Abstract
Background
Electrical cardioversion is an effective procedure for restoring normal sinus rhythm in the hearts of patients with irregular heart rhythms. It is important that the patient is not fully conscious during the procedure, as it can be painful and distressing. The drug used to make patients unaware of the procedure should rapidly achieve the desired level of sedation, should wear off quickly and should not cause cardiovascular or respiratory side effects.
Objectives
We aimed to compare the safety, effectiveness and adverse events associated with various anaesthetic or sedative agents used in direct current cardioversion for cardiac arrhythmia in both elective and emergency settings.
We sought answers to the following specific questions.
• Which drugs deliver the best outcomes for patients undergoing electrical cardioversion?
• Does using a particular agent confer advantages or disadvantages?
• Is additional analgesic necessary to prevent pain?
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) on 27 March 2014. Our search terms were relevant to the review question and were not limited by outcomes. We also carried out searches of clinical trials registers and forward and backward citation tracking.
Selection criteria
We considered all randomized controlled trials and quasi‐randomized and cluster‐randomized studies with adult participants undergoing electrical cardioversion procedures in the elective or emergency setting.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data, consulting with a third review author for disagreements. We used standard Cochrane methodological procedures, including assessment of risk of bias for all studies.
Main results
We included 23 studies with 1250 participants that compared one drug with one or more other drugs. Of these comparisons, 19 studies compared propofol with another drug. Seven of these compared propofol with etomidate (four of which combined the drugs with remifentanil or fentanyl), five midazolam, six thiopentone and two sevoflurane. Three studies compared etomidate with thiopentone, and three etomidate with midazolam. Two studies compared thiopentone with midazolam, one thiopentone with diazepam and one midazolam with diazepam. Drug doses and the time over which the drugs were given varied between studies. Although all studies were described as randomized, limited information was provided about the methods used for selection and group allocation. A high level of performance bias was observed across studies, as study authors had not attempted to blind the anaesthetist to group allocation. Similarly, study authors had rarely provided sufficient information on whether outcome assessors had been blinded.
Included studies presented outcome data for hypotension, apnoea, participant recall, success of cardioversion, minor adverse events of nausea and vomiting, pain at injection site and myoclonus, additional analgesia and participant satisfaction. We did not pool the data from different studies in view of the multiple drug comparisons, differences in definitions and reporting of outcomes, variability of endpoints and high or unclear risk of bias across studies.
Authors' conclusions
Few studies reported statistically significant results for our relevant outcomes, and most study authors concluded that both, or all, agents compared in individual studies were adequate for cardioversion procedures. It is our opinion that at present, there is no evidence to suggest that current anaesthetic practice for cardioversion should change.
Keywords: Humans, Anesthetics, Anesthetics/administration & dosage, Anesthetics/adverse effects, Apnea, Apnea/chemically induced, Diazepam, Diazepam/administration & dosage, Diazepam/adverse effects, Electric Countershock, Electric Countershock/adverse effects, Electric Countershock/methods, Etomidate, Etomidate/administration & dosage, Etomidate/adverse effects, Fentanyl, Fentanyl/administration & dosage, Fentanyl/adverse effects, Hypnotics and Sedatives, Hypnotics and Sedatives/administration & dosage, Hypnotics and Sedatives/adverse effects, Hypotension, Hypotension/chemically induced, Mental Recall, Methyl Ethers, Methyl Ethers/administration & dosage, Methyl Ethers/adverse effects, Midazolam, Midazolam/administration & dosage, Midazolam/adverse effects, Piperidines, Piperidines/administration & dosage, Piperidines/adverse effects, Propofol, Propofol/administration & dosage, Propofol/adverse effects, Randomized Controlled Trials as Topic, Remifentanil, Sevoflurane, Thiopental, Thiopental/administration & dosage, Thiopental/adverse effects
Plain language summary
Anaesthetic drugs for cardioversion
Background
Electrical cardioversion is a procedure by which pads on the chest aim to return the heart to a normal rhythm following disturbances. This procedure is painful and can be distressing for the patient; therefore drugs are used to make patients unaware of the procedure. We aimed to compare the safety and effectiveness of the drugs used in electrical cardioversion.
Study characteristics
Evidence is current to 27 March 2014. We found 23 relevant randomized controlled trials with 1250 participants undergoing cardioversion procedures. These studies compared one anaesthetic drug against one or more other drugs, including propofol, etomidate, thiopentone, sevoflurane, midazolam and diazepam.
Key results
Study authors considered clinical outcomes such as decreased blood pressure, interrupted breathing and whether cardioversion was successful, as well as patient relevant outcomes such as recall, nausea and vomiting, pain on injection and satisfaction with the procedures. In addition to a variety of drug comparisons between studies, differences in study methods were described, with drugs given in different doses and over different lengths of time. These differences meant that it was inappropriate to combine the results of these studies.
Quality of the evidence
We believe that the quality of these studies was not sufficiently high, and that it would be misleading to combine the findings of all studies within this review. Study authors had not taken enough steps to reduce the risk of differences in methods within the studies, for example, by masking doctors and assessors regarding which drug was given to each patient.
Conclusions
Most authors of individual studies concluded that all agents studied were adequate for making patients unaware during cardioversion. It is our opinion that at present, there is no evidence to suggest that drugs used by anaesthetists to make patients unaware of cardioversion should change.
Background
Description of the condition
In electrical cardioversion, electrical current is delivered to patients and is synchronized with their existing, irregular heartbeat with the aim of converting tachycardia (irregular heart rhythm) to regular sinus rhythm. This is an effective procedure, particularly when the patient's cardiovascular condition is unstable (Blomstrom 2003; Resuscitation Council 2010). Cardioversion is usually performed externally with the use of pads on the chest (external cardioversion), but it can be done via intravenous electrode to the heart (internal cardioversion) or via balloon electrode through the oesophagus (trans‐oesophageal). It may be performed as an elective day‐case procedure or as an urgent procedure in the emergency department. Patients undergoing elective cardioversion are usually optimally prepared for the procedure, haemodynamically stable and starved, and the procedure takes place in a hospital department with appropriate staffing and equipment. Patients undergoing emergency cardioversion may be haemodynamically unstable and may have eaten recently, and cardioversion may take place in settings where staff members may be less familiar with the side effects of anaesthesia and its associated drugs and equipment, such as coronary care units and emergency departments.
Electrical or direct current (DC) cardioversion is one of the most widely used and successful methods of treating cardiac arrhythmias such as atrial fibrillation (AF). In the UK, Hospital Episode Statistics for England (2011 to 2012) reveal 21,127 admissions for DC cardioversion, of which 16,380 were day‐case admissions (Hospital Episode Statistics 2011 to 2012). The success of the procedure depends on both patient‐related and technique‐related factors. Patient‐related factors that affect success include length of time in arrhythmia, antiarrhythmic drugs taken, dimensions of the atrium, degree of obesity and presence of pulmonary disorders. Technique‐related factors include skin preparation, pressure on the paddles used, electrode placement, bi‐phasic or mono‐phasic defibrillation and initial and total energy levels (Reiffel 2009).
Description of the intervention
It is important that the patient is made unaware of cardioversion, as the procedure is painful and can be very distressing (Kowey 1988). The drug used should rapidly achieve the desired level of impairment of consciousness, should wear off quickly and should not cause cardiovascular, respiratory or other side effects. Few people recall the procedure as sedation deepens; however, this advantage should be balanced against increased risk of airway problems or respiratory and cardiovascular instability.
The title of this review reflects common conceptions of the drugs used to obtund consciousness. These drugs are usually thought of in three groups.
Drugs classified as intravenous anaesthetic agents (e.g. etomidate, propofol, thiopentone, methohexital).
Drugs classified as inhaled anaesthetic agents (e,g, isoflurane, sevoflurane).
Drugs classified as sedative agents (e.g. midazolam, diazepam) and given via any route (i.e. intramuscular, subcutaneous, intravenous, rectal).
However, it is important to note that this distinction is artificial, as all listed drugs provide sedation at low doses and anaesthesia at higher doses, as these two states exist along a continuum of consciousness. A recent UK guideline on the provision of safe sedation (AoMRC 2013), issued after national incident reporting systems had revealed cases of oversedation (Smith 2009a), offered the following definitions.
Anaesthesia: state of unconsciousness with no arousal by painful stimuli, usually requiring airway management and ventilatory support.
Moderate sedation: state in which the individual is able to make a purposeful response to verbal commands alone or accompanied by light tactile stimulation.
Deep sedation: state in which the individual cannot easily be aroused but responds purposefully to repeated or painful stimulation. This may be accompanied by clinically significant ventilatory depression. The individual may require assistance maintaining a patent airway, as well as positive‐pressure ventilation (AoMRC 2013).
The state of 'anaesthesia' is easier to define than lesser degrees of impairment of consciousness; definitions vary. The speed of onset of action and the side effects of all agents vary with dose and method of administration, for example, bolus or infusion. Anaesthetic or sedative agents that do not have cardiovascular side effects are preferable for cardioversion, as many patients have underlying cardiovascular disease.
Analgesic agents such as opioids may be used in conjunction with anaesthetic agents. Premedication is seldom given, although atropine is sometimes used before the procedure to reduce the risk of vagus nerve–induced bradyarrhythmia (slow abnormal heart rhythm).
Why it is important to do this review
The practice of cardioversion varies between clinicians and countries and involves use of an anaesthetic agent (such as propofol, etomidate, thiopentone or methohexital) or a sedative agent (such as midazolam or diazepam) with or without additional analgesia. A survey of UK hospitals in 2003 confirmed that many different agents were used for cardioversion; 90% of hospitals used propofol, 9% etomidate and 43% an additional short‐acting opiate as part of the anaesthetic (James 2003). Factors that influence the choice of drug include speed of action and recovery time, patient recall or awareness of pain, adverse effects caused by the drug and the influence of the drug on the success of the procedure. Currently no systematic review has compared different agents across these outcomes.
Numerous studies have compared different drugs designed to temporarily impair consciousness for cardioversion in emergency and elective settings. Most studies compared propofol against one or more other agents. For example, Coll‐Vinent 2003, Herregods 2003, Hullander 1993 and Siedy 2010 compared propofol versus etomidate and other agents, with the general conclusion that whilst similarly effective, propofol is superior in terms of reduced side effects, such as myoclonus, nausea and vomiting, prolonged sedation and time to recovery. Gale 1993, Gupta 1990 and Parlak 2006 compared propofol versus midazolam and other agents, and although Gale 1993 and Parlak 2006 again agreed that the superiority of propofol is due to more rapid anaesthetic onset and reduced recovery time, Gupta 1990 concluded that thiopentone was preferable to both propofol and midazolam, reporting a significantly greater decrease in mean blood pressure, as well as low blood oxygen saturation (< 95%), which was more common among participants in the propofol group. Participant‐reported outcomes for propofol over thiopentone were favoured, however, in a study by Valtonen 1988, with propofol described as making the anaesthetic experience "more pleasant".
We are aware of only one systematic review that looked specifically at the choice of agent for cardioversion (Wood 2006); this brief (non‐Cochrane) report concluded that propofol, methohexital, thiopentone and etomidate were all good choices for sedation for cardioversion, but midazolam and diazepam had longer recovery periods and so should be considered as second‐line agents. No meta‐analysis was included in this review, and different outcomes were not considered separately. Other reviews compared different agents used within the emergency department for procedures in addition to cardioversion; whilst Symington 2006 again showed benefit for the use of propofol, Hohl 2008 reported no differences whether midazolam or propofol was used.
Two published Cochrane protocols are of relevance; both Morão 2011 and Wakai 2008 are concerned with sedation for a range of procedures including cardioversion ‐ one using midazolam and the other propofol ‐ restricted to emergency department procedures. Two further Cochrane protocols are relevant: "Capnography versus standard monitoring for emergency department procedural sedation and analgesia" (Wall 2013) and "Atropine therapy versus no atropine therapy for the prevention of adverse events in paediatric patients undergoing intubation" (Wilmott 2014).
The most recent guidelines for the management of atrial fibrillation and ventricular arrhythmias (Camm 2012; Fuster 2001; Fuster 2006; Link 2010; NICE CG36 2006; Zipes 2006) recommend the use of a rapid‐acting anaesthetic agent for direct current cardioversion ‐ especially in the emergency treatment setting, where delivering the shock as quickly as possible is important ‐ but do not state which exact agent should be used. Currently, no guidelines indicate which particular anaesthetic agent should be used for cardioversion.
A summary and systematic review of the existing literature will enable clinicians to become better informed about the advantages and disadvantages of different agents, so they can choose the best option for their patients.
