Abstract
Background
Sedation reduces patient levels of anxiety and stress, facilitates the delivery of care and ensures safety. Alpha‐2 agonists have a range of effects including sedation, analgesia and antianxiety. They sedate, but allow staff to interact with patients and do not suppress respiration. They are attractive alternatives for long‐term sedation during mechanical ventilation in critically ill patients.
Objectives
To assess the safety and efficacy of alpha‐2 agonists for sedation of more than 24 hours, compared with traditional sedatives, in mechanically‐ventilated critically ill patients.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 10, 2014), MEDLINE (1946 to 9 October 2014), EMBASE (1980 to 9 October 2014), CINAHL (1982 to 9 October 2014), Latin American and Caribbean Health Sciences Literature (1982 to 9 October 2014), ISI Web of Science (1987 to 9 October 2014), Chinese Biological Medical Database (1978 to 9 October 2014) and China National Knowledge Infrastructure (1979 to 9 October 2014), the World Health Organization international clinical trials registry platform (to 9 October 2014), Current Controlled Trials metaRegister of controlled trials active registers (to 9 October 2014), the ClinicalTrials.gov database (to 9 October 2014), the conference proceedings citation index (to 9 October 2014) and the reference lists of included studies and previously published meta‐analyses and systematic reviews for relevant studies. We imposed no language restriction.
Selection criteria
We included all randomized and quasi‐randomized controlled trials comparing alpha‐2 agonists (clonidine or dexmedetomidine) versus alternative sedatives for long‐term sedation (more than 24 hours) during mechanical ventilation in critically ill patients.
Data collection and analysis
Two review authors independently assessed study quality and extracted data. We contacted study authors for additional information. We performed meta‐analyses when more than three studies were included, and selected a random‐effects model due to expected clinical heterogeneity. We calculated the geometric mean difference for continuous outcomes and the risk ratio for dichotomous outcomes. We described the effects by values and 95% confidence intervals (CIs). We considered two‐sided P < 0.05 to be statistically significant.
Main results
Seven studies, covering 1624 participants, met the inclusion criteria. All included studies investigated adults and compared dexmedetomidine with traditional sedatives, including propofol, midazolam and lorazepam. Compared with traditional sedatives, dexmedetomidine reduced the geometric mean duration of mechanical ventilation by 22% (95% CI 10% to 33%; four studies, 1120 participants, low quality evidence), and consequently the length of stay in the intensive care unit (ICU) by 14% (95% CI 1% to 24%; five studies, 1223 participants, very low quality evidence). There was no evidence that dexmedetomidine decreased the risk of delirium (RR 0.85; 95% CI 0.63 to 1.14; seven studies, 1624 participants, very low quality evidence) as results were consistent with both no effect and appreciable benefit. Only one study assessed the risk of coma, but lacked methodological reliability (RR 0.69; 95% CI 0.55 to 0.86, very low quality evidence). Of all the adverse events included, the most commonly reported one was bradycardia, and we observed a doubled (111%) increase in the incidence of bradycardia (RR 2.11; 95% CI 1.39 to 3.20; six studies, 1587 participants, very low quality evidence). Our meta‐analysis provided no evidence that dexmedetomidine had any impact on mortality (RR 0.99; 95% CI 0.79 to 1.24; six studies, 1584 participants, very low quality evidence). We observed high levels of heterogeneity in risk of delirium (I² = 70%), but due to the limited number of studies we were unable to determine the source of heterogeneity through subgroup analyses or meta‐regression. We judged six of the seven studies to be at high risk of bias.
Authors' conclusions
In this review, we found no eligible studies for children or for clonidine. Compared with traditional sedatives, long‐term sedation using dexmedetomidine in critically ill adults reduced the duration of mechanical ventilation and ICU length of stay. There was no evidence for a beneficial effect on risk of delirium and the heterogeneity was high. The evidence for risk of coma was inadequate. The most common adverse event was bradycardia. No evidence indicated that dexmedetomidine changed mortality. The general quality of evidence ranged from very low to low, due to high risks of bias, serious inconsistency and imprecision, and strongly suspected publication bias. Future studies could pay more attention to children and to using clonidine.
Plain language summary
Dexmedetomidine and clonidine for long‐term sedation during mechanical ventilation in critically ill patients
Review question
We reviewed the evidence about the safety and efficacy of dexmedetomidine and clonidine (known as alpha‐2 agonists) for long‐term sedation during mechanical ventilation in critically ill patients in the intensive care unit (ICU).
Background
Sedation is an important treatment for critically ill patients who need a machine to support breathing, because it reduces anxiety and stress and facilitates the delivery of nursing care. However, some commonly‐used sedatives, such as propofol, midazolam and lorazepam, might decrease blood pressure, depress breathing, and delay awakening after a long‐term infusion. They may prolong breathing support time and length of stay in hospital. Dexmedetomidine and clonidine sedate but allow staff to interact with patients, and they ease pain but do not depress breathing. Those treated with them could be more easy to awake, and more able to communicate their discomfort and pain. These drugs are therefore attractive alternatives for long‐term sedation, and we planned to assess their efficacy and safety for long‐term (more than 24 hours) sedation, compared with traditional sedatives.
Study characteristics
We searched the databases until October 2014. We included seven randomized controlled trials, with a total of 1624 participants, comparing dexmedetomidine versus traditional sedatives. All the studies required participants to have an anticipated need for sedation of more than 24 hours. The alternative sedatives included propofol, midazolam and lorazepam. We found no eligible studies in children or for clonidine. Of the seven studies, six were funded by the drug manufacturer, and one did not state any conflict of interest.
Key results
Compared with traditional sedatives, dexmedetomidine reduced the breathing support time by approximately one‐fifth, and the length of stay time in ICU by one‐seventh. Dexmedetomidine was at least as effective as traditional sedatives for producing sedation and maintaining a light sedation level. There was no clear evidence in support of dexmedetomidine reducing the risk of delirium (a kind of acute confusion state), as results were consistent with both no effect and appreciable benefit. We had insufficient information to draw conclusions about reducing the risk of coma. Dexmedetomidine doubled the incidence of slow heartbeat, which was the most commonly reported adverse event. Our review provides no evidence that dexmedetomidine changed the overall death rate.
Quality of the evidence
The general quality of evidence ranged from very low to low, as most of the studies were at high risk of bias, serious inconsistency and imprecision, or strongly suspected publication bias.
Summary of findings
Summary of findings for the main comparison. Dexmedetomidine compared to traditional sedative agents for long‐term sedation during mechanical ventilation in critically ill patients.
| Dexmedetomidine compared to traditional sedative agents for long‐term sedation during mechanical ventilation in critically ill patients | ||||||
| Patient or population: Critically ill patients requiring long‐term sedation during mechanical ventilation Settings: ICUs Intervention: Dexmedetomidine Comparison: traditional sedative agents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| traditional sedative agents | Dexmedetomidine | |||||
| Duration of mechanical ventilation | Not estimable | Not estimable | Relative decrease 22% in the geometric mean (95% CI 10% to 33%) | 1120 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | Alpha‐2 agonists reduced geometric mean duration of mechanical ventilation by 0.25 (95% CI 0.10 to 0.40), corresponding to a reduction of 22% in the geometric mean (95% CI 10% to 33%). The relative change in geometric means usually produces similar results to the relative change in arithmetic means. |
| Risk of delirium | Study population | RR 0.85 (0.63 to 1.14) | 1624 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 3,4,5,6 | ‐ | |
| 266 per 1000 | 226 per 1000 (167 to 303) | |||||
| Low | ||||||
| 76 per 1000 8 | 65 per 1000 (48 to 87) 8 | |||||
| High | ||||||
| 824 per 1000 8 | 700 per 1000 (519 to 939) 8 | |||||
| Risk of coma | Study population | RR 0.69 (0.55 to 0.86) | 103 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2,7 | ‐ | |
| 922 per 1000 | 636 per 1000 (507 to 793) | |||||
| Incidence of bradycardia | Study population | RR 2.11 (1.39 to 3.2) | 1587 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 10,7,9 | ‐ | |
| 90 per 1000 | 189 per 1000 (124 to 287) | |||||
| Low | ||||||
| 39 per 1000 8 | 82 per 1000 (54 to 125) 8 | |||||
| High | ||||||
| 188 per 1000 8 | 397 per 1000 (261 to 602) 8 | |||||
| Duration of weaning | See comment | 85 (1 RCT) | ‐ | Only one study assessed this outcome and only reported median and range. We were not able to estimate a relative effect. | ||
| ICU length of stay | Not estimable | Not estimable | Relative decrease 14% in the geometric mean (95% CI 0.01% to 24%) | 1223 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,11,2 | Sedation using alpha‐2 agonists reduced geometric mean ICU LOS by 0.15 (95% CI 0.01 to 0.28), corresponding to a reduction of 14% in the geometric mean (95% CI 0.01% to 24%). Relative change in geometric means usually produces similar results to the relative change in arithmetic means. |
| Mortality | Study population | RR 0.99 (0.79 to 1.24) | 1584 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 10,5,9 | ‐ | |
| 205 per 1000 | 203 per 1000 (162 to 254) | |||||
| Moderate | ||||||
| 192 per 1000 12 | 190 per 1000 (152 to 238) 12 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by 1 point due to all the studies being at high risk of bias, which was considered very serious.
2Downgraded by 1 point because all the studies received funding support from pharmaceutical firms and thus publication bias was strongly suspected.
3Downgraded by 1 point due to 6 of the 7 studies being at high risk of bias, which was considered serious.
4Downgraded by 1 point because the heterogeneity is substantial and thus inconsistency was serious.
5Downgraded by 1 point because results were consistent with both no effect and appreciable benefit, and thus imprecision was serious.
6Downgraded by 1 point because 6 of the 7 studies received funding support from pharmaceutical firms and thus publication bias was strongly suspected.
7Downgraded by 1 point because the sample size was small (less than 2000) and thus inconsistency was serious.
8The baseline risk varies broadly among studies, and thus we chose the lowest and highest control group risk in the included studies.
9Downgraded by 1 point due to 5 of the 6 studies being at high risk of bias, which was considered serious.
10Downgraded by 1 point because 5 of the 6 studies received funding support from pharmaceutical firms and thus publication bias was strongly suspected.
11Downgraded by 1 point due to wide confidence interval, although there may still be enough precision to make decisions about the utility of the intervention.
12There was little variation in the baseline risk across the studies, and thus we calculated the median control group risk.
Background
Description of the condition
Sedation has become an important treatment in intensive care settings, especially for those undergoing mechanical ventilation. In the United States, half of patients with mechanical ventilation received intravenous sedation for more than 70% of their ventilation time (Wunsch 2009). Sedation could reduce levels of anxiety and stress, facilitate the delivery of nursing care, prevent self extubation and ensure synchrony with mechanical ventilation (Gehlbach 2002).
Most physicians choose propofol and benzodiazepines for long‐term sedation (Mehta 2011). Propofol is fast onset and offset, but decreases blood pressure, and may cause lipid metabolism disorder and fatal propofol infusion syndrome after long‐term use (Kam 2007; Miller 1998). Although benzodiazepines have little effect on blood pressure, they may cause apnoea and respiratory depression (Watling 1996). Benzodiazepines tend to be accumulated in body tissues and thus lead to delayed awakening (Shelly 1991). Furthermore, benzodiazepines might be associated with high risks of delirium and coma (Agarwal 2010; Pandharipande 2006; Pandharipande 2008;Watson 2008), which increase ventilation time, mortality and healthcare costs (Dubois 2001; Ely 2004).
Increasingly, many institutions use sedation strategies, such as nursing‐implemented sedation protocols and daily interruption to minimize the use of sedatives and limit the adverse events. These strategies reduce the dosage of sedatives, ventilation time and intensive care unit length of stay (ICU LOS) (Aitken 2012; Brook 1999; Burry 2014; Girard 2008), but lack of physicians' specific order and nursing acceptance have prevented them from being widely adopted. A survey of members of the Society of Critical Care Medicine showed that only 64% of respondents used sedation protocols, and 40% used interruption of sedation every day (Tanios 2009). Moreover, even when physicians applied sedation strategies, the choice of sedatives ‐ for example, using short‐acting, non‐benzodiazepine sedatives rather than benzodiazepines ‐ could still affect the ventilation time and hospital stay (Carson 2006; Pandharipande 2010). This is probably because these sedatives are easy to titrate and rapid to wake from, which in turn helps to finish sedation protocols and daily interruption.
Results from recent studies suggest that a strategy of light sedation could reduce ventilation time and ICU LOS (Shehabi 2012; Treggiari 2009). In addition, many investigators also recommend that any sedation strategy should incorporate analgesia, due to the difficulty of determining pain in critically ill patients (Fraser 2007) and the favourable results in shorter ventilation time, weaning time and ICU LOS (Breen 2005). A randomized controlled trial (RCT) found that when all the participants received intravenous analgesia (morphine), those with no infusion of sedatives had shorter ventilation time and ICU LOS, although more occurrences of delirium, than those with protocolized sedation and daily interruption (Strom 2010). The 'protocol of no sedation' in Strom 2010 was not really a protocol without any sedation, since morphine has a mild sedative effect, but it explored new challenges for sedation management: whenever possible, using the minimum level of sedation, providing adequate pain control and making a careful trade‐off between the level of sedation and the patient's comfort and safety. A sedative agent that provided lighter sedation, less pain and fewer adverse events would therefore be an attractive alternative.
Description of the intervention
Alpha‐2 agonists have a wide range of effects including sedation, analgesia and relief of anxiety (Mantz 2011; Pichot 2012). These effects are mainly mediated though adrenoceptors of the alpha‐2a subtype, which are distributed in the locus coeruleus (Gregoretti 2009; Mantz 2011). Unlike benzodiazepines and propofol, which inhibit the neurons in the central nervous system, alpha‐2 agonists reduce the activity but preserve the reactivity of neurons in the locus coeruleus. Alpha‐2 agonists therefore sedate but allow staff to interact with patients and do not suppress the respiratory drive. Alpha‐2 agonists might also be involved in neuroprotection and inflammatory responses, which may potentially help protect against the occurrence of delirium or coma (Mantz 2011). The adverse events of alpha‐2 agonists, such as bradycardia and hypotension, are mediated via adrenoceptors of the alpha‐2 in the medullary dorsal motor nucleus and motor complex, and are thus independent of sedative effect (Gregoretti 2009; Pichot 2012).
Clonidine and dexmedetomidine are two extensively‐studied alpha‐2 agonists, and both have similar mechanisms and adverse events. Although physicians have used clonidine to treat hypertension and acute withdrawal syndrome (Bohrer 1990; Gregoretti 2009), or have used it as an anaesthetic adjuvant (Dahmani 2010; Gregoretti 2009; Lambert 2014), its use for sedation remains 'off label' in many countries. Dexmedetomidine has a higher alpha‐2/alpha‐1 selectivity ratio (dexmedetomidine 1620:1, clonidine 220:1) (Virtanen 1988). The US Food and Drug Administration (FDA) authorized it for sedation during mechanical ventilation in 1999, and it can also be used as an analgesic adjuvant for acute postoperative pain control (Aho 1991; Jessen 2013). Dexmedetomidine is now available in the United States, Asia, the Middle East, Australia and Europe (Mantz 2011; Pichot 2012).
Clonidine is usually administered to adults intravenously at a dose of 2 mcg/kg per hour, or given as a bolus of 50 mcg to 150 mcg every eight hours (Ise 2002; Jamadarkhana 2010). The oral dose of clonidine is 50 mcg to 600 mcg every eight hours (Jamadarkhana 2010). The maintenance infusion rate of dexmedetomidine is 0.2 mcg/kg per hour to 0.7 mcg/kg per hour, for less than 24 hours (Precedex Prescribing Information).
How the intervention might work
Several RCTs showed alpha‐2 agonists for long‐term sedation reduced the duration of mechanical ventilation compared with traditional sedatives (Riker 2009; Ruokonen 2009; Spies 2003). Two aspects of alpha‐2 agonists might be related to this reduction: one could be that people treated with alpha‐2 agonists were more arousable, easier to communicate with and more able to express pain and needs (Martin 2003; Ruokonen 2009). The analgesia‐based sedation retained spontaneous respiration, and facilitated the delivery of care and the implementation of spontaneous breathing trials (Bailey 1991; Hall 2000; Hall 2001; Hsu 2004). A second aspect could be that alpha‐2 agonists were associated with fewer occurrence of delirium and coma (Pandharipande 2007; Riker 2009; Rubino 2010), probably because they work differently to propofol and benzodiazepines, and provide sleep‐like sedation (Huupponen 2008; Mason 2009).
Why it is important to do this review
Although some RCTs of long‐term sedation suggest that alpha‐2 agonists could reduce the duration of mechanical ventilation and delirium, other RCTs disagree: they found no improvement in ventilation time (Pandharipande 2007) and even a higher incidence of delirium (Ruokonen 2009). The FDA did not approve clonidine for sedation and only authorized dexmedetomidine infusions for less than 24 hours. There was therefore no way to know whether they were safe and effective for longer sedation. A previous meta‐analysis assessed the effects of dexmedetomidine in critically ill patients and found no difference in ventilation time or incidence of delirium compared with traditional sedatives or placebo (Tan 2010). But a number of RCTs have been published since its last search in 2009 (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Shehabi 2013), and a comment on that meta‐analysis pointed out flaws in methodology (for example, unit‐of‐analyses error, confusion of agitation with delirium, and inadequate investigation of heterogeneity), which weaken the value of its results (Tejani 2010). They included RCTs investigating both short‐term and long‐term sedation, but most were short‐term, and thus provided limited information on long‐term safety and efficacy.
Despite the theoretical advantages of alpha‐2 agonists, their safety and efficacy for long‐term use remain controversial. We also consider that people undergoing long‐term ventilation and sedation are substantially different from those receiving short‐term treatment; they have a different disease spectrum, are more critically ill, need more dosage of sedatives, are more exposed to the adverse events of sedatives, and are more likely to wake up later. A comprehensive synthesis is therefore needed to assess whether alpha‐2 agonists are safe and whether they have advantages over traditional sedatives for long‐term sedation, by reducing the duration of mechanical ventilation and the risks of delirium and coma.
Objectives
To assess the safety and efficacy of alpha‐2 agonists for sedation of more than 24 hours, compared with traditional sedatives, in mechanically‐ventilated critically ill patients.
Methods
Criteria for considering studies for this review
Types of studies
We include all randomized and quasi‐randomized controlled trials. Quasi‐randomized controlled trials include those trials that use inappropriate randomization strategies (e.g. alternation, birth date, hospital registration number, etc.) (Higgins 2011a).
We exclude cluster‐randomized, cross‐over and non‐randomized trials.
Types of participants
We include randomized controlled trials (RCTs) of critically ill participants of any age, except neonates, who required intensive care and invasive mechanical ventilation when recruited.
We exclude RCTs in which participants only had non‐invasive mechanical ventilation.
Types of interventions
We include studies comparing clonidine or dexmedetomidine versus alternative sedative agents. The alternative sedatives included benzodiazepines, such as midazolam or diazepam, propofol and other sedatives.
We include studies which explicitly report that each participant was anticipated to need sedation for more than 24 hours. There was no limitation for dose, frequency or route of administration of alpha‐2 agonists.
We exclude studies in which all participants were sedated for less than 24 hours or studies without an explicit statement that they required or anticipated participants to have sedation for more than 24 hours . We exclude studies that compare one alpha‐2 agonist with another.
Types of outcome measures
Primary outcomes
Duration of mechanical ventilation (as defined by authors of the studies).
