Abstract
Background
This is an updated version of the original Cochrane review published in Issue 10, 2011. Paracetamol (acetaminophen) is the most commonly prescribed analgesic for the treatment of acute pain. It may be administered orally, rectally, or intravenously. The efficacy and safety of intravenous (IV) formulations of paracetamol, IV paracetamol, and IV propacetamol (a prodrug that is metabolized to paracetamol), compared with placebo and other analgesics, is unclear.
Objectives
To assess the efficacy and safety of IV formulations of paracetamol for the treatment of postoperative pain in both adults and children.
Search methods
We ran the search for the previous review in May 2010. For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1), MEDLINE (May 2010 to 16 February 2016), EMBASE (May 2010 to 16 February 2016), LILACS (2010 to 2016), a clinical trials registry, and reference lists of reviews for randomized controlled trials (RCTs) in any language and we retrieved articles.
Selection criteria
Randomized, double‐blind, placebo‐ or active‐controlled single dose clinical trials of IV paracetamol or IV propacetamol for acute postoperative pain in adults or children.
Data collection and analysis
Two review authors independently extracted data, which included demographic variables, type of surgery, interventions, efficacy, and adverse events. We contacted study authors for additional information. We graded each included study for methodological quality by assessing risk of bias and employed the GRADE approach to assess the overall quality of the evidence.
Main results
We included 75 studies (36 from the original review and 39 from our updated review) enrolling a total of 7200 participants.
Among primary outcomes, 36% of participants receiving IV paracetamol/propacetamol experienced at least 50% pain relief over four hours compared with 16% of those receiving placebo (number needed to treat to benefit (NNT) = 5; 95% confidence interval (CI) 3.7 to 5.6, high quality evidence). The proportion of participants in IV paracetamol/propacetamol groups experiencing at least 50% pain relief diminished over six hours, as reflected in a higher NNT of 6 (4.6 to 7.1, moderate quality evidence). Mean pain intensity at four hours was similar when comparing IV paracetamol and placebo, but was seven points lower on a 0 to 100 visual analog scale (0 = no pain, 100 = worst pain imaginable, 95% CI ‐9 to ‐6, low quality evidence) in those receiving paracetamol at six hours.
For secondary outcomes, participants receiving IV paracetamol/propacetamol required 26% less opioid over four hours and 16% less over six hours (moderate quality evidence) than those receiving placebo. However, this did not translate to a clinically meaningful reduction in opioid‐induced adverse events.
Meta‐analysis of efficacy comparisons between IV paracetamol/propacetamol and active comparators (e.g., opioids or nonsteroidal anti‐inflammatory drugs) were either not statistically significant, not clinically significant, or both.
Adverse events occurred at similar rates with IV paracetamol or IV propacetamol and placebo. However, pain on infusion occurred more frequently in those receiving IV propacetamol versus placebo (23% versus 1%). Meta‐analysis did not demonstrate clinically meaningful differences between IV paracetamol/propacetamol and active comparators for any adverse event.
Authors' conclusions
Since the last version of this review, we have found 39 new studies providing additional information. Most included studies evaluated adults only. We reanalyzed the data but the results did not substantially alter any of our previously published conclusions. This review provides high quality evidence that a single dose of either IV paracetamol or IV propacetamol provides around four hours of effective analgesia for about 36% of patients with acute postoperative pain. Low to very low quality evidence demonstrates that both formulations are associated with few adverse events, although patients receiving IV propacetamol have a higher incidence of pain on infusion than both placebo and IV paracetamol.
Plain language summary
Intravenous paracetamol (acetaminophen) for pain after surgery in adults and children
Background
Pain is commonly experienced after surgical procedures and multiple medications (e.g., painkillers) are routinely used to control it. In February 2016, we searched for clinical trials looking at intravenous (IV) formulations (solutions that can be administered directly into a vein) of paracetamol (either IV paracetamol or IV propacetamol) and how they might manage pain after surgery.
Results and quality of the evidence
Our updated review included data from 75 studies of 7200 patients with moderate‐to‐severe pain after an operation. We found high quality evidence that IV paracetamol or IV propacetamol provided pain relief for four hours for about 36% of people versus 16% of those receiving placebo. Direct comparisons with other painkillers, such as morphine and anti‐inflammatories, did not show large differences (if any) in effectiveness, although this may have been due to the small numbers of patients studied.
Low quality evidence showed that IV paracetamol and IV propacetamol produced few side effects. However, patients receiving IV propacetamol complained of pain at the site their medication was infused at more often than those receiving placebo or IV paracetamol.
Due to the amount of data already included in our review, we think it is unlikely that any new studies will change our conclusions. However, we found very few studies that included children, so this is an area that requires further investigation.
Summary of findings
Summary of findings for the main comparison. Proportion of participants experiencing at least 50% of maximum pain relief at 4 hours.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol/propacetamol Comparison: placebo or other analgesics | |||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo see footnote1 | 156 per 1000 | 394 per 1000 (313 to 497) | RR 2.53 (2.01 to 3.19) | 1149 (11 studies) | ⊕⊕⊕⊕ high2,3 |
| Paracetamol vs placebo see footnote1 | 66 per 1000 | 317 per 1000 (152 to 661) | RR 4.8 (2.3 to 10) | 393 (5 studies) | ⊕⊕⊕⊝ moderate2,3,4 |
| Propacetamol vs placebo see footnote1 | 188 per 1000 | 411 per 1000 (327 to 520) | RR 2.19 (1.74 to 2.77) | 756 (8 studies) | ⊕⊕⊕⊝ moderate2,3,4 |
| Para/propacetamol vs NSAIDs see footnote1 | 599 per 1000 | 605 per 1000 (515 to 707) | RR 1.01 (0.86 to 1.18) | 353 (5 studies) | ⊕⊕⊝⊝ low4,5 |
| Paracetamol vs NSAIDs see footnote1 | 631 per 1000 | 568 per 1000 (454 to 713) | RR 0.9 (0.72 to 1.13) | 130 (2 studies) | ⊕⊝⊝⊝ very low4,5,6 |
| Propacetamol vs NSAIDs see footnote1 | 577 per 1000 | 624 per 1000 (496 to 774) | RR 1.08 (0.86 to 1.34) | 223 (3 studies) | ⊕⊝⊝⊝ very low2,4,5,6 |
| Paracetamol vs propacetamol see footnote1 | 428 per 1000 | 419 per 1000 (329 to 530) | RR 0.98 (0.77 to 1.24) | 361 (3 studies) | ⊕⊕⊕⊝ moderate4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IV: intravenous; NSAIDs: nonsteroidal anti‐inflammatory drugs; RR: risk ratio; SPID = summed pain intensity difference; TOTPAR = total pain relief; VAS: visual analog scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1TOTPAR or SPID using either VAS or categorical data, and calculating their corresponding percentage of theoretical maximum TOTPAR and SPID. 2Considerable unexplained heterogeneity exists between studies. 3Large effect. 4Total # events < 300. 5Different NSAIDs studied. 6Wide confidence interval that includes no effect and appreciable benefit and/or harm.
Summary of findings 2. Proportion of participants experiencing at least 50% of maximum pain relief at 6 hours.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol/propacetamol Comparison: placebo or other analgesics | |||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo see footnote1 | 97 per 1000 | 276 per 1000 (203 to 378) | RR 2.86 (2.1 to 3.91) | 1143 (10 studies) | ⊕⊕⊕⊝ moderate2,3,4 |
| Paracetamol vs placebo see footnote1 | 83 per 1000 | 304 per 1000 (179 to 517) | RR 3.65 (2.15 to 6.21) | 532 (6 studies) | ⊕⊕⊕⊝ moderate2,3,4 |
| Propacetamol vs placebo see footnote1 | 105 per 1000 | 252 per 1000 (172 to 367) | RR 2.4 (1.64 to 3.5) | 611 (6 studies) | ⊕⊕⊝⊝ low2,3,4,5,6 |
| Para/propacetamol vs NSAIDs see footnote1 | 632 per 1000 | 499 per 1000 (417 to 600) | RR 0.79 (0.66 to 0.95) | 355 (5 studies) | ⊕⊝⊝⊝ very low3,7,8 |
| Paracetamol vs NSAIDs see footnote1 | 623 per 1000 | 511 per 1000 (411 to 635) | RR 0.82 (0.66 to 1.02) | 212 (3 studies) | ⊕⊝⊝⊝ very low3,7,9,10 |
| Propacetamol vs NSAIDs see footnote1 | 649 per 1000 | 487 per 1000 (364 to 662) | RR 0.75 (0.56 to 1.02) | 143 (2 studies) | ⊕⊝⊝⊝ very low3,7,9,10 |
| Paracetamol vs propacetamol see footnote1 | 411 per 1000 | 386 per 1000 (300 to 493) | RR 0.94 (0.73 to 1.2) | 361 (3 studies) | ⊕⊕⊝⊝ low3,10 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNT = number needed to treat to benefit; NSAIDs: nonsteroidal anti‐inflammatory drugs; RR: risk ratio; SPID = summed pain intensity difference; TOTPAR = total pain relief; VAS: visual analog scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1TOTPAR or SPID using either VAS or categorical data, and calculating their corresponding percentage of theoretical maximum TOTPAR and SPID. 2Considerable unexplained heterogeneity exists between studies. 3Total # events <300. 4Large effect. 5One study data "not estimable" because of zero events in both groups. 6Publication bias favoring propacetamol; < 400 additional participants needed in studies with zero effect (relative benefit of one) required to change the NNT for at least 50% maximum pain relief to an unacceptably high level (in this case a NNT of 10). 7Different NSAIDs studied. 8Publication bias for superiority of NSAID; < 400 additional participants needed in studies with zero effect (relative benefit of one) required to change the NNT for at least 50% maximum pain relief to an unacceptably high level (in this case a NNT of 10). 9All individual studies < 100 participants. 10Wide confidence interval that includes no effect and appreciable benefit and/or harm.
Summary of findings 3. Mean pain intensity over a 4‐hour period.
| IV paracetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol Comparison: placebo or other analgesics | |||
| Comparison | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| Paracetamol vs placebo | The mean pain intensity over a 4‐hour period was: 1.21 lower (3.73 lower to 1.31 higher) | 485 (6 studies) | ⊕⊕⊝⊝ low1,2 |
| Paracetamol vs NSAIDs | The mean pain intensity over a 4‐hour period was: 5.02 higher (3.18 to 6.86 higher) | 350 (6 studies) | ⊕⊝⊝⊝ very low1,3,4,5,6 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAIDs: nonsteroidal anti‐inflammatory drugs | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1See 'Risk of bias' tables; several unclear assessments related to randomization; unclear to high risk for selective reporting. 2Wide confidence interval that includes no effect and appreciable benefit and/or harm. 3Total population size < 400. 4Majority of all individual studies had < 100 total participants. 5Considerable unexplained heterogeneity exists between studies. 6Different NSAIDs studied.
Summary of findings 4. Mean pain intensity over a 6‐hour period.
| IV paracetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol Comparison: placebo or other analgesics | |||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| Paracetamol vs placebo | The mean pain intensity over a 6‐hour period was: 7.48 lower (8.98 to 5.97 lower) | 837 (12 studies) | ⊕⊕⊝⊝ low1,2 |
| Paracetamol vs NSAIDs | The mean pain intensity over a 6‐hour period was: 2.95 higher (1.18 to 4.72 higher) | 524 (9 studies) | ⊕⊝⊝⊝ very low2,3,4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAIDs: nonsteroidal anti‐inflammatory drugs | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Majority of all individual studies had < 100 total participants. 2Considerable unexplained heterogeneity exists between studies. 3See 'Risk of bias' tables: several unclear assessments related to randomization; unclear to high risk for selective reporting. 4Different NSAIDs studied.
Summary of findings 5. Proportion of participants receiving additional analgesic medication.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol/propacetamol Comparison: placebo or other analgesics | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 820 per 1000 | 574 per 1000 (525 to 631) | RR 0.7 (0.64 to 0.77) | 859 (9 studies) | ⊕⊕⊝⊝ low1,2 |
| Paracetamol vs placebo | 892 per 1000 | 669 per 1000 (616 to 732) | RR 0.75 (0.69 to 0.82) | 655 (6 studies) | ⊕⊕⊝⊝ low1,2 |
| Propacetamol vs placebo | 625 per 1000 | 306 per 1000 (219 to 431) | RR 0.49 (0.35 to 0.69) | 204 (3 studies) | ⊕⊕⊝⊝ low1,2,3,4,5 |
| Para/propacetamol vs NSAIDs | 284 per 1000 | 338 per 1000 (247 to 463) | RR 1.19 (0.87 to 1.63) | 309 (5 studies) | ⊕⊝⊝⊝ very low1,3,4,6,7 |
| Paracetamol vs NSAIDs | 200 per 1000 | 216 per 1000 (118 to 396) | RR 1.08 (0.59 to 1.98) | 120 (2 studies) | ⊕⊝⊝⊝ very low1,3,4,6,7 |
| Propacetamol vs NSAIDs | 337 per 1000 | 414 per 1000 (290 to 596) | RR 1.23 (0.86 to 1.77) | 189 (3 studies) | ⊕⊝⊝⊝ very low1,3,4,6,7 |
| Propacetamol vs opioids | 86 per 1000 | 157 per 1000 (62 to 398) | RR 1.83 (0.72 to 4.64) | 139 (2 studies) | ⊕⊝⊝⊝ very low1,3,4,7 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAIDs: nonsteroidal anti‐inflammatory drugs; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1See 'Risk of bias' tables: several unclear assessments related to randomization and attrition bias; unclear to high risk for selective outcome reporting. 2Considerable unexplained heterogeneity exists between studies. 3Majority of all individual studies had < 100 total participants. 4Total # events < 300. 5Large effect. 6Different NSAIDs studied. 7Wide confidence interval that includes no effect and appreciable benefit and/or harm.
Summary of findings 6. Opioid consumption (IV morphine equivalents) over 6 hours.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol/propacetamol Comparison: placebo or other analgesics | |||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| Para/propacetamol vs placebo see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 1.92 lower (2.41 to 1.42 lower) | 777 (13 studies) | ⊕⊕⊕⊝ moderate2,3 |
| Paracetamol vs placebo see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 1.83 lower (2.35 to 1.31 lower) | 404 (8 studies) | ⊕⊕⊝⊝ low2,3,4 |
| Propacetamol vs placebo see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 2.67 lower (4.21 to 1.13 lower) | 373 (6 studies) | ⊕⊕⊝⊝ low2,4,5 |
| Para/propacetamol vs NSAIDs see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 0.12 lower (0.37 lower to 0.12 higher) | 540 (8 studies) | ⊕⊝⊝⊝ very low2,3,6 |
| Paracetamol vs NSAIDs see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 0.81 higher (0.87 lower to 2.49 higher) | 160 (3 studies) | ⊕⊝⊝⊝ very low3,5,6,7 |
| Propacetamol vs NSAIDs see footnote1 | The mean opioid consumption (IV morphine equivalents) over 6 hours was: 0.14 lower (0.39 lower to 0.11 higher) | 380 (5 studies) | ⊕⊝⊝⊝ very low5,6,7 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAIDs: nonsteroidal anti‐inflammatory drugs | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Mean opioid consumption (in mg) over 6 hours in each treatment arm converted into IV morphine‐equivalents, using commonly used and widely accepted opioid conversion tables. 2See 'Risk of bias' tables: several unclear assessments related to randomization, unclear risk for selective reporting. 3Majority of all individual studies had < 100 participants. 4Considerable unexplained heterogeneity exists between studies. 5Total population size < 400. 6Different NSAIDs studied. 7Wide confidence interval that includes no effect and appreciable benefit and/or harm.
Summary of findings 7. Proportion of participants vomiting.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: IV paracetamol/propacetamol Comparison: placebo or other analgesics | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 208 per 1000 | 145 per 1000 (118 to 181) | RR 0.7 (0.57 to 0.87) | 1414 (15 studies) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Paracetamol vs placebo | 263 per 1000 | 168 per 1000 (134 to 210) | RR 0.64 (0.51 to 0.8) | 1037 (13 studies) | ⊕⊝⊝⊝ very low1,3,4 |
| Propacetamol vs placebo | 45 per 1000 | 74 per 1000 (34 to 158) | RR 1.62 (0.75 to 3.48) | 377 (3 studies) | ⊕⊕⊝⊝ low3,5 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNH = number needed to treat to harm; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Majority of all individual studies had < 100 participants. 2Considerable unexplained heterogeneity exists between studies. 3Total # events < 300. 4Publication bias suspected in favor of a lower occurrence of vomiting in the paracetamol and/or propacetamol arm; NNH > 10. 5Wide confidence interval that includes no effect and appreciable benefit and/or harm.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 10, 2011) on 'Single dose intravenous propacetamol or intravenous paracetamol for postoperative pain’ (Tzortzopoulou 2011).
Description of the condition
Pain after surgery is common. Evidence indicates that a variety of populations experience suboptimal treatment and patients often return home with substantial ongoing pain (Apfelbaum 2003).
Description of the intervention
Paracetamol, known as acetaminophen in North America, is the most commonly prescribed analgesic for the treatment of acute pain (Sachs 2005). Its major advantages over nonsteroidal anti‐inflammatory drugs (NSAIDs) are its lack of interference with platelet function and its safe administration in patients with a history of peptic ulcers or asthma (Hyllested 2002). Its efficacy is influenced by baseline pain intensity and the origin of the pain (Juhl 2006). Paracetamol is less efficacious when baseline pain is severe than when it is of lesser intensity and is less efficacious when pain is secondary to orthopedic procedures versus dental procedures (Remy 2006). Systematic reviews of randomized controlled trials (RCTs) confirm the efficacy of oral paracetamol for acute pain (Perrott 2004; Toms 2008). For every four patients treated with oral paracetamol, one will experience at least 50% pain relief who would not have experienced it with placebo (Toms 2008). Oral paracetamol takes 60 minutes to provide peak pain relief and the non‐availability of the oral route immediately after surgery limits its value in treating immediate postoperative pain. Therefore, an intravenous formulation of paracetamol is an attractive option for the treatment of postsurgical pain. In adults, a mean peak concentration of 28.4 µg/ml is achieved with the parenteral formulation, at the end of a 15‐minute infusion (Cadence 2011). Plasma concentrations achieved are proportional to body weight; therefore, doses should be reduced in adults with low body weight (< 50 kg). Metabolism of the parent drug occurs in the liver. Hepatotoxicity can occur in patients with pre‐existing hepatic impairment or when supra‐therapeutic doses are administered to patients with normal hepatic function. Paracetamol is excreted by the kidneys. In patients with renal impairment (creatinine CL ≤ 30 ml/min), paracetamol should be administered at longer dosing intervals and at a reduced total daily dose.
Currently, there are two formulations of intravenous (IV) paracetamol: propacetamol, a prodrug of paracetamol; and the recently approved IV paracetamol. Propacetamol is hydrolyzed by plasma esterases to paracetamol within seven minutes after administration. A dose of 2 g of propacetamol is hydrolyzed to 1 g of paracetamol (Anderson 2005; Flouvat 2004). Propacetamol requires reconstitution, and allergic contact dermatitis caused by the N,N‐diethylglycidyl ester portion of the propacetamol molecule has been observed in healthcare personnel who have handled the drug (Barbaud 1995; Gielen 2001). Additionally, it causes pain for the patient at the site of injection. This discomfort can be reduced if it is infused slowly (Depre 1992). Conversely, IV paracetamol is presented as a ready‐to‐use solution. No incidences of contact dermatitis have been reported, and reports of its infusion causing discomfort are limited (Berl 1998; Moller 2005; Murat 2005).
How the intervention might work
The analgesic effect of oral or parenteral paracetamol, unlike NSAIDs, cannot be explained by the peripheral inhibition of cyclooxygenase 1 or 2 (COX‐1, COX‐2) (Greco 2003). Its mechanism of action may involve central inhibition of COX‐2 (Graham 2005; Kumpulainen 2007; Remy 2006), inhibition of nitric oxide generation via blockade of the N‐methyl‐D‐aspartate receptor (Björkman 1994), and the activation of descending serotonergic and cannabinoid pathways (Hama 2010; Mallet 2008). Previous theories about the inhibition of COX‐3 (a spliced variant of COX‐1) have largely been discounted (Agnes 2006; Chandrasekharan 2002; Chandrasekharan 2004; Lee 2007).
Why it is important to do this review
Although many clinical trials have evaluated the efficacy and safety of IV formulations of paracetamol for postoperative pain management, published systematic reviews have studied limited populations (Jebaraj 2013), or have analyzed only selected outcomes (Apfel 2013). While it is assumed that IV paracetamol and IV propacetamol would have similar safety profiles to oral paracetamol, evidence specific to parenteral administration from both case reports and drug use evaluations has demonstrated that patients are at increased risk of toxicity if IV dosing is not properly adjusted (NHS 2010). We therefore performed a systematic review to assess the efficacy and safety of IV formulations of paracetamol for the treatment of postoperative pain in both adults and children.
Objectives
To assess the efficacy and safety of IV formulations of paracetamol for the treatment of postoperative pain in both adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
We included:
blinded or unblinded RCTs;
studies that evaluated the analgesic efficacy of IV paracetamol or IV propacetamol for the treatment of postoperative pain, following any type of surgery, in children and in adults;
single dose or multiple‐dose studies (the latter were included only if the studies provided data for four to six hours after first dose administration);
studies that used placebo or another active treatment (e.g., NSAIDs, opioids) as control;
studies in which the interventions were administered intraoperatively or postoperatively alone or in addition to other analgesic treatment;
studies in which participants self reported pain relief or pain intensity;
studies that reported the outcomes of interest at four to six hours after administration of the study interventions.
Exclusion criteria
We excluded non‐randomized and cross‐over studies. We excluded the latter because the intensity of postoperative pain often changes rapidly. We excluded studies with less than four hours of follow‐up after IV propacetamol or IV paracetamol administration and studies in which pain was not self reported. We excluded multiple‐dose studies that did not separately report data for the first four to six hours after IV paracetamol or IV propacetamol administration since the review is restricted to this time period. For the updated review, we excluded studies that administered interventions as continuous infusions and studies with fewer than 10 participants in each arm. Conversely, we no longer excluded studies where all of the arms also received a NSAID postoperatively (assuming the same regimen in each arm), as we decided that this design reflects the current clinical practice of multimodal analgesia.
Types of participants
We included studies that evaluated children or adults with postoperative pain following any kind of surgery, including dental, who were able to self report pain intensity or pain relief.
Types of interventions
Intravenous paracetamol or IV propacetamol for postoperative pain relief and control interventions, either placebo or another analgesic (e.g., NSAIDS or opioids). Control interventions were subject to the same inclusion and exclusion criteria as for paracetamol and propacetamol; other than that they could be administered via any route.
The interventions had to be administered within the last 30 minutes before the end of surgery (i.e., not preoperatively or at induction of anesthesia), in the immediate postoperative period or at any time within the first three postoperative days.
Types of outcome measures
We assessed primary and secondary outcomes four to six hours after first administration of IV paracetamol or IV propacetamol.
Primary outcomes
Pain relief: number of participants experiencing at least 50% of maximum pain relief over four or six hours postintervention.
Pain intensity: we extracted mean pain intensity over both the four‐ and six‐hour postintervention periods in each treatment arm and their corresponding standard deviations (SD), and in turn calculated the mean pain difference between groups.
We accepted the use of any categorical or numerical pain intensity or pain relief scale.
Secondary outcomes
Time to achieve 50% pain relief: we intended to extract the mean time to achieve this degree of relief in each treatment arm and the corresponding SD and calculate the mean time difference between groups. However, no study reported these data, either in our original review or in our update.
Number of participants requiring rescue medication: we extracted the proportion of participants who received additional analgesic medication during the four to six hours after administration of the study drugs in each treatment arm and calculated the risk ratios (RRs) of receiving rescue medication and the number needed to treat to prevent (NNTp) re‐medication.
Time to rescue medication: we extracted the mean time to requiring rescue medication in each treatment arm and the corresponding SD, and calculated the mean time difference between groups.
Opioid consumption: in studies in which coadministration of opioids (including patient‐controlled analgesia (PCA)) was allowed, we extracted the mean opioid consumption (in mg) over both four hours and six hours postintervention in each treatment arm and the corresponding SD. We converted opioid requirements into IV morphine‐equivalents, using commonly used and widely accepted opioid conversion tables (Jacox 1994). To determine the opioid sparing effect of an intervention we calculated the mean difference in opioid requirements between treatment arms.
Patients' global evaluation of therapy: we used dichotomous information derived from categorical global evaluations (number of participants reporting the top two categories, e.g., good/satisfied or excellent/very satisfied versus all lower categories) to calculate RRs. For VAS ratings, we compared the means of each intervention.
Adverse events (AEs): we noted validated scales when used. When the only available information was subjective or observational for specific side effects (such as nausea or vomiting) or determined through asking general questions or merely noting the presence or absence of side effects, without any attempt at quantification, we documented these outcomes as such. We noted withdrawals or dropouts when adequately described, and if information was reported further characterized these as due to either lack of efficacy or to AEs. In addition, we extracted the number of participants reporting pain due to infusion of the study medication. We intended to extract mean pain intensity with infusion in each treatment arm and their corresponding SDs, and calculate the mean pain difference between groups. However, there were insufficient data for this outcome. For our updated review, we excluded the following AEs that had been included in our 2011 review: headache, vertigo/dizziness, fatigue, fever, gastrointestinal disorders, heart rate, malaise, bleeding, liver function test abnormalities, and hypotension. We excluded these analyses as each event occurred too infrequently for meaningful analysis. Last, in our updated review we added an analysis of the number of participants experiencing a serious adverse event.
Search methods for identification of studies
This search was run for the original review on 10 May 2010 and subsequent searches have been run on 16 February 2016.
Electronic searches
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1);
MEDLINE (OVID) (1950 to 16 February 2016);
EMBASE (1980 to 16 February 2016);
LILACS (1982 to 2016).
Both the original and updated search strategies for MEDLINE, CENTRAL, LILACS, and EMBASE can be found in Appendix 1; Appendix 2; Appendix 3; and Appendix 4, respectively. We did not apply any language restriction.
Searching other resources
We checked the reference lists of retrieved articles. We also checked the clinical trials registry http://www.clinicaltrials.gov in February 2016. Lastly, in May 2011, we contacted the US manufacturer of IV paracetamol (Cadence Pharmaceuticals at that time) for its internal reference list of studies. We did not re‐contact this manufacturer for the 2016 update as it was acquired by Mallinckrodt plc.
Data collection and analysis
Selection of studies
Two independent review authors screened each article identified in the electronic searches. We retrieved in full studies whose title or abstract referred to the administration of any formulation of IV paracetamol or IV propacetamol for postoperative analgesia, in both children and adults.
Data extraction and management
We performed data extraction and analysis in duplicate. We resolved any disagreement through discussion. If disagreement persisted, we sought agreement via consultation with a third review author. When studies did not provide sufficient data, we contacted study authors where possible. We performed all meta‐analyses using Review Manager 5.3 software (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of all included studies in this review using a domain‐based evaluation, outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following for each study:
Random sequence generation (selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g., random number table; computer random number generator); high risk of bias (any non‐random process, e.g., odd or even date of birth; hospital or clinic record number); unclear risk of bias (method not adequately described). We excluded studies that were not randomized.
Allocation concealment (selection bias). The method used to conceal allocation to interventions prior to assignment assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g., telephone or central randomization; consecutively numbered, sealed, opaque envelopes); high risk of bias (open random allocation; unsealed or non‐opaque envelopes); unclear risk of bias (method not adequately described).
Blinding (detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they stated that they were blinded and described the method used to achieve blinding (e.g., identical packaging; matched in appearance and color), or as unclear risk if they stated that they were blinded but did not provide an adequate description of how it was achieved. We included unblinded studies and assessed them as having a high risk of bias.
Incomplete outcome data (attrition bias). We assessed the methods used to handle missing outcome data as: low risk of bias (e.g., no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups); high risk of bias (reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization); unclear risk of bias (method not adequately described).
Selective reporting (reporting bias). We assessed the reporting of results as: low risk of bias (e.g., the study protocol was available and all of the study’s pre‐specified outcomes that were of interest in the review were reported in the pre‐specified way; the study protocol was not available but it is clear that the published reports included all expected outcomes, including those that were pre‐specified); high risk of bias (e.g., not all of the study’s pre‐specified primary outcomes were reported; one or more primary outcomes was reported using measurements, analysis methods or subsets of the data that were not pre‐specified); unclear risk of bias (insufficient information to permit judgement of low risk or high risk).
For our updated review, we also assessed risk of bias due to sample size: we considered studies to have a low risk of bias if they had ≥ 200 participants per treatment arm, an unclear risk if they had 50 to 199 participants per treatment arm, and a high risk if they had < 50 participants per treatment arm (AUREF 2012).
Measures of treatment effect
Dichotomous data
We used discrete events, such as the number of participants requiring rescue analgesia or with adverse events (AEs), to calculate the risk difference and/or risk ratio using Review Manager 5.3 software (RevMan 2014). When a statistically significant risk difference existed between interventions, we derived the number needed to treat for one additional beneficial outcome (NNT) or one additional harmful outcome (NNH) (Cook 1995). Additionally, we presented dichotomous outcomes in terms of both raw numbers and percentages of participants in each study arm benefiting from therapy or suffering AEs.
Continuous data
We undertook meta‐analyses when comparable data were available from continuous outcomes, such as pain intensity, analgesic consumption in mg of morphine equivalents, or intensity of a specific adverse event, using mean differences (MD).
Unit of analysis issues
Randomization was by individual participant. When two active treatment arms were compared with a placebo arm within the same meta‐analysis, we avoided double counting of participants in the placebo arm by splitting the total number between the active arms. This was an issue with only two studies (Moller 2005a; Sinatra 2005).
Dealing with missing data
Wherever possible we used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomized, received at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement. We also looked for information about methods of imputation for missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity by visually examining forest plots and quantified it by using the I2 statistic. The I2 statistic is a reliable and robust test to quantify heterogeneity, since it does not depend on the number of trials or on the between‐study variance. I2 measures the extent of inconsistency among studies' results, and can be interpreted as the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error. An I2 value of greater than 50% is considered to indicate substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess the impact of reporting bias we considered the number of additional participants needed in studies with zero effect (relative benefit of one) required to change the NNT for all statistically significant outcomes to an unacceptably high level (in this case the arbitrary NNT of 10) (Moore 2008). Where this number was less than 400 (equivalent to four studies with 100 participants per comparison, or 50 participants per group), we considered the results to be susceptible to publication bias and therefore unreliable (low quality evidence). We also attempted to mitigate the potential for publication bias by searching the website http://www.clinicaltrials.gov and by contacting the manufacturer of IV paracetamol for an internal reference list of completed studies.
Data synthesis
If not reported, we calculated the theoretical proportion of participants achieving at least 50% pain relief by extracting or calculating total pain relief (TOTPAR) or summed pain intensity difference (SPID) using either visual analog scale (VAS) or categorical data, and calculating their corresponding percentage of theoretical maximum TOTPAR and SPID using the formulas derived by Cooper and Moore (Cooper 1991; Moore 1997a; Moore 1997b; Appendix 5). If data were only presented graphically, we extracted them using xyExtract Graph Digitizer software (v 3.1, Wilton Pereira da Silva, Brazil) or WebPlotDigitizer software (Version 3.7, Ankit Rohatgi, http://arohatgi.info/WebPlotDigitizer). From these outcomes we calculated the number needed to treat to benefit (NNT) for at least 50% pain relief over the four‐ and six‐hour periods.
We employed a fixed‐effect model (Deeks 2011), using Review Manager 5.3, to combine outcomes data at comparable time points.
We included 'Summary of findings' tables as set out in the PaPaS author guide (AUREF 2012) and recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 4.6.6 (Higgins 2011). The 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7) include the outcomes of pain relief, pain intensity, number of participants requiring rescue medication, opioid consumption, and number of participants with occurrences of vomiting.
We assessed the overall quality of the evidence for each outcome using the GRADE system (GRADEpro GDT 2015), and presented this in the 'Summary of findings' tables. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grade of evidence:
High = further research is very unlikely to change our confidence in the estimate of effect.
Moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low = any estimate of effect is very uncertain.
We decreased GRADE if:
there was a serious (‐1) or very serious (‐2) limitation to study quality;
there was important inconsistency (‐1);
there was some (‐1) or major (‐2) uncertainty about directness;
there were imprecise or sparse data (‐1);
there was high probability of reporting bias (‐1).
Subgroup analysis and investigation of heterogeneity
Where possible we performed the following subgroup analyses in an attempt to explain heterogeneity:
IV paracetamol and IV propacetamol;
type of surgery.
Sensitivity analysis
We performed sensitivity analyses to investigate the effect of various study characteristics on the primary efficacy outcome by eliminating the following:
Studies enrolling children, defined as individuals less than 18 years of age.
Non‐blinded studies.
Studies with atypical designs. Most studies reporting data for our primary outcome administered interventions at the first report of moderate‐to‐severe pain postoperatively. A minority of studies enrolled participants on the day after surgery (Hynes 2006; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65+; Sinatra 2005; Wininger 2010; Zhou 2001), and a single study administered interventions immediately post‐surgery, but regardless of pain intensity (Koppert 2006).
We also performed a sensitivity analysis using a random‐effects model instead of our original fixed‐effect model.
Results
Description of studies
See: 'Characteristics of included studies', 'Characteristics of excluded studies', and 'Characteristics of studies awaiting classification' tables.
Results of the search
Our 2010 literature search yielded 366 references from CENTRAL, 292 references from MEDLINE, 483 studies from EMBASE, 47 from LILACS, and 43 from http://www.clinicaltrials.gov. None of the ongoing studies listed on clinicaltrials.gov met our inclusion criteria. Review of the abstracts identified 56 potentially relevant studies of which we included 36 in the analysis. The literature search covering 2010 to 2016 yielded an additional 1661 citations (568 from CENTRAL; 341 from MEDLINE; 745 from EMBASE; and 7 from LILACS), of which we selected 62 for possible inclusion (Figure 1). Eight studies identified from the 62 citations are awaiting classification. One of the 62 citations provided additional data for a study included in our 2011 review (Sinatra 2005). In addition to the new citations, we considered two studies that did not meet the criteria in our original review (due to all arms also receiving a NSAID), but potentially met the updated criteria, for inclusion (Salonen 2009; Uvarov 2008). We considered two studies from the internal reference list of Cadence Pharmaceuticals for inclusion. Finally, we discovered one potentially eligible study in the reference section of an included study (Koppert 2006). We found no completed or ongoing studies on clinicaltrials.gov, other than those already included from our database search.
