Abstract
Rationale: Characterization of patterns of wheezing and allergic sensitization in early life may allow for identification of specific environmental exposures impacting asthma development.
Objectives: To define respiratory phenotypes in inner-city children and their associations with early-life environmental exposures.
Methods: Data were collected prospectively from 442 children in the URECA (Urban Environment and Childhood Asthma) birth cohort through age 7 years, reflecting symptoms (wheezing), aeroallergen sensitization, pulmonary function, and body mass index. Latent class mixed models identified trajectories of wheezing, allergic sensitization, and pulmonary function. Cluster analysis defined nonoverlapping groups (termed phenotypes). Potential associations between phenotypes and early-life environmental exposures were examined.
Measurements and Main Results: Five phenotypes were identified and mainly differentiated by patterns of wheezing and allergic sensitization (low wheeze/low atopy; low wheeze/high atopy; transient wheeze/low atopy; high wheeze/low atopy; high wheeze/high atopy). Asthma was most often present in the high-wheeze phenotypes, with greatest respiratory morbidity among children with frequent wheezing and allergic sensitization. These phenotypes differentially related to early-life exposures, including maternal stress and depression, antenatal environmental tobacco smoke, house dust microbiome, and allergen content (all P < 0.05). Prenatal smoke exposure, maternal stress, and depression were highest in the high-wheeze/low-atopy phenotype. The high-wheeze/high-atopy phenotype was associated with low household microbial richness and diversity. Early-life aeroallergen exposure was low in high-wheeze phenotypes.
Conclusions: Patterns of wheezing, allergic sensitization, and lung function identified five respiratory phenotypes among inner-city children. Early-life environmental exposure to stress, depression, tobacco smoke, and indoor allergens and microbes differentially associate with specific phenotypes.
Keywords: childhood asthma, phenotypes, environmental exposures
At a Glance Commentary
Scientific Knowledge on the Subject
Recurrent wheezing and its subsequent evolution into asthma during the preschool years reflects a heterogeneity of conditions that represent multiple disease phenotypes. These phenotypes differ in age of onset, symptomatology, concomitant atopic characteristics, and long-term prognosis. Prior research has categorized early childhood wheezing phenotypes based on patterns of wheezing (timing of presentation and potential resolution); type of airway inflammation; presence or absence of risk factors, such as allergic sensitization; and presence or absence of pulmonary function testing abnormalities. A precise characterization of early childhood wheezing phenotypes can facilitate parent counseling regarding outcomes and prognosis, and could be used for targeted prevention.
What This Study Adds to the Field
Among inner-city children in the URECA (Urban Environment and Childhood Asthma) birth cohort study, we identified five respiratory phenotypes, defined primarily by patterns of wheezing and aeroallergen sensitization. Multiple early-life environmental exposures (maternal stress, depression, tobacco smoke, and indoor allergens and microbes) differentially associate with specific phenotypes. Furthermore, exposure to allergens, microbes, stress, and tobacco smoke may specifically modify the risk for high-wheeze respiratory phenotypes with and without allergic sensitization. These findings advance the identification of asthma endotypes, which may inform the development of mechanism-based strategies to prevent childhood asthma.
Asthma, the most common chronic disease of childhood (1), is a major public health problem associated with significant morbidity, mortality, and health care costs. Children living in the inner-city are particularly prone to develop asthma and to experience asthma morbidity (2–4). Reasons for the high asthma burden in this population include low socioeconomic status (5), prenatal tobacco smoke exposures (6), home exposures that promote asthma (7) including environments with low bacterial biodiversity (8), and high prevalence of maternal stress and depression (6).
Wheezing is often the first manifestation of childhood asthma, with approximately 50% of children experiencing at least one episode before the age of 6 years (9). Recurrent wheezing and its evolution into asthma among preschool children reflect heterogeneous conditions representing multiple disease phenotypes, differing in age of onset, expression of symptoms, presence of atopic characteristics, treatment response, and long-term outcomes (9–12). Early childhood wheezing phenotypes have been grouped based on the pattern of wheezing (timing of presentation and potential resolution); type of airway inflammation; presence or absence of risk factors, such as allergic sensitization; and presence or absence of pulmonary function testing abnormalities (13–15). A precise characterization of early childhood wheezing phenotypes can facilitate parent counseling regarding outcomes and prognosis (16), and could be used for phenotype-driven therapy selection to decrease early-life asthma morbidity (17).
Several patterns of early-life wheezing and their relationships to allergic sensitization have been described using supervised and unsupervised approaches (14, 15). However, no study to date has attempted to simultaneously integrate multiple relevant longitudinal trajectories of specific disease-related factors in defining early-life wheezing and asthma phenotypes. In addition, it remains unknown if inner-city children exhibit similar phenotypes as other populations. Based on these gaps of knowledge, we aimed to describe the relevant patterns of respiratory disease seen in early life in the URECA (Urban Environment and Childhood Asthma) study (18), a birth cohort of inner-city children at risk for asthma based on parental allergic disease and/or asthma. We hypothesized that having precisely defined early-life respiratory phenotypes should enable more directed investigations into specific factors associated with asthma causation, such as environmental exposure to allergens, microbes, stress, and tobacco smoke.
Some of the results of these studies have been previously reported in the form of an abstract (19).
Methods
Study Design and Participants
The URECA birth cohort study was initiated in 2005 in inner-city Baltimore, Boston, New York City, and St. Louis. Methods of the study have been previously described (6, 18). Pregnant women, whose children had a high risk of developing asthma because of a history of asthma, allergic rhinitis, or eczema in the mother or father, were recruited from prenatal clinics of the participating hospitals. Written informed consent was obtained from the women enrolled and, after birth, from the parent or legal guardian of the infant.
Data Collection
Maternal questionnaires were administered prenatally, and postnatal child health questionnaires were administered every 3 months through age 7 years. Annual visits included questionnaires, anthropomorphic measurements, pulmonary function testing, and phlebotomy. Questionnaires included the Perceived Stress Scale (20) and the Edinburgh Postpartum Depression Scale (21).
