To the Editor:
Limited long-term prospective data are available to characterize the effect of low-rate smoking on lung health outcomes, particularly in population-based cohorts. Prior studies have also differed in the measurement of cumulative smoking exposure, with many studies relying on imprecise measurement and long periods of retrospective reporting. Smoking exposure is traditionally indexed by pack-years, and individuals with lifetime smoking thresholds below 10 pack-years are typically excluded from clinical trials for chronic lung disease. However, recent studies have found smoking duration (i.e., years of cigarette smoking) to be superior to cigarettes per day or pack-years in predicting emphysema (1) and mortality (2). Low-rate smoking is increasingly prevalent, with 25% of smokers in the United States currently consuming fewer than 10 cigarettes per day, and a growing body of literature has identified negative health effects associated with low-rate smoking, including cardiovascular disease, cancer, and all-cause mortality (2–4). Using data from the CARDIA (Coronary Artery Risk Development in Young Adults) study, we studied how longitudinal patterns of smoking exposure, based on cigarettes per day reported annually over the course of 25 years of follow-up, are associated with loss of lung function, incident obstructive lung physiology, and computed tomography–measured emphysema.
Methods
CARDIA is a prospective cohort study of the evolution of cardiovascular disease risk factors in 5,115 young adults, initiated in 1985 (5). Participants were invited to complete follow-up exams at Years 2, 5, 7, 10, 15, 20, 25, and 30 with 91%, 86%, 81%, 79%, 74%, 72%, 72%, and 71% retention. Cigarette smoking was evaluated during each in-person CARDIA visit and at annual telephone assessments. We used group-based zero-inflated Poisson trajectory modeling (SAS PROC TRAJ) to identify distinct patterns of smoking among participants by including all ever-smokers who had data recorded on number of cigarettes smoked per day for at least 3 of the 26 annual queries taken from Year 0 to Year 25 (6). Trajectories of cigarettes per day were specified as a function of participant age, using third-order polynomials for both the cigarettes per day Poisson count model and the logit model for predicting extra zeros.
Incident obstructive lung physiology was defined as having a post-bronchodilator FEV1/FVC ratio <70% at the Year 30 examination but not at the time of peak lung function. Emphysema at Year 25 was determined by visual review of computed tomography scans (7).
We examined the associations of each lifetime smoking trajectory group with lung function decline and risk for future lung disease, relative to never-smokers. Logistic regression models examined smoking trajectory group as a predictor of emphysema on the Year 25 computed tomography scan and Year 30 obstructive lung physiology, adjusting for age, race–sex group, center, height, body mass index, physician-confirmed asthma, baseline cigarettes per day, and baseline pack-years.
Results
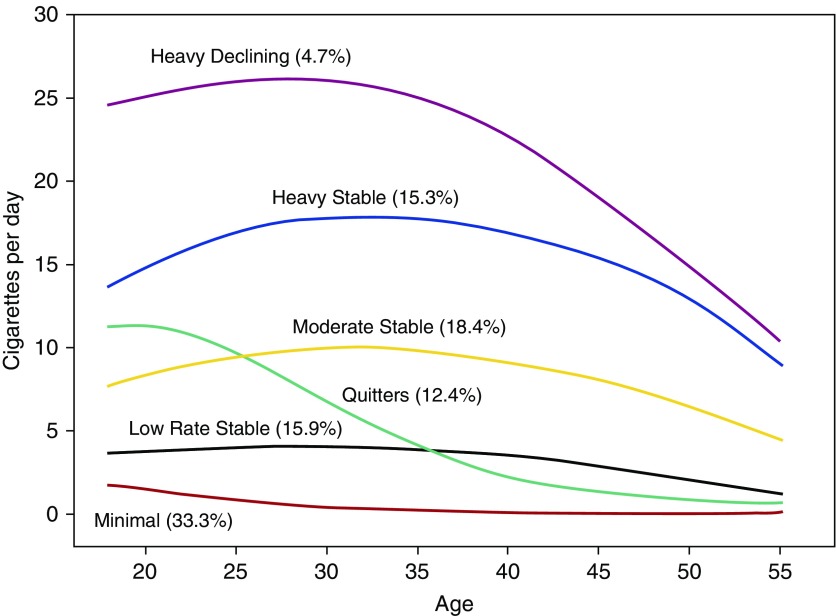
Life-course smoking trajectories are presented in Figure 1. Although the Bayesian Information Criterion indicated a slightly better fit of the seven- versus six-group model, visual inspection of the trajectory group plots suggested greater parsimony in the six-group model presented here. Trajectory group membership was associated with lung function decline in a stepwise manner by smoking exposure, with heavy stable smokers showing the greatest decline in FEV1 (−42.2 ml/yr; Table 1). Smoking trajectory groups differed in risk for incident obstructive lung physiology, with heavy stable smokers versus never-smokers demonstrating nearly eight times the odds of obstructive disease and more than 20 times the odds of computed tomography emphysema (Table 1). Among the two low-rate smoking groups (low-rate stable smokers and quitters), quitters showed less FEV1 decline (−33.8 vs. −35.7 ml/yr) and lower emphysema odds than low-rate stable smokers despite having greater mean lifetime pack-years (9.8 vs. 6.4 pack-years, using 20 cigarettes per pack).
