Abstract
Rationale: Airways obstruction with thick, adherent mucus is a pathophysiologic and clinical feature of muco-obstructive respiratory diseases, including chronic obstructive pulmonary disease, asthma, and cystic fibrosis (CF). Mucins, the dominant biopolymer in mucus, organize into complex polymeric networks via the formation of covalent disulfide bonds, which govern the viscoelastic properties of the mucus gel. For decades, inhaled N-acetylcysteine (NAC) has been used as a mucolytic to reduce mucin disulfide bonds with little, if any, therapeutic effects. Improvement of mucolytic therapy requires the identification of NAC deficiencies and the development of compounds that overcome them.
Objectives: Elucidate the pharmacological limitations of NAC and test a novel mucin-reducing agent, P3001, in preclinical settings.
Methods: The study used biochemical (e.g., Western blotting, mass spectrometry) and biophysical assays (e.g., microrheology/macrorheology, spinnability, mucus velocity measurements) to test compound efficacy and toxicity in in vitro and in vivo models and patient sputa.
Measurements and Main Results: Dithiothreitol and P3001 were directly compared with NAC in vitro and both exhibited superior reducing activities. In vivo, P3001 significantly decreased lung mucus burden in βENaC-overexpressing mice, whereas NAC did not (n = 6–24 mice per group). In NAC-treated CF subjects (n = 5), aerosolized NAC was rapidly cleared from the lungs and did not alter sputum biophysical properties. In contrast, P3001 acted faster and at lower concentrations than did NAC, and it was more effective than DNase in CF sputum ex vivo.
Conclusions: These results suggest that reducing the viscoelasticity of airway mucus is an achievable therapeutic goal with P3001 class mucolytic agents.
Keywords: mucins, mucus, reducing agents, mucociliary clearance, obstructive pulmonary diseases
At a Glance Commentary
Scientific Knowledge on the Subject
Inhaled mucolytic agents are designed to decrease the viscoelasticity of airway secretions, improve mucociliary clearance, and reduce the mucus burden in the lungs of patients suffering from muco-obstructive pulmonary diseases. Reducing agents break the disulfide bonds that connect mucin macromolecules by donating electrons to the thiol groups of mucin monomer cysteine residues, changing the rheology of mucin-rich secretions.
What This Study Adds to the Field
This study identified the deficiencies of the only approved inhaled reducing agent, N-acetylcysteine (NAC), and compared NAC with novel reducing agents. As observed in in vitro and in vivo studies, the low intrinsic reducing activity and short half-life of NAC on airway surfaces are responsible for its ineffectiveness. Next-generation reducing agents tested in preclinical assays exhibited significant improvements in reducing activity, restoration of mucus transport, and reduction of lung mucus burden in mouse models. This study supports the need for novel reducing agents and provides unique insights into new mucolytic agents as inhaled therapies to treat lung disease.
In healthy individuals, mucus is secreted by airway epithelial cells and submucosal glands to trap and clear inhaled particles from the lung. The biophysical properties of airway mucus are governed by the secreted polymeric mucins, MUC5AC and MUC5B, which are high-molecular-mass glycoproteins that organize into large multimers (2–100 MDa) via the formation of intermolecular disulfide bonds. In muco-obstructive diseases, including chronic bronchitis, asthma, and cystic fibrosis (CF), mucin hypersecretion, hyperconcentration, and increased mucin cross-linking slow mucus transport, producing persistent mucus plugging and airflow obstruction (1–3). Adding complexity to the pathophysiology of these diseases, recent studies suggest that the properties of MUC5AC and MUC5B differ and each mucin may be dominant in different muco-obstructive diseases; for example, MUC5AC dominates in asthma and MUC5B dominates in chronic obstructive pulmonary disease (COPD) and CF (4, 5).
For decades, mucolytic agents have been pursued as a therapeutic approach to treat muco-obstructive diseases. Reducing agents that disrupt the mucin intermolecular covalent disulfide bonds are predicted to disadhese mucus from airway surfaces and improve clearance. Mucomyst (N-acetylcysteine [NAC]) was approved as an inhaled therapy for patients with CF and chronic bronchitis in the 1960s. However, clinical data in CF and COPD do not support the efficacy of inhaled NAC with respect to improvement of lung function or reduction in pulmonary exacerbations (6–9). These negative clinical NAC studies have limited the enthusiasm for inhaled mucolytics as therapeutic options for muco-obstructive lung diseases. In contrast, recombinant human DNase (rhDNase or dornase alfa), an enzyme that breaks down the extracellular DNA released by dying neutrophils entrapped in the mucus, produced substantial clinical benefits in adult CF subjects (10–12). These positive results validated the concept that an inhaled drug that affects the rheology of airway secretions could improve health outcomes. However, rhDNase is only active in CF subjects with persistent inflammation and was ineffective in obstructive diseases other than CF (13–15). Recently, reducing agents have been reported to produce greater effects on CF sputum viscoelasticity than rhDNase in vitro (1, 16), suggesting they may benefit CF subjects and a broader population.
Our study was designed to elucidate the mechanisms underlying the clinical failure of NAC as an inhaled mucolytic and test whether novel candidates could overcome these deficiencies. Accordingly, NAC reducing activity was compared with dithiothreitol (DTT) and a novel mucolytic agent, P3001, in human bronchial epithelial (HBE) cell mucus, COPD patient sputa, and mouse BAL fluid (BALf). Mucolytic activities were tested in a series of biochemical and biophysical assays, including mucin agarose gel, macrorheology and microrheology, and mucus transport rates assays. The efficacy of P3001 was tested in wild-type (WT) and βENaC-overexpressing mice, an in vivo model of chronic muco-obstructive lung disease. Finally, we measured the pharmacokinetics and pharmacodynamics of NAC after a single administration of inhaled 20% NAC in CF subjects and compared P3001 effects to NAC and rhDNase in sputum obtained from these subjects. Some of these results have been previously reported in abstracts (17, 18).
