Abstract
Rationale: MMPs (Matrix metalloproteinases) and their endogenous tissue inhibitors may contribute to lung injury through extracellular matrix degradation and modulation of inflammation and fibrosis.
Objectives: To test for an association between MMP pathway proteins and inflammation, endothelial dysfunction, and clinical outcomes.
Methods: We measured MMPs in plasma collected on acute respiratory distress syndrome (ARDS) Day 1 from 235 children at five hospitals between 2008 and 2017. We used latent class analysis to identify patients with distinct MMP profiles and then associated those profiles with markers of inflammation (IL-1RA, -6, -8, -10, and -18; macrophage inflammatory protein-1α and -1β; tumor necrosis factor-α and -R2), endothelial injury (angiopoietin-2, von Willebrand factor, soluble thrombomodulin), impaired oxygenation (PaO2/FiO2 [P/F] ratio, oxygenation index), morbidity, and mortality.
Measurements and Main Results: In geographically distinct derivation and validation cohorts, approximately one-third of patients demonstrated an MMP profile characterized by elevated MMP-1, -2, -3, -7, and -8 and tissue inhibitor of metalloproteinase-1 and -2; and depressed active and total MMP-9. This MMP profile was associated with multiple markers of inflammation, endothelial injury, and impaired oxygenation on Day 1 of ARDS, and conferred fourfold increased odds of mortality or severe morbidity independent of the P/F ratio and other confounders (95% confidence interval, 2.1–7.6; P < 0.001). Logistic regression using both the P/F ratio and MMP profiles was superior to the P/F ratio alone in prognosticating mortality or severe morbidity (area under the receiver operating characteristic curve, 0.75; 95% confidence interval, 0.68–0.82 vs. area under the receiver operating characteristic curve, 0.66; 95% confidence interval, 0.58–0.73; P = 0.009).
Conclusions: Pediatric patients with ARDS have specific plasma MMP profiles associated with inflammation, endothelial injury, morbidity, and mortality. MMPs may play a role in the pathobiology of children with ARDS.
Keywords: matrix metalloproteinases, pediatric intensive care unit, pediatric acute respiratory distress syndrome, tissue inhibitor of metalloproteinases, pediatric acute lung injury
At a Glance Commentary
Scientific Knowledge on the Subject
MMPs (matrix metalloproteinases) and their endogenous tissue inhibitors may contribute to lung injury through extracellular matrix degradation and modulation of inflammation and fibrosis.
What This Study Adds to the Field
Approximately one-third of children with acute respiratory distress syndrome display an MMP profile of elevated MMP-1, -2, -3, -7, and -8 and tissue inhibitor of metalloproteinase-1 and -2; and depressed active and total MMP-9. This MMP profile was associated with multiple markers of inflammation, endothelial injury, and impaired oxygenation on Day 1 of acute respiratory distress syndrome, and conferred a fourfold increase in the adjusted odds of mortality or severe morbidity. Patients with this MMP profile represent a high-risk subgroup and merit further investigation.
In children, the acute respiratory distress syndrome (ARDS) is defined by clinical oxygenation impairment and radiographic evidence of new-onset noncardiogenic pulmonary edema (1). This disease is mediated by systemic inflammation, pulmonary endothelial and epithelial injury, and abnormal coagulation and fibrinolysis, resulting in 15–20% mortality and significant morbidity among survivors (2–5). As such, identification of novel biochemical pathways related to disease onset and severity is of paramount interest to clinicians and researchers.
MMPs (matrix metalloproteinases) represent a potential class of proteins involved in pediatric ARDS because of their predominant localization to neutrophils, monocytes, fibroblasts, and epithelial cells (6–8); their direct role in extracellular matrix degradation; and their ability to modulate inflammation by cleaving membrane-bound cytokines and chemokines (9–11). More than 20 proteins in this family have been described; individual members share overlapping functions as collagenases, gelatinases, stromelysins, matrilysins, and activators of a variety of proteins including pro–TNF-α (tumor necrosis factor-α), pro–IL-1β, and pro–TGF-β (transforming growth factor-β) (9). Elevated levels of many MMPs have been measured in lung fluid and plasma of animals and adults with ARDS and are associated with disease severity and mortality (12–14). Furthermore, pharmacologic or genetic inhibition of MMPs, such as MMP-3, has been associated with decreased severity of lung injury in preclinical models (15–17). However, after the initial inflammatory response to injury, some MMPs play an important role in the resolution of inflammation by downregulating chemokinesis, inducing leukocyte autophagy, and opposing the effects of TGF-β (18, 19). In several animal models of ARDS, pharmacologic or genetic inhibition of MMP-9 leads to prolonged, unresolved inflammation resulting in pulmonary fibrosis (20, 21). In adults, an imbalance in MMP-9 relative to its endogenous inhibitor TIMP-1 (tissue inhibitor of metalloproteinase-1) has been demonstrated in several fibrotic diseases, including idiopathic pulmonary fibrosis (22–24). However, to date, evidence linking MMP levels to pediatric ARDS pathobiology and clinical outcomes is lacking.
Therefore, the objective of this study was to interrogate a wide spectrum of the MMP family in the blood of children on Day 1 of ARDS and test for associations with clinical outcomes. Furthermore, the clinical observation that patients with similarly impaired oxygenation on ARDS Day 1 can progress to markedly different outcomes suggests that there may be differences in ARDS biology that cannot be observed at the bedside (25, 26). As such, we hypothesized that subgroups of pediatric ARDS patients might exist and could be distinguished using MMP measurements. Finally, we hypothesized that these biologically different groups would have specific patterns of inflammation, endothelial injury, and impaired oxygenation on ARDS Day 1 and disparate clinical outcomes.
Methods
Patients
As described previously (27), patients were enrolled with parent/surrogate consent in an ongoing, prospective cohort between 2008 and 2017 if they were between 30 days and 18 years of age, used positive pressure ventilation, and met the Berlin diagnostic criteria for ARDS (University of California San Francisco IRB 10-00206) (28). Patients younger than 36 weeks corrected gestational age and those with limited goals of care were excluded. We divided patients into a derivation cohort, consisting of patients enrolled at the primary site (University of California San Francisco Benioff Children’s Hospital), and a validation cohort, consisting of patients enrolled at collaborating sites (Oakland Children’s Hospital, Children’s Hospital Central California, Children’s Hospital Los Angeles, and American Family Children’s Hospital).