Allergen-specific IgE levels (ImmunoCAP, Phadia) for foods (milk, egg, and peanut) and German cockroach were measured yearly at ages 1, 2, 3, 5, and 7 years, and allergen-specific IgE for house dust mites, dog, cat, mouse, and Alternaria were measured at ages 2, 3, 5, and 7 years. Prick skin testing was performed at 3, 5, and 7 years of age for 14 common aeroallergens (6, 18). Positive tests were defined as a wheal greater than or equal to 3 mm larger than the saline control on prick skin testing or specific IgE greater than or equal to 0.35 kU/L.
Starting at age 3 years, spirometry and impulse oscillometry were performed annually in accordance with American Thoracic Society guidelines (22). We were able to obtain acceptable spirometry from 60% of 3 year olds and 89% of 5 year olds (23). At ages 6 and 7 years, reversibility was measured 15 minutes after four inhalations of albuterol hydrofluoroalkane metered-dose inhaler, 90 μg per inhalation. At a separate visit at age 7 years, a methacholine challenge was performed (6).
Cotinine levels were measured in cord blood plasma as previously described (18). First-year home dust samples were assayed for allergenic proteins, including Bla g 1 (German cockroach), Can f 1 (dog), Fel d 1 (cat), Der f 1 and Der p 1 (house dust mites), and Mus m 1 (mouse) by ELISA (Indoor Biotechnologies). A sum of allergen exposure index was calculated from levels of exposure to Bla g 1, Mus m 1, Can f 1, and Fel d 1, as previously described (6). House dust samples were profiled for bacterial microbiota using 16S rRNA biomarker sequencing, and diversity indices were calculated as previously described (6).
Asthma Definition
In earlier work (6), we classified children as having asthma at age 7 years if at least one of the following three conditions was met: 1) a parent-reported physician diagnosis of asthma between age 4 and 7 years, combined with asthma symptoms or the use of asthma controller medication for 6 of the past 12 months; 2) provocative concentration of methacholine causing a 20% fall in FEV1 less than or equal to 4 mg/ml or albuterol reversibility of FEV1 greater than or equal to 10%, combined with asthma symptoms or use of asthma controller medication for 6 of the past 12 months; or 3) the report in the past 12 months of two or more wheezing episodes, two or more doctor visits for asthma/wheeze, one or more hospitalization for asthma/wheeze, and/or the use of controller medications for 6 of the past 12 months.
Statistical Methods
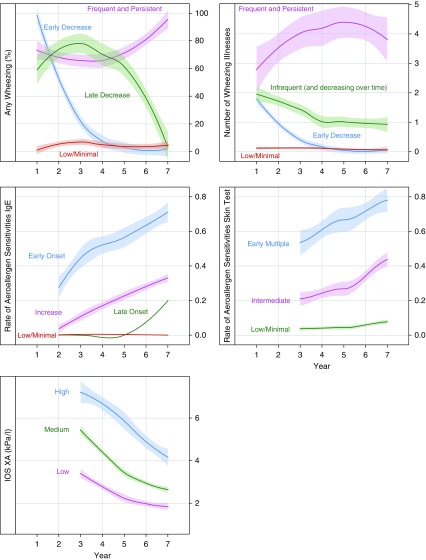
Figure 1 provides a graphical overview of the analytic approaches taken. Our goal for the present analysis was to identify phenotypes of early-life respiratory health, focusing on childhood wheeze and asthma, that incorporated and maximized the value of the longitudinal nature of the URECA follow-up data. Variables considered in this analysis included symptoms (wheezing), allergic sensitization (prick skin tests, serum allergen-specific IgE), lung function (impulse oscillometry and spirometry), and body mass index (BMI) (see Table E1 in the online supplement). To take advantage of our longitudinal data, we first examined the variable trajectories over time using latent class mixed models, examining two to five patterns or trajectories for each variable (Figures 1A and 1B). We then analyzed goodness of fit (Akaike information criterion) (Figure 1C) along with investigator consensus to determine the best number of trajectories for each variable (see online supplement for details). The latent class mixed models could not identify separate trajectory groups for certain variables (e.g., FEV1, FEV1/FVC ratio), so these were recorded for later analyses as mean and variance over time per child. As a data reduction strategy, trajectory variables were evaluated for collinearity by studying the association between variables. For variable trajectories that were highly collinear (e.g., allergen-specific IgE by skin testing, allergen-specific IgE by serum testing, blood eosinophil counts, and serum total IgE), variables were selected that best represented their domain.
Figure 1.
Pictorial representation of the methods used for determining URECA clusters/phenotypes. After construction of the latent class mixed models, we selected the optimal number of cluster solution for each variable under consideration. We subsequently combined all trajectories and nontrajectory variables into cluster analyses and determined the optimal solution using an ensemble model. AIC = Akaike information criterion; HA = high atopy; HW = high wheeze; IOS = impulse oscillometry; LA = low atopy; LW = low wheeze; TW = transient wheeze; URECA = Urban Environment and Childhood Asthma; XA = area of reactance.
The selected trajectory variables and other relevant predictors (e.g., FEV1/FVC ratio) were then included in a Gower distance matrix (Figure 1D), which can handle mixed data types and grouped children according to their similarities and differences. Five different clustering algorithms were used, with solutions examining 2 to 10 groups. The optimal solution was chosen using four criteria: 1) a generalization of the within clusters sum of squares, 2) average silhouette width, 3) Dunn index, and 4) the ratio of average within versus average between clusters (Figure 1E). In our case, Partitioning Around Medoids algorithm produced the best solution with five groups (i.e., phenotypes) (Figure 1F). Further details concerning the statistical analysis can be found in the online supplement. For visualizing the high-dimensional distance matrix in a two-dimensional space we used t-distributed stochastic neighbor embedding, a nonlinear dimension-reduction technique using a machine learning algorithm. Internal validation techniques and results for both the trajectory construction and for the clustering algorithm are provided in the online supplement.
Overall differences among the phenotypes were tested using the chi-square test for categorical variables and the F test for continuous variables. For pairwise differences, we used the low-wheeze (LW), low-atopy (LA) phenotype as the reference group in a multinomial logistic regression model to test the relationships of selected exposures in the first 3 years of life as predictors of the resultant phenotypes.