Methods
For detailed information on laboratory and clinical protocols, see online supplement.
Biological Specimens
COPD and CF sputa were spontaneously expectorated and processed for biophysical and biochemical measurements. HBE airway epithelial cells were grown at an air–liquid interface and washed weekly to provide mucus samples. Procedures involving human cells and sputum specimens were approved by the Institutional Review Board (approval nos. 03-1396, 12-2602, and 02-0948) at the University of North Carolina Chapel Hill.
WT and βENaC-overexpressing mice were used to test efficacy and/or toxicity of pharmacological compounds via Western blotting, mass spectrometry, cell counts, and morphometric measurements. Animal use was approved by the University of North Carolina Chapel Hill Institutional Animal Care and Use Committee.
Experimental Procedures
The biochemical methods included a colorimetric assay using an artificial S–S target (5′,5′-dithio-bis[2-nitrobenzoic acid] [DTNB]) substrate to characterize intrinsic and pH-dependent reductive activities of test molecules. Agarose gel electrophoreses and Western blotting measured the molecular mass of mucins in sputum, HBE secretions, and BALf from mice. Immunohistochemical methods were employed to characterize the mucin structure in COPD sputum. Measurements of NAC and NAC metabolite concentration in CF sputum were performed by mass spectroscopy.
The biophysical measurements of mucus properties included 1) a capillary break assay using an extension rheometer, 2) microbead rheology, and 3) macrorheology using a parallel plate oscillating rheometer.
Mice were dosed with test compounds via nasal instillation (15 μl) or via a modified ultrasonic aerosol generator designed to deliver ∼1 μl transnasally to mouse lower airways. BAL was used to sample mucins and deposited drug from mouse lungs. Morphometry was used to measure lung mucus burden.
Statistics
The effects of varying concentrations of agents were analyzed by a two-way ANOVA Dunnett test for multiple comparisons. Independent sample groups were analyzed by a Mann-Whitney U test. All tests are two-sided, and significance level is 0.05. All results are reported in terms of means ± SEM.
Results
Comparing Reducing Activity of NAC with Other Reducing Agents
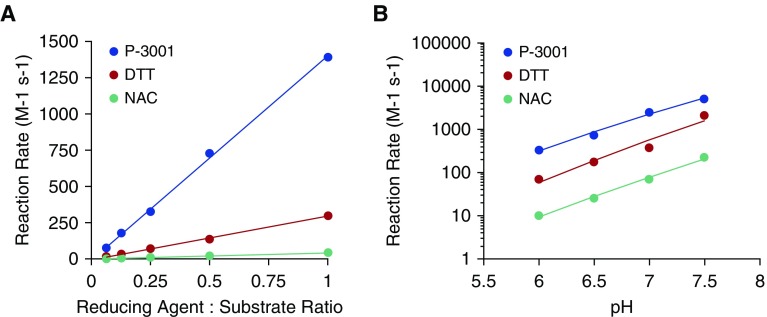
NAC was compared with two alternative reducing agents, DTT and the novel mucolytic agent P3001. DTT is a well-characterized (12), rapidly acting reducing agent. P3001 is predicted to be more effective than NAC based on a lower pKa of the active moiety (7.6) and more rapid kinetics of disulfide bond reduction. An artificial substrate (DTNB) was used to directly measure the kinetics of interaction between each candidate and its substrate, that is, DTNB S–S bond (Figure 1). As reported, NAC exhibited slow, pH-dependent reaction rates. DTT reaction rates were ∼10-fold faster than NAC and, again, pH dependent. P3001 exhibited an additional 10-fold pH-dependent increase in reaction rates over DTT, producing an increase in activity of ∼100-fold over NAC. Thus, NAC, DTT, and P3001 exhibit a spectrum of reductive activities suitable for testing in mucus environments.
Figure 1.
The reduction potential of P3001 exceeds that of dithiothreitol and N-acetylcysteine. Spectrophotometric studies monitored formation of reduced 5′,5′-dithio-bis(2-nitrobenzoic acid) over time. Reaction rates, generated using second-order kinetics, for each compound were compared as a (A) function of reducing agent concentration at pH 6.5 or (B) over a relevant pH range. The x-axis reflects the pseudo–second-order kinetics of the reducing reaction rates per molar per second. DTT = dithiothreitol; NAC = N-acetylcysteine.
Note that DTT is not considered as a clinical candidate because low concentrations of DTT permeate into cells, induce endoplasmic reticulum stress, and enhance H2O2-induced toxicity (19). In contrast to DTT or NAC, P3001 is odorless and only allows limited cellular uptake due to its size, polar surface, and hydrophilicity. Consistent with absence of cell permeation, HBE cultures exposed to P3001 (2 mM) via nebulization twice daily for 2 weeks did not exhibit cell stress as indexed by XBP1 splicing and/or cytokine assays and had no off-target effects on mucin expression or production (see Figure E1 in the online supplement).