Measurements
We collected plasma within 24 hours of ARDS diagnosis and used a customized multiplex immunoassay to measure MMP-1, -2, -3, -7, -8, and -9 (total) and TIMP-1 and -2 (Myriad RBM), and an ELISA to measure active MMP (aMMP)-9 (R&D). A subset of measurements was repeated to verify less than 15% intraassay variability.
Classification of MMP Profiles
We tested whether patients could be grouped according to similar MMP profiles using a model-based clustering approach called latent class analysis (LCA; Mplus version 7; see the Methods section of the online supplement). This method allows identification of patients that are biologically similar with respect to the MMP pathway measurements and unlike supervised clustering strategies, is agnostic toward any distal outcome, such as mortality. It also allows the inclusion of patients with partially missing data by using the Full Information Maximum Likelihood approach (29). The optimal number of patient groups was selected using previously described criteria (25, 26, 30). To determine whether MMP profiles found in the derivation cohort also existed in the validation cohort, we modeled latent groups in the derivation cohort with parsimonious linear regression, applied that regression to the validation cohort to assign latent group membership, and compared the assignments with those made by independent latent profile analysis.
Outcomes
Our primary outcome was hospital mortality and the secondary outcomes were morbidity, as estimated by the Pediatric Logistic Organ Dysfunction (PELOD) score, and the composite outcome of hospital mortality or severe morbidity. The PELOD score was taken from the highest individual score recorded on pediatric ICU Days 1–7, 14, 21, and 28 (31). Severe morbidity was defined as the top quartile of PELOD score among survivors (PELOD ≥30). To control for potential confounding, we tested for associations between mortality and age; sex; race; admission illness severity; lung injury subtype; a diagnosis of cancer or prior hematopoietic cellular transplantation (HCT), which we have previously shown to be strongly associated with pediatric ARDS outcomes (5, 32, 33); and white blood cell count, which we hypothesized might be directly associated with plasma MMP levels and ARDS outcomes. Admission illness severity was estimated by the PRISM-3 score and the worst PaO2/FiO2 (P/F) ratio and oxygenation index (OI) recorded in the first 24 hours after ARDS diagnosis.
Plasma Biomarkers
Measurement of inflammatory biomarkers (IL-1RA, -6, -8, -10, and -18; macrophage inflammatory proteins 1α and 1β; TNF-α; TNF-R2) and endothelial injury biomarkers (soluble thrombomodulin, angiopoietin-2, von Willebrand factor) in this cohort was described previously (4, 27, 34).
Statistics
Distributions of categorical and continuous variables were compared across MMP profiles using the Pearson chi-square and Wilcoxon rank sum tests, respectively. Multiple logistic regression was performed to test for associations between latent class assignment and the primary and secondary outcomes, and area under the receiver operating characteristic curve (AUROC) was compared using a nonparametric covariance matrix with significance estimated by a two-tailed chi-square statistic (35).
Results
Of 326 patients enrolled in the primary study, 235 patients consented to and had adequate plasma for MMP measurements and were included in this study (Table 1). Nonwhite race was more common among patients without biomarker measurements (see Supplemental Data 1 and Table E1 in the online supplement). Because of limitations in available blood volume, 170 of 235 patients had all nine biomarkers measured and 65 of 235 patients had a subset of the nine biomarkers measured.
Table 1.
Characteristics of Enrolled Patients with MMP Pathway Measurements
| Characteristics | All Patients (n = 235) | Survivors (n = 193) | Nonsurvivors (n = 42) | P Value |
|---|---|---|---|---|
| Age, yr, median (IQR) | 4.1 (1.0–11.5) | 3.7 (0.9–11.3) | 7.7 (2.5–12.6) | 0.141 |
| Sex, male, n (%) | 125 (53.2) | 98 (50.8) | 27 (64.3) | 0.112 |
| Race, n (%) | 0.712 | |||
| White | 160 (68.1) | 132 (68.4) | 28 (66.7) | |
| Unknown | 30 (12.8) | 24 (12.4) | 6 (14.3) | |
| Black | 17 (7.2) | 15 (7.8) | 2 (4.8) | |
| Asian/Pacific Islander | 15 (6.4) | 13 (6.7) | 2 (4.8) | |
| Multiple | 12 (5.1) | 8 (4.2) | 4 (9.5) | |
| American Indian | 1 (0.4) | 1 (0.5) | 0 (0) | |
| Ethnicity, n (%) | 0.568 | |||
| Hispanic/Latino | 84 (35.7) | 67 (34.7) | 17 (40.5) | |
| Not Hispanic/Latino | 139 (59.2) | 117 (60.6) | 22 (52.4) | |
| Unknown | 12 (5.1) | 9 (4.7) | 3 (7.1) | |
| Lung injury etiology, n (%) | 0.288 | |||
| Pneumonia | 127 (54.5) | 104 (54.2) | 23 (56.1) | |
| Sepsis | 49 (21.0) | 38 (19.8) | 11 (26.8) | |
| Other | 27 (11.6) | 26 (13.5) | 2 (4.9) | |
| Trauma | 13 (5.6) | 11 (5.7) | 2 (4.9) | |
| Aspiration | 12 (5.2) | 10 (5.2) | 2 (4.9) | |
| TRALI | 5 (2.2) | 3 (1.6) | 2 (4.9) | |
| Cancer/HCT, n (%) | <0.001 | |||
| Cancer | 26 (11.2) | 11 (5.7) | 15 (36.7) | |
| HCT | 29 (12.6) | 14 (7.4) | 15 (35.7) | |
| Cancer or HCT | 41 (17.5) | 20 (10.4) | 21 (50.0) | |
| WBC, median (IQR) | 7.8 (4.3–14) | 8.9 (5.1–14.4) | 3.9 (1.9–7.8) | <0.001 |
| Day 1 illness severity, median (IQR) | ||||
| P/F | 132 (84.3–220) | 145 (88.3–239) | 96.3 (72.2–157.3) | 0.005 |
| OI | 10.1 (6.1–19.6) | 9.3 (5.4–17.2) | 15.3 (9.8–30.4) | 0.002 |
| PRISM-3 | 12 (7–20) | 12 (6–19) | 17 (11–20) | 0.006 |
| Day 3 illness severity, median (IQR) | ||||
| P/F | 173.9 (116.1–264.8) | 194.7 (120–272) | 125 (98.5–162) | <0.001 |
| OI | 7.9 (4.5–14.5) | 6.9 (4.3–13.8) | 13.1 (9.7–21.7) | <0.001 |
| PELOD | 11 (1–12) | 11 (1–11) | 11 (10–21) | 0.002 |
Definition of abbreviations: HCT = hematopoietic cellular transplantation; IQR = interquartile range; MMP = matrix metalloproteinase; OI = oxygenation index; PELOD = Pediatric Logistic Organ Dysfunction score; P/F ratio = PaO2/FiO2 ratio; PRISM-3 = Pediatric Risk of Mortality-3; TRALI = transfusion-associated acute lung injury; WBC = white blood cell count.