1.
2016 Study flow diagram.
Included studies
We included 75 studies (36 from the original review and 39 from the interim) enrolling a total of 7200 participants in the review (see 'Characteristics of included studies' table). One of the 75 studies was conducted in Africa (Atef 2008), one in Australasia (Paech 2014), 15 in Asia (Chen 2011; Faiz 2014; Kamath 2014; Khajavi 2007; Khalili 2013; Khan 2007; Lee 2010; Ma 2003; Maghsoudi 2014; Mitra 2012; Mowafi 2012; Omar 2011; Sanyal 2014; Shimia 2014; Siddik 2001), and seven in the United States (Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65‐; Jahr 2012 Study 3, 65+; Sinatra 2005; Wininger 2010; Zhou 2001). The remaining 51 studies were conducted in Europe. Of note, 16 of the latter were conducted in Turkey, 14 of which we included in the updated review.
Enrollment ranged from 27 participants (Jahr 2012 Study 3, 65‐) to 550 participants (Aubrun 2003). Similarly, IV paracetamol/propacetamol arms ranged from 12 (Landwehr 2005) to 275 participants (Aubrun 2003).
Fifty studies administered IV paracetamol (Abdulla 2012a; Abdulla 2012b; Akarsu 2010; Akil 2014; Arici 2009; Arslan 2011; Arslan 2013; Atef 2008; Brodner 2011; Cakan 2008; Eremenko 2008; Faiz 2014; Hiller 2012; Inal 2006; Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65‐; Jahr 2012 Study 3, 65+; Juhl 2006; Kamath 2014; Kara 2010; Karaman 2010; Kemppainen 2006; Khalili 2013; Khan 2007; Kilicaslan 2010; Koppert 2006; Korkmaz 2010; Landwehr 2005; Lee 2010; Maghsoudi 2014; Marty 2005; Mitra 2012; Moller 2005a; Mowafi 2012; Ohnesorge 2009; Omar 2011; Oncul 2011; Oreskovic 2014; Paech 2014; Salonen 2009; Sanyal 2014; Shimia 2014; Sinatra 2005; Tiippana 2008; Togrul 2011; Tunali 2013; Tuncel 2012; Unal 2013; Wininger 2010), and 28 administered IV propacetamol (Aubrun 2003; Beaussier 2005; Chen 2011; Dejonckheere 2001; Delbos 1995; Farkas 1992; Fletcher 1997; Hahn 2003; Hans 1993; Hiller 2004; Hynes 2006; Jarde 1997; Kampe 2006; Khajavi 2007; Lahtinen 2002; Leykin 2008; Ma 2003; Marty 2005; Mimoz 2001; Moller 2005a; Moller 2005b; Peduto 1998; Siddik 2001; Sinatra 2005; Van Aken 2004; Varrassi 1999; Vuilleumier 1998; Zhou 2001). Three studies administered both (Marty 2005; Moller 2005a; Sinatra 2005). Of note, only one new study in our updated review assessed propacetamol (Chen 2011). This study did not contribute data to any of our analyses; therefore, results of the propacetamol analyses are unchanged (except where changes in methodology led to minor changes in data analysis).
All but nine studies administered the equivalent of 1 g paracetamol. The remaining studies administered 30 mg/kg propacetamol (Vuilleumier 1998), 10 mg/kg, 20 mg/kg or 40 mg/kg propacetamol (Hahn 2003), 15 mg/kg of IV paracetamol (Faiz 2014; Khalili 2013), 30 mg/kg of IV paracetamol (Hiller 2012), 2 g IV paracetamol (Paech 2014), a 2 g IV paracetamol arm in addition to 1 g (Juhl 2006; Salonen 2009), and a 650 mg IV paracetamol arm in addition to 1 g (Wininger 2010). In studies where there were two different paracetamol/propacetamol arms, we chose the arm administering the equivalent of 1 g of IV paracetamol for analysis.
The types of surgery performed included orthopedic (Delbos 1995; Hynes 2006; Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65‐; Jahr 2012 Study 3, 65+; Jarde 1997; Khalili 2013; Khan 2007; Koppert 2006; Oreskovic 2014; Peduto 1998; Sinatra 2005; Zhou 2001); obstetric/gynecologic (Akarsu 2010; Akil 2014; Arici 2009; Faiz 2014; Hahn 2003; Inal 2006; Kamath 2014; Kilicaslan 2010; Marty 2005; Mitra 2012; Omar 2011; Paech 2014; Sanyal 2014; Siddik 2001; Unal 2013; Varrassi 1999); eye/ear/nose and throat (Atef 2008; Hiller 2004; Karaman 2010; Kemppainen 2006; Landwehr 2005; Leykin 2008; Salonen 2009; Togrul 2011); back (Cakan 2008; Chen 2011; Fletcher 1997; Hans 1993; Hiller 2012; Korkmaz 2010; Shimia 2014; Tunali 2013); cardiovascular (Eremenko 2008; Farkas 1992; Lahtinen 2002); dental (Juhl 2006; Moller 2005a; Moller 2005b; Oncul 2011; Van Aken 2004); general (Abdulla 2012a; Abdulla 2012b; Arslan 2011; Arslan 2013; Beaussier 2005; Dejonckheere 2001; Kampe 2006; Kara 2010; Lee 2010; Maghsoudi 2014; Mimoz 2001; Mowafi 2012; Ohnesorge 2009; Tiippana 2008; Tuncel 2012; Wininger 2010); transplant (Khajavi 2007); and mixed (Aubrun 2003; Brodner 2011; Ma 2003; Vuilleumier 1998).
Three studies evaluated adults and adolescents together, with the youngest participant being 13 years of age (Atef 2008; Hiller 2004; Van Aken 2004). One study assessed children and adolescents (Hiller 2012). The remainder evaluated only adults. Most studies performed exclusively in children did not meet the inclusion criteria, primarily because pain was not patient‐reported.
Studies fell broadly into two designs: (1) those in which the intervention was automatically administered shortly before or immediately after the end of surgery and the primary outcome was opioid consumption (usually administered via PCA, but occasionally as on‐demand injections); or (2) those in which the intervention was administered only after a participant reported moderate‐to‐severe pain postsurgically, in which case the primary outcome was pain relief/pain intensity difference. The latter studies contributed the majority of data for our primary outcome of at least 50% pain relief (reported either in terms of pain relief or pain intensity) over either four or six hours, or both (Akarsu 2010; Farkas 1992; Hynes 2006; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65+; Jarde 1997; Juhl 2006; Marty 2005; Moller 2005a; Moller 2005b; Sinatra 2005; Van Aken 2004; Wininger 2010; Zhou 2001). However, three studies employing the former design (automatically administered interventions immediately post surgery, regardless of pain intensity) also reported data that we were able to use for our primary outcome (Akil 2014; Koppert 2006; Ma 2003).
Fourteen studies did not present efficacy data in a format that we were able to meta‐analyze, e.g., presenting data without standard deviations (Arici 2009; Atef 2008; Hiller 2004; Hiller 2012; Inal 2006; Kamath 2014; Kara 2010; Khajavi 2007; Khan 2007; Mitra 2012; Mowafi 2012; Omar 2011; Oncul 2011; Oreskovic 2014). In six studies we were unable to analyze either efficacy or safety data for similar reasons (Chen 2011; Eremenko 2008; Hahn 2003; Ohnesorge 2009; Sanyal 2014; Tuncel 2012).
Excluded studies
Forty‐one studies did not meet the inclusion criteria (see 'Characteristics of excluded studies' table). Reasons for exclusion included: pain assessments that were not patient‐reported; time periods that were not within those specified in our inclusion criteria; propacetamol being administered intramuscularly; IV paracetamol being administered via a continuous infusion; absence of pain or analgesic outcomes; comparisons of procedures rather than interventions; pre‐emptive administration of intervention or administration more than 30 minutes before the end of surgery; non‐randomization; all arms receiving IV paracetamol/IV propacetamol; or control groups not receiving either an active control or placebo.
Studies awaiting classification
For one study we were unable to retrieve the full article from any source (Rasheed 2007). This trial has been added to Studies awaiting classification. Seven additional studies identified in our 2016 update also await classification (Atashkhoyi 2014; Dawoodi 2014; Jabalameli 2014; Majumdar 2014; Pekmezci 2014; Ritchie 2015; Singla 2015).
Risk of bias in included studies
Our findings are summarized in Figure 2 and Figure 3.
2.
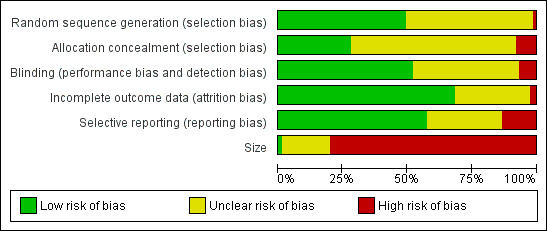
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
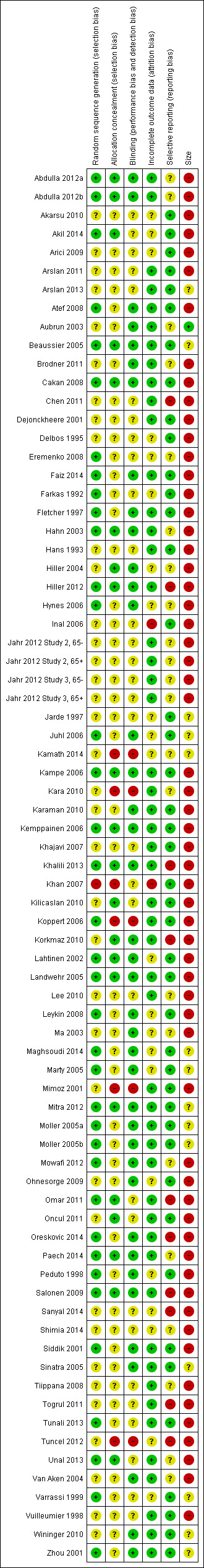
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirty‐seven studies used adequate randomization methods, either by using tables of random numbers, or by computer‐generated randomization. In 37 studies the method of randomization was unclear, usually because there was no description of the methods used. One study did not employ adequate randomization methods (Khan 2007). Participants were assigned to each intervention via the last digit of their medical record number, with odd receiving paracetamol and even receiving morphine. Fewer studies described attempts at allocation concealment. In 48 studies concealment was unclear as there was no description of any method used. Twenty‐one studies did employ adequate concealment methods. We assessed six studies as employing inadequate methods to conceal allocation, either because they were unblinded (Kamath 2014; Kara 2010; Koppert 2006; Mimoz 2001; Tuncel 2012), or because allocation could be deduced based on inadequate randomization methodology (Khan 2007).
Blinding
Thirty‐nine studies employed adequate methods to ensure blinding. Interventions were prepared by a party not directly involved in the study. Papers either stated that the interventions appeared identical, or where that was not possible, a double‐ or triple‐dummy technique was used. For the 31 studies in which the adequacy of blinding was unclear, most made some description of their method, e.g., a third party prepared the interventions, but did not provide enough information that we could be certain (e.g., no mention of whether treatments appeared identical). Five studies were reported or assumed to be unblinded, or were not double‐blinded (Kamath 2014; Kara 2010; Koppert 2006; Mimoz 2001; Tuncel 2012).
Incomplete outcome data
Generally, due to the acute nature of the studies, numbers of participants withdrawn were low and missing data minimal. We judged 51 studies to have a low risk of bias and 22 studies to have an unclear risk, due to not describing imputation methods for missing data, because they employed last observation carried forward for imputation, or because they only analyzed data from participants completing the study. While the latter method may be considered as generating a high risk of bias, the numbers of participants withdrawing were low, evenly balanced between groups, and the reasons for withdrawal generally unrelated to the true outcome. We assessed two studies as having a high risk of bias as it was unclear how many participants completed the studies and because pain data were presented without standard deviations (Inal 2006; Khan 2007).
Selective reporting
While we cannot rule out the possibility that data were eliminated from both the Methods and Results section (i.e., data that were part of the original study were not reported), the homogeneity of outcomes amongst studies suggests that data were not withheld in this manner. We judged 43 studies to have a low risk of bias, in that all of the outcomes mentioned in the Methods section were reported in full in the Results section and we judged 22 to have an unclear risk, primarily because they reported some secondary outcomes as not statistically significant, but did not present data. We assessed 10 studies, all from the updated search, as having a high risk of selective reporting, due to not reporting results for all of the outcomes described in the Methods section, not reporting AEs, or only displaying results graphically (Chen 2011; Hiller 2012; Khalili 2013; Korkmaz 2010; Omar 2011; Oreskovic 2014; Salonen 2009; Sanyal 2014; Togrul 2011; Tuncel 2012).
Other potential sources of bias
Study size
Only one study enrolled at least 200 participants in each arm of the study. Fourteen studies enrolled 50 to 199 participants per treatment arm (unclear risk of bias) and 60 enrolled fewer than 50 per treatment arm (high risk of bias).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
’Summary of findings’ tables are presented for the following outcomes: proportion of participants with > 50% pain relief at four hours; proportion of participants with > 50% pain relief at six hours; mean pain intensity over a four‐hour period; mean pain intensity over a six‐hour period; proportion of participants receiving additional analgesia; mean opioid consumption over six hours; proportion of participants vomiting (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7, respectively). Quality of evidence is reported with these results, based on GRADE criteria.
Number of participants experiencing at least 50% of maximum pain relief at four hours
See: Table 1. Of the various comparisons below, we only assessed the analysis that combined studies of paracetamol or propacetamol versus placebo as high quality. We downgraded other comparisons to moderate or lower, based on factors such as unexplained heterogeneity, small numbers of events, heterogeneity of comparators, and imprecision of results.
Intravenous (IV) paracetamol or propacetamol versus placebo
See Analysis 1.1 and Figure 4.
1.1. Analysis.
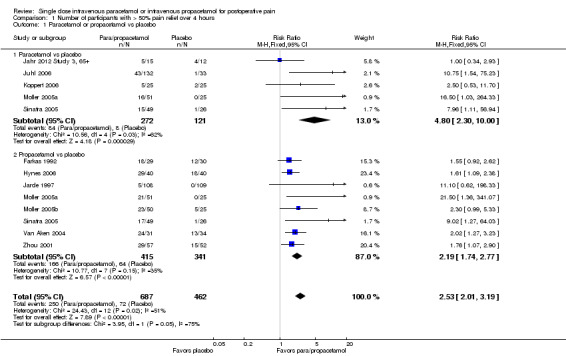
Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
4.
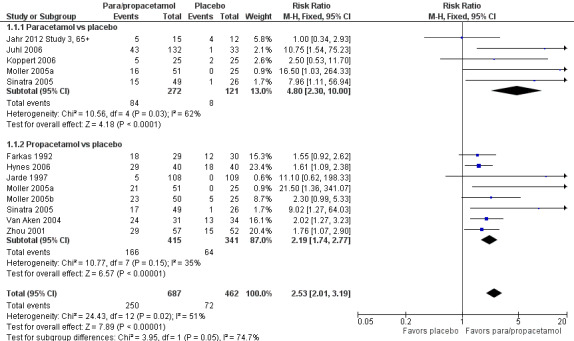
Forest plot of comparison: 1 Number of participants with > 50% pain relief over 4 hours, outcome: 1.1 Propacetamol or paracetamol versus placebo.
Eleven studies provided data (Farkas 1992; Hynes 2006; Jahr 2012 Study 3, 65+; Jarde 1997; Juhl 2006; Koppert 2006; Moller 2005a; Moller 2005b; Sinatra 2005; Van Aken 2004; Zhou 2001): five compared IV paracetamol versus placebo; eight compared IV propacetamol versus placebo (two studies reported both). There were 272 participants treated with IV paracetamol, 415 treated with propacetamol, and 462 treated with placebo.
The proportion of participants experiencing at least 50% pain relief over four hours with IV paracetamol was 31% (84/272) and with propacetamol was 40% (166/415). Combining data from both interventions, 36% had at least 50% pain relief.
The proportion of participants experiencing at least 50% pain relief over four hours with placebo was 16% (72/462).
The risk ratio (RR) for IV paracetamol versus placebo for at least 50% pain relief was 4.8 (95% confidence interval (CI) 2.3 to 10.0) and for propacetamol versus placebo was 2.2 (95% CI 1.7 to 2.8). Combining both interventions, the RR versus placebo was 2.5 (95% CI 2.0 to 3.2).
The derived NNT for at least 50% pain relief over four hours was 5 (95% CI 3.2 to 5.9), 5 (95% CI 3.7 to 5.9), and 5 (95% CI 3.7 to 5.6) for IV paracetamol, propacetamol, and the combined data, respectively. For every five participants treated with IV propacetamol or IV paracetamol one person would experience at least 50% pain relief who would not have had this with placebo.
Based on our assessment of the risk of publication bias (Table 8) these results are reliable and not subject to potential publication bias.
One of the included studies allowed rescue dosing (Koppert 2006). We performed a post hoc sensitivity analysis with this study removed. It had minimal effect on the size of effect.
1. Publication bias risk assessment: efficacy outcomes.
| Comparison/outcome | Number of studies | Number of participants | n with outcome/total | % with outcome | Risk difference | NNT | Susceptibility to publication bias | ||
| Active | Control | Active | Control | ||||||
| Comparison 1. Number of participants with > 50% pain relief over 4 hours | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 11 | 1149 | 250/687 | 72/462 | 36 | 16 | 0.23 (0.18 to 0.27) | 5 | 1492 |
| 1.1 Paracetamol vs placebo | 5 | 393 | 84/272 | 8/121 | 31 | 7 | 0.24 (0.17 to 0.31) | 5 | 549 |
| 1.2 Propacetamol vs placebo | 8 | 756 | 166/415 | 64/341 | 40 | 19 | 0.22 (0.17 to 0.27) | 5 | 906 |
| Comparison 2. Number of participants with > 50% pain relief over 6 hours | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 10 | 1143 | 200/708 | 42/435 | 28 | 10 | 0.18 (0.14 to 0.22) | 6 | 913 |
| 1.1 Paracetamol vs placebo | 6 | 532 | 109/364 | 14/168 | 30 | 8 | 0.22 (0.16 to 0.29) | 5 | 637 |
| 1.2 Propacetamol vs placebo | 6 | 611 | 91/344 | 28/267 | 26 | 10 | 0.15 (0.10 0.20) | 7 | 305 |
| 2. Paracetamol or propacetamol vs NSAIDs | 5 | 355 | 95/192 | 103/163 | 50 | 63 | ‐0.13 (‐0.23 to ‐0.03) | 8* | 107 |
| Comparison 5. Number of participants requiring rescue medication | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 9 | 859 | 295/476 | 314/383 | 62 | 82 | ‐0.25 (‐0.30 to ‐0.19) | 4 | 1289 |
| 1.1 Paracetamol vs placebo | 6 | 655 | 267/376 | 249/279 | 71 | 89 | ‐0.22 (‐0.28 to ‐0.17) | 5 | 785 |
| 1.2 Propacetamol vs placebo | 3 | 204 | 31/100 | 65/104 | 31 | 62 | ‐0.32 (‐0.44 to ‐0.19) | 4 | 449 |
| Comparison 9. Global evaluation rated as good/satisfied or excellent/very satisfied | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 16 | 2015 | 787/1100 | 529/915 | 72 | 58 | 0.19 (0.15 to 0.23) | 6 | 1816 |
| 1.1 Paracetamol vs placebo | 9 | 876 | 355/508 | 207/368 | 70 | 56 | 0.24 (0.19 to 0.29) | 5 | 1225 |
| 1.2 Propacetamol vs placebo | 9 | 1139 | 432/592 | 322/547 | 73 | 59 | 0.15 (0.10 to 0.21) | 7 | 569 |
| 2. Paracetamol or propacetamol vs NSAIDs | 11 | 795 | 306/410 | 313/385 | 75 | 81 | ‐0.06 (‐0.11 to ‐4.81) | 17* | NNT > 10 |
*NSAID superior
IV paracetamol or propacetamol versus nonsteroidal anti‐inflammatory drugs (NSAIDs)
See Analysis 1.2.
1.2. Analysis.
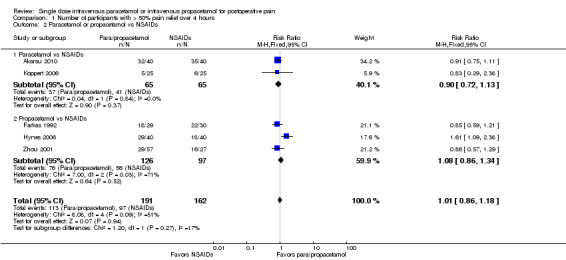
Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
Two studies provided analyzable data for IV paracetamol versus NSAIDs (Akarsu 2010; Koppert 2006) (130 participants). For IV propacetamol, three studies (223 participants) provided data (Farkas 1992; Hynes 2006; Zhou 2001).
The proportion of participants experiencing at least 50% pain relief over four hours with IV paracetamol was 57% (37/65) and with propacetamol was 60% (76/126).
The proportion of participants experiencing at least 50% pain relief over four hours with NSAIDs was 60% (97/162).
There was not a statistically significant difference between participants receiving IV paracetamol and/or propacetamol and those receiving NSAIDs.
IV propacetamol versus opioids
See Analysis 1.3.
1.3. Analysis.
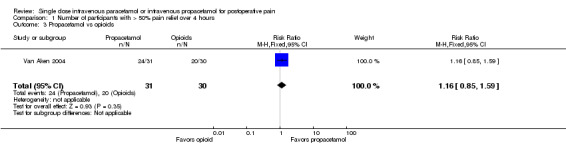
Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 3 Propacetamol vs opioids.
No studies provided analyzable data for IV paracetamol versus opioids. Only one study compared IV propacetamol versus opioids (Van Aken 2004, 61 participants). This single study did not show a statistically significant difference between IV propacetamol and morphine.
IV propacetamol versus IV paracetamol
See Analysis 1.4.
1.4. Analysis.
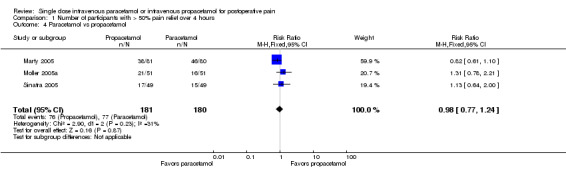
Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 4 Paracetamol vs propacetamol.
Three studies provided data from head‐to‐head studies, with a total of 361 participants. The proportion of participants achieving at least 50% pain relief over four hours was 42% (76/181) in the IV propacetamol arms and 43% (77/180) in those treated with IV paracetamol. There was not a statistically significant difference between the interventions.
Number of participants experiencing at least 50% of maximum pain relief at six hours
Outcomes measured over six hours produced similar results to those measured over four hours, but with some diminution of analgesic effect. We assessed the quality of these data as moderate or lower, based on similar limitations as described in the outcomes measured over four hours (see Table 2). In addition, some of the comparisons were susceptible to publication bias (Assessment of reporting biases).
IV paracetamol or propacetamol versus placebo
See Analysis 2.1 and Figure 5.
2.1. Analysis.
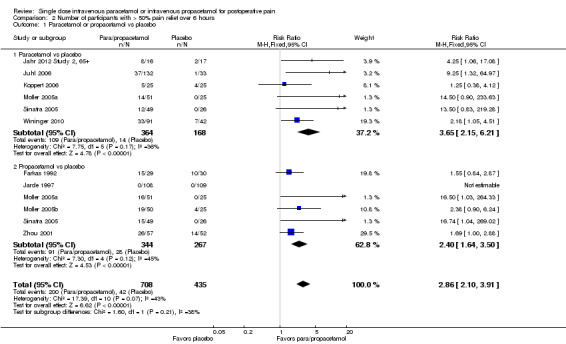
Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
5.
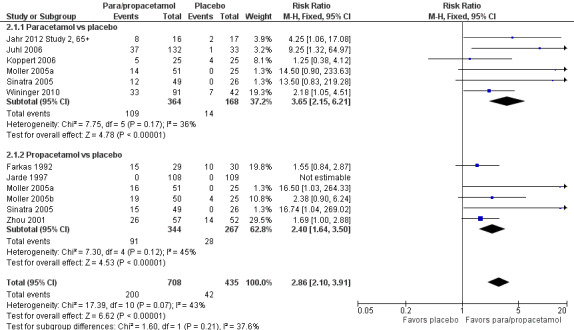
Forest plot of comparison: 2 Number of participants with > 50% pain relief over 6 hours, outcome: 2.1 Propacetamol or paracetamol versus placebo.
Ten studies provided data ‐ six compared IV paracetamol versus placebo, six compared propacetamol versus placebo (two studies reported both). There were 364 participants treated with IV paracetamol, 344 treated with IV propacetamol, and 435 treated with placebo.
The proportion of participants experiencing at least 50% pain relief over six hours with IV paracetamol was 30% (109/364) and with propacetamol was 26% (91/344). Combining data from both interventions, 28% had at least 50% pain relief.
The proportion of participants experiencing at least 50% pain relief over six hours with placebo was 10% (42/435).
The RR for IV paracetamol versus placebo was 3.7 (95% CI 2.2 to 6.2) and for propacetamol versus placebo was 2.4 (95% CI 1.6 to 3.5). Combining data from both interventions, the RR versus placebo was 2.9 (95% CI 2.1 to 3.9).
The derived NNT for at least 50% pain relief over six hours was 5 (95% CI 3.5 to 6.2), 7 (95% CI 5.0 to 10.0), and 6 (95% CI 4.6 to 7.1) for IV paracetamol, propacetamol, and their data combined, respectively. For every five participants treated with IV paracetamol and every seven treated with propacetamol one would experience at least 50% pain relief who would not have done so with placebo.
We judged the results from propacetamol versus placebo to have high susceptibility to publication bias (Table 8).
Sensitivity analysis with removal of Koppert 2006 led to a slight increase in RR for IV paracetamol versus placebo for participants experiencing at least 50% pain relief over six hours, but made little difference to the combined estimate of IV paracetamol and propacetamol. Removal of studies with atypical design (Jahr 2012 Study 2, 65+; Sinatra 2005; Wininger 2010) increased the RR (i.e., greater efficacy) for IV paracetamol (4.7, 95% CI 1.8 to 2.2), but reduced the RR for propacetamol (2.1, 95% CI 1.4 to 3.0).
IV paracetamol or propacetamol versus NSAIDs
See Analysis 2.2.
2.2. Analysis.
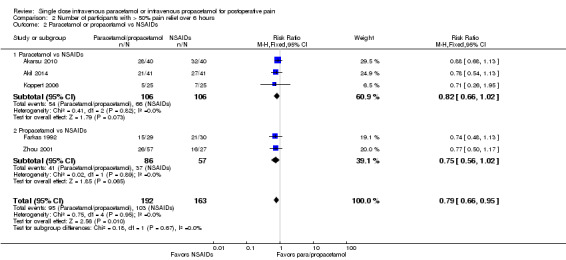
Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
Three studies with 212 participants provided data for IV paracetamol versus NSAIDs (Akarsu 2010; Akil 2014; Koppert 2006). For IV propacetamol, two studies with 143 participant provided data (Farkas 1992; Zhou 2001).
The proportion of participants experiencing at least 50% pain relief over six hours with IV paracetamol was 51% (54/106), with propacetamol was 48% (41/86), and with NSAIDs was 63% (103/163). This difference was statistically significant when data for IV paracetamol and propacetamol were combined, with a RR of 0.8, translating to a NNT of 8 (95% CI 4.3 to 33) in favor of NSAIDs. However, we assessed the data as being highly susceptible to publication bias, most likely due to the low overall numbers of participants (Table 8).
IV propacetamol versus opioids
See Analysis 2.3.
2.3. Analysis.
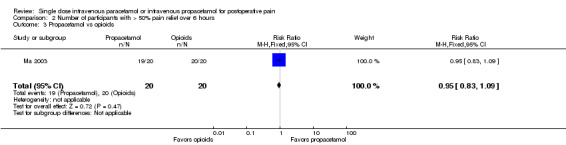
Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 3 Propacetamol vs opioids.
No studies provided data for IV paracetamol versus opioids. Only one study, enrolling 40 participants, compared IV propacetamol versus opioids (Ma 2003). This single study did not show a statistically significant difference between IV propacetamol and pethidine (meperidine).
Propacetamol versus IV paracetamol
See Analysis 2.4
2.4. Analysis.
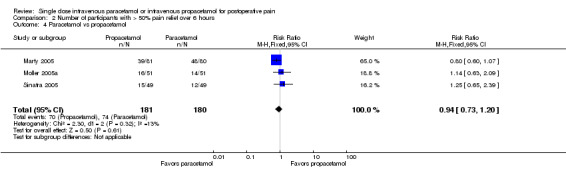
Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 4 Paracetamol vs propacetamol.
Three studies provided data with a total of 361 participants. The proportion of participants achieving at least 50% pain relief over six hours was 39% (70/181) in the IV propacetamol participants and 41% (74/180) in those treated with IV paracetamol. There was not a statistically significant difference between the interventions.
Pain intensity at four hours
No studies employing propacetamol contributed data to our analysis of pain intensity at either four hours or six hours. No included studies compared pain intensity at either time point with IV paracetamol versus opioids. One study enrolling 80 participants compared IV paracetamol with ketamine and reported a statistically significantly lower mean pain score in the paracetamol group (‐12, 95% CI ‐19 to ‐5) (Faiz 2014). However, the administered dose of ketamine, 0.15 mg/kg, is lower than that typically used clinically. There were insufficient data for subgroup analyses by type of surgery for any comparison.
We assessed the quality of the data as low to very low, based on risk of bias from studies, small study sizes, imprecision of results, and heterogeneity between studies (see Table 3).
IV paracetamol versus placebo
See Analysis 3.1.
3.1. Analysis.
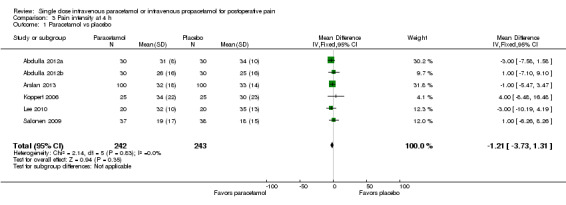
Comparison 3 Pain intensity at 4 h, Outcome 1 Paracetamol vs placebo.
Six studies enrolling 485 participants provided data. There was no difference either statistically or clinically between IV paracetamol and placebo. Studies consistently demonstrated no difference, as demonstrated by inspection of the forest plot and an I2 score of 0%.
IV paracetamol versus NSAIDs
See Analysis 3.2.
3.2. Analysis.
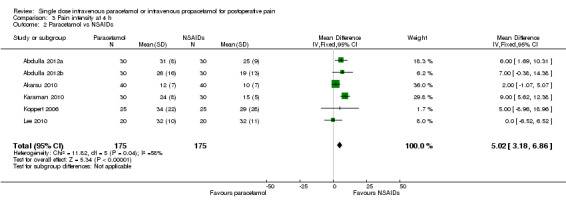
Comparison 3 Pain intensity at 4 h, Outcome 2 Paracetamol vs NSAIDs.
Six studies enrolling 350 participants compared IV paracetamol with various NSAIDs (Abdulla 2012a; Abdulla 2012b; Akarsu 2010; Karaman 2010; Koppert 2006; Lee 2010). Mean pain scores at four hours were 5 points lower (95% CI ‐3 to ‐7) on a 0 to 100 visual analog scale (VAS) in the NSAID arm versus IV paracetamol.
Pain intensity at six hours
There were insufficient data for subgroup analyses by type of surgery for any comparison. Only one study (Togrul 2011, 50 participants) compared paracetamol with opioids (tramadol in this study) and found no statistical or clinical difference between arms. One study (Faiz 2014, 80 participants) again reported lower mean scores in those administered paracetamol versus ketamine (‐13, 95% CI ‐18 to ‐8).
As with the data at four hours, we assessed the quality as low to very low (see Table 4).
IV paracetamol versus placebo
See Analysis 4.1.
4.1. Analysis.
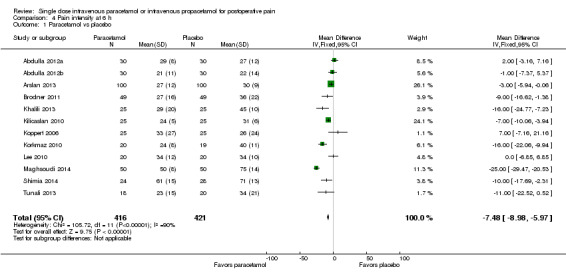
Comparison 4 Pain intensity at 6 h, Outcome 1 Paracetamol vs placebo.
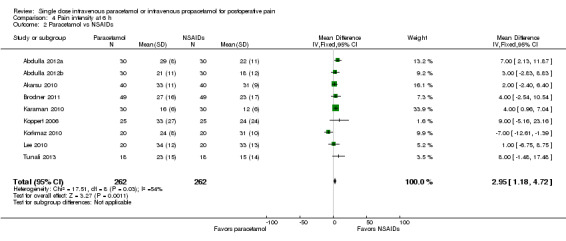
Twelve studies enrolling 837 participants provided data (Abdulla 2012a; Abdulla 2012b; Arslan 2013; Brodner 2011; Khalili 2013; Kilicaslan 2010; Koppert 2006; Korkmaz 2010; Lee 2010; Maghsoudi 2014; Shimia 2014; Tunali 2013). Overall, mean pain scores were seven points lower on a 0 to 100 VAS (95% CI ‐9 to ‐6) in the paracetamol arm. However, there was evidence of heterogeneity between studies, primarily due to differences in effect size, as illustrated by an I2 score of 90%.
IV paracetamol versus NSAIDs
See Analysis 4.2.