Results
Study Population
Between February 2005 and March 2007, a total of 1,850 families were screened, 776 met eligibility criteria, and 560 newborns with gestational age greater than or equal to 34 weeks were enrolled at birth (6). Of those enrolled, 442 (79%) were followed to the age of 7 years and had sufficient outcome data to classify whether they had developed asthma according to the three-part URECA criteria (6). The population was mostly minority (71.9% African American, 19.7% Hispanic), low income, and at high risk for developing asthma on the basis of parental history (see Table E2).
Selection of Variables for Clustering Algorithm
Analyses for longitudinal trajectories of the variables chosen to represent the domains of wheezing symptoms, sensitization to aeroallergens, lung function, and BMI revealed three or four trajectories for each variable, and these trajectories had varied patterns (Figure 2; see Figure E1). Next, the cluster analysis was performed using the categorical trajectory variables, along with the mean FEV1/FVC ratio and the variability of the FEV1/FVC ratio (reflected by the standard deviation of the FEV1/FVC) across ages 3 and 7 years. Trajectories of BMI changes over time did not contribute to the resolution of the resultant phenotypes and were therefore omitted from the clustering algorithm.
Figure 2.
Observed trajectories with SEs for variables used in clustering algorithm among URECA participants through age 7 years. Latent class mixed models were performed, and the optimal number of classes selected is plotted. Each model is constructed separately for each variable; thus, the classes are unrelated between variables. IOS = impulse oscillometry; URECA = Urban Environment and Childhood Asthma; XA = area of reactance.
Relationship of Respiratory Phenotypes to Variables Used in the Algorithm
The algorithm identified five clusters of children, which we termed respiratory phenotypes. We examined differences in the variables that went into the algorithm to understand the composition of each respiratory phenotype (Table 1). The first phenotype (LW-LA) included mainly children with low to minimal wheezing and low to minimal allergic sensitization, intermediate variability in lung function, and intermediate or high area of reactance (XA). The second phenotype (LW/high atopy [HA]) was characterized by allergic sensitization, most often intermediate with an increase over time, but with little or no wheezing, low variability in lung function, and intermediate or high XA. The third phenotype (transient wheeze [TW]/LA) reported frequent wheezing in early life that resolved early and most had no or minimal allergic sensitization, low variability in lung function, and intermediate or high XA. The remaining two phenotypes exhibited both the frequent and persistent wheezing and the infrequent and decreasing over time wheezing trajectories. The patterns of allergic sensitization differentiated these two phenotypes; phenotype four had high wheeze (HW) and low to minimal atopy (HW-LA), whereas phenotype five had HW-HA. The allergic sensitization trajectories for the HW-HA group were notable for either early onset multiple sensitizations (67.6%) or intermediate and increasing (27.9%) serum specific IgE trajectories, and either intermediate (70.6%) or early multiple (23.5%) trajectories of skin test reactivity (Table 1).
Table 1.
Association of Respiratory Phenotypes with Variables Used in Clustering Algorithm*
| LW-LA (n = 117) | LW-HA (n = 81) | TW-LA (n = 75) | HW-LA (n = 101) | HW-HA (n = 68) | P Value† | |
|---|---|---|---|---|---|---|
| Any wheezing | ||||||
| Low/minimal | 100 (85.5) | 57 (70.4) | 0 (0.00) | 0 (0.00) | 0 (0.00) | <0.01 |
| Early decrease | 9 (7.69) | 10 (12.3) | 71 (94.7) | 1 (0.99) | 0 (0.00) | |
| Late decrease | 6 (5.13) | 7 (8.64) | 4 (5.33) | 25 (24.8) | 16 (23.5) | |
| Frequent and persistent | 2 (1.71) | 7 (8.64) | 0 (0.00) | 75 (74.3) | 52 (76.5) | |
| Number of wheezing illnesses | ||||||
| Low/minimal | 117 (100) | 74 (91.4) | 2 (2.67) | 0 (0.00) | 2 (2.94) | <0.01 |
| Early resolution | 0 (0.00) | 0 (0.00) | 67 (89.3) | 3 (2.97) | 2 (2.94) | |
| Infrequent (and decreasing over time) | 0 (0.00) | 4 (4.94) | 6 (8.00) | 70 (69.3) | 46 (67.6) | |
| Frequent and persistent | 0 (0.00) | 3 (3.70) | 0 (0.00) | 28 (27.7) | 18 (26.5) | |
| Serum aeroallergen-specific IgE | ||||||
| Low/minimal | 104 (88.9) | 0 (0.00) | 48 (64.0) | 82 (81.2) | 0 (0.00) | <0.01 |
| Late onset | 10 (8.55) | 7 (8.64) | 4 (5.33) | 3 (2.97) | 3 (4.41) | |
| Intermediate | 0 (0.00) | 60 (74.1) | 14 (18.7) | 16 (15.8) | 19 (27.9) | |
| Early multiple | 3 (2.56) | 14 (17.3) | 9 (12.0) | 0 (0.00) | 46 (67.6) | |
| Aeroallergen-specific skin test responses | ||||||
| Low/minimal | 103 (88.0) | 26 (32.1) | 58 (77.3) | 95 (94.1) | 4 (5.88) | <0.01 |
| Intermediate | 14 (12.0) | 46 (56.8) | 15 (20.0) | 6 (5.94) | 48 (70.6) | |
| Early multiple | 0 (0.00) | 9 (11.1) | 2 (2.67) | 0 (0.00) | 16 (23.5) | |
| FEV1/FVC ratio‡ | ||||||
| Mean (Years 3–7) | 0.91 (0.88–0.94) | 0.90 (0.87–0.94) | 0.91 (0.88–0.93) | 0.90 (0.85–0.95) | 0.90 (0.86–0.93) | 0.50 |
| Variability (Years 3–7) | 0.09 (0.06–0.11) | 0.07 (0.05–0.11) | 0.07 (0.05–0.11) | 0.09 (0.06–0.12) | 0.10 (0.08–0.13) | <0.01 |
| IOS XA | ||||||
| Low | 29 (24.8) | 18 (22.2) | 9 (12.0) | 14 (13.9) | 9 (13.2) | 0.01 |
| Intermediate | 57 (48.7) | 46 (56.8) | 46 (61.3) | 45 (44.6) | 31 (45.6) | |
| High | 31 (26.5) | 17 (21.0) | 20 (26.7) | 42 (41.6) | 28 (41.2) | |
Definition of abbreviations: HA = high atopy; HW = high wheeze; IOS = impulse oscillometry; LA = low atopy; LW = low wheeze; TW = transient wheeze; XA = area of reactance.
