Abstract
Rationale: Obstructive sleep apnea (OSA) is associated with systemic hypertension. Either overnight intermittent hypoxia, or the recurrent arousals that occur in OSA, could cause the daytime increases in blood pressure (BP).
Objectives: To establish the role of intermittent hypoxia in the increased morning BP in patients with OSA.
Methods: Randomized, double-blinded, crossover trial assessing the effects of overnight supplemental oxygen versus air (sham) on morning BP, after continuous positive airway pressure (CPAP) withdrawal in patients with moderate to severe OSA. The primary outcome was the change in home morning BP after CPAP withdrawal for 14 nights, oxygen versus air. Secondary outcomes included oxygen desaturation index (ODI), apnea–hypopnea index (AHI), subjective sleepiness (Epworth Sleepiness Scale score), and objective sleepiness (Oxford Sleep Resistance Test).
Measurements and Main Results: Supplemental oxygen virtually abolished the BP rise after CPAP withdrawal and, compared with air, significantly reduced the rise in mean systolic BP (−6.6 mm Hg; 95% confidence interval [CI], −11.3 to −1.9; P = 0.008), mean diastolic BP (−4.6 mm Hg; 95% CI, −7.8 to −1.5; P = 0.006), and median ODI (−23.8/h; interquartile range, −31.0 to −16.3; P < 0.001) after CPAP withdrawal. There was no significant difference, oxygen versus air, in AHI, subjective sleepiness, or objective sleepiness.
Conclusions: Supplemental oxygen virtually abolished the rise in morning BP during CPAP withdrawal. Supplemental oxygen substantially reduced intermittent hypoxia, but had a minimal effect on markers of arousal (including AHI), subjective sleepiness, or objective sleepiness. Therefore intermittent hypoxia, and not recurrent arousals, appears to be the dominant cause of daytime increases in BP in OSA.
Keywords: obstructive sleep apnea, blood pressure, intermittent hypoxia
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is associated with systemic hypertension. In animal and human experimental models, intermittent hypoxia has been shown to cause diurnal blood pressure elevations. Previous trials assessing the effects of supplemental oxygen on blood pressure in OSA have had methodological limitations and therefore have not adequately addressed the role of intermittent hypoxia in daytime blood pressure elevations in OSA.
What This Study Adds to the Field
In this study, after CPAP withdrawal, supplemental oxygen virtually abolished the rise in morning blood pressure seen with supplemental air, over 14 days. Thus, intermittent hypoxia, which supplemental oxygen ameliorated, is likely to be the dominant cause of increased morning blood pressure in OSA. This is the first study to demonstrate the importance of intermittent hypoxia as the dominant cause of morning blood pressure increases in patients with OSA. It potentially has important implications for patients with OSA who have resistant hypertension and who cannot tolerate continuous positive airway pressure (CPAP), the standard treatment for OSA. As such, further work is required to assess whether the effects of supplemental oxygen on blood pressure after CPAP withdrawal translate into a therapeutic benefit for patients.
Obstructive sleep apnea (OSA) is associated with significant cardiovascular disease (1, 2), particularly with elevated daytime blood pressure (BP) and hypertension (3, 4). Continuous positive airway pressure (CPAP) has been shown to improve important markers of cardiovascular health; to improve BP (5–7), particularly in those with resistant hypertension (7); and to improve endothelial function (8). The potential underlying roles of intermittent hypoxia, versus sleep fragmentation, in the development of hypertension in OSA are not fully understood, and an improvement in this understanding may help to develop new treatments to reduce cardiovascular risk in OSA.
Hypertension is a key risk factor for cardiovascular disease (9). OSA causes both acute rises in BP overnight (10) and daytime elevations in BP (3). Acute rises in BP overnight are known to be due to arousals and not intermittent hypoxia (11, 12). However, the mechanisms underlying daytime elevations in BP are not fully understood. Experimental exposure to intermittent hypoxia increases awake blood pressure in rodents (13) and in healthy human subjects (14). In addition, canine models of OSA produce more marked daytime blood pressure elevations than sleep fragmentation in the same dogs, suggesting that either intermittent hypoxia, or another non–arousal-mediated mechanism, is responsible (15). However, we have previously argued that these experiments do not accurately model the intermittent hypoxia of OSA, and that sympathetic activation from arousals may be a more important cause of daytime BP elevations in OSA (16).
Supplemental oxygen therapy can abolish intermittent hypoxia in OSA (17), and therefore, if intermittent hypoxia is the dominant cause of daytime increased BP rather than recurrent arousals, it should lower daytime BP in OSA. Two previous randomized controlled trials showed no effect of supplemental oxygen on BP in OSA (18, 19). However, both had limitations; the flow rates of oxygen used were low at 2–3 L/min, patients with the most severe OSA and the most severe hypoxemia were excluded, and the average usage of supplemental oxygen was modest. Because of these limitations, these trials have not definitely established whether intermittent hypoxia or recurrent arousals are the dominant cause of daytime BP rises in OSA.
