To the Editor:
According to recently released World Health Organization data on mortality, in 2015 there were 5.9 million deaths among children under the age of 5 years, and 2.7 million occurred in the neonatal period (1). Globally, pneumonia was the leading cause of under-5 deaths, particularly in sub-Saharan Africa countries, where the decrease in child mortality from pneumonia from 2010 to 2015 was reduced compared with the already modest 6% observed globally (1). Although improvements in survival are directly linked to socioeconomic progress and concurrent improvements in healthcare systems in low-income settings, premature deaths among children can be prevented with access to simple and affordable interventions. In the case of preterm birth (2) and pediatric respiratory diseases (3), a particularly effective treatment is continuous positive airway pressure (CPAP), in particular bubble CPAP (4), with demonstrated efficacy in low-income countries (LICs) (5, 6). The simplest and cheapest way to provide CPAP is to use the central gas pressure source in a hospital, and as such, hospitals would seem to be the most suitable setting in LICs. Nevertheless, because the number of hospitals in LICs with central gas supply facilities is exceedingly low, CPAP is usually restricted to private medical facilities in urban settings and therefore is unavailable to the majority of the population receiving care in extremely underserviced regional hospitals and rural healthcare sites. Hence, readily extending potential CPAP treatment relies on the provision of stand-alone devices incorporating autonomous gas pressure sources.
There are three potential options to provide healthcare centers in LICs with the CPAP devices they cannot afford:
-
1.
Philanthropic donation of conventional devices. This option is expensive and has low effectiveness because 50% of donated equipment becomes unusable or remains unused, mainly due to lack of training in maintenance and inability to obtain spare parts (7).
-
2.
Provision of devices that do not include lesser-value “luxury” features included in conventional devices and are specifically designed for LICs (8, 9). This option may facilitate donations to and purchases by hospitals in LICs. Nevertheless, regardless of efforts to minimize costs, any conventional commercial process, even on a nonprofit basis, results in device pricing that is still unaffordable in most LIC healthcare settings.
-
3.
Construction and donation of custom-made devices (10, 11). This approach is hardly sustainable because it requires long-term commitments to ensure appropriate maintenance of the devices.
Regardless of their drawbacks, all of these approaches are valuable and should be promoted as much as possible considering the critical health needs in LICs. However, alternative procedures that would enable wide-scale availability of pediatric CPAP devices are warranted.
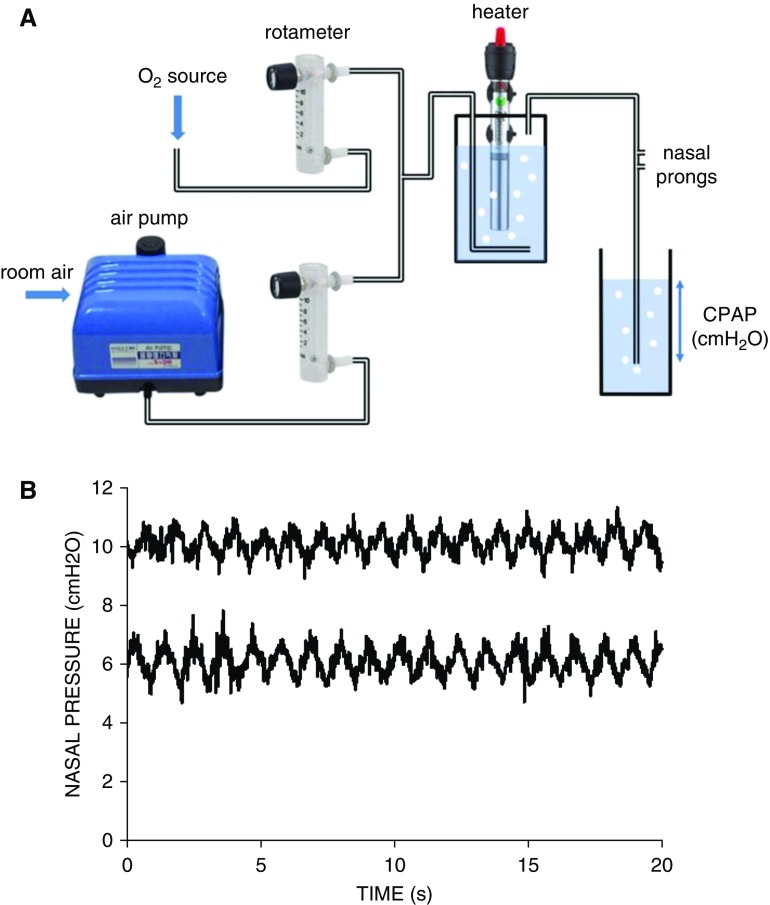
In this context, we herein describe a novel approach based on collaborative cocreation and design thinking (12), involving a civil engineering university team in Mozambique, initially unexperienced in CPAP, and a Western team with expertise in the technology and clinical application of this therapy. The key point of this inclusive innovation approach, which makes it original as compared with other proposals, is that it moves the design focus from high-income countries to LICs (13). Specifically, the aim was to produce a high-performing, stand-alone CPAP device setting that the team in Mozambique could autonomously build and further maintain at very low cost while using off-the-shelf components that are easy to acquire via e-commerce (Figure 1A). We implemented this novel system according to the conventional concept of bubble CPAP (10, 14). Specifically, room air flow generated by a domestic aquarium air pump was fed into a low-cost acrylic rotameter. The outlet of this rotameter was connected to the outlet of an identical one with its inlet potentially connected to an oxygen source. Regulating