Abstract
Rationale: In primary ciliary dyskinesia, factors leading to disease heterogeneity are poorly understood.
Objectives: To describe early lung disease progression in primary ciliary dyskinesia and identify associations between ultrastructural defects and genotypes with clinical phenotype.
Methods: This was a prospective, longitudinal (5 yr), multicenter, observational study. Inclusion criteria were less than 19 years at enrollment and greater than or equal to two annual study visits. Linear mixed effects models including random slope and random intercept were used to evaluate longitudinal associations between the ciliary defect group (or genotype group) and clinical features (percent predicted FEV1 and weight and height z-scores).
Measurements and Main Results: A total of 137 participants completed 732 visits. The group with absent inner dynein arm, central apparatus defects, and microtubular disorganization (IDA/CA/MTD) (n = 41) were significantly younger at diagnosis and in mixed effects models had significantly lower percent predicted FEV1 and weight and height z-scores than the isolated outer dynein arm defect (n = 55) group. Participants with CCDC39 or CCDC40 mutations (n = 34) had lower percent predicted FEV1 and weight and height z-scores than those with DNAH5 mutations (n = 36). For the entire cohort, percent predicted FEV1 decline was heterogeneous with a mean (SE) decline of 0.57 (0.25) percent predicted/yr. Rate of decline was different from zero only in the IDA/MTD/CA group (mean [SE], −1.11 [0.48] percent predicted/yr; P = 0.02).
Conclusions: Participants with IDA/MTD/CA defects, which included individuals with CCDC39 or CCDC40 mutations, had worse lung function and growth indices compared with those with outer dynein arm defects and DNAH5 mutations, respectively. The only group with a significant lung function decline over time were participants with IDA/MTD/CA defects.
Keywords: Kartagener syndrome, cilia, respiratory function tests
At a Glance Commentary
Scientific Knowledge on the Subject
In children with primary ciliary dyskinesia, abnormal ultrastructure and/or function of respiratory cilia leads to upper and lower respiratory tract disease that typically begins during infancy. Respiratory manifestations are heterogeneous, and ultrastructural and genotype–phenotype relationships are emerging in primary ciliary dyskinesia. The factors leading to longitudinal changes in lung disease are poorly understood in this population.
What This Study Adds to the Field
This multicenter, prospective study detected different clinical phenotypes based on ciliary ultrastructural defect group and the corresponding genotype. Children who had absence of inner dynein arm with central apparatus defects and microtubular disorganization had poorer lung function at all ages compared with those with isolated outer dynein arm defects. Furthermore, this was the only group that had a significant decline in lung function over time. Those with CCDC39 or CCDC40 genetic mutations also had significantly diminished lung function and growth parameters compared with those with genetic mutations in DNAH5.
In primary ciliary dyskinesia (PCD), abnormal function of motor cilia lining the respiratory epithelium leads to impaired mucociliary clearance, resulting in recurrent or chronic bacterial infections of the airways, paranasal sinuses, and middle ear. Lower and upper respiratory tract manifestations, such as recurrent or persistent bronchitis, sinusitis, and otitis media, typically begin during infancy (1–3). Chronic atelectasis, bronchiectasis, and airflow limitation are frequently apparent by early childhood (4–6).
Pathogenic variants in PCD-causing genes lead to specific ultrastructural defects, and genotype-phenotype relationships are emerging in PCD. Specifically, variability in the severity and progression of lung disease in PCD may be related to the underlying ultrastructural or genetic defect (5–7). In a cross-sectional study of children and adolescents with confirmed PCD (5), growth parameters and spirometry indices were worse in participants who had absent inner dynein arm (IDA), central apparatus abnormalities (CA), and microtubular disorganization (MTD) compared with participants with isolated outer dynein arm (ODA) or combined ODA/IDA defects. Most (75%) participants with IDA/CA/MTD ultrastructural defects had biallelic pathogenic variants in CCDC39 or CCDC40. However, there are little longitudinal data (6, 7) evaluating the impact of specific ultrastructural or genetic defects on progression of respiratory disease in children and adolescents with PCD.
Streptococcus pneumoniae, Staphylococcus aureus, and nontypable Haemophilus influenzae are common bacterial isolates in PCD sputum (8–10). Pseudomonas aeruginosa is reported in sputum samples from children, but is more prevalent in adults with PCD (5, 9, 10). However, the evolution of PCD pathogen prevalence with age and its relationship to ultrastructural or genetic defects has not been systematically evaluated in the pediatric population.
The primary objectives of this study are to describe the age-related progression of early lung disease in PCD and identify associations between genotypes and ultrastructural defects with clinical phenotypes in the pediatric population. We hypothesize that lung disease progresses over time and that specific ciliary ultrastructural abnormalities and their associated genetic defects will segregate into clinical phenotypes with different pathogen prevalence, severities, and trajectories of airway obstruction and growth metrics. In this era of precision medicine, elucidating the longitudinal course of this rare genetic lung disease is imperative to further the understanding of its pathogenesis and progression. Some of the results of this study have been previously reported in the form of an abstract (11).
Methods
Study Design
This was a prospective, longitudinal, multicenter, observational study.
