Abstract
Background
Arterial line cannulation in paediatric patients is traditionally performed by palpation or with Doppler auditory assistance in locating the artery before catheterization. It is not clear whether ultrasound guidance offers benefits over these methods.
Objectives
To assess first attempt success rates and complication rates when ultrasound guidance is used for arterial line placement in the paediatric population, as compared with traditional techniques (palpation, Doppler auditory assistance), at all potential sites for arterial cannulation (left or right radial, ulnar, brachial, femoral or dorsalis pedis artery).
Search methods
We searched CENTRAL, MEDLINE (Ovid) and Embase (Ovid). We also searched databases of ongoing trials (ClinicalTrials.gov (www.clinicaltrials.gov/), Current Controlled Trials metaRegister (www.controlled‐trials.com/), the EU Clinical Trials register (www.clinicaltrialsregister.eu/) and the WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/). We tried to identify other potentially eligible trials by searching the reference lists of retrieved included trials and related systematic or other reviews. We searched until January 2016.
Selection criteria
We included randomized controlled trials (RCTs) comparing ultrasound guidance versus palpation or Doppler auditory assistance to guide arterial line cannulation in paediatrics.
Data collection and analysis
Two review authors independently assessed the risk of bias of included trials and extracted data. We used standard Cochrane meta‐analytical procedures, and we applied the GRADE method to assess the quality of evidence.
Main results
We included five RCTs reporting 444 arterial cannulations in paediatric participants. Four RCTs compared ultrasound with palpation, and one compared ultrasound with Doppler auditory assistance.
Risk of bias varied across studies, with some studies lacking details of allocation concealment. It was not possible to blind practitioners in all of the included studies; this adds a performance bias that is inherent to the type of intervention studied in our review. Only two studies reported the rate of complications.
Meta‐analysis showed that ultrasound guidance produces superior success rates at first attempt (risk ratio (RR) 1.96, 95% confidence interval (CI) 1.34 to 2.85, 404 catheters, four RCTs, moderate‐quality evidence) and fewer complications, such as haematoma formation (RR 0.20, 95% CI 0.07 to 0.60, 222 catheters, two RCTs, moderate‐quality evidence). Our results suggest, but do not confirm, that a possible advantage of ultrasound guidance for the first attempt success rate over other techniques is more pronounced in infants and small children than in older children. Similarly, our results suggest, but do not confirm, the possibility of a positive influence of expertise in the use of ultrasound on the first attempt success rate. We also found improved success rates within two attempts (RR 1.78, 95% CI 1.25 to 2.51, 134 catheters, two RCTs, moderate‐quality evidence) with ultrasound guidance compared with other types of guidance. No studies reported data about ischaemic damage. We rated the quality of evidence for all outcomes as moderate owing to imprecision due to wide confidence intervals, modest sample sizes and limited numbers of events.
Authors' conclusions
We identified moderate‐quality evidence suggesting that ultrasound guidance for radial artery cannulation improves first and second attempt success rates and decreases the rate of complications as compared with palpation or Doppler auditory assistance. The improved success rate at the first attempt may be more pronounced in infants and small children, in whom arterial line cannulation is more challenging than in older children.
Plain language summary
Ultrasound use for insertion of arterial catheters in children
Background
An arterial catheter is a thin tube or line that can be inserted into an artery. Arterial catheters are used to monitor blood pressure during complex surgeries and during stays in intensive care. Ultrasound imaging (an image created with sound waves of soft tissue) allows anaesthesiologists and intensivists to see surrounding structures. Ultrasound can help medical practitioners accurately locate the artery and insert the catheter, and, particularly when surgeries involve children, ultrasound can prevent the need for multiple needle sticks. This reduces the occurrence of haematoma (a localized collection of blood outside the blood vessels) or damage to the artery, compared with other techniques such as palpation of the artery (feeling through the skin for the pulse) or Doppler auditory assistance (listening for a change to a higher pitch at the exact location of the artery). Our aim was to find out whether ultrasound offers any advantages over palpation of the artery or Doppler auditory assistance.
Study characteristics
The evidence is current to January 2016. We found five eligible studies ‐ four comparing ultrasound with palpation and one comparing ultrasound with Doppler auditory assistance.
Key results
We included in the review children aged one month to 18 years. We found that ultrasound increased the rate of successful cannulation at the first attempt and reduced the formation of haematomas. Ultrasound also increased the success rate within two attempts. It is likely that ultrasound is more useful for infants and small children than for older children. It is also likely that ultrasound is more useful if the practitioner is experienced in its use.
Quality of the evidence
We noted variation in the risk of bias of included studies. We rated the quality of evidence as moderate mainly because the number of studies was limited. For the same reason, we could not confirm the effect of age and expertise in ultrasound usage.
Conclusions
Our evidence suggests that ultrasound is superior to other techniques for arterial catheter insertion, particularly in babies and young children.
Summary of findings
Summary of findings for the main comparison. Summary of findings table.
| Ultrasound‐guided arterial cannulation compared with palpation or Doppler guidance for paediatric patients | ||||||
| Patient or population: paediatric patients Setting: participants undergoing various surgical procedures in operating rooms/ICU/emergency departments in university hospital settings in Germany, Japan and USA Intervention: US‐guided arterial cannulation Comparison: other techniques (palpation/Doppler) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with other techniques (palpation/Doppler) | Risk with US‐guided arterial cannulation | |||||
| Successful cannulation at the first attempt | Study population | RR 1.96 (1.34 to 2.85) | 404 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 209 per 1000 | 409 per 1000 (280 to 595) | |||||
| Rate of complications (haematoma or ischaemia) | Study population | RR 0.20 (0.07 to 0.60) | 222 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | ||
| 153 per 1000 | 31 per 1000 (11 to 92) | |||||
| Successful cannulation within the first 2 attempts | Study population | RR 1.78 (1.25 to 2.51) | 134 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | ||
| 358 per 1000 | 616 per 1000 (448 to 849) |
|||||
CI: confidence interval; ICU: intensive care unit; RCT: randomized controlled trial; RR: risk ratio; US: ultrasound.
aDowngraded by one level owing to imprecision (specifically, a relatively small number of events (n = 125)) and some level of risk of bias (specifically, performance bias and selective reporting bias).
bDowngraded by one level owing to imprecision (specifically, a relatively small number of events (n = 20)) and some level of risk of bias (specifically, performance bias).
cDowngraded by one level owing to imprecision (specifically, a relatively small number of events (n = 67)) and some level of risk of bias (specifically, performance bias).
Background
Description of the condition
Arterial line cannulation is an intervention that is commonly performed during major surgery and in the intensive care unit (ICU) for continuous blood pressure monitoring and arterial blood sampling in children. Cannulation is different for children than for adults because of the small size of their arteries. Therefore, arterial line cannulation can be more challenging in paediatric patients.
Description of the intervention
The site most commonly used for arterial cannulation is the radial artery; other sites include the femoral, axillary, brachial, ulnar, dorsalis pedis, tibial posterior and temporal arteries. Many techniques for arterial cannulation in the paediatric age group have been described (Ueda 2013). These techniques include palpation, ultrasound guidance and Doppler auditory assistance.
Palpation of the pulse
Pulse palpation to identify a landmark is the traditional approach for insertion of an arterial catheter. The site of cannulation is usually selected, positioned and prepped. The artery is localized by palpating the pulse, and the procedure is initiated. Accurate localization of the small artery is technically difficult, especially in small children and infants (Varga 2013). This may complicate placement and threading of the catheter (Schindler 2005). This situation can be further complicated by dehydration or haemodynamic instability, which makes the pulse weak and difficult to find.
Ultrasound guidance
Ultrasound guidance represents an alternative to the traditional palpation technique for insertion of arterial catheters. It is commonly used for placement of central venous catheters (CVCs). Numerous randomized controlled trials (RCTs) and meta‐analyses have found that the use of ultrasound reduces complications and increases first attempt success for CVC placement when compared with traditional landmark techniques (Hind 2003; Milling 2005; Randolph 1996).
Doppler auditory assistance
Doppler auditory assistance has been described as another alternative to the traditional palpation technique for insertion of arterial catheters. The Doppler tone changes to a higher pitch at the exact location of the artery, which might facilitate arterial cannulation. Success rates with this technique have been reported at 46% (Ueda 2013).
Potential complications
Although rare, devastating complications associated with arterial line cannulation, such as permanent ischaemic damage, sepsis and pseudoaneurysm formation, may occur (Scheer 2002). Arterial occlusion, haematoma and nerve injury are seen more frequently (King 2008).