Risk of delirium, measured with any diagnostic criteria, such as the confusion assessment method for the intensive care unit (CAM‐ICU).
Risk of coma (as defined by authors of the studies).
Secondary outcomes
-
Adverse events:
incidence of bradycardia (as defined by authors of the studies);
incidence of hypotension (as defined by authors of the studies);
incidence of hypertension (as defined by authors of the studies);
any other adverse events.
Proportion of sedation time spent at target sedation level (as defined by authors of the studies).
Duration of weaning (time from weaning to extubation as defined by authors of the studies).
intensive care unit length of stay (ICU LOS).
Mortality; if mortality is assessed at various follow‐up times (e.g. ICU mortality, 30‐day mortality, one‐year mortality), we will include only the one that most closely approximates to that used in the other included studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 10, 2014; see Appendix 1 for detailed search strategy), MEDLINE (Ovid SP, 1946 to 9 October 2014; see Appendix 2), EMBASE (Ovid SP, 1980 to 9 October 2014; see Appendix 3), CINAHL (EBSCO host, 1982 to 9 October 2014; see Appendix 4), Latin American and Caribbean Health Sciences Literature (LILACS, via BIREME interface, 1982 to 9 October 2014; see Appendix 5), ISI Web of Science (1987 to 9 October 2014; see Appendix 6), Chinese Biological Medical Database (CBM, 1978 to 9 October 2014; see Appendix 7) and China National Knowledge Infrastructure (CNKI, 1979 to 9 October 2014; see Appendix 8) for relevant studies.
We imposed no language and publication status restrictions. However, we excluded studies without detailed methodological information. Detailed methodological information means that the information obtained from full copies, abstracts or study authors should be sufficient to enable us to make judgements on the overall study quality.
Searching other resources
We searched for unpublished or ongoing trials on the World Health Organization international clinical trials registry platform WHO ICTRP (www.who.int/ictrp/search/en/), Current Controlled Trials metaRegister of controlled trials (www.isrctn.com/page/mrct) active registers, and the clinicaltrials.gov database.
We also searched the conference proceedings citation index.
We handsearched the reference lists of included studies and previously published meta‐analyses and systematic reviews for relevant studies (Lin 2012; Tan 2010; Xia 2013; Zhuo 2012). We contacted the first author and the corresponding author of the included studies for information on other published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (KC and YCX) independently scanned each title and abstract retrieved by the search strategy. We excluded irrelevant studies in this step. We excluded studies in abstract only and where no detailed methodological information could be obtained from the study authors. We obtained full copies of the remaining studies for further assessment. Our methodologist (YIC) resolved any disagreements unresolved by consensus. We used Endnote X7 to manage the search results and the articles. We noted the reasons for exclusion in Endnote X7. We were not blinded to the study authors, institutions or journals.
Data extraction and management
Two review authors (KC and YCX) independently extracted data from the articles using a modified version of the Cochrane Anaesthesia Review Group's (CARG) data extraction form (Appendix 9). We selected three articles randomly to test our form before formal extraction. We exported data from these articles to doc.google.com. Our statistician (YC) resolved inconsistent data extraction between the two review authors.
Assessment of risk of bias in included studies
Two review authors (KC and YCX) independently assessed the risk of bias using the Cochrane Collaboration’s tool (Higgins 2011b) in the following seven key domains:
Random sequence generation;
Allocation concealment;
Blinding of participants and personnel;
Blinding of outcome assessment;
Incomplete outcome data;
Selective reporting;
Other bias (whether the groups were balanced at baseline, whether the co‐interventions were similar, etc.).
We assessed blinding for each outcome as recommended. We judged each domain as 'low risk', 'high risk' or 'unclear risk'. We noted the summary of known facts and the source of this information for each domain. We asked the authors of the study for clarification if any details were unclear.
We tested the 'Risk of bias' assessment tool on some similar studies. When KC and YCX disagreed, we consulted our methodologist (YIC) and content specialists (SMP and ZJL). After reaching consensus, we classified each included study into one of three categories, as follows.
Low risk of bias: low risk of bias for all key domains.
Unclear risk of bias: unclear risk of bias for one or more key domains.
High risk of bias: high risk of bias for one or more key domains.
We generated a 'Risk of bias' graph and a 'Risk of bias' summary. We also reported the risk of selective outcome reporting in the Results section under Assessment of risk of bias in included studies.
We performed the kappa test to evaluate the interobserver reliability between two review authors (KC and YCX).
Measures of treatment effect
We calculated the geometric mean difference (GMD) for continuous outcomes and the risk ratio (RR) for dichotomous outcomes. We describe the effects by their values and 95% confidence intervals (CIs). We considered two‐sided P < 0.05 to be statistically significant.
Unit of analysis issues
We expected all the trials to be simple parallel‐group designs. The unit of analysis was a single measurement for each outcome from each participant.
Dealing with missing data
We tried to contact the study authors for missing data and further information twice by e‐mail. For those missing data that could not be obtained from authors, we did the following:
If we judged missing data to be 'missing at random', we only analysed the available data. We categorized the data as 'missing at random' if the loss was unrelated to the actual values of the missing data (Higgins 2011a). For example, if participants died before receiving any study drugs.
If we judged missing data to be 'not missing at random' and meta‐analysis was not feasible, we performed a qualitative synthesis.
If we could systematically meta‐analyse missing data, we imputed average measurements for continuous outcomes and we inferred bad outcomes for categorical outcomes.
Assessment of heterogeneity
We measured heterogeneity using both the Chi² statistic and the I² statistic. Values for I² of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity, respectively (Higgins 2003). We considered there to be substantial heterogeneity when a P value of Chi² < 0.10 or I² > 50%, or both (moderate and high degrees of heterogeneity). If we identified substantial heterogeneity, we checked the data entered into Review Manager 5 (RevMan 5.3). If I² exceeded 75%, we did not perform meta‐analyses.
Assessment of reporting biases
We had planned to generate funnel plots if there were more than 10 studies included in the meta‐analyses of primary outcomes (Higgins 2011a). For continuous outcomes, we would have conducted the Egger test to assess funnel plot asymmetry; for dichotomous outcomes, we would have conducted the arcsine test (Sterne 2011). We would have performed all tests using STATA/SE 12.0.
Data synthesis
We performed meta‐analyses only if we could include more than three studies. Otherwise, we undertook a qualitative synthesis. We selected a random‐effects model due to expected clinical heterogeneity. We used the Mantel‐Haenszel method to estimate dichotomous outcomes and the inverse variance method to estimate continuous outcomes. We did not perform a meta‐analysis for 'Proportion of sedation time spent at target sedation level' because there was no consistent outcome measure and statistical methods for pooling such percentage rates are not well developed (Higgins 2011a). We would have synthesized the data of adult studies and paediatric studies separately if both data were available. We performed all statistical analyses using Review Manager 5 software (RevMan 5.3).
The data for the durations of mechanical ventilation, weaning and ICU LOS are always skewed. We performed meta‐analyses on log‐transformed data. If we could obtain means and standard deviations of raw data without log‐transformation, we conducted a transformation using Method 1 mentioned in Higgins 2008. If means and standard deviations of raw data were not available, we estimated log‐transformed means using log‐transformed medians (Hozo 2005) and calculated approximate standard deviations by dividing the log‐transformed interquartile range (log‐transformed third quartile minus first quartile) by 1.35 (Higgins 2011b).
We did not make any assumptions about the distribution of censored data from time‐to‐event data (duration of mechanical ventilation and ICU LOS). Some of the studies analysed time‐to‐event data using, for instance, Cox's proportional‐hazards regression model and Kaplan‐Meier survival analyses. We undertook a narrative synthesis for these adjusted outcomes.
Subgroup analysis and investigation of heterogeneity
If there were adequate data we performed subgroup analyses as follows.
For duration of mechanical ventilation and duration of weaning
Dexmedetomidine compared with clonidine.
Class of alternative agents, classified according to the 'Anatomical Therapeutic Chemical' (ATC) classification system advocated by the World Health Organization’s collaborating centre for drugs statistics methodology.
Sedation with or without analgesics/pain control.
For risk of delirium
Different delirium scores used.
Class of alternative agents.
For risk of coma and adverse events
Class of alternative agents.
Dexmedetomidine compared with clonidine.
Rapid‐dosing regimens compared with slow‐dosing regimens, where rapid‐dosing regimens include intravenous bolus and continuous intravenous infusion with initial loading infusion, and where slow‐dosing regimens include continuous intravenous infusion without initial loading infusion and oral or nasogastric administration.
For ICU LOS and mortality
Class of alternative agents.
Dexmedetomidine compared with clonidine.
Type of ICU (e.g. mixed ICU, medical ICU, surgical ICU, coronary care unit (CCU), respiratory care unit, burn unit).
We determined whether there were differences of effect size among subgroups based on the P values from tests for subgroup differences (Higgins 2011a). If differences existed, we compared the direction and magnitude of effect size in these subgroups with each other.
If there were adequate data, we performed random‐effects meta‐regression to investigate how mean ventilation time (log‐transformed) of the included studies was associated with the risk of delirium (Thompson 2002). We performed meta‐regression using R 3.0.2 with 'Metafor' package (version 1.9‐2). We used a restricted maximum‐likelihood estimator method to calculate the amount of heterogeneity (Viechtbauer 2010). We determined whether this difference was statistically significant based on the P value of the coefficients.
Sensitivity analysis
If there were sufficient studies, we undertook sensitivity analyses by excluding studies as follows:
Studies at high or unclear risk of bias;
Studies of a non‐standardized design: RCTs without a target sedation level or using non‐intravenous administration.
We also undertook sensitivity analyses by pooling observed data only, without any imputation.
Summary of findings
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes: duration of mechanical ventilation, rate of delirium, rate of coma, rate of bradycardia, duration of weaning, ICU length of stay and mortality. We constructed a 'Summary of findings' table using GRADE software. The GRADE approach appraised the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence is derived from within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
Running the search strategy of electronic databases yielded 3009 citations: 1348 citations from western databases and 1661 citations from Chinese databases. We excluded 2956 citations based on abstract content alone. Three citations were in abstract only, and we failed to retrieve further information or to trace the original investigators. We performed full‐text review of 53 citations, and finally included six citations in our data synthesis (and one citation in Studies awaiting classification). One included citation reported on two independently‐conducted multi‐centre, randomized, double‐blind studies, the MIDEX trial (clinicaltrials.gov Identifier: NCT00481312) and the PRODEX trial (clinicaltrials.gov Identifier: NCT00479661). We considered them as two discrete studies during data synthesis (Jakob 2012 MIDEX: MIDEX trial; Jakob 2012 PRODEX: PRODEX trial). We found two potentially relevant unpublished/ongoing studies from clinical trial registries and emailed the contact investigators (NCT01059929; NCT01760967). One replied (NCT01760967), but provided no useful information (Characteristics of ongoing studies table shows detailed information about the ongoing studies). We did not obtain any information about unpublished or ongoing randomized controlled trials (RCTs) from authors of the included studies. Figure 1 presents the entire study selection process.
1.

Selection process for studies included in data synthesis.
Included studies
See Characteristics of included studies.
We include seven studies in our review. Six studies were of a two‐arm design. One used a four‐arm design that compared dexmedetomidine infusion alone, midazolam infusion alone, dexmedetomidine infusion combined with midazolam infusion and dexmedetomidine infusion combined with midazolam bolus (Xu 2012). We extracted the data only from the dexmedetomidine‐infusion‐alone group and the midazolam‐infusion‐alone group. Five of the included studies were international multi‐centre (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009; Ruokonen 2009; Shehabi 2013); one was multi‐centre, conducted in the United States (Pandharipande 2007); and one was single‐centre, conducted in China (Xu 2012). Six studies received funding support from pharmaceutical firms which manufacture dexmedetomidine (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013), and one did not state any potential conflict of interest (Xu 2012).
Types of participants
There were 1624 participants meeting the inclusion criteria. The sample sizes of studies ranged from 37 to 501. All the studies recruited adults from mixed intensive care units (ICUs) which may include medical, surgical and trauma patients. All the studies required participants to have an anticipated need for sedation of at least 24 to 36 hours. The main exclusion criteria of the studies include conditions as follows: neurological disease, severe bradycardia, second or third degree atrioventricular conduction block, hepatic impairment and pregnancy.
Types of interventions
We found no eligible studies for clonidine. All the included studies compared dexmedetomidine with traditional sedatives. The traditional sedatives could be midazolam (Jakob 2012 MIDEX; Riker 2009; Xu 2012), lorazepam (Pandharipande 2007), propofol (Jakob 2012 PRODEX), or standard care (either propofol or midazolam) (Ruokonen 2009; Shehabi 2013). A total of 871 participants (53.6%) received dexmedetomidine, 393 (24.2%) midazolam, 51 (3.1%) lorazepam, 249 (15.3%) propofol or 60 (3.7%) standard care. All studies administered sedative agents intravenously. The infusion rate of dexmedetomidine varied among trials, ranging from 0.15 to 1.5 mcg/kg per hour. Only one study routinely administered loading doses of dexmedetomidine (Xu 2012).
Only four studies explicitly stated that participants might receive sedation prior to the study treatment (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009): three reported that the sedation scores at the time of study treatment initiation were similar between groups (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007), and one started treatment only when the participants were within a prespecified range of sedation score (Riker 2009). Other studies reported no detailed information about sedation prior to the study treatment (Ruokonen 2009; Shehabi 2013; Xu 2012). All the studies stated that they titrated sedatives to a target sedation level: Jakob 2012 MIDEX and Jakob 2012 PRODEX set the target as a Richmond Agitation‐Sedation Scale (RASS) score of 0 to ‐3 and assessed it every two hours; Pandharipande 2007 set it as a "clinically individualized target" based on RASS and assessed it twice daily; Riker 2009 set it as a RASS score of ‐2 to 1 and assessed it every four hours; Ruokonen 2009 set it as a RASS score of ‐4 to 0 and assessed it every four hours; Shehabi 2013 set it as a RASS score of ‐2 to 1 and assessed it every four hours; Xu 2012 set it as a Motor Activity Assessment Scale (MAAS) score of 3 and assessed it every two hours. Three studies performed daily interruption as part of the study protocol (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Ruokonen 2009). Six studies routinely gave opioids to treat pain during sedation (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013) and one study did not report that they used analgesics (Xu 2012). Two studies used intravenous haloperidol to control delirium (Riker 2009; Shehabi 2013), and the others did not report any information about delirium treatment. Four studies stopped sedation after extubation (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009); two studies stopped it when the investigators thought it was no longer required (Riker 2009; Shehabi 2013); one study did not explicitly state the indication for sedation discontinuation (Xu 2012).
Types of outcome measures
Five studies reported on duration of mechanical ventilation (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009; Ruokonen 2009; Shehabi 2013). All the studies reported on the risk of delirium, based on the confusion assessment method for the intensive care unit (CAM‐ICU). Three studies explicitly described the frequency of delirium assessment: Riker 2009 assessed it daily during sedation interruption; Ruokonen 2009 assessed it daily; Shehabi 2013 assessed it when the RASS score was more than ‐3. Only two studies explicitly described the follow‐up period of delirium assessment: Pandharipande 2007 assessed it until hospital discharge or for 12 days; Riker 2009 assessed it during study treatment. One study reported on the risk of coma (Pandharipande 2007). The investigators defined coma as a RASS score of ‐4 or ‐5 and assessed it until hospital discharge or for 12 days.
Excluded studies
Of the 46 studies excluded during full‐text review, 44 were because they did not explicitly report the required or anticipated duration of sedation or did not meet the criteria, one because it was not an RCT and one because not all the participants were mechanically ventilated. The Characteristics of excluded studies table provides detailed reasons for the exclusions.
Risk of bias in included studies
Allocation
Five studies had adequate sequence generation (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Shehabi 2013). Two studies reported they used stratified randomization, but did not state in detail how they randomized the participants (Ruokonen 2009; Xu 2012). We contacted the authors of Ruokonen 2009 and Xu 2012 for additional methodological information, but no‐one replied. We therefore judged them to be at 'unclear risk of bias for random sequence generation. Five studies had adequate allocation concealment (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Shehabi 2013). Two studies did not state that they concealed the randomization sequence (Ruokonen 2009; Xu 2012), and we therefore judged them to be at 'high' risk of bias for allocation concealment. The two review authors were in complete agreement for this domain.
Figure 2 and Figure 3 illustrate the proportion of studies with those judgements and the judgement made for each study. See the Characteristics of included studies for details of those judgements. .
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Four studies used an adequate double‐blinded design and described the details of the blinding method (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009). One study used a double‐dummy design, but the blinding method was brittle and we believe that the study investigators could easily have broken the blinding (Ruokonen 2009). One study did not mention that the participants or the investigators were blinded, and therefore probably did not perform blinding (Xu 2012). One study stated that they were "unblinded" (Shehabi 2013). We believed blinding had little impact on these objective outcomes, and we therefore judged Ruokonen 2009, Xu 2012 and Shehabi 2013 to be at 'low' risk for blinding of outcome assessment in objective outcomes, but at 'high' risk for blinding of participants and personnel and blinding of outcome assessment in subjective outcomes. Agreement between the two review authors for blinding of participants and personnel was at 86% (kappa = 0.70). The two review authors were in complete agreement for blinding of outcome assessment, both subjective and objective outcomes.
Figure 2 and Figure 3 illustrate the proportion of studies with those judgements and the judgement made for each study. Characteristics of included studies showed the details for the judgements.
Incomplete outcome data
Five studies had low withdrawal rates, and reasons for withdrawal were explicitly reported and balanced across groups (Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013; Xu 2012). However, in Jakob 2012 MIDEX and Jakob 2012 PRODEX, withdrawal due to lack of efficacy was significantly more frequent in dexmedetomidine participants and would therefore inevitably bias the results. We judged them to be at 'high' risk of bias. The two review authors were in complete agreement on this domain.
Figure 2 and Figure 3 illustrate the proportion of studies with those judgements and the judgement made for each study. Characteristics of included studies showed the details for the judgements.
Selective reporting
There was no indications of selective reporting in any of the included studies. The two review authors were in complete agreement for this domain.
Other potential sources of bias
Six studies appeared to be free of other sources of bias (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009; Ruokonen 2009; Shehabi 2013; Xu 2012). One study used continuous infusion of lorazepam as a comparison intervention (Pandharipande 2007). Because of the long half‐life of lorazepam, such a study design might increase the risk of coma in the control group, and we suspected it to be a potential source of bias. Agreement between the two review authors for other potential sources of bias was at 86% (kappa = 0.70).
Figure 2 and Figure 3 illustrate the proportion of studies with those judgements and the judgement made for each study. Characteristics of included studies showed the details for the judgements.
Effects of interventions
See: Table 1
Duration of mechanical ventilation (Analysis 1.1; Analysis 1.2)
1.1. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 1 Duration of mechanical ventilation (subgroup: class of alternative agents).
1.2. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 2 Duration of mechanical ventilation (sensitivity analysis using data from 'time to extubation'").
