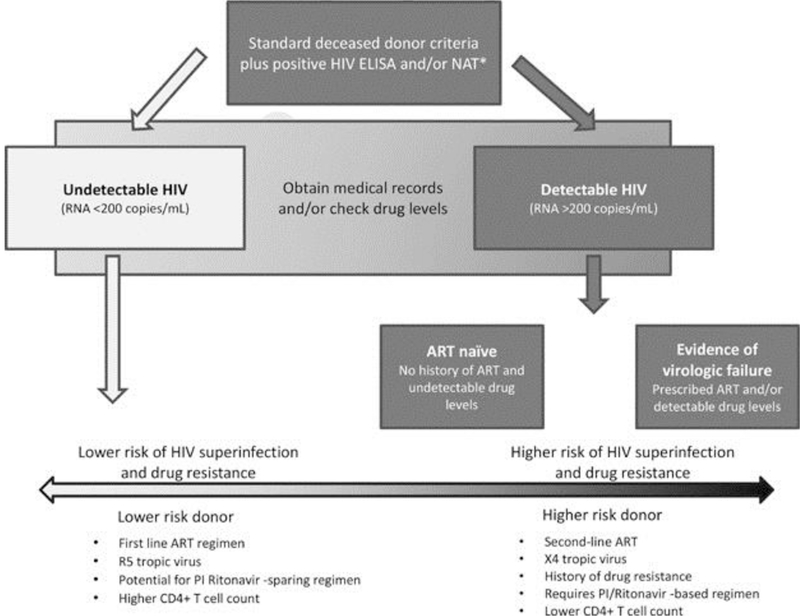
Patients on first-line ART regimens, infected with R5 tropic virus, who have potential for protease inhibitor ritonavir-sparing ART regimens, and who have higher CD4+ T-cell counts are at lower risk of causing HIV superinfection or spreading drug resistance.
However, use of organs from HIV-infected donors was illegal in the United States according to the National Organ Transplant Act (NOTA) of 1984/1988. In 2010, we estimated that the potential number of organs available from HIV+ donors was 350-600 per year (51, 52). This inspired us to write and advocate for the HIV Organ Policy Equity (HOPE) Act, which passed in 2013 (53, 54). Based on lessons from HIV+ kidney transplant recipients (46) and the results of HIV-to-HIV transplants in South Africa (47, 48) and Switzerland (55), the first clinical trial of HIV-to-HIV organ transplantation in the United States was developed (56). The multicenter “HOPE in Action” prospective trials will evaluate the safety of HIV-to-HIV kidney and liver transplantation and analyze graft survival, patient survival, and transplant-related and HIV-related complications compared to transplants from HIV- donors to HIV+ recipients.
Areas of future study include disparities in access to HIV-to-HIV transplant, adequate consent for candidates, optimal immunosuppression and antiretroviral therapy regimens, management of donor-derived transmission of a resistant HIV strain (Figure 4), and prevention of acute and chronic rejection (56-58). Additionally, the safety and feasibility of HIV-to-HIV transplantation using living donors is being studied (59).
HCV+ DONOR KIDNEYS
Organs from deceased donors infected with hepatitis C (HCV+) have historically been offered only to transplant recipients who were also HCV+. However, these organs are underutilized: a study of all HCV+ deceased donor kidney offers from 1995-2009 found that HCV+ kidneys were 2.6-times more likely to be discarded, despite the fact that only 29% of HCV+ recipients received HCV+ organs (60) and candidates who accepted HCV+ kidneys waited, on average, 310 days less than the average waiting time at their center and 395 days less than their counterparts at the same center waiting for HCV-negative kidneys (60). In liver transplantation, donor HCV status was not associated with risk of all-cause graft loss, meaning that HCV+ organs did not make recipients more likely to experience graft failure or death (61). Since 2013, use of HCV+ deceased donor livers for HCV+ recipients increased 1.7-fold, suggesting a promising trend in optimizing the use of these organs (61).
The dramatic, field-changing development of direct-acting antivirals (DAAs) that cure HCV infection has opened up new possibilities for the use of HCV+ organs for transplantation in patients without HCV infection (HCV-). Given that only 37% of HCV+ kidneys offered from 2005-2014 were transplanted (62), despite being from younger and otherwise healthier donors than the general population (63), the thousands of discarded kidneys could offer substantial benefit to HCV- candidates on the waitlist. Reese et al. estimated that these discarded kidneys could have benefited more than 4,000 patients and provided more than 12,000 years of graft life by five years post-transplant (62).
