Abstract
Background
Macrolide antibiotics (macrolides) are among the most commonly prescribed antibiotics worldwide and are used for a wide range of infections. However, macrolides also expose people to the risk of adverse events. The current understanding of adverse events is mostly derived from observational studies, which are subject to bias because it is hard to distinguish events caused by antibiotics from events caused by the diseases being treated. Because adverse events are treatment‐specific, rather than disease‐specific, it is possible to increase the number of adverse events available for analysis by combining randomised controlled trials (RCTs) of the same treatment across different diseases.
Objectives
To quantify the incidences of reported adverse events in people taking macrolide antibiotics compared to placebo for any indication.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which includes the Cochrane Acute Respiratory Infections Group Specialised Register (2018, Issue 4); MEDLINE (Ovid, from 1946 to 8 May 2018); Embase (from 2010 to 8 May 2018); CINAHL (from 1981 to 8 May 2018); LILACS (from 1982 to 8 May 2018); and Web of Science (from 1955 to 8 May 2018). We searched clinical trial registries for current and completed trials (9 May 2018) and checked the reference lists of included studies and of previous Cochrane Reviews on macrolides.
Selection criteria
We included RCTs that compared a macrolide antibiotic to placebo for any indication. We included trials using any of the four most commonly used macrolide antibiotics: azithromycin, clarithromycin, erythromycin, or roxithromycin. Macrolides could be administered by any route. Concomitant medications were permitted provided they were equally available to both treatment and comparison groups.
Data collection and analysis
Two review authors independently extracted and collected data. We assessed the risk of bias of all included studies and the quality of evidence for each outcome of interest. We analysed specific adverse events, deaths, and subsequent carriage of macrolide‐resistant bacteria separately. The study participant was the unit of analysis for each adverse event. Any specific adverse events that occurred in 5% or more of any group were reported. We undertook a meta‐analysis when three or more included studies reported a specific adverse event.
Main results
We included 183 studies with a total of 252,886 participants (range 40 to 190,238). The indications for macrolide antibiotics varied greatly, with most studies using macrolides for the treatment or prevention of either acute respiratory tract infections, cardiovascular diseases, chronic respiratory diseases, gastrointestinal conditions, or urogynaecological problems. Most trials were conducted in secondary care settings. Azithromycin and erythromycin were more commonly studied than clarithromycin and roxithromycin.
Most studies (89%) reported some adverse events or at least stated that no adverse events were observed.
Gastrointestinal adverse events were the most commonly reported type of adverse event. Compared to placebo, macrolides caused more diarrhoea (odds ratio (OR) 1.70, 95% confidence interval (CI) 1.34 to 2.16; low‐quality evidence); more abdominal pain (OR 1.66, 95% CI 1.22 to 2.26; low‐quality evidence); and more nausea (OR 1.61, 95% CI 1.37 to 1.90; moderate‐quality evidence). Vomiting (OR 1.27, 95% CI 1.04 to 1.56; moderate‐quality evidence) and gastrointestinal disorders not otherwise specified (NOS) (OR 2.16, 95% CI 1.56 to 3.00; moderate‐quality evidence) were also reported more often in participants taking macrolides compared to placebo.
The number of additional people (absolute difference in risk) who experienced adverse events from macrolides was: gastrointestinal disorders NOS 85/1000; diarrhoea 72/1000; abdominal pain 62/1000; nausea 47/1000; and vomiting 23/1000.
The number needed to treat for an additional harmful outcome (NNTH) ranged from 12 (95% CI 8 to 23) for gastrointestinal disorders NOS to 17 (9 to 47) for abdominal pain; 19 (12 to 33) for diarrhoea; 19 (13 to 30) for nausea; and 45 (22 to 295) for vomiting.
There was no clear consistent difference in gastrointestinal adverse events between different types of macrolides or route of administration.
Taste disturbances were reported more often by participants taking macrolide antibiotics, although there were wide confidence intervals and moderate heterogeneity (OR 4.95, 95% CI 1.64 to 14.93; I² = 46%; low‐quality evidence).
Compared with participants taking placebo, those taking macrolides experienced hearing loss more often, however only four studies reported this outcome (OR 1.30, 95% CI 1.00 to 1.70; I² = 0%; low‐quality evidence).
We did not find any evidence that macrolides caused more cardiac disorders (OR 0.87, 95% CI 0.54 to 1.40; very low‐quality evidence); hepatobiliary disorders (OR 1.04, 95% CI 0.27 to 4.09; very low‐quality evidence); or changes in liver enzymes (OR 1.56, 95% CI 0.73 to 3.37; very low‐quality evidence) compared to placebo.
We did not find any evidence that appetite loss, dizziness, headache, respiratory symptoms, blood infections, skin and soft tissue infections, itching, or rashes were reported more often by participants treated with macrolides compared to placebo.
Macrolides caused less cough (OR 0.57, 95% CI 0.40 to 0.80; moderate‐quality evidence) and fewer respiratory tract infections (OR 0.70, 95% CI 0.62 to 0.80; moderate‐quality evidence) compared to placebo, probably because these are not adverse events, but rather characteristics of the indications for the antibiotics. Less fever (OR 0.73, 95% 0.54 to 1.00; moderate‐quality evidence) was also reported by participants taking macrolides compared to placebo, although these findings were non‐significant.
There was no increase in mortality in participants taking macrolides compared with placebo (OR 0.96, 95% 0.87 to 1.06; I² = 11%; low‐quality evidence).
Only 24 studies (13%) provided useful data on macrolide‐resistant bacteria. Macrolide‐resistant bacteria were more commonly identified among participants immediately after exposure to the antibiotic. However, differences in resistance thereafter were inconsistent.
Pharmaceutical companies supplied the trial medication or funding, or both, for 91 trials.
Authors' conclusions
The macrolides as a group clearly increased rates of gastrointestinal adverse events. Most trials made at least some statement about adverse events, such as "none were observed". However, few trials clearly listed adverse events as outcomes, reported on the methods used for eliciting adverse events, or even detailed the numbers of people who experienced adverse events in both the intervention and placebo group. This was especially true for the adverse event of bacterial resistance.
Plain language summary
Adverse events in people taking macrolide antibiotics
Review question
We wanted to find out if people treated with a macrolide antibiotic experienced more adverse events than those treated with placebo.
Background
Macrolide antibiotics are a group of antibiotics that are commonly used to treat both acute and chronic infections. The four most frequently used macrolides are: azithromycin, clarithromycin, erythromycin, and roxithromycin. People taking macrolide antibiotics are at risk of experiencing adverse events such as nausea, diarrhoea, or rash.
Search date
We searched the literature up to May 2018.
Study characteristics
We included 183 studies with a total of 252,886 participants. Most studies were conducted in the hospital setting. Azithromycin and erythromycin were more commonly studied than clarithromycin and roxithromycin. Most studies (89%) reported some adverse events, or at least stated that no adverse events were observed.
Study funding sources
Drug companies supplied trial medications or funding, or both, in 91 studies. Funding sources were unclear in 59 studies.
Key results
People treated with a macrolide antibiotic experienced gastrointestinal adverse events such as nausea, vomiting, abdominal pain, and diarrhoea more often than those treated with placebo.
Taste disturbances were reported more often by people taking macrolides than those taking a placebo. However, as very few studies reported on these adverse events, these results should be interpreted with caution.
Hearing loss was reported more often by people taking macrolide antibiotics, however only four studies reported this outcome.
Macrolides caused less cough and fewer respiratory tract infections than placebo.
We did not find any evidence that macrolides caused more cardiac disorders, liver disorders, blood infections, skin and soft tissue infections, changes in liver enzymes, appetite loss, dizziness, headache, respiratory symptoms, itching, or rashes than placebo.
We did not find more deaths in people treated with macrolides than in those treated with placebo.
Very limited information was available to assess if people treated with a macrolide antibiotic were at greater risk of developing resistant bacteria than those treated with placebo. However, bacteria that did not respond to macrolide antibiotics were more commonly identified immediately after treatment in people taking a macrolide than in those taking a placebo, but differences in resistance thereafter were inconsistent.
Quality of the evidence
The quality of the evidence ranged from very low (cardiac disorders, change in liver enzymes, liver disorders) to low (abdominal pain, death, diarrhoea, dizziness, hearing loss, skin and soft tissue infections, taste disturbance, wheeze) to moderate (appetite loss, blood infection, cough, fever, headache, itching, nausea, rash, respiratory symptoms, respiratory tract infections, vomiting).
Summary of findings
Background
Description of the condition
Macrolide antibiotics, often referred to as macrolides, are among the most commonly prescribed antibiotics worldwide. Macrolides are often prescribed for the treatment of acute upper and lower respiratory infections (Laopaiboon 2015; van Driel 2016), pelvic inflammatory disease (Savaris 2017), skin and soft tissue infections (Dalal 2017), and to eradicate Helicobacter pylori (Ford 2016). Macrolides are frequently the drug of choice for people allergic to penicillin.
As well as antibiotic activity, macrolides have anti‐inflammatory and immunomodulatory activity (Spagnolo 2013), and are used to treat several chronic respiratory tract conditions such as diffuse panbronchiolitis (Lin 2015), cystic fibrosis (Southern 2012), bronchiectasis (Hnin 2015), asthma (Kew 2015), and chronic rhinosinusitis (Head 2016). Long‐term therapy has also been used for decades for the treatment of acne vulgaris, using both the antibacterial and anti‐inflammatory effects of macrolides (Dawson 2013). There are various other indications for treatment with macrolide antibiotics, such as gastroparesis (Enweluzo 2013), trachoma (Evans 2011), typhoid fever (Chandey 2012), and preventing Mycobacterium avium complex infection in people with HIV infection (Uthman 2013). Several other indications exist or are being tested.
Description of the intervention
Erythromycin, the first discovered macrolide antibiotic, has been in use since the early 1950s. A series of semisynthetic compounds were subsequently developed, with clarithromycin, roxithromycin, and azithromycin being the most commonly used clinically (Zuckerman 2009). The availability of these new macrolides has substantially reduced the use of erythromycin over recent years, as they have greater acid stability in the digestive tract, improved oral bioavailability, longer half‐lives, and diminished gastrointestinal adverse reactions (Dougherty 2012). In general, macrolides have a moderately broad spectrum of activity, which includes most gram‐positive but only selected gram‐negative organisms, as well as several bacteria responsible for intracellular infections, such as Mycoplasma spp,Chlamydia spp, and Legionella spp. Azithromycin has more potent antibacterial activity against gram‐negative organisms than erythromycin and has an exceptional ability to accumulate inside eukaryotic cells, resulting in a favourable profile against intracellular bacteria (Zuckerman 2009).
In the USA, macrolides are the most commonly prescribed antibiotics together with penicillins (Hicks 2013). In Europe, macrolides are also among the most commonly prescribed antibiotics in the community (ECDC 2017a). However, resistance to macrolides has become a major problem, and macrolides are no longer always effective in treating common infections, such as community‐acquired pneumonia (ECDC 2017b).
How the intervention might work
The most commonly used therapeutic macrolides are characterised by a 14‐, 15‐ or 16‐membered lactone ring, to which one or more sugars are attached (Dinos 2017). Macrolides are considered as bacteriostatic antibiotics. Macrolides are protein synthesis inhibitors, exerting their antimicrobial effect by preventing the bacterial ribosome from translating its messenger ribonucleic acid (RNA) into new proteins (Dougherty 2012). The immunomodulatory properties of macrolides are related to the lactone ring and are seen with the 14‐membered ring macrolides (erythromycin, clarithromycin, and roxithromycin) and the 15‐membered ring macrolides (azithromycin) (Spagnolo 2013). Although the precise mechanism of the immunomodulatory properties is unknown, it has been proposed that macrolides attenuate mucous hypersecretion, reduce production of pro‐inflammatory cytokines, and have a suppressive effect on lymphocytic activity (Sadarangani 2015).
Taking macrolides also exposes people to the risk of various adverse events. For example, gastrointestinal adverse reactions such as abdominal pain, nausea, vomiting, and diarrhoea are common. The mechanism underlying these reactions is believed to be partly motilin‐receptor agonism and consequently stimulation of stomach and gut motility (Abu‐Gharbieh 2004). Ototoxicity (hearing loss and tinnitus) and hepatotoxicity (e.g. raised liver enzymes, hepatitis, and intrahepatic cholestasis) have also been reported in people taking macrolides. Headache, taste disturbances, and haematologic toxicity such as leukopenia, thrombocytopenia, agranulocytosis, neutropenia, and neutrophilia are also seen. Allergic reactions such as eosinophilia, fever, and rashes are rarely reported, as is Candida overgrowth and pseudomembranous enterocolitis caused by Clostridium difficile (Dougherty 2012; Zuckerman 2009).
Cardiac toxicity may complicate the use of macrolides, as macrolide antibiotics inhibit the delayed rectifier potassium current (IKr), resulting in prolongation of cardiac repolarisation (prolongation of the QT interval), which can cause cardiac arrhythmias (Owens 2006). Observational studies have shown that both azithromycin and clarithromycin are associated with a significantly increased risk of cardiovascular death (Ray 2012; Svanström 2013; Svanström 2014). However, a Danish cohort study comparing azithromycin with penicillin V found that the former was not associated with a significantly increased risk, suggesting that the increased risk of cardiovascular death observed in people taking azithromycin compared with no antibiotic use was attributable to underlying patient factors that led to the prescription of antibiotics (Svanström 2013).
Finally, there is a well‐documented association between antibiotic consumption and the development of bacterial resistance at both the individual and community level, and people taking macrolides are at risk of becoming carriers of resistant bacteria (Bell 2014).
Definitions
An adverse event is an adverse outcome that occurs while a person is taking a drug, but the event is not (or not necessarily) attributable to the drug taken (Edwards 2000). It is recommended that the recording of adverse events in clinical trials should distinguish suspected adverse effects from suspected adverse reactions (Aronson 2013).
Adverse effects and adverse reactions have different manifestations by which they can be recognised (Aronson 2013):
adverse reactions are unwanted outcomes that the person experiences and that are detected by their clinical manifestations (symptoms or signs, or both);
adverse effects are unwanted outcomes of which the person is not aware; they are usually detected by laboratory tests (e.g. biochemical, haematological, immunological, radiological, pathological tests) or by clinical investigations (e.g. gastrointestinal endoscopy, cardiac catheterisation).
Serious adverse events are often reported separately. These are adverse events that occur at any dose and result in death or life‐threatening events; requirement for hospitalisation or prolongation of existing hospitalisation; persistent or significant disability; or congenital anomalies, or are events that are considered medically important (ICH 2003).
Why it is important to do this review
The current understanding of adverse events in people taking antibiotics is largely derived from observational studies, in which estimates may be biased because it is hard to distinguish adverse drug reactions from disease‐related symptoms. One way of addressing this problem is to investigate common adverse events encountered in randomised, placebo‐controlled trials of antibiotics. This study design controls for disease‐related symptoms, allowing for better quantification of antibiotic‐related adverse events.
However, most randomised controlled trials are set up to demonstrate the benefits of antibiotic treatment for specific infections, and these studies are often not powered to quantify adverse events (Vandenbroucke 2004). The Cochrane Handbook for Systematic Reviews of Interventions states that "many adverse events are too uncommon or too long‐term to be observed within randomised trials" (Higgins 2011). As a consequence, a typical systematic review of controlled trials focusing on a specific indication may not provide sufficient evidence on the adverse events profile of an intervention, for example antibiotics (Zorzela 2014). Because adverse events are not disease‐specific (with a very few exceptions, e.g. ampicillin rash in people with Epstein‐Barr virus acute infectious mononucleosis), it is possible to 'borrow strength' from studies using the same intervention for different diseases to better estimate adverse events (Chen 2014).
We undertook this review to quantify adverse events in people using macrolide antibiotics, independently of the indication or effects of the treatments. The intent is to support clinicians and patients in evaluating harms as well as benefits in the choice of management when antibiotics are contemplated.
Objectives
To quantify the incidences of reported adverse events in people taking macrolide antibiotics compared to placebo for any indication.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, placebo‐controlled trials of any of the four most commonly used macrolide antibiotics: azithromycin, clarithromycin, erythromycin, or roxithromycin. We included trials with more than two intervention arms if we could identify a macrolide arm and a placebo arm.
We excluded purely pharmacodynamic studies and purely pharmacokinetic studies, unless they also reported clinical measurements. We also excluded studies in which fewer than 20 participants were randomised to each arm.
Types of participants
We included individuals of all ages taking a macrolide antibiotic for any indication.
Types of interventions
We included trials of macrolides delivered by any route, including oral, topical, intravenous, and intramuscular. Use of concomitant medications was permitted.
Types of outcome measures
Primary outcomes
Any reported adverse event that occurred in 5% or more of any group (Zarin 2016).
Death.
Subsequent carriage of macrolide‐resistant bacteria.
Secondary outcomes
None.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 8 May 2018:
the Cochrane Central Register of Controlled Trials, which contains the Cochrane Acute Respiratory Infections Group Specialised Register (CENTRAL; 2018, Issue 4) in the Cochrane Library using the strategy in Appendix 1;
MEDLINE (Ovid) (from 1946 to 8 May 2018) using the search strategy in Appendix 1;
Embase (Elsevier) (from 2010 to 8 May 2018) using the search strategy in Appendix 2;
CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature) (from 1981 to 8 May 2018) using the search strategy in Appendix 3;
LILACS (BIREME) (Latin American and Caribbean Health Science Information database) (from 1982 to 8 May 2018) using the search strategy in Appendix 4; and
Web of Science (Clarivate Analytics) (from 1955 to 8 May 2018) using the search strategy in Appendix 5.
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Higgins 2011). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4), and Web of Science (Appendix 5).
We searched the following trial registries on 9 May 2018:
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov).
We did not restrict the results by language or publication status (published, unpublished, in press, or in progress).
Searching other resources
We checked the reference lists of all primary studies for additional trials by performing a backward citation (cited references) search in Web of Science. We adapted the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format, Higgins 2011, for use in EndNote 2016 on these results, before they were screened.
We searched the Cochrane Library (title, abstract, and keyword fields) using the following terms: macrolide, azithromycin, clarithromycin, erythromycin, or roxithromycin, to exploit the reference lists of previous Cochrane Reviews on macrolide antibiotics.
Data collection and analysis
Selection of studies
Two review authors (MPH and ST, AMcC, or AMS) independently screened the titles and abstracts of all studies identified by the searches for potential relevance. We retrieved full‐text copies of all potentially relevant articles for full‐text evaluation. Any disputes were resolved by consensus or by consulting a third review author (CDM).
We collated multiple reports of the same study to ensure that each study, rather than each report, was analysed. The process for selecting studies is detailed in a PRISMA flow chart (Figure 1) (Moher 2009).
1.
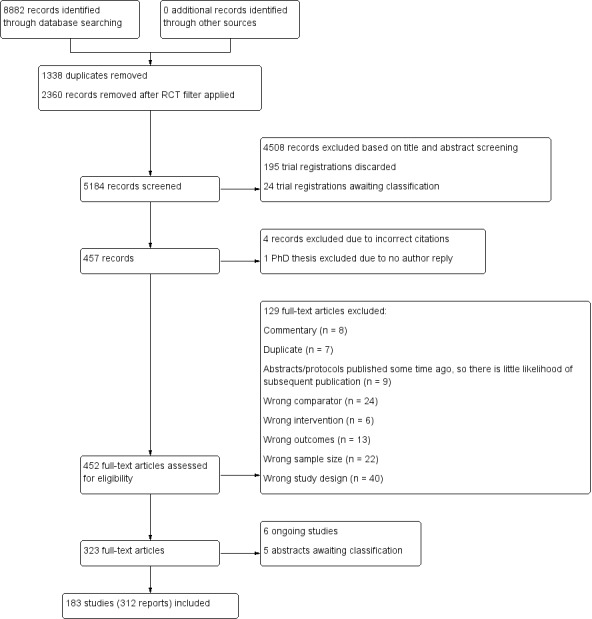
PRISMA study flow diagram.
Data extraction and management
Two review authors (MPH and AMcC or AMS) independently extracted data from the included studies using a standardised extraction form.
We extracted the following information.
Trial characteristics and methodological quality: year of publication, study design, number of participants, study setting, information for assessing risk of bias.
Participant characteristics: age, sex, concomitant medications if relevant.
Information about the intervention: indication for treatment, type of macrolide, route of administration, dose of treatment, duration of treatment, total treatment dose.
Outcome measures: whether adverse events were stated as an outcome, any reported adverse events (including death and data on antimicrobial resistance), method of eliciting adverse events.
Assessment of risk of bias in included studies
Two review authors (MPH and AMcC or AMS) independently assessed the risks of common biases for each of the included studies using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by consulting a third review author (CDM). We assessed risk of bias according to the following seven domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias); and
other sources of bias.
We assessed each domain as having a high, low, or unclear risk of bias and provided a justification for our judgement. Furthermore, we summarised the 'Risk of bias' judgements across different studies for each of the seven domains.
Measures of treatment effect
We expressed outcome measures as odds ratios (OR) with accompanying 95% confidence intervals (CI). When appropriate, odds ratios were also expressed as absolute risk differences (ARDs), based on average rates of adverse events in the control groups, and converted to number needed to treat for an additional harmful outcome (NNTH) to interpret the results from the meta‐analysis.
We calculated NNTH in the following manner:
NNTH = (PEER*(OR ‐ 1)) + 1/(PEER*(OR ‐ 1)*(1 ‐ PEER))
(where PEER = patient expected event rate (i.e. the rate of events in the control population), OR = odds ratio).
Unit of analysis issues
For each of the specific adverse events, including death, the participant was the unit of analysis. We used participants and isolates (colonies of bacteria grown microbiologically that arise from one or few individual bacteria) as units of analysis when reporting subsequent carriage of macrolide‐resistant bacteria. Reported data from the included large cluster‐randomised controlled trial were adjusted for clustering by the trial authors and no additional adjustments were performed (Keenan 2018).
Dealing with missing data
We contacted trial authors when adverse events were incompletely reported and contact details (an e‐mail address) were provided in the publication. In case of no reply or message undeliverable, we did not make a second attempt to contact authors. We did not contact authors if a study provided no information on adverse events.
Assessment of heterogeneity
We used the I² statistic to measure statistical heterogeneity, as recommended in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
Outcome reporting bias is particularly important for adverse events, as they are often not the primary outcome. For each study, we searched for information about whether adverse events was predefined as an outcome, the method of eliciting adverse events, and whether adverse events were reported or not. This information is provided in Characteristics of included studies.
Data synthesis
Classification of adverse events
Some adverse events are reported under different names but are subsets of the same phenomenon. To address this, we classified the adverse events using the Medical Dictionary for Regulatory Activities (MedDRA), developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (MedDRA 2018). MedDRA is a clinically validated and standardised hierarchy consisting of five levels, arranged from very specific to very general:
System Organ Class, e.g. gastrointestinal disorders;
High Level Group Term, e.g. gastrointestinal signs and symptoms;
High Level Term, e.g. nausea and vomiting symptoms;
Preferred Term, e.g. nausea;
Lowest Level Term, e.g. feeling queasy.
One review author (MPH) classified reported individual adverse events at the most specific level by means of the MedDRA Web‐Based Browser tool (MedDRA 2018), and then grouped them under the primary System Organ Class, according to the MedDRA coding system. There are 27 System Organ Classes, as follows.
Blood and lymphatic system disorders.
Cardiac disorders.
Congenital, familial, and genetic disorders.
Ear and labyrinth disorders.
Endocrine disorders.
Eye disorders.
Gastrointestinal disorders.
General disorders and administration site conditions.
Hepatobiliary disorders.
Immune system disorders.
Infections and infestations.
Injury, poisoning, and procedural complications.
Investigations.
Metabolism and nutrition disorders.
Musculoskeletal and connective tissue disorders.
Neoplasms benign, malignant, and unspecified.
Nervous system disorders.
Pregnancy, puerperium, and perinatal conditions.
Product issues.
Psychiatric disorders.
Renal and urinary disorders.
Reproductive system and breast disorders.
Respiratory, thoracic, and mediastinal disorders.
Skin and subcutaneous tissue disorders.
Social circumstances.
Surgical and medical procedures.
Vascular disorders.
Two review authors (MPH and AMcC or AMS) then attempted to reclassify the adverse events to a lower common hierarchical level within each System Organ Class to enable comparisons between studies. Adverse events were most often identified at the Preferred Term level (e.g. nausea or vomiting). However, some studies only reported at the High Level Term level (e.g. nausea and vomiting symptoms) or Lowest Level Term level (e.g. gastrointestinal disorder NOS).
We needed to manage a long list of infrequently reported adverse events that were unlikely to be clinically significant, and accordingly set a threshold of ≥ 5% to analyse (Zarin 2016). However, because it is possible that less frequent adverse events might be important, we extracted these to facilitate future analysis by interested investigators (Hansen 2018a; Hansen 2018b).
Analysis
When only one or two studies reported a specific adverse event, at any MedDRA level, we reported it simply as a percentage of events in each group, and calculated P values (reported as rarely reported adverse events). We undertook a meta‐analysis when ≥ 3 studies reported a specific adverse event. If studies reported more than one type of adverse event (e.g. sore throat and nasal congestion) within the same analysis (e.g. respiratory symptoms not otherwise specified), we included only the adverse event with the largest number of events in the meta‐analysis to avoid the risk of double‐counting. Haemoptysis is included in the meta‐analysis of cough, as both types of adverse events were coded in the same adverse event group (coughing and associated symptoms). When studies reported on deaths for several follow‐up periods, we used data from the follow‐up period that was mainly in line with the maximum follow‐up period used in most of the included studies for the meta‐analysis. We used Review Manager 5 to analyse data (Review Manager 2014). As we expected heterogeneity among the included studies, we used random‐effects meta‐analysis models (Higgins 2011). Some studies reported the adverse event data of macrolide resistance by isolates rather than by participants, and we modified the protocol to include those data. Whether the data were related to participants or isolates (which include studies limiting isolates to resistant streptococci), we have reported on the absolute difference, in percentage: ([absolute value of difference in macrolide‐resistant bacteria after treatment] – [absolute value of difference in macrolide‐resistant bacteria before treatment] and the relative difference: [difference in macrolide‐resistant bacteria after treatment] / [difference in macrolide‐resistant bacteria before treatment]).
'Summary of findings' table and GRADE
We created two ‘Summary of findings' tables. Table 1 presents the following gastrointestinal outcomes: not otherwise specified gastrointestinal disorders, abdominal pain, diarrhoea, nausea, and vomiting. Table 2 presents other outcomes: cardiac disorders, hearing loss, taste disturbance, hepatobiliary disorders, and deaths. We used GRADE to rate the overall quality of evidence of each of the outcomes as either high, moderate, low, or very low, employing the five GRADE considerations (study limitations, consistency of effect, indirectness, imprecision, and publication bias) (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015).
Summary of findings for the main comparison. Gastrointestinal adverse events in people taking macrolide antibiotics versus placebo for any indication.
| Gastrointestinal adverse events in people taking macrolide antibiotics versus placebo for any indication | ||||||
| Patient or population: any indication Setting: any setting Intervention: macrolide antibiotics (azithromycin, clarithromycin, erythromycin, or roxithromycin, administered by any route) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with macrolide antibiotics | |||||
| Gastrointestinal disorders not otherwise specified | 90 per 1000 | 176 per 1000 (133 to 228) | OR 2.16 (1.56 to 3.00) |
3295 (23 RCTs) |
⊕⊕⊕⊝ MODERATE1 | NNTH = 12 |
| Abdominal pain | 114 per 1000 | 176 per 1000 (135 to 225) | OR 1.66 (1.22 to 2.26) | 7776 (23 RCTs) | ⊕⊕⊝⊝ LOW1 2 | NNTH = 17 |
| Diarrhoea | 89 per 1000 | 143 per 1000 (116 to 175) | OR 1.70 (1.34 to 2.16) | 23,754 (37 RCTs) | ⊕⊕⊝⊝ LOW1 2 | NNTH = 19 |
| Nausea | 107 per 1000 | 162 per 1000 (142 to 186) | OR 1.61 (1.37 to 1.90) | 14,983 (28 RCTs) | ⊕⊕⊕⊝ MODERATE1 | NNTH = 19 |
| Vomiting | 94 per 1000 | 117 per 1000 (98 to 140 ) | OR 1.27 (1.04 to 1.56) | 5328 (15 RCTs) | ⊕⊕⊕⊝ MODERATE1 | NNTH = 45 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to imprecision. The outcome was reported in only a small proportion of the included studies.
2Downgraded one level due to inconsistency. I² = 59% for abdominal pain, I² = 74% for diarrhoea.
Summary of findings 2. Other adverse events in people taking macrolide antibiotics versus placebo for any indication.
| Other adverse events in people taking macrolide antibiotics versus placebo for any indication | ||||||
| Patient or population: any indication Setting: any setting Intervention: macrolide antibiotics (azithromycin, clarithromycin, erythromycin, or roxithromycin, administered by any route) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with macrolide antibiotics | |||||
| Cardiac disorders | 73 per 1000 | 64 per 1000 (41 to 99) | OR 0.87 (0.54 to 1.40) | 1715 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Hearing loss | 187 per 1000 | 230 per 1000 (187 to 281) | OR 1.30 (1.00 to 1.70) | 1369 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | NNTH = 24 |
| Taste disturbance | 27 per 1000 | 119 per 1000 (43 to 290) | OR 4.95 (1.64 to 14.93) | 932 (5 RCTs) | ⊕⊕⊝⊝ LOW 4 | NNTH = 11 |
| Hepatobiliary disorders | 48 per 1000 | 50 per 1000 (14 to 172) | OR 1.04 (0.27 to 4.09) | 443 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | |
| Deaths | 34 per 1000 | 32 per 1000 (29 to 35) | OR 0.96 (0.87 to 1.06) | 216,246 (52 RCTs) | ⊕⊕⊝⊝ LOW 1 6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to imprecision. The outcome was reported in only a small proportion of the included studies.
2Downgraded two levels due to risk of bias. High risk of reporting bias for Kim 2004, as they only report on major cardiac events and no other possible adverse events. Importantly, the study population consists of participants with acute coronary syndrome who underwent percutaneous coronary intervention. High risk of bias for Gupta 1997, as they report on adverse events as a total for both treatment regimens (azithromycin dose 1500 mg or 3000 mg). Importantly, the study population consists of male survivors of myocardial infarction, and the events are reported as adverse cardiovascular events.
3Downgraded one level due to risk of bias. High risk of reporting bias for Saiman 2003, as hearing loss (judged by audiology testing) was only reported for about 50% of participants assigned.
4Downgraded two levels due to very serious imprecision. The outcome was reported in only a very small proportion of the included studies, and there were large confidence intervals.
5Downgraded one level due to indirectness. Two out of four studies did not clearly state adverse events as an outcome or did not report on standardised adverse event ascertainment (Aly 2007; Black 2001).
6Downgraded one level due to indirectness. Death is reported in this review regardless if reported as a primary outcome or adverse event in the primary studies.
Subgroup analysis and investigation of heterogeneity
We decided a priori that if sufficient data were available we would undertake subgroup analyses according to:
age groups (children, adults, and elderly people);
type of macrolide (erythromycin, clarithromycin, roxithromycin, or azithromycin);
route of administration (topical, oral, intramuscular, intravenous);
antibiotic dosage (dose and frequency of administration); and
duration of therapy.
At least three studies were required for a subgroup analysis.
Sensitivity analysis
We decided a priori to perform sensitivity analyses by excluding studies with missing data on the outcome (adverse events). However, as no studies had more than 20% of randomised participants lost to follow‐up, none of the studies that provided data for the meta‐analyses were assessed as being at high risk of attrition bias.
Results
Description of studies
We presented information about the studies in Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
We retrieved a total of 8882 records from our database searches (electronic searches, n = 8663; trial registry searches, n = 219). We removed 1338 duplicates and an additional 2360 records when the randomised controlled trial (RCT) filter was applied to the backward citation searches.
We excluded 4508 records based on title and abstract screening and discarded 195 trial registrations as they were clearly not relevant or there was little likelihood of a subsequent publication.
We excluded another four records based on incorrect citations, and one PhD thesis due to no author reply. We assessed the remaining 452 full‐text articles for eligibility and excluded 129 full‐text articles, of which we have reported the reasons for exclusion for 17 key studies; see the Characteristics of excluded studies table. We included 312 full‐text records, comprising 183 studies (Figure 1).
A few of the included trials were published in languages other than English: Chinese (Wang 2012; Yang 2013), Farsi (Akhyani 2003; Paknejad 2010), German (Rozman 1984), Korean (Kim 2004), and Spanish (Garcia‐Burguillo 1996).
We identified 64 Cochrane Reviews on macrolide antibiotics. However, we did not include any additional studies based on our exploration of the reference lists of these Cochrane Reviews.
Included studies
We included 183 randomised placebo‐controlled trials involving a total of 252,886 participants.
Participants and settings
A total of 30 trials included only children aged up to 18 years; 61 trials included adults aged 18 to 64 years, and two trials included elderly people aged over 65 years; 16 trials included both children and adults; 64 trials included both adults and elderly people; three trials included children, adults, and elderly people; and seven trials did not specify the ages of participants.
Macrolide antibiotics were used for treatment or prevention of the following indications.
Acute respiratory infections (21 studies) (Bacharier 2015; Beigelman 2015; Brickfield 1986; Dunlay 1987; Grob 1981; Halperin 1999; Haye 1998; Hyde 2001; Kaiser 2001; King 1996; Kneyber 2008; Lildholdt 2003; Mandhane 2017; McCallum 2013; McCallum 2015; McDonald 1985; Moller 1990; Petersen 1997; Pinto 2012; Schalen 1993; Van Delden 2012).
Arthritis (4 studies) (Kvien 2004; Ogrendik 2007; Ogrendik 2011; Sadreddini 2009).
Bacterial carriage (3 studies) (Malhotra‐Kumar 2007a; Malhotra‐Kumar 2007b; Wilson 1977; Wilson 1979).
Cancer (2 studies) (Barkhordar 2018; Bergeron 2017).
Cardiovascular diseases (24 studies) (Anderson 1999; Berg 2005; Cercek 2003; Grayston 2005; Gupta 1997; Gurfinkel 1999; Hillis 2004; Ikeoka 2007; Jackson 1999; Jespersen 2006; Joensen 2008; Kaehler 2005; Karlsson 2009; Kim 2004; Leowattana 2001; Neumann 2001; O'Connor 2003; Parchure 2002; Sander 2002; Sinisalo 2002; Vainas 2005; Vammen 2001; Wiesli 2002; Zahn 2003).
Chronic respiratory diseases (39 studies) (Albert 2011; Altenburg 2013; Amali 2015; Anthony 2014; Ballard 2011; Banerjee 2004; Berkhof 2013; Black 2001; Branden 2004; Brill 2015; Brusselle 2013; Cameron 2013; Clement 2006; Corris 2015; Fonseca‐Aten 2006; Gibson 2017; Hahn 2006; Hahn 2012; Haxel 2015; Hodgson 2016; Johnston 2016; Kostadima 2004; Kraft 2002; Ozdemir 2011; Saiman 2003; Saiman 2010; Seemungal 2008; Serisier 2013; Shafuddin 2015; Simpson 2008; Uzun 2014; Valery 2013; Veskitkul 2017; Videler 2011; Vos 2011; Wallwork 2006; Wang 2012; Wolter 2002; Wong 2012).
Dental problems (15 studies) (Agarwal 2012; Agarwal 2017; Andere 2017; Bajaj 2012; Botero 2013; Bystedt 1980; Kathariya 2014; Martande 2015; Martande 2016; Paknejad 2010; Pradeep 2011; Pradeep 2013; Sampaio 2011; Shanson 1985; Smith 2002).
Eye infections (Yang 2013).
Gastrointestinal conditions (31 studies) (Altraif 2011; Aly 2007; Andremont 1981; Bala 2008; Berne 2002; Bonacini 1993; Carbonell 2006; Curry 2004; Czarnetzki 2015; Ehsani 2013; Frossard 2002; Gharpure 2001; Gokmen 2012; Jun 2014; Kalliafas 1996; Lanza 1998; Mandal 1984; Mathai 2007; Memis 2002; Narchi 1993; Ng 2007; Nuntnarumit 2006; Oei 2001; Patole 2000; Peterson 1996; Reignier 2002; Robins‐Browne 1983; Roy 1998; Sirinavin 2003; Smith 2000; Yeo 1993).
Infections associated with HIV infection (5 studies) (Currier 2000; El‐Sadr 2000; Jablonowski 1997; Oldfield 1998; Pierce 1996).
Improvement of immune responses (Grassly 2016).
Malaria (3 studies) (Andersen 1998; Heppner 2005; Taylor 1999).
Prevention of childhood mortality (Keenan 2018).
Sepsis (2 studies) (Giamarellos‐Bourboulis 2008; Giamarellos‐Bourboulis 2014).
Skin or soft tissue complaints (9 studies) (Ahmed 2014; Akhyani 2003; Amer 2006; Amland 1995; Avci 2013; Glass 1999; Pandhi 2014; Rozman 1984; Schwameis 2017)
Urogynaecological problems (22 studies) (Alger 1991; Eschenbach 1991; Garcia‐Burguillo 1996; Hooton 1990; Kaul 2004; Kenyon 2001a; Kenyon 2001b; Klebanoff 1995; Martin 1997; McCormack 1987; McGregor 1986; McGregor 1990; McGregor 1991; Mercer 1992; Paul 1998; Rajaei 2006; Roca 2016a; Sorensen 1992; Tita 2016; Van den Broek 2009; Walsh 1998; Winkler 1988).
Of the 183 included studies, 129 were conducted in secondary care, nine in primary care (Brickfield 1986; Dunlay 1987; Grob 1981; Hahn 2006; Hahn 2012; Haye 1998; King 1996; McDonald 1985; Petersen 1997), two in both primary and secondary care (Brill 2015; Johnston 2016), and 14 in dental care (Agarwal 2012; Agarwal 2017; Andere 2017; Bajaj 2012; Botero 2013; Kathariya 2014; Martande 2015; Martande 2016; Paknejad 2010; Pradeep 2011; Pradeep 2013; Sampaio 2011; Shanson 1985; Smith 2002). Another 22 trials were conducted in various settings, including: villages in sub‐Saharan Africa (Andersen 1998; Keenan 2018), among residents travelling to Mexico (Andremont 1981), centres or clinics not specified (Bacharier 2015; Hodgson 2016; Jablonowski 1997; Lanza 1998; O'Connor 2003; Pierce 1996; Walsh 1998), antenatal clinics in Southern Malawi (Van den Broek 2009), university‐based outpatient clinics (Currier 2000), households (Halperin 1999), remote forest and scrub‐covered foothills in Thailand (Heppner 2005), an urban slum area of Nairobi in Kenya (Kaul 2004), universities (Malhotra‐Kumar 2007a; Malhotra‐Kumar 2007b; Wilson 1977; Wilson 1979), food factories in Thailand (Sirinavin 2003), soldiers and civilians in Indonesia (Taylor 1999), community clinics in Australia and a tertiary paediatric hospital in New Zealand (Valery 2013), and infants living in the Vellore district in India (Grassly 2016). The setting was not specified clearly in seven trials (Cameron 2013; El‐Sadr 2000; Jackson 1999; Kraft 2002; Oldfield 1998; Rozman 1984; Schwameis 2017).
Interventions
Azithromycin was used as one of the treatment arms in 80 studies, erythromycin in 66 studies, clarithromycin in 23 studies, and roxithromycin in 14 studies. Five studies had two intervention arms, both using one of the four included macrolides. In Andersen 1998, one arm received azithromycin 250 mg per day for 10 weeks and one arm received azithromycin 1000 mg per week for 10 weeks. In Gupta 1997, both arms were treated with azithromycin for three or six days. Kostadima 2004 had two intervention arms, both treated with clarithromycin 250 mg, one twice, and one three times a day. In the study by Malhotra‐Kumar and colleagues, one arm received azithromycin 500 mg for three days (Malhotra‐Kumar 2007a), and the other arm received clarithromycin 1000 mg for seven days (Malhotra‐Kumar 2007b). In McCormack 1987, the form of erythromycin was changed from the estolate to the stearate about halfway through the study after reports of liver damage due to the former appeared; these two treatment arms were reported separately.
Some studies specified the form of erythromycin used: 12 studies used erythromycin base, 3 erythromycin estolate, 10 studies erythromycin ethylsuccinate, 11 studies erythromycin lactobionate, and 5 studies erythromycin stearate.
Macrolides were delivered orally in 154 studies, intravenously in 20 studies (Altraif 2011; Ballard 2011; Berne 2002; Bonacini 1993; Carbonell 2006; Czarnetzki 2015; Ehsani 2013; Frossard 2002; Gharpure 2001; Giamarellos‐Bourboulis 2008; Giamarellos‐Bourboulis 2014; Jun 2014; Kalliafas 1996; Narchi 1993; Ozdemir 2011; Reignier 2002; Smith 2000; Tita 2016; Van Delden 2012; Yeo 1993), and topically in nine studies (Agarwal 2012; Agarwal 2017; Bajaj 2012; Glass 1999; Kathariya 2014; Pradeep 2013; Rozman 1984; Schwameis 2017; Yang 2013). None of the included studies administered the macrolides intramuscularly.
In 131 of the 183 studies, the study participants used concomitant medications. One study advised participants not to use concomitant medications (Avci 2013). In 51 studies, the authors did not clearly specify if concomitant medications were permitted.
Outcomes
Adverse events were reported in 146 studies. Three of these studies reported only the number of adverse events, rather than the numbers of participants with adverse events (Andersen 1998; Bergeron 2017; Brusselle 2013), and were therefore excluded from the analyses to avoid the potential problem of double‐counting of events. In 17 studies, the authors stated that no adverse events were observed or reported (Agarwal 2012; Agarwal 2017; Altraif 2011; Andremont 1981; Bajaj 2012; Bala 2008; Carbonell 2006; Kathariya 2014; Mandal 1984; Martande 2016; Mathai 2007; McCallum 2013; Memis 2002; Moller 1990; Oei 2001; Vammen 2001; Veskitkul 2017). Twenty studies did not report adverse events (excluding data on death or resistant bacteria, or both) (Berg 2005; Ehsani 2013; Fonseca‐Aten 2006; Garcia‐Burguillo 1996; Grob 1981; Jablonowski 1997; Kalliafas 1996; Kneyber 2008; Leowattana 2001; Neumann 2001; Paknejad 2010; Parchure 2002; Paul 1998; Pinto 2012; Robins‐Browne 1983; Roy 1998; Sander 2002; Schalen 1993; Wang 2012; Winkler 1988).
A few studies provided additional information on adverse events (Ahmed 2014; Cameron 2013; Gibson 2017; Grassly 2016; Pradeep 2011; Roca 2016a), and when authors were contacted by e‐mail (Ahmed 2016 [pers comm]; Grassly 2017 [pers comm]; Kathariya 2016 [pers comm]; Powell 2018 [pers comm]; Roca 2016b [pers comm]; Thomsen 2016 [pers comm]).
Thirteen studies reported on participants with subsequent carriage of macrolide‐resistant bacteria; eight studies reported isolates with macrolide‐resistant bacteria; and three studies specifically reported the proportion of macrolide‐resistant streptococci. Fifty‐two studies reported on deaths.
Study funding sources
Funding sources of the 183 included studies are reported in the Characteristics of included studies table. Pharmaceutical companies supplied the trial medication, funding, or both for 91 of the included studies; 33 studies were non‐industry funded; and the funding sources were unclear in 59 studies.
Excluded studies
We excluded 129 studies. However, for brevity, we elected to report only 17 key studies. See the Characteristics of excluded studies table. We excluded these 17 studies for the following reasons.
Cross‐over trial, reporting adverse events only after cross‐over (Ferahbas 2004).
Only reported on pharmacodynamic outcomes (microbiome) (Doan 2017; Parker 2017).
Not placebo‐controlled (Pazoki‐Toroudi 2010; Rasi 2008; Weber 1993).
Not possible to identify if participants were treated with clarithromycin or azithromycin (Figueiredo‐Mello 2018).
Participants randomised to receive both a macrolide antibiotic and metronidazole (Aboud 2009).
Participants received erythromycin on top of placebo if feed failure (Makkar 2016).
Sample size too small (Ballard 2007; Gong 2014; Nielsen 2016).
The unit of randomisation was asthma episodes rather than participants (Stokholm 2016).
Quasi‐randomised or non‐randomised design (Batieha 2002; Sharma 2000; Yamamoto 1992; Zhang 2006).
Ongoing studies
We identified six ongoing studies (Chang 2012; Gonzalez‐Martinez 2017; Kobbernagel 2016; Mosquera 2016; Pavlinac 2017; Vermeersch 2016). The macrolide used in all six studies was azithromycin.
Studies awaiting classification
Twenty‐four trials identified by the clinical trial registry searches are awaiting classification and are listed in the Characteristics of studies awaiting classification table. We identified five abstracts based on four trials in the database searches (Dicko 2016; Gregersen 2017; Milito 2017; Ramsey 2017), however we were not able to locate peer‐reviewed publications of these trials.
Risk of bias in included studies
We assessed all 183 included studies using the six domains in the Cochrane ‘Risk of bias’ tool as described in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). Details of the 'Risk of bias' assessments are provided in Characteristics of included studies and summarised in Figure 2 and Figure 3.
2.