Figure 1.
Lifetime smoking trajectories by cigarettes per day reported annually over the course of 25 years. Group-based trajectory modeling was used to generate trajectories of lifetime cigarette smoking. Group compositions were as follows: heavy declining smokers (4.7%; n = 125; mean lifetime cumulative pack-years, 38.2), heavy stable smokers (15.3%; n = 406; mean lifetime cumulative pack-years, 28.2), moderate stable smokers (18.4%; n = 488; mean cumulative pack-years, 15.5), quitters (12.4%; n = 327; mean cumulative pack-years, 9.8), low-rate stable smokers (15.9%; n = 420; mean cumulative pack-years, 6.4), and minimal smokers (33.3%; n = 881; mean cumulative pack years, 2.1).
Table 1.
Smoking Trajectory Group, Mean FEV1 Decline, and Association (Covariate Adjusted Odds Ratio) with Incident Lung Disease
| Smoking Trajectory Group | FEV1 Decline (ml/yr) (SEM) | Incident Lung Disease |
||||
|---|---|---|---|---|---|---|
| Prebronchodilator Obstructive [OR (95% CI)] | Post-bronchodilator Obstructive [OR (95% CI)] | Centrilobular Emphysema [OR (95% CI)] | Paraseptal Emphysema [OR (95% CI)] | Any Emphysema (Paraseptal or Centrilobular) [OR (95% CI)] | ||
| Never smokers | −32.48 (0.35) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Minimal smokers | −32.34 (0.53) | 1.36 (0.98–1.88) | 1.42 (0.86–2.35) | 1.81 (0.64–5.15) | 1.59 (0.62–4.09) | 1.44 (0.66–3.13) |
| Low-rate stable smokers | −35.70 (0.83) | 2.44 (1.59–3.72) | 2.80 (1.55–5.06) | 9.60 (4.04–22.84) | 11.24 (5.42–23.31) | 8.45 (4.52–15.82) |
| Quitters | −33.80 (0.93) | 1.19 (0.69–2.03) | 2.03 (1.02–4.06) | 5.85 (2.13–16.04) | 3.79 (1.46–9.87) | 3.44 (1.53–7.71) |
| Moderate stable smokers | −38.46 (0.87) | 3.15 (2.06–4.81) | 5.34 (3.10–9.19) | 25.72 (11.63–56.88) | 21.27 (10.59–42.73) | 20.10 (11.15–36.22) |
| Heavy stable smokers | −42.19 (1.12) | 4.20 (2.52–6.98) | 7.44 (3.94–14.06) | 37.56 (15.87–88.89) | 25.37 (11.68–55.18) | 26.01 (13.36–50.63) |
| Heavy declining smokers | −41.29 (1.97) | 3.04 (1.36–6.76) | 7.54 (2.92–19.47) | 35.53 (11.33–111.38) | 26.98 (9.25–78.70) | 25.40 (9.82–65.67) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; ref = reference.
FEV1 decline values reflect mean (SEM) adjusted annualized decline in lung function from peak measurement to Year 30. Never-smokers were the reference group for all models. Obstructive lung disease indicates FEV1/FVC value <70% at the Year 30 examination but not at the time of peak lung function. Covariates are baseline age, race–sex group, center, height, baseline body mass index, physician-confirmed asthma, baseline cigarettes per day, and baseline pack-years.