CPAP withdrawal is a useful experimental model of OSA (20). The return of OSA during 2 weeks of CPAP withdrawal leads to a large rise in morning BP (approximately 9.0 mm Hg systolic and 5.4 mm Hg diastolic) (21). We hypothesized that if intermittent hypoxia is important in daytime BP elevations in OSA, then supplemental oxygen would attenuate the rise in BP seen during CPAP withdrawal, compared with supplemental air (sham).
Some of the results of these studies have been previously reported in the form of abstracts (22, 23).
Methods
The SOX (Supplemental Oxygen during CPAP Withdrawal) trial was a single, tertiary center, double-blind, crossover trial with randomized treatment order. It was prospectively registered (ISRCTN 17987510) and approved by the South Central Oxford B Research Ethics Committee (REC Reference 15/SC/0007).
Patients and Screening
Patients had an original diagnosis of moderate-to-severe OSA and had been treated with CPAP for more than 1 year, with average CPAP usage exceeding 4 h/night. After written informed consent had been obtained, patients underwent screening, which involved three nights of overnight pulse oximetry (300i; Konica Minolta) while receiving CPAP, followed by four nights of overnight pulse oximetry without CPAP. Full inclusion and exclusion criteria are reported in the online supplement; briefly, eligible patients had adequate control of OSA while receiving CPAP, with an ODI≥4% (nocturnal oxygen desaturation index of at least 4%) less than 10, and showed a return of OSA when not receiving CPAP, with an ODI≥4% exceeding 20 on at least one of four nights off CPAP. After screening, eligible patients resumed normal CPAP for at least 2 weeks before attending for randomization.
Randomization, Intervention, and Blinding
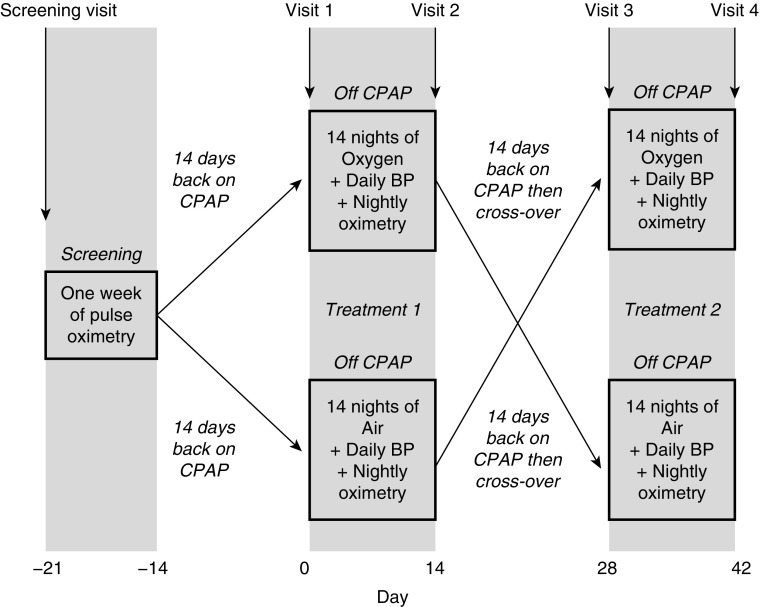
Figure 1 summarizes the trial visits and procedures. Briefly, patients were randomized at visit 1 to receive overnight supplemental oxygen or air (using a real or sham concentrator, respectively) at a rate of 5 L/min via either a nasal cannula or fitted face mask, instead of CPAP for 2 weeks. Following that, after a 2-week washout period back on CPAP, at visit 3, patients crossed over to receive supplemental air or oxygen for another 2 weeks, again instead of CPAP. A high flow rate of oxygen was chosen because in a previous randomized controlled trial an oxygen flow rate of 3 L/min showed only modest reductions in intermittent hypoxia in patients with severe OSA (18).
Figure 1.
SOX trial flow diagram. Patients initially underwent 1 week of overnight screening pulse oximetry. After screening, and after 14 days back on CPAP, patients were allocated either oxygen or air concentrators, with treatment order randomized. Between the two treatment arms patients had a washout period of at least 2 weeks back on CPAP. BP = blood pressure; CPAP = continuous positive airway pressure; SOX = Supplemental Oxygen during CPAP Withdrawal.
Treatment order was randomized 1:1, using permuted blocks of six to either oxygen–air or air–oxygen, via online randomization software (Sealed Envelope). Patients and researchers carrying out outcome assessments and data analyses were blinded to the study allocation, that is, oxygen or sham (air) arm. Oxygen and sham concentrators (NewLife Elite; AirSep) were identical in appearance and were labeled A (oxygen) or B (air) by an unblinded researcher not involved in outcome assessment or day-to-day trial management.