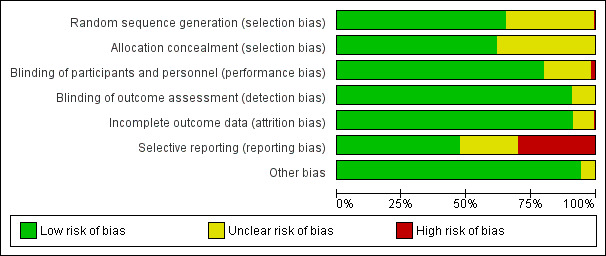
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assessed 119 studies, most of which used either computer‐generated randomisation or random number tables, as at low risk of bias. We assessed one study as at high risk of bias because randomisation was by lottery (Ahmed 2014). We assessed 63 studies that did not provide detailed information about the randomisation method used as at unclear risk of bias.
Allocation concealment
We assessed 112 studies as at low risk of bias for allocation concealment. Most studies used central allocation, but some also used sequentially numbered, identical drug containers, or sealed, opaque envelopes. We assessed studies with either insufficient or no information about allocation concealment as at unclear risk of bias.
Blinding
Blinding of participants and personnel
We assessed three studies as at high risk of bias for this domain (Brill 2015; Wilson 1977; Wilson 1979). Wilson 1977 and Wilson 1979 did not use an identical placebo. In the four‐armed study by Brill 2015, the placebo was given as one tablet daily, while the macrolide treatment was taken three times per week.
We assessed 34 studies as at unclear risk of bias because the placebo was not described in sufficient detail to judge whether blinding of participants and personnel was sufficient. The remaining studies used an identical placebo and were assessed as at low risk of bias.
Blinding of outcome assessment
We assessed 158 studies as at low risk of bias for blinding of outcome assessment. We assessed studies as at low risk of bias if blinding of all possible outcome assessors was judged sufficient; if studies only reported objective outcomes (death, data on antimicrobial resistance); or if no relevant outcomes were reported. We assessed 17 studies at unclear risk of bias because it was unclear if study participants, clinicians, and other possible outcome assessors were blinded.
Incomplete outcome data
We assessed one study as at high risk of bias for incomplete data reporting because over 20% of study participants were excluded from the final analysis without providing reasons (Paul 1998). We assessed 15 studies as at unclear risk of bias. We assessed most studies as at low risk of bias, with no or limited participant dropout, or with reasons for dropouts provided.
Selective reporting
We assessed 56 studies that either did not report adverse events or where reporting was incomplete as at high risk of selective reporting. We assessed 42 studies as at unclear risk of bias for this domain. We judged 85 studies, all of which reported on adverse events and most of which reported on the method for eliciting adverse events, as at low risk of bias.
Other potential sources of bias
We assessed 174 studies as at low risk of other bias. We assessed nine studies as at unclear risk of bias: four had an uneven distribution of participants allocated to the trial arms (Amali 2015; Lanza 1998; Peterson 1996; Taylor 1999), and five had baseline imbalances (Frossard 2002; Gokmen 2012; Gurfinkel 1999; Mathai 2007; Wolter 2002).
Effects of interventions
See Table 1 for adverse events in people taking macrolide antibiotics versus placebo for any indication.
Primary outcomes
1. Any reported adverse event that occurred in 5% or more of any group
Sufficient numbers of adverse events were reported to perform meta‐analyses for 11 of the 27 System Organ Classes.
i. Cardiac disorders
Seven studies reported cardiac disorders as adverse events, involving 1715 participants with 115 events (Albert 2011; Berkhof 2013; Gupta 1997; Kim 2004; Smith 2000; Vammen 2001; Vos 2011). The cardiovascular adverse events reported were arrhythmias, acute coronary syndrome, and not specified cardiac events. We found no difference in cardiac disorders in participants taking macrolide antibiotics compared to participants taking placebo (odds ratio (OR) 0.87, 95% confidence interval (CI) 0.54 to 1.40; I² = 9%; Analysis 1.1). We judged the evidence for cardiac disorders to be of very low‐quality due to high risk of reporting bias and imprecision.
1.1. Analysis.
Comparison 1 Cardiac disorders, Outcome 1 Cardiac disorders.
ii. Ear and labyrinth disorders
Hearing loss was reported in four studies, involving 1369 participants with 284 events (Albert 2011; Altenburg 2013; Hahn 2012; Saiman 2003). None of the studies explicitly stated if they reported on short‐ or long‐term hearing loss. Participants taking macrolides experienced hearing loss more often than those taking placebo (OR 1.30, 95% CI 1.00 to 1.70; I² = 0%; Analysis 2.1), although the findings are non‐significant. The absolute risk difference (ARD) of experiencing hearing loss was 42/1000 people, and the number of people treated with macrolides for one to experience the adverse event of hearing loss (number needed to treat for an additional harmful outcome (NNTH)) was 24 (95% CI 11 to infinity). We judged the evidence for hearing loss as of low‐quality due to high risk of reporting bias and imprecision.
2.1. Analysis.
Comparison 2 Ear and labyrinth disorders, Outcome 1 Hearing loss.
iii. Gastrointestinal disorders
Nausea was an outcome in 28 studies (14,983 participants), and vomiting an outcome of 15 studies (5328 participants). Participants taking macrolides had more nausea (OR 1.61, 95% CI 1.37 to 1.90; I² = 35%; Analysis 3.1) and vomiting (OR 1.27, 95% CI 1.04 to 1.56; I² = 6%; Analysis 3.4) than participants taking placebo. When reported together, macrolides were not associated with nausea and vomiting (High Level Term) (OR 0.92, 95% CI 0.60 to 1.42; I² = 0%; Analysis 3.7).
3.1. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 1 Nausea.
3.4. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 4 Vomiting.
3.7. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 7 Nausea and vomiting.
Compared to those taking placebo, participants taking a macrolide antibiotic more often experienced abdominal pain (OR 1.66, 95% CI 1.22 to 2.26; I² = 58%; Analysis 3.8); diarrhoea (OR 1.70, 95% CI 1.34 to 2.16; I² = 74%; Analysis 3.10); and gastrointestinal disorders not otherwise specified (NOS) (when gastrointestinal disorders were reported together) (OR 2.16, 95% CI 1.56 to 3.00; I² = 42%; Analysis 3.12).
3.8. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 8 Abdominal pain.
3.10. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 10 Diarrhoea.
3.12. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 12 Gastrointestinal disorders not otherwise specified.
The number of additional people who experienced adverse events from macrolides compared to placebo (ARD) was: gastrointestinal disorders NOS: 85/1000; diarrhoea: 72/1000; abdominal pain: 62/1000; nausea: 47/1000; and vomiting: 23/1000. The NNTH ranged from 12 (95% CI 8 to 23) for gastrointestinal disorders NOS to 17 (9 to 47) for abdominal pain; 19 (12 to 33) for diarrhoea; 19 (13 to 30) for nausea; and 45 (22 to 295) for vomiting.
We judged the evidence for abdominal pain and diarrhoea to be of low‐quality due to inconsistency and imprecision, and the evidence of nausea, vomiting, nausea and vomiting, and gastrointestinal disorders NOS to be of moderate quality due to imprecision.
iv. Nervous system disorders
There was insufficient evidence to determine whether macrolides caused dizziness based on the three studies reporting this outcome (376 participants, 31 events) (OR 1.83, 95% CI 0.85 to 3.95; I² = 0%; Analysis 4.1). Macrolides were not associated with headache in 12 trials with 1386 participants, 195 events (OR 0.81, 95% CI 0.58 to 1.11; I² = 0%; Analysis 4.2). However, macrolides did cause taste disturbance in five trials, involving 932 participants, reporting 81 instances (OR 4.95, 95% CI 1.64 to 14.93; I² = 46%; Analysis 4.3). The ARD of experiencing taste disturbances was 117/1000 people, and the number of people treated with macrolides for one to experience the adverse event of taste disturbance (NNTH) was 11 (4 to 62).
4.1. Analysis.
Comparison 4 Nervous system disorders, Outcome 1 Dizziness.
4.2. Analysis.
Comparison 4 Nervous system disorders, Outcome 2 Headache.
4.3. Analysis.
Comparison 4 Nervous system disorders, Outcome 3 Taste disturbance.
We judged the evidence for taste disturbance and dizziness as of low‐quality due to very serious imprecision, and the evidence for headache as moderate quality due to imprecision.
v. Skin and subcutaneous tissue disorders
Macrolides did not cause increased itching in four trials with 1388 participants reporting 99 events (OR 1.11, 95% CI 0.73 to 1.67; I² = 0%; Analysis 5.1) or rash in eight trials of 5314 participants reporting rash in 360 instances (OR 1.13, 95% CI 0.91 to 1.41; I² = 0%; Analysis 5.2). We judged the evidence of itching and rash as of moderate quality due to imprecision.
5.1. Analysis.
Comparison 5 Skin and subcutaneous tissue disorders, Outcome 1 Itching.
5.2. Analysis.
Comparison 5 Skin and subcutaneous tissue disorders, Outcome 2 Rash.
vi. General disorders and administration site conditions
Seven studies (2451 participants) reported fever (Bonacini 1993; Clement 2006; Grassly 2016; Heppner 2005; Roca 2016a; Saiman 2003; Saiman 2010). We found that fever was reduced in participants taking macrolides compared to placebo (OR 0.73, 95% CI 0.54 to 1.00; I² = 35%; Analysis 6.1), although the findings were non‐significant. We judged the evidence for fever as of moderate quality due to imprecision.
6.1. Analysis.
Comparison 6 General disorders and administration site conditions, Outcome 1 Fever.
vii. Hepatobiliary disorders
Four trials reported 23 hepatobiliary disorders as adverse events (cholestatic jaundice, cholangitis, or abnormal hepatic function) (Aly 2007; Black 2001; Nuntnarumit 2006; Yeo 1993). We did not find a difference in the occurrence of hepatobiliary disorders between the participants in the macrolides and placebo groups (OR 1.04, 95% CI 0.27 to 4.09; I² = 47%; Analysis 7.1). We judged the evidence for hepatobiliary disorders as of very low‐quality due to indirectness and very serious imprecision.
7.1. Analysis.
Comparison 7 Hepatobiliary disorders, Outcome 1 Hepatobiliary disorders.
viii. Infections and infestations
Four studies reported blood infections (356 participants with 99 events) (Aly 2007; Berne 2002; Ng 2007; Nuntnarumit 2006). We found no difference in the number of blood infections in participants taking macrolide antibiotics compared to those taking placebo (OR 0.83, 95% CI 0.52 to 1.34; I² = 0%; Analysis 8.1). Macrolides reduced respiratory tract infections (11 trials, 11,062 participants, 1078 events) (OR 0.70, 95% CI 0.62 to 0.80; I² = 0%; Analysis 8.2), while for skin and soft tissue infections (3 trials, 263 participants, and only 9 events) there was no difference between groups (OR 1.57, 95% CI 0.53 to 4.64; I² = 0%; Analysis 8.3). We judged the evidence for blood infections and respiratory tract infections as of moderate quality due to imprecision, and the evidence for skin and soft tissue infections as of low‐quality due to very serious imprecision.
8.1. Analysis.
Comparison 8 Infections and infestations, Outcome 1 Blood infection.
8.2. Analysis.
Comparison 8 Infections and infestations, Outcome 2 Respiratory tract infections.
8.3. Analysis.
Comparison 8 Infections and infestations, Outcome 3 Skin and soft tissue infections.
ix. Investigations
There was insufficient evidence to determine whether macrolides caused changes in liver enzymes (reported as either "elevated" or "abnormal") in the six trials reporting these adverse events (144 events among 1187 participants) (OR 1.56, 95% CI 0.73 to 3.37) because of wide confidence intervals and high heterogeneity (I² = 71%; Analysis 9.1). We judged the evidence for changes in liver enzymes as of very low‐quality due to inconsistency and very serious imprecision.
9.1. Analysis.
Comparison 9 Investigations, Outcome 1 Change in liver enzymes.
x. Metabolism and nutrition disorders
Five studies reported appetite loss (2183 participants with 248 events) (Eschenbach 1991; Heppner 2005; Martin 1997; Petersen 1997; Saiman 2003). We found no difference in appetite loss between participants taking macrolide antibiotics and those taking placebo (OR 1.10, 95% CI 0.84 to 1.43; I² = 16%; Analysis 10.1). We judged the evidence for appetite loss as of moderate quality due to imprecision.
10.1. Analysis.
Comparison 10 Metabolism and nutrition disorders, Outcome 1 Appetite lost.
xi. Respiratory, thoracic and mediastinal disorders
Six trials reported that macrolides reduced cough (1587 participants with 390 events) (OR 0.57, 95% CI 0.40 to 0.80; I² = 14%; Analysis 11.1). We did not find evidence that macrolides caused more respiratory symptoms NOS in eight trials of 2176 participants reporting 461 events (OR 1.02, 95% CI 0.82 to 1.25; I² = 0%; Analysis 11.2) or wheeze in three trials of 484 participants reporting 41 events (OR 2.20, 95% 0.74 to 6.52; I² = 49%; Analysis 11.3). We judged the evidence for cough and respiratory symptoms NOS as of moderate quality due to imprecision, and the evidence for wheeze as of low‐quality due to very serious imprecision.
11.1. Analysis.
Comparison 11 Respiratory, thoracic, and mediastinal disorders, Outcome 1 Cough.
11.2. Analysis.
Comparison 11 Respiratory, thoracic, and mediastinal disorders, Outcome 2 Respiratory symptoms not otherwise specified.
11.3. Analysis.
Comparison 11 Respiratory, thoracic, and mediastinal disorders, Outcome 3 Wheezing.
xii. Rarely reported adverse events
Rarely reported adverse events are presented in a separate table according to System Organ Classes (Table 3). No differences were observed for most rarely reported adverse events between the macrolides and placebo groups. The exceptions are listed below.
1. Rarely reported adverse events classified according to System Organ Classes.
| System Organ Class1 | Adverse event2 | Participants with an event | P value | |
| Macrolide N (%) | Placebo N (%) | |||
| Blood and lymphatic system disorders | Anaemia (Garcia‐Burguillo 1996) | 2 (7) | 3 (10) | 0.640 |
| Gastrointestinal disorders | Dental disorder NOS (Cameron 2013) | 0 | 2 (5) | 0.147 |
| Rectal disorder (Pierce 1996) | 27 (8) | 10 (3) | 0.004 | |
| Dry mouth (Ogrendik 2011) | 3 (6) | 2 (4) | 0.646 | |
| Dyspepsia (Lanza 1998) | 0 | 2 (7) | 0.040 | |
| Flatulence (Jespersen 2006) | 99 (5) | 29 (1) | 0.000 | |
| Frequent bowel movement (Frossard 2002) | 3 (6) | 0 | 0.071 | |
| Upset stomach (Jespersen 2006) | 232 (11) | 146 (7) | 0.000 | |
| Haemorrhoids (Cameron 2013) | 0 | 2 (5) | 0.147 | |
| Heartburn (Hodgson 2016) | 1 (5) | 1 (5) | 1.000 | |
| Necrotising enterocolitis (Aly 2007) | 3 (10) | 4 (13) | 0.688 | |
| Necrotising enterocolitis (Nuntnarumit 2006) | 1 (4) | 3 (13) | 0.295 | |
| Pancreatic fistula3 (Yeo 1993) | 5 (9) | 10 (17) | 0.190 | |
| General disorders and administration site conditions | Infusion site pain (Giamarellos‐Bourboulis 2014) | 26 (9) | 1 (0) | 0.000 |
| Swelling (Hahn 2012) | 0 | 2 (5) | 0.146 | |
| General disorders (Johnston 2016) | 16 (16) | 19 (19) | 0.693 | |
| Generally unwell (Saiman 2003) | 1 (5) | 1 (5) | 1.000 | |
| Malaise (Cameron 2013) | 1 (3) | 2 (5) | 0.541 | |
| Fatigue (Saiman 2003) | 24 (28) | 36 (37) | 0.185 | |
| Fatigue (Saiman 2010) | 9 (7) | 13 (10) | 0.353 | |
| Immune system disorders | Allergic reaction (Hyde 2001) | 4 (5) | 0 | 0.041 |
| Infections and infestations | Puerperal pyrexia (Tita 2016) | 51 (5) | 81 (8) | 0.001 |
| Gastroenteritis (Cameron 2013) | 7 (18) | 0 (0) | 0.006 | |
| Bacterial infection (Haxel 2015) | 13 (45) | 9 (31) | 0.279 | |
| Infection NOS (Roca 2016a) | 15 (4) | 38 (9) | 0.001 | |
| Viral infection (Cameron 2013) | 0 (0) | 2 (5) | 0.147 | |
| Chorioamnionitis (Garcia‐Burguillo 1996) | 3 (10) | 1 (3) | 0.301 | |
| Endometritis (Garcia‐Burguillo 1996) | 3 (10) | 2 (7) | 0.640 | |
| Urinary tract infection (Berne 2002) | 4 (13) | 8 (22) | 0.294 | |
| Vaginal candidiasis (Hahn 2012) | 4 (11) | 3 (8) | 0.719 | |
| Otitis (Cameron 2013) | 0 (0) | 7 (18) | 0.005 | |
| Injury, poisoning, and procedural complications | Accident4 (Valery 2013) | 2 (4) | 2 (5) | 0.982 |
| Drug dosage error (Valery 2013) | 3 (7) | 1 (2) | 0.317 | |
| Fall (Hodgson 2016) | 0 (0) | 1 (5) | 0.312 | |
| Investigations | Blood urea nitrogen increased (Uzun 2014) | 4 (9) | 10 (22) | 0.067 |
| Gastric residuals (Reignier 2002) | 7 (35) | 11 (55) | 0.204 | |
| Decreased lung function (Saiman 2003) | 13 (15) | 7 (7) | 0.088 | |
| Decreased lung function (Saiman 2010) | 8 (6) | 16 (12) | 0.080 | |
| Hearing test abnormal (Ballard 2011) | 20 (18) | 24 (22) | 0.458 | |
| Heart rate irregular (Mandhane 2017) | 10 (7) | 4 (3) | 0.103 | |
| Laboratory test abnormalities5 (Currier 2000) | 82 (25) | 104 (32) | 0.053 | |
| Metabolism and nutrition disorders | Hypochloraemia (Uzun 2014) | 6 (13) | 5 (11) | 0.807 |
| Musculoskeletal and connective tissue disorders | Back pain (Cameron 2013) | 2 (5) | 6 (16) | 0.125 |
| Back pain (Hodgson 2016) | 0 | 1 (5) | 0.312 | |
| Knee pain (Cameron 2013) | 2 (5) | 0 | 0.157 | |
| Myalgia (Heppner 2005) | 51 (30) | 30 (32) | 0.747 | |
| Rib pain (Hodgson 2016) | 0 | 1 (5) | 0.312 | |
| Nervous system disorders | Nervous system disorder NOS (Johnston 2016) | 14 (14) | 13 (13) | 0.728 |
| Impaired concentration (Peterson 1996) | 0 (0) | 2 (6) | 0.069 | |
| Sleepiness (Sampaio 2011) | 3 (15) | 3 (15) | 1.000 | |
| Psychiatric disorders | Psychiatric symptom NOS (Cameron 2013) | 4 (10) | 2 (5) | 0.414 |
| Renal and urinary disorders | Urine colour abnormal6 (McCormack 1987) | 21 (6) | 23 (6) | 0.977 |
| Reproductive system and breast disorders | Vaginal itching7 (Eschenbach 1991) | 55 (9) | 48 (9) | 0.714 |
| Skin and subcutaneous tissues disorders | Allergic skin reaction8 (Petersen 1997) | 7 (8) | 7 (8) | 1.000 |
| Cutaneous symptom (Kvien 2004) | 5 (6) | 3 (4) | 0.592 | |
| Dermatitis (Cameron 2013) | 1 (3) | 2 (5) | 0.541 | |
| Hives (Mandhane 2017) | 10 (7) | 16 (12) | 0.210 | |
| Skin ulcer (Heppner 2005) | 13 (8) | 14 (15) | 0.063 | |
| Surgical and medical procedures | Sinus operation NOS (Altenburg 2013) | 1 (2) | 2 (5) | 0.514 |
| Surgery9 (Valery 2013) | 3 (7) | 3 (7) | 0.977 | |
Abbreviations: MedDRA: Medical Dictionary for Regulatory Activities. NOS: not otherwise specified.
1System Organ Classes are groupings by aetiology, manifestation site, or purpose defined by MedDRA 2018.
2Best matching term identified in MedDRA 2018. 3Reported as a postoperative complication.
4Reported as accident, fracture, or foreign body.
5Participants who developed a severe or life‐threatening laboratory toxicity.
6Treated with erythromycin estolate or erythromycin stearate.
7Reported as "vaginal or rectal itching" ‐ coded as vaginal itching.
8Adverse events reported at day 3.
9Type of surgery not specified.
Adverse events significantly more common in people treated with a macrolide
Rectal disorder (P = 0.004) (Pierce 1996).
Flatulence (P < 0.001) (Jespersen 2006).
Upset stomach (P < 0.001) (Jespersen 2006).
Infusion site pain (P < 0.001) (Giamarellos‐Bourboulis 2014).
Allergic reactions (P = 0.041) (Hyde 2001).
Gastroenteritis (P = 0.006) (Cameron 2013).
Adverse events significantly more common in people taking a placebo
Dyspepsia (P = 0.040) (Lanza 1998).
Puerperal pyrexia (P = 0.001) (Tita 2016).
Infections NOS (P = 0.001) (Roca 2016a).
Otitis (P = 0.005) (Cameron 2013).
2. Death
Macrolides did not cause increased mortality in 52 studies with 216,246 participants reporting 6923 events (OR 0.96, 95% 0.87 to 1.06; I² = 11%; Analysis 12.1). Five studies reported on number of deaths at various time points; see Table 4 for details (Giamarellos‐Bourboulis 2008; Gurfinkel 1999; Jespersen 2006; Keenan 2018; Van den Broek 2009). We obtained number of deaths (all‐cause mortality) at 10‐year follow‐up of the CLARICOR trial, Jespersen 2006, by e‐mail correspondence with Winkel 2017 [pers comm]. We judged the evidence for death as of low‐quality due to indirectness and imprecision.
12.1. Analysis.
Comparison 12 Deaths, Outcome 1 Deaths ‐ overall.
2. Deaths.
| Indication for treatment | Study ID | Follow‐up period (days) | Participants who died | P value | |
| Macrolide N (%) | Placebo N (%) | ||||
| Acute respiratory tract infection | Van Delden 2012 | 71 | 9 (19) | 6 (13) | 0.450 |
| Cancer | Barkhordar 20182 | n/a | 11 (23) | 10 (21) | 0.804 |
| Bergeron 20173 | 730 | 95 (41) | 66 (29) | 0.006 | |
| Cardiovascular disease | Anderson 19994 | 730 | 5 (3) | 4 (3) | 0.720 |
| Berg 2005 | 730 | 10 (4) | 9 (4) | 0.837 | |
| Cercek 2003 | n/a | 23 (3) | 29 (4) | 0.417 | |
| Grayston 2005 | 1424 | 143 (7) | 132 (7) | 0.481 | |
| Gupta 19975 | n/a | 1 (3) | 1 (5) | 0.611 | |
| Gurfinkel 1999 | 30 | 0 | 2 (2) | 0.151 | |
| Gurfinkel 1999 | 90 | 0 | 4 (4) | 0.041 | |
| Gurfinkel 1999 | 180 | 2 (2) | 5 (5) | 0.238 | |
| Ikeoka 20076 | 183 | 2 (5) | 0 | 0.162 | |
| Jespersen 20067 | 949 | 212 (10) | 172 (8) | 0.023 | |
| Jespersen 20068 | 2190 | 497 (23) | 426 (19) | 0.004 | |
| Jespersen 20069 | 3650 | 866 (40) | 815 (37) | 0.055 | |
| Joensen 2008 | 767 | 28 (11) | 26 (10) | 0.693 | |
| Kaehler 2005 | 365 | 1 (1) | 1 (1) | 0.990 | |
| Karlsson 2009 | 548 | 5 (4) | 8 (6) | 0.418 | |
| Kim 200410 | 365 | 2 (3) | 2 (3) | 0.987 | |
| Leowattana 200111 | 90 | 1 (2) | 1 (2) | 0.973 | |
| Neumann 2001 | 365 | 16 (3) | 13 (3) | 0.579 | |
| Sander 200212 | 730 | 4 (3) | 5 (4) | 0.735 | |
| Sinisalo 200213 | 555 | 4 (5) | 1 (1) | 0.172 | |
| Vainas 2005 | 730 | 20 (8) | 25 (10) | 0.396 | |
| Vammen 2001 | 767 | 3 (7) | 2 (4) | 0.541 | |
| Wiesli 2002 | 986 | 1 (5) | 2 (10) | 0.548 | |
| Zahn 2003 | 365 | 28 (6) | 26 (6) | 0.739 | |
| Chronic respiratory disease | Albert 201114 | 344 | 18 (3) | 21 (4) | 0.629 |
| Anthony 201415 | 168 | 2 (5) | 0 | 0.152 | |
| Ballard 201116 | n/a17 | 20 (18) | 24 (22) | 0.458 | |
| Hahn 200618 | n/a | 0 | 1 (5) | 0.280 | |
| Ozdemir 201119 | n/a | 2 (5) | 4 (11) | 0.394 | |
| Seemungal 2008 | 365 | 0 | 1 (2) | 0.328 | |
| Shafuddin 2015 | 420 | 3 (3) | 5 (5) | 0.443 | |
| Uzun 201420 | 365 | 0 | 2 (4) | 0.144 | |
| Vos 201121 | 2555 | 5 (33) | 8 (62) | 0.136 | |
| Gastrointestinal condition | Aly 2007 | n/a | 5 (17) | 6 (20) | 0.739 |
| Berne 2002 | n/a | 2 (6) | 2 (6) | 0.903 | |
| Ehsani 2013 | n/a | 0 | 1 (5) | 0.311 | |
| Gokmen 2012 | 14 | 0 | 1 (4) | 0.302 | |
| Ng 2007 | n/a | 2 (2) | 4 (4) | 0.406 | |
| Nuntnarumit 200622 | n/a | 2 (9) | 0 | 0.148 | |
| Oei 200123 | n/a | 1 (4) | 1 (4) | 1.000 | |
| Reignier 2002 | n/a | 6 (30) | 8 (40) | 0.507 | |
| Robins‐Browne 1983 | 7 | 1 (3) | 1 (3) | 1.000 | |
| HIV | Currier 2000 | 483 | 3 (1) | 7 (2) | 0.201 |
| El‐Sadr 200024 | 386 | 5 (2) | 5 (2) | 0.980 | |
| Jablonowski 1997 | n/a | 1 (< 1) | 7 (2) | 0.033 | |
| Oldfield 1998 | n/a | 38 (45) | 38 (44) | 0.946 | |
| Pierce 1996 | 427/40225 | 107 (32) | 137 (41) | 0.017 | |
| Prevention of childhood mortality | Keenan 2018 | 726 | 4 (< 1) | 1 (< 1) | 0.195 |
| Keenan 2018 | 62127 | 2404 (2) | 2616 (3) | 0.000 | |
| Sepsis | Giamarellos‐Bourboulis 2008 | 28 | 31 (31) | 28 (28) | 0.642 |
| Giamarellos‐Bourboulis 2008 | 90 | 43 (43) | 60 (60) | 0.016 | |
| Giamarellos‐Bourboulis 2014 | 28 | 56 (19) | 51 (17) | 0.648 | |
| Skin and soft tissue complaints | Schwameis 2017 | 30 | 0 | 1 (< 1) | 0.318 |
| Urogynaecological conditions | Kaul 200428 | 801/76429 | 1 (< 1) | 2 (1) | 0.578 |
| Van den Broek 2009 | n/a30 | 1 (< 1) | 2 (< 1) | 0.563 | |
| Van den Broek 2009 | 4231 | 7 (1) | 3 (< 1) | 0.205 | |
Abbreviation: HIV: human immunodeficiency virus. n/a: not available.
1Post‐treatment.
2Death caused by relapse, infection, and other reasons. Relapse caused five and seven deaths in the macrolide and placebo groups, respectively.
3Relapse caused 52 and 23 deaths in the macrolide and placebo groups, respectively.
4Cardiovascular death.
5Cardiovascular death.
6Death caused by respiratory complications of chronic obstructive pulmonary disease or sepsis after limb revascularising surgery.
7All‐cause mortality.
8All‐cause mortality.
9All‐cause mortality. Data obtained by e‐mail correspondence with authors (Winkel 2017 [pers comm]).
10Cardiac death.
11Cardiac death.
12Incomplete reporting of death at 4‐year follow‐up. We contacted the authors but received no reply.
13Death caused by ischaemic heart disease or cancer.
14Death caused by chronic obstructive pulmonary disease, cardiovascular attacks, neoplasm, or other/unknown causes. Report on data from Sadatsafavi 2016, a secondary study of Albert 2011.
15Death caused by bronchopneumonia with underlying coronary artery disease.
16Death caused by hypoxic respiratory failure, confirmed sepsis and/or necrotising enterocolitis, pulmonary haemorrhage, or withdrawal of life support due to intraventricular haemorrhage.
17Data collected at days 3, 5, 7, then weekly for the duration of the study, and at discharge.
18Death caused by asthma‐related cause.
19Death caused by sepsis or necrotising enterocolitis.
20Death caused by respiratory failure due to exacerbation in chronic obstructive pulmonary disease.
21Report on patients that never received open‐label azithromycin. Report on data from Ruttens 2015, a secondary study of Vos 2011.
22Death caused by severe bronchopulmonary dysplasia or from necrotising enterocolitis.
23Death caused by necrotising enterocolitis and septicaemia.
24Death caused by liver failure, cardiovascular disease, cancer, an overdose of methadone, or wasting.
25Follow‐up reported separately for clarithromycin and placebo group.
26Deaths reported within one week of study drug administration.
27Follow‐up period estimated as person‐years (N = 323,302)/total number of children randomised (N = 190,238).
28Deaths caused by trauma.
29Follow‐up period reported separately for azithromycin and placebo groups.
30During pregnancy.
31During six weeks after delivery.
3. Subsequent carriage of macrolide‐resistant bacteria
Thirteen studies reported on participants with macrolide‐resistant bacteria following treatment with macrolide antibiotics (Table 5). The range of absolute increases across the studies in the numbers of participants carrying macrolide‐resistant organisms was 0% to 43%. No clear trend was observed in studies reporting on resistant bacteria at multiple time points: two trials showed an absolute decrease in resistance over time (Berg 2005; Valery 2013); one showed an absolute increase over time (Roca 2016a); and one initially reported an absolute increase followed by a decrease (Sirinavin 2003). Four studies reported a small (< 10%) relative increase in resistance (Bacharier 2015; Brusselle 2013; McCallum 2015; Wilson 1977), and three studies reported a small relative decrease in resistance (Berkhof 2013; Gibson 2017; Uzun 2014). Valery 2013 and Sirinavin 2003 showed an initial relative increase in resistance followed by a decrease over time.
3. Participants with macrolide‐resistant bacteria.
| Participants with macrolide‐resistant bacteria1: 13 studies | ||||||||
| Study ID |
Type of
macrolide (days of treatment) |
Time for follow‐up swabs | Macrolide‐resistant bacteria at baseline N (%) | Macrolide‐resistant bacteria after treatment2N (%) | Absolute increase in resistance with antibiotic (%) | Relative increase in resistance with antibiotic (%) | ||
| Macrolide | Placebo | Macrolide | Placebo | |||||
| Bacharier 20153 | AZM (5) | ≥ 14 days postintervention | 5 (12) | 4 (9) | 8 (20) | 7 (17) | 0 | 1 |
| Berg 20054 | CLM (16*) | Week 2 | 50 (34) | 50 (34) | 102 (69) | 46 (31) | 38 | N/A |
| Week 8 | 96 (65) | 55 (37) | 28 | N/A | ||||
| Berkhof 20135 | AZM (84) | Week 12 | 0 | 1 (2) | 1 (3) | 0 | 1 | ‐2 |
| Brusselle 20136 | AZM (182) | Week 26 | 11 (48) | 9 (39) | 20 (87) | 8 (35) | 43 | 6 |
| Gibson 20177 | AZM (336) | Week 48 | 14 (22) | 18 (26) | 20 (51) | 17 (41) | 6 | ‐3 |
| McCallum 20157,8 | AZM (21) | Day 23 | 8 (8) | 13 (12) | 7 (7) | 13 (12) | 1 | 1 |
| Pierce 19968,9 | CLM (315*) | Not specified | N/A | N/A | 11 (58) | 0 | N/A | N/A |
| Roca 2016a9,10 | AZM (1) | Day 3 | 12 (3) | 11 (3) | 19 (5) | 9 (2) | 3 | N/A |
| Day 6 | 25 (6) | 17 (4) | 2 | N/A | ||||
| Day 14 | 41 (11) | 15 (4) | 7 | N/A | ||||
| Day 28 | 56 (15) | 13 (3) | 12 | N/A | ||||
| Saiman 201010,11 | AZM (168) | Day 168 | 38 (29) | 50 (39) | 43 (N/A) | 9 (N/A) | N/A | N/A |
| Sirinavin 200311,12 | AZM (5) | Day 7 | 5 (5) | 4 (4) | 1 (33) | 5 (24) | 8 | 9 |
| Day 30 | 3 (18) | 0 | 17 | 18 | ||||
| Day 60 | 1 (4) | 3 (14) | 9 | ‐10 | ||||
| Day 90 | 10 (42) | 7 (37) | 4 | 5 | ||||
| Uzun 201412,13 | AZM (365) | 1 year | 5 (23) | 4 (20) | 3 (12) | 11 (41) | 26 | ‐10 |
| Valery 201313,14 | AZM (621*) | End of study | 10 (24) | 8 (22) | 19 (46) | 4 (11) | 33 | 18 |
| > 30 days and ≤ 12 months postintervention14,15 |
6 (17) | 3 (12) | 3 | 3 | ||||
| Wilson 1977 | ERY (7) | Post‐treatment | 0 | 1 (4) | 0 | 1 (4) | 0 | 1 |
Brill 2016 [pers comm] reported via email correspondence on both number of participants with resistant bacteria and the number of resistant isolates (unpublished data). We contacted the author again for information on what type of resistant bacteria they report on (macrolide‐resistant or ‘others’), and are awaiting author reply.
Smith 2002 present the mean number of colony forming units of azithromycin‐resistant streptococci per sample, and state that the number of streptococci resistant to 2 mg/L azithromycin was significantly higher in people who had taken azithromycin compared to placebo even at 22 weeks (data from Sefton 1996, a secondary study of Smith 2002). We contacted the author, but did not receive any reply.
Wallwork 2006 report on nasal swabs from participants treated with roxithromycin and state that no macrolide‐resistant organisms were noted to have developed. Data not given for placebo group.
Wong 2012 state that macrolide resistance testing was not routinely undertaken, but two (4%) participants in the azithromycin group developed macrolide‐resistant Streptococcus pneumoniae at six months.
Abbreviations: AZM: azithromycin. CLM: clarithromycin. ERY: erythromycin. N/A: not available.
*Mean duration of treatment.
1Bacterial isolates tested vary between studies. The most common ones were: Streptococcus pneumoniae,Haemophilus influenza,Moraxella catarrhalis,Pseudomonas aeruginosa, and Staphylococcus aureus.
2Some studies report on macrolide‐resistant bacteria during treatment.
3A subsample of participants (14%) was tested for resistant bacteria. The authors also report on the number of participants acquiring azithromycin‐resistant bacteria (6 in AZM group versus 4 in placebo group).
4Data from Figure 2 in Berg 2005. Only the percentages of participants with macrolide‐resistant bacteria are reported. We used the number of participants randomised and screened for culture of pathogens (N = 148 in both groups) to calculate the number of participants in each group.
5Data from Table 4 in Berkhof 2013.
6A subsample of participants (42%) was tested for resistant bacteria.
7Data from Table S8 and Table S9 in Gibson 2017. We only present data from nose swabs, as the same bacteria may be identified in the various samples (sputum, throat, nose). A subsample of participants was tested for resistant bacteria.
8Data from Table 3 in McCallum 2015. We have reported on any of the macrolide‐resistant bacteria.
9Report on people who contracted Mycobacterium avium complex infections.
10Data on mothers from Table 3 in Roca 2016a. We only present data from mothers’ nasopharyngeal swabs, as the same bacteria may be identified in the various samples (nasopharynx, milk, vagina).
11Data from Table 4 in Saiman 2010. Report on treatment‐emergent bacteria at day 168. Not possible to calculate the percentage of resistant bacteria at day 168, as the given denominator varies for each reported micro‐organism.
12Data from Table 4 in Sirinavin 2003. Report on participants with a Salmonella isolate. The denominator (number with available data) varied significantly (range 3 to 98) at days 7, 30, 60, and 90.
13Data from supplementary Table 2 in Uzun 2014. Number of participants with sputum samples used as denominator.
14Data from Table 4 in Valery 2013.
15Data on post‐intervention macrolide‐resistant bacteria are from Table 3 in Hare 2015, a secondary study of Valery 2013.
Eight studies reported on the proportion of macrolide‐resistant isolates following macrolide treatment. The absolute increase in resistance ranged from 0% to 55% for studies reporting on macrolide‐resistant isolates at a single follow‐up point (Albert 2011; Altenburg 2013; Berg 2005; Seemungal 2008; Tita 2016; Videler 2011; Wilson 1979). A single trial reported on macrolide‐resistant isolates at multiple time points, showing an initial absolute increase (at week 26) followed by a gradual decrease to 0% at week 78 (Lildholdt 2003). There was a mixed picture for relative increase in resistance, with three trials showing a small (< 10%) relative decrease in resistance (Albert 2011; Berg 2005; Videler 2011); one showing a small relative increase (Altenburg 2013); and one trial showing an initial relative increase followed by a decrease over time (Lildholdt 2003) (Table 6).
4. Isolates with macrolide‐resistant bacteria.
| Isolates with macrolide‐resistant bacteria1: 8 studies | ||||||||
| Study ID |
Type of macrolide (days of treatment) |
Time for follow‐up swabs |
Macrolide‐resistant bacteria at baseline N (%) |
Macrolide‐resistant bacteria after treatment2N (%) | Absolute increase in resistance with antibiotic (%) | Relative increase in resistance with antibiotic (%) | ||
| Macrolide | Placebo | Macrolide | Placebo | |||||
| Albert 20113 | AZM (365) | At enrolment and every 3 months | 23 (52) | 28 (57) | 38 (81) | 44 (41) | 35 | ‐8 |
| Altenburg 20134 | AZM (365) | Week 12 and 64 + exacerbations | 7 (35) | 8 (28) | 53 (88) | 29 (26) | 55 | 9 |
| Berg 20055 | CLM (16*) | “After therapy” | 27 (35) | 33 (38) | 51 (66) | 40 (45) | 18 | ‐7 |
| Lildholdt 20036 | AZM (183) | Week 26 | 1 (2) | 0 | 2 (14) | 0 | 12 | 7 |
| Week 43 | 1 (6) | 0 | 6 | 3 | ||||
| Week 60 | 1 (9) | 0 | 9 | 5 | ||||
| Week 78 | 0 | 0 | 0 | 0 | ||||
| Seemungal 20087 | ERY (365) | 12 months | 0 | 0 | 1 (4) | 0 | 4 | N/A |
| Tita 2016 | AZM (1) | Postpartum | N/A | N/A | 3 | 4 | N/A | N/A |
| Videler 2011 | AZM (84) | Day 84 | 2 (4) | 1 (2) | 1 (2) | 3 (7) | 3 | ‐3 |
| Wilson 1979 | ERY (7) | “Post‐treatment” | 0 | 0 | 0 | 0 | 0 | N/A |
Brill 2016 [pers comm] report via email correspondence on both the number of participants with resistant bacteria and the number of resistant isolates (unpublished data). We contacted the author again for information on what type of resistant bacteria they report on (macrolide‐resistant or ‘others’), and are awaiting author reply.
Van Delden 2012 state that azithromycin exposure did not lead to an MIC increase comparing the initial and last Pseudomonas aeruginosa isolates. Data not shown.
Abbreviations: AZM: azithromycin. CLM: clarithromycin. ERY: erythromycin. MIC: minimum inhibitory concentration. N/A: not available.
*Mean duration of treatment.
1Bacterial isolates tested vary between studies. The most common ones were: Staphylococcus aureus,Streptococcus pneumoniae,Moraxella catarrhalis, andHaemophilus influenzae.
2Some studies report on macrolide‐resistant bacteria during treatment.
3The denominator varies. At baseline: cultures from participants who had selected respiratory pathogens cultured at enrolment. During course: cultures from participants who became colonised with selected respiratory pathogens during the course of the study. Note: a much larger number of participants were colonised in the placebo group compared to the azithromycin group during the course of treatment (range: 44 to 108).
4Data from supplementary online content, eResults from Altenburg 2013. Number of pathogens tested is used as denominator.
5Data from Table 3 in Berg 2005. Denominator: total number of oropharyngeal Haemophilus parainfluenzae strains (sensitive, intermediate, resistant).
6Data from Table 2 in Lildholdt 2003. Denominator: number of positive cultures (range: 6 to 47).
7Report on one resistant Streptococcus pneumoniae, and state that all Haemophilus influenzae were resistant or assumed constitutionally resistant to erythromycin.
Three trials reported the proportion of macrolide‐resistant streptococci isolates (Brusselle 2013; Serisier 2013), of which one trial had two active treatment arms (Malhotra‐Kumar 2007a; Malhotra‐Kumar 2007b). Absolute increase in resistance decreased over time in Brusselle 2013, Malhotra‐Kumar 2007a, and Malhotra‐Kumar 2007b. Two trials also reported an initial relative increase in macrolide‐resistant bacteria followed by a decrease over time (Brusselle 2013; Malhotra‐Kumar 2007b); and Malhotra‐Kumar 2007a reported an initial decrease in relative resistance, but its magnitude decreased over time (Table 7).
5. Proportion of macrolide‐resistant streptococci.
| Proportion of macrolide‐resistant streptococci1 isolates: 3 studies | ||||||||
| Study ID |
Type of macrolide (days of treatment) |
Time for follow‐up swabs |
Proportion of resistant streptococci at baseline | Proportion of resistant streptococci after treatment | Absolute increase in resistance with antibiotic (%) | Relative increase in resistance with antibiotic (%) | ||
| Macrolide | Placebo | Macrolide | Placebo | |||||
| Brusselle 20132 | AZM (182) | Day 30 | 18 | 11 | 52 | 10 | 35 | 6 |
| Day 180 | 74 | 18 | 49 | 8 | ||||
| Day 210 | 44 | 12 | 25 | 5 | ||||
| Malhotra‐Kumar 2007a3 | AZM (3) | Day 4 | 26 | 28 | 87 | 33 | 52 | ‐27 |
| Day 8 | 83 | 34 | 47 | ‐25 | ||||
| Day 14 | 83 | 34 | 47 | ‐25 | ||||
| Day 28 | 80 | 33 | 45 | ‐24 | ||||
| Day 42 | 67 | 36 | 29 | ‐16 | ||||
| Day 180 | 46 | 23 | 21 | ‐12 | ||||
| Malhotra‐Kumar 2007b4 | CLM (7) | Day 8 | 30 | 25 | 81 | 31 | 45 | 10 |
| Day 14 | 71 | 31 | 35 | 8 | ||||
| Day 28 | 63 | 30 | 28 | 7 | ||||
| Day 42 | 59 | 28 | 26 | 6 | ||||
| Day 180 | 43 | 21 | 17 | 4 | ||||
| Serisier 20135 | ERY (336) | Week 48 | N/A | N/A | 29 | 0 | N/A | N/A |
Abbreviations: AZM: azithromycin. CLM: clarithromycin. ERY: erythromycin. N/A: not available.
1Denominator: number of streptococci.
2Data from Figure S3 in Brusselle 2013. A subsample of participants (42%) was tested for resistant bacteria.
3Data from Figure 2 in Malhotra‐Kumar 2007a. Note that only about 47% of participants attended follow‐up on day 180.
4Data from Figure 2 in Malhotra‐Kumar 2007b. Note that only about 47% of participants attended follow‐up on day 180.
5Data from eTable 2 in Serisier 2013. Results are presented for the intention‐to‐treat population. Report on median change in the proportion of macrolide‐resistant streptococci.
Subgroup analysis
The protocol prespecified the following subgroup analyses: age groups, type of macrolide, route of administration, antibiotic dosage, and duration of therapy. However, we were unable to undertake all planned subgroup analyses because either there were too few studies in the subgroup (< 3); data were confounded (e.g. subgroups not reported separately); or we decided against ‘duration of therapy’ from which, together with daily dose, we had hoped to estimate peak or steady‐state blood concentrations, but could not. We conducted the following subgroup analyses.
i. Nausea
Type of macrolide: the increased nausea caused by roxithromycin (OR 3.29, 95% CI 1.15 to 9.43) compared with either azithromycin (OR 1.66, 95% CI 1.27 to 2.16) or erythromycin (OR 1.58, 95% CI 1.23 to 2.04) was not significant (test for subgroup differences P = 0.41) (Analysis 3.2).
3.2. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 2 Nausea ‐ subgroup analysis by macrolide.
Route of administration: intravenous administration of macrolides (OR 3.04, 95% CI 0.69 to 13.51) was not significantly different from oral administration (OR 1.57, 95% CI 1.35 to 1.81; P = 0.38; Analysis 3.3).
3.3. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 3 Nausea ‐ subgroup analysis by route of administration.
ii. Vomiting
Type of macrolide: erythromycin was not significantly more likely to cause vomiting (OR 1.46, 95% CI 1.07 to 1.98) than azithromycin (OR 1.06, 95% CI 0.76 to 1.49; P = 0.17; Analysis 3.5).
3.5. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 5 Vomiting ‐ subgroup analysis by macrolide.