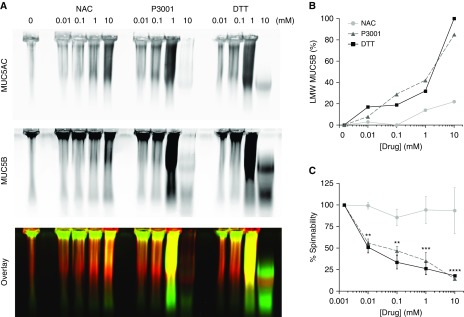
The reducing activities of the three compounds were next compared in COPD sputa via mucin agarose electrophoresis and spinnability (capillary break) assays. Three COPD specimens, selected for homogeneity, similar concentrations (5–8% solids), and low salivary and inflammatory cell content (limiting proteolytic activity), were exposed to increasing concentrations of NAC, DTT, or P3001 (0–10 mM for 30 min). Note that deposition models predict that 20% (1.27 M) NAC delivered by a conventional nebulizer does not exceed 10 mM on distal airway surfaces and will be cleared within 30 minutes (see Figure E2). The dose–response curves for DTT or P3001 versus low-molecular-weight (LMW) MUC5B production were significantly left-shifted compared with NAC (Figures 2B and E3). Spinnability measurements also showed that the dose–effect relationships for DTT or P3001 were significantly left-shifted compared with NAC (Figure 2C). Importantly, full mucin reduction by DTT or P3001 was not required to change mucus biophysical properties. At the lowest concentration tested (0.01 mM), DTT and P3001 generated only 19% and 7% LMW MUC5B production, respectively, which correlated with an ∼50% decrease in spinnability for both compounds. In contrast, 10 mM concentrations of NAC were required to produce a 20% LMW MUC5B production and a 24% decrease in spinnability within 30 minutes, consistent with slower reduction of intermolecular disulfide bonds by NAC. The observation that DTT and P3001 exhibited similar dose–effect relationships for MUC5B reduction and spinnability, juxtaposed to the 10-fold faster DTNB reduction kinetics of P3001 versus DTT, suggests that P3001 has more limited access to mucin S–S bonds than does DTT.
Figure 2.
Effect of N-acetylcysteine (NAC), dithiothreitol (DTT), and a novel reducing agent, P3001, on the biochemical and biophysical properties of chronic obstructive pulmonary disease sputum. Freshly harvested sputa were aliquoted and subjected to increasing concentrations (0.01–10 mM) of NAC, DTT, or P3001 at 37°C for 30 minutes and then quenched. (A) Electrophoretic separation reveals the biochemical effect of each compound on mucin multimerization. MUC5AC and MUC5B signals are shown individually or as an overlay (red indicates MUC5AC; green indicates MUC5B). (B) Graph indicates the degree of mucin reduction as percentage of monomeric signal intensity (e.g., full or 100% MUC5B reduction was achieved at 10 mM DTT). (C) Graph reveals changes in sputum biophysical properties as measured by spinnability, also referred to as capillary break. n = 6 for NAC and P3001 and n = 4 for DTT. **P < 0.01, ***P < 0.0001, ****P < 0.00001, two-way ANOVA Dunnett test. LMW = low molecular weight.
Because impaired mucus strand detachment was shown to disrupt mucociliary clearance in CF models (20), we tested the effects of P3001 on submucosal gland mucus. Western blotting revealed that P3001 was at least 10-fold more potent than NAC at breaking down submucosal gland mucus, and scanning electron microscopy images established that P3001 disrupted methacholine-stimulated mucus strands (Figure E4).
Physical Alterations of the Mucin Network in Response to Reducing Agents
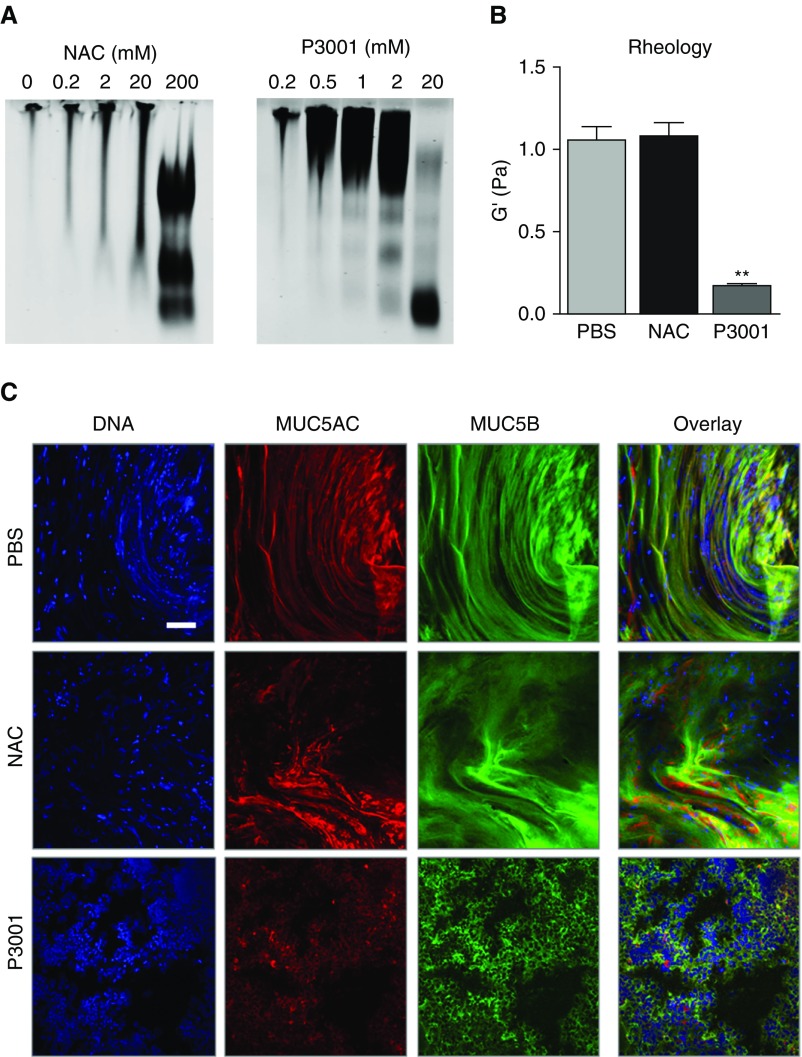
To measure the effects of mucin reduction on macroscopic mucus properties, COPD sputum was exposed to increasing concentrations of NAC (0.2–200 mM) or P3001 (0.2–20 mM) and analyzed by agarose mucin gel, immunostaining, and cone-and-plate rheometry. Western blotting confirmed that treatment with 20 mM P3001 caused full reduction of MUC5B, whereas 20 mM NAC produced partial reduction (Figure 3A). The marked changes in the migration pattern of sputum exposed to 20 mM P3001 correlated with an ∼10-fold decrease in the elastic modulus (Figure 3B). In comparison, NAC at the same concentration had a negligible impact on sputum biophysical properties. The electrophoretic and biophysical responses to NAC versus P3001 were paralleled by the immunochemical characterizations of mucin network topologies that showed relaxation of interwoven structures with P3001 (Figure 3C).