Procedures
Full details of the study procedures can be found in Methods in the online supplement. Patients recorded their BP in triplicate at home for 5 days before and on the 14 days of each treatment arm. In addition, office BP was recorded in triplicate at all four study visits. During each treatment arm, patients recorded overnight pulse oximetry on Nights 1–13 and performed home respiratory polygraphy (Stowood Scientific Instruments) on Night 14. To assess daytime sleepiness, the Epworth Sleepiness Scale (ESS) score and maintenance of wakefulness test equivalent, the Oxford Sleep Resistance (OSLER) test (see Methods in the online supplement), was recorded at each trial visit. On Night 14 of each treatment arm, patients collected overnight urine for urinary metadrenaline and normetadrenaline measurements. At each trial visit, serum was collected to measure high-sensitivity CRP (hsCRP). Venous base excess, as an integrated measure of carbon dioxide levels (24), was measured at all visits for the last 12 patients.
Outcome Measures
The primary outcome measure was the difference in the change in home morning BP from baseline to follow-up at 2 weeks, between the oxygen and air arms (see Blood Pressure Recording in the online supplement). Triplicate home early morning BPs were averaged over the 3 days before each visit. Secondary outcome measures were the absolute, or change in, the following: home morning heart rate, office BP and heart rate, overnight urinary normetadrenaline and metadrenaline (metabolites of sympathetic hormones noradrenaline and adrenaline, respectively), ESS score, OSLER test, overnight pulse oximetry parameters, and the apnea–hypopnea index (AHI), oxygen versus air. Pulse oximetry parameters were averaged from Night 8 to Night 13 for each arm. Pulse oximetry parameters were as follows: the ODI≥4%, percentage time with oxygen saturations less than 90%, mean overnight heart rate, and heart rate rise index (as a surrogate marker of “arousals” at the brainstem level, so-called “autonomic” arousals) (25). Home respiratory polygraphy was recorded on Night 14 of each treatment arm (see Home Respiratory Polygraphy in the online supplement) to calculate the AHI. There was no oxygen desaturation requirement in our hypopnea definition, as this would have introduced an obvious bias between the treatment arms. The relative changes in venous base excess and serum hsCRP were included as exploratory outcomes.
Statistics
In a meta-analysis of previous CPAP withdrawal trials, sham CPAP led to a +9.7–mm Hg (SD, 14) rise in home morning systolic BP when compared with continued CPAP (19). Thus, we calculated that the number of participants required in order not to miss a 6–mm Hg difference in home morning systolic BP (approximately two-thirds the size of the BP rise seen between sham CPAP and continued CPAP) was 24, in a crossover design using a paired t test, assuming a SD of the change of 10 mm Hg (approximately two-thirds the size of the SD in BP change between sham CPAP and continued CPAP), for a power of 80% and a significance level of less than 0.05. In previous parallel withdrawal trials, there were low dropout rates of approximately 1% (21). Therefore, to allow for increased dropouts due to the more arduous crossover design, a sample size of 30 was initially proposed. Because of a significantly higher actual dropout rate, with nine of the first 23 patients dropping out (39%), the protocol was amended partway through the trial to allow increased recruitment to 50 patients, allowing for this higher dropout rate and still providing 24 complete data sets for analysis.
Outcome measures were assessed for normality. Normally distributed data are expressed as mean ± SD and nonnormally distributed data as median (interquartile range [IQR]). Where normally distributed, the primary and secondary outcome measures were assessed using paired t tests, and when not normally distributed, using Wilcoxon rank tests. Statistical analyses were conducted with SPSS (version 20; IBM).
Results
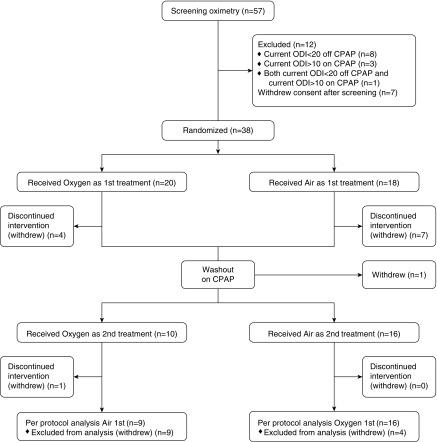
Patients were recruited from June 2015 until June 2017, when enough complete data sets were available. Details of screening, randomization, and withdrawals are shown in the CONSORT diagram in Figure 2. Twenty patients received treatment order oxygen–air and 18 patients received treatment order air–oxygen. After receiving the first trial intervention, there were seven patients who withdrew consent while receiving supplemental air, five patients who withdrew consent while receiving supplemental oxygen, and one patient who withdrew consent during the washout period while receiving CPAP because of an unrelated illness.
Figure 2.
CONSORT diagram showing the flow of the 57 patients who entered screening and the 38 patients who were randomized in the SOX (Supplemental Oxygen during CPAP Withdrawal) trial. CONSORT = Consolidated Standards of Reporting Trials; CPAP = continuous positive airway pressure; ODI = oxygen desaturation index.