Route of administration: intravenous administration of macrolides (OR 1.21, 95% CI 0.88 to 1.66) was not significantly different from oral administration (OR 1.32, 95% CI 0.97 to 1.78; P = 0.70; Analysis 3.6).
3.6. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 6 Vomiting ‐ subgroup analysis by route of administration.
iii. Abdominal pain
Type of macrolide: erythromycin and azithromycin caused similar increases of abdominal pain (OR 3.16, 95% CI 1.14 to 8.75) and (OR 1.47, 95% CI 1.01 to 2.13), respectively; P = 0.16 (Analysis 3.9).
3.9. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 9 Abdominal pain ‐ subgroup analysis by macrolide.
iv. Diarrhoea
Type of macrolide: clarithromycin did not cause diarrhoea significantly more often (OR 2.09, 95% CI 1.70 to 2.56) than azithromycin (OR 1.96, 95% CI 1.37 to 2.81), erythromycin (OR 1.36, 95% CI 0.94 to 1.98), or roxithromycin (OR 0.88, 95% CI 0.38 to 2.07); P = 0.07 (Analysis 3.11).
3.11. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 11 Diarrhoea ‐ subgroup analysis by macrolide.
v. Gastrointestinal NOS
Type of macrolide: erythromycin was not significantly more likely to cause gastrointestinal adverse events NOS (OR 4.00, 95% 1.83 to 8.74) than azithromycin (OR 1.77, 95% CI 1.30 to 2.42); P = 0.06 (Analysis 3.13).
3.13. Analysis.
Comparison 3 Gastrointestinal disorders, Outcome 13 Gastrointestinal disorders not otherwise specified ‐ subgroup analysis by macrolide.
vi. Deaths
Type of macrolide: roxithromycin did not cause death significantly more often (OR 1.03, 95% CI 0.76 to 1.41) than azithromycin (OR 0.97, 95% 0.85 to 1.10), clarithromycin (OR 0.86, 95% 0.59 to 1.24), or erythromycin (OR 0.73, 95% 0.38 to 1.40); P = 0.74 (Analysis 12.2).
12.2. Analysis.
Comparison 12 Deaths, Outcome 2 Deaths ‐ subgroup analysis by type of macrolide.
Route of administration: intravenous administration of macrolides (OR 0.83, 95% CI 0.63 to 1.10) was not significantly different from oral administration (OR 0.98, 95% CI 0.88 to 1.10); P = 0.28 (Analysis 12.3).
12.3. Analysis.
Comparison 12 Deaths, Outcome 3 Deaths ‐ subgroup analysis by route of administration.
Sensitivity analyses
We decided a priori to perform sensitivity analyses by excluding those studies with missing data on the outcome (adverse events). However, none of the studies that provided data for the meta‐analyses had more than 20% of randomised participants lost to follow‐up, that is were assessed as being at high risk of attrition bias.
Supplementary data
In this Cochrane Review we have reported on any reported adverse event that occurred in 5% or more of any group. However, we extracted all adverse events and grouped them by primary System Organ Class, according to the MedDRA coding system (MedDRA 2018). See adverse events by System Organ Classes: threshold ≥ 5%, Hansen 2018a, and adverse events by System Organ Classes < 5%, Hansen 2018b.
Discussion
Summary of main results
This multi‐indication review included 183 randomised, placebo‐controlled trials (RCTs) involving a total of 252,886 participants. The indications for macrolide antibiotics varied greatly, with most studies using macrolides for the treatment or prevention of acute respiratory tract infections, cardiovascular diseases, chronic respiratory diseases, gastrointestinal conditions, or urogynaecological problems. Azithromycin and erythromycin were more commonly studied than clarithromycin and roxithromycin.
The most commonly reported adverse events were gastrointestinal. Participants taking macrolide antibiotics experienced vomiting, nausea, diarrhoea, abdominal pain, and gastrointestinal disorders NOS significantly more often than those taking a placebo.
We found low‐quality evidence that macrolides caused taste disturbances, although there were wide confidence intervals and moderate heterogeneity.
Participants taking macrolides experienced hearing loss more often than those taking a placebo, although the findings were non‐significant.
We did not find any evidence that macrolides caused more cardiac disorders, hepatobiliary disorders, or changes in liver enzymes compared to placebo.
In the overall meta‐analysis there was no evidence of an increase in deaths in participants treated with macrolides compared to those treated with placebo.
Very few of the included studies reported on macrolide‐resistant bacteria. Macrolide‐resistant bacteria were more commonly identified among participants immediately after exposure to the antibiotic, as expected, but there was little pattern of the decay of resistance thereafter.
Pharmaceutical companies supplied the trial medication or provided funding, or both, for about 50% of the included studies.
Overall completeness and applicability of evidence
Some of the outcomes were based on very few studies, despite the large total (183 trials) of included studies. However, most studies did report on some adverse events, and only 20 studies did not report on any adverse events.
The strengths of this review include the large set of RCTs to analyse. Randomised controlled trials avoid the complexity of attempting to distinguish symptoms caused by the treatment (antibiotics) or the disease (for which antibiotics were used), which makes observational studies weak for answering this question. Additionally, we included trials that allowed concomitant medications (when they were equally available in the placebo group), which might have caused drug interactions, and possibly have amplified any adverse event rates, which is an advantage when generalising to normal use.
One limitation is the assumptions made to decide what outcomes are adverse events and which are disease outcomes (for trials testing antibiotic efficacy); deaths, cardiac disorders, and symptoms of acute respiratory infections are examples. Furthermore, it was not possible to test dose effects because of the confusion surrounding the different forms of macrolide, especially erythromycin (which was used in estolate, stearate, base, and ethylsuccinate forms). A failure of most studies to report participant age groups’ data discretely meant that we could not analyse the effect of age on adverse events.
When trial authors reported adverse events, it was not always obvious if they reported the numbers of adverse events or the numbers of participants with adverse events. Consequently, there is a risk of double‐counting when performing a systematic review reporting adverse events data. In this systematic review, we aimed to report only adverse events from trials that reported the numbers of participants with adverse events. However, some of the included studies did not clearly specify if they reported on participants with adverse events, and in those cases our assessments have been based on inferences made by comparing the total numbers of participants and events they reported.
We tried to collect information on the follow‐up period for reporting on adverse events from all of the included studies. However, in most cases it was not possible to calculate the follow‐up period for the reporting of adverse events, as most trial authors only clearly reported the follow‐up period for the main outcome(s) and not for adverse events.
We did not plan to perform a subgroup analysis based on indications for macrolide treatment, as we anticipated that adverse events are not disease‐specific. However, different populations might experience different adverse events. For example, people with certain susceptibility factors have an increased risk of arrhythmias in response to macrolides (Albert 2014). Nevertheless, such differences need not necessarily be related to different indications for treatment rather than differences in individual susceptibility.
Quality of the evidence
The quality of evidence according to GRADE assessment ranged from very low (cardiac disorders, change in liver enzymes, hepatobiliary disorders) to low (abdominal pain, death, diarrhoea, dizziness, hearing loss, skin and soft tissue infections, taste disturbance, wheeze) to moderate (appetite loss, blood infection, cough, fever, gastrointestinal disorders NOS, headache, itching, nausea, nausea and vomiting, rash, respiratory symptoms NOS, respiratory tract infections, vomiting). We downgraded the quality of the evidence due to high risk of reporting bias, inconsistency, indirectness, and imprecision.
Potential biases in the review process
The interpretation of an adverse event differed significantly between trial authors. For example, some authors reported on pneumonia as a complication and wheezing as a disease‐specific symptom, while others reported on these as an adverse event. When extracting data from the included trials, two review authors independently searched for any information that could be interpreted as an adverse event, regardless of how this was reported in the original trial. Consequently, this review may report on outcomes that some trial authors did not consider to be an adverse event. An exception was the study by Andremont 1981, which we excluded from the meta‐analysis on diarrhoea as the trial tested a macrolide antibiotic versus placebo for the prevention of traveller’s diarrhoea and reported on diarrhoea as a primary outcome. We assessed the reported cases of diarrhoea (four participants in the placebo group) as caused by virus/bacteria, rather than by treatments.
Less than one‐third of the included RCTs reported on death (52 studies), and even fewer reported on data on macrolide‐resistant bacteria (24 studies). There is strong evidence that much of the information on adverse events remains unpublished, and that the number ‐ and range ‐ of adverse events is higher in unpublished versions of the same study (Golder 2016). We searched six databases, the reference lists of included trials, the World Health Organization (WHO) International Clinical Trials Registry Platform and ClinicalTrials.gov for ongoing trials, and exploited the reference lists of previous Cochrane Reviews on macrolide antibiotics. We also contacted authors if they reported incompletely on adverse events and contact details (an e‐mail address) were available. However, we did not contact each of the 183 trial authors asking for unpublished data on adverse events, and consequently it is possible that we missed information on adverse events, including death and data on macrolide‐resistant bacteria.
The methods used for eliciting adverse events varied greatly between the included trials and included spontaneous reporting, asking participants, use of a questionnaire, identification during a clinical examination, and/or laboratory testing. Also, many studies did not provide any information on how the information on adverse events was obtained. A newly published Cochrane Review raises concerns that methods used for eliciting adverse events may influence the detection of these data (Allen 2018). The review authors found that there was a risk for underdetection of adverse events in studies using a more general elicitation method compared to those using a comprehensive method (Allen 2018). This possible underdetection of adverse events might have compromised our ability to pool data, as we required at least three studies reporting on a specific adverse event in order to perform a meta‐analysis.
Agreements and disagreements with other studies or reviews
This Cochrane Review is the first multi‐indication review on adverse events in people taking macrolides that includes studies using the same intervention for different diseases (Chen 2014). However, several other reviews have presented data on adverse events in people taking macrolides for various indications. Some reviews, such as Ni 2015, have only presented the total number of adverse events, whilst other authors have presented data for specific adverse events (Shi 2014). Shi 2014 studied macrolides for bronchiectasis, presenting both efficacy and adverse outcomes, and finding abdominal pain (risk ratio (RR) 6.2, 95% CI 1.43 to 26.83) and diarrhoea (RR 2.89, 95% CI 1.13 to 7.35) significantly more often in participants treated with a macrolide than in those treated with a placebo. Also, in line with our findings, that review found no increased risk of headache in participants treated with a macrolide (RR 0.62, 95% CI 0.17 to 2.29). Reporting of other adverse events in the Cochrane Review by Shi and colleagues was limited by lack of statistical power (Shi 2014).
The absence of a signal of liver damage in this review contrasts with older reports that macrolide antibiotics, erythromycin in particular, can cause two different types of liver damage ‐ changes in liver enzymes and cholestatic jaundice (Braun 1976; Ginsburg 1976). There are several possible explanations for the dissonance between our review and the previous reports. Because many of the older reports were anecdotal, the associations may have occurred purely by chance; alternatively, newer formulations of erythromycin may be less hepatotoxic; previous observational studies may have been confounded by indication, hepatobiliary adverse effects having been caused by the infections being treated; or the risk of hepatotoxicity may be real but too small to have met our eligibility entry requirement that adverse events should have affected ≥ 5% of participants. Settling this question may need interrogation of large data sets beyond the remit of this review.
Findings when cardiovascular adverse events are reported in people taking macrolide antibiotics are contradictory. Observational studies have shown that treatment with macrolide antibiotics is associated with an increased risk of cardiovascular outcomes, including cardiovascular deaths, myocardial infarction, and arrhythmias (Wong 2017). In contrast, meta‐analyses of RCTs did not show an increased cardiovascular risk (Wong 2017). Our findings concur with the RCT‐derived data, as we did not find evidence of an increased risk of cardiac disorders in participants taking a macrolide antibiotic compared with placebo.
Authors' conclusions
Implications for practice.
Antimicrobial resistance is one of the key global health problems facing our generation, with antibiotic use being the main driver (O'Neill 2014; WHO 2018). Most antibiotics used in humans are used in primary care (DANMAP 2016), and particularly in general practice (Aabenhus 2016). For some infections, such as acute respiratory infections, the benefits of antibiotic treatment are minimal, if any. We undertook this systematic review to quantify adverse events in people using macrolide antibiotics, independently of the indication or effects of treatment, and found that macrolides as a group increased rates of gastrointestinal adverse events. The intention of this review is to support clinicians and patients in evaluating harms as well as benefits in the choice of management when antibiotics are contemplated.
Implications for research.
Poor and inconsistent reporting of adverse events in clinical trials is well known (Hodkinson 2013). Most trials reported on some adverse events, or at least stated that no adverse events were observed. Nonetheless, trial authors are encouraged to clearly state adverse events (including data on resistant bacteria) as outcomes; to report on the methods used for eliciting adverse events; and preferably to report both the number of each specific adverse event and the number of people with each event in both the intervention and control groups.
Most systematic reviews of antimicrobial treatments ignore the problem of antimicrobial resistance (Leibovici 2003), and a framework for addressing antibiotic resistance in systematic reviews has recently been proposed for use in Cochrane Review protocols and Cochrane Reviews (Leibovici 2016). A revised version of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) framework was published in 2016 to increase the appropriateness of reporting for epidemiological studies, focusing on the link between resistant bacteria and antibiotic use (Tacconelli 2016). Only 24 (13%) of the trials included in our review provided useful data on macrolide‐resistant bacteria. Consequently, not only review authors, but also authors conducting primary research on antimicrobial treatments are encouraged to measure and report on resistance data in future research projects.
Acknowledgements
We wish to thank the staff and editors of the Cochrane Acute Respiratory Infections Group for their invaluable support during the review process.
We also wish to thank Jane Knight from the MedDRA Maintenance and Support Services Organization for her kind support in using the MedDRA classification system. MedDRA® trademark is registered by the International Federation of Pharmaceutical Manufacturers & Associations on behalf of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
We gratefully acknowledge the translators: Oyungerel Byambasuren (Russian), Loai Albarqouni (German), Athanasios Raikos (Greek), Chia Ming Len (Chinese), Jing Liu (Chinese), and Seyedehzoya Ashabi (Persian).
We sincerely wish to thank the following trial authors for providing additional data or responding to the review authors' queries, or both: Ricardo Mosquera, Anne Chang, Helene Kobbernagel, Jane Lindschou, Per Winkel, Nicholas Grassly, Gagandeep Kang, Jacob John, Guy Brusselle, Anwar Batieha, Neil C Thomsen, Najia Ahmed, Annette Hoegh, Steen Vammen, Ramazan Ozdemir, Richard Martin, Wim Janssens, Simon Brill, Anna Roca, Patricia Pavlinac, Rashida Ferrand, and Heather Powell.
Finally, we thank the following people for commenting on the draft of this review: Ankur Barua, Jeffrey Linder, Mutsuo Yamaya, Conor Teljeur, Tom Fahey, and Chris Cates. We thank Lisa Winer for copy‐editing the final draft.
Appendices
Appendix 1. MEDLINE (Ovid) and Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
1 exp Macrolides/ 2 macrolide*.tw,nm,ot. 3 (azithromycin* or clarithromycin* or erythromycin* or roxithromycin*).tw,nm,ot. 4 or/1‐3 5 exp Placebos/ 6 placebo*.tw,nm,ot. 7 5 or 6 8 4 and 7
Appendix 2. Embase (Elsevier) search strategy
#13 #8 AND #11 AND [1‐1‐2010]/sd NOT [22‐8‐2015]/sd (690)
#12 #8 AND #11 (2,267)
#11 #9 OR #10 (1,401,271)
#10 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR factorial:ab,ti OR volunteer*:ab,ti OR allocat*:ab,ti OR assign*:ab,ti OR ((singl* OR doubl*) NEAR/2 blind*):ab,ti AND [embase]/lim (1,246,381)
#9 'single blind procedure'/de OR 'double blind procedure'/de OR 'crossover procedure'/exp OR 'randomized controlled trial'/de (421,654)
#8 #4 AND #7 (5,008)
#7 #5 OR #6 (328,717)
#6 placebo*:ab,ti AND [embase]/lim (204,119)
#5 'placebo'/de AND [embase]/lim (263,844)
#4 #1 OR #2 OR #3 (129,809)
#3 azithromycin*:ab,ti OR clarithromycin*:ab,ti OR erythromycin*:ab,ti OR roxithromycin*:ab,ti AND [embase]/lim (31,108)
#2 macrolide*:ab,ti AND [embase]/lim (14,020)
#1 'macrolide'/exp AND [embase]/lim (126,714)
Appendix 3. CINAHL (EBSCO) search strategy
| S19 | S8 AND S18 |
| S18 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 |
| S17 | (MH "Quantitative Studies") |
| S16 | TI placebo* OR AB placebo* |
| S15 | (MH "Placebos") |
| S14 | (MH "Random Assignment") |
| S13 | TI random* OR AB random* |
| S12 | TI ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) |
| S11 | TI clinic* trial* OR AB clinic* trial* |
| S10 | PT clinical trial |
| S9 | (MH "Clinical Trials+") |
| S8 | S4 AND S7 |
| S7 | S5 OR S6 |
| S6 | TI placebo* OR AB placebo* |
| S5 | (MH "Placebos") |
| S4 | S1 OR S2 OR S3 |
| S3 | TI ( azithromycin* or clarithromycin* or erythromycin* or roxithromycin* ) OR AB ( azithromycin* or clarithromycin* or erythromycin* or roxithromycin* ) |
| S2 | TI macrolide* OR AB macrolide* |
| S1 | (MH "Antibiotics, Macrolide+") |
Appendix 4. LILACS (BIREME) search strategy
(mh:macrolides OR macrolide* OR macrólidos OR macrolídeos or mh:d02.540.505* OR mh:d02.540.576.500* OR mh:d04.345.674.500* OR mh:azithromycin OR azithromycin* OR azitromicina OR mh:d02.540.505.250.050* OR mh:clarithromycin OR clarithromycin* OR mh:claritromicina* OR mh:d02.540.505.250.100* OR mh:erythromycin OR erythromycin* OR eritromicina or mh:d02.540.505.250* OR mh:roxithromycin OR roxithromycin* OR roxitromicina OR mh:d02.540.505.250.630*) AND (mh:placebos OR placebo*)
Appendix 5. Web of Science (Clarivate Analytics) search strategy
| #6 | 71 | #4 AND #3 Refined by: publication years: (2015 OR 2016 ) Indexes = SCI‐EXPANDED, SSCI,A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
| #5 | 1254 | #4 AND #3 Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
| #4 | 1,797,642 |
TOPIC: (random* or placebo* or crossover* or "cross over" or allocat* or ((doubl* or singl*) NEAR/1 blind*)) ORTITLE: (trial) Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
| #3 | 1254 | #2 AND #1 Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
| #2 | 198,122 |
TOPIC: (placebo*) Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
| #1 | 40,012 |
TOPIC: (macrolide* or azithromycin* or clarithromycin* or erythromycin* or roxithromycin*) Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan = 1985‐2016 |
Data and analyses
Comparison 1. Cardiac disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac disorders | 7 | 1715 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.54, 1.40] |
Comparison 2. Ear and labyrinth disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hearing loss | 4 | 1369 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.70] |
Comparison 3. Gastrointestinal disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 28 | 14983 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.37, 1.90] |
| 2 Nausea ‐ subgroup analysis by macrolide | 26 | 10572 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [1.39, 2.00] |
| 2.1 Azithromycin | 10 | 5437 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.27, 2.16] |
| 2.2 Erythromycin | 13 | 4625 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [1.23, 2.04] |
| 2.3 Roxithromycin | 3 | 510 | Odds Ratio (M‐H, Random, 95% CI) | 3.29 [1.15, 9.43] |
| 3 Nausea ‐ subgroup analysis by route of administration | 28 | 14983 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.37, 1.90] |
| 3.1 Intravenous | 3 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 3.04 [0.69, 13.51] |
| 3.2 Peroral | 25 | 14587 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [1.35, 1.81] |
| 4 Vomiting | 15 | 5328 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [1.04, 1.56] |
| 5 Vomiting ‐ subgroup analysis by macrolide | 13 | 5147 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [1.00, 1.60] |
| 5.1 Azithromycin | 6 | 2692 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.76, 1.49] |
| 5.2 Erythromycin | 7 | 2455 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.07, 1.98] |
| 6 Vomiting ‐ subgroup analysis by route of administration | 15 | 5328 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [1.04, 1.56] |
| 6.1 Intravenous | 5 | 2354 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.88, 1.66] |
| 6.2 Peroral | 10 | 2974 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.97, 1.78] |
| 7 Nausea and vomiting | 8 | 1053 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.60, 1.42] |
| 8 Abdominal pain | 23 | 7776 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.22, 2.26] |
| 9 Abdominal pain ‐ subgroup analysis by macrolide | 20 | 7506 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.21, 2.34] |
| 9.1 Azithromycin | 14 | 6072 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [1.01, 2.13] |
| 9.2 Erythromycin | 6 | 1434 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [1.14, 8.75] |
| 10 Diarrhoea | 37 | 23754 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [1.34, 2.16] |
| 11 Diarrhoea ‐ subgroup analysis by macrolide | 37 | 23754 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [1.34, 2.16] |
| 11.1 Azithromycin | 22 | 15144 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [1.37, 2.81] |
| 11.2 Clarithromycin | 4 | 4540 | Odds Ratio (M‐H, Random, 95% CI) | 2.09 [1.70, 2.56] |
| 11.3 Erythromycin | 8 | 3711 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.94, 1.98] |
| 11.4 Roxithromycin | 3 | 359 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.38, 2.07] |
| 12 Gastrointestinal disorders not otherwise specified | 23 | 3295 | Odds Ratio (M‐H, Random, 95% CI) | 2.16 [1.56, 3.00] |
| 13 Gastrointestinal disorders not otherwise specified ‐ subgroup analysis by macrolide | 22 | 3238 | Odds Ratio (M‐H, Random, 95% CI) | 2.19 [1.56, 3.09] |
| 13.1 Azithromycin | 13 | 2396 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [1.30, 2.42] |
| 13.2 Erythromycin | 9 | 842 | Odds Ratio (M‐H, Random, 95% CI) | 4.00 [1.83, 8.74] |
Comparison 4. Nervous system disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dizziness | 3 | 376 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [0.85, 3.95] |
| 2 Headache | 12 | 1386 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.11] |
| 3 Taste disturbance | 5 | 932 | Odds Ratio (M‐H, Random, 95% CI) | 4.95 [1.64, 14.93] |
Comparison 5. Skin and subcutaneous tissue disorders.
Comparison 6. General disorders and administration site conditions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever | 7 | 2451 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 1.00] |
Comparison 7. Hepatobiliary disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatobiliary disorders | 4 | 443 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.27, 4.09] |
Comparison 8. Infections and infestations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood infection | 4 | 356 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2 Respiratory tract infections | 11 | 11062 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.62, 0.80] |
| 3 Skin and soft tissue infections | 3 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.53, 4.64] |
Comparison 9. Investigations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in liver enzymes | 6 | 1187 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.73, 3.37] |
Comparison 10. Metabolism and nutrition disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Appetite lost | 5 | 2183 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.84, 1.43] |
Comparison 11. Respiratory, thoracic, and mediastinal disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cough | 6 | 1587 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.80] |
| 2 Respiratory symptoms not otherwise specified | 8 | 2176 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.25] |
| 3 Wheezing | 3 | 484 | Odds Ratio (M‐H, Random, 95% CI) | 2.20 [0.74, 6.52] |
Comparison 12. Deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths ‐ overall | 52 | 216246 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 2 Deaths ‐ subgroup analysis by type of macrolide | 52 | 216246 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 2.1 Azithromycin | 24 | 204719 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.10] |
| 2.2 Clarithromycin | 8 | 7216 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.24] |
| 2.3 Erythromycin | 10 | 718 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.40] |
| 2.4 Roxithromycin | 10 | 3593 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.41] |
| 3 Deaths ‐ subgroup analysis by route of administration | 51 | 214875 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.1 Intravenous | 8 | 1334 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 3.2 Peroral | 43 | 213541 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 61 adults (macrolide n = 31, placebo n = 30) Age in years (range): 30 to 50 Setting: dental care |
|
| Interventions | Indication: chronic periodontitis Type of macrolide: clarithromycin Route: topical Dose per day: 0.5% gel x 1 Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Ascertainment of adverse events: unclear Adverse events: states that no adverse events were observed or reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment. However, report on adverse events |
| Other bias | Low risk | None were identified. |
Agarwal 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 63 adults (macrolide n = 31, placebo n = 32) Age in years (range): 30 to 50 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis in people with type 2 diabetes mellitus Type of macrolide: azithromycin Route: topical Dose per day: 0.2 mL of 0.5% gel Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Ascertainment of adverse events: participants asked Adverse event: authors state that no adverse events were observed or reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. Authors thank supplying companies. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome. However, clear ascertainment and report on (no) adverse events |
| Other bias | Low risk | None were identified. |
Ahmed 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 children and adults (macrolide n = 30, placebo n = 30) Age in years (mean ± SD): macrolide: 23.13 ± 10.34, placebo: 21.67 ± 7.42 Setting: secondary care |
|
| Interventions |
Indication: pityriasis rosea Type of macrolide: clarithromycin Route: per oral Dose per day: 250 mg (child)/500 mg (adult) x 2 Duration of treatment: 7 days Total treatment dose: 7000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Ascertainment of adverse events: unclear Adverse event: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was done by lottery method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Akhyani 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 46 children and adults (macrolide n = 23, placebo n = 23) Age in years (mean (range)): 21.5 (11 to 36) Setting: secondary care |
|
| Interventions |
Indication: pityriasis rosea Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Ascertainment of adverse events: unclear Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Albert 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1142 adults and elderly (macrolide n = 570, placebo n = 572) Age in years (mean ± SD): macrolide: 65 ± 9, placebo: 66 ± 8 Setting: secondary care |
|
| Interventions |
Indication: prevention of an exacerbation in people with chronic obstructive pulmonary disease Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg Duration of treatment: 1 year Total treatment dose: 91,250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked and clinical examination/laboratory tests Adverse events: data reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Funded by the National Institutes of Health. Several authors are on pharmaceutical boards. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome in the protocol, and participants were asked about adverse events/were examined. |
| Other bias | Low risk | None were identified. |
Alger 1991.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 84 children and adults (macrolide n = 40, placebo n = 44) Age in years (mean ± SD): macrolide: 21.7 ± 4.2, placebo: 21.3 ± 4.0 Setting: secondary care |
|
| Interventions |
Indication: antenatal Chlamydia trachomatis infection Type of macrolide: erythromycin base Route: per oral Dose per day: 1332 mg Duration of treatment: 14 days Total treatment dose: 18,648 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a grant from the Upjohn Company. Role of funding source unclear. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if the placebo group was generated from another trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome and unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Altenburg 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 89 adults and elderly (macrolide n = 45, placebo n = 44) Age in years (mean ± SD): macrolide: 59.9 ± 12.3, placebo: 64.6 ± 9.1 Setting: secondary care |
|
| Interventions |
Indication: prevention of pulmonary exacerbations in people with non‐cystic fibrosis bronchiectasis Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg Duration of treatment: 12 months Total treatment dose: 91,250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant diary and clinical examination/laboratory tests Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | A research grant from the Foreest Medical School was used for paying salaries. The study was supported by an unrestricted grant from GlaxoSmithKline. Azithromycin tablets were supplied by Teva Netherlands. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Numbers on the boxes matched a treatment allocation, in accordance with a computer‐generated allocation sequence that was kept in a safe place in the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, adverse events reported. |
| Other bias | Low risk | None were identified. |
Altraif 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 102 adults and elderly (macrolide n = 53, placebo n = 49) Age in years (mean ± SD): macrolide: 62.3 ± 9.8, placebo: 62.7 ± 14.7 Setting: secondary care |
|
| Interventions |
Indication: variceal bleeding in people with liver cirrhosis Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 125 mg Duration of treatment: 1 day Total treatment dose: 125 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: states that no adverse events were observed or reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and endoscopist/clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome. However, unclear ascertainment and states that no adverse events were observed. |
| Other bias | Low risk | None were identified. |
Aly 2007.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 children (macrolide n = 30, placebo n = 30) Age in days (median (range)): macrolide: 2.0 (2.0 to 24.0), placebo: 2.0 (2.0 to 10.0) Setting: secondary care |
|
| Interventions |
Indication: feeding intolerance in preterm infants Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 3 mg/kg Duration of treatment: the study medicine was to stop once the primary endpoint was achieved Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by cards provided in consecutively numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both active drug and placebo were mixed thoroughly into the milk feeds by designated staff not involved in the clinical management of the infants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. Adverse events/complications reported. |
| Other bias | Low risk | None were identified. |
Amali 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 66 children and adults (macrolide n = 22, placebo n = 44) Age in years (mean ± SD (range)): macrolide: 34.9 ± 9.2 (18 to 57), placebo: 39.1 ± 10.7 (15 to 62) Setting: secondary care |
|
| Interventions |
Indication: chronic rhinosinusitis Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg Duration of treatment: 84 days Total treatment dose: 21,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, and individuals analysing data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups. Reasons for dropout given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, incomplete reporting of adverse events. |
| Other bias | Unclear risk | 2:1 randomisation design |
Amer 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 49 children (macrolide n = 25, placebo n = 24) Age in years (mean): macrolide: 8.0, placebo: 8.4 Setting: secondary care |
|
| Interventions |
Indication: pityriasis rosea Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg (maximum) Duration of treatment: 5 days Total treatment dose: 2500 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a grant from Pfizer Inc. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment of adverse events at each follow‐up visit and adverse events reported. |
| Other bias | Low risk | None were identified. |
Amland 1995.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 339 children, adults, and elderly (macrolide n = 171, placebo n = 168) Age in years mean (range): male: macrolide: 30 (7 to 85), placebo: 28 (6 to 84); female: macrolide 33 (6 to 84), placebo: 33 (7 to 82) Setting: secondary care |
|
| Interventions |
Indication: prevention of postoperative wound infections Type of macrolide: azithromycin Route: per oral Dose per day: 1000 mg (maximum) Duration of treatment: 1 day Total treatment dose: 1000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Reports that the study was supported by Pfizer, who provided the study medication | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 10 using a computer‐generated chart. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Andere 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults (macrolide n = 20, placebo n = 20) Age in years (mean (range)): 32.2 (22 to 35) Setting: dental care |
|
| Interventions |
Indication: generalised aggressive periodontitis Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 3 days Total treatment dose: 3000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Ascertainment of adverse events: participant diary Adverse event: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Funded by Research Funding Agency from Sao Paulo State and National Council for Science and Technological Development | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome. However, clear ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Andersen 1998.
| Methods | Design: randomised, placebo‐controlled, 4‐armed trial | |
| Participants |
Number assigned: 177 adults (macrolide (daily dose) n = 59, macrolide (weekly dose) n = 58, placebo n = 60) Age in years (range): 18 to 55 Setting: 2 villages in western Kenya |
|
| Interventions |
Indication: malaria prophylaxis Type of macrolide: azithromycin Route: per oral Dose per day/week: arm 1: 250 mg/day; arm 2: 1000 mg/week Duration of treatment: 10 weeks Total treatment dose: arm 1: 17,500 mg; arm 2: 10,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination/lab tests Adverse events: data reported. Adverse events are reported as "number of events" and not as "patients with events". Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Kenya Medical Research Institute through the US Army Medical Material Development Activity and Pfizer Central Research. Pfizer provided the study drugs and placebo. | |
| Notes |
Concomitant medication: yes Note: a 4th group of people were treated with doxycyclin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Separate placebos were used for different treatment groups to preserve the blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome and were assessed by a daily symptom questionnaire. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Anderson 1999.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 302 adults and elderly (macrolide n = 150, placebo n = 152) Age in years (mean ± SD): macrolide: 64 ± 10, placebo: 63 ± 11 Setting: secondary care |
|
| Interventions |
Indication: secondary prevention in people with coronary artery disease and seropositivity to Chlamydia pneumoniae Type of macrolide: azithromycin Route: per oral Dose: 500 mg/day for 3 days, then 500 mg/week for 3 months Duration of treatment: 93 days Total treatment dose: 7500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse event: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported in part by a grant from the Deseret Foundation, LDS Hospital, Salt Lake City, Utah. Azithromycin and placebo purchased from pharmacies. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (alternating blocks of 4 and 6) |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome. Unclear ascertainment, but adverse events reported |
| Other bias | Low risk | None were identified. |
Andremont 1981.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 48 adults (macrolide n = 24, placebo n = 24) Age in years: N/A Setting: healthy US residents travelling to Mexico to attend a professional meeting |
|
| Interventions |
Indication: prevention of traveller's diarrhoea Type of macrolide: erythromycin base Route: per oral Dose per day: 1000 mg Duration of treatment: mean days of treatment 6 (range 4 to 13 days) Total treatment dose: 6000 mg (mean) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: states that no adverse events were reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a "contrat de recherche clinique" from Institut Gustave Roussy and a grant from Roussel‐Uclaf Laboratories. | |
| Notes |
Concomitant medication: unclear Note: gastrointestinal symptoms were reported as the primary outcome in this study and not reported/regarded as an adverse event. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. However, states that no adverse events were reported. |
| Other bias | Low risk | None were identified. |
Anthony 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 78 adults and elderly (macrolide n = 39, placebo n = 39) Age in years (mean ± SD): azithromycin: 65.94 ± 11.77, placebo: 59.75 ± 15.03 Setting: secondary care |
|
| Interventions |
Indication: bronchiectasis Type of macrolide: azithromycin Route: per oral Dose: 1000 mg/week Duration of treatment: 12 weeks Total treatment dose: 12,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by a grant approved by the Ministry of Health of Malaysia. Study medication was manufactured and provided by Pfizer Inc. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout in both groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment at each follow‐up visit and adverse events reported. |
| Other bias | Low risk | None were identified. |
Avci 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 adults and elderly (macrolide n = 30, placebo n = 30) Age in years (mean age ± SD (range)): 50.68 ± 12.92 (18 to 78) Setting: secondary care |
|
| Interventions |
Indication: erythrasma Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 14 days Total treatment dose: 14,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. Authors thank supplying companies. | |
| Notes | Concomitant medication: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment of adverse events at each follow‐up visit and adverse events reported. |
| Other bias | Low risk | None were identified. |
Bacharier 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 607 children (macrolide n = 307, placebo n = 300) Age in months (mean ± SD): 41.5 ± 16.5 Setting: 9 US academic medical centres in the National Heart, Lung, and Blood Institute's AsthmaNet network |
|
| Interventions |
Indication: recurrent severe lower respiratory tract illness Type of macrolide: azithromycin Route: per oral Dose per day: 12 mg/kg Duration of treatment: 5 days (per treatment course) Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: spontaneously reporting + clinical examination Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by the National Heart, Lung, and Blood Institute as part of AsthmaNet. Several authors have received personal fees and grants from various pharmaceutical companies. | |
| Notes |
Concomitant medication: yes Note: during the 78‐week follow‐up included children could use the study treatment during a maximum of 4 treated respiratory tract infection episodes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled, double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17% and 18% withdrew for reasons other than "early termination" or were lost to follow‐up, respectively. Reasons not given. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Bajaj 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 63 adults (macrolide n = 32, placebo n = 31) Age in years (range): 30 to 50 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis in people with type 2 diabetes mellitus Type of macrolide: clarithromycin Route: topical Dose per day: 0.5% gel once Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: states that no adverse events were observed or reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors thank Micro Labs, India, and Purac Biomaterials, the Netherlands, for providing active drug and placebo. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo gel used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both the participant and the clinician, who provided treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment. Authors state that no adverse events were reported. |
| Other bias | Low risk | None were identified. |
Bala 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): macrolide: 28 ± 10.2, placebo: 35 ± 10.4 Setting: secondary care |
|
| Interventions |
Indication: gastric fluid pH and volume during surgery Type of macrolide: erythromycin Route: per oral Dose per day: 250 mg Duration of treatment: 1 day Total treatment dose: 250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: authors state that "no adverse effects could be attributed to the test drugs". Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Institutional funding | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by statistician off‐site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. Authors report that no adverse events were observed. |
| Other bias | Low risk | None were identified. |
Ballard 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 220 children (macrolide n = 111, placebo n = 109) Age in weeks (mean ± SD): macrolide: 25.7 ± 1.5, placebo: 26 ± 1.6 Setting: secondary care |
|
| Interventions |
Indication: prevention of bronchopulmonary dysplasia in preterm infants Type of macrolide: azithromycin Route: intravenous (study drugs were initially administered intravenously, but switched to enteral route once the infant reached full enteral feeds) Dose per day: 10 mg/kg for 7 days, followed by 5 mg/kg for 5 weeks Duration of treatment: 42 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of caretakers and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Audiometry and lab tests performed, however not complete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Banerjee 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 67 adults and elderly (macrolide n = 31, placebo n = 36) Age in years (mean ± SE): macrolide: 65.1 ± 1.4, placebo: 68.1 ± 1.2 Setting: secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: clarithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 90 days Total treatment dose: 45,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + sputum Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Funded by a research grant from Abbott Laboratories Ltd, Maidenhead, UK | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Participants were contacted and asked about adverse events regularly, however no reporting of adverse events in published paper. |
| Other bias | Low risk | None were identified. |
Barkhordar 2018.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 96 adults (macrolide n = 48, placebo n = 48) Age in years (mean ± SD (range)): macrolide: 35.5 ± 12.0 (16 to 62), placebo: 36.1 ± 11.5 (18 to 62) Setting: secondary care |
|
| Interventions |
Indication: prevention of graft versus host disease in people with acute leukaemia Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 18 days Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no (mortality stated as outcome) Ascertainment of adverse events: unclear Adverse event: not reported. States that "the medication was well tolerated by all patients" Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants, nursing staff, outcome assessor, and attending physician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups, reasons given |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Beigelman 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 children (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): 3.8 ± 2.9 Setting: secondary care |
|
| Interventions |
Indication: respiratory syncytial virus bronchiolitis Type of macrolide: azithromycin Route: per oral Dose per day: 10 mg/kg once daily for 7 days, followed by 5 mg/kg once daily for an additional 7 days Duration of treatment: 14 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: contacting participants' families 3 times a week during the treatment period Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Washington University Institute of Clinical and Translational Sciences grant from the National Center for Advancing Translational Sciences and the Children’s Discovery Institute of Washington University and St Louis Children’s Hospital. Supported in part by CTSA grant and Siteman Comprehensive Cancer Center and NCI Cancer Center support grant | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of all participants, their families, investigators, and study staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Berg 2005.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 473 adults and elderly (macrolide n = 238, placebo n = 235) Age in years (mean ± SD): macrolide: 64.9 ± 8.7, placebo: 63.8 ± 10.8 Setting: secondary care |
|
| Interventions |
Indication: coronary artery disease Type of macrolide: clarithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 16 days (mean) Total treatment dose: 8000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Unrestricted grant from Abbott Pharmaceuticals | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and research physician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Bergeron 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 480 adults and elderly (macrolide n = 243, placebo n = 237, excluded n = 15) Age in years: median (IQR): macrolide: 57.5 (45.0 to 63.6), placebo: 55.6 (40.3 to 63.2) Setting: secondary care |
|
| Interventions |
Indication: improvement of airflow decline‐free survival after allogenic haematopoietic stem cell transplant Type of macrolide: azithromycin Route: per oral Dose: 250 mg 3 times per week Duration of treatment: 730 days Total treatment dose: 78,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Ascertainment of adverse events: participants asked + clinical examination Adverse event: data reported. Adverse events are reported as "number of events" and not as "patients with events". Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by the French Ministry of Health, SFGM‐TC Capucine association, and SOS Oxygene | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adverse events reported for all allocated participants (safety population). |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome, clear ascertainment. However, only serious adverse events are reported on. |
| Other bias | Low risk | None were identified. |
Berkhof 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 84 adults and elderly (macrolide n = 42, placebo n = 42) Age in years (mean ± SD): macrolide: 67 ± 9, placebo: 68 ± 10 Setting: secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: azithromycin Route: per oral Dose: 750 mg/week Duration of treatment: 12 weeks Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + lab tests Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | None stated. However, the authors thank Stichting Astma Bestrijding for financial support and Teva Pharma for providing the azithromycin tablets. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of investigators, research nurses, and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Higher dropout in azithromycin group because of adverse events, however they are reported |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Berne 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 68 adults (macrolide n = 32, placebo n = 36) Age in years (mean): macrolide: 40.0, placebo: 34.1 Setting: secondary care |
|
| Interventions |
Indication: gastric emptying in critically trauma participants Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 1000 mg Duration of treatment: 2 days Total treatment dose: 2000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported. Report on 1 participant developing a penicillin‐resistant Streptococcus pneumoniae pneumonia Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Black 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 232 adults (macrolide n = 105, placebo n = 114, excluded n = 13) Age in years (mean ± SD): macrolide: 40 ± 11.6, placebo: 42 ± 11.9 Setting: secondary care |
|
| Interventions |
Indication: asthma participants infected with Chlamydia pneumoniae Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 42 days Total treatment dose: 12,600 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Aventis Pharma. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of both participant and examiner/clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Bonacini 1993.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 80 adults and elderly (macrolide n = 41, placebo n = 36, excluded n = 3) Age in years (median (range)): macrolide: 42 (18 to 80), placebo: 40 (18 to 81) Setting: secondary care |
|
| Interventions |
Indication: postoperative ileus Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 750 mg Duration of treatment: 3 days Total treatment dose: 2250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. However, 3 assigned participants (4%) were excluded from analysis based on unclear reasons. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Botero 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 70 adults and elderly (macrolide n = 33, placebo n = 37) Age in years (mean ± SD): macrolide: 55.9 ± 12.6, placebo: 58.2 ± 11.1 Setting: dental care |
|
| Interventions |
Indication: periodontitis in people with diabetes mellitus Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: spontaneously reporting (participants were instructed to report any side effects) Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Partially supported by a grant from Colgate‐Palmolive and the Universidad de Antioquia. Authors thank supplying companies. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed, and coded envelopes used for allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Branden 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 103 adults and elderly (macrolide n = 51, placebo n = 52) Age in years (mean ± SD): macrolide: 61.1 ± 10.5, placebo: 59.8 ± 13.4 Setting: secondary care |
|
| Interventions |
Indication: chronic Chlamydia pneumoniae‐infected participants with longstanding airway and/or pharyngeal symptoms Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 15 days in total (5 days treatment, repeated 3 times with 23‐day intervals) Total treatment dose: 7500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + lab tests Reporting of adverse events: yes Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Karolinska Institutet, Stockholm, Sweden and the Swedish Heart and Lung Foundation. Pfizer AB, Sweden supplied the study medication. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Adverse events leading to discontinuation reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome, standardised ascertainment of adverse events at follow‐up visits. Adverse events clearly presented in a table. |
| Other bias | Low risk | None were identified. |
Brickfield 1986.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 52 adults (macrolide n = 27, placebo n = 25) Age in years (mean): macrolide: 32.0, placebo: 32.5 Setting: primary care |
|
| Interventions |
Indication: acute bronchitis Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 7 days Total treatment dose: 6993 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting. However, no contact details for author. Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a grant from the American Academy of Family Physicians. Authors acknowledge supplying companies. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent company generated numbered, sealed bottles containing tablets of placebo or erythromycin. |
| Allocation concealment (selection bias) | Low risk | Participants received a numbered, sealed bottle with tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant withdrew from each group, no reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Brill 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 49 adults and elderly (macrolide n = 25, placebo n = 24) Age in years (mean ± SD): macrolide: 67.9 ± 8.6, placebo: 68.7 ± 9.8 Setting: participants were recruited from both primary and secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: azithromycin Route: per oral Dose: 250 mg 3 times a week Duration of treatment: 13 weeks Total treatment dose: 9750 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + swabs Adverse events: incomplete reporting, author contacted Antimicrobial resistance: incomplete reporting, author contacted Death: not reported |
|
| Funding sources | Study supported by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme and the NIHR Royal Brompton Respiratory Biomedical Research Unit. Many of the authors have received honoraria, consulting, and board membership fees from pharmaceutical companies. Authors state that the study presents independent research. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Azithromycin was taken 3 times per week, while placebo was given as 1 tablet per day. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors probably not blinded. However, only report on AMR data, which is an objective outcome and not influenced by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | High risk | Adverse events stated as an outcome and standardised ascertainment. However, incomplete reporting of adverse events including data on antimicrobial resistance. |
| Other bias | Low risk | None were identified. |
Brusselle 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 109 adults and elderly (macrolide n = 55, placebo n = 54) Age in years median (range): macrolide: 53 (19 to 76), placebo: 53 (20 to 74) Setting: secondary care |
|
| Interventions |
Indication: severe asthma Type of macrolide: azithromycin Route: per oral Dose: 250 mg per day for 5 days, then 250 mg 3 times/week for 25 weeks Duration of treatment: 26 weeks Total treatment dose: 20,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported. Adverse events are reported as "number of events" and not as "patients with events". Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | The study was funded by the Agency for Innovation by Science and Technology, Flanders, Belgium. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a central, web‐based tool |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo not described in detail. However, both active treatment and placebo were formulated at the same pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded trial and presumably matching placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Adverse events resulting in discontinuation are reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, unclear ascertainment. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Bystedt 1980.
