Abstract
Background
There is no consensus regarding whether the peritoneum should be closed or left open during non‐obstetric operations involving laparotomy. Neither is there consensus about the method of closure of the peritoneum (continuous suture versus interrupted suture). If closing the peritoneum could be omitted without complications, or even with benefit for patients, this could result in reductions in the cost of abdominal operations by reducing both the number of sutures used and the operating time.
Objectives
To compare the benefits and harms of parietal peritoneal closure compared with no parietal peritoneal closure in patients undergoing non‐obstetric abdominal operations.
Search methods
In February 2013 we searched the The Cochrane Wounds Group Specialised Register (searched 14 February 2013); The Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1); The Database of Abstracts of Reviews of Effects (2013, Issue 1); Ovid MEDLINE (1946 to February Week 1, 2013); Ovid EMBASE (1974 to 2013 Week 06); and EBSCO CINAHL 1982 to 8 February 2013).
Selection criteria
We included only randomised controlled trials (RCTs) comparing peritoneal closure with no peritoneal closure in patients (adults and children) undergoing non‐obstetric abdominal operations. All relevant RCTs irrespective of language, publication status, publication year, or sample size were included in the analysis.
Data collection and analysis
Two review authors independently identified trials and extracted data. We calculated the risk ratio (RR) with 95% confidence intervals (CI) for comparing the binary outcomes between the groups, and mean difference (MD) with 95% CI for comparing the continuous outcomes. We performed the meta‐analysis using both a fixed‐effect model and a random‐effects model. Intention‐to‐treat analysis was performed whenever possible.
Main results
Five trials involving 836 participants randomised to peritoneal closure (410 participants) and no peritoneal closure (426 participants) were included in this review. All the trials were at high risk of bias. All the trials included participants undergoing laparotomy (open surgery). Four of the five trials used catgut or chromic catgut for peritoneal closure. Three trials involved vertical incisions and two trials involved transverse incisions. None of the trials reported 30‐day mortality. There was no significant difference in the one‐year mortality between the two groups (RR 1.11; 95% CI 0.56 to 2.19) in the only trial that reported this outcome. The only serious peri‐operative adverse event reported was burst abdomen, which was reported by three trials. Overall, 10/663 (1.5%) of participants developed burst abdomen. There was no significant difference in the proportion of participants who developed burst abdomen between the two groups (RR 0.71; 95% CI 0.22 to 2.35). The same three trials reported the proportion of participants who developed incisional hernia. Details of the follow‐up period were only available for one trial, and so we were unable to calculate the incidence rate. Overall, 17/663 (2.5%) of participants developed incisional hernia. There was no significant difference in the proportion of participants who developed incisional hernia between the two groups (RR 0.92; 95% CI 0.37 to 2.28). None of the trials reported quality of life; the incidence rate of, or proportion of participants who developed, intestinal obstruction due to adhesions; or re‐operation due to incisional hernia or adhesions. Only one trial reported the length of hospital stay, and this trial did not include readmissions in its calculations. There was no significant difference in the length of hospital stay between the two groups (MD 0.40 days; 95% CI ‐0.51 to 1.31).
Authors' conclusions
There is no evidence for any short‐term or long‐term advantage in peritoneal closure for non‐obstetric operations. If further trials are performed on this topic, they should have an adequate period of follow‐up and adequate measures should be taken to ensure that the results are not subject to bias.
Plain language summary
Surgical closure of the lining of the abdominal cavity versus no closure for reducing wound complications after operations unrelated to childbirth
The peritoneum is the inner lining of the abdomen (tummy). After surgery, when closing the abdomen, some surgeons stitch the peritoneum together because they think this increases the strength of the wound. Others do not stitch the peritoneum together because they think it is unnecessary, increases operating costs through use of additional stitching material, increases operating time, and may increase pain. So, whether to close the peritoneum, and method of closure (continuous running stitches versus interrupted stitches) are controversial in operations not related to childbirth. We addressed these controversies by performing a thorough search of the medical literature for trials that compared closing and not closing the peritoneum after abdominal operations not related to childbirth. We included only randomised controlled trials without limiting trials according to language or year of publication, or number of participants in the study. Two review authors independently identified the trials and extracted information.
We identified five trials involving 836 participants who had open abdominal operations. Peritoneal closure was done in 410 participants and not done in 426. All trials had a high risk of bias. Only one trial reported the proportion of participants who died up to one year after the operation, and there was no significant difference between the closure and non‐closure groups. Three trials reported major wound breakdown (burst abdomen), which requires emergency surgery. Overall, 10/663 participants (1.5%) developed burst abdomen, with no significant difference in proportions between the two groups. Three trials reported minor wound breakdown (incisional hernia), that may require surgery. Overall, 17/663 participants (2.5%) developed incisional hernia; again there was no significant difference between the two groups.
None of the trials reported on important outcomes, such as quality of life; the occurrence of intestinal obstruction (caused by intestines sticking to themselves and the abdominal wall (adhesions)); or the proportion of participants who had surgery to fix incisional hernia or adhesions. Only one trial reported length of hospital stay, and showed no significant difference between the groups, but did not include readmissions in its calculations. There does not appear to be any evidence for a short‐term or long‐term advantage in peritoneal closure in operations not related to childbirth. However, the trials were at high risk of bias, which can lead to false conclusions. Interestingly, our findings are similar to those of another research group who performed a similar review for operations related to childbirth.
Summary of findings
Summary of findings for the main comparison. Peritoneal closure versus no peritoneal closure for decreasing wound complications after non‐obstetric abdominal operations.
| Peritoneal closure versus no peritoneal closure for decreasing wound complications after non‐obstetric abdominal operations | |||||
|
Patient or population: patients having non‐obstetric abdominal operations
Settings: secondary care
Intervention: peritoneal closure Control: no peritoneal closure | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No peritoneal closure | Peritoneal closure | ||||
| Mortality | 169 per 1000 | 187 per 1000 (95 to 370) | RR 1.11 (0.56 to 2.19) | 152 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Deep wound dehiscence | 18 per 1000 | 13 per 1000 (4 to 42) | RR 0.71 (0.22 to 2.35) | 663 (3 studies) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Incisional hernia | 27 per 1000 | 24 per 1000 (10 to 61) | RR 0.92 (0.37 to 2.28) | 663 (3 studies) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Length of hospital stay | The mean length of hospital stay in the control groups was 7.83 days | The mean length of hospital stay in the intervention groups was 0.4 higher (0.51 lower to 1.31 higher) | 79 (1 study) | ⊕⊝⊝⊝ very low1,4,5,6 | |
| *The basis for the assumed risk was the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||||
1 The trial(s) was/were of high risk of bias 2 The confidence interval overlaps 1 and 0.75 or 1.25 3 There were fewer than 300 events in total in the analysis 4 We were unable to assess reporting bias by funnel plot. The outcome was not reported in some of the trials included in this review 5 The confidence intervals overlap 0 and minimal clinically important difference (1 day for hospital stay) 6 There were fewer than 400 patients in total
Background
Description of the condition
In open operations on abdominal organs, the multilayered abdominal wall that surrounds the organs has to be divided (cut into) and this is called laparotomy. In a laparotomy, the parietal peritoneum, a layer of tissue that lines the inner wall of the abdomen, has to be opened before the abdominal cavity can be entered. By comparison, laparoscopy is key‐hole abdominal surgery in which instruments are introduced through small incisions (1 cm or less) to perform the operation, this also involves opening the parietal peritoneum. Non‐obstetric abdominal operations that breach the parietal peritoneum are common surgical procedures, with over 800,000 operations performed during the course of a year on the bowel, liver, gallbladder, bile ducts, pancreas and spleen in the NHS in England between 2009 and 2010 (NHS Information Centre 2011).