Baseline Characteristics
The baseline characteristics are shown in Table 1 for all 25 patients who completed the trial and for each order of treatment. Patients allocated to the treatment order air–oxygen had by chance a lower median ODI during pretrial screening of 26.7/h (25.2 to 34.3/h), compared with those allocated to oxygen–air (median, 42.7/h [30.5 to 54.3/h]). Otherwise the two treatment orders were well matched.
Table 1.
Baseline Characteristics for Patients Who Underwent Randomization and Completed the Trial
| All Patients (n = 25) | Treatment Order Oxygen–Air (n = 16) | Treatment Order Air–Oxygen (n = 9) | |
|---|---|---|---|
| Age, yr | 62.7 ± 6.9 | 61.0 ± 7.4 | 65.8 ± 4.8 |
| Male sex | 21 (84%) | 14 (88%) | 7 (78%) |
| BMI, kg/m2 | 35.3 ± 6.7 | 36.1 ± 7.1 | 34.0 ± 6.3 |
| Neck circumference, cm | 44.2 ± 4.1 | 45.3 ± 3.7 | 42.4 ± 4.2 |
| ODI≥4% at diagnosis, per hour | 48.0 (25.3–68.2) | 50.0 (26.1–70.1) | 45.7 (24.1–65.7) |
| ODI≥4% off CPAP in screening, per hour | 34.5 (26.3–46.5) | 42.7 (30.5–54.3) | 26.7 (25.2–34.3) |
| CPAP usage, h/night | 6.5 ± 0.2 | 6.6 ± 0.3 | 6.3 ± 0.2 |
| Receiving regular antihypertensives | 16 (64%) | 11 (69%) | 5 (56%) |
Definition of abbreviations: BMI = body mass index; CPAP = continuous positive airway pressure; ODI≥4% = oxygen desaturation index of at least 4%.
Data are shown as mean ± SD, median (interquartile range), or n (%). Characteristics are shown for all patients and divided into groups by treatment order.
Concentrator Usage
The mean operation hours of the concentrators during the treatment arms were 7.2 ± 1.0 and 7.4 ± 0.8 h/night for oxygen and air, respectively.
Morning Blood Pressure
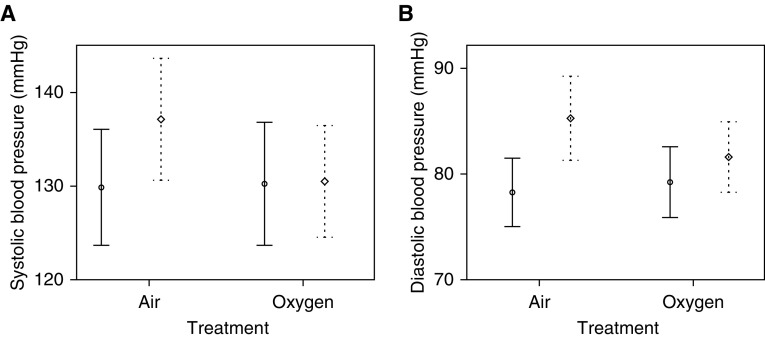
Supplemental oxygen abolished the usual rise in home BP caused by 2 weeks of CPAP withdrawal (21). Compared with the supplemental air (sham) arm, the effect size for oxygen was −6.6 mm Hg systolic (95% confidence interval [CI], −11.3 to −1.9; P = 0.008) and −4.6 mm Hg diastolic (95% CI, −7.8 to −1.5; P = 0.006) (Table 2 and Figure 3). Adjustments for treatment order and potential modifiers essentially did not alter these relationships (see Blood Pressure Analysis in the online supplement).
Table 2.
Home Blood Pressure (Primary Outcome) and Home Heart Rate (Secondary Outcome) Data at Baseline/Run-in and at 2-Week Follow-up for the Supplemental Oxygen and Air (Sham) Arms
| Oxygen |
Air |
Difference in Mean Change, Oxygen vs. Air (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Baseline/Run-In | Follow-Up | Baseline/Run-in | Follow-up | |||
| Systolic BP, mm Hg | 129.6 ± 15.1 | 129.8 ± 13.6 | 129.2 ± 14.1 | 136.1 ± 14.9 | −6.6 (−11.3 to −1.9) | 0.008 |
| Diastolic BP, mm Hg | 79.3 ± 8.0 | 81.6 ± 8.0 | 78.3 ± 7.8 | 85.3 ± 9.6 | −4.6 (−7.8 to −1.5) | 0.006 |
| Heart rate, beats/min | 61.9 ± 9.4 | 64.1 ± 9.1 | 61.7 ± 8.3 | 64.9 ± 8.9 | −1.0 (−3.9 to +1.9) | 0.50 |
Definition of abbreviations: BP = blood pressure; CI = confidence interval.
Baseline/run-in and follow-up data are displayed as means ± SD. P values and 95% CIs were calculated using paired t tests. P < 0.05 highlighted in boldface.
Figure 3.