In the past year, the THINKER and EXPANDER-1 trials have suggested that HCV+ organs can be successfully transplanted into HCV- organs without persistent viremia (64, 65). Early results from these trials, which report results for a total of 20 HCV- recipients of HCV+ organs, demonstrated that all patients were cured of HCV infection based on a sustained virologic response 12 weeks after the end of treatment (Figure 5) (64, 65). These findings are exciting, although longer follow-up is necessary to monitor graft function and long-term sequelae and analyze survival benefit from HCV+ transplantation. Though only in its early stages, expanded use of HCV+ organs for HCV- recipients has the potential to enlarge the donor pool without compromising recipient outcomes.
Figure 5. Hepatitis C viral loads in hepatitis C-negative recipients of hepatitis C-positive kidneys.
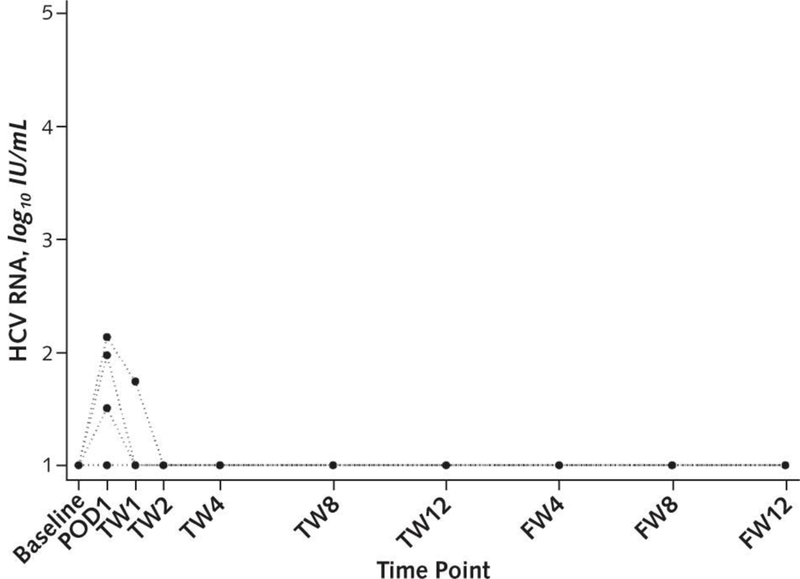
Baseline levels of hepatitis C virus (HCV) RNA confirm that all recipients were HCV-negative prior to transplantation. Some recipients had increased HCV RNA detected in their blood immediately after transplant and even during the first two weeks post-transplant. However, at subsequent time points, all recipients had undetectable HCV RNA levels.
CONCLUSION
Reevaluation of previously underutilized organ sources has helped to increase the deceased donor transplant rate and decrease the size of the transplant waiting list for the first time in over a decade. High-KDPI organs, IRD organs, HIV-positive organs, and HCV-positive organs all remain underutilized, but progress made in these areas is promising. These advances underscore the need for critical reevaluation of discard practices and recognition that remaining on dialysis may be riskier than accepting an offer of a “higher risk” organ.
BULLET POINTS.
Kidneys with a high Kidney Donor Profile Index (KDPI) or from increased risk donors (IRDs) provide a survival benefit to transplant waitlist candidates compared to remaining on dialysis.
Prospective trials of transplantation of HCV-infected kidneys into HCV-negative recipients have had promising preliminary results.
Changes in legislation through the HOPE Act have legalized transplantations from HIV-positive deceased donors to HIV-positive recipients for the first time in the U.S., creating a new source of deceased donor organs.
ACKNOWLEDGEMENTS
FINANCIAL SUPPORT AND SPONSORSHIP
This work was supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The first author is supported by a Doris Duke Clinical Research Foundation grant.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- DAA
direct-acting antivirals
- ECD
expanded criteria donor
- EXPANDER-1
Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients
- HCV
hepatitis C virus
- HCV+
hepatitis C virus-positive
- HCV-
hepatitis C virus-negative
- HIV
human immunodeficiency virus
- HIV+
human immunodeficiency virus-positive
- HIV-
human immunodeficiency virus-negative
- HOPE Act
HIV Organ Policy Equity Act
- IRD
increased risk donor
- KDPI
Kidney Donor Profile Index
- NOTA
National Organ Transplant Act
- OPO
organ procurement organization
- PHS
Public Health Service
- SCD
standard criteria donor
- THINKER
Transplanting Hepatitis C Kidneys into Negative KidnEy Recipients
Footnotes
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Current Opinions in Nephrology and Hypertension.