| Methods | Design: randomised, placebo‐controlled, 7‐armed trial | |
| Participants |
Number assigned: 40 children, adults, and elderly (macrolide n = 20, placebo n = 20) Age in years (mean (range)): 29 (17 to 79) Setting: secondary care |
|
| Interventions |
Indication: impacted mandibular 3rd molars Type of macrolide: erythromycin stearate Route: per oral Dose per day: 500 mg at day 1, then 250 mg x 4 for 7 days Duration of treatment: 8 days Total treatment dose: 7500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: incomplete reporting. However, no contact details for author. Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome, standardised ascertainment, adverse events not reported. |
| Other bias | Low risk | None were identified. |
Cameron 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 77 adults (macrolide n = 39, placebo n = 38) Age in years (mean ± SD): macrolide: 46.4 ± 8.8, placebo: 42.8 ± 9.4 Setting: unclear |
|
| Interventions |
Indication: smokers with chronic asthma Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg Duration of treatment: 84 days Total treatment dose: 21,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study funded by the Medical Research Council UK and supported financially by NHS Research Scotland (NRS), through the Scottish Primary Care Research Network. Authors purchased study medication with an educational grant from AstraZeneca. Some authors were on advisory boards, received consultancy fee or grants for institutions from pharmaceutical companies. |
|
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. However, information on adverse events was clearly presented upon contacting authors. |
| Other bias | Low risk | None were identified. |
Carbonell 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 100 adults and elderly (macrolide n = 50, placebo n = 50) Age in years (mean ± SD): macrolide: 59.3 ± 14.6, placebo: 57.0 ± 13.4 Setting: secondary care |
|
| Interventions |
Indication: endoscopy for acute upper gastrointestinal bleeding Type of macrolide: erythromycin Route: intravenous Dose per day: 250 mg Duration of treatment: 1 day Total treatment dose: 250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination Adverse events: states that no adverse events were observed Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by Assistance Publique Hopitanx de Paris, France. Erythromycin produced by Abbott France. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active treatment or placebo was mixed with saline before infusion and administered intravenously. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout, except 1 participant randomised to erythromycin who was withdrawn before treatment as he had advanced hepatocellular carcinoma |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Cercek 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1439 adults and elderly (macrolide n = 716, placebo n = 723) Age in years (mean ± SE): macrolide: 65.2 ± 0.5, placebo: 64.7 ± 0.5 Setting: secondary care |
|
| Interventions |
Indication: unstable angina or acute myocardial infarction Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg day 1 followed by 250 mg/day for 4 days Duration of treatment: 5 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: laboratory tests Reporting of adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Funded by The Heart Foundation at Cedars‐Sinai (formerly the Steven S Cohen Heart Fund) and institutional funds of the participating centres | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, tamper‐evident envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active drugs and matched placebo delivered in identical bottles. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded evaluators and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, except for liver function tests. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Clement 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 82 children (macrolide n = 40, placebo n = 42) Age in years (mean ± SD): macrolide: 10.9 ± 3.5, placebo: 11.1 ± 3.2 Setting: secondary care |
|
| Interventions |
Indication: cystic fibrosis Type of macrolide: azithromycin Route: per oral Dose: 250 mg if < 40 kg or 500 mg if ≥ 40 kg, 3 days/week Duration of treatment: 1 year Total treatment dose: 78,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinical examination/lab tests Reporting of adverse events: yes Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by the Cystic Fibrosis Association Vaincre la Mucoviscidose, Paris, France. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and study investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment and adverse events presented clearly. However, liver function measured but not reported. |
| Other bias | Low risk | None were identified. |
Corris 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 48 adults (macrolide n = 25, placebo n = 23) Age in years (median (IQR)): macrolide: 51.0 (35 to 56), placebo: 51.0 (44 to 59) Setting: secondary care |
|
| Interventions |
Indication: bronchiolitis obliterans syndrome post‐lung transplantation Type of macrolide: azithromycin Route: per oral Dose: 250 mg on alternate days Duration of treatment: 84 days Total treatment dose: 10,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: data reported Death: no deaths during the study period |
|
| Funding sources | Study funded by a Medical Research Council project grant and a British Lung Foundation Trevor Clay Award. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in a 1:1 ratio using random permuted blocks within strata |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo not described in detail. However, active treatment and placebo were formulated by the same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. 1 adverse event leading to discontinuation reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome and unclear ascertainment. Adverse events not presented clearly. |
| Other bias | Low risk | None were identified. |
Currier 2000.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 644 adults (macrolide n = 322, placebo n = 321, mistakenly enrolled n = 1) Age in years (median): 40 Setting: AIDS clinical trial study sites at university‐based outpatient clinics |
|
| Interventions |
Indication: mycobacterium avium complex infection in people with AIDS and increased CD4+ cell counts Type of macrolide: azithromycin Route: per oral Dose: 1200 mg/week Duration of treatment: 69 weeks (median) Total treatment dose: 82,800 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by the AIDS Clinical Trials Group and National Institute of Allergy and Infectious Diseases and in part by Pfizer Inc. 1 of the authors was a representative for Pfizer Inc and reviewed the protocol, statistical reports, and manuscript. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in permuted blocks of 4 within each stratification level |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups. Discontinuation due to adverse events was larger in azithromycin group than in placebo group (8% versus 2%), but this is reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Curry 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 62 children (macrolide n = 32, placebo n = 30) Age in weeks (mean ± SD): macrolide: 36.3 ± 2.1, placebo: 36.3 ± 1.1 Setting: secondary care |
|
| Interventions |
Indication: infants with gastroschisis Type of macrolide: erythromycin Route: per oral Dose per day: 12 mg/kg Duration of treatment: 13 days (mean) Total treatment dose: 377 mg (mean weight used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: laboratory tests and ECG Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | The BAPS Multicentre Research Fellow was funded by Dunhill Medical Trust. Authors acknowledge supplying company (Rosemont Pharmaceuticals). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of investigators and caretakers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome, laboratory tests and ECG performed regularly. However, unclear reporting of adverse events |
| Other bias | Low risk | None were identified. |
Czarnetzki 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 132 adults (macrolide n = 66, placebo n = 66) Age in years (median (IQR)): macrolide: 40.5 (31 to 58), placebo: 45.0 (29 to 55) Setting: secondary care |
|
| Interventions |
Indication: gastric emptying in people undergoing general anaesthesia for emergency surgery Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 3 mg/kg Duration of treatment: 1 day Total treatment dose: 223.5 mg (mean weight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by institutional funds from the Division of Anestesiology, Geneva University Hospitals. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Dunlay 1987.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 63 adults (macrolide n = 32, placebo n = 31) Age in years (mean): macrolide: 43.0, placebo: 44.0 Setting: primary care |
|
| Interventions |
Indication: acute bronchitis Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 10 days Total treatment dose: 9990 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary used Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. Authors acknowledge supplying company (Upjohn Company). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered, identical drug containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, physician, and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, discontinuation due to adverse events reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, however unclear reporting of adverse events as only reported on how many participants withdrew due to adverse events |
| Other bias | Low risk | None were identified. |
Ehsani 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults and elderly (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): macrolide: 61 ± 15, placebo: 62 ± 17 Setting: secondary care |
|
| Interventions |
Indication: upper gastrointestinal bleeding Type of macrolide: erythromycin Route: intravenous Dose per day: 3 mg/kg in 100 mL saline Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding as placebo not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if participants and staff were blinded, however the only reported outcome is death, which is objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome. States that adverse events were recorded, but unclear ascertainment, and adverse events not reported. |
| Other bias | Low risk | None were identified. |
El‐Sadr 2000.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 520 adults (macrolide n = 258, placebo n = 262) Age in years (mean ± SD): macrolide: 41.7 ± 7.4, placebo: 41.9 ± 8.5 Setting: not specified |
|
| Interventions |
Indication: mycobacterium avium complex infection in people with HIV and increased CD4+ cell counts Type of macrolide: azithromycin Route: per oral Dose: 1200 mg/week Duration of treatment: 12.7 months (median in azithromycin group) Total treatment dose: 66,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by a grant from the National Institute of Allergy and Infectious Diseases. Authors acknowledge supplying company (Pfizer). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, discontinuation due to adverse events reported |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, unclear ascertainment. Adverse events presented clearly. |
| Other bias | Low risk | None were identified. |
Eschenbach 1991.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1181 adults (macrolide n = 605, placebo n = 576) Age in years (mean ± SD): macrolide: 23.9 ± 5.3, placebo: 23. 6 ± 5.6 Setting: secondary care |
|
| Interventions |
Indication: pregnant women with Ureaplasma urealyticum Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: maximum of 70 days (starting between 26 and 30 weeks' gestation and continuing through 35 completed weeks of pregnancy. Instructed to take the medication for 10 weeks or until the end of the 35th week of pregnancy, whichever came first) Total treatment dose: 69,930 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: spontaneously + asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported for babies |
|
| Funding sources | Study supported by the National Institutes of Health. Authors acknowledge supplying company (The Upjohn Company). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, and adverse events presented clearly. |
| Other bias | Low risk | None were identified. |
Fonseca‐Aten 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 43 children (macrolide n = 22, placebo n = 21) Age in months (median (range)): macrolide: 112.5 (62 to 187), placebo: 100 (50 to 181) Setting: emergency department of Children's Medical Center |
|
| Interventions |
Indication: acute exacerbation of recurrent wheezing or asthma Type of macrolide: clarithromycin Route: per oral Dose per day: 15 mg/day, in 2 divided doses (maximum of 1000 mg) Duration of treatment: 5 days Total treatment dose: 5000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by Abbott Laboratories and Children's Medical Center of Dallas Research Advisory committee | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo not described, however active treatment and placebo prepared by the same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No outcomes reported. Children, caretakers, and staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up not reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and adverse events not reported. |
| Other bias | Low risk | None were identified. |
Frossard 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 105 adults and elderly (macrolide n = 51, placebo n = 54) Age in years (mean ± SD): macrolide: 59.2 ± 15, placebo: 64.5 ± 16 Setting: secondary care |
|
| Interventions |
Indication: acute upper gastrointestinal bleeding Type of macrolide: erythromycin Route: intravenous Dose per day: 250 mg (mixed with 50 mL saline) Duration of treatment: 1 day Total treatment dose: 250 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: spontaneously Adverse events: data reported Antimicrobial resistance: not reported Death: incomplete reporting |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Allocation done off‐site at a central pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Unclear risk | Small, significant age difference between the 2 groups |
Garcia‐Burguillo 1996.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 adults (macrolide n = 30, placebo n = 30) Age in years (mean ± SD): macrolide: 28.3 ± 5.9, placebo: 27.4 ± 6 Setting: secondary care |
|
| Interventions |
Indication: preterm rupture of the amniotic membranes Type of macrolide: erythromycin ethyl succinate Route: per oral Dose per day: 2000 mg Duration of treatment: 8 days (mean duration of treatment in erythromycin group) Total treatment dose: 16,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: reported for babies of treated mothers |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding as placebo not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only reported on death in babies, which is an objective outcome not influenced by blinding or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, adverse events not reported (only death in babies). |
| Other bias | Low risk | None were identified. |
Gharpure 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 74 children (macrolide n = 37, placebo n = 37) Age in years (mean (range)): macrolide: 3.5 (0.1 to 16), placebo: 1.8 (0.1 to 17) Setting: secondary care |
|
| Interventions |
Indication: tube placement in critically ill children Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 10 mg/kg for every 6 hours (maximum 3 doses) Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: continuous electrocardiogram monitoring and adverse events defined before study start Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Children’s Research Center of Michigan. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saline used as placebo and equal amounts. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of children, parents, and staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, report on reason for discontinuation |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, adverse events defined before study start and reported. |
| Other bias | Low risk | None were identified. |
Giamarellos‐Bourboulis 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 200 adults and elderly (macrolide n = 100, placebo n = 100) Age in years (mean ± SD): macrolide: 58.4 ± 20.7, placebo: 58.4 ± 17.4 Setting: secondary care |
|
| Interventions |
Indication: sepsis associated with ventilator‐associated pneumonia Type of macrolide: clarithromycin Route: intravenous Dose per day: 1000 mg Duration of treatment: 3 days Total treatment dose: 3000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: clinical examination (lab tests, ECG) Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Abbott Laboratories. No information about their role in the study | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was generated by an independent biostatistician and stratified by study site. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. Report on reasons for discontinuation |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome. Only serious adverse events reported; info on QTc interval not presented even though ECG was performed. |
| Other bias | Low risk | None were identified. |
Giamarellos‐Bourboulis 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 600 adults and elderly (macrolide n = 302, placebo n = 298) Age in years (mean ± SD): macrolide: 67.8 ± 19.3, placebo: 65.9 ± 19.9 Setting: secondary care |
|
| Interventions |
Indication: suspected gram‐negative sepsis Type of macrolide: clarithromycin Route: intravenous Dose per day: 1000 mg Duration of treatment: 4 days Total treatment dose: 4000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Abbott Laboratories (Hellas) SA. The first author serves as an advisor of Astellas Hellas and The Medicines Company and has received honoraria from AbbVie. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was generated by an independent biostatistician and stratified by study site. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. Report on reasons for discontinuation |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome and unclear ascertainment. However, adverse events reported in detail. |
| Other bias | Low risk | None were identified. |
Gibson 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 420 adults and elderly (macrolide n = 213, placebo n = 207) Age in years (median (IQR)): macrolide: 60.01 (49.58 to 67.98), placebo: 61.02 (50.62 to 68.74) Setting: secondary care |
|
| Interventions |
Indication: persistent uncontrolled asthma Type of macrolide: azithromycin Route: per oral Dose: 500 mg 3 times per week Duration of treatment: 336 days Total treatment dose: 72,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Ascertainment of adverse events: participants asked + clinical examination Adverse event: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Supported by the National Health and Medical Research Council of Australia and the John Hunter Hospital Charitable Trust | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | About 20% of participants in each group were withdrawn, however reasons (including adverse events) for withdrawal were provided. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome and clear ascertainment. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Glass 1999.
| Methods | Design: randomised, placebo‐controlled, 4‐armed trial | |
| Participants |
Number assigned: 80 children and adults (macrolide n = 39, placebo n = 41) Age in years (mean ± SD): macrolide: 18.8 ± 2.5, placebo: 18.3 ± 1.9 Setting: secondary care |
|
| Interventions |
Indication: acne vulgaris Type of macrolide: erythromycin Route: topical Dose per day: 2% gel twice a day Duration of treatment: 84 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinician assessment Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo not described (4 arms in study). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants or staff or both were blinded to treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment and adverse events reported. However, only report "overall" on participants with adverse events. |
| Other bias | Low risk | None were identified. |
Gokmen 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 47 children (macrolide n = 24, placebo n = 23) Gestational age in weeks (median (range)): macrolide: 28.5 (26 to 32), placebo: 27 (25 to 30) Setting: secondary care |
|
| Interventions |
Indication: preventing feeding intolerance and liver function abnormalities in premature infants Type of macrolide: erythromycin Route: per oral Dose per day: 12.5 mg/kg (mixed into milk feeds) Duration of treatment: 14 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: electrocardiography was performed before drug treatment began and after the 1st and 2nd week of treatment to assess the QTc intervals Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None reported. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo solution was given as an equivalent volume of normal saline. All the medications were mixed thoroughly into milk feeds to mask their appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medicine or placebo addition to the milk was performed by a dietitian so that the neonatal nurses were blinded to the particular intervention in each infant. Death is an objective outcome, not influenced by blinding or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, none caused by adverse events |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. State that ECG was performed during study period, but no reporting of ECG measures |
| Other bias | Unclear risk | Infants in the macrolide group had higher gestational age and birthweight than those assigned placebo. |
Grassly 2016.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 754 children (macrolide n = 376, placebo n = 378) Age in months (mean ± SE): azithromycin: 7.46 ± 0.08, placebo: 7.49 ± 0.08 Setting: healthy infants living in 14 blocks of Vellore district, India |
|
| Interventions |
Indication: improve immune response to oral poliovirus vaccination Type of macrolide: azithromycin Route: per oral Dose per day: 10 mg/kg Duration of treatment: 3 days Total treatment dose: 219 mg (mean weight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Bill & Melinda Gates Foundation. 1 author declared unrelated collaborations with pharmaceutical companies. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children, parents, and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome. However, unclear ascertainment and incomplete reporting of adverse events (do not report on each adverse event separately). |
| Other bias | Low risk | None were identified. |
Grayston 2005.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 4012 adults and elderly (macrolide n = 2004, placebo n = 2008) Age in years (mean): macrolide: 65, placebo: 65 Setting: secondary care |
|
| Interventions |
Indication: secondary prevention in people with stable coronary heart disease Type of macrolide: azithromycin Route: per oral Dose: 600 mg/week Duration of treatment: 1 year Total treatment dose: 31,200 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported (part of composite primary outcome) |
|
| Funding sources | Study supported by the National Heart, Lung, and Blood Institute and Pfizer. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, none due to adverse events |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Grob 1981.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 91 children (macrolide n = 52, placebo n = 39) Age in years (range): 0 to 8 Setting: primary care |
|
| Interventions |
Indication:Bordetella pertussis prevention Type of macrolide: erythromycin ethyl succinate Route: per oral Dose per day: 500 mg if aged < 2 years and 1000 mg if aged 2 to 8 years Duration of treatment: 14 days Total treatment dose: 14,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study funded by the Medical Research Council. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and adverse events not reported. |
| Other bias | Low risk | None were identified. |
Gupta 1997.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 60 adults and elderly (macrolide (3‐day course) n = 28, macrolide (6‐day course) n = 12, placebo n = 20) Age in years (mean ± SD): macrolide (both arms): 58 ± 7, placebo: 60 ± 9 Setting: secondary care |
|
| Interventions |
Indication: male survivors of myocardial infarction Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: arm 1: 3 days, arm 2: 6 days Total treatment dose: arm 1: 1500 mg, arm 2: 3000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported (note: do not report on "common adverse events") Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by the British Heart Foundation. Authors acknowledge supplying company (Pfizer Ltd). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo and azithromycin supplied by the same company. However, unclear if placebo matched the single course of azithromycin (3 days) or the 2 courses (2 x 3 days). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only report on objective outcomes (death/myocardial infarction) not influenced by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | High risk | Adverse events stated as an outcome. Unclear ascertainment. Report on outcomes for the 2 treatment regimens as 1 group and do not report on common adverse events. |
| Other bias | Low risk | None were identified. |
Gurfinkel 1999.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 202 adults and elderly (macrolide n = 102, placebo n = 100) Age in years (mean ± SD): macrolide: 61 ± 12, placebo: 61 ± 12 Setting: secondary care |
|
| Interventions |
Indication: non‐Q‐wave coronary syndrome Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 30 days Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: electrocardiogram Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study funded by the Favaloro Foundation. Authors acknowledge supplying company (Hoechst Marion Roussel). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff blinded to treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome and unclear ascertainment, except from ECG. However, adverse events reported. |
| Other bias | Unclear risk | More participants with diabetes were randomised to macrolide group. |
Hahn 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 45 adults (macrolide n = 24, placebo n = 21) Age in years (mean ± SD): macrolide: 50 ± 14, placebo: 45 ± 12 Setting: primary care |
|
| Interventions |
Indication: asthma Type of macrolide: azithromycin Route: per oral Dose: 600 mg/day for 3 days, followed by 600 mg weekly Duration of treatment: 6 weeks Total treatment dose: 4800 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by the National Institutes of Health, the American Academy of Family Physicians Foundation Joint Grant Awards Program, the Wisconsin Academy of Family Physicians, under the auspices of the Wisconsin Research Network, the Dean Foundation for Health Research and Education. Study supported by an unrestricted educational grant from Pfizer. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study physicians, research staff, participants, and data analysts were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated clearly as an outcome, however standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Hahn 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 75 adults (macrolide n = 38, placebo n = 37) Age in years (mean ± SD): macrolide: 45.7 ± 15.5, placebo: 47.4 ± 14.2 Setting: primary care |
|
| Interventions |
Indication: asthma Type of macrolide: azithromycin Route: per oral Dose: 600 mg/day for 3 days, followed by 600 mg weekly Duration of treatment: 12 weeks Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the Wisconsin Academy of Family Physicians, the American Academy of Family Physicians Foundation, the Dean Foundation for Health Research and Education, and private donors provided financial support for direct costs of AZMATICS (AZithroMycin/Asthma: Trial in Community Settings). Authors acknowledge supplying company (Pfizer Inc). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42% lost to follow‐up in azithromycin group versus 30% in placebo group. However, authors report on adverse events for 92% to 95% of participants in macrolide group and 92% in placebo group. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Halperin 1999.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 362 children and adults (macrolide n = 170, placebo n = 192) Age in years (mean): macrolide: 26.6, placebo: 24.9 Setting: community based (households) |
|
| Interventions |
Indication:Bordetella pertussis prevention Type of macrolide: erythromycin estolate Route: per oral Dose per day: 40 mg/kg (max 1000 mg) Duration of treatment: 10 days Total treatment dose: 10,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the National Health Research and Development Program, Health Canada. Authors acknowledge supplying company (Eli Lilly Canada Inc). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Haxel 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number: 58 adults (macrolide n = 29, placebo n = 29) Age in years (mean ± SD): macrolide: 45.7 ± 12.8, placebo: 47.7 ± 12.5 Setting: secondary care |
|
| Interventions |
Indication: chronic rhinosinusitis Type of macrolide: erythromycin Route: per oral Dose per day: 250 mg Duration of treatment: 90 days Total treatment dose: 22,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. More participants dropped out in macrolide group. However, adverse events reported. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome. However, unclear ascertainment and authors only report on gastrointestinal adverse events, although it reads as there might have been other kinds of adverse events to report ("Adverse events such as gastrointestinal disorders..."). |
| Other bias | Low risk | None were identified. |
Haye 1998.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 169 adults and elderly (macrolide n = 87, placebo n = 82) Age in years (mean (range)): macrolide: 40.2 (21 to 70), placebo: 43.2 (18 to 68) Setting: primary care |
|
| Interventions |
Indication: acute maxillary sinusitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout and no participants discontinued treatment due to adverse events |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated clearly as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Heppner 2005.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 292 adults (macrolide n = 190, placebo n = 102) Age in years (mean ± SD): macrolide: 29.3 ± 8, placebo: 29.1 ± 8 Setting: the remote forest and scrub‐covered foothills at the AFRIMS–Kwai River Christian Hospital field site in western Thailand |
|
| Interventions |
Indication: Plasmodium vivax malaria prophylaxis Type of macrolide: azithromycin Route: per oral Dose per day: loading dose on day 1 of 750 mg, then 250 mg per day Duration of treatment: 74 days (on average) Total treatment dose: 19,000 mg (average duration of treatment used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the US Army Medical Materiel Development Activity and by the Military Infectious Diseases Research Program. Azithromycin and placebo were provided by Pfizer Central Research. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and trial personnel blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 28% (macrolide) versus 25% (placebo). However, adverse events reported for > 90% of participants. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Unclear risk | 2:1 randomisation design |
Hillis 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 141 adults and elderly (macrolide n = 72, placebo n = 69) Age in years (mean ± SD): macrolide: 66 ± 11, placebo: 65 ± 12 Setting: secondary care |
|
| Interventions |
Indication: survivors of acute coronary syndrome Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 5 days Total treatment dose: 2500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: data reported, but only those resulting in discontinuation Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the British Heart Foundation. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant dropped out in each group. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated clearly as an outcome. Standardised ascertainment. However, only adverse events resulting in discontinuation were reported on. |
| Other bias | Low risk | None were identified. |
Hodgson 2016.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 44 adults and elderly (macrolide n = 22, placebo n = 22) Age in years (mean ± SD): macrolide: 59.6 ± 11.0, placebo: 56.9 ± 9.0 Setting: respiratory clinics |
|
| Interventions |
Indication: chronic cough Type of macrolide: azithromycin Route: per oral Dose: 500 mg daily for 3 days, followed by 250 mg 3 times/week Duration of treatment: 59 days Total treatment dose: 7500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear (ECG and phlebotomy prior to study entry) Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a National Institute for Health Research Biomedical Research fellowship. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups. Reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome. Unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Hooton 1990.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 87 adults (macrolide n = 36, placebo n = 41) Age in years (mean ± SD): macrolide: 26 ± 6, placebo: 29 ± 8 Setting: secondary care |
|
| Interventions |
Indication: non‐gonococcal urethritis Type of macrolide: erythromycin estolate Route: per oral Dose per day: 1000 mg Duration of treatment: 21 days Total treatment dose: 21,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Erythromycin and placebo were provided by The Upjohn Company. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome. Unclear ascertainment, although follow‐up visits scheduled. Adverse events reported. |
| Other bias | Low risk | None were identified. |
Hyde 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 147 adults (macrolide n = 73, placebo n = 74) Age in years (mean (range)): macrolide: 44 (25 to 63), placebo: 46 (19 to 64) Setting: secondary care |
|
| Interventions |
Indication: Mycoplasma pneumoniae prophylaxis Type of macrolide: azithromycin Route: per oral Dose: 500 mg on day 1, followed by 250 mg on days 2 to 5 Duration of treatment: 5 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Pfizer). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Residents and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome, unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Ikeoka 2007.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 90 adults and elderly (macrolide n = 42, placebo n = 40, excluded n = 8) Age in years (mean ± SD): macrolide: 62 ± 10, placebo: 59 ± 9 Setting: secondary care |
|
| Interventions |
Indication: stable coronary disease Type of macrolide: azithromycin Route: per oral Dose: 500 mg x 1 for 3 days in week 1, followed by 500 mg x 1 weekly for 12 weeks, then 500 mg x 1 for 3 days in week 14 Duration of treatment: 14 weeks Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Pfizer). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated clearly as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Jablonowski 1997.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 682 adults (macrolide n = 341, placebo n = 341) Age in years (range): 20 to 60 Setting: multicentre trial |
|
| Interventions |
Indication: mycobacterium avium‐intracellulare complex prophylaxis in HIV‐infected individuals Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: N/A Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: no surveillance system was used to study the emergence of resistant bacteria. However, authors state that there were no reports of infections with clarithromycin‐resistant organisms during the study, and no pneumonia due to a clarithromycin‐resistant organism was observed. Death: data reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only report on objective outcomes, blinding not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, adverse events not reported. |
| Other bias | Low risk | None were identified. |
Jackson 1999.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 88 adults and elderly (macrolide n = 44, placebo n = 44) Age in years (mean (range)): 57 (37 to 79) Setting: unclear |
|
| Interventions |
Indication: coronary artery disease Type of macrolide: azithromycin Route: per oral Dose: 500 mg on days 1 and 2, then 250 mg on days 3 to 28 Duration of treatment: 28 days Total treatment dose: 8000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Pfizer Inc). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo not described in detail. However, active treatment and placebo were formulated by the same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, adverse events reported. |
| Other bias | Low risk | None were identified. |
Jespersen 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 4373 adults and elderly (macrolide n = 2172, placebo n = 2201) Age in years (mean ± SD): macrolide: 65.4 ± 10.3, placebo: 65.2 ± 10.4 Setting: secondary care |
|
| Interventions |
Indication: stable coronary heart disease Type of macrolide: clarithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 14 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant diary used Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by the Danish Heart Foundation, Copenhagen Hospital Corporation, Danish Research Council, and 1991 Pharmacy Foundation. Authors acknowledge supplying company (Abbott Laboratories). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded, death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | About 1% in each group did not return the participant diary. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Joensen 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 507 adults and elderly (macrolide n = 250, placebo n = 257) Age in years (mean ± SD): macrolide: 64.8 ± 8.8, placebo: 66.6 ± 10.1 Setting: secondary care |
|
| Interventions |
Indication: peripheral arterial disease Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 28 days Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported (primary outcome) |
|
| Funding sources | Study supported by the Danish Heart Foundation, the Rosa and Asta Jensen Foundation, the Danish Medical Research Council, and the Health Department of Viborg County. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Nurses at the department gave participants a glass of pills (unaware of content). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse, other team members, and participants blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Johnston 2016.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 199 adults (macrolide n = 97, placebo n = 102) Age in years (median (IQR)): macrolide: 39.1 (28.9 to 49.5), placebo: 36.2 (25.4 to 49.3) Setting: 30 secondary care hospitals and 1 primary centre |
|
| Interventions |
Indication: acute asthma exacerbation Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary used Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study funded by the Efficacy and Mechanisms Evaluation programme of the Medical Research Council, in partnership with the National Institute for Health Research. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | ID numbers assigned sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants included in safety assessments, numbers not stated. However, authors report that 80% attended all follow‐up visits. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events are reported. |
| Other bias | Low risk | None were identified. |
Jun 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 116 adults and elderly (macrolide n = 58, placebo n = 58) Age in years (mean ± SD): macrolide: 56.6 ± 10.3, placebo: 59 ± 11.6 Setting: secondary care |
|
| Interventions |
Indication: subtotal gastrectomy Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 125 mg Duration of treatment: 1 day Total treatment dose: 125 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Business of Globalization for Science and Technology, Seoul, Republic of South Korea. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both groups received infusion of saline (+/‐ antibiotics). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. Some adverse events reported (nausea, vomiting). |
| Other bias | Low risk | None were identified. |
Kaehler 2005.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 327 adults and elderly (macrolide n = 165, placebo n = 162) Age in years (mean ± SD): macrolide: 62 ± 16, placebo: 63 ± 14 Setting: secondary care |
|
| Interventions |
Indication: coronary artery disease Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 42 days Total treatment dose: 12,600 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Aventis Pharma GmbH, Germany. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if identical‐appearing placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No adverse events reported, death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Kaiser 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 269 adults and elderly (macrolide n = 133, placebo n = 132, excluded n = 4) Age in years (median (range)): 35 (18 to 93) Setting: secondary care |
|
| Interventions |
Indication: common cold and acute rhinosinusitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by a grant from Pfizer AG, Switzerland. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome and unclear ascertainment. However, gastrointestinal adverse events reported. |
| Other bias | Low risk | None were identified. |
Kalliafas 1996.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 57 adults and elderly (macrolide n = 31, placebo n = 26) Age in years (mean (range)): macrolide: 54.7 (19 to 84), placebo: 57.8 (19 to 86) Setting: secondary care |
|
| Interventions |
Indication: critically ill individuals assessed as needing nutrition support Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 200 mg Duration of treatment: 1 day Total treatment dose: 200 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saline used as placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No reporting of relevant outcomes. Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, adverse events not reported. |
| Other bias | Low risk | None were identified. |
Karlsson 2009.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 247 elderly (macrolide n = 122, placebo n = 125) Age in years (median (IQR)): macrolide: 71 (67 to 74), placebo: 71 (67 to 76) Setting: secondary care |
|
| Interventions |
Indication: abdominal aortic aneurysms Type of macrolide: azithromycin Route: per oral Dose per day: 600 mg x 1 daily for 3 days, then 600 mg once weekly for 15 weeks Duration of treatment: 16 weeks Total treatment dose: 10,800 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by County of Gävleborg Research and Development Center, Gore Swedish Research Foundation, Pfizer AB Sweden, Schyberg medical research fund, and Zoega medical research fund. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 person in each group was lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and only non‐specific adverse events are reported on. |
| Other bias | Low risk | None were identified. |
Kathariya 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 100 adults (macrolide n = 50, placebo n = 50) Age in years (mean ± SD): macrolide: 39.3 ± 7.4, placebo: 37.4 ± 7.3 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis Type of macrolide: clarithromycin Route: topical Dose per day: 0.5% gel once Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: states that no adverse events were observed or reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Self funded project | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation done by a study co‐ordinator not involved in the clinical treatment/assessments. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants lost to follow‐up in placebo group: 1 migrated and 1 was unwilling to continue. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and states that no adverse events were observed or reported. |
| Other bias | Low risk | None were identified. |
Kaul 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 466 adults (macrolide n = 230, placebo n = 236) Age in years (mean ± SD): macrolide: 29.1 ± 7.8, placebo: 28.1 ± 7.7 Setting: urban slum area of Nairobi, Kenya |
|
| Interventions |
Indication: prevention of sexually transmitted infections and HIV‐1 infection Type of macrolide: azithromycin Route: per oral Dose: 1000 mg once a month Duration of treatment: 26 months (on average) Total treatment dose: 26,000 mg (on average) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by the Rockefeller Foundation, the European Commission, the Canada Research Chairs Program, Ontario HIV Treatment Network, the Canadian Institutes of Health Research, and the Canadian Infectious Disease Society. Authors acknowledge supplying company (Pfizer Inc). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Clinic staff assigned study numbers consecutively at enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 20% lost to follow‐up after 2 years in the 2 groups, but adverse events as a source of dropout reported. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment. However, adverse events considered to be possibly or likely related to treatments are reported on. |
| Other bias | Low risk | None were identified. |
Keenan 2018.
| Methods | Design: cluster‐randomised placebo‐controlled trial | |
| Participants |
Number assigned: 1533 communities (macrolide n = 767 communities (97,047 children), placebo n = 766 communities (93,191 children), excluded n = 20 communities, declined n = 1 community) Age in months (range): 1 to 59 Setting: communities in Malawi, Niger, and Tanzania |
|
| Interventions |
Indication: mass distribution of antibiotics to reduce mortality Type of macrolide: azithromycin Route: per oral Dose: minimum 20 mg/kg once. Repeated twice yearly Duration of treatment: 4 years Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Ascertainment of adverse events: parents asked Adverse event: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by a grant from the Bill & Melinda Gates Foundation. Pfizer provided both the azithromycin and the placebo. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants, observers, and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for exclusion of 20 communities explained, no communities were lost to follow‐up after the initial census. |
| Selective reporting (reporting bias) | High risk | Unclear if adverse events were stated as an outcome, standardised ascertainment. Report on very few adverse events in a large trial population |
| Other bias | Low risk | None were identified. |
Kenyon 2001a.
| Methods | Design: randomised, placebo‐controlled, factorial trial | |
| Participants |
Number assigned: 3180 adults (macrolide n = 1611, placebo n = 1569) Age in years (mean ± SD): macrolide: 26.5 ± 6.1, placebo: 26.7 ± 5.7 Setting: secondary care |
|
| Interventions |
Indication: spontaneous preterm labour Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 10 days (or until delivery) Total treatment dose: 10,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: report on death of babies born to women with preterm labour |
|
| Funding sources | Study supported by the UK Medical Research Council. Authors acknowledge supplying company (Parke‐Davis). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Each woman was assigned a sequentially numbered study‐drug pack. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, clinicians, and trial staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups (note 50% completion at 7 years follow‐up) |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and adverse events not reported. |
| Other bias | Low risk | None were identified. |
Kenyon 2001b.
| Methods | Design: randomised, placebo‐controlled, factorial trial | |
| Participants |
Number assigned: 2422 adults (macrolide n = 1197, placebo n = 1225) Age in years (mean ± SD): macrolide: 27.5 ± 6.1, placebo: 27.9 ± 6.1 Setting: secondary care |
|
| Interventions |
Indication: preterm pre‐labour rupture of foetal membranes Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 10 days (or until delivery) Total treatment dose: 10,000 (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: report on death of babies born to women with PPROM |
|
| Funding sources | Study supported by the UK Medical Research Council. Authors acknowledge supplying company (Parke‐Davis). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Each woman was assigned a sequentially numbered study‐drug pack. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, clinicians, and trial staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups (69% completion at 7 years follow‐up) |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment. Most adverse events presented as a total, and it was not possible to determine how many there were in each of the 4 groups (erythromycin, erythromycin and co‐amoxiclav, co‐amoxiclav, or placebo). |
| Other bias | Low risk | None were identified. |
Kim 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 129 adults and elderly (macrolide n = 64, placebo n = 65) Age in years (mean ± SD): macrolide: 60.0 ± 10.0, placebo: 59.6 ± 10.1 Setting: secondary care |
|
| Interventions |
Indication: acute coronary syndrome who underwent PCI Type of macrolide: azithromycin Route: per oral Dose: 500 mg daily for 3 days before and after PCI, followed by 500 mg/week Duration of treatment: 3 weeks Total treatment dose: 4000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (lab tests) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Not stated | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if matching placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No adverse events reported, death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 months follow‐up in 95% of participants. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated clearly as an outcome, unclear ascertainment, adverse events not reported (only adverse cardiac outcomes are reported on). |
| Other bias | Low risk | None were identified. |
King 1996.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 91 adults (macrolide n = 49, placebo n = 42) Age in years (mean ± SD): macrolide: 36.0 ± 13, placebo: 38.2 ± 14.5 Setting: primary care |
|
| Interventions |
Indication: acute bronchitis Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 10 days Total treatment dose: 10,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the Division of Primary Care of the Agency for Health Care Policy and Research. Authors acknowledge supplying company (Parke‐Davis, Morris Plane, New Jersey). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | > 20% lost to follow‐up, unclear from which groups. |
| Selective reporting (reporting bias) | Low risk | Adverse events were stated clearly as an outcome. Standardised ascertainment and adverse events presented. |
| Other bias | Low risk | None were reported. |
Klebanoff 1995.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 938 women (macrolide n = 469, placebo n = 469) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: pregnant women colonised with group B streptococci Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 10 weeks or until the end of the 35th week of pregnancy, whichever came first Total treatment dose: 69,930 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: spontaneously Adverse events: data reported Antimicrobial resistance: not reported Death: report on death in babies of mothers treated |
|
| Funding sources | Study supported by the National Institutes of Health. Authors acknowledge supplying company (The Upjohn Company). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 1% of women not included in reporting of adverse events. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated clearly as an outcome. Standardised ascertainment. However, adverse events not presented clearly. |
| Other bias | Low risk | None were identified. |
Kneyber 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 71 children (macrolide n = 32, placebo n = 39) Age in months (mean (IQR)): macrolide: 3.0 (1.0 to 4.0), placebo: 3.6 (1.0 to 6.0) Setting: secondary care |
|
| Interventions |
Indication: respiratory syncytial virus lower respiratory tract disease Type of macrolide: azithromycin Route: per oral Dose per day: 10 mg/kg Duration of treatment: 3 days Total treatment dose: 276 mg (mean weight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. Children, parents, and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child in placebo group dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Kostadima 2004.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 75 adults (macrolide (twice a day) n = 25, macrolide (3 times a day) n = 25, placebo n = 25) Age in years (mean ± SD): macrolide (twice a day): 48 ± 16, macrolide (3 times a day): 42 ± 12, placebo: 41 ± 16 Setting: secondary care |
|
| Interventions |
Indication: asthma Type of macrolide: clarithromycin Route: per oral Dose per day: arm 1: 500 mg, arm 2: 750 mg Duration of treatment: 8 weeks Total treatment dose: arm 1: 28,000 mg, arm 2: 42,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (lab tests) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Allocation done by an independent nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that the placebo tablets were indistinguishable from the clarithromycin tablets. However, there are 2 active groups with 2 or 3 doses/day, unclear how many placebo tablets/day. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | High risk | Adverse events not stated clearly as an outcome. States that laboratory assessment was done, however values/changes not reported. Incomplete reporting of adverse events |
| Other bias | Low risk | None were identified. |
Kraft 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 52 adults (macrolide n = 26, placebo n = 26) Age in years (mean ± SD): 33.4 ± 1.2 Setting: unclear |
|
| Interventions |
Indication: asthma Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 6 weeks Total treatment dose: 42,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events reported: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the American Lung Association Asthma Research Center Grant and Abbott Laboratories. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Kvien 2004.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 152 adults (macrolide n = 81, placebo n = 71) Age in years (mean ± SD): macrolide: 33.0 ± 9.8, placebo: 34.7 ± 8.9 Setting: secondary care |
|
| Interventions |
Indication: reactive arthritis Type of macrolide: azithromycin Route: per oral Dose: 1000 mg per week (starting after a single 1 g dose of azithromycin) Duration of treatment: 12 weeks Total treatment dose: 13,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Pfizer. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout was 30% and 34% in macrolide and placebo groups, respectively. However, reasons reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Lanza 1998.