Wound‐related complications of laparotomy can include burst abdomen (2% to 3%) (Albertsmeier 2012; Gupta 2008), surgical site infection (1% to 10%) (Albertsmeier 2012; Davis 2011; Sajid 2011), and incisional hernia (8% to 25%) (Albertsmeier 2012; Gupta 2008; Sajid 2011). Wound‐related complications of laparoscopy include surgical site infection (1% to 9%) (Davis 2011; Trastulli 2012), and incisional hernia (2% to 3%) (Bunting 2010; Trastulli 2012).
There are many possible ways of performing an abdominal operation, through different sites and sizes of incision and use of different operative techniques and types of suture material. The term 'sutures' refers to the material used for stitching and the method used for stitching. These may depend on many factors including the disease being treated and the surgeon’s preference (Brown 2004; Keus 2010). When closing the abdomen after laparotomy, the parietal peritoneum may be closed or left unsutured.
Description of the intervention
Traditionally, closure of the parietal peritoneum at laparotomy either as a separate layer or as part of an abdominal mass closure (closure of all the layers of the abdominal wall apart from the skin) has been practised by many surgeons (Kendall 1991; van 't Riet 2002). Closure of the parietal peritoneum is usually performed using absorbable (dissolved by body fluids) or delayed absorbable sutures, and can be done with interrupted or continuous sutures. The interrupted method involves making each suture individually using a separate piece of material, whereas the continuous technique involves a series of sutures made using a single, uninterrupted piece of material. The peritoneum may be closed in a similar way after laparoscopy. The decision to close the peritoneum after laparotomy or laparoscopy is an arbitrary decision made by the surgeon based on his or her preference. The decision to close the peritoneum does not generally alter the method of suturing of other layers of the abdominal wall.
How the intervention might work
Possible reasons for peritoneal closure include restoration of anatomy by re‐approximation (bringing together) of tissues (which may facilitate improved closure of the fibrous layers), reduction of risk of wound infection by re‐establishing an anatomical barrier, and reduction of risk of wound dehiscence (wound breakdown), wound herniation, haemorrhage and adhesions (Bamigboye 1999; Bamigboye 2003; Duffy 1994).
Possible reasons for not undertaking peritoneal closure include rapid healing of the peritoneum without re‐approximation (Duffy 1994); and reductions in operating time, postoperative analgesic requirements, wound infection, length of hospital stay (Bamigboye 2003), and adhesion formation (Kapur 1979; Kyzer 1986).
Why it is important to do this review
There is no consensus regarding whether the peritoneum should be closed or left open after non‐obstetric operations involving laparotomy, and no systematic review has been conducted. Neither is there consensus about the method of closure of the peritoneum (continuous suture versus interrupted suture). If closing the peritoneum could be omitted without complications ‐ or even with benefit for patients ‐ this could result in reductions in the cost of abdominal operations through reductions in operating time and number of sutures used.
Objectives
To compare the benefits and harms of parietal peritoneal closure compared with no parietal peritoneal closure in patients undergoing non‐obstetric abdominal operations.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), irrespective of their use of blinding, language of publication, publication status, date of publication, study setting or sample sizes. We planned to include cluster randomised clinical trials if the effect estimate was available after adjusting for clustering effect. We excluded other study designs.
Types of participants
All adults and children undergoing non‐obstetric abdominal closure irrespective of the reason for surgery, whether the surgery was performed as an elective or emergency procedure, or the size of the incision. We have excluded obstetric operations because the closure of the peritoneum in those has been assessed in another review (Bamigboye 2003), and the anatomy of closure is different, as it involves the visceral peritoneum that lines the abdominal organs. Obstetric operations are generally considered to generate 'clean' wounds compared to non‐obstetric operations, which can have 'clean', 'clean‐contaminated', 'contaminated' or 'dirty' wounds (Garner 1986) (Appendix 1). The incidence of postoperative wound complications is lower in clean operations (Garner 1986). We included trials in which the closure or non‐closure of the parietal peritoneum was the only systematic difference between treatment groups.
Types of interventions
Parietal peritoneal closure (either as part of an abdominal mass closure or as a separate layer) compared with no parietal peritoneal closure.
Types of outcome measures
Primary outcomes
All‐cause mortality (within 30 days of surgery).
Other serious peri‐operative adverse events (within 30 days of surgery) (such as complete wound dehiscence (burst abdomen), bowel obstruction due to adhesions, deep surgical site infection, organ space infection, postoperative chest infection) are defined as any event that would increase mortality; is life‐threatening; requires inpatient hospitalisation or prolongation of existing hospitalisation; results in a persistent or significant disability or incapacity, or any important medical event, which might have jeopardised the patient or requires intervention to prevent it (ICH‐GCP 1996).
Quality of life (long‐term; however defined by authors).
Secondary outcomes
Incidence of incisional hernia (long‐term; however defined by authors).
Incidence of intestinal obstruction due to adhesions (long‐term; however defined by authors).
Incidence of requirement for re‐operation due to incisional hernia or adhesions (long‐term; however defined by authors).
Length of hospital stay (including any readmissions for adhesions or incisional hernia; long‐term; however defined by authors).
Search methods for identification of studies
Electronic searches
In February 2013 we searched the following electronic databases for potentially relevant trials:
The Cochrane Wounds Group Specialised Register (searched 14 February 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1);
The Database of Abstracts of Reviews of Effects (DARE) (2013, Issue 1);
Ovid MEDLINE (1946 to February Week 1, 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, February 13, 2013);
Ovid EMBASE (1974 to 2013 Week 06);
EBSCO CINAHL (1982 to 8 February 2013).
We used the following search strategy in CENTRAL and DARE:
#1 MeSH descriptor: [Sutures] explode all trees 662 #2 MeSH descriptor: [Wound Closure Techniques] explode all trees 1533 #3 (closure or close or closing or sutur*):ti,ab,kw 13372 #4 #1 or #2 or #3 13581 #5 MeSH descriptor: [Peritoneum] explode all trees 213 #6 peritone*:ti,ab,kw 2501 #7 #5 or #6 2501 #8 MeSH descriptor: [Abdomen] explode all trees and with qualifiers: [Surgery ‐ SU] 1298 #9 MeSH descriptor: [Laparotomy] explode all trees 664 #10 MeSH descriptor: [Abdominal Injuries] explode all trees 127 #11 MeSH descriptor: [Abdominal Cavity] explode all trees 472 #12 MeSH descriptor: [Abdominal Wall] explode all trees 73 #13 (abdom* near/5 surg*):ti,ab,kw 3763 #14 laparotom*:ti,ab,kw 1512 #15 #8 or #9 or #10 or #11 or #12 or #13 or #14 5711 #16 #4 and #7 and #15 119
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). We did not restrict studies with respect to language, date of publication or study setting.
We searched the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/) and ICTRP (International Clinical Trials Registry Platform) (http://apps.who.int/trialsearch/). The metaRegister includes the ISRCTN Register and NIH ClinicalTrials.gov Register among other registers. The ICTRP portal includes these trial registers along with trial registry data from a number of countries.
Searching other resources
We searched the references of included trials to identify further relevant trials. We contacted Ethicon and Dolphin sutures to enquire about any trials of which they were aware.
Data collection and analysis
We performed the systematic review following the instructions in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors (KSG and ECD) identified the trials for inclusion independently by screening titles and abstracts and retrieving full text copies of those that potentially met the inclusion criteria. We have listed the excluded studies with reasons for their exclusion (Characteristics of excluded studies). We resolved any differences in opinion through discussion amongst the review authors.
Data extraction and management
Both review authors independently extracted the following data:
Year and language of publication.
Country of conduct of the trial.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Sample size.
Details of suture material used and surgical technique used if the peritoneum was closed.
Outcomes (as described above).
Risk of bias (as described below).
We sought further information from authors when information was not available. Had multiple reports been identified for a trial, we planned to examine all the reports for information. We planned to seek clarification for any unclear or missing information by contacting the authors of individual trials. If there had been any doubt about whether the trials shared the same participants (by identifying common authors and centres), we planned to contact the study authors to check whether the trial report had been duplicated. We resolved any differences in opinion through discussion amongst the review authors.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). According to empirical evidence (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008), we planned to assess the risk of bias of included trials based on the following risk of bias domains:
Sequence generation
Low risk of bias: the method used is either adequate (e.g. computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding.