(A and B) Mean systolic (A) and diastolic (B) blood pressure in both treatment arms. Mean values are represented by circles (baseline) and diamonds (follow-up), and error bars represent the 95% confidence intervals (solid lines at baseline and dashed lines at 2-wk follow-up).
There was a significant effect of supplemental oxygen compared with air on office BP changes, recorded later in the morning, as shown in Table 3. There was no significant effect of supplemental oxygen compared with air on either home or office morning heart rate (Tables 2 and 3, respectively).
Table 3.
Secondary and Exploratory Outcome Data for the Supplemental Oxygen and Air Arms at Baseline/Run-in and at 2-Week Follow-up
| Oxygen |
Air |
Between-Group Difference, Oxygen vs. Air | P Value | |||
|---|---|---|---|---|---|---|
| Baseline/Run-in | Follow-up | Baseline/Run-in | Follow-up | |||
| Office BP and heart rate recordings | ||||||
| Systolic BP, mm Hg | 132.4 ± 16.7 | 130.9 ± 15.3 | 128.0 ± 13.7 | 134.8 ± 15.5 | −8.3 (−15.3 to −1.3) | 0.02 |
| Diastolic BP, mm Hg | 80.8 ± 9.4 | 79.6 ± 8.2 | 78.9 ± 9.9 | 84.0 ± 9.2 | −6.3 (−11.0 to −1.6) | 0.01 |
| Heart rate, beats/min | 66.3 ± 13.5 | 67.6 ± 13.0 | 65.6 ± 11.9 | 67.8 ± 14.0 | −0.8 (−4.1 to +2.5) | 0.61 |
| Overnight pulse oximetry and sleep study parameters | ||||||
| ODI, per hour | — | 6.4 (4.0 to 14.7) | — | 32.5 (25.6 to 47.0) | −23.8 (−31.0 to −16.3) | <0.001 |
| Mean oxygen saturations, % | — | 96.9 ± 1.2 | — | 92.6 ± 1.8 | +4.3 (+3.6 to +4.9) | <0.001 |
| Time O2 sats < 90%, % | — | 2.0 (0.3 to 3.9) | — | 14.3 (5.9 to 21.2) | −9.8 (−16.7 to −4.3) | <0.001 |
| Heart rate rises < 6 beats/min index, per hour | — | 27.1 (21.4 to 37.4) | — | 31.9 (24.0 to 44.3) | −3.7 (−9.1 to −0.8) | 0.006 |
| Mean heart rate, beats/min | — | 61.5 (53.5 to 64.0) | — | 62.0 (55.4 to 65.1) | −1.2 (−2.8 to +1.1) | 0.12 |
| AHI, per hour | — | 30.4 (23.6 to 42.6) | — | 34.4 (22.7 to 44.4) | −3.6 (−10.2 to +10.1) | 0.98 |
| Measures of sleepiness | ||||||
| ESS, score | 7.0 ± 4.8 | 9.0 ± 4.2 | 6.3 ± 3.8 | 8.8 ± 4.5 | −0.6 (−2.5 to +1.4) | 0.56 |
| OSLER, s | 2,400 (1,285 to 2,400) | 1,746 (770 to 2,400) | 2,400 (1,286 to 2,400) | 2,025 (956 to 2,400) | 0 (−425 to +14) | 0.50 |
| Urinary measurements | ||||||
| Rate of urine production, ml/h | 66.7 (48.6 to 95.8) | 85.1 (59.0 to 111.8) | 66.7 (46.8 to 105.1) | 95.8 (56.3 to 116.7) | +0.4 (−18.2 to +19.0) | 0.97 |
| Normetadrenaline, nmol/μmol | 121.7 (85.2 to 145.3) | 140.0 (116.8 to 186.2) | 107.5 (79.7 to 137.6) | 134.7 (113.7 to 199.7) | −12.8 (−35.3 to +9.6) | 0.25 |
| Metadrenaline, nmol/μmol | 34.1 (28.3 to 44.6) | 37.9 (27.1 to 43.2) | 35.1 (24.8 to 43.6) | 37.3 (26.9 to 46.8) | −0.6 (−6.9 to +5.7) | 0.84 |
| Blood measurements | ||||||
| hsCRP, mg/L | 2.7 (0.9 to 3.5) | 2.0 (0.7 to 3.8) | 1.1 (0.5 to 2.8) | 1.7 (0.9 to 2.6) | −0.4 (−3.7 to +0.6) | 0.12 |
| Venous base excess, mM | +1.6 ± 2.5 | +3.2 ± 2.2 | +3.0 ± 2.3 | +1.5 ± 1.9 | +3.1 (+1.8 to +4.4) | <0.001 |
Definition of abbreviations: AHI = apnea–hypopnea index; BP = blood pressure; CI = confidence interval; ESS = Epworth Sleepiness Scale; hsCRP = high-sensitivity C-reactive protein; ODI = oxygen desaturation index; OSLER = Oxford Sleep Resistance; sats = saturations.