How the intervention might work
Intervention
Real‐time ultrasound guidance technique
Through an out‐of‐plane technique, the artery is centred in the middle of the screen, with the probe held with the left hand perpendicular to the skin. A cannula of an appropriate size is introduced with the right hand below the ultrasound probe at its centre, and tissue movement is observed on the ultrasound screen. The cannula is redirected or the manoeuvre repeated until adequate arterial flow allows easy insertion of the guidewire or the cannula.
Comparator
Palpation technique
With this approach, the non‐dominant hand palpates the artery, while the dominant hand manipulates the intravascular needle or catheter, which is inserted at a 30 to 45 degree angle and is advanced slowly until pulsatile blood flow returns. The outer cannula is then advanced into the artery directly from the needle or with the aid of a guidewire.
Doppler auditory assistance
The Doppler probe identifies the artery by locating the area with maximum sound. During cannulation, the Doppler probe is used to identify the exact position of the artery and to guide needle or cannula insertion.
Ultrasound guidance may improve the success rate and reduce potential complications of arterial line cannulation in children.
Why it is important to do this review
The importance of this Cochrane systematic review stems from the large number of arterial lines placed in paediatric patients undergoing major surgery or hospitalised in an intensive care unit, or both. UK guidelines for placement of CVCs have recommended use of an ultrasound‐guided technique, given associated reductions in the rate of failure and in mechanical complications (NICE 2002). The American Society of Anesthesiology Task Force has issued practice guidelines for central venous access, in which they recommended that real‐time ultrasound guidance should be used for vessel localization and venipuncture when the internal jugular vein is selected for cannulation (ASA 2012). The use of ultrasound for arterial line insertion has been controversial, in that some studies advocate for its use (Schwemmer 2006), but others oppose it (Ganesh 2009). No guidelines are available for use of ultrasound for arterial line placement in the paediatric population. Randomized controlled trials have published findings on this topic (Ganesh 2009; Ishii 2013; Schwemmer 2006; Ueda 2013), but meta‐analyses for the paediatric population are not available. This Cochrane systematic review will provide an objective assessment of the benefits and harms of using an ultrasound guidance technique as compared with traditional techniques (palpation, Doppler auditory assistance, and others) for arterial line placement in the paediatric population. This information will serve to assist doctors in making educated choices and reducing potential complications that may stem from arterial line placement.
Objectives
To assess first attempt success rates and complication rates when ultrasound guidance is used for arterial line placement in the paediatric population, as compared with traditional techniques (palpation, Doppler auditory assistance), at all potential sites for arterial cannulation (left or right radial, ulnar, brachial, femoral or dorsalis pedis artery).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled clinical trials (RCTs).
Types of participants
We limited participants of interest to paediatric patients, infants and adolescents (one month to 18 years of age) undergoing arterial line placement. We excluded neonates.
Types of interventions
Ultrasound guidance
Pulse palpation
Doppler auditory assistance
Types of outcome measures
Primary outcomes
First attempt success rate
-
Rate of complications
Haematoma
Ischaemic damage
Secondary outcomes
Rate of successful cannulation
Time to successful cannulation
Number of attempts to successful cannulation
Number of cannulas used
Need for assistance by another operator (primary operator fails when attempting to insert and asks for help)
However, most of the secondary outcomes listed above could not be analysed because they were not listed in the included studies, because they were listed in only one study or because definitions of the outcomes were not consistent across studies. Therefore, we identified the following new outcome as relevant to our analysis.
Successful cannulation within the first two attempts.
Search methods for identification of studies
Electronic searches
We used the Ovid platform to search the following sources from inception to January 2016: the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), MEDLINE and Embase. We also searched databases of registered trials from inception to January 2016: ClinicalTrials.gov (www.clinicaltrials.gov/), Current Controlled Trials metaRegister (www.controlled‐trials.com/), the EU Clinical Trials register (www.clinicaltrialsregister.eu/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/). We provided information, including trial identifiers, for potentially relevant ongoing studies in the Ongoing studies table.
Please see Appendix 1, Appendix 2 and Appendix 3 for details of our searches.
We continuously applied the basic search strategy of the ’My NCBI’ (National Center for Biotechnology Information) email alert service of PubMed to identify newly published studies. We performed a completely updated search of all specified databases in January 2016.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, related systematic or other reviews and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (CR, SS) assessed independently, and in duplicate, every retrieved citation for potential eligibility. We retrieved the full texts for all citations judged by at least one of the two review authors as potentially eligible. The two review authors then assessed the full texts for eligibility in a duplicate and independent manner, using a standardized and pilot‐tested screening form (Appendix 4). We compared results and resolved disagreements by consensus, or with the help of a third review author (MAM) when needed. Before starting the selection process, CR and SS conducted calibration exercises to ensure the validity of the process.
Data extraction and management
Two review authors (CR, SS) independently, and in duplicate, abstracted relevant data, using standard data extraction forms. Abstracted data included characteristics of the population, interventions, controls and outcomes. We also abstracted statistical data needed for the meta‐analysis. We resolved disagreements by discussion or, if required, by consultation with a third review author (MAM). We contacted one study author to clarify information and provide additional data. When data extraction forms were completed, two review authors (CR, SS) entered the data into Review Manager software (RevMan 2014).
Dealing with duplicate publications and companion papers
In the event of duplicate publications, companion documents or multiple reports of a primary study, we planned to maximize the yield of information by collating all available data. We planned to resolve remaining uncertainties by attempting to contact study authors when possible.
Assessment of risk of bias in included studies
Two review authors (CR, SS) assessed the risk of bias of each included study independently and in duplicate. We resolved disagreements by consensus or by consultation with a third review author (EA). We assessed risk of bias by using the risk of bias tool of The Cochrane Collaboration (Higgins 2011a; Higgins 2011b), which includes the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, providers, data collectors, outcome adjudicators and data analysts (performance bias and detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (outcome reporting bias).
Other bias.
We assessed outcome reporting bias (Kirkham 2010) by comparing outcomes listed in a trial protocol, at registration and in the Methods section versus outcomes for which data were reported in the Results section. We judged trials as having ’low risk’, ’high risk’ or ’unclear risk’ of bias and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data), we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We planned to consider the implications of missing outcome data for individual participants.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs). We planned to express continuous data as mean differences (MDs) with 95% CIs when all studies reported the outcome using the same scale, and as standardized mean differences (SMDs) when studies reported the outcome using different scales.
Unit of analysis issues
The unit of analysis is catheterization of the radial artery. We took into account the level at which randomization occurred, such as cluster‐randomized trials and multiple observations for the same outcome.
Dealing with missing data
We planned to use a complete case approach in the main analysis and to conduct sensitivity analyses using plausible assumptions about the outcomes of participants with missing outcome data, to test the robustness of statistically significant results, as outlined in Akl 2013 and Ebrahim 2013. However, no missing data were reported.
Assessment of heterogeneity
We assessed statistical heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi2 test with a significance level of 0.1. In view of the low power of this test, we also considered the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We considered an I2 statistic of 50% or more as indicative of a considerable level of statistical heterogeneity (Higgins 2011a).
We planned to conduct subgroup analyses to explore whether any clinical or methodological factor could explain cases of considerable statistical heterogeneity (see Subgroup analysis and investigation of heterogeneity below). If the subgroup analysis identified a subgroup effect (i.e. statistical heterogeneity was explained), we planned to present results stratified by relevant subgroups. If the subgroup analysis did not identify a subgroup effect (i.e. statistical heterogeneity remained unexplained), we planned to refrain from meta‐analysis of studies.
We expected the following characteristics to introduce clinical heterogeneity.
Expertise of the anaesthesiologist.
Academic versus non‐academic setting.
Age group of participants (infants vs children vs adolescents).
Site of cannulation (radial or other arteries).
Expertise in ultrasound usage.
Studies at low vs high risk of bias.
In a post hoc decision, we decided to conduct subgroup analyses that we judged clinically relevant even in the absence of statistical heterogeneity.
Assessment of reporting biases
We planned to examine funnel plots to assess the potential for publication bias if we found 10 or more studies reporting on a particular outcome (Sterne 2011). However, we constructed no funnel plots because the number of included studies was limited.
Data synthesis
We synthesized and analysed data using RevMan 5.3. We calculated agreement between the two independent review authors for assessment of full‐text eligibility using the kappa statistic. For categorical data, we calculated the risk ratio separately for each study for the event rate of outcomes by treatment arm. Then, we pooled the results of different studies using a random‐effects model. For continuous data, we planned to pool data from different studies using a random‐effects models and the inverse variance approach.