Uncertain risk of bias: there is insufficient information to assess whether the method used is likely to introduce confounding.
High risk of bias: the method used is improper and likely to introduce confounding (e.g. quasi‐randomised studies).
Allocation concealment
Low risk of bias: the method used is unlikely to induce bias on the final observed effect (e.g. central allocation).
Uncertain risk of bias: there is insufficient information to assess whether the method used is likely to induce bias on the estimate of effect.
High risk of bias: the method used (e.g. open random allocation schedule) is likely to induce bias on the final observed effect.
Blinding of participants and personnel
It is impossible to blind the healthcare personnel who suture the wound. However, this is unlikely to result in bias provided that the personnel are not involved in any other aspect of patient care apart from suturing the subcutaneous layer of the wound. Blinding of personnel refers to healthcare personnel involved in all other patient care apart from suturing the subcutaneous layer of the wound.
Low risk of bias: blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding.
Uncertain risk of bias: there is insufficient information to assess whether the type of blinding used is likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding.
Uncertain risk of bias: there is insufficient information to assess whether the type of blinding used is likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data.
Uncertain risk of bias: there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects will clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data are unsatisfactory (e.g. complete case estimate).
Selective outcome reporting
Low risk of bias: the trial protocol is available and all of the trial's pre‐specified outcomes that are of interest in the review have been reported; if the trial protocol is not available, all the primary outcomes in this review are reported.
Uncertain risk of bias: there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting.
High risk of bias: not all of the trial's pre‐specified primary outcomes have been reported.
We considered trials that were classified as low risk of bias in all the above domains to be low bias‐risk trials. We considered the other trials to be high bias‐risk trials.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). The dichotomous outcomes include short‐term mortality. If the event is very rare (less than 1% occurrence), we planned to use the Peto odds ratio and 95% CI for dichotomous variables (Higgins 2011d). We planned to calculate the rate ratio (RaR) with 95% CI for serious adverse events (since more than one serious adverse event can develop in the same patient), such as incidence of incisional hernia, incidence of adhesions and the incidence of requirement for re‐operation if the annual incidence was available, but none of these were available. So, we have calculated the risk ratio for incisional hernia, adhesions and requirement for re‐operation, ignoring the difference in time between the different trials, clearly aware that this may be a source of heterogeneity. For continuous variables, we calculated the mean difference (MD) with 95% CI for outcomes that can be quantified, such as hospital stay, and planned to calculate the standardised mean difference (SMD) with 95% CI for quality of life (where different assessment scales might be used).
Unit of analysis issues
The unit of analysis was individual patients undergoing laparotomy or laparoscopy.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). We imputed data for binary outcomes using various scenarios such as best‐best scenario, best‐worst scenario, worst‐best scenario, and worst‐worst scenario (Gurusamy 2009). In the best‐best scenario, the outcomes of patients with missing data in both groups were assumed to be good. In the best‐worst scenario, the outcomes of patients with missing data in the intervention group were assumed to be good while the outcomes were assumed to be bad for those with missing data in the control group. The worst‐best scenario was the opposite of the best‐worst scenario, i.e. the outcomes of patients with missing data in the intervention group were assumed to be bad while they were assumed to be good for those in the control group with missing data. In the worst‐worst scenario, the outcomes of patients with missing data in both groups were assumed to be bad.
For continuous outcomes, we used available‐case analysis. We have imputed the standard deviation from P values according to the instructions in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), and we planned to use the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we imputed the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation will decrease the weight of the study for calculation of mean differences and bias the effect estimate to no effect in case of standardised mean difference (Higgins 2011d).
Assessment of heterogeneity
We explored heterogeneity using the Chi2 test with significance set at P value 0.10, and measured the quantity of heterogeneity using the I2 statistic (Higgins 2002).
Thresholds for the interpretation of the I2 statistic can be misleading. A rough guide to interpretation is as follows (Deeks 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
We took factors such as clinical and methodological heterogeneity into account while interpreting the I2 statistic, particularly where there was overlapping of ranges.
Assessment of reporting biases
We planned to explore reporting bias using visual asymmetry on the funnel plot (Egger 1997; Macaskill 2001). We planned to perform linear regression as described by Egger 1997 to determine funnel plot asymmetry. We explored reporting bias from selective outcome reporting of the trials.
Data synthesis
We performed the meta‐analysis using RevMan 5 software (RevMan 2011), and following the recommendations of The Cochrane Collaboration (Deeks 2011). We used both a random‐effects model (DerSimonian 1986), which provides an average of the intervention effect, and a fixed‐effect model (DeMets 1987), which provides the average intervention effect, for meta‐analyses. Where there was discrepancy between the two models identified from the pooled estimates and their confidence intervals resulting in a change in interpretation of information, we reported both results; otherwise we reported the results of the fixed‐effect model. With regard to dichotomous outcomes, risk ratio calculations do not include trials in which no events occurred in either group in the meta‐analysis, whereas risk difference calculations do. We planned to report the risk difference (RD) if the results using this association measure were different from risk ratio in terms of statistical significance. However, risk ratio is the measure that we used to arrive at conclusions, since risk ratios perform better when there are differences in the control event rate (proportion of patients who develop the event in the control).
Summary of findings
We have presented a 'Summary of findings' table for all the primary and secondary outcomes (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Peritoneal closure with different types of suture material.
Peritoneal closure using continuous sutures versus interrupted sutures.
Trials with adults compared to trials with children.
Trials with clean wounds versus clean‐contaminated wounds versus contaminated wounds versus dirty wounds (Garner 1986) (Appendix 1).
Laparotomy versus laparoscopy.
Vertical versus transverse incisions.
We planned to use a P value of less than 0.05 for the Chi2 test to identify the differences (heterogeneity in the effect estimates) between subgroups.
Sensitivity analysis
We planned to perform a sensitivity analysis by excluding trials at high risk of bias. We planned to perform sensitivity analysis by imputing data for dichotomous outcomes using various scenarios including best‐best analysis, best‐worst analysis, worst‐best analysis, and worst‐worst analysis scenarios (Gurusamy 2009). In each of these analyses, the first component provides information on the way we planned to impute the outcome for intervention group and the second component provides information on the way we planned to impute the outcome for the control group. The word 'best' indicates that the participant with the missing outcome had a favourable event and the word 'worst' indicates that the participant with the missing outcome had an unfavourable event. We also planned to perform sensitivity analysis by excluding the trials in which the mean and the standard deviation were imputed.
Results
Description of studies
Results of the search
We identified a total of 265 unique references through electronic searches. We excluded 256 clearly irrelevant references through reading titles and abstracts. We retrieved nine references in full for further assessment. We did not identify any additional references to trials by scanning reference lists of included trials. We did not identify any new trials through other searches, or by contacting suture manufacturers. We excluded four references for reasons given in the Characteristics of excluded studies section. Five references for five completed trials met the inclusion criteria and were included in this review (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012). Four trials provided data for this review (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990). Three trials could be included in the meta‐analysis (Ellis 1977; Gilbert 1987; Hugh 1990). The reference flow is shown in Figure 1.
1.
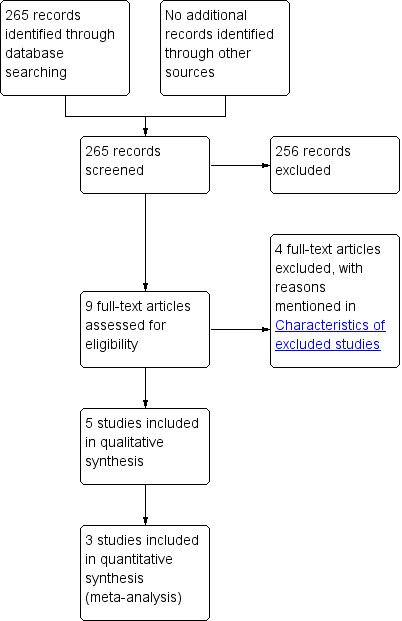
Study flow diagram.