gas flow in both rotameters allowed us to set up an air-oxygen mixture (0–10 L/min range) at the desired concentration of oxygen, which was fed into a closed receptacle containing water, and a submerged stainless-steel heater/controller for a domestic aquarium. The conditioned air was fed into conventional flexible tubing connected to one inlet of newborn nasal prongs. The nasal-prong inlet was connected to similar conventional tubing, which was ended by a rigid tube with a multiorifice piece. CPAP was set by adequately submerging this piece in an open receptacle with water. To provide robustness, the whole setting, including a low-cost automatic power switch (220-VAC/12-VCC), was assembled within a box (28 × 20 × 24 cm) including the required electrical and gas connections.
Figure 1.
(A) Diagram of the continuous positive airway pressure (CPAP) setting, including its basic components: a domestic aquarium air pump (Hailea V-20; 15W; >300 cm H2O; 20 L/min, 2.5 kg; 230 × 185 × 180 mm), low-cost rotameters (CNBTR LZQ-6 0-10LPM; 65 × 22 × 90 mm) with precision flow delivery within ±0.2 L/min when compared with a reference Fleisch pneumotachograph, and a domestic aquarium water heater/controller (RS Electrical RS-139; 25W; 22.5 cm length) providing up to 10 L/min output air at 36°C from room air at 20°C. The retail sale cost by internet of these three components was $60, $15, and $10, respectively. (B) Nasal pressures actually applied at the newborn nasal prongs by the novel device for CPAP settings of 6 and 10 cm H2O. The simulated newborn infant was breathing with a Vt of 20 ml and frequency of 55 breaths/min while 8 L/min of humidified heated airflow circulated through the circuit.
The performance of the CPAP setting was tested in the bench under well-controlled conditions by connecting the newborn nasal prongs to a patient simulator that was able to reproduce the conventional pediatric Vts and frequencies (15). Figure 1B shows real-time recordings of the pressures achieved at the nasal prongs for CPAP settings of 6 and 10 cm H2O when the simulated newborn was breathing at 55 breaths/min with a Vt of 20 ml. Nasal pressures were stable when compared with other reported bubble CPAP devices (10, 11), in terms of both noise induced by bubbling and fluctuations caused by breathing oscillations. Consistent results emerged when breathing of a 10-kg body weight infant (35 breaths/min, 100 ml Vt) was simulated through nasal prongs of the corresponding size. It is important to note that our setting included heated humidification, which was not included in other reports (10, 11), because it could be relevant particularly among premature and full-term infants. However, depending on the specific application, this component can be eliminated. Also, the setting can be simplified if it is used in healthcare centers without oxygen availability (the majority of which are found in rural areas) because one of the rotameters can be excluded, still providing a realistically feasible rescue CPAP setting. Interestingly, the retail cost of components (≤$100) should be further reduced in case of wholesale acquisition. We should point out that our proposed CPAP setting uses components that are not designed and commercialized for medical use, and therefore have not undergone medical device regulatory approval. However, the current solution is not intended to replace approved medical devices if such are available, but rather to give patients access to treatment in situations in which the alternative would be to simply leave the patient unattended, along with the inherent potentially devastating consequences. Our proposed system application de facto complies with conditions similar to those conventionally agreed upon for compassionate use (life-threatening disease, no other treatment option, and potential benefit justifying potential risks). Obviously, eventual safety issues would have to be discussed and agreed upon by the healthcare team while considering pertinent ethical criteria as applicable to each individual instance.