Subgroup analysis and investigation of heterogeneity
We planned to attempt to determine potential reasons for heterogeneity by conducting subgroup analyses. We planned to investigate interactions by conducting subgroup analyses based on the following characteristics.
Expertise of the anaesthesiologist.
Academic versus non‐academic setting.
Age group of participants (infants vs children vs adolescents).
Site of cannulation (radial or other arteries).
Expertise in ultrasound usage.
However, we found that data for some of the above mentioned characteristics were insufficient: Years of experience varied widely among the included studies; all studies were performed in university hospitals; and all sites of cannulation were radial arteries. Also, we could not obtain from one study author additional data regarding age groups of participants. Therefore, we analysed data according to age groups of participants and expertise in ultrasound usage.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect size.
Restricting the analysis to published studies.
Restricting the analysis to studies with low risk of bias.
Making plausible assumptions about the outcomes of participants with missing data.
Summary of findings table and GRADE
We graded the overall quality (certainty) of the evidence for each outcome by using the GRADE approach (Guyatt 2011a), which classifies the quality of evidence into four categories: high, moderate, low and very low. This approach takes into account the study design, as well as the following factors: risk of bias, imprecision, inconsistency, indirectness, publication bias, large effect size, dose‐response effect and confounding. We used the principles of the GRADE system to assess the quality of the body of evidence associated with the following specific outcomes in our review.
First attempt success rate.
-
Rate of complications.
Haematoma.
Ischaemic damage.
Successful cannulation within the first two attempts.
We used GRADE software to construct a 'Summary of findings’ (SoF) table. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See Characteristics of included studies.
Results of the search
Of the 241 records that we identified as a result of the database search (excluding duplicates), we selected 58 abstracts or titles as potentially relevant studies. Independent scrutiny of these 58 titles and abstracts yielded five potentially relevant studies, all of which met the criteria for inclusion. We excluded no studies and identified one ongoing study (Siddik‐Sayyid 2014). The kappa statistic for full‐text screening was one.
We have further illustrated these findings in the study flow diagram (Figure 1) (Liberati 2009).
1.

Study flow diagram.
Included studies
We included five studies published between 2006 and 2015, all in English. The five included studies involved a total of 444 radial artery catheterizations, including 218 ultrasound‐assisted arterial catheterizations, 174 palpation‐assisted catheterizations and 52 Doppler‐assisted catheterizations.
All five included studies were randomized controlled trials of radial artery catheterizations. Four of the included studies (Ganesh 2009; Ishii 2013; Schwemmer 2006; Tan 2015) compared ultrasound‐guided arterial catheterization versus palpation‐guided arterial catheterization, and the fifth study (Ueda 2013) compared ultrasound‐guided arterial catheterization versus Doppler‐guided arterial catheterization. Individual studies reported catheterization sample sizes of 30 (Schwemmer 2006), 104 (Ueda 2013), 118 (Ishii 2013), 152 (Ganesh 2009) and 40 (Tan 2015). These studies took place in university hospital settings in Germany, Japan, USA and Canada.
The five studies included participants of both sexes, with ages ranging from two months (Ueda 2013) to 18 years (Ganesh 2009). The five included studies excluded patients with any injury near the site of insertion (such as skin erosion or haematoma, a visible recent catheterization scar or an arterial puncture site one month earlier) (Ishii 2013; Ueda 2013), prominent differences in arterial pressure between left and right arms (Ishii 2013) or anticipated circulatory instability after anesthesia induction, such as pulmonary hypertension or severe heart failure (Tan 2015). Three of the included studies listed the type of surgery performed, which included elective congenital heart disease surgery (Ishii 2013), major neurosurgery (Schwemmer 2006) and cardiac and non‐cardiac surgeries (Ueda 2013).
Operators of catheterization consisted of inexperienced anaesthesiology fellows (Tan 2015), paediatric subspecialty trainee anaesthesiologists with a minimum of two (Ueda 2013) or three years' training in anaesthesia (Ganesh 2009; Ishii 2013), a mix of consultant paediatric anaesthesiologists and trainees (Ganesh 2009) and cardiac anaesthesia fellows (Ueda 2013). Operators included individuals with minimal (Ganesh 2009; Ueda 2013; Tan 2015) to advanced experience in ultrasound‐guided and Doppler‐assisted techniques (Ishii 2013; Schwemmer 2006).
Excluded studies
We excluded no studies.
Ongoing studies
We identified one ongoing study (Siddik‐Sayyid 2014; see the Characteristics of ongoing studies table for details).
Awaiting classification
We included no studies awaiting classification.
Risk of bias in included studies
Figure 2 shows the risk of bias summary, which reflects judgements about each risk of bias item for each included study.
2.
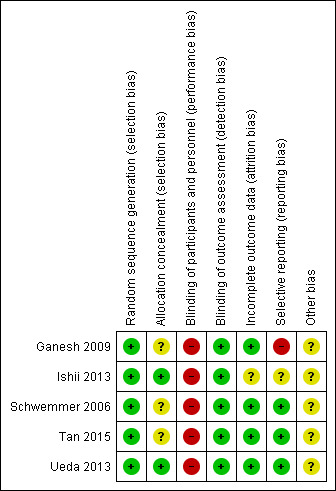
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The five included studies utilized different methods of random sequence generation.
Ganesh 2009 and Tan 2015 used computer‐generated random number sequence for assignment to one of two groups, whereas Ishii 2013 utilized the envelope method. Schwemmer 2006 tossed a coin and allocated "heads" for the ultrasound technique and "tails" for the palpation technique. Ueda 2013assigned participants by randomized block design to the Doppler‐assisted technique group or the ultrasound‐guided technique group. Ishii 2013 and Ueda 2013 ensured allocation concealment via the envelope method, whereby assignments were contained in prepared opaque envelopes that were opened just before cannulation. However, Ganesh 2009, Schwemmer 2006 and Tan 2015 did not mention the method of concealment.
Blinding
Risk of performance bias for participants in all five included studies was low because all participants underwent induction of general anaesthesia before catheter insertion. As for the operator, the risk of performance bias was high because the anaesthesiologist cannot be blinded during the intervention and is aware of what technique he or she will perform before performing arterial catheterization. Study authors classified the procedure as successful when the artery was cannulated and an arterial waveform was recorded, which is a non‐equivocal endpoint. Therefore, the risk for detection bias was low.
Ganesh 2009 considered aspiration of blood from the distal end of the arterial cannula as the endpoint, and both Ishii 2013 and Ueda 2013 classified the procedure as successful when the artery was cannulated and an arterial waveform was recorded. Tan 2015 classified the procedure as successful when the artery was cannulated. Schwemmer 2006 mentioned only that in the ultrasound technique, when the cannula appeared to be within the vessel, the transducer was removed and catheterization was considered successful, but investigators failed to mention what was considered an endpoint when the palpation technique was applied.
Incomplete outcome data
Risk of attrition bias was low in four studies (Ganesh 2009; Ishii 2013; Schwemmer 2006; Tan 2015) because outcome data were complete and no participants withdrew or were lost to follow‐up. However, two cases were withdrawn and were counted as failures according to the intention‐to‐treat analysis reported in Ueda 2013. The first of these occurred because an unintentional femoral arterial cannulation was performed on a participant who had been allocated to the ultrasound‐guided technique; in the second case, the participant dropped out because the operator who would have performed the procedure was unavailable once the participant had been randomized to the Doppler‐assisted group.
Selective reporting
Four of the included studies (Ganesh 2009; Ishii 2013; Schwemmer 2006; Ueda 2013) reported our primary outcome ‐ success rate at first attempt. Only two studies (Ishii 2013; Ueda 2013) reported the second primary outcome ‐ rate of complications, and this might indicate selective reporting bias. Although the methods of Ganesh 2009 included stratification according to age group (younger than two years, two to five years, older than five years), investigators did not report results according to this stratification.
Other potential sources of bias
Differences in the definitions of outcome measures among the included studies may be another source of bias. Ganesh 2009 did not define a specific duration, or number of attempts, before calling a method unsuccessful, whereas the other study authors did. It is also unclear how researchers calculated the number of attempts and if the reported number included cases in which the needle was redirected within the same skin puncture (Ganesh 2009; Ishii 2013; Schwemmer 2006; Tan 2015; Ueda 2013). Differences in the definitions of secondary outcomes made it impossible for review authors to include them in our analysis. In Ueda 2013, two potential sources of bias were present, in that haemodynamic manipulation of the size of a radial artery (by volume load or vasopressor effect) might improve the success rate of cannulation; and the trial was prematurely terminated after only 50% of the original sample size was enrolled.
