To the Editor:
Monoclonal antibodies that block type 2 cytokines (e.g., IL-4, IL-5, and IL-13) are promising new therapies for asthma (1). However, these agents need to be targeted to selected patients with asthma, where biomarker assessment of airway samples may support stratification of patients into molecular endotypes (2). Thus, we measured cytokine and chemokine mediator levels in nasosorption samples and by using a novel method of sputosorption from induced sputum. Nasal and sputum mediators were then compared with sputum eosinophil levels. A group of 29 allergic asthmatics (AAs) not receiving oral, inhaled, or intranasal corticosteroids or leukotriene modifiers in the last 3 months (with positive skin prick tests, mean baseline FEV1 of 79.3 ± 18.0, and FEV1 reversibility), one of whom did not report a history of allergic rhinitis, were recruited. Dust mite extracts were the most commonly sensitized antigens: Dermatophagoides pteronyssinus (93.1%), Dermatophagoides farinae (79.3%), and Blomia tropicalis (75.8%). These were compared with a control group of 17 nonatopic healthy control patients (HC) with no history of allergic rhinitis and a mean baseline FEV1 of 94.1 ± 11.4. Participants were nonsmokers and were free of upper or lower respiratory infections for the last 4 weeks. Subjects were excluded if they could not safely provide specimens; by the presence of other medical conditions; because of use of concomitant medication, tobacco, or illicit drugs; and if women were pregnant or breastfeeding. The study was approved by the Ethical Committee of Universidade Federal de Santa Catarina (Brazil) on March 10, 2008.
Methods
Nasosorption was performed by placing two strips of synthetic absorptive matrix (Leukosorb, Pall Life Sciences), measuring 7 × 30 mm each, against the inferior turbinate for 2 minutes (Conformité Européene–marked nasosorption sampling devices are now available sterile and allergen-free from Mucosal Diagnostics Ltd.) (3, 4). Sputum was induced by inhalation of hypertonic saline, after which the sample was poured onto a Petri dish, and the viscid portions apparently free of salivary contamination were macroscopically selected. One portion of the selected material was used for cytospin preparation and differential cell count. The remaining portion was used for sputosorption, whereby two synthetic absorptive matrix strips, 7 × 30 mm each, overlaid the sputum for 2 minutes.
Once removed, the synthetic absorptive matrixes were immersed in 200 μl assay buffer (Millipore) + 4 μl DPP Protease Inhibitor-IV (Millipore), placed in the filter cup within a microcentrifuge tube, and centrifuged (10 min, 16,000 × g, 4°C); the supernatant aliquots were stored at −80°C. Mediators were quantified using a Luminex 200 IS Analyzer (Luminex Corp.), and results were expressed in picograms per milliliter. Continuous data were evaluated by the Shapiro-Wilk normality test and presented as median and interquartile ranges and tested for significance by Mann-Whitney U tests. Correlations were performed by Spearman rank correlation tests with Benjamini-Hochberg post hoc correction.
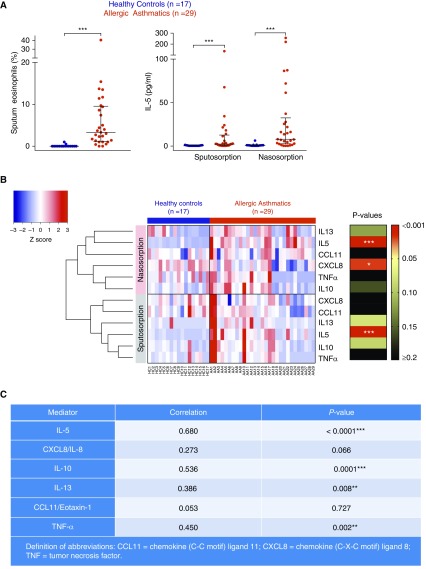
Sputum eosinophil percentage levels from AAs were significantly higher than those of HCs (Figure 1A, left; P < 0.001). A panel of cytokines and chemokines were measured in nasosorption and sputosorption samples, and the corresponding levels are shown for individual patients (Figure 1B), noting that sputum and nasal IL-5 levels were significantly higher in AAs relative to HCs (Figure 1A, right; P < 0.001), whereas AAs exhibited significantly lower levels of nasosorption CXCL8/IL-8 (Figure 1B; P < 0.05). Levels of IL-10, IL-13, tumor necrosis factor-α, and particularly IL-5 were significantly positively correlated between matched nasosorption and sputosorption samples (Figure 1C).
Figure 1.
Sputosorption and nasosorption reveal IL-5 to be selectively increased in the asthmatic airway. (A) Sputum eosinophil levels (left) and nasosorption and sputosorption IL-5 levels (right) in healthy control patients (HCs; n = 17) and patients with asthma (n = 29). Bars represent median and interquartile range. Between-group comparisons by Mann-Whitney U test; ***P < 0.001. (B) Heat map of nasosorption and sputosorption levels of a panel of cytokines and chemokines (IL-5, IL-10, IL-13, tumor necrosis factor-α, CXCL8/IL-8, and CCL11) in HCs and allergic asthmatics (AAs). Mediator levels were normalized (Z scores) and were hierarchically clustered. Levels of mediators between HCs and AAs were compared using Mann-Whitney test and corresponding P values summarized: *P < 0.05; ***P < 0.001. (C) Spearman rank correlations between matched sputosorption and nasosorption samples in HCs and AAs: **P < 0.01; ***P < 0.001.