Objectives
We aimed to compare the safety, effectiveness and adverse events associated with various anaesthetic or sedative agents used in direct current cardioversion for cardiac arrhythmia in both elective and emergency settings.
We sought answers to the following specific questions.
Which drugs deliver the best outcomes for patients undergoing electrical cardioversion?
Does using a particular agent confer advantages or disadvantages?
Is additional analgesia necessary to prevent pain?
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) including quasi‐randomized studies and cluster‐randomized studies.
Types of participants
We included studies that were performed in participants aged 16 years or older who were undergoing elective or emergency electrical cardioversion with the use of sedation or anaesthesia with or without supplemental analgesia.
We excluded trials of cardioversion that took place during other procedures or operations (e.g. during cardiac surgery, during cardiopulmonary resuscitation).
We included trials that examined a mixed participant population, such as some participants younger than 16 years or some participants undergoing additional procedures, only if the results pertaining to our population were reported separately.
Types of interventions
We included all studies that administered the following drugs to people undergoing electrical cardioversion.
Drugs classified as intravenous anaesthetic agents (e.g. etomidate, propofol, thiopentone, methohexital).
Drugs classified as inhaled anaesthetic agents (e.g. isoflurane, sevoflurane).
Drugs classified as sedative agents (e.g. midazolam, diazepam) administered via any route (i.e. intramuscular, subcutaneous, intravenous, rectal).
We included studies that compared different drugs between or within the above groups, or different doses of the same agent.
We examined each drug–drug comparison individually, so that only studies that compared the same agents, for example, propofol versus midazolam, would be considered for pooling.
Types of outcome measures
We reconsidered and changed our outcomes from the original protocol; further details are provided in Differences between protocol and review.
Primary outcomes
-
Major adverse events including the following.
Hypotension (defined as during the procedure; definitions such as > 20% decrease in systolic blood pressure, > 20 mmHg fall in systolic blood pressure to < 100 mmHg or the need for fluid intervention).
Unintended apneic episode (definitions such as no spontaneous respiration for > 20 seconds or the need for manual ventilation).
Patient awareness or recall of pain during the procedure.
Secondary outcomes
-
Minor adverse effects including general and drug‐specific effects and time profile of the drug used.
Nausea and vomiting.
Pain at the injection site.
Myoclonus for studied agents including etomidate.
All‐cause mortality within 30 days of the procedure.
Success of cardioversion: return to sinus rhythm, number of shocks required, energy required, length of time the participant remains in sinus rhythm.
Need for additional analgesia to prevent pain.
Patient satisfaction with the procedure. We did not wish to restrict potential available data by prespecifying the exact definitions used.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014 Issue 3;seeAppendix 1 for search strategy), MEDLINE via Ovid (1946 to March 2014; see Appendix 2), EMBASE via Ovid (1974 to March 2014; see Appendix 3) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO (see Appendix 4). We applied the highly sensitive Cochrane filter for RCTs in MEDLINE and EMBASE searches. We also searched trial registers such as www.clinicaltrials.gov and the Current Controlled Clinical Trials website (http://www.controlled‐trials.com/) for ongoing trials.
We limited the start date of searches based on the introduction date of DC electrical cardioversion in 1962. We did not restrict searches by language or location.
Searching other resources
We undertook forward and backward citation tracing for key review articles and eligible articles identified from electronic resources.
Data collection and analysis
Selection of studies
We merged the results of our searches using Endnote (Endnote), and Sharon R Lewis (SRL) removed by hand any duplicates not removed during the initial process.
Two review authors ‐ SRL and Amanda Nicholson (AN) ‐ independently sifted the initial search results and used a study eligibility form (see Appendix 5) to screen selected full‐text articles for potential inclusion. We referred disagreements that could not be resolved to Andrew F Smith (AFS) or Phil Alderson (PA). We recorded the numbers of papers retrieved and exclusions at each stage, along with reasons, in a PRISMA flowchart. We summarized the details of papers that were well known or were apparently eligible in the Characteristics of excluded studies table.
Data extraction and management
Two review authors ‐ SRL and Johnny Kenth (JK) ‐ used data extraction forms (see Appendix 6) to independently extract data from the included studies. We reviewed this form after data from the first three papers had been entered and modified it as required. If relevant information or data were not available in the paper, we contacted the lead study author to request the additional details. Again, we referred any disagreements that we could not resolve by discussion to AFS or PA for resolution.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to assess the quality of study design and the extent of potential bias (Higgins 2011). We considered the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcomes assessors.
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
We anticipated that It would be difficult for anaesthetists to be blinded completely to the agent, as they need to know in case of specific agent complications, but we noted whether attempts were made to blind other study personnel for outcomes such as complications.
We paid particular attention to sources of funding and the role of pharmaceutical companies and documented these details in the Characteristics of included studies.
We completed a 'Risk of bias' table for each included study.
Measures of treatment effect
Outcomes in this review included dichotomous outcomes (mortality, complications) and continuous outcomes (number of shocks, energy required). We did not enter data into RevMan 5.2 as specified in our protocol. See Differences between protocol and review.
Unit of analysis issues
We included studies that reported more than one comparison, for example, a group allocated to propofol compared with both a midazolam group and a sevoflurane group. As we did not combine our results, it was not necessary to perform single pair‐wise comparisons.
For cluster trials included in the review, we would have extracted data directly from the publication only if the analysis used accounted for the cluster design by incorporating a method such as multi‐level modelling or generalized estimating equations. If these adjustments were not made within the report, we would have undertaken approximate analyses by recalculating standard errors or sample sizes based on the design effect.
Dealing with missing data
We contacted study authors to request missing outcome data.
Assessment of heterogeneity
We expected that the findings for any given outcome may differ between studies included in the review. This heterogeneity may be due to:
expertise of the clinician;
the drug used;
use of a combination of drugs versus a single drug;
anticipated difficulty with cardioversion (e.g. pre‐existing cardiac or other medical problems in participant, unstable cardiovascular status before cardioversion);
type of cardioversion ‐ emergency or elective; or
mode of cardioversion ‐ external, internal or trans‐oesophageal.
Had we carried out meta‐analyses, Chi2 P value < 0.1 or I2 > 50% would have been considered as important heterogeneity, which may reflect differences in study populations, interventions or design. This heterogeneity would have informed our choice of analytical model (random‐effects or fixed‐effect).
Assessment of reporting biases
Had we pooled data, we would have examined funnel plots to assess the potential for publication bias. We would have used visual assessment to detect asymmetry. Heterogeneity between studies may lead to asymmetry, and we would have considered this possibility when reviewing results.
In addition to studies with no published results, reporting bias may be present within a study that provides data on some outcomes collected but not reported. If a report or the study protocol suggested that outcomes had not been reported, we would have contacted the study author to request outcome data.
Results
Description of studies
We provided summary details for each study in the Characteristics of included studies table.
Results of the search
We identified a total of 992 studies through electronic searches, 118 studies through forward citation searches and a further 39 through backward citation searches. We also identified studies from clinical trial databases. Having removed duplicates, we considered a total of 833 unique titles and abstracts and then assessed a further 95 full texts, when available, for eligibility. We performed data extraction and risk of bias assessment on 23 studies. See Figure 1 for the search flow diagram.
1.
Study flow diagram.
Included studies
A total of 23 studies with 1250 participants aged over 16 years met our inclusion criteria. We included one study (Coll‐Vinent 2003) that specified recruitment of adult participants but had an age range for one its comparison groups of 15 to 71 years. All participants were scheduled for cardioversion for arrhythmias such as atrial fibrillation, atrial flutter or supraventricular tachycardia. All procedures were elective, with the exception of Coll‐Vinent 2003, which dealt only with emergency procedures, and Parlak 2006 and Sternlo 1991, which included participants for both emergency and elective procedures.
The target level of consciousness varied between studies. Two studies defined the target as a score of two on the OAA/S scale (observer's assessment of alertness/sedation; level 2: "Responds only after mild prodding or shaking") (Akcaboy 2007; Altinoren 2005); two studies referred to the Ramsay Sedation Scale, with one setting a target level of four ("Patient exhibits brisk response to light glabellar tap or loud auditory stimulus") (Broch Porcar 1999) and one of five ("Patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus") (Parlak 2006). Six studies relied on loss of eyelash reflex (sometimes described as 'lid reflex') (Canessa 1991; Gupta 1990; Jan 1995; Karthikeyan 2002; Kick 1996; one of these equated this sign with 'clinical anaesthesia' (Sternlo 1991)). Conversely, 'general anaesthesia' was declared as 'degradation of the lid reflex' plus the inability to follow commands by one (Siedy 2010) of three studies (Gale 1993; Kalogridaki 2011), which defined their endpoint by these two signs. Loss of responsiveness to verbal commands or questions was an endpoint in many studies (Ford 1991; Hullander 1993; Mitchell 2003; Munoz 2002; Orko 1976a; Sharafudeen 2010; Valtonen 1988); however, 'loss of consciousness' (Herregods 2003) and 'deep sedation' (Coll‐Vinent 2003) were also used, without further definition. One study (Dellinger 1988) did not specify an endpoint.
Propofol was the intervention or comparison drug in 19 included studies (Akcaboy 2007; Altinoren 2005; Broch Porcar 1999; Canessa 1991; Coll‐Vinent 2003; Gale 1993; Gupta 1990; Herregods 2003; Hullander 1993; Jan 1995; Kalogridaki 2011; Karthikeyan 2002; Kick 1996; Munoz 2002; Parlak 2006; Sharafudeen 2010; Siedy 2010; Sternlo 1991; Valtonen 1988). In four of these studies, propofol was given with remifentanil (Akcaboy 2007; Altinoren 2005) or fentanyl (Kalogridaki 2011; Parlak 2006). In Canessa 1991, participants were given fentanyl three minutes before they were given propofol.
Etomidate was compared with propofol in seven studies (Broch Porcar 1999; Canessa 1991; Coll‐Vinent 2003; Herregods 2003; Kick 1996; Munoz 2002; Hullander 1993). In four studies, etomidate was given with remifentanil (Akcaboy 2007; Altinoren 2005) or fentanyl (Kalogridaki 2011; Siedy 2010) and was compared with propofol.
Midazolam was compared with propofol in six studies (Broch Porcar 1999; Canessa 1991; Coll‐Vinent 2003; Gale 1993; Gupta 1990; Parlak 2006).
Thiopentone/thiopental was compared with propofol in six studies (Canessa 1991; Dellinger 1988; Gupta 1990; Jan 1995; Sternlo 1991;Valtonen 1988).
Sevoflurane was compared with propofol in two studies (Karthikeyan 2002; Sharafudeen 2010).
Other drug comparisons included etomidate versus thiopentone (three studies: Dellinger 1988; Ford 1991; Canessa 1991), etomidate versus midazolam (three studies: Broch Porcar 1999; Canessa 1991; Coll‐Vinent 2003), thiopentone versus midazolam (two studies: Gupta 1990;Canessa 1991), thiopentone versus diazepam (Orko 1976a) and midazolam versus diazepam (Mitchell 2003).
Dose and timing of administration of each drug varied between studies. This detail is provided in Characteristics of included studies.
Excluded studies
We excluded studies that used an incorrect design or population group. We had identified several potential studies from backward citation that were conducted in the 1960s; many of these were not RCTs or did not include a drug comparison. We identified two studies that were relevant but compared a drug that was no longer in use for cardioversion (Orko 1976b; Tiongson 1978). Another study aimed specifically to look at cardioversion procedures following coronary artery bypass graft and was therefore excluded (Yildirim 2007). Also see Characteristics of excluded studies.
Studies awaiting classification
One study (Sawas 2013) was identified as possibly relevant for inclusion ‐ an RCT comparing propofol with ketofol (a combination of propofol with ketamine) for emergency department procedures. Of these, seven were cardioversions; however, the data were derived from an abstract only, and the results for cardioversion procedures were not reported separately. We were unable to contact the study authors. See Characteristics of studies awaiting classification.
Ongoing studies
We identified one ongoing study from our clinical trials register search. This RCT compares propofol with ketofol for emergency department procedures to include cardioversion (NCT01211158). Also see Characteristics of ongoing studies.
Risk of bias in included studies
A summary of the 'Risk of bias' results can be found in Figure 2 and Figure 3. Details are provided in Characteristics of included studies.
2.