Values are counts (percentages) or medians (interquartile range).
Chi-square for categorical data, F test for continuous data.
For FEV1/FVC ratio we were unable to obtain a trajectory solution. Instead, these data were analyzed as continuous variables rather than group trajectories. To account for both the overall average and its variability over the last 4 years, for each participant, we derived his/her mean and its SD, which are presented under mean and variability.
Although differences in lung function variables among the respiratory phenotypes were less pronounced, the greatest impairment in lung function, a high XA reflecting peripheral airway obstruction (24), was found in the two HW phenotypes (HW-LA, HW-HA) (Table 1; see Table E3). FEV1 and FEV1/FVC differed across the five phenotypes, with the greatest impairments noted among the HW-HA group (Table 2).
Table 2.
Association of Respiratory Phenotypes with Variables Not Used in Clustering Algorithm*
| LW-LA (n = 117) | LW-HA (n = 81) | TW-LA (n = 75) | HW-LA (n = 101) | HW-HA (n = 68) | P Value† | |
|---|---|---|---|---|---|---|
| Variables with trajectories | ||||||
| BMI (percentile) | ||||||
| Low | 30 (25.6) | 19 (23.5) | 18 (24.0) | 22 (21.8) | 13 (19.1) | 0.76 |
| Nonlinear rate increase | 37 (31.6) | 28 (34.6) | 29 (38.7) | 26 (25.7) | 25 (36.8) | |
| Linear rate increase | 27 (23.1) | 17 (21.0) | 15 (20.0) | 22 (21.8) | 15 (22.1) | |
| Nonlinear high rate increase | 23 (19.7) | 17 (21.0) | 13 (17.3) | 31 (30.7) | 15 (22.1) | |
| Blood eosinophil count | ||||||
| Low | 98 (83.8) | 44 (54.3) | 57 (76.0) | 83 (82.2) | 23 (33.8) | <0.01 |
| High | 19 (16.2) | 37 (45.7) | 18 (24.0) | 18 (17.8) | 45 (66.2) | |
| Total IgE level | ||||||
| Low | 63 (53.8) | 10 (12.3) | 29 (38.7) | 47 (46.5) | 3 (4.41) | <0.01 |
| Intermediate | 45 (38.5) | 42 (51.9) | 39 (52.0) | 46 (45.5) | 24 (35.3) | |
| High | 9 (7.69) | 29 (35.8) | 7 (9.33) | 8 (7.92) | 41 (60.3) | |
| Food-specific IgE | ||||||
| Low/minimal | 77 (65.8) | 28 (34.6) | 46 (61.3) | 58 (57.4) | 15 (22.1) | <0.01 |
| Decrease | 17 (14.5) | 11 (13.6) | 11 (14.7) | 13 (12.9) | 6 (8.82) | |
| Increase | 17 (14.5) | 21 (25.9) | 15 (20.0) | 21 (20.8) | 23 (33.8) | |
| High | 6 (5.13) | 21 (25.9) | 3 (4.00) | 9 (8.91) | 24 (35.3) | |
| | ||||||
| Variables without trajectories | ||||||
| Sex | 0.01 | |||||
| Female | 67 (57.3) | 39 (48.1) | 39 (52.0) | 50 (49.5) | 21 (30.9) | |
| Male | 50 (42.7) | 42 (51.9) | 36 (48.0) | 51 (50.5) | 47 (69.1) | |
| Race | ||||||
| Black | 87 (74.4) | 53 (65.4) | 57 (76.0) | 69 (68.3) | 52 (76.5) | 0.52 |
| Hispanic | 23 (19.7) | 21 (25.9) | 12 (16.0) | 19 (18.8) | 12 (17.6) | |
| Mixed/other | 7 (5.98) | 7 (8.64) | 6 (8.00) | 13 (12.9) | 4 (5.88) | |
| Gestational age, wk, mean | 39.1 (1.40) | 39.0 (1.50) | 38.8 (1.43) | 38.5 (1.48) | 38.7 (1.67) | 0.03 |
| Maternal depression (3 yr) ‡ | 3.00 (0.00–6.00) | 2.50 (0.00–6.00) | 6.00 (0.50–12.0) | 7.50 (2.00–14.0) | 3.00 (0.00–9.00) | <0.01 |
| Maternal perceived stress (3 yr) § | 3.67 (1.50–5.75) | 4.29 (2.06–6.31) | 5.00 (2.69–7.25) | 5.75 (3.50–7.75) | 4.12 (2.50–6.81) | <0.01 |
| Cotinine detected (yes first year) | 19 (17.9) | 10 (13.2) | 7 (10.0) | 28 (28.9) | 7 (11.1) | 0.01 |
| FEV1/FVC ratio (7 yr) | 0.84 ± 0.06 | 0.84 ± 0.07 | 0.84 ± 0.07 | 0.82 ± 0.08 | 0.80 ± 0.08 | 0.01 |
| FEV1% predicted (7 yr) | 102 ± 13.0 | 102 ± 14.8 | 104 ± 13.3 | 101 ± 14.9 | 95.8 ± 18.1 | 0.09 |
| Any eczema (first year) | 55 (47.8) | 50 (62.5) | 31 (41.9) | 53 (53.5) | 52 (77.6) | <0.01 |
| Sum of allergen exposures index (first year)|| | 3.9 ± 2.20 | 3.7 ± 2.17 | 3.8 ± 2.45 | 3.0 ± 2.33 | 3.2 ± 2.38 | 0.02 |
| Microbiota phylogenetic diversity (first year) | 181 (156–209) | 194 (160–219) | 210 (185–233) | 195 (164–221) | 159 (135–196) | 0.004 |
| Microbiota richness (first year) | 5,326 (4,402–6,246) | 5,556 (4,534–6,358) | 6,160 (5,446–7,026) | 5,642 (4,607–6,610) | 4,611 (3,848–5,990) | 0.005 |
| | ||||||
| Measures of asthma disease severity (age 7) | ||||||
| Bronchodilator reversibility ≥10% | 19 (25.3) | 27 (50.0) | 10 (23.8) | 26 (41.9) | 26 (56.5) | <0.01 |
| PC20 ≤ 4 mg/ml | 31 (34.8) | 27 (45.8) | 28 (49.1) | 34 (45.9) | 20 (41.7) | 0.44 |
| Inhaled corticosteroids in past year | 2 (1.7) | 4 (5.0) | 0 (0.0) | 22 (21.8) | 24 (35.3) | <0.01 |
| Oral corticosteroids in past year | 5 (4.3) | 4 (5.0) | 2 (2.7) | 14 (13.9) | 19 (27.9) | <0.01 |
| Hospitalization for wheeze in past year | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (5.9) | <0.01 |
| Asthma diagnosis per URECA criteria | 2 (1.7) | 12 (14.8) | 0 (0.0) | 68 (67.3) | 48 (70.6) | <0.01 |
Definition of abbreviations: BMI = body mass index; HA = high atopy; HW = high wheeze; LA = low atopy; LW = low wheeze; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1; TW = transient wheeze; URECA = Urban Environment and Childhood Asthma.