4.2. Analysis.
Comparison 4 Pain intensity at 6 h, Outcome 2 Paracetamol vs NSAIDs.
Nine studies enrolling 524 participants compared IV paracetamol with various NSAIDs (Abdulla 2012a; Abdulla 2012b; Akarsu 2010; Brodner 2011; Karaman 2010; Koppert 2006; Korkmaz 2010; Lee 2010; Tunali 2013). Mean pain scores at six hours were 3 points lower (95% CI ‐1 to ‐5) on a 0 to 100 VAS in the NSAID arm versus IV paracetamol.
Use of rescue medication
Number of participants using rescue medication
We rated the quality of data for the analyses below as low to very low, based on heterogeneity, small numbers of participants, the small number of total events, and imprecision (see Table 5).
IV paracetamol or propacetamol versus placebo
See Analysis 5.1.
5.1. Analysis.
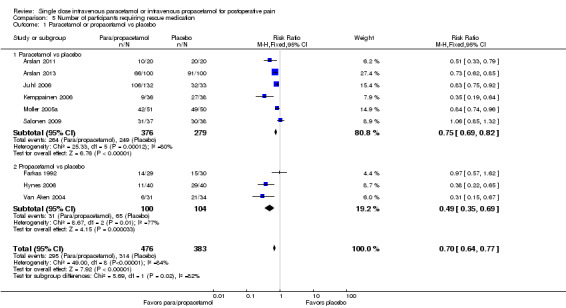
Comparison 5 Number of participants requiring rescue medication, Outcome 1 Paracetamol or propacetamol vs placebo.
Nine studies with a total of 859 participants reported the numbers of participants requiring rescue medication (Arslan 2011; Arslan 2013; Farkas 1992; Hynes 2006; Juhl 2006; Kemppainen 2006; Moller 2005a; Salonen 2009; Van Aken 2004). For combined IV paracetamol and propacetamol data, the proportion of participants using rescue medication was 62% (295/476) versus 82% (314/383) for those administered placebo. This gives a number needed to treat to prevent (NNTp) re‐medication of 4 (95% CI 3.3 to 5.3). Four participants need to be treated with IV paracetamol or propacetamol to prevent one using rescue medication within the study period of four to six hours that would have needed rescue with placebo. Based on our assessment of risk of publication bias (Table 8) these results are reliable and not subject to potential publication bias. Similar results were demonstrated when we performed subgroup analyses by intervention (IV paracetamol or propacetamol), with both sub‐analyses also not subject to potential publication bias.
IV paracetamol or propacetamol versus NSAIDs
See Analysis 5.2.
5.2. Analysis.
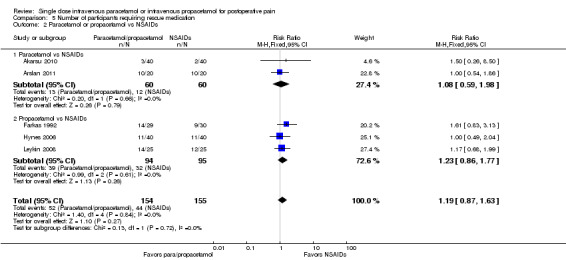
Comparison 5 Number of participants requiring rescue medication, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
Five studies with a total of 309 participants compared IV paracetamol or propacetamol versus NSAIDs (Akarsu 2010; Arslan 2011; Farkas 1992; Hynes 2006; Leykin 2008). Thirty‐four per cent (52/154) of participants receiving IV paracetamol or propacetamol required rescue analgesia versus 28% (44/155) receiving NSAIDs. The difference was not statistically significant for either combined IV paracetamol/propacetamol or when the interventions were compared separately.
IV propacetamol versus opioids
See Analysis 5.3.
5.3. Analysis.
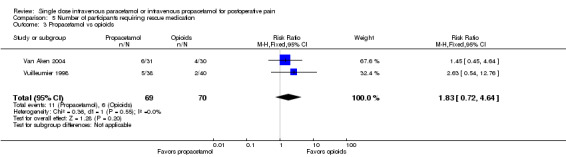
Comparison 5 Number of participants requiring rescue medication, Outcome 3 Propacetamol vs opioids.
Two studies with 139 participants provided data comparing IV propacetamol with opioids. Sixteen per cent (11/69) of participants receiving IV propacetamol required rescue analgesia versus 9% (6/70) receiving opioids. The difference was not statistically significant. No studies compared IV paracetamol with opioids for this outcome.
IV paracetamol versus propacetamol
See Analysis 5.4.
5.4. Analysis.
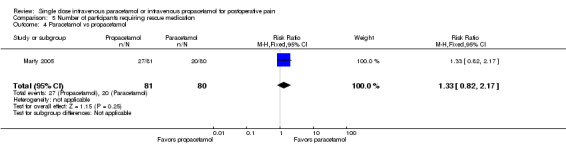
Comparison 5 Number of participants requiring rescue medication, Outcome 4 Paracetamol vs propacetamol.
Only one study with 161 participants provided head‐to‐head data on the number of participants requiring rescue for IV paracetamol versus propacetamol (Marty 2005). Thirty‐three per cent (27/81) of participants receiving IV propacetamol versus 25% (20/80) receiving IV paracetamol required rescue analgesia. The difference was not statistically significant.
Time to rescue medication
IV paracetamol or propacetamol versus placebo
See Analysis 6.1; Analysis 6.2.
6.1. Analysis.
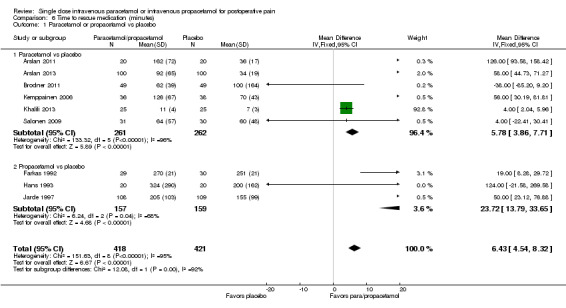
Comparison 6 Time to rescue medication (minutes), Outcome 1 Paracetamol or propacetamol vs placebo.
6.2. Analysis.
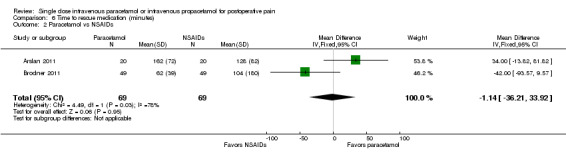
Comparison 6 Time to rescue medication (minutes), Outcome 2 Paracetamol vs NSAIDs.
Nine studies provided data comparing IV paracetamol or propacetamol versus placebo (Arslan 2011; Arslan 2013; Brodner 2011; Farkas 1992; Hans 1993; Jarde 1997; Kemppainen 2006; Khalili 2013; Salonen 2009). Two hundred and sixty‐one participants received IV paracetamol, 418 propacetamol, and 421 placebo. The mean difference in time to use of rescue medication was 6 minutes (95% CI 5 to 8) longer for participants receiving either IV paracetamol or propacetamol versus placebo. However, there was substantial heterogeneity, as demonstrated by an overall I2 score of 95%. There were large differences between studies in both the time to rescue among active groups and in differences between active and placebo groups. There were also large differences when subgroup analysis by test drug was performed, with participants receiving paracetamol having similar results to those demonstrated overall, but those receiving propacetamol requiring rescue 23 minutes (95% CI 14 to 34) later than those receiving placebo.
Two studies compared IV paracetamol with NSAIDs (Arslan 2011; Brodner 2011) and found no difference between groups (Analysis 6.2). No studies compared propacetamol with NSAIDs. There were no comparisons of either IV paracetamol or propacetamol with opioids for this outcome.
Opioid consumption
As with the comparisons of the proportion of participants requiring rescue medication, we assessed the quality of the data as low to very low, with the exception of the analysis of IV paracetamol or propacetamol versus placebo, which we assessed as of moderate quality (see Table 6).
IV paracetamol or propacetamol versus placebo
See Analysis 7.1; Analysis 8.1.
7.1. Analysis.
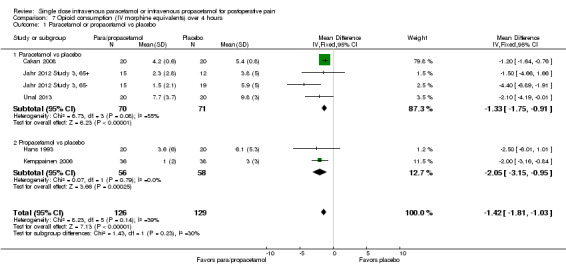
Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
8.1. Analysis.
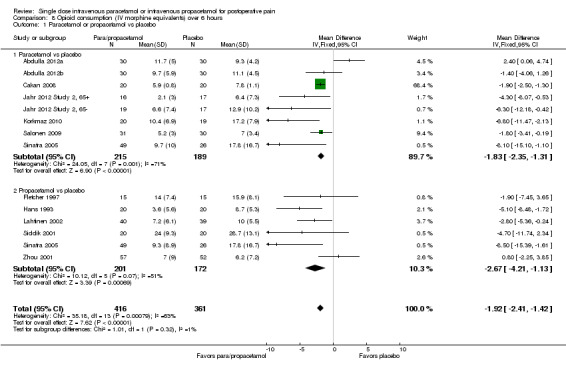
Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
Data were available for the time periods 0 to 4 hours and 0 to 6 hours. For the former, six studies reported data on IV paracetamol or propacetamol, with 70 participants receiving IV paracetamol, 56 propacetamol, and 129 placebo (Cakan 2008; Hans 1993; Jahr 2012 Study 3, 65‐; Jahr 2012 Study 3, 65+; Kemppainen 2006; Unal 2013). Overall, participants receiving IV propacetamol or IV paracetamol required 1.4 mg (95% CI 1.0 to 1.8) less IV morphine equivalents than those receiving placebo. Participants receiving placebo required an average of 5.4 mg of morphine; therefore, administration of IV paracetamol or propacetamol produced a 26% reduction in opioid requirements. For the time period 0 to 6 hours, 13 studies reported data on IV paracetamol, propacetamol, or both, with 215 participants receiving IV paracetamol, 201 IV propacetamol, and 361 placebo (Abdulla 2012a; Abdulla 2012b; Cakan 2008; Fletcher 1997; Hans 1993; Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Korkmaz 2010; Lahtinen 2002; Salonen 2009; Siddik 2001; Sinatra 2005; Zhou 2001). Overall, participants receiving IV paracetamol or propacetamol required 1.9 mg (95% CI 1.4 to 2.4) less IV morphine than those receiving placebo. Participants receiving placebo required an average of 11.8 mg of morphine, therefore administration of IV propacetamol or IV paracetamol provided a 16% reduction in opioid requirements.
IV paracetamol or propacetamol versus NSAIDs
See Analysis 7.2; Analysis 8.2.
7.2. Analysis.
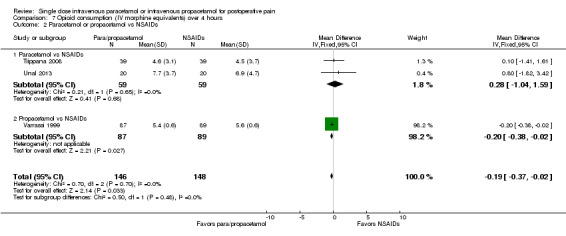
Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
8.2. Analysis.
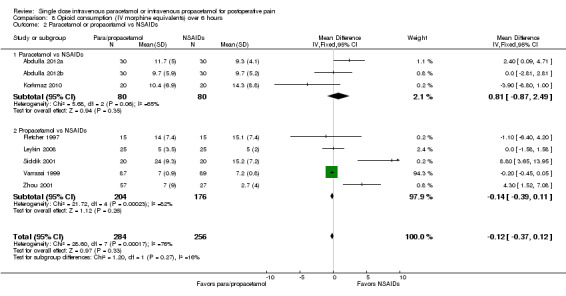
Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
Three studies supplied data for the time period 0 to 4 hours, with 59 participants receiving IV paracetamol, 87 receiving propacetamol and 148 a NSAID (Tiippana 2008; Unal 2013; Varrassi 1999). Those receiving IV propacetamol or IV paracetamol required 0.2 mg (95% CI 0.0 to 0.4) less IV morphine than those receiving a NSAID. Over six hours, eight studies provided data on 80 participants receiving IV paracetamol, 204 receiving propacetamol, and 256 receiving NSAIDs. There was not an overall statistically significant difference between groups.
IV paracetamol or propacetamol versus opioids
See Analysis 7.3; Analysis 8.3.
7.3. Analysis.
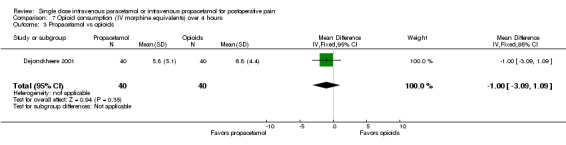
Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 3 Propacetamol vs opioids.
8.3. Analysis.
Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 3 Propacetamol vs opioids.
Only one study supplied data for the periods 0 to 4 hours and 0 to 6 hours (Dejonckheere 2001). Forty participants received IV propacetamol and 40 received tramadol. There was no statistical difference between the groups for either time period.
IV paracetamol versus propacetamol
See Analysis 8.4.
8.4. Analysis.
Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 4 Paracetamol vs propacetamol.
One study supplied data, and only for the time period 0 to 6 hours (Sinatra 2005). Forty‐nine participants were assessed in each group, with no statistical difference in opioid consumption.
Global evaluation
Global evaluation was predominately assessed using categorical scales, but was assessed with numerical rating scales in four studies (Abdulla 2012a; Dejonckheere 2001; Jarde 1997; Van Aken 2004).
IV paracetamol or propacetamol versus placebo
See Analysis 9.1; Analysis 10.1.
9.1. Analysis.
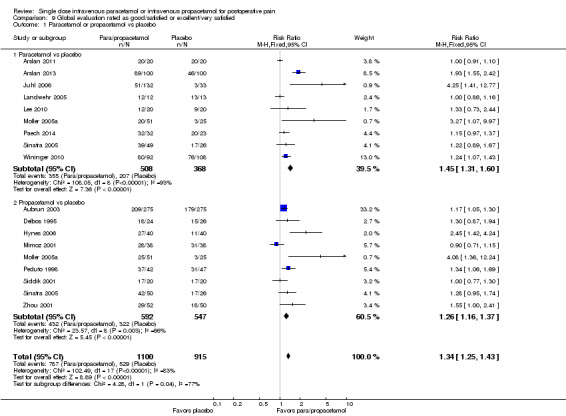
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 1 Paracetamol or propacetamol vs placebo.
10.1. Analysis.
Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 1 Paracetamol or propacetamol vs placebo.
Sixteen studies provided data on categorical rating of global evaluation versus placebo, for either IV paracetamol, propacetamol, or both. Five hundred and eight participants receiving IV paracetamol evaluated therapy, 592 propacetamol, and 915 placebo. Overall, 72% (787/1100) of participants receiving IV paracetamol or propacetamol rated therapy as "good/satisfied" or better versus 58% (529/915) receiving placebo. The overall RR of IV paracetamol or propacetamol versus placebo was 1.3 (95% CI 1.3 to 1.4). The derived overall NNT for a global evaluation of "good/satisfied" or better was 6 (95% CI 4.3 to 6.7). For every six participants treated with IV paracetamol or propacetamol, one would rate their analgesia as "good/satisfied" or better who would not have done so with placebo. Based on our assessment of risk of publication bias (Table 8) these results are reliable and not subject to potential publication bias.
Three studies employed numerical rating scale scores for global evaluation (Abdulla 2012a; Jarde 1997; Van Aken 2004). We mathematically converted each scale to a 0 to 10 scale. Thirty participants received IV paracetamol, 139 propacetamol, and 173 placebo. Overall there was a 0.4‐point (95% CI 0.0 to 0.7) superiority for participants receiving IV paracetamol or propacetamol compared with placebo.
IV paracetamol or propacetamol versus active comparators
Eleven studies with 705 participants compared IV paracetamol or propacetamol with a NSAID (Analysis 9.2). One compared IV propacetamol with an opioid (Analysis 9.3). Neither analysis was statistically significant. Seventy‐five per cent (306/410) of participants receiving IV paracetamol/propacetamol rated their analgesia as "good/satisfied" or better versus 81% (313/385) receiving a NSAID. Of note, while not directly compared, 92% of participants receiving IV paracetamol rated as "good/satisfied" or better versus only 67% of those receiving propacetamol.
9.2. Analysis.
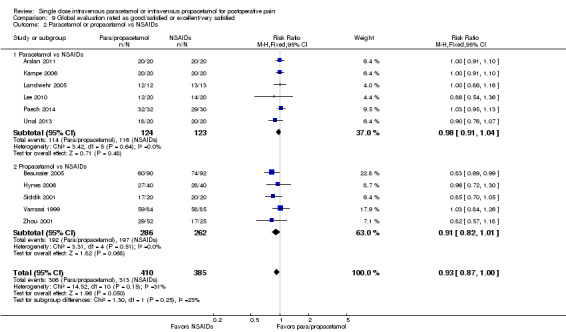
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
9.3. Analysis.
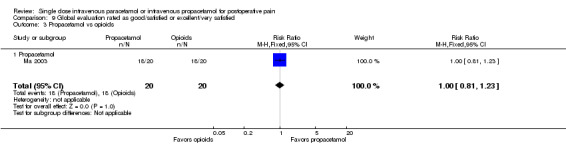
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 3 Propacetamol vs opioids.
Additionally, one study compared IV paracetamol with a NSAID (Abdulla 2012a) (Analysis 10.2) and two studies compared IV propacetamol with opioids using a VAS (Dejonckheere 2001; Van Aken 2004) (Analysis 10.3). Again, comparisons were not statistically significantly different.
10.2. Analysis.
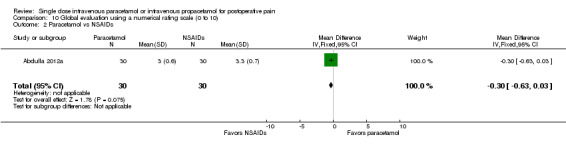
Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 2 Paracetamol vs NSAIDs.
10.3. Analysis.
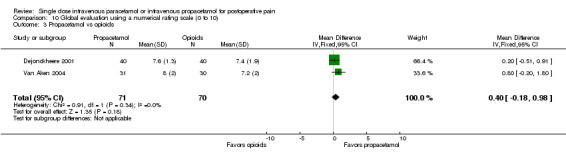
Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 3 Propacetamol vs opioids.
IV paracetamol versus propacetamol
See Analysis 9.4.
9.4. Analysis.
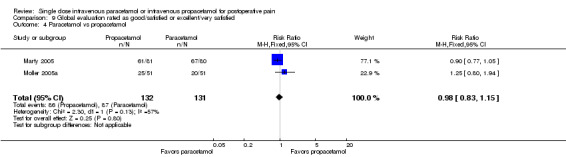
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 4 Paracetamol vs propacetamol.
Only two studies with 263 participants assessed global evaluation in head‐to‐head comparisons of IV paracetamol and propacetamol (Marty 2005; Moller 2005a), with opposite findings. In the former, 75% (61/81) evaluated analgesia as "good/satisfied" or better in the propacetamol arm versus 84% (67/80) in the IV paracetamol arm. In the latter, 49% of propacetamol participants rated their analgesia highly versus 39% of those receiving IV paracetamol.
Adverse events (AEs)
The time over which AE data were collected varied from four hours to seven days, with the majority of studies reporting data at 24 hours. In only 13 studies was it clear that AE data collection was confined to the four‐ to six‐hour postoperative period, i.e., the same period over which we assessed efficacy (Akarsu 2010; Dejonckheere 2001; Farkas 1992; Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Jarde 1997; Kemppainen 2006; Khan 2007; Lee 2010; Ma 2003; Marty 2005; Vuilleumier 1998; Zhou 2001). No studies indicated whether AE data continued to be collected after rescue medication had been taken.
We assessed all adverse event outcomes as low or very low quality, in large part because of the infrequency with which many events occurred, the susceptibility to publication bias of statistically significant results, and the heterogeneity in assessment methodology.
Total number of participants reporting adverse events
IV paracetamol or propacetamol versus placebo
See Analysis 11.1.
11.1. Analysis.
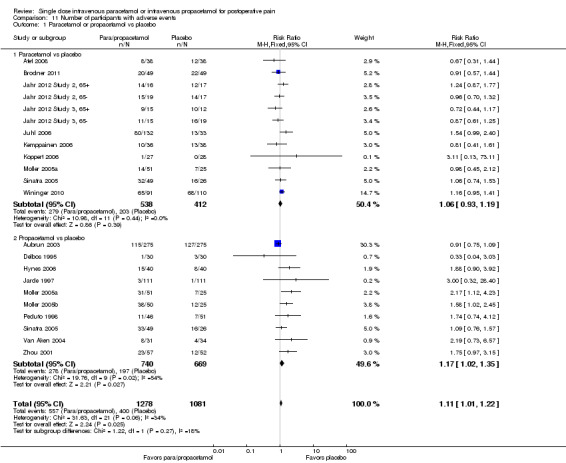
Comparison 11 Number of participants with adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.
Twenty studies with 2359 participants reported the number of participants with any AE. Twelve studies provided data on IV paracetamol versus placebo (Atef 2008; Brodner 2011; Jahr 2012 Study 2, 65‐; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65‐; Jahr 2012 Study 3, 65+; Juhl 2006; Kemppainen 2006; Koppert 2006; Moller 2005a; Sinatra 2005; Wininger 2010), and 10 studies provided data for propacetamol versus placebo (Aubrun 2003; Delbos 1995; Hynes 2006; Jarde 1997; Moller 2005a; Moller 2005b; Peduto 1998; Sinatra 2005; Van Aken 2004; Zhou 2001) (two studies provided data on both).
There was no statistical difference in the rate of AEs in those participants receiving IV paracetamol (52%, 279/538) versus those receiving placebo (49%, 203/412). Meta‐analysis of those studies comparing propacetamol versus placebo demonstrated an increase in AEs in the propacetamol group, with 38% (278/740) of participants receiving IV propacetamol reporting an AE versus 29% (197/669) of those receiving placebo. This finding translates to a RR of 1.2 (95% CI 1.0 to 1.4), i.e., barely statistically significant and, therefore, unreliable.
IV paracetamol or propacetamol versus active comparators
See Analysis 11.2; Analysis 11.3.
11.2. Analysis.
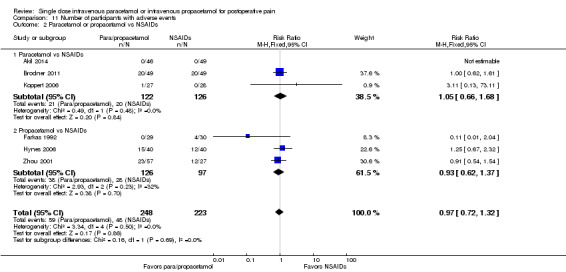
Comparison 11 Number of participants with adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
11.3. Analysis.
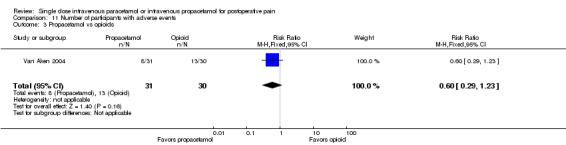
Comparison 11 Number of participants with adverse events, Outcome 3 Propacetamol vs opioids.
Three studies with 248 participants provided data for IV paracetamol (Akil 2014; Brodner 2011; Koppert 2006), and three studies with 223 participants provided data for propacetamol (Farkas 1992; Hynes 2006; Zhou 2001) versus NSAIDs. One study compared propacetamol versus opioids (Van Aken 2004) (61 participants). Neither the meta‐analysis of the NSAID studies, nor the single opioid study demonstrated a statistically significant difference between IV paracetamol ± propacetamol and active comparator.
Number of participants with serious adverse events (SAEs), withdrawing due to adverse events, or withdrawing due to lack of efficacy
There were insufficient numbers of participants with any of the above to allow meaningful analysis.
In 10 studies (910 participants) comparing IV paracetamol or propacetamol with placebo, 2/370 (0.5%) participants receiving IV paracetamol, 0/192 receiving propacetamol, and 2/408 (0.5%) receiving placebo were assessed as suffering a SAE (Analysis 12.1). Rates were similarly low when comparing IV paracetamol or propacetamol with either NSAIDs or opioids (Analysis 12.2; Analysis 12.3).
12.1. Analysis.
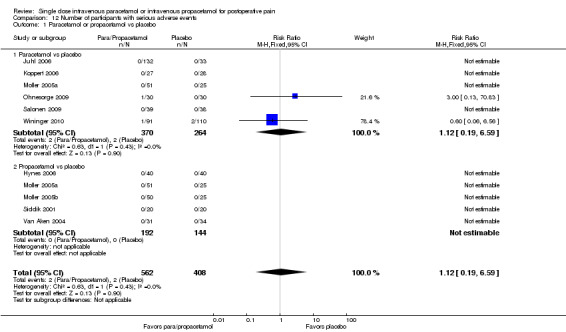
Comparison 12 Number of participants with serious adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.
12.2. Analysis.
Comparison 12 Number of participants with serious adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
12.3. Analysis.
Comparison 12 Number of participants with serious adverse events, Outcome 3 Paracetamol or propacetamol vs opioids.
Withdrawal rates due to AEs were also low. Thirty‐seven studies (2654 participants) provided data for IV paracetamol or propacetamol versus placebo (Analysis 13.1). Seven of 1024 (0.7%) participants receiving IV paracetamol, 4/404 (1.0%) receiving IV propacetamol, and 7/1226 (0.6%) receiving placebo withdrew. Again, rates were similarly low when IV paracetamol or propacetamol were compared with either NSAIDs, opioids, or ketamine (Analysis 13.2; Analysis 13.3; Analysis 13.4).
13.1. Analysis.
Comparison 13 Number of participants withdrawing due to adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.
13.2. Analysis.
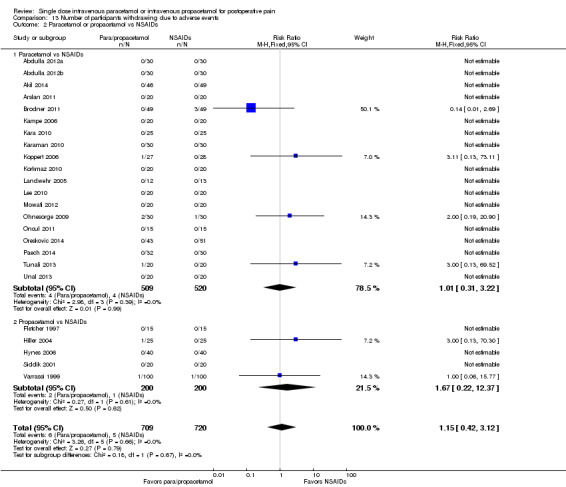
Comparison 13 Number of participants withdrawing due to adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
13.3. Analysis.
Comparison 13 Number of participants withdrawing due to adverse events, Outcome 3 Paracetamol or propacetamol vs opioids.
13.4. Analysis.
Comparison 13 Number of participants withdrawing due to adverse events, Outcome 4 Paracetamol vs ketamine.
Thirty‐eight studies (2600 participants) provided data for IV paracetamol or propacetamol versus placebo for the comparison of withdrawals due to lack of efficacy. One of 933 (0.1%) participants receiving IV paracetamol and 25/477 (5%) receiving propacetamol withdrew for this reason. Placebo rates of withdrawal varied greatly based on whether the active intervention was IV paracetamol or propacetamol. In IV paracetamol studies, 3/778 (0.4%) receiving placebo withdrew due to lack of efficacy, whereas in propacetamol studies, the withdrawal rate with placebo was 47/412 (11%), perhaps reflecting differences in study design. When propacetamol was compared with placebo, there was a statistically significant lower withdrawal rate in the active group (5% versus 11%, P value = 0.001); however the overall rates of withdrawal were low, rendering these findings unreliable (Analysis 14.1). Rates were similar and differences not statistically significant when IV paracetamol or propacetamol were compared with either NSAIDs, opioids, or ketamine (Analysis 14.2; Analysis 14.3; Analysis 14.4).
14.1. Analysis.
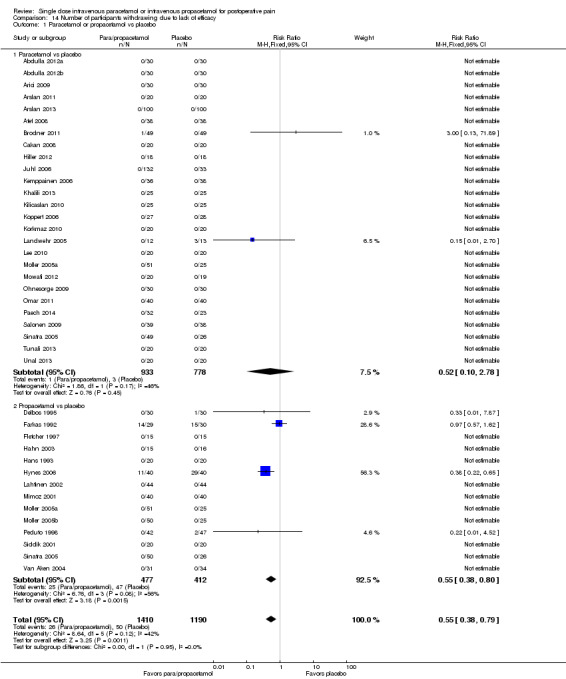
Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 1 Paracetamol or propacetamol vs placebo.
14.2. Analysis.
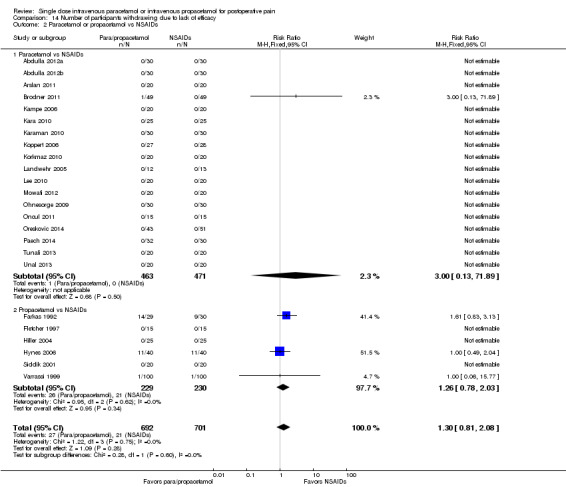
Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
14.3. Analysis.
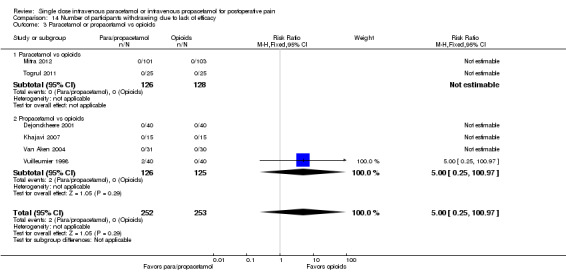
Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 3 Paracetamol or propacetamol vs opioids.
14.4. Analysis.
Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 4 Paracetamol vs ketamine.
Pain on infusion
IV propacetamol is reported to cause more pain on infusion than IV paracetamol. We analyzed data from IV propacetamol versus placebo studies (645 participants), IV paracetamol versus placebo studies (467 participants), and any studies that performed head‐to‐head comparisons of IV propacetamol and IV paracetamol (Marty 2005; Moller 2005a; Sinatra 2005) (362 participants). Our analysis of IV propacetamol versus placebo demonstrated that 23% (75/333) of participants reported pain on infusion with IV propacetamol versus 1% (4/312) of those receiving placebo (Analysis 15.2, P value < 0.00001). This translates to a NNH of 5 (95% CI 4.2 to 6.2). Based on our calculation that a study (or studies) enrolling 625 participants with zero treatment effect would be required to increase the NNH to above 10, we are confident that these data are not susceptible to publication bias (Table 9). Conversely, comparison of IV paracetamol versus placebo showed similarly low rates of pain on infusion, with 3% (8/272) of participants receiving IV paracetamol and 1% (2/195) of participants receiving placebo reporting pain (Analysis 15.1). In head‐to‐head comparisons of IV propacetamol and IV paracetamol, more participants reported pain on infusion when receiving IV propacetamol (39%, 71/182) than those receiving IV paracetamol (4%, 8/180) (P value < 0.00001) (Analysis 15.3; Figure 6). This translates to a NNH of 3 (95% CI 2.4 to 3.7). Based on our calculation, these data are also not susceptible to publication bias (Table 9).
15.2. Analysis.
Comparison 15 Number of participants with pain on infusion, Outcome 2 Propacetamol vs placebo.