Four studies (1448 participants) compared the duration of mechanical ventilation (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009; Ruokonen 2009), and showed the median time of ventilation in the dexmedetomidine group was less than control. Jakob 2012 MIDEX, Jakob 2012 PRODEX and Ruokonen 2009 analysed the data using Cox’s proportional‐hazards regression and Riker 2009 analysed it using Kaplan‐Meier survival analysis. Three of them were statistically significant (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009), but one was not (Ruokonen 2009) (calculations from median/interquartile range to geometric mean/standard deviation (SD) are shown in Table 2). Riker 2009 reported the median but no interquartile range. We were unable to obtain the raw data, and thus could not estimate the standard deviation and add the data into our meta‐analysis. We obtained data for Shehabi 2013 from the authors and pooled it with the other three studies (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Ruokonen 2009). Meta‐analysis of 1119 participants yielded a random‐effects estimate of the geometric mean difference (MD) of ‐0.25 log hours (95% confidence interval (CI) ‐0.40 to ‐0.10, P = 0.001), corresponding to a reduction of 22% in the geometric mean (95% CI 10% to 33%). Neither the I² nor the Chi² statistics indicated evidence of statistical heterogeneity across the trials, (I² = 0%; Chi² = 0.42, P = 0.94).
1. Calculation from median/interquartile range to geometric mean/standard deviation (SD).
| Study ID | Outcome | Study group | Median | Interquartile range | Geometric mean | Geometric SD |
| Jakob 2012 MIDEX | Duration of mechanical ventilation | Intervention | 123 hours | 67 ‐ 337 hours | 4.812 | 1.197 |
| Control | 164 hours | 92 ‐ 380 hours | 5.1 | 1.051 | ||
| Jakob 2012 PRODEX | Duration of mechanical ventilation | Intervention | 97 hours | 45 ‐ 257 hours | 4.575 | 1.291 |
| Control | 118 hours | 48 ‐ 327 hours | 4.771 | 1.421 | ||
| Ruokonen 2009 | Duration of mechanical ventilation | Intervention | 77.2 hours | 17.5 – 338.8 hours | 4.346 | 2.195 |
| Control | 110.6 hours | 20.1 – 675.0 hours | 4.706 | 2.603 | ||
| Jakob 2012 MIDEX | ICU LOS | Intervention | 8.8 days (211 hours) | 4.8 ‐ 34.6 days (115 ‐ 831 hours) | 2.174 | 1.465 |
| Control | 10.1 days (243 hours) | 5.8 ‐ 26.2 days (140 ‐ 630 hours) | 2.315 | 1.114 | ||
| Jakob 2012 PRODEX | ICU LOS | Intervention | 6.8 days (164 hours) | 3.8 ‐ 20.0 days (90 ‐ 480 hours) | 1.922 | 1.24 |
| Control | 7.7 days (185 hours) | 3.9 ‐ 21.7 days (93 ‐ 520 hours) | 2.042 | 1.275 | ||
| Pandharipande 2007 | ICU LOS | Intervention | 7.5 days | 5 ‐ 19 days | 2.015 | 0.989 |
| Control | 9 days | 6 ‐ 15 days | 2.197 | 0.679 | ||
| Ruokonen 2009 | ICU LOS | Intervention | 5.5 days | 1.7 – 19.5 days | 1.705 | 1.807 |
| Control | 5.7 days | 1.7 – 29.0 days | 1.74 | 2.101 |
ICU: intensive care unit LOS: length of stay
We performed subgroup analysis for different classes of alternatives. The subgroups were small, with one or two studies in each. The results from all subgroups favoured dexmedetomidine, and we detected no significant difference between them (P = 0.84). We did not perform subgroup analyses for dexmedetomidine compared with clonidine or sedation with or without pain control, because of inadequate data.
Jakob 2012 MIDEX and Jakob 2012 PRODEX reported not only 'duration of mechanical ventilation', which included non‐invasive ventilation time, but also 'time to extubation' which also matched our definition. We performed a post hoc sensitivity analysis to assess how the result would be changed if we extracted the data from 'time to extubation' instead of 'duration of mechanical ventilation'. The sensitivity analysis yielded a geometric mean difference of ‐0.34 log hours (95% CI ‐0.49 to ‐0.20, P < 0.00001), corresponding to a reduction of 29% in the geometric mean (95% CI 18% to 39%). We did not undertake sensitivity analyses by excluding studies at high risk of bias or studies with non‐standardized design because of the small number of studies.
We were not able to conduct the Egger test because of the small number of studies, but we suspected reporting bias for this outcome, because these four studies received funding support from pharmaceutical firms which manufacture dexmedetomidine.
Risk of delirium (Analysis 1.3; Analysis 1.4)
1.3. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 3 Risk of delirium (subgroup: class of alternative agents).
1.4. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 4 Risk of delirium (sensitivity analysis for observed data only).
The data for meta‐analysis were available from all the studies, with 1624 participants (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013; Xu 2012). The risk of delirium was numerically lower in the dexmedetomidine group than in the control group (risk ratio (RR) 0.85; 95% CI 0.63 to 1.14), but was not statistically significant (P = 0.27). The heterogeneity was high across the trials (I² = 70%).
We performed subgroup analyses for different classes of alternative agents. The number of studies within subgroups ranged from one to four, and the difference in effect size was significant among subgroups (P = 0.02). Pooled results from subgroups showed the risk of delirium was lower in participants with dexmedetomidine than in those with benzodiazepine derivatives (RR 0.81; 95% CI 0.59 to 1.09; 1007 participants) or propofol (RR 0.37; 95% CI 0.16 to 0.87; 495 participants), but higher than in participants with standard care (RR 1.44; 95% CI 0.86 to 2.41; 122 participants). There was a high level of heterogeneity in the subgroup of benzodiazepine derivatives (I² = 72%), but a low level in the subgroup of standard care (I² = 6%). Because all the studies assessed delirium based on the confusion assessment method for the intensive care unit (CAM‐ICU), subgroup analyses for different delirium scores were not possible.
We performed meta‐regression on log‐transformed mean ventilation time using data from six studies (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013) (Table 3). The meta‐regression did not show that the log‐transformed mean ventilation time was associated with the risk of delirium (P = 0.94) and was unable to explain the heterogeneity (R² = 0.00%).
2. Meta‐regression for incidence of delirium on geometric mean ventilation time.
| tau² (estimated amount of residual heterogeneity) | 0.1460 (SE = 0.1596) |
| tau (square root of estimated tau² value) | 0.3820 |
| I² (residual heterogeneity / unaccounted variability) | 70.09% |
| H² (unaccounted variability / sampling variability) | 3.34 |
| R² (amount of heterogeneity accounted for) | 0.00% |
| Intercept | 0.0895 (95% CI ‐5.4218 to 5.6007, P = 0.9746) |
| Coefficient for log‐transformed mean ventilation time | ‐0.0442 (95% CI ‐1.1989 to 1.1105, P = 0.9403) |
We undertook a sensitivity analysis by pooling observed data without any imputation, and confirmed a nearly identical result (RR 0.84; 95% CI 0.63 to 1.14, P = 0.27). We did not undertake sensitivity analyses by excluding studies at high risk of bias or studies with non‐standardized design, due to the small number of studies.
Two studies also reported on the number of delirium‐free days during study treatment (Riker 2009) or a 12‐day period (Pandharipande 2007). Riker 2009 showed participants treated with dexmedetomidine had more delirium‐free days than those treated with midazolam (2.5 days vs 1.7 days, P = 0.02), but Pandharipande 2007 did not (dexmedetomidine: 9 days, lorazepam: 7 days, P = 0.09).
We were not able to conduct the arcsine test because of the small number of studies, but we suspected reporting bias for this outcome because six studies received funding support from pharmaceutical firms which manufacture dexmedetomidine (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013).
Risk of coma
Because only one study investigated the risk of coma (Pandharipande 2007; 103 participants), meta‐analysis was impossible. In Pandharipande 2007, the risk of coma was 63% in the dexmedetomidine group and 92% in the lorazepam group (P < 0.001). Participants with dexmedetomidine also had more coma‐free days than those in the lorazepam group (10 days vs 8 days, P < 0.001).
We were not able to conduct the arcsine test, but we suspected reporting bias for this outcome because Pandharipande 2007 received funding support from pharmaceutical firms which manufacture dexmedetomidine.
Adverse events
Incidence of bradycardia (Analysis 1.5; Analysis 1.6; Analysis 1.7)
1.5. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 5 Incidence of bradycardia (subgroup: class of alternative agents).
1.6. Analysis.
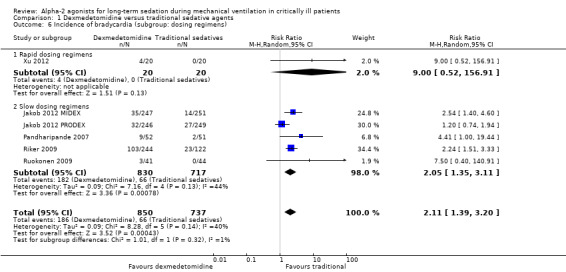
Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 6 Incidence of bradycardia (subgroup: dosing regimens).
1.7. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 7 Incidence of bradycardia (sensitivity analysis for observed data only).
Six studies, covering 1587 participants, reported on the incidence of bradycardia (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Xu 2012). Compared with traditional sedatives, those participants allocated to dexmedetomidine had a 111% significantly higher incidence of bradycardia (RR 2.11; 95% CI 1.39 to 3.20, P = 0.0004). The statistical heterogeneity was moderate across the trials (Chi² = 8.28, P = 0.14; I² = 40%).
We conducted two subgroup analyses, one for the class of alternative agents and one for the dosing regimens:
For subgroup analyses of different classes of alternative agents, the difference in effect size was significant (P = 0.04). Although all the pooled results from subgroups showed a higher incidence associated with dexmedetomidine, the standard care group had the highest risk ratio (RR 7.50; 95% CI 0.40 to 140.91; 85 participants) and the propofol group had the lowest (RR 1.20; 95% CI 0.74 to 1.94; 495 participants).
For subgroup analyses of different dosing regimens (rapid or slow), no between‐groups difference was detected (P = 0.32).
We undertook a sensitivity analysis by pooling the observed data without any imputation, and confirmed almost the same result (RR 2.11; 95% CI 1.39 to 3.21). We did not undertake sensitivity analyses by excluding studies at high risk of bias or studies with non‐standardized designs, due to the small number of studies.
Incidence of hypotension (Analysis 1.8; Analysis 1.9)
1.8. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 8 Incidence of hypotension (subgroup: class of alternative agents).
1.9. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 9 Incidence of hypotension (subgroup: dosing regimens).
Six studies, covering 1587 participants, reported on the incidence of hypotension (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Xu 2012). The risk of hypotension was higher with dexmedetomidine, but this difference was not statistically significant (RR 1.22; 95% CI 0.86 to 1.74, P = 0.26). The level of heterogeneity was high (I² = 53%, P = 0.07).
We conducted two subgroup analyses, one for the class of alternative agents and one for the dosing regimens, but none of them indicated differences in effect size between subgroups (class of alternative agents, P = 0.57; dosing regimens, P = 0.17).
We undertook a sensitivity analysis by pooling the observed data without any imputation, and confirmed almost the same result (RR 1.22; 95% CI 0.86 to 1.73, P = 0.27). We did not undertake sensitivity analyses by excluding trials at high risk of bias or trials with non‐standardized designs, due to the small number of trials.
Incidence of hypertension
Three trials, covering 1356 participants, reported on the incidence of hypertension (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009). None of the trials reported a difference between dexmedetomidine and alternatives: in Jakob 2012 MIDEX, the incidence of hypertension was 21.5% in the dexmedetomidine group and 20.8% in the midazolam group (P = 0.913). In Jakob 2012 PRODEX, the incidence of hypertension was 21.1% in the dexmedetomidine group and 15.0% in the propofol group (P = 0.08). In Riker 2009, the incidence of hypertension was 18.9% in the dexmedetomidine group and 29.5% in the midazolam group (P = 0.91).
Other adverse events possibly caused by alpha‐2 agonist
We added other alpha‐2‐associated adverse events that were reported to be significant in at least one study. Other adverse events included tachycardia, first‐degree atrioventricular block, hyperglycaemia and hypoglycaemia.
1. Tachycardia
Jakob 2012 PRODEX reported tachycardia as a significant adverse event, and we therefore include it in our assessment. Four studies, covering 1462 participants, reported on the incidence of tachycardia (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009). One of them indicated that dexmedetomidine was more likely to induce tachycardia than propofol (19.5% vs 11.3%, respectively; P = 0.02) (Jakob 2012 PRODEX). Two studies showed that participants allocated to dexmedetomidine compared with midazolam had a statistically significantly lower risk of tachycardia (Jakob 2012 MIDEX: dexmedetomidine 13.8%, midazolam 21.6%, P = 0.025; Riker 2009: dexmedetomidine 25.4%, midazolam 44.3%, P < 0.001), but one study comparing dexmedetomidine with lorazepam did not find a significant difference (dexmedetomidine 69%, lorazepam 73%, P = 0.71) (Pandharipande 2007). When we tried to pool these data, we detected an extremely high level of statistical heterogeneity (I² = 84%), and therefore abandoned the analysis.
2. First‐degree atrioventricular block
Jakob 2012 PRODEX reported first‐degree atrioventricular block as a significant adverse event. Two studies, covering 993 participants, reported on the incidence of first‐degree atrioventricular block (Jakob 2012 MIDEX; Jakob 2012 PRODEX). In Jakob 2012 PRODEX, the incidence was significantly higher in the dexmedetomidine group than in the propofol group (0.8% vs 3.7%, respectively; P = 0.04), but in Jakob 2012 MIDEX, comparing dexmedetomidine with midazolam (1.2% in each group; P = 0.99), we did not find any difference.
3. Hyperglycaemia
Riker 2009 reported hyperglycaemia as a significant adverse event. Three studies, totaling 1359 participants, reported on the incidence of hyperglycaemia (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Riker 2009). Only in Riker 2009 was the incidence higher in the dexmedetomidine group than in the midazolam group (56.6% vs 42.6%, respectively; P = 0.02). In the other studies, the incidence was low or absent, and did not reach statistical significance (Jakob 2012 MIDEX: 2% in dexmedetomidine group and 2% in midazolam group; Jakob 2012 PRODEX: 0.8% in dexmedetomidine group and 0% in propofol group).
4. Hypoglycaemia
Jakob 2012 MIDEX reported hypoglycaemia as a significant adverse event. Two studies, covering 993 participants, reported on the incidence of hypoglycaemia (Jakob 2012 MIDEX; Jakob 2012 PRODEX). Jakob 2012 MIDEX, comparing dexmedetomidine with midazolam, showed that the incidence in the dexmedetomidine group was higher (4% vs 0.8%, respectively; P = 0.02), while Jakob 2012 PRODEX compared dexmedetomidine with propofol, and showed the same incidence in both groups (1.2%; P = 0.99)
Proportion of sedation time spent at target sedation level (Analysis 1.11)
1.11. Analysis.
Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 11 Proportion of sedation time spent at target sedation level.
| Proportion of sedation time spent at target sedation level | ||
|---|---|---|
| Study | Dexmedetomidine | Traditional sedatives |
| Jakob 2012 MIDEX | 60.7% | 56.6% |
| Jakob 2012 PRODEX | 64.6% | 64.7% |
| Pandharipande 2007 | 80% (nurse); 67% (physician) | 67% (nurse); 55% (physician) |
| Riker 2009 | 77.3% | 75.1% |
| Ruokonen 2009 | 64% | 63% |
| Shehabi 2013 | 66% | 38% |
| Xu 2012 | 52.8% | 48.6% |
All the studies evaluated the proportion of sedation time spent at target sedation level. Pandharipande 2007 reported that participants treated with dexmedetomidine spent a higher proportion of time at the target sedation level than did those treated with lorazepam. Both physicians and nurses assessed the sedation level in this study: the proportion assessed by nurses was 80% for the dexmedetomidine group and 67% for the lorazepam group (P = 0.04); the proportion assessed by physicians was 67% for the dexmedetomidine group and 55% for the lorazepam group (P = 0.008). Similarly, in Shehabi 2013, there were more Richmond Agitation‐Sedation Scale (RASS) assessment scores in the target range in the dexmedetomidine group (66%) than in the standard sedation group (38%) during the first 48 hours of the trial (P = 0.01). The other five trials showed similar proportions of time between study groups: in Jakob 2012 MIDEX, 60.7% for the dexmedetomidine group and 56.6% for the midazolam group (P = 0.15); in Jakob 2012 PRODEX, 64.6% for the dexmedetomidine group and 64.7% for the propofol group (P = 0.97); in Riker 2009, 77.3% for the dexmedetomidine group and 75.1% for the midazolam group (P = 0.18); in Ruokonen 2009, 64% for the dexmedetomidine group and 63% for the standard care group; in Xu 2012, 52.8% for the dexmedetomidine group and 48.6% for the midazolam group. Ruokonen 2009 also separately calculated the proportion of participants with a different sedation target: RASS ‐4 and RASS ‐3 to 0. Of those with RASS ‐4, participants on dexmedetomidine spent 42% of the time at the target level, compared with 62% for participants on standard care; of those with RASS ‐3 to 0, participants on dexmedetomidine spent 74% of the time at the target level, compared with 64% for participants on standard care.
Duration of weaning
One study reported on the duration of weaning (Ruokonen 2009; 85 participants). The data for Ruokonen 2009, sourced from the electronic supplementary material which was available online, show that the median weaning times were 59.4 hours for the dexmedetomidine group (range 0 ‐ 259 hours) and 78.0 hours for the standard care group (either propofol or midazolam) (range 0 ‐ 565 hours), but the difference was not statistically significant (P = 0.270).
Intensive care unit length of stay (ICU LOS) (Analysis 1.12)
1.12. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 12 ICU length of stay (LOS) (subgroup: class of alternative agents).
Six trials, covering 1589 participants, compared intensive care unit (ICU) lengths of stay (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013). Jakob 2012 MIDEX, Jakob 2012 PRODEX and Ruokonen 2009 analysed the data using Cox’s proportional‐hazards regression, Riker 2009 and Pandharipande 2007 analysed it using Kaplan‐Meier survival analysis and Shehabi 2013 analysed it using Wilcoxon rank‐sum tests. All the studies showed that the median ICU length of stay was shorter in the dexmedetomidine groups than in the control groups, but none of the studies showed statistically significant results. Since Riker 2009 reported only the median without the interquartile range, we carried out meta‐analysis only on the other five trials (calculations from median/interquartile range to geometric mean/SD are shown in Table 2). Meta‐analysis yielded a random‐effects estimate of the geometric mean difference of ‐0.15 log days (95% CI ‐0.28 to ‐0.01, P = 0.04), a statistically significant finding corresponding to a reduction of 14% in the geometric mean (95% CI 1% to 24%). Neither the I² test (0%) nor the Chi² test (0.28, P = 0.99) indicate the presence of statistical heterogeneity. Subgroup analyses for different classes of alternative agents did not find any significant difference in effect size between subgroups (P = 0.93). We did not undertake sensitivity analyses by excluding studies at high risk of bias or studies with non‐standardized designs, due to the small number of studies.
Mortality (Analysis 1.13; Analysis 1.14)
1.13. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 13 Mortality (subgroup: class of alternative agents).
1.14. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 14 Mortality (sensitivity analysis for observed data only).
Six studies, covering 1584 participants, assessed mortality (Jakob 2012 MIDEX; Jakob 2012 PRODEX; Pandharipande 2007; Riker 2009; Ruokonen 2009; Shehabi 2013). We did not observed a significant difference between the dexmedetomidine and traditional sedative agents (RR 0.99; 95% CI 0.79 to 1.24, P = 0.92). The level of heterogeneity was low (Chi² = 6.05, P = 0.30; I² = 17%). We undertook subgroup analyses of different classes of alternative agents and found no significant differences in effect size among subgroups (P = 0.42). A sensitivity analysis pooling observed data only, without any imputation, did not change the result (RR 0.99; 95% CI 0.78 to 1.24, P = 0.90) (Analysis 1.14). We did not undertake sensitivity analyses by excluding studies at high risk of bias or studies with non‐standardized designs, due to the small number of studies.