Figure 3.
Alterations of the mucin network following treatment of chronic obstructive pulmonary disease sputum. The same patient sputum was treated with N-acetylcysteine (NAC) or P3001 for 30 minutes at 37°C and analyzed to compare the structural changes of the mucin network. (A) Breakdown of multimeric MUC5B was assessed by agarose gel electrophoresis. Increasing concentration of NAC (0.2–200 mM) and P3001 (0.2–20 mM) were tested. (B) Effect of 20 mM NAC and P3001 on sputum elastic modulus (G′) as measured by a cone-and-plate rheometer and compared with PBS. **P < 0.01. n = 3 repeats per condition. (C) Effect of 20 mM NAC and P3001 on the mucin network topology as examined by immunocytochemistry and compared with PBS. Confocal images captured the disruption of MUC5AC (red) and MUC5B (green) threads as a result of sputum reduction. Nuclei are shown in blue (DAPI). Scale bar, 100 μm. PBS = phosphate-buffered saline.
In Vitro Effect of NAC and P3001 on Mucus Transport
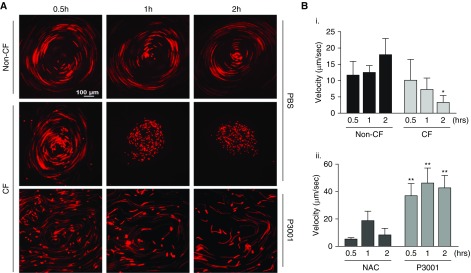
HBE cells grown at an air–liquid interface coordinate ciliary beating and organize mucus transport in a rotational fashion (i.e., “mucus hurricanes”). To mimic patient dosing and maintain HBE surfaces under thin-film conditions, HBE cultures were exposed to aerosols generated by an eFlow nebulizer modified to deposit 3 μl/cm2 phosphate-buffered saline (PBS; with/without drugs) during 10 seconds, which contained fluorescent beads for tracking. Non–CF cells maintained transport throughout the 2-hour post-PBS nebulization period, whereas, as previously reported, mucus transport ceased 2 hours after PBS nebulization for CF cultures (21, 22). CF cultures exposed to NAC (5 mM nebulized) exhibited modestly increased mucus transport at 1 hour, but the effect waned by 2 hours. In contrast, P3001 (5 mM nebulized) induced a large and sustained increase in CF cultures mucus velocity that persisted for 2 hours and was ∼10-fold on average above CF cells exposed to PBS (Figure 4).
Figure 4.
Effect of N-acetylcysteine and P3001 on cystic fibrosis (CF) human bronchial epithelial mucus transport. Human bronchial epithelial cells grown on air–liquid interface typically coordinate cilia beating and transport secreted material in a rotational manner (e.g., mucus hurricane). Mucus transport or velocity was tracked using fluorescently labeled microbeads (1 μm) while cells were maintained under thin-film conditions. (A) Long-exposure images of non-CF and CF cells nebulized with PBS (3 μl/cm2). Additionally, the same CF cells were nebulized with P3001 (5 mM). After P3001 treatment, beads cleared away from the initial anchor point (e.g., disruption of the mucus aggregate) but maintained the same overall directionality (e.g., clockwise rotational movement). Mucus transport was monitored for 2 hours. (B) Graphs show mucus velocity of non-CF and CF cells treated with PBS (i) or CF cells treated with 5 mM N-acetylcysteine or P3001 (ii). n = 3. *P < 0.05, **P < 0.01. NAC = N-acetylcysteine; PBS = phosphate-buffered saline.
In Vitro Effect of NAC and P3001 on Murine Muc5b in BALf
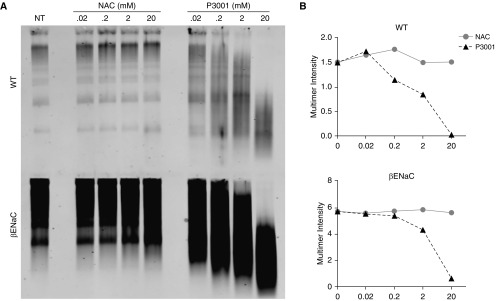
Prior to in vivo testing, NAC and P3001 effectiveness were assessed on mucins harvested by BAL from WT and βENaC-transgenic (Tg) mice. Muc5b, the dominant mucin expressed in murine lungs, is about fourfold more concentrated in βENaC-transgenic (Tg) than in WT BALf. Because both mucolytic agents exhibit a stoichiometry of one molecule per one disulfide bond, it is important for therapy of lung diseases with increased mucin concentrations to control for drug/target stoichiometry in dose–response experiments. Dose–effect studies of NAC and P3001 (0.02–20 mM) in WT mouse BALf (Figure 5) revealed that P3001 produced dose-dependent Muc5b reduction whereas NAC did not. Similar relationships were observed for NAC and P3001 in βENaC mouse BALf. However, a modest rightward shift was noted when comparing P3001 in WT versus βENaC BALf, for example, 2 mM P3001 generated 43% LMW Muc5b in WT mice compared with ∼24% LMW Muc5b in βENaC BALf, reflecting increased βENaC mucin concentration. Accordingly, the mass of a reducing agent delivered to patients will need to be adjusted according to the pulmonary mucus burden.