Values are counts (percentages), means ± SD, or medians (interquartile range). Because some data may be missing on the variables without a trajectory, the denominators may not be exactly the ones in the column headers.
Chi-square for categorical data, F test for continuous data.
Edinburgh Postnatal Depression Score, range 0–30, higher scores denote higher risk for clinical depression with scores greater than or equal to 12 suggesting a need for further screening.
Perceived Stress Scale, range 4–16, higher scores denote higher perceived stress.
Sum of allergen exposures is calculated by combining cat, mouse, cockroach, and dog into a single index based on quartiles of exposure to individual allergens, resulting in a range of 0–12. House dust mite exposure levels were not used for the index given the low prevalences in this population.
Relationship of Respiratory Phenotypes to Other Variables
The five respiratory phenotypes differed by sex distribution (Table 2); the LW-LA phenotype had a slight excess in the proportion of girls (57.3%), whereas the HW-HA phenotype included more boys (69.1%). Other phenotypes, including HW-LA, had an equal representation of boys and girls. As expected, the two phenotypes with substantial allergic sensitization (LW-HA and HW-HA) had higher proportions of children with high eosinophil trajectories (45.7% and 66.2%, respectively) and high total IgE trajectories (35.8% and 60.3%, respectively), compared with the three LA groups (Table 2). Bronchodilator reversibility (≥10%) was about twice as frequent in the LW-HA (50%), HW-LA (41.9%), and HW-HA (56.5%) phenotypes, compared with the LA-LW (25.3%) and TW-LA (23.8%) phenotypes. At age 7 years, FEV1 and the FEV1/FVC ratio were lowest in the HW-HA group, whereas methacholine reactivity was similar among the phenotypes (Table 2). Finally, other variables, including race/ethnicity and BMI trajectories, were not different among the five phenotypes.
Relationship of Respiratory Phenotypes to Asthma
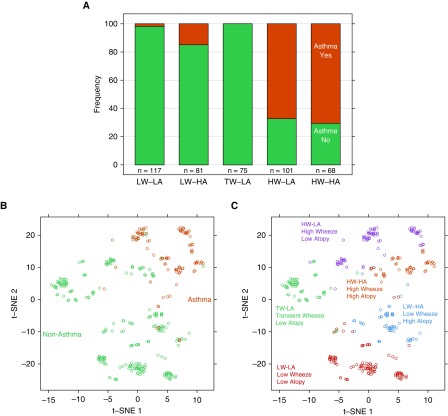
We next evaluated the relationships between the respiratory phenotypes and our a priori yes/no definition of childhood asthma (6). Asthma was present in 67.3% of the HW-LA subjects and 70.6% of the HW-HA subjects, whereas the LW-LA or TW-LA phenotypes had infrequent (1.7%) or no asthma, respectively (Table 2, Figure 3A). The LW-HA phenotype had a low prevalence (14.8%) of asthma. To illustrate similarities in children classified by respiratory phenotype versus asthma yes/no diagnosis, a two-dimensional figure was created where individuals were separated by a distance metric derived from the wheeze, allergic sensitization, and lung function clustering variables. Evaluation of asthma yes/no definition yielded relatively homogeneous clustering between subjects with asthma and subjects without asthma (Figure 3B). The respiratory phenotypes also formed distinct clusters (Figure 3C), with most asthma cases coinciding with the two HW phenotypes (HW-HA, HW-LA). Notably, the TW-LA phenotype was quite distinct from each of the others.
Figure 3.
Distributions of asthma diagnosis by URECA clusters. (A) The frequency (%) of asthma was greater in the high-wheeze/low-atopy and high-wheeze/high-atopy clusters (P < 0.01). (B and C) A two-dimensional distance matrix used in the construction of childhood URECA phenotype by asthma (B) and cluster group (C) is described by using t-distributed stochastic neighbor embedding, a novel dimension-reduction technique for high-dimensional data. HA = high atopy; HW = high wheeze; LA = low atopy; LW = low wheeze; t-SNE = t-distributed stochastic neighbor embedding; TW = transient wheeze; URECA = Urban Environment and Childhood Asthma.
Children with HW-HA and HW-LA were more likely to have used inhaled (35.3% and 21.8%, respectively; P < 0.01) and oral (27.9% and 13.9%; P < 0.01) corticosteroids during the last year than participants in the remaining three phenotypes (<5% for inhaled or oral corticosteroids use in any group). The HW-HA phenotype contained the only participants (5.9% of HW-HA, 0% for the other four groups; P < 0.01) who were hospitalized during the study period for wheezing illnesses (Table 2; pairwise comparisons are shown in Table E4).