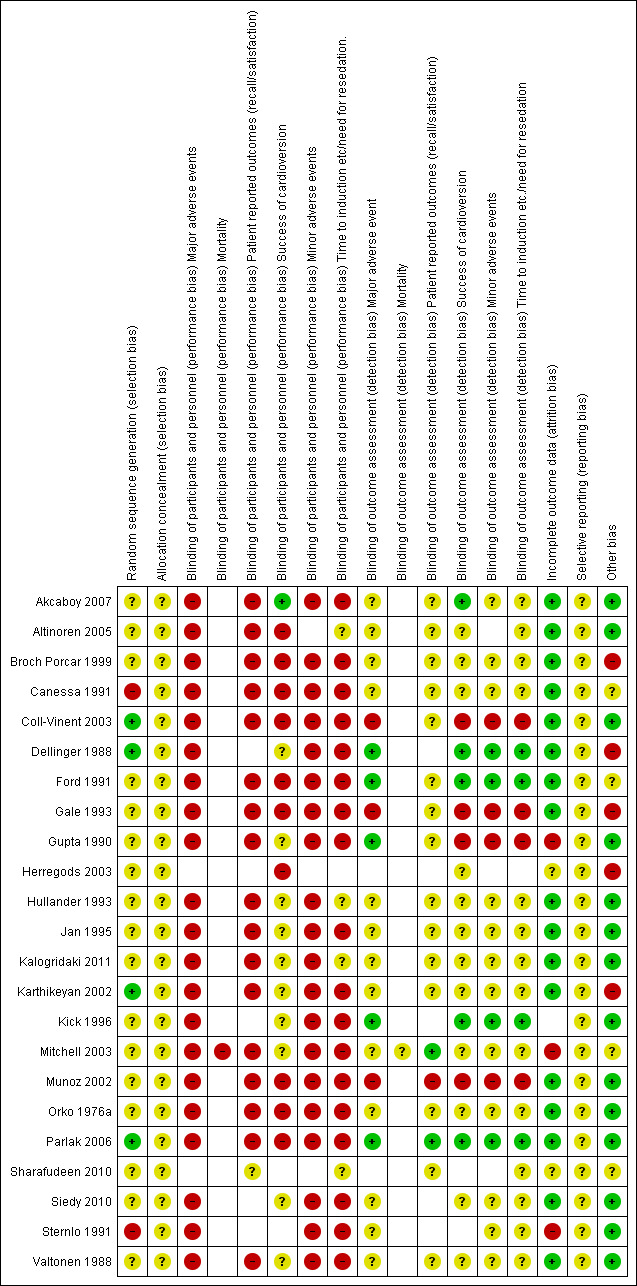
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
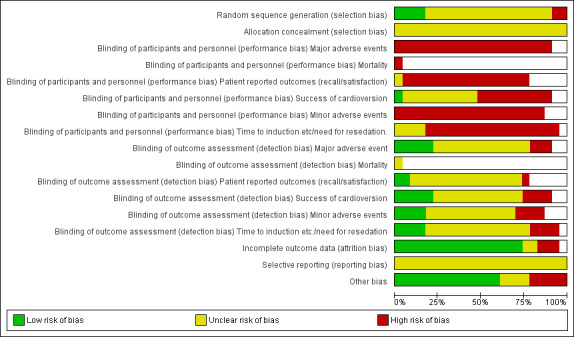
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
For Sharafudeen 2010, we had only information taken from an abstract; therefore much of the information required to make judgements about risk of bias was not available. We recorded domains as unclear in the 'Risk of bias' table.
Allocation
Only two of the 23 studies were not described as randomized by the study authors. These were quasi‐randomized studies in which study drugs were allocated according to the last digit of the participant record (Canessa 1991) or alternatively for each cardioversion procedure (Sternlo 1991). We judged both of these methods of allocation as having high risk of selection bias. Most studies did not provide sufficient detail on the randomization methods for us to be able to judge the risk of selection bias appropriately and are therefore recorded in the 'Risk of bias' tables as unclear (see Characteristics of included studies). Four studies included an adequate method of allocation using random number tables or computer software (Coll‐Vinent 2003; Dellinger 1988; Karthikeyan 2002; Parlak 2006).
No studies provided sufficient detail on how allocation was concealed; therefore we were unable to make a judgement and recorded allocation concealment in the 'Risk of bias' tables as unclear.
Blinding
Blinding was a particular issue for this review. Although some studies had stated specifically that personnel had not been blinded, we assumed that blinding had not taken place in the remaining studies and therefore judged performance bias for those outcomes that could be affected by the behaviour of the anaesthetist to be at high risk of bias. For the outcome 'success of cardioversion', we assumed that the clinician responsible for this procedure had the potential to introduce performance bias. Unless it was clearly stated in the paper that the clinician had not been blinded, we did not make a judgement on this and reported risk of bias as unclear.
Six papers provided greater detail on blinding of outcome assessors; we were able to judge these studies as having low risk of detection bias for those outcomes when specified (Akcaboy 2007; Dellinger 1988; Ford 1991; Kick 1996; Mitchell 2003; Parlak 2006). Unless it was stated in the paper that personnel or participants had not been blinded, we did not make a judgement on detection bias for particular outcomes and reported risk of bias as unclear.
Incomplete outcome data
Little attrition bias was apparent in the studies. We judged Gupta 1990 as having high risk of attrition bias specifically for the patient‐reported outcomes for which outcome assessors had been unable to contact 50% of participants in the midazolam group. We judged Mitchell 2003 as having high risk of attrition bias specifically for the 'time to awakening' outcome, for which 15 participants had been excluded from data analysis. We judged Sternlo 1991 as having high risk of bias for excluding participants from data analysis who had been given cardioversion as an emergency procedure.
Selective reporting
Although outcomes from the methods section appeared to be reported for all studies, we did not search specifically for any prepublished protocols for the included studies; therefore we were unable to make a judgement on this. We recorded risk of reporting bias as unclear.
Other potential sources of bias
We considered baseline imbalances of participants within studies as having potential bias; therefore we judged these studies as having high risk of bias. Differences in Broch Porcar 1999, Dellinger 1988 and Gale 1993 were apparent, and information was insufficient to permit a judgement of whether bias had been introduced for Ford 1991, Herregods 2003, Mitchell 2003 and Sharafudeen 2010.
Only one study declared support from external funding (Dellinger 1988); it is unclear whether this funding introduced bias.
We considered the number of anaesthetists providing anaesthesia/sedation to participants relevant to bias assessment ‐ the greater the number of anaesthetists involved in the study, the greater was the degree of bias. Most studies failed to report how many anaesthetists were involved in the study process. Of those that did report this information, Akcaboy 2007, Broch Porcar 1999, Gupta 1990 and Valtonen 1988 used only one anaesthetist/nurse and were therefore considered to have low risk of bias.
Effects of interventions
Substantial heterogeneity was observed between studies with regard to drugs, doses, timing of administration and target levels of consciousness. We judged that risk of bias across many domains was unclear or high in most studies. We therefore did not combine data in this review as intended.
We reconsidered our outcomes as identified in our protocol (see Differences between protocol and review) and therefore reported only on the outcomes described below. We presented the data for each study in Characteristics of included studies, and we summarized available data for the primary outcomes in a single table (Table 1). This table presents each drug comparison, starting with the most frequently studied, along with different doses and timings of each agent. It should be noted, however, that further differences between studies were identified in the definitions of outcomes; these differences are detailed below. When outcomes are reported as having a statistically significant effect, including a P value, we have presented these values below and in Table 1. None off these studies had presented these data with effect estimates.
1. Primary outcome data.
| Intervention (dose; timing) | Comparison (dose; timing) | Study | Hypotension* | Apnoea* | Patient recall* |
| 1. Propofol (1.5 mg/kg, additional supplements of 0.25 mg/kg; iv over 30 seconds) | 2. Etomidate (0.25 mg/kg, additional supplements of 0.03 mg/kg; iv over 30 seconds) | Kick 1996 | 1. 6/20 2. 15/20 P value < 0.05 |
||
| 1. Propofol (50 mg/min + 2.5% (0.5 mg/kg) lidocaine at start of induction; iv over 30 seconds) | 2. Etomidate (8 mg/min + 2.5% (0.5 mg/kg) lidocaine at start of induction; iv over 30 seconds) | Hullander 1993 | 1. 2/20 2. 1/20 |
1. 0/20 2. 0/20 |
|
| 1. Propofol + fentanyl (50 µg fentanyl, then 60 seconds later 0.5 mg/kg propofol; iv over 30 seconds) | 2. Etomidate + fentanyl (50 µg fentanyl, then 60 seconds later 0.1 mg/kg etomidate; iv over 30 seconds) | Kalogridaki 2011 | 1. 5/25 2. 0/21 P value 0.054 |
1. 7/25 2. 10/21 |
1. 3/25 2. 1/21 |
| 1. Propofol (0.5 mg/kg) + remifentanil (0.75 µg/kg); propofol iv over 15 seconds. Remifentanil iv over 90 seconds | 2. Etomidate (0.1 mg/kg + remifentanil (0.75 µg/kg); etomidate iv over 15 seconds. Remifentanil iv over 90 seconds | Akcaboy 2007 | 1. 2/20 2. 0/20 |
1. 1/20 2. 0/20 |
|
| 1. Propofol (0.5 mg/kg) + remifentanil (0.75 µg/kg); propofol iv over 15 seconds. Remifentanil iv over 90 seconds | 2. Etomidate (0.1 mg/kg + remifentanil (0.75 µg/kg); etomidate iv over 15 seconds. Remifentanil iv over 90 seconds | Altinoren 2005 | 1. 8/20 2. 0/20 |
1. 1/20 2. 0/20 |
|
| 1. Propofol (1 mg/kg + additional at 0.2 mg/kg; bolus) | 2. Etomidate + fentanyl (1 µg/kg fentanyl, then 0.15 mg/kg etomidate + additional 0.03 mg/kg; iv) | Siedy 2010 | 1. 3/50 2. 2/50 |
||
| 1. Propofol (1 mg/kg; iv over 1 minute) | 2. Etomidate (0.15 mg/kg) + Midazolam (1 mg); iv over 1 minute | Munoz 2002 | 1. 3/25 2. 4/25 |
1. 0/25 2. 1/25 |
|
| 1. Propofol (1 mg/kg 50% initially then boluses of 25%; iv over 1 minute) | 2. Etomidate (0.2 mg/kg 50% initially, then boluses of 25%; iv over 1 minute) 3. Midazolam (0.05 mg/kg 50% initially, then boluses of 25%; iv over 1 minute) |
Broch Porcar 1999 |
Mean % drop in arterial pressure 1. 24 (8) 2. 0.3 (8) 3. 14 (8) |
1. 1/13 2. 0/13 3. 1/12 |
1. 4/13 2. 1/13 3. 0/12 |
| 1. Propofol (1.5 mg/kg; iv over 20 seconds) | 2. Etomidate (0.2 mg/kg; iv over 20 seconds) 3. Midazolam (0.2 mg/kg; iv over 20 seconds) 4. Midazolam + flumazenil (0.5 mg followed by 0.5 mg in iv perfusion; perfusion over 1 hour post procedure) |
Coll‐Vinent 2003 | 1. 2/9 2. 2/9 3. 3/8 4. 1/6 |
||
| 1. Propofol (1.5 mg/kg; over 30 seconds). Plus 1.5 µg/kg fentanyl iv, 3 minutes before induction | 2. Thiopental (3 mg/kg; over 30 seconds) 3. Etomidate (0.15 mg/kg; over 30 seconds) 4. Midazolam (0.15 mg/kg; over 30 seconds) Plus 1.5 µg/kg fentanyl iv, 3 minutes before induction in all groups |
Canessa 1991 | 1. 7/12 2. 2/12 3. 1/10 4. 1/10 P <0.05 |
1. 0/12 2. 0/12 3. 0/10 4. 0/10 |
|
| 1. Propofol (iv infusion initiated at 10 mL/min and maintained at 10 mL/min) | 2. Midazolam (0.5 mg/mL iv infusion initiated and maintained at a rate of 10 mL/min) | Gale 1993 | 1. 2/10 2. 1/10 |
1. 2/10 2. 0/10 |
|
| 1. Propofol (20 mg) + fentanyl (1 µg) < 65 years; iv over 20‐30 seconds, then 20 mg propofol every 2 minutes 2. Propofol (20 mg) + fentanyl (0.5 µg) ≥ 65 years; iv over 20‐30 seconds, then 20 mg propofol every 2 minutes |
3. Midazolam (2 mg) + fentanyl (1 µg) < 65 years; iv over 20‐30 seconds, then 2 mg midazolam every 2 minutes 4. Midazolam (2 mg) + fentanyl (0.5 µg) ≥ 65 years; iv over 20‐30 seconds, then 2 mg midazolam every 2 minutes |
Parlak 2006 | 1. 1/11 2. 2/22 3. 1/12 4. 6/25 |
1. 0/11 2. 1/22 3. 1/12 4. 4/25 |
|
| 1. Propofol (mean dose 2.2 mg/kg; iv over 1 minute) | 2. Thiopentone (mean dose 5.2 mg/kg; iv over 1 minute) 3. Midazolam (mean dose 0.24 mg/kg; 5 mg injected over 1 minute, then 2‐mg increments) |
Gupta 1990 | 1. 3/10 2. 3/10 3. 0/10 |
1. 0/10 2. 0/10 3. 0/5 |
|
| 1. Propofol (1 mg/kg; iv over 30 seconds, then 2 mg/min infusion rate). Plus fentanyl (2 µg/kg) as premedication 3 minutes before induction | 2. Thiopentone (1.5 mg/kg; iv over 30 seconds, then 16 mg/min infusion rate). Plus fentanyl (2 µg/kg) as premedication 3 minutes before induction | Jan 1995 | 1. 7/12 2. 7/12 |
1. 0/12 2. 0/12 |
|
| 1. Propofol (no dose given) | 2. Thiopentone (no dose given) | Sternlo 1991 | 1. 2/23 2. 2/21 |
||
| 1. Propofol (2.5 mg/kg; iv over 45 seconds) | 2. Thiopentone (5 mg/kg; iv over 45 seconds) | Valtonen 1988 | 1. 5/15 2. 9/15 |
1. 0/15 2. 0/15 |
|
| 1. Propofol (6 µg/mL + nitrous oxide as co‐induction + gycopyrronium 200 µg; TCI throughout procedure) | 2. Sevoflurane (8% in 50% oxygen/nitrous oxide + nitrous oxide as co‐induction + gycopyrronium 200 µg; inhalation) | Karthikeyan 2002 | 1. 8/31 2. 5/30 |
||
| 1. Propofol (2% at 67 mg/min; infusion pump) | 2. Sevoflurane (8% in 10 L/min oxygen; inhalation) | Sharafudeen 2010 | Study authors state: "None of the patients reported awareness" | ||
| 1. Etomidate (0.3 mg/kg followed by 0.15 mg/kg. 10 mg diazepam premedication; iv over 30 seconds) | 2. Thiopental (3 mg/kg followed by 1.5 mg/kg if required. 10 mg diazepam premedication; iv over 30 seconds) | Dellinger 1988 | 1. 31/40 2. 22/40 P value 0.046 |
1. 17/40 2. 27/40 P value 0.02 |
|
| 1. Etomidate (0.20%; 2‐mL aliquots iv every 15 seconds) | 2. Thiopental (2.50%; 2‐mL aliquots iv every 15 seconds) | Ford 1991 | 1. 0/8 2. 0/8 |
1. 0/8 2. 0/8 |
1. 1/8 2. 1/8 |
| 1. Midazolam (2 mg/kg; 5 mg iv bolus followed by aliquots of 1‐2 mg each min to max 30 mg) | 2. Diazepam (5‐10 mg iv bolus followed by aliquots of 5‐10 mg each min to max 70 mg) | Mitchell 2003 | 1. 14/71 2. 5/70 |
1. 0/71 2. 1/70 |
|
| 1. Diazepam (dose not given; iv over 1 minute) | 2. Thiopentone (2.5% solution; iv over 1 minute) | Orko 1976a | 1. 2/50 2. 25/50 P value < 0.001 |
1. 15/41 2. 1/40 P value < 0.001 |
*Unless otherwise stated, data given as number of participant events per total number of participants in group.