Associations were tested with Fisher exact test for categorical variables and Wilcoxon rank sum for nonnormally distributed continuous variables. On Day 1, P/F ratio n = 219; OI n = 198; PRISM-3 n = 198. On Day 3, P/F ratio n = 208; OI n = 181, PELOD n = 235. Bold P values indicate statistical significance with an a priori set threshold of P < 0.05.
Plasma Levels of Individual MMP Pathway Proteins Are Associated with Morbidity and Mortality
Compared with survivors, nonsurvivors had higher Day 1 MMP-3 and TIMP-1 and had lower aMMP-9 and aMMP-9/TIMP-1 ratio (Table 2). Among ICU survivors, MMP-1, -2, -3, and -7 and TIMP-1 and -2 were each positively associated with greater peak organ dysfunction as assessed by the PELOD score (see Table E2).
Table 2.
Plasma MMP Levels Are Associated with Mortality in Pediatric ARDS
| Biomarker | Survivors (n = 193) (ng/ml) [Median (IQR)] | Nonsurvivors (n = 42) (ng/ml) [Median (IQR)] | P Value | Adjusted OR (95% CI) | Adjusted P Value |
|---|---|---|---|---|---|
| MMP-1 | 1,092.4 (662.5–2,324.3) | 1,665.5 (618.6–2,798.0) | 0.215 | 1.2 (0.6–2.8) | 0.688 |
| MMP-2 | 486.1 (358.5–727.7) | 517.9 (405.6–649.3) | 0.704 | 1.2 (0.2–6.3) | 0.856 |
| MMP-3 | 3.3 (1.9–7.8) | 4.8 (3.0–14.0) | 0.044 | 2.3 (1.0–5.5) | 0.048 |
| MMP-7 | 420.4 (173.0–1,039.8) | 461.2 (185.3–1,472.2) | 0.224 | 1.0 (0.5–2.0) | 0.967 |
| MMP-8 | 29.6 (13.0–57.7) | 31.3 (9.0–89.0) | 0.877 | 1.1 (0.5–2.4) | 0.780 |
| MMP-9 | 116.0 (64–248) | 89.0 (46–186) | 0.123 | 1.0 (0.3–3.2) | 0.972 |
| aMMP-9 | 242.3 (126.9–510.2) | 135.1 (76.9–274.5) | 0.007 | 0.8 (0.3–1.7) | 0.493 |
| TIMP-1 | 274.5 (138–487) | 475.5 (335–996) | <0.001 | 4.5 (1.3–15.7) | 0.019 |
| TIMP-2 | 78.4 (60.9–98.1) | 81.1 (70.4–97.1) | 0.369 | 2.3 (0.2–26.4) | 0.495 |
| MMP-9/TIMP-1 | 0.49 (0.17–1.11) | 0.14 (0.07–0.54) | <0.001 | 0.5 (0.2–1.1) | 0.101 |
| aMMP-9/TIMP-1 | 0.98 (0.34–2.27) | 0.26 (0.11–0.74) | <0.001 | 0.5 (0.2–0.9) | 0.044 |
Definition of abbreviations: aMMP = active MMP; ARDS = acute respiratory distress syndrome; CI = confidence interval; IQR = interquartile range; MMP = matrix metalloproteinase; OR = odds ratio; TIMP = tissue inhibitor of metalloproteinase.
Of 235 patients, n = 170 patients have all nine MMPs, n = 38 patients have just MMP-3/-9/TIMP1, n = 27 patients have just MMP-1/-2/-7/-8/-9a/TIMP2. Therefore, n = 208 for MMP-3, MMP-9, TIMP-1, and MMP-9/TIMP-1 ratio; n = 197 for MMP-1, MMP-2, MMP-7, MMP-8, aMMP-9, and TIMP-2; and n = 170 for aMMP-9/TIMP-1. Univariate significance was tested using the Wilcoxon rank sum test. Adjustment for multiple comparisons using the Benjamini-Hochberg procedure confirmed significantly different distributions of aMMP-9, TIMP-1, MMP-9/TIMP-1, and aMMP-9/TIMP-1 but not MMP-3 among survivors versus nonsurvivors. Adjusted associations were measured using logistic regression for the outcome of hospital survival with the predictor of log10-transformed plasma biomarker and confounding variables of PaO2/FiO2 ratio and cancer/hematopoietic cellular transplantation status. Mortality OR with 95% CIs and P values are reported. Bold P values indicate statistical significance with an a priori set threshold of P < 0.05.
Adjusted analysis
Measures of pulmonary dysfunction (P/F ratio, OI), multiorgan dysfunction (PRISM-3), a history of cancer/HCT, and lower white blood cell count were each associated with ARDS mortality and multiple MMP pathway proteins (see Tables E3–E7). After adjustment for the P/F ratio and cancer/HCT, mortality remained strongly associated with Day 1 plasma MMP-3, TIMP-1, and the aMMP-9/TIMP-1 ratio (Table 2). aMMP-9 was associated with mortality independent of the P/F ratio but not independent of cancer/HCT status.