Discussion
Summary of main results
In this systematic review, we assessed evidence from seven studies, involving 1624 critically ill participants, to investigate the effectiveness and safety of alpha‐2 agonists for long‐term sedation during mechanical ventilation. All the included studies investigated adults, and compared dexmedetomidine with traditional sedatives. Our meta‐analyses show that the use of dexmedetomidine in people who need more than 24 hours sedation was associated with a reduction of 22% in the geometric mean for ventilation time (95% confidence interval (CI) 10% to 33%). Because the relative change in geometric means usually produces results similar to the relative change in arithmetic means (Friedrich 2012), dexmedetomidine could cut approximately one‐fifth of the ventilation time. Our meta‐analysis provides no clear evidence that dexmedetomidine could reduce the risk of delirium. We found only one study reporting the risk of coma, but some methodological flaws prevented us from drawing a robust conclusion (Pandharipande 2007).
The results of our meta‐analyses, along with subgroup analyses for different classes of alternatives, showed a consistent trend across all the studies indicating a reduction in ventilation time. Two studies, Jakob 2012 MIDEX and Jakob 2012 PRODEX, which contributed most weight to the meta‐analysis, assessed both invasive and noninvasive ventilation time, and thus offer a wider application for this outcome. As a likely consequence of less ventilation time, we also observed a 14% reduction in intensive care unit (ICU) length of stay. However, the reason for this effect was unclear. One potential explanation might be that dexmedetomidine could provide adequate pain control. Early studies in rats and healthy volunteers suggested that dexmedetomidine offered additive or synergist analgesic effects (Hall 2000; Jaakola 1991; Meert 1994; Tham 2005). Results from previous meta‐analyses on perioperative use of dexmedetomidine for postoperative pain also found that dexmedetomidine decreased pain intensity and opioid consumption (Blaudszun 2012; Schnabel 2013). The importance of analgesia in reducing ventilation time and hospital stay has been confirmed by other randomized controlled trials (RCTs) (Breen 2005; Strom 2010). In our review, Ruokonen 2009 compared the visual analogue scale (VAS) scores of nurses’ assessments in those patients who were able to communicate, and found that the mean VAS scores in the dexmedetomidine group were lower than in the standard care group (30.6 vs 47.5; P < 0.001). Both Jakob 2012 MIDEX and Jakob 2012 PRODEX confirmed better pain scores in the dexmedetomidine group, although Ruokonen 2009 showed no difference in the cumulative dose of fentanyl for pain control between both groups, probably because the dexmedetomidine participants might request more additional analgesics. In Jakob 2012 MIDEX and Jakob 2012 PRODEX, participants on dexmedetomidine were more able to communicate pain, more arousable and more co‐operative. This characteristic of dexmedetomidine might promote the rational use of opioids and facilitate the delivery of patient care and medical examination. Another candidate explanation was suggested by a subgroup analysis of Pandharipande 2007. This post hoc analysis revealed that dexmedetomidine provided greater improvement in ventilation time among septic participants than among non‐septic participants, with the investigators attributing this effect to the anti‐inflammatory, organ‐protective and anti‐sympathetic properties of dexmedetomidine.
We found no evidence for the risk of delirium, with a high level of statistical heterogeneity. Two studies compared the number of ICU days without delirium but demonstrated inconsistent results (Pandharipande 2007; Riker 2009). Subgroup analysis of different classes of alternative agents found that both the benzodiazepine derivatives subgroup and the propofol subgroup favoured dexmedetomidine, while the standard care subgroup favoured the control condition. Because standard care participants used midazolam or propofol (both drugs were analysed alone in other subgroups), we were unable to attribute this difference to the characteristics of the control drugs. One possible explanation could be that in standard care both studies were at high risk for blinding of personnel and outcome assessment, which may have biased the results. Meta‐regression on geometric mean ventilation time found little statistical evidence to explain the heterogeneity, probably due to the small number of studies. We noted considerable variation among the studies for risk of delirium, which might be another important source of heterogeneity. There were several reasons for this: firstly, the time in ICU prior to study treatment ranged from 12 hours to 96 hours between the studies, which therefore altered the baseline risk of delirium; secondly, although all the studies used the confusion assessment method for the intensive care unit (CAM‐ICU), the frequency of assessments and the follow‐up periods were different; thirdly, even within same study the frequency might vary between groups, because a successful CAM‐ICU assessment was highly dependent on the participant's sedation level (within a RASS score of ‐3 or ehighermrhigher), which may explain in Ruokonen 2009 why more CAM‐ICU assessments were performed in the dexmedetomidine group than in the standard care group. Furthermore, because the sensitivity of the CAM‐ICU is poor (Van Eijk 2011), interpreting a positive CAM‐ICU assessment as an occurrence of delirium might overestimate the incidence and increase the uncertainty of the intervention effects.
Pandharipande 2007 showed that dexmedetomidine reduced the risk of coma and increased the number of ICU days without coma. However, because of its long half‐life, continuous infusion of lorazepam, which was used in the control group, could result in higher concentrations and could induce deeper sedation (De Wit 2006). On the one hand, such methodological limitation might increase the risk of iatrogenic coma (oversedation) in the control group, especially where the investigators assessed the sedation level infrequently (twice daily), but on the other hand dexmedetomidine caused less oversedation, even without frequent adjustment.
Bradycardia was the most commonly‐reported adverse event. Our meta‐analysis found that dexmedetomidine doubled the incidence of bradycardia. Subgroup analysis indicated that the risk ratio might differ depending on which alternatives were used as the comparator. For example, the risk ratio in the propofol subgroup was lower than in the benzodiazepine derivatives subgroup, probably because propofol itself could also induce bradycardia (Hashiba 2003). Riker 2009 showed the risk of bradycardia requiring an intervention (including readjusting or interruption of study drugs and use of atropine) in the dexmedetomidine group was low (4.9%) and not significantly increased. The bradycardiac effect of dexmedetomidine might therefore be mild. We did not find an increase in the risk of hypotension or hypertension. We included tachycardia, first‐degree atrioventricular block, hyperglycaemia and hypoglycaemia, which were reported to be significant in at least one study, as outcomes of adverse events post hoc. The results of the post hoc adverse events were inconsistent among studies, probably due to the different comparators. Because of the small number of studies, there was insufficient power to investigate any impact of these rare adverse events.
With respect to other secondary outcomes, we found dexmedetomidine was at least as effective as traditional sedatives on maintaining the target sedation level. In Ruokonen 2009, among participants with a target RASS score of ‐4 compared with ‐3 to 0, those on dexmedetomidine spent less time at target level than did those on standard care, which implies that dexmedetomidine may be a poor sedative choice when deep sedation is needed. Additionally, in both Jakob 2012 MIDEX and Jakob 2012 PRODEX, approximately one in every eight to ten participants ceased participation due to lack of efficacy, even on the maximum dose, and hence these two studies may overestimate the effectiveness of dexmedetomidine. Lack of blinding in some studies (Ruokonen 2009; Shehabi 2013; Xu 2012) would also have an unpredictable impact on this subjective outcome. We failed to identify any evidence for reducing duration of weaning.
Our meta‐analysis provides no evidence that dexmedetomidine had any impact on overall mortality. On the one hand the adverse events which might potentially influence haemodynamic stability, such as bradycardia and hypotension, did not contribute to an increase in mortality, while on the other it would seem that choosing sedatives still occurs 'downstream' in a framework of intensive care, and has limited effect on the prognosis for survival.
When we compared dexmedetomidine sedation in our review to 'no sedation protocol' in Strom 2010, we found some common patterns which might result in reduced ventilation time: using analgesia‐based sedation, retaining some degree of participant's cognitive function, and providing on‐demand pain control. It is also clear that dexmedetomidine was a more 'professional' sedative than the morphine used in the 'no sedation protocol'. A cost‐minimization analysis of Riker 2009 showed that dexmedetomidine could reduce total intensive care unit costs by about USD 10,000, because of decreased mechanical ventilation costs and ICU stay costs (Dasta 2010). All these properties of dexmedetomidine seemed to make it a cost‐effective choice for ICU sedation. But we must also consider any negative aspects to offset the beneficial effects. Firstly, patients might request additional sedatives or analgesics because they could express discomfort (Corbett 2005; Jakob 2012 MIDEX; Jakob 2012 PRODEX; Maclaren 2013). Secondly, despite a theoretical advantage of dexmedetomidine in providing nature‐like sleep, patients on dexmedetomidine were more likely to have sleeping difficulties (Corbett 2005; Oto 2012). Thirdly, a recent study focusing on psychological outcomes showed that dexmedetomidine was associated with greater recall of ICU experiences and higher risk of acute stress disorder (Maclaren 2013). As more frequent assessment of discomfort or pain and appropriate supplemental sedatives or analgesic would be needed, dexmedetomidine for sedation might potentially increase the workload for medical staff.
Overall completeness and applicability of evidence
Although the results of our meta‐analyses seem to support beneficial outcomes for the use of alpha‐2 agonists for long‐term sedation, there were several limitations. Firstly, the included studies investigated only adults treated with dexmedetomidine. Our findings therefore should not be extrapolated to children or to patients treated with clonidine. Secondly, none of the studies described in sufficient detail how they predicted that a patient would require long‐term ventilation and sedation. We believe that clinicians in different healthcare system may have different opinions on this point, so that the participants in our review might be substantially different from those in other healthcare systems. Thirdly, most studies excluded people with severe bradycardia, second‐ or third‐degree atrioventricular conduction block or hepatic impairment, and used a dose of dexmedetomidine of no more than 1.5 mcg/kg per hour. Thus, it was not clear whether dexmedetomidine could be used safely in these excluded patients, or whether it could safely be used in higher doses. Fourthly, most studies incorporated new ICU sedation strategies which were recommended by recent guideline (Barr 2013): light level of sedation protocols, daily interruptions, analgesia‐based sedation and monitoring of delirium. It is unclear whether our findings can be generalized to other healthcare systems where these revised sedation strategies do not apply.
Quality of the evidence
In our systematic review, six of the seven studies were at high risk of bias across the six domains (Figure 3). As a consequence, the general quality of evidence ranged from very low to low, based on the GRADE system (Table 1). We noted several methodological limitations in the included studies. Firstly, the risk of breaking blinding might be high, even when double‐blinding and a double‐dummy design were used. Wunsch 2012 has pointed out that when the comparator was propofol, even a single drop visible at the end of an infusion would break the blinding. We also suspect that a sophisticated clinician could easily guess the identity of the study drugs from the bradycardiac effect of dexmedetomidine. Secondly, high withdrawal rates in some studies might bias the overall results, especially in Jakob 2012 MIDEX and Jakob 2012 PRODEX, where more than 20% of participants withdrew from each study group. Most reasons for withdrawal were unbalanced between the groups and were related to outcomes, such as lack of efficacy and adverse events, which might lead to an underestimate of the adverse consequences and might have unpredictable impacts on the other outcomes. Thirdly, some participants from Jakob 2012 MIDEX, Jakob 2012 PRODEX and Ruokonen 2009 received continuous sedation prior to the study treatment. The carry‐over effect of preceding sedation might reduce the required dosages of dexmedetomidine and have unpredictable impacts on the intervention effect.
Potential biases in the review process
There were several potential biases in the review process which should be reiterated. Firstly, our systematic review includes only seven studies, and thus we were not able to generate funnel plots or to assess publication bias by using the Egger test and the arcsine test. Secondly, we emailed the first and corresponding authors of all seven included studies twice, but received only two responses (Pandharipande 2007; Shehabi 2013), with one providing study data (Shehabi 2013). In the absence of individual patient data or log‐transformed data, we estimated means using medians. We do not consider that this estimation would excessively influence the results of the meta‐analyses, since log‐transformed means will be approximately equal to log‐transformed median under the assumption that the log‐transformation of the raw data may substantially reduce skew. Thirdly, due to small numbers of included studies, our predefined subgroup analyses might be underpowered to explore and eliminate heterogeneity.
Agreements and disagreements with other studies or reviews
Previous meta‐analyses have investigated the effect of dexmedetomidine in an ICU setting. Tan 2010 reached similar conclusions to our findings for the ICU length of stay, risk of delirium and adverse events, but not for duration of mechanical ventilation. Two meta‐analyses comparing propofol head‐to‐head with dexmedetomidine both suggested that dexmedetomidine did not reduce ventilation time (Xia 2013; Zhuo 2012). One reported that dexmedetomidine reduced the risk of delirium (Zhuo 2012), which was confirmed in our subgroup analysis. Another meta‐analysis (Lin 2012) compared dexmedetomidine with placebo or traditional sedatives in the setting of a post‐cardiac surgery unit and found that dexmedetomidine reduced ventilation time and risk of delirium. However, this review included both randomized controlled trials and cohort studies, which may have increased heterogeneity and the risk of publication bias.
Authors' conclusions
Implications for practice.
In this review, we found the use of dexmedetomidine for long‐term sedation in critically ill, mechanically‐ventilated adults could help to reduce ventilation time and intensive care unit (ICU) length of stay. The premise behind this beneficial effect might be that medical staff carefully administered dexmedetomidine based on sedation level assessment, used a light level of sedation protocols, treated pain during sedation, and routinely monitored delirium. There was no evidence to support the use of dexmedetomidine in reducing risk of delirium, and inadequate evidence for it reducing the risk of coma. The most common adverse event of dexmedetomidine was bradycardia, but in most cases the bradycardiac effect was harmless and did not need to be treated. Dexmedetomidine was as effective as traditional sedatives for producing sedation and maintaining a light sedation level (a RASS score greater than or equal to ‐3). However, it might be a poor choice for a deeper sedation; even for light sedation, dexmedetomidine might still result in insufficient sedation in approximately one in every eight to 10 patients. We failed to identify any evidence concerning reducing duration of weaning or overall mortality.
Implications for research.
In this review, we found no eligible studies investigating clonidine, although a pilot randomized controlled trial (RCT) has shown clonidine to be as safe and effective as dexmedetomidine in critically ill patients (Machado 2011). There is therefore a need for a larger prospective study which could evaluate the efficacy, safety and cost effectiveness of clonidine for long‐term sedation. We found no studies in children, although many retrospective studies have shown the efficacy and safety of alpha‐2 agonists in children (Arenas 2004; Carney 2013; Czaja 2009; Gupta 2012). Future RCTs of alpha‐2 agonists could be conducted in children. Although our systematic review includes a broad range of populations, some confounding factors, such as participants' physical characteristics, medical conditions and reasons for ventilation, might affect the magnitude of the intervention effects. For example, participants with sepsis might benefit more from dexmedetomidine than those without sepsis (Pandharipande 2010). Future studies could focus more directly on specific populations, such as people with chronic obstructive pulmonary disease (COPD), people with adult respiratory distress syndrome (ARDS) or brain‐injured people. We observed a high level of heterogeneity for the risk of delirium, which may be associated with the imprecision of the screening tool. Future studies evaluating the efficacy of alpha‐2 agonists for ICU delirium could stratify randomized participants by similar risks, could use more validated diagnostic tools, and could focus on long‐term cognitive outcomes.
Feedback
Suggest standardized definition for delirium and more transparency in how data is extracted
Summary
Comment one
Given the significant heterogeneity observed in the incidence of delirium data, we would suggest using a standardized definition for delirium. For example, the Riker 2009 study defined delirium as the presence of a positive CAM‐ICU score. However, the Jakob 2012 PRODEX and Jakob 2012 MIDEX authors reported delirium separately in two ways:
positive CAM‐ICU score, and
clinician‐reported delirium.
The data for these methods of reporting were not concordant. That is, many patients with positive CAM‐ICU scores were not counted as having delirium by the more subjective clinician‐reported method. In the meta‐analysis, data for clinician‐reported delirium in the PRODEX, MIDEX, and Ruokonen 2009 studies was used, but the presence of a positive CAM‐ICU score to determine delirium was used in the remaining studies. We feel that counting delirium in two separate ways introduces avoidable heterogeneity.
Comment two
Additionally, we suggest more transparency in how data is extracted. We noticed some inconsistencies in data obtained from the PRODEX and MIDEX trials. For example, in Analysis 1.3, delirium was recorded as having occurred in 7/246 dexmedetomidine patients and 19/249 propofol patients. However, in the PRODEX dataset, 7 (2.8%) dexmedetomidine patients were reported to have developed delirium while 17 (6.9%) developed delirium in the propofol group. We were unable to identify where the numbers of patients who developed delirium were obtained in the Cochrane meta‐analysis. To improve reader comprehension, we suggest that a base analysis be performed using raw extracted values from the study with a best and worst case sensitivity analysis performed to assess differences between the exposed population and all randomized patients.
Reply
Reply to comment one
We were aware that the Jakob 2012 PRODEX and Jakob 2012 MIDEX trials reported the rate of both positive CAM‐ICU score and clinician‐reported delirium (which was also based on the CAM‐ICU score). We agree that patients with positive CAM‐ICU scores might not be counted as having delirium by the clinician‐reported method. However, the rate of positive CAM‐ICU score was only counted at a single time point of 48 hours after stopping study sedation rather than whole study follow‐up. We aimed to compare the delirium rate during ventilation time or sedation time, and thus we did not considered it as representative of the delirium rate. Although the clinician‐reported delirium rate during study follow‐up was more subjective, we thought it was more appropriate to be included in our analyses
Reply to comment two
In the Jakob 2012 PRODEX trial, two patients in the propofol group were excluded because they 'withdrew consent’ (this was clarified in the Characteristics of included studies). We judged the data of the two patients to be "not missing at random”, and thus we inferred bad outcomes (having delirium) and performed analyses using both imputed data and raw extracted data (sensitivity analyses).
Contributors
Summary authors
Alice Hou, B.Sc.(Pharm.) Ying (Joane) Tang, B.Sc.(Pharm.) Angus Kinkade, PharmD, MSc.
We certify that we have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of our feedback.
Reply author
Dr Ken Chen Anesthesiologist Department of Anesthesiology Rui Jin Hospital Lu Wan Branch, Shanghai Jiao Tong University School of Medicine Shanghai China
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 12, 2012 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 19 August 2015 | Feedback has been incorporated | see Feedback 1 |
Acknowledgements
We would like to thank Andrew Smith (content editor), Nathan Pace (statistical editor), Hannah Wunsch, Lisa Burry, Souhayl Dahmani (peer reviewers) for their help and editorial advice during the preparation of this review.
We would like to thank Yahya Shehabi (Professor of Intensive Care Medicine, Medical Director ACCC Program Director Intensive Care Research) for providing additional information on his study (Shehabi 2013).
We would like to thank John Carlisle (content editor), Cathal Walsh (statistical editor), Lisa Burry, Hannah Wunsch and Souhayl Dahmani (peer reviewers) for their help and editorial advice during the preparation of this protocol for the systematic review. We would also like to thank Xiaoli Ge (Physician, Emergency Department, Xinhua Hospital), Jie Zhao (Physician, Emergency Department, Xinhua Hospital) and Zengbin Wu (Physician, Emergency Department, Xinhua Hospital) for their comments on our protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Adrenergic alpha‐Agonists] explode all trees #2 MeSH descriptor: [Dexmedetomidine] explode all trees #3 MeSH descriptor: [Clonidine] explode all trees #4 alpha?2 adrenergic agonist* or dexmed*or precede or clonidin* or catapresan* or isoglancon or ST‐155 #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Respiration, Artificial] explode all trees #7 MeSH descriptor: [Ventilation] this term only #8 ((invasive or intubat* or mechanical or artificial) near (ventilat* or respire*)) or ventilat*:ti,ab #9 #6 or #7 or #8 #10 #5 and #9
Appendix 2. OVID MEDLINE search strategy
1. exp Adrenergic alpha‐Agonists/ or exp Dexmedetomidine/ or exp Clonidine/ or alpha?2 adrenergic agonist*.af. or (dexmed*or precede* or clonidin* or catapresan* or isoglancon or ST‐155).mp.