Study Sites and Participants
As previously described (5), study participants were enrolled at seven sites participating in the Genetic Disorders of Mucociliary Clearance Consortium between 2006 and 2011. At enrollment, participants were less than 19 years of age with a diagnosis of confirmed, probable, or possible PCD. Study visits occurred annually for up to 5 years (six study visits). The cohort for the current analysis was limited to those with confirmed PCD (abnormal ciliary ultrastructure by transmission electron microscopy and/or genetic mutations consistent with PCD along with compatible clinical features) who had two or more study visits. Institutional review board approval was obtained at each site. Informed consent and assent were acquired from parents and participants, when appropriate. An observational safety monitoring board reviewed and approved all clinical protocols.
Study Procedures
Study procedures have been described previously (5). Briefly, standardized procedures were used for diagnostic testing (evaluation of ciliary ultrastructure from transmission electron micrographs [EM] and mutation screening for >30 PCD-causing genes) (5) and for annual assessment of clinical features including medical history, physical examination, spirometry (preschool spirometry in those 3–5 yr of age and standard spirometry in those >5 yr of age), and a respiratory culture from a deep oropharyngeal swab or expectorated sputum. All EMs were reviewed in a blinded fashion by three expert reviewers at the University of North Carolina at Chapel Hill (5). To confirm an isolated IDA defect, two to three nasal epithelial biopsies must be conducted at different time points. Between 25% and 30% of IDA defects on the first biopsy are normal on repeat biopsy (12, 13). To date, no genetic defect has been associated with an IDA defect alone. These annual assessments were conducted when the participants were healthy or free from acute illness for 4 weeks. All spirometry measurements were over-read for participants less than 7 years of age.
Statistical Analysis
Descriptive statistics are presented for the entire cohort and for five ciliary ultrastructure defect groups: 1) isolated ODA defects, 2) ODA/IDA defects, 3) absence of IDA in conjunction with CA defects and MTD, 4) normal ultrastructure on EM with genetic mutations consistent with PCD, and 5) other defects (oligocilia and isolated CA defects).
Baseline characteristics were compared between the ODA defect group (the most prevalent group) and the other ciliary ultrastructural defect groups using general linear models for continuous characteristics and logistic regression for dichotomous outcomes. To evaluate the association between ciliary ultrastructure defect group and clinical features (percent predicted FEV1 [14] and weight-for-age z-scores and height-for-age z-scores), we used linear mixed effects models for all repeated measurements from each participant, including random slope (within subject change) and random intercept (subject specific difference) (15). We first examined a model including ultrastructure defect group, age in years, and the interaction between them as fixed effects. When the interaction term was not statistically significant, it was removed from the model, and we estimated the overall association between the clinical feature and defect group, assuming it was not different by age. We also performed a sensitivity analysis eliminating all spirometric measurements for participants 3–5 years of age due to potential differences between preschool spirometry and standard spirometry. Generalized estimating equations with a logit link were used to compare respiratory pathogen prevalence between defect groups. Two-sided P values less than 0.05 were considered statistically significant. Analyses were performed using SAS version 9.4.
Results
Participant Characteristics
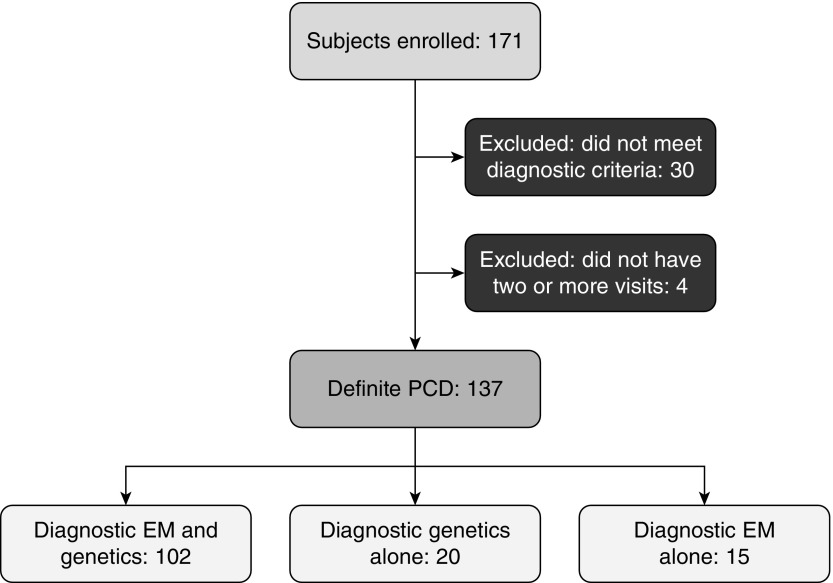
Of the 171 study participants, 137 had a definite diagnosis of PCD and participated in at least two study visits; these individuals comprised the cohort for the current analysis (Figure 1). These participants completed 732 study visits, with a median of six visits per participant (range, 2–6); 91 participants completed all six annual study visits. Median (range) follow-up was 6 (1–6) years. The ciliary ultrastructural defects and genetic mutations (two pathogenic or likely pathogenic variants in a known PCD-associated gene; or, for X-linked cases, one pathogenic variant in the X chromosome in males) are shown in Table 1: a total of 55 had isolated ODA defects, 20 had ODA + IDA defects, 41 had IDA/CA/MTD defects, 12 had normal ciliary ultrastructure, and nine had other defects (oligocilia [n = 4] and isolated CA defect [n = 5]). Of note, the genetic mutations for the isolated ODA and ODA/IDA groups included multiple PCD genes; however, the genetic mutations for the IDA/CA/MTD group (CCDC39 or CCDC40) and for the normal ultrastructure group (DNAH11 or RPGR) were each limited to two genes (Table E1 in the online supplement outlines diagnostic genetic variants for each participant).