Included studies
Five trials involving 836 participants randomised to peritoneal closure (410 participants) and to no peritoneal closure (426 participants) were included in this review (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012). The average ages of participants was 47 years, 55 years, and 25 years in the three trials that stated this information (Demirel 2005; Ellis 1977; Suresh 2012). The proportion of females was 100% and 51% in the two trials that stated this information (Demirel 2005; Ellis 1977). One of the trials was performed in women undergoing gynaecological surgery (Demirel 2005).
Four trials stated the suture material that was used (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990). All these trials used either catgut (Demirel 2005), or chromic catgut (Ellis 1977; Gilbert 1987; Hugh 1990), which generally provide strength to the wound for about five to seven days and 10 to 14 days respectively (Swanson 1982). Three trials stated that continuous sutures were used (Demirel 2005; Ellis 1977; Hugh 1990). The remaining two trials did not state whether continuous or interrupted sutures were used. One trial included only adult participants (Ellis 1977). One trial included adults and children over 12 years of age (Suresh 2012). Information on whether children were included was not available in the remaining trials (Demirel 2005; Gilbert 1987; Hugh 1990). All the trials included a wide range of operations, but did not report the outcomes separately for clean wounds, clean‐contaminated wounds, contaminated wounds and dirty wounds. All the trials included people undergoing laparotomy. Three trials explicitly stated that they included elective and emergency operations (Gilbert 1987; Hugh 1990; Suresh 2012). This information was not available for the remaining two trials (Demirel 2005; Ellis 1977). The incisions were vertical incisions in three trials (Ellis 1977; Gilbert 1987; Hugh 1990), and transverse (or oblique) incisions in the remaining two (Demirel 2005; Suresh 2012). There were no trials in people undergoing laparoscopy. The peritoneum was closed separately in all the trials, i.e. two‐layered closure of abdominal wall was used in the peritoneal closure group while a single‐layered closure was used in the no peritoneal closure group (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012). Further details of the included studies are shown in Characteristics of included studies.
Excluded studies
None of the excluded studies met the inclusion criteria as shown in Characteristics of excluded studies. In three trials, the visceral peritoneum was not closed. It is difficult to determine whether the effect (benefit or harm, if any) was due to closure of visceral peritoneum or parietal peritoneum (Al‐Inany 2004; Franchi 1997; Kadanali 1996). One study was a quasi‐randomised study (Dorfman 1997).
Risk of bias in included studies
The overall risk of bias in the different trials is shown in Figure 2. The risk of bias in each domain in each trial is shown in Figure 3. As seen in Figure 3, all the trials were at high risk of bias.
2.
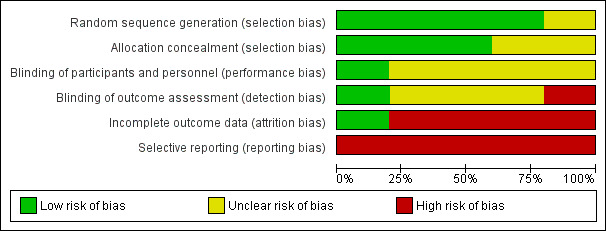
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
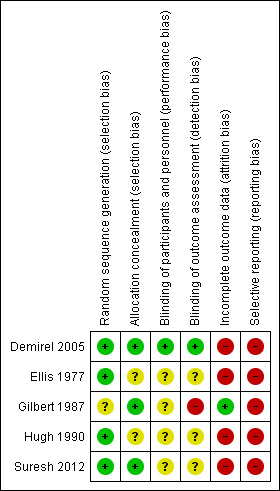
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials had low risk of selection bias, i.e. the randomisation procedure was considered to be adequate (Demirel 2005; Suresh 2012). The other trials had unclear random‐sequence generation (Gilbert 1987), or allocation concealment (Ellis 1977; Hugh 1990).
Blinding
Only one trial had both a low risk of performance and detection bias (Demirel 2005). The remaining trials had an unclear risk of performance bias (Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012), and either an unclear (Ellis 1977; Hugh 1990; Suresh 2012), or high risk of detection bias (Gilbert 1987).
Incomplete outcome data
Only one trial had low risk of attrition bias (Gilbert 1987). The remaining trials had post‐randomisation drop‐outs and were at high risk of attrition bias (Demirel 2005; Ellis 1977; Hugh 1990; Suresh 2012).
Selective reporting
None of the trials reported the mortality and morbidity that would normally have been recorded in such a trial. The protocols were not available for any of the trials to verify whether all the pre‐specified outcomes in the protocol had been reported. So, all the trials suffered from reporting bias.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
We included five trials (836 participants) randomised to peritoneal closure (410 participants) and no peritoneal closure (426 participants) in this review (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012). The available results are presented in the Table 1.
Primary outcomes
All‐cause mortality
None of the trials reported 30‐day mortality. We obtained information on long‐term mortality, which was a post hoc decision. This was reported in only one trial (Gilbert 1987), where the follow‐up period was at least one year in most participants.There was no significant difference in the mortality between the two groups (RR 1.11; 95% CI 0.56 to 2.19) (Analysis 1.1). Since this was the only trial included in this outcome, the issue of performing meta‐analysis using a fixed‐effect or random‐effects model did not arise. There was no change in the interpretation of results using the risk difference as the effect estimate. This trial was at high risk of bias; we did not perform a sensitivity analysis excluding this trial. Since there were no post‐randomisation drop‐outs in this trial (Gilbert 1987), we did not impute their outcomes using different scenarios.
1.1. Analysis.
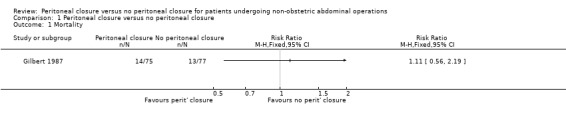
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 1 Mortality.
Other serious peri‐operative adverse events
The only serious peri‐operative adverse event reported in three trials was burst abdomen (Ellis 1977; Gilbert 1987; Hugh 1990). There was no significant difference in the proportion of participants who developed burst abdomen between the two groups (RR 0.71; 95% CI 0.22 to 2.35) (Analysis 1.2). There was no significant heterogeneity noted (I2 = 0%; P value 0.49). There was no change in the interpretation of results by adopting the random‐effects model or by using risk difference as the effect estimate. All three trials were at high risk of bias, so we did not perform a sensitivity analysis excluding these trials. One of the three trials did not report whether there were any post‐randomisation drop‐outs (Gilbert 1987). In another there were six post‐randomisation drop‐outs in the peritoneal closure group and 11 post‐randomisation drop‐outs in the no peritoneal closure group (Ellis 1977). In another trial, there were eight post‐randomisation drop‐outs in the peritoneal closure group and seven in the no peritoneal closure group (Hugh 1990). Imputing the outcomes using the different scenarios showed that the interpretation of results, and hence the recommendation, changes with different imputations (Analysis 1.3).
1.2. Analysis.
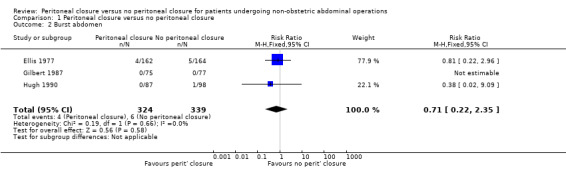
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 2 Burst abdomen.