2. exp Respiration, Artificial/ or Ventilation/ or ((invasive or intubat* or mechanical or artificial) adj3 (ventilat* or respire*)).mp. or ventilat*.ti,ab.
3. 1 and 2
4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp alpha adrenergic receptor stimulating agent/ or exp dexmedetomidine/ or exp clonidine/ or alpha?2 adrenergic agonist*.af. or (dexmed*or precede* or clonidin* or catapresan* or isoglancon or ST‐155).mp. 2. exp artificial ventilation/ or air conditioning/ or ((invasive or intubat* or mechanical or artificial) adj3 (ventilat* or respire*)).mp. or ventilat*.ti,ab. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh 5. 3 and 4
Appendix 4. CINAHL (EBSCO host) search strategy
S1 (MM "Adrenergic Alpha‐Agonists") OR (MM "Clonidine") OR alpha?2 adrenergic agonist* OR dexmed*or precede or clonidin* or catapresan* or isoglancon or ST‐155 S2 (MM "Respiration, Artificial") OR (MM "Ventilation") OR ((invasive or intubat* or mechanical or artificial) N3 (ventilat* or respire*)) OR AB ventilat*S3 S1 AND S2
Appendix 5. LILACS (BIREME) search strategy
alpha 2 adrenergic agonist$ or dexmed$ or precede$ or clonidin$ or catapresan$ or isoglancon or ST‐155 [Palavras] and ((invasive or intubat$ or mechanical or artificial) and (ventilat$ or respire$)) or ventilat$ [Palavras]
Appendix 6. ISI Web of Science search strategy
#1 TS=(alpha?2 adrenergic agonist* or dexmed*or precede* or clonidin* or catapresan* or isoglancon or ST‐155) #2 TS=((invasive or intubat* or mechanical or artificial) SAME (ventilat* or respire*)) or TI=ventilat* #3 #2 AND #1
Appendix 7. Chinese Biological Medical Database (CBM) search strategy
("肾上腺素能α激动剂"[主题词] OR "右美托咪啶"[主题词] OR "可乐定"[主题词] OR "肾上腺素能α激动剂"[全字段] OR "dexmedetomidine"[全字段] OR "右美托咪啶"[全字段] OR "艾贝宁"[全字段] OR "clonidine"[全字段] OR "可乐定"[全字段]) AND ("通气机, 机械"[主题词] OR "Ventilators, Mechanical"[全字段] OR "机械通气"[全字段] OR "肺通气机"[全字段] OR "呼吸机"[全字段] OR "呼吸器"[全字段] OR "辅助通气"[全字段] OR "人工气道"[全字段] OR "intubation"[全字段] OR "气管插管"[全字段]) AND (治疗应用 OR 随机 OR 对照)
Appendix 8. China National Knowledge Infrastructure (CNKI) search strategy
((SU=α_2肾上腺素受体激动药 OR SU=右美托咪啶 OR SU=可乐定) OR (FT=α_2肾上腺素受体激动药 OR FT=α_2受体激动药 OR FT=右美托咪啶 OR FT=可乐定 OR FT=艾贝宁)) AND (SU=机械通气 OR (FT=机械通气 OR FT=肺通气机 OR FT=呼吸器 OR FT=呼吸机 OR FT=辅助通气 OR FT=人工气道 OR FT=气管插管)) AND (TI=多中心 OR TI=疗效 OR TI=随机 OR AB=多中心 OR AB=疗效 OR AB=随机)
Appendix 9. Data extraction form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/ abstract/ report that data are extracted from) |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
2. Study Eligibility
| Study Characteristics | Eligibility criteria | Yes | No | Unclear | |
| Type of study | Randomized controlled trial or quasi‐randomized controlled Trial? | ||||
| Simple parallel group design? | |||||
|
Participants |
Were the participants of any age, except neonates, who required intensive care and invasive mechanical ventilation when recruited? | ||||
| Types of intervention | Did the study compare clonidine or dexmedetomidine versus alternative sedative agents? | ||||
| Did the study explicitly report that each participant is anticipated to need long‐term sedation (> 24 hours)? | |||||
| Types of outcome measures | Did the study report any one of
|
||||
| The study will be excluded if any of the above answers are “No”. | |||||
| INCLUDE □ | EXCLUDE □ | ||||
|
Notes: | |||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Setting and methods
| Description | |||
| Country/countries | |||
| Setting | □ Single‐centre □ Multi‐centre (centre number = ) |
||
| Type of ICU | □ Mixed intensive care unit □ Medical intensive care unit □ Surgical intensive care unit □ Coronary care unit □ Respiratory care unit □ Burn unit □ Other (specify: ) |
||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Informed consent obtained | Yes □ | No □ | Unclear □ |
| Ethical approval needed/ obtained for study | Yes □ | No □ | Unclear □ |
|
Notes: | |||
4. Risk of Bias assessment
See Chapter 8 of the Cochrane Handbook
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: duration of mechanical ventilation |
||||
|
Outcome group: rate of delirium |
|||||
| Outcome group: rate of coma | |||||
|
Outcome group: adverse events |
|||||
| Outcome group: proportion of sedation time spent in target sedation level | |||||
|
Outcome group: duration of weaning |
|||||
|
Outcome group: ICU Length of Stay (LOS) |
|||||
|
Outcome group: mortality |
|||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: duration of mechanical ventilation |
||||
|
Outcome group: rate of delirium |
|||||
| Outcome group: rate of coma | |||||
|
Outcome group: adverse events |
|||||
|
Outcome group: duration of weaning |
|||||
| Outcome group: proportion of sedation time spent in target sedation level | |||||
|
Outcome group: ICU Length of Stay (LOS) |
|||||
|
Outcome group: mortality |
|||||
|
Incomplete outcome data (attrition bias) |
Outcome group: duration of mechanical ventilation |
||||
|
Outcome group: rate of delirium |
|||||
| Outcome group: rate of coma | |||||
|
Outcome group: adverse events |
|||||
| Outcome group: proportion of sedation time spent in target sedation level | |||||
|
Outcome group: duration of weaning |
|||||
|
Outcome group: ICU Length of Stay (LOS) |
|||||
|
Outcome group: Mortality |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias (whether the groups were balanced at baseline, whether the co‐interventions were avoided or similar) |
|||||
|
Quality classification |
Low risk of bias □ Unclear risk of bias □ High risk of bias □ | ||||
|
Notes: | |||||
5. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
|
| Total no. analysed | |
|
Withdrawals and exclusions |
|
|
Age (mean or median) |
|
|
Co‐morbidities |
|
|
Notes: | |
6. Intervention groups
Copy and paste table if more than two groups
|
Description as stated in report/paper |
||
|
Anticipated duration of sedation (if no limitation of anticipated duration of sedation, please mark "no limitation") |
||
| No. randomized to group | Intervention group: |
Control group 1: |
|
No. included in analyses |
Intervention group: | Control group 1: |
| Sedative agent | Intervention group: |
Control group 1: |
|
Description of route, dose and frequency |
Intervention group: |
Control group 1: |
|
Target sedation level and sedation level measurement (if no target sedation level, please mark "no target sedation level") |
||
|
Rescue medication to reach target sedation level (if no rescue medication, please mark "/") |
Intervention group: |
Control group 1: |
| Study design | □ Standardized design | □ Non‐standardized design |
|
Daily interruption |
Yes □ No □ |
|
|
Description of pain control (if no pain control, please mark "no pain control") |
|
|
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | Intervention group: | Intervention group: |
|
Notes: | ||
7. Outcomes
|
Outcome name |
Outcome reported |
Outcome definition or measurement as stated in report/paper | |
|
Duration of mechanical ventilation |
Yes □ No □ |
||
| Risk of delirium | Yes □ No □ |
||
| Risk of coma | Yes □ No □ |
||
|
Adverse events |
Incidence of bradycardia | Yes □ No □ |
|
| Incidence of hypotension | Yes □ No □ |
||
| Incidence of hypertension | Yes □ No □ |
||
| Other | (specify: ) | ||
| Proportion of sedation time spent in target sedation level | Yes □ No □ | ||
|
Duration of weaning |
Yes □ No □ |
||
|
ICU Length of Stay (LOS) |
Yes □ No □ |
||
|
Mortality |
Yes □ No □ |
||
| Other | (specify: ) | ||
|
Notes: | |||
8. Results
Copy and paste table if more than two groups
Dichotomous outcome
| Outcome | Intervention group | Control group 1 | Follow‐up time point | |||||||
| No. events | No. participants | No. missing participants | Other information | No. events | No. participants | No. missing participants | Other information | |||
| Risk of delirium | ||||||||||
| Risk of coma | ||||||||||
|
Adverse events |
Incidence of bradycardia | |||||||||
| Incidence of hypotension | ||||||||||
| Incidence of hypertension | ||||||||||
| Other (specify: ) |
||||||||||
| Mortality | ||||||||||
|
Notes: | ||||||||||
Continuous outcome
| Outcome | Log scale | Intervention group | Control group 1 | ||||||
| No. participants | Mean (SD) |
95% CI | Other information | No. participants | Mean (SD) |
95% CI | Other information | ||
| Duration of mechanical ventilation | Yes/No |
||||||||
| Duration of weaning | Yes/No |
||||||||
| ICU Length of Stay (LOS) | Yes/No |
||||||||
|
Notes: | |||||||||
Other outcome
| Outcome | Result stated in report/paper |
| Proportion of sedation time spent in target sedation level | |
| Notes: | |
9. Applicability
| Yes | No | Unclear | |
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | |||
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | |||
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
|||
| Notes: | |||
10. Other information
|
Description as stated in report/paper |
|
| Key conclusions of study authors | |
| References to other relevant studies | |
| Correspondence required for further study information(from whom, what and when) | |
| Notes: | |
Data and analyses
Comparison 1. Dexmedetomidine versus traditional sedative agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation (subgroup: class of alternative agents) | 4 | 1120 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.40, ‐0.10] |
| 1.1 Benzodiazepine derivatives | 1 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.49, ‐0.09] |
| 1.2 Propofol | 1 | 498 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.43, 0.04] |
| 1.3 Standard care (propofol and midazolam) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.26, 0.64] |
| 2 Duration of mechanical ventilation (sensitivity analysis using data from 'time to extubation'") | 4 | 1120 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.49, ‐0.20] |
| 3 Risk of delirium (subgroup: class of alternative agents) | 7 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 3.1 Benzodiazepine derivatives | 4 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.59, 1.09] |
| 3.2 Propofol | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.87] |
| 3.3 Standard care (propofol and midazolam) | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.86, 2.41] |
| 4 Risk of delirium (sensitivity analysis for observed data only) | 7 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.14] |
| 5 Incidence of bradycardia (subgroup: class of alternative agents) | 6 | 1587 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.39, 3.20] |
| 5.1 Benzodiazepine derivatives | 4 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.77, 3.36] |
| 5.2 Propofol | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.74, 1.94] |
| 5.3 Standard care (propofol and midazolam) | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 7.50 [0.40, 140.91] |
| 6 Incidence of bradycardia (subgroup: dosing regimens) | 6 | 1587 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.39, 3.20] |
| 6.1 Rapid dosing regimens | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 6.2 Slow dosing regimens | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.35, 3.11] |
| 7 Incidence of bradycardia (sensitivity analysis for observed data only) | 6 | 1584 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.39, 3.21] |
| 8 Incidence of hypotension (subgroup: class of alternative agents) | 6 | 1587 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.74] |
| 8.1 Benzodiazepine derivatives | 4 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.77, 2.53] |
| 8.2 Propofol | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.62, 1.54] |
| 8.3 Standard care (propofol and midazolam) | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.20, 22.79] |
| 9 Incidence of hypotension (subgroup: dosing regimens) | 6 | 1587 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.74] |
| 9.1 Rapid dosing regimens | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 9.2 Slow dosing regimens | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.86, 1.62] |
| 10 Incidence of hypotension (sensitivity analysis for observed data only) | 6 | 1584 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.73] |
| 11 Proportion of sedation time spent at target sedation level | Other data | No numeric data | ||
| 12 ICU length of stay (LOS) (subgroup: class of alternative agents) | 5 | 1223 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.28, ‐0.01] |
| 12.1 Benzodiazepine derivatives | 2 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.03] |
| 12.2 Propofol | 1 | 498 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.10] |
| 12.3 Standard care (propofol and midazolam) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.69, 0.26] |
| 13 Mortality (subgroup: class of alternative agents) | 6 | 1584 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 13.1 Benzodiazepine derivatives | 3 | 967 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 13.2 Propofol | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.55, 1.21] |
| 13.3 Standard care (propofol and midazolam) | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.67, 3.11] |
| 14 Mortality (sensitivity analysis for observed data only) | 6 | 1581 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.24] |
1.10. Analysis.

Comparison 1 Dexmedetomidine versus traditional sedative agents, Outcome 10 Incidence of hypotension (sensitivity analysis for observed data only).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jakob 2012 MIDEX.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
249 randomized to receive dexmedetomidine; 252 randomized to receive midazolam, 2 in the dexmedetomidine group and 1 in the midazolam group were withdrawn but did not receive any study drugs. We excluded them from our analyses of dichotomous outcomes but not for continuous outcomes. This is because in the absence of raw data, we were unable to distinguish the continuous data from them. The analyses of continuous outcomes, duration of mechanical ventilation and ICU LOS, were based on intention‐to‐treat analysis (249 in the dexmedetomidine group included and 251 in midazolam group included, 1 in midazolam group excluded due to 'withdrew consent'). The median age of the dexmedetomidine group was 65 years. The median age of the midazolam group was 65 years. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | June 2007 to August 2009 | |
| Setting and Country | Setting: intensive care units Country: Belgium, Estonia, Finland, France, Germany, Netherlands, Norway, Switzerland, United Kingdom | |
| Funding source and Declarations of interest | Sources of funding: Orion Corporation, Orion Pharma Declaration of investigators’ conflicts of interest: On behalf of Drs Jakob and Takala, a grant and consulting fees were paid to the Bern University Hospital Department of Intensive Care Medicine to cover the costs of performing the study. The department was reimbursed for the authors’ travel costs to publication committee meetings. Dr Jakob served as an expert for preparing the regulatory filing of the drug; the money was also paid into a departmental fund. Dr Grounds received a consulting fee from Orion Pharma during planning and execution of the study. He and members of his clinical staff at St George’s Hospital received monetary support from Orion for travel to study‐related meetings to ensure compliance with the study protocol. His department received payment of a fee per patient recruited to the study, as well as drugs used in the study and support personnel to assist with data input. Dr Ruokonen’s institution, Kuopio University, received a grant from Orion to carry out the study, as well as consulting fees and reimbursement of travel funds. Messrs Sarapohja, Garratt, and Bratty were full‐time employees of Orion Pharma through‐out the period of the project. Their routine travel expenses were reimbursed as part of their employment. Dr Pocock of the London School of Hygiene and Tropical Medicine received a consulting fee from Orion in connection with the study, and his institution received a grant. London School of Hygiene and Tropical Medicine also received a grant for independent statistical review performed by Dr Duolao Wang. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified for study centre in blocks of 4." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible study participants were randomized 1:1 by a central interactive voice‐response system funded by the sponsor to either continue their current standard care (midazolam [MIDEX trial] or propofol [PRODEX trial]) or switch to dexmedetomidine." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatments were administered in a double‐dummy design, with 0.9% sodium chloride as dummy for all treatments." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcome: risk of delirium, proportion of sedation time spent at target sedation level Quote: "Treatments were administered in a double‐dummy design, with 0.9% sodium chloride as dummy for all treatments." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: duration of mechanical ventilation, adverse events, ICU length of stay (LOS), mortality Quote: "Treatments were administered in a double‐dummy design, with 0.9% sodium chloride as dummy for all treatments." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dexmedetomidine group: 249 included in intention‐to‐treat analyses; 60/249 withdrawals (23 due to 'lack of efficacy'; 23 due to 'adverse or serious adverse event'; 2 due to 'protocol violation'; 16 due to 'other reasons'); 227 included in per‐protocol analyses, 22 excluded (8 due to 'missing inclusion criteria'; 8 due to 'incorrect dosing'; 1 due to 'received excluded medication'; 5 due to 'missing assessments'); Midazolam group: 251 included in intention‐to‐treat analyses, 1 excluded due to 'withdrew consent'; 51/252 withdrawals (10 due to 'lack of efficacy'; 19 due to 'adverse or serious adverse event'; 2 due to 'protocol violation'; 21 due to 'other reasons'); 233 included in per‐protocol analyses, 18 excluded (7 due to 'missing inclusion criteria'; 1 due to 'met exclusion criteria'; 6 due to 'incorrect dosing'; 2 due to 'received excluded medication'; 2 due to 'missing assessments') Comment: > 20% participants in each group withdrew. Reasons for withdrawal and exclusion were not balanced between study groups. These missing data would inevitably bias the results. |
| Selective reporting (reporting bias) | Low risk | Comment: All the outcomes listed in Methods section are reported. |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias. |
Jakob 2012 PRODEX.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria: Age > 18 years
Exclusion criteria:
251 randomized to receive dexmedetomidine; 249 randomized to receive propofol, 5 in dexmedetomidine group were withdrawn but did not receive any study drugs. We excluded them in our analyses of dichotomous outcomes but not for continuous outcomes. This is because in the absence of raw data, we were unable to distinguish the continuous data from them. The analyses of continuous outcomes, duration of mechanical ventilation and ICU LOS, were based on intention‐to‐treat analysis (251 in dexmedetomidine group included and 247 in propofol group included, 2 in propofol group excluded due to 'withdrew consent'). The median age of the dexmedetomidine group was 65 years; the median age of the propofol group was 65 years. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | May 2007 to March 2010 | |
| Setting and Country | Setting: intensive care units Country: Belgium, Finland, Germany, Netherlands, Russian Federation, Switzerland, United Kingdom | |
| Funding source and Declarations of interest | Sources of funding: Orion Corporation, Orion Pharma Declaration of investigators’ conflicts of interest: On behalf of Drs Jakob and Takala, a grant and consulting fees were paid to the Bern University Hospital Department of Intensive Care Medicine to cover the costs of performing the study. The department was reimbursed for the authors’ travel costs to publication committee meetings. Dr Jakob served as an expert for preparing the regulatory filing of the drug; the money was also paid into a departmental fund. Dr Grounds received a consulting fee from Orion Pharma during planning and execution of the study. He and members of his clinical staff at St George’s Hospital received monetary support from Orion for travel to study‐related meetings to ensure compliance with the study protocol. His department received payment of a fee per patient recruited to the study, as well as drugs used in the study and support personnel to assist with data input. Dr Ruokonen’s institution, Kuopio University, received a grant from Orion to carry out the study, as well as consulting fees and reimbursement of travel funds. Messrs Sarapohja, Garratt, and Bratty were full‐time employees of Orion Pharma through‐out the period of the project. Their routine travel expenses were reimbursed as part of their employment. Dr Pocock of the London School of Hygiene and Tropical Medicine received a consulting fee from Orion in connection with the study, and his institution received a grant. London School of Hygiene and Tropical Medicine also received a grant for independent statistical review performed by Dr Duolao Wang. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified for study centre in blocks of 4." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible study participants were randomized 1:1 by a central interactive voice‐response system funded by the sponsor to either continue their current standard care (midazolam [MIDEX trial] or propofol [PRODEX trial]) or switch to dexmedetomidine." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Propofol and propofol dummy were prepared, connected, and removed by independent personnel and infused with nontransparent black syringes, infusion tubings, and connectors." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcome: risk of delirium, proportion of sedation time spent at target sedation level Quote: "Propofol and propofol dummy were prepared, connected, and removed by independent personnel and infused with nontransparent black syringes, infusion tubings, and connectors." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: duration of mechanical ventilation, adverse events, ICU length of stay (LOS), mortality Quote: "Propofol and propofol dummy were prepared, connected, and removed by independent personnel and infused with nontransparent black syringes, infusion tubings, and connectors." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dexmedetomidine group: 251 included in intention‐to‐treat analyses; 71/251 withdrawals (36 due to 'lack of efficacy'; 29 due to 'adverse or serious adverse event'; 1 due to ‘Nonpharmacological intervention’; 1 due to 'protocol violation'; 7 due to 'other reasons'); 223 included in per‐protocol analyses, 28 excluded (3 due to 'missing inclusion criteria'; 1 due to 'met exclusion criteria'; 7 due to 'incorrect dosing'; 1 due to 'received excluded medication'; 16 due to 'missing assessments') Propofol group: 247 included in intention‐to‐treat analyses, 2 excluded due to 'withdrew consent'; 60/249 withdraws from propofol group (13 due to 'lack of efficacy'; 28 due to 'adverse or serious adverse event'; 4 due to 'nonpharmacological intervention'; 3 due to 'protocol violation'; 16 due to 'other reasons'); 214 included in per‐protocol analyses, 33 excluded (7 due to 'missing inclusion criteria'; 2 due to 'met exclusion criteria'; 10 due to 'incorrect dosing'; 6 due to 'received excluded medication'; 8 due to 'missing assessments') Comment: > 20% participants in each group withdrew. Reasons for withdrawal and exclusions were not balanced between study groups. These missing data would inevitably bias the results. |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in Methods section are reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Pandharipande 2007.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