2. Publication bias risk assessment: safety outcomes.
| Comparison/outcome | Number of studies | Number of participants | n with outcome/total | % with outcome | Risk difference | NNH | Susceptibility to publication bias | ||
| Active | Control | Active | Control | ||||||
| Comparison 11. Number of participants with adverse events | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 20 | 2359 | 557/1278 | 400/1081 | 44 | 37 | 0.04 (0.01 to 0.08) | 25 | NNH > 10 |
| 1.2 Propacetamol vs placebo | 10 | 1409 | 278/740 | 197/669 | 38 | 29 | 0.05 (0.01 to 0.10) | 20 | NNH > 10 |
| Comparison 14. Number of participants withdrawing due to lack of efficacy | |||||||||
| 1.2 Propacetamol vs placebo | 14 | 889 | 25/477 | 47/412 | 5 | 11 | ‐0.05 (‐0.08 to ‐0.02) | 20* | NNH > 10 |
| Comparison 15. Number of participants with pain on infusion | |||||||||
| 2. Propacetamol vs placebo | 6 | 645 | 75/333 | 4/312 | 23 | 1 | 0.20 (0.16 to 0.24) | 5 | 645 |
| 3. Propacetamol vs paracetamol | 3 | 362 | 71/182 | 8/180 | 39 | 4 | 0.35 (0.27 to 0.42) | 3 | 904 |
| Comparison 16. Individual adverse events: paracetamol or propacetamol vs placebo | |||||||||
| 1. Nausea: Paracetamol or propacetamol vs placebo | 15 | 1267 | 189/660 | 213/607 | 29 | 35 | ‐0.05 (‐0.10 to ‐0.01) | 20* | NNH > 10 |
| 1.1. Nausea: Paracetamol vs placebo | 13 | 1037 | 153/520 | 196/517 | 29 | 38 | ‐0.09 (‐0.14 to ‐0.04) | 11* | NNH > 10 |
| 2. Vomiting: Paracetamol or propacetamol vs placebo | 15 | 1414 | 103/721 | 144/693 | 14 | 21 | ‐0.06 (‐0.10 to ‐0.03) | 17* | NNH > 10 |
| 2.1. Vomiting: Paracetamol vs placebo | 13 | 1037 | 88/520 | 136/517 | 17 | 26 | ‐0.10 (‐0.14 to ‐0.05) | 10* | NNH > 10 |
| Comparison 18. Individual adverse events: paracetamol or propacetamol vs opioids | |||||||||
| 1. Nausea: Paracetamol or propacetamol vs opioids | 6 | 545 | 19/272 | 48/273 | 7 | 18 | ‐0.11 (‐0.16 to ‐0.05) | 10* | 55 |
| 1.1 Nausea: Paracetamol vs opioids | 4 | 438 | 12/219 | 39/219 | 5 | 18 | ‐0.12 (‐0.18 to ‐0.07) | 9* | 88 |
| 2. Vomiting: Paracetamol or propacetamol vs opioids | 5 | 495 | 6/247 | 20/248 | 2 | 8 | ‐0.06 (‐0.09 to ‐0.02) | 17* | NNH > 10 |
| 2.1. Vomiting: Paracetamol vs opioids | 3 | 388 | 4/194 | 16/194 | 2 | 8 | ‐0.06 (‐0.10 to ‐0.02) | 17* | NNH > 10 |
| 6.1. Sedation: Paracetamol vs opioids | 3 | 354 | 2/176 | 26/178 | 1 | 15 | ‐0.14 (‐0.18 to ‐0.09) | 8* | 142 |
*lower occurrence of adverse event in paracetamol and/or propacetamol arm
15.1. Analysis.
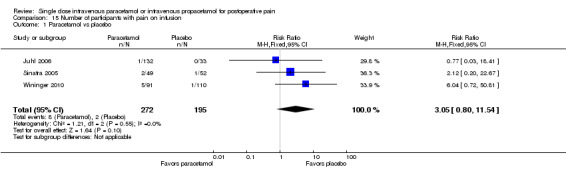
Comparison 15 Number of participants with pain on infusion, Outcome 1 Paracetamol vs placebo.
15.3. Analysis.
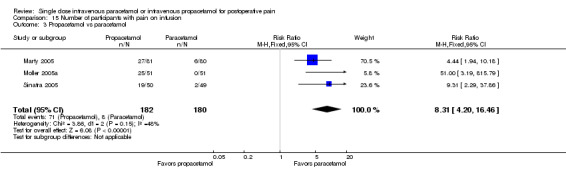
Comparison 15 Number of participants with pain on infusion, Outcome 3 Propacetamol vs paracetamol.
6.
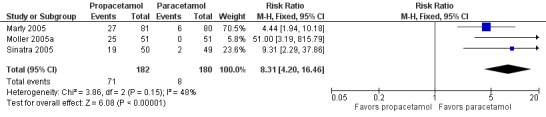
Forest plot of comparison: 15 Pain on infusion, outcome: 15.3 Propacetamol versus paracetamol.
Individual adverse events
We analyzed individual AEs in both IV paracetamol and propacetamol groups versus both placebo and active comparator (NSAID, opioid, ketamine) groups. All of the statistically significant results reported below (Analysis 16.1; Analysis 16.2; Analysis 18.1; Analysis 18.2; Analysis 18.6) had either NNHs above 10 or were highly susceptible to publication bias (Table 9), i.e., they were probably not clinically meaningful or we had low confidence in the robustness of the data, or both.
16.1. Analysis.
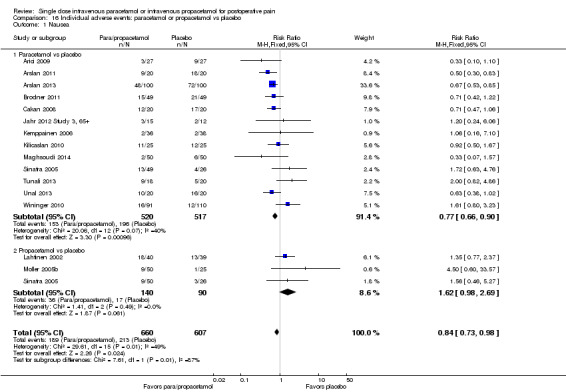
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 1 Nausea.
16.2. Analysis.
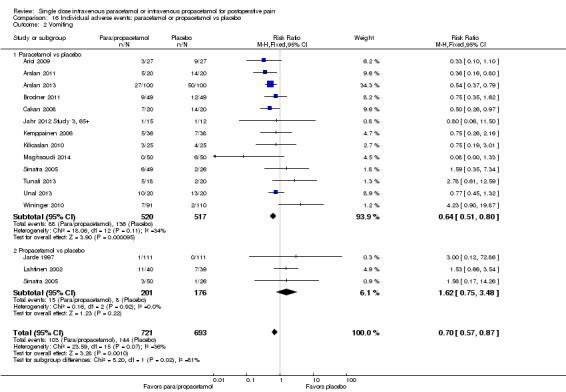
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 2 Vomiting.
18.1. Analysis.
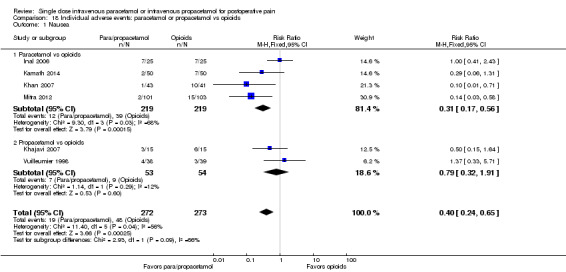
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 1 Nausea.
18.2. Analysis.
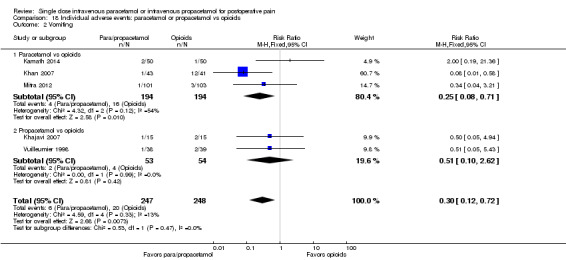
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 2 Vomiting.
18.6. Analysis.
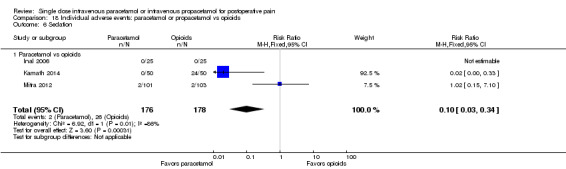
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 6 Sedation.
IV paracetamol or propacetamol versus placebo
Of the many individual AEs reported, only analyses of rates of nausea and vomiting demonstrated a statistically significant difference between IV paracetamol or propacetamol and placebo, both demonstrating higher rates in those receiving placebo. Analysis of 12 studies enrolling 1267 participants demonstrated that 29% (189/660) of participants treated with IV paracetamol or propacetamol reported nausea versus 35% (213/607) of those receiving placebo (NNTp = 20; 95% CI 10.0 to 100.0) (Analysis 16.1). For analysis of vomiting, 14% (103/721) of participants receiving IV paracetamol or propacetamol vomited versus 21% (144/693) of those receiving placebo (NNTp = 17; 95% CI 10.0 to 33.3) (Analysis 16.2; Table 7). Subgroup analyses demonstrated that IV paracetamol, but not propacetamol, also statistically reduced rates of nausea and vomiting versus placebo.
IV paracetamol or propacetamol versus NSAIDs
We were able to meta‐analyze data for eight different individual AEs (nausea, vomiting, nausea/vomiting, pruritus, respiratory depression, sedation, urinary retention, and allergy/skin reaction/rash) when comparing IV paracetamol or propacetamol versus a NSAID. There were no statistically significant differences between interventions for any analysis.
IV paracetamol or propacetamol versus opioids
There were data comparing rates of six different AEs when assessing IV propacetamol versus opioids (nausea, vomiting, nausea/vomiting, pruritus, respiratory depression, and sedation). Of these comparisons, rates of nausea and vomiting were lower in the combined IV paracetamol/propacetamol arms, and subgroup analysis demonstrated a reduction in the rate of sedation in participants receiving IV paracetamol versus those receiving opioids. Seven per cent (19/272) of those receiving IV paracetamol or propacetamol reported nausea versus 18% of those receiving opioids (NNTp = 10; 95% CI 6.2 to 20.0) (Analysis 18.1). Two per cent (6/247) of those receiving IV paracetamol or propacetamol vomited versus 8% (20/248) of those receiving opioids (NNTp = 17; 95% CI 11.1 to 50.0) (Analysis 18.2). Last, 1% (2/176) of participants receiving IV paracetamol reported sedation versus 15% (26/178) of those receiving opioids, translating to a NNTp of 8 (95% CI 5.6 to 11.1) (Analysis 18.6).
IV paracetamol or propacetamol versus ketamine
A single study, Faiz 2014, enrolling 80 participants reported rates of nausea, vomiting, and sedation for participants receiving IV paracetamol versus those receiving ketamine, but found no statistical difference between interventions for any of these AEs (Analysis 19.1; Analysis 19.2; Analysis 19.3).
19.1. Analysis.
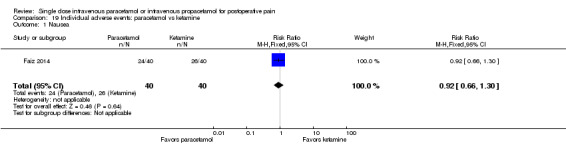
Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 1 Nausea.
19.2. Analysis.
Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 2 Vomiting.
19.3. Analysis.
Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 3 Sedation.
Subgroup analyses and sensitivity analyses
We intended to perform subgroup and sensitivity analyses based on various study characteristics.
Subgroup analyses
IV propacetamol and IV paracetamol. Each comparison shows subtotals for IV paracetamol versus control, IV propacetamol versus control, and their data combined, with one exception: we did not combine analyses for pain on infusion, as IV paracetamol is reported to produce a lower incidence of this side effect. As reported above, meta‐analysis confirms that there is a lower incidence of pain on infusion in participants receiving IV paracetamol. Where data were available, meta‐analyses of all efficacy and safety data outcomes were generally similar for IV propacetamol and IV paracetamol. However, as reported above, analyses demonstrated a statistically significant increase in the number of participants treated with IV propacetamol reporting AEs versus placebo, whereas comparison of IV paracetamol versus placebo did not demonstrate a significant difference (Analysis 11.1).
Type of surgery. There were insufficient numbers of studies of the various types of surgeries to enable us to perform subgroup analyses. However, despite expected differences in pain intensity and duration, evidence suggests that analgesic response and derived NNTs are similar when comparing dental and other postsurgical models, and that it is legitimate to extrapolate efficacy from one pain model to another (Barden 2004).
Sensitivity analyses
Adults and children. Only one included study enrolled exclusively pediatric participants (Hiller 2012), and in those studies where both adults and children were enrolled, data were not reported separately. Removal of the single pediatric study from the three analyses to which it contributed data (Analysis 13.1; Analysis 14.1; Analysis 16.3) made no difference to either the effect size or the statistical significance of any outcome.
Blinded and non‐blinded studies. Five studies were reported or assumed to be unblinded, or were not double‐blinded (Kamath 2014; Kara 2010; Koppert 2006; Mimoz 2001; Tuncel 2012). Removing data from these studies made no difference to either the direction or the statistical significance of any comparison, and only minor differences in the effect size, for the primary outcome (number of participants with > 50% pain relief over four or six hours).
Studies with atypical design. Seven studies included in the primary outcome analyses administered interventions on the day after surgery (Hynes 2006; Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65+; Sinatra 2005; Wininger 2010; Zhou 2001), or administered the intervention without requiring that the patient report moderate‐to‐severe pain (Koppert 2006). Sensitivity analyses with these studies removed increased risk ratios for each analysis of IV paracetamol or propacetamol versus placebo (i.e., increased superiority of intervention versus placebo). For the analysis of number of participants with at least 50% pain relief at four hours (Analysis 1.1), the RR for IV paracetamol versus placebo was 7.8 (2.5 to 24.4) and for propacetamol versus placebo was 2.42 (95% CI 1.7 to 3.5). Combining both interventions, the RR versus placebo was 3.1 (95% CI 2.2 to 4.3). There were similar increases in RR when six‐hour data were analyzed with these studies removed. Removal of the above studies from analyses versus active comparators made no difference in statistical significance or direction of effect, and only minimal differences in any effect size.
16.3. Analysis.
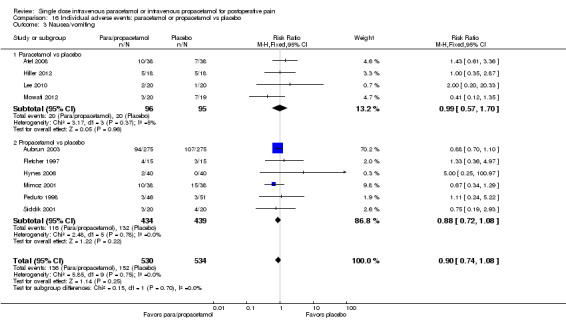
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 3 Nausea/vomiting.
Additionally, due to evidence of heterogeneity in many of our comparisons, we performed a sensitivity analysis using a random‐effects model as opposed to our original fixed‐effect model. None of the estimates for the primary outcome changed in direction, statistically significant analyses remained so, and changes in effect size were minimal. However, 95% confidence intervals were, predictably, generally wider with the random‐effects model. For secondary efficacy outcomes, three group/subgroup analyses changed from demonstrating statistical significance to no longer being statistically significant: pain intensity at six hours (paracetamol versus NSAIDs); number of participants requiring rescue medication (propacetamol versus placebo); and global evaluation using VAS (paracetamol or propacetamol versus placebo). In all cases, the point estimates remained similar, but 95% confidence intervals contained a point of no difference. For the analysis of time to rescue medication (Analysis 6.1; Analysis 6.2) effect sizes (minutes) versus placebo increased substantially, in large part because of the reduced weight assigned to one study (Khalili 2013). When this study was removed, estimates based on fixed‐effect versus random‐effects models were similar. Last, several AE analyses also changed from demonstrating statistical significance to no longer being statistically significant.
In one of the included trials, Akil 2014, the study population consisted of women who had episiotomy or perineal tear after vaginal delivery that required repair with suturing. We decided to include the study, despite the nature of the procedure being dissimilar to the other included studies. Post‐hoc sensitivity analysis demonstrated that removal of the study from all of the meta‐analyses to which it contributed data made no difference to the statistical significance or effect size of any.
Discussion
In this update, we added 39 studies to the analysis of the 36 studies from the original review. The number of studies more than doubled for orthopedic, obstetric/gynecologic, back, and general surgeries. As in our original analysis, the use of IV paracetamol and IV propacetamol was compared with either placebo or an active comparator such as an opioid or NSAID. In a clinical setting, it is unlikely that IV paracetamol/IV propacetamol would be used instead of an opioid, except in surgeries expected to produce only mild‐to‐moderate pain. This somewhat limits the relevance and applicability of the head‐to‐head studies of IV propacetamol/IV paracetamol versus opioids (Dejonckheere 2001; Inal 2006; Khajavi 2007; Khan 2007; Ma 2003; Mimoz 2001; Vuilleumier 1998; Van Aken 2004). Rather, for moderate‐to‐severe postoperative pain, it is assumed that IV paracetamol or IV propacetamol would be utilized as part of a multimodal pain strategy that includes an opioid. Therefore, the comparisons of IV paracetamol/IV propacetamol with placebo or NSAID may more readily be extrapolated to clinical practice. The majority of studies included in our update employed this design. Studies that utilized a NSAID in addition to (as opposed to in comparison with) IV paracetamol/propacetamol were included, assuming the same regimen was used in both arms. The majority of studies administered interventions automatically, no earlier than 30 minutes before the end of surgery or immediately postoperatively, reflecting common clinical practice.
In our original review, the majority of studies employed IV propacetamol. In our updated review, all but one study, Chen 2011, utilized IV paracetamol. This increased interest in IV paracetamol is likely a result of the wider availability of IV paracetamol in addition to its ease of administration and superior tolerability versus propacetamol.
Summary of main results
Efficacy
Primary outcomes
As in our previous analysis, meta‐analyses demonstrate that IV paracetamol and IV propacetamol are statistically superior to placebo for the outcome of the proportion of participants achieving at least 50% pain relief over four or six hours. Estimates of the minimum reduction in acute pain intensity that patients describe as meaningful vary between 30% and 50%, with larger absolute reductions required when baseline pain is more severe (Campbell 1998; Cepeda 2003; Toms 2008). Similar to the original review, the proportion of participants with at least 50% pain relief appears to decrease at six hours in both active groups (and in the placebo groups). Over four hours, 31%, 40%, and 36% of participants receiving IV paracetamol, IV propacetamol, or overall, respectively, had at least 50% pain relief versus 16% in those receiving placebo. Inspection of forest plots suggests low to moderate heterogeneity exists amongst the placebo‐controlled studies, quantified by the I2 statistic of 51% (P value = 0.00001) and 43% (P value = 0.0003) at four and six hours, respectively; however heterogeneity was lower than in the original review.
Heterogeneity may, in part, be explained by the different types of surgeries performed. Placebo rates in dental surgery have been shown to be lower than in other types of surgery (Gray 2005). In the four studies included in our primary analysis that employed the dental model (Juhl 2006; Moller 2005a; Moller 2005b; Van Aken 2004), placebo rates were indeed very low, with the exception of the study by Van Aken and colleagues. Efficacy was also affected by study design. Five studies (three additional studies from the updated review) (Jahr 2012 Study 2, 65+; Jahr 2012 Study 3, 65+; Sinatra 2005; Wininger 2010; Zhou 2001), enrolled participants on the first postoperative day and allowed them to use patient‐controlled analgesia (PCA). One study administered the intervention without requiring that the patient report moderate‐to‐severe pain (Koppert 2006). All other studies were started at first report of moderate‐to‐severe pain and participants had to request rescue analgesia (Hynes 2006 also enrolled participants on the first postoperative day, but participants had to request analgesia). Sensitivity analysis, with these studies removed, suggests that IV paracetamol and/or propacetamol may have greater efficacy when administered on the day of surgery.
To assess for publication bias, we calculated the number of additional participants needed in studies with zero effect to increase the NNT for at least 50% pain relief to 10 or greater, which is what we considered to be clinically insignificant (Moore 2008). If the number of additional participants required was less than 400, we considered the result to be susceptible to publication bias. We established through these calculations that our analysis of IV propacetamol versus placebo for the number of participants with > 50% pain relief at six hours was susceptible to publication bias.
When assessing the clinical significance of the above findings, it is possible to indirectly compare the NNT for a single dose of IV paracetamol and/or IV propacetamol with that of a single dose of other analgesics (Bandolier 2010). In this update, the NNTs for combined IV paracetamol and IV propacetamol data (5 at four hours, 6 at six hours) are similar to those seen with various single doses of oral paracetamol (Toms 2008), but inferior to most orally or parenterally administered opioids. While these indirect comparisons are not surprising, the data should be interpreted with caution. The efficacy of the other analgesics in this 'league table' is measured over four to six hours, rather than discretely at four and six hours as we performed in our analyses. As demonstrated above, NNTs may increase (i.e., analgesia diminishes) if measured over six hours in drugs with a short duration of effect. Although NNTs for IV and oral paracetamol are similar, the studies included in each analysis would almost certainly have enrolled different populations. First, participants in the oral studies would have to be capable of taking oral medication immediately postoperatively. Oral administration of medications postoperatively is frequently problematic in that participants may be nauseated or vomiting or may have absorption issues, such as postoperative ileus. Second, participants in the oral studies may have had lower baseline pain. When baseline pain is low, a smaller absolute reduction in intensity is required to effect a clinically important change (Cepeda 2003).
For direct comparisons versus other analgesics, the combined analysis of IV paracetamol or propacetamol versus NSAIDs at six hours showed statistical superiority of NSAIDs. However, these data were highly susceptible to publication bias and we assessed the quality of evidence as very low according to GRADE.
Mean pain intensity at four and six hours was not presented in the original review because no studies reported these data. For this update, no studies utilizing propacetamol contributed data to pain intensity at either time point. We assessed the data as being of low to very low quality. Comparisons of IV paracetamol versus placebo demonstrated no difference at four hours and statistically significant, but clinically minor reductions in pain at six hours. This may be a consequence of availability of rescue medication. Comparison of IV paracetamol with NSAIDs showed statistical superiority of NSAIDs at both time points, although differences were minor. Analyses exhibited moderate heterogeneity quantified by the I2 statistic of 58% and 54% at four and six hours, respectively.
Secondary outcomes
Secondary efficacy outcomes included time to achieve 50% pain relief (however, no study reported these data, either in our original review or in our update), number of participants requiring rescue analgesia, time to rescue, opioid consumption, and global evaluation.
Data related to rescue medication demonstrated that fewer participants receiving IV paracetamol or IV propacetamol required rescue analgesia in the four‐ to six‐hour time period than those receiving placebo, and those that did require rescue analgesia waited an average of six minutes longer before requesting it than those receiving placebo. This delay in requiring rescue medication is unlikely to be clinically meaningful, and inspection of forest plots suggests substantial heterogeneity exists amongst the placebo‐controlled studies, quantified by the I2 statistic of 95% (P value < 0.00001). When we re‐analyzed data using a random‐effects model, the result of the analysis of number of participants requiring rescue medication (propacetamol versus placebo) was no longer statistically significant.
As in our original review, in the majority of studies included in the comparison 'opioid consumption', participants self administered opioid via PCA. Results showed that analgesic effectiveness of IV paracetamol or IV propacetamol appears to diminish between four and six hours, illustrated by the fact that over four hours there was a 26% reduction in opioid requirements compared with placebo versus only a 16% reduction over six hours. Evidence suggests that a reduction in opioid consumption of 30% to 40% is required in order to produce a reduction in opioid‐induced side effects (Marret 2005; Remy 2005); therefore these reductions would appear to fall short of those required to make a clinical difference (see 'Agreements and disagreements with other studies or reviews'). This shortfall is also confirmed by the lack of reliable data demonstrating a reduction in opioid‐induced AEs when comparing IV paracetamol/IV propacetamol to placebo (see 'Safety' below). Meta‐analyses did not demonstrate a statistical difference in opioid consumption between either IV paracetamol or IV propacetamol and NSAIDs.
Global evaluations demonstrated that 72% of participants rated their therapy as "good/satisfied" or better when receiving IV paracetamol/IV propacetamol versus 58% receiving placebo. When patient global evaluation was measured using a VAS, there was a 0.4‐point improvement on a 0 to 10 scale for those receiving IV paracetamol or IV propacetamol, although the analysis included only three studies. When data we re‐analyzed data using a random‐effects model, the result of the analysis of global evaluation using NRS was no longer statistically significant. We are not aware of a definition of clinically meaningful superiority in global evaluation when comparing interventions in acute pain; however, the limited NRS data demonstrate less than 20% absolute and relative improvement between IV paracetamol or propacetamol and placebo.
Meta‐analyses did not demonstrate clinically important differences between either IV paracetamol or IV propacetamol and NSAIDs or opioids for any other secondary pain outcome. There were fewer participants in head‐to‐head comparisons of analgesics than in the placebo‐controlled studies, which suggests that rather than demonstrating lack of difference there may be insufficient data to demonstrate a difference ('lack of evidence of efficacy' versus 'evidence of lack of efficacy'). Also, the nature of comparators, even within the same class of drugs, may vary considerably. For example, in one comparison three different NSAIDs were employed as comparators at doses that may not be equivalent.
Safety
In our updated review we added analyses of the number of participants that experienced a serious adverse event and the number of participants withdrawing due to AEs and removed analysis of several individual AEs (i.e., headache, vertigo/dizziness, fatigue, fever, gastrointestinal disorders, heart rate, malaise, bleeding, liver function test abnormalities, and hypotension). The rationale for removing these AEs was that each occurred too infrequently to allow for meaningful interpretation and analysis.
Reported AEs in a postoperative pain study may reflect a number of factors. The AEs may be due to the interventions themselves, or due to residual effects of anesthesia and surgery. Alternatively, they may be due to side effects of postoperative opioids, in which case one might expect to see a reduction in their incidence when an effective analgesic that lowers opioid consumption is administered, i.e., the AEs are a reflection of differences in efficacy rather than safety. Our analyses showed that despite demonstrating a reduction in morphine consumption at four and six hours, IV paracetamol and IV propacetamol did not produce statistically and/or clinically meaningful reductions versus placebo in the rate of AEs that were likely opioid‐induced or in the number of participants withdrawing due to AEs. Additionally, no statistical or clinical difference was noted for IV paracetamol or IV propacetamol versus active comparators. Lastly, inclusion of studies administering other analgesics in both treatment arms might affect the absolute rates of AEs and increase heterogeneity.
The analyses of participants withdrawing due to lack of efficacy included insufficient numbers of participants to allow for meaningful analysis. Our analyses and the outcomes of individual head‐to‐head studies (Marty 2005; Moller 2005a; Sinatra 2005) demonstrate that IV paracetamol and IV propacetamol have similar efficacy and safety, with the exception being that the incidence of pain on infusion is much higher in participants receiving IV propacetamol. The number of participants reporting pain with IV paracetamol was similar to placebo. Although most studies do not report the intensity of pain on infusion, it appears to be in the moderate‐to‐severe range (Jarde 1997) and may lead to interruption of the infusion (Moller 2005). Coupled with the fact that IV propacetamol requires reconstitution (with issues of contact dermatitis), whereas IV paracetamol comes ready to use, IV paracetamol would appear preferable, assuming its cost is justifiable based on improved outcomes and other cost avoidance. Subramanyam 2014 performed a cost‐effectiveness analysis of intraoperative use of IV acetaminophen in combination with opioids versus opioids alone in pediatric tonsillectomies and found the combination was overall less costly due to reduced requirement for rescue analgesia and shortened length of stay in the post‐anesthesia care unit.
There were few differences in the proportion of participants with individual AEs when IV paracetamol or IV propacetamol were compared to placebo or active comparators. The incidences of both nausea and vomiting were statistically lower with paracetamol as compared to placebo but the quality of evidence was low and the difference assessed as not clinically meaningful based on a NNH > 10 for both comparisons. There were no statistically significant differences in the rate of individual AEs when IV paracetamol or propacetamol were compared with NSAIDs. Comparisons with opioids demonstrated lower rates of nausea and vomiting in combined IV paracetamol/propacetamol arms; however, these data were highly subject to publication bias or were clinically insignificant. Statistically lower rates of sedation were found in the comparison of IV paracetamol versus opioids, but this analysis involved only three studies, demonstrated substantial heterogeneity (I2 86%, P value = 0.0003), and was again highly susceptible to publication bias. While our analyses showed little difference between IV formulations of paracetamol and NSAIDs or opioids, it is generally acknowledged that paracetamol has a superior safety profile than both in a controlled setting. Other than in situations of accidental overdose where hepatotoxicity may occur, AEs with paracetamol are rare (> 1/10,000 to < 1/1000) including malaise, hypotension, and increased levels of hepatic transaminases, or very rare (< 1/10,000) including hypersensitivity, thrombocytopenia, leucopenia, and neutropenia (EMC 2010). This illustrates that AE data from randomized controlled trials should be interpreted with caution. Studies are routinely underpowered to detect differences in AEs, and do not capture rare, but potentially catastrophic events. Additionally, as mentioned above only 13 studies confined AE data to the initial four‐ to six‐hour period from which we measured efficacy.
Overall completeness and applicability of evidence
Our analysis compared IV paracetamol and IV propacetamol with either placebo or an active comparator, which in turn could be an opioid, NSAID, or other analgesic. As discussed above, in a clinical setting, it is unlikely that IV paracetamol/IV propacetamol would be used instead of an opioid, except in surgeries expected to produce only mild‐to‐moderate pain. This somewhat limits the relevance and applicability of the head‐to‐head studies of IV paracetamol/IV propacetamol versus opioids. A more likely scenario is that either intervention would be used in addition to an opioid, either combined with a NSAID or, in participants considered to be at risk of bleeding, instead of a NSAID. Therefore, the comparisons of IV paracetamol/IV propacetamol with placebo or NSAID may more readily be extrapolated to clinical practice.
As described above, studies fell broadly into two designs: those in which the intervention was administered shortly before or after the end of surgery (prevention of pain) and the primary outcome was opioid consumption; or those in which the intervention was administered only if the participant reported moderate‐to‐severe pain post‐surgically (treatment of pain) and the primary outcome was pain relief/pain intensity difference. The former design may not accurately reflect a drug's efficacy in that some participants may never have developed moderate‐to severe‐pain. The latter studies offer proof of concept, i.e., they demonstrate that IV paracetamol or IV propacetamol has analgesic efficacy and allow us to make direct or indirect comparisons with other analgesics. However, they do not necessarily reflect practice, as it is unlikely that paracetamol alone would provide sufficient analgesia in moderate‐to‐severe pain for the majority of participants. Conversely, the former studies more accurately reflect clinical practice. Participants would routinely be administered postoperative analgesia rather than not receiving it until reporting severe pain.
As in the previous analysis, efficacy and AE outcomes were not consistently reported across the studies, and this limited our analyses to some extent. Our data have limited generalizability to the pediatric population as most included studies evaluated adults only, due to our inclusion criteria stipulating that pain be self reported.
Quality of the evidence
As with all quantitative systematic reviews, meta‐analyses are only as good as the data that are reported in each study. Efficacy data were analyzed over the time periods of either four or six hours, whereas most safety analyses used data from 24 hours or later.
When assessing the quality of findings using GRADE, we ranked quality from very low to high across the different efficacy outcomes. Lower rankings were primarily due to inconsistency (unexplained heterogeneity), imprecision (low sample sizes, low numbers of events, or wide confidence intervals), or publication bias (< 400 participants in studies of zero effect required to render result clinically insignificant). Regarding imprecision, only one of the 75 studies enrolled at least 200 participants in each arm of the study, i.e., was considered to be at low risk of sample size bias. Small studies are likely underpowered to detect statistical differences in what may be clinically relevant effects (Derry 2012). Lastly, indirectness of evidence was an issue for studies that utilized NSAIDs as the active comparator, as the NSAID utilized in studies varied. We included all NSAIDs regardless of cyclooxygenase selectivity, potency, and safety profile.
As previously stated our analyses of AEs are likely underpowered to detect notable differences, and do not capture rare, but potentially catastrophic events.
Potential biases in the review process
The review was restricted to randomized studies, thus limiting the potential for selection bias; however, we did include non‐blinded studies. Studies that do not blind both the participant and the investigator risk overestimating efficacy. We thought that blinding may be challenging given the various formulations and methods of administration of active comparators. Therefore, we decided to include both blinded and non‐blinded studies with the intention of performing a sensitivity analysis with non‐blinded studies removed. As stated above (see Sensitivity analysis), only five studies were defined as non‐blinded (Kamath 2014; Kara 2010; Koppert 2006; Mimoz 2001; Tuncel 2012). Removing data from these studies made no difference to either the direction or the statistical significance of any efficacy or safety outcome.
Other possible sources of bias that could have affected the review included:
Combining of studies from different surgical populations. As mentioned above, there was evidence of heterogeneity in our primary analyses. We planned to conduct a sensitivity analysis according to type of surgery for the primary outcome; however, there were insufficient numbers of studies in various types of surgeries for us to perform meaningful analyses. As previously mentioned, despite expected differences in pain intensity and duration, evidence suggests that analgesic response and derived NNTs are similar when comparing dental and other postsurgical models, and that it is legitimate to extrapolate efficacy from one pain model to another (Barden 2004).
The inclusion of nine studies that did not administer the equivalent of 1 g of paracetamol in at least one arm of the study. For three of these studies no data were used in the primary efficacy analyses (Hahn 2003; Hiller 2012; Paech 2014). One study employed a dose of 30 mg/kg propacetamol (Vuilleumier 1998). The average weight of participants receiving propacetamol was 69 kg, translating to an average of 2.07 g propacetamol or the equivalent of 1.04 g paracetamol.
Using a fixed‐effect model for statistical analysis. As stated in the results section, the point estimates of all of the primary analyses changed minimally when we employed a random‐effects model, and all statistically significant meta‐analyses remained so. The only difference, as expected, was that 95% confidence intervals widened with the random‐effects model. Of the secondary efficacy outcomes, only three of the analyses/subgroup analyses changed from being statistically significant to being non‐significant. In all of these situations the point estimate remained similar but the 95% confidence interval included no difference. Only the analysis of time to rescue medication resulted in substantial increases in the point estimate but this was attributed to one large study; when we removed this study both the fixed‐effect and random‐effects analyses were similar. Overall, therefore, we believe the conclusions of the review remain sound.
The possibility of publication bias (unpublished trials showing no benefit of IV paracetamol or IV propacetamol over placebo) may exist. We attempted to limit this by searching the clinical trials registry http://www.clinicaltrials.gov, reviewing internal reference lists from industry, and conducting additional analyses to aid our assessment. As previously mentioned, to assess for publication bias we calculated the number of additional participants needed in studies with zero effect required to change the NNT to 10, which is what we considered to be the threshold for a clinically meaningful effect.
Few manuscripts stated whether analyses were performed on intention‐to‐treat (ITT) or per‐protocol populations, and in those that did, imputation methods used when participants withdrew were (when stated at all) last observation carried forward. However, given the short duration of the time period of interest, there were few dropouts, if any, in the vast majority of studies.