1.3. Analysis.
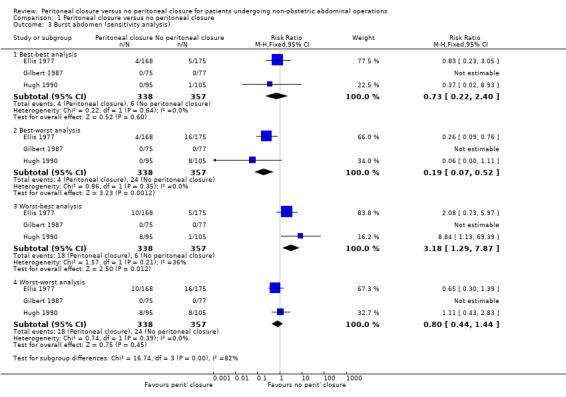
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 3 Burst abdomen (sensitivity analysis).
Quality of life
None of the trials reported quality of life.
Secondary outcomes
Incisional hernia
We were able to calculate the proportion of participants who developed incisional hernia, but could not calculate the incidence rate from trials. Three trials reported the proportion of participants who developed incisional hernia (Ellis 1977; Gilbert 1987; Hugh 1990). There was no significant difference in the proportion of participants who developed incisional hernia between the two groups (RR 0.92; 95% CI 0.37 to 2.28) (Analysis 1.4). There was no significant heterogeneity noted (I2 = 0%; P value 0.81).There was no change in the interpretation of results by adopting the random‐effects model or by using risk difference as the effect estimate. All three trials were of high risk of bias, so we did not perform a sensitivity analysis excluding these trials. The post‐randomisation drop‐outs in these three trials are stated under the outcome 'Other serious peri‐operative adverse events'. Imputing the outcomes using the different scenarios showed that the interpretation of results, and hence the recommendation, changes with different imputations (Analysis 1.5).
1.4. Analysis.
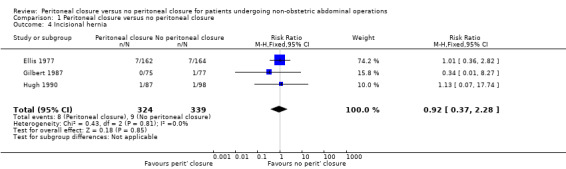
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 4 Incisional hernia.
1.5. Analysis.
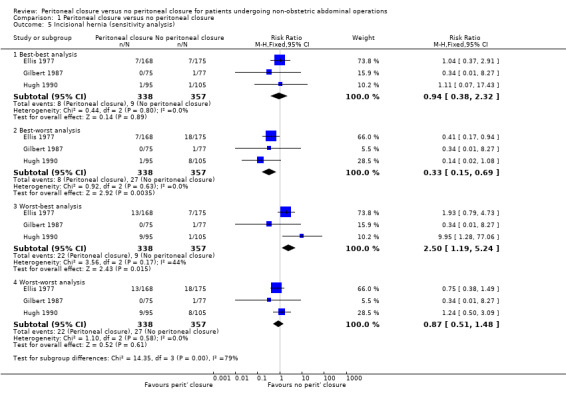
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 5 Incisional hernia (sensitivity analysis).
Incidence of intestinal obstruction due to adhesions
None of the trials reported the incidence rate of intestinal obstruction due to adhesions, or the proportion of participants who developed them.
Incidence of requirement for re‐operation due to incisional hernia or adhesions
None of the trials reported the requirement for re‐operation due to incisional hernia or adhesions.
Length of hospital stay
Only one trial reported the length of hospital stay, but did not include readmissions in its calculations (Demirel 2005). There was no significant difference in the length of hospital stay between the two groups (MD 0.40 days; 95% CI ‐0.51 to 1.31) (Analysis 1.6). Since this was the only trial that included this outcome, the issue of performing meta‐analysis using a fixed‐effect or random‐effects model did not arise. This trial was at high risk of bias, so we did not perform a sensitivity analysis excluding this trial. This trial reported the standard deviation, so we did not perform a sensitivity analysis excluding the trials that did not report the standard deviation.
1.6. Analysis.
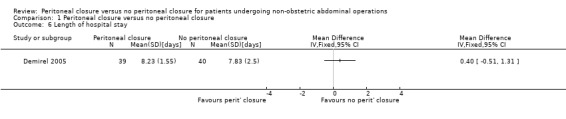
Comparison 1 Peritoneal closure versus no peritoneal closure, Outcome 6 Length of hospital stay.
Subgroup analysis
Only two of the outcomes (burst abdomen, incisional hernia) were reported in more than one trial (Ellis 1977; Gilbert 1987; Hugh 1990). We considered our planned sub‐group analyses in relation to these outcomes, but did not conduct these because of the following reasons:
Peritoneal closure with different types of suture material: all three trials used chromic catgut (Ellis 1977; Gilbert 1987; Hugh 1990).
Peritoneal closure using continuous sutures versus interrupted sutures: two trials used continuous sutures (Ellis 1977; Hugh 1990), and the third trial did not report whether continuous or interrupted sutures were used.
Trials with adults compared to trials with children: one trial included only adult participants (Ellis 1977): information on whether children were included was not available in the remaining two trials (Gilbert 1987; Hugh 1990).
Trials with clean wounds versus clean‐contaminated wounds versus contaminated wounds versus dirty wounds (Garner 1986): all three trials included a mixture of participants (Ellis 1977; Gilbert 1987; Hugh 1990). Separate results categorised by the contamination of wounds was not available in these three trials.
Laparotomy versus laparoscopy: all three trials only included participants who underwent laparotomy (Ellis 1977; Gilbert 1987; Hugh 1990).
Vertical versus transverse incisions: all three trials used vertical incisions (Ellis 1977; Gilbert 1987; Hugh 1990).
Reporting bias
We did not use the funnel plot to explore bias since the number of trials included for each outcome was fewer than 10. As shown in Figure 3, all the trials suffered from selective outcome reporting bias.
Discussion
Summary of main results
This review has shown that there is no evidence of any clinical advantage in closing the peritoneum. There was no significant difference in mortality between the peritoneal closure groups and the no peritoneal closure groups. The only serious adverse event reported in the trials was burst abdomen and there was no significant difference between the groups in the proportion of participants who developed burst abdomen. Vertical incisions were used on participants in the three trials that reported burst abdomen. Of these, one trial included median (midline) and paramedian incisions (parallel to the midline) and the wound was closed in a single layer in the no peritoneal closure group and in two layers in the peritoneal closure group (the additional layer closed being the parietal peritoneal layer) (Ellis 1977). In another trial, paramedian incisions were used to perform the laparotomy and the wound was closed in a single layer in the no peritoneal closure group and in two layers in the peritoneal closure group (the additional layer closed being the fascial tissue behind the rectus muscles) (Gilbert 1987). In the third trial, the participants underwent laparotomy by a midline incision and the wound was closed in a single layer in the no peritoneal closure group and in two layers in the peritoneal closure group (the additional layer closed being the parietal peritoneal layer) (Hugh 1990). Burst abdomen can be a disastrous event from the patient's point of view, particularly if the bowels protrude through the wound. It appears from current evidence that the suturing of an additional layer of the wound, i.e. the peritoneum does not offer any protection from this.
There was no significant difference in the proportion of people who developed incisional hernia. Supporters of peritoneal closure claim that it facilitates improved closure of the fibrous layers and hence decreases incisional hernia. Only one trial stated the time of measurement of outcomes (Gilbert 1987); the follow‐up period was at least one year in this trial. However, it should be noted that incisional hernia can develop many years after the surgery. This may be one of the reasons for lack of evidence for difference in the proportion of incisional hernia. There is no evidence to suggest any differences in occurrence of short‐term incisional hernia and there are no potential mechanisms by which peritoneal closure can decrease the long‐term incidence of incisional hernia without affecting the short‐term incidence of incisional hernia. So, there appears to be no evidence to suggest that peritoneal closure decreases the incisional hernia incidence either in the short‐term or long‐term.
One trial reported length of hospital stay, for which there was no significant difference between the two groups (Analysis 1.6). One would expect a difference in hospital stay if one of the intervention groups had more serious complications or more pain than the other group. However, there is currently no evidence for any difference in hospital stay between the peritoneal closure group and no peritoneal closure group.