For office BP and heart rate, measures of sleepiness, urinary measurements, and blood measurements, the difference in the change in values from baseline to follow-up was compared, oxygen versus air. For overnight oximetry and home sleep study parameters, the absolute differences in values at follow-up were compared, oxygen versus air. Normetadrenaline and metadrenaline excretions were corrected by urinary creatinine concentrations. Baseline and follow-up data are expressed as mean ± SD or median (interquartile range) and for oxygen–air differences, data are expressed as mean (95% CI, lower limit–upper limit) or median (interquartile range). Differences were compared using paired t tests or Wilcoxon rank tests as appropriate. Bold indicates P < 0.05.
Overnight Pulse Oximetry and Respiratory Polygraphy
As expected, supplemental oxygen significantly and markedly attenuated measures of intermittent hypoxia, with a median reduction in ODI of −23.8/h (P < 0.001; IQR, −31.0 to −16.3), and an absolute median reduction of the percentage time with oxygen saturations less than 90% of −9.8% (P < 0.001; IQR, −16.7 to −4.3), compared with air. There was a small significant reduction in the heart rate rise index (a measure of autonomic arousals), with a median reduction of −3.7/h (P = 0.006; IQR, −9.1 to −0.8), oxygen versus air. The small reduction in median AHI of −3.6/h (P = 0.98; IQR, −10.2 to +10.1), oxygen versus air, was not statistically significant.
Daytime Sleepiness and Sympathetic Activation
There were no significant differences in the change in either subjective or objective sleepiness, oxygen versus air. There were no significant differences in the change in mean overnight urinary volume or overnight urinary creatinine concentration, oxygen versus air. The small reduction in mean overnight urinary normetadrenaline levels of −12.8 nmol/μmol (P = 0.25; 95% CI, −35.3 to +9.6), oxygen versus air, was not statistically significant. There was no significant difference in overnight urinary metadrenaline or normetadrenaline production, oxygen versus air, whether correcting either for the rate of urinary production or urinary creatinine levels (see Table E3 in the online supplement).
hsCRP and Venous Blood Gases
Changes in hsCRP and venous base excess were exploratory outcomes. There were no significant differences in the change in hsCRP levels, oxygen versus air. Mean venous base excess from baseline to follow-up increased by +3.1 mM (P < 0.001; 95% CI, +1.8 to +4.4), oxygen versus air.
Discussion
Supplemental oxygen virtually abolished the rise in morning BP during CPAP withdrawal, compared with air. This suggests that intermittent hypoxia is responsible for the increased morning blood pressure in OSA. As expected, supplemental oxygen markedly attenuated intermittent hypoxia, while having only a small effect on heart rate rises and the AHI, surrogate markers of arousal. This suggests that intermittent hypoxia, rather than arousals, is responsible for the increased morning BP in OSA. The partial reduction in overnight urinary normetadrenaline was not significant, and it is thus not clear in patients with OSA whether overnight intermittent hypoxia increases morning BP via changes in sympathetic activity or via another mechanism. Unexpectedly, venous base excess was significantly increased with supplemental oxygen therapy and needs to be monitored in patients with OSA given such therapy.
The effects of supplemental oxygen during CPAP withdrawal on BP contrast with the findings of a previous randomized controlled trial (19). Gottlieb and colleagues found no effect of supplemental oxygen on BP during 12 weeks of therapy in patients with OSA, compared with control subjects. However, Gottlieb and colleagues applied supplemental oxygen at a low flow rate of 2 L/min delivered by nasal cannula, monitoring ambulatory BP by an intermittent method that itself causes arousals and elevations in nocturnal BP (26); furthermore, they excluded patients with the most severe OSA or hypoxia (those with either an AHI > 50 or with >10% time with oxygen saturations < 85%). In our study we used a higher supplemental oxygen flow rate of 5 L/min, monitored morning awake BP, and did not exclude patients based on OSA or hypoxia severity. The exclusion of patients with severe OSA in previous studies is a key difference, as a previous meta-analysis has shown that greater severity of returning OSA during CPAP withdrawal leads to larger increases in morning blood pressure (21). We also used a more powerful crossover CPAP withdrawal design, including patients with moderate to severe OSA with known previous response to CPAP, rather than treatment-naive patients. In addition, the mean usage of oxygen was lower in the Gottlieb study, at 4.8 h/night compared with 7.2 h/night in our study. It is likely that these methodological issues explain the differences in our findings. Gottlieb and colleagues also examined the effects of oxygen over a longer time period of 12 weeks rather than 2 weeks. Although it is possible that the effect of supplemental oxygen on BP may decrease over time we did not observe any decrease in the attenuating effect of supplemental oxygen on BP over 2 weeks (see Table E2 and Figures E1 and E2 in the online supplement).
As has been the case previously (17), supplemental oxygen did substantially reduce intermittent hypoxia in the SOX trial, which makes it likely to be responsible for the changes in BP. The reductions in overnight heart rate rises and the AHI (surrogates of arousal) that we observed were small, and therefore unlikely to be a significant alternative explanation for the virtual abolition of BP rises observed with supplemental oxygen during CPAP withdrawal.
