Abstract
Background
Ovulation induction with follicle stimulating hormone (FSH) is a second‐line treatment in women with polycystic ovary syndrome (PCOS) who do not ovulate or conceive on clomiphene citrate.
Objectives
To compare the effectiveness and safety of gonadotrophins as a second‐line treatment for ovulation induction in women with clomiphene citrate‐resistant polycystic ovary syndrome (PCOS), and women who do not ovulate or conceive after clomiphene citrate.
Search methods
In January 2018, we searched the Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, the World Health Organisation clinical trials register, Clinicaltrials.gov, LILACs, and PubMed databases, and Google Scholar. We checked references of in all obtained studies. We had no language restrictions.
Selection criteria
All randomised controlled trials reporting data on clinical outcomes in women with PCOS who did not ovulate or conceive on clomiphene citrate, and undergoing ovulation induction with urinary‐derived gonadotrophins, including urofollitropin (uFSH) in purified FSH (FSH‐P) or highly purified FSH (FSH‐HP) form, human menopausal gonadotropin (HMG) and highly purified human menopausal gonadotrophin (HP‐HMG), or recombinant FSH (rFSH), or continuing clomiphene citrate. We included trials reporting on ovulation induction followed by intercourse or intrauterine insemination. We excluded studies that described co‐treatment with clomiphene citrate, metformin, luteinizing hormone, or letrozole.
Data collection and analysis
Three review authors (NW, EK, and MvW) independently selected studies for inclusion, assessed risk of bias, and extracted study data. Primary outcomes were live birth rate per woman and multiple pregnancy per woman. Secondary outcomes were clinical pregnancy, miscarriage, incidence of ovarian hyperstimulation syndrome (OHSS) per woman, total gonadotrophin dose, and total duration of stimulation per woman. We combined data using a fixed‐effect model to calculate the risk ratio (RR). We summarised the overall quality of evidence for the main outcomes using GRADE criteria.
Main results
The review included 15 trials with 2387 women. Ten trials compared rFSH with urinary‐derived gonadotrophins (three compared rFSH with human menopausal gonadotrophin, and seven compared rFSH with FSH‐HP), four trials compared FSH‐P with HMG. We found no trials that compared FSH‐HP with FSH‐P. One trial compared FSH with continued clomiphene citrate.
Recombinant FSH (rFSH) versus urinary‐derived gonadotrophins
There may be little or no difference in the birth rate between rFSH and urinary‐derived gonadotrophins (RR 1.21, 95% confidence interval (CI) 0.83 to 1.78; five trials, N = 505; I² = 9%; low‐quality evidence). This suggests that for the observed average live birth per woman who used urinary‐derived FSH of 16%, the chance of live birth with rFSH is between 13% and 28%. There may also be little or no difference between groups in incidence of multiple pregnancy (RR 0.86, 95% CI 0.46 to 1.61; eight trials, N = 1368; I² = 0%; low‐quality evidence), clinical pregnancy rate (RR 1.05, 95% CI 0.88 to 1.27; eight trials, N = 1330; I² = 0; low‐quality evidence), or miscarriage rate (RR 1.20, 95% CI 0.71 to 2.04; seven trials, N = 970; I² = 0; low‐quality evidence). We are uncertain whether rFSH reduces the incidence of OHSS (RR 1.48, 95% CI 0.82 to 2.65, ten trials, n=1565, I² = 0%, very low‐quality evidence).
Human menopausal gonadotrophin (HMG) or HP‐HMG versus uFSH
When compared to uFSH, we are uncertain whether HMG or HP‐HMG improves live birth rate (RR 1.28, 95% CI 0.65 to 2.52; three trials, N = 138; I² = 0%; very low quality evidence), or reduces multiple pregnancy rate (RR 2.13, 95% CI 0.51 to 8.91; four trials, N = 161; I² = 0%; very low quality evidence). We are also uncertain whether HMG or HP‐HMG improves clinical pregnancy rate (RR 1.31, 95% CI 0.66 to 2.59; three trials, N = 102; I² = 0; very low quality evidence), reduces miscarriage rate (RR 0.33, 95% CI 0.06 to 1.97; two trials, N = 98; I² = 0%; very low quality evidence), or reduces the incidence of OHSS (RR 7.07, 95% CI 0.42 to 117.81; two trials, N = 53; very low quality evidence) when compared to uFSH.
Gonadotrophins versus continued clomiphene citrate
Gonadotrophins resulted in more live births than continued clomiphene citrate (RR 1.24, 95% CI 1.05 to 1.46; one trial, N = 661; I² = 0%; moderate‐quality evidence). This suggests that for a woman with a live birth rate of 41% with continued clomiphene citrate, the live birth rate with FSH was between 43% and 60%. There is probably little or no difference in the incidence of multiple pregnancy between treatments (RR 0.89, 95% CI 0.33 to 2.44; one trial, N = 661; I² = 0%; moderate‐quality evidence). Gonadotrophins resulted in more clinical pregnancies than continued clomiphene citrate (RR 1.31, 95% CI 1.13 to 1.52; one trial, N = 661; I² = 0%; moderate‐quality evidence), and more miscarriages (RR 2.23, 95% CI 1.11 to 4.47; one trial, N = 661; I² = 0%; moderate‐quality evidence). None of the women developed OHSS.
Authors' conclusions
There may be little or no difference in live birth, incidence of multiple pregnancy, clinical pregnancy rate, or miscarriage rate between urinary‐derived gonadotrophins and recombinant follicle stimulating hormone in women with polycystic ovary syndrome. For human menopausal gonadotropin or highly purified human menopausal gonadotrophin versus urinary follicle stimulating hormone we are uncertain whether one or the other improves or lowers live birth, incidence of multiple pregnancy, clinical pregnancy rate, or miscarriage rate. We are uncertain whether any of the interventions reduce the incidence of ovarian hyperstimulation syndrome. We suggest weighing costs and convenience in the decision to use one or the other gonadotrophin. In women with clomiphene citrate failure, gonadotrophins resulted in more live births than continued clomiphene citrate without increasing multiple pregnancies.
Plain language summary
Gonadotrophins to induce ovulation in women with polycystic ovary syndrome (PCOS)
Review question
To compare the effectiveness and safety of gonadotrophins, hormones that regulate the reproductive system, as a second‐line treatment to stimulate ovulation in women with PCOS who do not ovulate or conceive on clomiphene citrate.
Background
Infertility due to ovulation disorders is the most common reason for women to seek counselling or treatment. These women are treated by stimulating ovulation with medication, so‐called 'ovulation induction'. This is usually done with tablets containing clomiphene citrate, as the first line of treatment. If women do not react to this medication, the most common second‐line treatment in these women is ovulation induction with gonadotrophins, which are injectable drugs. Various types of gonadotrophin have been developed: urinary‐derived products, available in purified (FSH‐P), and highly purified (FSH‐HP) form, and human menopausal gonadotrophin, also available in highly purified form (HP‐HMG). Finally, recombinant FSH (rFSH) was developed artificially to obtain even higher purity.
Women who do react, but do not conceive within six ovulatory clomiphene citrate cycles, may continue with clomiphene citrate or switch to gonadotrophins.
Study characteristics
The review includes 15 trials, covering 2387 women. Ten trials compared urinary‐derived gonadotrophins with rFSH. Of these, three trials compared rFSH with human menopausal gonadotrophin, and seven trials compared rFSH with FSH‐HP. Four trials compared FSH‐P with human menopausal gonadotrophin. One trial compared gonadotrophins with continued clomiphene citrate. We found no trials that compared rFSH with FSH‐P, or FSH‐HP with FSH‐P. The evidence is current to January 2018.
Key results
There may be little or no difference in live birth, multiple pregnancy, clinical pregnancy, or miscarriage rate between urinary‐derived gonadotrophins and recombinant FSH. We are uncertain whether human menopausal gonadotrophin or urinary follicle stimulating hormone improves pregnancy outcomes in women with PCOS. We are uncertain whether the interventions decrease the incidence of ovarian hyperstimulation syndrome.
When compared to continued treatment with clomiphene citrate, gonadotrophins resulted in more live births without increasing the rate of multiple pregnancies. Gonadotrophins resulted in more clinical pregnancies, but also in more miscarriages than clomiphene citrate, while there were no cases of ovarian hyperstimulation syndrome.
Quality of the evidence
The quality of the evidence was low to very low for outcomes from rFSH versus urinary gonadotrophins, and human menopausal gonadotrophin versus FSH‐P. The quality of the evidence was moderate for outcomes from gonadotrophins versus continued clomiphene citrate.
Ten of the fifteen studies included in this review reported a commercial sponsor.
Summary of findings
Summary of findings for the main comparison. Recombinant follicle stimulating hormone versus urinary‐derived gonadotrophins for ovulation induction in women with polycystic ovarian syndrome.
| Recombinant follicle stimulating hormone versus urinary‐derived gonadotrophins for ovulation induction in women with polycystic ovarian syndrome | |||||
|
Patient or population: women with polycystic ovarian syndrome (PCOS) undergoing ovulation induction
Settings: women visiting the outpatient clinic
Intervention: recombinant follicle stimulating hormone (rFSH) Comparison: urinary‐derived gonadotrophins | |||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Risk with urinary‐derived gonadotrophins | Risk with rFSH | ||||
| Live birth rate per woman | 157 per 1000 | 190 per 1000 (130 to 279) | RR 1.21 (0.83 to 1.78) | 505 (5 studies) | ⊕⊕⊝⊝ LOW a,b |
| Incidence of multiple pregnancy (per woman) | 30 per 1000 | 25 per 1000 (14 to 48) | RR 0.86 (0.46 to 1.61) | 1368 (8 studies) | ⊕⊕⊝⊝ LOW a,b |
| Clinical pregnancy rate per woman | 239 per 1000 | 251 per 1000 (210 to 303) | RR 1.05 (0.88 to 1.27) | 1330 (8 studies) | ⊕⊕⊝⊝ LOW a,b |
| Miscarriage rate per woman | 47 per 1000 | 56 per 1000 (33 to 95) | RR 1.20 (0.71 to 2.04) | 970 (7 studies) | ⊕⊕⊝⊝ LOW a,b |
| Incidence of OHSS per woman | 22 per 1000 | 33 per 1000 (12 to 96) | RR 1.48 (0.82 to 2.65) | 1565 (10 studies) | ⊕⊝⊝⊝ VERY LOW a,b,c |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OHSS: ovarian hyperstimulation syndrome | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded one level for imprecision around the absolute effect bDowngraded one level for inconsistency in results across studies cDowngraded one level for inconsistent definition or for the lack of definition of OHSS
Summary of findings 2. Human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus urinary follicle stimulating hormone for ovulation induction in women with polycystic ovarian syndrome.
| Human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus urinary follicle stimulating hormone for ovulation induction in women with polycystic ovarian syndrome | |||||
|
Patient or population: women with polycystic ovarian syndrome (PCOS) undergoing ovulation induction
Settings: women visiting the outpatient clinic
Intervention: Human menopausal gonadotrophin (HMG) or highly purified HMG Comparison: urinary follicle stimulating hormone (uFSH) | |||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) | |
| Risk with uFSH | Risk with HMG or HP‐HMG | ||||
| Live birth rate per woman | 179 per 1000 | 230 per 1000 (117 to 452) | RR 1.28 (0.65 to 2.52) |
138 (3 studies) | ⊕⊝⊝⊝ VERY LOW a,b |
| Incidence of multiple pregnancy (per woman) | 23 per 1000 | 48 per 1000 (12 to 203) | RR 2.13 (0.51 to 8.91) | 161 (4 studies) | ⊕⊝⊝⊝ VERY LOW a,b |
| Clinical pregnancy rate per woman | 203 per 1000 | 266 per 1000 (134 to 527) | RR 1.31 (0.66 to 2.59) | 102 (3 studies) | ⊕⊝⊝⊝ VERY LOW a,b |
| Miscarriage rate per woman | 82 per 1000 | 27 per 1000 (5 to 161) | RR 0.33 (0.06 to 1.97) | 98 (2 studies) | ⊕⊝⊝⊝ VERY LOW a,b |
| Incidence of OHSS per woman | No events c | 4/28 c | RR 7.07 (0.42 to 117.81) | 53 (2 studies) | ⊕⊝⊝⊝ VERY LOW a,b,d |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OHSS: ovarian hyperstimulation syndrome | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngarded two levels for serious imprecision around the absolute effect (wide CI and small sample size) bDowngraded one level for inconsistency in results across studies cEvent rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group
d Downgraded one level for inconsistent definition or for the lack of definition of OHSS; two of four studies did not report this outcome
Summary of findings 3. Gonadotrophins compared to continued clomiphene citrate for ovulation induction.
| Gonadotrophins compared to continued clomiphene citrate for ovulation induction | ||||||
| Patient or population: anovulatory women with clomiphene citrate‐failure Setting: women visiting the outpatient clinic Intervention: gonadotrophins Comparison: continued clomiphene citrate (CC) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with continued CC | Risk with gonadotrophins | |||||
| Live birth rate per woman | 413 per 1000 | 512 per 1000 (434 to 603) | RR 1.24 (1.05 to 1.46) | 661 (1 study) | ⊕⊕⊕⊝ MODERATE a | |
| Incidence of multiple pregnancy per woman | 24 per 1000 | 21 per 1000 (8 to 57) | RR 0.89 (0.33 to 2.40) | 661 (1 study) | ⊕⊕⊕⊝ MODERATE a | |
| Clinical pregnancy rate per woman | 446 per 1000 | 584 per 1000 (504 to 678) | RR 1.31 (1.13 to 1.52) | 661 (1 study) | ⊕⊕⊕⊝ MODERATE a | |
| Miscarriages per woman | 33 per 1000 | 73 per 1000 (37 to 147) | RR 2.23 (1.11 to 4.47) | 661 (1 study) | ⊕⊕⊝⊝ LOW a,b,c | There may be little or no difference when expressed per clinical pregnancy |
| Incidence of OHSS per woman | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 661 (1 study) | ⊕⊕⊝⊝ LOW a,b | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OHSS: ovarian hyperstimulation syndrome | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for risk of bias – no blinding performed
b Downgraded one level for imprecision in result
c Downgraded one level for inconsistency in outcome, i.e. there were more clinical pregnancies in the gonadotrophin group; there may be little or no difference when expressing miscarriage per clinical pregnancy
Background
Description of the condition
Subfertility occurs in one in 10 couples world‐wide. In about one‐third of couples, this is based on polycystic ovary syndrome (PCOS). PCOS is characterised by oligo‐anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries (Rotterdam consensus group 2004a; Rotterdam consensus group 2004b). The syndrome affects approximately 6% to 10% of women of childbearing age.
Infertility due to chronic anovulation is the most common reason for women with PCOS to seek counselling or treatment. First line treatment for these women is ovulation induction with clomiphene citrate, with or without metformin. A recent review showed that letrozole is an effective alternative to clomiphene citrate (Franik 2018).
About 20% of women do not ovulate on clomiphene citrate, and require alternative or second‐line ovulation induction strategies. This failure to ovulate with clomiphene citrate is termed ‘clomiphene resistance’. The most common treatment in women with clomiphene citrate‐resistant PCOS is ovulation induction with gonadotrophins (Balen 2013), or laparoscopic electrocautery of the ovaries as an effective alternative treatment (Farquhar 2012).
Of the women ovulating on clomiphene citrate, only half of these women conceive within six months of treatment leads. If women fail to conceive with clomiphene citrate, despite regular ovulatory cycles, the term ‘clomiphene‐failure’ is used. Also in these women, clomiphene citrate or letrozole treatment is often changed to second‐line ovulation induction with gonadotrophins.
Description of the intervention
The strategy of stimulating follicle development and growth with exogenous gonadotrophins for ovulation induction in women with clomiphene citrate‐resistant PCOS or clomiphene citrate‐failure is well established.
Follicle‐stimulating hormone (FSH) is found in the pituitary gland, and circulates in the bloodstream in various molecular forms. This molecular heterogeneity is due to the variation in the structures of the carbohydrate moieties, in particular of sialic acid. It is the configuration of these carbohydrate moieties that determines the FSH isoform. The configuration depends on which glycosylation enzymes are available in the cell during synthesis (Wide 1997). Each molecular glycoform has a different molecular weight, net charge, circulating half‐life, and metabolic clearance (Baenziger 1988; Gray 1988; Stockell Hartree 1992; Wilson 1990). Gonadotrophins were originally extracted from pituitary glands (Gemzell 1958), and later from the urine of postmenopausal women (Lunenfeld 1960).
Over the last five decades, various urinary‐derived FSH products, or urofollitropins, have been developed. Menotropin (human menopausal gonadotrophin) has been available since the early 1960s and contains FSH, luteinising hormone (LH) and large quantities of potentially allergenic urinary proteins. Purified urofollitropin has been available since the mid‐1980s. Purified FSH is devoid of LH, but still contains urinary proteins. Highly purified urofollitropin has been available since the mid‐1990s and contains very small amounts of urinary proteins. The absence of urinary proteins reduces rare adverse reactions, such as local allergy or hypersensitivity (Albano 1996; Biffoni 1994). The most recent development in urinary gonadotrophins is highly purified menotropin (highly purified human menopausal gonadotrophin), containing equal amounts of FSH and LH activity.