Figure 5.
In vitro testing of N-acetylcysteine (NAC) and P3001 potency on mouse BAL fluid. BAL fluid from wild-type (WT) and βENaC-transgenic mice were subjected to increasing concentration of NAC and P3001 (0.02–20 mM). Samples were incubated at 37°C for 15 minutes and then quenched. (A) Western blotting was used to assess NAC and P3001 potency at different concentrations and at different stoichiometric ratios because mucin concentration was increased fourfold in βENaC mice as compared with WT animals. (B) Reduction of high-molecular-weight multimer intensity that reflects partial of full Muc5b reduction is graphed for NAC (gray circles) and P3001 (black squares) in the two models. NT = no treatment.
In Vivo Effect of NAC and P3001 on WT Mice
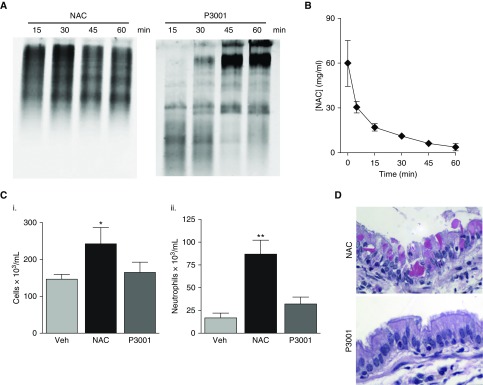
WT mice were treated via intratracheal instillation (15 μl) of NAC at a concentration mimicking clinical doses, for example, 10% or ∼600 mM, or concentrations of P3001 ∼50-fold lower than NAC that produced significant mucin reduction in in vitro assays, that is, 12 mM. No reduction of Muc5b after NAC delivery was detected by Western blotting during a 1-hour period (Figure 6A). In contrast, P3001 produced full reduction of Muc5b within 15 minutes after treatment. Interestingly, reduced mucins were cleared from the lungs with time and replaced by newly synthetized, intact polymeric mucins during 60 minutes (Figure 6A).
Figure 6.
Comparing N-acetylcysteine (NAC) and P3001 efficacy and toxicity in wild-type mice. Wild-type mice were treated via oropharyngeal aspiration (15 μl) with NAC at 50 mg/kg or P3001 at 1.5 mg/kg. BAL fluid was collected at various time points (15, 30, 45, and 60 min and 6 h). (A) NAC and P3001 efficacy to reduce Muc5b in vivo was assessed by agarose gel electrophoresis during a 60-minute period. (B) NAC pharmacokinetic properties in the murine lungs were established by measuring NAC concentrations in BAL fluid via mass spectrometry. n = 3–5 animals per time point. (C) Inflammatory responses were measured 6 hours after treatment and are displayed as total cell count (i) and percentage neutrophils (ii). n = 4–6 mice per group. *P < 0.05, **P < 0.01. (D) Representative images of hematoxylin and eosin–stained sections of mouse lungs harvested 30 minutes after treatment with NAC and P3001. Veh = vehicle.
Investigations into the failure of NAC to reduce Muc5b in vivo revealed that NAC was rapidly cleared/absorbed from murine lungs; that is, ∼70% of the drug was cleared within 15 minutes and little was detected in BALf 60 minutes after administration (Figure 6B). Analyses of BALf for NAC toxicity revealed an increase in total cell number (Figure 6C), neutrophils, and histologic evidence of epithelial injury, for example, appearance of eosin-positive crystals (Figure 6D), suggesting that the inefficiency of NAC could not be overcome by increasing the dose. In contrast, mice exposed to lower doses of P3001 showed no signs of inflammation or epithelial damage (Figures 6C and 6D).
In Vivo Effects of Aerosolized Agents in βENaC-Tg Mice
βENaC-Tg mice were exposed via a nose-only nebulizer to small volumes (∼1 μl deposited) of normal saline (NS), NAC, or P3001 during a 15-minute interval. Equal drug concentrations (200 mM) were placed in the aerosol generator. Mass spectrometry of BALf collected immediately after inhalation confirmed that 1.5 mg/kg (∼12 mM) of P3001 was deposited into the lungs using this technique.
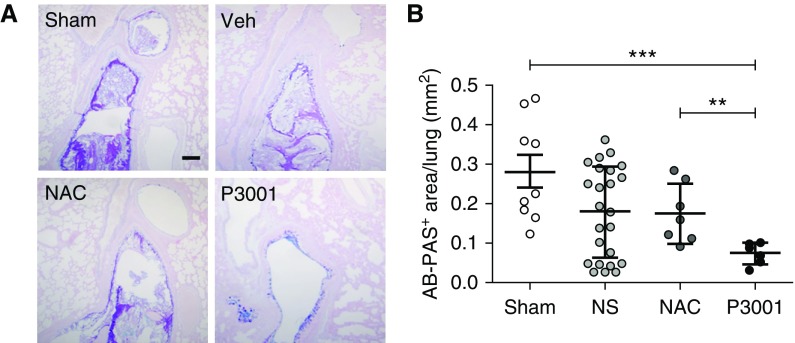
Morphometric analysis of AB/PAS histological sections revealed a robust mucus burden in sham-treated βENaC-Tg mice (Figure 7). Hydration via normal saline aerosolization produced an overall reduction in the AB/PAS mucus burden, as reported previously (23, 24). NS-treated mice showed a trend toward bimodal distribution. Hence, the number of animals was increased to demonstrate that some mice (30%) responded to NS administration and others did not (Figure E5), suggesting that more tenacious plugs require a mucolytic drug for removal. The NAC-exposed group was not different from the sham group (Figure 7B). However, the P3001-exposed group exhibited significantly reduced airway mucus obstruction compared with sham-treated (P < 0.001) or NAC-treated (P = 0.01) groups.