None of the trials reported quality of life, proportion of participants with symptomatic adhesions, or re‐operations for incisional hernia or symptomatic adhesions. These are important patient‐oriented outcomes that could play an important role in terms of making a decision to close the peritoneum or leave it open. It should be noted that all these outcomes are long‐term outcomes and future trials should follow participants for at least three to five years in order to conclude whether peritoneal closure is beneficial or harmful. Although patients may develop symptomatic adhesions decades after surgery, they may have mild symptoms related to adhesions, and, evidence of such symptoms within a period of three to five years from surgery may suggest whether patients are likely to require hospital admissions and re‐operations related to adhesions.
Closure of the peritoneum involves additional operating time and use of additional suture material, both of which can increase the costs of the operation. However, it does not appear to provide any benefit. Based on current evidence, peritoneal closure is not necessary during non‐obstetric abdominal operations.
Overall completeness and applicability of evidence
This review included patients undergoing abdominal operations. Both elective and emergency operations were included in the trials in the review. Both vertical and transverse incisions were included. However, all the information relating to the outcomes of burst abdomen and incisional hernia were from trials in which vertical incisions were performed. A Cochrane review has shown that fewer people developed incisional hernia with transverse incisions compared to vertical incisions (Brown 2005). Considering the purpose of the peritoneal closure, i.e. to establish an additional anatomic layer to prevent wound disruption, peritoneal closure is unlikely to decrease wound complications significantly in transverse incisions in which the wound complications are fewer. The overall proportion of children included in this review is not known. However, there is no reason to suggest that children's wounds behave differently from adults' in terms of peritoneal closure. The review included only participants having open abdominal operations. There is no evidence to suggest that the peritoneal closure may be beneficial for people undergoing laparoscopy. So, the findings of this review are applicable to all abdominal operations.
Quality of the evidence
All the trials were at high risk of bias. Most of the outcomes, except for mortality and burst abdomen, were subjective. Absence of blinding may affect the effect estimates because of preference for one or other treatment by the participant or the surgeon. Different scenarios for imputation of outcomes of post‐randomisation drop‐out participants resulted in different conclusions. This shows that the influence of the post‐randomisation drop‐outs could be significant in this review. There was no significant heterogeneity in the outcomes in which more than one trial was included (burst abdomen and incisional hernia). However, there was serious imprecision in all the outcomes indicating that the lack of difference between the groups may be due to lack of evidence of effect rather than lack of effect. Although we could not explore reporting biases by a funnel plot because of the low number of trials included in this review, none of the trials reported all the important clinical outcomes, suggesting that the effect estimates may be unreliable because of selective outcome reporting bias. Overall, the quality of evidence was very low in all the outcomes as shown in Table 1.
Potential biases in the review process
Two review authors searched all the electronic databases for the relevant trials. While it is unlikely that we missed any relevant trials identified by the electronic searches, one has to be wary of the fact that some trials with negative results (as perceived by the person conducting the trial or the journal editors) may not have been reported.
Agreements and disagreements with other studies or reviews
This is the first systematic review of peritoneal closure versus no peritoneal closure in non‐obstetric operations. We agree with all the authors of the individual trials who either concluded that there was no benefit in closing the peritoneum or recommended against closing the peritoneum (Demirel 2005; Ellis 1977; Gilbert 1987; Hugh 1990; Suresh 2012). It must be noted that there is no evidence to justify peritoneal closure in caesarean sections either (Bamigboye 2003).
Authors' conclusions
Implications for practice.
There is no evidence for any short‐term or long‐term advantage in peritoneal closure for non‐obstetric operations. However, the confidence intervals of the included trials were wide, and further randomised controlled trials are necessary.
Implications for research.
If further trials are performed on this topic, adequate measures should be taken to ensure that the results are not subject to bias.
The trials should be based on outcomes important for patients, and participants should be followed up for an adequate period of time ‐ at least three to five years.
Acknowledgements
To the Cochrane Wounds Group.
The review authors would like to thank Cochrane Wounds Group referees (Sally Bell‐Syer, Ruth Foxlee, Robert Wyllie, Ajima Olaghere), Editors (Andrew Jull) and Statistician (Jane Burch, Marialena Trivella) and Rachel Richardson, for their comments on this review. The authors would also like to thank the Copy Editor (Elizabeth Royle).
This project was funded by the National Institute for Health Research.
Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health'.
Appendices
Appendix 1. Classification of wounds
Clean wound
|
Clean‐contaminated wound
|
Contaminated wound
|
Dirty wound
|
Appendix 2. Search strategies for Ovid MEDLINE, Ovid EMBASE & EBSCO CINAHL
Ovid MEDLINE
1 exp Sutures/ (11902) 2 exp Wound Closure Techniques/ (37366) 3 (closure or close or closing or sutur*).tw. (295185) 4 or/1‐3 (318050) 5 exp Peritoneum/ (33603) 6 peritone*.tw. (82363) 7 or/5‐6 (102156) 8 4 and 7 (3833) 9 exp Abdomen/su [Surgery] (15758) 10 exp Laparotomy/ (14553) 11 exp Abdominal Injuries/ (16385) 12 exp Abdominal Cavity/ (40426) 13 exp Abdominal Wall/ (3125) 14 (abdom* adj5 surg*).tw. (19574) 15 laparotom*.tw. (33384) 16 or/9‐15 (117499) 17 8 and 16 (2027) 18 randomized controlled trial.pt. (330201) 19 controlled clinical trial.pt. (84375) 20 randomized.ab. (233876) 21 placebo.ab. (132230) 22 clinical trials as topic.sh. (160871) 23 randomly.ab. (168558) 24 trial.ti. (101112) 25 or/18‐24 (765087) 26 (animals not (humans and animals)).sh. (3644610) 27 25 not 26 (705342) 28 17 and 27 (116) Ovid EMBASE
1 exp suture/ (18289) 2 exp wound closure/ (7615) 3 (closure or close or closing or sutur*).tw. (269589) 4 or/1‐3 (277210) 5 exp peritoneum/ (18458) 6 peritone*.tw. (62412) 7 or/5‐6 (74023) 8 4 and 7 (3687) 9 exp abdomen/su [Surgery] (3204) 10 exp laparotomy/ (37213) 11 exp abdominal injury/ (65766) 12 exp peritoneal cavity/ (10517) 13 exp abdominal wall/ (11095) 14 (abdom* adj5 surg*).tw. (18069) 15 laparotom*.tw. (29205) 16 or/9‐15 (141208) 17 8 and 16 (1544) 18 Randomized controlled trials/ (26219) 19 Single‐Blind Method/ (15552) 20 Double‐Blind Method/ (85854) 21 Crossover Procedure/ (31844) 22 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (941534) 23 (doubl$ adj blind$).ti,ab. (90196) 24 (singl$ adj blind$).ti,ab. (9662) 25 or/18‐24 (976081) 26 animal/ (724423) 27 human/ (8628708) 28 26 not 27 (484255) 29 25 not 28 (943761) 30 17 and 29 (147)
EBSCO CINAHL
S26 S13 and S25 S25 S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 MH "Quantitative Studies" S23 TI placebo* or AB placebo* S22 MH "Placebos" S21 TI random* allocat* or AB random* allocat* S20 MH "Random Assignment" S19 TI randomi?ed control* trial* or AB randomi?ed control* trial* S18 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S17 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S16 TI clinic* N1 trial* or AB clinic* N1 trial* S15 PT Clinical trial S14 MH "Clinical Trials+" S13 S7 and S12 S12 S8 or S9 or S10 or S11 S11 TI abdom* N5 surg* OR AB abdom* N5 surg* S10 (MH "Abdominal Injuries+") S9 (MH "Laparotomy") S8 (MH "Abdomen+/SU") S7 S3 and S6 S6 S4 or S5 S5 TI peritone* OR AB peritone* S4 (MH "Peritoneum+") S3 S1 or S2 S2 TI ( closure or close or closing or sutur* ) OR AB ( closure or close or closing or sutur* ) S1 (MH "Sutures")
Data and analyses
Comparison 1. Peritoneal closure versus no peritoneal closure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Burst abdomen | 3 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.35] |
| 3 Burst abdomen (sensitivity analysis) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Best‐best analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.22, 2.40] |
| 3.2 Best‐worst analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.52] |
| 3.3 Worst‐best analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [1.29, 7.87] |
| 3.4 Worst‐worst analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.44, 1.44] |
| 4 Incisional hernia | 3 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.37, 2.28] |
| 5 Incisional hernia (sensitivity analysis) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Best‐best analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.38, 2.32] |
| 5.2 Best‐worst analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.69] |
| 5.3 Worst‐best analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.19, 5.24] |
| 5.4 Worst‐worst analysis | 3 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.48] |
| 6 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Demirel 2005.