Agreements and disagreements with other studies or reviews
Remy 2005 meta‐analyzed opioid consumption via PCA in participants receiving multiple doses of IV or oral paracetamol over 24 hours and found an overall reduction in morphine consumption of 20%. In turn, they analyzed whether this led to a reduction in opioid‐induced side effects (nausea, vomiting, sedation, urinary retention, and respiratory depression) and concluded that this modest reduction in opioid consumption made no clinical difference. Another systematic review, focusing on use of IV paracetamol following orthopedic surgery, concluded that there is a lack of data to support a reduction in opioid‐induced adverse effects with IV paracetamol (Jebaraj 2013). In our larger analysis, we compared incidence of the following AEs that could be considered to be opioid‐induced: nausea, vomiting, pruritus, respiratory depression, sedation, allergy/rash, and urinary retention. As with Remy 2005, we found no reliable evidence supporting a reduction in side effects in the IV paracetamol/IV propacetamol arms versus placebo, despite a small reduction in opioid requirements. In contrast, meta‐analyses of NSAIDs used in combination with PCA demonstrate a relative reduction in postoperative nausea and vomiting by 30%, nausea alone by 12%, vomiting alone by 32%, and sedation by 29% (Elia 2005; Marret 2005). It is not surprising, then, that our analyses did not demonstrate a reduction in morphine consumption in participants receiving IV propacetamol or IV paracetamol versus those receiving NSAIDs. This was in contrast to our previous review; however, the difference in our prior review was derived from limited, heterogenous data, was only significant at four hours, and lacked clinical significance (0.2 mg at four hours).
Previous meta‐analyses have used patient global evaluation as a surrogate marker for number of patients with at least 50% pain relief (Toms 2008). We chose to analyze it separately, as we believe that global evaluation is not only a measure of a drug's effectiveness, but also of its tolerability, and thus consider it to be a measure of patient's preference (Collins 2001). Patient‐controlled analgesia is routinely employed despite the only substantial difference in outcomes between it and as‐needed opioid analgesia being that patients prefer it (McNicol 2015). We found a difference of around 14% in the number of participants rating their medication as "good/satisfied" or better in those receiving IV paracetamol or IV propacetamol (or 0.4 on a 0 to 10 VAS). This modest difference may be in part due to the high proportion of participants receiving placebo who expressed satisfaction with their intervention (58%), which in turn could be due to the fact that those in the placebo group had access to rescue medication, or simply that inclusion in a trial may lead to participants being more closely monitored.
Apfel 2013 completed a systematic review and meta‐analysis to evaluate the impact of IV acetaminophen on the primary outcome of nausea and vomiting postoperatively. From the 30 studies analyzed, results demonstrated that acetaminophen was associated with a relative risk of 0.73 (95% CI 0.60 to 0.88) for nausea and 0.63 (95% CI 0.45 to 0.88) for vomiting. Heterogeneity was high among the studies, and a sensitivity analysis of investigator‐initiated versus industry‐sponsored studies showed differences in these effects. In the investigator‐initiated studies, nausea and vomiting reductions were significant. In the industry‐sponsored studies, nausea was not reduced (RR 1.12, 95% CI 0.85 to 1.48) and vomiting was increased (RR 1.41, 95% CI 1.02 to 1.96). Upon further review, the authors noted that in the investigator‐initiated studies, acetaminophen was often started prophylactically (before surgery and intraoperatively) as opposed to industry‐sponsored studies where administration was usually the day following surgery. Though a reduction in postoperative opioid consumption did not impact nausea and vomiting, a reduction in pain score was associated with significantly reduced nausea. Similarly, in our analyses most studies evaluated prophylactic administration of IV paracetamol with statistical reductions in nausea and vomiting despite only marginal reductions in opioid consumption. Evidence regarding the effectiveness of IV paracetamol in reducing the incidence and severity of nausea and/or vomiting, therefore, remains unclear. Perhaps, as others have suggested, there are still unknown mechanisms behind the antiemetic effect of paracetamol (Apfel 2013).
Authors' conclusions
Implications for practice.
We identified a large amount of additional data for this update; however our original conclusions remain largely unchanged.
For people with postoperative pain
Data of various quality demonstrate that a higher number of patients have clinically meaningful pain relief and that more patients are satisfied with treatment versus placebo. Patients should expect pain relief to be superior to that achieved with placebo, with a similar degree of side effects. Most patients will receive intravenous (IV) paracetamol or IV propacetamol as part of a multi‐modal pain regimen.
For clinicians
Our meta‐analysis includes high to very low quality evidence that IV paracetamol and IV propacetamol provide superior analgesia in comparison to placebo. Neither IV paracetamol nor IV propacetamol were clinically superior for any efficacy outcome versus other analgesic agents, such as nonsteroidal anti‐inflammatory drugs (NSAIDs) or opioids. Given alone, they are unlikely to provide sufficient analgesia after surgeries that produce moderate‐to‐severe pain. If used in combination with opioids they reduce opioid consumption, but this reduction does not appear sufficient to reduce opioid‐induced adverse effects (AEs). Both offer an advantage over oral paracetamol due to their faster onset of action and in that many patients are unable to tolerate oral medication postsurgically. Intravenous paracetamol may prove a better option versus IV propacetamol as reconstitution is not required and because the incidence of pain on infusion is reduced.
For policy makers
The availability of either IV paracetamol or IV propacetamol varies by country. The decision to add either formulation to a hospital formulary should take into account how adding one would affect current policies for analgesic algorithms, additional workload, and patient satisfaction.
For funders
The cost of IV paracetamol and IV propacetamol also varies by country. In the United States, IV paracetamol is considerably more expensive than either the oral formulation or than parenteral formulations of other analgesics. There are few studies comparing oral versus parenteral formulations of paracetamol; however, given the postoperative setting, the utility of orally administered analgesia may be limited. Our findings do not demonstrate superiority in efficacy or safety of IV paracetamol versus other analgesics that would justify increased cost. However, given that hospital reimbursement is, in part, contingent on patient satisfaction data in some countries, increases in direct costs may be offset by such policies.
Implications for research.
General
More studies that assess self reported pain in pediatric patients are required. Self report pain assessment tools such as the Faces Pain Scale and the Color Analog Scale are validated for use in children as young as three years of age.
Design
Our analyses, based on limited evidence, suggest that IV propacetamol or IV paracetamol reduce opioid consumption, but not to a sufficient degree to reduce opioid‐induced AEs. Larger trials that accurately and prospectively assess adverse events, and that are sufficiently powered to demonstrate a difference, are required to confirm or contradict this finding. Many of the included studies may have been underpowered to show a difference between interventions where one actually exists.
Measurement (endpoints)
Few included studies reported power calculations ‐ future studies should include them. One of the included studies tested a dose of 2 g of IV paracetamol and demonstrated superior analgesic efficacy versus 1 g (Juhl 2006). Further studies at this higher dose may provide evidence that IV paracetamol reduces opioid consumption to an extent that opioid‐induced AEs are reduced. Equally, they may show an increase in paracetamol‐induced AEs.
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2019 | Amended | Contact details updated. |
| 13 May 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 2 October 2015 | New citation required but conclusions have not changed | Results from original primary outcomes unchanged. Some changes in secondary outcome results. New comparisons added related to safety and withdrawals did not produce enough data for meaningful analysis. Some safety outcomes excluded. New safety outcomes added. New efficacy outcome (mean pain at 4 hours and 6 hours) added. Minor changes in inclusion and exclusion criteria. |
| 2 October 2015 | New search has been performed | Search updated 16 February 2016. 1179 new studies identified; 39 additional studies included in update; 7200 participants in total. 'Risk of bias' tables expanded and 'Summary of findings' tables added. |
| 1 May 2008 | Amended | New authorship and Methods section updated |
| 17 April 2008 | Amended | Converted to new review format |
| 7 December 2007 | New citation required and major changes | Substantive amendment |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Although we identified eight studies that may be eligible for inclusion (see Characteristics of studies awaiting classification), we suspect they will be small and of low quality and therefore unlikely to change our confidence in the estimate of effect. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review again if substantial new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We appreciated the contributions of the authors of the original review, Dr. Soledad Cepeda, Dr. Aikaterini Tzortzopoulou, Dr. Marie Belle Francia and Dr. Tamman Farhat. We are grateful to Zehui He, PhD from the Department of Big Medical Data, Guangzhou University of Chinese Medicine, Guangzhou, China, for translation and extraction of a Chinese language article. Lastly, we would like to thank Joanne Abbott, TSC, from the Cochrane Pain, Palliative and Supportive Care Group for running and compiling all of the literature searches for our review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE & MEDLINE in Process (OVID) May 2010 to 16 February 2016
1. Acetaminophen/
2. (paracetamol or acetaminophen).tw.
3. Infusions, Intravenous/
4. (intravenous or intra‐venous).ti.
5. (intravenous or intra‐venous).ab.
6. "IV".ti.
7. "IV".ab.
8. 1 or 2
9. 3 or 4 or 5 or 6 or 7
10. 8 and 9
11. propacetamol.tw.
12. 10 or 11
13. exp Pain/
14. exp Pain, Postoperative/
15. pain.tw.
16. analgesi*.tw.
17. exp Specialties, Surgical/
18. surgery.tw.
19. exp Surgery, Oral/
20. or/13‐19
21. 12 and 20
22 randomized controlled trial.pt.
23 controlled clinical trial.pt.
24 randomized.ab.
25 placebo.ab.
26 drug therapy.fs.
27 randomly.ab.
28 trial.ab.
29 groups.ab.
30 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31 exp animals/ not humans.sh.
32 30 not 31
33 21 and 32
| MEDLINE (run 10 May 2010) |
| 1 RANDOMIZED CONTROLLED TRIAL.pt. (290189) |
| 2 CONTROLLED CLINICAL TRIAL.pt. (81509) |
| 3 RANDOMIZED CONTROLLED TRIALS.sh. (0) |
| 4 RANDOM ALLOCATION.sh. (68229) |
| 5 DOUBLE BLIND METHOD.sh. (106417) |
| 6 SINGLE BLIND METHOD.sh. (13917) |
| 7 1 or 2 or 3 or 4 or 5 or 6 (431925) |
| 8 (ANIMALS not HUMAN).sh (4555526) |
| 9 7 not 8 (387859) |
| 10 CLINICAL TRIAL.pt. (461595) |
| 11 exp CLINICAL TRIAL/ (609241) |
| 12 (clin$ adj25 trial$).ti,ab. (173685) |
| 13 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (106621) |
| 14 PLACEBOS.sh. (28814) |
| 15 placebo$.ti,ab. (122893) |
| 16 random$.ti,ab. (480583) |
| 17 RESEARCH DESIGN.sh. (59058) |
| 18 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (1033443) |
| 19 18 not 8 (908539) |
| 20 19 or 9 (921089) |
| 21 paracetamol.mp. or exp Acetaminophen/ (13307) |
| 22 ACETAMINOPHEN/ (11522) |
| 23 INFUSIONS, INTRAVENOUS/ (42133) |
| 24 (intravenous or intra‐venous).mp. or "IV" (ti,ab) [mp=title, original title, abstract, name of substance word, subject heading word] (474942) |
| 25 infusion& (ti,ab,kw) (161134) |
| 26 21 or 22 (13307) |
| 27 23 or 24 or 25 (571958) |
| 28 26 and 27 (1064) |
| 29 propacetamol (ti,ab,kw) (139) |
| 30 28 or 29 (1120) |
| 31 exp Pain/ or exp Pain, Postoperative/ (248648) |
| 32 pain$ (ti,ab,kw) (324242) |
| 33 exp SURGERY, ORAL/ (6041) |
| 34 ORAL SURGICAL PROCEDURES.mp. or exp Oral Surgical Procedures/ (42154) |
| 35 analgesi$ (ti,ab) (67402) |
| 36 31 or 32 or 33 or 34 or 35 (512066) |
| 37 20 and 30 and 36 (292) |
Appendix 2. CENTRAL search strategy
CENTRAL Issue 1 of 12, 2016 (The Cochrane Library) (searched 2010‐2016)
#1 MeSH descriptor: [Acetaminophen] this term only
#2 (paracetamol or acetaminophen):ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Infusions, Intravenous] this term only
#4 (intravenous or intra‐venous):ti,ab,kw (Word variations have been searched)
#5 "IV":ti,ab,kw (Word variations have been searched)
#6 (#1 or #2) and (#3 or #4 or #5)
#7 propacetamol:ti,ab,kw (Word variations have been searched)
#8 #6 or #7
#9 MeSH descriptor: [Pain] explode all trees
#10 MeSH descriptor: [Pain, Postoperative] explode all trees
#11 pain:ti,ab,kw (Word variations have been searched)
#12 analgesi*:ti,ab,kw (Word variations have been searched)
#13 MeSH descriptor: [Specialties, Surgical] explode all trees
#14 surgery:ti,ab,kw (Word variations have been searched)
#15 MeSH descriptor: [Surgery, Oral] explode all trees
#16 #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #8 and #16 Publication Year from 2010 to 2014
| CENTRAL (run 10 May 2010) |
| 1 ACETAMINOPHEN/ (1462) |
| 2 paracetamol.mp. or acetaminophen (ti,ab,kw) [mp=title, original title, abstract, mesh headings, heading words, keyword] (2761) |
| 3 INFUSIONS, INTRAVENOUS/ (7592) |
| 4 (intravenous or intra‐venous).mp. or IV.in. [mp=title, original title, abstract, mesh headings, heading words, keyword] (37164) |
| 5 1 or 2 (3017) |
| 6 3 or 4 (37164) |
| 7 5 and 6 (405) |
| 8 propacetamol (ti,ab,kw) (118) |
| 9 7 or 8 (479) |
| 10 pain.mp. or exp Pain/ or exp Pain, Postoperative/ (50055) |
| 11 pain$ (ti,ab) (41517) |
| 12 postoperative pain (ti,ab) (5190) |
| 13 postsurgical pain (ti,ab) (114) |
| 14 analgesi$ (ti,ab) (18117) |
| 15 10 or 11 or 12 or 13 or 14 (56219) |
| 16 exp Surgery/ (187) |
| 17 oral surgery.mp. or exp Surgery, Oral/ (660) |
| 18 ORAL SURGICAL PROCEDURES.mp. [mp=title, original title, abstract, mesh headings, heading words, keyword] (327) |
| 19 dental surgery.mp. [mp=title, original title, abstract, mesh headings, heading words, keyword] (196) |
| 20 16 or 17 or 18 or 19 (1278) |
| 21 15 or 20 (56993) |
| 22 9 and 21 (366) |
Appendix 3. LILACS search strategy
LILACS (Birme) 2010 to 2016
propacet$ OR (paracetamol and intraven$) OR (paracetamol and infus$) OR (acetaminophen and intraven$) OR (acetaminophen and infus$) [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
| LILACS (run 10 May 2010) |
| 1. propacet$ OR paracetamol intraven$ OR paracetamol infus$ OR acetaminophen intraven$ OR acetaminophen infus$ (2272) |
| 2. Limit 1 to humans (1335) |
| 3. Limit 2 to Controlled Clinical trials (47) |
Appendix 4. EMBASE search strategy
EMBASE (OVID) May 2010 to 16/2/16
1. paracetamol/
2. (paracetamol or acetaminophen).tw.
3. intravenous drug administration/
4. (intravenous or intra‐venous).ti.
5. (intravenous or intra‐venous).ab.
6. "IV".ti.
7. "IV".ab.
8. 1 or 2
9. 3 or 4 or 5 or 6 or 7
10. 8 and 9
11. propacetamol.tw.
12. 10 or 11
13. exp Pain/
14. exp postoperative pain/
15. pain.tw.
16. analgesi*.tw.
17. exp surgery/
18. surgery.tw.
19. exp oral surgery/
20. or/13‐19
21. 12 and 20
22. random$.tw.
23. factorial$.tw.
24. crossover$.tw.
25. cross over$.tw.
26. cross‐over$.tw.
27. placebo$.tw.
28. (doubl$ adj blind$).tw.
29. (singl$ adj blind$).tw.
30. assign$.tw.
31. allocat$.tw.
32. volunteer$.tw.
33. Crossover Procedure/
34. double‐blind procedure.tw.
35. Randomized Controlled Trial/
36. Single Blind Procedure/
37. or/22‐36
38. (animal/ or nonhuman/) not human/
39. 37 not 38
40. 21 and 39
| EMBASE (1980 to 2010 week 18) |
| 1 Paracetamol/ (41655) |
| 2 (paracetamol or acetaminophen).ti,sh,ab. (42813) |
| 3 (intravenous or intra‐venous or "IV").ti,sh,ab. (346916) |
| 4 (1 or 2) and 3 (2410) |
| 5 Propacetamol/ (487) |
| 6 propacetamol.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (504) |
| 7 5 or 6 (504) |
| 8 4 or 7 (2815) |
| 9 Postoperative Pain/ (22153) |
| 10 ((Pain$ adj6 (postoperat$ or post‐operat$ or "after operat$" or postsurgery or "post surgery" or "post surgical" or post‐surg$ or "after surg$" or "following surg$" or "follow$ operat$")) or post‐operative‐pain or (pain‐control$ adj6 ("following surgery" or "after operat$" or postoperat$ or postsurgery or post‐surg$ or "post surg$")) or ("pain relief" adj6 ("after surgery" or postoperative$ or "following surgery")) or (pain‐relief adj6 ("after surgery" or postoperative$ or post‐operativ$ or "following surgery"))).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (34456) |
| 11 (analgesi$ adj6 (postoperat$ or post‐operat$ or "after operat$" or postsurgery or "following surgery" or "after operat$" or "post surgery" or "post surgical" or post‐surg$ or "after surg$" or "following surg$" or "follow$ operat$")).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (19842) |
| 12 9 or 10 or 11 (40326) |
| 13 8 and 12 (837) |
| 14 random*.ti,ab. (437492) |
| 15 factorial*.ti,ab. (9360) |
| 16 (crossover* or cross over* or cross‐over*).ti,ab. (42161) |
| 17 placebo*.ti,ab. (118485) |
| 18 (doubl* adj blind*).ti,ab. (90128) |
| 19 (singl* adj blind*).ti,ab. (8106) |
| 20 assign*.ti,ab. (119788) |
| 21 allocat*.ti,ab. (38303) |
| 22 volunteer*.ti,ab. (105881) |
| 23 CROSSOVER PROCEDURE.sh. (22900) |
| 24 DOUBLE‐BLIND PROCEDURE.sh. (77599) |
| 25 RANDOMIZED CONTROLLED TRIAL.sh. (187136) |
| 26 SINGLE BLIND PROCEDURE.sh. (9365) |
| 27 or/14‐26 (724675) |
| 28 ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ (3671107) |
| 29 HUMAN/ (6992618) |
| 30 28 and 29 (608727) |
| 31 28 not 30 (3062380) |
| 32 27 not 31 (630733) |
| 33 13 and 32 (483) |
Appendix 5. Calculations for deriving number of participants experiencing at least 50% of maximum pain relief
Categorical total pain relief
% with at least 50% of maximum pain relief = 1.33 (TOTPAR x 100/ MAX TOTPAR) ‐ 11.52
VAS total pain relief
% with at least 50% of maximum pain relief = 1.15 (TOTPAR x 100/ MAX TOTPAR) ‐ 8.51
Categorical summed pain intensity difference
% with at least 50% of maximum pain relief = 1.36 (SPID x 100/ MAX SPID) ‐ 2.3
VAS summed pain intensity difference
% with at least 50% of maximum pain relief = 1.18 (SPID x 100/ MAX SPID) ‐ 2.2
MAX = maximum; SPID = summed pain intensity difference; TOTPAR = total pain relief
Data and analyses
Comparison 1. Number of participants with > 50% pain relief over 4 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 11 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [2.01, 3.19] |
| 1.1 Paracetamol vs placebo | 5 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.80 [2.30, 10.00] |
| 1.2 Propacetamol vs placebo | 8 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.77] |
| 2 Paracetamol or propacetamol vs NSAIDs | 5 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.18] |
| 2.1 Paracetamol vs NSAIDs | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.34] |
| 3 Propacetamol vs opioids | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.59] |
| 4 Paracetamol vs propacetamol | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
Comparison 2. Number of participants with > 50% pain relief over 6 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 10 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [2.10, 3.91] |
| 1.1 Paracetamol vs placebo | 6 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.65 [2.15, 6.21] |
| 1.2 Propacetamol vs placebo | 6 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.64, 3.50] |
| 2 Paracetamol or propacetamol vs NSAIDs | 5 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.95] |
| 2.1 Paracetamol vs NSAIDs | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
| 2.2 Propacetamol vs NSAIDs | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.02] |
| 3 Propacetamol vs opioids | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 4 Paracetamol vs propacetamol | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
Comparison 3. Pain intensity at 4 h.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol vs placebo | 6 | 485 | Mean Difference (IV, Fixed, 95% CI) | ‐1.21 [‐3.73, 1.31] |
| 2 Paracetamol vs NSAIDs | 6 | 350 | Mean Difference (IV, Fixed, 95% CI) | 5.02 [3.18, 6.86] |
| 3 Paracetamol vs opioids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Paracetamol vs ketamine | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐19.23, ‐4.77] |
3.4. Analysis.
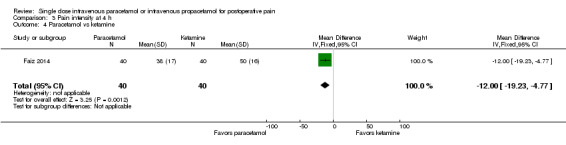
Comparison 3 Pain intensity at 4 h, Outcome 4 Paracetamol vs ketamine.
Comparison 4. Pain intensity at 6 h.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol vs placebo | 12 | 837 | Mean Difference (IV, Fixed, 95% CI) | ‐7.48 [‐8.98, ‐5.97] |
| 2 Paracetamol vs NSAIDs | 9 | 524 | Mean Difference (IV, Fixed, 95% CI) | 2.95 [1.18, 4.72] |
| 3 Paracetamol vs opioids | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.57, 7.57] |
| 4 Paracetamol vs ketamine | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐18.28, ‐7.72] |
4.3. Analysis.
Comparison 4 Pain intensity at 6 h, Outcome 3 Paracetamol vs opioids.
4.4. Analysis.
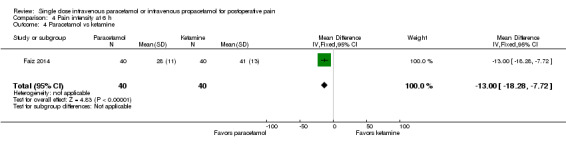
Comparison 4 Pain intensity at 6 h, Outcome 4 Paracetamol vs ketamine.
Comparison 5. Number of participants requiring rescue medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 9 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.77] |
| 1.1 Paracetamol vs placebo | 6 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.82] |
| 1.2 Propacetamol vs placebo | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.35, 0.69] |
| 2 Paracetamol or propacetamol vs NSAIDs | 5 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| 2.1 Paracetamol vs NSAIDs | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.98] |
| 2.2 Propacetamol vs NSAIDs | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 3 Propacetamol vs opioids | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.72, 4.64] |
| 4 Paracetamol vs propacetamol | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.82, 2.17] |
Comparison 6. Time to rescue medication (minutes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 9 | 839 | Mean Difference (IV, Fixed, 95% CI) | 6.43 [4.54, 8.32] |
| 1.1 Paracetamol vs placebo | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | 5.78 [3.86, 7.71] |
| 1.2 Propacetamol vs placebo | 3 | 316 | Mean Difference (IV, Fixed, 95% CI) | 23.72 [13.79, 33.65] |
| 2 Paracetamol vs NSAIDs | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐36.21, 33.92] |
Comparison 7. Opioid consumption (IV morphine equivalents) over 4 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 6 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.81, ‐1.03] |
| 1.1 Paracetamol vs placebo | 4 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.75, ‐0.91] |
| 1.2 Propacetamol vs placebo | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.15, ‐0.95] |
| 2 Paracetamol or propacetamol vs NSAIDs | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.37, ‐0.02] |
| 2.1 Paracetamol vs NSAIDs | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.04, 1.59] |
| 2.2 Propacetamol vs NSAIDs | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 3 Propacetamol vs opioids | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.09, 1.09] |
Comparison 8. Opioid consumption (IV morphine equivalents) over 6 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 13 | 777 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐2.41, ‐1.42] |
| 1.1 Paracetamol vs placebo | 8 | 404 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐2.35, ‐1.31] |
| 1.2 Propacetamol vs placebo | 6 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐4.21, ‐1.13] |
| 2 Paracetamol or propacetamol vs NSAIDs | 8 | 540 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.12] |
| 2.1 Paracetamol vs NSAIDs | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐0.87, 2.49] |
| 2.2 Propacetamol vs NSAIDs | 5 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 3 Propacetamol vs opioids | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.01, 2.01] |
| 4 Paracetamol vs propacetamol | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.15, 3.35] |
Comparison 9. Global evaluation rated as good/satisfied or excellent/very satisfied.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 16 | 2015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.25, 1.43] |
| 1.1 Paracetamol vs placebo | 9 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.31, 1.60] |
| 1.2 Propacetamol vs placebo | 9 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 2 Paracetamol or propacetamol vs NSAIDs | 11 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 2.1 Paracetamol vs NSAIDs | 6 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2.2 Propacetamol vs NSAIDs | 5 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 3 Propacetamol vs opioids | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 3.1 Propacetamol | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 4 Paracetamol vs propacetamol | 2 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.15] |
Comparison 10. Global evaluation using a numerical rating scale (0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 3 | 342 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.04, 0.66] |
| 1.1 Paracetamol vs placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 1.2 Propacetamol vs placebo | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | 1.64 [1.04, 2.25] |
| 2 Paracetamol vs NSAIDs | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.63, 0.03] |
| 3 Propacetamol vs opioids | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.18, 0.98] |
Comparison 11. Number of participants with adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 20 | 2359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| 1.1 Paracetamol vs placebo | 12 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.19] |
| 1.2 Propacetamol vs placebo | 10 | 1409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 2 Paracetamol or propacetamol vs NSAIDs | 6 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.32] |
| 2.1 Paracetamol vs NSAIDs | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.37] |
| 3 Propacetamol vs opioids | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.23] |
Comparison 12. Number of participants with serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 10 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| 1.1 Paracetamol vs placebo | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| 1.2 Propacetamol vs placebo | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Paracetamol or propacetamol vs NSAIDs | 9 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.65] |
| 2.1 Paracetamol vs NSAIDs | 4 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 2.2 Propacetamol vs NSAIDs | 5 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 3 Paracetamol or propacetamol vs opioids | 3 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 3.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
Comparison 13. Number of participants withdrawing due to adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 37 | 2654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.56, 2.84] |
| 1.1 Paracetamol vs placebo | 27 | 1912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.17] |
| 1.2 Propacetamol vs placebo | 12 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.25, 6.68] |
| 2 Paracetamol or propacetamol vs NSAIDs | 24 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.42, 3.12] |
| 2.1 Paracetamol vs NSAIDs | 19 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.22] |
| 2.2 Propacetamol vs NSAIDs | 5 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.22, 12.37] |
| 3 Paracetamol or propacetamol vs opioids | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Paracetamol vs ketamine | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Number of participants withdrawing due to lack of efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol or propacetamol vs placebo | 38 | 2600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.79] |
| 1.1 Paracetamol vs placebo | 26 | 1711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.78] |
| 1.2 Propacetamol vs placebo | 14 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.80] |
| 2 Paracetamol or propacetamol vs NSAIDs | 24 | 1393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.81, 2.08] |
| 2.1 Paracetamol vs NSAIDs | 18 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.89] |
| 2.2 Propacetamol vs NSAIDs | 6 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.78, 2.03] |
| 3 Paracetamol or propacetamol vs opioids | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 4 Paracetamol vs ketamine | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Number of participants with pain on infusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol vs placebo | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.80, 11.54] |
| 2 Propacetamol vs placebo | 6 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.07 [5.35, 31.98] |
| 3 Propacetamol vs paracetamol | 3 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.31 [4.20, 16.46] |
Comparison 16. Individual adverse events: paracetamol or propacetamol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 15 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.98] |
| 1.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 1.2 Propacetamol vs placebo | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.98, 2.69] |
| 2 Vomiting | 15 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| 2.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.80] |
| 2.2 Propacetamol vs placebo | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.75, 3.48] |
| 3 Nausea/vomiting | 10 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.08] |
| 3.1 Paracetamol vs placebo | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.57, 1.70] |
| 3.2 Propacetamol vs placebo | 6 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 4 Pruritus | 7 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.60, 1.40] |
| 4.1 Paracetamol vs placebo | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.72] |
| 4.2 Propacetamol vs placebo | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.51] |
| 5 Respiratory depression | 11 | 1082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.92] |
| 5.1 Paracetamol vs placebo | 6 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.65] |
| 5.2 Propacetamol vs placebo | 5 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.35, 2.80] |
| 6 Sedation | 10 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.51] |
| 6.1 Paracetamol vs placebo | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.01] |
| 6.2 Propacetamol vs placebo | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
| 7 Urinary retention | 8 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 7.1 Paracetamol vs placebo | 5 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.66] |
| 7.2 Propacetamol vs placebo | 3 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.65, 1.66] |
| 8 Allergy/skin rash/local reaction | 7 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.61, 3.91] |
| 8.1 Paracetamol vs placebo | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.24, 4.34] |
| 8.2 Propacetamol vs placebo | 4 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.57, 6.73] |
16.4. Analysis.
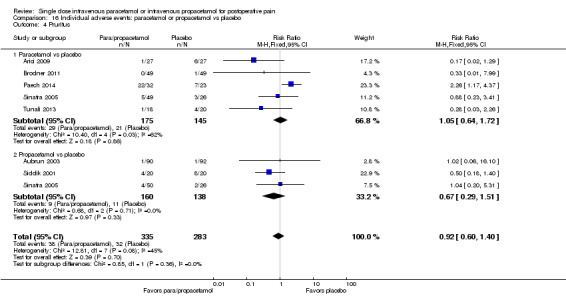
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 4 Pruritus.
16.5. Analysis.
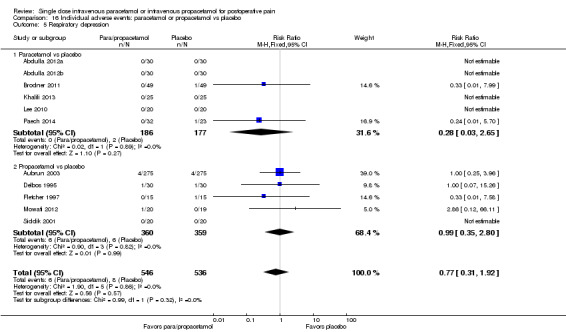
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 5 Respiratory depression.
16.6. Analysis.
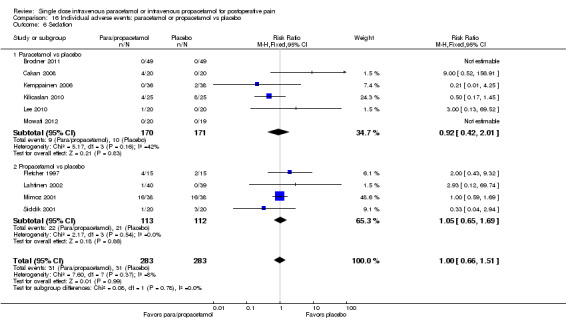
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 6 Sedation.
16.7. Analysis.
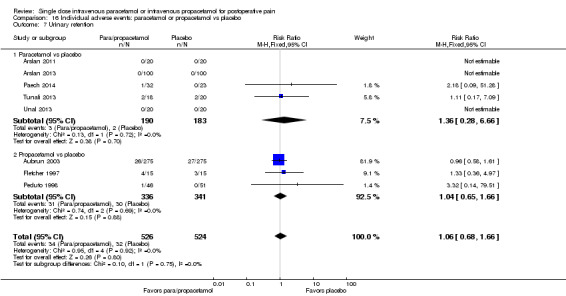
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 7 Urinary retention.
16.8. Analysis.
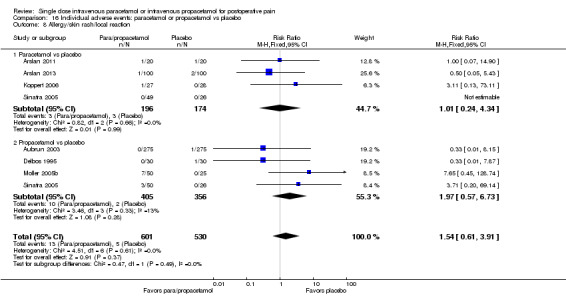
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 8 Allergy/skin rash/local reaction.
Comparison 17. Individual adverse events: paracetamol or propacetamol vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.28] |
| 1.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.31] |
| 1.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.44] |
| 2 Vomiting | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.89, 1.55] |
| 2.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.81] |
| 3 Nausea/vomiting | 8 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.97] |
| 3.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.42, 2.39] |
| 3.2 Propacetamol vs NSAIDs | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.60] |
| 4 Pruritus | 8 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.34] |
| 4.1 Paracetamol vs NSAIDs | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.51] |
| 4.2 Propacetamol vs NSAIDs | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.60] |
| 5 Respiratory depression | 9 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| 5.1 Paracetamol vs NSAIDs | 8 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| 5.2 Propacetamol vs NSAIDs | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation | 6 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.63, 10.75] |
| 6.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 6.2 Propacetamol vs NSAIDs | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.63, 21.22] |
| 7 Urinary retention | 6 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.51, 2.32] |
| 7.1 Paracetamol vs NSAIDs | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 4.02] |
| 7.2 Propacetamol vs NSAIDs | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.47, 2.78] |
| 8 Allergy/skin rash/local reaction | 8 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.26] |
| 8.1 Paracetamol vs NSAIDs | 6 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.02] |
| 8.2 Propacetamol vs NSAIDs | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.16] |
17.1. Analysis.
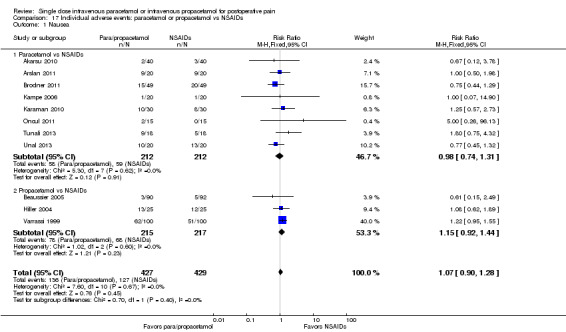
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 1 Nausea.