Figure 7.
Comparing N-acetylcysteine (NAC) and P3001 efficacy to remove persistent mucus plugs from βENaC-overexpressing mice by aerosolization. βENaC-overexpressing mice were slightly anesthetized with isoflurane and subjected to repeated 15-minute nebulizations (nose only, three times, 2-h intervals) with normal saline, NAC, or P3001 at 200 mM. Lung sections were stained with alcian blue–periodic acid/Schiff (AB-PAS) and morphometric analysis was performed. (A) Representative images of AB-PAS sections of animals exposed to sham (n = 9) or treated with saline (n = 24), NAC (n = 6), and P3001 (n = 6). Images display the proximal region of the left main stem bronchus, a site routinely presenting significant mucus obstruction in βENaC-transgenic animals. Scale bar, 100 μm. (B) Morphometric measurement of AB-PAS–positive signal in whole-lung sections. **P < 0.01, ***P < 0.001, Mann-Whitney U test. NS = normal saline; Veh = vehicle.
Pharmacokinetics and Pharmacodynamics of Mucolytic Agents
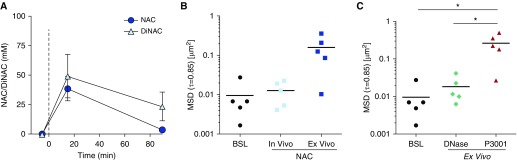
To test the hypothesis that delivered mass and residency time of NAC were inadequate for mucin reduction, spontaneous sputa were collected from five CF adult subjects at baseline and at 15 minutes and 90 minutes after Mucomyst (4 ml of 20% NAC) aerosolization. Sputum pharmacokinetics measurements of NAC and the reduced N,N′-diacetyl-l-cystine metabolite revealed peaks 15 minutes after aerosolization of 37 and 50 mM, respectively, suggesting that of the total deposited NAC (∼100 mM) only half had reacted with S–S bonds at t = 15 min (Figure 8A). Concentrations of NAC were not detectable 90 minutes after administration, and NAC/N,N′-diacetyl-l-cystine fell to ∼25% of its 15-minute concentration.
Figure 8.
N-acetylcysteine (NAC) pharmacokinetics and mucolytic effects on cystic fibrosis (CF) sputum measured by microrheology. Five individuals with CF were treated with 20% (1.27 M) NAC via inhalation. Spontaneously expectorated sputa were collected 0, 15, and 90 minutes after treatment. (A) Sputum drug concentrations measured using mass spectrometry at 0, 15, and 90 minutes. (B) Sputum microrheology or mean square displacement (MSD) measured at baseline and 90 minutes after NAC treatment (in vivo) by tracking 1-μm fluorescence beads mixed in sputum specimens. In parallel, aliquots of sputa collected at baseline were incubated at 37°C with 100 mM NAC for 90 minutes (ex vivo). (C) MSD of baseline sputa incubated ex vivo at 37°C with 0.1 mg/ml rhDNase or 10 mM P3001 for 15 minutes. Passive bead diffusion (or MSD) is shown as scatter plot of MSDτ=0.83s for tracer particles in sputum for each treatment condition. *P < 0.05. BSL = baseline; DiNAC = N,N′-diacetyl-l-cystine.
As shown in Figure 8B, passive bead diffusion measurements revealed no detectable increase in mean square displacement (MSD; a correlate of viscoelastic properties) in CF sputum following NAC inhalation. This result was consistent with Western blot analyses of sputum samples (Figure E6). In contrast, NAC (100 mM) administration to sputa ex vivo for 90 minutes, to mimic the total NAC exposure/concentration in vivo, produced an increase in MSD and increased MUC5B mobility on Western blots (Figures 8B and E6). These data demonstrate that NAC could be an effective mucolytic if it remained longer on the airways, acted faster, and/or was present in higher concentrations.
In comparison, P3001 produced a significant increase in MSD following incubation at 10-fold lower concentrations (10 mM) than NAC over a 6-fold shorter period (15 min) (Figure 8C). Importantly, the P3001 effect on microrheology was significantly greater than rhDNase at 15 minutes. Therefore, P3001 is predicted to act faster and at low enough concentrations to be clinically effective.
Discussion
In pulmonary medicine, there has been a long-standing need to treat diseases associated with mucus accumulation in the lungs. The overarching goal of an inhaled therapy is to clear the hyperconcentrated, adherent mucus that causes airflow obstruction, inflammation, and infection. Reducing the molecular mass of mucin gel polymers is a straightforward approach to treat a broad spectrum of muco-obstructive diseases and was tested several decades ago with NAC. Studies from the 1960s suggest that high concentrations of NAC could remove mucus plugs in subjects with CF or tracheostomies when delivered topically via bronchoscopy (25–28). Studies from the early 1970s described modest improvements in lung function following inhalation NAC treatment, which may have reflected the osmotic activity of the high NAC concentrations (20%, 1,200 mOsm) aerosolized. Indeed, human studies (29, 30) showed that the effects of NAC on mucociliary clearance were mimicked by equiosmotic concentrations of NaCl. Our studies in WT mice paralleled these findings, demonstrating that delivery of high concentrations of NAC induced epithelial cell damage and acute neutrophilic responses that may in part have reflected the large osmotic load deposited (Figure 6). The limited efficacy of NAC, coupled with the off-target irritation effects, including cough and bronchospasm, appear responsible for its failure in clinical pulmonary medicine as an inhaled mucolytic (6–9).