REFERENCES
- 1.United Network for Organ Sharing (UNOS). UNOS Information: Matching Organs, Saving Lives; 2018.
- 2.OPTN/SRTR 2016 Annual Data Report: Introduction. American Journal of Transplantation 2018;18(S1):10–17. [DOI] [PubMed] [Google Scholar]
- 3.Massie AB, Gentry SE, Montgomery RA, Bingaman AA, Segev DL. Center-level utilization of kidney paired donation. Am J Transplant 2013;13(5):1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA 2005;293(15):1883–1890. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery RA, Zachary AA, Ratner LE, Segev DL, Hiller JM, Houp J et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. Jama 2005;294(13):1655–1663. [DOI] [PubMed] [Google Scholar]
- 6.Melcher ML, Blosser CD, Baxter-Lowe LA, Delmonico FL, Gentry SE, Leishman R et al. Dynamic challenges inhibiting optimal adoption of kidney paired donation: findings of a consensus conference. Am J Transplant 2013;13(4):851–860. [DOI] [PubMed] [Google Scholar]
- 7.Melancon JK, Cummings LS, Graham J, Rosen-Bronson S, Light J, Desai CS et al. Paired kidney donor exchanges and antibody reduction therapy: novel methods to ameliorate disparate access to living donor kidney transplantation in ethnic minorities. J Am Coll Surg 2011;212(4):740–745; discussion 746-747. [DOI] [PubMed] [Google Scholar]
- 8.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. New England Journal of Medicine 2016;374(10):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garonzik-Wang JM, Berger JC, Ros RL, Kucirka LM, Deshpande NA, Boyarsky BJ et al. Live donor champion: finding live kidney donors by separating the advocate from the patient. Transplantation 2012;93(11):1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron AM, Massie AB, Alexander CE, Stewart B, Montgomery RA, Benavides NR et al. Social Media and Organ Donor Registration: The Facebook Effect. American Journal of Transplantation 2013;13(8):2059–2065. [DOI] [PubMed] [Google Scholar]
- 11.Waterman AD, McSorley A-MM, Peipert JD, Goalby CJ, Peace LJ, Lutz PA et al. Explore Transplant at Home: a randomized control trial of an educational intervention to increase transplant knowledge for Black and White socioeconomically disadvantaged dialysis patients. In. BMC nephrology. 2015: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzaale AD, Massie AB, Wang M, et al. Risk of end-stage renal disease following live kidney donation. JAMA 2014;311(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massie AB, Muzaale AD, Luo X, Chow EKH, Locke JE, Nguyen AQ et al. Quantifying Postdonation Risk of ESRD in Living Kidney Donors. J Am Soc Nephrol 2017;28(9):2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. New England Journal of Medicine;0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberu J, Bakr MA et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017;101(8S Suppl 1):S1–s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durand CM, Bowring MG, Thomas AG, et al. The drug overdose epidemic and deceased-donor transplantation in the united states: A national registry study. Annals of Internal Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OA O. Expanded Criteria Donors: Process and Outcomes. Seminars in Dialysis 2005;18(6):463–468. [DOI] [PubMed] [Google Scholar]
- 18.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. Jama 2005;294(21):2726–2733. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Womer KL, Ugarte RM, Desai NM, Montgomery RA, Segev DL. Listing for Expanded Criteria Donor Kidneys in Older Adults and Those with Predicted Benefit. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2010;10(4):802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart DE, Garcia VC, Aeder MI, Klassen DK. New Insights Into the Alleged Kidney Donor Profile Index Labeling Effect on Kidney Utilization. Am J Transplant 2017;17(10):2696–2704. [DOI] [PubMed] [Google Scholar]
- 21.Network OPaT. Kidney Donor Profile Index (KDPI) Guide for Clinicians. 2018. [cited 2018; Available from: https://optn.transplant.hrsa.gov/resources/guidance/kidney-donor-profile-index-kdpi-guide-for-clinicians/
- 22.Massie AB, Luo X, Chow EKH, Alejo JL, Desai NM, Segev DL. Survival Benefit of Primary Deceased Donor Transplantation With High‐KDPI Kidneys. American Journal of Transplantation 2014;14(10):2310–2316. [DOI] [PubMed] [Google Scholar]