iv = intravenously; TCI = target controlled infusion; max = maximum.
Whilst Sharafudeen 2010 met our inclusion criteria and is included in our Characteristics of included studies, this conference abstract provided no denominator figures. We were unable to contact the study authors and therefore have not included any of the data in this narrative.
Primary outcomes
Major adverse events
Five studies with a total of 322 participants compared anaesthetic agents for cardioversion and reported numbers of events of hypotension, decreased systolic arterial blood pressure of more than 20% or decreased systolic arterial blood pressure of more than 20 mmHg (Broch Porcar 1999; Dellinger 1988; Ford 1991; Kalogridaki 2011; Mitchell 2003). Only Dellinger 1988 reported a result that was statistically significant, with more participants in the etomidate group than the thiopentone group having hypotension (P value 0.046). An additional 13 studies presented data for changes in systolic and diastolic blood pressure at different intervals (Akcaboy 2007; Altinoren 2005; Canessa 1991; Coll‐Vinent 2003; Gale 1993; Gupta 1990; Hullander 1993; Jan 1995; Karthikeyan 2002; Kick 1996; Siedy 2010; Sternlo 1991; Valtonen 1988). These studies did not present data as number of events per group, but in a variety of graphs and figures with some statements of difference in blood pressure between groups. When applicable, direct quotes from the study reports have been given in Characteristics of included studies.
A total of 20 studies with a total of 1015 participants measured apnoea. Definitions of apnoea were given at different points: need for assisted ventilation for longer than 10 seconds (Munoz 2002); longer than 20 seconds (Coll‐Vinent 2003; Kick 1996; Parlak 2006); or longer than 30 seconds (Canessa 1991; Gale 1993; Gupta 1990; Jan 1995; Orko 1976a; Sternlo 1991; Valtonen 1988). Other studies did not provide a definition (Akcaboy 2007; Altinoren 2005; Broch Porcar 1999; Dellinger 1988; Ford 1991; Hullander 1993; Kalogridaki 2011; Karthikeyan 2002; Siedy 2010). Four studies reported a result that was statistically significant: In Canessa 1991, more participants in the propofol group had apnoea than in the thiopental, etomidate or midazolam group (P value < 0.05); in Dellinger 1988, more in the thiopental group than in the etomidate group (P value 0.02); in Kick 1996, more in the etomidate group than in the propofol group (P value < 0.05) and in Orko 1976a, more in the thiopentone group than in the diazepam group (P value < 0.001). See Table 1.
Patient awareness or recall
In all, 16 studies with a total of 859 participants measured patient awareness or recall (Akcaboy 2007; Altinoren 2005; Canessa 1991; Broch Porcar 1999; Ford 1991; Gale 1993; Gupta 1990; Hullander 1993; Jan 1995; Kalogridaki 2011; Mitchell 2003; Munoz 2002; Orko 1976a; Parlak 2006; Sharafudeen 2010; Valtonen 1988). Only one study reported a statistically significant effect for this outcome. Orko 1976a reported that more participants were included in the diazepam group than in the thiopentone group (P value < 0.001). See Table 1.
Secondary outcomes
Minor adverse events
Eight studies with 477 participants reported nausea and vomiting (Akcaboy 2007; Altinoren 2005; Ford 1991; Gupta 1990; Hullander 1993; Karthikeyan 2002; Orko 1976a; Siedy 2010). Of these, Hullander 1993 reported data for nausea only, and Siedy 2010 reported data for nausea and vomiting separately. In Siedy 2010, the etomidate group had statistically less nausea and less vomiting than the propofol group (P value < 0.05 for both outcomes).
In all, 15 studies with 628 participants reported pain at the injection site (Akcaboy 2007; Altinoren 2005; Canessa 1991; Coll‐Vinent 2003; Dellinger 1988; Ford 1991; Gale 1993; Hullander 1993; Jan 1995; Kalogridaki 2011; Karthikeyan 2002; Kick 1996; Siedy 2010; Sternlo 1991; Valtonen 1988). Four studies reported results that were statistically significant for pain at the injection site. Dellinger 1988 reported a statistically significant difference in the number of participants reporting pain, with more included in the etomidate group than in the thiopental group (P value 0.002). In Gale 1993, more participants were included in the propofol group than in the midazolam group (P value <0.05). In both Kick 1996 and Siedy 2010, more participants were included in the etomidate group than in the propofol group (P value < 0.05).
Nine studies with 377 participants reported myoclonus without further definition (Akcaboy 2007; Altinoren 2005; Broch Porcar 1999; Canessa 1991; Coll‐Vinent 2003; Dellinger 1988; Ford 1991; Hullander 1993; Kalogridaki 2011). Karthikeyan 2002 reported the incidence of "movements" as a complication for participants, Kick 1996 reported the incidence of "involuntary movements", Orko 1976a reported "excitary side effects, such as muscular tension or involuntary movements" and Siedy 2010 reported "severe involuntary muscle movements during anaesthesia requiring midazolam". We reported data for these four studies as 'myoclonus' in the Characteristics of included studies. Two studies reported results that were statistically significant for myoclonus ‐ Dellinger 1988 reported more participants with myoclonus in the etomidate group than in the thiopental group (P value < 0.002), and Kalogridaki 2011 reported more in the etomidate group than in the propofol group (P value 0.0004).
Mortality
Only one study (Mitchell 2003) reported no mortality at one month. No other studies reported on this outcome.
Success of cardioversion
Six studies with 283 participants reported the number of participants who had returned to sinus rhythm following cardioversion (Altinoren 2005; Canessa 1991; Dellinger 1988; Herregods 2003; Jan 1995; Karthikeyan 2002).
Three studies reported the number of shocks required for successful cardioversion and reported data as one, two or three shocks (Akcaboy 2007; Altinoren 2005; Kick 1996). Orko 1976a reported the mean number of shocks required, and Coll‐Vinent 2003 reported the median number of shocks required.
Canessa 1991 and Coll‐Vinent 2003 reported the amount of energy, in Joules, required for successful cardioversion.
Twelve studies with 717 participants reported the number of successful cardioversion procedures without further definition (Broch Porcar 1999; Ford 1991; Gale 1993; Gupta 1990; Hullander 1993; Kalogridaki 2011; Kick 1996; Mitchell 2003; Munoz 2002; Orko 1976a; Sternlo 1991; Valtonen 1988).
Additional analgesia
Only one study reported data on the need for additional analgesics (Mitchell 2003). In this study, 6% of participants in the diazepam group required additional analgesia, with none in the midazolam group.
Patient satisfaction
Eight studies with 492 participants reported patient satisfaction (Akcaboy 2007; Altinoren 2005; Canessa 1991; Coll‐Vinent 2003; Karthikeyan 2002; Mitchell 2003; Parlak 2006 ;Sharafudeen 2010). A variety of questions and scales were used before patient discharge to determine this outcome; these are presented in Characteristics of included studies.
Discussion
Summary of main results
We found 23 studies of drugs given to obtund consciousness during electrical cardioversion. Drug comparisons were made between propofol, etomidate, thiopentone, sevoflurane, midazolam and diazepam, of which 18 studies compared propofol. We found that the desired sedative endpoints and methods used to administer anaesthetics varied between studies, even with the same drug comparisons, with different doses used and with different timing of administration. We believed it was not appropriate to pool the data because of these differences. Studies measured our primary outcomes of hypotension, apnoea and recall, as well as secondary outcomes of minor adverse events, mortality, success of cardioversion, additional analgesia and participant satisfaction. Only one study reported a statistically significant result for hypotension, with more participants in the etomidate group than in the thiopentone group having hypotension. Four studies reported statistically significant results for apnoea. One study described more apnoea events in the propofol group than in the thiopental, etomidate or midazolam group (P value < 0.05); one reported more events in the thiopental group than in the etomidate group (P value 0.02); one documented more events in the etomidate group than in the propofol group (P value ≤ 0.05) and one discussed more in the thiopentone group than in the diazepam group (P value < 0.001). Only one study reported a statistically significant difference for recall, with more patients having recall in the diazepam and thiopentone group. All studies that reported statistically significant differences for our primary outcomes were older studies (from 1976, 1988 and 1996).
Whilst studies collected data on potential drawbacks of each agent, few studies reported statistically significant results for relevant outcomes. Consistent with this, many of the study authors concluded that both, or all, of their study drugs were acceptable for use during cardioversion procedures. What is likely to be important is not so much the drug chosen, but how it is used; this is a key component of anaesthetic expertise (Smith 2009b; Smith 2011). Those that did report significant results for our primary outcomes had poor risk of bias and were old studies (Canessa 1991; Dellinger 1988; Kick 1996; Orko 1976a).
Overall completeness and applicability of evidence
We carried out a thorough search and identified a reasonable number of studies with 1250 participants relevant to our review. We were able to include studies with the relevant population group that measured our identified interventions and outcomes, although only one study obtained data solely from the emergency department.
Most studies compared propofol with another drug; this is reflected in current practice in the UK, where propofol is seen as the current drug of choice (James 2003).