Discussion
In a longitudinal, community-based study, we identified a dose–response relationship of smoking exposure with lung function decline and lung disease risk. Trajectory analyses indicated distinct patterns of life-course smoking, which were differentially associated with lung disease risk. Among the two low-rate groups, quitters preserved more lung function and reduced their lung disease risk relative to low-rate stable smokers. These results highlight that there is no safe threshold of sustained smoking with regard to lung disease risk.
The current manuscript extends prior findings on the utility of smoking duration as a predictor of lung health outcomes. Measurement of smoking using pack-years is imprecise (8) and raises concerns of inaccurate reporting and recall bias, especially for individuals whose smoking rate is low or fluctuating. Years smoking may be a more sensitive, reliable, and efficient operational index of smoking exposure in predicting lung disease risk.
Our findings add to a growing body of research on associations between smoking duration and disease risk (2). We identified marked increases in lung disease risk among ever-smokers versus never-smokers, even among low-rate stable smokers, for whom there was nearly three times the risk for incident obstruction and more than an eightfold increase in computed tomography emphysema risk. As missing data were more common among participants with greater smoking exposure, our analyses are biased toward the null, and effect sizes likely underestimate the true disease risk among heavy smokers. Further, although our approach adjusted for participant-level peak lung function, we were unable to examine the main effect of peak lung function on subsequent disease risk (9, 10).
Given that an increasing proportion of individuals smoke at a low or intermittent rate (3), our findings have important public health implications. As compared with never-smokers, low-rate smokers demonstrated increased disease risk, despite a relatively low threshold of lifetime smoking exposure (6.4 pack-years). Targeted messaging is needed to reiterate the lung health risk of sustained smoking at any level. In addition, healthcare providers should underscore the lung health benefit of smoking cessation over and above smoking reduction (i.e., cutting down on cigarettes per day without intention to quit). Although smoking reduction may be a positive initial step toward cessation, prospective studies demonstrate limited lung health benefits of smoking reduction alone (11). This is likely a result of changes in smoking topography, in which smokers inhale more deeply and/or smoke more puffs of each cigarette to compensate for a lower number of cigarettes per day. Our findings are consistent with the message that quitting, and not cutting down, is the most effective method of reducing lung disease risk.
Smoking influenced lung disease risk in a dose-dependent manner. There was no safe threshold for smoking intensity on lung disease risk, and even low-rate smokers were at increased risk for future lung disease. These results underscore the benefit of complete abstinence from smoking, even among low-rate smokers, on respiratory outcomes.
Supplementary Material
Footnotes
The Coronary Artery Risk Development in Young Adults Study is supported by grant contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the NHLBI, the Intramural Research Program of the National Institute on Aging, and an intraagency agreement between the National Institute on Aging and the NHLBI (AG0005).
Author Contributions: A.R.M. contributed to study conception and wrote the first draft of the manuscript; S.P.B., N.B.A., D.R.J., R.A., G.R.W., and M.T.D. interpreted the data and revised the manuscript; L.A.C. analyzed and interpreted the data and revised the manuscript; B.H. contributed to study conception, interpreted the data, and revised the manuscript; G.R.W. supervised data collection, interpreted the data, and revised the manuscript; R.K. conceived the study, supervised data collection, interpreted the data, and revised the manuscript; and all authors contributed intellectually to the content of the paper and approved the final published version.
Originally Published in Press as DOI: 10.1164/rccm.201808-1568LE on September 14, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Bhatt SP, Kim Y-i, Harrington KF, Hokanson JE, Lutz SM, Cho MH, et al. Smoking duration alone provides stronger risk estimates of chronic obstructive pulmonary disease than pack-years. Thorax. 2018;73:414–421. doi: 10.1136/thoraxjnl-2017-210722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health–AARP Diet and Health Study. JAMA Intern Med. 2017;177:87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:53. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j5855. doi: 10.1136/bmj.j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 6.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 7.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR, Jr, Thyagarajan B, et al. Respiratory symptoms in young adults and future lung disease: the CARDIA Lung Study. Am J Respir Crit Care Med. 2018;197:1616–1624. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etter J-F, Perneger T. Measurement of self reported active exposure to cigarette smoke. J Epidemiol Community Health. 2001;55:674–680. doi: 10.1136/jech.55.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 10.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee PN. The effect of reducing the number of cigarettes smoked on risk of lung cancer, COPD, cardiovascular disease and FEV1 – A review. Regul Toxicol Pharmacol. 2013;67:372–381. doi: 10.1016/j.yrtph.2013.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.