| Methods | Design: randomised, placebo‐controlled, 4‐armed trial | |
| Participants |
Number assigned: 89 adults and elderly (macrolide n = 60, placebo n = 29) Age in years (mean (range)): macrolide: 45.0 (28 to 76), placebo: 49.9 (24 to 78) Setting: "47‐Center U.S study" |
|
| Interventions |
Indication: duodenal ulcer Type of macrolide: clarithromycin Route: per oral Dose per day: 1500 mg Duration of treatment: 14 days Total treatment dose: 21,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study sponsored by Glaxo Wellcome Inc. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adverse events reported for all randomised participants. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Unclear risk | Uneven distribution of number of participants in the 2 arms (2:1 allocation) |
Leowattana 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 84 adults and elderly (macrolide n = 43, placebo n = 41) Age in years (mean ± SD): macrolide: 62.9 ± 9.6, placebo: 60.4 ± 12.6 Setting: secondary care |
|
| Interventions |
Indication: secondary prevention of acute coronary syndrome Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 30 days Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Siriraj Grant for Research Development and Medical Education. Authors acknowledge supplying company (Hoechst Marion Roussel). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identically appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Lildholdt 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 124 children and adults (macrolide n = 53, placebo n = 57, excluded n = 10) Age in years (mean (range)): 23.4 (6 to 58) Setting: secondary care |
|
| Interventions |
Indication: recurrent acute tonsillitis Type of macrolide: azithromycin Route: per oral Dose: 500 mg/week Duration of treatment: 26 weeks Total treatment dose: 13,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by Pfizer APS, Denmark. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, unclear in which group |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. However, adverse events are reported. |
| Other bias | Low risk | None were identified. |
Malhotra‐Kumar 2007a.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 112 adults (macrolide n = 74, placebo n = 38) Age in years: (mean (range)): macrolide: 24 (19 to 56), placebo: 24 (18 to 57) Setting: volunteers were selected from the University of Antwerp, Belgium |
|
| Interventions |
Indication: pharyngeal carriage of macrolide‐resistant streptococci in healthy volunteers Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes (only AMR) Adverse events ascertainment: clinical examination (oral swabs) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by Abbott Laboratories. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Volunteers allocated by an administrator with no further role in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 2 placebo groups (1 for each of the macrolide arms) were used to ensure complete blinding (Malhotra‐Kumar 2007b). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Volunteers and trial staff blinded. Objective outcomes (data on AMR) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Standardised ascertainment and subsequent carriage of resistant bacteria reported. However, no reporting on other adverse events. |
| Other bias | Low risk | None were identified. |
Malhotra‐Kumar 2007b.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 112 adults (macrolide n = 74, placebo n = 38) Age in years (mean (range)): macrolide: 24 (19 to 58), placebo: 24 (18 to 57) Setting: volunteers were selected from the University of Antwerp, Belgium |
|
| Interventions |
Indication: pharyngeal carriage of macrolide‐resistant streptococci in healthy volunteers Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes (only AMR) Adverse events ascertainment: clinical examination (oral swabs) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by Abbott Laboratories. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Volunteers allocated by an administrator with no further role in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 2 placebo groups (1 for each of the macrolide arms) were used to ensure complete blinding (Malhotra‐Kumar 2007a). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Volunteers and trial staff blinded. Objective outcomes (data on AMR) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Standardised ascertainment and subsequent carriage of resistant bacteria reported. However, no reporting on other adverse events. |
| Other bias | Low risk | None were identified. |
Mandal 1984.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 80 children and adults (macrolide n = 35, placebo n = 37, excluded n = 8) Age in years (mean ± SD): macrolide: 31.93 ± 16.59, placebo: 31.18 ± 21.15 Setting: secondary care |
|
| Interventions |
Indication: Campylobacter jejuni infection Type of macrolide: erythromycin Route: per oral Dose per day: 50 mg/kg/child, 1000 mg/adult Duration of treatment: 5 days Total treatment dose: 5000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: authors state that "no incidence of adverse drug reaction was recorded". Nausea, vomiting, and abdominal pain are reported as a primary outcome and are not considered to be adverse events. Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Abbott Laboratories). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded, none experienced adverse events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, unclear which group |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and authors state that no adverse events were noted. |
| Other bias | Low risk | None were identified. |
Mandhane 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 300 children (macrolide n = 150, placebo n = 150) Age in months (mean ± SD): macrolide: 34.8 ± 13.6, placebo: 30.5 ± 13.9 Setting: secondary care |
|
| Interventions |
Indication: wheezing Type of macrolide: azithromycin Route: per oral Dose per day: 10 mg/kg for 1 day, then 5 mg/kg for 4 days Duration of treatment: 5 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Ascertainment of adverse events: participant diary Adverse event: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by The Lung Association ‐ Alberta and Northwest Territories ‐ TLA‐IKON Pediatric Team Grant | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of children/parents and study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on adverse events provided for 93% of participants in each group, reasons for dropouts given. |
| Selective reporting (reporting bias) | Low risk | Unclear if adverse events were stated as an outcome and unclear ascertainment. However, protocol clearly states times for adverse event monitoring, and adverse events are reported. |
| Other bias | Low risk | None were identified. |
Martande 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 70 adults (macrolide n = 35, placebo n = 35) Age in years (range): 20 to 60 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 5 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Micro Labs). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment. However, incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Martande 2016.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 70 adults (macrolide n = 35, placebo n = 35) Age in years (mean ± SD): macrolide: 32.6 ± 5.4, placebo: 33.3 ± 7.3 Setting: dental care |
|
| Interventions |
Indication: Aggregatibacter actinomycetemcomitans‐associated periodontitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: authors state that "(n)one of the individuals reported any adverse effect due to the medications". Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors thank supplying companies (Micro Labs, Government College of Pharmacy, Bangalore, India). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. Clinicians and participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and authors state that no adverse events were identified. |
| Other bias | Low risk | None were identified. |
Martin 1997.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 414 children and adults (macrolide n = 205, placebo n = 209) Age in years (mean ± SD): macrolide: 21.5 ± 4.2, placebo: 21.1 ± 4.3 Setting: secondary care |
|
| Interventions |
Indication: pregnant women infected with Chlamydia trachomatis Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: N/A Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported on death in babies of treated mothers |
|
| Funding sources | Study supported by the National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases. Authors acknowledge supplying company (The Upjohn Company). | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment. However, adverse events not presented clearly. |
| Other bias | Low risk | None were identified. |
Mathai 2007.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 50 children (macrolide n = 27, placebo n = 23) Age in weeks (mean): macrolide: 35.5, placebo: 37.2 Setting: secondary care |
|
| Interventions |
Indication: gastric emptying of low‐birthweight babies Type of macrolide: erythromycin Route: per oral Dose per day: 6 mg/kg Duration of treatment: 4 days Total treatment dose: 47 mg (used mean birthweight in erythromycin group) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinician assessment + clinical examination Adverse events: authors state that "no side effects of the drug were seen". Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the office of Director General Armed Forces Medical Services. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment, and authors state that no adverse events were identified. |
| Other bias | Unclear risk | Infants in the erythromycin group had lower gestational age and birthweight than those in the placebo group. |
McCallum 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 97 children (macrolide n = 50, placebo n = 47) Age in months (median (IQR)): macrolide: 5.3 (3 to 9.4), placebo: 5 (3 to 8.5) Setting: secondary care |
|
| Interventions |
Indication: bronchiolitis Type of macrolide: azithromycin Route: per oral Dose per day: 30 mg/kg Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: clinician assessment Adverse events: authors state that "there were no adverse events or serious adverse events". Antimicrobial resistance: not reported Death: no deaths reported |
|
| Funding sources | Funded by the National Health and Medical Research Council, the Channel 7 Foundation, and the Financial Markets Foundation for Children | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical drug containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as a primary outcome, and adverse events monitored by study staff every 12 hours until discharge. |
| Other bias | Low risk | None were identified. |
McCallum 2015.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 219 children (macrolide n = 106, placebo n = 113) Age in months (median (IQR)): macrolide: 5.7 (3 to 10), placebo: 5.6 (3 to 9) Setting: secondary care |
|
| Interventions |
Indication: bronchiolitis Type of macrolide: azithromycin Route: per oral Dose: 30 mg/kg once weekly Duration of treatment: 3 weeks Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination (swabs) Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by the National Health and Medical Research Council and by a Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children, parents, and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% and 3% did not attend the day 21 follow‐up interview in the macrolide and placebo groups, respectvely. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
McCormack 1987.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 825 women (macrolide arm 1, n = 174; macrolide arm 2, n = 224; placebo, n = 427) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: pregnant women harbouring genital Ureaplasma urealyticum or Mycoplasma hominis, or both Type of macrolide: arm 1: erythromycin estolate, arm 2: erythromycin stearate Route: per oral Dose per day: 1000 mg (both arms) Duration of treatment: 6 weeks (both arms) Total treatment dose: 42,000 mg (both arms) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the National Institute of Child Health and Human Development. | |
| Notes |
Concomitant medication: unclear Note: type of erythromycin used is changed roughly halfway through the study period (stearate to estolate) due to the reporting of many adverse events. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large dropout in all 3 groups ‐ only about 40% of women completed the study. However, adverse events presented for 91% of participants. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. Standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
McDonald 1985.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 114 adults (macrolide n = N/A, placebo n = N/A) Age in years: N/A Setting: primary care |
|
| Interventions |
Indication: non‐streptococcal pharyngitis Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary used Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the National Institute of Allergy and Infectious Diseases. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons given for 16 dropouts, unclear in what groups. Unclear how many participants are actually included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, however incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
McGregor 1986.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 58 women (macrolide n = 29, placebo n = 29) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: idiopathic preterm labour Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 7 days Total treatment dose: 6993 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by The Upjohn Company, Kalamazoo, Michigan. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical drug bottles. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active drug and placebo supplied by the same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant lost to follow‐up in each group. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and unclear reporting of adverse events. |
| Other bias | Low risk | None were identified. |
McGregor 1990.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 235 children and adults (macrolide n = 119, placebo n = 110, excluded n = 6) Age in years (mean (range)): macrolide: 23.0 (13 to 37), placebo: 23.2 (16 to 34) Setting: secondary care |
|
| Interventions |
Indication: impact on cervicovaginal microflora and pregnancy outcomes Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 7 days Total treatment dose: 6993 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: only intrauterine foetal death is reported on. |
|
| Funding sources | Funding not stated. The Upjohn Company prepared the treatments. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants lost to follow‐up (3%), unclear in which group. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
McGregor 1991.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 65 adults (macrolide n = 28, placebo n = 27, excluded n = 10) Age in years (mean (range)): macrolide: 25.4 (18 to 41), placebo: 24.2 (18 to 38) Setting: secondary care |
|
| Interventions |
Indication: preterm premature rupture of the membranes Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: until active labour or for maximum 7 days Total treatment dose: 6993 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: only foetal or neonatal death reported on. |
|
| Funding sources | Funding not stated. The Upjohn Company prepared the treatments. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pregnant women and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons stated |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome and unclear ascertainment. However, adverse events are reported. |
| Other bias | Low risk | None were identified. |
Memis 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults and elderly (macrolide n = 20, placebo n = 20) Age in years (mean (SD)): macrolide: 47 (22), placebo: 49 (16) Setting: secondary care |
|
| Interventions |
Indication: effect of preoperative erythromycin on gastric acidity and volume Type of macrolide: erythromycin Route: per oral Dose per day: 200 mg Duration of treatment: 1 day Total treatment dose: 200 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: authors state that "there were no side‐effects observed in any of the groups". Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs prepared by the same pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. Standardised ascertainment for 24 hours after surgery, and authors report that no adverse events were observed. |
| Other bias | Low risk | None were identified. |
Mercer 1992.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 220 adults (macrolide n = 106, placebo n = 114) Age in years (mean (SD)): macrolide: 23.7 (5.7), placebo: 24.1 (5.6) Setting: secondary care |
|
| Interventions |
Indication: preterm premature rupture of the membranes Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: N/A (until delivery) Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: only death in babies of treated mothers reported on. |
|
| Funding sources | None stated. Boots Pharmaceuticals supplied the treatments. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, participant caregivers, and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up (1%). |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment, and only gastrointestinal discomfort mentioned as a possible adverse event. |
| Other bias | Low risk | None were identified. |
Moller 1990.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 147 children (macrolide n = 69, placebo n = 72, excluded n = 6) Age in years (range): 1 to 15 Setting: secondary care |
|
| Interventions |
Indication: otitis media with effusion Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 50 mg/kg Duration of treatment: 14 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events reported: stated that no adverse events were reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% dropout, unclear in which group |
| Selective reporting (reporting bias) | Unclear risk | Unclear if adverse events were stated as an outcome, unclear ascertainment. Authors state that no adverse events were reported. |
| Other bias | Low risk | None were identified. |
Narchi 1993.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 50 adults (macrolide n = 25, placebo n = 25) Age in years (mean ± SD): macrolide: 33 ± 5, placebo: 36 ± 9 Setting: secondary care |
|
| Interventions |
Indication: gastric acidity and volume in people scheduled for diagnostic laparoscopy Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 500 mg Duration of treatment: 1 day Total treatment dose: 500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo appears similar. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Neumann 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1010 adults and elderly (macrolide n = 506, placebo n = 504) Age in years (mean ± SD): macrolide: 64.6 ± 11.4, placebo: 64.3 ± 11.4 Setting: secondary care |
|
| Interventions |
Indication: restenosis after coronary stent replacement Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 28 days Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by funds from the Medical Faculty of Technische Universität München. Aventis provided the study medication and funded participant insurance and cost of reagents for titre assays. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and adverse events not presented. |
| Other bias | Low risk | None were identified. |
Ng 2007.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 182 children (macrolide n = 91, placebo n = 91) Age in weeks (median (range)): macrolide: 28.6 (27.3 to 30.5), placebo: 28.9 (26.6 to 30.6) Setting: secondary care |
|
| Interventions |
Indication: parenteral nutrition‐associated cholestasis in preterm, very low‐birthweight infants Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 50 mg/kg Duration of treatment: 14 days Total treatment dose: 767 mg (mean birthweight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: clinician assessment + clinical examination (ECG, lab tests) Adverse events: authors state that "no serious adverse effects were associated with erythromycin treatment", data on complications reported. Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by Department of Pediatrics, Chinese University of Hong Kong, Research Grant Council of the Government of Hong Kong SAR and by the HM Lui Memorial Fund | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both active drug and normal saline (placebo) were mixed thoroughly into the milk feeds. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome. Standardised ascertainment. However, only complications were reported. |
| Other bias | Low risk | None were identified. |
Nuntnarumit 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 46 children (macrolide n = 23, placebo n = 23) Age in weeks (median (range)): macrolide: 30 (29 to 32), placebo: 29 (28 to 31) Setting: secondary care |
|
| Interventions |
Indication: feeding intolerance in preterm infants Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 40 mg/kg/day for 2 days, then 16 mg/kg/day for 5 days Duration of treatment: 7 days Total treatment dose: 176 mg (median birthweight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: clinician assessment + clinical examination (ECG, lab tests) Adverse events: authors state that "(n)o significant adverse effects related to erythromycin were observed". Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by Ramathibodi Fund. Authors acknowledge supplying company (Siam Pharmaceutical Ltd). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (by age) |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents, participant‐care team, and assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome, standardised ascertainment, however only complications reported. |
| Other bias | Low risk | None were identified. |
O'Connor 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 7747 adults and elderly (macrolide n = 3879, placebo n = 3868) Age in years (mean): 62 Setting: clinical practices in North America, Europe, Argentina, and India |
|
| Interventions |
Indication: coronary artery disease and known Chlamydia pneumoniae exposure Type of macrolide: azithromycin Route: per oral Dose per day: 600 mg/day for 3 days during week 1, then 600 mg/week during weeks 2 to 12 Duration of treatment: 84 days Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study was sponsored by Pfizer Global Research and Development. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Identical drug containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, clinical site monitors, and the sponsor project team were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Adverse events resulting in discontinuation are reported. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome, standardised ascertainment. Authors only report on gastrointestinal complaints, not lab tests. Adverse events are reported as %, not numbers, assume that this is out of the total analysed. |
| Other bias | Low risk | None were identified. |
Oei 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 50 children (macrolide n = 25, placebo n = 25) Gestational age in weeks (mean (range)): macrolide: 28.6 (24 to 32), placebo: 29.3 (27 to 32) Setting: secondary care |
|
| Interventions |
Indication: feeding intolerance in preterm infants Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 10 mg/day Duration of treatment: 10 days Total treatment dose: 123 mg (mean birthweight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: authors state that no adverse events were noted during the trial. Vomiting is reported as a primary outcome and is not considered to be an adverse event. Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. Authors acknowledge supplying company (Abbott Australasia Ltd). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome, unclear ascertainment. However, authors state that no adverse events were noted. |
| Other bias | Low risk | None were identified. |
Ogrendik 2007.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 81 adults (macrolide n = 41, placebo n = 40) Age in years (mean ± SD): macrolide: 42 ± 9, placebo: 38 ± 10 Setting: secondary care |
|
| Interventions |
Indication: rheumatoid arthritis Type of macrolide: clarithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 6 months Total treatment dose: 90,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: reported that no deaths occurred |
|
| Funding sources | Supported by Sanovel, Istanbul | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, most discontinued because of lack of efficacy of treatments |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, only most frequently reported adverse events reported (5% cut‐off). |
| Other bias | Low risk | None were identified. |
Ogrendik 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 100 adults (macrolide n = 50, placebo n = 50) Age in years (mean ± SD): macrolide: 49 ± 7, placebo: 45 ± 8 Setting: secondary care |
|
| Interventions |
Indication: rheumatoid arthritis Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 6 months Total treatment dose: 54,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: reported that no deaths occurred |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, most discontinued because of lack of efficacy of treatments |
| Selective reporting (reporting bias) | Low risk | Unclear if adverse events were stated as an outcome. Standardised ascertainment, only most frequently reported adverse events reported (5% cut‐off). |
| Other bias | Low risk | None were identified. |
Oldfield 1998.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 182 adults (macrolide n = 89, placebo n = 93) Age in years (mean (range)): macrolide: 41.1 (24 to 63), placebo: 38.2 (24 to 61) Setting: unclear |
|
| Interventions |
Indication: prevention of Mycobacterium avium complex infection in people with AIDS Type of macrolide: azithromycin Route: per oral Dose: 1200 mg once a week Duration of treatment: 400 days (mean duration of therapy in macrolide group) Total treatment dose: 68,571 mg (used mean days in macrolide group) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked + clinical examination (biaural audiograms) Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by Pfizer and the Military Medical Consortium for Applied Retroviral Research | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many are analysed for various outcomes. Reported n = 90 in adverse events section, although only 89 people were randomised. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Ozdemir 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 74 children (macrolide n = 37, placebo, n = 37) Gestational age in years (mean ± SD): macrolide: 27.4 ± 1.3, placebo: 27.3 ± 1.8 Setting: secondary care |
|
| Interventions |
Indication: prevention of bronchopulmonary dysplasia in Ureaplasma urealyticum–positive preterm infants Type of macrolide: clarithromycin Route: intravenous Dose per day: 20 mg/kg Duration of treatment: 10 days Total treatment dose: 198 mg (mean birthweight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted. Author reply: "We didn't see any adverse events in both groups" Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only objective outcomes (death) reported on. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Paknejad 2010.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults (macrolide n = 20, placebo n = 20) Age in years (min to max): 18.0 to 46.7 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, unclear which group, reasons given |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Pandhi 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 70 children and adults (macrolide n = 35, placebo n = 35) Age in years (mean ± SD): macrolide: 23.00 ± 8.96, placebo: 23.66 ± 8.35 Setting: secondary care |
|
| Interventions |
Indication: pityriasis rosea Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg (maximum) Duration of treatment: 5 days Total treatment dose: 2500 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Parchure 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults and elderly (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): macrolide: 56 ± 9, placebo: 54 ± 10 Setting: secondary care |
|
| Interventions |
Indication: coronary artery disease and antibodies positive to Chlamydia pneumoniae Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg for 3 days, then 500 mg once a week for an additional 4 weeks Duration of treatment: 5 weeks Total treatment dose: 3500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by the British Heart Foundation | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Patole 2000.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 73 children (macrolide n = 36, placebo n = 37) Gestational age in weeks (median (IQR)): macrolide: 29 (27 to 30), placebo: 30 (27 to 31) Setting: secondary care |
|
| Interventions |
Indication: full enteral feeds in preterm infants Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 48 mg/kg Duration of treatment: until full feeds or maximum of 14 days Total treatment dose: 230 mg (mean birthweight in macrolide group and median time taken to full feeds used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, however no contact information for author Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Authors acknowledge Abbott Australasia. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Paul 1998.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 437 women (macrolide n = 219, placebo n = 218) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: low birthweight and preterm delivery Type of macrolide: erythromycin stearate Route: per oral Dose per day: 1000 mg Duration of treatment: 6 weeks Total treatment dose: 42,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27% and 24% excluded from the final analysis in the macrolide and placebo groups, respectively; 29 lost to follow‐up. Reasons not given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Petersen 1997.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 212 adults (macrolide n = 93, placebo n = 93, excluded n = 26) Age in years (median): macrolide: 25, placebo: 26 Setting: primary care |
|
| Interventions |
Indication: pharyngitis not caused by group A Streptococcus Type of macrolide: erythromycin base Route: per oral Dose per day: 999 mg Duration of treatment: 10 days Total treatment dose: 9990 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary used Adverse events: data reported on day 1, 3, and 6 Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by Henry J Kaiser Foundation and The Upjohn Company | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar between groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. Reported on adverse events as %, not numbers, assume that this is out of the total analysed. |
| Other bias | Low risk | None were identified. |
Peterson 1996.
| Methods | Design: randomised, placebo‐controlled, 4‐armed trial | |
| Participants |
Number assigned: 89 adults and elderly (macrolide n = 55, placebo n = 34) Age in years (mean (range)): macrolide: 51.7 (26 to 77), placebo: 48.4 (22 to 76) Setting: secondary care |
|
| Interventions |
Indication: duodenal ulcer Type of macrolide: clarithromycin Route: per oral Dose per day: 1500 mg Duration of treatment: 14 days Total treatment dose: 21,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: physical + clinical examination (lab tests) Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Glaxo Wellcome Inc. | |
| Notes |
Concomitant medication: yes Note: this is a 4‐armed randomised controlled trial (placebo, clarithromycin, ranitidine bismuth citrate, ranitidine bismuth citrate + clarithromycin). Importantly, the participants in both the macrolide and the placebo group received a placebo at some time to ensure blinding in all groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts due to adverse events reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Unclear risk | Participants were assigned in a 2:1 ratio. |
Pierce 1996.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 682 adults and elderly (macrolide n = 341, placebo n = 341) Age in years (mean (range)): macrolide: 37.5 (22 to 60), placebo: 37.6 (20 to 65) Setting: unclear |
|
| Interventions |
Indication: prevention of disseminated Mycobacterium avium complex infection in people with AIDS Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 315 days (mean duration of treatment in macrolide group used) Total treatment dose: 315,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Supported by a grant from Abbott Laboratories | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost to follow‐up. Withdrawal due to adverse events reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome. Unclear ascertainment, but clear statement about the approach used to summarise adverse events. Adverse events reported in detail. Authors only present adverse events as % ‐ calculations done on all participants enrolled/treated. |
| Other bias | Low risk | None were identified. |
Pinto 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 185 children (macrolide n = 88, placebo n = 97) Age in months (mean ± SD): macrolide: 3.08 ± 2.23, placebo: 3.12 ± 2.29 Setting: secondary care |
|
| Interventions |
Indication: acute bronchiolitis Type of macrolide: azithromycin Route: per oral Dose per day: 10 mg/kg Duration of treatment: 7 days Total treatment dose: 394 mg (current weight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by Fundacao de Amparo a Pesquisa do Estado do Rio Grande do Sul | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in placebo group lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Pradeep 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults (macrolide n = 20, placebo n = 20) Age in years (mean ± SD (range)): macrolide: 35.2 ± 6.0 (26 to 45), placebo: 37.3 ± 5.7 (29 to 48) Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 3 days Total treatment dose: 3000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Stated that project is self funded | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiner and participant blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% and 5% of participants were lost to follow‐up in the macrolide and placebo groups, respectively. |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Pradeep 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 61 adults (macrolide n = 31, placebo n = 30) Age in years (range): 30 to 50 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis in smokers Type of macrolide: azithromycin Route: topical Dose per day: 0.5% gel Duration of treatment: 1 day Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge Micro Labs and Purac Biomaterials for providing samples of gel and antibiotics. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo gel not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No adverse events reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, but incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Rajaei 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 94 children and adults (macrolide n = 38, placebo n = 42, excluded n = 12) Age in years (mean ± SD): macrolide: 23.87 ± 4.99, placebo: 22.59 ± 5.06 Setting: secondary care |
|
| Interventions |
Indication: idiopathic preterm labor Type of macrolide: erythromycin Route: per oral Dose per day: 1600 mg Duration of treatment: 10 days Total treatment dose: 16,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No adverse events reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants had no follow‐up, and a further 3 stopped medication (9%). Reasons not given, unclear in which group. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, but incomplete reporting of adverse event. |
| Other bias | Low risk | None were identified. |
Reignier 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 48 adults and elderly (macrolide n = 25, placebo n = 23) Age in years (mean ± SD): macrolide: 70 ± 2, placebo: 66 ± 3 Setting: secondary care |
|
| Interventions |
Indication: enteral feeding in mechanically ventilated, critically ill individuals Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 1000 mg Duration of treatment: 5 days Total treatment dose: 5000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded. Death is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Robins‐Browne 1983.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 78 children (macrolide n = 39, placebo n = 39) Age in months: (mean): macrolide: 9.1, placebo: 7.4 Setting: secondary care |
|
| Interventions |
Indication: acute non‐specific gastroenteritis Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 40 mg/kg Duration of treatment: 5 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported (only at baseline) Death: data reported |
|
| Funding sources | Study supported by the South African Medical Research Council, the University of Natal, and Abbott Laboratories. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical drug containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Paediatricians, nurses, and children/parents blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar between groups. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Roca 2016a.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 829 adults (macrolide n = 414, placebo n = 415) Age in years (median (IQR)): macrolide: 26.0 (22.0 to 30.0), placebo: 25.0 (22.0 to 30.0) Setting: secondary care |
|
| Interventions |
Indication: bacterial carriage in mothers and their offspring Type of macrolide: azithromycin Route: per oral Dose per day: 2000 mg Duration of treatment: 1 day Total treatment dose: 2000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination Adverse events: incomplete reporting, author contacted Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Study supported by the UK Medical Research Council, the UK Department for International Development, and the EDCTP2 programme supported by the European Union. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mothers and clinicians blinded. Death and AMR objective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% and 4% dropouts in the macrolide and placebo groups, respectively. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome, standardised ascertainment, but incomplete reporting of adverse events (complete after author reply). |
| Other bias | Low risk | None were identified. |
Roy 1998.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 94 children (macrolide n = 46, placebo n = 48) Age in months (mean ± SD): macrolide: 43.5 ± 12.2, placebo: 43.6 ± 10.6 Setting: secondary care |
|
| Interventions |
Indication: cholera Type of macrolide: erythromycin Route: per oral Dose per day: 50 mg/kg Duration of treatment: 3 days Total treatment dose: 1560 mg (mean weight in macrolide group used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (lab tests) Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by the International Centre for Diarrhoeal Disease Research, Bangladesh | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Dropouts not reported. However, it seems like all participants are included in the final analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No adverse events reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, but no adverse events reported. |
| Other bias | Low risk | None were identified. |
Rozman 1984.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 282 participants (macrolide n = 146, placebo n = 136) Age in years: N/A Setting: unclear |
|
| Interventions |
Indication: acne Type of macrolide: erythromycin Route: topical Dose per day: 1% gel/cream twice a day Duration of treatment: 3 months Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7% dropout, unclear in which group. Reasons unclear |
| Selective reporting (reporting bias) | Low risk | Adverse events not stated as an outcome, unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Sadreddini 2009.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 108 adults and elderly (macrolide n = 54, placebo n = 54) Age in years (mean ± SD): macrolide: 55.71 ± 11.19, placebo: 52.73 ± 10.25 Setting: secondary care |
|
| Interventions |
Indication: knee effusion due to osteoarthritis Type of macrolide: erythromycin Route: per oral Dose per day: 800 mg Duration of treatment: 12 weeks Total treatment dose: 67,200 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% and 2% dropout in the macrolide and placebo groups, respectively. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome and unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Saiman 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 185 children and adults (macrolide n = 87, placebo n = 98) Age in years (mean ± SD): macrolide: 20.2 ± 7.9, placebo: 20.6 ± 8.6 Setting: secondary care |
|
| Interventions |
Indication: people with cystic fibrosis chronically infected with Pseudomonas aeroginosa Type of macrolide: azithromycin Route: per oral Dose per week: 1500 mg (maximum) Duration of treatment: 168 days Total treatment dose: 36,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by the Cystic Fibrosis Foundation. Authors acknowledge supplying company (Pfizer Pharmaceuticals). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active treatment and placebo supplied from the same company and packed identically. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% and 6% lost to follow‐up in the macrolide and placebo groups, respectively. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events stated as an outcome. Standardised ascertainment, adverse events reported. However, adverse events were only reported if at least 15% of participants in the macrolide group experienced the adverse event. |
| Other bias | Low risk | None were identified. |
Saiman 2010.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 263 children (macrolide n = 131, placebo n = 132) Age in years (mean ± SD): macrolide: 10.7 ± 3.25, placebo: 10.6 ± 3.10 Setting: secondary care |
|
| Interventions |
Indication: cystic fibrosis (uninfected with Pseudomonas aeruginosa) Type of macrolide: azithromycin Route: per oral Dose per week: 1500 mg (maximum) Duration of treatment: 168 days Total treatment dose: 36,000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study funded by CF Foundation Therapeutics Inc. Authors acknowledge supplying company (Pfizer). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups |
| Selective reporting (reporting bias) | High risk | Adverse events stated as an outcome, standardised ascertainment, and reporting of adverse events. However, adverse events were only reported on if at least 10% of participants in either of the groups experienced the adverse event. |
| Other bias | Low risk | None were identified. |
Sampaio 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): macrolide: 44.40 ± 7.42, placebo: 43.52 ± 5.90 Setting: dental care |
|
| Interventions |
Indication: chronic periodontitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 5 days Total treatment dose: 2500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Independent person did the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiners, participants, and biostatisticians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Sander 2002.
| Methods | Design: randomised, placebo‐controlled, 4‐armed trial | |
| Participants |
Number assigned: 272 adults and elderly (macrolide n = 136, placebo n = 136) Age in years (range): 61 to 69 Setting: secondary care |
|
| Interventions |
Indication: carotid atherosclerosis Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 30 days Total treatment dose: 9000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. | |
| Notes |
Concomitant medication: yes Note: within the 2 groups (macrolide versus placebo) Chlamydia pneumoniae positive and negative are presented as 1 group ‐ i.e. 2 arms instead of 4 arms. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and clinicians. Only report on objective outcome (death) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 5% dropout during the 4‐year follow‐up. All reported as deaths. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Schalen 1993.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 106 adults (macrolide n = 53, placebo n = 53) Age in years (mean): macrolide: 33.6, placebo: 38.3 Setting: secondary care |
|
| Interventions |
Indication: acute laryngitis Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 5 days Total treatment dose: 5000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by Abbott Scandinavia AB, Sweden. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. No relevant outcome reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% dropout, unclear which group. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, no reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Schwameis 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1371 adults (macrolide n = 685, placebo n = 686) Age in years (mean ± SD): macrolide: 44.2 ± 15.3, placebo: 43.7 ± 14.8 Setting: unclear |
|
| Interventions |
Indication: prevention of Lyme borreliosis in people bitten by European ticks Type of macrolide: azithromycin Route: topical Dose per day: N/A (10% gel twice per day) Duration of treatment: 3 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Ixodes AG. | |
| Notes |
Concomitant medication: unclear Note: trial stopped early as a futility analysis showed that the prespecified primary endpoint was not reached in the intention‐to‐treat population. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and trial staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adverse events reported for all allocated participants. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Seemungal 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 109 adults and elderly (macrolide n = 53, placebo n = 56) Age in years (mean ± SD): macrolide: 66.54 ± 8.10, placebo: 67.79 ± 9.08 Setting: secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: erythromycin stearate Route: per oral Dose per day: 500 mg Duration of treatment: 1 year Total treatment dose: 182,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Supported by the British Lung Foundation | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% and 18% dropout in the macrolide and placebo groups, respectively. However, reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Serisier 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 117 adults and elderly (macrolide n = 59, placebo n = 58) Age in years (mean ± SD): macrolide: 63.5 ± 9.5, placebo: 61.1 ± 10.5 Setting: secondary care |
|
| Interventions |
Indication: non‐cystic fibrosis bronchiectasis Type of macrolide: erythromycin ethylsuccinate Route: per oral Dose per day: 800 mg Duration of treatment: 336 days Total treatment dose: 268,800 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: clinical examination (laboratory tests, audiometry) Adverse events: data reported Antimicrobial resistance: not reported Death: reported that no deaths occurred |
|
| Funding sources | Study funded by Mater Adult Respiratory Research Trust Fund. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, trial supervisors, and all staff directly involved in participant care were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Adverse events resulting in discontinuation are reported. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, adverse events reported. |
| Other bias | Low risk | None were identified. |
Shafuddin 2015.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 191 adults and elderly (macrolide n = 97, placebo n = 94) Age in years (mean ± SD): macrolide: 67.6 ± 7.85, placebo: 66.7 ± 8.7 Setting: secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 84 days Total treatment dose: 25,200 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant diary + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Sanofi‐Aventis Australia Pty. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and active treatment supplied by same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Shanson 1985.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 109 adults and elderly (macrolide n = 56, placebo n = 53) Age in years (range): 18 to 78 Setting: dental care |
|
| Interventions |
Indication: prophylaxis of streptococcal bacteraemia after dental extraction Type of macrolide: erythromycin stearate Route: per oral Dose per day: 1500 mg Duration of treatment: 1 day Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant diary used Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by a grant from Abbott Laboratories | |
| Notes |
Concomitant medication: yes Note: randomised participants were also allocated alternatively for different measurement methods for adverse events (1 with leading questions about adverse events and 1 without). However, adverse events are reported as a total. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Coded envelopes were used with identical‐appearing content. Allocation done by nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainments, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Simpson 2008.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 46 adults and elderly (macrolide n = 23, placebo n = 23) Age in years (mean (range)): macrolide: 60 (27 to 80), placebo: 55 (27 to 77) Setting: secondary care |
|
| Interventions |
Indication: refractory asthma Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 8 weeks Total treatment dose: 56,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by the National Health and Medical Research Council of Australia | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. No adverse events reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in placebo group was withdrawn as the participant did not complete first week treatment. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment. However, incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Sinisalo 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 152 adults and elderly (macrolide n = 74, placebo n = 74, excluded n = 4) Age in years (mean ± SD): macrolide: 64 ± 10, placebo: 63 ± 11 Setting: secondary care |
|
| Interventions |
Indication: unstable angina or non‐Q‐wave myocardial infarction Type of macrolide: clarithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 85 days Total treatment dose: 42,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (ECG, lab tests) Adverse events reported: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by the Aarno Koskelo Foundation and the Finnish Foundation for Cardiovascular Research. Authors acknowledge Abbott Laboratories for supplying trial medication. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. Reasons for dropouts given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome, however standardised ascertainment and reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Sirinavin 2003.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 191 children and adults (macrolide n = 95, placebo n = 96) Age in years (mean (range)): macrolide: 25 (15 to 55), placebo: 22 (15 to 48) Setting: 4 food factories in Thailand |
|
| Interventions |
Indication: eradication of non‐typhoidal Salmonella Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 5 days Total treatment dose: 2500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination (swabs) Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Supported by Bureau of General Communicable Diseases, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if matching placebo used. Two placebo groups in lieu of 2 different antibiotic regimens (azithromycin, norfloxacin) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participant and trial investigators were blinded for assessment of adverse events. Data on AMR should be considered an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% of participants missed more than 1 follow‐up visit, however reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Smith 2000.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 150 adults and elderly (macrolide n = 75, placebo n = 75) Age in years (mean ± SD): macrolide: 63.2 ± 12.6, placebo: 61.4 ± 11.7 Setting: secondary care |
|
| Interventions |
Indication: postoperative ileus after colorectal surgery Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 800 mg Duration of treatment: 5 days (maximum) Total treatment dose: 4000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (ECG) Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Smith 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 46 adults (macrolide n = 23, placebo n = 21, excluded n = 2) Age in years (mean ± SD): macrolide: 41.87 ± 7.09, placebo: 43.57 ± 10.22 Setting: dental care |
|
| Interventions |
Indication: periodontitis Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (lab tests) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: incomplete reporting, author contacted Death: not reported |
|
| Funding sources | Study supported by Pfizer Ltd Sandwich. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% dropout, reasons given |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, however incomplete reporting of adverse events, including AMR. |
| Other bias | Low risk | None were identified. |
Sorensen 1992.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 432 children and adults (macrolide n = 216, placebo n = 216) Age in years (median (range)): macrolide: 28 (14 to 46), placebo: 27 (14 to 46) Setting: secondary care |
|
| Interventions |
Indication: prevention of postabortal pelvic inflammatory disease Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7.5 days Total treatment dose: 7500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, however no contact details for author Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. Abbott supplied treatments. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active treatment and placebo supplied by the same company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Taylor 1999.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 225 adults (macrolide n = 148, placebo n = 77) Age in years (median (range)): macrolide: 27 (18 to 52), placebo: 26 (20 to 50) Setting: army soldiers and civilians in Indonesia |
|
| Interventions |
Indication: malaria prophylaxis Type of macrolide: azithromycin Route: per oral Dose per day: loading dose on day 1 of 750 mg, then 250 mg per day Duration of treatment: 141 days Total treatment dose: 35,750 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked + clinical examination (lab tests) Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by the US Army Medical Materiel Development Activity and the US Naval Medical Research and Development Command. Authors acknowledge supplying company (Pfizer Central Research). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical drug containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and trial staff blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21% and 17% dropout in the macrolide and placebo groups, respectively. Reasons (including withdrawal due to adverse events) given. However, unclear how many people adverse events data were based on, and numbers change throughout the reporting. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome, however standardised ascertainment. Incomplete reporting of adverse events. |
| Other bias | Unclear risk | 2:1 allocation to macrolide and placebo group. |
Tita 2016.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 2013 adults (macrolide n = 1019, placebo n = 994) Age in years (mean ± SD): macrolide: 28.2 ± 6.1, placebo: 28.4 ± 6.5 Setting: secondary care |
|
| Interventions |
Indication: non‐elective Caesarean delivery Type of macrolide: azithromycin Route: intravenous Dose per day: 500 mg Duration of treatment: 1 hour Total treatment dose: 500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: medical records review and participant asked Adverse events: data reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo saline |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts for reporting of adverse events |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events are reported. |
| Other bias | Low risk | None were identified. |
Uzun 2014.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 92 adults and elderly (macrolide n = 47, placebo n = 45) Age in years (mean ± SD): macrolide: 64.7 ± 10.2, placebo: 64.9 ± 10.2 Setting: secondary care |
|
| Interventions |
Indication: chronic obstructive pulmonary disease Type of macrolide: azithromycin Route: per oral Dose: 500 mg 3 times a week Duration of treatment: 52 weeks Total treatment dose: 78,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination (lab tests, swabs) Adverse events: data reported Antimicrobial resistance: data reported Death: data reported |
|
| Funding sources | Supported by SoLong Trust | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and trial staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% and 16% withdrew in the macrolide and placebo groups, respectively. However, reasons given, including adverse events. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Vainas 2005.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 509 adults and elderly (macrolide n = 257, placebo n = 252) Age in years (mean ± SD): macrolide: 64.4 ± 9.9, placebo: 65.5 ± 9.7 Setting: secondary care |
|
| Interventions |
Indication: peripheral arterial disease Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 3 days Total treatment dose: 1500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary used Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by the Netherlands Heart Foundation | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, attending surgeons, and the co‐ordinating scientist blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% and 95% completed treatments in the macrolide and placebo groups, respectively. Reasons for dropouts given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Valery 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 89 children (macrolide n = 45, placebo n = 44) Age in years (mean ± SD): macrolide: 3.99 ± 2.14, placebo: 4.22 ± 2.30 Setting: community clinics in central and northern Australia, and urban Maori and Pacific Island children from a tertiary paediatric hospital in Auckland, New Zealand |
|
| Interventions |
Indication: bronchiectasis Type of macrolide: azithromycin Route: per oral Dose: 30 mg/kg (max 600 mg) once weekly Duration of treatment: 24 months (maximum) Total treatment dose: 62,400 (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked + clinical examination (swabs) Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Supported by the National Health and Medical Research Council of Australia and Health Research Council, New Zealand | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, double‐sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, families, health professionals, and study personnel blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% and 21% dropouts in the macrolide and placebo groups, respectively. However, reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Vammen 2001.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 92 elderly (macrolide n = 43, placebo n = 49) Age in years (mean ± SD): macrolide: 72 ± 3.7, placebo: 73 ± 3.7 Setting: secondary care |
|
| Interventions |
Indication: abdominal aortic aneurysms Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: annual 4 weeks' treatment. Followed/treated annually for a mean of 5.27 years Total treatment dose: 44,268 mg (mean follow‐up used) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: unclear Adverse events: stated that no participants stopped their medication due to side effects and that no adverse events were observed Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Supported by the Danish Heart Foundation, the Foundation of Asta and Rosa Jensen, and the Health Department of Viborg County. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Alocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout. Stated that no participants stopped their medication due to side effects. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome. Unclear ascertainment, but reported that no adverse events were observed. |
| Other bias | Low risk | None were identified. |
Van Delden 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 92 adults and elderly (macrolide n = 47, placebo n = 45) Age in years (mean ± SD): macrolide: 59.3 ± 16.98, placebo: 59.7 ± 15.18 Setting: secondary care |
|
| Interventions |
Indication: prevention of Pseudomonas aeruginosa ventilator‐associated pneumonia Type of macrolide: azithromycin Route: intravenous Dose per day: 300 mg Duration of treatment: 20 days (maximum) Total treatment dose: 6000 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: spontaneously Adverse events: data reported Antimicrobial resistance: stated that azithromycin did lead to an increase in minimum inhibitory concentration when comparing initial and last P aeruginosa isolate. Death: data reported |
|
| Funding sources | Study supported by Anbics Corporation, the Swiss Ministry of Technolog, and the Swiss National Science Foundation. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo (saline). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator, staff, participants, and monitor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, similar across groups. |
| Selective reporting (reporting bias) | Low risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Van den Broek 2009.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 2297 children and adults (macrolide n = 1149, placebo n = 1148) Age in years (mean ± SD): azithromycin: 22.8 ± 5.1, placebo: 23.0 ± 5.2 Setting: 3 rural and 1 peri‐urban antenatal clinic in southern Malawi |
|
| Interventions |
Indication: preterm birth Type of macrolide: azithromycin Route: per oral Dose: 1000 mg given 1 time between 16 to 24 weeks and 1 time between 28 to 32 weeks Duration of treatment: N/A Total treatment dose: 2000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Reporting of adverse events: yes Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study funded by Wellcome Trust. Authors acknowledge supplying company (Pfizer) and state that Pfizer had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo not described in detail, however drug and placebo were supplied by the same pharmaceutical company. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants, study midwives, and trial statistician |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar dropouts across groups. Unclear reasons for loss to follow‐up: "Missed visit, could not be traced, declined to continue and did not attend". Possibly missed reporting on some adverse events as discontinuation due to adverse events was not reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. However, adverse events reported. |
| Other bias | Low risk | None were identified. |
Veskitkul 2017.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 children (macrolide n = 20, placebo n = 20) Age in years (median (range)): macrolide: 5.8 (5.0 to 9.2), placebo: 5.9 (5.0 to 12.3) Setting: secondary care |
|
| Interventions |
Indication: recurrent acute rhinosinusitis Type of macrolide: azithromycin Route: per oral Dose per day: 5 mg/kg/day for 3 days/week Duration of treatment: 12 months Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Ascertainment of adverse events: participants/parents asked Adverse event: stated that "adverse events were not reported in either group" Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by a Siriraj Grant for Research Development from the Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described in detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Low risk | Unclear if adverse events were stated as an outcome. However, standardised ascertainment and reported on (no) adverse events. |
| Other bias | Low risk | None were identified. |
Videler 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 adults and elderly (macrolide n = 29, placebo n = 31) Age in years (median (range)): macrolide: 49 (20 to 70), placebo: 49 (20 to 70) Setting: secondary care |
|
| Interventions |
Indication: chronic rhinosinusitis Type of macrolide: azithromycin Route: per oral Dose: 500 mg once a day for 3 days for the first week, then once a week for 11 weeks Duration of treatment: 12 weeks Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked + clinical examination (swabs) Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | None stated. Authors acknowledge Pliva Hrvatska d.o.o., Zagreb, Croatia for supplying treatments. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% and 10% dropout at follow‐up 2 weeks after treatment finished in the macrolide and placebo groups, respectively. However, reasons given. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly stated as an outcome. Standardised ascertainment and adverse events reported. However, lab tests for liver function were performed but the results were not provided. |
| Other bias | Low risk | None were identified. |
Vos 2011.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 83 adults (macrolide n = 40, placebo n = 43) Age in years (median (range)): macrolide: 56.1 (47.7 to 61.2), placebo: 55.1 (44.2 to 59.4) Setting: secondary care |
|
| Interventions |
Indication: prevention of bronchiolitis obliterans syndrome post‐lung transplantation Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg daily for 5 days, followed by 250 mg 3 times a week for 2 years Duration of treatment: 2 years Total treatment dose: 79,250 mg (maximum) |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70% and 41.9% completed 2 years' treatment in the macrolide and placebo groups, respectively. However, reasons given for discontinuation/entering open‐label treatment. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. Standardised ascertainment, and adverse events reported. |
| Other bias | Low risk | None were identified. |
Wallwork 2006.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 64 participants (macrolide n = 29, placebo n = 35) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: chronic rhinosinusitis Type of macrolide: roxithromycin Route: per oral Dose per day: 150 mg Duration of treatment: 3 months Total treatment dose: 13,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: clinical examination (swabs) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: authors state that "no macrolide‐resistant organisms were noted to develop". Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% and 9% withdrew in the macrolide and placebo groups, respectively. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Unclear ascertainment, only swabs mentioned. Reported solely on adverse events leading to discontinuation. |
| Other bias | Low risk | None were identified. |
Walsh 1998.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 1985 adults (macrolide n = 996, placebo n = 989) Age in years (mean ± SD): macrolide: 30.4 ± 6.3, placebo: 30.5 ± 6.5 Setting: 11 clinics in Los Angeles County, USA. Clinics represented several provider types. |
|
| Interventions |
Indication: intrauterine device insertion Type of macrolide: azithromycin Route: per oral Dose per day: 500 mg Duration of treatment: 1 day Total treatment dose: 500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: data reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Supported by the National Institute of Child Health and Human Development, National Institutes of Health | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, identical, opaque, sealed pill bottles |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians, research personnel, and participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% lost to follow‐up in both groups. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Wang 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 45 adults and elderly (macrolide n = 23, placebo n = 22) Age in years (mean (range)): macrolide: 60 (27 to 80), placebo: 55 (27 to 80) Setting: secondary care |
|
| Interventions |
Indication: non‐eosinophilic refractory asthma Type of macrolide: clarithromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 56 days Total treatment dose: 56,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participants asked Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant dropped out, reason unclear. |
| Selective reporting (reporting bias) | High risk | Adverse events not clearly stated as an outcome. Standardised ascertainment. However, no reporting about adverse events. |
| Other bias | Low risk | None were identified. |
Wiesli 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 40 adults and elderly (macrolide n = 20, placebo n = 20) Age in years (mean ± SD): macrolide: 72.4 ± 7.7, placebo: 70.3 ± 9.1 Setting: secondary care |
|
| Interventions |
Indication: peripheral arterial occlusive disease in Chlamydia pneumoniae seropositive men Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 28 days Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported |
|
| Funding sources | Study supported by Aventis Pharma AG, Switzerland and the Lixmar foundation, Switzerland | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians and participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not stated as an outcome and unclear ascertainment. However, adverse events are reported. |
| Other bias | Low risk | None were identified. |
Wilson 1977.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 51 adults (macrolide n = 26, placebo n = 25) Age in years: N/A Setting: healthy volunteers at the Baylor College of Medicine |
|
| Interventions |
Indication: nasal carriage of Staphylococcus aureus Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by the EI duPont de Nemours and Company and the National Institute of Allergy and Infectious Diseases. | |
| Notes |
Concomitant medication: unclear Note: a third group of people were treated with josamycin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo not identical appearing, orange vs pink tablet. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who was blinded. Data on AMR assessed as an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 4 dropouts in the 3 arms before medication was given, unclear in which groups |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Wilson 1979.
| Methods | Design: randomised, placebo‐controlled, 3‐armed trial | |
| Participants |
Number assigned: 57 adults (macrolide n = 27, placebo n = 30) Age in years (range): 18 to 43 Setting: healthy volunteers at the Baylor College of Medicine |
|
| Interventions |
Indication: nasal carriage of Staphylococcus aureus Type of macrolide: erythromycin Route: per oral Dose per day: 1000 mg Duration of treatment: 7 days Total treatment dose: 7000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant diary + clinical examination/lab tests Adverse events: data reported Antimicrobial resistance: data reported Death: not reported |
|
| Funding sources | Study supported by Schering Laboratories, The Council for Tobacco Research, and the National Institutes of Health. | |
| Notes |
Concomitant medication: unclear Note: a third group of people were treated with rosaramicin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear if placebo was identical appearing. Authors state only that the placebo was identical in appearance to the rosaramicin capsules (the third arm). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who was blinded. Data on AMR assessed as an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% and 3% dropout in the macrolide and placebo groups, respectively. Reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Winkler 1988.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 43 pregnant women (macrolide n = 20, placebo n = 23) Age in years: N/A Setting: secondary care |
|
| Interventions |
Indication: preterm delivery Type of macrolide: erythromycin Route: per oral Dose per day: 1200 mg Duration of treatment: 7 days Total treatment dose: 8400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: not reported Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo was identical appearing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and adverse events not reported. |
| Other bias | Low risk | None were identified. |
Wolter 2002.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 60 adults (macrolide n = 30, placebo n = 30) Age in years (mean (range)): 27.9 (18 to 44) Setting: secondary care |
|
| Interventions |
Indication: cystic fibrosis Type of macrolide: azithromycin Route: per oral Dose per day: 250 mg Duration of treatment: 90 days Total treatment dose: 22,500 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | Study supported by the John P Kelly Mater Research Foundation and the Mater Hospital Private Practice Fund. Authors thank supplying company (Pfizer). | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomised by independent pharmacy staff, and participants were automatically dispensed the next allocated treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants, clinicians, and statistician. No relevant outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants (25%) and 9 participants (30%) did not complete the treatment in the macrolide and placebo groups, respectively. However, adverse events are reported for 3 participants, while the remainder dropped out due to non‐compliance or personal request. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment, and incomplete reporting of adverse events. |
| Other bias | Unclear risk | The placebo group contained more men, and they were also taller, heavier, and had a better lung function. |
Wong 2012.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 141 adults and elderly (macrolide n = 71, placebo n = 70) Age in years (mean ± SD): macrolide: 60.9 ± 13.6, placebo: 59.0 ± 13.3 Setting: secondary care |
|
| Interventions |
Indication: non‐cystic fibrosis bronchiectasis Type of macrolide: azithromycin Route: per oral Dose: 500 mg 3 times a week Duration of treatment: 6 months Total treatment dose: 39,000 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: reported on participants diagnosed with macrolide‐resistant Streptococcus pneumoniae following macrolide treatment Death: not reported |
|
| Funding sources | Study funded by the Health Research Council of New Zealand and the Auckland District Health Board Charitable Trust. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, clinicians, and investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% in macrolide group versus 10% in placebo group withdrew. However, reasons for dropout are clearly presented. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events stated as an outcome, standardised ascertainment, and adverse events reported. Note that only adverse events with an incidence of more than 2.5% in either group were presented. |
| Other bias | Low risk | None were identified. |
Yang 2013.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 180 children, adults, and elderly (macrolide n = 89, placebo n = 91) Age in years (mean (range)): 41 (9 to 87) Setting: secondary care |
|
| Interventions |
Indication: bacterial conjunctivitis Type of macrolide: azithromycin Route: topical Dose: a 1% drop of gel twice a day for 2 days, then 1 drop once a day for the next 3 to 7 days Duration of treatment: 7 days Total treatment dose: N/A |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: yes Adverse events ascertainment: participants asked + clinical examination (swabs) Adverse events: incomplete reporting, author contacted Antimicrobial resistance: not reported Death: not reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. No outcomes reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events stated as an outcome, standardised ascertainment. However, incomplete reporting of adverse events. |
| Other bias | Low risk | None were identified. |
Yeo 1993.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 128 adults and elderly (macrolide n = 58, placebo n = 60) Age in years (mean ± SD): macrolide: 65.6 ± 1.6, placebo: 63.7 ± 1.4 Setting: secondary care |
|
| Interventions |
Indication: gastric emptying after pancreaticoduodenectomy Type of macrolide: erythromycin lactobionate Route: intravenous Dose per day: 800 mg Duration of treatment: 8 days Total treatment dose: 6400 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: unclear Adverse events ascertainment: participant asked Adverse events: data reported Antimicrobial resistance: not reported Death: no deaths reported |
|
| Funding sources | None stated. | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nursing staff, physicians, and participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants (8%) excluded from analysis, unclear which group. However, reasons given. |
| Selective reporting (reporting bias) | Low risk | Adverse events not clearly stated as an outcome. However, standardised ascertainment and adverse events reported. |
| Other bias | Low risk | None were identified. |
Zahn 2003.