To obtain even higher purity, gonadotrophins were developed with recombinant DNA technology (recombinant FSH) in 1988 (Howles 1996; Keene 1989). The production of recombinant FSH is independent of urine collection, thus guaranteeing a high availability of a biochemical pure FSH preparation that is free from LH and urinary protein contaminants. The production process also yields FSH with high specific bioactivity (roughly 100 times higher than for urine‐derived FSH products), minimal batch‐to‐batch discrepancies (Bergh 1999), and low immunogenicity. There is evidence that recombinant FSH has a higher bioactivity than urinary products (Andersen 2004).
At present, two preparations of recombinant FSH are available: follitropin alpha and follitropin beta. Both preparations are similar to pituitary and urinary FSH, although they show minor differences in the structure of the carbohydrate side chains, and contain more basic and fewer acidic isohormones than the urinary‐derived gonadotrophin preparations (De Leeuw 1996; Hard 1990; Lambert 1995).
Continued clomiphene citrate is taken for five days at the dose on which the woman ovulates. This is usually 50 mg, 100 mg, or 150 mg.
How the intervention might work
In the follicular phase of an ovulatory menstrual cycle, between 10 and 20 antral follicles develop. Of this cohort, one follicle will obtain dominance over the others, and will continue to grow until ovulation. In women with PCOS, this dominance does not occur. The aim of ovulation induction is to induce growth of preferably one follicle, and not more than three follicles. This can be accomplished by ovulation induction with gonadotrophins. Too forceful a regimen will result in overstimulation. and hence, in an increased risk of multiple pregnancy and ovarian hyperstimulation syndrome (OHSS); a stimulation regimen with too low a dosage of gonadotrophins will not result in a dominant follicle, and thereby, will not lead to ovulation.
Why it is important to do this review
Gonadotrophins are the standard drugs in medical ovulation induction for women with PCOS, who did not ovulate or conceive on clomiphene citrate. In women who do ovulate on clomiphene citrate, continued clomiphene citrate for another six cycles is an option. Knowlegde on effectiveness and safety of these treatment options will enable informed treatment decisions. The present review is an update and extension of two previous Cochrane reviews (Bayram 2001; Nugent 2000). Bayram 2001 compared rFSH with purified FSH and highly purified FSH; Nugent 2000 compared human menopausal gonadotrophin with purified FSH. No Cochrane review has yet compared human menopausal gonadotrophin with recombinant FSH in clomiphene citrate‐resistant women. Summarising the evidence on the effectiveness and safety of the various gonadotrophins will help gynaecologists and women to make informed decisions on the best regimen for ovulation induction.
Objectives
To compare the effectiveness and safety of gonadotrophins as a second‐line treatment for ovulation induction in women with clomiphene citrate‐resistant polycystic ovary syndrome (PCOS), and women who do not ovulate or conceive after clomiphene citrate.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. We excluded quasi‐randomised controlled trials in which allocation was, for example, by alternation, reference to case record numbers, or to dates of birth. We also excluded cross‐over trials, which are not appropriate in this context (Vail 2003).
Types of participants
Subfertile women with clomiphene citrate‐resistant PCOS undergoing ovulation induction. We defined clomiphene citrate‐resistance as a failure to ovulate with clomiphene citrate doses of at least 100 mg/day for at least five days.
Subfertile women with PCOS and clomiphene citrate‐failure undergoing ovulation induction. We defined clomiphene citrate‐failure as a failure to conceive after three cycles of ovulation induction with clomiphene citrate.
Women with prior treatment with metformin with or without clomiphene citrate.
Women with prior treatment with electrocautery of the ovaries.
Types of interventions
Ovulation induction with recombinant follicle‐stimulating hormone (FSH) versus any other urinary gonadotrophin (human menopausal gonadotrophin, purified FSH, highly purified FSH)
Ovulation induction with highly purified FSH versus purified FSH
Ovulation induction with human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus purified FSH or highly purified FSH
Ovulation induction with gonadotrophins or continued clomiphene citrate
For all interventions, ovulation induction could be followed by intercourse or intrauterine insemination. We excluded trials involving co‐treatment with clomiphene citrate, metformin, luteinising hormone, letrozole or different gonadotrophins.
Types of outcome measures
Primary outcomes
1. Live birth rate per woman
2. Multiple pregnancy per woman
Secondary outcomes
3. Clinical pregnancy rate (per woman)
4. Miscarriage rate (per woman) or miscarriages per woman
5. Incidence of ovarian hyperstimulation syndrome (OHSS; (per woman))
6. Total gonadotrophin dose per woman (IU)
7. Total duration of stimulation per woman
Search methods for identification of studies
This review has drawn on the search strategy developed for the Cochrane Gynaecology and Fertility group (CGF) as a whole.
Electronic searches
Marian Showell (CGF Group Information Specialist) developed the search strategies. See Appendix 1, Appendix 2Appendix 3, Appendix 4, Appendix 5, Appendix 6.
1) We searched the following electronic sources:
Cochrane Gynaecology and Fertility Group specialised Register of Controlled Trials (searched 15 January 2018; Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL Register of Studies Online (CRSO; searched 15 January 2018; Appendix 2))
MEDLINE (1946 to 15 January 2018; Appendix 3)
Embase (1980 to 15 January 2018; Appendix 4)
PsycINFO (1806 to 15 January 2018; Appendix 5)
CINAHL (1961 to 15 January 2018; Appendix 6)
2) Other electronic sources included:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 15 January 2018)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/; searched 15 January 2018)
LILACS (Latin American and Caribbean Health Science Information database) and other Spanish and Portuguese language databases (pesquisa.bvsalud.org/portal/; 1982 to 15 January 2018)
OpenGrey for unpublished literature from Europe (www.opengrey.eu/; searched 15 January 2018)
Searching other resources
We searched the following conference abstracts:
American Society for Reproductive Medicine and Canadian Fertility and Andrology Society (ASRM/CFAS) Conjoint Annual Meeting (2001 to 2018), Abstracts of the Scientific Oral and Poster Sessions, Program Supplement;
European Society of Human Reproduction and Embryology (ESHRE) Annual Meeting (2001 to 2018), Abstracts of the Scientific Oral and Poster Sessions, Program Supplement.
We handsearched the references cited in all obtained studies. We searched PubMed and Google for any recent trials that had not yet been indexed in MEDLINE.
We asked Serono Benelux BV (Merck Group), Ferring, and IBSA, the manufacturers of gonadotrophins, for ongoing studies and unpublished data.
Data collection and analysis
Selection of studies
Three review authors (NW, EK, and MvW) independently examined the electronic search results for reports of possibly relevant trials, and retrieved these reports in full. All review authors independently applied the selection criteria to the trial reports, rechecking trial eligibility and resolving disagreements by discussion with the other authors.
Data extraction and management
Three review authors (NW, EK, and MvW) independently extracted the outcome data and information on funding, location, clinical and design details, and participants. We resolved any differences by discussion. We entered details of the studies into the 'Characteristics of included studies' table. We presented studies that appeared to meet the inclusion criteria but were excluded from the review in the 'Characteristics of excluded studies' table, briefly stating the reason for exclusion, but giving no further information.
Assessment of risk of bias in included studies
Three review authors (NW, EK, and MvW) extracted information regarding the risk of bias (threats to internal validity) under six domains (also see the Cochrane 'Risk of bias' assessment tool in Appendix 7; (Higgins 2011)). We resolved any differences by discussion.
1. Sequence generation. Evidence that an unpredictable random process was used.
2. Allocation concealment. Evidence that the allocation list was not available to anyone involved in the recruitment process.
3. Blinding of participants, clinicians, and outcome assessors. Evidence that knowledge of allocation was not available to those involved in subsequent treatment decisions or follow‐up efforts.
4. Completeness of outcome data. Evidence that any losses to follow‐up were low and comparable between groups.
5. Selective outcome reporting. Evidence that major outcomes had been reported in sufficient detail to allow analysis, independently of their apparent statistical significance.
6. Other potential sources. Evidence of miscellaneous errors or circumstances that might influence the internal validity of trial results.
We sought missing details from the authors of the original publications. We present all details in the 'Risk of bias' table following each included study.
Measures of treatment effect
We summarised all binary outcomes using relative risk ratio (RR) with a 95% confidence interval (CI). In cases of no events, we also calculated a risk difference (RD) with a 95% CI.
We treated ordinal scales, such as amount of gonadotrophin used and duration of ovarian stimulation, as continuous outcomes. We abstracted, calculated, or requested means and standard deviations and calculated the mean difference with 95% CI for these outcomes.
Unit of analysis issues
We expressed all outcomes per woman randomised, and multiple pregnancy per clinical pregnancy.
Dealing with missing data
Where there was insufficient information in the published report, we attempted to contact the authors for clarification. If missing data became available, we included them in the analysis. We anticipated that trials conducted over 10 years ago might not have data on live birth rates. We analysed data extracted from the trials on an intention‐to‐treat basis. Where randomised participants were missing from outcome assessment, we contacted the authors for additional data. If further data were not available, we assumed that missing participants had failed to achieve pregnancy and had not suffered any of the reported adverse events.
Assessment of heterogeneity
The presence of statistical heterogeneity of treatment effect among trials was determined using the I² statistic (Higgins 2003). We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I² statistic. We took an I² measurement greater than 50% to indicate substantial heterogeneity, in which case, we tested the effect of using a random‐effects model (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies, and by being alert for duplication of data. If we had included 10 or more studies in an analysis, we had planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
When multiple studies were available on a similar comparison, we used Review Manager 5.3 software to perform the meta‐analyses, using the Mantel‐Haenszel method with a fixed‐effect model (Review Manager 2014). For reporting purposes, we translated primary outcomes to absolute risks. We combined results for continuous outcomes using the mean difference.
Subgroup analysis and investigation of heterogeneity
If excessive heterogeneity existed within strata, we had planned to explore this informally using the clinical and design details recorded in the 'Characteristics of included studies' table.
Prospectively, we had planned to undertake three different stratifications of the primary outcomes: type of urinary gonadotrophin (human menopausal gonadotrophin, purified FSH and highly purified FSH); single or multiple cycles; sponsorship (commercial, non‐commercial (Lexchin 2003)).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made about study eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
we had used a random‐effects model
we had reported odds ratios rather than relative risk ratios
Overall quality of the body of evidence: 'Summary of findings' table
We generated 'Summary of findings' tables using GRADEpro software and Cochrane methods (GRADEpro GDT 2015; Higgins 2011). These tables present the overall quality of the body of evidence for main review outcomes (live birth, multiple pregnancy, clinical pregnancy, miscarriage, and OHSS) for the main review comparison (recombinant FSH versus urinary‐derived gonadotrophins) using GRADE criteria: study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias. We also presented tables for our other comparisons: human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus urinary FSH, and gonadotrophins versus continued clomiphene citrate. We justified judgements about evidence quality (high, moderate or low), documented them, and incorporated them into the reporting of results for each outcome.
Results
Description of studies
For details of the studies please see: Characteristics of included studies; Characteristics of excluded studies
Results of the search
For this update, we screened 588 titles and identified an additional five studies for eligibility assessment. From these five studies, we included one trial, we excluded two studies, and we listed two studies as studies awaiting classification.
See Figure 1.
1.

Study flow diagram
Included studies
We included 15 trials in this update.
1. Ten studies compared the effects of recombinant follicle‐stimulating hormone (rFSH) versus urinary derived gonadotrophins (human menopausal gonadotrophin: Balen 2007; Platteau 2006; Revelli 2006; urinary follicle‐stimulating hormone (uFSH): Coelingh Bennink 1998; Feigenbaum 2001; Gerli 2004; Loumaye 1996; Szilágyi 2004; Taketani 2010; Yarali 1999). Loumaye 1996 was described in a review on human gonadotrophins produced by recombinant DNA technology. The authors of the 2001 Cochrane Review collected the data for this trial by personal communication, and we used them again in this update (Bayram 2001).
2. There were no studies that compared highly purified FSH with purified FSH.
3. Four studies compared purified FSH with human menopausal gonadotrophin (Gadir 1990: McFaul 1990; Sagle 1991; Seibel 1985). Gadir 1990 made an extra comparison with laparoscopic electrocautery of the ovaries.
4. One study compared gonadotrophins and continued clomiphene citrate during six cycles (Weiss 2018).
One trial also included normo‐ovulatory women with unexplained subfertility (Revelli 2006). For this review, we used only the data of women with polycystic ovary syndrome (PCOS). For Seibel 1985, we included pre‐cross‐over data.
Eight trials reported data on live birth, and thirteen trials reported on multiple pregnancy (Balen 2007; Coelingh Bennink 1998; Feigenbaum 2001; Gadir 1990; Gerli 2004; McFaul 1990; Platteau 2006; Revelli 2006; Sagle 1991; Seibel 1985; Taketani 2010; Weiss 2018; Yarali 1999).
All studies that compared types of gonadotrophins included women who were clomiphene citrate‐resistant; seven of them also included women with clomiphene citrate‐failure (Balen 2007; Coelingh Bennink 1998; Gerli 2004; Platteau 2006; Seibel 1985; Yarali 1999). The study that compared gonadotrophins with continuous included only women with clomiphene citrate‐failure (Weiss 2018). None of the women included in this review had been treated with electrocautery in the past. Ten trials analysed more than one cycle per woman, whereas five trials only analysed one cycle per woman (Balen 2007; Feigenbaum 2001; Platteau 2006; Revelli 2006; Taketani 2010). In four trials, intrauterine insemination was performed in some cases (Balen 2007; Gerli 2004; Platteau 2006; Weiss 2018). All trials used a low‐dose step‐up protocol, but the protocol used in Loumaye 1996 was unknown. Ten trials reported a commercial sponsor (Balen 2007; Coelingh Bennink 1998; Feigenbaum 2001; Loumaye 1996; Platteau 2006; Sagle 1991; Seibel 1985; Szilágyi 2004; Taketani 2010; Yarali 1999).
Six trials reported a power calculation (Balen 2007; Coelingh Bennink 1998; Loumaye 1996; Platteau 2006; Revelli 2006; Weiss 2018).
Excluded studies
We excluded six trials: one trial because the outcome measure was the effect of FSH on haemostasis (Ricci 2004); two studies because the outcome 'pregnancy' was not defined, and this outcome was only presented per cycle (Homburg 1990; Jacobs 1987); one study because it was a cross‐over design, and it was not possible to extract the pre‐cross‐over data per woman (Larsen 1990); one study had the wrong intervention (cotreatment with clomiphene citrate (Rashidi 2016)); and one study reported a wrong comparator (Zhou 2016).
Risk of bias in included studies
We summarised the risks of bias in the included studies in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
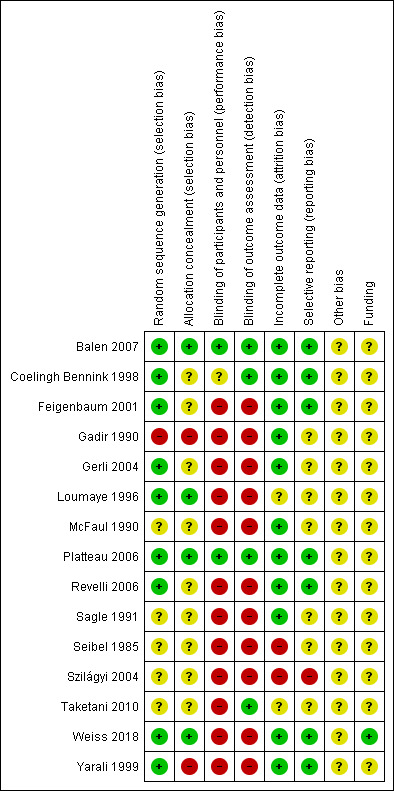
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Allocation to the intervention or control group was adequately concealed in four trials (Balen 2007; Loumaye 1996; Platteau 2006; Weiss 2018). The allocation concealment was inadequate in two trials (Gadir 1990; Gerli 2004), and unclear in the remaining trials.
Blinding
Four trials were assessor‐blinded (Balen 2007; Coelingh Bennink 1998; Platteau 2006; Taketani 2010). Blinding was not performed in the remaining studies.
Incomplete outcome data
Two trials had a high risk of attrition bias (Seibel 1985; Szilágyi 2004). For another two trials, this was unclear (Loumaye 1996; Taketani 2010). All other trials had a low risk of bias for this domain.
Selective reporting
We rated six studies as having a low risk of selective reporting bias; eight as having an unclear risk of bias in this domain, and one study as having high risk (Szilágyi 2004).
Other potential sources of bias
We rated this as unclear for all studies. Some studies provided too few details to make a judgement. Within all the trials, the baseline characteristics appeared balanced over the two treatment groups. Only six of the 15 trials mentioned the duration of the trial (Balen 2007; Coelingh Bennink 1998; Loumaye 1996; Platteau 2006; Taketani 2010; Weiss 2018).