Studies were reported over a wide time range: 1976 to 2011. Some drug comparisons from the earlier studies were not reported in the review results, as these drugs were no longer in common use; two potentially relevant studies were excluded for this reason. This reflects the change in anaesthetic practice over time. It is also likely that anaesthetic technique and equipment will have changed from the time of earlier studies, and changes in the quality of study design may have occurred.
Whilst we identified studies that measured our primary and secondary outcomes, we believe that these studies were of insufficient quality for review authors to report all results in data tables or in the body of the review; instead we provided this information in individual Characteristics of included studies tables.
Quality of the evidence
Evidence was generally of poor quality, with very few studies providing sufficient information to demonstrate adequate randomization of participants. It was assumed that no studies had attempted to blind the anaesthetist from the anaesthetic agent, and this approach could have been carried out using pre‐prepared unmarked syringes for the agents (as in an excluded but equivalent study design; Maltepe 2006). Observers/outcome assessors could have been blinded to study allocation; however few studies had described such blinding. Most studies were assessed as having high risk of bias or as providing insufficient information to allow a decision across risk of bias domains.
Pooling of results would be inappropriate with evidence of such quality.
Potential biases in the review process
We are confident that we identified the relevant studies for this review through a thorough search that was not limited by language and that included studies conducted since cardioversion was introduced into medical practice.
However, we did not contact investigators in the field to enquire about unpublished studies. We did not seek additional information from study authors regarding their study protocol, methods and results to clarify risk of bias, and we judged all studies equally on the information provided in the full report.
Agreements and disagreements with other studies or reviews
We found three other reviews in this topic area. Wood 2006 included seven relevant studies, all of which were included in our systematic review (Canessa 1991; Coll‐Vinent 2003; Ford 1991; Gale 1993; Herregods 2003; Mitchell 2003; Valtonen 1988), and concluded that propofol, methohexital (not considered in our review as not currently in use), thiopentone and etomidate "all appear to be good choices for procedural sedation in patients requiring electrical cardioversion". This review author had not presented a meta‐analysis for the data and had drawn conclusions from the findings of individual studies. However, no assessment of quality or bias was performed in this review. Hohl 2008 prepared a review specific to the emergency department and to a comparison of midazolam and propofol. This review included data from Coll‐Vinent 2003 and Parlak 2006, and review authors concluded that there was no difference in the safety profile of these two agents when used in the emergency department. Symington 2006 also reviewed propofol in the emergency department and included data from Coll‐Vinent 2003; remaining studies involved a paediatric population or procedures other than cardioversion.
Authors' conclusions
Implications for practice.
Whilst we did not combine results from included studies, and as data on adverse events were limited, it is our opinion that at present, there is no evidence to indicate that current anaesthetic practice for cardioversion should change.
Implications for research.
This review highlights the lack of good‐quality large studies that have explored the use of anaesthetic agents for electrical cardioversion. It would be beneficial for future systematic reviews to focus on the effectiveness of one particular agent, for example, propofol, against other agents, using methodological rigour, with particular attention to blinding of personnel.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
We would like to thank Harald Herkner (Content Editor), Cathal Walsh (Statistical Editor) and Charles Deakin, Ben Gibbison and Afshin Gholipour Baradari (Peer Reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review (Reed 2013).
Acknowledgements
We would like to thank Harald Herkner (Content Editor), Cathal Walsh and Nathan Pace (Statistical Editors) and Charles Deakin, Ben Gibbison and Afshin Gholipour Baradari (Peer Reviewers) for help and editorial advice provided during preparation of this systematic review. We would like to thank Zeynep Kayhan for translations of sections of the full text of Altinoren 2005.
Appendices
Appendix 1. CENTRAL, The Cochrane Library search strategy
#1 MeSH descriptor: [Electric Countershock] explode all trees #2 MeSH descriptor: [Defibrillators] explode all trees #3 cardiover* or defibrill* #4 #1 or #2 or #3 #5 MeSH descriptor: [Anesthesia and Analgesia] explode all trees #6 MeSH descriptor: [Anesthesia, Intravenous] explode all trees #7 MeSH descriptor: [Conscious Sedation] explode all trees #8 MeSH descriptor: [Midazolam] explode all trees #9 MeSH descriptor: [Etomidate] explode all trees #10 MeSH descriptor: [Propofol] explode all trees #11 MeSH descriptor: [Thiopental] explode all trees #12 MeSH descriptor: [Methohexital] explode all trees #13 MeSH descriptor: [Isoflurane] explode all trees #14 MeSH descriptor: [Xenon] explode all trees #15 MeSH descriptor: [Deep Sedation] explode all trees #16 MeSH descriptor: [Anesthetics, Inhalation] explode all trees #17 MeSH descriptor: [Benzodiazepinones] explode all trees #18 midazolam or etomidate or propofol or thiopentone or methohexital or isoflurane or desflurane or xenon or sevoflurane or diazepam #19 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #4 and #19
Appendix 2. MEDLINE via Ovid search strategy
1. exp Electric Countershock/ or cardiover*.mp. or exp defibrillators/ or defibrill*.mp. 2. exp "anesthesia and analgesia"/ or exp Anesthesia, Intravenous/ or exp Conscious Sedation/ or midazolam.mp. or exp Midazolam/ or etomidate.mp. or exp Etomidate/ or propofol.mp. or exp Propofol/ or thiopentone.mp. or exp Thiopental/ or methohexital.mp. or exp Methohexital/ or isoflurane.mp. or exp Isoflurane/ or desflurane.mp. or xenon.mp. or exp Xenon/ or exp Deep Sedation/ or sevoflurane.mp. or exp "Anesthetics, Inhalation"/ or exp Benzodiazepinones/ or diazepam.mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or randomised.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. EMBASE via Ovid search strategy
1. exp cardioversion/ or cardiover*.mp. or exp defibrillator/ or defibrill*.mp. 2. exp anesthesia/ or exp analgesia/ or exp intravenous anesthesia/ or exp conscious sedation/ or midazolam.mp. or exp midazolam/ or etomidate.mp. or exp etomidate/ or propofol.mp. or exp propofol/ or thiopentone.mp. or exp thiopental/ or methohexital.mp. or exp methohexital/ or isoflurane.mp. or exp isoflurane/ or desflurane.mp. or xenon.mp. or exp xenon/ or exp deep sedation/ or sevoflurane.mp. or exp inhalation anesthetic agent/ or exp benzodiazepine derivative/ or diazepam.mp. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. CINAHL via EBSCO search strategy
| S1 | (MH "Cardioversion") or "electrical countershock" or "cardiover*" |
| S2 | (MH "Defibrillators") or defibrill* |
| S3 | (MH "Anesthesia and Analgesia (Non‐Cinahl)+") |
| S4 | (MH "Anesthesia, Intravenous") |
| S5 | (MH "Conscious Sedation") |
| S6 | (MH "Midazolam") or midazolam |
| S7 | (MH "Etomidate") or etomidate |
| S8 | (MH "Propofol") or propofol |
| S9 | (MH "Thiopental") or thiopentone |
| S10 | methohexital |
| S11 | (MH "Isoflurane") or isoflurane |
| S12 | (MH "Sedation") |
| S13 | (MH "Sevoflurane") or sevoflurane |
| S14 | (MH "Anesthetics, Inhalation+") |
| S15 | (MH "Antianxiety Agents, Benzodiazepine+") |
| S16 | (MH "Diazepam") or diazepam |
| S17 | TI (anesth* or anaesth* or sedat* or analges*) |
| S18 | S1 OR S2 |
| S19 | S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 |
| S20 | (MH "Clinical Trials+") |
| S21 | PT Clinical trial |
| S22 | TX clinic* n1 trial* |
| S23 | TX ((singl* n1 blind*) or (singl* n1 mask*)) or TX ((doubl* n1 blind*) or (doubl* n1 mask*)) or TX ((tripl* n1 blind*) or (tripl* n1 mask*)) or TX ((trebl* n1 blind*) or (trebl* n1 mask*)) |
| S24 | TX randomi* control* trial* |
| S25 | (MH "Random Assignment") |
| S26 | TX random* allocat* |
| S27 | TX placebo* |
| S28 | (MH "Placebos") |
| S29 | (MH "Quantitative Studies") |
| S30 | TX allocat* random* |
| S31 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 |
| S32 | S18 AND S19 AND S31 |
| S33 | S18 AND S19 |
Appendix 5. Study eligibility form
Anaesthetic and sedative agents used for electrical cardioversion
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) |
|
|
Publication type (e.g. full report, abstract, letter) |
|
| Study characteristics | Eligibility criteria | Y/N or unclear | Details and location |
| Type of study | Randomized controlled trial | ||
| Controlled clinical trial (quasi‐randomized trial and cluster‐randomized trial) |
|||
|
Participants |
Adults > 16 years undergoing electrical cardioversion in the emergency or elective setting | ||
|
Types of intervention and comparison We will include studies comparing 2 different anaesthetic agents or different doses of the same agent Groups may include the additional use of an analgesic (e.g. fentanyl, alfentanil) by its use alone or by dose |
Comparison of 1 of: | ||
| intravenous anaesthetic agents (e.g. etomidate, propofol, thiopentone, methohexital); | |||
| inhaled anaesthetic agents (e.g. isoflurane, sevoflurane); | |||
| sedative agents (e.g. midazolam, diazepam) via any route (intramuscular, subcutaneous, intravenous, rectal); or | |||
| analgesic (e.g. fentanyl, alfentanil). | |||
| With one of: | |||
| intravenous anaesthetic agents (e.g. etomidate, propofol, thiopentone, methohexital); | |||
| inhaled anaesthetic agents (e.g. isoflurane, sevoflurane); or | |||
| sedative agents (e.g. midazolam, diazepam) via any route (intramuscular, subcutaneous, intravenous, rectal). |
| Types of outcome measures | Details of outcomes and location in text | |||
| 1. Major adverse events. | ||||
| a) Hypotension. | ||||
| b) Apneic episodes. | ||||
| c) Other arrhythmias. | ||||
| d) Abandoned procedure. | ||||
| 2. All‐cause mortality within 30 days | ||||
| 3. Patient awareness or recall of procedure | ||||
| 4. Success of cardioversion | ||||
| 5. Minor adverse effects. | ||||
| a) Nausea and vomiting, | ||||
| b) Pain at injection site. | ||||
| c) Myoclonus. | ||||
| 6. Need for re‐sedation | ||||
| 7. Time from start of induction to: | ||||
| a) loss of consciousness/target level of sedation; | ||||
| b) first shock; | ||||
| c) awakening time; or | ||||
| d) full recovery. | ||||
| 8. Patient satisfaction with procedure | ||||
| Outcomes are not part of the eligibility criteria ‐ so a study that meets design, participant and intervention criteria is included | ||||
| INCLUDE | EXCLUDE | |||
| Reason for exclusion | ||||
Appendix 6. Data extraction form
ANAESTHESIA FOR CARDIOVERSION
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Study ID (surname of first author and year first full report of study was published, e.g. Smith 2001) |
|
|
Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
2. Population and setting
|
Description |
Location in text (pg & ¶/fig/table) |
|
|
Population description (type of arrhythmia and cardiovascular disease; time in arrhythmia) |
||
|
Location of study (town, country) |
||
|
Setting of cardioversion (in or out of hospital; ED, ward or CCU) |
||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Method/s of recruitment of participants | ||
| Informed consent obtained |
3. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Aim of study | ||
| Design(e.g. parallel, cross‐over, cluster) | ||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
||
| Start date | ||
| End date | ||
| Total study duration | ||
| Ethical approval obtained |
4. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total population at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Emergency or elective | ||
|
Details of cardioversion procedure (external, internal, via oesophagus) |
||
| Current used and details of shock protocol | ||
|
Use of adjunct agents (e.g. fentanyl, other opioid) |
||
| Other details | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported |
5. Intervention groups
5.1 Intervention group ‐ repeat as required
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name (sedation, general anaesthesia) |
||
| No. randomly assigned to group | ||
|
Description of drug (name, dose & timing) |
||
|
Method of induction (IV, inhaled, etc.) |
||
| Co‐interventions (e.g. additional opioid given) | ||
| Type of staff administering anaesthetic |
5.2 Comparison group ‐ repeat as required
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name (sedation, general anaesthesia) |
||
| No. randomly assigned to group | ||
|
Description of drug (name, dose and timing) |
||
|
Method of induction (IV, inhaled, etc.) |
||
| Co‐interventions (e.g. additional opioid given) | ||
| Type of staff administering anaesthetic |
6. Outcomes
| TYPES OF OUTCOME MEASURES | MEASURED | REPORTED | FORM COMPLETED |
| Primary outcomes | |||
| 1. Major adverse events. | |||
| a) Hypotension. | |||
| b) Apneic episodes. | |||
| c) Other arrhythmia. | |||
| d) Abandoned procedure. | |||
| 2. All‐cause mortality within 30 days of procedure. | |||
| 3. Patient awareness or recall of the procedure. | |||
| 4. Success of cardioversion ‐ defined as return to sinus rhythm; however, if the data are published, we may consider number of shocks required; energy needed; length of time remaining in sinus rhythm. | |||
| Secondary outcomes | |||
| 1. Minor adverse effects. | |||
| a) Nausea and vomiting. | |||
| b) Pain at site of injection. | |||
| c) Myoclonus. | |||
| 2. Need for re‐sedation. | |||
| 3. Time from start of induction to: | |||
| a) loss of consciousness/target level of sedation; | |||
| b) first shock; | |||
| c) awakening time; and | |||
| d) full recovery. | |||
| 4. Patient satisfaction with procedure. |
For each outcome ticked, please complete a separate outcome form.
|
Description as stated in report/paper |
Location in text |
|
|
Outcome name (number of attempts, pain) |
||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Scales: levels, upper and lower limits(indicate whether high or low score is good) | ||
| Is outcome/tool validated? | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| RESULTS | Description as stated in report/paper |
Location in text |
| Comparison | ||
| Outcome | ||
| Subgroup | ||
| Time point (specify whether from start or end of intervention) | ||
| Post intervention or change from baseline? | ||
| Results: intervention* | ||
| Results: comparison* | ||
| No. missing participants and reasons | ||
| No. participants moved from other group and reasons | ||
| Any other results reported | ||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||
| Reanalysis required?(specify) | ||
| Reanalysed results |
*Results for continuous outcome: mean: SD (or other variance): total number of participants.