Pediatric ARDS Patients Have Two Distinct MMP Profiles
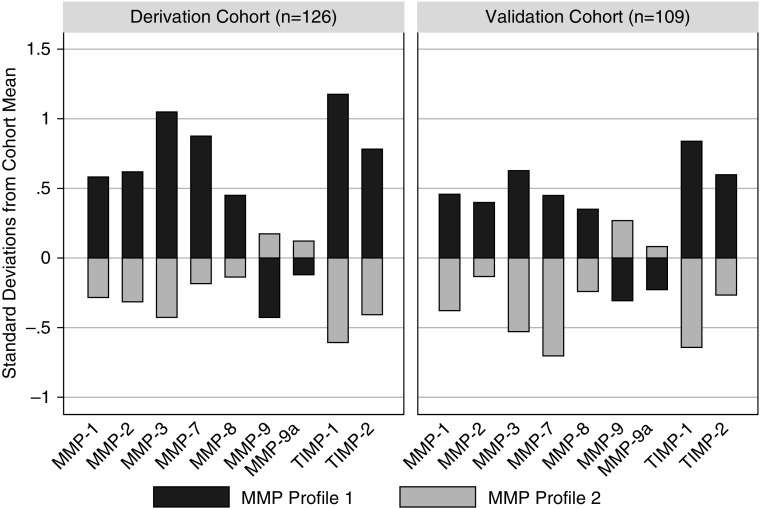
To understand the broader relationship between various MMP family proteins in pediatric ARDS patients, we tested for the presence of latent classes of patients with similar MMP profiles using derivation and validation cohorts (n = 126 and n = 109, respectively). The derivation and validation cohorts differed in distribution of race and reason for ARDS but not in illness severity, mortality, or the composite outcome of mortality or severe morbidity (see Supplemental Data 2 and Table E8). LCA of the derivation cohort identified two groups of patients with distinct MMP profiles (see Tables E9 and E10). The group with MMP profile 1 (n = 43) had elevated MMP-1, -2, -3, -7, and -8 and TIMP-1 and -2, and depressed active and total MMP-9 relative to the group with MMP profile 2 (n = 83) (Figure 1; see Table E11). Although the distributions of each of the nine measured MMP proteins differed between the two groups, most of the variation between the groups could be explained by levels of MMP-2, -3, -7, and -9 and TIMP-1 (adjusted R2 = 0.816) (see Table E12).
Figure 1.
MMP (matrix metalloproteinase) profiles in pediatric acute respiratory distress syndrome. Each bar represents the mean level for each log10-transformed MMP protein, standardized to the cohort mean of 0 and SD of 1. In both the derivation (n = 126) and validation (n = 109) cohorts, patients separated into two profiles using MMP-1, -3, -7, -8, and -9, and TIMP-1 (tissue inhibitor of metalloproteinase-1) and -2 (MMP-2 and active MMP-9 were not used in latent class generation because of collinearity with TIMP-2 and total MMP-9, respectively). MMP profile 1 demonstrated elevated MMP-1, -2, -3, -7, and -8 and TIMP-1 and -2, and depressed active and total MMP-9 relative to MMP profile 2.
We then independently applied LCA to the validation cohort and again saw patients form two groups, with profile 1 demonstrating elevated MMP-1, -2, -3, -7, and -8 and TIMP-1 and -2, and depressed MMP-9 relative to profile 2 (n = 42 and n = 67, respectively) (see Supplemental Data 3 and Tables E13 and E14). Although latent groups in the derivation and validation cohorts demonstrated similar profiles, these profiles cannot be directly compared across populations. Therefore, we fit a linear regression model associating MMP-3, -7, and -9 and TIMP-1 and -2 with latent group assignment in the derivation cohort, and then applied this model to the validation cohort (see Table E15). In all but three patients, latent group assignments using this method were identical to those made by LCA, suggesting external validity of the latent profiles across the derivation and validation cohorts. These data suggest that two distinct MMP profiles exist in pediatric ARDS, and that the probability of any individual patient belonging to MMP profile 1 can be accurately modeled using just MMP-3, -7, and -9, and TIMP-1 and -2 levels.
MMP Profiles Are Associated with Elevated Inflammation, Endothelial Injury, and Impaired Oxygenation
In both the derivation and validation cohorts, patients with MMP profile 1 had significantly higher concentrations of proinflammatory and antiinflammatory markers, including IL-1RA, -6, -8, -10, and -18; macrophage inflammatory protein-1α and -1β; and TNF-α and -R2 (see Table E16). Similarly, patients with MMP profile 1 had significantly higher concentrations of markers of endothelial injury, including angiopoietin-2, von Willebrand Factor, and soluble thrombomodulin. However, there was only weak evidence of impaired oxygenation in patients with MMP profile 1. These data associate MMPs with inflammation and endothelial injury on the first day of ARDS diagnosis and support the broader biochemical uniqueness of the two MMP profiles.
MMP Profiles Are Associated with Mortality and Survivor Morbidity
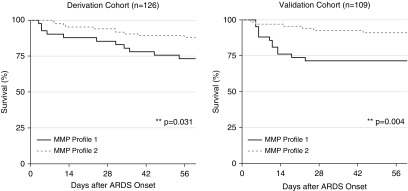
In both the derivation and validation cohorts, patients with MMP profile 1 had significantly greater hospital mortality than patients with MMP profile 2 (26.7% vs. 12.7%, P = 0.047 and 34.5% vs. 8.0%, P < 0.001, respectively). Mortality rates for patients with MMP profiles 1 versus 2 diverged within 1 week of ARDS diagnosis (Figure 2).
Figure 2.
Pediatric acute respiratory distress syndrome survival stratified by Day 1 plasma MMP (matrix metalloproteinase) profile. Kaplan-Meier survival estimates are plotted according to MMP profile. Survival estimates were compared using the log-rank test of equality of survivor functions. By hospital discharge, mortality rates for patients with MMP profile 1 exceeded those of patients with MMP profile 2 (derivation cohort: 26.7% vs. 12.7%, P = 0.047; validation cohort: 34.5% vs. 8.0%, P < 0.001). ARDS = acute respiratory distress syndrome.
Adjusted analysis
Cancer/HCT patients were more likely than noncancer/HCT patients to display MMP profile 1 in both the derivation and validation cohorts (48% vs. 29%, P = 0.070 and 94% vs. 29%, P < 0.001, respectively). After controlling for the P/F ratio and cancer/HCT, MMP profile 1 remained independently associated with both mortality (odds ratio, 2.31; 95% confidence interval [CI], 1.02–5.23; P = 0.046) and the composite outcome of mortality or severe morbidity (odds ratio, 4.0; 95% CI, 2.1–7.6; P < 0.001) in the combined study population.