Effects of interventions
See: Table 1; Table 2; Table 3
1 Recombinant follicle‐stimulating hormone (rFSH) versus urinary‐derived gonadotrophins
1.1 Live birth rate per woman
4.

Forest plot of comparison 1. Recombinant FSH (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), outcome: 1.1 Live birth rate per woman by urinary gonadotrophins
1.1. Analysis.

Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 1 Live birth rate per woman by urinary gonadotrophins.
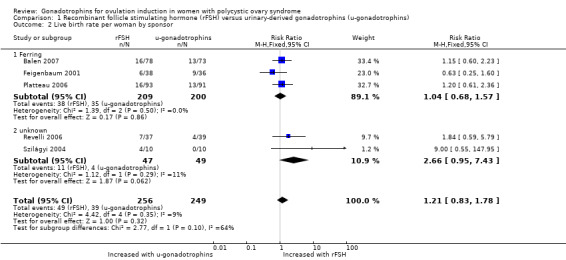
Five trials, including 505 women, reported on live birth (Balen 2007; Feigenbaum 2001; Platteau 2006; Revelli 2006; Szilágyi 2004). After pooling the results, the overall risk ratio (RR) per woman was 1.21 (95% confidence interval (CI) 0.83 to 1.78; five RCTs, N = 505; I² = 9%, low‐quality evidence) indicating there may be little or no difference between the intervention and the comparison. Translated into absolute risks, this means that for a woman with a 16% chance of achieving a live birth with the use of urinary‐derived FSH, the chance of a live birth with the use of rFSH would be between 13% and 28%. Statistical heterogeneity for this outcome was low. The live birth rate varied from 16% to 40% in the rFSH group, and from 0% to 25% in the urinary gonadotrophin group.
When we divided the urinary‐derived gonadotrophins into subgroups (three trials compared rFSH versus highly purified human menopausal gonadotrophin, two trials compared rFSH versus highly purified FSH), we found that there may be little or no difference between the subgroups (P = 0.10). The RR for rFSH versus highly purified human menopausal gonadotrophin or human menopausal gonadotrophin was 1.04 (95% CI 0.68 to 1.57; three RCTs, N = 409; I² = 0%; low‐quality evidence), and for rFSH versus highly purified FSH was 2.66 (95% CI 0.95 to 7.43; two RCTs, N = 96; I² = 11%; low‐quality evidence).
1.2 Live birth rate per woman ‐ stratified by sponsor
All trials comparing rFSH and highly purified human menopausal gonadotrophin were sponsored by Ferring; the other two trials comparing rFSH and purified FSH did not report the sponsor. Therefore, subgrouped results per sponsor were similar to the gonadotrophin comparison, i.e. when we divided into subgroups, we found little or no difference between subgroups (P = 0.1; Analysis 1.2).
1.2. Analysis.
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 2 Live birth rate per woman by sponsor.
1.3 Incidence of multiple pregnancy per woman
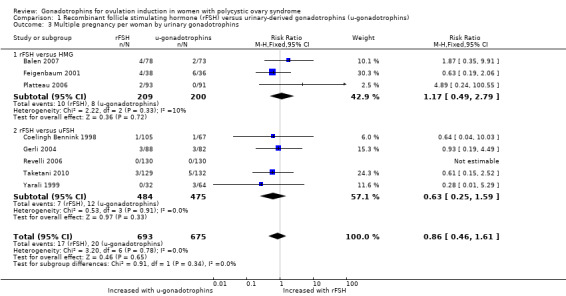
Eight studies, including 1368 women, reported on multiple pregnancy (Balen 2007; Coelingh Bennink 1998; Feigenbaum 2001; Gerli 2004; Platteau 2006; Revelli 2006; Taketani 2010; Yarali 1999). There may be little or no difference in multiple pregnancy per woman between groups (RR 0.86, 95% CI 0.46 to 1.61; eight RCTs, N = 1368; I² = 0%; low‐quality evidence; Analysis 1.3).
1.3. Analysis.
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 3 Multiple pregnancy per woman by urinary gonadotrophins.
When we divided the urinary‐derived gonadotrophins into subgroups (three trials compared rFSH versus highly purified human menopausal gonadotrophin, five trials compared rFSH versus highly purified FSH), there was no evidence of a difference between the subgroups (P = 0.34).
1.4 Incidence of multiple pregnancy per woman ‐ stratified per sponsor
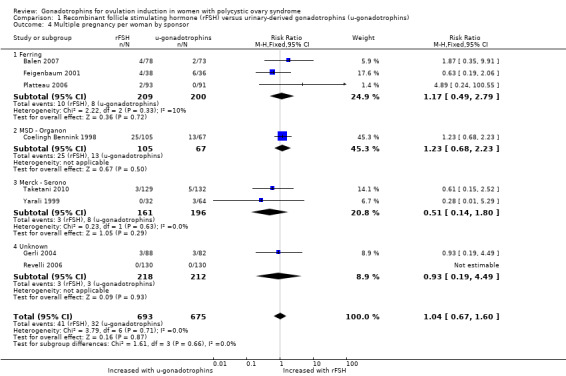
When we subgrouped by sponsor, we found little or no difference between subgroups (P = 0.86; Analysis 1.4).
1.4. Analysis.
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 4 Multiple pregnancy per woman by sponsor.
1.5 Clinical pregnancy rate per woman
Eight studies, including 1330 women, reported on clinical pregnancy (Balen 2007; Coelingh Bennink 1998; Feigenbaum 2001; Gerli 2004; Loumaye 1996; Platteau 2006; Taketani 2010; Yarali 1999). There may be little or no difference in clinical pregnancy (RR 1.05, 95% CI 0.88 to 1.27; eight RCTs, N = 1330; I² = 0%; low‐quality evidence; Analysis 1.5).
1.5. Analysis.
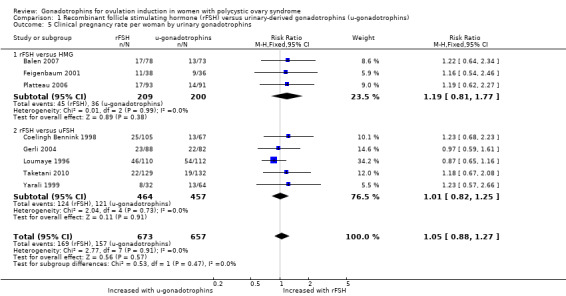
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 5 Clinical pregnancy rate per woman by urinary gonadotrophins.
When we divided the urinary‐derived gonadotrophins into subgroups (three trials compared rFSH versus highly purified human menopausal gonadotrophin, five trials compared rFSH versus highly purified FSH), there was no evidence of a difference between the subgroups (P = 0.47). The RR for rFSH versus HP‐human menopausal gonadotrophin was 1.19 (95% CI 0.81 to 1.77, three RCTs, N = 409; I² = 0%; low‐quality evidence), and for rFSH versus highly purified FSH was 1.01 (95% CI 0.82 to 1.25; five RCTs, N = 921; I² = 0%; low‐quality evidence).
1.6 Incidence of multiple pregnancy per clinical pregnancy
We found that there may be little or no difference in multiple pregnancy per clinical pregnancy (RR 0.75, 95% CI 0.43 to 1.32; eight RCTs, 315 pregnancies; I² = 0%; Analysis 1.6).
1.6. Analysis.
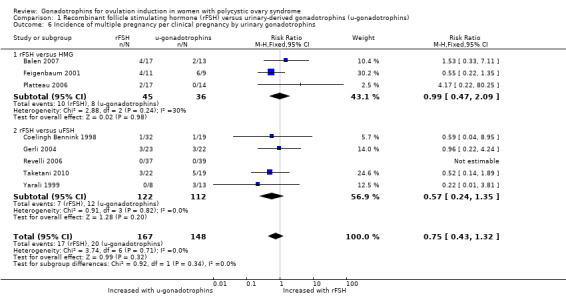
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 6 Incidence of multiple pregnancy per clinical pregnancy by urinary gonadotrophins.
1.7 Miscarriage rate per woman
Seven studies, including 970 women, reported on miscarriage (Balen 2007; Coelingh Bennink 1998; Gerli 2004; Loumaye 1996; Platteau 2006; Szilágyi 2004; Yarali 1999). There may be little or no difference in miscarriage rate (RR 1.20, 95% CI 0.71 to 2.04; seven RCTs, N = 970; I² = 0%; low‐quality evidence; Analysis 1.7).
1.7. Analysis.
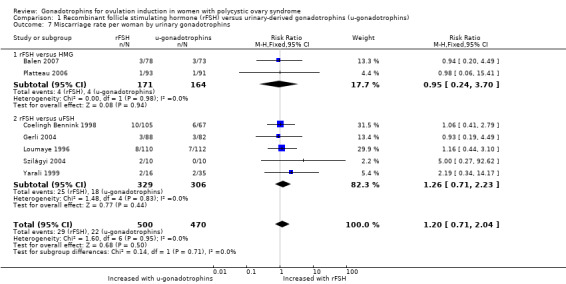
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 7 Miscarriage rate per woman by urinary gonadotrophins.
When we divided the urinary‐derived gonadotrophins into subgroups (two trials compared rFSH versus highly purified human menopausal gonadotrophin, five trials compared rFSH versus highly purified FSH), we found no evidence of a difference between the subgroups (P = 0.71).
1.8 Incidence of ovarian hyperstimulation syndrome (OHSS) per woman
5.

Forest plot of comparison 1. Recombinant FSH (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), outcome: 1.8 Incidence of OHSS per woman by urinary gonadotrophins
1.8. Analysis.

Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 8 Incidence of OHSS per woman by urinary gonadotrophins.
Ten studies, including 1565 women, reported OHSS (Balen 2007; Coelingh Bennink 1998; Feigenbaum 2001; Gerli 2004; Loumaye 1996; Platteau 2006; Revelli 2006; Szilágyi 2004; Taketani 2010; Yarali 1999). After pooling the results, the overall RR for OHSS per woman was 1.48 (95% CI 0.82 to 2.65; 10 RCTs, N = 1565; I² = 0%; very low‐quality evidence), indicating we could not be certain whether rFSH reduced the incidence of OHSS (Analysis 1.8). This means that for a woman with a 2.2% chance of OHSS with the use of urinary‐derived gonadotrophins, the chance of OHSS with the use of rFSH would be between 1.2% and 9.6%. The OHSS rate varied from 0% to 20% in both groups.
When we divided the urinary‐derived gonadotrophins into subgroups (three trials compared rFSH versus highly purified human menopausal gonadotrophin, seven trials compared rFSH versus highly purified FSH), we found no evidence of a difference between the subgroups (P = 0.53). The RR for rFSH versus highly purified human menopausal gonadotrophin was 1.11 (95% CI 0.39 to 3.20; three RCTs, N = 409; I² = 0%; very low‐quality evidence), and for rFSH versus highly purified FSH was 1.67 (95% CI 0.82 to 3.39; seven RCTs, N = 1156; I² = 0%; very low‐quality evidence).
1.9 Mean total gonadotrophin dose per woman
We found that rFSH required a lower dose than urinary‐derived gonadotrophins to stimulate ovulation (MD ‐105.44 IU, 95% CI ‐154.21 to ‐56.68; six RCTs, N = 1046; I² = 81%). When we used a random‐effects model, in view of the high statistical heterogeneity, we found there may be little or no difference (MD ‐127.4 IU, 95% CI ‐258.06 to 3.26; Analysis 1.9).
1.9. Analysis.
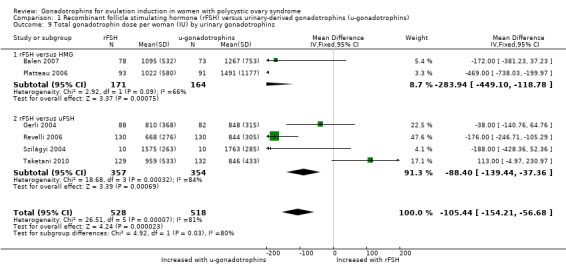
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 9 Total gonadotrophin dose per woman (IU) by urinary gonadotrophins.
1.10 Total duration of stimulation per woman (days)
We found that rFSH required a shorter time to stimulate ovulation than urinary‐derived gonadotrophins (MD ‐0.66 days, 95% CI ‐1.04 to ‐0.28; six RCTs, N = 1122; I² = 72%). When we used a random‐effects model, in view of the high statistical heterogeneity, we found there may be little or no difference (MD ‐0.80 days, 95% CI ‐1.66 to 0.05; Analysis 1.10).
1.10. Analysis.
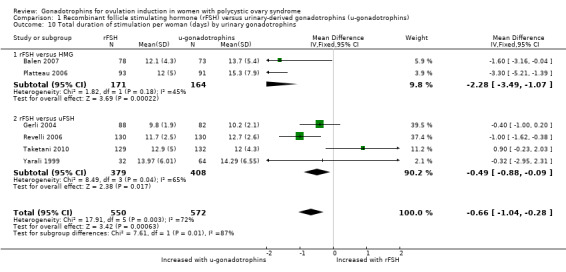
Comparison 1 Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins), Outcome 10 Total duration of stimulation per woman (days) by urinary gonadotrophins.
2 Human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus urinary FSH (uFSH)
2.1 Live birth per woman
Three trials, including 138 women, reported on live birth (Gadir 1990; McFaul 1990; Sagle 1991). We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin improved live birth rate (RR 1.28, 95% CI 0.65 to 2.52; three RCTs, N = 138; I² = 0%; very low‐quality evidence; Analysis 2.1; Figure 6).
2.1. Analysis.

Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 1 Live birth rate per woman.
6.
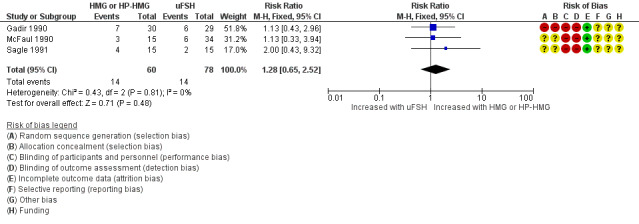
Forest plot of comparison 2. Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), outcome: 2.1 Live birth rate per woman
2.2. Incidence of multiple pregnancy per woman
We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin led to a higher multiple pregnancy rate per woman (RR 2.13, 95% CI 0.51 to 8.91; four RCTs, N = 161; I² = 0%; very low‐quality evidence; Analysis 2.2). As two of the four studies had no multiple pregnancies, we also calculated the risk difference (RD 0.03, 95% CI ‐0.05 to 0.11).
2.2. Analysis.

Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 2 Multiple pregnancy per woman.
2.3 Incidence of multiple pregnancy per clinical pregnancy
We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin led to a higher multiple pregnancy rate per clinical pregnancy (RR 4.20, 95% CI 0.21 to 83.33; four RCTs, N = 161; I² = 0%; Analysis 2.3). As two of the four studies had no multiple pregnancies, we also calculated the risk difference (RD 0.11, 95% CI ‐0.22 to 0.45).
2.3. Analysis.

Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 3 Multiple pregnancy per clinical pregnancy.
2.4 Clinical pregnancy rate per woman
One study reported clinical pregnancy rate per woman (Sagle 1991). McFaul 1990 presented pregnancy rates without defining this outcome. For this study, we calculated the clinical pregnancy rates by adding the number of live births to the number of miscarriages in each group. Seibel 1985 reported conception rates, which we used as clinical pregnancy rate. After pooling the data, we are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin improved clinical pregnancy rate (RR 1.31, 95% CI 0.66 to 2.59; three RCTs, N = 102; I² = 0%; very low‐quality evidence; Analysis 2.4).
2.4. Analysis.
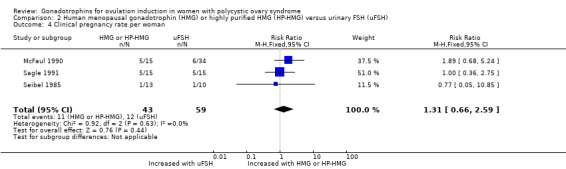
Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 4 Clinical pregnancy rate per woman.
2.5 Miscarriage rate per woman
We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin reduced miscarriage rate (RR 0.33, 95% CI 0.06 to 1.97; two RCTs, N = 98; I² = 0%; very low‐quality evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 5 Miscarriage rate per woman.
2.6 Incidence of OHSS per woman
Two studies, including 53 women, reported OHSS (Sagle 1991; Seibel 1985). We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin reduced the incidence of OHSS (RR 7.07, 95% CI 0.42 to 117.81; two RCTs, N = 53; very low‐quality evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2 Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH), Outcome 6 Incidence of OHSS per woman.
2.7 Mean total gonadotrophin dose per woman
Gadir 1990 and McFaul 1990 reported mean values for total doses, but they did not state standard deviations. Mean total does for human menopausal gonadotrophin or highly purified human menopausal gonadotrophin versus uFSH were 1568 IU versus 1478 IU in Gadir 1990, and 1770 IU versus 1995 IU in McFaul 1990. The authors reported that they found no significant difference between groups.