In conclusion, we have effectively implemented a collaborative procedure for health technology transfer to a team in LICs, enabling autonomous construction and maintenance, thereby facilitating adequate provision of pediatric CPAP settings in markedly underserved regions. The approach is based on a concept that is usually overlooked when providing health technology support, namely, to empower the final users in LICs to fully control the procedure and adapt it to the specific local conditions. The low-cost and geographical distribution facilities currently provided by e-commerce are also cardinal features of our approach because they facilitate end-user access and the flexibility to adapt and update the components in response to market availability. Importantly, the approach described here does not exclude continued support and advice from experienced teams regionally or internationally in higher-resource settings. In addition, it provides opportunities for more cost-effective philanthropic donations (in cash or components) aimed at reducing the overwhelming child mortality caused by respiratory diseases in LICs.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Miguel A. Rodríguez-Lázaro (Universitat de Barcelona) for his excellent technical support.
Footnotes
Supported in part by the Spanish Ministry of Economy and Competitiveness (SAF2017-85574-R, DPI2017-83721-P; AEI/FEDER, UE), and the CERCA Program of Generalitat de Catalunya. D.G. is supported by NIH grant HL130984.
Originally Published in Press as DOI: 10.1164/rccm.201808-1452LE on September 28, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Association of Perinatal Medicine. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 3.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome—2016 update. Neonatology. 2017;111:107–125. doi: 10.1159/000448985. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Maria A, Roy MK, Verma A. A randomized trial comparing efficacy of bubble and ventilator derived nasal CPAP in very low birth weight neonates with respiratory distress. J Clin Diagn Res. 2016;10:SC09–SC12. doi: 10.7860/JCDR/2016/20584.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin S, Duke T, Davis P. Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2014;99:F495–F504. doi: 10.1136/archdischild-2013-305519. [DOI] [PubMed] [Google Scholar]
- 6.Rezzonico R, Caccamo LM, Manfredini V, Cartabia M, Sanchez N, Paredes Z, et al. Impact of the systematic introduction of low-cost bubble nasal CPAP in a NICU of a developing country: a prospective pre- and post-intervention study. BMC Pediatr. 2015;15:26. doi: 10.1186/s12887-015-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howie SRC, Hill SE, Peel D, Sanneh M, Njie M, Hill PC, et al. Beyond good intentions: lessons on equipment donation from an African hospital. Bull World Health Organ. 2008;86:52–56. doi: 10.2471/BLT.07.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health OrganizationWHO compendium of innovative health technologies for low resource settings; 2014 [accessed 2018 Nov 27]. Available from: http://www.who.int/medical_devices/innovation/compendium/en/
- 9.DePasse JW, Caldwell A, Santorino D, Bailey E, Gudapakkam S, Bangsberg D, et al. Affordable medical technologies: bringing value-based design into global health. BMJ Innov. 2016;2:4–7. [Google Scholar]
- 10.Brown J, Machen H, Kawaza K, Mwanza Z, Iniguez S, Lang H, et al. A high-value, low-cost bubble continuous positive airway pressure system for low-resource settings: technical assessment and initial case reports. PLoS One. 2013;8:e53622. doi: 10.1371/journal.pone.0053622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DJ, Carroll RW, Kacmarek RM. Evaluation of a low-cost bubble CPAP system designed for resource-limited settings. Respir Care. 2018;63:395–403. doi: 10.4187/respcare.05762. [DOI] [PubMed] [Google Scholar]
- 12.Ranger BJ, Mantzavinou A. Design thinking in development engineering education: a case study oncreating prosthetic and assistive technologies for the developing world. Dev Eng. 2018;3:166–174. [Google Scholar]
- 13.Clifford KL, Zaman MH. Engineering, global health, and inclusive innovation: focus on partnership, system strengthening, and local impact for SDGs. Glob Health Action. 2016;9:30175. doi: 10.3402/gha.v9.30175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli JA, Richardson CP, DiBlasi RM. Volume oscillations delivered to a lung model using 4 different bubble CPAP systems. Respir Care. 2015;60:371–381. doi: 10.4187/respcare.03432. [DOI] [PubMed] [Google Scholar]
- 15.Farré R, Montserrat JM, Rigau J, Trepat X, Pinto P, Navajas D. Response of automatic continuous positive airway pressure devices to different sleep breathing patterns: a bench study. Am J Respir Crit Care Med. 2002;166:469–473. doi: 10.1164/rccm.2111050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.