Association of Early Childhood Exposures with Respiratory Phenotypes
We previously reported that several early-life exposures (maternal stress and depression, antenatal environmental tobacco smoke exposure [cord blood cotinine], house dust bacterial microbiota, and allergen content) were associated with the risk of wheezing and asthma in the URECA population (8, 25). We next tested the hypothesis that these exposures would be differentially associated with the URECA respiratory phenotypes. Indicators of maternal perceived stress and maternal depression differed between the phenotypes and were both greatest in the HW-LA children (Figures 4A and 4B). Umbilical cord plasma cotinine levels differed significantly across the phenotypes (Table 2; P = 0.01), and was most often detectable in the HW-LA, and least often detected in the TW-LA phenotype (Figure 4C). The sum of indoor allergen exposure (cockroach, mouse, cat, and dog) over the first year of life differed significantly across the five phenotypes (P = 0.02), with the highest cumulative exposure among LW-LA, whereas the two HW phenotypes (HW-LA and HW-HA) had the lowest cumulative exposures (Figure 4D). Finally, house dust microbial richness (P = 0.005) and phylogenetic diversity (P = 0.004) at 3 months of age differed significantly among the phenotypes (Table 2) and were lowest in HW-HA and highest in TW-LA (Figures 4E and 4F; see Table E4).
Figure 4.
Observed results from Perceived Stress Scale (A), Edinburgh Postnatal Depression Scale (B), detection levels of cotinine (C), sum of Allergen Exposure Index at first year (D), microbiota dust exposure richness (E), and phylogenetic diversity (F). All show a significant difference (P < 0.01) between phenotypes. HA = high atopy; HW = high wheeze; LA = low atopy; LW = low wheeze; TW = transient wheeze.
Discussion
Childhood asthma is better regarded as a syndrome rather than a disease, and several phenotypes of asthma have been described based on patterns of symptoms, allergy, and lung function. To determine whether asthma and respiratory phenotypes could be related to early-life environmental exposures, we evaluated longitudinal and prospectively acquired data from a high-risk birth cohort of children growing up in low-income urban environments. After considering longitudinal trajectories of wheeze, allergic sensitization, and lung function, we have identified five distinct patterns of early-life respiratory health (i.e., phenotypes) that were differentiated primarily based on patterns of wheezing and development of aeroallergen sensitization. Notably, these respiratory phenotypes were differentially related to early-life exposures including maternal stress and depression, cord blood cotinine, and exposure to microbes and allergens in the home. Collectively, these findings suggest that there are specific respiratory phenotypes associated with distinct patterns of clinical characteristics, which are related to specific risk factors and perhaps differential causal pathways (i.e., endotypes).
Multiple patterns of early-life wheezing, and their varying natural histories, have been described. Martinez and colleagues (9) first described three patterns of early-life wheezing (transient early wheeze, late-onset wheeze, and persistent wheeze) among participants in the Tucson Children’s Respiratory Study. More recently, investigators have used nonbiased statistical approaches on large cohorts, generally confirming, and in some cases further refining, the wheezing patterns described by Martinez (10–12, 26–28). Similarly, several developmental trajectories of atopy have been described, highlighting patterns of highly sensitized and multiple early sensitization as significant risk factors for asthma (29–31). Although unsupervised clustering approaches have defined several patterns of asthma and atopy development, often referred to as phenotypes, our approach was novel in several respects. First, our approach simultaneously included trajectories of multiple variables rather than focusing on one central variable, such as allergic sensitization. The inclusion of such trajectories also maximally incorporates a major strength of this study, longitudinally acquired data. Second, our cohort was comprised of high-risk children raised in urban environments who are particularly vulnerable to asthma development. Finally, we tested for associations between respiratory phenotypes and multiple early-life environmental risk factors.
Patterns of acquisition of allergic sensitization served as important differentiators between phenotypes, including both wheezing and nonwheezing groups. Notably, early onset and multiple sensitization trajectories were strongly associated with HW-HA, whereas the intermediate sensitization trajectory was more common in LW-HA. Allergic sensitization was also an important differentiating feature among children with wheezing. The HW-LA group was surprisingly large (n = 101) and outnumbered the HW-HA group (n = 68). However, although wheeze trajectories were similar between the two groups, the HA-HW group had the greatest expression of respiratory disease morbidity, including greater use of inhaled and oral corticosteroids, and included all of the children who were hospitalized for wheeze and asthma. These findings are consistent with other studies demonstrating allergic sensitization as a risk factor for asthma severity and exacerbations (32). This relationship may be caused by greater airway inflammation in highly sensitized children, together with increased susceptibility to rhinovirus infections and virus-induced wheeze (33–35). These findings are consistent with other cohorts, indicating that allergic sensitization should be considered as a continuous variable, and more significant aeroallergen sensitization in early life strongly predicts subsequent asthma.
Although lung function differences have been demonstrated in a cross-sectional study of inner-city children with a range of asthma severities (36) and among children with unremitting and multitrigger wheeze (10), measurements of lung function trajectories among the URECA participants during the first 7 years of life did not provide much discrimination between phenotypes, possibly related to the relatively short period of time over which testing was performed (ages 3–7 yr) and the relatively mild asthma among affected individuals. However, similar to other birth cohorts (37, 38), persistent wheeze phenotypes in URECA had lower FEV1 and FEV1/FVC at age 7 years compared with never/infrequent wheeze phenotypes. Furthermore, bronchodilator reversibility of at least 10% at age 6–7 years occurred least often in the LW-LA and TW-LA phenotypes, whereas approximately half of subjects in the LW-HA, HW-LA, and HW-HA phenotypes demonstrated reversibility. Despite this, the prevalence of bronchial hyperresponsiveness to methacholine at age 7 years did not differ between the groups, although more frequent use of inhaled corticosteroids in the HW groups may have resulted in lesser degrees of bronchial hyperresponsiveness in these groups. Ongoing longitudinal follow-up through late childhood/adolescence will help to further define the natural course of respiratory morbidity among these phenotypes.