Figure 1.
Flowchart outlining the enrolled participants. PCD = primary ciliary dyskinesia; EM = electron micrographs.
Table 1.
Ultrastructural and Genetic Findings
| PCD-Causing Gene | ODA | ODA/IDA | IDA/CA/MTD | Normal EM | Other: Oligocilia | Other: CA | Total |
|---|---|---|---|---|---|---|---|
| None identified | 4 | 3 | 7 | — | — | 1 | 15 |
| DNAH5 | 36 | — | — | — | — | — | 36 |
| DNAI1 | 7 | — | — | — | — | — | 7 |
| DNAI2 | 5 | — | — | — | — | — | 5 |
| CCDC114 | 2 | — | — | — | — | — | 2 |
| ARMC4 | 1 | — | — | — | — | — | 1 |
| LRRC6 | — | 3 | — | — | — | — | 3 |
| DYX1C1 | — | 3 | — | — | — | — | 3 |
| CCDC103 | — | 2 | — | — | — | — | 2 |
| HEATR2 | — | 2 | — | — | — | — | 2 |
| SPAG1 | — | 2 | — | — | — | — | 2 |
| DNAAF1 | — | 2 | — | — | — | — | 2 |
| DNAAF2 | — | 1 | — | — | — | — | 1 |
| DNAAF3 | — | 1 | — | — | — | — | 1 |
| PIH1D3 (X-linked) | — | 1 | — | — | — | — | 1 |
| CCDC40 | — | — | 18 | — | — | — | 18 |
| CCDC39 | — | — | 16 | — | — | — | 16 |
| DNAH11 | — | — | — | 11 | — | — | 11 |
| RPGR (X-linked) | — | — | — | 1 | — | — | 1 |
| CCNO | — | — | — | — | 4 | — | 4 |
| RSPH4A | — | — | — | — | — | 2 | 2 |
| RSPH1 | — | — | — | — | — | 1 | 1 |
| RSPH9 | — | — | — | — | — | 1 | 1 |
| Total | 55 | 20 | 41 | 12 | 4 | 5 | 137 |
Definition of abbreviations: CA = central apparatus; EM = electron micrographs; IDA = inner dynein arm; MTD = microtubular disorganization; ODA = outer dynein arm; PCD = primary ciliary dyskinesia.
The 20 participants with diagnosis confirmed by genetics alone are classified into the appropriate ciliary defect group based on their PCD-causing gene mutations.
Baseline characteristics of the entire cohort and categorized by ciliary ultrastructure defect are shown in Table 2. Participants with IDA/CA/MTD defects were younger at age of initial diagnosis and at enrollment, and had significantly lower growth parameters (weight and body mass index percentile) and percent predicted FEV1 than participants with isolated ODA defects. There were no differences in baseline characteristics between the other ciliary defect groups and the ODA defect group.
Table 2.
Baseline Demographic and Clinical Characteristics by Ciliary Ultrastructural Defect Group
| Overall (n = 137) | ODA (n = 55) | ODA/IDA (n = 20) | IDA/CA/MTD (n = 41) | Normal EM (n = 12) | Other* (n = 9) | |
|---|---|---|---|---|---|---|
| Male, n (%) | 67 (49) | 27 (49) | 12 (60) | 21 (51) | 3 (25) | 4 (44) |
| Race, n (%) | ||||||
| White | 112 (82) | 48 (87) | 13 (65) | 32 (78) | 10 (83) | 9 (100) |
| Black | 3 (2) | — | 1 (5) | 2 (5) | — | — |
| Asian | 16 (12) | 4 (7) | 6 (30) | 4 (10) | 2 (17) | — |
| Native American | 1 (<1) | 1 (2) | — | — | — | — |
| Multiracial | 3 (2) | 2 (4) | — | 1 (2) | — | — |
| Not reported | 2 (2) | — | — | 2 (5) | — | — |
| Hispanic or Latino, n (%) | 13 (9) | 3 (5) | 1 (5) | 5 (12) | 2 (17) | 2 (22) |
| Age at initial diagnosis, yr, mean (SD) | 3.9 (3.8) | 4.8 (4.4) | 3.2 (3.4) | 2.5 (2.9)‡ | 5.2 (2.8) | 4.8 (3.1) |
| Age at enrollment, yr, mean (SD) | 7.8 (4.6) | 8.9 (4.3) | 7.5 (5.6) | 6.4 (4.4)‡ | 9.0 (4.4) | 7.1 (4.2) |
| Height percentile, mean (SD) | 47.9 (30.5) | 52.4 (31.4) | 44.7 (32.8) | 41.6 (25.8)‡ | 56.5 (35.3) | 38.4 (29.5) |
| Weight percentile, mean (SD) | 52.6 (31.2) | 60.8 (32.6) | 49.9 (34.7) | 40.4 (24.3)‡ | 54.4 (31.6) | 59.6 (29.5) |
| BMI percentile, mean (SD) | 55.4 (31.1) | 62.5 (30.7) | 50.2 (34.8) | 45.5 (26.6)‡ | 52.1 (33.9) | 65.3 (32.0) |
| FEV1 percent predicted (SD)† | 82.7 (19.1) | 88.3 (17.4) | 85.5 (21.4) | 71.5 (18.4)‡ | 86.1 (16.4) | 81.0 (9.5) |
| Nasal NO, nl/min, mean (SD) | 18.2 (20.1) | 14.0 (9.1) | 21.6 (24.2) | 17.8 (14.0) | 31.3 (48.4)‡ | 27.4 (21.1) |
| Clinical features, n (%) | ||||||
| Laterality defect | 71 (52) | 32 (58) | 10 (50) | 21 (51) | 8 (67) | 0 |
| Neonatal respiratory distress | 111 (81) | 43 (78) | 18 (90) | 36 (88) | 9 (75) | 5 (56) |
| Chronic cough | 136 (99) | 55 (100) | 20 (100) | 40 (98) | 12 (100) | 9 (100) |
| Chronic nasal congestion | 134 (98) | 54 (98) | 19 (95) | 40 (98) | 12 (100) | 9 (100) |
| Chronic otitis media | 127 (93) | 52 (95) | 18 (90) | 37 (90) | 11 (92) | 9 (100) |
Definition of abbreviations: BMI = body mass index; CA = central apparatus; EM = electron micrographs; IDA = inner dynein arm; MTD = microtubular disorganization; ODA = outer dynein arm.