MMP Profiles Improve Prognostication of Clinical Outcomes
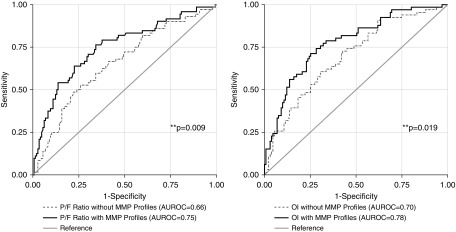
Individually, the P/F ratio, OI, and MMP profile each demonstrated similar AUROC for the outcome of mortality and for the composite outcome of mortality or severe morbidity (see Table E17). Given the finding that MMP profile 1 was associated with clinical outcome independent of the P/F ratio, we sought to quantify the extent to which MMP latent profiles might improve the ability of the P/F ratio to identify high-risk patients on ARDS Day 1. A logistic regression model incorporating both the P/F ratio and MMP profile on ARDS Day 1 was superior to the logistic model using the P/F ratio alone in predicting the composite outcome of mortality or severe morbidity (AUROC, 0.75; 95% CI, 0.68–0.82 vs. AUROC, 0.66; 95% CI 0.58–0.73; P = 0.009) (Figure 3). Similarly, a logistic regression model incorporating both the OI and MMP profile on ARDS Day 1 was superior to the logistic model using the OI alone (AUROC, 0.78; 95% CI, 0.71–0.85 vs. AUROC, 0.69; 95% CI, 0.62–0.77; P = 0.019). The composite outcome of mortality or severe morbidity occurred in 53.1% of patients in the top quartile of risk according to the OI alone (26/49; positive likelihood ratio, 2.26; 95% CI, 1.40–3.64), whereas the composite outcome occurred in 67.4% of patients in the top quartile of risk according to the OI and MMP profile model (33/49; positive likelihood ratio, 4.09; 95% CI, 2.44–6.88), demonstrating a doubling of the positive likelihood ratio and a 27% relative enrichment in the outcome when stratifying patients using the combined model. These data demonstrate that MMP profiles on ARDS Day 1 have the potential to improve the prognostication of clinical outcomes.
Figure 3.
Prognostication of mortality or severe morbidity in pediatric acute respiratory distress syndrome. In the combined cohorts, logistic regression for the composite outcome of mortality or severe morbidity was performed with the Day 1 PaO2/FiO2 (P/F) ratio and then again with both the Day 1 P/F ratio and Day 1 plasma MMP (matrix metalloproteinase) profile. The model using both Day 1 P/F ratio and the Day 1 plasma MMP profile demonstrated greater area under the receiver operating characteristic curve (AUROC) than the model using Day 1 P/F ratio alone (AUROC, 0.75; 95% confidence interval [CI], 0.68–0.82 vs. AUROC, 0.66; 95% CI, 0.58–0.73; P = 0.009). Similar findings occurred when replacing the P/F ratio with the oxygenation index (AUROC, 0.78; 95% CI, 0.71–0.85 vs. AUROC, 0.69; 95% CI, 0.62–0.77; P = 0.019). OI = oxygenation index.
Discussion
In this study, we measured a wide spectrum of MMP family proteins in the plasma of children with ARDS, discovered associations with mortality and survivor morbidity, and identified two latent groups of patients with discrete MMP profiles. MMP profile 1, characterized by elevated MMP-1, -2, -3, -7, and -8 and TIMP-1 and -2 and depressed active and total MMP-9, was associated with markers of elevated inflammation, endothelial injury, and impaired oxygenation. Although only one third of the cohort demonstrated MMP profile 1, it was strongly associated with morbidity and mortality independent of known confounders. Inclusion of the plasma MMP profile improved the prognostic discrimination of the Day 1 P/F ratio and OI for predicting the composite outcome of mortality or severe morbidity. Furthermore, the MMP profile 1 was more prevalent among the cancer/HCT patients, suggesting a possible dysregulated profile that might contribute to the poor outcomes of this high-risk subgroup.
Individual Plasma MMP Pathway Proteins Are Associated with Morbidity and Mortality
The results of this study indicate that early elevated plasma MMP levels are associated with adverse clinical outcomes in pediatric ARDS and augment similar associations drawn from animal models and adult cohorts, with one notable exception (14, 22, 36, 37). Our data demonstrated that patients with MMP profile 1 displayed lower plasma MMP-9 on ARDS Day 1 than patients with MMP profile 2, despite progressing to worse clinical outcomes. A recently published animal model of ARDS demonstrated that intratracheal instillation of lipopolysaccharide resulted in peak plasma MMP-9 levels within 24 hours with resolution by 48 hours (38). In our study, MMP-9 levels were measured within 24 hours after ARDS onset, but the initial pulmonary insult may have preceded ARDS by several days, precluding us from recapitulating the plasma kinetics demonstrated in animal models.
Whereas plasma MMP-9 seems to normalize quickly after an insult, several investigators have reported that MMP-9 levels within pulmonary fluid continued to rise beyond 96 hours and are associated with resolution of extravascular lung water in adult ARDS and protection from the development of chronic lung disease in neonates (39–41). Conversely, MMP-9-deficient animal models have shown an inability to self-limit pulmonary inflammation, leading to pulmonary fibrosis and death (20, 21, 42–44). Further research contrasting daily plasma versus pulmonary MMP profiles might identify longitudinal patterns of interest. For example, on ARDS Day 3, resolution of depressed plasma MMP-9 in conjunction with sustained elevation in pulmonary MMP-9 might be associated with a survival advantage, although this hypothesis needs to be tested in a future cohort.
Pediatric ARDS Patients Display Distinct MMP Profiles
Using LCA, we identified that approximately one-third of both the derivation and validation cohorts demonstrated a specific signature, MMP profile 1, that was characterized by elevated MMP-1, -2, -3, -7, and -8 and TIMP-1 and -2, and depressed active and total MMP-9. Although it was not possible to determine the relative contribution of each MMP to each patient’s overall clinical outcome, we did note that levels of TIMP-1 and MMP-3 were the strongest determinants of MMP profile, which was in turn strongly associated with worse outcome. Potential mechanisms linking TIMP-1 and mortality include inhibition of pro-MMP-9 activation and upregulation of myofibroblast growth; potential mechanisms linking MMP-3 and mortality include upregulation of epithelial mesenchymal transition, TGF-β activation, and endostatin release (18, 45, 46). However, the function of each MMP is cell type specific and varies according to the time from the original insult, making the exact mechanisms of tissue injury and fibrosis challenging to isolate in this cohort.