One of the principal expectations and potential advantages for defining respiratory phenotypes is that more precise definitions of respiratory outcomes in URECA would reveal specific associations between early-life risk factors and respiratory phenotypes. In fact, house dust microbiota, home allergens, maternal stress, and prenatal tobacco smoke each had unique associations. For example, low house dust microbial richness and diversity were associated with the HW-HA phenotype. These findings support and extend previous findings from the URECA cohort that house dust microbial richness was inversely associated with atopy at age 3 years (8). Notably, these summary measures of house dust microbiome composition were not related to the asthma binary definition (yes/no) at 7 years of age in URECA (6), underscoring the need to more specifically define respiratory phenotypes in these populations to understand the early-life exposures that lead to their development. We also previously reported that maternal stress in the first few years was associated with recurrent wheeze at age 3 years (25) and asthma at age 7 years (6). Of the respiratory phenotypes, high maternal stress was specifically associated with HW-LA. Also, intrauterine smoke exposure, reflected by cord blood cotinine levels, was greatest and most frequent among children in the HW-LA phenotype. This may reflect lower lung function among infants with greatest intrauterine smoke exposure (39, 40), which predisposes these children to recurrent wheezing without significant allergic sensitization. Finally, reduced exposure to indoor allergens was associated with the HW-LA and HW-HA phenotypes, suggesting a specific association with persistent wheeze and asthma regardless of allergic sensitization. These findings, which are consistent with observations at age 3 years in URECA (8), suggest that microbes and allergens have distinct and complementary effects on the expression of allergic sensitization, wheeze, and ultimately asthma.
Several limitations should be considered in interpreting these findings. The URECA cohort consists of children born at high risk for the development of atopic disease and who are being raised in four low-income, urban areas in the United States; thus, these findings may not be generalizable to other populations. However, this may be less of a concern as we identified several parallels (e.g., trajectories of wheeze and atopy) with other birth cohorts (9, 29, 30). In addition, most asthma in birth cohorts is of mild severity, and thus information about the early-life risk factors for moderate or severe asthma phenotypes is limited. Assessment of environmental tobacco smoke exposure was limited to prenatal exposure. Larger samples sizes may be required to identify interactions between respiratory phenotypes and BMI. Finally, the microbiota data represented a single sample reflective of house dust–associated microbes rather than the participants’ personal microbiome (i.e., fecal or respiratory). We expect to learn more from repeated assessments of early-life microbial exposure and colonization. Ongoing longitudinal follow-up of this cohort will also shed light on the long-term natural histories of these phenotypes, including the risk of asthma among the LW-HA phenotype.
In conclusion, our findings support the premise that identifying respiratory phenotypes adds clarity to the search for the contributions of environmental risk factors to distinct forms of childhood asthma. In particular, the URECA data indicate that exposures to allergens, microbes, stress, and environmental tobacco smoke may specifically modify the risk for HW respiratory phenotypes with and without allergic sensitization. These findings move us closer toward the identification of asthma endotypes, with the ultimate goal of developing mechanism-based strategies to prevent childhood asthma.
Supplementary Material
Acknowledgments
Urban Environment and Childhood Asthma Study collaborators: The Urban Environment and Childhood Asthma Study is a collaboration of the following institutions, investigators, and staff (principal investigators are indicated by an asterisk; protocol chair is indicated by double asterisks). Johns Hopkins University, Baltimore, M.D.: R. Wood,* E. Matsui, H. Lederman, F. Witter, S. Leimenstoll, D. Scott, M. Cootauco, and P. Jones. Boston University School of Medicine, Boston, M.A.: G. O’Connor,* W. Cruikshank, M. Sandel, A. Lee-Parritz, C. Jordan, E. Gjerasi, P. Price-Johnson, L. Gagalis, L. Wang, N. Gonzalez, and M. Tuzova. Harvard Medical School, Boston, M.A.: D. Gold and R. Wright. Columbia University, New York, N.Y.: M. Kattan,* C. Lamm, N. Whitney, P. Yaniv, and M. Pierce. Mount Sinai School of Medicine, New York, N.Y.: H. Sampson, R. Sperling, and N. Rivers. Washington University School of Medicine, St Louis, M.O.: G. Bloomberg,* L. Bacharier,* Y. Sadovsky, E. Tesson, C. Koerkenmeier, R. Sharp, K. Ray, J. Durrange, I. Bauer, A. Freie, and V. Morgan. Statistical and Clinical Coordinating Center, Rho, Inc., Chapel Hill, N.C.: C. Visness,* P. Zook, M. Yaeger, J. Martin, A. Calatroni, K. Jaffee, W. Taylor, R. Budrevich, and H. Mitchell. Scientific Coordination and Administrative Center—University of Wisconsin, Madison, W.I.: W. Busse,* J. Gern,** P. Heinritz, C. Sorkness, K. Hernandez, Y. Bochkov, K. Grindle, A. Dresen, T. Pappas, M. Renneberg, and B. Stoffel. National Institute of Allergy and Infectious Diseases, Bethesda, M.D.: P. Gergen, A. Togias, E. Smartt, and K. Thompson.
Footnotes
Funded by the National Institute of Allergy and Infectious Diseases, NIH (contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, HHSN272201000052I, 1 UM1AI114271-01, and UM2AI117870). Additional support was provided by the National Center for Research Resources, NIH (grants RR00052, M01RR00533, 1 UL1RR025771, M01RR00071, 1 UL1RR024156, and 5 UL1RR024992-02) and the National Center for the Advancement of Translational Research, NIH (grants UL1TR001079 and UL1TR000040).