Effects of interventions
See: Table 1
Primary outcomes
1. First attempt success rate
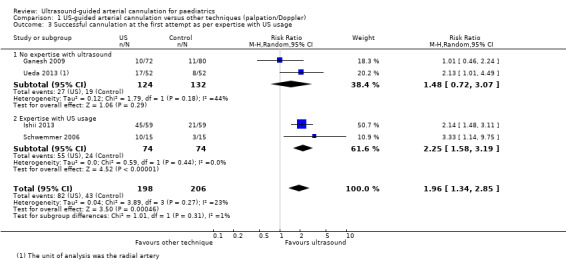
The main analysis revealed that ultrasound guidance significantly increased the success rate of cannulation at the first attempt in the paediatric population as compared with palpation or the Doppler technique (Ganesh 2009; Ishii 2013; Schwemmer 2006; Ueda 2013; four studies, 404 catheters, RR 1.96, 95% CI 1.34 to 2.85) (Figure 3). We judged the quality of evidence as moderate owing to imprecision and some level of risk of bias (Table 1).
3.

Subgroup analysis based on age
We conducted subgroup analysis based on age (Figure 4). Only Ganesh 2009 (152 catheters) reported data on older children. This study included children from a wide age group, but most were older children, with a mean age of 99 months. Investigators reported a success rate at first attempt of 14% for both palpation and ultrasound groups. Three studies reported data on infants and small children(Ishii 2013; Schwemmer 2006; Ueda 2013) showing that ultrasound guidance significantly increased successful radial artery cannulation at the first attempt (three studies, 252 catheters, RR 2.22, 95% CI 1.62 to 3.06). However, the test for subgroup effects revealed that age‐related subgroup differences were not statistically significant (P = 0.07).
4.
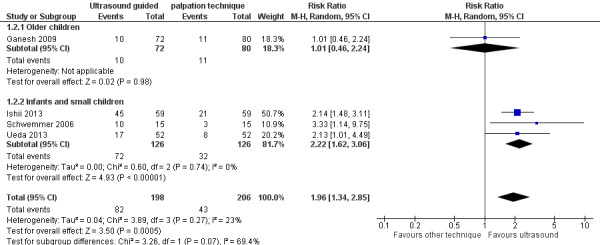
Subgroup analysis based on experience of anaesthesiologist performing arterial cannulation
Minimal experience
We also conducted subgroup analysis based on experience of the anaesthesiologist performing the arterial cannulation (Figure 5). In the subgroup of studies with minimal experience, anaesthesiologists had experience with fewer than 10 ultrasound‐guided arterial cannulations (Ganesh 2009) and fewer than five ultrasound‐guided arterial cannulations (Ueda 2013). We found that ultrasound guidance did not significantly increase the success of cannulation at the first attempt in the paediatric population as compared with palpation or the Doppler technique when the operator had minimal experience in performing ultrasound‐guided or Doppler‐assisted radial artery cannulation (two studies, 256 catheters, RR 1.48, 95% CI 0.72 to 3.07).
5.
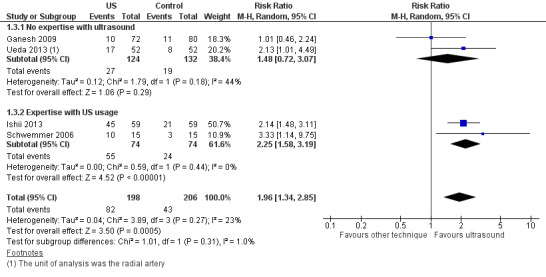
More experience
In the subgroup of studies in which anaesthesiologists had more experience, those performing arterial cannulation in Ishii 2013 were familiar with the ultrasound‐guided technique for central venous catheterization in adults and children, and anaesthesiologists in Schwemmer 2006 had experience that included more than 20 paediatric ultrasound‐guided arterial cannulation procedures. We found that ultrasound guidance significantly increased the success of cannulation at the first attempt in the paediatric population as compared with the palpation technique when an operator with experience in performing ultrasound‐guided radial artery cannulation completed the procedure (two studies, 148 catheters, RR 2.25, 95% CI 1.58 to 3.19). However, the test for subgroup effects showed no statistically significant differences related to experience with ultrasound procedures (P = 0.31).
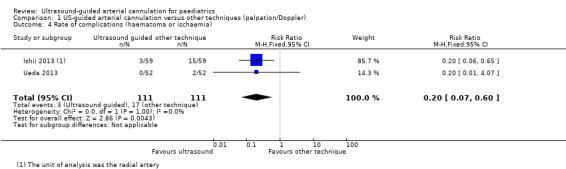
2. Rate of complications (haematoma or ischaemia)
Researchers found that ultrasound guidance significantly decreased the rate of complications such as haematoma during radial artery cannulation in the paediatric population as compared with palpation or the Doppler technique (two studies, 222 catheters, RR 0.20, 95% CI 0.07 to 0.60) (Figure 6). We judged the quality of evidence as moderate owing to imprecision and some level of risk of bias (Table 1).
6.

None of the included studies reported ischaemia as an outcome.
Secondary outcomes
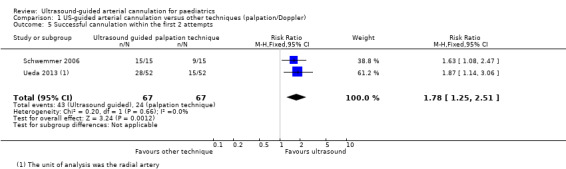
1. Successful cannulation within the first two attempts
Investigators in two studies (Schwemmer 2006; Ueda 2013) reported that ultrasound guidance significantly increased successful radial artery cannulation within the first two attempts in the paediatric population as compared with palpation or the Doppler technique (134 catheters, RR 1.78, 95% CI 1.25 to 2.51, P = 0.002). We judged the quality of evidence as moderate owing to imprecision and some level of risk of bias (Table 1).
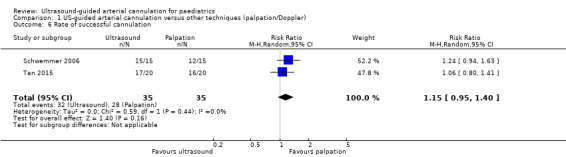
2. Rate of successful cannulation
Two studies (Schwemmer 2006; Tan 2015) reported that ultrasound guidance did not significantly increase the rate of successful cannulation in the paediatric population as compared with palpation (70 catheters, RR 1.15, 95% CI 0.95 to 1.40, P = 0.16).
3. Time to successful cannulation
Two studies (Schwemmer 2006; Tan 2015) reported time to successful cannulation. Schwemmer 2006 reported a significantly shorter time for the ultrasound group compared with the palpation group (64.5 ± 54.2 seconds vs 150.8 ± 130.2 seconds, respectively, P < 0.05, 30 catheters). Tan 2015 reported a mean of 7.8 minutes for the ultrasound group and 12.7 minutes for the palpation group and provided no standard deviations and no P values (40 catheters). Therefore, review authors could not perform a meta‐analysis.
4. Number of attempts to successful cannulation
Schwemmer 2006 reported that the mean number of attempts to successful cannulation was 1.3 ± 0.5 in the ultrasound group versus 2.3 ± 0.9 in the palpation group (30 catheters), whereas Ishii 2013 reported a median of 1 (1‐1) in the ultrasound group versus 2 (1‐2) in the palpation group (P = 0.001, 118 catheters).
5. Number of cannulas used
Two studies (Ganesh 2009; Tan 2015) reported the number of cannulas used for successful cannulation. Ganesh 2009 reported a median of one cannula in each group (152 catheters), and Tan 2015 used 52 cannulas for 20 catheterizations in the ultrasound group and 57 cannulas for 20 catheterizations in the palpation group. Review authors could not perform a meta‐analysis.
6. Need for assistance from another operator (primary operator fails when attempting to insert and asks for help)
The need for assistance from another operator, that is, when the primary operator fails when attempting to insert and asks for help from another operator, was reported at a rate of 30.6% in the ultrasound group versus 33.7% in the palpation group (Ganesh 2009) (P = 0.73, 152 catheters).
Review authors did not conduct a sensitivity analysis because we retrieved no unpublished studies, overall risk of bias for all included studies was low and studies did not report missing data.