Sagle 1991 also reported no significant difference between groups. They reported values in mean total dose per cycle: human menopausal gonadotrophin or highly purified human menopausal gonadotrophin 1080 IU (range: 525 to 1950 IU) versus uFSH 1447.5 IU (range: 675 to 2887.5 IU).
2.8 Total duration of stimulation per woman (days)
McFaul 1990 reported no significant mean difference between human menopausal gonadotrophin (11.8 days) and uFSH (11.9 days). They did not provide standard deviations.
3 Gonadotrophins versus continued clomiphene citrate
One trial, including 661 women, measured all outcomes (Weiss 2018).
3.1 Live birth rate per woman
One trial, including 666 women reported on live birth (Weiss 2018). We found that gonadotrophins resulted in more live births than continued clomiphene citrate (RR 1.24, 95% CI 1.05 to 1.46; one trial, N = 661; moderate‐quality evidence; Analysis 3.1). This suggests that for a woman with a live birth rate of 41% with continued clomiphene citrate, the live birth rate with FSH was 43% to 60%.
3.1. Analysis.
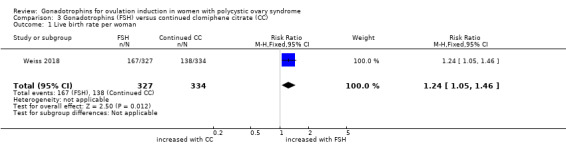
Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 1 Live birth rate per woman.
3.2. Incidence of multiple pregnancy per woman
There is probably little or no difference in the multiple pregnancy rate per woman (RR 0.89, 95% CI 0.33 to 2.44; one trial, N = 661; moderate‐quality evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 2 Multiple pregnancy per woman.
3.3 Incidence of multiple pregnancy per clinical pregnancy
There is probably little or no difference in the multiple pregnancy rate per clinical pregnancies (RR 0.68, 95% CI 0.25 to 1.84; one trial, N = 661, moderate‐quality evidence; Analysis 3.3).
3.3. Analysis.
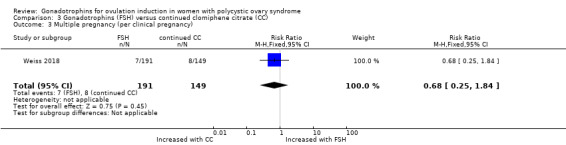
Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 3 Multiple pregnancy (per clinical pregnancy).
3.4 Clinical pregnancy rate per woman
Gonadotrophins resulted in more clinical pregnancies than continued clomiphene citrate (RR 1.31, 95% CI 1.13 to 1.52; one trial, N = 661; moderate‐quality evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 4 Clinical pregnancy per woman.
3.5 Miscarriage rate per woman
The number of miscarriages was higher in the group treated with gonadotrophins than in the clomiphene citrate group (RR 2.23, 95% CI 1.11 to 4.47; one trial, N = 661; low‐quality evidence). When expressed per clinical pregnancy, there was probably little or no difference in miscarriage rate (RR 1.70, 95% 0.86 to 3.36; Analysis 3.5)
3.5. Analysis.
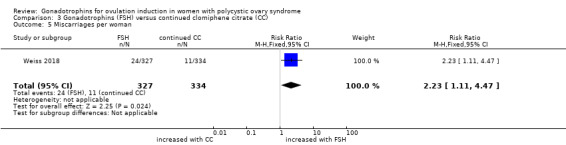
Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 5 Miscarriages per woman.
3.6 Incidence of OHSS per woman
OHSS did not occur in any of the women, therefore, we could not calculate the RR. The estimate for the risk difference was (0.00, 95% CI ‐0.01 to 0.01; one trial, N = 661; low‐quality evidence; Analysis 3.6).
3.6. Analysis.

Comparison 3 Gonadotrophins (FSH) versus continued clomiphene citrate (CC), Outcome 6 Incidence of OHSS per woman.
Discussion
Summary of main results
This review compared the effectiveness and safety of gonadotrophins as a second‐line treatment for ovulation induction in women with polycystic ovary syndrome (PCOS) who did not ovulate or conceive on clomiphene citrate. We found 10 studies that compared recombinant follicle‐stimulating hormone (rFSH) with urinary‐derived gonadotrophins, four trials that compared urinary FSH (uFSH) with human menopausal gonadotrophin, and one trial that compared gonadotrophins with continued clomiphene citrate. There may be little or no difference in pregnancy outcomes when rFSH was compared to urinary gonadotrophins as a whole. We are uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin improved pregnancy outcomes when compared with uFSH. We are uncertain whether there was any difference observed in ovarian hyperstimulation syndrome (OHSS) for any of the comparisons. We found no trials that compared rFSH and purified FSH, or highly purified FSH and purified FSH. The use of gonadotrophins resulted in higher live birth rates without increasing multiple pregnancy rates when compared to continued clomiphene citrate.
Overall completeness and applicability of evidence
For the trials that compared rFSH and urinary‐derived gonadotrophins, outcome data needed to make the planned comparisons were largely available; these trials were all published after 1996. The data from trials that compared rFSH and purified uFSH and highly purified uFSH were incomplete, probably because these trials had been published between 1985 and 1991, when there were no CONSORT or PRISMA guidelines, and clinical pregnancy or ovulation rates were still accepted endpoints. The outcome data for the gonadotrophin versus continued clomiphene citrate trial was complete.
Seven trials did not define the outcome OHSS. The remaining studies used very different definitions (see Characteristics of included studies). It is common to categorise cases of OHSS by three degrees; mild, moderate, or severe (Youssef 2014). Since this ranking was almost never used in the included studies of this review, it may be inappropriate to pool the data on OHSS. Also, different starting dosages were used, varying from 50 to 150 IU per day, with various criteria outlined to withhold an injection of human chorionic gonadotrophin. This may influence the incidence of OHSS, regardless of the type of gonadotrophin used. Nowadays, OHSS is not a common finding in ovulation induction. OHSS is mainly a complication that occurs after treatment with in vitro fertilisation (Youssef 2014).
The data on gonadotrophin dose used and duration of stimulation were never presented per woman randomised, and showed high statistical heterogeneity. Therefore, these outcomes are likely to be biased, and one should not draw conclusions on the basis of these data.
Four of the included studies comparing gonadotrophins used intrauterine insemination (IUI) in addition to ovulation induction with gonadotrophins. IUI may or may not have increased the pregnancy rate, but since these studies always provided IUI in both study arms, its effect on differential pregnancy rates was likely to be small. In the study comparing gonadotrophins with continued clomiphene citrate, women had also been randomised to IUI or intercourse. This study found little or no differences in the effect of IUI on any of the pregnancy outcomes (Weiss 2018).
For the studies comparing gonadotrophins, the included population represented women with PCOS who were either clomiphene citrate‐resistant or had failed to conceive with clomiphene citrate. The evidence is broadly applicable as a second‐line treatment for ovulation induction in these women. The study comparing gonadotrophins and continued clomiphene citrate included only women who had ovulated on previous clomiphene citrate cycles but failed to conceive.
Quality of the evidence
Using GRADE assessment, we found that evidence for most outcomes was of low to very low quality, due to the limited number of studies comparing gonadotrophins, small study size, statistical heterogeneity, and the risk of bias in the individual studies.
For the study comparing gonadotrophins with continuous clomiphene citrate, we assessed evidence for live birth and clinical pregnancy to be of moderate quality.
Potential biases in the review process
Strengths of this review include comprehensive systematic searching for eligible studies, rigid inclusion criteria for RCTs and data extraction, and independent analysis by three review authors. The possibility of publication bias was minimised by including both published and unpublished studies (such as abstracts from meetings). However, as with any review, we cannot guarantee that we found all eligible studies.
Agreements and disagreements with other studies or reviews
Our results are in line with the outcomes of the previous Cochrane review of Bayram 2001, in concluding that rFSH and urinary‐derived gonadotrophins are equally effective for ovulation induction in women with PCOS, in terms of ovulation rate, pregnancy rate, miscarriage rate, and multiple pregnancy rate. Our results are also in line with the outcomes of the previous Cochrane Review of Nugent 2000, who concluded that comparing FSH and human menopausal gonadotrophin showed little or no difference in pregnancy rates. Nugent 2000 did find a significant reduction in OHSS rate per cycle in women treated with purified FSH compared to human menopausal gonadotrophin. We focused on OHSS rate per woman, and found little or no difference, although only two trials were available for this analysis.
Bayram 2001 and Nugent 2000 did not evaluate the outcome of live birth. We found there may be little or no difference in live birth rate for the comparison of rFSH versus urinary gonadotrophins. We were uncertain whether human menopausal gonadotrophin or highly purified human menopausal gonadotrophin improved live birth rate when compared to uFSH.
Another review compared rFSH with urinary‐derived FSH products (Nahuis 2009). The authors found that follitropin alpha, beta, and urinary FSH products appeared to be similarly effective in live birth rates, and clinical, ongoing, and multiple pregnancy rates. Nahuis 2009 did not pool data on OHSS.
Weiss 2018 was the first to compare gonadotrophins and continuous clomiphene citrate in anovulatory women with clomiphene citrate‐failure.
Authors' conclusions
Implications for practice.
There may be little or no difference in live birth, incidence of multiple pregnancy, clinical pregnancy rate, or miscarriage rate between urinary‐derived gonadotrophins and recombinant follicle stimulating hormone in women with polycystic ovary syndrome. For human menopausal gonadotropin or highly purified human menopausal gonadotrophin versus urinary follicle stimulating hormone we are uncertain wether one or the other improves or lowers live birth, incidence of multiple pregnancy, clinical pregnancy rate, or miscarriage rate. We are uncertain whether any of the interventions reduce the incidence of ovarian hyperstimulation syndrome. We suggest weighing costs and convenience in the decision to use one or the other gonadotrophin. In women with clomiphene citrate failure, gonadotrophins resulted in more live births than continued clomiphene citrate without increasing multiple pregnancies.
Implications for research.
New research on the effectiveness of gonadotrophins should be specifically directed at preventing multiple pregnancies while retaining the highest live birth chances. Another reason for the need for new research is the high risk of bias in most of the included studies in this review. To reduce the risk of performance and detection bias, future trials should implement blinding of study participants, personnel, and outcome assessors. We need trials that study ovulation induction with letrozole in clomiphene citrate‐resistant women, or ovulation induction with letrozole to treat naive women over 12 cycles. We also need to study the effect of body mass index on the effectiveness of all ovulation induction treatments. According to a network meta‐analysis, letrozole or clomiphene citrate plus metformin are most effective, specifically in obese women (Wang 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2018 | New citation required but conclusions have not changed | The addition of one new study (Weiss 2018) did not lead to a change in the conclusions of this review. |
| 23 May 2018 | New search has been performed | We updated the literature search. We did not find any new studies that compared different gonadotrophins. We included one study that compared gonadotrophins with continued ovulation induction with clomiphene citrate. We changed OHSS from a primary safety outcome to a secondary outcome. This was advised by several gynaecologists as OHSS occurs very rarely. We added multiple pregnancy as a primary outcome. |
Acknowledgements
This review incorporates the Cochrane reviews of Nugent 2000 and Bayram 2001. We would like to acknowledge the previous work done by the lead authors David Nugent and Neriman Bayram. Also, we would like to thank Neriman Bayram for contributing to the previous version of this review.
The authors of the 2018 review thank referees Emily Liu and Katie Stocking for commenting on the draft.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register search strategy
Searched 16 January 2018
PROCITE platform
Keywords CONTAINS "polycystic ovary syndrome" or "PCOS" or "anovulation" or "amenorrhea" or "amenorrhoea" or "ovarian dysfunction" or "ovarian failure" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligoanovulatory" or "oligoamenorrhea" or "oligo‐ovulatory" or Title CONTAINS "polycystic ovary syndrome" or "PCOS" or "anovulation" or "amenorrhea" or "amenorrhoea" or "ovarian dysfunction" or "ovarian failure" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligoanovulatory" or "oligoamenorrhea "or "oligo‐ovulatory"
AND
Keywords CONTAINS "urinary FSH" or "urofollitropin" or "FSH" or "follitropin" or "Follitropin A" or "follitropin alfa" or "Follitropin B" or "recombinant FSH" or "recombinant hFSH" or "r‐FSH" or "r‐hFSH" or "follicle stimulating hormone" or "rh‐FSH" or "rFSH" or "rh‐FSH" or "rhFSH" or "human menopausal gonadotrophin" or "human menopausal gonadotrophins" or "human menopausal gonadotropins" or "Menopur" or "menotrophin" or "menotropin" or "pergonal" or "pergonol" or "HMG" or "HP hMG" or "hpHMG" or "humegon" or "normegon" or "ovulation induction" or "ovulation stimulation" or "ovarian hyperstimulation" or "ovarian stimulation " or "ovarian stimulation controlled ovarian stimulation" or Title CONTAINS "urinary FSH" or "urofollitropin" or "FSH" or "follitropin" or "Follitropin A" or "follitropin alfa" or "Follitropin B" or "recombinant FSH" or "recombinant hFSH" or "r‐FSH" or "r‐hFSH" or "follicle stimulating hormone" or "rh‐FSH" (757 hits)
Appendix 2. CENTRAL Register of Studies Online (CRS‐O) search strategy
Searched 16 January 2018
Web platform
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 962
#2 (Polycystic Ovar*):TI,AB,KY 2073
#3 PCOS:TI,AB,KY 1636
#4 PCOD:TI,AB,KY 25
#5 (stein‐leventhal or leventhal):TI,AB,KY 18
#6 anovulat*:TI,AB,KY 527
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 2543
#8 MESH DESCRIPTOR Follicle Stimulating Hormone EXPLODE ALL TREES 1785
#9 (Follicle Stimulating Hormone* or FSH or rFSH or recFSH):TI,AB,KY 4100
#10 (recombinant FSH):TI,AB,KY 495
#11 (recombinant human):TI,AB,KY 4214
#12 (uFSH or puregon or metrodin):TI,AB,KY 157
#13 (urinary FSH):TI,AB,KY 95
#14 (urinary follicle):TI,AB,KY 47
#15 (r FSH or u‐FSH or rhFSH or uhFSH):TI,AB,KY 140
#16 (Follitropin or Urofollitropin):TI,AB,KY 1480
#17 MESH DESCRIPTOR Urofollitropin EXPLODE ALL TREES 10
#18 Bravelle*:TI,AB,KY 22
#19 (FSH‐HP or FSH‐P):TI,AB,KY 67
#20 (recombinant gonadotropin*):TI,AB,KY 9
#21 (recombinant gonadotrophin*):TI,AB,KY 11
#22 HP‐uFSH:TI,AB,KY 9
#23 MESH DESCRIPTOR Menotropins EXPLODE ALL TREES 383
#24 Menopur:TI,AB,KY 36
#25 HMG:TI,AB,KY 1471
#26 Menogon:TI,AB,KY 2
#27 menotropin:TI,AB,KY 33
#28 pergonal:TI,AB,KY 19
#29 (human menopausal gonadotrop?in*):TI,AB,KY 622
#30 humegon:TI,AB,KY 8
#31 normegon:TI,AB,KY 6
#32 (gonadotrop?in* adj3 ovulat*):TI,AB,KY 111
#33 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 9878
#34 #7 AND #33 707
Appendix 3. MEDLINE search strategy
Searched from 1946 to 16 January 2018