Includes oligocilia (n = 4) and central apparatus defect (n = 5).
Baseline spirometry available from 92 participants; others were too young to perform spirometry at enrollment.
P < 0.05 for comparison with ODA defect group.
Age-Specific Respiratory Microbiology
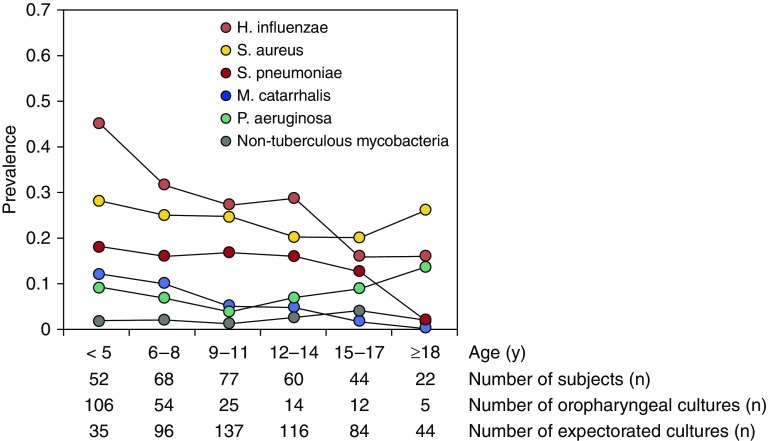
Respiratory cultures were obtained at 728 of the 732 (99.4%) study visits with a median of six cultures per participant (range, 2–6). Most (70.3%) samples were expectorated sputum; deep pharyngeal samples (29.7%) were obtained from those unable to expectorate, predominantly young children less than or equal to 5 years of age (Figure 2). Figure 2 is a cross-sectional plot of the prevalence of bacteria isolated from respiratory cultures by age category. Each participant contributed data in each age category in which culture results were available.
Figure 2.
Serial cross-sectional plot of the prevalence of bacteria isolated from respiratory cultures by age category.
The most common bacterial isolates across all ages were H. influenzae and S. aureus. P. aeruginosa and nontuberculous mycobacteria were relatively less common. During the study period, P. aeruginosa was recovered from 40 participants, and mucoid P. aeruginosa was isolated from seven participants, including four who had ODA defects (four DNAH5), two with IDA/CA/MTD defects (one CCDC39, one CCDC40), and one with an other defect (RSPH4A). The youngest age for P. aeruginosa recovery was 1.4 years (deep oropharyngeal culture yielding mucoid and nonmucoid P. aeruginosa in a participant with IDA/CA/MTD- CCDC39). P. aeruginosa infection was persistent (recovered in sputum culture from at least two consecutive annual visits) in 13 participants, including five participants with ODA defects (four DNAH5, one DNAI1), five with IDA/CA/MTD defects (one CCDC39, three CCDC40, one with no gene identified), two with normal axonemal ultrastructure (two DNAH11), and one with a CA defect (RSPH4A). The youngest age for persistent P. aeruginosa was 4 years (positive culture at 4 and 5 yr of age). Generalized estimating equations analyses did not reveal a difference in pathogen prevalence between defect groups.
Association of Ciliary Ultrastructural Defects with Lung Function and Growth Parameters
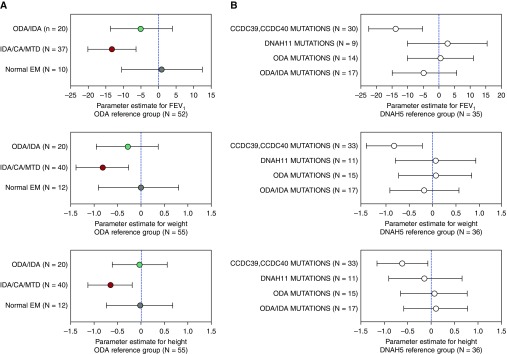
Figure 3A compares percent predicted FEV1 and weight-for-age and height-for-age z-scores between ultrastructural defect groups from linear mixed effects models. Compared with those with isolated ODA defects, participants with IDA/CA/MTD defects had lower percent predicted FEV1 and growth measures. We also compared percent predicted FEV1 and growth parameters between PCD genetic groups (Figure 3B). Compared with participants with DNAH5 mutations, participants with genetic mutations in CCDC39 or CCDC40 had lower percent predicted FEV1 and weight and height z-scores. When preschool spirometry was removed, the FEV1 values for the IDA/CA/MTD group and for the participants with CCDC39 and CCDC40 mutations remained significantly lower than the ODA (P = 0.0002) and DNAH5 (P = 0.001) reference groups, respectively (see the online supplement).