Discussion
Summary of main results
Results of our review indicate that ultrasound usage for radial line cannulation significantly improves the first attempt success rate and the success rate after two attempts, as compared with traditional techniques (palpation or Doppler auditory assistance). Moreover, haematoma formation, which was the only reported complication, was significantly reduced in the ultrasound group compared with groups treated with traditional methods. The rate of successful cannulation was not improved by the use of ultrasound. However, this outcome is not clinically meaningful in that ultimately all practitioners will succeed in placing an arterial line after multiple attempts. What is more relevant than the success rate is the number of attempts needed to secure successful cannulation.
Our subgroup analysis per operator’s experience with ultrasound usage showed that the operator’s experience did not influence the first attempt success rate (P = 0.31). However, lack of proof of the usefulness of expertise in ultrasound usage might be related to lack of adequate power. Our subgroup analysis per age group included only one study in which participants were children with a high mean age and weight (Ganesh 2009) and three studies in which participants were infants and small children (Ishii 2013; Schwemmer 2006; Ueda 2013). However, the study with older children (Ganesh 2009) was the only one of the four studies included in the meta‐analysis that did not show a difference between ultrasound and palpation techniques. This observation supports the possibility of a subgroup effect.
Overall completeness and applicability of evidence
We carried out a thorough search of appropriate electronic databases. We also used citation tracking and searched clinical trials registers. We attempted to contact study authors for additional study details.
We did not restrict the systematic review to a particular arterial site. Yet eligible studies included cannulation of the radial artery site only. Therefore, the results of our review are directly applicable to cannulation of the radial artery in infants and children.
We did not limit our comparator to the palpation technique. However, most of the included studies compared ultrasound against palpation, and only one study compared ultrasound versus Doppler assistance. Given the small number of relevant studies, we could not explore a subgroup effect related to different comparators.
Quality of the evidence
Risk of bias in the included studies varied across assessed factors. Details of allocation concealment were not consistent across studies. In addition, as it is impossible to blind the anaesthesiologist or the intensivist to the method of arterial line insertion, all studies are at increased risk of performance bias. Another bias could have been introduced by the lack of a standardized definition of the primary outcome. It is not clear whether a “first pass successful arterial cannulation” includes or excludes redirection of the needle. Moreover, some studies (e.g. Ganesh 2009), included children with a broad age interval, and we were unable to obtain additional data from study authors.
We graded the evidence as moderate quality for all outcomes owing to imprecision, given the relatively wide confidence intervals, the small number of events and the small sample sizes for these outcomes (Guyatt 2011b), along with some level of risk of bias.
Potential biases in the review process
We identified one article written in Chinese by cross‐checking the reference lists of identified articles. However, we were unable to find or retrieve this article (Liu 2013). One meta‐analysis mentioned this article, and its results seem to be consistent with our findings. Study authors reported a higher first attempt success rate in the ultrasound group (25 out of 30) than in the palpation group (18 out of 30). Therefore, it is unlikely that its inclusion would have modified our results.
Agreements and disagreements with other studies or reviews
This is the first meta‐analysis comparing real‐time ultrasound use versus palpation or Doppler guidance for arterial cannulation exclusively in children. Our results are consistent with those of previous meta‐analyses confirming the superiority of ultrasound over palpation techniques for insertion of a radial artery in the adult population (Gao 2015; Gu 2014; Shiloh 2011; Tang 2014; White 2016). These meta‐analyses, which gathered data from both adult and paediatric populations, show an improved first attempt success rate with the use of ultrasound guidance as compared with palpation. .
Authors' conclusions
Implications for practice.
Anesthisiologists should consider using ultrasound for radial line cannulation, given that it likely improves first and second attempt success rates, while reducing complication rates. Ultrasound could provide greater value in the care of infants and small children, in whom arterial line cannulation is more challenging than in older children. However, evidence is lacking regarding the benefits of ultrasound for the cannulation of larger arteries, such as the femoral artery. In addition, researchers must provide practitioners with defined training on the use of ultrasound in performing cannulation and must define cost/benefit ratios for ultrasound use.
Implications for research.
Future studies must use a standardized definition of each outcome measure and must clearly state whether redirection of the needle within the same entry point is considered an additional attempt. Furthermore, future studies should be stratified by age to confirm differences related to infants and small children. These studies should include larger numbers of well‐defined age groups. Investigators must confirm the contribution of expertise in ultrasound usage to the success of arterial cannulation and must highlight the usefulness of ultrasound as a “rescue technique” following multiple attempts when palpation guidance fails. What is applicable for the radial artery, which is small, might not be applicable for the larger femoral artery. Therefore, future studies must examine cannulation of arteries at different sites. Moreover, ultrasound might be particularly useful in difficult clinical scenarios, such as the presence of hypotension, oedema or obesity, and in children with congenital cardiac disease who are subjected to multiple arterial cannulations.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We wish to thank Karen Hovannisyan (former TSC Cochrane Anaesthesia, Critical and Emergency Care Group) for helping with the search strategy.
We would like to thank Rodrigo Cavallazzi (Content Editor), Cathal Walsh (Statistical Editor) and Jin‐Tae Kim, Claude Abdallah and George Bikhazi (Peer Reviewers) for help and editorial advice provided during preparation of the protocol (Aouad‐Maroun 2014) for this systematic review.
We would like to thank Rodrigo Cavallazzi (Content Editor); Vibeke E Horstmann (Statistical Editor); Massimo Lamperti, Jin‐Tae Kim and Claude Abdallah (Peer Reviewers) and Odie Geiger (Consumer Referee) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL (The Cochrane Library) search strategy
#1 MeSH descriptor: [Catheterization] explode all trees #2 MeSH descriptor: [Arteries] explode all trees #3 (arter* near (cannula* or catheter*)) or (#1 and #2) #4 MeSH descriptor: [Ultrasonography] explode all trees #5 ultrasound* #6 #4 or #5 #7 pediatr* or child* or adolescent* or infant* #8 #3 and #6 and #7
Appendix 2. MEDLINE (Ovid SP) search strategy
1 ((exp Catheterization/ and exp Arteries/) or (arteria* adj3 (cannula* or catheter*)).mp.) and (exp Ultrasonography/ or ultrasound.ti,ab. or (ultrasound* adj3 (guid* or insert*)).mp.) and (pediatr* or child* or adolescent* or infant*).af. 2 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 3 1 and 2
Appendix 3. Embase (Ovid SP) search strategy
1. ((exp catheterization/ and exp artery/) or (arteria* adj3 (cannula* or catheter*)).mp.) and (exp echography/ or ultrasound.ti,ab. or (ultrasound* adj3 (guid* or insert*)).mp.) and (pediatr* or child* or adolescent* or infant*).af.
2. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh.
3. 1 and 2
Appendix 4. Data extraction form
CARG
Data collection form
Intervention review – RCTs only
Notes on using a data extraction form.
Be consistent in the order and style you use to describe the information for each report.
Record any missing information as unclear or not described, to make it clear that the information was not found in the study report(s) ‐ not that you forgot to extract it.
Include any instructions and decision rules on the data collection form, or in an accompanying document. It is important to practice using the form and to give training to any other review authors using the form.
| Review title or ID |
| Study ID(surname of first study author and year first full report of study was published, e.g. Smith 2001) |
| Report IDs of other reports in this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) |
| Name/ID of person extracting data |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
Report ID (ID for this paper/abstract/report) |
| Reference details |
| Report author contact details |
|
Publication type (e.g. full report, abstract, letter) |
|
Study funding sources (including role of funders) |
|
Possible conflicts of interest (for study authors) |
| Notes: |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial | |||||
| Controlled clinical trial (quasi‐randomized trial) |
||||||
| Participants | ||||||
| Types of interventions | ||||||
| Types of outcome measures | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, clusters/groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/obtained for study | Yes No Unclear | ||
| Notes: | |||
5. 'Risk of bias' assessment
See Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear risk | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All | ||||
| (if required) | Outcome group: | ||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All | ||||
| (if required) | Outcome group: | ||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total population at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Total no. undergoing ultrasound‐guided arterial cannulation | ||
| Total no. undergoing arterial cannulation by palpation technique | ||
| Total no. undergoing arterial cannulation with Doppler auditory assistance | ||
| Other methods used | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group.