OVID platform 1 exp Polycystic Ovary Syndrome/ (14039) 2 Polycystic Ovary Syndrome.tw. (11235) 3 PCOS.tw. (9889) 4 polycystic ovar$.ti,ab,sh. (18088) 5 PCOD.ti,ab,sh. (345) 6 (stein‐leventhal or leventhal).tw. (790) 7 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (88) 8 anovulat$.tw. (5762) 9 or/1‐8 (22407) 10 exp Follicle Stimulating Hormone/ (39612) 11 Follicle Stimulating Hormone$.tw. (19554) 12 recombinant FSH.tw. (909) 13 recombinant human.tw. (41361) 14 (rFSH or uFSH).tw. (625) 15 (puregon or metrodin).tw. (171) 16 urinary FSH.tw. (249) 17 urinary follicle.tw. (163) 18 (recFSH or r‐FSH).tw. (182) 19 (u‐FSH or rhFSH or uhFSH).tw. (188) 20 Follitropin$.tw. (653) 21 exp Urofollitropin/ (23) 22 Urofollitropin.tw. (47) 23 Bravelle$.tw. (15) 24 FSH.tw. (34846) 25 FSH‐HP.tw. (34) 26 FSH‐P.tw. (476) 27 recombinant gonadotropin$.tw. (99) 28 recombinant gonadotrophin$.tw. (65) 29 HP‐uFSH.tw. (7) 30 exp Menotropins/ (3799) 31 Menopur.tw. (27) 32 HP‐HMG.tw. (66) 33 HMG.tw. (15337) 34 Menogon.tw. (5) 35 menotropin$.tw. (246) 36 pergonal.tw. (178) 37 human menopausal gonadotrop?in$.tw. (2518) 38 humegon.tw. (21) 39 normegon.tw. (5) 40 or/10‐39 (112054) 41 randomized controlled trial.pt. (516400) 42 controlled clinical trial.pt. (101760) 43 randomized.ab. (453289) 44 placebo.tw. (216065) 45 clinical trials as topic.sh. (202635) 46 randomly.ab. (312328) 47 trial.ti. (203673) 48 (crossover or cross‐over or cross over).tw. (83438) 49 or/41‐48 (1290958) 50 exp animals/ not humans.sh. (4815681) 51 49 not 50 (1188906) 52 9 and 40 and 51 (582)
Appendix 4. Embase search strategy
Searched from 1980 to 16 January 2018
OVID platform
1 exp ovary polycystic disease/ or exp stein leventhal syndrome/ (22744) 2 Polycystic Ovar$.tw. (19178) 3 PCOS.tw. (13595) 4 PCOD.tw. (369) 5 (stein‐leventhal or leventhal).tw. (557) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (89) 7 anovulat$.tw. (5995) 8 or/1‐7 (29802) 9 exp Follitropin/ (51573) 10 Follicle Stimulating Hormone$.tw. (18411) 11 recombinant FSH.tw. (1387) 12 recombinant human.tw. (46551) 13 (rFSH or uFSH).tw. (1129) 14 (puregon or metrodin).tw. (2130) 15 urinary FSH.tw. (293) 16 urinary follicle.tw. (144) 17 (recFSH or r‐FSH).tw. (351) 18 u‐fsh.tw. (31) 19 (u‐fsh or r‐FSH).tw. (258) 20 (rhFSH or uhFSH).tw. (228) 21 Follitropin$.tw. (741) 22 exp urofollitropin/ (1642) 23 Urofollitropin.tw. (71) 24 Bravelle$.tw. (89) 25 FSH.tw. (38965) 26 FSH‐HP.tw. (46) 27 FSH‐P.tw. (446) 28 recombinant gonadotropin$.tw. (129) 29 recombinant gonadotrophin$.tw. (81) 30 exp human menopausal gonadotropin/ (8631) 31 Menopur.tw. (515) 32 HMG.tw. (17156) 33 HP‐HMG.tw. (161) 34 HP‐uFSH.tw. (10) 35 Menogon.tw. (320) 36 menotropin$.tw. (250) 37 pergonal.tw. (1912) 38 humegon.tw. (742) 39 normegon.tw. (22) 40 human menopausal gonadotrop?in$.tw. (2204) 41 or/9‐40 (130433) 42 8 and 41 (6618) 43 Clinical Trial/ (962428) 44 Randomized Controlled Trial/ (479673) 45 exp randomization/ (76644) 46 Single Blind Procedure/ (30038) 47 Double Blind Procedure/ (142304) 48 Crossover Procedure/ (53731) 49 Placebo/ (302872) 50 Randomi?ed controlled trial$.tw. (170118) 51 Rct.tw. (26475) 52 random allocation.tw. (1711) 53 randomly allocated.tw. (28610) 54 allocated randomly.tw. (2271) 55 (allocated adj2 random).tw. (789) 56 Single blind$.tw. (20076) 57 Double blind$.tw. (177577) 58 ((treble or triple) adj blind$).tw. (726) 59 placebo$.tw. (259211) 60 prospective study/ (416492) 61 or/43‐60 (1839556) 62 case study/ (51379) 63 case report.tw. (343137) 64 abstract report/ or letter/ (1013729) 65 or/62‐64 (1400044) 66 61 not 65 (1792698) 67 42 and 66 (1405)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 16 January 2018
OVID platform
1 PCOS.tw. (238) 2 polycystic ovar$.tw. (369) 3 PCOD.tw. (6) 4 (stein‐leventhal or leventhal).tw. (289) 5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (0) 6 anovulat$.tw. (145) 7 or/1‐6 (806) 8 exp follicle stimulating hormone/ (93) 9 Follicle Stimulating Hormone$.tw. (522) 10 recombinant FSH.tw. (1) 11 recombinant human.tw. (421) 12 (rFSH or uFSH).tw. (0) 13 (puregon or metrodin).tw. (0) 14 urinary FSH.tw. (2) 15 urinary follicle.tw. (2) 16 (recFSH or r‐FSH).tw. (0) 17 rFSH.tw. (0) 18 uFSH.tw. (0) 19 (u‐FSH or rhFSH or uhFSH).tw. (0) 20 Follitropin$.tw. (1) 21 Urofollitropin.tw. (0) 22 Bravelle$.tw. (0) 23 FSH.tw. (444) 24 FSH‐HP.tw. (0) 25 FSH‐P.tw. (5) 26 recombinant gonadotropin$.tw. (0) 27 recombinant gonadotrophin$.tw. (0) 28 HP‐uFSH.tw. (0) 29 exp Gonadotropic Hormones/ (4096) 30 Menopur.tw. (0) 31 HP‐HMG.tw. (0) 32 HMG.tw. (205) 33 Menogon.tw. (0) 34 menotropin$.tw. (1) 35 pergonal.tw. (2) 36 human menopausal gonadotrop?in$.tw. (5) 37 humegon.tw. (0) 38 normegon.tw. (0) 39 or/8‐38 (5113) 40 7 and 39 (35) 41 random.tw. (52010) 42 control.tw. (401391) 43 double‐blind.tw. (21220) 44 clinical trials/ (10764) 45 placebo/ (5053) 46 exp Treatment/ (704448) 47 or/41‐46 (1094480) 48 40 and 47 (11)
Appendix 6. CINAHL search strategy
Searched from 1961 to 16 January 2018
EBSCO platform
| # | Query | Results |
| S43 | S30 AND S42 | 76 |
| S42 | S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 1,189,654 |
| S41 | TX allocat* random* | 7,669 |
| S40 | (MH "Quantitative Studies") | 17,068 |
| S39 | (MH "Placebos") | 10,551 |
| S38 | TX placebo* | 49,038 |
| S37 | TX random* allocat* | 7,669 |
| S36 | (MH "Random Assignment") | 45,100 |
| S35 | TX randomi* control* trial* | 138,334 |
| S34 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 926,310 |
| S33 | TX clinic* n1 trial* | 216,926 |
| S32 | PT Clinical trial | 85,271 |
| S31 | (MH "Clinical Trials+") | 228,391 |
| S30 | S4 AND S29 | 201 |
| S29 | S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 | 5,835 |
| S28 | TX human menopaus* gonadotrop* | 47 |
| S27 | TX human menopaus* gonadotrop* | 47 |
| S26 | TX pergonal | 3 |
| S25 | TX HMG | 837 |
| S24 | TX HP‐HMG | 5 |
| S23 | TX Menopur | 1 |
| S22 | TX HP‐uFSH | 1 |
| S21 | TX recombinant gonadotrophin* | 8 |
| S20 | TX recombinant gonadotropin* | 23 |
| S19 | TX FSH‐P | 899 |
| S18 | TX FSH‐HP | 4 |
| S17 | TX FSH | 899 |
| S16 | TX Bravelle | 0 |
| S15 | TX Follitropin | 22 |
| S14 | TX (u‐FSH or rhFSH or uhFSH) | 4 |
| S13 | TX (recFSH or r‐FSH) | 7 |
| S12 | TX urinary follicle | 19 |
| S11 | TX urinary FSH | 27 |
| S10 | TX (puregon or metrodin) | 3 |
| S9 | TX (rFSH or uFSH) | 24 |
| S8 | TX recombinant human | 2,939 |
| S7 | TX recombinant FSH | 36 |
| S6 | TX Follicle Stimulating Hormone* | 1,807 |
| S5 | (MM "Follicle‐Stimulating Hormone") | Display |
| S4 | S1 OR S2 OR S3 | 3,763 |
| S3 | TX Polycystic Ovar* | 2,661 |
| S2 | TX PCOS | 2,241 |
| S1 | (MM "Polycystic Ovary Syndrome") | 1,624 |
Appendix 7. Cochrane 'Risk of bias' assessment tool
Cochrane's tool for assessing risk of bias
| Domain | Support for judgement | Review authors’ judgement |
| Selection bias | ||
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Performance bias | ||
| Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Detection bias | ||
|
Blinding of outcome assessment Assessments should be made for each main outcome (or class of outcomes). |
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Attrition bias | ||
|
Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes). |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition or exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to amount, nature, or handling of incomplete outcome data. |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions or entries were pre‐specified in the review’s protocol, responses should be provided for each question or entry. |
Bias due to problems not covered elsewhere in the table. |
Data and analyses
Comparison 1. Recombinant follicle stimulating hormone (rFSH) versus urinary‐derived gonadotrophins (u‐gonadotrophins).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman by urinary gonadotrophins | 5 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.78] |
| 1.1 rFSH versus HMG | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.68, 1.57] |
| 1.2 rFSH versus uFSH | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.95, 7.43] |
| 2 Live birth rate per woman by sponsor | 5 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.78] |
| 2.1 Ferring | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.68, 1.57] |
| 2.2 unknown | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.95, 7.43] |
| 3 Multiple pregnancy per woman by urinary gonadotrophins | 8 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.61] |
| 3.1 rFSH versus HMG | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.49, 2.79] |
| 3.2 rFSH versus uFSH | 5 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.59] |
| 4 Multiple pregnancy per woman by sponsor | 8 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.60] |
| 4.1 Ferring | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.49, 2.79] |
| 4.2 MSD ‐ Organon | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.68, 2.23] |
| 4.3 Merck ‐ Serono | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.14, 1.80] |
| 4.4 Unknown | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.19, 4.49] |
| 5 Clinical pregnancy rate per woman by urinary gonadotrophins | 8 | 1330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.27] |
| 5.1 rFSH versus HMG | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.81, 1.77] |
| 5.2 rFSH versus uFSH | 5 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| 6 Incidence of multiple pregnancy per clinical pregnancy by urinary gonadotrophins | 8 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.32] |
| 6.1 rFSH versus HMG | 3 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.09] |
| 6.2 rFSH versus uFSH | 5 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.35] |
| 7 Miscarriage rate per woman by urinary gonadotrophins | 7 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.71, 2.04] |
| 7.1 rFSH versus HMG | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.24, 3.70] |
| 7.2 rFSH versus uFSH | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.23] |
| 8 Incidence of OHSS per woman by urinary gonadotrophins | 10 | 1565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.82, 2.65] |
| 8.1 rFSH versus HMG | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.39, 3.20] |
| 8.2 rFSH versus uFSH | 7 | 1156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.82, 3.39] |
| 9 Total gonadotrophin dose per woman (IU) by urinary gonadotrophins | 6 | 1046 | Mean Difference (IV, Fixed, 95% CI) | ‐105.44 [‐154.21, ‐56.68] |
| 9.1 rFSH versus HMG | 2 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐283.94 [‐449.10, ‐118.78] |
| 9.2 rFSH versus uFSH | 4 | 711 | Mean Difference (IV, Fixed, 95% CI) | ‐88.40 [‐139.44, ‐37.36] |
| 10 Total duration of stimulation per woman (days) by urinary gonadotrophins | 6 | 1122 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.04, ‐0.28] |
| 10.1 rFSH versus HMG | 2 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐2.28 [‐3.49, ‐1.07] |
| 10.2 rFSH versus uFSH | 4 | 787 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.88, ‐0.09] |
Comparison 2. Human menopausal gonadotrophin (HMG) or highly purified HMG (HP‐HMG) versus urinary FSH (uFSH).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman | 3 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.65, 2.52] |
| 2 Multiple pregnancy per woman | 4 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.51, 8.91] |
| 3 Multiple pregnancy per clinical pregnancy | 3 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 83.33] |
| 4 Clinical pregnancy rate per woman | 3 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.66, 2.59] |
| 5 Miscarriage rate per woman | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.97] |
| 6 Incidence of OHSS per woman | 2 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.42, 117.81] |
Comparison 3. Gonadotrophins (FSH) versus continued clomiphene citrate (CC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.05, 1.46] |
| 2 Multiple pregnancy per woman | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.33, 2.44] |
| 3 Multiple pregnancy (per clinical pregnancy) | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.25, 1.84] |
| 4 Clinical pregnancy per woman | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 5 Miscarriages per woman | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.11, 4.47] |
| 6 Incidence of OHSS per woman | 1 | 661 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balen 2007.
| Methods | Randomised, open‐label, assessor‐blind, parallel‐group, multinational controlled non‐inferiority trial Duration, timing, and location of the trial: between November 2002 and October 2003 in 22 fertility centres (12 in Belgium, 7 in Denmark, 3 in the UK) Sample size calculation: 2‐sided significance level of 0.05 and a power of 80%. 126 women were needed for the study 151 women randomised 1 cycle/woman Ratio between FSH‐HP and rFSH was 1:1. A per protocol and intention‐to‐treat analysis was performed |
|
| Participants | Clomiphene citrate‐resistant WHO Group II chronic anovulatory women (see Notes) and women who failed to conceive on clomiphene citrate Mean age (± SD) of the women was 28.9 (3.5) for the FSH‐HP group and 29.0 (3.9) for the rFSH group Body mass index (± SD) was 25.0 (4.4) and 24.7 (4.7) respectively Duration of infertility in years (± SD) was 2.8 (1.5) and 2.8 (1.8) respectively Number of women with primary infertility was 65.8% and 62.8% respectively LH:FSH ratio was (± SD) 1.3 (0.8) and 1.4 (0.9) respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, testosterone) and semen analysis. In all cases, there was at least 1 patent fallopian tube documented within 3 years prior to screening |
|
| Interventions | rFSH versus FSH‐HP as second‐line treatment Treatment was started 2 to 5 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 75 IU daily and maintained for 7 days. After this, the dose was maintained or increased by 37.5 IU according to individual response. The maximum allowed daily dose was 225 IU, and participants were treated for a maximum of 6 weeks hCG (5000 IU, Profasi) was given when a single follicle of ≥ 17 mm, or 2 to 3 follicles of ≥ 15 mm developed. Timed intercourse was advised or IUI performed. hCG was not given in cases of no follicular response, ≥ 4 follicles of ≥ 15 mm, or serum estradiol levels > 2000 pg/mL |
|
| Outcomes | Ovulation rate (see Notes) Clinical pregnancy rate Ongoing pregnancy rate Live birth rate Singleton live birth rate Number of follicles Endometrial thickness at the time of hCG administration Total FSH dose and duration of FSH treatment Incidence of OHSS (see Notes) Multiple pregnancies Number of cancellations |
|
| Notes | Clomiphene‐resistant: failure to ovulate with clomiphene citrate doses of at least 100 mg/day for at least 5 days, or failure to conceive after 3 cycles of ovulation induction with clomiphene citrate Chronic anovulation: amenorrhoea, or oligomenorrhoea, or anovulatory cycles based on progesterone levels in women with cycle lengths of 21 to 35 days Ovulation: mid‐luteal serum progesterone concentration of ≥ 25 nmol/L Clinical pregnancy: transvaginal ultrasound showing at least 1 intrauterine gestation sac with foetal heart beat 7 ± 2 weeks after hCG administration Ongoing pregnancy: transvaginal ultrasound showing at least 1 viable foetus 12 ± 2 weeks after hCG administration OHSS: Categorised as mild, moderate, or severe according to classification of Golan 1989 Sponsored by Ferring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated randomisation list prepared by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Computerised allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and sponsor study staff were blinded to treatment allocation. The treatment code was not unblinded for any participant during the study. Gonadotrophin distribution was handled by research nurses. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported; 4/73 in the uFSH group, 6/78 in the rFSH group (participants were withdrawn after randomisation because of adverse events, non‐compliance, excessive response, personal reasons and other). No further loss to follow‐up. intention‐to‐treat (ITT) analysis. |
| Selective reporting (reporting bias) | Low risk | Per protocol and ITT analyses were performed. Data on all outcomes available |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored by Ferring |
Coelingh Bennink 1998.