Results for dichotomous outcome: number participants with outcome: total number of participants.
7. Risk of bias assessment
| Domain |
Risk of bias : high/low/unclear |
Support for judgement |
Location in text |
|
Random sequence generation (selection bias) |
|||
|
Allocation concealment (selection bias) |
|||
|
Blinding of participants and personnel (performance bias) |
|||
|
Blinding of outcome assessment (detection bias) |
|||
|
Incomplete outcome data (attrition bias) |
|||
|
Selective outcome reporting? (reporting bias) |
|||
|
Other bias (baseline characteristics for cluster‐randomized, carryover for cross‐over trials) |
8. Applicability
| Yes/No/Unclear | Support for judgement | |
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | ||
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | ||
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
9. Other information
|
Description as stated in report/paper |
Location in text |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akcaboy 2007.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Supplemental doses of propofol (10 mg) or etomidate (2 mg) given if sedation not adequate to start cardioversion Aim: for patient to be sedated to OAA/S score of 2 |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | All participants had the same anaesthetist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized via sealed envelope assignment”. No further details |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Cardiologist had no information on drugs used. However, assume anaesthetist was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Assume anaesthetist was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Low risk | Cardiologist had no information on drugs used |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Assume anaesthetist was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Assume anaesthetist was aware of drug allocation |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | Unclear who assessed outcomes. Participant, cardiologist and nurse were unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | Unclear who assessed outcomes. Participant, cardiologist and nurse were unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Low risk | Assume assessed by cardiologist who was unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | Unclear who assessed outcomes. Participant, cardiologist and nurse were unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | Unclear who assessed outcomes. Participant, cardiologist and nurse were unaware of drug allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods reported. Prepublished protocol not sought |
| Other bias | Low risk |
Baseline characteristics: largely comparable Anaesthetists: all participants given anaesthetic by single anaesthetist External funding: no apparent funding/conflicts of interest |
Altinoren 2005.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: for patient to be sedated to OAA/S score of 2 |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | Abstract only, with limited detail, and no detail on baseline characteristics Although study authors and study methods are the same as Akcaboy 2007, results in the abstract are different, so assumed to be different study Duplicate of full paper in Turkish (Altınören 2008) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized. No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of blinding given. Assume participants and personnel were not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No details of blinding given. Assume clinician was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | Unclear risk | No details of whether observers were blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of whether observers were blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether observers were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of whether observers were blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of whether observers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable. No external funding or apparent external sources of support |
Broch Porcar 1999.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
For all 3 drugs, 50% of total quantity given initially, then boluses of 25% thereafter until patient was sedated. If more than calculated dose was necessary, a further 25% was given at 60‐second intervals Aim: for patient to be sedated to Ramsey Sedation Scale level 4 |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate vs Midazolam
|
|
| Notes | Anaesthetic given by same nurse for all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Assume nurse giving anaesthetic drugs was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Assume nurse giving anaesthetic drugs was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No details. Assume all personnel were not blinded. |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Assume nurse giving anaesthetic drugs was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Assume nurse giving anaesthetic drugs was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details as to who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details as to who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details as to who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details as to who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details as to who assessed outcomes and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocol not sought |
| Other bias | High risk |
Baseline imbalance: imbalance between groups of participants with NYHA class I (propofol 7.7%, etomidate 45.5%, midazolam 27.3%) Anaesthetists: All anaesthetics were given by 1 nurse External funding: no apparent funding/conflicts of interest |
Canessa 1991.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
One‐third of induction dose was added if eyelash reflex was not lost within 2 minutes All participants also received1.5 µg/kg fentanyl iv, 3 minutes before induction Aim: loss of eyelash reflex |
|
| Outcomes |
|
|
| Results |
Thiopental vs Etomidate vs Propofol vs Midazolam
|
|
| Notes | No effect estimates provided with data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignment done by last digit of clinical record |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No details of blinding given. Assume cardiologist was not blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of who assessed outcomes and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported from methods section. Prepublished protocol not sought |
| Other bias | Unclear risk | Baseline characteristics: largely comparable External funding: no apparent funding or conflicts of interests stated Anaesthetists: no details as to how many anaesthetists were involved in study |
Coll‐Vinent 2003.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: deep sedation in all patients |
|
| Outcomes |
|
|
| Results |
Etomidate vs Propofol vs Midazolam vs Midazolam + Flumazenil
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered envelopes used. No further details given |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No blinding |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No blinding |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No blinding |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No blinding |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Major adverse event | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details given as to whether participants were blinded to drug allocation |
| Blinding of outcome assessment (detection bias) Success of cardioversion | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Minor adverse events | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Nausea/vomiting and recall not reported, although methods section states that this was measured Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics appear mostly comparable – although some differences in age range Anaesthetists: no details as to how many anaesthetists were involved in study |
Dellinger 1988.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Both groups given premedication of 10 mg diazepam, oral, 2‐4 hours before procedure; and oxygen by mask, 3 minutes before induction Aim: not detailed specifically, but assume aim for general anaesthesia as sedation is not mentioned in text |
|
| Outcomes |
|
|
| Results |
Thiopental vs Etomidate
|
|
| Notes | Work subsidized by Janssen Laboratories No effect estimates provided with data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomized and stratified by groups, using 2 random number tables |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding of anaesthetist. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details of blinding of anaesthetist or personnel responsible for carrying out cardioversion procedure |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding of anaesthetist. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding of anaesthetist. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Low risk | Outcomes assessed by an independent observer unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Low risk | Outcomes assessed by an independent observer unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Minor adverse events | Low risk | Outcomes assessed by an independent observer unaware of drug allocation |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Low risk | Outcomes assessed by an independent observer unaware of drug allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant lost from each group |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section appear to be reported. Prepubished protocol not sought |
| Other bias | High risk | Baseline characteristics comparable except for NYHA class, with higher graded cases in etomidate group External funding: work subsidized by Janssen Laboratories Anaesthetists: no details as to how many anaesthetists were involved in study |
Ford 1991.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: until patient no longer responded to verbal commands |
|
| Outcomes |
|
|
| Results |
Etomidate vs Thiopental
|
|
| Notes | Insufficient information supplied for baseline characteristics. No female participants in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Prospectively randomized into two study groups”. No further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Clinician and anaesthesiologist not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Clinician and anaesthesiologist not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | Clinician and anaesthesiologist not blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Clinician and anaesthesiologist not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Clinician and anaesthesiologist not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Low risk | Observer blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | Participants blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Low risk | Observer blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Low risk | Observer blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Low risk | Observer blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported Prepublished protocol not sought |
| Other bias | Unclear risk | Baseline characteristics – insufficient information supplied. Also only males included in study External funding: no funding/conflicts of interest reported Anaesthetists: no details as to how many anaesthetists were involved in study |
Gale 1993.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: "until patient was no longer able to follow simple commands (i.e. hold up two fingers on command) and degradation of the lid reflex was noted" |
|
| Outcomes |
|
|
| Results |
Propofol vs Midazolam
|
|
| Notes | One participant from each group required additional sedation during procedure because of repeated efforts at cardioversion. These participants were removed from analysis of haemodynamic and time data No effect estimates provided with data Data from methohexital group not included, as this drug is no longer in use for cardioversion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No blinding of personnel |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No blinding of personnel |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No blinding of personnel |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No blinding of personnel |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No blinding of personnel |
| Blinding of outcome assessment (detection bias) Major adverse event | High risk | No blinding of personnel |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details as to whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | High risk | No blinding of personnel |
| Blinding of outcome assessment (detection bias) Minor adverse events | High risk | No blinding of personnel |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | High risk | No blinding of personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data not taken from 3 participants because of difficulties with cardioversion, but low number |
| Selective reporting (reporting bias) | Unclear risk | All outcomes in methods section appear to be reported Prepublished protocol not sought |
| Other bias | High risk | Baseline characteristics mostly comparable, although some differences in ASA status between groups |
Gupta 1990.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: "loss of eyelash reflex" |
|
| Outcomes |
|
|
| Results |
Propofol vs Thiopentone vs Midazolam
|
|
| Notes | Participants in midazolam group given flumazenil (0.3–0.5 mg) at 15‐30 minutes after induction to waken them. Response to flumazenil occurred within 2‐3 minutes but was short lasting. Five out of 10 participants were asleep at time of interview 4 hours later | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated. No further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details as to whether anaesthetist was blinded but assume not |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details as to whether anaesthetist was blinded but assume not |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details as to whether cardiologist was blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details as to whether anaesthetist was blinded but assume not |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details as to whether anaesthetist was blinded but assume not |
| Blinding of outcome assessment (detection bias) Major adverse event | Low risk | “Observer had no information about the drug used” |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details as to whether participant was blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | High risk | No details as to whether cardiologist was blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | High risk | “Observer had no information about the drug used” |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | High risk | “Observer had no information about the drug used” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50% loss in midazolam group for participant interview, although losses were not reported for other outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics ‐ some differences in gender, otherwise comparable Same anaesthetist for all procedures External funding: no apparent funding or conflicts of interest |
Herregods 2003.