Figure 3.
(A) Forest plots of association of percent predicted FEV1 and weight and height z-scores with ciliary ultrastructural defects: ODA (reference group, n = 55), ODA/IDA (n = 20), IDA/CA/MTD (n = 40), and normal EM (n = 12). Linear mixed effects models including data from all study visits were used to estimate the overall association between clinical feature and defect group. (B) Forest plots of association of percent predicted FEV1 and weight and height z-scores with primary ciliary dyskinesia mutations. DNAH5 (reference group, n = 36), CCDC39 and CCDC40 (n = 33), DNAH11 (n = 11), other ODA (n = 15), and ODA/IDA (n = 17). Linear mixed effects models including data from all study visits were used to estimate the overall association between clinical feature and genetic defect group. CA = central apparatus; EM = electron micrographs; IDA = inner dynein arm; MTD = microtubular disorganization; ODA = outer dynein arm.
Longitudinal Changes in Lung Function and Growth Status
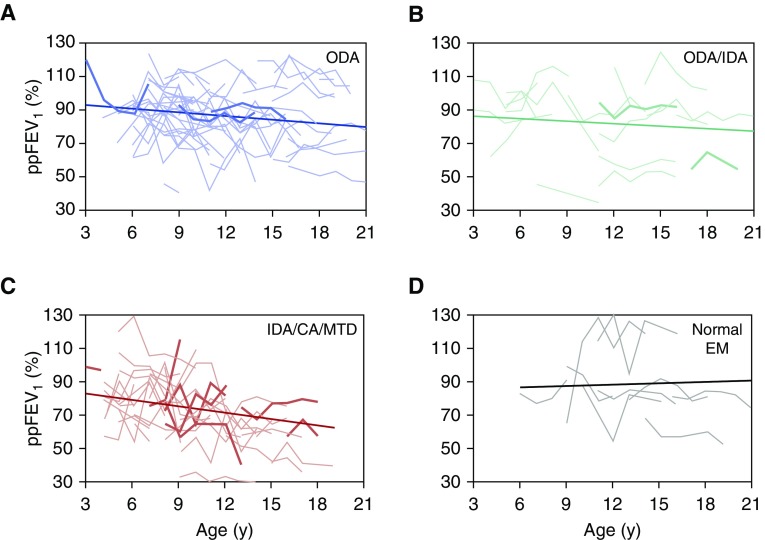
The mean annual change in percent predicted FEV1 was −0.57% per year (SE, 0.25; P = 0.03) for the cohort as a whole. Figure 4 shows the variability in individual patterns in percent predicted FEV1 change over time for each defect group and the estimated slopes from linear mixed effects models. Slopes (mean [SE] predicted/yr) were as follows: ODA (−0.73 [0.38]; P = 0.06), ODA/IDA (−0.48 [0.65]; P = 0.46), IDA/CA/MTD (−1.11 [0.48]; P = 0.02), and normal EM (0.29 [0.88]; P = 0.75). The rates of decline in the ODA, ODA/IDA, and IDA/CA/MTD groups were not significantly different from each other (P = 0.56). When preschool spirometry was removed for participants between the ages of 3 and 5 years, the decline in FEV1 for the IDA/CA/MTD group remained significant (see the online supplement). Weight-for-age and height-for-age z-scores did not decline with age for any of the ultrastructural defect groups (data not shown).
Figure 4.
Estimated mean annual change in percent predicted FEV1 (ppFEV1) for each ciliary ultrastructural defect. (A–D) Change in lung function with age for each individual in each defect group, with the estimated slope for that defect group superimposed. (A) ODA, ppFEV1 = 95.54 − 0.73 × age (yr). (B) ODA + IDA, ppFEV1 = 88.22 − 0.48 × age (yr). (C) IDA/CA/MTD, ppFEV1 = 85.54 − 1.1 × age (yr). (D) Normal EM, ppFEV1 = 85.77 + 0.29 × age (yr). Estimated slopes are from linear mixed effects models including ultrastructure defect group, age in years, and the interaction between them as fixed effects. Individuals with the bold lines are those who were diagnosed based on an ultrastructural defect without a corresponding genetic mutation. For definition of abbreviations, see Figure 3.
Discussion
In this prospective, multicenter, longitudinal North American study of children and young adults with confirmed PCD, we were able to detect different clinical phenotypes based on ciliary ultrastructural defect group and corresponding genotype. Specifically, we found differences in lung function and growth parameters, but not pathogen prevalence. Participants with IDA/CA/MTD defects had poorer lung function at the time of enrollment and at all ages compared with those with isolated ODA defects. Furthermore, this was the only group that had a significant decline in lung function over time. Participants with biallelic CCDC39 or CCDC40 pathogenic variants also had lower FEV1 values and weight and height z-scores compared with those with genetic mutations in DNAH5. In this cohort, growth parameters only seemed to be affected in those with IDA/CA/MTD defects, who had worse weight and height z-scores at every age compared with those with ODA defects. The most common respiratory organisms isolated were H. influenzae and S. aureus. P. aeruginosa was relatively uncommon in this young cohort, but its prevalence rose during adolescence. The prevalence of specific organisms did not differ based on ultrastructural defect. Participants with IDA/CA/MTD defects were younger at age of initial diagnosis, reinforcing the severity of disease in this group.