Our studies were designed to elucidate the mechanisms for the clinical failure of NAC and test whether a novel molecule in the thiol-reducing class could overcome these deficiencies. Small-molecule reducing agents reduce protein disulfide bonds via a stoichiometric bimolecular chemical reaction. The speed of the reaction is directly dependent on their intrinsic activity and access to the target S–S bonds. The efficacy of reduction is also dependent on the residence time on airway surfaces of a given agent. As indicated by the DTNB studies, NAC has a low intrinsic reducing activity (Figure 1). This deficiency is compounded by the alkaline pKa of its thiol group (∼9.5). The thiolate anion form (S−) of the NAC sulfur is required for attack on mucin S–S bonds. On a normal airway surface with a pH of ∼7.0–7.2, NAC is then 99% in the inactive protonated form. Although NAC will cycle from SH (inactive) to S− (active) forms over time in airway mucus, this process is slow and requires NAC to be retained on the airway surfaces for long time periods to react to completion (Figures 8 and E6). However, NAC is rapidly cleared and/or absorbed from epithelial surfaces (Figures 6B, 8A, and E2) and, consequently, not present at sufficient concentrations or durations to be efficacious in human airways. Consistent with these analyses, NAC administered at maximum tolerated doses (20%) did not produce mucus reduction in CF subjects (Figures 8 and E6).
The paucity of mucolytic drugs in development relates to the fact that the mechanisms mediating mucus accumulation in the lungs have remained elusive. However, a recent polymer physics formulation has emphasized the interplay between the cell surface–tethered mucins, the secreted mucins within the mucus layer, and mucus transport. The two-gel model, which emphasizes the importance of mucus layer concentration in determining transport versus failure to transport from the lung (2), has provided a framework for understanding the role of mucus-hydrating agents (e.g., inhaled hypertonic saline, ion transport modulators) to restore mucus transport. Previous predictions and recent data have also suggested that intermucin disulfide bonds can be formed under conditions of inflammation and oxidant stress (1, 3). Such bonds would be predicted to limit hydration-induced swelling and clearance, providing a rationale for mucin-targeted mucolytic agents.
NAC chemical profiling suggests that properties of novel mucolytic molecules should include: 1) high efficiency/rates of disulfide reduction and/or 2) prolonged residence times on airway surfaces. P3001 is both more intrinsically active than NAC and exhibits significant activity at pH 7 (Figure 1). The increased activity was reflected in P3001 being more effective than NAC in reducing mucins in vitro (Figures 2–5 and E3). Note that P3001 reduced MUC5AC and MUC5B equally well, suggesting that P3001 may have activity against mucus accumulation in a broad range of respiratory diseases. Similarly, the increased activity of P3001 was reflected in studies of in vivo mucin reduction in WT mice (Figure 6). Importantly, these in vivo effects were achieved without evidence in vitro or in vivo of P3001 toxicity.
Three approaches were used to test the therapeutic utility of P3001 in muco-obstructive diseases. First, the CF mucus transport studies demonstrated restoration of mucus clearance with P3001 but not NAC administration (Figures 4 and 7). Note that these experiments were performed with compounds nebulized in a PBS/NS solution; hence, they may reflect the effect of the mucolytic agent plus hydration. Reducing agents formulated as dry powder would test whether mucolytic therapies alone are active. Second, the studies of inhaled NAC versus P3001 in βENaC-Tg mice provided in vivo evidence that an effective mucolytic agent could reduce the mucus burden in mice with muco-obstructive lung disease. Strikingly, clearance of mucus plugs was observed with three administrations of P3001 in a single day (Figure 7). Third, P3001 was more effective in reducing viscosity than a clinically relevant concentration of DNase in CF sputum in vitro (Figure 8C).
A key consideration in developing novel mucolytics for human subjects with muco-obstructive diseases is outcome measures. Relief from symptoms and improvement in airflow will be pivotal. However, biomarkers may be important in early studies searching for activity and dose optimization. Biochemical assays, for example, Western blotting, directly measure the biochemical activity of this class of mucolytics. However, the goal of therapy is to improve the biophysical properties of mucus that relate to transportability. A variety of biophysical assays are available and applicable to sputum samples, including microbead/macrorheology and spinnability. Interestingly, the spinnability assay revealed more profound effects of reducing agents on mucus than did Western blots (Figure 2). Thus, it will be important in clinical early development programs to compare biochemical and biophysical measures of mucus with outcomes of clinical benefit.
In summary, our analyses of NAC suggested that its failure reflected low intrinsic activity and limited residence time on airway surfaces. Improving these properties, as exemplified in P3001, demonstrated that reducing viscoelasticity of airway mucus with mucolytic agents may be an achievable therapeutic goal. Targeted treatment of pathologic airway mucus in the future may improve symptoms of cough and dyspnea, decrease the frequency of disease-related exacerbations, and slow disease progression in a broad range of muco-obstructive diseases.
Supplementary Material
Acknowledgments
Acknowledgement
The authors thank Agathe Ceppe for help with biostatistics, Dr. Carla Ribeiro for advice on cellular stress assays, Kimberly Burns for all histological preparations, Troy D. Rogers for technical assistance with treatment of βENaC-Tg mice, Margret Powell for sputum collection, and Parion Sciences for providing compounds.
Footnotes
Supported by grants from the NHI/NHLBI (5 UH3 HL 123645, 5 P01 HL 108808, and 1 R43 HL112436-01A1), the NIH/NIDDK (2 P30 DK 065988-11), the Cystic Fibrosis Foundation (EHRE07XX0 and EHRE16XX0), and the NIH/NIEHS (P30-ES10126).