| Methods | RCT, cross‐over design | |
| Participants |
|
|
| Interventions |
Aim: "loss of consciousness" |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | Cross‐over trial at 7 days. Data taken only from initial cardioversion for which results were available for those in whom cardioversion was unsuccessful Insufficient baseline characteristics detailed by group because of cross‐over design |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Sealed envelope randomization”. No further details |
| Allocation concealment (selection bias) | Unclear risk | “Sealed envelope randomization”. No further details |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No details on whether any personnel were blinded. Assume no blinding |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details on whether any personnel were blinded. Assume no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 participants excluded |
| Selective reporting (reporting bias) | Unclear risk | All outcomes in methods section appear to be reported Prepublished protocol not sought |
| Other bias | High risk | Baseline imbalances – available only for those participants for whom cardioversion was successful. Also not broken down by group. Therefore no evidence of effective randomization for the purposes of the data that we have used |
Hullander 1993.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
2.5% lidocaine (0.5 mg/kg) iv within 2 minutes of start of induction infusion for both groups. To avoid pain on injection Aim: "patient lost consciousness as determined by cessation of response to verbal commands" |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were “randomly assigned”. No further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details as to whether any personnel/cardiologist were blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | Unclear risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who was responsible for outcome assessment and whether blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of who was responsible for outcome assessment and whether blinded. No details as to whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of who was responsible for outcome assessment and whether blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who was responsible for outcome assessment and whether blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of who was responsible for outcome assessment and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section appear to be reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics largely comparable |
Jan 1995.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Both groups given fentanyl 2 µg/kg as premedication 3 minutes before study drug. Aim: loss of eyelash reflex |
|
| Outcomes |
|
|
| Results |
Thiopentone vs Propofol
|
|
| Notes | Only male participants were randomly assigned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly allocated” but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details of blinding of personnel/cardiologist |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details as to whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of who assessed outcomes and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable |
Kalogridaki 2011.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions | Both groups given fentanyl 50 µg iv
Aim: for patient to no longer respond to commands and loss of eyelash reflex |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | No effect estimates provided with data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly allocated to two groups”. No further details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details as to whether any personnel/cardiologist were blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | Unclear risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether they were blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details as to whether any personnel were blinded. Assume anaesthetist was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section appear to be reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable |
Karthikeyan 2002.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Both groups given nitrous oxide as co‐induction. Also both given glycopyrronium 200 µg iv during 3‐minute preoxygenation period Aim: loss of eyelash reflex |
|
| Outcomes |
|
|
| Results |
Propofol vs Sevoflurane
|
|
| Notes | Propofol/nitrous oxide dose high | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of whether anaesthetist was blinded. Assumed not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of whether anaesthetist was blinded. Assumed not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details of whether cardiologist/clinician was blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of whether anaesthetist was blinded. Assumed not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of whether anaesthetist was blinded. Assumed not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | Time to awakening measured by a recovery nurse unaware of group allocation However, no details of who reported other time outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported Prepublished protocol not sought |
| Other bias | High risk | Baseline characteristics comparable. However dose of propofol/nitrous oxide is high, which could bias the results |
Kick 1996.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: loss of lid reflex |
|
| Outcomes |
|
|
| Results |
Etomidate vs Propofol
|
|
| Notes | No effect estimates provided with data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization done but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details as to whether anaesthetist was blinded. Assumed was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details as to whether anaesthetist or personnel responsible for cardioversion procedure were blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details as to whether anaesthetist was blinded. Assumed not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details as to whether anaesthetist was blinded. Assumed not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Low risk | Observations done by assessor who knew neither group allocation nor anaesthetic drug given |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Low risk | Observations done by assessor who knew neither group allocation nor anaesthetic drug given |
| Blinding of outcome assessment (detection bias) Minor adverse events | Low risk | Observations done by assessor who knew neither group allocation nor anaesthetic drug given |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Low risk | Observations done by assessor who knew neither group allocation nor anaesthetic drug given |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported. Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics largely comparable |
Mitchell 2003.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Both groups given additional analgesics if required (such as diamorphine, morphine and pethidine) Aim: "Adequate sedation was determined by loss of response to verbal stimulus or tactile stimulus" |
|
| Outcomes |
|
|
| Results |
Midazolam vs Diazepam
|
|
| Notes | Anaesthetic administered by attending doctor (anaesthetist available within 5 minutes). This is no longer standard practice in UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Mortality | High risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Attending doctor who administered anaesthetic was aware of drug allocation |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details as to who recorded all outcome assessments |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | No details as to who recorded all outcome assessments. Assume not blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Low risk | Participant blinded to study allocation |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details as to who recorded all outcome assessments. Assume not blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details as to who recorded all outcome assessments. Assume not blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details as to who recorded all outcome assessments. Assume not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 participants (11 in midazolam group; 4 in diazepam group) given flumazenil and excluded from awakening data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes from methods appear to be reported Prepublished protocol not sought |
| Other bias | Unclear risk | No details presented for all baseline characteristics – although described as comparable |
Munoz 2002.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Both given in a bolus slowly over 1 minute. Etomidate group also given 1 mg midazolam Aim: loss of response to verbal or tactile stimulus |
|
| Outcomes |
|
|
| Results |
Propofol vs Etomidate
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Study described by study authors as not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Study described by study authors as not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | Study described by study authors as not blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Study described by study authors as not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Study described by study authors as not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | High risk | Study described by study authors as not blinded |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | High risk | Study described by study authors as not blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | High risk | Study described by study authors as not blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | High risk | Study described by study authors as not blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | High risk | Study described by study authors as not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant excluded from data analysis because of extravasation of the drug |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocol not sought |
| Other bias | Low risk |
Baseline imbalances: all comparable External funding: no apparent funding with potential sources of conflict |
Orko 1976a.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
All groups given atropine (0.01 mg/kg given iv 2 minutes) before anaesthesia Aim: "when patient did not respond to questions, the level of anaesthesia was considered adequate" |
|
| Outcomes |
|
|
| Results |
Diazepam vs Thiopentone
|
|
| Notes | No effect estimates provided with data Propanidid is no longer in use; therefore data were not taken from this group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Assume anaesthetist was aware of group allocation |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | No details of blinding given. Old study and assume cardiologist/surgeon was not blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding given. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details given as to who recorded outcome data, although arrhythmias were analysed by someone unaware of group allocation |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether participant was blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details given as to who recorded outcome data, although arrhythmias were analysed by someone unaware of group allocation |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details given as to who recorded outcome data, although arrhythmias were analysed by someone unaware of group allocation |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details given as to who recorded outcome data, although arrhythmias were analysed by someone unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes appear to be reported from methods section Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable |
Parlak 2006.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
All groups given fentanyl citrate for preprocedural analgesia Aim: participant sedated to Ramsey Sedation Scale level 5 |
|
| Outcomes |
|
|
| Results |
< 65 years Midazolam vs < 65 years Propofol vs ≥65 years Midazolam vs ≥65 years Propofol
Unsure if satisfied: 0/12 vs 0/11 vs 2/25 vs 2/22 |
|
| Notes | Four groups for 2 drug comparisons divided by age group (< 65 years, ≥ 65 years) Anaesthetic administered by 2 final year medical residents from Emergency department and Anesthetic department |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification by age and then computer software to generate random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details of how allocation was concealed |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | Medical resident who administered anaesthetic aware of group allocation |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | Medical resident who administered anaesthetic aware of group allocation |
| Blinding of participants and personnel (performance bias) Success of cardioversion | High risk | Medical resident who administered anaesthetic aware of group allocation |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | Medical resident who administered anaesthetic aware of group allocation |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | Medical resident who administered anaesthetic aware of group allocation |
| Blinding of outcome assessment (detection bias) Major adverse event | Low risk | Researcher who collected data was blinded to participant treatment allocation. Blinding achieved by obscuring participant's arm |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Low risk | Researcher who collected data was blinded to participant treatment allocation. Blinding achieved by obscuring participant's arm |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Low risk | Researcher who collected data was blinded to participant treatment allocation. Blinding achieved by obscuring participant's arm |
| Blinding of outcome assessment (detection bias) Minor adverse events | Low risk | Researcher who collected data was blinded to participant treatment allocation. Blinding achieved by obscuring participant's arm |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Low risk | Researcher who collected data was blinded to participant treatment allocation. Blinding achieved by obscuring participant's arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants excluded “because of difficulties in data collection”. Only 5% |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics largely comparable – given stratification by age |
Sharafudeen 2010.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: for patient to stop tapping finger continuously on chest during induction |
|
| Outcomes |
|
|
| Results | No denominator figures reported. Study authors quote in abstract: "None of the patients reported awareness and satisfaction scores were similar in both groups" | |
| Notes | Conference abstract with limited detail. No detail on participant number per group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients randomly assigned to two groups”. No further details |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Insufficient detail to make judgement |
| Other bias | Unclear risk | Insufficient detail on baseline characteristics |
Siedy 2010.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: general anaesthesia as determined by inability to open eyes on command and lack of eyelash reflex |
|
| Outcomes |
|
|
| Results |
Propofol vs Fentanyl + Etomidate
|
|
| Notes | No effect estimates provided with data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization took place but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details of blinding of personnel/cardiologist |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether blinding took place |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details of who assessed outcomes and whether blinding took place |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who assessed outcomes and whether blinding took place |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of who assessed outcomes and whether blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as in methods section Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics well documented and largely comparable |
Sternlo 1991.
| Methods | RCT, parallel design | |
| Participants |
|
|
| Interventions |
Aim: clinical anaesthesia as indicated by loss of eyelid reflexes |
|
| Outcomes |
|
|
| Results |
Thiopentone vs Propofol
|
|
| Notes | Quasi‐randomized ‐ by alternating participants Final statistical analysis included only elective participants Most anaesthetics given by trained anaesthetic nurses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating participants |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding of personnel. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details of who assessed outcomes and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants for emergency cardioversion not included in data analysis. No details as to how many participants this has excluded. Five thiopentone participants and three propofol participants missing from blood pressure data; reason given is "some incomplete protocols were discarded" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods appear to be reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable |
Valtonen 1988.
| Methods | RCT, cross‐over design | |
| Participants |
|
|
| Interventions |
Aim: patient no longer responded to command |
|
| Outcomes |
|
|
| Results |
Propofol vs Thiopentone
|
|
| Notes | Cross‐over study with no details of a wash‐out period between different interventions. Therefore only data from first set of procedures used in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Major adverse events | High risk | No details of blinding. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Patient reported outcomes (recall/satisfaction) | High risk | No details of blinding. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Success of cardioversion | Unclear risk | No details of blinding of cardiologist/personnel performing cardioversion |
| Blinding of participants and personnel (performance bias) Minor adverse events | High risk | No details of blinding. Assume anaesthetist was not blinded |
| Blinding of participants and personnel (performance bias) Time to induction etc/need for resedation. | High risk | No details of blinding. Assume anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) Major adverse event | Unclear risk | No details as to who was responsible for outcome assessment and if blinded to group allocation |
| Blinding of outcome assessment (detection bias) Patient reported outcomes (recall/satisfaction) | Unclear risk | No details of whether participants were blinded |
| Blinding of outcome assessment (detection bias) Success of cardioversion | Unclear risk | No details as to who was responsible for outcome assessment and if blinded to group allocation |
| Blinding of outcome assessment (detection bias) Minor adverse events | Unclear risk | No details as to who was responsible for outcome assessment and if blinded to group allocation |
| Blinding of outcome assessment (detection bias) Time to induction etc./need for resedation | Unclear risk | No details as to who was responsible for outcome assessment and if blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section appear to be reported Prepublished protocol not sought |
| Other bias | Low risk | Baseline characteristics comparable Same anaesthetist used for all procedures |
RCT = randomized controlled trial, ASA = American Society of Anesthesiologists, OAA/S = observer's assessment of alertness/sedation, PONV = postoperative nausea and vomiting, vs = versus, NYHA = New York Heart Association, ICU = intensive care unit, SpO2 = oxygen saturation, iv = intravenous, USA = United States of America.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Huter 2007 | RCT comparing midazolam with placebo as pretreatment for etomidate |
| Maltepe 2006 | RCT comparing remifentanil with fentanyl. Both intervention and comparison groups given propofol |
| Orko 1976b | RCT comparing thiopental with althesin. Althesin no longer available |
| Prieto 1995 | Insufficient reporting of outcome data in abstract. No known published full text. No contact details for study authors |
| Tiongson 1978 | RCT comparing diazepam with sodium methohexital. Methohexital no longer in use for cardioversion |
| Yildirim 2007 | RCT. Cardioversion procedure done following coronary artery bypass grafting |
RCT = randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Sawas 2013.
| Methods | RCT, parallel design |
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes | Unclear whether data for 7 cardioversion participants are reported separately. Abstract only, no contact details for study authors |
ASA = American Society of Anesthesiologists,
RCT = randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01211158.
| Trial name or title | Randomized double‐blind trial to evaluate ketamine‐propofol combination vs propofol alone for procedural sedation and analgesia in the emergency department |
| Methods | RCT, parallel design |
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 28, 2010 |
| Contact information | G Andolfatto, Lions Gate Hospital, University of British Columbia Department of Emergency Medicine |
| Notes | clinicaltrials.gov register number: NCT01211158 |
RCT = randomized controlled trial.
Differences between protocol and review
Review information
Johnny Kenth (JK) was added as a new review author after publication of the protocol (Reed 2013).
Types of outcome measures
We reconsidered and reordered our outcomes. Our original outcomes to measure 'time to loss of consciousness, first shock, awakening and full recovery', as well as the outcome 'need for re‐sedation', are dependent on dose, choice of agent and time over which the agent is given, and we removed these outcomes. We also did not report 'other arrhythmia', nor 'abandoned procedure', which are dependent on individual participant factors. We re‐classified 'mortality' and 'success of cardioversion' as secondary outcomes. We added a new outcome (need for additional analgesia to prevent pain during or after the procedure) to meet the objectives in our protocol, but this outcome was omitted from this list. We maintained all outcomes that were relevant to the patient.
Searching other resources
We did not contact other investigators known to be involved in previous studies to enquire about ongoing or unpublished studies.