54 randomized to receive dexmedetomidine; 52 randomized to receive lorazepam, 2 in the dexmedetomidine group and 1 in the lorazepam group were withdrawn but did not receive any study drugs and therefore were excluded in our review. These participants were not included in intention‐to‐treat analysis. The median age of the dexmedetomidine group was 60 years. The median age of the lorazepam group was 59 years. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | August 2004 to August 2007 | |
| Setting and Country | Setting: intensive care units Country: United States | |
| Funding source and Declarations of interest | Sources of funding: Hospira Inc, Lake Forest, Illinois Declaration of investigators’ conflicts of interest: This investigator‐initiated study was aided by the receipt of the study drug and an unrestricted research grant for laboratory and investigational studies from Hospira Inc. Dr Pandharipande is the recipient of the ASCCA‐FAER‐Abbott Physician Scientist Award and the Vanderbilt Physician Scientist Development Award. Dr Girard is supported by the Hartford Geriatrics Health Outcomes Research Scholars Award Program and the Vanderbilt Physician Scientist Development Program. Dr Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award) and a grant from the National Institutes of Health (AG0727201). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized using computer‐generated, permuted block randomization (known only to the investigational pharmacists) and stratified by site to receive sedation with either dexmedetomidine or lorazepam." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized using computer‐generated, permuted block randomization (known only to the investigational pharmacists) and stratified by site to receive sedation with either dexmedetomidine or lorazepam." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We used infusion instead of bolus dosing to preserve the blinding and to minimize potential adverse events. The study drug was prepared in clear bags containing either dexmedetomidine (prepared for a final concentration of 0.15 μg/kg per mL) or lorazepam (1 mg/mL). The study drug infusion was started at 1 mL/h (0.15 μg/kg per hour dexmedetomidine or 1 mg/h lorazepam) and titrated by the bedside nurse to a maximum of 10 mL/h (1.5 μg/kg per hour dexmedetomidine or 10 mg/h lorazepam) to achieve the sedation goal set by the patient's medical team using the Richmond Agitation‐Sedation Scale (RASS)." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcome: risk of delirium, risk of coma, proportion of sedation time spent at target sedation level Quote: "We used infusion instead of bolus dosing to preserve the blinding and to minimize potential adverse events. The study drug was prepared in clear bags containing either dexmedetomidine (prepared for a final concentration of 0.15 μg/kg per mL) or lorazepam (1 mg/mL). The study drug infusion was started at 1 mL/h (0.15 μg/kg per hour dexmedetomidine or 1 mg/h lorazepam) and titrated by the bedside nurse to a maximum of 10 mL/h (1.5 μg/kg per hour dexmedetomidine or 10 mg/h lorazepam) to achieve the sedation goal set by the patient's medical team using the Richmond Agitation‐Sedation Scale (RASS)." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: ventilator‐free days, adverse effects, ICU LOS, mortality Quote: "We used infusion instead of bolus dosing to preserve the blinding and to minimize potential adverse events. The study drug was prepared in clear bags containing either dexmedetomidine (prepared for a final concentration of 0.15 μg/kg per mL) or lorazepam (1 mg/mL). The study drug infusion was started at 1 mL/h (0.15 μg/kg per hour dexmedetomidine or 1 mg/h lorazepam) and titrated by the bedside nurse to a maximum of 10 mL/h (1.5 μg/kg per hour dexmedetomidine or 10 mg/h lorazepam) to achieve the sedation goal set by the patient's medical team using the Richmond Agitation‐Sedation Scale (RASS)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals from dexmedetomidine group (due to 'withdrawn by family'), 52 completed study protocol; 1 withdrawal from lorazepam group (due to 'withdrawn by family'), 51 completed study protocol. Comment: Reasons for withdrawal were explicitly reported and balanced across study groups. |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in Methods section are reported. |
| Other bias | High risk | Comment: This study used continuous infusion of lorazepam as a comparator, did not mandate daily interruption, and assessed sedation level infrequently. Such a study design might potentially increase the risk of coma in the control group and was considered to be a source of bias. |
Riker 2009.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
250 randomized to receive dexmedetomidine; 125 randomized to receive midazolam, 6 in dexmedetomidine group and 3 in midazolam group were withdrawn but did not receive any study drugs and therefore excluded in our review. These participants were not included in intention‐to‐treat analysis. The mean age of the dexmedetomidine group was 61.5 years; the mean age of the midazolam group was 62.9 years |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | March 2005 to August 2007 | |
| Setting and Country | Setting: intensive care units Country: United States | |
| Funding source and Declarations of interest | Sources of funding: Hospira Inc, Lake Forest, Illinois Declaration of investigators’ conflicts of interest: Dr Riker reports that he has received honoraria and/or grant support from Aspect Medical Systems Inc, AstraZeneca, Eli Lilly, Hospira, Takeda, and the Academy for Continued Healthcare Learning. Dr Shehabi reports that he has received honoraria and/or grant support from Hospira, Edward Life‐ sciences, Theravance, and the Intensive Care Foundation. Dr Ceraso reports that he has received honoraria and/or grant support from Hospira. Dr Koura reports that he has received honoraria and/or grant support from Altana, Artisan Pharma, Boehringer‐Ingelheim, CSL, Hospira, Ortho‐McNeil, Sepracor, Schering‐Plough, and United BioScience Corp. Dr Whitten reports that he has received honoraria and/or grant support from Hospira. Dr Margolis reports that he has received honoraria and/or grant support from Hospira. Mr Byrne reports that he was paid a consulting fee for serving as the independent statistical reviewer by the sponsor. Dr Ely reports that he has received honoraria and/or grant support from Hospira, Pfizer, Eli Lilly, GlaxoSmithKline, and Aspect Medical, and is an advisor to Healthways. Dr Rocha reports that he has received honoraria and/or grant sup‐ port from Hospira, Theravance, Altana, Novartis and the Canadian Institute of Health Research. Dr Bokesch and Mr Wisemandle reported no disclosures. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomized 2:1 to receive dexmedetomidine to obtain more comprehensive safety data during prolonged dexmedetomidine use."; "All patients were centrally randomized using an interactive voice‐response system and a computer‐generated schedule." |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients were centrally randomized using an interactive voice‐response system and a computer‐generated schedule." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "Optional blinded loading doses (up to 1 μg/kg dexmedetomidine or 0.05 mg/kg midazolam) could be administered at the investigator’s discretion. The starting maintenance infusion dose of blinded study drug was 0.8 μg/kg per hour for dexmedetomidine and 0.06 mg/kg per hour for midazolam, corresponding to the mid‐point of the allowable infusion dose range." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcome: risk of delirium, proportion of sedation time spent at target sedation level Quote: "Optional blinded loading doses (up to 1 μg/kg dexmedetomidine or 0.05 mg/kg midazolam) could be administered at the investigator’s discretion. The starting maintenance infusion dose of blinded study drug was 0.8 μg/kg per hour for dexmedetomidine and 0.06 mg/kg per hour for midazolam, corresponding to the mid‐point of the allowable infusion dose range." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: duration of mechanical ventilation, adverse events, ICU LOS, mortality Quote: "Optional blinded loading doses (up to 1 μg/kg dexmedetomidine or 0.05 mg/kg midazolam) could be administered at the investigator’s discretion. The starting maintenance infusion dose of blinded study drug was 0.8 μg/kg per hour for dexmedetomidine and 0.06 mg/kg per hour for midazolam, corresponding to the mid‐point of the allowable infusion dose range." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 375 eligible patients were randomized and 366 patients received study drug, comprising the primary analyses study population (244 patients received dexmedetomidine, 122 received midazolam). Nine patients randomized (6 in the dexmedetomidine group, 3 in the midazolam group) never received study drug" 194 included in long‐term use analyses in dexmedetomidine group, 50 excluded (received study drug ≤ 24 hours) (21 due to "extubated"; 17 due to "adverse event"; 7 due to "lack of efficacy"; 3 due to "withdrew consent"; 1 due to "entry criteria" (patient had new information after consent that identified an exclusion criterion (e.g., need for general anaesthesia, unexpected liver or cardiac disease)); 1 due to "investigator opinion" (investigator felt that patient no longer met entry criteria (e.g., extubated, no longer required sedation, required deeper sedation)); 103 included in long‐term use analyses in midazolam group, 19 excluded (5 due to "adverse event"; 5 due to "lack of efficacy"; 4 due to "withdrew consent"; 4 due to "entry criteria"; 1 due to "investigator opinion") Comment: Reasons for withdrawal and exclusion were explicitly reported and balanced across study groups |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in Methods section are reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ruokonen 2009.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
41 randomized to receive dexmedetomidine; 44 randomized to receive midazolam/propofol (previous standard care). The median age of the dexmedetomidine was 64 years; the median age of the standard care group was 68 years |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | October 2005 to July 2006 | |
| Setting and Country | Setting: intensive care units Country: Finland, Switzerland | |
| Funding source and Declarations of interest | Sources of funding: Orion Pharma, Helsinki, Finland Declaration of investigators’ conflicts of interest: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified for study centre, current sedative, sedation target (Richmond Agitation–Sedation Scale; RASS) 0 to ‐3 vs. RASS ‐4, and admission type (medical vs. postoperative/trauma)." Comment: The authors reported they used stratified randomization, but did not state how they randomly divide participants in detail. |
| Allocation concealment (selection bias) | High risk | Comment: The authors did not explicitly state how they concealed randomization sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind"; "Dexmedetomidine and dexmedetomidine dummy (0.9% NaCl), propofol 2% and propofol dummy (0.9% NaCl) and midazolam (0.1%) and midazolam dummy (0.9% NaCl) were prepared by personnel not involved in the study or the patient’s care; infusion systems for propofol and its dummy were nontransparent."; "Depending on standard care at time of randomization, midazolam was given either as intravenous boluses (1–2 mg), starting at 3 boluses per hour for 1 h, and thereafter 1–4 boluses per hour, and if not sufficient as continuous infusion of 0.2 mg/kg/h, or as a continuous infusion at 0.12 mg/kg/h for 1 h, followed by adjustments at 0.04, 0.08, 0.12, 0.16, and 0.20 mg/kg/h." Comment: Bolus injection of midazolam usually acts much faster than continuous infusion of dexmedetomidine and therefore blinding could be easily broken. It is therefore possible that the investigators’ decisions and actions could have been influenced. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective outcome: risk of delirium, proportion of sedation time spent at target sedation level Quote: "Dexmedetomidine and dexmedetomidine dummy (0.9% NaCl), propofol 2% and propofol dummy (0.9% NaCl) and midazolam (0.1%) and midazolam dummy (0.9% NaCl) were prepared by personnel not involved in the study or the patient’s care; infusion systems for propofol and its dummy were nontransparent."; "Depending on standard care at time of randomization, midazolam was given either as intravenous boluses (1–2 mg), starting at 3 boluses per hour for 1 h, and thereafter 1–4 boluses per hour, and if not sufficient as continuous infusion of 0.2 mg/kg/h, or as a continuous infusion at 0.12 mg/kg/h for 1 h, followed by adjustments at 0.04, 0.08, 0.12, 0.16, and 0.20 mg/kg/h." Comment: Blinding could be easily broken. Lack of blinding might bias the assessment of subjective outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: duration of mechanical ventilation, adverse events, duration of weaning, ICU LOS, mortality Comment: Objective outcomes were not likely to be biased by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "85 were randomized (intention‐to‐treat patients) to receive dexmedetomidine (DEX; n = 41) or to continue their previous standard care (SC; n = 44)" 31 completed study treatment in dexmedetomidine group, 10 withdraws (6 due to "lack of efficacy"; 3 due to "adverse event"; 1 due to "discharge to another hospital"); 37 completed study treatment in standard group, 7 withdraws (1 due to "lack of efficacy"; 5 due to "adverse event"; 1 due to "protocol violation") Imputation roles:
Comment: Reasons for withdrawal were explicitly reported and generally balanced across study groups. Conservative imputation rules were applied. |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in Methods section are reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Shehabi 2013.
| Methods | Parallel group randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
21 randomized to receive dexmedetomidine; 16 randomized to receive midazolam/propofol The mean age of the dexmedetomidine group was 61.6 years; the mean age of the standard care group was 65 years. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Dates | July 2011 to December 2011 | |
| Setting and Country | Setting: Intensive care units Country: Australia, New Zealand | |
| Funding source and Declarations of interest | Sources of funding: Hospira, Lake For‐ est, IL Declaration of investigators’ conflicts of interest: Dr Shehabi has received unrestricted Grant‐In‐Aid research grants from Hospira Inc. (Lake Forest, IL); research grants from Roche Diagnostics and Thermofisher Scientific; competitive research funding grants from the National Health and Medical Research Council, Australia. Dr Shehabi's research department has received payment for article preparation for being part of SEDCOM delirium manuscript review 2009; and speakers’ honoraria and consulting fee from Hospira and Roche Diagnostics. He was on an advisory board for Hospira and GSK and has received payment from GSK for the development of educational material approved by College of Intensive Care Medicine of ANZ. Dr Reade has received a consulting fee and research grants from Hospira. Dr McArthur has received grant support, travel reimbursements, and provisions for writing assistance from Hospira Australia. Dr Seppelt was on an advisory board in Intensive Care supported by Hospira; has received competitive research funding grants from National Health and Medical Research Council, Australia; and has received payment for the development of educational presentations from Asklepios Medical Education. Dr Webb has consulted for Aalix Healthcare Services Consulting, Ibis Biosciences, Astra Zeneca, Jansen Cilag, and has received grant support from Fresenius Kabi. The remaining authors have not disclosed any potential conflicts of interest. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Block randomization was undertaken with concealed envelopes.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Block randomization was undertaken with concealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “unblinded” Comment: It was possible that the investigators’ decisions and actions could have been influenced. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective outcome: rate of delirium, proportion of sedation time spent at target sedation level Quote: “unblinded” Comment: Lack of blinding might influence the subjective outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: ICU LOS, mortality Comment: Objective outcomes were not likely to be biased by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no losses to follow‐up, no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in Methods are reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Xu 2012.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria:
Exclusion criteria:
20 randomized to dexmedetomidine infusion alone, 20 randomized to midazolam infusion alone, 20 randomized to dexmedetomidine infusion combined with midazolam infusion and 20 randomized to dexmedetomidine infusion combined with midazolam bolus. We only included dexmedetomidine‐infusion‐alone group and midazolam‐infusion‐alone group in our review. The mean age of the dexmedetomidine was 59.52 years; the mean age of the midazolam group was 58.77 years. |
|
| Interventions |
|
|
| Outcomes | Risk of delirium: Delirium was assessed using the CAM‐ICU Adverse events: Hypotension, bradycardia, nausea Proportion of sedation time spent at target sedation level: Proportion of sedation time within MAAS target |
|
| Dates | February 2010 to September 2012 | |
| Setting and Country | Setting: intensive care units Country: China | |
| Funding source and Declarations of interest | Sources of funding: Not reported Declaration of investigators’ conflicts of interest: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The authors stated that they used stratified randomization, but they did not state how they did this. |
| Allocation concealment (selection bias) | High risk | Comment: The authors did not state how they concealed their randomization sequence. Allocation concealment was probably not done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Subjective outcome: risk of delirium, proportion of sedation time spent at target sedation level. Comment: The authors did not state they used blinded methods. It was possible that the investigators’ decisions and actions could have been influenced. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: The authors did not state they used blinded methods. Lack of blinding might bias the assessment of subjective outcomes, but not objective outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome: adverse events. Comment: Objective outcomes were not likely to be biased by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the study protocol. |
| Selective reporting (reporting bias) | Low risk | All the outcomes listed in the Methods section are reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
CAM‐ICU: confusion assessment method for the intensive care unit FDA: Food and Drug Administration ICU: intensive care unit LOS: length of stay MAAS: Motor Activity Assessment Scale RASS: Richmond Agitation‐Sedation Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arenas 2004 | Non‐RCT |
| Corbett 2005 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Fang 2012 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Feng 2012 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Guo 2012 | Duration of sedation was < 6 hours |
| Herr 2003 | Duration of sedation was limited to 6 ‐ 24 hours |
| Hu 2013 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Jiang 2012 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Kadoi 2008 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Kaneko 2008 | Duration of sedation was < 24 hours |
| Li 2011 | Duration of sedation was < 24 hours |
| Li 2012 | Duration of sedation was < 24 hours |
| Li 2013 | Not all the participants were mechanically ventilated |
| Linj 2012 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Liu 2013a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Liu 2013b | Study did not require or anticipate participants to have more than 24 hours sedation |
| Luo 2012a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Luo 2012b | Study did not require or anticipate participants to have more than 24 hours sedation |
| Luo 2012c | Duration of sedation was limited to 12 hours |
| Peng 2011 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Pu 2012 | Duration of sedation was limited to 24 hours |
| Qu 2011 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Su 2014 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Tasdogan 2009 | Duration of sedation was < 24 hours |
| Tian 2010 | Duration of sedation was limited to 12 hours |
| Tobias 2004 | The use of dexmedetomidine was limited to up to 24 hours |
| Venn 2001 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Wan 2011 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Wang 2011a | Duration of sedation was limited to 24 hours |
| Wang 2011b | Study did not require or anticipate participants to have more than 24 hours sedation |
| Wang 2011c | Study did not require or anticipate participants to have more than 24 hours sedation |
| Wang 2012a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Wang 2012b | Duration of sedation was limited to 24 hours |
| Wang 2014 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Xiao 2013 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Yang 2013 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Yao 2010 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Yuan 2014 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhang 2012a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhang 2012b | Duration of sedation was limited to 12 ‐ 72 hours |
| Zhang 2013 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhang 2014a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhang 2014b | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zheng 2012 | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhou 2012a | Study did not require or anticipate participants to have more than 24 hours sedation |
| Zhou 2012b | Study did not require or anticipate participants to have more than 24 hours sedation |
Characteristics of studies awaiting assessment [ordered by study ID]
Tao 2011.
| Methods | Randomized controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
30 randomized to receive dexmedetomidine; 30 randomized to receive midazolam. |
| Interventions |
|
| Outcomes |
|
| Notes | This study appears to duplicate Riker 2009 |
CAM‐ICU: confusion assessment method for the intensive care unit ICU: intensive care unit LOS: length of stay
Characteristics of ongoing studies [ordered by study ID]
NCT01059929.
| Trial name or title | A double‐blinded randomized controlled trial of dexmedetomidine versus propofol for sedation in mechanically ventilated medical intensive care unit patients |
| Methods | Randomized control trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures
|
| Starting date | September 2009 |
| Contact information | Contact: Anne S Pohlman 773‐702‐3804 apohlman@medicince.bsd.uchicago.edu |
| Notes |
NCT01760967.
| Trial name or title | Effect of dexmedetomidine on mortality, duration of mechanical ventilation and multi‐organ function in sepsis patients under lighter sedation by randomized control trial |
| Methods | Randomized control trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary Outcome Measures:
|
| Starting date | January 2013 |
| Contact information | Contact: Yu Kawazoe +81‐73‐441‐0603 ukz411@gmail.com Contact: Kyohei Miyamoto +81‐73‐441‐0603 go.go.kyohei.miyamoto@gmail.com |
| Notes |
ICU: intensive care unit LOS: length of stay RASS: Richmond Agitation‐Sedation Scale
Differences between protocol and review
We did not limit the follow‐up time for mortality in the protocol (Chen 2012). However, some studies assessed mortality at various follow‐up times (e.g. 30‐day mortality, one‐year mortality). We only included the follow‐up that was most comparable to those used in other studies.