17.2. Analysis.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 2 Vomiting.
17.3. Analysis.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 3 Nausea/vomiting.
17.4. Analysis.
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 4 Pruritus.
17.5. Analysis.
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 5 Respiratory depression.
17.6. Analysis.
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 6 Sedation.
17.7. Analysis.
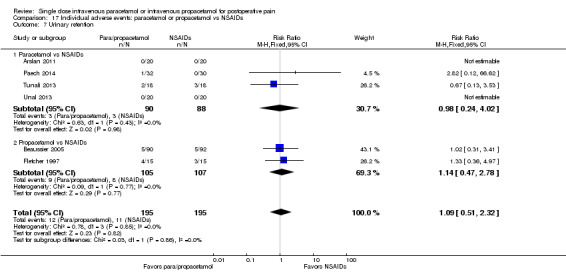
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 7 Urinary retention.
17.8. Analysis.
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 8 Allergy/skin rash/local reaction.
Comparison 18. Individual adverse events: paracetamol or propacetamol vs opioids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 6 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.65] |
| 1.1 Paracetamol vs opioids | 4 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.56] |
| 1.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 2 Vomiting | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.72] |
| 2.1 Paracetamol vs opioids | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.71] |
| 2.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.62] |
| 3 Nausea/vomiting | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| 3.1 Propacetamol vs opioids | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| 4 Pruritus | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.43] |
| 4.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| 4.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.10, 1.19] |
| 5 Respiratory depression | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| 6.1 Paracetamol vs opioids | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
18.3. Analysis.
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 3 Nausea/vomiting.
18.4. Analysis.
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 4 Pruritus.
18.5. Analysis.
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 5 Respiratory depression.
Comparison 19. Individual adverse events: paracetamol vs ketamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.30] |
| 2 Vomiting | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.22, 1.33] |
| 3 Sedation | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulla 2012a.
| Methods | Double‐blind, placebo and active‐controlled, multiple dose, over 24 h First dose administered 15 min prior to extubation |
|
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 30/30 Age (mean, SD): 44.5 ± 15.1 Sex (male, %): 16.7% Placebo Entered/completing: 30/30 Age (mean, SD): 47.9 ± 11.8 Sex (male, %): 10.0% Parecoxib Entered/completing: 30/30 Age (mean, SD): 48.3 ± 14.2 Sex (male, %): 23.3% Metamizole Entered/completing: 30/30 Age (mean, SD): 43.8 ± 13.7 Sex (male, %): 16.7% |
|
| Interventions | 1 g paracetamol in 100 ml normal saline over 15 min Placebo, parecoxib 40 mg, or metamizole 1 g: all in 100 ml NS over 15 min |
|
| Outcomes | Primary: accumulated opioid consumption (piritramide via PCA) Secondary: Pain intensity (VAS) Pain relief (VRS) Patient satisfaction (VRS) |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (http://www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope, only opened in emergency |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The study solutions were clear so that they could not be recognized by the anesthesiologists collecting the data and were prepared by one of the researchers who was not involved in the intraoperative and postoperative treatment of these patients”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or protocol violations – complete data set obtained for all 4 groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but side effects not monitored adequately |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo, 30 parecoxib, 30 metamizole) |
Abdulla 2012b.
| Methods | Double‐blind, placebo and active‐controlled, multiple dose, over 24 h First dose administered 15 min prior to extubation |
|
| Participants | Type of surgery: laparoscopic cholecystectomy Paracetamol group Entered/completing: 30/30 Age (mean, SD): 52.5 ± 15.8 Sex (male, %): 23.3% Placebo Entered/completing: 30/30 Age (mean, SD): 47.1 ± 13.9 Sex (male, %): 20.0% Parecoxib Entered/completing: 30/30 Age (mean, SD): 54.9 ± 13.0 Sex (male, %): 26.7% Metamizole Entered/completing: 30/30 Age (mean, SD): 52.4 ± 15.6 Sex (male, %): 30.0% |
|
| Interventions | 1 g paracetamol in 100 ml normal saline over 15 min Placebo, parecoxib 40 mg, or metamizole 1 g: all in 100 ml NS over 15 min |
|
| Outcomes | Primary: accumulated opioid consumption (piritramide via PCA) Secondary: Pain intensity (VAS) Pain relief (VRS) Patient satisfaction (VRS) |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (http://www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | The group assignment code was retained until the conclusion of the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The study solutions were prepared by one of the researchers who were not involved in the intraoperative and postoperative treatment of these patients. Postoperative data were collected by anesthesiologists who were blinded as to the treatment used. Other caretakers were also unaware of the analgesic drug that would be used for each patient during the study”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or protocol violations – complete data set obtained for all 4 groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but side effects not monitored adequately and satisfaction score results not fully described |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo, 30 parecoxib, 30 metamizole) |
Akarsu 2010.
| Methods | Randomized, double‐blind, parallel, active‐controlled. Pain evaluated up to 6 h after dose administered. | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 40/unclear Age (mean, SD): 24.2 ± 1.1 Sex (male, %): 0 Control group Entered/completing: 40/unclear Age (mean, SD): 24.4 ± 1.2 Sex (male, %): 0 |
|
| Interventions | Paracetamol 1 g IV over 15 min at first complaint of pain Diclofenac 75 mg IM as above |
|
| Outcomes | Primary: time to first rescue analgesic (1 mg/kg IM pethidine) Secondary: VAS pain scores at 30 min, 1, 2, 4 and 6 h; adverse events |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes ‐ all P values > 0.05: demographic characteristics, week of pregnancy, newborn's weight, Apgar scores, operation time, time to first postoperative analgesic requirement | |
| Details of preoperative pain | Participants with previous continuous analgesic use were excluded | |
| Notes | Translated from Turkish using Google Translate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used envelopes to randomly divide 2 groups – no additional details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details but stated as double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of participants dropping out |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 diclofenac) |
Akil 2014.
| Methods | Parallel, double‐blind, double dummy, active‐controlled study Interventions administered 5 min after perineal repair and at 6 h |
|
| Participants | Type of surgery: repair of episiotomy or perineal tear post vaginal delivery using cut and suturing Paracetamol group Entered/completing: 46/41 Age (mean, SD): 24.71 ± 4.91 Sex (male, %): 0 Control group Entered/completing: 49/41 Age (mean, SD): 24.71 ± 5.83 Sex (male, %): 0 |
|
| Interventions | Paracetamol 1 g/100 ml via slow infusion q6 x 2 doses Dexketoprofen 50 mg IV also via slow infusion q6 x 2 doses |
|
| Outcomes | Primary: VAS (0 to 10 0 to 100?) scores at 1 h Secondary: VAS at 6 and 12 h, adverse events. VAS at 2 and 3 also measured and reported, but not listed as outcomes. |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Only difference was gravidity (P value = 0.02). No significant differences in sociodemographic data or baseline parameters (parity, age, the length of the labour, birth weight, dose of the local anesthetic used during the repair of episiotomy or perineal tears (lidocaine) and VAS 0. | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Block randomization was achieved by using a computer‐generated random number chart” |
| Allocation concealment (selection bias) | Low risk | “Consecutive numbers generated by the computer were written on the numbered opaque envelopes, which were sealed by someone other than those involved in the study” |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded, but no details other than that participants in both of the groups had also placebo injections mimicking the active drug |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in paracetamol group and 2 participants in dexketoprofen group not included in final analysis due to incomplete data. 4 participants in paracetamol group and 6 in dexketoprofen group excluded due to need for extra analgesic. Despite flow chart, unclear if these participants were excluded before or after receiving interventions. |
| Selective reporting (reporting bias) | Low risk | Adverse events assessed but reported that none occurred in any participant |
| Size | High risk | Fewer than 50 participants per arm of the study (46 paracetamol, 49 dexketoprofen) |
Arici 2009.
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled Medications administered prior to skin closure |
|
| Participants | Type of surgery: elective total abdominal hysterectomy by laparotomy Paracetamol group Entered/completing: 30/27 Age (mean, SD): 47.73 ± 7.20 Sex (male, %): 0 Placebo group Entered/completing: 30/27 Age (mean, SD): 49.90 ± 6.40 Sex (male, %): 0 |
|
| Interventions | Intervention: paracetamol 1 g/100 ml IV at skin closure Placebo: normal saline 100 ml Third group received paracetamol preemptively (not included in analysis) |
|
| Outcomes | Pain intensity at rest and movement (VAS) Opioid consumption (morphine) All other outcomes reported at 24 h only |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, height, weight, ASA status, time of operation | |
| Details of preoperative pain | Not reported | |
| Notes | Preemptive group had lower morphine consumption versus postsurgical group at all time intervals post 2 to 4 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Same number of dropouts in both groups (n = 3), but reasons for dropouts not given |
| Selective reporting (reporting bias) | Low risk | Full reporting of primary outcome data, some secondary outcomes listed only as "no significant difference" |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo) |
Arslan 2011.
| Methods | Double‐blind, placebo and active‐controlled, single dose, over 24 h Interventions administered at end of procedure |
|
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 49.2 ± 13.3 Sex (male, %): 10.0% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 43.9 ± 9.5 Sex (male, %): 20.0% Placebo group Entered/completing: 20/20 Age (mean, SD): 48.7 ± 12.3 Sex (male, %): 15.0% |
|
| Interventions | Paracetamol 1 g over 10 min Lornoxicam 8 mg or placebo (100 ml NS) over 10 min |
|
| Outcomes | Primary: analgesic consumption (tramadol, n/N) at 0 to 6, 6 to 12 and 12 to 24 h and mg total Secondary: VAS pain scores at 15 min, and 1, 2, 4, 6, 8, 12, 18, and 24 h postoperatively Time to first request for analgesia Adverse events |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Patients using analgesics long‐term were excluded from the study | |
| Notes | Translated from Turkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Withdrawal of a card. No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all participants completed the study and that all data were collected |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | 60 participants (20 paracetamol, 20 placebo, 20 lornoxicam) |
Arslan 2013.
| Methods | Randomized, placebo‐controlled, single dose, over 24 h Interventions administered preemptively (not included in this review) or at end of surgery |
|
| Participants | Type of surgery: elective lap cholecystectomy Paracetamol group Entered/completing: 100/100 Age (mean, SD): 41.5 ± 7.8 Sex (male, %): 32 Control group Entered/completing: 100/100 Age (mean, SD): 44.5 ± 6.5 Sex (male, %): 34 |
|
| Interventions | Paracetamol 1 g/100 ml IV over 10 min Placebo: saline as above |
|
| Outcomes | Primary: time to first rescue dose and cumulative amount of rescue analgesic (tramadol 100 mg IV for VAS pain score > 4, up to 400 mg max) Secondary: VAS pain scores at several time points up to 24 h, numbers of participants in each group requiring rescue medication within various postoperative intervals and cumulatively, adverse events, patient satisfaction at 24 h (0 = poor to 4 = excellent) |
|
| Source of funding | No funding | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | None – participants with history of usage of paracetamol, opioids, or NSAIDs for 3 months were excluded | |
| Notes | Third group receiving paracetamol preemptively not included in this review. All participants received fentanyl 1 µg/kg at induction of anesthesia, which had a total duration of about 100 min in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (100 paracetamol, 100 placebo) |
Atef 2008.
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered at the end of surgery |
|
| Participants | Type of surgery: elective standard bipolar diathermy tonsillectomy Paracetamol group Entered/completing: 38/38 Age (mean, SD): 27 ± 4 Sex (male, %): 50 Placebo group Entered/completing: 38/38 Age (mean, SD): 25 ± 5 Sex (male, %): 47 |
|
| Interventions | Intervention: paracetamol 1 g IV in 100 ml normal saline over 15 min Placebo: 100 ml normal saline |
|
| Outcomes | Pain intensity at rest and on swallowing (VAS 0 to 100) Pain relief (defined as a VAS score of < 30 mm at rest and < 50 mm on swallowing Opioid consumption (pethidine) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes ‐ patient characteristics and duration of operation | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Nurse not involved in the study prepared the study solutions. Similar appearance of the study infusions assured blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts; figures suggest that all participants reported data |
| Selective reporting (reporting bias) | Low risk | Primary outcome stated and fully reported. Stated secondary outcomes fully reported. |
| Size | High risk | Fewer than 50 participants per arm of the study (38 paracetamol, 38 placebo) |
Aubrun 2003.
| Methods | Randomized, placebo‐controlled, double‐blind Medications administered at the beginning of skin closure |
|
| Participants | Type of surgery: orthopedic, abdominal, general, or gynecological surgery Propacetamol group Entered/completing: ?/275 Age (mean, SD): 44 (range 18 to 85) Sex (male, %): 46 Placebo group Entered/completing: ?/275 Age (mean, SD): 45 (18 to 72) Sex (male, %): 42 |
|
| Interventions | Intervention: 2 g propacetamol over 15 min Placebo: 125 ml saline |
|
| Outcomes | Morphine related AEs Opioid consumption (morphine) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: patient characteristics, type and duration of surgery, expected postoperative pain, ASA status and type of anesthesia | |
| Details of preoperative pain | Not mentioned, but anesthetists were asked to exclude patients who were expected to have no postoperative pain and those who were expected to have very severe postoperative pain requiring prolonged epidural and/or spinal postoperative analgesia or prolonged postoperative sedation and/or patient‐controlled analgesia (PCA). | |
| Notes | Minor protocol violation occurred in 80 (15%) participants. There were no significant differences between the 2 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified according to centers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Each vial was prepared immediately before administration by a nurse who was not involved in the care or pain assessment of the patient. Vials containing 2 g of propacetamol (yielding 1 g of acetaminophen) or saline were administered IV over 15 min. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% of randomized participants were not included in analysis, but reasons for exclusion suggest outcome would be unaffected |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome, morphine‐related adverse events, reported in full, except for incidence of bronchospasm. All secondary outcomes reported, but VAS and pain relief only reported as "no significant difference" between groups. |
| Size | Low risk | >/= 200 participants in each arm of the study |
Beaussier 2005.
| Methods | Randomized, double‐blind, double‐dummy, active‐controlled Medications administered at the beginning of wound closure |
|
| Participants | Type of surgery: inguinal hernia repair Propacetamol group Entered/completing: 90/90 Age (mean, SD): 46 ± 14 Sex (male, %): 94 Parecoxib group Entered/completing: 92/90 Age (mean, SD): 42 ± 14 Sex (male, %): 91 |
|
| Interventions | Intervention: 2 g propacetamol over 15 min Control: parecoxib 40 mg IV |
|
| Outcomes | Primary: opioid consumption (morphine) Pain intensity at rest and while coughing (VRS, VAS) and derived summary measures |
|
| Source of funding | Pfizer SA | |
| Were treatment groups comparable at baseline? | Yes: intraoperative opioid consumption and time to tracheal extubation did not differ between groups; demographics (age, sex, weight, height) similar | |
| Details of preoperative pain | Patients with chronic pain excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization performed centrally as blocks of 4 and with a 2:2 treatment ratio. In each center, treatment allocation was performed on the basis of one complete treatment block. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy design employed. Propacetamol was administered by slow infusion over 15 min whereas parecoxib was injected by rapid bolus. Each participant received both an active product and the placebo of the other product. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For primary outcome, data reported for both modified‐ITT population and per‐protocol population. Secondary outcomes reported data for ITT population. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported with mean data or raw numbers |
| Size | Unclear risk | 50 to 199 participants per arm of the study (90 propacetamol, 92 parecoxib) |
Brodner 2011.
| Methods | Prospective, double‐blind, placebo‐ and active‐controlled study Multiple‐dose study, first dose administered 30 min before the end of surgery. Outcomes assessed for at least 48 h and up to 1 week in patients not discharged. |
|
| Participants | Type of surgery: plastic surgery (breast surgery, inguinal or axillary dissections), oral and maxillofacial surgery (correction of retrognathism and prognathism), gynecological (laparoscopy, breast surgery), and urological (cystoscopy, transurethral prostatectomy) surgery and orthopedic surgery (hip endoprosthesis for coxarthrosis) Paracetamol group Entered/completing: 49/45 Age (mean, SD): 50.5 ± 17.5 Sex (male, %): 26.5% Dipyrone group Entered/completing: 49/41 Age (mean, SD): 45.5 ± 17.9 Sex (male, %): 44.9% Parecoxib group Entered/completing: 49/44 Age (mean, SD): 49.4 ± 14.6 Sex (male, %): 26.5% Placebo group Entered/completing: 49/45 Age (mean, SD): 42.8 ± 16.8 Sex (male, %): 49.0% |
|
| Interventions | Paracetamol 1 g/100 ml NS over 15 min every 6 h for at least 48 h Dipyrone 1 g every 6 h, parecoxib 40 mg every 12 h (saline every 6 h between doses), or placebo (0.9% saline) every 6 h as above |
|
| Outcomes | Primary: dynamic VAS (0 to 100) for pain localized to the site of surgery Secondary: time to first piritramide PCA bolus and piritramide consumption as quantified by the number of boluses demanded and administered; satisfaction rated as 1, excellent; 2, good; 3, moderate; 4, insufficient; and 5, poor; adverse events (respiratory depression, N/V, sedation, itching sweating) |
|
| Source of funding | Bristol‐Myers Squibb, Munich, Germany, and Pfizer Pharma GmbH, Karlsruhe, Germany | |
| Were treatment groups comparable at baseline? | Yes, with 3 exceptions: participants of group 3 parecoxib had a significantly shorter duration of anesthesia and needed significantly less intraoperative sufentanil compared to group 4 placebo, and there were more women in group 1 paracetamol and group 3 parecoxib than in group 2 dipyrone and group 4 placebo | |
| Details of preoperative pain | Surgical area (0 to 100 VAS): paracetamol 9.2 ± 17.1, dipyrone 10.8 ± 17.2, parecoxib 13.3 ± 16.6, placebo 6.0 ± 13.2 | |
| Notes | Numbers completing based on number of participants discontinued after at least 2 doses of intervention. ITT analysis employed – no participants lost to follow‐up at 42 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “assigned by random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “All study drugs were prepared by the hospital pharmacy in identical glass bottles as infusions of 100 ml. The bottles were labelled with patient number and time of administration. Infusions were administered by a blinded attending physician”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. ITT analysis performed, but no mention of how missing data imputed. |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcome, patient satisfaction, reported as mean across all groups and statement that all participants were satisfied with their pain treatment. No mean data for each group and no details of what point on scale was defined as satisfied. |
| Size | High risk | Fewer than 50 participants per arm of the study (49 paracetamol, 49 dipyrone, 49 parecoxib, 49 placebo) |
Cakan 2008.
| Methods | Randomized, double‐blind, placebo‐controlled, over 24 h Medications administered during skin closure |
|
| Participants | Type of surgery: elective lumbar laminectomy and discectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 41 ± 10 Sex (male, %): 60 Placebo group Entered/completing: 20/20 Age (mean, SD): 44 ± 10 Sex (male, %): 55 |
|
| Interventions | Intervention: paracetamol 1 g IV over 15 min Placebo: 100 ml normal saline |
|
| Outcomes | Pain intensity at rest or movement (VAS) Opioid consumption (morphine) All other outcomes reported at 24 h only |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographic data (sex, age, duration of surgery, intraoperative opioid use) and vital signs | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo administered in same solution over same time period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo) |
Chen 2011.
| Methods | RCT, 60 participants were randomly divided into 2 groups, with 30 participants in each group Participants in Group I were treated with 2 g propacetamol 15 min before the end of operation |
|
| Participants | Type of surgery: lumbar spine surgery Propacetamol group Entered/completing: 30 Age (mean, SD): 48, 13 Sex (male, %): 13, 43% Control group Entered/completing: 30 Age (mean, SD): 53, 14 Sex (male, %): 14, 47% |
|
| Interventions | 2 g propacetamol in 100 ml saline, intravenous injection, 15 min before the end of operation Placebo: 100 ml saline, intravenous injection, 15 min before the end of operation |
|
| Outcomes | The authors did not point out which were primary outcomes Vomiting frequency in 48 h after the operation VAS pain score (0 to 10), Ramsay sedation score(0, 1, 2, 3), breathing frequency, heart rate and mean arterial pressure were observed at the time of 0 min, 30 min, 1 h, 2 h, 4 h, 6 h, 12 h, 24 h, 36 h, 48 h after the operation |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: sex, age, weight, height | |
| Details of preoperative pain | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported as no incomplete outcome data in the study |
| Selective reporting (reporting bias) | High risk | There were only comparisons of VAS pain scores and Ramsay sedation scores between the 2 groups as shown in Table 2 and 3 |
| Size | High risk | Fewer than 50 participants per arm of the study (30 propacetamol, 30 placebo) |
Dejonckheere 2001.
| Methods | Randomized, blinded, active‐controlled Medications administered on request in the PACU |
|
| Participants | Type of surgery: thyroidectomy Propacetamol group Entered/completing: 40/40 Age (mean, SD): 46.9 ± 2.1 Sex (male, %): 15 Tramadol group Entered/completing: 40/40 Age (mean, SD): 44.1 ± 1.8 Sex (male, %): 10 |
|
| Interventions | Intervention: 2 g IV propacetamol Control: tramadol 1.5 mg/kg |
|
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS) |
|
| Source of funding | SearΙe Continental Pharma Ιnc provided the trial drugs and statistical assistance | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; intraoperative opioid use; nausea, vomiting, and drowsiness incidence pre‐interventions | |
| Details of preoperative pain | Not reported ‐ participants with chronic opioid use were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 tramadol) |
Delbos 1995.
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered in the recovery room |
|
| Participants | Type of surgery: knee ligamentoplasty Propacetamol group Entered/completing: 30/29 Age (mean, SD): 25.5 ± 5.6 Sex (male, %): 97 Placebo group Entered/completing: 30/28 Age (mean, SD): 26.3 ± 5.8 Sex (male, %): 90 |
|
| Interventions | Intervention: 2 g propacetamol in 125 ml 5% dextrose over 15 min Placebo: 125 ml 5% dextrose |
|
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS 0 to 100 and 5‐point VRS) and derived summary measures Global efficacy (5‐point VRS) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data presented for some outcomes from all participants, but for other outcomes from only those apparently completing the study |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (30 propacetamol, 30 placebo) |
Eremenko 2008.
| Methods | Randomized, blinded, placebo‐controlled Medications administered 30 min before extubation |
|
| Participants | Type of surgery: coronary bypass surgery Paracetamol group Entered/completing: 22/? Age (mean, SD): unclear Sex (male, %): 77 Placebo group Entered/completing: 23/? Age (mean, SD): unclear Sex (male, %): 73 |
|
| Interventions | Intervention: paracetamol 1 g/100 ml IV (every 6 h x 4 doses) Placebo: 100 ml normal saline |
|
| Outcomes | Inspiratory volume Pain intensity (VRS) AEs not reported All other outcomes described over duration of study (18 h) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics | |
| Details of preoperative pain | Not reported | |
| Notes | Russian language study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded, but no mention of whether single‐ or double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Size | High risk | Fewer than 50 participants per arm of the study (22 paracetamol, 23 placebo) |
Faiz 2014.
| Methods | Randomized, double‐blind, active‐controlled. Evaluated up to 24 h after surgery. Medications administered prior to skin closure |
|
| Participants | Type of surgery: elective abdominal hysterectomy Paracetamol group Entered/completing: 40/40 Age (mean, SD): 49.9 ± 6.9 Sex (male, %): 0 Control group Entered/completing: 40/40 Age (mean, SD): 47.2 ± 7.2 Sex (male, %): 0 |
|
| Interventions | Paracetamol 15 mg/kg in 100 ml NS, single dose over 15 min Ketamine 0.15 mg/kg administered as above |
|
| Outcomes | Primary: pain (VAS 0 to 10) in recovery room and at 4, 6, 12 and 24 h postop Secondary: sedation (Ramsay scale), adverse events (nausea, vomiting, respiratory complications, hemodynamic changes), rescue analgesia (pethidine 15 mg for VAS pain > 3) |
|
| Source of funding | Iran University of Medical Sciences | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of surgery | |
| Details of preoperative pain | Not reported, but participants currently using opioids were excluded | |
| Notes | Low dose of ketamine used in comparator group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Both medication solutions were prepared by the research pharmacist in 100 ml of normal saline and were administered by the anesthesia care team within a 15‐minute time period. The administering team was blinded to the nature of the infusate”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts; tables suggest that all participants reported data |
| Selective reporting (reporting bias) | Low risk | All planned primary and secondary outcomes reported, although total amount of rescue analgesia not reported. Confirmed on trials registry (http://www.irct.ir/searchresult.php?id=11319&number=1). |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 ketamine) |
Farkas 1992.
| Methods | Randomized, double‐blind, double‐placebo, placebo‐ and active‐controlled Medication administered when baseline pain reached at least moderate intensity |
|
| Participants | Type of surgery: abdominal aortic repair Propacetamol group Entered/completing: 29/15 Age (mean, SD): 64.3 ± 2.2 Sex (male, %): 3 Dipyrone group Entered/completing: 30/21 Age (mean, SD): 62.4 ± 1.7 Sex (male, %): 20 Placebo group Entered/completing: 30/15 Age (mean, SD): 64.3 ± 1.8 Sex (male, %): 3 |
|
| Interventions | Intervention: 2 g propacetamol over 2 min Control: 2.5 g dipyrone plus 0.01 g pitofenone IV Placebo: not described |
|
| Outcomes | Pain intensity (5‐point VRS) Requirement for rescue analgesia (morphine) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, baseline postoperative pain score | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Investigator who did not know the nature of the products, infused the test products to each patient according to their number of entry into the trial. The patients were then monitored in the recovery room over six hours by the same investigator who did not know the nature of the product administered". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐rescue assessments imputed using LOCF; imbalance amongst groups in number of participants withdrawn due to requirement for rescue analgesia |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (29 propacetamol, 30 dipyrone, 30 placebo) |
Fletcher 1997.
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled Medications administered at skin closure (and repeated every 6 h x 48 h) |
|
| Participants | Type of surgery: surgery of one herniated lumbar disc Propacetamol group Entered/completing: 15/14 Age (mean, SD): 41.8 ± 2.7 Sex (male, %): 53 Ketoprofen group Entered/completing: 15/14 Age (mean, SD): 49.7 ± 2.9 Sex (male, %): 53 Placebo group Entered/completing: 15/15 Age (mean, SD): 41.8 ± 2.4 Sex (male, %): 60 |
|
| Interventions | Intervention: 2 g propacetamol in 125 ml dextrose 5% Control: ketoprofen 50 mg Control: combination of ketoprofen with propacetamol (not included in our analysis) Placebo: 125 ml dextrose 5% |
|
| Outcomes | Pain intensity at rest and with movement (VAS) Sedation (4‐point categorical scale) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; preoperative pain; duration of surgery | |
| Details of preoperative pain | Incidence of preoperative leg and/or back pain similar between groups. Mean severity of preoperative pain similar (ranged from 42 to 51/100) between groups. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before the study began, a random number table was used to generate a randomized schedule specifying the group to which each patient would be assigned upon entry into the trial |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All drugs were administered IV after dilution in 125 ml dextrose 5% labeled with the randomization number of the participant. Participants in all groups received 2 injections to assure blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate balanced amongst groups |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (15 propacetamol, 15 ketoprofen, 15 placebo) |
Hahn 2003.
| Methods | Randomized, double‐blinded, placebo‐controlled Medications administered after surgery and immediately before extubation |
|
| Participants | Type of surgery: laparoscopic sterilization Propacetamol group Entered/completing: 15/15 Age (mean, SD): 36 ± 4 Sex (male, %): 0 Placebo group Entered/completing: 16/16 Age (mean, SD): 37 ± 4 Sex (male, %): 0 |
|
| Interventions | Intervention: 40 mg/kg propacetamol Control: 20 mg/kg propacetamol (not included in our analysis) Control: 10 mg/kg propacetamol (not included in our analysis) 1 g propacetamol was dissolved in 5 ml of contained solvent and administered as bolus Placebo: normal saline |
|
| Outcomes | Opioid consumption (alfentanil via PCA) Postoperative pain at rest and with movement (10 cm VAS) |
|
| Source of funding | SmithKline Beecham, Denmark supported the study. The infusion pumps were supplied by Baxter, Denmark. | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of anesthesia | |
| Details of preoperative pain | Patients were excluded if they had a history of chronic pain | |
| Notes | 40 mg/kg dose chosen in analysis as, based on participant weights, this would be closest dose to standard 2 g of propacetamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs were administered by an anesthetist who had no further contact with the participant or study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal number of dropouts; it appears that all other data reported for remaining participants and at all relevant time points |
| Selective reporting (reporting bias) | Unclear risk | Time points not specified in Methods section for primary outcome; data pooled for different dosing regimens post 3 h |
| Size | High risk | Fewer than 50 participants per arm of the study (15 propacetamol, 16 placebo) |
Hans 1993.
| Methods | Randomized, placebo‐controlled Medications administered at end of surgery |
|
| Participants | Type of surgery: lumbar disc surgery Propacetamol group Entered/completing: 20/20 Age (mean, SD): 38.3 ± 9.4 Sex (male, %): 80 Placebo group Entered/completing: 20/20 Age (mean, SD): 38.2 ± 10.8 Sex (male, %): 55 |
|
| Interventions | Intervention: propacetamol 2 g over 20 min Placebo: saline |
|
| Outcomes | Opioid consumption (piritramide on request) Pain intensity (VAS) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 placebo) |
Hiller 2004.
| Methods | Randomized, double‐blind, active‐controlled Medications administered immediately after induction of anesthesia (surgeries averaged around 30 min) |
|
| Participants | Type of surgery: elective tonsillectomy Propacetamol group Entered/completing: 26/25 Age (mean, SD): 29 ± 11 Sex (male, %): 52 Diclofenac group Entered/completing: 25/25 Age (mean, SD): 27 ± 7 Sex (male, %): 44 |
|
| Interventions | Intervention: 2 g propacetamol in 100 ml normal saline Control: 75 mg diclofenac Control: 2 g propacetamol plus 75 mg diclofenac (not included in our analysis) |
|
| Outcomes | Opioid consumption (oxycodone) Pain intensity at rest and on swallowing (VRS, VAS) Patient satisfaction (VAS) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery, intraoperative opioid use; blood loss | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Nurse preparing solutions did not participate in the study. To maintain double‐blind design volumes infused were equal. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Various reasons for dropouts amongst groups, data analyzed in completers only, but minimal missing data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified in Methods section; possibility of additional post‐hoc analyses cannot be ruled out |
| Size | High risk | Fewer than 50 participants per arm of the study (26 propacetamol, 25 diclofenac) |
Hiller 2012.
| Methods | Randomized, double‐blind, placebo‐controlled, over 24 h Medications administered during skin closure |
|
| Participants | Type of surgery: major spine (posterior and/or anterior correction) Paracetamol group Entered/completing: 18/18 Age (mean, SD): 15.1 ± 2.0 Sex (male, %): 5, 27.8% Placebo group Entered/completing: 18/18 Age (mean, SD): 14.4 ± 1.9 Sex (male, %): 6, 33.3% |
|
| Interventions | Paracetamol 30 mg/kg IV (3 ml/kg) over 15 min, max dose 1.5 g, q8h x 3 doses Placebo (NS) as above |
|
| Outcomes | Primary: pain scores at rest on the surgical ward q1h for 24 h as the highest VAS 0 to 10 score during the preceding hour Secondary: time to first and total PCA oxycodone dose; supplemental analgesia (for VAS ≥ 6 oxycodone 0.05 mg/kg IV, if VAS score still ≥ 6, parecoxib 20 to 40 mg IV); adverse events (nausea, vomiting, and pruritus); sedation (Michigan sedation scale: 0, awake; 4, unresponsive to painful stimulus); plasma levels of acetaminophen and metabolites |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; intraoperative analgesia; blood loss; spinal pathology and type of procedure | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “An unblinded anesthesia nurse who did not participate in peri‐ or postoperative care opened an envelope and prepared the study medications (acetaminophen or placebo)”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis (method of imputation not specified); reasons for protocol violations specified and similar between groups, and results reported to be similar if these participants were excluded. |
| Selective reporting (reporting bias) | High risk | All outcomes from Methods section reported in Results section (other than number of participants with pruritus), but many only presented graphically and without SDs |
| Size | High risk | Fewer than 50 participants per arm of the study (18 paracetamol, 18 placebo) |
Hynes 2006.