- 23.Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in Discard Rate After the Introduction of the Kidney Donor Profile Index (KDPI). Am J Transplant 2016;16(7):2202–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese PP, Harhay MN, Abt PL, Levine MH, Halpern SD. New Solutions to Reduce Discard of Kidneys Donated for Transplantation. J Am Soc Nephrol 2016;27(4):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant 2013;13(1):67–75. [DOI] [PubMed] [Google Scholar]
- 26.Kasiske BL, McBride MA, Cornell DL, Gaston RS, Henry ML, Irwin FD et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant 2012;12(8):1988–1996. [DOI] [PubMed] [Google Scholar]
- 27.Garonzik-Wang JM, James NT, Weatherspoon KC, Deshpande NA, Berger JA, Hall EC et al. The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant 2012;12(2):400–408. [DOI] [PubMed] [Google Scholar]
- 28.Network OPaT. Understanding the Risk of Transmission of HIV, Hepatitis B, and Hepatitis C from U.S. PHS Increased Risk Donors. 2017. [cited 2018; Available from: https://optn.transplant.hrsa.gov/media/2270/dtac_guidance_risks_201706.pdf
- 29.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ et al. Risk of window period hepatitis-C infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant 2011;11(6):1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ et al. Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant 2011;11(6):1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow EKH, Massie AB, Muzaale AD, Singer AL, Kucirka LM, Montgomery RA et al. Identifying Appropriate Recipients for CDC Infectious Risk Donor Kidneys. American Journal of Transplantation 2013;13(5):1227–1234. [DOI] [PubMed] [Google Scholar]
- 32.Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucirka LM, Ros RL, Subramanian AK, Montgomery RA, Segev DL. Provider response to a rare but highly publicized transmission of HIV through solid organ transplantation. Archives of surgery (Chicago, Ill : 1960) 2011;146(1):41–45. [DOI] [PubMed] [Google Scholar]
- 34.Gordon EJ, Mullee J, Beauvais N, Warren E, Theodoropoulos N, McNatt G et al. Education and informed consent about increased risk donor kidneys: a national survey of non‐physician transplant providers. Transplant Infectious Disease 2014;16(2):251–260. [DOI] [PubMed] [Google Scholar]
- 35.Ison MG, Stosor V. Transplantation of high‐risk donor organs: a survey of US solid organ transplant center practices as reported by transplant infectious diseases physicians. Clinical Transplantation 2009;23(6):866–873. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Mandhani A, Verma BS, Srivastava A, Gupta A, Sharma RK et al. EXPANDING THE LIVING RELATED DONOR POOL IN RENAL TRANSPLANTATION: USE OF MARGINAL DONORS. The Journal of Urology 2000;163(1):33–36. [PubMed] [Google Scholar]
- 37.Ros RL, Kucirka LM, Govindan P, Sarathy H, Montgomery RA, Segev DL. Patient attitudes toward CDC high infectious risk donor kidney transplantation: inferences from focus groups. Clinical Transplantation 2012;26(2):247–253. [DOI] [PubMed] [Google Scholar]
- 38.Reese PP, Tehrani T, Lim MA, Asch DA, Blumberg EA, Simon MK et al. Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection. Clin J Am Soc Nephrol 2010;5(5):917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrenn SM, Callas PW, Kapoor T, Aunchman AF, Paine AN, Pineda JA et al. Increased risk organ transplantation in the pediatric population. Pediatric Transplantation 2017;21(8):e13041. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer EJ, Perencevich EN, Philosophe B, Bartlett ST. Estimated Benefits of Transplantation of Kidneys from Donors at Increased Risk for HIV or Hepatitis C Infection. American Journal of Transplantation 2007;7(6):1515–1525. [DOI] [PubMed] [Google Scholar]
- 41.Bowring MG, Holscher CM, Zhou S, Massie AB, Garonzik‐Wang J, Kucirka LM et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. American Journal of Transplantation 2018;18(3):617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kucirka LM, Singer AL, Segev DL. High infectious risk donors: what are the risks and when are they too high? Curr Opin Organ Transplant 2011;16(2):256–261. [DOI] [PubMed] [Google Scholar]