Measures of treatment effect
We did not enter data into RevMan 5.2, and we did not calculate risk ratios or mean differences, because of heterogeneity between studies.
Assessment of reporting bias
We did not use funnel plots to assess reporting bias.
Data synthesis, subgroup analysis and sensitivity analysis
We did not pool data because of heterogeneity between studies and therefore did not carry out any subgroup or sensitivity analyses.
Summary of findings
We did not prepare a GRADE (Grades of Recommendation, Assessment, Development and Evaluation) 'Summary of findings' table because of the levels of heterogeneity observed between studies and because of our decision to refrain from pooling the results.
Contributions of authors
Sharon R Lewis (SRL), Amanda Nicholson (AN), Stephanie S Reed (SSR), Johnny J Kenth (JK), Phil Alderson (PA), Andrew F Smith (AFS).
Conceiving of the review: Jane Cracknell and the Cochrane Anaesthesia Review Group (CARG) Editorial Team.
Co‐ordinating the review: SRL.
Undertaking manual searches: AN and SRL, with support from CARG.
Screening search results: AN and SRL.
Organizing retrieval of papers: SRL and AN.
Screening retrieved papers against inclusion criteria: AN, SRL, SSR and AFS.
Appraising quality of papers: SRL and AN, with AFS resolving disagreements.
Abstracting data from papers: SRL and JK with AFS resolving disagreements.
Writing to authors of papers for additional information: SRL.
Obtaining and screening data on unpublished studies: SRL and AN.
Providing data management for the review: SRL.
Entering data into Review Manager (RevMan 5.2): SRL.
Interpretating data: SRL, PA and AFS.
Making statistical inferences: n/a.
Writing the review: all review authors.
Securing funding for the review: AFS.
Acting as guarantor for the review (one review author): AFS.
Reading and checking the review before submission: SRL.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant: Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK., UK.
This grant funds the work of SRL, AN, AFS and PA performed for this review.
Declarations of interest
Stephanie S Reed: During my time spent working on this Cochrane review, I was employed by the local hospital trust as a clinical fellow in anaesthetics (20%) and research (80%).
Sharon R Lewis: See Sources of support.
Amanda Nicholson: From March to August 2011, I worked for the Cardiff Research Consortium, which provides research and consultancy services to the pharmaceutical industry. Cardiff Research Consortium has no connection with my work with The Cochrane Collaboration. My husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio. (See also Sources of support.)
Johnny J Kenth: During my time spent working on this Cochrane review, I was employed full time as an NIHR Academic Clinical Fellow in Anaesthesia with the North West Deanery and the University of Lancaster. I am in full‐time clinical employment at University Hospital South Manchester.
Phil Alderson: My time on this review is funded as part of a grant by the UK NIHR to prepare Cochrane reviews relevant to perioperative care (see Sources of support).
Andrew F Smith: (see Sources of support).
Edited (no change to conclusions)
References
References to studies included in this review
Akcaboy 2007 {published data only}
- Akcaboy ZN, Akcaboy EY, Altinoren B, Karabulut E, Gogus N. Adding remifentanil to propofol and etomidate in cardioversion anesthesia. Saudi Medical Journal 2007; Vol. 28, issue 10:1550‐4. [PUBMED: 17914519] [PubMed]
Altinoren 2005 {published data only}
- Altinoren B, Akcaboy ZN, Arkan G, Akcaboy Y, Mutlu NM, Gogus N. Combining remifentanil to propofol and etomidate in cardioversion anaesthesia. European Journal of Anaesthesiology 2005;22:131. [Google Scholar]
Broch Porcar 1999 {published data only}
- Broch Porcar MJ, Valentin Segura V, Murcia Llacer B, Bartual Lobato E. Comparative assessment of etomidate, propofol and midazolam in elective cardioversion. [Spanish]. Medicina Intensiva 1999; Vol. 23, issue 5:209‐15.
Canessa 1991 {published data only}
Coll‐Vinent 2003 {published data only}
Dellinger 1988 {published data only}
Ford 1991 {published data only}
Gale 1993 {published data only}
Gupta 1990 {published data only}
Herregods 2003 {published data only}
Hullander 1993 {published data only}
Jan 1995 {published data only}
- Jan KT, Wang KY, Lo Y, Lu BK, Liu K. Anesthesia for elective cardioversion: a comparison of thiopentone and propofol. Acta Anaesthesiologica Sinica 1995; Vol. 33, issue 1:35‐9. [PUBMED: 7788197] [PubMed]
Kalogridaki 2011 {published data only}
- Kalogridaki M, Souvatzis X, Mavrakis HE, Kanoupakis EM, Panteli A, Kasotaki S, et al. Anaesthesia for cardioversion: a prospective randomised comparison of propofol and etomidate combined with fentanyl. Hellenic Journal of Cardiology 2011; Vol. 52, issue 6:483‐8. [PUBMED: 22143010] [PubMed]
Karthikeyan 2002 {published data only}
Kick 1996 {published data only}
Mitchell 2003 {published data only}
Munoz 2002 {published data only}
- Munoz Martinez T, Castedo Gonzalez JF, Castaneda Saiz A, Dudagoitia Otaolea JL, Poveda Hernandez Y, Iribarren Diarasarri S. Sedation for electrical cardioversion. A comparison of low doses of propofol and etomidate. [Spanish]. Medicina Intensiva 2002; Vol. 26, issue 3:98‐103.
Orko 1976a {published data only}
- Orko R, Malmivuo J. Anaesthesia for cardioversion: immediate haemodynamics in patients anaesthetized with thiopental or althesin. Annals of Clinical Research 1976; Vol. 8, issue 4:248‐53. [PUBMED: 999210] [PubMed]
Parlak 2006 {published data only}
Sharafudeen 2010 {published data only}
- Sharafudeen S, Rashid A, Khan SN, Parsloe M. BIS monitored day‐case cardioversion: a comparison of sevoflurane with propofol. Anaesthesia 2010; Vol. (1):94.
Siedy 2010 {published data only}
- Siedy J, Knapik P, Saucha W, Gross M. Comparison of propofol and etomidate anaesthesia for elective electrical cardioversion. Kardiologia Polska 2010; Vol. 68, issue 11:1249‐55. [PUBMED: 21108204] [PubMed]
Sternlo 1991 {published data only}
Valtonen 1988 {published data only}
References to studies excluded from this review
Huter 2007 {published data only}
Maltepe 2006 {published data only}
Orko 1976b {published data only}
Prieto 1995 {published data only}
- Prieto MJ, Martínez JM, Enríquez P, Collado J, Gonzalez R, Citores R, et al. Comparación entre pentotal, midazolam y tiopental en la cardioversión eléctrica electiva. Medicina Intensiva 1995, issue Suppl 1:40.
Tiongson 1978 {published data only}
- Tiongson JG Jr, Helmuth J, Austin M, Linde C. Comparison of diazepam and sodium methohexital in elective DC cardioversion. Delaware Medical Journal 1978; Vol. 50, issue 11:601‐3. [PUBMED: 365621] [PubMed]
Yildirim 2007 {published data only}
- Yildirim V, Doganci S, Bolcal C, Oz BS, Kucukarslan N, Cosar A, et al. Combination sedoanalgesia with remifentanil and propofol versus remifentanil and midazolam for elective cardioversion after coronary artery bypass grafting. Advances in Therapy 2007; Vol. 24, issue 3:662‐70. [PUBMED: 17660177] [DOI] [PubMed]
References to studies awaiting assessment
Sawas 2013 {published data only}
References to ongoing studies
NCT01211158 {published data only}
- Randomized double‐blind trial to evaluate ketamine‐propofol combination vs propofol alone for procedural sedation and analgesia in the emergency department. Ongoing study September 28, 2010.
Additional references
Altınören 2008
- Altınören B, Akçaboy ZN, Akçaboy EY, Arkan G, Aksu C, Göğüş N. Elektif elektrokardiyoversiyon anestezisinde propofol ve etomidata remifentanil eklenmesi. Genel Tip Dergisi 2008; Vol. 18, issue 4.
AoMRC 2013
- Academy of Medical Royal Colleges. Safe Sedation Practices of Healthcare Procedures. Standards and Guidance. http://www.aomrc.org.uk/doc_details/9737‐safe‐sedation‐practice‐for‐healthcare‐procedures‐standards‐and‐guidance October 2013 (accessed 15 May 2014).
Blomstrom 2003
- Blomstrom‐Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias ‐ executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation 2003;108:1871–909. [PUBMED: 14557344] [DOI] [PubMed] [Google Scholar]
Camm 2012
- Camm AJ, Lip GYH, Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European Heart Journal 2012;33:2719–47. [PUBMED: 22922413] [DOI] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote X5. Version X5. Thomson Reuters, 2011.
Fuster 2001
- Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the North American Society of Pacing and Electrophysiology. Journal of the American College of Cardiology 2001;38(4):1231‐66. [PUBMED: 11583910] [DOI] [PubMed] [Google Scholar]
Fuster 2006
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation ‐ executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). Journal of the American College of Cardiology 2006;48(4):854‐906. [PUBMED: 16904574] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hohl 2008
- Hohl CM, Sadatsafavi M, Nosyk B, Anis AH. Safety and clinical effectiveness of midazolam versus propofol for procedural sedation in the emergency department: a systematic review. Academic Emergency Medicine 2008;15(1):1‐8. [PUBMED: 18211306] [DOI] [PubMed] [Google Scholar]
Hospital Episode Statistics 2011 to 2012
- Health and Social Care Information Centre. Hospital Episode Statistics (HES). www.hscic.gov.uk/hes (accessed 26 June 2013).
James 2003
Kowey 1988
- Kowey PR. The calamity of cardioversion of conscious patients. The American Journal of Cardiology 1988;61(13):1106‐7. [PUBMED: 3364364] [DOI] [PubMed] [Google Scholar]
Link 2010
- Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, et al. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 Suppl 3):S706‐19. [PUBMED: 20956222] [DOI] [PubMed] [Google Scholar]
Morão 2011
- Morão S, Ratilal Bernardo O, Santos H, Sampaio C. Midazolam for sedation before procedures. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009491] [DOI] [Google Scholar]
NICE CG36 2006
- National Institute for Health and Clinical Excellence. CG36. Atrial fibrillation: the management of atrial fibrillation. National Institute for Health and Clinical Excellence 2006; Vol. CG36.
Reiffel 2009
- Reiffel JA. Cardioversion for atrial fibrillation: treatment options and advances. Pacing and Clinical Electrophysiology: PACE 2009;32(8):1073‐84. [PUBMED: 19659629] [DOI] [PubMed] [Google Scholar]
Resuscitation Council 2010
- Resuscitation Council. Peri‐arrest arrhythmias. Resuscitation Guidelines 2010. www.resus.org.uk. London: Resuscitation Council (UK), 2010:81‐91. [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Smith 2009a
- Smith AF, Mahajan RM. National critical incident reporting: improving patient safety. British Journal of Anaesthesia 2009;103:623‐5. [PUBMED: 19837805] [DOI] [PubMed] [Google Scholar]
Smith 2009b
- Smith AF. In search of excellence in anesthesiology. Anesthesiology 2009;110:4‐5. [PUBMED: 19104159] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith AF, Glavin R, Greaves JD. Defining excellence in anaesthesia: the role of personal qualities and practice environment. British Journal of Anaesthesia 2011;106:38‐43. [PUBMED: 21118845] [DOI] [PubMed] [Google Scholar]
Symington 2006
- Symington L, Thakore S. A review of the use of propofol for procedural sedation in the emergency department. Emergency Medicine Journal: EMJ 2006;23(2):89‐93. [PUBMED: 16439733] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakai 2008
- Wakai A, Staunton P, Cummins F, O'Sullivan R. The use of propofol for procedural sedation in emergency departments. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 2013
- Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilmott 2014
- Wilmott AR, Thompson GC, Lang E, Powelson S, Wakai A, Vandermeer B, et al. Atropine therapy versus no atropine therapy for the prevention of adverse events in paediatric patients undergoing intubation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010898] [DOI] [Google Scholar]
Wood 2006
- Wood J, Ferguson C. Best evidence topic report. Procedural sedation for cardioversion. Emergency Medicine Journal: EMJ 2006;23(12):932‐4. [PUBMED: 17130605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zipes 2006
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Journal of the American College of Cardiology 2006;48(5):e247‐346. [PUBMED: 16949478] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Reed 2013
- Reed SS, Lewis SR, Nicholson A, Alderson P, Smith AF. Anaesthetic and sedative agents used for electrical cardioversion. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010824] [DOI] [PMC free article] [PubMed] [Google Scholar]