Lung function change over time was highly variable in our cohort, similar to past reports (6, 16). In the entire cohort, there was a significant decline in FEV1 over time (mean, −0.57% predicted per year). The fact that we were only able to detect a significant rate of decline in the IDA/CA/MTD group, despite these participants having a younger age of diagnosis and presumably earlier access to specialized care, highlights the increased risk for disease progression in this defect group. Furthermore, the seven individuals with the IDA/CA/MTD ultrastructural defect without genetic confirmation also had similar FEV1 values as the entire defect group. A recently published single-center study corroborated our findings, reporting that children and adults with PCD who had the MTD ultrastructural defect had lower spirometric indices and worse lung clearance index values when compared with those with dynein arm defects or normal ciliary ultrastructure (17). The findings in our study should be interpreted with caution, because our analyses had limited statistical power because of small sample sizes within each defect group. A past single-center study demonstrated stabilization of lung function in patients who received a standardized approach to care (16). However, our patients were followed at multiple clinical sites with different approaches to management.
Recent studies have shown that children with PCD can have growth deficiencies (18), but the association between axonemal ultrastructure defect or genotype and longitudinal growth parameters has not previously been reported. We found that growth was generally normal in all groups, except participants with IDA/CA/MTD ultrastructural defects, who had lower weight and height z-scores. Growth status did not progressively decline with time in any defect group. It is possible that the impaired growth is related to increased energy expenditure secondary to greater work of breathing in this cohort, which has been implicated in other suppurative airway diseases (19, 20).
Another potential explanation for poorer growth is greater upper airway involvement leading to anosmia and reduced appetite (21). A limitation of this prospective study is that we did not collect detailed data on upper airway disease. Future studies evaluating the impact of ultrastructural defects on the upper airway would further elucidate potential mechanisms for poor growth. The mechanism may be unclear, but a more aggressive approach to nutritional management may be required in these patients. As reported previously, a higher body mass index is also associated with higher FEV1 and FVC z-scores in PCD (22).
As previously reported (8–10), the most common pathogens were H. influenzae, S. aureus, and S. pneumoniae. The higher prevalence of these organisms in the younger participants may reflect the predominantly upper airway source of their respiratory cultures. As previously reported in pediatric PCD cohorts, we saw a low overall prevalence of P. aeruginosa. The predominance of P. aeruginosa in adults with PCD has been reported to be higher and we saw a rise in P. aeruginosa during adolescence. The observed decline in prevalence of certain organisms with age may also reflect overgrowth of cultures by P. aeruginosa in older patients or differences in yield from oropharyngeal cultures versus expectorated sputum.
Historically, the progression of PCD lung disease has been thought to be milder than that of cystic fibrosis (CF), although some patients with PCD have lung disease severe enough to require lung transplant in adulthood (23). Few studies have directly compared the two conditions (24–26). In our cohort, mean FEV1 was 83–93% predicted at age 5 depending on the defect group, potentially slightly lower than that of contemporary young patients with CF (27). However, great caution must be taken in relating observed lung function in PCD to CF cohorts. Comparisons require careful matching and the same analytic methods in both disease cohorts. Compared with pediatric CF cohorts (27), the prevalence of airway infection with P. aeruginosa is lower, although children with PCD seem to be more symptomatic at a younger age, with daily productive cough and persistent nasal congestion that begins in early infancy (5). The relative preservation of cough clearance in PCD may delay or prevent chronic colonization with P. aeruginosa during childhood. Alternatively, there are likely differences in airway surface liquid composition, mucus rheology, or innate airway defenses between CF and PCD that could explain the apparent difference in predilection for P. aeruginosa infection.
Understanding the underlying mechanisms for more severe airway disease in individuals with IDA/CA/MTD defects, often associated with mutations in CCDC39 or CCDC40, is imperative for developing interventions. CCDC39 and CCDC40 function as cilia “rulers,” directing the spacing and arrangement of the IDAs and radial spokes (28). CCDC39 and CCDC40 are integral components within the nexin-dynein regulatory complex, which is central for controlling the rhythmic motion of cilia (29–31). Genetic mutations in either CCDC39 or CCDC40 are associated with an abnormal ciliary beat pattern, in which most (up to 75%) cilia are static and the rest move with a rigid and ineffective beat (32, 33). For ODA defects, a smaller proportion of cilia are static and those that move have a stiff beat pattern, but not as rigid as the ciliary beat for IDA/CA/MTD defects (32–34). It seems unlikely that these differences in ciliary motion could account for the increased severity of lung disease in patients with IDA/CA/MTD defects. Conceivably, CCDC39 and CCDC40 may have a role in other nonepithelial cells that affect lung health. Early studies showed that neutrophils extracted from some individuals with PCD may have defective motility causing impaired chemotaxis and bacterial killing (35, 36). Because these studies were performed before the advent of genetic testing, it is unclear whether the neutrophil phenotype is related to specific PCD genotypes. A recent study demonstrated that neutrophils from patients with PCD with different ultrastructural and genetic defects had reduced chemotaxis compared with healthy control subjects (37), but only one of these individuals had a genetic defect in CCDC39 or CCDC40.