Fletcher’s group conducted elegant experiments in rodents showing that intermittent hypoxia leads to diurnal BP rises (13); furthermore, these diurnal BP rises were shown to be dependent on the carotid body, the adrenal medulla, peripheral sympathetic nervous system, and the renin–angiotensin system (13, 27). Intermittent hypoxia also increases daytime BP and muscle sympathetic nerve activity in healthy volunteers (14). These experiments suggest that intermittent hypoxia, sensed by the carotid chemoreceptors, leads to increased daytime BP by increased sympathetic activity. In the SOX trial overnight urinary normetadrenaline levels, used as a marker of sympathetic activity, were only slightly reduced and this change was not statistically significant. This is perhaps not a surprising result as supplemental oxygen had little effect on the AHI or heart rate rises, and therefore arousal-mediated sympathetic activity was presumably unaffected by supplemental oxygen. Previously, supplemental oxygen has been shown to decrease daytime, but not nighttime, noradrenaline levels in patients with OSA (18). It is therefore still possible that reductions in daytime sympathetic activity underlie attenuated BP rises during CPAP withdrawal, but this is uncertain.
Whereas supplemental oxygen markedly attenuated the rise in BP with CPAP withdrawal, it had no effect on the rise in morning heart rate. If supplemental oxygen attenuates BP rises by attenuating sympathetic activation, similar attenuation in heart rate rises might have been expected. However, baroreceptor-mediated vagal activation from the rise in BP might have prevented significant rises in heart rate in the air arm (28). In addition, the mechanisms by which intermittent hypoxia/sympathetic activation lead to BP rises and heart rate rises are different. In animal models intermittent hypoxia-mediated BP rises were dependent on the renin–angiotensin system (27), and diurnal heart rate increases were not observed with intermittent hypoxia (29). Vagal tone is also important in determining heart rate and there is evidence of altered sympathetic/vagal balance with reductions in vagal modulation in OSA (30), which could be mediated by other mechanisms.
In the SOX trial, supplemental oxygen also had no effect on either objective or subjective sleepiness. Similarly, supplemental oxygen in OSA has previously been shown not to affect daytime sleepiness (31). Arousal-mediated sleep fragmentation therefore seems likely to cause daytime sleepiness in OSA, rather than intermittent hypoxia. Supplemental oxygen also had no effect on nocturia, suggesting hypoxia is not a driver of this consequence of OSA.
Previous studies have shown that supplemental oxygen could increase the size of myocardial injury, potentially by increasing oxidative stress, when given to normoxic patients after myocardial infarction (32). We have not measured oxidative stress in this study but did not see any downstream changes in systemic inflammation, measured by hsCRP. This potential deleterious effect of supplemental oxygen is not directly related to our primary outcome in this physiological mechanistic study but would need consideration in future research studies assessing any therapeutic effects of supplemental oxygen.
Supplemental oxygen caused significant increases in venous base excess, compared with air. Venous bicarbonate was used as an integrated marker of hypercapnia (24). The longer-term safety of oxygen therapy in OSA therefore needs careful consideration as, although hypercapnia was not particularly marked in this study, oxygen has the potential to worsen/lead to hypercapnic respiratory failure in some individuals, particularly those with OSA/obesity hypoventilation overlap (33).
There was a higher dropout rate in this trial compared with previous withdrawal trials (26% vs. 1%) (21). This is probably explained by the longer duration and crossover design of the SOX trial. Patients who withdrew after randomization were more obese and had more severe OSA (Table E1), and there were slightly more patients who withdrew while receiving supplemental air than when receiving supplemental oxygen (seven vs. five; not significant). However, adjustments for treatment order and baseline characteristics essentially did not alter the primary outcome (see Adjustments for Treatment Order and Modifiers in the online supplement). Although a higher dropout rate would have been of concern in a clinical trial of therapy, this is of less importance in a physiological mechanistic study.
BP was recorded only in the early and later morning, and the effect of oxygen therapy on the 24-hour BP profile is not known. Morning BP was chosen as an outcome as OSA is associated with a “nondipping” pattern in BP, which is an important risk factor for cardiovascular disease (34). Standard 24-hour BP monitoring techniques have limitations and have been shown to cause arousals from sleep and thus raise BP (26). Changes in office BP, which were measured later than the home measurement (at approximately 10 a.m.), were of similar magnitude to changes in home BP. This suggests the BP rise is not isolated to a brief period after waking.