| Methods | RCT | |
| Participants | Country: Turkey Sample size: 80 Post‐randomisation drop‐out(s): 1 (1.3%) Revised sample size: 79 Females: 79 (100%) Mean age: 47 years Inclusion criteria: people having gynaecological abdominal surgery through Pfannensteil incision | |
| Interventions | Participants were randomised to the following groups: Group 1 (n = 40): peritoneal closure with continuous running 2/0 catgut Group 2 (n = 40): no peritoneal closure | |
| Outcomes | Length of hospital stay until discharge | |
| Notes | We made contact with the authors in August 2012. The author provided the following details:
1. Post‐randomisation drop‐out was due to complications 2. The study was funded by the National Social Insurance system as an integral part of the Health Insurance System |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was generated by computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each continuous numbered envelope contained a note instructing the surgeon to leave the peritoneum open or to close it" Comment: authors confirmed that the envelope was opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the attending midwives, nor the patients knew of the group allocation". "The involved doctors who were involved in patient care were not aware of the patient groups" (author replies) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the attending midwives, nor the patients knew of the group allocation". "The involved doctors who were involved in patient care were no aware of the patient groups" (author replies) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there was one post‐randomisation drop‐out. |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes were not reported |
Ellis 1977.
| Methods | RCT | |
| Participants | Country: UK Sample size: 343 Post‐randomisation drop‐out(s): 17 (5%) Revised sample size: 326 Females: 166 (50.9%) Mean age: 55 years Inclusion criteria: adults having laparotomy through vertical incisions | |
| Interventions | The participants were randomised to the following groups: Group 1 (n = 162): peritoneal closure performed with 1 continuous chromic catgut sutures Group 2 (n = 164): no peritoneal closure | |
| Outcomes | The outcomes reported were burst abdomen and incisional hernia Time of measurement of outcomes: not stated | |
| Notes | Reason for post‐randomisation drop‐out(s): died within the first 10 days (10) or required re‐exploration for obstruction, infection or other complications in the early postoperative period Information about funding was not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . were randomized by means of random number lists into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes were not reported |
Gilbert 1987.
| Methods | RCT | |
| Participants | Country: UK Sample size: 152 Post‐randomisation drop‐out(s): 0 (0%) Revised sample size: 152 Females: not stated Mean age: not stated Inclusion criteria: people having laparotomy through a lateral paramedian incision (both elective and emergency). Exclusion criteria: 1. Laparotomy performed through the scar tissue of a previous abdominal operation 2. Patients in whom urgency precluded the necessary dissection | |
| Interventions | Participants were randomised to the following groups; Group 1 (n = 75): peritoneal closure, peritoneal layer closed with 1‐0 chromic catgut (no details of whether continuous or interrupted) Group 2 (n = 77): no peritoneal closure | |
| Outcomes | Mortality, burst abdomen and incisional hernia Time of measurement of outcomes: most participants were followed at least for 1 year | |
| Notes | We attempted to contact the authors in August 2012 Information about funding was not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized into two groups for closure of the wound by a random allocation held by a third party" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The type of closure was not masked from the assessors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes were not reported |
Hugh 1990.
| Methods | RCT | |
| Participants | Country: Australia Sample size: 200 Post‐randomisation drop‐out(s): 15 (7.5%) Revised sample size: 185 Females: not stated Mean age: not stated Inclusion criteria: people having laparotomy, both elective and emergency, via a midline incision Exclusion criteria: 1. People whose wounds were at the site of a previous incision 2. People in whom delayed primary closure of the wound was employed 3. Participants who required a second laparotomy or who died within month of the initial operation 4. People who had a second abdominal incision at the time of the midline laparotomy were excluded from the pain component of the study 5. People whose general physical and mental condition precluded pain assessment were excluded from the pain component of the study. | |
| Interventions | Participants were randomised to the following groups: Group 1 (n = 87): peritoneal closure using continuous 0 chromic catgut Group 2: no peritoneal closure (n = 98) | |
| Outcomes | Burst abdomen and incisional hernia Time of measurement: not stated | |
| Notes | We attempted to contact the authors in August 2012.
Post‐randomisation drop‐out(s) due to early postoperative death, need for early relaparotomy, or lost to follow‐up Funding: this study was supported by a grant from Autosuture Company, Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was by random numbers tables" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was done by opening a sealed trial envelope immediately before closure of the wound" Comment: further details of the sealed envelope method were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes were not reported |
Suresh 2012.
| Methods | RCT | |
| Participants | Country: India Sample size: 100 Post‐randomisation drop‐out(s): 6 (6%) Revised sample size: 94 Females: not stated Mean age: 25 years Inclusion criteria: people having emergency or elective open appendectomy with proven ultrasonographic findings. Exclusion criteria: 1. Children younger than 12 years 2. Neurotic or psychiatric people 3. Complicated appendicitis 4. People who were operated under anaesthesia other than spinal anaesthesia 5. People who had additional pathology intra‐operatively or those who underwent additional procedure 6. People who developed wound infection | |
| Interventions | Participants were randomised to the following groups: Group 1 (n = 47): peritoneal closure, no further details available Group 2 (n = 47): no peritoneal closure | |
| Outcomes | This trial reported no outcomes of interest for this review | |
| Notes | We attempted to contact the authors in August 2012
Post‐randomisation drop‐out(s) due to participants who had additional pathology, underwent additional procedures or had wound infections Information about funding was not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By using computer generated random numbers, with the use of opaque sealed envelopes, the patients were randomly allocated to one of the two groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "By using computer generated random numbers, with the use of opaque sealed envelopes, the patients were randomly allocated to one of the two groups " Comment: probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: mentioned that it was a double‐blinded trial, but further details on blinding were not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: mentioned that it was a double‐blinded trial, but further details on blinding were not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes were not reported |
Abbreviation
RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Inany 2004 | The visceral peritoneum was also not closed in the control group. It is difficult to determine if the effect (benefit or harm, if any) was due to closure of visceral peritoneum or parietal peritoneum |
| Dorfman 1997 | Quasi‐randomised study (allocation by hospital numbers) |
| Franchi 1997 | The visceral peritoneum was also not closed in the control group. It is difficult to determine if the effect (benefit or harm, if any) was due to closure of visceral peritoneum or parietal peritoneum |
| Kadanali 1996 | The visceral peritoneum was also not closed in the control group. It is difficult to determine if the effect (benefit or harm, if any) was due to closure of visceral peritoneum or parietal peritoneum |
Differences between protocol and review
Information on long‐term mortality was obtained. Since symptomatic adhesions may necessitate surgery, it was felt that the information on long‐term mortality would be useful for both the patients and the healthcare funders in decision making on this issue.
Contributions of authors
Kurinchi Gurusamy: conceived, designed and co‐ordinated the review; extracted data, checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data, performed statistical analysis and checked its quality. Completed the first draft of the review, did part of the editing; made an intellectual contribution to and approved the final review prior to submission, and secured funding. Wrote to study authors, experts and companies, performed translations and acts as guarantor of the review. Etienne Cassar Delia: extracted data; checked quality of data extraction; undertook quality assessment and approved the final review prior to submission. Brian R Davidson: conceived and designed review; performed part of writing or editing; made an intellectual contribution to the review; secured funding, and advised on the review.