The strengths of this study are its prospective, longitudinal, multicenter study design and the use of rigorous, standardized diagnostic criteria, based on hallmark ultrastructure defects and/or genetic testing, which has allowed us to uncover ultrastructural and genotype-phenotype relationships in PCD. Nevertheless, PCD is still a largely underrecognized, rare disease. The relatively small sample size for each defect group limited our ability to detect differences between groups in clinical parameters, particularly FEV1 decline. Moreover, environmental factors and treatment strategies may have varied across sites, thus potentially impacting outcomes, especially if particular ultrastructural and/or genetic defects were overrepresented or underrepresented at a particular site. All participants in this study received clinical care at local institutions and were referred to the research sites for participation in this study. Because of relatively small numbers at each site, we were unable to assess this potential bias. A larger, international approach to studying rare lung diseases, such as PCD, is critical for future investigations to better identify predictors of bronchiectasis and lung function decline. Earlier identification of patients at higher risk for severe disease could alter early management and potentially change outcomes. In addition, an improved understanding of the impact of nutrition on respiratory disease severity among patients with PCD could aid management. Next steps in our cohort include refining the genotype and ultrastructural relationships to lung function, structure and growth parameters, and evaluating predictors of rate of lung function decline, such as nutritional status.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the children and families that participated in this study. They acknowledge Susan Minnix, Caroline LaFave, and Kathy Thurlow (research coodinators); Whitney Wolf (research technician) for performing DNA extractions, processing, and sequencing; Kimberly Burns (research technician) for processing the ciliary biopsies for ultrastructural analysis; and Robin Johnson (respiratory therapist), all from the University of North Carolina at Chapel Hill. Charles Clem (respiratory therapist, Indiana University) assisted in overreading lung function test results. They also appreciate the effort and skills provided by Jane Quante (research coordinator, Washington University); Carol Kopecky and Shelley Mann (research coordinators, University of Colorado); Melody Miki (research coordinator, The Hospital for Sick Children, Toronto); Liz Cochrane, Molly Elliott, Sharon McNamara, and Robert Johnson (research coordinators, Children’s Hospital and Regional Medical Center, Seattle); Jackie Zirbes (research coordinator, Stanford University); and Lou Ann Epperson (research coordinator, Indiana University). They also acknowledge Shrikant Mane, Francesc Lopez-Giraldez, and Weilai Dong (Yale Center for Mendelian Genomics; [UM1 HG006504]) for providing whole-exome sequencing and bioinformatics support; and Jay Shendure, Deborah Nickerson, and Michael Bamshad (University of Washington School of Medicine, Seattle [NIH/NHGRI grant U54HG0006493] and Seattle GO Sequencing project [HL-102926]) and the family studies project team for providing whole-exome sequencing and bioinformatics support. Finally, they thank all participants and families who were part of this study.
Footnotes
The Genetic Disorders of Mucociliary Clearance (U54HL096458) is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network. Rare Diseases Clinical Research Network is an initiative of the Office of Rare Diseases Research, NCATS, funded through a collaboration between NCATS and NHLBI; Clinical and Translational Science Awards NIH/NCATS UNC ULTR000083; R01HL071798; NIH/NCATS Colorado Clinical and Translational Science Awards grant number UL1 TR001082; and Intramural Research Program of NIH/National Institute of Allergy and Infectious Diseases.
Author Contributions: S. D. Davis, M.R., M.R.K., and M.W.L. designed the study. S. D. Davis, M.R., T.W.F., M.R.K., and M.W.L. prepared the manuscript. H.-S.L. performed statistical analysis. M.W.L. and M.R.K. reviewed electron photomicrographs. S. D. Davis, M.R., T.W.F., S.D.S., S. D. Dell, C.M., J.E.P., A.J.S., K.M.S., and M.W.L. recruited and evaluated patients at Genetic Disorders of Mucociliary Clearance Consortium sites. J.P.K. coordinated web-based data entry. K.R.N. and M.A.Z. coordinated and validated genetic studies.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201803-0548OC on August 1, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: the Genetic Disorders of Mucociliary Clearance Consortium
References
- 1.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullowney T, Manson D, Kim R, Stephens D, Shah V, Dell S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics. 2014;134:1160–1166. doi: 10.1542/peds.2014-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann Am Thorac Soc. 2016;13:1305–1313. doi: 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43:514–516. doi: 10.1002/ppul.20792. [DOI] [PubMed] [Google Scholar]
- 5.Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–324. doi: 10.1164/rccm.201409-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marthin JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181:1262–1268. doi: 10.1164/rccm.200811-1731OC. [DOI] [PubMed] [Google Scholar]
- 7.Maglione M, Bush A, Nielsen KG, Hogg C, Montella S, Marthin JK, et al. Multicenter analysis of body mass index, lung function, and sputum microbiology in primary ciliary dyskinesia. Pediatr Pulmonol. 2014;49:1243–1250. doi: 10.1002/ppul.22984. [DOI] [PubMed] [Google Scholar]