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered on postoperative day 1, when baseline pain reached moderate‐to‐severe intensity |
|
| Participants | Type of surgery: total hip arthroplasty Propacetamol group Entered/completing: 40/40 Age (mean, SD): 65.7 ± 9.8 Sex (male, %): 40 Diclofenac group Entered/completing: 40/40 Age (mean, SD): 65.6 ± 7.6 Sex (male, %): 55 Placebo group Entered/completing: 40/40 Age (mean, SD): 66.1 ± 7.1 Sex (male, %): 45 |
|
| Interventions | Intervention: 2 g propacetamol IV Control: 75 mg diclofenac IM Placebo: double‐dummy not described |
|
| Outcomes | Pain intensity (VRS, VAS) Pain relief (categorical) Time to request for rescue medication Global assessment (categorical) |
|
| Source of funding | Supported by Bristοl‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of anesthesia; baseline postoperative pain | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/40 missing pain assessment data at 5 h in intervention group due to lack of efficacy and administration of rescue dose (29/40 in placebo group), but all 40 included in efficacy analysis. Data were imputed using LOCF. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but time to rescue was instead reported as number of participants requesting rescue |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 diclofenac, 40 placebo) |
Inal 2006.
| Methods | Parallel, active‐controlled, randomized, double‐blind, single dose over 24 h | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 25/unclear Age (mean, SD): 30.6 ± 4.23 Sex (male, %): 0 Pethidine group Entered/completing: 25/unclear Age (mean, SD): 29.6 ± 3.51 Sex (male, %): 0 |
|
| Interventions | Paracetamol 1 g/100 ml single dose over 15 min, 30 min before end of surgery pethidine 100 mg IV as above |
|
| Outcomes | Primary: VAS pain intensity at 0, 1, 5, 30 min and 1, 2, 4, 6, 8 and 24 h after surgery Secondary: Side effects Total rescue analgesic use (unspecified) over 24 h for pain > 7/10 |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, height, weight, duration of surgery, duration of anesthesia | |
| Details of preoperative pain | Participants with chronic abdominal pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many participants completed the study; mean pain data did not have SDs; 24 h rescue analgesic use did not specify analgesic administered |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section were reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 pethidine) |
Jahr 2012 Study 2, 65+.
| Methods | Randomized, double‐blind, placebo‐controlled single dose study evaluated 6 h postop Study entry occurred the day after surgery |
|
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 16/16 Age (mean, SD): 74.6 (5.7) Sex (male, %): 8 (50%) Placebo Entered/completing: 17/17 Age (mean, SD): 73.9 (6.2) Sex (male, %): 5 (29.4%) |
|
| Interventions | Paracetamol: 1000 mg IV as a single dose Placebo Each arm had free access to PCA (details not specified including if it could be different opioids in PCA) |
|
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID6 Secondary: adverse events |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. Participants were required to have moderate pain the day after surgery. Baseline PI scores were not statistically different between groups (VAS 0 to 100). Details regarding drugs used/dosing of opioid PCA for all participants are lacking. (“…each arm having free access to PCA opioids.”) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (16 paracetamol, 17 placebo) |
Jahr 2012 Study 2, 65‐.
| Methods | Randomized, double‐blind, placebo‐controlled, single dose study evaluated 6 h postop Study entry occurred the day after surgery. |
|
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 19/19 Age (mean, SD): 52.6 (7.9) Sex (male, %): 9 (47.4%) Placebo Entered/completing: 17/17 Age (mean, SD): < 65: 57.2 (6.4) Sex (male, %): 8 (47.1%) |
|
| Interventions | Paracetamol: 1000 mg IV as a single dose Placebo Each arm had free access to PCA (details not specified including if it could be different opioids in PCA) |
|
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID6 Secondary: adverse events |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. Participants were required to have moderate pain the day after surgery. Baseline PI scores were not statistically different between groups (VAS 0 to 10). Details regarding drugs used/dosing of opioid PCA for all participants are lacking. (“…each arm having free access to PCA opioids.”) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (19 paracetamol, 17 placebo) |
Jahr 2012 Study 3, 65+.
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter repeated dose study evaluated up to 16 h postop Study entry occurred the day after surgery. Participants were required to have moderate postop pain for eligibility |
|
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 15/15 Age (mean, SD): 71.4 +/‐ 4.7 Sex (male, %): 9 (60%) Placebo Entered/completing: 12/12 Age (mean, SD): 68.4 +/‐ 3.5 Sex (male, %): 6 (50%) |
|
| Interventions | Paracetamol: 1000 mg IV administered at 0, 4, 10, 16 h Placebo |
|
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID4, patient satisfaction Secondary: adverse events |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Only published as an abstract. Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 12 placebo) |
Jahr 2012 Study 3, 65‐.
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter repeated dose study evaluated up to 16 h postop Study entry occurred the day after surgery. Participants were required to have moderate postop pain for eligibility. |
|
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 15/unclear Age (mean, SD): 54.1 +/‐ 6.2 Sex (male, %): 11 (73.3%) Placebo Entered/completing: 19/unclear Age (mean, SD): 53.4 +/‐ 9.3 Sex (male, %): 14 (73.7%) |
|
| Interventions | Paracetamol: 1000 mg IV administered at 0, 4, 10, 16 h Placebo |
|
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID4, patient satisfaction Secondary: adverse events |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Only published as an abstract. Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 19 placebo) |
Jarde 1997.
| Methods | Randomized, double‐blind, placebo‐ and active‐controlled Medication administered immediately after surgery in patients with at least moderate pain |
|
| Participants | Type of surgery: hallux valgus Propacetamol group Entered/completing: 108/108 Age (mean, SD): 52.2 ± 13.0 Sex (male, %): 11 Placebo group Entered/completing: 109/109 Age (mean, SD): 51.9 ± 13.6 Sex (male, %): 8 |
|
| Interventions | Intervention: propacetamol 2 g in 125 ml dextrose 5% over 15 min Control: oral paracetamol 1 g (not included in our analysis) Placebo: 125 ml dextrose 5% and tablet |
|
| Outcomes | Pain intensity (categorical) and derived pain intensity difference, SPID and maximum pain intensity difference Time to rescue medication Global evaluation (categorical) |
|
| Source of funding | Supported by UPSA Laboratories | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; baseline postoperative pain | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Inadequately described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants requesting rescue medication had LOCF in efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (108 propacetamol, 109 placebo) |
Juhl 2006.
| Methods | Randomized, double‐blind, double‐dummy, active‐ and placebo‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 6 h of surgery |
|
| Participants | Type of surgery: third molar extraction Paracetamol group Entered/completing: 132/132 Age (mean, SD): 25.0 ± 2.6 Sex (male, %): 41 Placebo group Entered/completing: 33/33 Age (mean, SD): 25.2 ± 2.8 Sex (male, %): 55 |
|
| Interventions | Intervention: IV paracetamol 1 g Control: IV paracetamol 2 g (not included in our analysis) Placebo: 100 ml solution All interventions administered in 100 ml solution for each 1 g of paracetamol (or placebo) over 15 min |
|
| Outcomes | Pain relief (VAS and VRS) and derived TOTPAR Pain intensity (VAS and VRS) Time to request of rescue medication Global evaluation (categorical) |
|
| Source of funding | Supported by Bristol‐Myers Squibb Company | |
| Were treatment groups comparable at baseline? | Yes: demographics; ASA classification; number of teeth removed; baseline postoperative pain intensity; surgical trauma No: longer duration of surgery in the IV paracetamol 1 g group in comparison with the IV paracetamol 2 g and placebo groups |
|
| Details of preoperative pain | Participants with other painful physical conditions that might confound pain assessment were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization 4:4:1, each block n = 9 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A double‐dummy method was used to assure double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis using LOCF. Unclear how many participants had data imputed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section, but SPID was only calculated using categorical pain intensity despite being measured with both categorical and VAS scales |
| Size | Unclear risk | 50 to 199 participants per arm of the study (132 paracetamol, 33 placebo) |
Kamath 2014.
| Methods | Randomized, parallel, active‐controlled trial; multiple doses evaluated for 24 h | |
| Participants | Type of surgery: cesarean sections and gynecological surgeries Paracetamol group Entered/completing: 51/50 Age (mean, SD): not reported Sex (male, %): 100% female Butorphanol group Entered/completing: 50/50 Age (mean, SD): not reported Sex (male, %): 100% female |
|
| Interventions | Paracetamol: 1 g IV every 8 h Butorphanol 2 mg IV every 12 h |
|
| Outcomes | Primary: pain intensity Secondary: administration of rescue medication (tramadol), timing of rescue medication, adverse effects |
|
| Source of funding | ICMR under STS program | |
| Were treatment groups comparable at baseline? | Not reported | |
| Details of preoperative pain | Not reported | |
| Notes | Poster presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described; due to the fact that the study was likely unblinded we categorized this as high risk also |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described; assume to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One participant was not accounted for in the presentation of graphical results |
| Selective reporting (reporting bias) | Unclear risk | Timing of rescue medication was not presented in Results. No data for pain scores reported. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (51 paracetamol, 50 placebo) |
Kampe 2006.
| Methods | Randomized, double‐blind, active‐controlled Medications administered 30 min before the end of surgery |
|
| Participants | Type of surgery: breast cancer (breast conserving or total mastectomy, balanced between groups) Propacetamol group Entered/completing: 20/20 Age (mean, SD): 52 ± 10.2 Sex (male, %): 0 Dipyrone group Entered/completing: 20/20 Age (mean, SD): 55.9 ± 8.7 Sex (male, %): 0 |
|
| Interventions | Intervention: 1 g propacetamol in 100 ml solution over 15 min Control: 1 g dipyrone |
|
| Outcomes | Pain intensity at rest and on coughing (VAS) Opioid consumption (piritramide via PCA) Patient satisfaction (categorical) |
|
| Source of funding | In part supported by a grant from BristoΙ‐Myers Squibb GmbFΙ, München, Germany, with publication support provided by the Department of Anaesthesiology, University of Cologne | |
| Were treatment groups comparable at baseline? | Yes: demographics, type of procedure | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on a computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Randomization results were sealed in sequentially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The infusions were made to look identical; participants and investigators were blinded to the study treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The data for all patients were eligible for statistical analysis." |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 dipyrone) |
Kara 2010.
| Methods | Randomized, active‐controlled study, multiple dose, evaluated up to 2 days postop Medication administered at the end of the operation, without being contingent upon pain intensity Rescue medication (meperidine/pethidine 1 mg/kg IM) given to both groups as rescue medication for VAS > 4 |
|
| Participants | Type of surgery: trans‐urethral resection of prostate Paracetamol group Entered/completing: 25/25 Age (mean, SD): only median age reported (64.3) Sex (male, %): 25 (100%) Diclofenac group Entered/completing: 25/25 Age (mean, SD): only median reported (66.8) Sex (male, %): 25 (100%) |
|
| Interventions | Paracetamol 1 g/100 ml IV over 15 min twice daily Diclofenac IM 75 mg at the end of the operation, followed by 75 mg IM for 24 h. Time interval between first two 75 mg doses not reported, but the authors describe this regimen as 150 mg once per day = 150 mg per 24 h. |
|
| Outcomes | Primary: pain intensity (VAS) Secondary: hemoglobin levels, hemostatic variables (bleeding time PT, INR), adverse effects, rescue opioid use (pethidine) |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; transrectal ultrasound volume | |
| Details of preoperative pain | Similar at baseline | |
| Notes | No statistically/clinically significant differences in postoperative hemoglobin, hemostatic parameters or bleeding events between placebo, paracetamol, and diclofenac groups. Diclofenac dosing (2 x 75 mg given in presumably quick succession, then repeated as a 150 mg dose 24 h later) is highly idiosyncratic, high. This regimen could bias results towards a greater diclofenac effect in the first portion of the 24 h dosing interval, and a lesser effect towards the end, compared with a more conventional dosing regimen of 75 mg IM every 12 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described. Assessed as high risk based on assumption of non‐blinding. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described; assume to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or protocol violations |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section; opioid use reported in Results but not specifically mentioned in Methods. No SD reported for VAS data. |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 diclofenac) |
Karaman 2010.
| Methods | Randomized, double‐blind, active‐controlled study, multiple dose, 24 h Medication was administered at the end of surgery after skin closure |
|
| Participants | Type of surgery: ENT surgery (nasal/sinus, otologic, head/neck) Paracetamol group Entered/completing: 30/30 Age (mean, SD): 48.5 +/‐ 12.1 Sex (male, %): 16 (53%) Dexketoprofen Entered/completing: 30/30 Age (mean, SD): 54.8 +/‐ 8.6 Sex (male, %): 16 (53%) |
|
| Interventions | Paracetamol: 1 g IV at the end of surgery then at 6, 12, 18 h (4 g total) Dexketoprofen: 50 mg IV at the end of surgery then repeated twice at an 8‐h interval (150 mg total) Metamizol: 1 g IV at the end of surgery then repeated twice at an 8‐h interval (3 g total, not included in our analysis) |
|
| Outcomes | Primary: VAS (0 to 10) and VRS (0 to 3) pain intensity Secondary: adverse events, sedation score, use of rescue medication (pethidine 1 mg/kg for VAS ≥ 30 mm) |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery | |
| Details of preoperative pain | Not reported ‐ participants were excluded if they had received analgesics within 12 h before surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and anesthetists were unaware of treatment assignments. All outcome measurements were recorded by the same anesthesia resident who was blinded to assignments. “All medicines were prepared by a nurse who had no other involvement in the study” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or protocol violations – complete data set obtained for all groups |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section although no data provided for sedation assessment |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 dexketoprofen) |
Kemppainen 2006.
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered at completion of surgery over 15 min |
|
| Participants | Type of surgery: endoscopic sinus Paracetamol group Entered/completing: 36/36 Age (mean, SD): not reported Sex (male, %): not reported Placebo group Entered/completing: 38/38 Age (mean, SD): not reported Sex (male, %): not reported |
|
| Interventions | Intervention: paracetamol 1 g IV Placebo: 100 ml normal saline |
|
| Outcomes | Pain intensity (NRS) Time to rescue medication Opioid consumption (oxycodone) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No details | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Opaque envelope method |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The nurse preparing the infusions did not participate in the study. Preparation of infusions of identical volumes (100 ml) to assure blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All the patients asked agreed to participate and there were no dropouts during the study". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (36 paracetamol, 38 placebo) |
Khajavi 2007.
| Methods | Randomized, double‐blind, active‐controlled Medications administered directly before skin closure |
|
| Participants | Type of surgery: renal transplant Paracetamol group Entered/completing: 15/15 Age (mean, SD): 40.47 ± 11.2 Sex (male, %): 53 Morphine group Entered/completing: 15/15 Age (mean, SD): 40.2 ± 11.6 Sex (male, %): 60 |
|
| Interventions | Intervention: propacetamol 2 g IV over 10 min Control: morphine 5 mg IV |
|
| Outcomes | Pain intensity (VRS) Pain relief (VRS) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No statistical analysis, but groups appear balanced for demographics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Anesthesiologist blinded to the drug administered assessed pain score, blood pressure, heart rate, lab tests, etc. No description of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table suggests that all participants (15 in each group) reported data at all time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 15 morphine) |
Khalili 2013.
| Methods | Randomized, double‐blind placebo‐controlled, single dose, over 24 h IV acetaminophen was administered before skin closure versus a control group that received normal saline as placebo; preemptive group receiving 15 mg/kg 0.5 h preoperatively, not reported here |
|
| Participants | Type of surgery: orthopedic, lower extremity Paracetamol group Entered/completing: 25/25 Age (mean, SD): 36.8 +/‐ 14.8 Sex (male, %): 21 (84%) Placebo group Entered/completing: 25/25 Age (mean, SD): 37.8 +/‐ 12.9 Sex (male, %): 17 (68%) |
|
| Interventions | Paracetamol: 15 mg/kg in 100 ml of IV normal saline prior to skin closure Placebo (normal saline) 100 ml prior to skin closure |
|
| Outcomes | Primary: pain intensity according to VRS Secondary: Timing, # participants requesting and dose of rescue medication (pethidine) Adverse effects (sedation, hypotension, etc.) |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; site of surgery; postoperative VRS scores | |
| Details of preoperative pain | Patients with a history of opioid use in the past 48 h or chronic pain were excluded; baseline pain scores not significantly different | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized (random number generator) |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and anesthesiologists were blinded by “creating treatments that looked identical” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or protocol violations – complete data set obtained for both groups |
| Selective reporting (reporting bias) | High risk | Not all outcomes from Methods section reported in Results section (e.g., sedation scores, patient satisfaction) |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 placebo) |
Khan 2007.
| Methods | Parallel, single dose, active comparator, quasi‐randomized, double‐blinded over 4 h Dose administered just before reversal of general anesthesia |
|
| Participants | Type of surgery: diagnostic knee arthroscopic procedures Paracetamol group Entered/completing: 43/unclear (appears to be 43 from Figures) Age (mean, SD): range (entire population) 18 to 69 years Sex (male, %): 90% (entire population) Morphine group Entered/completing: 41/unclear (appears to be 41 from Figures) Age (mean, SD): see above Sex (male, %): see above |
|
| Interventions | Paracetamol 1 g IV over 15 min Morphine 0.1 mg/kg IV bolus |
|
| Outcomes | Primary: VRS pain intensity at 0, 1, 2, 3, and 4 h Secondary: adverse effects |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No data presented | |
| Details of preoperative pain | Not reported | |
| Notes | Both interventions given along with 0.5% bupivacaine 20 ml intra‐articular injection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization via last number of medical record number, with odd receiving paracetamol and even receiving morphine |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study mentioned once it was double‐blinded without further description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No SDs for pain scores, unclear how many participants completed study |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (43 paracetamol, 41 morphine) |
Kilicaslan 2010.
| Methods | Paracetamol Randomized, placebo‐controlled, multiple dose study evaluated 24 h postop Medication was administered 15 min before the end of surgery |
|
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 25/25 Age (mean, SD): 28.8 +/‐ 4.8 Sex (male, %): 0 (100% female) Placebo Entered/completing: 25/25 Age (mean, SD): 27.6 +/‐ 5.4 Sex (male, %): 0 (100% female) |
|
| Interventions | Paracetamol: 1 g in 100 ml 15 min before the end of surgery and every 6 h x 24 h Placebo: saline 100 ml 15 min before the end of surgery and every 6 h x 24 h All participants received IV PCA (tramadol) – 20 mg bolus; 10 min lockout |
|
| Outcomes | Primary: pain score (VAS 0 to 10), tramadol consumption Secondary: sedation scores, nausea/vomiting scores, adverse effects |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; weeks pregnant | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Anaesthesiologists and participants were blinded. No other description provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data provided for all participants. No one was excluded from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in Methods are discussed in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 placebo) |
Koppert 2006.
| Methods | Randomized, double‐blind, controlled, multiple dose study; evaluated outcomes over at least 3 days. Medication was administered immediately after surgery upon arrival in the PACU. | |
| Participants | Type of surgery: hip replacement or surgery of the femoral shaft Paracetamol group Entered/completing: 27/25 Age (mean, SD): 76.7 +/‐ 8.9 Sex (male, %): 15 (55.6%) Parecoxib group Entered/completing: 28/25 Age (mean, SD): 76 +/‐ 8 Sex (male, %): 11 (39.3%) Placebo group (saline) Entered/completing: 28/25 Age (mean, SD): 76.7 +/‐ 8.6 Sex (male, %): 12 (42.9%) |
|
| Interventions | Paracetamol: IV infusion of 1000 mg paracetamol (Perfalgan) over 10 min; admin at 6‐h intervals for at least 3 days Parecoxib: 40 mg IV over 10 min (Dynastat); admin at 12‐h intervals for at least 3 days Placebo: saline IV over 10 min |
|
| Outcomes | Primary: renal function: blood samples (serum Cystatin C, creatinine, blood urea nitrogen, liver biochemistry); urine samples (creatinine clearance, urinary excretion of sodium, potassium, albumin, alpha1‐microglobulin; fluid balance (CVP) Secondary: pain intensity (NRS 0 to 10), rescue medication usage including morphine equianalgesic dosages |
|
| Source of funding | Supported by Bristol‐Meyers Squibb | |
| Were treatment groups comparable at baseline? | “All groups were comparable with regard to age, weight, height, distribution of sex, preexisting diseases, and ASA status…. Type and lengths of surgical and anesthetic procedures across the treatment groups were similar… Furthermore, consumption of crystalloids and colloids were similar.” | |
| Details of preoperative pain | Not reported | |
| Notes | “If a patient had received NSAIDs or COX‐2 inhibitors, there was a washout period of at least 72 h and the weak opioid, tramadol, was provided as a substitute.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization of the study medication (parecoxib versus paracetamol versus saline) was performed by computer‐generated codes maintained in sequentially numbered, opaque envelopes. Additional envelopes were provided if participants had to be excluded after recruitment and randomization.” |
| Allocation concealment (selection bias) | High risk | Despite use of sequentially numbered envelopes, participants and nursing staff on ward were unblinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Anesthesiologist, nursing staff, and investigators were blinded. All study medication solutions were prepared by a hospital pharmacist who was not involved in the data collection. On the ward, participants and nursing staff were unblinded. How blinding was maintained was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All participants that did not complete the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (27 paracetamol, 28 parecoxib, 28 placebo) |
Korkmaz 2010.
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled, multiple dose, 24 h Medications administered at time of wound closure |
|
| Participants | Type of surgery: lumbar disc Paracetamol group Entered/completing: 20/20 Age (mean, SD): 46.0 ± 11.0 Sex (male, %): 11, 55% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 46.7 ± 12.8 Sex (male, %): 12, 60% Placebo group Entered/completing: 20/19 Age (mean, SD): 44.5 ± 14.4 Sex (male, %): 9, 47.4% |
|
| Interventions | Paracetamol 1 g in 100 ml NS over 15 min every 6 h Metamizole 1 g (not included in our analysis), lornoxicam 8 mg or saline. All administered as above (lornoxicam every 12 h). Placebo: normal saline 100 ml infused over 15 min every 6 h |
|
| Outcomes | Primary: VAS (0 to 10) pain intensity over 24 h Secondary: sedation (Ramsay score); morphine consumption via PCA, other adverse events, vital signs |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; smoking status; history of postoperative nausea and vomiting; duration of anesthesia; type of surgery; number of herniated discs; experience of surgeon | |
| Details of preoperative pain | Not reported | |
| Notes | All participants received morphine PCA 100 mg in 100 ml normal saline for 24 h postop (1 mg bolus, lockout 7 min) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The study solutions were prepared by a nurse, whereas postoperative data were collected by a blinded anaesthesiologist. The colour of lornoxicam solution is yellow; to maintain blinding, all solutions were covered by aluminium foil during administration”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis only, but number of dropouts small and apparently unrelated to interventions |
| Selective reporting (reporting bias) | High risk | Adverse events only reported as similar between groups, with no accompanying data. Morphine requirements were reported in Results and not specifically mentioned in Methods. Blood pressure, heart rate, and sedation not reported in Results. |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 lornoxicam, 20 placebo) |
Lahtinen 2002.
| Methods | Randomized, double‐blinded, placebo‐controlled Medications administered immediately after arrival in the PACU |
|
| Participants | Type of surgery: cardiac Propacetamol group Entered/completing: unclear/40 Age (mean, SD): 59 ± 6 Sex (male, %): 85 Placebo group Entered/completing: unclear/39 Age (mean, SD): 58 ± 7 Sex (male, %): 90 |
|
| Interventions | Intervention: 2 g propacetamol in 100 ml normal saline Placebo: 100 ml normal saline |
|
| Outcomes | Opioid consumption (oxycodone via PCA and rescue) Pain intensity at rest and during deep breath (VAS) Patient satisfaction (categorical) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of anesthesia and surgery; intraoperative opioid use | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers and a balanced design with a computer program |
| Allocation concealment (selection bias) | Low risk | Randomization/blinding performed in pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The propacetamol and placebo ampoules were supplied in identical packages. The code remained blinded until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/88 participants withdrew for various reasons ‐ unclear which arm participants withdrew from and if this was after receiving intervention |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 39 placebo) |
Landwehr 2005.
| Methods | Randomized, double‐blinded, placebo‐ and active‐controlled Medications administered 30 min before arrival in the recovery area |
|
| Participants | Type of surgery: retinal Propacetamol group Entered/completing: 12/12 Age (mean, SD): 52 ± 18 Sex (male, %): 67 Metamizole group Entered/completing: 13/13 Age (mean, SD): 60 ± 19 Sex (male, %): 31? (data reported in error) Placebo group Entered/completing: 13/13 Age (mean, SD): 58 ± 22 Sex (male, %): 69 |
|
| Interventions | Intervention: 1 g paracetamol in 100 ml over 15 min Active control: 1 g metamizol Placebo: 100 ml normal saline |
|
| Outcomes | Pain intensity at rest and on coughing (VRS, VAS) Opioid consumption (tilidine) Patient satisfaction (categorical) |
|
| Source of funding | The study was in part financed by a grant from Bristol‐Myers Squibb GmbH, München, Germany | |
| Were treatment groups comparable at baseline? | Yes: demographics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Code prepared at a remote site and sealed in sequentially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Infusions were made to look identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants in placebo group had incomplete data, imputed by LOCF. It appears that all other participants contributed data at all time points for all outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section. Some adverse event data listed only as P values. |
| Size | High risk | Fewer than 50 participants per arm of the study (12 propacetamol, 13 metamizole, 13 placebo) |
Lee 2010.
| Methods | Randomized, controlled, single dose study evaluating outcomes 6 h postop Medication was administered 30 min before the end of surgery |
|
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 44.7 +/‐ 7.3 Sex (male, %): 0 Control (normal saline) Entered/completing: 20/20 Age (mean, SD): 46.3 +/‐ 9.5 Sex (male, %): 0 Ketorolac Entered/completing: 20/20 Age (mean, SD): 46.1 +/‐ 9.9 Sex (male, %): 0 |
|
| Interventions | Paracetamol: 1 g IV over 15 min administered 30 min before the end of surgery Normal saline; ketorolac 30 mg; paracetamol 700 mg/morphine 3 mg (not included in our analysis). All administered 30 min before end of surgery. |
|
| Outcomes | Primary: degree of pain (VAS 0 to 10) Secondary: side effects, respiratory depression, degree of satisfaction, incidence of rescue medication |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia | |
| Details of preoperative pain | Participants excluded if medicated with drugs that could affect analgesic effect before the operation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Anesthesiologists were blinded. Manuscript did not specifically state if participants were blinded. Also, no description of blinding methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts ‐ complete data set obtained for all participants. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section. Incidence of rescue medication reported in Results but not mentioned in Methods. |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 ketorolac) |
Leykin 2008.
| Methods | Randomized, double‐blind, active‐controlled study Medications administered 15 min before discontinuation of anesthesia |
|
| Participants | Type of surgery: functional endoscopic sinus Propacetamol group Entered/completing: 25/25 Age (mean, SD): 32 ± 10 Sex (male, %): 72 Parecoxib group Entered/completing: 25/25 Age (mean, SD): 34 ± 12 Sex (male, %): 76 |
|
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 40 mg IV parecoxib |
|
| Outcomes | Pain intensity (VAS) and derived SPID Opioid consumption (morphine) Patient satisfaction (categorical) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; type of procedure. Intraoperative and postoperative hemodynamic variables also reported to be similar, but no data shown. | |
| Details of preoperative pain | Participants with chronic pain requiring major analgesics, sedatives, or corticosteroids were excluded | |
| Notes | Participants had only mild pain at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study drugs mixed by physician not involved in study. Double‐dummy technique employed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses on ITT population, but no mention of imputation method |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (25 propacetamol, 25 parecoxib) |
Ma 2003.
| Methods | Randomized, double‐blinded, double‐dummy, active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity |
|
| Participants | Type of surgery: thoracic and abdominal elective Propacetamol group Entered/completing: 20/20 Age (mean, SD): unclear Sex (male, %): unclear Pethidine group Entered/completing: 20/20 Age (mean, SD): unclear Sex (male, %): unclear |
|
| Interventions | Intervention: 2 g propacetamol in 100 ml saline Control: 50 mg pethidine IM |
|
| Outcomes | Pain intensity (VAS, VRS) and derived SPID Pain relief (VAS, VRS) and derived TOTPAR Time to onset and duration of analgesia Global evaluation (categorical) |
|
| Source of funding | Unclear ‐ one author was an employee of Squibb Pharmaceuticals | |
| Were treatment groups comparable at baseline? | Yes: demographics, disease categories, operation categories, anesthesia methods and duration, vital signs, hepatorenal function, and blood cell count | |
| Details of preoperative pain | Unclear | |
| Notes | Chinese language article with abstract and data in English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Allocation concealment (selection bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Chinese article ‐ unable to ascertain |
| Selective reporting (reporting bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 pethidine) |
Maghsoudi 2014.
| Methods | Randomized, double‐blind, placebo‐controlled multiple dose, 24‐hour study Medication was administered 30 min after extubation |
|
| Participants | Type of surgery: percutaneous nephrolithotomy Paracetamol group Entered/completing: 50/50 Age (mean, SD): 44.48 +/‐ 12.92 Sex (male, %): 34 (68%) Placebo group Entered/completing: 50/50 Age (mean, SD): 42.56 +/‐ 13.57 Sex (male, %): 40 (80%) |
|
| Interventions | Paracetamol: 100 ml normal saline and 1 g paracetamol IV 30 min after extubation and every 8 h until 24 h (4 g total) 100 ml IV normal saline 30 min after extubation and every 8 h until 24 h |
|
| Outcomes | Primary: pain intensity (VAS) over first 6 h and 24 h after extubation, demand for opioid analgesia (pethidine 25 to 50 mg IM up to 200 mg per day), total pethidine dose consumed Secondary: adverse effects |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes ‐ age, BMI, stone size, operative time, baseline VAS. No mention if # of males balanced – 40 versus 34. | |
| Details of preoperative pain | Participants were excluded if they reported use of a NSAID or other analgesic less than 12 h before prescribing the study medications. Also excluded if painful physical conditions that may affect pain assessment after percutaneous nephrolithotomy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced blocked randomization; randomization schedule was prepared by someone that was blinded to the study. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Serums containing placebo and paracetamol, identical in color and appearance were prepared by an assistant and administered by nursing personnel blinded to the study.” Other group blinded was not specifically stated but assumed to be participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per Results, 2 participants did not complete the study but no additional information was provided. No deviations from the protocol was also noted. |
| Selective reporting (reporting bias) | Low risk | Frequency of VAS < or > 4 was not mentioned as an outcome in Methods (Table 2) |
| Size | Unclear risk | 50 to 199 participants per arm of the study (50 paracetamol, 50 placebo) |
Marty 2005.
| Methods | Randomized, single dose, double‐blind, active‐controlled parallel‐group Medication administered when baseline pain reached moderate‐to‐severe intensity |
|
| Participants | Type of surgery: gynecological Paracetamol group Entered/completing: 80/80 Age (mean, SD): 38.3 ± 12.8 Sex (male, %): 0 Propacetamol group Entered/completing: 81/81 Age (mean, SD): 33.9 ± 12.0 Sex (male, %): 0 |
|
| Interventions | Intervention: paracetamol 1g IV in 100 ml solution over 15 min Active control: propacetamol 2 g in 100 ml solution |
|
| Outcomes | Primary outcome: tolerability, including pain at infusion site Pain intensity (VRS, VAS) Number of participants requesting rescue medication Patient satisfaction (categorical) |
|
| Source of funding | Not mentioned, but senior author was employee of Bristol Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: weight, type of surgery, baseline pain intensity at surgical site. No ‐ age: 38.3 paracetamol versus 33.9 propacetamol. | |
| Details of preoperative pain | Patients with any painful physical condition (other than postoperative pain) were excluded. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1 ratio according to a computer‐generated list of numbers to either group |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study drugs mixed by pharmacist or nurse not involved in the study, were administered as a 100 ml solution infused over 15 min |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 163 women were enrolled and 161 received the single infusion of study medication. All remaining 161 patients including 2 patients (1 in each group) who did not meet eligibility criteria, were included in the ITT population and analyses of demographic characteristics, tolerability, and efficacy". Not clear if data were imputed. |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting. All outcomes from Methods section reported in Results section. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (80 paracetamol, 81 propacetamol) |
Mimoz 2001.
| Methods | Randomized, placebo‐ and active‐controlled Medications administered at completion of surgery |
|
| Participants | Type of surgery: hepatic resection Propacetamol group Entered/completing: unclear/38 Age (median, range): 49 (28 to 75) Sex (male, %): 40 Nefopam group Entered/completing: unclear/36 Age (median, range): 57 (21 to 75) Sex (male, %): 53 Placebo group Entered/completing: unclear/38 Age (median, range): 57 (27 to 75) Sex (male, %): 58 |
|
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 20 mg nefopam over 60 min Placebo: no treatment |
|
| Outcomes | Primary: opioid consumption (morphine via PCA) Pain intensity (VAS) Patient satisfaction (categorical) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: sex, weight, height, ASA physical status, duration of surgery and anesthesia, sufentanil and midazolam cumulative doses, VAS score at extubation, and morphine dose for titration Participants in the propacetamol group were younger versus other 2 groups. Compared with the propacetamol group, participants in the nefopam group had lower VAS scores at extubation and longer intervals between the completion of hepatic resection and extubation. |
|
| Details of preoperative pain | Participants receiving chronic anaΙgesic or anti‐inflammatory treatment were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Open study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From 120 participants 8 were withdrawn for various reasons. Data from all remaining participants were used in the analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (38 propacetamol, 36 nefopam, 38 placebo) |
Mitra 2012.
| Methods | Randomized, double‐blind, parallel‐group controlled trial, multiple dose, active‐controlled. First dose after spinal block regression to T10. | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 101/101 Age (mean, SD): 25.92 ± 3.09 Sex (male, %): 0 Tramadol group Entered/completing: 103/103 Age (mean, SD): 26.04 ± 3.65 Sex (male, %): 0 |
|
| Interventions | Diclofenac 100 mg suppository for all participants starting at the ‘end of surgery’ and every 8 h for 24 h Paracetamol IV in 10 cc of NS (no mention of injection time), 1 g every 6 h beginning at block regression to T10 Tramadol 75 mg IV in 10 cc NS per the above protocol |
|
| Outcomes | Primary: summed pain intensities during the entire observation period, calculated as the sum of time‐weighted pain intensity scores as an area under the curve (AUC). NRS at rest and movement at 0, 1, 2, 4, 8, 12, 24 h Secondary: use of supplementary rescue analgesic (pethidine 30 mg IV, administered if the participant’s NRS scores > 4) |
|
| Source of funding | Grant received from the Department of Science & Technology (DST), Ministry of Science & Technology, Government of India | |
| Were treatment groups comparable at baseline? | Yes ‐ age BMI, surgical duration, blood loss, NRS at rest and movement. No ‐ BP and HR lower in acetaminophen group | |
| Details of preoperative pain | Participants receiving long‐term analgesics were excluded | |
| Notes | Primary analysis plan was not pursued because of a non normal distribution of pain scores. Instead medians and interquartile ranges were reported and compared for each time point at rest and at movement. This includes time at 4 h. Use of rescue medications over the entire time period was summarized without specific information as to the time for request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Coded, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both the test drugs (tramadol and acetaminophen) were drawn up in similar (Dispovan, Faridabad, Haryana, India) 10 ml coded syringes and diluted with normal saline so as to make the final volume of injection to 10 ml. Participants and assessors were blinded to assignment, but blinding success not tested. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (101 paracetamol, 103 tramadol) |
Moller 2005a.