The identification of latent classes of patients using clinical and biomarker measurements has previously been used in ARDS (25, 26, 30) and is an appealing alternative to traditional hierarchical clustering approaches because it groups patients based on shared biology rather than shared outcome (47). In an analysis of the ARMA and ALVEOLI trials, Calfee and coworkers (25, 30) identified a latent class of patients with a hyperinflammed profile associated with mortality and a differential response to mechanical ventilation strategy. Famous and coworkers (26) replicated this finding in the FACTT and demonstrated an association between a hyperinflammed profile and mortality as well as response to conservative fluid management. Whereas our analysis represents a first step toward incorporating this approach into pediatric ARDS, future studies are needed to assess whether the MMP profile 1 bears any association with differential response to therapeutic strategies in children.
Plasma MMP Profiles Are Associated with Biochemical Evidence of Inflammation, Endothelial Injury, and Impaired Oxygenation
In our analysis, plasma MMP profile 1 was strongly associated with multiple inflammatory cytokines on ARDS Day 1. This finding is concordant with previous studies that have associated elevated TNF-α with TIMP-1, and have demonstrated that IL-1β and IL-6/STAT pathways induce MMP-1, MMP-3, and TIMP-1 expression via JAK/STAT and ERK/MAPK pathways (48–51). Our finding that MMP profile 1 also demonstrates an endothelial injury signature is also concordant with studies showing that release of angiopoietin-2 induces MMP expression (52, 53). These data provide evidence for the biochemical uniqueness of the MMP profiles described here and also fill in a key missing piece of pathobiology regarding the evolution of ARDS in children.
Plasma MMP Profiles Improve Mortality Prognostication
The ability to predict poor clinical outcomes early in the course of pediatric ARDS is crucial so that new therapies can be applied before the development of irreversible lung injury or multiorgan dysfunction syndrome (54). In our study, incorporation of Day 1 plasma MMP profile in a logistic regression model predicting mortality or severe morbidity demonstrated superior performance relative to a model with just the Day 1 P/F or OI. In other words, were patients to be enrolled in a clinical trial on ARDS Day 1, stratification enhanced with MMP measurements would result in a 27% relative enrichment in the outcome of mortality. Progress in identifying and evaluating potential new therapies may depend on early and accurate identification of such high-risk patients (54).
Pediatric HCT Patients with ARDS Have a High Burden of MMP Profile 1
In this study, we found that MMP profile 1 was common among pediatric cancer/HCT patients. Emerging evidence suggests that monocyte myeloid-derived suppressor cells produce significant quantities of MMP-9 post-HCT and that neutropenic mice are deficient in pulmonary MMP-9; we therefore speculate that post-transplant immune reconstitution of these cellular lineages may play a role in ARDS (44, 55). In our study, aMMP-9 was associated with mortality independent of P/F ratio but not independent of cancer/HCT status, and because total and aMMP-9 levels were strongly depressed in cancer/HCT patients, this suggests a potential pathobiologic pattern largely unique to this patient group. Furthermore, we speculate that HCT patients may have endogenously upregulated TIMP-1 in response to chronic post-transplant inflammation and endothelial injury (56, 57), which might inhibit the compensatory antiinflammatory response and thus play a role in the propagation of pulmonary fibrosis. Interestingly, both animals and humans with bleomycin-induced pulmonary fibrosis have demonstrated decreased MMP-9/TIMP-1 ratios, and treatment with the antifibrotic drug pirfenidone has been associated with restoration of the MMP-9/TIMP-1 ratio in the lungs, downregulation of the TGF-β pathway, and clinical improvement (18, 58–61). Other pharmacotherapeutics that have reported efficacy in modulating the MMP-9/TIMP-1 ratio in patients with pulmonary fibrosis include aliskiren, artusenate, bosentan, doxycycline, and induced pluripotent stem cells, although further studies are needed (62–66).
The analyses we present here have several strengths. First, we analyzed a wide spectrum of the MMP pathway and benchmarked our results to clinically relevant outcomes including mortality and survivor morbidity. Second, we characterized the role of individual MMP proteins while also identifying subgroups of patients with specific MMP profiles. Third, the MMP profiles were derived and validated in geographically distinct cohorts with a rigorous statistical method that is agnostic to patient outcomes. One limitation of this work is that the MMP distributions among survivors and nonsurvivors partially overlapped, suggesting that MMP profiles may account for some but not all of the pathways critical to ARDS pathobiology. In addition, after ARDS diagnosis, treatment decisions pertaining to supportive care were not mandated, and thus different provider practice patterns may have influenced outcomes. A future study in pediatric ARDS needs to be done to validate the proposed MMP profiles for their associations with clinical outcomes.
Conclusions
In pediatric ARDS, MMPs and their endogenous inhibitors are independently associated with Day 1 illness severity, inflammation, endothelial injury, morbidity, and mortality. Pediatric ARDS patients demonstrate specific patterns of MMP concentration in the plasma, and these MMP profiles correlate with clinical outcomes independent of confounders. Additional investigation into the pathobiologic significance of MMPs in pediatric ARDS is warranted.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients and their families for participating in this research. They also thank the following collaborators for their assistance recruiting patients for this study: Heidi Flori, M.D., Children’s Hospital Oakland; Robinder Khemani, M.D., Children’s Hospital Los Angeles; Ana Graciano, M.D., Children’s Hospital Central California; and Juan Boriosi, M.D., American Family Children’s Hospital.
Footnotes
Supported by the NIH National Institute of Child Health and Human Development (K12HD000850, M.S.Z.; 5K12HD047349 and 5K08HL119359-02, M.Y.K.) and the NIH NHLBI (K23HL085526 and R01HL114484, A.S.; R37HL51856, M.A.M.; R35HL140026, C.S.C.).