- 43.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial 2005;18(1):52–61. [DOI] [PubMed] [Google Scholar]
- 44.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J et al. Outcomes of Kidney Transplantation in HIV-Infected Recipients. New England Journal of Medicine 2010;363(21):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locke JE, Mehta S, Reed RD, MacLennan P, Massie A, Nellore A et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol 2015;26(9):2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kucirka LM, Durand CM, Bae S, Avery RK, Locke JE, Orandi BJ et al. Induction Immunosuppression and Clinical Outcomes in Kidney Transplant Recipients Infected With Human Immunodeficiency Virus. Am J Transplant 2016;16(8):2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller E, Kahn D, Mendelson M. Renal Transplantation between HIV-Positive Donors and Recipients. New England Journal of Medicine 2010;362(24):2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller E, Barday Z, Mendelson M, Kahn D. HIV-Positive-to-HIV-Positive Kidney Transplantation — Results at 3 to 5 Years. New England Journal of Medicine 2015;372(7):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller E, Barday Z. HIV-Positive Kidney Donor Selection for HIV-Positive Transplant Recipients. J Am Soc Nephrol 2018;29(4):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller E, Barday Z, Mendelson M, Kahn D. Renal transplantation between HIV-positive donors and recipients justified. In. S Afr Med J. 2012: 497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyarsky BJ, Hall EC, Singer AL, Montgomery RA, Gebo KA, Segev DL. Estimating the Potential Pool of HIV‐Infected Deceased Organ Donors in the United States. American Journal of Transplantation 2011;11(6):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richterman A, Sawinski D, Reese PP, Lee DH, Clauss H, Hasz RD et al. An Assessment of HIV‐Infected Patients Dying in Care for Deceased Organ Donation in a United States Urban Center. American Journal of Transplantation 2015;15(8):2105–2116. [DOI] [PubMed] [Google Scholar]
- 53.Boyarsky BJ, Durand CM, Palella FJ, Segev DL. Challenges and Clinical Decision‐Making in HIV‐to‐HIV Transplantation: Insights From the HIV Literature. American Journal of Transplantation 2015;15(8):2023–2030. [DOI] [PubMed] [Google Scholar]
- 54.Boyarsky BJ, Segev DL. From Bench to Bill: How a Transplant Nuance Became 1 of Only 57 Laws Passed in 2013. Ann Surg 2016;263(3):430–433. [DOI] [PubMed] [Google Scholar]
- 55.Calmy A, Delden C, Giostra E, Junet C, Brandt LR, Yerly S et al. HIV‐Positive‐to‐HIV‐Positive Liver Transplantation. American Journal of Transplantation 2016;16(8):2473–2478. [DOI] [PubMed] [Google Scholar]
- 56.Doby BL, Tobian AAR, Segev DL, Durand CM. Moving from the HIV Organ Policy Equity Act to HIV Organ Policy Equity in action: changing practice and challenging stigma. Curr Opin Organ Transplant 2018;23(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand CM, Segev D, Sugarman J. Realizing hope: The ethics of organ transplantation from hiv-positive donors. Annals of Internal Medicine 2016;165(2):138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haidar G, Singh N. The Times, They are a-Changing: HOPE for HIV-to-HIV Organ Transplantation. Transplantation 2017;101(9):1987–1995. [DOI] [PubMed] [Google Scholar]
- 59.Malani P HIV and Transplantation: New Reasons for HOPE. Jama 2016;316(2):136–138. [DOI] [PubMed] [Google Scholar]
- 60.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant 2010;10(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 61.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. American Journal of Transplantation 2017;17(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. New England Journal of Medicine 2015;373(4):303–305. [DOI] [PubMed] [Google Scholar]
- 63.Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 64.Durand CM, Bowring MG, Brown DM, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis c virus-infected donors to noninfected recipients: An open-label nonrandomized trial. Annals of Internal Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. New England Journal of Medicine 2017;376(24):2394–2395. [DOI] [PubMed] [Google Scholar]