Conclusions
Supplemental oxygen virtually abolished the rise in morning BP during 2 weeks of CPAP withdrawal. Supplemental oxygen markedly attenuated intermittent hypoxia while having a minimal effect on the AHI and autonomic arousals. Supplemental oxygen had no effect on morning heart rate, daytime sleepiness, hsCRP, and urinary volume, and only partially reduced overnight sympathetic activity. Although the exact mechanisms underlying the attenuation of BP rises by oxygen during CPAP withdrawal are not clear, intermittent hypoxia, and not arousal-mediated sympathetic activation, appears to be the likely dominant cause of daytime increases in BP in OSA. This was a physiological mechanistic study and future research is needed to see whether this translates into a potential clinical benefit. Previous trials assessing supplemental oxygen in OSA excluded patients with the most severe hypoxia, and this is probably the group most likely to benefit from oxygen treatment. CPAP has a greater effect on BP in patients with resistant hypertension. Thus, the effect of supplemental oxygen on BP should be assessed in patients with OSA and resistant hypertension, with significant nocturnal intermittent hypoxia, where CPAP is not indicated or tolerated, but with careful monitoring of carbon dioxide levels.
Supplementary Material
Footnotes
Supported by the Oxford Radcliffe Hospital Charitable Funds and ResMed UK. This research was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily of the NHS, the NIHR, or the Department of Health. N.P. was supported by an NIHR Academic Clinical Lectureship.
Author Contributions: C.D.T., M.K., N.P., and J.R.S. were responsible for study design. C.D.T., N.P., and D.S. performed data collection. C.D.T., N.P., and J.R.S. performed data analysis. C.D.T. performed literature searches. All authors contributed to data interpretation and manuscript writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201802-0240OC on July 20, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 3.Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case–control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55:736–740. doi: 10.1136/thorax.55.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Pepperell JCT, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 6.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2015;17:215–222. doi: 10.1111/jch.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta-analysis. Respirology. 2015;20:889–895. doi: 10.1111/resp.12573. [DOI] [PubMed] [Google Scholar]
- 9.Lawes CMM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 10.Davies RJ, Crosby J, Vardi-Visy K, Clarke M, Stradling JR. Non-invasive beat to beat arterial blood pressure during non-REM sleep in obstructive sleep apnoea and snoring. Thorax. 1994;49:335–339. doi: 10.1136/thx.49.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringler J, Basner RC, Shannon R, Schwartzstein R, Manning H, Weinberger SE, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1985;1990:2143–2148. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 12.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–1532. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 13.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia: influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 14.Tamisier R, Pépin JL, Rémy J, Baguet JP, Taylor JA, Weiss JW, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 15.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 17.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9:271–279. doi: 10.5664/jcsm.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 1985;2006:343–348. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler M, Stoewhas A-C, Ayers L, Senn O, Bloch KE, Russi EW, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–1199. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz EI, Schlatzer C, Rossi VA, Stradling JR, Kohler M. Effect of CPAP withdrawal on BP in OSA: data from three randomized controlled trials. Chest. 2016;150:1202–1210. doi: 10.1016/j.chest.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull CD, Petousi N, Sen D, Stradling JR, Kohler M. The effects of supplemental oxygen on blood pressure in obstructive sleep apnoea during CPAP withdrawal [abstract] Thorax. 2017;72:A202. doi: 10.1164/rccm.201802-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petousi N, Turnbull CD, Sen D, Kohler M, Stradling J.Effects of overnight supplemental oxygen on morning blood pressure in a randomised controlled CPAP withdrawal trial in patients with OSA [abstract]. Am J Respir Crit Care Med 2018197A1045 [Google Scholar]
- 24.Norman RG, Goldring RM, Clain JM, Oppenheimer BW, Charney AN, Rapoport DM, et al. Transition from acute to chronic hypercapnia in patients with periodic breathing: predictions from a computer model. J Appl Physiol. 1985;2006:1733–1741. doi: 10.1152/japplphysiol.00502.2005. [DOI] [PubMed] [Google Scholar]
- 25.Pitson DJ, Stradling JR. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnoea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998;7:53–59. doi: 10.1046/j.1365-2869.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 26.Davies RJ, Jenkins NE, Stradling JR. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994;308:820–823. doi: 10.1136/bmj.308.6932.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren P, Manning J. Decrease in cardiac activity by carotid sinus baroceptor reflex. Acta Physiol Scand. 1965;63:401–408. doi: 10.1111/j.1748-1716.1965.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 30.Balachandran JS, Bakker JP, Rahangdale S, Yim-Yeh S, Mietus JE, Goldberger AL, et al. Effect of mild, asymptomatic obstructive sleep apnea on daytime heart rate variability and impedance cardiography measurements. Am J Cardiol. 2012;109:140–145. doi: 10.1016/j.amjcard.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loredo JS, Ancoli-Israel S, Kim E-J, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;29:564–571. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 32.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, et al. AVOID Investigators. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 33.Hollier CA, Harmer AR, Maxwell LJ, Menadue C, Willson GN, Unger G, et al. Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomised crossover study. Thorax. 2014;69:346–353. doi: 10.1136/thoraxjnl-2013-204389. [DOI] [PubMed] [Google Scholar]
- 34.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70:1062–1069. doi: 10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.