Author Contributions: L.B.B., A.B., A.C., D.J.J., P.J.G., and J.E.G. made substantial contributions to the conception or design of the work reported. L.B.B., A.B., G.T.O’C., M.K., R.A.W., M.T.S., C.M.V., S.V.L., H.B., and J.E.G. participated in the acquisition of reported data. A.C., K.E.F., D.W.F., and C.A.S. participated in the analysis of reported data. L.B.B., A.B., A.C., D.J.J., P.J.G., C.M.V., S.V.L., and J.E.G. participated in the interpretation of reported data and helped to draft the manuscript. All authors reviewed and/or critically revised the manuscript for important intellectual content and provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201801-0190OC on August 4, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the NIAID-sponsored Inner-City Asthma Consortium, R. Wood, E. Matsui, H. Lederman, F. Witter, S. Leimenstoll, D. Scott, M. Cootauco, P. Jones, G. O’Connor, W. Cruikshank, M. Sandel, A. Lee-Parritz, C. Jordan, E. Gjerasi, P. Price-Johnson, L. Gagalis, L. Wang, N. Gonzalez, M. Tuzova, D. Gold, R. Wright, M. Kattan, C. Lamm, N. Whitney, P. Yaniv, M. Pierce, H. Sampson, R. Sperling, N. Rivers, G. Bloomberg, L. Bacharier, Y. Sadovsky, E. Tesson, C. Koerkenmeier, R. Sharp, K. Ray, J. Durrange, I. Bauer, A. Freie, V. Morgan. C. Visness, P. Zook, M. Yaeger, J. Martin, A. Calatroni, K. Jaffee, W. Taylor, R. Budrevich, H. Mitchell, W. Busse, J. Gern, P. Heinritz, C. Sorkness, K. Hernandez, Y. Bochkov, K. Grindle, A. Dresen, T. Pappas, M. Renneberg, B. Stoffel, P. Gergen, A. Togias, E. Smartt, and K. Thompson
References
- 1.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303:623–630. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- 2.Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. Am J Public Health. 1992;82:59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crain EF, Weiss KB, Bijur PE, Hersh M, Westbrook L, Stein RE. An estimate of the prevalence of asthma and wheezing among inner-city children. Pediatrics. 1994;94:356–362. [PubMed] [Google Scholar]
- 4.Weiss KB, Gergen PJ, Crain EF. Inner-city asthma. The epidemiology of an emerging US public health concern. Chest. 1992;101(Suppl. 6):362S–367S. doi: 10.1378/chest.101.6.362s. [DOI] [PubMed] [Google Scholar]
- 5.Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol. 1999;28:394–401. doi: 10.1002/(sici)1099-0496(199912)28:6<394::aid-ppul2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and the risk of developing asthma among inner-city children. J Allergy Clin Immunol. 2018;141:1468–1475. doi: 10.1016/j.jaci.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110:939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 10.Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvärinen A, Kaulek V, et al. PASTURE Study Group. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189:129–138. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 11.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–980. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127:1505–1512. doi: 10.1016/j.jaci.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Guilbert TW, Mauger DT, Lemanske RF., Jr Childhood asthma-predictive phenotype. J Allergy Clin Immunol Pract. 2014;2:664–670. doi: 10.1016/j.jaip.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Cowan K, Guilbert TW. Pediatric asthma phenotypes. Curr Opin Pediatr. 2012;24:344–351. doi: 10.1097/MOP.0b013e32835357ab. [DOI] [PubMed] [Google Scholar]
- 15.Reddy MB, Covar RA. Asthma phenotypes in childhood. Curr Opin Allergy Clin Immunol. 2016;16:127–134. doi: 10.1097/ACI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 16.Granell R, Henderson AJ, Sterne JA.Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Children: a population-based birth cohortJ Allergy Clin Immunol 20161381060–70 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigelman A, Bacharier LB. Management of preschool recurrent wheezing and asthma: a phenotype-based approach. Curr Opin Allergy Clin Immunol. 2017;17:131–138. doi: 10.1097/ACI.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O’Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramratnam SK, Calatroni A, Bacharier LB, Jackson DJ, Beigelman A, Wood RA, et al. Maternal stress and depression are associated with development of a high-wheeze, low-atopy phenotype in their young offspring. AAAAI Annual Meeting / World Allergy Congress Orlando, Florida. J Allergy Clin Immunol 20181412 SupplAB7 [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 21.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Kattan M, Bacharier LB, O’Connor GT, Cohen R, Sorkness RL, Morgan W, et al. Spirometry and impulse oscillometry in preschool children: acceptability and relationship to maternal smoking in pregnancy. J Allergy Clin Immunol Pract. 2018;6:1596–1603. doi: 10.1016/j.jaip.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146:841–847. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 25.Ramratnam SK, Visness CM, Jaffee KF, Bloomberg GR, Kattan M, Sandel MT, et al. Relationships among maternal stress and depression, type 2 responses, and recurrent wheezing at age 3 years in low-income urban families. Am J Respir Crit Care Med. 2017;195:674–681. doi: 10.1164/rccm.201602-0272OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spycher BD, Henderson J, Granell R, Evans DM, Smith GD, Timpson NJ, et al. Genome-wide prediction of childhood asthma and related phenotypes in a longitudinal birth cohort. J Allergy Clin Immunol. 2012;130:503–509. doi: 10.1016/j.jaci.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sbihi H, Koehoorn M, Tamburic L, Brauer M. Asthma trajectories in a population-based birth cohort. Impacts of air pollution and greenness. Am J Respir Crit Care Med. 2017;195:607–613. doi: 10.1164/rccm.201601-0164OC. [DOI] [PubMed] [Google Scholar]
- 28.Panico L, Stuart B, Bartley M, Kelly Y. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One. 2014;9:e111922. doi: 10.1371/journal.pone.0111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havstad S, Johnson CC, Kim H, Levin AM, Zoratti EM, Joseph CL, et al. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol. 2014;134:722–727. doi: 10.1016/j.jaci.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson A, Tan VY, Winn J, Svensén M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 31.Hose AJ, Depner M, Illi S, Lau S, Keil T, Wahn U, et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. 2017;139:1935–1945. doi: 10.1016/j.jaci.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 32.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138:1030–1041. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, et al. Effects of omalizumab on rhinovirus infections, illnesses and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196:985–992. doi: 10.1164/rccm.201701-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138:1016–1029. doi: 10.1016/j.jaci.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savenije OE, Mahachie John JM, Granell R, Kerkhof M, Dijk FN, de Jongste JC, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol. 2014;134:170–177. doi: 10.1016/j.jaci.2013.12.1080. [DOI] [PubMed] [Google Scholar]
- 38.Belgrave DC, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A. Trajectories of lung function during childhood. Am J Respir Crit Care Med. 2014;189:1101–1109. doi: 10.1164/rccm.201309-1700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. doi: 10.1183/09031936.97.10081774. [DOI] [PubMed] [Google Scholar]
- 40.Hoo A-F, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.