Author Contributions: Study concept and design, M.S.Z. and A.S. Acquisition of data, M.S.Z., B.E.O., A.S.S., M.F.A., A.E.R., and A.S. Analysis and interpretation of data, M.S.Z., K.L.D., and A.S. Drafting of the manuscript, M.S.Z. and A.S. Critical revision of the manuscript for important intellectual content, M.S.Z., K.L.D., M.Y.K., B.E.O., A.S.S., M.J.L., M.F.A., A.E.R., A.V.M., P.S.M., C.C.D., C.S.C., M.A.M., and A.S. Statistical analysis, M.S.Z., A.S., and K.L.D. Administrative, technical, or material support, M.S.Z., B.E.O., A.S.S., M.F.A., A.E.R., A.V.M., P.S.M., and A.S. Study supervision, A.S. Approval of final manuscript, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0678OC on August 16, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 3.Sapru A, Flori H, Quasney MW, Dahmer MK Pediatric Acute Lung Injury Consensus Conference Group. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(Suppl. 5):S6–S22. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinter MS, Spicer A, Orwoll BO, Alkhouli M, Dvorak CC, Calfee CS, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol. 2016;310:L224–L231. doi: 10.1152/ajplung.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spicer AC, Calfee CS, Zinter MS, Khemani RG, Lo VP, Alkhouli MF, et al. A simple and robust bedside model for mortality risk in pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17:907–916. doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torii K, Iida K, Miyazaki Y, Saga S, Kondoh Y, Taniguchi H, et al. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:43–46. doi: 10.1164/ajrccm.155.1.9001287. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Choeng HC, Ahn C, Cho SH. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med J. 2009;50:68–77. doi: 10.3349/ymj.2009.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey A, McAuley DF, O’Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J. 2011;38:959–970. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita CM, Radisky DC, Aschner Y, Downey GP. The importance of matrix metalloproteinase-3 in respiratory disorders. Expert Rev Respir Med. 2014;8:411–421. doi: 10.1586/17476348.2014.909288. [DOI] [PubMed] [Google Scholar]
- 11.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 13.Kong MY, Li Y, Oster R, Gaggar A, Clancy JP. Early elevation of matrix metalloproteinase-8 and -9 in pediatric ARDS is associated with an increased risk of prolonged mechanical ventilation. PLoS One. 2011;6:e22596. doi: 10.1371/journal.pone.0022596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hästbacka J, Linko R, Tervahartiala T, Varpula T, Hovilehto S, Parviainen I, et al. Serum MMP-8 and TIMP-1 in critically ill patients with acute respiratory failure: TIMP-1 is associated with increased 90-day mortality. Anesth Analg. 2014;118:790–798. doi: 10.1213/ANE.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 15.Chetty A, Cao GJ, Severgnini M, Simon A, Warburton R, Nielsen HC. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L584–L592. doi: 10.1152/ajplung.00441.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg J, Halter J, Schiller HJ, Dasilva M, Landas S, Gatto LA, et al. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. 2003;111:185–195. doi: 10.1016/s0022-4804(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 17.Carney DE, McCann UG, Schiller HJ, Gatto LA, Steinberg J, Picone AL, et al. Metalloproteinase inhibition prevents acute respiratory distress syndrome. J Surg Res. 2001;99:245–252. doi: 10.1006/jsre.2001.6180. [DOI] [PubMed] [Google Scholar]
- 18.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aschner Y, Zemans RL, Yamashita CM, Downey GP. Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS. Chest. 2014;146:1081–1091. doi: 10.1378/chest.14-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albaiceta GM, Gutiérrez-Fernández A, Parra D, Astudillo A, García-Prieto E, Taboada F, et al. Lack of matrix metalloproteinase-9 worsens ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L535–L543. doi: 10.1152/ajplung.00334.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2009;41:59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 22.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 23.Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, et al. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med. 2003;31:536–542. doi: 10.1097/01.CCM.0000048626.02184.F8. [DOI] [PubMed] [Google Scholar]
- 24.Roderfeld M, Weiskirchen R, Wagner S, Berres ML, Henkel C, Grötzinger J, et al. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006;20:444–454. doi: 10.1096/fj.05-4828com. [DOI] [PubMed] [Google Scholar]
- 25.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. ARDS Network. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinter MS, Orwoll BE, Spicer AC, Alkhouli MF, Calfee CS, Matthay MA, et al. Incorporating inflammation into mortality risk in pediatric acute respiratory distress syndrome. Crit Care Med. 2017;45:858–866. doi: 10.1097/CCM.0000000000002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Wothke W. Longitudinal and multi-group modeling with missing data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple group data: practical issues, applied approaches and specific examples. Mahwah, NJ: Lawrence Erlbaum Publishers; 1998. pp. 197–210. [Google Scholar]
- 30.Delucchi K, Famous KR, Ware LB, Parsons PE, Thompson BT, Calfee CS. ARDS Network. Stability of ARDS subphenotypes over time in two randomised controlled trials. Thorax. 2018;73:439–445. doi: 10.1136/thoraxjnl-2017-211090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 32.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. 2015;43:1986–1994. doi: 10.1097/CCM.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536–1544. doi: 10.1007/s00134-014-3389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orwoll BE, Spicer AC, Zinter MS, Alkhouli MF, Khemani RG, Flori HR, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): a prospective observational cohort study. Crit Care. 2015;19:435. doi: 10.1186/s13054-015-1145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 36.Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, et al. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. 2006;37:422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Brand KH, Ahout IM, de Groot R, Warris A, Ferwerda G, Hermans PW. Use of MMP-8 and MMP-9 to assess disease severity in children with viral lower respiratory tract infections. J Med Virol. 2012;84:1471–1480. doi: 10.1002/jmv.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu AT, Barrett CD, DeBusk GM, Ellson CD, Gautam S, Talmor DS, et al. Kinetics and role of plasma matrix metalloproteinase-9 expression in acute lung injury and the acute respiratory distress syndrome. Shock. 2015;44:128–136. doi: 10.1097/SHK.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gushima Y, Ichikado K, Suga M, Okamoto T, Iyonaga K, Sato K, et al. Expression of matrix metalloproteinases in pigs with hyperoxia-induced acute lung injury. Eur Respir J. 2001;18:827–837. doi: 10.1183/09031936.01.00049201. [DOI] [PubMed] [Google Scholar]