Intervention group 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomized to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Operator’s experience (e.g. no., profession, training, ethnicity, etc., if relevant) |
||
| Co‐interventions | ||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||||||
| Reanalysis possible? | Yes No Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | Yes No Unclear |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
| Notes: | |
Data and analyses
Comparison 1. US‐guided arterial cannulation versus other techniques (palpation/Doppler).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successful cannulation at the first attempt | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.34, 2.85] |
| 2 Successful cannulation at the first attempt as per age group | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.34, 2.85] |
| 2.1 Older children | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.24] |
| 2.2 Infants and small children | 3 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.62, 3.06] |
| 3 Successful cannulation at the first attempt as per expertise with US usage | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.34, 2.85] |
| 3.1 No expertise with ultrasound | 2 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.72, 3.07] |
| 3.2 Expertise with US usage | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.58, 3.19] |
| 4 Rate of complications (haematoma or ischaemia) | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.07, 0.60] |
| 5 Successful cannulation within the first 2 attempts | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.25, 2.51] |
| 6 Rate of successful cannulation | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
1.1. Analysis.
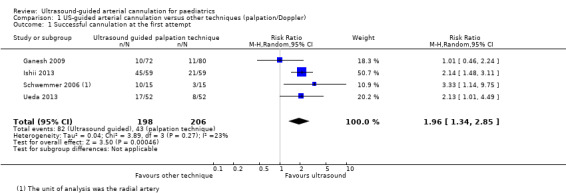
Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 1 Successful cannulation at the first attempt.
1.2. Analysis.

Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 2 Successful cannulation at the first attempt as per age group.
1.3. Analysis.
Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 3 Successful cannulation at the first attempt as per expertise with US usage.
1.4. Analysis.
Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 4 Rate of complications (haematoma or ischaemia).
1.5. Analysis.
Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 5 Successful cannulation within the first 2 attempts.
1.6. Analysis.
Comparison 1 US‐guided arterial cannulation versus other techniques (palpation/Doppler), Outcome 6 Rate of successful cannulation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ganesh 2009.
| Methods | Prospective randomized study | |
| Participants |
Number: 152 radial arteries Number per intervention
Inclusion criteria: Patients younger than 18 years of age scheduled for radial arterial catheterization were enrolled in this prospective, randomized, observational study. Exclusion criteria: not mentioned Surgery: not specified Baseline characteristics Ultrasound
Palpation
|
|
| Interventions |
Randomization: Participants were randomized to (1) US guidance technique (intervention) or (2) palpation (control) for radial artery cannulation. Intervention: The ultrasound‐guided technique was performed as follows: The HST/10‐5 25‐mm broadband linear array transducer (5–10 MHz) for the portable US device (SonoSite 180plus, SonoSite, Bothell, WA, USA) was placed in a sterile sheath and then was applied to the skin to localize the radial artery. After localization of the radial artery, the age appropriate‐sized catheter over a needle was inserted just distal to the transducer and was directed according to the US image. Control: The palpation‐guided technique was performed as follows: The position of the artery was identified by palpation, and the cannula was directed by continuous or intermittent palpation of arterial pulsation. Co‐intervention: All participants underwent induction of general anaesthesia via inhalation agents or intravenous induction. After induction of anaesthesia and endotracheal intubation, cannulation was performed according to the randomized method. Skin at the insertion site was cleaned and disinfected as per the standard protocol for arterial catheterization. The wrist was extended over a roll, and the hand and forearm were taped to maintain wrist extension. Skin puncture marked the start, and successful cannulation was the endpoint of the procedure. Failure of either technique and use of a cross‐over technique were determined by the consultant anaesthesiologist assigned to the case. The decision to use a new catheter or to request additional help was left to the consultant anaesthesiologist. Expertise of user: All catheterizations were performed by paediatric subspecialty trainee anaesthesiologists who had completed a minimum of 3 years' training in anaesthesia or by consultant paediatric anaesthesiologists. No operator had performed more than 10 US‐guided arterial cannulations before the time of the study. |
|
| Outcomes |
Primary endpoint:time to successful cannulation by the first operator at the first site of arterial puncture
Secondary endpoints
|
|
| Notes | Supported by departmental funds. Study authors disclosed no potential conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by a computer‐generated random number sequence to 1 of 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | No details were mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Participants: All participants underwent induction of general anaesthesia. Low risk of bias Personnel: The anaesthesiologist knows what technique he or she will use before performing arterial catheterization. High risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Aspiration of blood from the distal end of the inserted cannula is the endpoint of the procedure in both techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data were noted. |
| Selective reporting (reporting bias) | High risk | All planned outcomes were reported, but the results were not stratified according to the age groups to which participants were originally randomized (< 2 years, 2‐5 years, > 5 years). |
| Other bias | Unclear risk | No specific time or number of attempts was defined before a method was labelled unsuccessful. |
Ishii 2013.
| Methods | Prospective randomized study | |
| Participants |
Number: 118 radial arteries Number per intervention
Inclusion criteria: infants and small children weighing 3–20 kg Exclusion criteria: skin erosions or haematomas at or near the insertion site, visible recent catheterization scars, prominent differences in arterial pressure between left and right arms, as in the case of aortic coarctation Surgery type: elective cardiac surgery for congenital heart disease Baseline characteristics: In each participant, right and left radial arteries were randomly assigned to cannulation by the ultrasound‐guided technique vs the usual palpation technique via the envelope method
|
|
| Interventions |
Randomization: For each participant, right and left radial arteries were randomly assigned to cannulation by the ultrasound‐guided technique (ultrasound group) vs the usual palpation technique (palpation group) via the envelope method. The ultrasound‐guided group included 28 right and 31 left radial arteries, whereas the palpation‐guided group included 31 right and 28 left radial arteries. Intervention: The arterial puncture was guided by a SonoSite 180 ultrasound imaging device (SonoSite, Bothell, WA, USA) with a 2‐ to 7‐MHz linear array transducer in real time.The artery was imaged in its short axis. Control: The operator used the pulsation of the radial artery as a guide for cannulation. Co‐intervention: Patients’ demographic and medical information was recorded. The electrocardiogram, pulse oximetry and blood pressure were monitored non‐invasively. Blood pressure was measured from the arm just before the catheterization attempt. After induction of general anaesthesia, cannulation was attempted with standard 24‐G JELCO cannulas (Smith's Medical, Dublin, OH, USA). A pillow was placed under the wrist to keep the arm slightly extended. The insertion site was disinfected and no local anaesthetic was used. Expertise of the US user: Arterial catheterization procedures were performed by trainees in anaesthesiology who had completed 3 or more years of clinical training and were familiar with the ultrasound‐guided technique for central venous catheterization in adults and children. |
|
| Outcomes |
Primary study endpoints
Secondary study endpoints
|
|
| Notes | No information was provided regarding funding. Dr Sawa received royalties from The Reagents from the University of California. The remaining study authors have disclosed that they have no potential conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the right and left radial arteries were randomly assigned to cannulation by the ultrasound‐guided technique (ultrasound group) versus the usual palpation technique (palpation group), using the envelope method" |
| Allocation concealment (selection bias) | Low risk | "the right and left radial arteries were randomly assigned to cannulation by the ultrasound‐guided technique (ultrasound group) versus the usual palpation technique (palpation group), using the envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Partcipants: All participants underwent induction of general anaesthesia before arterial line cannulation. Low risk of bias Personnel: The anaesthesiologist knows what technique he or she will use before performing arterial catheterization. High risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The procedure was classified as successful when the artery was cannulated and an arterial waveform was recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study author did not mention participant withdrawal. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported. |
| Other bias | Unclear risk | ‐ |
Schwemmer 2006.