Author Contributions: C.E. designed experiments, performed immunohistochemistry, mouse treatment, tissue collection, and macrorheology, and participated in data collection, statistical analyses, and manuscript preparation. Z.L.R. performed Western blotting and animal tissue processing. B.W. performed experiments on human bronchial epithelial cells, including cell maintenance, cell washing, cell imaging, and ELISA. L.N.H. performed particle bead tracking and measurements of mucus velocity. C.B.M. performed Western blotting, analyzed blot intensity, and provided assistance with data management and graphs. N.C.F. processed sputa and performed particle bead tracking for mean square displacement. M.R.M. performed statistical analyses of treated mice. M.F.D. performed scanning electron microscopy. T.K. provided submucosal gland mucus. D.V. provided reagents for treatment. W.R.T. performed 5′,5′-dithio-bis(2-nitrobenzoic acid) assay and aerosol deposition prediction. C.R.E. performed and analyzed data from mass spectrometry. D.B.H. advised and supervised biophysical experiments. B.R.G. directed studies on βENaC-transgenic–treated mice with nose-only nebulizer. A.L.-B. isolated βENaC-transgenic lungs after nebulization and performed morphometric measurements. S.H.D. designed human protocol, enrolled patients, and assisted with patient treatment and sputum collection. R.C.B. advised on overall experimental design and manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201802-0245OC on September 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Vilar J, Boucher RC. Reevaluating gel-forming mucins’ roles in cystic fibrosis lung disease. Free Radic Biol Med. 2004;37:1564–1577. doi: 10.1016/j.freeradbiomed.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;(7):CD007168. doi: 10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash EF, Stephenson A, Ratjen F, Tullis E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2009;(1):CD007168. doi: 10.1002/14651858.CD007168.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Rogers DF. Mucoactive drugs for asthma and COPD: any place in therapy? Expert Opin Investig Drugs. 2002;11:15–35. doi: 10.1517/13543784.11.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(7):CD001287. doi: 10.1002/14651858.CD001287.pub5. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey BW, Astley SJ, Aitken ML, Burke W, Colin AA, Dorkin HL, et al. Efficacy and safety of short-term administration of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. Am Rev Respir Dis. 1993;148:145–151. doi: 10.1164/ajrccm/148.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Shah PI, Bush A, Canny GJ, Colin AA, Fuchs HJ, Geddes DM, et al. Recombinant human DNase I in cystic fibrosis patients with severe pulmonary disease: a short-term, double-blind study followed by six months open-label treatment. Eur Respir J. 1995;8:954–958. [PubMed] [Google Scholar]
- 12.Yang C, Chilvers M, Montgomery M, Nolan SJ. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2016;4:CD001127. doi: 10.1002/14651858.CD001127.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Wills PJ, Wodehouse T, Corkery K, Mallon K, Wilson R, Cole PJ. Short-term recombinant human DNase in bronchiectasis. Effect on clinical state and in vitro sputum transportability. Am J Respir Crit Care Med. 1996;154:413–417. doi: 10.1164/ajrccm.154.2.8756815. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell AE, Barker AF, Ilowite JS, Fick RB rhDNase Study Group. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest. 1998;113:1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson M, Sugumar K, Milan SJ, Hart A, Crockett A, Crossingham I. Mucolytics for bronchiectasis. Cochrane Database Syst Rev. 2014;(5):CD001289. doi: 10.1002/14651858.CD001289.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsley A, Rousseau K, Ridley C, Flight W, Jones A, Waigh TA, et al. Reassessment of the importance of mucins in determining sputum properties in cystic fibrosis. J Cyst Fibros. 2014;13:260–266. doi: 10.1016/j.jcf.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehre C, Rushton Z, Yu J, Gentzsch M, Esther CR, Hill DB, et al. Pharmacological approaches to clear mucus from the lungs. Pediatr Pulmonol. 2015;50:225–226. [Google Scholar]
- 18.Ehre C, Rushton Z, Veazey R, Esther CR, Hill DB, Thelin B, et al. Improving mucolytics to remove adherent mucus from the lungs: a comparative preclinical study. Pediatr Pulmonol. 2014;49:262–263. [Google Scholar]
- 19.Held KD, Biaglow JE. Mechanisms for the oxygen radical-mediated toxicity of various thiol-containing compounds in cultured mammalian cells. Radiat Res. 1994;139:15–23. [PubMed] [Google Scholar]
- 20.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 23.Graeber SY, Zhou-Suckow Z, Schatterny J, Hirtz S, Boucher RC, Mall MA. Hypertonic saline is effective in the prevention and treatment of mucus obstruction, but not airway inflammation, in mice with chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2013;49:410–417. doi: 10.1165/rcmb.2013-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mall MA, Graeber SY, Stahl M, Zhou-Suckow Z. Early cystic fibrosis lung disease: role of airway surface dehydration and lessons from preventive rehydration therapies in mice. Int J Biochem Cell Biol. 2014;52:174–179. doi: 10.1016/j.biocel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Webb WR. New mucolytic agents for sputum liquefaction. Postgrad Med. 1964;36:449–453. doi: 10.1080/00325481.1964.11695324. [DOI] [PubMed] [Google Scholar]
- 26.Matthews LW, Doershuk CF. Inhalation therapy and postural drainage for the treatment of cystic fibrosis. Bibl Paediatr. 1967;86:297–314. [PubMed] [Google Scholar]
- 27.Miller WF. Aerosol therapy in acute and chronic respiratory disease. Arch Intern Med. 1973;131:148–155. [PubMed] [Google Scholar]
- 28.Dietzsch HJ, Gottschalk B, Heyne K, Leupoid W, Wunderlich P. Cystic fibrosis: comparison of two mucolytic drugs for inhalation treatment (acetylcysteine and arginine hydrochloride) Pediatrics. 1975;55:96–100. [PubMed] [Google Scholar]
- 29.Clarke SW, Thomson ML, Pavia D. Effect of mucolytic and expectorant drugs on tracheobronchial clearance in chronic bronchitis. Eur J Respir Dis Suppl. 1980;110:179–191. [PubMed] [Google Scholar]
- 30.Pavia D, Sutton PP, Lopez-Vidriero MT, Agnew JE, Clarke SW. Drug effects on mucociliary function. Eur J Respir Dis Suppl. 1983;128:304–317. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.