- 40.O’Kane CM, McKeown SW, Perkins GD, Bassford CR, Gao F, Thickett DR, et al. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med. 2009;37:2242–2249. doi: 10.1097/CCM.0b013e3181a5506c. [DOI] [PubMed] [Google Scholar]
- 41.Dik WA, van Kaam AH, Dekker T, Naber BA, Janssen DJ, Kroon AA, et al. Early increased levels of matrix metalloproteinase-9 in neonates recovering from respiratory distress syndrome. Biol Neonate. 2006;89:6–14. doi: 10.1159/000088193. [DOI] [PubMed] [Google Scholar]
- 42.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, et al. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol. 2001;24:537–544. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]
- 43.Buckley S, Driscoll B, Shi W, Anderson K, Warburton D. Migration and gelatinases in cultured fetal, adult, and hyperoxic alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L427–L434. doi: 10.1152/ajplung.2001.281.2.L427. [DOI] [PubMed] [Google Scholar]
- 44.Blázquez-Prieto J, López-Alonso I, Amado-Rodríguez L, Huidobro C, González-López A, Kuebler WM, et al. Impaired lung repair during neutropenia can be reverted by matrix metalloproteinase-9. Thorax. 2018;73:321–330. doi: 10.1136/thoraxjnl-2017-210105. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992;267:4583–4591. [PubMed] [Google Scholar]
- 46.Strongin AY, Collier IE, Krasnov PA, Genrich LT, Marmer BL, Goldberg GI. Human 92 kDa type IV collagenase: functional analysis of fibronectin and carboxyl-end domains. Kidney Int. 1993;43:158–162. doi: 10.1038/ki.1993.26. [DOI] [PubMed] [Google Scholar]
- 47.Feuillet F, Bellanger L, Hardouin JB, Victorri-Vigneau C, Sébille V. On comparison of clustering methods for pharmacoepidemiological data. J Biopharm Stat. 2015;25:843–856. doi: 10.1080/10543406.2014.920855. [DOI] [PubMed] [Google Scholar]
- 48.Sollazzo D, Forte D, Polverelli N, Romano M, Perricone M, Rossi L, et al. Crucial factors of the inflammatory microenvironment (IL-1β/TNF-α/TIMP-1) promote the maintenance of the malignant hemopoietic clone of myelofibrosis: an in vitro study. Oncotarget. 2016;7:43974–43988. doi: 10.18632/oncotarget.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng X, Xu M, Yao B, Wang C, Jia Y, Liu Q. IL-6/STAT3 axis initiated CAFs via up-regulating TIMP-1 which was attenuated by acetylation of STAT3 induced by PCAF in HCC microenvironment. Cell Signal. 2016;28:1314–1324. doi: 10.1016/j.cellsig.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, Suzuki N, et al. IL-6 and soluble IL-6 receptor stimulate the production of MMPs and their inhibitors via JAK-STAT and ERK-MAPK signalling in human chondrocytes. Cell Biol Int. 2012;36:367–376. doi: 10.1042/CBI20110150. [DOI] [PubMed] [Google Scholar]
- 51.Weise S, Kralisch S, Sommer G, Lossner U, Bluher M, Stumvoll M, et al. Tissue inhibitor of metalloproteinase-1 mRNA production and protein secretion are induced by interleukin-1 beta in 3T3-L1 adipocytes. J Endocrinol. 2008;198:169–174. doi: 10.1677/JOE-07-0631. [DOI] [PubMed] [Google Scholar]
- 52.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate mmp-9 expression and retinal neovascularization. Lab Invest. 2003;83:1637–1645. doi: 10.1097/01.lab.0000097189.79233.d8. [DOI] [PubMed] [Google Scholar]
- 53.Bezuidenhout L, Zilla P, Davies N. Association of Ang-2 with integrin beta 2 controls Ang-2/PDGF-BB-dependent upregulation of human peripheral blood monocyte fibrinolysis. Inflammation. 2009;32:393–401. doi: 10.1007/s10753-009-9148-9. [DOI] [PubMed] [Google Scholar]
- 54.Matthay MA, Liu KD. New strategies for effective therapeutics in critically ill patients. JAMA. 2016;315:747–748. doi: 10.1001/jama.2016.0661. [DOI] [PubMed] [Google Scholar]
- 55.Lee SE, Lim JY, Kim TW, Jeon YW, Yoon JH, Cho BS, et al. Matrix metalloproteinase-9 in monocytic myeloid-derived suppressor cells correlate with early infections and clinical outcomes in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:32–42. doi: 10.1016/j.bbmt.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Doring M, Cabanillas Stanchi KM, Mezger M, Erbacher A, Feucht J, Pfeiffer M, et al. Cytokine serum levels during post-transplant adverse events in 61 pediatric patients after hematopoietic stem cell transplantation. BMC Cancer. 2015;15:607. doi: 10.1186/s12885-015-1616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomura S, Ishii K, Inami N, Kimura Y, Uoshima N, Ishida H, et al. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:766–774. doi: 10.1016/j.bbmt.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Meyer KC, Decker CA. Role of pirfenidone in the management of pulmonary fibrosis. Ther Clin Risk Manag. 2017;13:427–437. doi: 10.2147/TCRM.S81141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbel M, Lanchou J, Germain N, Malledant Y, Boichot E, Lagente V. Modulation of airway remodeling-associated mediators by the antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol. 2001;426:113–121. doi: 10.1016/s0014-2999(01)01209-2. [DOI] [PubMed] [Google Scholar]
- 60.Tian XL, Yao W, Guo ZJ, Gu L, Zhu YJ. Low dose pirfenidone suppresses transforming growth factor beta-1 and tissue inhibitor of metalloproteinase-1, and protects rats from lung fibrosis induced by bleomycina. Chin Med Sci J. 2006;21:145–151. [PubMed] [Google Scholar]
- 61.Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood. 2017;129:2570–2580. doi: 10.1182/blood-2017-01-758854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abuelezz SA, Hendawy N, Osman WM. Aliskiren attenuates bleomycin-induced pulmonary fibrosis in rats: focus on oxidative stress, advanced glycation end products, and matrix metalloproteinase-9. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:897–909. doi: 10.1007/s00210-016-1253-3. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Huang G, Mo B, Wang C. Artesunate modulates expression of matrix metalloproteinases and their inhibitors as well as collagen-IV to attenuate pulmonary fibrosis in rats. Genet Mol Res. 2016;15(2) doi: 10.4238/gmr.15027530. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, He Z, Gao Y, Zheng R, Zhang X, Zhao L, et al. Induced pluripotent stem cells inhibit bleomycin-induced pulmonary fibrosis in mice through suppressing TGF-β1/Smad-mediated epithelial to mesenchymal transition. Front Pharmacol. 2016;7:430. doi: 10.3389/fphar.2016.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuo WL, Zhao JM, Huang JX, Zhou W, Lei ZH, Huang YM, et al. Effect of bosentan is correlated with MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed Rep. 2017;6:201–205. doi: 10.3892/br.2016.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X, Abdalla T, Bratcher PE, Jackson PL, Sabbatini G, Wells JM, et al. Doxycycline improves clinical outcomes during cystic fibrosis exacerbations. Eur Respir J. 2017;49:1601102. doi: 10.1183/13993003.01102-2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.