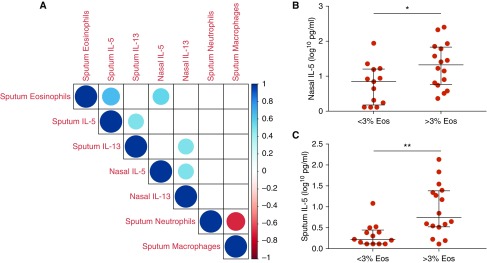
In the AA group, levels of type 2 cytokines (IL-5 and IL-13) were positively correlated in nasosorption (rs = 0.500; P = 0.021) and sputosorption (rs = 0.502; P = 0.021) samples. In the AAs, levels of IL-13 were also positively correlated between nasosorption and sputosorption samples (rs = 0.509; P = 0.021). Similarly, sputum eosinophils were positively correlated with nasal IL-5 (rs = 0.560; P = 0.01) and sputum IL-5 (rs = 0.657; P = 0.001) levels in AAs. In addition, sputum macrophages were negatively correlated with sputum neutrophils (rs = −0.673; P = 0.001; Figure 2A) in AAs. Eosinophilic individuals with asthma (those with ≥3% sputum eosinophils) had higher nasal IL-5 (Figure 2B; P = 0.037) and sputum IL-5 (Figure 2C; P = 0.001) levels. These findings support the United Airways Concept (5) and demonstrate the potential of minimally invasive nasal IL-5 measurement as a promising biomarker, representative of lower airway eosinophilia in patients with asthma. Despite the known role of CCL11 in eosinophil recruitment, no significant correlation was observed between sputum eosinophils and levels of nasal or sputum CCL11 (rs = 0.341 [P = 0.07] and rs = 0.108 [P = 0.576], respectively). This may be because β2-agonists can inhibit tumor necrosis factor-α–mediated CCL11 induction (6).
Figure 2.
Airway IL-5 levels are closely associated with eosinophilic asthma. (A) Correlation matrix of nasosorption and sputosorption ILs and sputum eosinophils, neutrophils, and macrophages in patients with asthma (n = 29); blank squares denote insignificant correlations; color denotes Spearman R value. (B) Nasosorption IL-5 levels in allergic asthmatics stratified on a 3% cut-off for defining sputum eosinophilia (Eos). (C) Sputosorption IL-5 levels in allergic asthmatics with the 3% threshold for defining sputum eosinophilia. (<3% Eos: n = 13, >3%Eos: n = 16). *P = 0.037; **P = 0.001.
Discussion
The utility of nasosorption and sputosorption IL-5 levels as a biomarker for eosinophilic asthma will require direct comparison with existing biomarkers, including fractional exhaled nitric oxide, peripheral blood eosinophil counts, IgE levels, and findings on mucosal bronchial biopsy. In smaller clinical studies, eosinophil counts in induced sputum have been used for the selection of patients with asthma who could benefit from anti-IL-5 therapy (7), whereas peripheral blood eosinophilia has been extensively used in phase III studies as a surrogate for sputum eosinophilia (8). However, blood eosinophil counts not only undergo diurnal variation and increase after exercise (9) but also fail to accurately predict sputum eosinophilia in patients with asthma (10).
As patients in this study were not treated with corticosteroids, the effect of these common therapeutics for asthma on nasosorption and sputosorption IL-5 levels, and the potential consequences for patient stratification, will require further study.
In conclusion, nasosorption and sputosorption sampling methods were minimally invasive and rapidly performed to permit measurement of cytokine levels that reflect lower airway sputum eosinophilia. Although extensive further validation will be required, nasosorption and sputosorption samples have promise for stratification and selection of patients with asthma to receive highly specific therapy with biologics.
Supplementary Material
Footnotes
T.T., T.T.H., and R.S.T are funded by Imperial’s Health Protection Research Unit in Respiratory Infection and the National Institute for Health Research Imperial Biomedical Research Centre at Imperial College Healthcare NHS Trust.
Originally Published in Press as DOI: 10.1164/rccm.201807-1279LE on October 18, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–976. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 2.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2018;391:350–400. doi: 10.1016/S0140-6736(17)30879-6. [DOI] [PubMed] [Google Scholar]
- 3.Hansel TT, Tunstall T, Trujillo-Torralbo MB, Shamji B, Del-Rosario A, Dhariwal J, et al. A comprehensive evaluation of nasal and bronchial cytokines and chemokines following experimental rhinovirus infection in allergic asthma: increased interferons (IFN-γ and IFN-λ) and type 2 inflammation (IL-5 and IL-13) EBioMedicine. 2017;19:128–138. doi: 10.1016/j.ebiom.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thwaites RS, Jarvis HC, Singh N, Jha A, Pritchard A, Fan H, et al. Absorption of nasal and bronchial fluids: precision sampling of the human respiratory mucosa and laboratory processing of samples J Vis Exp 2018. e56413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.Nie M, Knox AJ, Pang L. beta2-Adrenoceptor agonists, like glucocorticoids, repress eotaxin gene transcription by selective inhibition of histone H4 acetylation. J Immunol. 2005;175:478–486. doi: 10.4049/jimmunol.175.1.478. [DOI] [PubMed] [Google Scholar]
- 7.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 8.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 9.Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for “eosinophilic asthma?”. J Asthma. 2012;49:807–810. doi: 10.3109/02770903.2012.713428. [DOI] [PubMed] [Google Scholar]
- 10.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.