| Methods | Prospective, multicenter, assessor‐blind, randomised, clinical trial Duration, timing, and location of the trial: between June 1992 and March 1994 in 12 centres throughout Europe. Sample size calculation: not stated 178 women randomised 3 cycles/woman Ratio between uFSH and rFSH was 2:3 An intention‐to‐treat analysis was performed |
|
| Participants | Clomiphene citrate‐resistant WHO Group II chronic anovulatory women (see Notes) Mean age (± SD) of the women in years was 29.4 (3.9) for the uFSH group and 28.9 (4.2) for the rFSH group Body mass index (±SD) was 24.3 (3.1) and 24.5 (3.4) respectively. Duration of infertility in years (± SD) was 4.5 (2.7) and 3.9 (2.4) respectively Number of women with primary infertility was 76.1% and 55.2% respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, testosterone, TSH) and semen analysis. In all cases, there was at least 1 patent fallopian tube documented |
|
| Interventions | uFSH versus rFSH as second‐line treatment Treatment was started within 5 days after a spontaneous, or progesterone‐induced menstrual bleed A stepwise increasing dosing scheme was used, starting with 75 IU daily, and maintained for up to 14 days. The maximum allowed daily dose was 225 IU, and participants were treated for a maximum of 6 weeks hCG (10000 IU, Pregnyl) was given when a follicle of ≥ 18 mm, or 2 to 3 follicles of ≥ 15 mm developed. hCG was not given in case of no follicular response, > 3 follicles of ≥ 15 mm |
|
| Outcomes | Cumulative ovulation rate after 3 cycles Ongoing pregnancy rate Miscarriage rate Total FSH dose and duration of FSH treatment Number of follicles Number of cancellations Incidence of OHSS Multiple pregnancies Presence of antibodies to FSH |
|
| Notes | Clomiphene‐resistant: failure to ovulate during 3 previous cycles with clomiphene citrate or failure to conceive during 6 cycles with CC. Ovulation: mid‐luteal serum progesterone concentration of ≥ 25 nmol/L on at least 1 occasion Ongoing pregnancy: vital pregnancy at least 12 weeks after hCG administration OHSS: not defined ("according to criteria of the investigator") Sponsored study (Organon) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: women received a subject number from a randomisation list corresponding with patient boxes in which the medication was kept |
| Allocation concealment (selection bias) | Unclear risk | Women received a subject number from a randomisation list corresponding with patient boxes in which the medication was kept |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Preparation and administration of the medication was done by a study co‐ordinator who took no part in any decision concerning the FSH dose during treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported; 2/69 in the uFSH group, 4/109 in the rFSH group. Reasons for dropout were not clarified |
| Selective reporting (reporting bias) | Low risk | No missing data, all outcomes reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored by Organon |
Feigenbaum 2001.
| Methods | Randomised, open‐label, prospective, multicentre trial
Duration, timing, and location of the trial: 15 private and academic centres A sample size with power calculation was performed 111 women randomised 1 cycle per woman Ratio between recombinant and urinary FSH was 1:1 Parallel design An intention‐to‐treat analysis was performed. |
|
| Participants | Clomiphene citrate‐resistant, normogonadotropic, chronic anovulatory women (see Notes). Mean age (± SD) of the women in years was 28.2 (3.4) for the rFSH group and 29.3 (3.7) for the urinary FSH group Body mass index (± SD) of the women was 30.2 (5.3) and 29.0 (6.7), respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, TSH, testosterone, androstenedione, dehydroepiandrosterone, 17‐OH‐progesterone), a HSG and a semen analysis | |
| Interventions | Recombinant FSH (Follistim®) versus urinary FSH‐HP (Bravelle®) as second‐line treatment Follicular phase (long‐protocol) pituitary down‐regulation with daily leuprolide acetate with addition of up to 12 days of Bravelle® SC (N = 36), Bravelle® IM (N = 37), or Follistim® SC (N = 38) followed by IM hCG administration Treatment was started after successful down‐regulation for 29 days Starting dose was 75 IU FSH/d, SC or IM, for the first 5 days. After this period, dose could be adjusted by 75 IU to 150 IU every other day. Maximum dose was 450 IU/day Treatment was discontinued after a maximum of 12 stimulation days hCG (10,000 IU, Pregnyl) was given when a follicle of ≥ 14 mm developed, and acceptable E2 levels |
|
| Outcomes | Live birth rate OHSS rate Ovulation rate Clinical pregnancy rate Multiple pregnancy rate |
|
| Notes | Clomiphene‐resistant: failure to ovulate during 3 previous medication cycles, or to conceive during 6 cycles with ovulation induced by clomiphene citrate
Chronic anovulation: diagnosed on the basis of cycle length > 35 days, amenorrhoea, E2 and progesterone concentrations, and other.
Ovulation: progesterone concentration of at least 10 mmol/L 6 to 9 days after hCG injection
Clinical pregnancy: foetal heartbeat at vaginal ultrasound 5 weeks after hCG injection Sponsor: Ferring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: block‐of‐3 design using SAS software |
| Allocation concealment (selection bias) | Unclear risk | No details known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were complete and presented according to ITT |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, results presented for all preplanned outcomes |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored by Ferring |
Gadir 1990.
| Methods | Pseudo‐randomised trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 59 women randomised 6 cycles/woman Ratio between HMG and uFSH was 1:1 |
|
| Participants | Clomiphene citrate‐resistant women attributable to PCOS (see Notes). Mean age (± SD) of the women in years was 26.5 (0.73) for the HMG group and 27.0 (0.66) for the uFSH group Body mass index (± SD) was 28.5 (0.95) and 29.2 (0.75) respectively Duration of menstrual dysfunction in years (± SD) was 11.6 (0.85) and 12.2 (0.85) respectively Number of women with primary infertility: not stated LH (IU/L (± SD)) was 15.3 (1.42) and 18.5 (3.58) respectively FSH (IU/L (± SD)) was 6.1 (0.28) and 5.1 (0.33) respectively Infertility work‐up consisted of endocrinology (TSH, DHEAS, prolactin), hysterosalpingography, laparoscopy, and repeated semen analysis |
|
| Interventions | HMG versus uFSH as second‐line treatment Treatment was started on the first or second day of each cycle Starting dose was 75 IU uFSH (uFSH group) or 75 IU uFSH with 75 IU LH (HMG group) daily. Adjustment in dosages was decided for each woman individually according to serum oestradiol (E2), cervical mucus assessment, and ultrasonic monitoring hCG (5000 IU) was given when a single follicle of 18 mm and serum estradiol levels > 1000 pg/mL per follicle of 15 mm or more existed. hCG was not given in case of > 3 follicles of 15 mm or more |
|
| Outcomes | Number of ovulatory cycles Pregnancy rate Cumulative pregnancy rate Endocrine levels during treatment Duration of follicle phase and luteal phase Total dose and duration of FSH and HMG treatment Mean ovarian volume Miscarriages Live birth rate Multiple pregnancies |
|
| Notes | PCOS: diagnosis made on criteria of Yen 1980 and Adams 1986 Clomiphene‐resistant: failure to ovulate with clomiphene citrate doses of at least 150 mg/day for at least 5 days for 3 cycles Ovulation: ultrasonic visualisation of a corpus luteum or disappearance of a dominant follicle in cycles which showed progressive rise of serum E2 Pregnancy: not defined Miscarriage: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random allocation with serial entry was performed |
| Allocation concealment (selection bias) | High risk | Serial entry was used, meaning that no true randomisation was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women who consented to participate were randomised and follow‐up was complete |
| Selective reporting (reporting bias) | Unclear risk | Not sure, this is an old study and it is not certain whether all intended outcomes are reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Unclear whether this was a sponsored trial |
Gerli 2004.
| Methods | Randomised controlled trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 170 women randomised > 1 cycle/woman Ratio between uFSH and rFSH was 1:1 |
|
| Participants | Clomiphene citrate‐resistant PCOS women (see notes) or PCOS women who failed to conceive with clomiphene citrate within 6 to 12 months. All women had a history of at least 2 years of infertility Mean age (± SD) of the women in years was 28.6 (2.7) for the uFSH group and 29.1 (2.4) for the rFSH group Body mass index (± SD) was 23.1 (2.1) and 23.7 (2.0) respectively Number of women with primary infertility: not stated Infertility work‐up consisted of gynaecological and ultrasound examination, semen analysis, hormonal assessment, and hysterosalpingogram |
|
| Interventions | rFSH versus FSH‐HP as second‐line treatment with IUI Treatment was started 2 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 50 IU (rFSH) or 75 IU (uFSH) daily, and maintained for 6 to 7 days. After this, the dose was adjusted according to the women's response hCG (10000 IU, Profasi) was given when a single follicle of ≥ 18 mm developed. hCG was not given in case of > 5 follicles of ≥ 17 mm A single IUI was performed 32 to 40 hours after the injection of hCG |
|
| Outcomes | Number of follicles Total FSH dose and duration of FSH treatment Biochemical pregnancy rate Clinical pregnancy rate Costs per cycle Miscarriages Incidence of OHSS Multiple pregnancies Number of cancellations |
|
| Notes | PCOS women: clinical or biochemical hyperandrogenism (or both), chronic anovulation, and exclusion of related disorders Clomiphene‐resistant: not defined Ovulation: adequate mid‐luteal serum progesterone concentration (not specified) Biochemical pregnancy: small and transient increase in hCG concentrations Clinical pregnancy: ultrasound showing an embryo with cardiac activity at 6 to 7 weeks of pregnancy OHSS: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was prepared by computer |
| Allocation concealment (selection bias) | Unclear risk | Nothing stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported; 2/82 in the uFSH group, 3/88 in the rFSH group (Participants were withdrawn after randomisation because of personal reasons) |
| Selective reporting (reporting bias) | Unclear risk | Only outcomes up to clinical pregnancy; not sure whether all intended outcomes were reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Unclear whether this was a sponsored trial |
Loumaye 1996.
| Methods | Randomised, comparative, open‐label, multinational trial Duration, timing, and location of the trial: Between 1992 and 1994, multinational, European study Sample size calculation: 2‐sided significance level of 0.05 and a power of 90% to detect a difference of 20% in cumulative ovulation rate 222 women randomised 3 cycles/woman Ratio between uFSH and rFSH was 1:1. Parallel design No intention‐to‐treat analysis was performed |
|
| Participants | Clomiphene citrate‐resistant WHO Group II chronic anovulatory women (see Notes) Baseline characteristics not stated |
|
| Interventions | uFSH versus rFSH as second‐line treatment Treatment was started within 5 days after a spontaneous, or progesterone‐induced menstrual bleed |
|
| Outcomes | Cumulative ovulation rate Cumulative pregnancy rate (per woman) Miscarriage rate (per woman) Incidence of OHSS Multiple pregnancy |
|
| Notes | Clomiphene‐resistant: not defined Chronic anovulation: not defined Ovulation: mid‐luteal serum progesterone concentration of ≥ 30 nmol/L Clinical pregnancy: positive hCG Sponsored study (Serono) OHSS: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Truly randomised using sealed opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Truly randomised using sealed opaque numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Publication is a monograph and many details were missing |
| Selective reporting (reporting bias) | Unclear risk | It is a monograph and many details were missing, and not certain whether all intended outcomes were reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored by Serono |
McFaul 1990.
| Methods | Randomised controlled trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 49 women randomised Cycles/woman: not stated Ratio between uFSH and rFSH: not stated |
|
| Participants | Clomiphene citrate‐resistant women with PCOS (see Notes) Mean age of the women: not stated. Mean body mass index was 29.3 for the uFSH group and 28.4 for the HMG group (NS; non‐significant) Mean duration of infertility in years was 5.6 and 6.3 respectively (NS) Number of women with primary infertility: not specified Complete fertility work‐up was performed, including semen analysis and laparoscopy 5 couples with male subfertility were inseminated with washed semen or donor sperm |
|
| Interventions | uFSH versus HMG as second‐line treatment Starting dose was 150 IU daily. The dose was increased by 150 IU in case there was no response based on serum E2 level. If necessary, the dose was increased by another 150 IU every 3 or 4 days hCG (5000 IU, Profasi) was given when a follicle of ≥ 18 mm was measured. hCG was not given in case of 4 or more primary follicles |
|
| Outcomes | Ovulation rate Maximum serum E2 level Pregnancy rate Number of follicles Total FSH dose and duration of FSH treatment Incidence of hyperstimulation (OHSS) Live birth Multiple pregnancies Miscarriages Cumulative pregnancy rate |
|
| Notes | PCOS: women with a history of oligomenorrhoea or amenorrhoea, LH:FSH ratio of at least 3:1 in postmenstrual phase, elevated testosterone and androstenedione levels, polycystic ovaries on ultrasonography Clomiphene‐resistant: failure to ovulate with clomiphene citrate doses of a maximum of 200 mg/day for 5 days in at least 3 treatment cycles Ovulation: mid‐luteal serum progesterone concentration of > 30 nmol/L Hyperstimulation: graded using the standards of Jewelewicz 1973 Pregnancy: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated; method was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up of all treatment cycles was complete. |
| Selective reporting (reporting bias) | Unclear risk | Not sure, this is an old study and it is not certain whether all intended outcomes are reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Probably supported by pharmaceutical company |
Platteau 2006.
| Methods | Randomised, open‐label, assessor‐blind, parallel‐group, multicentre, multinational controlled non‐inferiority trial Duration, timing, and location of the trial: between May 2003 and June 2004 in 29 fertility centres (8 in Belgium, 9 in Denmark, 5 in Sweden, 7 in the UK). Sample size calculation: 2‐sided significance level of 0.05 and a power of 80%. 126 women were needed for the study. 184 women randomised 1 cycle/woman Ratio between HP‐HMG and rFSH was 1:1. Per protocol and intention‐to‐treat analyses were performed |
|
| Participants | Clomiphene citrate‐resistant WHO Group II chronic anovulatory women (see Notes) Mean age (± SD) of the women in years was 29.0 (4.2) for the HP‐HMG group and 29.2 (3.8) for the rFSH group Body mass index (±SD) was 26.5 (5.2) and 25.0 (4.2) respectively. Duration of infertility in years (± SD) was 2.9 (1.8) and 3.0 (2.1) respectively Number of women with primary infertility was 57.1% and 64.5% respectively LH:FSH ratio was (± SD) 1.6 (1.2) and 1.6 (1.1) respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, testosterone) and semen analysis. In all cases, there was at least 1 patent fallopian tube documented within 3 years prior to screening |
|
| Interventions | HP‐HMG versus rFSH as second‐line treatment Treatment was started 2 to 5 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 75 IU daily, and maintained for 7 days. After this, the dose was maintained or increased by 37.5 IU, according to individual response. The maximum allowed daily dose was 225 IU, and participants were treated for a maximum of 6 weeks hCG (5000 IU, Profasi) was given when a single follicle of ≥ 17 mm, or 2 to 3 follicles of ≥ 15 mm developed. Timed intercourse was advised or IUI performed. hCG was not given in case of no follicular response or ≥ 4 follicles of ≥ 15 mm. |
|
| Outcomes | Ovulation rate Clinical pregnancy rate Ongoing pregnancy rate Live birth rate Singleton live birth rate Number of follicles Endometrial thickness at the time of hCG administration Total FSH dose, duration of FSH treatment and threshold dose Incidence of OHSS Multiple pregnancies Number of cancellations |
|
| Notes | Clomiphene‐resistant: failure to ovulate with clomiphene citrate doses of at least 100 mg/day for at least 5 days, or failure to conceive after 3 cycles of ovulation induction with clomiphene citrate Chronic anovulation: amenorrhea or oligomenorrhoea, or anovulatory cycles based on progesterone levels in women with cycle lengths of 21 to 35 days Ovulation: mid‐luteal serum progesterone concentration of ≥ 25 nmol/L Clinical pregnancy: transvaginal ultrasound showing at least 1 intrauterine gestation sac with foetal heart beat 7 ± 2 weeks after hCG administration Ongoing pregnancy: transvaginal ultrasound showing at least 1 viable foetus 12 ± 2 weeks after hCG administration OHSS: categorised as mild, moderate, or severe according to classification of Golan 1989 Sponsored by Ferring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated randomisation list prepared by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Central computerised allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and sponsor study staff were blinded to treatment allocation. The treatment code was not unblinded for any participant during the study. Gonadotrophin distribution was handled by research nurses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinding was performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts accounted for. Per protocol and intention‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | Low risk | No indication. Intended outcomes reported according to protocol |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored by Ferring |
Revelli 2006.
| Methods | Prospective, randomised trial Location of the trial: Reproductive Medicine and IVF Unit of the University of Turin. Duration and timing: not stated. Sample size calculation: 2‐sided significance level of 0.05 and a power of 80%. 130 women were needed for the study. 260 women randomised 1 cycle/woman Ratio between rFSH and FSH‐HP was 1:1 A cost‐minimisation analysis was performed |
|
| Participants | Normo‐ovulatory women with unexplained infertility (N = 184) and clomiphene citrate‐resistant women with PCOS (N = 76) . Mean age (± SD) of the women in years was 33.0 (3.6) for the FSH‐HP group and 32.3 (4.0) for the rFSH group Body mass index (± SD) was 21.2 (3.0) and 21.3 (3.1) respectively Duration of infertility in years (± SD) was 2.7 (1.4) and 2.5 (1.4) respectively Number of women with primary infertility was 74.6% and 76.1% respectively LH:FSH ratio (± SD) was 1.3 (0.9) and 1.4 (0.9) respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, testosterone), tubal tests by hysterosalpingography or laparoscopy, and semen analysis |
|
| Interventions | HP‐HMG versus rFSH as second‐line treatment in women with PCOS Treatment was started 3 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 75 IU daily. If no ovarian response was detected after 2 weeks, the daily dose was increased to 112.5 IU. The maximum allowed daily dose was 225 IU per day hCG (10.000 IU, Profasi HP) was given when a single follicle of ≥ 18 mm, or 2 to 3 follicles of ≥ 18 mm (without other follicles ≥ 12 mm) developed. hCG was not given in cases of no follicular response or ≥ 3 follicles of ≥ 18 mm Luteal phase was supported by vaginal progesterone at a daily dose of 200 mg for 12 days starting on day 2 following hCG administration |
|
| Outcomes | Cost of therapy per delivered baby Monofollicular ovulation rate Total FSH dose Length of follicular phase Number of developing follicles (> 12 mm) Number of cancellations Endometrial thickness at the time of hCG administration Incidence of OHSS Multiple pregnancies Delivery rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on a computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not clear how allocation was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. Canceled cycles were reported; 20/39 in the uFSH group, 16/37 in the rFSH group. Per protocol and intention‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. Intended outcomes reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Unclear whether this was a sponsored trial |
Sagle 1991.