We had planned to generate funnel plots and conduct the Egger test or arcsine test in the protocol. However, there were fewer than 10 studies included, and thus we were not able to perform them.
For these continuous outcomes, we planned to estimate log‐transformed means and standard deviations by using Method 1 mentioned in Higgins 2008 in the protocol, if we could obtain means and standard deviations of raw data. However, we were not able to obtain these data from study authors. Consequently, we estimated means using medians (Hozo 2005), and calculated approximate standard deviations by dividing the log‐transformed interquartile range by 1.35 (Higgins 2011b). We calculated the geometric mean difference rather than standardized mean difference for continuous outcomes.
We did not perform subgroup analyses on different follow‐up time points, since the cut‐off time point (72 hours) used to divide the subgroups was arbitrary, and subgroup analyses were insufficient to explore how the risk of delirium changed with follow‐up time. Instead, because most studies only measure delirium during mechanical ventilation, we performed a meta‐regression to investigate how log‐transformed mean ventilation time was associated with risk of delirium.
We had planned to undertake sensitivity analyses by excluding studies with imputed data to explore the influence of our imputation rule in the protocol. However, we found that excluding a whole study with imputed data would lead to massive loss of information. Thus, we undertook sensitivity analyses by pooling observed data only, without any imputation.
We had planned to use the principles of the GRADE system to assess the quality of the body of evidence associated with the proportion of sedation time spent at the target sedation level in the protocol. However, we did not perform a meta‐analysis for 'proportion of sedation time spent at target sedation level' and thus we were not able to calculate a relative effect. Bradycardia was the most commonly reported adverse event but we did not list it as one of the outcomes for our 'Summary of findings' in the protocol. Thus, we decided to use the GRADE system to assess the quality of evidence associated with 'incidence of bradycardia' instead of 'proportion of sedation time spent at target sedation level'.
Jakob 2012 MIDEX and Jakob 2012 PRODEX reported not only 'duration of mechanical ventilation', but also 'time to extubation', which matched our definition of duration of mechanical ventilation. We performed a post hoc sensitivity analysis to assess how the result would change if we extracted the data from 'time to extubation' instead of 'duration of mechanical ventilation'.
Contributions of authors
Ken Chen (KC), Zhijun Lu (ZJL), Yi Chun Xin (YCX), Yong Cai (YC), Yi Chen (YIC), Shu Ming Pan (SMP)
Conceiving the review: KC Co‐ordinating the review: SMP and KC Undertaking manual searches: KC and YCX Screening search results: KC and YCX Organizing retrieval of papers: KC Screening retrieved papers against inclusion criteria: KC, YCX and YIC Appraising quality of papers: KC, YCX, YIC, SMP and ZJL Abstracting data from papers: KC and YCX Writing to authors of papers for additional information: KC Providing additional data about papers: KC, SMP and ZJL Obtaining and screening data on unpublished studies: KC and YCX Data management for the review: KC Entering data into Review Manager 5 (RevMan) (RevMan 5.3): KC and YCX RevMan statistical data: KC and YC Other statistical analyses not using RevMan: KC and YC Interpretation of data: KC, SMP and ZJL Statistical inferences: KC and YC Writing the review: KC Securing funding for the review: SMP and ZJL Performing previous work that was the foundation of the present study: None Guarantor for the review (one author): KC Person responsible for reading and checking review before submission: KC and SMP
Sources of support
Internal sources
Rui Jin Hospital, Lu Wan Branch, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
External sources
No sources of support supplied
Declarations of interest
Ken Chen: none known Zhijun Lu: none known Yi Chun Xin: none known Yong Cai: none known Yi Chen: none known Shu Ming Pan: none known
Edited (no change to conclusions)
References
References to studies included in this review
Jakob 2012 MIDEX {published data only}
- Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012;307(11):1151‐60. [PUBMED: 22436955] [DOI] [PubMed] [Google Scholar]
Jakob 2012 PRODEX {published data only}
- Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012;307(11):1151‐60. [PUBMED: 22436955] [DOI] [PubMed] [Google Scholar]
Pandharipande 2007 {published data only}
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298(22):2644‐53. [PUBMED: 18073360] [DOI] [PubMed] [Google Scholar]
Riker 2009 {published data only}
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301(5):489‐99. [PUBMED: 19188334] [DOI] [PubMed] [Google Scholar]
Ruokonen 2009 {published data only}
- Ruokonen E, Parviainen I, Jakob SM, Nunes S, Kaukonen M, Shepherd ST, et al. Dexmedetomidine versus propofol/midazolam for long‐term sedation during mechanical ventilation. Intensive Care Medicine 2009;35(2):282‐90. [PUBMED: 18795253] [DOI] [PubMed] [Google Scholar]
Shehabi 2013 {published data only}
- Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early goal‐directed sedation versus standard sedation in mechanically ventilated critically III patients: A pilot study. Critical Care Medicine 2013;41(8):1983‐91. [PUBMED: 23863230] [DOI] [PubMed] [Google Scholar]
Xu 2012 {published data only}
- Xu JB, Wang YZ, Shi QS. A combined protocol for dexmedetomidine used in prolonged sedation in intensive care unit [右美托咪啶联合用药新方案用于重症医学科长时间镇静的研究]. Modern Medicine Journal of China 2012;14(12):20‐2. [Google Scholar]
References to studies excluded from this review
Arenas 2004 {published data only}
- Arenas‐Lopéz S, Riphagen S, Tibby SM, Durward A, Tomlin S, Davies G, et al. Use of oral clonidine for sedation in ventilated paediatric intensive care patients. Intensive Care Medicine 2004;30(8):1625‐9. [PUBMED: 15197439] [DOI] [PubMed] [Google Scholar]
Corbett 2005 {published data only}
- Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Critical Care Medicine 2005;33(5):940‐5. [PUBMED: 15891317] [DOI] [PubMed] [Google Scholar]
Fang 2012 {published data only}
- Fang SD, Zhu YS, Xu H, Jiang H. Dexmedetomidine for sedation during intubation period in postoperative patients receiving orthognathic surgery in intensive care unit [右美托咪定用于重症监护病房正颌外科术后留置气管插管患者的镇静]. Chinese Journal of New Drugs and Clinical Remedies 2012;31(8):454‐7. [Google Scholar]
Feng 2012 {published data only}
- Feng JH, Li KP, Deng ZB. Comparison of dexmedetomidine and propofol for sedation of flail chest complicated with acute pulmonary contusion patients in intensive care unit [右美托咪定与丙泊酚用于ICU连枷胸合并急性肺损伤患者镇静的效果比较]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2012;20(11):1772‐3. [Google Scholar]
Guo 2012 {published data only}
- Guo JY, Deng Q, Guo XS, Liu SQ, Zhang YH, He ZJ. Study of sedation with dexmedetomidine in postoperative patients with severe obstructive sleep apnea syndrome [右美托咪定对重症睡眠呼吸暂停综合征患者术后镇静作用的研究]. Medical Journal of Chinese People's Liberation Army 2012;37(6):649‐52. [Google Scholar]
Herr 2003 {published data only}
- Herr DL, Sum‐Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine‐based versus propofol‐based sedation regimens. Journal of Cardiothoracic and Vascular Anesthesia 2003;17(5):576‐84. [PUBMED: 14579210] [DOI] [PubMed] [Google Scholar]
Hu 2013 {published data only}
- Hu MJ. The sedative effect of dexmedetomidine and midazolam in mechanical ventilated patients [右美托咪啶与咪达唑仑在机械通气患者中镇静作用的研究]. Strait Pharmaceutical Journal 2013;25:3. [Google Scholar]
Jiang 2012 {published data only}
- Jiang L, Ding S, Zhang YH, Zhang LP, Chen X, Zhang JB. Dexmedetomidine vs midazolam/fentanyl for sedation of children with pulmonary artery hypertension after congenital cardiac surgery [右旋美托咪啶和咪达唑仑联合芬太尼镇静治疗在先心病肺动脉高压患儿术后的对比研究]. Chinese Journal of Thoracic and Cardiovascular Surgery 2012;28(8):485‐7. [Google Scholar]
Kadoi 2008 {published data only}
- Kadoi Y, Saito S, Kawauchi C, Hinohara H, Kunimoto F. Comparative effects of propofol vs dexmedetomidine on cerebrovascular carbon dioxide reactivity in patients with septic shock. British Journal of Anaesthesia 2008;100(2):224‐9. [PUBMED: 18178608] [DOI] [PubMed] [Google Scholar]
Kaneko 2008 {published data only}
- Kaneko T. Postoperative management of carotid endarterectomy with dexmedetomidine ‐ a comparison with propofol [デクスメデトミジンを用いた頸動脈内膜剥離術後管理 一プロポフォールとの比較一]. Japanese Journal of Anesthesiology 2008;57:696‐703. [PUBMED: 18546896] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li HQ, Hou DP, Jiang CH, Han CH. Use of dexmedetomidine in the postoperative patients with mechanical ventilation [右美托咪定在术后机械通气患者中的应用]. Chinese Journal of Critical Care Medicine 2011;31(8):697‐700. [Google Scholar]
Li 2012 {published data only}
- Li JQ, Xiong XM, Chen XW, Chen SL, Zhou HF, Chen HW, et al. A study of dexmedetomidine in treatment of mechanically ventilated patients with acute respiratory distress syndrome [右美托咪定在急性呼吸窘迫综合征机械通气患者中的应用]. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 2012;19(2):79‐82. [Google Scholar]
Li 2013 {published data only}
- Li ZY. The influence of dexmedetomidine on patients' renal function [右美托咪定用于计划镇静时对病人肾脏功能的影响]. Chinese Selected Doctoral Dissertations and Master's Theses Full‐Text Databases 2013; Vol. 2.
Linj 2012 {published data only}
- Lin JP, Wen JS, Yang JP, Lin F, Chen Y. The use of dexmedetomidine in postoperative elder patients with mechanical ventilation [右美托咪定和咪达唑仑用于术后老年患者机械通气镇静的临床观察]. Fujian Medical Journal 2012;34:76‐7. [Google Scholar]
Liu 2013a {published data only}
- Liu P, Xiu GH, Gu YF, Ling B. Right dexmedetomidine compared with midazolam mechanical ventilation sedative efficacy analysis [咪达唑仑对比右美托咪啶用于机械通气镇静临床疗效分析]. National Medical Frontiers of China 2013;12:31. [Google Scholar]
Liu 2013b {published data only}
- Liu P, Guo XC, WangD. Observation of sedative effect for ICU patients sedation using dexmedetomidine and midazolam [右美托咪定与咪达唑仑对ICU危重患者镇静效果观察]. Jilin Medical Journal 2013;32:6708‐9. [Google Scholar]
Luo 2012a {published data only}
- Luo J, Tan XF, Deng XK, Wu HB. The use of dexmedetomidine hydrochloride in mechanical ventilated patients [盐酸右美托咪定在机械通气患者中的应用]. Hebei Medical Journal 2012;34:989‐90. [Google Scholar]
Luo 2012b {published data only}
- Luo L, Zeng M, Kuan KP. Comparison of the effects of dexmedetomidine with midazolam on the respiration and circulation of mechanically ventilated patients [右美托咪啶和咪达唑仑对机械通气患者呼吸和循环影响的比较]. Chinese Journal of Emergency Medicine 2012;21(3):295‐8. [Google Scholar]
Luo 2012c {published data only}
- Luo ZZ, Xiao YF. The sedative effect research of dexmedetomidine in the ICU patients [右美托咪啶对重症治疗室患者镇静效果研究]. China Modern Medicine 2012;19(279):74‐5. [Google Scholar]
Peng 2011 {published data only}
- Peng M, Fang WQ, Yu XT, Cai JY. Clinical application of dexmedetomidine in intensive care unit [右美托咪定在重症监护病房镇静中的临床应用]. Chinese Journal of Critical Care Medicine 2011;31(3):197‐9. [Google Scholar]
Pu 2012 {published data only}
- Pu N, Wu Y, Cao QT. Clinical observation of sedate treatment of dexmedetomidine on ICU patients [右美托咪定在重症监护患者镇静中的临床观察]. Guide of China Medicine 2012;10:24‐5. [Google Scholar]
Qu 2011 {published data only}
- Qu G. The clinical observation of dexmedetomidine for sedation of the patients after CABG in the ICU [右美托咪啶用于CABG术后患者ICU镇静的临床观察]. Chinese Selected Doctoral Dissertations and Master's Theses Full‐Text Databases 2011; Vol. 10.
Su 2014 {published data only}
- Su J, Li Y. Observation of the sedative effects and adverse reactions of dexmedetomidine on the patients with craniocerebral injury [右美托咪定对颅脑损伤患者的镇静效果及不良反应观察]. Chinese Journal of Pharmacoepidemiology 2014;6:348‐50. [Google Scholar]
Tasdogan 2009 {published data only}
- Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. Journal of Clinical Anesthesia 2009;21(6):394‐400. [PUBMED: 19833271] [DOI] [PubMed] [Google Scholar]
Tian 2010 {published data only}
- Tian ST, Huang H. The clinical effect of dexmedetomidine in ventilated patients sedation [盐酸右美托咪定用于机械通气患者镇静的临床观察]. Chinese Journal of Pharmacovigilance 2010;7(45):515‐7. [Google Scholar]
Tobias 2004 {published data only}
- Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. Southern Medical Journal 2004;97(5):451‐5. [PUBMED: 15180019] [DOI] [PubMed] [Google Scholar]
Venn 2001 {published data only}
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. British Journal of Anaesthesia 2001;87(5):684‐90. [PUBMED: 11878517] [DOI] [PubMed] [Google Scholar]
Wan 2011 {published data only}
- Wan LJ, Huang QQ, Yue JX, Lin L, Li SH. Comparison of sedative effect of dexmedetomidine and midazolam for post‐operative patients undergoing mechanical ventilation in surgical intensive care unit [右美托咪啶与咪达唑仑用于外科重症监护病房术后机械通气患者镇静的比较研究]. Chinese Critical Care Medicine 2011;23:543‐6. [PUBMED: 21944176] [PubMed] [Google Scholar]
Wang 2011a {published data only}
- Wang CY, Shang M, Cheng L, Zhou QS. Dexmedetomidine vs midazolam for sedation in critically ill patients [右美托咪啶和咪达唑仑用于危重症患者镇静效应的比较]. Journal of Hainan Medical University 2011;17(96):695‐7. [Google Scholar]
Wang 2011b {published data only}
- Wang HL, Liu HT, Yu KJ. Comparison of dexmedetomidine and propofol for sedation of eclampsia patients in intensive care unit [右美托咪定与丙泊酚用于重症监护病房子痫患者镇静的效果比较]. Shanghai Medical Journal 2011;34(8):613‐5. [Google Scholar]
Wang 2011c {published data only}
- Wang KM, Sun YH, Hou YQ. Clinical observation of dexmedetomidine hydrochloride on sedation and analgesia of ICU patients [盐酸右美托咪定用于重症监护患者镇静及镇痛的临床观察]. Journal of Henan University of Science & Technology (Medical Science) 2011;29(4):267‐8, 271. [Google Scholar]
Wang 2012a {published data only}
- Wang HB, Li KP, Deng ZB. The sedation effect comparison of dexmedetomidine and midazolam to treat patients with flail chest complicated with pulmonary injury in ICU [右美托咪定与咪达唑仑用于重症监护病房连枷胸并肺损伤患者镇静的效果比较]. Journal of Heze Medical College 2012;24(90):4‐5, 60. [Google Scholar]
Wang 2012b {published data only}
- Wang H. Curative effect observation of dexmedetomidine in ICU sedation [右美托嘧啶在ICU中镇静的疗效观察]. Guide of China Medicine 2012;10:153‐4. [Google Scholar]
Wang 2014 {published data only}
- Wang S, Zhu L. Efficacy of dexmedetomidine and midazolam combined with fentanyl on sedation of postoperative and mechanically ventilated ICU patients [右美托咪定和咪达唑仑复合芬太尼对ICU术后机械通气患者镇静的影响]. Practical Pharmacy and Clinical Remedies 2014;3:308‐10. [Google Scholar]
Xiao 2013 {published data only}
- Xiao Y, Wang Y, Wu Y. Evaluation on sedation effect of dexmedetomidine used in ICU [右美托咪啶用于重症监护病房镇静的效果评价]. China Pharmaceuticals 2013;20:37‐9. [Google Scholar]
Yang 2013 {published data only}
- Yang S, Xie J, Huo K, Xu F, Chen L. Dexmedetomidine versus midazolam for sedation of acute exacerbation of chronic obstructive pulmonary disease underwent mechanical ventilation [右美托咪啶与咪达唑仑对慢性阻塞性肺疾病急性加重期机械通气患者镇静的比较研究]. Chinese Journal of Respiratory and Critical Care Medicine 2013;5:481‐4. [Google Scholar]
Yao 2010 {published data only}
- Yao L, Zhou XM, Zhao JJ. The role of dexmedetomidine in treatment of serious patients in intensive care unit [右美托咪啶在重症监护病房应用的研究]. Chinese Critical Care Medicine 2010;22(10):632‐4. [PUBMED: 20977851] [PubMed] [Google Scholar]
Yuan 2014 {published data only}
- Yuan L, Ma HY. Comparasion of dexmedetomidine and midazolam for ICU patients with mechanical ventilation [右美托咪定与咪达唑仑用于ICU机械通气患者镇静疗效比较]. Jilin Medical Journal 2014;12:2555. [Google Scholar]
Zhang 2012a {published data only}
- Zhang BC, Zhong ZY, Li HZ, Liang XJ, Li J, Shen J. Comparison of the sedative effects of dexmedetomidine and midazolam for critically Ill patients [右美托咪定与咪达唑仑对危重病患者镇静效果的比较]. Chinese Journal of Clinical Medicine 2012;19(97):290‐2. [Google Scholar]
Zhang 2012b {published data only}
- Zhang J, Lou TZ, Zhang N, Xu JL, Jiu H, Qiu ZL, et al. Feasibility of dexmedetomidine in sedation for chronic obstructive pulmonary diseases patients undergoing mechanical ventilation [右美托咪定在慢性阻塞性肺疾病需机械通气患者镇静中的应用]. Chinese Journal of New Drugs and Clinical Remedies 2012;31:451‐4. [Google Scholar]
Zhang 2013 {published data only}
- Zhang C, Wang Y, Du Z, Zhang S, Chang X, Zhang W. Clinical study of dexmedetomidine combined with propofol for post‐operative patients in intensive care unit [右美托咪定联合丙泊酚在ICU中的应用]. Medical Journal of Wuhan University 2013;3:395‐8. [Google Scholar]
Zhang 2014a {published data only}
- Zhang W, Zhang B, Liu N. Analysis of curative effect of dexmedetomidine hydrochloride in tube drawing from patients with mechanical ventilation [盐酸右美托咪定用于机械通气患者撤机拔管的疗效分析]. Journal of Clinical Pulmonary Medicine 2014;5:828‐30. [Google Scholar]
Zhang 2014b {published data only}
- Zhang W, Tan J, Guan R, Xie S. Clinical study of dexmedetomidine hydrochloride in mechanical ventilation for critical patients [盐酸右美托咪定在危重症患者机械通气的临床研究]. China Medicine and Pharmacy 2014;12:82‐3. [Google Scholar]
Zheng 2012 {published data only}
- Zheng M, Tian G, Li HN, Liu Y, Wang Q. Effect evaluation of dexmedetomidine and midazolam in infants in mechanical ventilation [婴幼儿机械通气时使用右旋美托咪定和咪达唑仑的效果评价]. Progress in Modern Biomedicine 2012;12:2342‐4, 2348. [Google Scholar]
Zhou 2012a {published data only}
- Zhou J. Clinical research of sedation with dexmedetomidine for surgical patients requiring mechanical ventilation [右美托咪定用于外科手术后需机械通气患者镇静治疗的临床观察]. Chinese Selected Doctoral Dissertations and Master's Theses Full‐Text Databases 2012; Vol. 11.