| Methods | Design: randomised, placebo‐controlled, parallel‐group trial | |
| Participants |
Number assigned: 872 adults and elderly (macrolide n = 433, placebo n = 439) Age in years (mean (IQR)): macrolide: 60.4 (51.3 to 69.1), placebo: 61.0 (52.2 to 68.6) Setting: secondary care |
|
| Interventions |
Indication: acute myocardial infarction Type of macrolide: roxithromycin Route: per oral Dose per day: 300 mg Duration of treatment: 42 days Total treatment dose: 12,600 mg |
|
| Outcomes |
Adverse events stated as an outcome in trial registration/protocol/paper: no Adverse events ascertainment: unclear Adverse events: data reported Antimicrobial resistance: not reported Death: data reported (death is reported as a primary outcome) |
|
| Funding sources | Supported by Aventis Pharma GmbH | |
| Notes | Concomitant medication: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18% and 11% dropouts in the macrolide and placebo groups, respectively. Reasons given. |
| Selective reporting (reporting bias) | High risk | Adverse events not stated as an outcome, unclear ascertainment. Only adverse events resulting in discontinuation were reported. |
| Other bias | Low risk | None were identified. |
AMR: antimicrobial resistance ECG: electrocardiogram IQR: interquartile range N/A: not applicable PCI: percutaneous coronary intervention PPROM: preterm pre‐labour rupture of membrane SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboud 2009 | Participants in treatment group were randomised to receive both a macrolide (erythromycin) and metronidazole. |
| Ballard 2007 | Too‐small sample size. 19 infants were allocated to macrolide treatment and 16 infants were allocated to placebo. |
| Batieha 2002 | Quasi‐randomised trial. Participants were allocated by alternate assignment to either macrolide or placebo group. |
| Doan 2017 | Only report on pharmacodynamic outcomes (microbiome) |
| Ferahbas 2004 | Cross‐over trial. Adverse events were only reported after cross‐over. |
| Figueiredo‐Mello 2018 | Participants in the intervention group were allocated to 1 of 2 types of macrolides (clarithromycin or azithromycin). However, it was not possible to identify those participants treated with clarithromycin and those treated with azithromycin. |
| Gong 2014 | Too‐small sample size. Only 17 participants were allocated in each arm. |
| Makkar 2016 | Not possible to extract data on participants only treated with placebo. Participants allocated to placebo also received erythromycin if feed failure. |
| Nielsen 2016 | Too‐small sample size. Only 12 participants were allocated in each arm. |
| Parker 2017 | Only report on pharmacodynamic outcomes (microbiome) |
| Pazoki‐Toroudi 2010 | Not placebo controlled. Participants allocated to topical macrolide gel were treated for 12 weeks, while participants allocated to topical placebo gel were treated for 4 weeks. |
| Rasi 2008 | Not placebo controlled. Participants allocated to macrolides were treated with tablets, while participants allocated to placebo were treated with an emollient cream. |
| Sharma 2000 | Quasi‐randomised trial. Participants were allocated by alternate assignment to either macrolide treatment or placebo. |
| Stokholm 2016 | Asthma‐like episodes, not participants, randomised to either macrolide treatment or placebo. |
| Weber 1993 | Not placebo controlled. Participants allocated to macrolides were treated with a cream, while participants allocated to placebo were treated with tablets. |
| Yamamoto 1992 | Participants were not randomly assigned to treatment or placebo group. |
| Zhang 2006 | Quasi‐randomised trial. Participants were allocated by alternate assignment to either macrolide treatment or placebo. |
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12617000531314.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with chronic periodontitis |
| Interventions |
Arm 1: azithromycin (+ non‐surgical periodontal scaling and root planing + use of mouthwashes) Arm 2: placebo (+ non‐surgical periodontal scaling and root planing + use of mouthwash) |
| Outcomes | Adverse events, antimicrobial resistance, and death |
| Notes |
ChiCTR‐INR‐17013272.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Women having Caesarean section |
| Interventions |
Arm 1: azithromycin (+ usual antibiotic regimen = cefuroxime) Arm 2: placebo (+ usual antibiotic regimen = cefuroxime) |
| Outcomes | Adverse events, antimicrobial resistance, and death |
| Notes |
ChiCTR‐IOR‐16008820.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with chronic obstructive pulmonary disease |
| Interventions |
Arm 1: erythromycin Arm 2: placebo |
| Outcomes | Adverse events, antimicrobial resistance, and death |
| Notes |
CTRI/2017/07/009017.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children with acute diarrhoea |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
Dicko 2016.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | African children |
| Interventions |
Arm 1: azithromycin (+ usual malaria prevention = sulfadoxine/pyrimethamine + amodiaquine) Arm 2: placebo (+ usual malaria prevention = sulfadoxine/pyrimethamine + amodiaquine) |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
EUCTR2011‐004351‐39‐IT.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adolescents and adults with primary immunodeficiency and chronic obstructive pulmonary disease |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
EUCTR2012‐002792‐34‐GB.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults and elderly with bronchiectasis |
| Interventions |
Arm 1: erythromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
EUCTR2015‐004306‐42‐SI.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with chronic periodontitis |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
Gregersen 2017.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults and elderly with multiple myeloma |
| Interventions |
Arm 1: clarithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes | Extended abstract identified. However, we could not identify a peer‐reviewed publication of this study. |
IRCT2015052322383N1.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults residing in endemic area of leptospirosis and working in the paddy field |
| Interventions |
Arm 1: azithromycin Arm 2: doxycycline Arm 3: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
KCT0002373.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Ureaplasma‐positive preterm infants |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
Milito 2017.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children and adults with primary antibody deficiency and chronic obstructive pulmonary disease with recurrent exacerbations |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT01270074.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children with cystic fibrosis |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT01778634.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Preterm infants with indwelling intravenous line for drug administration |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes | Study results posted on ClinicalTrials.gov in May 2018. However, we could not identify a peer‐reviewed publication of this study. |
NCT02003911.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children hospitalised with acute asthma exacerbations |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02307825.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with chronic rhinosinusitis |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02336516.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children diagnosed with postdiarrhoeal haemolytic and uraemic syndrome |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02677701.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children and adults with cystic fibrosis and chronic airway infection with Pseudomonas aeruginosa |
| Interventions |
Arm 1: azithromycin + tobramycin Arm 2: placebo + tobramycin |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02756403.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Women having a first trimester abortion |
| Interventions |
Arm 1: azithromycin Arm 2: doxycycline Arm 3: metronidazole Arm 4: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02911935.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children hospitalised with respiratory syncytial virus bronchiolitis |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT02960503.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with sickle cell disease |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT03130114.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children with severe diarrhoea |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT03233880.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Healthy primigravidae: prevention of pre‐eclampsia |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT03248297.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | High‐risk labouring women in low‐income countries |
| Interventions |
Arm 1: azithromycin Arm 2: azithromycin + amoxicillin Arm 3: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT03341273.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with a suspected lower respiratory tract infection |
| Interventions |
Arm 1: azithromycin (+ procalcitonin test) Arm 2: placebo (+ procalcitonin test) |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
NCT03345992.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with sepsis and respiratory and multiple organ dysfunction syndrome |
| Interventions |
Arm 1: clarithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
Ramsey 2017.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Children with cystic fibrosis with early Pseudomonas aeruginosa |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
RBR‐9pqqpb.
| Methods | Randomised, placebo‐controlled clinical trial |
| Participants | Adults with eosinophilic nasosinusinal polyposis |
| Interventions |
Arm 1: azithromycin Arm 2: placebo |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Chang 2012.
| Trial name or title | A randomised, double‐blind, placebo‐controlled trial of azithromycin versus amoxicillin‐clavulanic acid to treat mild to moderate respiratory exacerbations in children with non‐cystic fibrosis bronchiectasis, study one |
| Methods | Randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group trial |
| Participants | Children aged less than 18 years, diagnosed with non‐cystic fibrosis bronchiectasis |
| Interventions | Arm 1: oral azithromycin 5 mg/kg x 1 for 14 days Arm 2: oral amoxicillin‐clavulanic acid 22.5 mg/kg x 2 for 14 days Arm 3: oral placebo for 14 days |
| Outcomes | Adverse events including data on antimicrobial resistance |
| Starting date | 15 March 2012 |
| Contact information | annechang@ausdoctors.net |
| Notes |
Author reply in April 2018: Dr Anne Chang reports that the trial has completed recruitment and data are being analysed. No publication yet. Trial registration: Australia and New Zealand Clinical Trials Register ACTRN12612000011886 |
Gonzalez‐Martinez 2017.
| Trial name or title | Azithromycin versus placebo for the treatment of HIV‐associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Children and adolescents aged 6 to 19 years, diagnosed with HIV‐associated chronic lung disease |
| Interventions | Arm 1: oral azithromycin (10 to 19.9 kg, 250 mg; 20 to 29.9 kg, 500 mg; 30 to 39.9 kg, 750 mg; > 40 kg, 1250 mg) once a week for 12 months Arm 2: oral placebo for 12 months |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Starting date | June 2016 |
| Contact information | rashida.ferrand@lshtm.ac.uk |
| Notes |
Author reply in June 2018: Dr Rashida Ferrand reports that the trial will be completed shortly and that they plan to publish the results in 2019. Trial registration: ClinicalTrials.gov NCT02426112 |
Kobbernagel 2016.
| Trial name or title | Randomised controlled trial to determine the efficacy and safety of azithromycin maintenance for 6 months in participants with primary ciliary dyskinesia ‐ a double‐blind, parallel‐group study |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Children and adults aged 7 to 50 years, diagnosed with primary ciliary dyskinesia |
| Interventions | Arm 1: oral azithromycin 250 mg/500 mg (according to body weight) x 1, 3 times a week for 6 months Arm 2: oral placebo for 6 months |
| Outcomes | Adverse events including data on antimicrobial resistance |
| Starting date | 26 August 2014 |
| Contact information | helene_kobber@hotmail.com |
| Notes |
Author reply in April 2018: Dr Helene Kobbernagel reports that the trial has completed recruitment and data are being analysed. No publication yet. Trial registration: EU Clinical Trials Register EudraCT 2013‐004664‐58 |
Mosquera 2016.
| Trial name or title | The anti‐inflammatory effect of prophylactic macrolides on children with chronic lung disease |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Children aged 6 months to 6 years with chronic lung disease secondary to bronchopulmonary dysplasia |
| Interventions | Arm 1: oral azithromycin 5 mg/kg x 1, 3 times a week for 3 to 6 months Arm 2: oral placebo for 3 to 6 months |
| Outcomes | Adverse events |
| Starting date | October 2015 |
| Contact information | Richardo.A.Mosquera@uth.tmc.edu |
| Notes |
Author reply in April 2018: Dr Richardo Mosquera reports that the trial has completed recruitment and data are being analysed. No publication yet. Trial registration: ClinicalTrials.gov NCT02544984 |
Pavlinac 2017.
| Trial name or title | Azithromycin to prevent post‐discharge morbidity and mortality in Kenyan children: a protocol for a randomised, double‐blind, placebo‐controlled trial (the Toto Bora trial) |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Children aged 1 to 59 months discharged from hospitals |
| Interventions | Arm 1: oral azithromycin, 10 mg/kg on day 1, followed by 5 mg/kg for days 2 to 5 Arm 2: oral placebo for 5 days |
| Outcomes | Adverse events including data on antimicrobial resistance and death |
| Starting date | 28 June 2016 |
| Contact information | ppav@uw.edu |
| Notes |
Author reply in June 2018: Dr Patricia Pavlinac reports that they are still recruiting patients and anticipate publishing results in late 2019/late 2020. Trial registration: ClinicalTrials.gov NCT02414399 |
Vermeersch 2016.
| Trial name or title | Belgian trial with azithromycin during acute COPD exacerbations |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Adults aged 18 years or older hospitalised for an acute exacerbation in chronic obstructive pulmonary disease (COPD) |
| Interventions | Arm 1: oral azithromycin: 500 mg x 1 for 3 days, followed by 250 mg once every 2 days for the remainder of the 90‐day treatment period Arm 2: oral placebo for 90 days |
| Outcomes | Adverse events including data on deaths |
| Starting date | 1 August 2014 |
| Contact information | wim.janssens@uzleuven.be |
| Notes |
Author reply in April 2018: Dr Wim Janssens reports that the trial has completed recruitment and data are being analysed. No publication yet. Trial registration: ClinicalTrials.gov NCT02135354 |
Differences between protocol and review
The review differs from the protocol, Hansen 2015, in the following ways.
Objectives and Types of outcome measures: while conducting this review we realised that it would be most appropriate to present each of the specific reported adverse events separately. Consequently, instead of handling the adverse events as adverse effects, adverse reactions, and serious adverse events, as stated in the protocol, we have presented each of the adverse events separately. We have reported on adverse events that occurred in ≥ 5% in any of the groups (macrolide or placebo) (Zarin 2016). However, all reported adverse events are available: adverse events by System Organ Classes: threshold ≥ 5%, Hansen 2018a, and adverse events by System Organ Classes < 5%, Hansen 2018b.
Trial authors very seldom referred to a specific definition of how they classified severe adverse events, and consequently we did not find it appropriate to report these as a composite outcome labelled 'severe adverse events'. However, every single adverse event reported in all of the included studies, regardless of how it was labelled by the trial authors, was extracted, and data are available (Hansen 2018a; Hansen 2018b).
'Subsequent carriage of resistant bacteria' has been refined to 'subsequent carriage of macrolide‐resistant bacteria'.
Types of studies: we clarified that we included trials with more than two intervention arms, if it was possible to identify a macrolide arm and a placebo arm. After the protocol was published, we decided to exclude purely pharmacodynamic and pharmacokinetic studies, unless they also reported clinical parameters. We also excluded studies with fewer than 20 participants randomised to each arm. We made these decisions after starting the title and abstract screening, when we realised that many of these small pharmacodynamic or pharmacokinetic studies posed a high risk of reporting drug‐drug interactions of macrolides or non‐macrolide‐related adverse events.
Searching other resources and Dealing with missing data: in the protocol we stated that we would contact authors of trials if adverse events data were not published. However, as this evolved into an unexpectedly large review with generally very poor reporting of adverse events, we contacted only trial authors if adverse events were incompletely reported and an e‐mail address was available in the publication.
Data collection and analysis: we stated in the protocol that MPH and ST would assess all studies identified by the searches, extract data, and assess risk of bias for each of the included studies. However, the size of the review necessitated involvement of additional authors. ST participated in the process of selecting studies, while both AMcC and AMS participated in the selection of studies, data extraction, and 'Risk of bias' assessments. Uniform data collection was ensured by the participation of MPH at all stages and by having CDM as the third review author in resolving any discrepancies.
Measures of treatment effect: in the protocol we planned to express all outcomes as Peto odds ratios (OR) as we expected that the included trials would report on few adverse events. However, Peto OR mandates fixed‐effect models, which would not be appropriate to apply to our data as several sources of heterogeneity that might undermine the use of a fixed‐effect approach exist in this review.
Unit of analysis issues: we deviated from the protocol by including both participants and bacterial isolates as units of analysis when reporting subsequent carriage of macrolide‐resistant bacteria.
Data synthesis: as trial authors used a wide range of terms when reporting adverse events, we categorised the reported adverse events using a clinically validated, standardised medical classification system, the Medical Dictionary for Regulatory Activities (MedDRA). We added a section describing the classification system to the review and how we analysed adverse events. To deal with an enormous long tail of (mostly irrelevant) adverse events described in tiny numbers, we decided that we would undertake a meta‐analysis when ≥ 3 studies reported a specific adverse event.
Subgroup analysis and investigation of heterogeneity: as in the case of meta‐analyses of the primary outcomes, at least three studies were required for subgroup analyses.
Contributions of authors
Malene Plejdrup Hansen (MPH) contributed to the selection of studies, data extraction, 'Risk of bias' assessment, data analysis, and was responsible for drafting the review.
Anna M Scott (AMS) contributed to the selection of studies, data extraction, 'Risk of bias' assessment, data analysis, and the drafting of the review.
Amanda McCullough (AMcC) contributed to the selection of studies, data extraction, 'Risk of bias' assessment, and contributed to the final version of the review.
Sarah Thorning (ST) and Justin Clark (JC) performed the searches. ST contributed to the selection of studies, and both ST and JC contributed to the final version of the review.
Jeffrey K Aronson (JKA) provided methodological expertise on dealing with adverse events, and contributed to the final version of the review.
Elaine M Beller (EMB) provided statistical expertise and contributed to the final version of the review.
Paul P Glasziou (PG) and Tammy C Hoffmann (TH) contributed to the final version of the review.
Chris B Del Mar (CDM) conceived the original idea for this review. CDM resolved disagreements at any stage in the review process and contributed to the writing of the review.
Sources of support
Internal sources
Bond University, Gold Coast, Australia.
Copenhagen University, Copenhagen, Denmark.
Aalborg University, Aalborg, Denmark.
External sources
National Health and Medical Research Council (1044904), Australia.
Cochrane Review Support Programme, UK.
Declarations of interest
Malene Plejdrup Hansen: senior research fellow at the Research Unit for General Practice in Aalborg funded by the Research Foundation of General Practice in Denmark. From 2014 to 2016 she was a postdoctoral fellow at the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections (CREMARA) funded by the National Health and Medical Research Council (NHMRC), Australia (1044904).
Anna M Scott: senior research fellow at the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections (CREMARA) funded by the National Health and Medical Research Council (NHMRC), Australia (1044904).
Amanda McCullough: postdoctoral fellow at the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections (CREMARA) funded by the National Health and Medical Research Council (NHMRC), Australia (1044904).
Sarah Thorning: none known.
Jeffrey K Aronson: is a President Emeritus of the British Pharmacological Society and a member of the Advisory Board of the British National Formulary; was until recently a member of a Technology Appraisal Committee of the UK’s National Institute for Health and Care Excellence (NICE); and is editor of textbooks on adverse drug reactions, including Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. He has published in peer‐reviewed journals on different aspects of adverse drug reactions.
Elaine M Beller: co‐investigator on the National Health and Medical Research Council (NHMRC)‐funded Centre for Research Excellence grant on Antibiotic Resistance.
Paul P Glasziou: co‐investigator on the National Health and Medical Research Council (NHMRC)‐funded Centre for Research Excellence grant on Antibiotic Resistance.
Tammy C Hoffmann: co‐investigator on the National Health and Medical Research Council (NHMRC)‐funded Centre for Research Excellence grant on Antibiotic Resistance.
Justin Clark: Information Specialist of the Cochrane Acute Respiratory Infections Group and partly funded by the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections (CREMARA) funded by the National Health and Medical Research Council (NHMRC), Australia (1044904).
Chris B Del Mar: Co‐ordinating Editor of the Cochrane Acute Respiratory Infections Group and chief investigator at the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections (CREMARA), both funded by the National Health and Medical Research Council (NHMRC), Australia. He has received royalties from BMJ Books and Elsevier for activities unrelated to this submitted work.
New
References
References to studies included in this review
Agarwal 2012 {published data only}
- Agarwal E, Pradeep AR, Bajaj P, Naik SB. Efficacy of local drug delivery of 0.5% clarithromycin gel as an adjunct to non‐surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2012;83(9):1155‐63. [DOI] [PubMed] [Google Scholar]
Agarwal 2017 {published data only}
- Agarwal E, Bajaj P, Naik SB, Pradeep AR. Locally delivered 0.5% azithromycin as an adjunct to non‐surgical treatment in patients with chronic periodontitis with type 2 diabetes: a randomized controlled clinical trial. Journal of Periodontology 2017;88(12):1281‐7. [DOI] [PubMed] [Google Scholar]
Ahmed 2014 {published data only}
- Ahmed N, Iftikhar N, Bashir U, Rizvi SD, Sheikh ZI, Manzur A. Efficacy of clarithromycin in pityriasis rosea. Journal of the College of Physicians and Surgeons Pakistan 2014;24(11):802‐5. [PubMed] [Google Scholar]
Akhyani 2003 {published data only}
- Akhyani M. The efficacy of oral erythromycin in the treatment of patients with pityriasis rosea: a randomized double blind, placebo controlled clinical trial. Iranian Journal of Dermatology 2003;7:3. [Google Scholar]
Albert 2011 {published data only}
- Albert RK, Bailey WC, Casaburi R, Connett J, Cooper A, Criner GJ, et al. Chronic azithromycin decreases the frequency of chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2011;183:A6416. [Google Scholar]
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2011;365(8):689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Criner GJ, et al. Long‐term azithromycin decreases COPD exacerbations. Respirology 2013;18:5. [Google Scholar]
- Albert RK, Connett J, Curtis JL, Martinez FJ, Han MK, Lazarus SC, et al. Mannose‐binding lectin deficiency and acute exacerbations of chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:767‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Connett J, Voelker H, Criner GJ, Han MK, Make BJ, et al. Chronic azithromycin therapy decreases the risk of re‐hospitalization in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A4383. [Google Scholar]
- OReilly P, Jackson P, Dransfield MT, Blalock JE. Azithromycin treatment reduces levels of PGP and MPO in sputum of subjects with COPD. American Journal of Respiratory and Critical Care Medicine 2012;185:A1290. [Google Scholar]
- Sadatsafavi M, Sin DD, Zafari Z, Criner G, Connett JE, Lazarus S, et al. The association between rate and severity of exacerbations in chronic obstructive pulmonary disease: an application of a joint frailty‐logistic model. American Journal of Epidemiology 2016;184(9):681‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alger 1991 {published data only}
- Alger LS, Lovchik JC. Comparative efficacy of clindamycin versus erythromycin in eradication of antenatal Chlamydia trachomatis. American Journal of Obstetrics and Gynecology 1991;165(2):375‐81. [DOI] [PubMed] [Google Scholar]
Altenburg 2013 {published data only}
- Altenburg J, Graaff C, Werf T, Boersma W. Long term azithromycin treatment: a randomised placebo‐controlled trial in non‐CF bronchiectasis; results from the BAT trial. European Respiratory Journal 2011;38(Suppl 55):1924. [Google Scholar]
- Altenburg J, Wolf R, Go S, Rijn P, Boersma W, Werf T. Changes of computed tomography features of bronchiectasis during one year of azithromycin treatment. European Respiratory Journal 2016;48(Suppl 60):OA278. [Google Scholar]
- Altenburg J, Graaff CS, Stienstra Y, Sloos JH, Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251‐9. [DOI] [PubMed] [Google Scholar]
- Boersma W, Altenburg J, Werf T. Evaluation of symptoms score and QoL in azithromycin maintenance treatment: results of a RCT in patients with bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;185:A3658. [Google Scholar]
Altraif 2011 {published data only}
- Altraif I, Handoo FA, Aljumah A, Alalwan A, Dafalla M, Saeed AM, et al. Effect of erythromycin before endoscopy in patients presenting with variceal bleeding: a prospective, randomized, double‐blind, placebo‐controlled trial. Gastrointestinal Endoscopy 2011;73(2):245‐50. [DOI] [PubMed] [Google Scholar]
- Altraif IH. Effect of intravenous bolus infusion of erythromycin prior to endoscopy in patients presenting with variceal bleeding: a prospective, randomized, placebo controlled, double blind trial. Hepatology 2009;50:402A. [DOI] [PubMed] [Google Scholar]
Aly 2007 {published data only}
- Aly H, Abdel‐Hady H, Khashaba M, El‐Badry N. Erythromycin and feeding intolerance in premature infants: a randomized trial. Journal of Perinatology 2007;27(1):39‐43. [DOI] [PubMed] [Google Scholar]
Amali 2015 {published data only}
- Amali A, Saedi B, Rahavi‐Ezabadi S, Ghazavi H, Hassanpoor N. Long‐term postoperative azithromycin in patients with chronic rhinosinusitis: a randomized clinical trial. American Journal of Rhinology & Allergy 2015;29(6):421‐4. [DOI] [PubMed] [Google Scholar]
Amer 2006 {published data only}
- Amer A, Fischer H. Azithromycin does not cure pityriasis rosea. Pediatrics 2006;117(5):1702‐5. [DOI] [PubMed] [Google Scholar]
Amland 1995 {published data only}
- Amland PF, Andenaes K, Samdal F, Lingaas E, Sandsmark M, Abyholm F, et al. A prospective, double‐blind, placebo‐controlled trial of a single dose of azithromycin on postoperative wound infections in plastic surgery. Plastic and Reconstructive Surgery 1995;96(6):1378‐83. [DOI] [PubMed] [Google Scholar]
Andere 2017 {published data only}
- Andere N, dos Santos NC, Araujo CF, Mathias IF, Taiete T, Casarin RC, et al. Clarithromycin as an adjunct to one‐stage full‐mouth ultrasonic periodontal debridement in generalized aggressive periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2017;88(12):1244‐52. [DOI] [PubMed] [Google Scholar]
Andersen 1998 {published data only}
- Andersen SL, Oloo AJ, Gordon DM, Ragama OB, Aleman GM, Berman JD, et al. Successful double‐blinded, randomized, placebo‐controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in western Kenya. Clinical Infectious Diseases 1998;26(1):146‐50. [DOI] [PubMed] [Google Scholar]
Anderson 1999 {published data only}
- Anderson JL, Muhlestein JB. The ACADEMIC study in perspective (azithromycin in coronary artery disease: elimination of myocardial infection with Chlamydia). Journal of Infectious Diseases 2000;181(3):S569‐71. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Muhlestein JB, Carlquist J, Allen A, Trehan S, Nielson C, et al. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection: the azithromycin in coronary artery disease: elimination of myocardial infection with chlamydia (ACADEMIC) study. Circulation 1999;99(12):1540‐7. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Anderson JL, Carlquist JF, Salunkhe K, Horne BD, Pearson RR, et al. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease: primary clinical results of the ACADEMIC study. Circulation 2000;102(15):1755‐60. [DOI] [PubMed] [Google Scholar]
- Semaan HB, Gurbel PA, Anderson JL, Muhlestein JB, Carlquist JF, Horne BD, et al. The effect of chronic azithromycin therapy on soluble endothelium‐derived adhesion molecules in patients with coronary artery disease. Journal of Cardiovascular Pharmacology 2000;36(4):533‐7. [DOI] [PubMed] [Google Scholar]
Andremont 1981 {published data only}
- Andremont A, Tancrede C. Reduction of the aerobic gram negative bacterial flora of the gastro‐intestinal tract and prevention of traveller's diarrhea using oral erythromycin. Annales de Microbiologie 1981;132 B(3):419‐27. [PubMed] [Google Scholar]
Anthony 2014 {published data only}
- Anthony AI, Muthukumaru U. Efficacy of azithromycin in the treatment of bronchiectasis. Respirology 2014;19(8):1178‐82. [DOI] [PubMed] [Google Scholar]
Avci 2013 {published data only}
- Avci O, Tanyildizi T, Kusku E. A comparison between the effectiveness of erythromycin, single‐dose clarithromycin and topical fusidic acid in the treatment of erythrasma. Journal of Dermatological Treatment 2013;24(1):70‐4. [DOI] [PubMed] [Google Scholar]
Bacharier 2015 {published data only}
- Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA 2015;314(19):2034‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2012 {published data only}
- Bajaj P, Pradeep AR, Agarwal E, Kumari M, Naik SB. Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well‐controlled type 2 diabetes: a randomized controlled clinical trial. Journal of Investigative and Clinical Dentistry 2012;3(4):276‐83. [DOI] [PubMed] [Google Scholar]
Bala 2008 {published data only}
- Bala I, Prasad K, Bhukal I, Nakra D, Pratap M. Effect of preoperative oral erythromycin, erythromycin‐ranitidine, and ranitidine‐metoclopramide on gastric fluid pH and volume. Journal of Clinical Anesthesia 2008;20(1):30‐4. [DOI] [PubMed] [Google Scholar]
Ballard 2011 {published data only}
- Ballard HO, Bernard P, Whitehead V, Grider D, England J, Tucker M, et al. Use of azithromycin for the early treatment of Ureaplasma spp in preterm infants: a randomized, double‐blind, placebo controlled trial. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore (MD). 2009.
- Ballard HO, Bernard PA, Hayes D, Anstead MI, Desai NS, Smart EJ, et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia: a randomized, double‐blind, placebo controlled trial. American Journal of Respiratory and Critical Care Medicine 2009;179:A4126. [Google Scholar]
- Ballard HO, Shook LA, Bernard P, Anstead MI, Kuhn R, Whitehead V, et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double‐blind, placebo controlled trial. Pediatric Pulmonology 2011;46(2):111‐8. [DOI] [PubMed] [Google Scholar]
Banerjee 2004 {published data only}
- Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate‐to‐severe stable COPD: a randomized controlled trial. Treatments in Respiratory Medicine 2004;3(1):59‐65. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Khair OA, Honeybourne D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respiratory Medicine 2005;99(2):208‐15. [DOI] [PubMed] [Google Scholar]
Barkhordar 2018 {published data only}
- Barkhordar M, Mohammadi M, Shamshiri AR, Hadjibabaie M, Ghavamzadeh A. Azithromycin for prevention of graft‐versus‐host disease: a randomized placebo‐controlled trial. International Journal of Hematology‐Oncology and Stem Cell Research 2018;12(2):77‐83. [PMC free article] [PubMed] [Google Scholar]
Beigelman 2015 {published data only}
- Beigelman A, Bacharier LB, Buller R, Mason S, Baty J, Schechtman KB, et al. The effect of azithromycin on RSV load and recurrent wheezing among infants hospitalized with RSV bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2015;191:A2634. [Google Scholar]
- Beigelman A, Isaacson‐Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL‐8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. Journal of Allergy and Clinical Immunology 2015;135(5):1171‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 2005 {published data only}
- Berg HF, Maraha B, Scheffer GJ, Peelers MF, Kluytmans J. Effect of clarithromycin on inflammatory markers in patients with atherosclerosis. Clinical and Diagnostic Laboratory Immunology 2003;10(4):525‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HF, Maraha B, Scheffer GJ, Quarles‐van Ufford M, Vandenbroucke‐Grauls CM, Peeters MF, et al. Treatment with clarithromycin prior to coronary artery bypass graft surgery does not prevent subsequent cardiac events. Clinical Infectious Diseases 2005;40(3):358‐65. [DOI] [PubMed] [Google Scholar]
- Berg HF, Maraha B, Zee A, Gielis SK, Roholl PJ, Scheffer GJ, et al. Effect of clarithromycin treatment on Chlamydia pneumoniae in vascular tissue of patients with coronary artery disease: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Microbiology 2005;43(3):1325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HF, Tjhie JH, Scheffer GJ, Peeters MF, Keulen PH, Kluytmans JA, et al. Emergence and persistence of macrolide resistance in oropharyngeal flora and elimination of nasal carriage of Staphylococcus aureus after therapy with slow‐release clarithromycin: a randomized, double‐blind, placebo‐controlled study. Antimicrobial Agents and Chemotherapy 2004;48(11):4183‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergeron 2017 {published data only}
- Bergeron A, Chevret S, Granata A, Chevallier P, Vincent L, Huynh, A, et al. Effect of azithromycin on airflow decline‐free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO randomized clinical trial. JAMA 2017;318(6):557‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkhof 2013 {published data only}
- Berkhof FF, Doornewaard‐ten Hertog NE, Uil SM, Kerstjens HA, Berg JW. Azithromycin and cough‐specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respiratory Research 2013;14(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhof FF, Hertog NE, Uil SM, Kerstjens HA, Berg JK. Randomized controlled trial of prophylactic azithromycin on cough‐specific health status in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187:A2449. [Google Scholar]
Berne 2002 {published data only}
- Berne JD, Norwood SH, McAuley CE, Vallina VL, Villareal D, Weston J, et al. Erythromycin reduces delayed gastric emptying in critically ill trauma patients: a randomized, controlled trial. Journal of Trauma 2002;53(3):422‐5. [DOI] [PubMed] [Google Scholar]
Black 2001 {published data only}
- Allegra L, Blasi F. Chlamydia pneumoniae and bronchial asthma. 16th World Congress of Asthma; 1999 Oct 17‐20; Buenos Aires. 1999:186‐9.
- Black PN, Blasi F, Jenkins CR, Scicchitano R, Mills GD, Rubinfeld AR, et al. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. American Journal of Respiratory and Critical Care Medicine 2001;164(4):536‐41. [DOI] [PubMed] [Google Scholar]
Bonacini 1993 {published data only}
- Bonacini M, Quiason S, Reynolds M, Gaddis M, Pemberton B, Smith O. Effect of intravenous erythromycin on postoperative ileus. American Journal of Gastroenterology 1993;88(2):208‐11. [PubMed] [Google Scholar]
Botero 2013 {published data only}
- Botero JE, Yepes FL, Ochoa SP, Hincapie JP, Roldan N, Ospina CA, et al. Effects of periodontal non‐surgical therapy plus azithromycin on glycemic control in patients with diabetes: a randomized clinical trial. Journal of Periodontal Research 2013;48(6):706‐12. [DOI] [PubMed] [Google Scholar]
- Hincapie JP, Castrillon CA, Yepes FL, Roldan NB, Becerra MA, Moreno SM, et al. Microbiological effects of periodontal therapy plus azithromycin in patients with diabetes: results from a randomized clinical trial. Acta Odontologica Latinoamericana 2014;27(2):89‐95. [DOI] [PubMed] [Google Scholar]
Branden 2004 {published data only}
- Branden E, Koyi H, Gnarpe J, Gnarpe H, Tornling G. Intermittent azithromycin treatment for respiratory symptoms in patients with chronic Chlamydia pneumoniae infection. Scandinavian Journal of Infectious Diseases 2004;36(11‐12):811‐6. [DOI] [PubMed] [Google Scholar]
Brickfield 1986 {published data only}
- Brickfield FX, Carter WH, Johnson RE. Erythromycin in the treatment of acute bronchitis in a community practice. Journal of Family Practice 1986;23(2):119‐22. [PubMed] [Google Scholar]
Brill 2015 {published data only}
- Brill S, James P, Cuthbertson L, Cookson W, Moffatt M, Wedzicha J. Haemophilus dominance of the stable COPD microbiome is associated with greater bacterial load and inflammation and is modulated by prophylactic antibiotic therapy. European Respiratory Journal 2015;46(Suppl 59):OA4746. [Google Scholar]
- Brill SE, Law M, El‐Emir E, Allinson JP, James P, Maddox V, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax 2015;70(10):930‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brusselle 2013 {published data only}
- Brusselle GG, VanderStichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double‐blind placebo‐controlled trial. Thorax 2013;68(4):322‐9. [DOI] [PubMed] [Google Scholar]
- Lavens M, Brusselle G, Verleden G, Wever W, Coolen J, Verschakelen J. Air‐trapping on HRCT as a marker for patient outcome in severe asthmatics treated with azithromycin. European Respiratory Journal 2014;44(Suppl 58):P656. [Google Scholar]
Bystedt 1980 {published data only}
- Bystedt H, Nord CE, Nordenram A. Effect of azidocillin, erythromycin, clindamycin and doxycycline on postoperative complications after surgical removal of impacted mandibular third molars. International Journal of Oral Surgery 1980;9(3):157‐65. [DOI] [PubMed] [Google Scholar]
Cameron 2013 {published data only}
- Cameron EJ, Chaudhuri R, Mair F, McSharry C, Greenlaw N, Weir CJ, et al. Randomised controlled trial of azithromycin in smokers with asthma. European Respiratory Journal 2013;42(5):1412‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EJ, Chaudhuri R, McSharry C, Greenlaw N, Weir CJ, Jolly L, et al. Effects of azithromycin on asthma control, airway inflammation and bacterial colonisation in smokers with asthma: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;185:A3965. [Google Scholar]
Carbonell 2006 {published data only}
- Carbonell N, Godard V, Serfaty L, Best N, Poupon R. IV erythromycin improves gastric lavage in acute upper gastrointestinal bleeding: a randomized controlled trial. Journal of Hepatology 2005;42:78. [Google Scholar]
- Carbonell N, Pauwels A, Serfaty L, Boelle PY, Becquemont L, Poupon R. Erythromycin infusion prior to endoscopy for acute upper gastrointestinal bleeding: a randomized, controlled, double‐blind trial. American Journal of Gastroenterology 2006;101(6):1211‐5. [DOI] [PubMed] [Google Scholar]
Cercek 2003 {published data only}
- Cercek B, Shah PK, Noc M, Zahger D, Zeymer U, Matetzky S, et al. Effect of short‐term treatment with azithromycin on recurrent ischaemic events in patients with acute coronary syndrome in the azithromycin in acute coronary syndrome (AZACS) trial: a randomised controlled trial. Lancet 2003; Vol. 361, issue 9360:809‐13. [DOI] [PubMed]
Clement 2006 {published data only}
- Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax 2006;61(10):895‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement A, Tamalet A, Leroux E, Ravilly S, Jais J. Long term effects and clinical outcome of low‐dose azithromycin in young patients with cystic fibrosis: a multicenter, randomized, double‐blind, placebo‐controlled trial. Pediatric Pulmonology 2005;40(Suppl 28):285. [Google Scholar]
- Clement A, Tamalet A, Roux E, Ravilly S, Jais JP. Long term effects and clinical outcome of low‐dose azithromycin in young patients with cystic fibrosis: a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal of Cystic Fibrosis 2005;4(Suppl 1):S68. [Google Scholar]
Corris 2015 {published data only}
- Corris P, Small T, Ryan V, Lordan J, Fisher AJ, Meachery G, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. American Journal of Respiratory and Critical Care Medicine 2012;185:A5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corris PA, Ryan VA, Small T, Lordan J, Fisher AJ, Meachery G, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax 2015;70(5):442‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corris PA, Small T, Ryan VA, Lordan J, Fisher AJ, Meachery G, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Journal of Heart and Lung Transplantation 2012;31:S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C, Johnson G, Ryan V, Small T, Lordan J, Fisher A, et al. BAL neutrophil levels in a randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome post lung transplantation. European Respiratory Journal 2012;40(Suppl 56):1859. [Google Scholar]
Currier 2000 {published data only}
- Currier JS, Williams PL, Koletar SL, Cohn SE, Murphy RL, Heald AE, et al. Discontinuation of Mycobacterium avium complex prophylaxis in patients with antiretroviral therapy‐induced increases in CD4+ cell count. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2000;133(7):493‐503. [DOI] [PubMed] [Google Scholar]
Curry 2004 {published data only}
- Curry JI, Lander AD, Stringer MD. A multicenter, randomized, double‐blind, placebo‐controlled trial of the prokinetic agent erythromycin in the postoperative recovery of infants with gastroschisis. Journal of Pediatric Surgery 2004;39(4):565‐9. [DOI] [PubMed] [Google Scholar]
Czarnetzki 2015 {published data only}
- Czarnetzki C, Elia N, Frossard JL, Giostra E, Spahr L, Waeber JL, et al. Erythromycin for gastric emptying in patients undergoing general anesthesia for emergency procedures. Swiss Medical Weekly 2014;144:S5. [DOI] [PubMed] [Google Scholar]
- Czarnetzki C, Elia N, Frossard JL, Giostra E, Spahr L, Waeber JL, et al. Erythromycin for gastric emptying in patients undergoing general anesthesia for emergency surgery: a randomized clinical trial. JAMA Surgery 2015;150(8):730‐7. [DOI] [PubMed] [Google Scholar]
Dunlay 1987 {published data only}
- Dunlay J, Reinhardt R, Roi LD. A placebo‐controlled, double‐blind trial of erythromycin in adults with acute bronchitis. Journal of Family Practice 1987;25(2):137‐41. [PubMed] [Google Scholar]
Ehsani 2013 {published data only}
- Ehsani Ardakani MJ, Zare E, Basiri M, Mohaghegh Shalmani H. Erythromycin decreases the time and improves the quality of EGD in patients with acute upper GI bleeding. Gastroenterology and Hepatology From Bed to Bench 2013;6(4):195‐201. [PMC free article] [PubMed] [Google Scholar]
El‐Sadr 2000 {published data only}
- El‐Sadr WM, Burman WJ, Grant LB, Matts JP, Hafner R, Crane L, et al. Discontinuation of prophylaxis for Mycobacterium avium complex disease in HIV‐infected patients who have a response to antiretroviral therapy. New England Journal of Medicine 2000;342(15):1085‐92. [DOI] [PubMed] [Google Scholar]
Eschenbach 1991 {published data only}
- Eschenbach DA, Nugent RP, Rao AV, Cotch MF, Gibbs RS, Lipscomb KA, et al. A randomized placebo‐controlled trial of erythromycin for the treatment of Ureaplasma urealyticum to prevent premature delivery. The Vaginal Infections and Prematurity Study Group. American Journal of Obstetrics and Gynecology 1991; Vol. 164, issue 3:734‐42. [DOI] [PubMed]
Fonseca‐Aten 2006 {published data only}
- Fonseca‐Aten M, Okada PJ, Bowlware KL, Chavez‐Bueno S, Mejias A, Rios AM, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double‐blind, randomized, placebo‐controlled trial. Annals of Allergy, Asthma & Immunology 2006; Vol. 97, issue 4:457‐63. [DOI] [PubMed]
Frossard 2002 {published data only}
- Frossard JL, Spahr L, Queneau PE, Giostra E, Burckhardt B, Ory G, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: a randomized, controlled, double‐blind trial. Gastroenterology 2002;123(1):17‐23. [DOI] [PubMed] [Google Scholar]
Garcia‐Burguillo 1996 {published data only}
- Garcia‐Burguillo A, Hernandez‐Garcia JM, Fuente P. Erythromycin prophylaxis in preterm pregnancies with rupture of the amniotic membranes [Profilaxis con eritromicina en gestaciones pretérmino con rotura prematura de las membranas amnióticas]. Clinica e Investigacion en Ginecologia y Obstetricia 1996; Vol. 23, issue 3:96‐100.