| Methods | Prospective randomized study | |
| Participants |
Number: 30 radial arteries Number per intervention
Inclusion criteria: small children Exclusion criteria: not mentioned Surgery: major neurosurgery Baseline characteristics
|
|
| Interventions |
Randomization: The technique to be used for radial artery puncture and insertion of the catheter was selected by tossing a coin: heads for ultrasound guidance and tails for palpation. Co‐Intervention: A peripheral intravascular line was inserted the evening before, and crystalloids were given during the night, to keep a normovolaemic status. For all participants, a linear transducer connected to an ultrasound system with small parts imaging capability (Sonos 5000; Hewlett‐Packard, Andover, MA, USA) was used to identify the radial artery. The probe generates ultrasound waves with a frequency of 15 MHz and has a focal length positioned 1.8 cm from the cap. The image is displayed on a 12‐inch monitor with 30.5‐cm diagonal dimension. The cross‐sectional area of the artery was measured at the head of the radius with and without dorsiflexion of the hand by about 45 degrees. A trackball system was used to trace the vessel wall, and the area was obtained from automated planimetry of the ultrasound system. The skin at the insertion site was cleaned and disinfected. The transducer or the physician’s fingertip was applied to the skin, and the radial artery was identified as the pulsating vessel. Following further local disinfection, the vessel was approached with standard 24‐G cannulas (Becton Dickinson, Helsinborg, Sweden) via 1 of the 2 techniques. Intervention: The radial artery was first localized by ultrasound in its short cross‐section. The cannula was advanced to perforate the skin slightly distally to the transducer. It was then directed toward the vessel at an angle of approximately 45 degrees. Further advancement was guided by minimal ultrasound scanning of the artery and its close vicinity. When the cannula appeared to be within the vessel, the transducer was removed and catheterization was accomplished. Control
Expertise of the US user: " staff personnel with experience of more than 20 paediatric arterial catheterizations" |
|
| Outcomes |
|
|
| Notes | No information was provided regarding funding, and no conflicts of interest were declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The technique to be used for radial artery puncture and insertion of the catheter was selected by tossing a coin: heads for ultrasound guidance and tails for palpation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Partcipants: All participants underwent induction of general anaesthesia. Low risk of bias Personnel: The anaesthesiologist knows what technique he or she will use before performing arterial catheterization. High risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "When the cannula appeared to be within the vessel, the transducer was removed and catheterization was accomplished" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the 30 children were withdrawn. |
| Selective reporting (reporting bias) | Low risk | All outcomes were addressed. |
| Other bias | Unclear risk | ‐ |
Tan 2015.
| Methods | Prospective randomized study | |
| Participants |
Number: 40 radial arteries Number per intervention:
Inclusion criteria: infants younger than 24 months of age undergoing elective surgical procedure where indwelling arterial catheterization was indicated Exclusion criteria: refusal of consent from parents or attending anaesthesiologist, anticipated circulatory instability after anaesthesia induction as in those with pulmonary hypertension (defined as estimated pulmonary arterial pressure ≥ 66% of systemic blood pressure) or severe heart failure Surgery: not specified Baseline characteristics Ultrasound
Palpation
|
|
| Interventions | Patients were randomized to (1) US guidance technique (intervention) or (2) palpation (control) for radial artery cannulation. Intervention: The ultrasound‐guided technique was performed with a SonoSite M‐Turbo (SonoSite, Bothell, WA, USA) SLAX “hockey stick” probe. Control: The position of the artery was identified by palpation. Co‐Intervention: After induction of anaesthesia, cannulation was performed according to the randomized method. After failure of either technique, cross‐over was allowed after 3 attempts. Expertise of user: All catheterizations were performed by anaesthesiology fellows. To facilitate learning of US‐assisted arterial cannulation, each fellow underwent practice with customized age‐specific forearm and femoral phantoms until self reported comfort with the technique before actual participant recruitment was commenced |
|
| Outcomes |
Primary endpoint: time to successful cannulation within 3 attempts
Secondary endpoints
|
|
| Notes | Supported by departmental funds. Study authors have disclosed no potential conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by a computer‐generated random number sequence to 1 of 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | No details were mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Participants: All participants underwent induction of general anaesthesia. Low risk of bias Personnel: The anaesthesiologist knows what technique he or she will use before performing arterial catheterization. High risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Successful arterial cannulation is the endpoint of the procedure for both techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data were noted. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported. |
| Other bias | Unclear risk | ‐ |
Ueda 2013.
| Methods | Prospective randomized study | |
| Participants |
Number: 104 radial arteries Number per intervention
Inclusion criteria: children weighing ≥ 3 and ≤ 12 kg Exclusion criteria: signs of skin infection or a wound near the puncture site, abnormal circulation of the hand, recent arterial puncture 1 month earlier, patients requiring emergency surgery Surgery Ultrasound
Doppler
Baseline characteristics Ultrasound group
Doppler group
|
|
| Interventions |
Randomization: Participants were assigned by randomized block design to the Doppler‐assisted technique group or the US‐guided technique group. Assignments were contained in prepared opaque envelopes that were opened just before cannulation. To ensure balance between operators for each study procedure, each operator was randomly assigned procedures in blocks of 4. Each block had a random arrangement of 2 US‐guided and 2 Doppler‐guided techniques. Once an operator participated, he or she was required to complete 2 to 3 blocks (i.e. each operator performed the Doppler‐assisted technique 4 or 6 times and the US‐guided technique 4 or 6 times). Intervention: After anaesthetic induction, the participant's hand was secured on an armboard in a neutral position without a wrist roll. US (HD 11 XE; Andover, MA, USA) via a linear transducer (L15‐7io) was utilized to measure the diameter of the radial artery. The side of the artery to be used was determined by operator discretion or surgical indication. The frequency of the transducer was set to 15 MHz with depth of 1.0 cm. The diameter of the radial artery was measured 3 times and was averaged at the level of the radial head without dorsiflexion of the wrist. The field was then prepped and draped. After the transducer was prepared with a sterile cover, the radial artery was identified by US (HD 11 XE) with a linear transducer (L15‐7io) in the short‐axis view. Approximately 0.5 cm distal to the probe, a small cut in the epidermis and dermis was made at the insertion site with an 18 G needle. A 24 G catheter (Jelco, Smith Medical International Ltd, Rossendale, UK) was advanced at a 15 to 30 degree angle until the tip of the needle was seen on the image. Fine adjustments were made with the needle until the tip was seen in contact with the anterior wall of the artery. The needle was then advanced until the artery collapsed and re‐expanded, or until blood appeared in the hub. The metal stylet was removed, and if the flash of blood continued, a wire was inserted through the catheter and was advanced into the artery via the Seldinger technique. If no flash of blood was seen after the stylet was removed, the cannula was withdrawn until blood flow was observed. The catheter was then replaced with a 22 G catheter (Cook Medical Inc., Bloomington, IN, USA) over a guidewire. Control
Expertise of the US user: The operator was a clinical anaesthesia year 2 or 3 (CA‐2, CA‐3) resident or a cardiac anaesthesia fellow. None of the participants had previously performed US‐guided or Doppler‐assisted radial artery cannulation in paediatric patients more than 5 times. Before starting the study, each operator received a formal demonstration on the use of each technique on a simulated paediatric radial artery. |
|
| Outcomes |
|
|
| Notes | Supported by departmental funds. Study authors have disclosed no potential conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by randomized block design to the Doppler‐assisted technique group or the US‐guided technique group. To ensure balance between operators for each study procedure, each operator was randomly assigned procedures in blocks of 4. Each block had a random arrangement of 2 US‐guided and 2 Doppler‐guided techniques. Once an operator participated, he or she was required to complete 2 to 3 blocks. |
| Allocation concealment (selection bias) | Low risk | Assignments were contained in prepared opaque envelopes that were opened just before cannulation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Partcipants: All participants underwent induction of general anaesthesia before the time of arterial line cannulation. Low risk of bias Personnel: The anaesthesiologist knows what technique he or she will use before performing arterial catheterization. High risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An arterial waveform is seen on the monitor after the catheter is connected to a transducer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 cases were withdrawn. Two cases were counted as failures according to the intention‐to‐treat principle: (1) an unintentional femoral arterial cannulation while the faculty was trying the femoral venous cannulation before the radial arterial cannulation was attempted, which was allocated to the US‐guided technique, and (2) unavailability of the operator to perform the procedure once the participant had been randomized to the Doppler‐assisted group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were addressed. |
| Other bias | Unclear risk |
|
CA: clinical anaesthesia.
cm: centimetre.
G: gauge.
kg: kilogram.
MHz: megahertz.
mm: millimetre.
US: ultrasound.
Characteristics of ongoing studies [ordered by study ID]
Siddik‐Sayyid 2014.
| Trial name or title | Ultrasound‐Guided vs Landmark Technique for Femoral Arterial Cannulation in Pediatric Cardiac Surgery |
| Methods | Randomized study |
| Participants | Paediatric cardiac patients, neonates up to 12 years of age |
| Interventions | Femoral arterial line cannulation via ultrasound vs palpation |
| Outcomes | First attempt success rate, time to successful cannulation |
| Starting date | July 2014 |
| Contact information | Sahar Siddik‐Sayyid, MD; ss01@aub.edu.lb |
| Notes | ‐ |
Differences between protocol and review
Changes between the published protocol (Aouad‐Maroun 2014) and the review are as follows.
In the section “Unit of analysis”, we changed the unit of analysis from the “individual patient” to “the catheterization of the radial artery" because in one of the studies (Ishii 2013), each participant underwent catheterization of two radial arteries; thus the unit was not the participant per se but the radial artery catheterization.