Zhou 2012b {published data only}
- Zhou LX, Yang TF. The sedative effect of dexmedetomidine in ICU patients receiving mechanical ventilation [右美托咪定用于ICU机械通气患者的镇静效果及护理]. Strait Pharmaceutical Journal 2012;24(150):187‐8. [Google Scholar]
References to studies awaiting assessment
Tao 2011 {published data only}
- Tao SY, Li WX, Qi XF. Sedative effects of dexmedetomidine on patients in intensive care unit [右美托咪定在ICU患者镇静中的疗效分析]. Chinese Journal of Critical Care Medicine 2011;31(12):1103‐5. [Google Scholar]
References to ongoing studies
NCT01059929 {published data only}
- NCT01059929. Dexmedetomidine Versus Propofol in the Medical Intensive Care Unit (MICU). clinicaltrials.gov/ct2/show/NCT01059929 accessed 10 December 2014.
NCT01760967 {published data only}
- NCT01760967. Dexmedetomidine for Sepsis in ICU Randomized Evaluation Trial (DESIRE). clinicaltrials.gov/ct2/show/NCT01760967 accessed 10 December 2014.
Additional references
Agarwal 2010
- Agarwal V, O'Neill PJ, Cotton BA, Pun BT, Haney S, Thompson J, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. Journal of Burn Care & Research 2010;31(5):706‐15. [PUBMED: 20647937] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aho 1991
- Aho MS, Erkola OA, Scheinin H, Lehtinen AM, Korttila KT. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesthesia and Analgesia 1991;73(2):112‐8. [PUBMED: 1854025] [DOI] [PubMed] [Google Scholar]
Aitken 2012
- Aitken LM, Bucknall T, Kent B, Mitchell M, Burmeister E, Keogh SJ. Protocol directed sedation versus non‐protocol directed sedation to reduce duration of mechanical ventilation in mechanically ventilated intensive care patients. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009771] [DOI] [PubMed] [Google Scholar]
Anatomical Therapeutic Chemical
- Anatomical Therapeutic Chemical Classification System. WHO collaborating centre for drugs statistics methodology. International language for drug utilization research. www.whocc.no/ (Accessed 31st October 2012).
Bailey 1991
- Bailey PL, Sperry RJ, Johnson GK, Eldredge SJ, East KA, East TD, et al. Respiratory effects of clonidine alone and combined with morphine, in humans. Anesthesiology 1991;74(1):43‐8. [PUBMED: 1898841] [DOI] [PubMed] [Google Scholar]
Barr 2013
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine 2013;41(1):263‐306. [PUBMED: 23269131] [DOI] [PubMed] [Google Scholar]
Blaudszun 2012
- Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta‐analysis of randomized controlled trials. Anesthesiology 2012 Jun;116(6):1312‐22. [PUBMED: 22546966] [DOI] [PubMed] [Google Scholar]
Bohrer 1990
- Bohrer H, Bach A, Layer M, Werning P. Clonidine as a sedative adjunct in intensive care. Intensive Care Medicine 1990;16(4):265‐6. [PUBMED: 2358560] [DOI] [PubMed] [Google Scholar]
Breen 2005
- Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL, et al. Decreased duration of mechanical ventilation when comparing analgesia‐based sedation using remifentanil with standard hypnotic‐based sedation for up to 10 days in intensive care unit patients: a randomised trial. Critical Care 2005;9(3):R200‐10. [PUBMED: 15987391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 1999
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, et al. Effect of a nursing‐implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine 1999;27(12):2609‐15. [PUBMED: 10628598] [DOI] [PubMed] [Google Scholar]
Burry 2014
- Burry L, Rose L, McCullagh I, Ferguson ND, Ferguson D, Mehta S. Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009176.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2013
- Carney L, Kendrick J, Carr R. Safety and Effectiveness of Dexmedetomidine in the Pediatric Intensive Care Unit (SAD‐PICU). Canadian Society of Hospital Pharmacists 2013;66(1):21‐7. [PUBMED: 23467635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2006
- Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell‐Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Critical Care Medicine 2006;34(5):1326‐32. [PUBMED: 16540958] [DOI] [PubMed] [Google Scholar]
Czaja 2009
- Czaja AS, Zimmerman JJ. The use of dexmedetomidine in critically ill children. Paediatric Critical Care Medicine 2009;10(3):381‐6. [PUBMED: 19325505] [DOI] [PubMed] [Google Scholar]
Dahmani 2010
- Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiologica Scandinavica 2010;54(4):397‐402. [PUBMED: 20085541] [DOI] [PubMed] [Google Scholar]
Dasta 2010
- Dasta JF, Kane‐Gill SL, Pencina M, Shehabi Y, Bokesch PM, Wisemandle W, et al. A cost‐minimization analysis of dexmedetomidine compared with midazolam for long‐term sedation in the intensive care unit. Critical Care Medicine 2010;38(2):497‐503. [PUBMED: 19789442] [DOI] [PubMed] [Google Scholar]
De Wit 2006
- Wit M, Best AM, Epstein SK, Greenblatt DJ. Lorazepam concentrations, pharmacokinetics and pharmacodynamics in a cohort of mechanically ventilated ICU patients. International Journal of Clinical Pharmacology and Therapeutics 2006;44(10):466‐73. [PUBMED: 17063976] [DOI] [PubMed] [Google Scholar]
Dubois 2001
- Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Medicine 2001;27(8):1297‐304. [PUBMED: 11511942] [DOI] [PubMed] [Google Scholar]
Ely 2004
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FEJ, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753‐62. [PUBMED: 15082703] [DOI] [PubMed] [Google Scholar]
Fraser 2007
- Fraser GL, Riker RR. Sedation and analgesia in the critically ill adult. Current Opinion in Anaesthesiology 2007;20(2):119‐23. [PUBMED: 17413394] [DOI] [PubMed] [Google Scholar]
Friedrich 2012
- Friedrich JO, Adhikari NK, Beyene J. Ratio of geometric means to analyze continuous outcomes in meta‐analysis: comparison to mean differences and ratio of arithmetic means using empiric data and simulation. Statistic in Medicine 2012;31(17):1857‐86. [PUBMED: 22438170] [DOI] [PubMed] [Google Scholar]
Gehlbach 2002
- Gehlbach BK, Kress JP. Sedation in the intensive care unit. Current Opinion in Critical Care 2002;8(4):290‐8. [PUBMED: 12386488] [DOI] [PubMed] [Google Scholar]
Girard 2008
- Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. The Lancet 2008;371(9607):126–34. [PUBMED: 18191684] [DOI] [PubMed] [Google Scholar]
Gregoretti 2009
- Gregoretti C, Moglia B, Pelosi P, Navalesi P. Clonidine in perioperative medicine and intensive care unit: more than an anti‐hypertensive drug. Current Drug Targets 2009;10(8):799‐814. [PUBMED: 19702526] [DOI] [PubMed] [Google Scholar]
Gupta 2012
- Gupta P, Whiteside W, Sabati A, Tesoro TM, Gossett JM, Tobias JD, et al. Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease. Pediatric Critical Care Medicine 2012;13(6):660‐6. [PUBMED: 22791093] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2000
- Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small‐dose dexmedetomidine infusions. Anesthesia and Analgesia 2000;90(3):699‐705. [PUBMED: 10702460] [DOI] [PubMed] [Google Scholar]
Hall 2001
- Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. British Journal of Anaesthesia 2001;86(1):5‐11. [PUBMED: 11575409] [DOI] [PubMed] [Google Scholar]
Hashiba 2003
- Hashiba E, Hirota K, Suzuki K, Matsuki A. Effects of propofol on bronchoconstriction and bradycardia induced by vagal nerve stimulation. Acta Anaesthesiologica Scandinavica 2003;47(9):1059‐63. [PUBMED: 12969095] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Anzures‐Cabrera J. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine 2008;27(29):6072‐92. [PUBMED: 18800342] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboratio, 2011. available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2004
- Hsu YW, Cortinez LI, Robertson KM, Keifer J‐C, Sum‐Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 2004;101(5):1066‐76. [PUBMED: 15505441] [DOI] [PubMed] [Google Scholar]
Huupponen 2008
Ise 2002
- Ise T, Yamashiro M, Furuya H. Clonidine as a drug for intravenous conscious sedation. Odontology/the Society of the Nippon Dental University 2002;90(1):57‐63. [PUBMED: 12955567] [DOI] [PubMed] [Google Scholar]
Jaakola 1991
- Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine‐‐a novel alpha 2‐adrenoceptor agonist‐‐in healthy volunteers. Pain 1991 Sep;46(3):281‐5. [PUBMED: 1684653] [DOI] [PubMed] [Google Scholar]
Jamadarkhana 2010
- Jamadarkhana S, Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. Journal of Anaesthesiology, Clinical Pharmacology 2010;26(4):439‐45. [PUBMED: 21547166] [PMC free article] [PubMed] [Google Scholar]
Jessen 2013
- Jessen Lundorf L, Korvenius Jørgensen H, Møller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kam 2007
- Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia 2007;62(7):690‐701. [PUBMED: 17567345] [DOI] [PubMed] [Google Scholar]
Lambert 2014
- Lambert P, Cyna AM, Knight N, Middleton P. Clonidine premedication for postoperative analgesia in children. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009633.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2012
- Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post‐cardiac surgery patients? A meta‐analysis. Critical Care 2012;16(5):R169. [PUBMED: 23016926] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2011
- Machado FO, Moritz RD, Berbigier EJ, Berbigier RJ. Comparison between clonidine and dexmedetomidine for sedation and analgesia in critically ill patients. Intensive Care Medicine. 2011; Vol. Conference: 24th Annual Congress of the European Society of Intensive Care Medicine, ESICM LIVES 2011 Berlin Germany.
Maclaren 2013
- Maclaren R, Preslaski CR, Mueller SW, Kiser TH, Fish DN, Lavelle JC, et al. A randomized, double‐blind pilot study of dexmedetomidine versus midazolam for intensive care unit sedation: patient recall of their experiences and short‐term psychological outcomes. Journal of Intensive Care Medicine 2013 [Epub ahead of print]. [PUBMED: 24227448] [DOI] [PubMed]
Mantz 2011
- Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. European Journal of Anaesthesiology 2011;28(1):3‐6. [PUBMED: 20881501] [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin E, Ramsay G, Mantz J, Sum‐Ping ST. The role of the alpha2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. Journal of Intensive Care Medicine 2003;18(1):29‐41. [PUBMED: 15189665] [DOI] [PubMed] [Google Scholar]
Mason 2009
- Mason KP, O'Mahony E, Zurakowski D, Libenson MH. Effects of dexmedetomidine sedation on the EEG in children. Paediatric Anaesthesia 2009;19(12):1175‐83. [PUBMED: 20017865] [DOI] [PubMed] [Google Scholar]
Meert 1994
- Meert TF, Kock M. Potentiation of the analgesic properties of fentanyl‐like opioids with alpha 2‐adrenoceptor agonists in rats. Anesthesiology 1994 Sep;81(3):677‐88. [PUBMED: 7916547] [DOI] [PubMed] [Google Scholar]
Mehta 2011
- Mehta S, McCullagh I, Burry L. Current sedation practices: lessons learned from international surveys. Critical Care Clinics 2011;29(4):607‐24. [PUBMED: 19576525] [DOI] [PubMed] [Google Scholar]
Miller 1998
- Miller LJ, Wiles‐Pfeifler R. Propofol for the long‐term sedation of a critically ill patient. American Journal of Critical Care 1998;7(1):73‐6. [PUBMED: 9429686] [PubMed] [Google Scholar]
Oto 2012
- Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Medicine 2012;38(12):1982‐9. [PUBMED: 22961436] [DOI] [PubMed] [Google Scholar]
Pandharipande 2006
- Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006;104(1):21‐6. [PUBMED: 16394685] [DOI] [PubMed] [Google Scholar]
Pandharipande 2008
- Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JAJ, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. Journal of Trauma‐Injury Infection & Critical Care 2008;65(1):34‐41. [PUBMED: 18580517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandharipande 2010
- Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori‐designed analysis of the MENDS randomized controlled trial. Critical Care 2010;14(2):R38. [PUBMED: 20233428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pichot 2012
- Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second‐to‐first‐line sedative agents in the critical care setting?. Journal of Intensive Care Medicine 2012;27(4):219‐37. [PUBMED: 21525113] [DOI] [PubMed] [Google Scholar]
Precedex Prescribing Information
- Precedex prescribing information. www.precedex.com/safety/full‐prescribing‐information (Accessed 31st October 2012).
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubino 2010
- Rubino AS, Onorati F, Caroleo S, Galato E, Nucera S, Amantea B, et al. Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type‐A aortic dissection: results of a pilot study. Interactive Cardiovascular and Thoracic Surgery 2010;10(1):58‐62. [PUBMED: 19854793] [DOI] [PubMed] [Google Scholar]
Schnabel 2013
- Schnabel A, Meyer‐Frießem CH, Reichl SU, Zahn PK, Pogatzki‐Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta‐analysis of randomized controlled trials. Pain 2013 Jul;154(7):1140‐9. [PUBMED: 23706726] [DOI] [PubMed] [Google Scholar]
Shehabi 2012
- Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. American Journal of Respiratory and Critical Care Medicine 2012;186(8):724‐31. [PUBMED: 22859526] [DOI] [PubMed] [Google Scholar]
Shelly 1991
- Shelly MP, Sultan MA, Bodenham A, Park GR. Midazolam infusions in critically ill patients. European Journal of Anaesthesiology 1991;8(1):21‐7. [PUBMED: 1678701] [PubMed] [Google Scholar]
Spies 2003
- Spies CD, Otter HE, Huske B, Sinha P, Neumann T, Rettig J, et al. Alcohol withdrawal severity is decreased by symptom‐orientated adjusted bolus therapy in the ICU. Intensive Care Medicine 2003;29(12):2230‐8. [PUBMED: 14557857] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Strom 2010
- Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010;375(9713):475‐80. [PUBMED: 20116824] [DOI] [PubMed] [Google Scholar]
Tan 2010
- Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta‐analysis. Intensive Care Medicine 2010;36(6):926‐39. [PUBMED: 20376429] [DOI] [PubMed] [Google Scholar]
Tanios 2009
- Tanios MA, Wit M, Epstein SK, Devlin JW. Perceived barriers to the use of sedation protocols and daily sedation interruption: a multidisciplinary survey. Journal of Critical Care 2009;24(1):66‐73. [PUBMED: 19272541] [DOI] [PubMed] [Google Scholar]
Tejani 2010
- Tejani AM, Schwenger E. Comment on dexmedetomidine systematic review and meta‐analysis methodology. Intensive Care Medicine 2010;36(11):1974; author reply 1975. [PUBMED: 20689913] [DOI] [PubMed] [Google Scholar]
Tham 2005
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2‐adrenoceptor agonists in acute pain models in mice. British Journal of Pharmacology 2005 Mar;144(6):875‐84. [PUBMED: 15778704] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [PUBMED: 12111920] [DOI] [PubMed] [Google Scholar]
Treggiari 2009
- Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Critical Care Medicine 2009;37(9):2527‐34. [PUBMED: 19602975] [DOI] [PubMed] [Google Scholar]
Van Eijk 2011
- Eijk MM, Boogaard M, Marum RJ, Benner P, Eikelenboom P, Honing ML, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. American Journal of Respiratory and Critical Care Medicine 2011;184(3):340‐4. [PUBMED: 21562131] [DOI] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer W. Conducting meta‐analyses in R with the metafor package.. Journal of Statistical Software 2010;36(3):1‐48. [Google Scholar]
Virtanen 1988
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2‐adrenoceptor agonist. European Journal of Pharmacology 1988;150(1‐2):9‐14. [PUBMED: 2900154] [DOI] [PubMed] [Google Scholar]
Watling 1996
- Watling SM, Johnson M, Yanos J. A method to produce sedation in critically ill patients. The Annals of Pharmacotherapy 1996;30(11):1227‐31. [PUBMED: 8913400] [DOI] [PubMed] [Google Scholar]
Watson 2008
- Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Critical Care Medicine 2008;36(12):3171‐7. [PUBMED: 19020432] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wunsch 2009
- Wunsch H, Kahn JM, Kramer AA, Rubenfeld GD. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Critical Care Medicine 2009;37(12):3031‐9. [PUBMED: 19633543] [DOI] [PubMed] [Google Scholar]
Wunsch 2012
- Wunsch H. Weighing the costs and benefits of a sedative. JAMA 2012;307(11):1195‐7. [PUBMED: 22436960] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2013
- Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH, Liu KX. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta‐analysis of randomized clinical trials. Journal of Surgical Research 2013;185(2):833‐43. [PUBMED: 23910886] [DOI] [PubMed] [Google Scholar]
Zhuo 2012
- Zhuo S, Zheng J, Llu B. Sedative effect of dexmedetomidine versus propofol on postoperative patients in ICU: a systematic review. Chinese Journal of Evidence‐Based Medicine 2012;12(6):686‐93. [EMBASE: 2012409765] [Google Scholar]
References to other published versions of this review
Chen 2012
- Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha‐2 agonists for long‐term sedation during mechanical ventilation in critically ill patients. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD010269] [DOI] [PMC free article] [PubMed] [Google Scholar]