Gharpure 2001 {published data only}
Giamarellos‐Bourboulis 2008 {published data only}
- Giamarellos‐Bourboulis EJ. New antibiotic targets: virulence and quorum sensing. Inflammation Research 2010;59:S156. [Google Scholar]
- Giamarellos‐Bourboulis EJ, Pechere JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, et al. Effect of clarithromycin in patients with sepsis and ventilator‐associated pneumonia. Clinical Infectious Diseases 2008;46(8):1157‐64. [DOI] [PubMed] [Google Scholar]
- Giamarellos‐Bourboulis EJ, Routsi C, Raftogiannis M, Kollias S, Baziaka F, Zervakis D, et al. Effect of clarithromycin on septic patients. Archives of Hellenic Medicine 2007;24(2):170‐7. [Google Scholar]
- Raftogiannis M, Antonopoulou A, Baziaka F, Koutoukas P, Tsaganos T, Pelekanou A, et al. Clarithromycin reverses sepsis‐induced immunoparalysis of monocytes. Critical Care 2009;13(Suppl 4):P25. [Google Scholar]
- Spyridaki A, Raftogiannis M, Antonopoulou A, Tsaganos T, Routsi C, Baziaka F, et al. Effect of clarithromycin in inflammatory markers of patients with ventilator‐associated pneumonia and sepsis caused by gram‐negative bacteria: results from a randomized clinical study. Antimicrobial Agents and Chemotherapy 2012;56(7):3819‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaganos T, Raftogiannis M, Pratikaki M, Christodoulou S, Kotanidou A, Papadomichelakis E, et al. Clarithromycin leads to long‐term survival and cost benefit in ventilator‐associated pneumonia and sepsis. Antimicrobial Agents and Chemotherapy 2016;60(6):3640‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giamarellos‐Bourboulis 2014 {published data only}
- Giamarellos‐Bourboulis E, Lymberopoulou K, Tsangaris I, Antonopoulou A, Marioli A, Leonidou L, et al. Effect of clarithromycin in patients with Gram‐negative sepsis: subgroup analysis of a randomized trial. Critical Care 2014;18(Suppl 1):P242. [Google Scholar]
- Giamarellos‐Bourboulis EJ, Mylona V, Antonopoulou A, Tsangaris I, Koutelidakis I, Marioli A, et al. Effect of clarithromycin in patients with suspected Gram‐negative sepsis: results of a randomized controlled trial. Journal of Antimicrobial Chemotherapy 2014;69(4):1111‐8. [DOI] [PubMed] [Google Scholar]
Gibson 2017 {published data only}
- Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Azithromycin reduces exacerbations in adults with persistent symptomatic eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2017;195:A4679. [Google Scholar]
- Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Azithromycin reduces exacerbations in adults with persistent symptomatic eosinophilic asthma. Respirology 2017;22:24. [Google Scholar]
- Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390(10095):659‐68. [DOI] [PubMed] [Google Scholar]
Glass 1999 {published data only}
- Clark SM. A double blind parallel group, clinical evaluation of the efficacy and safety of isotretinoin (0.05%) gel, erythromycin (2%) gel, a gel containing isotretinoin (0.05%) and erythromycin (2%) and a gel base (placebo) in the topical treatment of acne vulgaris. British Journal of Dermatology 1996;135(Suppl 47):28‐45. [Google Scholar]
- Clark SM, Cunliffe WJ. Placebo controlled studies of topical isotretinoin/erythromycin gels in acne. Journal of Investigative Dermatology 1997;108(3):393. [Google Scholar]
- Glass D, Boorman GC, Stables GI, Cunliffe WJ, Goode K. A placebo‐controlled clinical trial to compare a gel containing a combination of isotretinoin (0.05%) and erythromycin (2%) with gels containing isotretinoin (0.05%) or erythromycin (2%) alone in the topical treatment of acne vulgaris. Dermatology 1999;199(3):242‐7. [DOI] [PubMed] [Google Scholar]
Gokmen 2012 {published data only}
- Gokmen T, Oguz SS, Bozdag S, Erdeve O, Uras N, Dilmen U. A comparison of erythromycin and ursodeoxycholic acid in preventing liver function abnormalities during parenteral nutrition in VLBW infants. Early Human Development 2010;86:S83‐4. [Google Scholar]
- Gokmen T, Oguz SS, Bozdag S, Erdeve O, Uras N, Dilmen U. A controlled trial of erythromycin and UDCA in premature infants during parenteral nutrition in minimizing feeding intolerance and liver function abnormalities. Journal of Perinatology 2012;32(2):123‐8. [DOI] [PubMed] [Google Scholar]
Grassly 2016 {published data only}
- Grassly NC, Praharaj I, Babji S, Kaliappan SP, Giri S, Venugopal S, et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double‐blind randomised placebo‐controlled trial in seronegative Indian infants. Lancet Infectious Diseases 2016;16(8):905‐14. [DOI] [PubMed] [Google Scholar]
Grayston 2005 {published data only}
- Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, et al. Azithromycin for the secondary prevention of coronary events. New England Journal of Medicine 2005; Vol. 352, issue 16:1637‐45. [DOI] [PubMed]
- Jackson LA. Description and status of the azithromycin and coronary events study (ACES). Journal of Infectious Diseases 2000;181 S(Suppl 3):S579‐81. [DOI] [PubMed] [Google Scholar]
Grob 1981 {published data only}
- Grob PR, Spencely M, Lambert HP. Prophylactic erythromycin for whooping‐cough contacts. Lancet 1981;317(8223):772‐3. [PubMed] [Google Scholar]
Gupta 1997 {published data only}
- Gupta S, Leatham EW, Carrington D, Mendall MA, Kaski JC, Camm AJ. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 1997;96(2):404‐7. [DOI] [PubMed] [Google Scholar]
Gurfinkel 1999 {published data only}
- Gurfinkel E, Bozovich G, Beck E, Testa E, Livellara B, Mautner B. Treatment with the antibiotic roxithromycin in patients with acute non‐Q‐wave coronary syndromes. The final report of the ROXIS study. European Heart Journal 1999;20(2):121‐7. [DOI] [PubMed] [Google Scholar]
- Gurfinkel E, Bozovich G, Daroca A, Beck E, Mautner B. Randomised trial of roxithromycin in non‐Q‐wave coronary syndromes: ROXIS pilot study. ROXIS Study Group. Lancet 1997;350(9075):404‐7. [DOI] [PubMed] [Google Scholar]
Hahn 2006 {published data only}
- Hahn DL, Plane MB, Mahdi OS, Byrne GI. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clinical Trials 2006; Vol. 1, issue 2:e11. [DOI] [PMC free article] [PubMed]
Hahn 2012 {published data only}
- Hahn D, Grasmick M, Hetzel S. Pragmatic controlled trial of azithromycin for asthma in adults. European Respiratory Journal 2011;38(Suppl 55):1877. [Google Scholar]
- Hahn DL, Grasmick M, Hetzel S, Yale S. Azithromycin for bronchial asthma in adults: an effectiveness trial. Journal of the American Board of Family Medicine 2012;25(4):442‐59. [DOI] [PubMed] [Google Scholar]
Halperin 1999 {published data only}
- Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, Serres G. A randomized, placebo‐controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture‐positive Bordetella pertussis infection. Pediatrics 1999;104(4):e42. [DOI] [PubMed] [Google Scholar]
Haxel 2015 {published data only}
- Haxel BR, Clemens M, Karaiskaki N, Dippold U, Kettern L, Mann WJ. Controlled trial for long‐term low‐dose erythromycin after sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125(5):1048‐55. [DOI] [PubMed] [Google Scholar]
Haye 1998 {published data only}
- Haye R, Lingaas E, Hoivik HO, Odegard T. Azithromycin versus placebo in acute infectious rhinitis with clinical symptoms but without radiological signs of maxillary sinusitis. European Journal of Clinical Microbiology & Infectious Diseases 1998;17(5):309‐12. [DOI] [PubMed] [Google Scholar]
Heppner 2005 {published data only}
- Heppner DG, Walsh DS, Uthaimongkol N, Tang DB, Tulyayon S, Permpanich B, et al. Randomized, controlled, double‐blind trial of daily oral azithromycin in adults for the prophylaxis of Plasmodium vivax malaria in Western Thailand. American Journal of Tropical Medicine and Hygiene 2005;73(5):842‐9. [PubMed] [Google Scholar]
Hillis 2004 {published data only}
- Hillis GS, Pearson C, Dawson P, Stirling D, Ludlam CA, Fox KA, et al. The effects of azithromycin on soluble cell adhesion molecules and markers of inflammation: a randomized double blind placebo controlled study. European Heart Journal 2002;23:260. [Google Scholar]
- Hillis GS, Pearson CV, Harding SA, Sutherland S, Ludlam CA, Marioni JC, et al. Effects of a brief course of azithromycin on soluble cell adhesion molecules and markers of inflammation in survivors of an acute coronary syndrome: a double‐blind, randomized, placebo‐controlled study. American Heart Journal 2004;148(1):72‐9. [DOI] [PubMed] [Google Scholar]
Hodgson 2016 {published data only}
- Hodgson D, Anderson J, Reynolds C, Oborne J, Meakin G, Bailey H, et al. The effects of azithromycin in treatment‐resistant cough: a randomized, double‐blind, placebo‐controlled trial. Chest 2016;149(4):1052‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooton 1990 {published data only}
- Hooton TM, Wong ES, Barnes RC, Roberts PL, Stamm WE. Erythromycin for persistent or recurrent nongonococcal urethritis. A randomized, placebo‐controlled trial. Annals of Internal Medicine 1990;113(1):21‐6. [DOI] [PubMed] [Google Scholar]
Hyde 2001 {published data only}
- Hyde TB, Gilbert M, Schwartz SB, Zell ER, Watt JP, Thacker WL, et al. Azithromycin prophylaxis during a hospital outbreak of Mycoplasma pneumoniae pneumonia. Journal of Infectious Diseases 2001;183(6):907‐12. [DOI] [PubMed] [Google Scholar]
Ikeoka 2007 {published data only}
- Ikeoka DT, Lemos PA, Vieira CZ, Strabelli TV, Silva EE, Perin MA, et al. Anti‐Chlamydia azithromycin therapy for the prevention of coronary renarrowing after stenting (ACTOR trial): a randomized, double‐blinded, controlled trial. International Journal of Atherosclerosis 2007;2:63‐7. [Google Scholar]
- Ikeoka DT, Vieira CZ, Lemos PA, Strabelli TV, Silva EE, Perin MA, et al. Azithromycin does not prevent six‐month myointimal proliferation but attenuates the transient systemic inflammation occurring after coronary stenting. Clinical Research in Cardiology 2009;98(1):44‐51. [DOI] [PubMed] [Google Scholar]
Jablonowski 1997 {published data only}
- Jablonowski H, Fatkenheuer G, Youle M, Newell T, Lines S, Craft JC. Ancillary benefits of Mycobacterium avium‐intracellulare complex prophylaxis with clarithromycin in HIV‐infected patients. Drugs 1997;54(Suppl 2):16‐22. [DOI] [PubMed] [Google Scholar]
Jackson 1999 {published data only}
- Jackson LA, Stewart DK, Wang SP, Cooke DB, Cantrell T, Grayston JT. Safety and effect on anti‐Chlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. Journal of Antimicrobial Chemotherapy 1999;44(3):411‐4. [DOI] [PubMed] [Google Scholar]
Jespersen 2006 {published data only}
- Gluud C, Als‐Nielsen B, Damgaard M, Fischer Hansen J, Hansen S, Helo OH, et al. Clarithromycin for 2 weeks for stable coronary heart disease: 6‐year follow‐up of the CLARICOR randomized trial and updated meta‐analysis of antibiotics for coronary heart disease. Cardiology 2008;111(4):280‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Als‐Nielsen B, Damgaard M, Helo OH, Petersen L, Jespersen CM. Intervention with clarithromycin in patients with stable coronary heart disease: the CLARICOR trial design. Heart Drug 2001;1(1):14‐9. [Google Scholar]
- Hilden J, Lind I, Kolmos HJ, Als‐Nielsen B, Damgaard M, Hansen JF, et al. Chlamydia pneumoniae IgG and IgA antibody titers and prognosis in patients with coronary heart disease: results from the CLARICOR trial. Diagnostic Microbiology and Infectious Disease 2010;66(4):385‐92. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Hilden J, Als‐Nielsen B, Damgaard M, Hansen JF, Hansen S, et al. Statin treatment prevents increased cardiovascular and all‐cause mortality associated with clarithromycin in patients with stable coronary heart disease. Journal of Cardiovascular Pharmacology 2010;55(2):123‐8. [DOI] [PubMed] [Google Scholar]
- Jespersen CM, Als‐Nielsen B, Damgaard M, Hansen JF, Hansen S, Helo OH, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ 2006;332(7532):22‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen CM, Kolmos HJ, Frydendall N, Hilden J, Gluud C, Hansen JF. Compliance with and short‐term adverse events from clarithromycin versus placebo in patients with stable coronary heart disease: the CLARICOR trial. Journal of Antimicrobial Chemotherapy 2009;64(2):411‐5. [DOI] [PubMed] [Google Scholar]
- Lyakishev AA. Randomized placebo‐controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease. Results of the CLARICOR study. Kardiologiya 2006;46:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel P, Hilden J, Fischer Hansen J, Hildebrandt P, Kastrup J, Kolmos HJ, et al. Excess sudden cardiac deaths after short‐term clarithromycin administration in the CLARICOR trial: why is this so, and why are statins protective?. Cardiology 2011;118(1):63‐7. [DOI] [PubMed] [Google Scholar]
- Winkel P, Hilden J, Fischer Hansen J, Kastrup J, Kolmos HJ, Kjøller E, et al. Clarithromycin for stable coronary heart disease increases all‐cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. International Journal of Cardiology 2015;182:459‐65. [DOI] [PubMed] [Google Scholar]
Joensen 2008 {published data only}
- Joensen JB, Juul S, Henneberg E, Thomsen G, Ostergaard L, Lindholt JS. Can long‐term antibiotic treatment prevent progression of peripheral arterial occlusive disease? A large, randomized, double‐blinded, placebo‐controlled trial. Atherosclerosis 2008;196(2):937‐42. [DOI] [PubMed] [Google Scholar]
Johnston 2016 {published data only}
- Johnston SL, Szigeti M, Cross M, Brightling C, Chaudhuri R, Harrison T, et al. A randomised, double‐blind, placebo‐controlled study to evaluate the efficacy of oral azithromycin as a supplement to standard care for adult patients with acute exacerbations of asthma (the AZALEA trial). www.ncbi.nlm.nih.gov/books/NBK390972/ (accessed prior to 6 January 2019). [DOI: 10.3310/eme03080] [DOI] [PubMed]
- Johnston SL, Szigeti M, Cross M, Brightling C, Chaudhuri R, Harrison T, et al. Azithromycin for acute exacerbations of asthma: the AZALEA randomized clinical trial. JAMA Internal Medicine 2016;176(11):1630‐7. [DOI] [PubMed] [Google Scholar]
Jun 2014 {published data only}
- Choi MG, Jun B, Lim CH, Park JM, Kim JS, Kim SW, et al. Premedication of erythromycin improves endoscopic mucosal visualization in patients with subtotal gastrectomy. Gastrointestinal Endoscopy 2013;77(Suppl 5):AB144. [Google Scholar]
- Jun BY, Choi MG, Lee JY, Baeg MK, Moon SJ, Lim CH, et al. Premedication with erythromycin improves endoscopic visualization of the gastric mucosa in patients with subtotal gastrectomy: a prospective, randomized, controlled trial. Surgical Endoscopy 2014;28(5):1641‐7. [DOI] [PubMed] [Google Scholar]
Kaehler 2005 {published data only}
- Kaehler J, Haar A, Schaps KP, Gaede A, Carstensen M, Schalwat I, et al. A randomized trial in patients undergoing percutaneous coronary angioplasty: roxithromycin does not reduce clinical restenosis but angioplasty increases antibody concentrations against Chlamydia pneumoniae. American Heart Journal 2005;150(5):987‐93. [DOI] [PubMed] [Google Scholar]
Kaiser 2001 {published data only}
- Kaiser L, Morabia A, Stalder H, Ricchetti A, Auckenthaler R, Terrier F, et al. Role of nasopharyngeal culture in antibiotic prescription for patients with common cold or acute sinusitis. European Journal of Clinical Microbiology & Infectious Diseases 2001;20(7):445‐51. [DOI] [PubMed] [Google Scholar]
- Lacroix JS, Ricchetti A, Lew D, Delhumeau C, Morabia A, Stadler H, et al. Symptoms and clinical and radiological signs predicting the presence of pathogenic bacteria in acute rhinosinusitis. Acta Oto‐Laryngologica 2002;122(2):192‐6. [DOI] [PubMed] [Google Scholar]
Kalliafas 1996 {published data only}
- Kalliafas S, Choban PS, Ziegler D, Drago S, Flancbaum L. Erythromycin facilitates postpyloric placement of nasoduodenal feeding tubes in intensive care unit patients: randomized, double‐blinded, placebo‐controlled trial. Journal of Parenteral and Enteral Nutrition 1996;20(6):385‐8. [DOI] [PubMed] [Google Scholar]
Karlsson 2009 {published data only}
- Karlsson L, Gnarpe J, Bergqvist D, Lindback J, Parsson H. The effect of azithromycin and Chlamydophilia pneumonia infection on expansion of small abdominal aortic aneurysms ‐ a prospective randomized double‐blind trial. Journal of Vascular Surgery 2009;50(1):23‐9. [DOI] [PubMed] [Google Scholar]
Kathariya 2014 {published data only}
- Kathariya R, Pradeep AR, Raghavendra NM, Gaikwad R. Evaluation of subgingivally delivered 0.5% clarithromycin as an adjunct to nonsurgical mechanotherapy in the management of chronic periodontitis: a short‐term double blinded randomized control trial. Journal of Investigative and Clinical Dentistry 2014;5(1):23‐31. [DOI] [PubMed] [Google Scholar]
Kaul 2004 {published data only}
- Fonck K, Kaul R, Kimani J, Keli F, MacDonald KS, Ronald AR, et al. A randomised, placebo‐controlled trial of monthly azithromycin prophylaxis to prevent sexually transmitted infections and HIV‐1 in Kenyan sex workers: study design and baseline findings. International Journal of STD & AIDS 2001;11(12):804‐11. [DOI] [PubMed] [Google Scholar]
- Kaul R, Kimani J, Nagelkerke NJ, Fonck K, Ngugi EN, Keli F, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV‐1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 2004;291(21):2555‐62. [DOI] [PubMed] [Google Scholar]
Keenan 2018 {published data only}
- Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, et al. Azithromycin to reduce childhood mortality in sub‐Saharan Africa. New England Journal of Medicine 2018;378(17):1583‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenyon 2001a {published data only}
- Kenyon S, Brocklehurst P, Jones D, Marlow N, Salt A, Taylor D. MRC ORACLE Children Study. Long term outcomes following prescription of antibiotics to pregnant women with either spontaneous preterm labour or preterm rupture of the membranes. BMC Pregnancy and Childbirth 2008;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7‐year follow‐up of the ORACLE I trial. Lancet 2008;372(9646):1310‐8. [DOI] [PubMed] [Google Scholar]
- Kenyon S, Taylor DJ, Tarnow‐Mordi WO. ORACLE ‐ antibiotics for preterm prelabour rupture of the membranes: short‐term and long‐term outcomes. Acta Paediatrica Supplement 2002;91(437):12‐5. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Taylor DJ, Tarnow‐Mordi W, Oracle Collaborative Group. Broad‐spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001;357(9261):979‐88. [DOI] [PubMed] [Google Scholar]
Kenyon 2001b {published data only}
- Kenyon S, Brocklehurst P, Jones D, Marlow N, Salt A, Taylor D. MRC ORACLE Children Study. Long term outcomes following prescription of antibiotics to pregnant women with either spontaneous preterm labour or preterm rupture of the membranes. BMC Pregnancy and Childbirth 2008;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet 2008;372(9646):1319‐27. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Taylor DJ, Tarnow‐Mordi W. Broad‐spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. Lancet 2001;357(9261):989‐94. [DOI] [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim W, Jeong MH, Hong YJ, Lee SH, Lim SY, Hong SN. A randomized trial for the secondary prevention by azithromycin in Korean patients with acute coronary syndrome after percutaneous coronary intervention. Korean Circulation Journal 2004;34(8):743‐51. [Google Scholar]
King 1996 {published data only}
- King DE, Williams WC, Bishop L, Shechter A. Effectiveness of erythromycin in the treatment of acute bronchitis. Journal of Family Practice 1996;42(6):601‐5. [PubMed] [Google Scholar]
Klebanoff 1995 {published data only}
- Klebanoff MA, Regan JA, Rao AV, Nugent RP, Blackwelder WC, Eschenbach DA, et al. Outcome of the vaginal infections and prematurity study: results of a clinical trial of erythromycin among pregnant women colonized with group B streptococci. American Journal of Obstetrics and Gynecology 1995;172(5):1540‐5. [DOI] [PubMed] [Google Scholar]
Kneyber 2008 {published data only}
- Kneyber MC, Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatric Pulmonology 2008;43(2):142‐9. [DOI] [PubMed] [Google Scholar]
Kostadima 2004 {published data only}
Kraft 2002 {published data only}
- Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 2002;121(6):1782‐8. [DOI] [PubMed] [Google Scholar]
Kvien 2004 {published data only}
- Kvien TK, Gaston JS, Bardin T, Butrimiene I, Dijkmans BA, Leirisalo‐Repo M, et al. Three month treatment of reactive arthritis with azithromycin: a EULAR double blind, placebo controlled study. Annals of the Rheumatic Diseases 2004;63:1113‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanza 1998 {published data only}
- Lanza FL, Sontag SJ, Ciociola AA, Sykes DL, Heath A, McSorley DJ. Ranitidine bismuth citrate plus clarithromycin: a dual therapy regimen for patients with duodenal ulcer. Helicobacter 1998;3(3):212‐21. [DOI] [PubMed] [Google Scholar]
Leowattana 2001 {published data only}
- Leowattana W, Bhuripanyo K, Singhaviranon L, Akaniroj S, Mahanonda N, Samranthin M, et al. Roxithromycin in prevention of acute coronary syndrome associated with Chlamydia pneumoniae infection: a randomized placebo controlled trial. Journal of the Medical Association of Thailand 2001;84(Suppl 3):S669‐75. [PubMed] [Google Scholar]
Lildholdt 2003 {published data only}
- Lildholdt T, Doessing H, Lyster M, Outzen KE. The natural history of recurrent acute tonsillitis and a clinical trial of azithromycin for antibiotic prophylaxis. Clinical Otolaryngology and Allied Sciences 2003;28(4):371‐3. [DOI] [PubMed] [Google Scholar]
Malhotra‐Kumar 2007a {published data only}
- Malhotra‐Kumar S, Lammens C, Coenen S, Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide‐resistant streptococci in healthy volunteers: a randomised, double‐blind, placebo‐controlled study. Lancet 2007; Vol. 369, issue 9560:482‐90. [DOI] [PubMed]
Malhotra‐Kumar 2007b {published data only}
- Malhotra‐Kumar S, Lammens C, Coenen S, Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide‐resistant streptococci in healthy volunteers: a randomised, double‐blind, placebo‐controlled study. Lancet 2007; Vol. 369, issue 9560:482‐90. [DOI] [PubMed]
Mandal 1984 {published data only}
- Mandal BK, Ellis ME, Dunbar EM, Whale K. Double‐blind placebo‐controlled trial of erythromycin in the treatment of clinical campylobacter infection. Journal of Antimicrobial Chemotherapy 1984;13(6):619‐23. [DOI] [PubMed] [Google Scholar]
Mandhane 2017 {published data only}
- Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, Williamson J, Lee BE, Spier S, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo‐controlled randomized trial. PLOS ONE 2017;12(8):e0182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martande 2015 {published data only}
- Martande SS, Pradeep AR, Kumari M, Priyanka N, Singh SP, Naik SB, et al. Clinical and microbiological efficacy of systemic roxithromycin as an adjunct to non‐surgical periodontal therapy in treatment of chronic periodontitis. A randomized, double‐blinded, placebo‐controlled clinical trial. American Journal of Dentistry 2015;28(3):137‐42. [PubMed] [Google Scholar]
Martande 2016 {published data only}
- Martande SS, Pradeep AR, Singh SP, Kumari M, Naik SB, Suke DK, et al. Clinical and microbiological effects of systemic azithromycin in adjunct to nonsurgical periodontal therapy in treatment of Aggregatibacter actinomycetemcomitans associated periodontitis: a randomized placebo‐controlled clinical trial. Journal of Investigative and Clinical Dentistry 2016;7(1):72‐80. [DOI] [PubMed] [Google Scholar]
Martin 1997 {published data only}
- Martin DH, Eschenbach DA, Cotch MF, Nugent RP, Rao AV, Klebanoff MA. Double‐blind placebo‐controlled treatment trial of Chlamydia trachomatis endocervical infections in pregnant women. Infectious Diseases in Obstetrics and Gynecology 1997; Vol. 5, issue 1:10‐7. [DOI] [PMC free article] [PubMed]
Mathai 2007 {published data only}
- Mathai SS, Bawa KS, Bhandari A. Effect of erythromycin on gastric emptying time of low birth weight babies. Medical Journal, Armed Forces India 2007;63(3):226‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCallum 2013 {published data only}
- McCallum G, Morris PS, Chatfield MD, Maclennan C, White A, Versteegh L, et al. A single dose of azithromycin did not improve clinical outcomes in children ≤ 18 months hospitalized with acute bronchiolitis: a doubleblind, placebo‐controlled randomized trial. Respirology 2013;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum G, Versteegh L, Maclennan C, Wilson C, Pizzutto S, Morris PS, et al. Randomized controlled trial to reduce morbidity of bronchiolitis in young children admitted to Royal Darwin Hospital in the NT. Respirology 2011;16:71. [Google Scholar]
- McCallum GB, Morris PS, Chatfield M, MacLennan C, White A, Versteegh L, et al. A single dose of azithromycin did not improve clinical outcomes in children ≤ 18 months hospitalized with acute bronchiolitis: a doubleblind, placebo‐controlled randomized trial. Pediatric Respiratory Reviews 2012;13:S47. [Google Scholar]
- McCallum GB, Morris PS, Chatfield MD, Maclennan C, White AV, Sloots TP, et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo‐controlled trial. PLOS ONE 2013;8(9):e74316. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCallum 2015 {published data only}
- Chang AB, Grimwood K, White AV, Maclennan C, Sloots TP, Sive A, et al. Randomized placebo‐controlled trial on azithromycin to reduce the morbidity of bronchiolitis in Indigenous Australian infants: rationale and protocol. Trials 2011;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum G, Morris P, Grimwood K, Maclennan C, White A, Sloots T, et al. Improving the management of indigenous children hospitalised with bronchiolitis: a multicenter RCT. Respirology 2015;20:30. [Google Scholar]
- McCallum GB, Morris PS, Grimwood K, Maclennan C, White AV, Chatfield MD, et al. Three‐weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: a multicentre, randomized, placebo‐controlled trial. Frontiers in Pediatrics 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum GB, Morris PS, Grimwood K, Sloots TP, White AV, Maclennan C, et al. Does 3 weeks of azithromycin improve clinical outcomes of indigenous children hospitalised with bronchiolitis: a placebo‐controlled randomised trial. Pediatric Pulmonology 2014;49:S85‐6. [Google Scholar]
McCormack 1987 {published data only}
- McCormack WM, George H, Donner A, Kodgis LF, Alpert S, Lowe EW, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrobial Agents and Chemotherapy 1977;12(5):630‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack WM, Rosner B, Lee YH, Munoz A, Charles D, Kass EH. Effect on birth weight of erythromycin treatment of pregnant women. Obstetrics and Gynecology 1987;69(2):202‐7. [PubMed] [Google Scholar]
McDonald 1985 {published data only}
- McDonald CJ, Tierney WM, Hui SL, French ML, Leland DS, Jones RB. A controlled trial of erythromycin in adults with nonstreptococcal pharyngitis. Journal of Infectious Diseases 1985;152(2):1093‐4. [DOI] [PubMed] [Google Scholar]
McGregor 1986 {published data only}
- McGregor JA, French JI, Reller LB, Todd JK, Makowski EL. Adjunctive erythromycin treatment for idiopathic preterm labor: results of a randomized, double‐blinded, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1986;154(1):98‐103. [DOI] [PubMed] [Google Scholar]
McGregor 1990 {published data only}
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Franco‐Buff A. Prospective, double‐blinded, randomized, placebo‐controlled trial of short‐course erythromycin (E) base in women at high risk for preterm birth. Society for Gynecologic Investigation. 1988.
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double‐blind, placebo‐controlled trial of erythromycin treatment. American Journal of Obstetrics and Gynecology 1990;163(5):1580‐91. [DOI] [PubMed] [Google Scholar]
McGregor 1991 {published data only}
- McGregor JA, French JI. Double‐blind, randomized, placebo controlled, prospective evaluation of the efficacy of short course erythromycin in prolonging gestation among women with preterm rupture of membranes. 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans (LA). 1989.
- McGregor JA, French JI, Seo K. Antimicrobial therapy in preterm premature rupture of membranes: results of a prospective, double‐blind, placebo‐controlled trial of erythromycin. American Journal of Obstetrics and Gynecology 1991;165(3):632‐40. [DOI] [PubMed] [Google Scholar]
Memis 2002 {published data only}
- Memis D, Turan A, Karamanlioglu B, Guler T, Yurdakoc A, Pamukcu Z, et al. Effect of preoperative oral use of erythromycin and nizatidine on gastric pH and volume. Anaesthesia and Intensive Care 2002;30(4):428‐32. [DOI] [PubMed] [Google Scholar]
Mercer 1992 {published data only}
- Mercer BM, Moretti ML, Prevost RR, Sibai BM. Erythromycin therapy in preterm premature rupture of the membranes: a prospective, randomized trial of 220 patients. American Journal of Obstetrics and Gynecology 1992;166(3):794‐802. [DOI] [PubMed] [Google Scholar]
Moller 1990 {published data only}
- Moller P, Dingsor G. Otitis media with effusion: can erythromycin reduce the need for ventilating tubes?. Journal of Laryngology and Otology 1990;104(3):200‐2. [DOI] [PubMed] [Google Scholar]
Narchi 1993 {published data only}
- Narchi P, Benhamou D, Elhaddoury M, Locatelli C, Fernandez H. Interactions of pre‐operative erythromycin administration with general anaesthesia. Canadian Journal of Anaesthesia 1993;40(5):444‐7. [DOI] [PubMed] [Google Scholar]
Neumann 2001 {published data only}
- Costa CP, Neumann FJ, Kastrati A, Stallforth I, Schmid M, Joghetai N, et al. Role of IgG‐seropositivity to Chlamydia pneumoniae in early thrombotic events after coronary stent placement. Atherosclerosis 2003;166(1):171‐6. [DOI] [PubMed] [Google Scholar]
- Neumann F, Kastrati A, Miethke T, Pogatsa‐Murray G, Mehilli J, Valina C, et al. Treatment of Chlamydia pneumoniae infection with roxithromycin and effect on neointima proliferation after coronary stent placement (ISAR‐3): a randomised, double‐blind, placebo‐controlled trial. Lancet 2001; Vol. 357, issue 9274:2085‐9. [DOI] [PubMed]
Ng 2007 {published data only}
- Ng PC, Lee CH, Wong SP, Lam HS, Liu FY, So KW, et al. High‐dose oral erythromycin decreased the incidence of parenteral nutrition‐associated cholestasis in preterm infants. Gastroenterology 2007;132(5):1726‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, So KW, Fung KS, Lee CH, Fok TF, Wong E, et al. Randomised controlled study of oral erythromycin for treatment of gastrointestinal dysmotility in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;84(3):F177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuntnarumit 2006 {published data only}
O'Connor 2003 {published data only}
- Dunne MW. Rationale and design of a secondary prevention trial of antibiotic use in patients after myocardial infarction: the WIZARD (weekly intervention with zithromax [azithromycin] for atherosclerosis and its related disorders) trial. Journal of Infectious Diseases 2000;181(Suppl 3):S572‐8. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD Study: a randomized controlled trial. JAMA 2003;290(11):1459‐66. [DOI] [PubMed] [Google Scholar]
Oei 2001 {published data only}
- Oei J, Lui K. A placebo‐controlled trial of low‐dose erythromycin to promote feed tolerance in preterm infants. Acta Paediatrica 2001;90(8):904‐8. [PubMed] [Google Scholar]
Ogrendik 2007 {published data only}
- Ogrendik M. Effects of clarithromycin in patients with active rheumatoid arthritis. Current Medical Research and Opinion 2007;23(3):515‐22. [DOI] [PubMed] [Google Scholar]
Ogrendik 2011 {published data only}
- Ogrendik M, Karagoz N. Treatment of rheumatoid arthritis with roxithromycin: a randomized trial. Postgraduate Medicine 2011;123(5):220‐7. [DOI] [PubMed] [Google Scholar]
Oldfield 1998 {published data only}
- Oldfield EC, Fessel WJ, Dunne MW, Dickinson G, Wallace MR, Byrne W, et al. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double‐blind, placebo‐controlled multicenter trial. Clinical Infectious Diseases 1998;26(3):611‐9. [DOI] [PubMed] [Google Scholar]
Ozdemir 2011 {published data only}
- Ozdemir R, Erdeve O, Dizdar EA, Oguz SS, Uras N, Karabulut E, et al. Efficacy of clarithromycin treatment in the prevention of chronic lung disease in preterm infants with birthweight < 1,250 g and Ureaplasma urealyticum colonization. Neonatology 2011;99(4):368‐9. [Google Scholar]
- Ozdemir R, Erdeve O, Dizdar EA, Oguz SS, Uras N, Saygan S, et al. Clarithromycin in preventing bronchopulmonary dysplasia in Ureaplasma urealyticum‐positive preterm infants. Pediatrics 2011;128:e1496‐501. [DOI] [PubMed] [Google Scholar]
Paknejad 2010 {published data only}
- Paknejad M, Khorsand A, Veisi N, Moslemi N, Kharazifard MJ. Azithromycin as an adjunct approach in non‐surgical treatment of chronic periodontitis. Journal of Islamic Dental Association of Iran 2010;22(2):100‐7. [Google Scholar]
Pandhi 2014 {published data only}
- Pandhi D, Singal A, Verma P, Sharma R. The efficacy of azithromycin in pityriasis rosea: a randomized, double‐blind, placebo‐controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2014;80(1):36‐40. [DOI] [PubMed] [Google Scholar]
Parchure 2002 {published data only}
- Parchure N, Zouridakis EG, Kaski JC. Effect of azithromycin treatment on endothelial function in patients with coronary artery disease and evidence of Chlamydia pneumoniae infection. Circulation 2002;105(11):1298‐303. [DOI] [PubMed] [Google Scholar]
Patole 2000 {published data only}
- Patole SK, Almonte R, Kadalraja R, Tuladhar R, Muller R, Whitehall JS. Can prophylactic oral erythromycin reduce time to full enteral feeds in preterm neonates?. International Journal of Clinical Practice 2000; Vol. 54, issue 8:504‐8. [PubMed]
- Patole SK, Kadalraja R, Tuladhar R, Almonte R, Muller R, Whitehall JS. Benefits of a standardised feeding regimen during a clinical trial in preterm neonates. International Journal of Clinical Practice 2000;54:429‐31. [PubMed] [Google Scholar]
Paul 1998 {published data only}
- Paul VK, Singh M, Buckshee K. Erythromycin treatment of pregnant women to reduce the incidence of low birth weight and preterm deliveries. International Journal of Gynecology & Obstetrics 1998;62(1):87‐8. [DOI] [PubMed] [Google Scholar]
Petersen 1997 {published data only}
- Petersen K, Phillips RS, Soukup J, Komaroff AL, Aronson M. The effect of erythromycin on resolution of symptoms among adults with pharyngitis not caused by group A streptococcus. Journal of General Internal Medicine 1997;12(2):95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 1996 {published data only}
- Peterson WL, Ciociola AA, Sykes DL, McSorley DJ, Webb DD, Adams L, et al. Ranitidine bismuth citrate plus clarithromycin is effective for healing duodenal ulcers, eradicating H‐pylori and reducing ulcer recurrence. Alimentary Pharmacology and Therapeutics 1996;10(3):251‐61. [DOI] [PubMed] [Google Scholar]
Pierce 1996 {published data only}
- Craft JC, Notario GF, Grosset JH, Heifets LB. Clarithromycin resistance and susceptibility patterns of Mycobacterium avium strains isolated during prophylaxis for disseminated infection in patients with AIDS. Clinical Infectious Diseases 1998;27(4):807‐12. [DOI] [PubMed] [Google Scholar]
- Pierce M, Crampton S, Henry D, Heifets L, LaMarca A, Montecalvo M, et al. A randomized trial of clarithromycin as prophylaxis against disseminated Mycobacterium avium complex infection in patients with advanced acquired immunodeficiency syndrome. New England Journal of Medicine 1996;335(6):384‐91. [DOI] [PubMed] [Google Scholar]
Pinto 2012 {published data only}
- D'Azevedo Silveira V, Roza CA, Luisi F, Pitrez PM, Tetelbom S, Pinto LA. Azithromycin therapy in infants with bronchiolitis reduces recurrent wheezing 3 months after hospitalization: a randomized, placebo‐controlled trial. Pediatric Pulmonology 2016;51(Suppl 42):S9. [Google Scholar]
- Pinto LA, Jones MH, Pitrez PM, Stein RT. Azithromycin administered at the time of severe bronchiolitis has a protective effect on subsequent wheezing in infants. Pediatric Pulmonology 2018;52:S165‐6. [Google Scholar]
- Pinto LA, Pitrez PM, Luisi F, Coutinho S, Mello PP, Gerhardt M, et al. Azithromycin does not reduce length of hospitalization in infants with acute bronchiolitis: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;185:A5489. [Google Scholar]
- Pinto LA, Pitrez PM, Luisi F, Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double‐blinded, and placebo‐controlled clinical trial. Journal of Pediatrics 2012;161(6):1104‐8. [DOI] [PubMed] [Google Scholar]
Pradeep 2011 {published data only}
- Pradeep AR, Kathariya R. Clarithromycin, as an adjunct to non surgical periodontal therapy for chronic periodontitis: a double blinded, placebo controlled, randomized clinical trial. Archives of Oral Biology 2011;56(10):1112‐9. [DOI] [PubMed] [Google Scholar]
Pradeep 2013 {published data only}
- Pradeep AR, Bajaj P, Agarwal E, Rao NS, Naik SB, Kalra N, et al. Local drug delivery of 0.5% azithromycin in the treatment of chronic periodontitis among smokers. Australian Dental Journal 2013;58(1):34‐40. [DOI] [PubMed] [Google Scholar]
Rajaei 2006 {published data only}
- Rajaei M, Sultani M, Zare S. A randomized controlled trial of adjunctive erythromycin in women with idiopathic preterm labor. Journal of Maternal‐Fetal & Neonatal Medicine 2006;19(1):17‐20. [DOI] [PubMed] [Google Scholar]
Reignier 2002 {published data only}
- Reignier J, Bensaid S, Perrin‐Gachadoat D, Burdin M, Boiteau R, Tenaillon A. Erythromycin and early enteral nutrition in mechanically ventilated patients. Critical Care Medicine 2002;30(6):1237‐41. [DOI] [PubMed] [Google Scholar]
Robins‐Browne 1983 {published data only}
- Robins‐Browne RM, Coovadia HM, Bodasing MN, Mackenjee MK. Treatment of acute nonspecific gastroenteritis of infants and young children with erythromycin. American Journal of Tropical Medicine and Hygiene 1983;32(4):886‐90. [DOI] [PubMed] [Google Scholar]
Roca 2016a {published data only}
- Burr SE, Camara B, Oluwalana C, Bojang E, Bottomley C, Bojang A. Does azithromycin given to women in labour decrease ocular bacterial infection in neonates? A double‐blind, randomized trial. BMC Infectious Diseases 2017;17(1):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwalana C, Camara B, Bottomley C, Goodier S, Bojang A, Kampmann B, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double‐blind trial. Pediatrics 2017;139(2):e20162281. [DOI] [PubMed] [Google Scholar]
- Roca A, Oluwalana C, Bojang A, Camara B, Kampmann B, Bailey R, et al. Oral azithromycin given during labour decreases bacterial carriage in the mothers and their offspring: a double‐blind randomized trial. Clinical Microbiology and Infection 2016;22(6):565.e1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca A, Oluwalana C, Camara B, Bojang A, Burr S, Davis TM, et al. Prevention of bacterial infections in the newborn by pre‐delivery administration of azithromycin: study protocol of a randomized efficacy trial. BMC Pregnancy and Childbirth 2015;15:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 1998 {published data only}
- Roy SK, Islam A, Ali R, Islam KE, Khan RA, Ara SH, et al. A randomized clinical trial to compare the efficacy of erythromycin, ampicillin and tetracycline for the treatment of cholera in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(4):460‐2. [DOI] [PubMed] [Google Scholar]
Rozman 1984 {published data only}
- Rozman TA, Klovekorn G, Kompa H. Double‐blind group comparison of topical meclocycline, erythromycin and placebo in the treatment of papulo‐pustulosa acne [Doppelblinder gruppenvergleich von topischem meclocyclin, erythromycin und placebo in der behandlung der akne papulo‐pustolosa]. Zeitschrift fur Hautkrankheiten 1984;59(23):1623‐34. [PubMed] [Google Scholar]
Sadreddini 2009 {published data only}
- Sadreddini S, Noshad H, Molaeefard M, Moloudi R, Ardalan MR, Ghojazadeh M. A double blind, randomized, placebo controlled study to evaluate the efficacy of erythromycin in patients with knee effusion due to osteoarthritis. International Journal of Rheumatic Diseases 2009;12(1):44‐51. [DOI] [PubMed] [Google Scholar]
Saiman 2003 {published data only}
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290(13):1749‐56. [DOI] [PubMed] [Google Scholar]
Saiman 2010 {published data only}
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in CF patients with early lung disease. Journal of Cystic Fibrosis 2014;13(1):74‐9. [DOI] [PubMed] [Google Scholar]
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in patients 6‐18 years old with CF and mild lung disease uninfected with Pseudomonas aeruginosa. Pediatric Pulmonology 2010;45:377‐8. [Google Scholar]
- Green N, Burns JL, Mayer‐Hamblett N, Kloster M, Lands LC, Anstead M, et al. Lack of association of small‐colony‐variant Staphylococcus aureus strains with long‐term use of azithromycin in patients with cystic fibrosis. Journal of Clinical Microbiology 2011;49(7):2772‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L, Anstead M, Mayer‐Hamblett N, Lands LC, Kloster M, Hocevar‐Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010;303(17):1707‐15. [DOI] [PubMed] [Google Scholar]
Sampaio 2011 {published data only}
- Sampaio E, Rocha M, Figueiredo LC, Faveri M, Duarte PM, Gomes Lira EA, et al. Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: a randomized placebo‐controlled clinical trial. Journal of Clinical Periodontology 2011;38(9):838‐46. [DOI] [PubMed] [Google Scholar]
Sander 2002 {published data only}
- Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Progression of early carotid atherosclerosis is only temporarily reduced after antibiotic treatment of Chlamydia pneumoniae seropositivity. Circulation 2004;109(8):1010‐5. [DOI] [PubMed] [Google Scholar]
- Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Reduced progression of early carotid atherosclerosis after antibiotic treatment and Chlamydia pneumoniae seropositivity. Circulation 2002;106(19):2428‐33. [DOI] [PubMed] [Google Scholar]
Schalen 1993 {published data only}
- Schalen L, Eliasson I, Fex S, Kamme C, Schalen C. Acute laryngitis in adults: results of erythromycin treatment. Acta Oto‐Laryngologica Supplementum 1992;492:55‐7. [DOI] [PubMed] [Google Scholar]
- Schalen L, Eliasson I, Kamme C, Schalen C. Erythromycin in acute laryngitis in adults. Annals of Otology, Rhinology & Laryngology 1993;102(3):209‐14. [DOI] [PubMed] [Google Scholar]
Schwameis 2017 {published data only}
- Schwameis M, Kündig T, Huber G, Bidder L, Meinel L, Weisser R, et al. Topical azithromycin for the prevention of Lyme borreliosis: a randomised, placebo‐controlled, phase 3 efficacy trial. Lancet Infectious Diseases 2017;17(3):322‐9. [DOI] [PubMed] [Google Scholar]
Seemungal 2008 {published data only}
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long‐term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2008;178(11):1139‐47. [DOI] [PubMed] [Google Scholar]
Serisier 2013 {published data only}
- Chen A, Martin M, Lourie R, Burr L, Hasnain S, Bowler S, et al. Clinical benefits of long‐term, low‐dose erythromycin in bronchiectasis are not due to modulation of sputum mucin content. Respirology 2014;19(Suppl 2):88. [Google Scholar]
- Leong L, Choo J, Seresier D, Rogers G. Long‐term erythromycin therapy affects microbiota composition and antibiotic resistance gene prevalence in the oropharynx of bronchiectasis patients. Respirology 2015;20:29. [Google Scholar]
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2014;2(12):988‐96. [DOI] [PubMed] [Google Scholar]
- Serisier DJ, Bowler SD, McGuckin M, Chen A, Lourie R, Martin ML. The bronchiectasis and low‐dose erythromycin study (BLESS). American Journal of Respiratory and Critical Care Medicine 2012;185:A6862. [Google Scholar]
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309(12):1260‐7. [DOI] [PubMed] [Google Scholar]
Shafuddin 2015 {published data only}
- Shafuddin E, Mills GD, Holmes MD, Poole PJ, Mullins PR, Black PN. A double‐blind, randomised, placebo‐controlled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. Journal of Negative Results in Biomedicine 2015;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shanson 1985 {published data only}
- Shanson DC, Akash S, Harris M, Tadayon M. Erythromycin stearate, 1.5 g, for the oral prophylaxis of streptococcal bacteraemia in patients undergoing dental extraction: efficacy and tolerance. Journal of Antimicrobial Chemotherapy 1985;15(1):83‐90. [DOI] [PubMed] [Google Scholar]
Simpson 2008 {published data only}
- Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Anti‐inflammatory effects of clarithromycin in refractory non‐eosinophilic asthma. Respirology 2007;12:A11. [Google Scholar]
- Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. American Journal of Respiratory and Critical Care Medicine 2008;177(2):148‐55. [DOI] [PubMed] [Google Scholar]
Sinisalo 2002 {published data only}
- Paju S, Pussinen PJ, Sinisalo J, Mattila K, Dogan B, Ahlberg J, et al. Clarithromycin reduces recurrent cardiovascular events in subjects without periodontitis. Atherosclerosis 2006;188(2):412‐9. [DOI] [PubMed] [Google Scholar]
- Sinisalo J, Mattila K, Valtonen V, Anttonen O, Juvonen J, Melin J, et al. Effect of 3 months of antimicrobial treatment with clarithromycin in acute non‐q‐wave coronary syndrome. Circulation 2002;105(13):1555‐60. [DOI] [PubMed] [Google Scholar]
Sirinavin 2003 {published data only}
- Sirinavin S, Thavornnunth J, Sakchainanont B, Bangtrakulnonth A, Chongthawonsatid S, Junumporn S. Norfloxacin and azithromycin for treatment of nontyphoidal Salmonella carriers. Clinical Infectious Diseases 2003;37(5):685‐91. [DOI] [PubMed] [Google Scholar]
Smith 2000 {published data only}
- Smith AJ, Nissan A, Lanouette NM, Shi W, Guillem JG, Wong WD, et al. Prokinetic effect of erythromycin after colorectal surgery: randomized, placebo‐controlled, double‐blind study. Diseases of the Colon and Rectum 2000;43(3):333‐7. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Sefton AM, Maskell JP, Beighton D, Whiley A, Shain H, Foyle D, et al. Azithromycin in the treatment of periodontal disease ‐ effect on microbial flora. Journal of Clinical Periodontology 1996;23(11):998‐1003. [DOI] [PubMed] [Google Scholar]
- Smith SR, Foyle DM, Daniels J, Joyston‐Bechal S, Smales FC, Sefton A, et al. A double‐blind placebo‐controlled trial of azithromycin as an adjunct to non‐surgical treatment of periodontitis in adults: clinical results. Journal of Clinical Periodontology 2002;29(1):54‐61. [DOI] [PubMed] [Google Scholar]