- 8.Alanin MC, Nielsen KG, von Buchwald C, Skov M, Aanaes K, Hoiby N, et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect. 2015;21:1093 e1091–1097. doi: 10.1016/j.cmi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Wijers CD, Chmiel JF, Gaston BM. Bacterial infections in patients with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chron Respir Dis. 2017;14:392–406. doi: 10.1177/1479972317694621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 11.Davis SD, Rosenfeld M, Lee H, Ferkol TW, Sagel SD, Dell SD, et al. Primary ciliary dyskinesia: longitudinal study of lung disease progression by ultrastructure and genotype. Am J Respir Crit Care Med. 2018. [DOI] [PMC free article] [PubMed]
- 12.Shoemark A, Ives A, Becker-Heck A, Burgoyne T, Dixon M, Bilton D, et al. Inner dynein arm defects in primary ciliary dyskinesia. J Genet Syndr Gene Ther. 2013;4:163. [Google Scholar]
- 13.O’Callaghan C, Rutman A, Williams GM, Hirst RA. Inner dynein arm defects causing primary ciliary dyskinesia: repeat testing required. Eur Respir J. 2011;38:603–607. doi: 10.1183/09031936.00108410. [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, N.J.: Wiley; 2011. [Google Scholar]
- 16.Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J. 1997;10:2376–2379. doi: 10.1183/09031936.97.10102376. [DOI] [PubMed] [Google Scholar]
- 17.Irving S, Dixon M, Fassad MR, Frost E, Hayward J, Kilpin K, et al. Primary ciliary dyskinesia due to microtubular defects is associated with worse lung clearance index. Hai. 2018;196:231–238. doi: 10.1007/s00408-018-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svobodova T, Djakow J, Zemkova D, Cipra A, Pohunek P, Lebl J. Impaired growth during childhood in patients with primary ciliary dyskinesia. Int J Endocrinol. 2013;2013:731423. doi: 10.1155/2013/731423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd RW, Holt TL, Vasques-Velasquez L, Coward WA, Prentice A, Lucas A. Increased energy expenditure in young children with cystic fibrosis. Lancet. 1988;1:1300–1303. doi: 10.1016/s0140-6736(88)92119-8. [DOI] [PubMed] [Google Scholar]
- 20.Vaisman N, Pencharz PB, Corey M, Canny GJ, Hahn E. Energy expenditure of patients with cystic fibrosis. J Pediatr. 1987;111:496–500. doi: 10.1016/s0022-3476(87)80107-5. [DOI] [PubMed] [Google Scholar]
- 21.Pifferi M, Bush A, Rizzo M, Tonacci A, Di Cicco M, Piras M, et al. Olfactory dysfunction is worse in primary ciliary dyskinesia compared with other causes of chronic sinusitis in children. Thorax. 2018;73:980–982. doi: 10.1136/thoraxjnl-2017-210661. [DOI] [PubMed] [Google Scholar]
- 22.Goutaki M, Halbeisen FS, Spycher BD, Maurer E, Belle F, Amirav I, et al. Swiss PCDG, French Reference Centre for Rare Lung D. Growth and nutritional status, and their association with lung function: a study from the international Primary Ciliary Dyskinesia Cohort. Eur Respir J. 2017;50:1701659. doi: 10.1183/13993003.01659-2017. [DOI] [PubMed] [Google Scholar]
- 23.Knowles MR, Zariwala M, Leigh M. Primary ciliary dyskinesia. Clin Chest Med. 2016;37:449–461. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maglione M, Montella S, Mollica C, Carnovale V, Iacotucci P, De Gregorio F, et al. Lung structure and function similarities between primary ciliary dyskinesia and mild cystic fibrosis: a pilot study. Ital J Pediatr. 2017;43:34. doi: 10.1186/s13052-017-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Cymberknoh M, Simanovsky N, Hiller N, Hillel AG, Shoseyov D, Kerem E. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest. 2014;145:738–744. doi: 10.1378/chest.13-1162. [DOI] [PubMed] [Google Scholar]
- 26.Ratjen F, Waters V, Klingel M, McDonald N, Dell S, Leahy TR, et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J. 2016;47:829–836. doi: 10.1183/13993003.01390-2015. [DOI] [PubMed] [Google Scholar]
- 27.2016 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation Patient Registry; 2016. [Google Scholar]
- 28.Oda T, Yanagisawa H, Kamiya R, Kikkawa M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science. 2014;346:857–860. doi: 10.1126/science.1260214. [DOI] [PubMed] [Google Scholar]
- 29.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79–84. doi: 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merveille AC, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43:72–78. doi: 10.1038/ng.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, et al. Uk10k. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–472. doi: 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, Loges NT, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014;44:1579–1588. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
- 34.Chilvers MA, Rutman A, O’Callaghan C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol. 2003;112:518–524. doi: 10.1016/S0091-6749(03)01799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valerius NH, Knudsen BB, Pedersen M. Defective neutrophil motility in patients with primary ciliary dyskinesia. Eur J Clin Invest. 1983;13:489–494. doi: 10.1111/j.1365-2362.1983.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 36.Kantar A, Oggiano N, Giorgi PL, Fiorini R. Membrane fluidity of polymorphonuclear leukocytes from children with primary ciliary dyskinesia. Pediatr Res. 1993;34:725–728. doi: 10.1203/00006450-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Cockx M, Gouwy M, Godding V, De Boeck K, Van Damme J, Boon M, et al. Neutrophils from patients with primary ciliary dyskinesia display reduced chemotaxis to CXCR2 ligands. Front Immunol. 2017;8:1126. doi: 10.3389/fimmu.2017.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.