| Methods | Randomised controlled trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 30 women randomised 3 cycles/woman Ratio between uFSH and HMG was 1:1 Per protocol and intention‐to‐treat analyses were performed |
|
| Participants | Women with anovulatory PCOS unresponsive to clomiphene citrate (see Notes) Women were < 38 years of age Women with a body mass index over 30, tubal disease, or abnormal male function tests (total count of < 40 million or motility of < 50%, or both) were not entered in the study Mean serum concentration of LH and FSH (± SD) was 9.1 (6.5) and 4.6 (1.6) in the uFSH group and 10.6 (6.2) and 4.4 (2.0) in the HMG group Duration of infertility: not specified Number of women with primary infertility: not specified |
|
| Interventions | uFSH versus HMG as second‐line treatment Treatment was started 2 or 3 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 75 IU daily. If no ovarian response was detected after 2 weeks, the daily dose was increased to 112.5 IU. The dose was increased by 37.5 IU every week until a follicle of ≥ 12 mm was observed hCG (5000 IU) was given when the dominant follicle was ≥ 18 mm, and if a progressive increase in endometrial thickness had been observed. hCG was not given in cases when more than 3 follicles ≥ 15 mm were seen |
|
| Outcomes | Ovulation rate Incidence of OHSS Total FSH dose Pregnancy rate Miscarriage rate Live birth Multiple pregnancies LH and FSH levels during treatment |
|
| Notes | PCOS: 10 or more follicles 2 to 10 mm in diameter observed on ultrasound in 1 plane, and either an ovarian volume ≥ 9 cm³, or an increased stromal area (or both), combined with elevated LH, testosterone, or both Unresponsive to clomiphene citrate: failure to ovulate (lack of follicular development demonstrated on ultrasound, and low serum progesterone) at a maximum dosage of 150 mg/day. Ovulation: mid‐luteal progesterone of > 30 nmol/L Pregnancy: serum hCG level of > 25 IU/L Clinical pregnancy: ultrasound showing a gestational sac OHSS: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated; method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts or cancelled cycles reported. Follow‐up of all treatment cycles was complete |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all intended outcomes reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Had commercial sponsor |
Seibel 1985.
| Methods | Randomised controlled (partly cross‐over) trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 23 women randomised Number of cycles/woman: not stated |
|
| Participants | Women diagnosed with classic PCOD (see Notes) who failed to ovulate or failed to conceive with clomiphene citrate Baseline characteristics: not stated |
|
| Interventions | HMG versus uFSH as second‐line treatment HMG group: starting dose was 2 to 3 ampoules daily for 4 days. Total duration of treatment was 8 to 14 days hCG (5000IU) was given when the leading follicle measured 18 mm and serum E2 levels reached a preovulatory window. hCG was not given in cases of ≥ 3 preovulatory follicles, or excessively high serum E2 levels uFSH group: Starting dose was 40 to 50 IU daily, and maintained for 7 days. If the leading follicle was < 10 mm, the dose was increased by 50 IU per day. The maximum allowed daily dose was 150 IU. Total duration of treatment was 13 to 36 days. In this group, no hCG was given. Among the 10 women who received uFSH, 7 also received HMG and hCG for 11 cycles |
|
| Outcomes | Ovulation rate Conception rate (not defined) Incidence of mild hyperstimulation (see Notes) Number of follicles |
|
| Notes | Classic PCOD: amenorrhoeic women with LH levels > 30 mIU/mL and low to low‐normal FSH levels Clomiphene failure: no ovulation or no conception after at least 6 cycles of clomiphene citrate Ovulation: ultrasound criteria, a biphasic basal body temperature chart and serum progesterone concentration of > 4 ng/mL Mild hyperstimulation: ovaries measured between 5 and 7 cm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated, method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 cycles were cancelled because of anticipated hyperstimulation, multiple births, or both. Follow‐up for all other cycles was complete. No ITT |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all intended outcomes reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Had commercial sponsor |
Szilágyi 2004.
| Methods | Multicentre randomised controlled trial Duration, timing, and location of the trial: 2 centres in Italy Sample size calculation: not stated 20 women randomised Up to 3 cycles/woman Ratio between uFSH and rFSH was 1:1 |
|
| Participants | PCOS women (see Notes) who failed to ovulate with clomiphene citrate (100 mg/day for 5 days), administered for at least 3 months Mean age, body mass index, and duration of infertility were not stated Content of infertility work‐up: not stated |
|
| Interventions | rFSH versus uFSH as second‐line treatment Starting dose was 75 IU. The dose was administered for 14 days, with an increment of 37.5 IU every 7 days until there was active follicular development, and an endometrial thickness of at least 8 mm. The maximum allowed daily dose was 150 IU hCG (10000 IU, Profasi) was given when 1 to 3 follicles of ≥ 16 mm developed, with an endometrium thickness of > 8 mm. hCG was not given if serum estradiol level was > 4000 pmol/L, or > 3 follicles developed of ≥ 16 mm (or both), or if no follicular growth occurred after 35 days of treatment |
|
| Outcomes | Ovulation rate Total FSH dose and duration of FSH treatment Estradiol and progesterone levels Live birth rate Miscarriages Incidence of OHSS |
|
| Notes | PCOS: enlarged ovaries with multiple cysts on ultrasound, oligo‐ or amenorrhoea, hirsutism, and infertility. Elevated serum LH with (sub)normal FSH concentrations and elevated testosterone, androstenedione or dehydroepiandrosterone sulfate levels (or both) Ovulation: not defined OHSS: Grade I, II, and III; not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated; method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 treatment cycles were cancelled because of unsuccessful stimulation. No dropouts were reported. No ITT |
| Selective reporting (reporting bias) | High risk | Unclear whether all intended outcomes reported |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Had commercial sponsor |
Taketani 2010.
| Methods | Randomised, single‐blind, prospective, multicentre trial Duration, timing, and location of the trial: 21 centres in Japan, between February 2007 and December 2007 A sample size with 95% power calculation was performed 1 cycle/woman Ratio between the 2 groups was 1:1 Parallel design An intention‐to‐treat analysis was performed. There were 4 dropouts. |
|
| Participants | 265 women with amenorrhoea or anovulatory cycles including PCOS who failed to ovulate or get pregnant despite 2 or more cycles of anti‐oestrogen therapy Mean age of women was 31.9 years (range 21 ‐ 39) Mean body mass index was 21.2 (range 17.0 ‐ 28.0) |
|
| Interventions | Women received either subcutaneous follitropin alfa or urofollitropin (Fertinorm HP) as second‐line treatment in a low‐dose step‐up regimen of maximum 28 days. The starting dose was 75 IU/day and increased with 37.5 IU every 7 days as required to a maximum of 187.5 IU | |
| Outcomes | Multiple pregnancy rate Ovulation rate Clinical pregnancy rate Ovarian hyperstimulation syndrome (OHSS) rate Total gonadotrophin dose Mean duration of stimulation days |
|
| Notes | Ovulation: a mid‐luteal serum progesterone ≥ 5 ng/mL Sponsor: Serono OHSS: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, unclear from abstract |
| Allocation concealment (selection bias) | Unclear risk | Not described, unclear from abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blinded for personnel and outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not sure, seems complete and ITT |
| Selective reporting (reporting bias) | Unclear risk | Unclear from abstract |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | Sponsored study |
Weiss 2018.
| Methods | Two‐by‐two factorial, multicentre, randomised controlled clinical trial Duration, timing, and location of the trial: Between Dec 8, 2008 and Dec 16, 2015 in 48 Dutch hospitals Sample size calculation: alpha of 5% and a power of 88% at three degrees of freedom; 600 women were needed for the study 666 women randomised The mean number of cycles per woman (± SD) ranged from 3.3 to 4 An intention‐to‐treat analysis was performed |
|
| Participants | Subfertile women with WHO class II anovulation who were ovulatory on clomiphene citrate, but had not conceived in 6 ovulatory cycles (see Notes) Mean age (± SD) of the women in years was 29.5 (3.7) for the gonadotrophins + IUI group, 29.9 (3.7) for the gonadotrophins + intercourse group, 30.0 (3.6) for the clomiphene citrate + IUI group, and 29.9 (4.0) for the clomiphene citrate + intercourse group Body mass index (± SD) was 25.4 (5.1) for the gonadotrophins + IUI group, 25.6 (5.6) for the gonadotrophins + intercourse group, 25.0 (4.9) for the clomiphene citrate + IUI group, and 25.4 (5.0) for the clomiphene citrate + intercourse group Duration of infertility in months (± SD) was 26.3 (14.9) for the gonadotrophins + IUI group, 24.5 (12.5) for the gonadotrophins + intercourse group, 24.5 (15.5) for the clomiphene citrate + IUI group, and 25.9 (19.0) for the clomiphene citrate + intercourse group Number of women with primary infertility: not stated LH (IU/L ± SD) was 9.7 (7.4) for the gonadotrophins + IUI group, 10.6 (7.8) for the gonadotrophins + intercourse group, 10.6 (7.6) for the clomiphene citrate + IUI group, and 10.9 (10.8) for the clomiphene citrate + intercourse group FSH (IU/L ± SD) was 5.7 (2.1) for the gonadotrophins + IUI group, 5.7 (1.7) for the gonadotrophins + intercourse group, 6.2 (2.2) for the clomiphene citrate + IUI group, and 6.0 (2.2) for the clomiphene citrate + intercourse group Infertility workup included semen analysis and endocrinology screening to rule out hyperprolactinaemia and uncorrected thyroid dysfunction |
|
| Interventions | Gonadtrophins vs clomiphene citrate Gonadotrophin treatment was started on the third to fifth day of a menstrual bleed. Treatment was not started if ultrasound showed ovarian cysts bigger than 25 mm in mean diameter. uFSH or rFSH was used with a starting dose of 50 IU or 75 IU daily. hCG (5000 IU or 10 000 IU) was given when at least one follicle with a diameter of at least 16 mm was present Clomiphene citrate treatment was started on the third to fifth day of a menstrual bleed; dosage varied between 50 mg and 150 mg daily, for 5 days. If ovulation did not occur, the dosage was increased in increments of 50 mg, to a maximum of 150 mg daily in the next cycles |
|
| Outcomes | Live birth rate (see Notes) Ongoing pregnancy Multiple pregnancy Clinical pregnancy Miscarriage (see Notes) OHSS (see Notes) Ectopic pregnancy Gestational age Fetal birthweight Pregnancy complications — i.e. hypertensive disorders, gestational diabetes, and preterm labour Costs |
|
| Notes | WHO Class II anovulation: menstrual cycle > 35 days, normogonadotropic, normo‐oestrogenic, oligo‐anovulation, or anovulation Live birth: conception leading to live birth within 8 months after randomisation, defined as any baby born alive with a gestational age beyond 24 weeks Clinical pregnancy: defined as any registered heart beat at sonography Multiple pregnancy: defined as a registered heart beat of at least two fetuses at 12 weeks of gestation Miscarriage: defined as loss of an intrauterine pregnancy confirmed by ultrasound or histological examination before the 20th week of pregnancy OHSS not defined. None of the women were hospitalised and none were registered with OHSS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was prepared by an independent statistician with a variable block size (maximum block size of 8) |
| Allocation concealment (selection bias) | Low risk | Women were randomly allocated by means of a central password‐protected internet‐based randomisation programme |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis; exclusions were reported |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate, costs, and gestational age will be reported elsewhere |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Low risk | Funded by The Netherlands Organization for Health Research and Development. The funder of the study had no involvement in study design, data collection, analysis, or interpretation. |
Yarali 1999.
| Methods | Prospective, randomised trial Duration, timing, and location of the trial: not stated Sample size calculation: not stated 51 women randomised 3 cycles/woman Ratio between uFSH and rFSH was approximately 2:1. Per protocol and intention‐to‐treat analyses were performed |
|
| Participants | Clomiphene citrate‐resistant WHO Group II chronic anovulatory women (see Notes) Mean age (± SD) of the women in years was 27.8 (4.8) for the uFSH group and 30.0 (5.8) for the rFSH group Body mass index (± SD) was 27.1 (5.5) and 27.1 (3.7), respectively Duration of infertility in years (± SD) was 7.0 (5.6) and 9.0 (4.2), respectively Number of women with primary infertility was 57.1% and 64.5%, respectively LH:FSH ratio (± SD) was 2.4 (1.3) and 3.4 (5.5), respectively Infertility work‐up consisted of endocrinology (FSH, prolactin, TSH, testosterone), tubal tests by hysterosalpingography or laparoscopy, or hysteroscopy, and semen analysis |
|
| Interventions | uFSH versus rFSH as second‐line treatment Treatment was started 3 to 5 days after a spontaneous, or progesterone‐induced menstrual bleed Starting dose was 75 IU daily, and was maintained for up to 14 days unless follicular maturity was reached. After this, the dose was maintained, or increased by 37.5 IU according to individual response. The maximum allowed daily dose was 225 IU hCG (10,000 IU, Profasi HP) was given when a single follicle of ≥ 17 mm was detected. hCG was not given in cases of > 4 follicles of ≥ 15 mm |
|
| Outcomes | Ovulation rate Clinical pregnancy rate Number of follicles Endometrial thickness at the time of hCG administration Duration of luteal phase Incidence of OHSS Total FSH dose and duration of stimulation FSH level on day of hCG administration Miscarriages Multiple pregnancies Number of cancellations |
|
| Notes | Clomiphene‐resistant: consistent failure to ovulate with incremental doses of clomiphene citrate up to 150 mg/day in 3 previous cycles, or failure to conceive with the ovulatory dose during 6 previous cycles Ovulation: mid‐luteal serum progesterone concentration of > 5 ng/mL Clinical pregnancy: transvaginal ultrasound showing at least 1 gestational sac OHSS: Not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a participant number from a randomisation list corresponding with patient drug codes |
| Allocation concealment (selection bias) | High risk | Participant number from a randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 treatment cycles were cancelled because of > 4 follicles of > 15 mm, or a lack of response. ITT and per protocol analysis |
| Selective reporting (reporting bias) | Low risk | Intended outcomes reported. |
| Other bias | Unclear risk | Insufficient information was available to evaluate this risk |
| Funding | Unclear risk | rFSH was provided by Ares‐Serono |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Homburg 1990 | Pregnancy not defined and data presented per cycle only |
| Jacobs 1987 | Pregnancy not defined and data presented per cycle only |
| Larsen 1990 | Cross‐over study, not possible to extract data per woman |
| Rashidi 2016 | Ineligible intervention (co‐treatment with clomiphene citrate) |
| Ricci 2004 | Outcome measure was the effect of FSH on haemostasis |
| Zhou 2016 | Ineligible comparator (rFSH from two different pharmaceutical companies) |
E2: estradiol
DHEAS: dehydroepiandrosterone sulfate
FSH: follicle stimulating hormone
FSH‐HP: highly purified follicle stimulating hormone
hCG: human chorionic gonadotropin
HP‐HMG: highly purified human menopausal gonadotrophin
HMG: human menopausal gonadotropin
HSG: hysterosalpingogram
IM: intramuscular
IUI: intrauterine insemination
LH: luteinising hormone
OHSS: ovarian hyperstimulation syndrome
PCOD: polycystic ovarian disease
PCOS: polycystic ovary syndrome
rFSH: recombinant follicle stimulating hormone
SC: subcutaneous
SD: standard deviation
TSH: thyroid‐stimulating hormone
WHO: World Health Organization
Characteristics of studies awaiting assessment [ordered by study ID]
Bejarano Velazquez 2016.
| Methods | A randomised clinical trial, open label Duration: from January to December 2015 152 cycles: Group A (rFSH+rLH) 51 cycles, Group B (rFSH) 53 cycles, Group C (hMG) 48 cycles |
| Participants | Women aged ≤35 years with unexplained infertility or anovulation, BMI ≤30 kg/m2, unilateral or bilateral tubal permeability, normal thyroid function, baseline serum FSH level ≤10 UI/l |
| Interventions | rFSH+rLH vs rFSH vs hMG |
| Outcomes | Ovulatory cycles Cancelled cycles Number of follicles of 18–23 mm Estradiol levels on day 10 Days of stimulation Total units administered Endometrial morphology Pregnancy rate |
| Notes | We have contacted the authors seeking further information |
NCT01923194.
| Methods | Interventional randomised clinical trial Duration: October 2013 ‐ August 2015 |
| Participants | Chinese women between the ages of 20 and 39 years Duration of infertility: at least 1 year before screening WHO type II anovulatory infertility with chronic anovulation |
| Interventions | Highly purified urofollitropin versus recombinant human follitropin alfa |
| Outcomes | Ovulation rate Positive serum progesterone rate Positive serum β‐hCG/hCG rate Clinical pregnancy rate Ongoing pregnancy rate Follicular development Endometrial thickness Total FSH administered Number of FSH treatment days Frequency and severity of adverse events Frequency and severity of injection site reactions Serum estradiol (E2) levels |
| Notes |
Differences between protocol and review
In the 2018 update:
One comparison was added: gonadotrophin versus continued clomiphene citrate.