Sorensen 1992 {published data only}
- Sorensen JL, Thranov I, Hoff G, Dirach J, Damsgaard MT. A double‐blind randomized study of the effect of erythromycin in preventing pelvic inflammatory disease after first trimester abortion. British Journal of Obstetrics and Gynaecology 1992;99(5):434‐8. [DOI] [PubMed] [Google Scholar]
Taylor 1999 {published data only}
- Taylor WR, Richie TL, Fryauff DJ, Ohrt C, Picarima H, Tang D, et al. Tolerability of azithromycin as malaria prophylaxis in adults in northeast Papua, Indonesia. Antimicrobial Agents and Chemotherapy 2003;47(7):2199‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Richie TL, Fryauff DJ, Picarima H, Ohrt C, Tang D, et al. Malaria prophylaxis using azithromycin: a double‐blind, placebo‐controlled trial in Irian Jaya, Indonesia. Clinical Infectious Diseases 1999;28(1):74‐81. [DOI] [PubMed] [Google Scholar]
Tita 2016 {published data only}
- Boggess K, Tita A, Jauk V, Saade G, Longo S, Clark E, et al. Clinical risk factors for post‐cesarean surgical site infection despite pre‐incision azithromycin‐based extended spectrum antibiotic prophylaxis. American Journal of Obstetrics and Gynecology 2016;214(1):S118‐9. [Google Scholar]
- Boggess K, Tita A, Jauk V, Saade G, Longo S, Clark E, et al. Risk factors for postcesarean maternal infection in a trial of extended‐spectrum antibiotic prophylaxis. Obstetrics and Gynecology 2017;129(3):481‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. Neonatal outcomes in term and preterm infants following adjunctive azithromycin antibiotic prophylaxis for non‐elective cesarean delivery. American Journal of Obstetrics and Gynecology 2017;216(1):S195. [DOI] [PubMed] [Google Scholar]
- Pasko DN, Tita AT. Does the effect of adjunctive azithromycin on cesarean infection vary with cefazolin dose?. American Journal of Obstetrics and Gynecology 2018;218(1):S522. [Google Scholar]
- Saade G. Incidence and risk factors for hospital readmission after unscheduled cesarean. American Journal of Obstetrics and Gynecology 2017;216(1):S416‐7. [Google Scholar]
- Tita A. Impact of azithromycin‐based extended spectrum antibiotic prophylaxis on non‐infectious cesarean wound complications. American Journal of Obstetrics and Gynecology 2017;216(1):S116. [Google Scholar]
- Tita A, Szychowski J, Boggess K, Saade G, Longo S, Clark E, et al. Azithromycin‐based extended spectrum antibiotic prophylaxis for non‐elective cesarean delivery: a pragmatic multicenter placebo‐controlled double‐blind RCT. American Journal of Obstetrics and Gynecology 2016;214(1):S3. [Google Scholar]
- Tita A, Szychowski JM, Boggess K, Saade G, Longo S, Clark E, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. New England Journal of Medicine 2016;375(13):1231‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tita A, Waites K, Jauk V, Biggio J, Sutton A, Szychowski J, et al. RCT of azithromycin‐based extended‐spectrum antibiotic prophylaxis for cesarean delivery: role of placental colonization with ureaplasma or mycoplasma. American Journal of Obstetrics and Gynecology 2016;214(1):S70‐1. [DOI] [PubMed] [Google Scholar]
Uzun 2014 {published data only}
- Djamin RS, Uzun S, Ermens AM, Kertens R, Hoogsteden HC, Aerts JG, et al. Which predictors in COPD patients with the frequent exacerbator phenotype predict the treatment response to maintenance therapy with azithromycin?. European Respiratory Journal 2016;48(Suppl 60):PA3713. [Google Scholar]
- Uzun S, Djamin RS, Aerts JG, Eerden MM. Patients with COPD GOLD C & D: the effect of long‐term treatment with azithromycin on exacerbation risk assessed by the GOLD framework. American Journal of Respiratory and Critical Care Medicine 2014;189:A5967. [Google Scholar]
- Uzun S, Djamin RS, Kluytmans J, Van't Veer NE, Ermens AA, Pelle AJ, et al. Influence of macrolide maintenance therapy and bacterial colonisation on exacerbation frequency and progression of COPD (COLUMBUS): study protocol for a randomised controlled trial. Trials 2012;13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double‐blind, placebo‐controlled trial. Lancet Respiratory Medicine 2014;2(5):361‐8. [DOI] [PubMed] [Google Scholar]
- Uzun S, Djamin RS, Mulder PG, Kluytmans JA, 't Veer NE, Pelle AJ, et al. Effect of azithromycin maintenance treatment in patients with frequent exacerbations of COPD (COLUMBUS): a randomized, double‐blind, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A2884. [DOI] [PubMed] [Google Scholar]
Vainas 2005 {published data only}
- Vainas T, Kitslaar PJ. The effect of antibiotics on atherosclerosis. Results of a randomized study in patients with peripheral arterial disease [Beïnvloeding van atherosclerose door antibiotica]. Hart Bulletin 2005;36(5):131‐3. [Google Scholar]
- Vainas T, Stassen FR, Schurink GW, Tordoir JH, Welten RJ, Akker LH, et al. Secondary Prevention of Atherosclerosis through Chlamydia pneumoniae Eradication (SPACE Trial): a randomised clinical trial in patients with peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2005;29(4):403‐11. [DOI] [PubMed] [Google Scholar]
Valery 2013 {published data only}
- Hare KM, Grimwood K, Chang AB, Chatfield MD, Valery PC, Leach AJ, et al. Nasopharyngeal carriage and macrolide resistance in Indigenous children with bronchiectasis randomized to long‐term azithromycin or placebo. European Journal of Clinical Microbiology & Infectious Diseases 2015;34(11):2275‐85. [DOI] [PubMed] [Google Scholar]
- Singleton R, Morris P, Leach A, Roseby R, White A. Multicentre bronchiectasis study; a collaboration and international study of bronchiectasis in indigenous children. Respirology 2007;12(Suppl 4):A192. [Google Scholar]
- Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, et al. Long‐term azithromycin for Indigenous children with non‐cystic‐fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double‐blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1(8):610‐20. [DOI] [PubMed] [Google Scholar]
- Valery PC, Morris PS, Grimwood K, Torzillo PJ, Byrnes CA, Masters IB, et al. Azithromycin for Indigenous children with bronchiectasis: study protocol for a multi‐centre randomized controlled trial. BMC Pediatrics 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vammen 2001 {published data only}
- Hogh A, Vammen S, Ostergaard L, Joensen JB, Henneberg EW, Lindholt JS. Intermittent roxithromycin for preventing progression of small abdominal aortic aneurysms: long‐term results of a small clinical trial. Vascular and Endovascular Surgery 2009;43(5):452‐6. [DOI] [PubMed] [Google Scholar]
- Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double‐blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. British Journal of Surgery 2001;88(8):1066‐72. [DOI] [PubMed] [Google Scholar]
- Vammen S, Lindholt JS, Ostergaard LJ, Fasting H, Henneberg EW. Reduction of the expansion rate of small abdominal aortic aneurysms with roxithromycin. Results from a randomized controlled trial [Hæmning af små abdominale aortaaneurismers ekspansion med roxithromycin. Resultater fra et randomiseret klinisk forsøg]. Ugeskrift for Laeger 2002;164:5916‐9. [PubMed] [Google Scholar]
Van Delden 2012 {published data only}
- Köhler T, Perron GG, Buckling A, Delden C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathogens 2010;6(5):e1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delden C, Kohler T, Brunner‐Ferber F, Francois B, Carlet J, Pechere JC. Azithromycin to prevent Pseudomonas aeruginosa ventilator‐associated pneumonia by inhibition of quorum sensing: a randomized controlled trial. Intensive Care Medicine 2012;38(7):1118‐25. [DOI] [PubMed] [Google Scholar]
Van den Broek 2009 {published data only}
- Broek NR, White SA, Goodall M, Ntonya C, Kayira E, Kafulafula G, et al. The APPLe study: a randomized, community‐based, placebo‐controlled trial of azithromycin for the prevention of preterm birth, with meta‐analysis. PLoS Medicine 2009;6(12):e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veskitkul 2017 {published data only}
- Veskitkul J, Wongkaewpothong P, Thaweethamchareon T, Ungkanont K, Visitsunthorn N, Pacharn P, et al. Recurrent acute rhinosinusitis prevention by azithromycin in children with nonallergic rhinitis. Journal of Allergy and Clinical Immunology: In Practice 2017;5(6):1632‐8. [DOI] [PubMed] [Google Scholar]
Videler 2011 {published data only}
- Videler WJ, Badia L, Harvey RJ, Gane S, Georgalas C, Meulen FW, et al. Lack of efficacy of long‐term, low‐dose azithromycin in chronic rhinosinusitis: a randomized controlled trial. Allergy 2011;66(11):1457‐68. [DOI] [PubMed] [Google Scholar]
Vos 2011 {published data only}
- Ruttens D, Verleden SE, Vandermeulen E, Bellon H, Vanaudenaerde BM, Somers J, et al. Prophylactic azithromycin therapy after lung transplantation: post hoc analysis of a randomized controlled trial. American Journal of Transplantation 2016;16(1):254‐61. [DOI] [PubMed] [Google Scholar]
- Vos R, Herck A, Vanaudenaerde BM, Verleden SE, Raemdonck DE, Frick A, et al. A prospective, randomized, placebo‐controlled trial of pre‐transplant and prompt post‐transplant treatment with azithromycin to improve early allograft function and outcome after lung transplantation (NCT01915082). Journal of Heart and Lung Transplantation 2018;37(4):S88. [Google Scholar]
- Vos R, Vanaudenaerde BM, Vleeschauwer SI, Schoonis A, Raemdonck DE, Dupont LJ, et al. Randomized, double‐blind, placebo‐controlled trial of azithromycin in lung transplantation: first interim results. Journal of Heart and Lung Transplantation 2009;28(2):S119. [Google Scholar]
- Vos R, Vanaudenaerde BM, Schoonis A, Raemdonck DE, Dupont LJ, Verleden GM. Azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Journal of Heart and Lung Transplantation 2010;29(2):S94. [Google Scholar]
- Vos R, Vanaudenaerde BM, Verleden SE, Vleeschauwer SI, Willems‐Widyastuti A, Raemdonck DE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. European Respiratory Journal 2011;37(1):164‐72. [DOI] [PubMed] [Google Scholar]
- Vos R, Vanaudenaerde BM, Verleden SE, Dupont LJ, Raemdonck DE, Verleden GM. Evaluation of FVC and FEV1 in both cohorts previously included in a randomized controlled trial of azithromycin to prevent bronchiolitis obliterans syndrome after lung transplantation. Journal of Heart and Lung Transplantation 2012;31:S123. [Google Scholar]
Wallwork 2006 {published data only}
- Wallwork B, Coman W, Mackay‐Sim A, Greiff L, Cervin A. A double‐blind, randomized, placebo‐controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006;116(2):189‐93. [DOI] [PubMed] [Google Scholar]
Walsh 1998 {published data only}
- Walsh T, Grimes D, Frezieres R, Nelson A, Bernstein L, Coulson A, et al. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. IUD study group. Lancet 1998;351(9108):1005‐8. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang Y, Zhang SL, Qu Y. Effect of clarithromycin on non‐eosinophilic refractory asthma [克拉霉素治疗非嗜酸粒细胞型难治性哮喘的疗效分析]. Journal of Clinical Pulmonary Medicine 2012;17:1948‐51. [Google Scholar]
Wiesli 2002 {published data only}
- Krayenbuehl PA, Wiesli P, Maly FE, Vetter W, Schulthess G. Progression of peripheral arterial occlusive disease is associated with Chlamydia pneumoniae seropositivity and can be inhibited by antibiotic treatment. Atherosclerosis 2005;179(1):103‐10. [DOI] [PubMed] [Google Scholar]
- Wiesli P, Czerwenka W, Meniconi A, Maly FE, Hoffmann U, Vetter W, et al. Roxithromycin treatment prevents progression of peripheral arterial occlusive disease in Chlamydia pneumoniae seropositive men: a randomized, double‐blind, placebo‐controlled trial. Circulation 2002;105(22):2646‐52. [DOI] [PubMed] [Google Scholar]
Wilson 1977 {published data only}
- Wilson SZ, Martin RR, Putman M. In vivo effects of josamycin, erythromycin, and placebo therapy on nasal carriage of Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 1977;11(3):407‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1979 {published data only}
- Wilson SZ, Martin RR, Putman M, Greenberg SB, Wallace RJ, Jemsek JG. Quantitative nasal cultures from carriers of Staphylococcus aureus: effects of oral therapy with erythromycin, rosamicin, and placebo. Antimicrobial Agents and Chemotherapy 1979;15(3):379‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkler 1988 {published data only}
- Winkler M, Baumann L, Ruckhaberle KE, Schiller EM. Erythromycin therapy for subclinical intrauterine infections in threatened preterm delivery ‐ a preliminary report. Journal of Perinatal Medicine 1988;16(3):253‐6. [PubMed] [Google Scholar]
Wolter 2002 {published data only}
- Bowler SD, Masel PJ, Bell SC, Seeney SL, Wolter JM, McCormack JG. A prospective, randomised trial of long term azithromycin (AZM) versus placebo in cystic fibrosis: impact on clinical, laboratory and quality of life (QOL) outcomes. Fourteenth Annual North American Cystic Fibrosis Conference; 2000 Nov 9‐12; Baltimore (MD). Wiley‐Liss, Div John Wiley & Sons, 2000.
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57(3):212‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Jayaram L, Wong CA, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin decreases exacerbations in noncystic fibrosis bronchiectasis. Respirology 2012;17(Suppl 1):35. [Google Scholar]
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis (EMBRACE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;380(9842):660‐7. [DOI] [PubMed] [Google Scholar]
- Wong CA, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin decreases exacerbations in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;185:A3657. [Google Scholar]
Yang 2013 {published data only}
- Yang SS, Pan XJ, Wang HG, Zhao GQ. A randomized, double‐blind and placebo‐controlled clinical trail of topical administration of 1% azithromycin eye drops for acute bacterial conjunctivitis. Zhonghua Shiyan Yanke Zazhi [Chinese Journal of Experimental Ophthalmology] 2013;31(2):182‐5. [Google Scholar]
Yeo 1993 {published data only}
- Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy: a prospective, randomized, placebo‐controlled trial. Annals of Surgery 1993;218(3):229‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zahn 2003 {published data only}
- Burkhardt U, Zahn R, Hoffler U, Siegler KE, Frilling B, Weber M, et al. Antibody levels against Chlamydia pneumoniae and outcome of roxithromycin therapy in patients with acute myocardial infarction. Results from a sub‐study of the randomised Antibiotic Therapy in Acute Myocardial Infarction (ANTIBIO) trial [Antikörper gegen Chlamydia pnuemoniae und roxitromycin therapie bei patienten mit einem akuten myokardinfarkt ‐ ergebnisse einer substudie der randomisierten antibiotischen therapie beim akuten myokardinfarkt (ANTIBIO) studie]. Zeitschrift Fur Kardiologie 2004;93(9):671‐8. [DOI] [PubMed] [Google Scholar]
- Zahn R, Schneider S, Frilling B, Seidl K, Tebbe U, Weber M, et al. Antibiotic therapy after acute myocardial infarction: a prospective randomized study. Circulation 2003;107(9):1253‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboud 2009 {published data only}
- Aboud S, Msamanga G, Read JS, Wang L, Mfalila C, Sharma U, et al. Effect of prenatal and perinatal antibiotics on maternal health in Malawi, Tanzania, and Zambia. International Journal of Gynecology & Obstetrics 2009;107(3):202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2007 {published data only}
- Ballard HO, Anstead MI, Shook LA. Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respiratory Research 2007;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batieha 2002 {published data only}
- Batieha A, Yahia G, Mahafzeh T, Omari M, Momani A, Dabbas M. No advantage of treating acute respiratory tract infections with azithromycin in a placebo‐controlled study. Scandinavian Journal of Infectious Diseases 2002;34(4):243‐7. [DOI] [PubMed] [Google Scholar]
Doan 2017 {published data only}
- Doan T, Arzika AM, Ray KJ, Cotter SY, Kim J, Maliki R, et al. Gut microbial diversity in antibiotic‐naive children after systemic antibiotic exposure: a randomized controlled trial. Clinical Infectious Diseases 2017;64(9):1147‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferahbas 2004 {published data only}
- Ferahbas A, Utas S, Aykol D, Borlu M, Uksal U. Clinical evaluation of roxithromycin: a double‐blind, placebo‐controlled and crossover trial in patients with acne vulgaris. Journal of Dermatology 2004;31(1):6‐9. [DOI] [PubMed] [Google Scholar]
Figueiredo‐Mello 2018 {published data only}
- Figueiredo‐Mello C, Naucler P, Negra MD, Levin AS. Ceftriaxone versus ceftriaxone plus a macrolide for community‐acquired pneumonia in hospitalized patients with HIV/AIDS: a randomized controlled trial. Clinical Microbiology and Infection 2018;24(2):171‐4. [DOI] [PubMed] [Google Scholar]
Gong 2014 {published data only}
- Gong Y, Lu J, Ding X, Yu Y. Effect of adjunctive roxithromycin therapy on interleukin‐1β, transforming growth factor‐β1 and vascular endothelial growth factor in gingival crevicular fluid of cyclosporine A‐treated patients with gingival overgrowth. Journal of Periodontal Research 2014;49(4):448‐57. [DOI] [PubMed] [Google Scholar]
Makkar 2016 {published data only}
- Makkar J, Gauli B, Jain K, Jain D, Batra YK. Comparison of erythromycin versus metoclopramide for gastric feeding intolerance in patients with traumatic brain injury: a randomized double‐blind study. Saudi Journal of Anaesthesia 2016;10(3):308‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2016 {published data only}
- Nielsen HL, Kirk KF, Bodilsen J, Ejlertsen T, Nielsen H. Azithromycin vs. placebo for the clinical outcome in Campylobacter concisus diarrhoea in adults: a randomised, double‐blinded, placebo‐controlled clinical trial. PLOS ONE 2016;11(11):e0166395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2017 {published data only}
- Parker EP, Praharaj I, John J, Kaliappan SP, Kampmann B, Kang G, et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo‐controlled trial among infants in south India. Scientific Reports 2017;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pazoki‐Toroudi 2010 {published data only}
- Pazoki‐Toroudi H, Nassiri‐Kashani M, Tabatabaie H, Ajami M, Habibey R, Shizarpour M, et al. Combination of azelaic acid 5% and erythromycin 2% in the treatment of acne vulgaris. Journal of Dermatological Treatment 2010;21(3):212‐6. [DOI] [PubMed] [Google Scholar]
Rasi 2008 {published data only}
- Rasi A, Tajziehchi L, Savabi‐Nasab S. Oral erythromycin is ineffective in the treatment of pityriasis rosea. Journal of Drugs in Dermatology 2008; Vol. 7, issue 1:35‐8. [PubMed]
Sharma 2000 {published data only}
- Sharma PK, Yadav TP, Gautam RK, Taneja N, Satyanarayana L. Erythromycin in pityriasis rosea: a double‐blind, placebo‐controlled clinical trial. Journal of the American Academy of Dermatology 2000;42(2 Pt 1):241‐4. [DOI] [PubMed] [Google Scholar]
Stokholm 2016 {published data only}
- Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma‐like symptoms in young children aged 1‐3 years: a randomised, double‐blind, placebo‐controlled trial. Lancet Respiratory Medicine 2016;4(1):19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 1993 {published data only}
- Weber K, Thurmayr R, Meisinger A. A topical erythromycin preparation and oral tetracycline for the treatment of perioral dermatitis: a placebo‐controlled trial. Journal of Dermatological Treatment 1993; Vol. 4:57‐9.
Yamamoto 1992 {published data only}
- Yamamoto M. Therapeutic effects of erythromycin on diffuse panbronchiolitis. A multicenter, double blind placebo‐controlled trial. Sarcoidosis 1992;9:633‐6. [Google Scholar]
Zhang 2006 {published data only}
- Zhang H, Kandel RP, Atakari HK, Dean D. Impact of oral azithromycin on recurrence of trachomatous trichiasis in Nepal over 1 year. British Journal of Ophthalmology 2006;90(8):943‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12617000531314 {unpublished data only}
- ACTRN12617000531314. Clinical and microbiological evaluation of nonsurgical treatment of chronic periodontitis with systemically administered azithromycin [Clinical and microbiological evaluation of one‐stage full mouth disinfection in conjunction with systemically administered azithromycin: a randomised controlled clinical trial in patients with moderate to advanced chronic periodontitis]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000531314 (first received 13 March 2017).
ChiCTR‐INR‐17013272 {unpublished data only}
- ChiCTR‐INR‐17013272. Adjunctive azithromycin prophylaxis for preventing cesarean scar defect [The infectious etiology of the cesarean scar defect and the prevention effect of the application of azithromycin in caesarean section]. www.chictr.org.cn/showproj.aspx?proj=22739 (first received 7 November 2017).
ChiCTR‐IOR‐16008820 {unpublished data only}
- ChiCTR‐IOR‐16008820. Effect of low‐dose erythromycin on the treatment of COPD [Effect of low‐dose erythromycin on the treatment of COPD]. www.chictr.org.cn/showprojen.aspx?proj=14443 (first received 11 July 2016).
CTRI/2017/07/009017 {unpublished data only}
- CTRI/2017/07/009017. Improved diarrhoea management for children with high risk of mortality [Antibiotics for Children with Severe Diarrhoea (ABCD) Trial]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15841 (first received 7 July 2017).
Dicko 2016 {published data only}
- Dicko A, Ouedraogo JB, Zongo I, Sagara I, Cairns M, Kuepfer I, et al. A trial of seasonal malaria chemoprevention plus azithromycin in African children. American Journal of Tropical Medicine and Hygiene 2016;95(5):480‐1. [Google Scholar]
EUCTR2011‐004351‐39‐IT {unpublished data only}
- EUCTR2011‐004351‐39‐IT. Phase II, randomized, double arm, multi‐center study evaluating the efficacy and safety of azithromycin for the long term prophylactic treatment of COPD in primary antibody deficiency patients with clinical and spirometrically confirmed COPD suffering from repeated acute exacerbations [Phase II, randomized, double arm, multi‐center study evaluating the efficacy and safety of azithromycin for the long term prophylactic treatment of COPD in primary antibody deficiency patients with clinical and spirometrically confirmed COPD suffering from repeated acute exacerbations]. www.clinicaltrialsregister.eu/ctr‐search/trial/2011‐004351‐39/IT (first received 13 March 2012).
EUCTR2012‐002792‐34‐GB {unpublished data only}
- EUCTR2012‐002792‐34‐GB. The characterisation of bronchiectasis over 2 years with a trial of a low dose antibiotic in the second year with the aim of identifying characteristics that mean people show the most improvement whilst on the drug [Phenotyping bronchiectasis based on aetiology, exacerbation characteristics and response to erythromycin]. www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐002792‐34/GB (first received 13 March 2014).
EUCTR2015‐004306‐42‐SI {unpublished data only}
- EUCTR2015‐004306‐42‐SI. Comparison of the efficacy of treatment of chronic periodontitis with scaling and root‐planning alone or in combination with azithromycin ‐ a prospective, double blind, randomised clinical trial [Comparison of the efficacy of treatment of chronic periodontitis with scaling and root‐planning alone or in combination with azithromycin ‐ a prospective, double blind, randomised clinical trial]. www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐004306‐42/SI (first received 17 December 2015).
Gregersen 2017 {published data only}
- Gregersen H, Abildgaard N, Hieu Do T, Kristensen IB, Frølund UC, Andersen NF, et al. A randomized placebo‐controlled phase II study of clarithromycin or placebo combined with VCD induction therapy prior to high‐dose melphalan with stem cell support in patients with newly diagnosed multiple myeloma. Blood 2017;130(Suppl 1):3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2015052322383N1 {unpublished data only}
- IRCT2015052322383N1. Clinical trial azithromycin versus doxycycline chemoprophylaxis in leptospirosis in farmers [A randomized double blind placebo‐controlled trial: comparison of azithromycin with doxycycline prophylaxis against leptospirosis in human in an endemic area]. www.en.irct.ir/trial/19314 (first received 7 July 2016).
KCT0002373 {unpublished data only}
- KCT0002373. The efficacy and safety of azithromycin in preventing bronchopulmonary dysplasia in Ureaplasma‐positive preterm infants [The efficacy and safety of azithromycin in preventing bronchopulmonary dysplasia in Ureaplasma‐positive preterm infants: prospective, randomized, double‐blind, placebo‐controlled study]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=10848 (first received 7 July 2017).
Milito 2017 {published data only}
- Milito C, Pulvirenti F, Tabolli S, Carello R, Quinti I. Antibiotic prophylaxis in primary antibody deficiency patients: study design. Journal of Clinical Immunology 2017;37:240‐1. [Google Scholar]
- Milito C, Pulvirenti F, Tabolli S, Carrabba M, Fabio G, Pietrogrande M, et al. Antibiotic prophylaxis in primary antibody deficiency patients: study design. Allergy: European Journal of Allergy and Clinical Immunology 2017;72:251‐2. [Google Scholar]
NCT01270074 {unpublished data only}
- NCT01270074. Prevention of bronchiectasis in infants with cystic fibrosis [A Phase 3 multi‐centre randomised placebo‐controlled study of azithromycin in the primary prevention of radiologically‐defined bronchiectasis in infants with cystic fibrosis]. clinicaltrials.gov/ct2/show/record/NCT01270074 (first received 23 December 2010).
NCT01778634 {unpublished data only}
- NCT01778634. Trial of intravenous azithromycin to eradicate Ureaplasma respiratory tract infection in preterm infants [A Phase IIb randomized, placebo‐controlled, double‐blind trial of azithromycin to eradicate Ureaplasma respiratory tract infection in preterm infants]. clinicaltrials.gov/ct2/show/study/NCT01778634 (first received 22 January 2013).
NCT02003911 {unpublished data only}
- NCT02003911. Azithromycin for children hospitalized with asthma [A double‐blind, randomized, placebo‐controlled trial of azithromycin in children hospitalized with acute asthma exacerbations]. clinicaltrials.gov/ct2/show/NCT02003911 (first received 21 November 2013).
NCT02307825 {unpublished data only}
- NCT02307825. Azithromycin for patients with chronic rhinosinusitis failing medical and surgical therapy [Azithromycin as add‐on therapy in patients failing medical and surgical treatment for chronic rhinosinusitis: a double‐blind, randomized, placebo‐controlled trial]. clinicaltrials.gov/ct2/show/record/NCT02307825 (first received 11 November 2014).
NCT02336516 {unpublished data only}
- NCT02336516. Azithromycin in post diarrheal haemolytic and uremic syndrome [Azithromycin in post diarrheal haemolytic and uremic syndrome]. clinicaltrials.gov/ct2/show/record/NCT02336516 (first received 8 January 2015).
NCT02677701 {unpublished data only}
- NCT02677701. Testing the effect of adding chronic oral azithromycin to inhaled tobramycin in people with CF [TEACH trial: testing the effect of adding chronic azithromycin to inhaled tobramycin. A randomized, placebo‐controlled, double‐blinded trial of azithromycin 500mg thrice weekly in combination with inhaled tobramycin]. clinicaltrials.gov/ct2/show/record/NCT02677701 (first received 29 January 2016).
NCT02756403 {unpublished data only}
- NCT02756403. A randomized controlled trial of three antibiotic regimens for first trimester abortions [A randomized controlled trial of three prophylactic antibiotic regimens for first trimester surgical abortion]. clinicaltrials.gov/ct2/show/record/NCT02756403 (first received 20 March 2016).
NCT02911935 {unpublished data only}
- NCT02911935. Azithromycin to prevent wheezing following severe RSV bronchiolitis‐II [Azithromycin to prevent wheezing following severe RSV bronchiolitis‐II]. clinicaltrials.gov/ct2/show/record/NCT02911935 (first received 18 September 2016).
NCT02960503 {unpublished data only}
- NCT02960503. Macrolide therapy to improve forced expiratory volume in 1 second in adults with sickle cell disease [Macrolide therapy to improve forced expiratory volume in 1 second in adults with sickle cell disease: a feasibility trial]. clinicaltrials.gov/ct2/show/record/NCT02960503 (first received 2 November 2016).
NCT03130114 {unpublished data only}
- NCT03130114. Antibiotics for children with severe diarrhoea [Antibiotics for children with severe diarrhoea]. clinicaltrials.gov/ct2/show/record/NCT03130114 (first received 23 April 2017).
NCT03233880 {unpublished data only}
- NCT03233880. Impact of antichlamydial treatment on the rate of preeclampsia [Impact of antichlamydial treatment on the rate of preeclampsia among Egyptian primigravidae: a randomized controlled trial]. clinicaltrials.gov/ct2/show/record/NCT03233880 (first received 23 July 2017).
NCT03248297 {unpublished data only}
- NCT03248297. Antibiotic prophlaxis for high‐risk laboring women in low income countries [Azithromycin with or without amoxicillin to prevent peripartum infection and sepsis in laboring high‐risk women: 3‐arm RCT]. clinicaltrials.gov/ct2/show/record/NCT03248297 (first received 25 July 2017).
NCT03341273 {unpublished data only}
- NCT03341273. A randomized double‐blinded, placebo‐controlled trial of antibiotic therapy in patients with lower respiratory tract infection (LRTI) and a procalcitonin level [Targeted reduction of antibiotics using procalcitonin in a multi‐center, randomized, double‐blinded, placebo‐controlled non‐inferiority study of azithromycin treatment in outpatient adults with suspect lower respiratory tract infection (LRTI) and a procalcitonin (PCT) level of < /= 0.25 ng/mL (TRAP‐LRTI)]. clinicaltrials.gov/ct2/show/record/NCT03341273 (first received 9 November 2017).
NCT03345992 {unpublished data only}
- NCT03345992. Benefit of clarithromycin in patients with severe infections through modulation of the immune system [A double‐blind, randomized, placebo‐controlled clinical study of the efficacy of intravenous clarithromycin as adjunctive treatment in patients with sepsis and respiratory and multiple organ dysfunction syndrome]. clinicaltrials.gov/ct2/show/record/NCT03345992 (first received 9 November 2017).
Ramsey 2017 {published data only}
- Ramsey BW, Retsch‐Bogart GZ, Kloster M, Buckingham R, Hamblett NM. Efficacy and safety of azithromycin for treatment of early pseudomonas in cystic fibrosis: the optimize trial. Pediatric Pulmonology 2017;52:380‐1. [Google Scholar]
RBR‐9pqqpb {unpublished data only}
- RBR‐9pqqpb. Azithromycin in the treatment of chronic sinusitis: clinical and biopsy evaluation in a controlled study [Azithromycin in the treatment of eosinophilic nasossinusal polypose: clinical and histomorphological analysis in a randomized masked study with placebo]. ensaiosclinicos.gov.br/rg/RBR‐9pqqpb (first received 11 September 2017).
References to ongoing studies
Chang 2012 {published data only}
- Chang AB, Grimwood K, Robertson CF, Wilson AC, Asperen PP, O'Grady KA, et al. Antibiotics for bronchiectasis exacerbations in children: rationale and study protocol for a randomised placebo‐controlled trial. Trials 2012;13:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Martinez 2017 {published data only}
- Gonzalez‐Martinez C, Kranzer K, McHugh G, Corbett EL, Mujuru H, Nicol MP, et al. Azithromycin versus placebo for the treatment of HIV‐associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial. Trials 2017;18(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobbernagel 2016 {published data only}
- Kobbernagel HE, Buchvald FF, Haarman EG, Casaulta C, Collins AA, Hogg C, et al. Study protocol, rationale and recruitment in a European multi‐centre randomized controlled trial to determine the efficacy and safety of azithromycin maintenance therapy for 6 months in primary ciliary dyskinesia. BMC Pulmonary Medicine 2016;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosquera 2016 {published data only}
- Mosquera RA, Gomez‐Rubio AM, Harris T, Yadav A, McBeth K, Gonzales T, et al. Anti‐inflammatory effect of pro macrolides on children with chronic lung disease: a protocol for a double‐blinded randomised controlled trial. BMJ Open 2016;6:e012060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavlinac 2017 {published data only}
- Pavlinac PB, Singa BO, John‐Stewart GC, Richardson BA, Brander RL, McGrath CJ, et al. Azithromycin to prevent post‐discharge morbidity and mortality in Kenyan children: a protocol for a randomised, double‐blind, placebo‐controlled trial (the Toto Bora trial). BMJ Open 2017;7:e019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeersch 2016 {published data only}
- Vermeersch K, Gabrovska M, Deslypere G, Demedts IK, Slabbynck H, Aumann J, et al. The Belgian trial with azithromycin for acute COPD exacerbations requiring hospitalization: an investigator‐initiated study protocol for a multicenter, randomized, double‐blind, placebo‐controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:687‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aabenhus 2016
- Aabenhus R, Siersma V, Hansen MP, Bjerrum L. Antibiotic prescribing in Danish general practice 2004‐13. Journal of Antimicrobial Chemotherapy 2016;71(8):2286‐94. [DOI] [PubMed] [Google Scholar]
Abu‐Gharbieh 2004
- Abu‐Gharbieh E, Vasina V, Poluzzi E, Ponti F. Antibacterial macrolides: a drug class with a complex pharmacological profile. Pharmacological Research 2004;50(3):211‐22. [DOI] [PubMed] [Google Scholar]
Ahmed 2016 [pers comm]
- Ahmed N. One patient from group B (clarithromycin group) complained of heartburn at follow up after one week [personal communication]. Email to: MP Hansen 12 March 2016.
Albert 2014
- Albert RK, Schuller JL. Macrolide antibiotics and the risk of cardiac arrhythmias. American Journal of Respiratory and Critical Care Medicine 2014;189(10):1173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2018
- Allen EN, Chandler CIR, Mandimika N, Leisegang C, Barnes K. Eliciting adverse effects data from participants in clinical trials. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.MR000039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aronson 2013
- Aronson JK. Distinguishing hazards and harms, adverse drug effects and adverse drug reactions: implications for drug development, clinical trials, pharmacovigilance, biomarkers, and monitoring. Drug Safety 2013;36(3):147‐53. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2014
- Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta‐analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infectious Diseases 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 1976
- Braun P. Hepatotoxicity of erythromycin. Journal of Infectious Diseases 1969;119(3):300‐6. [DOI] [PubMed] [Google Scholar]
Brill 2016 [pers comm]
- Brill S. Report on number of participants with resistant bacteria and the number of resistant isolates, however still awaiting reply on what type of resistant bacteria they report on (macrolide‐resistant or ‘others’) [personal communication]. Email to: Hansen MP 21 June 2016.
Chandey 2012
- Chandey M, Multani AS. A comparative study of efficacy and safety of azithromycin and ofloxacin in uncomplicated typhoid fever: a randomised, open labelled study. Journal of Clinical and Diagnostic Research 2012;6(10):1736‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014
- Chen YF, Hemming K, Chilton PJ, Gupta KK, Altman DG, Lilford RJ. Scientific hypotheses can be tested by comparing the effects of one treatment over many diseases in a systematic review. Journal of Clinical Epidemiology 2014;67(12):1309‐19. [DOI] [PubMed] [Google Scholar]
Dalal 2017
- Dalal A, Eskin‐Schwartz M, Mimoui D, Ray S, Days W, Hodak E, et al. Interventions for the prevention of recurrent erysipelas and cellulitis. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD009758.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
DANMAP 2016
- The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP). DANMAP 2016 ‐ Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. www.danmap.org (accessed 17 April 2017).
Dawson 2013
- Dawson AL, Dellavalle RP. Acne vulgaris. BMJ 2013;346:f2634. [DOI] [PubMed] [Google Scholar]
Dinos 2017
- Dinos GP. The macrolide antibiotic renaissance. British Journal of Pharmacology 2017;174(18):2967‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougherty 2012
- Dougherty TJ, Pucci MJ (editors). Antibiotic Discovery and Development. Vol. 1, New York: Springer, 2012. [Google Scholar]
ECDC 2017a
- European Centre for Disease Prevention and Control. Summary of the latest data on antibiotic consumption in EU: 2017. www.ecdc.europa.eu/en/publications‐data/summary‐latest‐data‐antibiotic‐consumption‐eu‐2017 (accessed 20 April 2018).
ECDC 2017b
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal 2017;15(2):4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2000
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356(9237):1255‐9. [DOI] [PubMed] [Google Scholar]
EndNote 2016 [Computer program]
- Thomson Reuters. EndNote. Version X8. Thomson Reuters, 2016.
Enweluzo 2013
- Enweluzo C, Aziz F. Gastroparesis: a review of current and emerging treatment options. Clinical and Experimental Gastroenterology 2013;6:161‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2011
- Evans JR, Solomon AW. Antibiotics for trachoma. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001860.pub3] [DOI] [PubMed] [Google Scholar]
Ford 2016
- Ford AC, Gurusamy KS, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori‐positive people. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD003840.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsburg 1976
- Ginsburg CM, Eichenwald HF. Erythromycin: a review of its uses in pediatric practice. Journal of Pediatrics 1976;89(6):872‐84. [DOI] [PubMed] [Google Scholar]
Golder 2016
- Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLOS Medicine 2016;13(9):e1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 10 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grassly 2017 [pers comm]
- Grassly N. A list of observed adverse events provided [personal communication]. Email to: Hansen MP 30 October 2017.
Hansen 2018a
- Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, et al. Adverse events by System Organ Classes: threshold ≥ 5%. Supplementary data set A. research.bond.edu.au/en/datasets/adverse‐events‐in‐patients‐taking‐macrolide‐antibiotics‐versus‐pl (accessed 12 December 2018). [DOI: 10.26139/5c10510a28064] [DOI]
Hansen 2018b
- Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, et al. Adverse events by System Organ Classes < 5%. Supplementary data set B. research.bond.edu.au/en/datasets/adverse‐events‐in‐patients‐taking‐macrolide‐antibiotics‐versus‐pl‐2 (accessed 12 December 2018). [DOI: 10.26139/5c10748decd2e] [DOI]
Head 2016
- Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder A, et al. Systemic antibiotics for chronic rhinosinusitis without nasal polyps in adults. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD008233.pub3] [DOI] [Google Scholar]
Hicks 2013
- Hicks LA, Taylor TH, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. New England Journal of Medicine 2013;368(15):1461‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Wiley‐Blackwell.
Hnin 2015
- Hnin K, Nguyen C, Carson‐Chahhoud KV, Evans DJ, Greenstone M, Smith BJ. Prolonged antibiotics for non‐cystic fibrosis bronchiectasis in children and adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD001392.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodkinson 2013
- Hodkinson A, Kirkham JJ, Tudur‐Smith C, Gamble C. Reporting of harms data in RCTs: a systematic review of empirical assessments against the CONSORT harms extension. BMJ Open 2013;3:e003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 2003
- International Conference on Harmonisation. ICH harmonised tripartite guideline post‐approval safety data management: definitions and standards for expedited reporting. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2D/Step4/E2D_Guideline.pdf (accessed 16 April 2018).
Kathariya 2016 [pers comm]
- Kathariya R. Only one patient in the control group reported unpalatable taste. The other two were from the test group. (One single patient reported both unpalatable taste and gastric intolerance and another reported only of unpalatable taste) [personal communication]. Email to: Hansen MP 28 April 2016.
Kew 2015
- Kew KM, Undela K, Kotorsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD002997.pub4] [DOI] [PubMed] [Google Scholar]
Laopaiboon 2015
- Laopaiboon M, Panpanich R, Swa Mya K. Azithromycin for acute lower respiratory tract infections. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD001954.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leibovici 2003
- Leibovici L, Soares‐Weiser K, Paul M, Goldberg E, Herxheimer A, Garner P. Considering resistance in systematic reviews of antibiotic treatment. Journal of Antimicrobial Chemotherapy 2003;52(4):564‐71. [DOI] [PubMed] [Google Scholar]
Leibovici 2016
- Leibovici L, Paul M, Garner P, Sinclair DJ, Afshari A, Pace NL, et al. Addressing resistance to antibiotics in systematic reviews of antibiotic interventions. Journal of Antimicrobial Chemotherapy 2016;71(9):2367‐9. [DOI] [PubMed] [Google Scholar]
Lin 2015
- Lin X, Lu J, Yang M, Dong BR, Wu HM. Macrolides for diffuse panbronchiolitis. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD007716.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
MedDRA 2018
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Medical Dictionary for Regulatory Activities (MedDRA). www.meddra.org (accessed 19 March 2018).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Ni 2015
- Ni W, Shao X, Cai X, Wei C, Cui J, Wang R, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta‐analysis. PLOS ONE 2015;10(3):e0121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J. Review on antimicrobial resistance: tackling a crisis for the health and wealth of nations. www.amr‐review.org/Publications.html (accessed 18 April 2018).
Owens 2006
- Owens RC Jr, Nolin TD. Antimicrobial‐associated QT interval prolongation: pointes of interest. Clinical Infectious Diseases 2006;43(12):1603‐11. [DOI] [PubMed] [Google Scholar]
Powell 2018 [pers comm]
- Powell H. Provide clarifying information about adverse events and macrolide‐resistant bacteria [personal communication]. Email to: Hansen MP 20 August 2018.
Ray 2012
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. New England Journal of Medicine 2012;366(20):1881‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roca 2016b [pers comm]
- Roca A. The urticarial rash was considered related to the intervention. It was in the azithromycin arm [personal communication]. Email to: Hansen MP 23 May 2016.
Sadarangani 2015
- Sadarangani SP, Estes LL, Steckelberg JM. Non‐anti‐infective effects of antimicrobials and their clinical applications: a review. Mayo Clinic Proceedings 2015;90(1):109‐27. [DOI] [PubMed] [Google Scholar]
Savaris 2017
- Savaris RF, Fuhrich DG, Duarte RV, Franik S, Ross J. Antibiotic therapy for pelvic inflammatory disease. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD010285.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2014
- Shi ZL, Peng H, Hu XW, Hu JG. Effectiveness and safety of macrolides in bronchiectasis patients: a meta‐analysis and systematic review. Pulmonary Pharmacology & Therapeutics 2014;28(2):171‐8. [DOI] [PubMed] [Google Scholar]
Southern 2012
- Southern KW, Barker PM, Solis‐Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002203.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spagnolo 2013
- Spagnolo P, Fabbri LM, Bush A. Long‐term macrolide treatment for chronic respiratory disease. European Respiratory Journal 2013;42(1):239‐51. [DOI] [PubMed] [Google Scholar]
Svanström 2013
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. New England Journal of Medicine 2013;368(18):1704‐12. [DOI] [PubMed] [Google Scholar]
Svanström 2014
- Svanström H, Pasternak B, Hviid A. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ 2014;349:g4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tacconelli 2016
- Tacconelli E, Cataldo MA, Paul M, Leibovici L, Kluytmans J, Schroder W, et al. STROBE‐AMS: recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open 2016;6(2):e010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 2016 [pers comm]
- Thomsen N. A list of adverse events enclosed [personal communication]. Email to: Hansen MP 8 March 2016.
Uthman 2013
- Uthman MM, Uthman OA, Yahaya I. Interventions for the prevention of mycobacterium avium complex in adults and children with HIV. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007191.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Driel 2016
- Driel ML, Sutter AI, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD004406.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandenbroucke 2004
- Vandenbroucke JP. When are observational studies as credible as randomised trials?. Lancet 2004;363(9422):1728‐31. [DOI] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization (WHO). Antibiotic resistance. www.who.int/mediacentre/factsheets/fs194/en/ (accessed 19 April 2018).
Winkel 2017 [pers comm]
- Winkel P. Per December 2009 there was 866 deaths in the clarithromycin group an 815 deaths in the placebo group in the CLARICOR study [personal communication]. Email to: Hansen MP 5 August 2017.
Wong 2017
- Wong AY, Chan EW, Anand S, Worsley AJ, Wong IC. Managing cardiovascular risk of macrolides: systematic review and meta‐analysis. Drug Safety 2017;40(8):663‐77. [DOI] [PubMed] [Google Scholar]
Zarin 2016
- Zarin DA, Tse T, Williams RJ, Carr S. Trial reporting in ClinicalTrials.gov ‐ the final rule. New England Journal of Medicine 2016;375(20):1998‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorzela 2014
- Zorzela L, Golder S, Liu Y, Pilkington K, Hartling L, Joffe A, et al. Quality of reporting in systematic reviews of adverse events: systematic review. BMJ 2014;348:f7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuckerman 2009
- Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infectious Disease Clinics of North America 2009;23(4):997‐1026. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hansen 2015
- Hansen M, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, et al. Adverse events in patients taking macrolide antibiotics versus placebo for any indication. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011825] [DOI] [PMC free article] [PubMed] [Google Scholar]