Contributions of editorial base:
Nicky Cullum: edited the protocol, advised on methodology, interpretation and review content. Joan Webster, Editor: approved the final review prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review. Ruth Foxlee: designed the search strategy, ran the searches and edited the search methods section.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
National Institute for Health Research, the health research wing of the UK Government Department of Health funds K Gurusamy to complete this review.
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
Kurinchi Gurusamy: none known. Etienne Cassar Delia: none known. Brian R Davidson: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Demirel 2005 {published data only}
- Demirel Y, Gursoy S, Duran B, Erden O, Cetin M, Balta O, et al. Closure or nonclosure of the peritoneum at gynecological operations. Effect on postoperative pain. Saudi Medical Journal 2005;26(6):964‐8. [PubMed] [Google Scholar]
Ellis 1977 {published data only}
- Ellis H, Heddle R. Does the peritoneum need to be closed at laparotomy?. British Journal of Surgery 1977;64(10):733‐6. [DOI] [PubMed] [Google Scholar]
Gilbert 1987 {published data only}
- Gilbert J, Ellis H, Foweraker S. Peritoneal closure after lateral paramedian incision. British Journal of Surgery 1987;74(2):113‐5. [DOI] [PubMed] [Google Scholar]
Hugh 1990 {published data only}
- Hugh T, Nankivell C, Meagher A, Li B. Is closure of the peritoneal layer necessary in the repair of midline surgical abdominal wounds?. World Journal of Surgery 1990;14(2):231‐3. [DOI] [PubMed] [Google Scholar]
Suresh 2012 {published data only}
- Suresh B, Ambi U, Anilkumar G, Shaileshl E, Lamani Y. Post‐operative analgesic requirement in non‐closure and closure of peritoneum during open appendectomy‐ a randomized controlled study. Journal of Clinical and Diagnostic Research 2012;6(2):264‐6. [Google Scholar]
References to studies excluded from this review
Al‐Inany 2004 {published data only}
- Al‐Inany H. Peritoneal closure vs. non‐closure: estimation of pelvic fluid by transvaginal ultrasonography after abdominal hysterectomy. Gynecologic and Obstetric Investigation 2004;58(4):183‐5. [DOI] [PubMed] [Google Scholar]
Dorfman 1997 {published data only}
- Dorfman S, Rincón A, Shortt H. Cholecystectomy via kocher incision without peritoneal closure. Investigacion Clinica 1997;38(1):3‐7. [PubMed] [Google Scholar]
Franchi 1997 {published data only}
- Franchi M, Ghezzi F, Zanaboni F, Scarabelli C, Beretta P, Donadello N. Nonclosure of peritoneum at radical abdominal hysterectomy and pelvic node dissection: a randomized study. Obstetrics and Gynecology 1997;90(4 Pt 1):622‐7. [DOI] [PubMed] [Google Scholar]
Kadanali 1996 {published data only}
- Kadanali S, Erten O, Kücüközkan T. Pelvic and periaortic peritoneal closure or non‐closure at lymphadenectomy in ovarian cancer: effects on morbidity and adhesion formation. European Journal of Surgical Oncology 1996;22(3):282‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Albertsmeier 2012
- Albertsmeier M, Seiler CM, Fischer L, Baumann P, Husing J, Seidlmayer C, et al. Evaluation of the safety and efficacy of MonoMax(R) suture material for abdominal wall closure after primary midline laparotomy‐a controlled prospective multicentre trial: ISSAAC [NCT005725079]. Langenbeck's Archives of Surgery 2012;397(3):363‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bamigboye 1999
- Bamigboye AA, Buchman E, Hofmeyr GJ. Closure of peritoneum at laparotomy: a survey of gynecological practice. South African Medical Journal 1999;89(3):332‐5. [Google Scholar]
Bamigboye 2003
- Bamigboye AA, Hofmeyr GJ. Closure versus non‐closure of the peritoneum at caesarean section. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000163] [DOI] [PubMed] [Google Scholar]
Brown 2004
- Brown SR, Goodfellow PJ, Adam IJ, Shorthouse AJ. A randomised controlled trial of transverse skin crease vs. vertical midline incision for right hemicolectomy. Techniques in Coloproctology 2004;8(1):15‐8. [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown SR, Goodfellow PB. Transverse verses midline incisions for abdominal surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005199.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bunting 2010
- Bunting DM. Port‐site hernia following laparoscopic cholecystectomy. Journal of the Society of Laparoendoscopic Surgeons 2010;14(4):490‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2011
- Davis JM, Kuo YH, Ahmed N, Kuo YL. Report card on Surgical Care Improvement Project (SCIP): nationwide inpatient sample infection data 2001‐2006. Surgical Infections 2011;12(6):429‐34. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Duffy 1994
- Duffy DM, diZerega GS. Is peritoneal closure necessary?. Obstetrical and Gynecological Survey 1994;49(12):817‐22. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garner 1986
- Garner JS. CDC guideline for prevention of surgical wound infections, 1985. Infection Control 1986;7(3):193‐200. [DOI] [PubMed] [Google Scholar]
Gupta 2008
- Gupta H, Srivastava A, Menon GR, Agrawal CS, Chumber S, Kumar S. Comparison of interrupted versus continuous closure in abdominal wound repair: a meta‐analysis of 23 trials. Asian Journal of Surgery 2008;31(3):104‐14. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
- Higgins JPT, Deeks JJ, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation and ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Kapur 1979
- Kapur BML, Daneswar A, Chopra P. Evaluation of peritoneal closure at laparotomy. American Journal of Surgery 1979;137(5):650‐2. [DOI] [PubMed] [Google Scholar]
Kendall 1991
- Kendall SW, Brennan TG, Guillou PJ. Suture length to wound length ratio and the integrity of midline and lateral paramedian incisions. British Journal of Surgery 1991;78(6):705‐7. [DOI] [PubMed] [Google Scholar]
Keus 2010
- Keus F, Gooszen HG, Laarhoven CJ. Open, small‐incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato‐Biliary Group reviews. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008318] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kyzer 1986
- Kyzer S, Bayer I, Turani H, Chaimoff C. The influence of peritoneal closure on formation of intraperitoneal adhesions: an experimental study. International Journal of Tissue Reactions 1986;8(5):355‐9. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors) on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NHS Information Centre 2011
- The NHS Information Centre, Hospital Episode Statistics for England. Inpatient statistics, 2009‐10. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=204 2011 (accessed on 16 August 2011).
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sajid 2011
- Sajid MS, Parampalli U, Baig MK, McFall MR. A systematic review on the effectiveness of slowly‐absorbable versus non‐absorbable sutures for abdominal fascial closure following laparotomy. International Journal of Surgery 2011;9(8):615‐25. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (editors) on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2011
- Scottish Intercollegiate Guidelines Network. Search filters. http://www.sign.ac.uk/methodology/filters.html (accessed 2 September 2011).
Swanson 1982
- Swanson NA, Tromovitch TA. Suture materials, 1980s: properties, uses, and abuses. International Journal of Dermatology 1982;21(7):373‐8. [DOI] [PubMed] [Google Scholar]
Trastulli 2012
- Trastulli S, Cirocchi R, Listorti C, Cavaliere D, Avenia N, Gulla N, et al. Laparoscopic versus open resection for rectal cancer: a meta‐analysis of randomized clinical trials. Colorectal Disease 2012;14(6):e277‐96. doi: 10.1111/j.1463‐1318.2012.02985.x.. [DOI] [PubMed] [Google Scholar]
van 't Riet 2002
- 't Riet M, Steyerberg EW, Nellensteyn J, Bonjer HJ, Jeekel J. Meta‐analysis of techniques for closure of midline abdominal incisions. British Journal of Surgery 2002;89(11):1350‐6. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