We changed ovarian hyperstimulation syndrome (OHSS) from a primary outcome to a secondary outcome. In turn, we listed multiple pregnancy as a primary outcome. Due to the improvement of stimulation protocols, OHSS has become a very rare outcome. Moreover, OHSS is often poorly defined. Furthermore, multiple pregnancy remains a very important safety outcome.
Dichotomous outcomes were summarised using Risk Ratio (RR) rather than Odds Ratio (OR).
Contributions of authors
MvW developed the protocol. NW, MN, NB, BM, and FV read the protocol, commented upon it, and agreed with its content.
NW, EK, and MvW conducted the literature searches for the review, selected relevant trials, procured data and information about studies, assessed the validity and checked the data extraction for each trial, entered all study information, data, and text into Review Manager 5, performed the analyses, wrote the abstract, background, methods, results, and conclusion sections of the review, and gave approval to the final version.
MN, BM, and FV took part in writing the abstract, background, methods, results, and conclusion sections of the review, and gave approval to the final version.
Sources of support
Internal sources
Center for Reproductive Medicine, VU Medical Center and Academic Medical Center, Netherlands.
External sources
No sources of support, Netherlands.
Declarations of interest
Nienke Weiss is the lead author of one of the included studies (Weiss 2018).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Balen 2007 {published data only}
- Balen A, Platteau P, Nyboe Andersen A, Devroey P, Helmgaard L, Arce J. Highly purified FSH is as efficacious as recombinant FSH for ovulation induction in women with WHO Group II anovulatory infertility: a randomized controlled non‐inferiority trial. Human Reproduction 2007;22(7):1816‐23. [DOI] [PubMed] [Google Scholar]
Coelingh Bennink 1998 {published data only}
- Coelingh Bennink H, Fauser B, Out H. Recombinant follicle‐stimulating hormone (FSH; Puregon) is more efficient than urinary FSH (Metrodin) in women with clomiphene‐resistant, normogonadotropic, chronic anovulation; a prospective, multicenter, assessor‐blind, randomized, clinical trial. Fertility and Sterility 1998;69(1):19‐25. [DOI] [PubMed] [Google Scholar]
Feigenbaum 2001 {published data only}
- Feigenbaum SL, Miller P, Kaufmann R, Elkind‐Hirsch K, Fein SH, Marshall DC. A new highly purified human‐derived FSH, Bravelle, is as effective and well tolerated as recombinant follitropin beta in ovulation induction in infertile women with ovulatory dysfunction. Today's Therapeutic Trends 2001;19(4):297‐313. [Google Scholar]
Gadir 1990 {published data only}
- Gadir A, Mofawi R, Alnaser H, Alrashid A, Alonezi O, Shaw R. Ovarian electrocautery versus human menopausal gonadotrophins and pure follicle stimulating hormone therapy in the treatment of patients with polycystic ovarian disease. Clinical Endocrinology 1990;33(5):585‐92. [DOI] [PubMed] [Google Scholar]
Gerli 2004 {published data only}
- Gerli S, Casini ML, Unfer V, Costabile L, Mignosa M, Renzo GC. Ovulation induction with urinary FSH or recombinant FSH in polycystic ovary syndrome patients: a prospective randomized analysis of cost‐effectiveness. Reproductive BioMedicine Online 2004;9(5):494‐9. [DOI] [PubMed] [Google Scholar]
Loumaye 1996 {published and unpublished data}
- Loumaye E, Martineau I, Piazzi A, O'Dea L, Ince S, Howles C, et al. Clinical assessment of human gonadotrophins produced by recombinant DNA technology. Human Reproduction 1996;11(1):95‐107. [DOI] [PubMed] [Google Scholar]
McFaul 1990 {published data only}
- McFaul P, Traub A, Thomson W. Treatment of clomiphene citrate‐resistant polycystic ovarian syndrome with pure follicle‐stimulating hormone or human menopausal gonadotropin. Fertility and Sterility 1990;53(5):792‐7. [DOI] [PubMed] [Google Scholar]
Platteau 2006 {published data only}
- Platteau P, Nyboe Andersen A, Balen A, Devroey P, Sørensen P, Helmgaard L, et al. Similar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled study. Human Reproduction 2006;21(7):1798‐804. [DOI] [PubMed] [Google Scholar]
Revelli 2006 {published data only}
- Revelli A, Poso F, Gennarelli G, Moffa F, Grassi G, Massobrio M. Recombinant versus highly‐purified, urinary follicle‐stimulating hormone (rFSH vs. HP‐uFSH) in ovulation induction: a prospective, randomized study with cost‐minimization analysis. Reproductive Biology and Endocrinology 2006;4:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagle 1991 {published data only}
- Sagle M, Hamilton‐Fairley D, Kiddy D, Franks S. A comparative, randomized study of low‐dose human menopausal gonadotropin and follicle‐stimulating hormone in women with polycystic ovarian syndrome. Fertility and Sterility 1991;55(1):56‐60. [DOI] [PubMed] [Google Scholar]
Seibel 1985 {published data only}
- Seibel MM, McArdle C, Smith D, Taymor ML. Ovulatoin induction in polycystic ovary syndrome with urinary follicle‐stimulating hormone or human menopausal gonadotropin. Fertility and Sterility 1985;43(5):703‐7. [DOI] [PubMed] [Google Scholar]
Szilágyi 2004 {published data only}
- Szilágyi A, Bártfai G, Mánfai A, Koloszár S, Pál A, Szabó I. Low‐dose ovulation induction with urinary gonadotrophins or recombinant follicle stimulating hormone in patients with polycystic ovary syndrome. Gynaecological Endocrinology 2004;18(1):17‐22. [DOI] [PubMed] [Google Scholar]
Taketani 2010 {published data only}
- Taketani Y, Kelly E, Yoshimura Y, Hoshiai H, Irahara M, Mizunuma H, et al. Recombinant follicle stimulating hormone (follitropin alfa) versus purified urinary follicle stimulating hormone in a low dose step‐up regimen to induce ovulation in Japanese women with anti‐estrogen‐ineffective oligo‐ or anovulatory infertility. Reproductive Medicine and Biology 2010;9:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2018 {published and unpublished data}
- Weiss NS, Nahuis MJ, Bordewijk E, Oosterhuis JE, Smeenk JM, Hoek A, et al. Gonadotrophins versus clomiphene citrate with or without intrauterine insemination in women with normogonadotropic anovulation and clomiphene failure (M‐OVIN): a randomised, two‐by‐two factorial trial. Lancet 2018;391(10122):758–65. [DOI] [PubMed] [Google Scholar]
Yarali 1999 {published data only}
- Yarali H, Bukulmez O, Gurgan T. Urinary follicle‐stimulating hormone (FSH) versus recombinant FSH in clomiphene citrate‐resistant, normogonadotropic, chronic anovulation: a prospective randomized study. Fertility and Sterility 1999;72(2):276‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Homburg 1990 {published data only}
- Homburg R, Eshel A, Kilborn J, Adams J, Jacobs HS. Combined luteinizing hormone releasing hormone analogue and exogenous gonadotrophins for the treatment of infertility associated with polycystic ovaries. Human Reproduction 1990;5(1):32‐5. [DOI] [PubMed] [Google Scholar]
Jacobs 1987 {published data only}
- Jacobs H, Porter R, Eshel A, Craft I. Profertility uses of LHRH agonist analogues. In: Vickery BH, Nestor JJ Jr editor(s). LHRH and its Analogues; Contraceptive and Therapeutic Application. Part 2. Lancaster: MTP Press, 1987:303‐22. [Google Scholar]
Larsen 1990 {published data only}
- Larsen T, Larsen JF, Schiøler V, Bostofte E, Felding C. Comparison of urinary human follicle‐stimulating hormone and human menopausal gonadotropin for ovarian stimulation in polycystic ovarian syndrome. Fertility and Sterility 1990;53(3):426‐31. [DOI] [PubMed] [Google Scholar]
Rashidi 2016 {published data only}
- Rashidi M, Najmi Z, Mobasseri A. Advantages of recombinant follicle‐stimulating hormone over human menopausal gonadotropin in intrauterine insemination: a randomized clinical trial in polycystic ovary syndrome‐associated infertility. Gynecologic and Obstetric Investigation 2016;81(2):118‐23. [DOI] [PubMed] [Google Scholar]
Ricci 2004 {published data only}
- Ricci G, Cerneca F, Simeone R, Pozzobon C, Guarnieri S, Sartore A, et al. Impact of highly purified urinary FSH and recombinant FSH on haemostasis: an open‐label, randomized, controlled trial. Human Reproduction 2004;19(4):838‐48. [DOI] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou YZ, Shen H, Zuo WL, Xu YH, Deng XH, Chen YL, et al. A randomized, single‐blind, parallel‐controlled and multicentre study: compare the efficacy and safety of domestic and imported human recombinant FSH in WHO group II anovulatory infertility. Chung‐Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics & Gynecology) 2016; Vol. 51, issue 4:258‐63. [DOI] [PubMed]
References to studies awaiting assessment
Bejarano Velazquez 2016 {unpublished data only}
- Bejarano Velázquez D, O Pérez LO, Treviño Báez JD, Gonzalez Díaz OA. A randomized trial comparing the efficacy and safety of rFSH + rLH versus rFSH alone and HMG to induce ovulation in cycles for low complexity techniques. Human Reproduction 2016;31(Suppl_1):i304. [Google Scholar]
NCT01923194 {unpublished data only}
- NCT01923194. Evaluation of efficacy and safety of highly purified urofollitropin for ovulation induction in Chinese females (Compass). clinicaltrials.gov/ct2/show/NCT01923194 (first posted 15 August 2013).
Additional references
Adams 1986
- Adams J, Polson DW, Franks S. Prevelance of polycystic ovaries in women with anovulation and idiopathic hirsutism. British Medical Journal (Clinical Research ed.) 1986;293(6543):355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albano 1996
- Albano C, Smitz J, Camus M, Coelingh Bennink H, Steirteghem AC, Devroey P. Pregnancy and birth in an in‐vitro fertilization cycle after controlled ovarian stimulation in a women with a history of allergic reaction to human menopausal gonadotrophin. Human Reproduction 1996;11(8):1632‐4. [DOI] [PubMed] [Google Scholar]
Andersen 2004
- Andersen CY, Westergaard LG, Wely M. FSH isoform composition of commercial gonadotrophin preparations: a neglected aspect?. Reproductive Biomedicine Online 2004;9(2):231‐6. [DOI] [PubMed] [Google Scholar]
Baenziger 1988
- Baenziger JU, Green ED. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of asparagine‐linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochimica et Biophysica Acta 1988;947(2):287‐306. [DOI] [PubMed] [Google Scholar]
Balen 2013
- Balen AH. Ovulation induction in the management of anovulatory polycystic ovary syndrome. Molecular and Cellular Endocrinology 2013;373(1‐2):77‐82. [DOI] [PubMed] [Google Scholar]
Bergh 1999
- Bergh C. What are the clinical benefits of recombinant gonadotrophins? Recombinant follicle stimulating hormone. Human Reproduction 1999;14(6):1418‐20. [DOI] [PubMed] [Google Scholar]
Biffoni 1994
- Biffoni M, Battaglia A, Borrelli F, Cantelmo A, Galli G, Eshkol A. Allergenic potential of gonadotrophic preparations in experimental animal: Relevance of purity. Human Reproduction 1994;9(10):1845‐8. [DOI] [PubMed] [Google Scholar]
De Leeuw 1996
- Leeuw R, Mulders J, Voortman G, Rombout F, Damm J, Kloosterboer L. Structure‐function relationship of recombinant follicle stimulating hormone (Puregon). Molecular Human Reproduction 1996;2(5):361‐9. [DOI] [PubMed] [Google Scholar]
Farquhar 2012
- Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD001122.pub4] [DOI] [PubMed] [Google Scholar]
Franik 2018
- Franik S, Kremer JA, Nelen WL, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD010287.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gemzell 1958
- Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle‐stimulating hormone (FSH). Journal of Clinical Endocrinology and Metabolism 1958;18(12):1333‐48. [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A, Ron‐el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical & Gynecological Survey 1989;44:430‐40. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 17 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gray 1988
- Gray CJ. Glycoprotein gonadotrophins. Structure and synthesis. Acta Endocrinologica. Supplementum 1988;288:20‐7. [PubMed] [Google Scholar]
Hard 1990
- Hard K, Mekking A, Damm JB, Kamerling JP, Boer W, Wijnands RA, et al. Isolation and structure determination of the intact sialylated N‐linked carbohydrate chains of recombinant human follitropin (hFSH) expressed in Chinese hamster ovary cells. European Journal of Biochemistry 1990;193(1):263‐71. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Howles 1996
- Howles CM. Genetic engineering of human FSH (Gonal F). Human Reproduction Update 1996;2(2):172‐91. [DOI] [PubMed] [Google Scholar]
Jewelewicz 1973
- Jewelewicz R, Dyrenfurth I, Warren MP, Wiele RL. Gonadotrophin therapy. Female Infertility. Amsterdam: Excerpta Medica, 1973. [Google Scholar]
Keene 1989
- Keene JL, Matzuk MM, Otani T, Fauser BC, Galway AB, Hsueh AJ, et al. Expression of biologically active human follitropin in Chinese hamster ovary cells. Journal of Biological Chemistry 1989;264(9):4769‐75. [PubMed] [Google Scholar]
Lambert 1995
- Lambert A, Rodgers M, Mitchell R, Wood AM, Wardle C, Hilton B, et al. In‐vitro biopotency and glycoform distribution of recombinant human follicle stimulating hormone (Org 32489), Metrodin and Metrodin‐HP. Human Reproduction 1995;10(7):1928‐35. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunenfeld 1960
- Lunenfeld B, Menzi A, Volet B. Clinical effects of a human postmenopausal gonadotropin. La Rassegna di Clinica, Terapia e Scienze Affini 1960;59:213‐6. [PubMed] [Google Scholar]
Nahuis 2009
- Nahuis M, Veen F, Oosterhuis J, Mol BW, Hompes P, Wely M. Review of the safety, efficacy, costs and patient acceptability of recombinant follicle‐stimulating hormone for injection in assisting ovulation induction in infertile women. International Journal of Women's Health 2009;1:205‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rotterdam consensus group 2004a
- Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction 2004;19(1):41‐7. [DOI] [PubMed] [Google Scholar]
Rotterdam consensus group 2004b
- Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004;81(1):19‐25. [DOI] [PubMed] [Google Scholar]
Stockell Hartree 1992
- Stockell Hartree AS, Renwick AG. Molecular structures of glycoprotein hormones and functions of their carbohydrate components. Biochemical Journal 1992;287(Part 3):665‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
Wang 2017
- Wang R, Kim BV, Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta‐analysis. BMJ 2017;356:j138. [doi: https://doi.org/10.1136/bmj.j138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wide 1997
- Wide L. Isoforms of human gonadotrophins under different physiological conditions. In: Kahn JA editor(s). Gonadotrophin Isoforms: Facts and Future. Copenhagen: Ciconia Foundation, 1997:43‐8. [Google Scholar]
Wilson 1990
- Wilson CA, Leigh AJ, Chapman AJ. Gonadotrophin glycosylation and function. Journal of Endocrinology 1990;125(1):3‐14. [DOI] [PubMed] [Google Scholar]
Yen 1980
- Yen SS. The polycystic ovary syndrome. Clinical Endocrinology 1980;12:177‐207. [DOI] [PubMed] [Google Scholar]
Youssef 2014
- Youssef MA, Veen F, Al‐Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist‐assisted reproductive technology. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008046.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bayram 2001
- Bayram N, Wely M, Veen F. Recombinant FSH versus urinary gonadotrophins or recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002121.pub2] [DOI] [PubMed] [Google Scholar]
Nugent 2000
- Nugent D, Vanderkerchove P, Hughes E, Arnot M, Lilford R. Gonadotrophin therapy for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000410.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2015
- Weiss NS, Nahuis M, Bayram N, Mol BW, Veen F, Wely M. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD010290.pub2] [DOI] [PubMed] [Google Scholar]