In the section "Assessment of heterogeneity", we added the following sentence: "In a post hoc decision, we decided to conduct subgroup analyses that we judged clinically relevant even in the absence of statistical heterogeneity" because we believed that despite the lack of heterogeneity, it was clinically relevant and important to the reader to conduct subgroup analysis per age group and per expertise in ultrasound usage.
In the section "Summary of findings", we did not include rate of successful cannulation, time to successful cannulation, number of attempts to successful cannulation, number of cannulas used and need for assistance from another operator (primary operator fails when attempting to insert and asks for help) in the 'Summary of findings' table because they were not relevant.
Conversely, we found the following outcome to be relevant to our study: successful cannulation after two attempts. Hence, we added it to our secondary outcomes.
Contributions of authors
Marie Aouad‐Maroun (MAM), Christian K Raphael (CR), Samia K Sayyid (SS), Fadi Farah (FF), Elie A Akl (EA).
Conceiving the review: FF, MAM, EA.
Co‐ordinating the review: CR, SS.
Undertaking manual searches: FF, CR, SS.
Screening search results: FF, CR, SS.
Organizing retrieval of papers: CR, SS.
Screening retrieved papers against inclusion criteria: CR, SS, MAM.
Appraising quality of papers: CR, SS.
Abstracting data from papers: CR, SS.
Writing to authors of papers for additional information: MAM.
Providing additional data about papers: MAM.
Obtaining and screening data on unpublished studies: CR, SS.
Managing data for the review: CR, SS, EA.
Entering data into Review Manager (RevMan 2014): MAM.
Analysing RevMan statistical data: EA.
Performing other statistical analysis not using RevMan: EA.
Interpreting data: EA, MAM.
Making statistical inferences: EA.
Writing the review: MAM, CR, SS.
Securing funding for the review: no funds for the review.
Performing previous work that was the foundation of the present study: MAM.
Serving as guarantor for the review (one review author): MAM.
Taking responsibility for reading and checking the review before submission: EA, MAM.
Sources of support
Internal sources
No sources of support, Other.
External sources
No sources of support supplied
Declarations of interest
Marie Aouad‐Maroun: no conflict of interest.
Christian K. Raphael: no conflict of interest.
Samia K. Sayyid: no conflict of interest.
Fadi Farah: no conflict of interest.
Elie A Akl: no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Ganesh 2009 {published data only}
- Ganesh A, Kaye R, Cahill AM, Stern W, Pachikara R, Gallagher P, et al. Evaluation of ultrasound‐guided radial artery cannulation in children. Pediatric Critical Care Medicine 2009;10(1):45‐8. [PUBMED: 19057451] [DOI] [PubMed] [Google Scholar]
Ishii 2013 {published data only}
- Ishii S, Shime N, Shibasaki M, Sawa T. Ultrasound‐guided radial artery catheterization in infants and small children. Pediatric Critical Care Medicine 2013;14(5):471‐3. [PUBMED: 23628835] [DOI] [PubMed] [Google Scholar]
Schwemmer 2006 {published data only}
- Schwemmer U, Arzet HA, Trautner H, Rauch S, Roewer N, Greim CA. Ultrasound‐guided arterial cannulation in infants improves success rate. European Journal of Anaesthesiology 2006;23(6):476‐80. [PUBMED: 16512974] [DOI] [PubMed] [Google Scholar]
Tan 2015 {published data only}
- Tan TYS, Petersen JAK, Zhao X, Taylor L. Randomized controlled trial of ultrasound versus palpation method for arterial cannulation in infants less than 24 months of age. Symbiosis Open Access Journals Anesthesiology and Pain Management 2015;2(2):1‐3. [Google Scholar]
Ueda 2013 {published data only}
- Ueda K, Puangsuvan S, Hove MA, Bayman EO. Ultrasound visual image‐guided vs Doppler auditory‐assisted radial artery cannulation in infants and small children by non‐expert anaesthesiologists: a randomized prospective study. British Journal of Anaesthesia 2013;110(2):281‐6. [PUBMED: 23151422] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Siddik‐Sayyid 2014 {unpublished data only}
- Siddik S. Ultrasound‐guided vs landmark technique for femoral arterial cannulation in pediatric cardiac surgery. Clinicaltrials.gov. [NCT02183857]
Additional references
Akl 2013
- Akl EA, Johnston BC, Alonso‐Coello P, Neumann I, Ebrahim, Briel M, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One 2013;8(2):1‐7. [PUBMED: 23451162] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASA 2012
- American Society of Anesthesiology. Practice guidelines for central venous access. A report by the American Society of Anesthesiologists Task Force on central venous access. Anesthesiology 2012;116(3):539‐73. [PUBMED: 22307320] [DOI] [PubMed] [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [PUBMED: 23774111] [DOI] [PubMed] [Google Scholar]
Gao 2015
- Gao Y, Yan J, Gao F, Pan L, Wang X, Lv C. Effects of ultrasound‐guided radial artery catheterization: an updated meta‐analysis. American Journal of Emergency Medicine 2015;33:50‐5. [PUBMED: 25453476] [DOI] [PubMed] [Google Scholar]
Gu 2014
- Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound‐guided radial artery catheterization: a systematic review and meta‐analysis of randomized controlled trials. Critical Care 2014;18(3):R93. [PUBMED: 24887241] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614 ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hind 2003
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, et al. Ultrasonic locating devices for central venous cannulation: meta‐analysis. BMJ 2003;327(7411):361. [PUBMED: 12919984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [PUBMED: 23359047] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2008
- King MA, Garrison MM, Vavilala MS, Zimmerman JJ, Rivara FP. Complications associated with arterial catheterization in children. Pediatric Critical Care Medicine 2008;9(4):367‐71. [PUBMED: 18496411] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365; PUBMED: 20156912] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [PUBMED: 19621070] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2013
- Liu GL, Zheng TH, Lv H. Value of ultrasound‐guided radial artery catheterization in infant patients. Chinese Journal of Anesthesiology 2013;33(10):1272‐3. [Google Scholar]
Milling 2005
- Milling TJ, Rose J, Briggs WM, Birkhahn R, Gaeta TJ, Bove JJ, et al. Randomized, controlled clinical trial of point‐of‐care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP‐3) Trial. Critical Care Medicine 2005;33(8):1764‐9. [PUBMED: 16096454] [DOI] [PubMed] [Google Scholar]
NICE 2002
- National Institute for Clinical Excellence. Guidance on the use of ultrasound locating devices for placing central venous catheters. www.nice.org.uk/TA49 (accessed 19 March 2014).
Randolph 1996
- Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature. Critical Care Medicine 1996;24(12):2053‐8. [8968276] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scheer 2002
- Scheer BV, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Critical Care 2002;6(3):198‐204. [PUBMED: 12133178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schindler 2005
- Schindler E, Kowald B, Swess H, Niehaus‐Borquez B, Tausch B, Brecher A. Catheterization of the radial or brachial artery in neonates and infants. Paediatric Anaesthesia 2005;15(8):677‐82. [PUBMED: 16029403] [DOI] [PubMed] [Google Scholar]
Shiloh 2011
- Shiloh Al, Savel RH, Paulin LM, Eisen LA. Ultrasound‐guided catheterization of the radial artery: a systematic review and meta‐analysis of randomized controlled trials. Chest 2011;139(3):524‐9. [PUBMED: 20724734] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomized controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Tang 2014
- Tang L, Wang F, Li X, Zhao L, Xi H, Guo Z, et al. Ultrasound guidance for radial artery catheterization: an updated meta‐analysis of randomized controlled trials. PLoS ONE 2014;9(11):e111527. [PUBMED: 25375152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varga 2013
- Varga EQ, Candiotti KA, Saltzman B, Gayer S, Giquel J, Castillo‐Pedraza C, et al. Evaluation of distal radial artery cross‐sectional internal diameter in pediatric patients using ultrasound. Paediatric Anaesthesia 2013;23(5):460‐2. [PUBMED: 23577822] [DOI] [PubMed] [Google Scholar]
White 2016
- White L, Halpin A, Turner M, Wallace L. Ultrasound‐guided radial artery cannulation in adult and paediatric populations: a systematic review and meta‐analysis. British Journal of Anaesthesia 2016;116(5):610‐7. [PUBMED: 27106964 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Aouad‐Maroun 2014
- Aouad‐Maroun M, Farah F, Akl EA, Raphael CK, Sayyid SK. Ultrasound‐guided arterial cannulation for paediatric patients. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011364] [DOI] [PMC free article] [PubMed] [Google Scholar]