| Methods | Randomized, double‐dummy, placebo and active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 4 h after surgery |
|
| Participants | Type of surgery: third molar extraction Paracetamol group Entered/completing: 51/51 Age (mean, SD): 24.5 ± 2.9 Sex (male, %): 69 Propacetamol group Entered/completing: 51/51 Age (mean, SD): 24.3 ± 3.6 Sex (male, %): 57 Placebo group Entered/completing: 50/50 Age (mean, SD): 24.5 ± 2.8 Sex (male, %): 68 |
|
| Interventions | Intervention: IV paracetamol 1 g Control: propacetamol 2 g Placebo: 100 ml saline, or 100 ml solution (double‐dummy) |
|
| Outcomes | Primary outcome: pain relief (VRS) Maximum pain relief, time of maximum pain relief, time to onset of pain relief, TOTPAR Pain intensity (VRS, VAS) and derived summary measures Time to rescue medication (oral ibuprofen 400 mg) Global evaluation (categorical) |
|
| Source of funding | Supported by the Bristol‐Myers Squibb Company | |
| Were treatment groups comparable at baseline? | Yes: demographics and baseline postoperative pain | |
| Details of preoperative pain | Participants with other painful physical conditions were excluded | |
| Notes | Propacetamol and paracetamol were compared to placebo and then propacetamol was compared to paracetamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatments were allocated according to block randomization (each block, n = 6) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Treatments were randomized among 152 patients. No patients withdrew from the study and all patients were evaluated for efficacy and safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (51 paracetamol, 51 propacetamol, 50 placebo) |
Moller 2005b.
| Methods | Randomized, double‐blinded, triple‐dummy, active‐ and placebo‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 4 h after surgery |
|
| Participants | Type of surgery: third molar extraction Propacetamol group Entered/completing: 50/50 Age (mean, SD): 24.2 (range 18 to 39) Sex (male, %): 46 Placebo group Entered/completing: 25/25 Age (mean, SD): 23.4 (range 20 to 29) Sex (male, %): 44 |
|
| Interventions | Intervention: 2 g propacetamol 15 min infusion Intervention: 2 g propacetamol 2 min bolus (not included in our analysis) Control: oral acetaminophen (not included in our analysis) Placebo: triple‐dummy, exact details not described |
|
| Outcomes | Primary: time to analgesia onset (double‐click stopwatch method) Pain relief (categorical) and derived summary scores Pain intensity (VAS) and derived summary scores Global evaluation (categorical) Duration of analgesia (time when 50% of participants in a group requested rescue medication, oral ibuprofen 600 mg) |
|
| Source of funding | The study was supported by a grant from Bristol–Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: sex, weight, baseline pain intensity. Age appears to be similar between the 2 groups included in our analysis. | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization schedule assigned treatments to sequential patients |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A triple‐dummy technique was employed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Treatments were randomized between 175 patients. No patients withdrew from the study and all 175 patients were evaluated by the intent‐to‐treat analyses and for safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (50 propacetamol, 25 placebo) |
Mowafi 2012.
| Methods | Randomized, investigator‐blinded, parallel‐group study with active and placebo control, first dose at skin closure, outcomes X 24 h | |
| Participants | Type of surgery: variety of lower abdominal surgery (bowel resection, abdominal hysterectomy, abdominal myomectomy, radical prostatectomy) Paracetamol group Entered/completing: 20/20 Age (mean, SD): 49.4 ± 18.4 Sex (male, %): 20% Placebo group Entered/completing: 20/19 Age (mean, SD): 48.5 ± 14.4 Sex (male, %): 25% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 52.8 ± 16.1 Sex (male, %): 30% |
|
| Interventions | Paracetamol: 1 g every 6 h in 100 cc IV x 24 h Placebo: 100 cc IV every 6 h x 24 h Lornoxicam: 16 mg in 100 cc saline at time 0 and 8 mg at time 12 h All: PCA pump containing morphine was attached to the participant in a separate IV cannula. The pumps were programmed to administer morphine 1 mg boluses at 10 min intervals and total of 20 mg through 4 h limits. |
|
| Outcomes | Primary: pain score via VPS during rest and coughing at 1st, 2nd, 4th, 8th, 12th and 24th postoperative h Secondary: heart rate, blood pressure, respiratory rate, and morphine consumption of the participants were assessed at 1st, 2nd, 4th, 8th, 12th and 24th postoperative h Adverse effects, including nausea, vomiting, itching, sweating, urinary retention, sedation, respiratory depression, hypotension, tachycardia, bradycardia, gastric irritation, increased bleeding from the wound, hematemesis, and melena were recorded and managed accordingly. The Ramsay sedation score [16] was used to evaluate the level of sedation. |
|
| Source of funding | Deanship of Scientific Research of Dammam University | |
| Were treatment groups comparable at baseline? | Reports no significant differences for study groups including operative time but states P value < 0.05 for all demographics, likely an error | |
| Details of preoperative pain | Patients who received pain medications on the day prior to surgery and chronic drug abusers were excluded | |
| Notes | Sample size based on internal pilot and VPS difference of 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online research randomizer (www.randomizer.org) |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of participants blinding. “The anesthetist who provided anesthesia and the on who followed up with the patients in the ward for assessment were blinded to the study drug given. Sealed and enclosed 100 ml bags containing either normal saline or the study drugs were used. The color of lornoxicam solution is yellow; to maintain blinding, the containers for all solutions were covered with aluminium foil during administration”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one dropout in entire study |
| Selective reporting (reporting bias) | Unclear risk | Opioid consumption measured at 1, 2 and 4 h, but not reported No results available on clinicaltrials.gov |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 lornoxicam) |
Ohnesorge 2009.
| Methods | Randomized, double‐blind, placebo‐ and active‐controlled Medications administered 20 min before the end of surgery (and at 4, 10 and 16 h postoperatively) |
|
| Participants | Type of surgery: breast cancer (segmental or mastectomy) Paracetamol group Entered/completing: 30/27 Age (mean, SD): 56 ± 13 Sex (male, %): 41 Metamizole group Entered/completing: 30/26 Age (mean, SD): 52 ± 12 Sex (male, %): 55 Placebo group Entered/completing: 30/26 Age (mean, SD): 58 ± 14 Sex (male, %): 55 |
|
| Interventions | Intervention: IV paracetamol 1 g/100 ml normal saline over 10 to 15 min Control: metamizole 1 g IV Placebo: 100 ml normal saline |
|
| Outcomes | Pain intensity (NRS) Cognitive function (TDT, DSST) All other outcomes assessed at 24 h |
|
| Source of funding | Supported by Department of Anesthesiology and Bristol‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, nature of surgery, ASA risk category | |
| Details of preoperative pain | Not reported | |
| Notes | From 2 h postoperatively onwards, average pain ratings were below 2.5/10 in all groups. No data could be used in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatments appeared identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27/30 paracetamol and 26/30 in other groups completed study ‐ dropouts for various reasons specified. It appears that only data from those completing study were analyzed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section. Minor omissions of complete data for some safety outcomes. |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 metamizole, 30 placebo) |
Omar 2011.
| Methods | Blinded, randomized, controlled, parallel‐group, multidose trial. Intervention start at the ‘end of surgery’. | |
| Participants | Type of surgery: elective cesarean section Paracetamol group Entered/completing: 40/40 Age (mean, SD): 30.80 ± 4.79 Sex (male, %): 0 Normal saline group Entered/completing: 40/40 Age (mean, SD): 29.60 ± 5.20 Sex (male, %):0 |
|
| Interventions | Paracetamol: 1 g IV in 100 cc start at end of surgery, then every 6 h x 24 h Control: 100 cc NS start at end of surgery, then every 6 h x 24 h All: every participant received 0.2 mg intrathecal morphine at the time of spinal placement. Clinically this intervention has an expected duration of action of up to 18 h. Pethidine for rescue analgesia |
|
| Outcomes | Primary: number of participants requiring rescue analgesic drug use x 24 h Secondary: visual analog scale (VAS) was used to evaluate pain level (0 = no pain to 10 = worst pain) at 6, 12 and 24 h postoperatively by a resident and nurse who did not know about the treatment protocols. Satisfaction was evaluated at 12 and 24 h postoperatively (1 = very unsatisfied to 5 = very satisfied). |
|
| Source of funding | None mentioned | |
| Were treatment groups comparable at baseline? | Yes, but no data on surgical duration or whether or not a participant had a repeat cesarean section | |
| Details of preoperative pain | Participants with history of chronic abdominal pain or treated with analgesics were excluded from the study | |
| Notes | No enrolment flow chart. When a spinal failed, a participant was dropped, likewise for participants with intraoperative complications for example, but unclear when participants were enrolled or randomized. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant blinding not addressed. Personnel blinding per statement except that the chief resident was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported. Appears that all participants completed the study. |
| Selective reporting (reporting bias) | High risk | Very limited outcomes (rescue analgesia, pain, and satisfaction scores). No side effects addressed at all. |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 placebo) |
Oncul 2011.
| Methods | Double‐blind, double‐dummy, parallel‐group, active‐controlled randomized study | |
| Participants | Type of surgery: bimaxillary osteotomy Paracetamol group Entered/completing: 15/15 Age (median, range): 21 (16 to 38) Sex (male, %): 27 Diclofenac group Entered/completing: 15/15 Age (median, range): 24 (17 to 42) Sex (male, %): 27 |
|
| Interventions | All: 160 mg of articaine LA with epinephrine to site of surgery at the start of case. General anesthesia, no opioids. Postoperative on demand rescue analgesic of 75 mg diclofenac IM. Observation period x 24 h. Paracetamol: intravenous solution of 1 g x 1 within 15 min of mucosal closure + IM placebo Diclofenac: IV solution of 1 g of placebo x 1 within 15 min of mucosal closure + IM 75 mg diclofenac |
|
| Outcomes | Primary: the severity of postoperative pain was evaluated on the VAS after 30 min, and then at 1, 2, 4, 6, 8, 12, and 24 h Secondary: systolic blood pressures and heart rates were also recorded at the same times. The number and time of diclofenac rescue were also recorded. Early and late side effects during the first 30 min and after 24 h, such as nausea, vomiting, hypotension, sedation, cyanosis, hypertension, facial oedema, and urticaria, were also recorded. Patients’ satisfaction was assessed 24 h postoperatively using a 3‐point scale (1 = not satisfied, 2 = satisfied, 3 = very satisfied). |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, BMI, duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation to 2 groups, no further info |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “a staff nurse premixed an intravenous solution containing either paracetamol or placebo 1 g. The same nurse gave an intramuscular injection of diclofenac 75 mg or placebo as assigned”. No mention of whether placebo and intervention appeared identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears all participants completed study and data were collected on all |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 15 diclofenac) |
Oreskovic 2014.
| Methods | Double‐blind, randomized, active‐controlled, parallel‐group study with intervention at end of surgery x 24 h for outcomes | |
| Participants | Type of surgery: total hip arthroplasty under spinal anesthesia Paracetamol group Entered/completing: 43/43 Age (mean, SD): 57.7 (13.8) Sex (male, %): 39.5 Metamizol group Entered/completing:51/51 Age (mean, SD): 62.2 (12.4) Sex (male, %):29.4 All: morphine PCA at baseline |
|
| Interventions | Paracetamol: IV 1 g paracetamol every 8 h x 24 h, start at ICU admission Metamizol: IV 1.5 g every 8h x 24 h, start at ICU admission All: PCA morphine with continuous setting 1 to 2 mg/h (based on participant weight), 1 mg bolus with 15 min lockout x 24 h |
|
| Outcomes | Primary: pain intensity in the first 24 h after surgery. Total pain over the study period was calculated as area under the pain/time curve. VAS 0 to 100 with a 10 cm ruler. Secondary: pain was assessed at time points of 1, 2, 3, 4, 6, 8, 10, 14, 18 and 22 h post‐baseline Amount of morphine consumption in 24 h |
|
| Source of funding | No financial support | |
| Were treatment groups comparable at baseline? | Yes: demographics, estimated blood loss, duration of surgery | |
| Details of preoperative pain | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Research team and the patients were not familiar with the information about products that patients received. Drugs were prescribed by an independent doctor and administered by nurses not involved in the research. Nurses not involved in the research recorded VAS scale results. Independent blinded researchers performed clinical observations”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants completed study. No mention of how missing data were imputed. |
| Selective reporting (reporting bias) | High risk | No reporting of adverse events |
| Size | High risk | Fewer than 50 participants per arm of the study (43 paracetamol, 51 metamizol) |
Paech 2014.
| Methods | Randomized, double‐blind, double‐dummy, parallel‐group, placebo‐controlled clinical trial x 24 h, starting with intervention immediately postoperatively | |
| Participants | Type of surgery: elective cesarean delivery under spinal anesthesia Paracetamol group Entered/completing: 32/32 Age (median, IQR): 31 (28 to 34) Sex (male, %): 0 Control group Entered/completing:23/23 Age (median, IQR): 30 (28 to 35) Sex (male, %): 0 Parecoxib group Entered/completing: 30/30 Age (median, IQR): 30 (26 to 35) Sex (male, %): 0 |
|
| Interventions | IV solutions (200 ml for infusion over 15 min or 2 ml for bolus injection after delivery) and oral capsules of identical appearance (for administration after surgery). Study regimen started immediately after delivery. All: 1. Pethidine patient‐controlled epidural analgesia x at least 24 h, 20 mg on demand, 15 min lockout 2. If the verbal numerical rating score for pain was > 6 with movement or > 3 at rest at any time, supplementary analgesia was available in the form of immediate‐release tramadol 50 to 100 mg oral 2‐hourly on demand (maximum dose 600 mg across 24 h) 3. Thereafter, analgesia was at the discretion of the attending anesthetist or acute pain service 4. Postoperative pruritus was treated with IV ondansetron 4 mg 6‐hourly on demand or, if this was ineffective, IV naloxone 50 µg hourly on demand Paracetamol: 2 g IV x 1 after delivery, then oral 1 g at 6, 12 and 18 h and appropriate placebos Parecoxib: 40 mg IV x 1 after delivery, then oral celecoxib 400 mg at 12 h and placebos Parecoxib + paracetamol: 40 mg and 2 g IV after delivery, then paracetamol 1 g oral at 6, 12 and 18 h + celecoxib 400 mg oral at 12 h (not included in our analysis) Control: saline placebos and capsules after delivery and at 6, 12 and 18 h respectively as appropriate |
|
| Outcomes |
Primary: 24‐hour postoperative patient‐controlled epidural pethidine use Secondary: the main secondary outcomes were the 0 to 24 h AUC pain scores with movement, the quality of recovery score and the "SDQ" score 1. Postoperative pain measured as verbal numerical rating score (VRS) of 0 to 10 at rest and movement at 6, 12, 24 and 48 h 2. VRS sedation scores at the same times. Area under the curve (AUC) for rest and movement pain scores over 0 to 24 h 3. Presence of gastrointestinal upset, nausea or epigastric pain at 24 h 4. Satisfaction with analgesia (0 to 10 VRS and ratings of excellent, good, fair, or poor) at 24 h 5. Severity of overall nausea, sedation, and pruritus (VRS) at 24 h 6. OR score, the opioid related "SDQ" score and a Modified Brief Pain Inventory (short‐form) 7. At 48 h, pain and sedation scores were recorded and the presence of urinary retention post‐catheter removal or observed respiratory depression (respiratory rate < 8 breaths per minute or sedation score of 3 representing 'difficult to rouse') assessed |
|
| Source of funding | Investigator‐initiated research grant from Pfizer Australia | |
| Were treatment groups comparable at baseline? | Yes: age, ASA II, BMI, gravidity, parity, previous cesarean section | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A randomization sequence for four groups in a 1:1:1:1 ratio was generated by the hospital Pharmacy Department using a computer‐generated random number sequence” |
| Allocation concealment (selection bias) | Low risk | “Allocation was by selection of the next sealed and coded study drug package and occurred intraoperatively”. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All observers were blinded to study group allocation. Medications prepared to look identical. No further detail. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis with LOCF, plus per‐protocol analysis and no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Did not report all adverse event data |
| Size | High risk | Fewer than 50 participants per arm of the study (32 paracetamol, 23 placebo, 30 parecoxib) |
Peduto 1998.
| Methods | Randomized, placebo‐controlled, double‐blind Medications administered after extubation |
|
| Participants | Type of surgery: orthopedic Propacetamol group Entered/completing: 46/41 Age (mean, SD): 62.6 ± 8.3 Sex (male, %): 31 Placebo group Entered/completing: 51/45 Age (mean, SD): 60.5 ± 9.6 Sex (male, %): 32 |
|
| Interventions | Intervention: propacetamol 2 g in 100 ml 5% dextrose over 15 min Placebo: 5% dextrose 100 ml |
|
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VRS, VAS) Global efficacy (VRS) |
|
| Source of funding | Financially supported by UΡSA MEDΙCA SPA | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, baseline pain intensity, pre‐intervention morphine use | |
| Details of preoperative pain | None | |
| Notes | Opioid consumption and global efficacy assessed at 24 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block 2:2 randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical vials for further dilution |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate intention‐to‐treat. "A total of 97 patients entered the study and 89 of them were evaluated". 8 were withdrawn from efficacy analyses due to malfunctioning of PCA. All of these 89 patients were used in the efficacy analyses including 3 cases of premature discontinuation of the study due to lack of efficacy and 1 withdrawal of consent. Time point of premature discontinuation is not defined |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (46 propacetamol, 51 placebo) |
Salonen 2009.
| Methods | Prospective, randomized, double‐blinded and placebo‐controlled add‐on study with 3 parallel groups, paracetamol given 5 min after ketoprofen that was given after surgery, both likely within 1 hour of surgery end, but exact time not given | |
| Participants | Type of surgery: tonsillectomy Paracetamol 1 g group Entered/completing: 39/39 Age (mean, SD): 22 (6) Sex (male, %): 15, 40% Placebo group Entered/completing: 38/38 Age (mean, SD): 38 (10) Sex (male, %): 18, 47% |
|
| Interventions | Paracetamol 1 g: 15 min infusion, 5 min after ketoprofen IV x 1 Paracetamol 2 g: 15 min infusion, 5 min after ketoprofen IV x 1 (not included in our analysis) Normal Saline: 15 min infusion 5 min after ketoprofen IV X 1 ALL: 2 µg/kg fentanyl intraoperatively, ketoprofen 1 mg/kg in 10 cc saline 5 min after surgery, oxycodone 2 mg IV was provided for rescue analgesia if VAS rest was > 30/100 mm or VAS swallowing > 50/100 mm. The oxycodone dose was repeated at 15‐min intervals until pain had diminished (VASr < 30mm and VASs < 50mm) x 6 h |
|
| Outcomes | Primary: proportion of patients requiring oxycodone for rescue analgesia to maintain VASr (resting) < 30 mm and VASs (swallowing) < 50 mm over the first 6 h Secondary: VASr&s at 1, 2, 3, 4, and 6 h after the surgery and at discharge. Length of time until the first dose of rescue analgesic, the number of oxycodone doses during the first 6 h after surgery, the sedation score at discharge, and the incidence of adverse effects. |
|
| Source of funding | Absence of external funding specifically mentioned | |
| Were treatment groups comparable at baseline? | Unclear: no statistical analysis was provided for the group characteristics | |
| Details of preoperative pain | Not reported | |
| Notes | 119 participants enrolled. 5 withdrew consent before randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “To ensure blinding, the syringes were prepared by a nurse otherwise not involved in the study, and the patients, surgeons and study nurses obtaining the outcome data were blinded to the treatment arms”. “Thus, we used an approach where all patients were provided similar infusions prepared by the study nurse. Because a paracetamol infusion is colorless, does not contain visible particles and does not irritate veins, we believe that the blinding should have performed sufficiently. Unfortunately, we did not test this in the present study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants completed the study and appear to have contributed data for all outcomes |
| Selective reporting (reporting bias) | High risk | 6 h pain scores measured but not reported, patient characteristics analyzed but no statistical comparison provided, insufficient detail regarding adverse effects, including nausea. Adverse events were not reported group‐specific but in aggregate for the entire cohort. |
| Size | High risk | Fewer than 50 participants per arm of the study (39 paracetamol, 38 placebo) |
Sanyal 2014.
| Methods | Prospective, randomized, double‐blind study, parallel‐group, active control x 24 h | |
| Participants | Type of surgery: elective total abdominal hysterectomy with or without bilateral salpingo‐oophorectomy Paracetamol group Entered/completing: not stated Age (mean, SD): not stated Sex (male, %): 0 Diclofenac group Entered/completing: not stated Age (mean, SD): not stated Sex (male, %): 0 |
|
| Interventions | Paracetamol: 1 g IV postoperatively every 8 h x 24 h Diclofenac: 75 mg IM every 8 h x 24 h |
|
| Outcomes | Primary: requirement of rescue analgesic (time component not described) Secondary: VAS (scale not mentioned) for pain at least at 4 and 12 h postoperatively, time until first rescue analgesic administration, patient satisfaction score (scale not mentioned), nausea, vomiting, bronchospasm |
|
| Source of funding | None mentioned | |
| Were treatment groups comparable at baseline? | Not described | |
| Details of preoperative pain | Not described | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants completed the study or what method of analysis was used |
| Selective reporting (reporting bias) | High risk | No data reported |
| Size | High risk | Fewer than 50 participants per arm of the study |
Shimia 2014.
| Methods | Double‐blind, randomized, placebo‐controlled. Single dose of intravenous paracetamol within the last 20 min of surgery or placebo. | |
| Participants | Type of surgery: lumbar discectomy Paracetamol group Entered/completing: dropouts not reported; presumably 24/24 Age (mean, SD): 46.50 ± 14.07 Sex (male, %): 46.2% for both groups Placebo group Entered/completing: dropouts not reported; presumably 28/28 Age (mean, SD): 52.25 ± 11.46 Sex (male, %): 46.2% for both groups |
|
| Interventions | Paracetamol: 1 g in 100 ml normal saline. Duration of administration not stated. 100 ml normal saline. Duration of administration not stated. |
|
| Outcomes | Primary: pain on 0 to 10 VAS at 1, 6, 12, 18, 24 h after surgery Morphine dosage for the first 24 h postop Secondary: Adverse effects |
|
| Source of funding | Tabriz University of Medical Sciences | |
| Were treatment groups comparable at baseline? | Yes: age, sex | |
| Details of preoperative pain | Patients with preoperative treatment with narcotics, benzodiazepines, or clonidine were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | All local anesthetic solutions and adjuvant drugs were prepared by an anesthesiologist who was not involved in the performance of the study agents, patient care, or data collection. No mention that interventions appeared identical. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects assessment mentioned in methods, but article states there were no side effects related to treatment (without any detail) |
| Size | High risk | Fewer than 50 participants per arm of the study (24 paracetamol, 28 placebo) |
Siddik 2001.
| Methods | Randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled Medications administered immediately after surgery |
|
| Participants | Type of surgery: cesarean delivery Propacetamol group Entered/completing: 20/20 Age (mean, SD): 31 ± 4.6 Sex (male, %): 0 Diclofenac group Entered/completing: 20/20 Age (mean, SD): 31.4 ± 6 Sex (male, %): 0 Placebo group Entered/completing: 20/20 Age (mean, SD): 30.6 ± 5.1 Sex (male, %): 0 |
|
| Interventions | Intervention: 2 g propacetamol Control: 100 mg rectal diclofenac Control: combination of 100 mg rectal diclofenac and 2 g propacetamol (not included in our analysis) Placebo: double‐dummy, exact details not described |
|
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity at rest and on coughing (VAS 0 to 10) Global assessment (categorical, at 24 h) |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, weight, height, parity, and gestationaΙ age | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Patients and staff were unaware of the patients' group assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "to ensure blinding of both the parturients and the anaesthesiologist, patients in all groups received both an IV injection and a suppository during the same period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From 80 patients one was excluded due to technical problems of the PCA device. Data obtained from the 79 remaining patients were used for the analysis of all outcomes and time points. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 diclofenac, 20 placebo) |
Sinatra 2005.
| Methods | Randomized, double‐blind, double‐dummy, placebo‐controlled Medications administered on postoperative day 1, when baseline pain reached moderate‐to‐severe intensity (after PCA disconnected) |
|
| Participants | Type of surgery: total hip arthroplasty Paracetamol group Entered/completing: 49/46 Age (mean, SD): 61.7 ± 16.9 Sex (male, %): 57 Propacetamol group Entered/completing: 50/44 Age (mean, SD): 59.5 ± 14.2 Sex (male, %): 54 Placebo group Entered/completing: 52/47 Age (mean, SD): 59.2 ± 13.4 Sex (male, %): 42 |
|
| Interventions | Intervention: 1 g IV paracetamol in 100 ml solution over 15 min Control: 2 g propacetamol Placebo: 100 ml solution |
|
| Outcomes | Primary: pain relief (VRS) Pain intensity (VAS, VRS) Time to rescue medication (morphine) Opioid consumption (morphine) Patient global evaluation |
|
| Source of funding | Supported by Bristol‐Myers Squibb Company, Rueil‐Malmaison, France | |
| Were treatment groups comparable at baseline? | Yes: demographics, anesthetic and surgical procedure and baseline pain intensity | |
| Details of preoperative pain | "The overwhelming majority of patients had symptoms of severe debilitating or painful osteoarthritis". Not mentioned if this was similar between groups. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unblinded pharmacist was not involved in the study. All study medications were administered as a 100 ml solution infused over 15 min. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From a total of 156 randomized patients 151 included. "All of these 151 patients were included in the intent‐to‐treat population and analyzed for demographics, efficacy and safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (49 paracetamol, 50 propacetamol, 52 placebo) |
Tiippana 2008.
| Methods | Randomized, double‐blind, active‐controlled Medications administered at removal of gall bladder, around 30 min from end of surgery |
|
| Participants | Type of surgery: laparoscopic cholecystectomy Paracetamol group Entered/completing: 40/39 Age (mean, SE): 38.9 ± 1.8 Sex (male, %): 18 Parecoxib group Entered/completing: 40/39 Age (mean, SE): 42.9 ± 1.7 Sex (male, %): 28 |
|
| Interventions | Intervention: paracetamol 1 g IV Control: paracetamol 1 g IV with dexamethasone 10 mg IV (not included in our analysis) Control: parecoxib 40 mg IV Control: parecoxib 40 mg IV with dexamethasone 10 mg IV (not included in our analysis) |
|
| Outcomes | Pain intensity at rest and with movement (VAS) Time to rescue medication (oxycodone) Opioid consumption (at 24 h onwards only) |
|
| Source of funding | Pfizer Finland supplied parecoxib. No other details. | |
| Were treatment groups comparable at baseline? | Yes: age, weight, gender, ASA physical status, the duration of the operation, or the length of stay in hospital | |
| Details of preoperative pain | Patients regularly using analgesics were excluded | |
| Notes | Combination groups not analyzed. Contact author confirmed that doses of paracetamol/parecoxib were administered within half an hour of end of surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study drugs administered by nurse not otherwise involved in the study, but no further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/40 in each analyzed group excluded from analysis. It appears that all other participants contributed data for all time points of interest (more participants dropped out after day 1 of study, but were not part of our analysis). |
| Selective reporting (reporting bias) | Unclear risk | Pain at rest and with motion was recorded every 20 min in PACU 1 and every 30 min in PACU 2, but data not presented for time points 1.5 h, 2 h and 2.5 h |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 parecoxib) |
Togrul 2011.
| Methods | Double‐blind, random allocation to IV paracetamol 30 min before surgery versus IV tramadol 20 min before end of surgery | |
| Participants | Type of surgery: septo‐rhinoplasty Paracetamol group Entered/completing: 25/25 Age (mean, SD): 31.5 ± 11 Sex (male, %): 64 Tramadol group Entered/completing: 25/25 Age (mean, SD): 31.8 ± 10 Sex (male, %): 64 |
|
| Interventions | Paracetamol infusion 1 g given 30 min before end of surgery IV tramadol given 30 min (in abstract written 20 min) before end of surgery, dose not stated. In abstract ‐ 1 mg/kg dose is mentioned. |
|
| Outcomes | Primary: Pain intensity on 10 cm VAS Secondary: Patient satisfaction, drug side effects, analgesic need |
|
| Source of funding | Not stated | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, duration of surgery, and anesthesia | |
| Details of preoperative pain | Not reported | |
| Notes | Postoperatively participants could take 500 mg oral paracetamol, up to 3 g/day, and if required, tramadol 0.5 mg/kg as IV bolus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Last paragraph of introduction states it was double‐blind but no additional details were provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants have completed |
| Selective reporting (reporting bias) | High risk | Objective state adverse effect and patient satisfaction investigation. Adverse effect not reported. Only nausea % in one group is reported, stating it is higher than in the other group, but no quantitative data. Patient satisfaction not reported. |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 tramadol) |
Tunali 2013.
| Methods | Participants randomized to 3 groups to receive interventions at the time of wound closure (supposedly within 30 min from end of surgery) | |
| Participants | Type of surgery: microsurgical lumbar discectomy and/or laminectomy Paracetamol group Entered/completing: 20/18 Age (mean, SD): 46.39 ± 10.06 Sex (male, %): 33 Placebo group Entered/completing: 20/20 Age (mean, SD): 48.35 ± 9.93 Sex (male, %): 55 Dexketoprofen group Entered/completing: 20/18 Age (mean, SD): 39.17 ± 11.10 Sex (male, %): 38.9 |
|
| Interventions | All administered IV in 100 ml infused over 15 min All participants used IV PCA with morphine Paracetamol: 1 g every 6 h for 24 h The study states paracetamol but in discussion says “Paracetamol is an IV formulation of a prodrug of acetaminophen and used as a supplemental analgesic to reduce postoperative pain” Dexketoprofen: 50 mg IV every 8 h for 24 h Placebo: 100 ml normal saline every 8 h for 24 h |
|
| Outcomes | Primary: pain intensity on 0 to 10 VAS at 0, 1, 2, 6, 12, 24 h post‐op but primary outcome not defined Secondary: sedation on Ramsay score, cumulative PCA morphine consumption at the above time points, adverse effects |
|
| Source of funding | No funding | |
| Were treatment groups comparable at baseline? | No: age in dexketoprofen group lower than in 2 other groups; pain at time 0 in dexketoprofen group substantially lower | |
| Details of preoperative pain | Not reported | |
| Notes | Frequency of administration different: every 6 h for paracetamol and every 8 h for dexketoprofen and saline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1 person prepared sealed envelopes and another person drew an envelope for each case |
| Allocation concealment (selection bias) | Unclear risk | Unclear if solutions looked any different |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The treatment frequency is different (3 times daily versus 4 times daily), true blinding not likely, although study data were collected by a blinded anesthesiologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were excluded, with reasons given. Outcomes seem to be reported in full. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 dexketoprofen) |
Tuncel 2012.
| Methods | Local wound infiltration versus IV paracetamol versus IV lornoxicam 30 min before extubation. Additional analgesia with tramadol and as required pethidine. | |
| Participants | Type of surgery: laparoscopic renal and adrenal surgery Paracetamol group Entered/completing: 20/not reported Age (mean, SD): not reported Sex (male, %): not reported Lornoxicam group Entered/completing: 20/not reported Age (mean, SD): not reported Sex (male, %): not reported |
|
| Interventions | Paracetamol 1 g 30 min before extubation, then 5 g in 24 h postop, frequency/timing not reported 0.25% levobupivacaine infiltration to trocar incisions (not included in our analysis) Lornoxicam: 8 mg IV 30 min before extubation and another 8 mg during 24 h postop. Frequency not reported. |
|
| Outcomes | Primary: pain VAS scores Secondary: cumulative tramadol and pethidine consumption |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Not reported | |
| Details of preoperative pain | Not reported | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported, and not stated to be unblinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated that study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants completed the study |
| Selective reporting (reporting bias) | High risk | Most of the outcomes not reported |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 lornoxicam) |
Unal 2013.
| Methods | Administration of IV paracetamol versus IV dexketoprofen versus placebo during incision closure | |
| Participants | Type of surgery: total abdominal hysterectomy Paracetamol group Entered/completing: 21/20 Age (mean, SD): 48.1 ± 3.6 Sex (male, %): 0 Dexketoprofen group Entered/completing: 22/20 Age (mean, SD): 47.7 ± 5.9 Sex (male, %): 0 Placebo group Entered/completing: 21/20 Age (mean, SD): 48.1 ± 4.5 Sex (male, %): 0 |
|
| Interventions | Paracetamol 1 g/100 ml in 15 min IV infusion, then every 6 h for 24 h Dexketoprofen: 50 mg in 100 ml 15 min IV infusion, then every 8 h for 24 h Placebo: normal saline 100 ml 15 min IV infusion, then every 6 h for 24 h |
|
| Outcomes | Primary: differences in cumulative 24 h morphine consumption Secondary: VAS pain scores, adverse events, patient satisfaction |
|
| Source of funding | None | |
| Were treatment groups comparable at baseline? | Yes: weight, height, BMI, ASA 1/2, anesthesia duration, surgery duration | |
| Details of preoperative pain | Participants with preoperative pain or regular analgesic use excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block random allocation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded person preparing drugs, blinded person assessed outcomes. Unclear if the solutions looked the same. Drug administration frequency different among groups (3 versus four times daily), so blinding might have been compromised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of dropouts low, evenly distributed among groups and reasons for dropout do not appear to be related to treatment |
| Selective reporting (reporting bias) | Unclear risk | Incomplete description of methodology for patient satisfaction assessment and incomplete presentation of data |
| Size | High risk | Fewer than 50 participants per arm of the study (21 paracetamol, 22 dexketoprofen, 21 placebo) |
Van Aken 2004.
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 3 h after regaining consciousness |
|
| Participants | Type of surgery: dental Propacetamol group Entered/completing: 31/31 Age (mean, SD): 20.0 ± 4.9 Sex (male, %): 25 Morphine group Entered/completing: 30/30 Age (mean, SD): 18.8 ± 4.3 Sex (male, %): 39 Placebo group Entered/completing: 34/34 Age (mean, SD): 20.9 ± 6.6 Sex (male, %): 29 |
|
| Interventions | Intervention: 2 g propacetamol in 150 ml normal saline over 15 min Control: 10 mg morphine Placebo: 150 ml normal saline |
|
| Outcomes | Pain intensity (VRS, VAS) and derived summary measures Pain relief (VRS) Proportion of patients requesting and time to rescue medication (morphine) Global assessment (at 10 h) |
|
| Source of funding | Supported by a grant from Bristol‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, baseline pain intensity No: the morphine group had a larger proportion of ASA II participants than the placebo group (P value < 0.01) |
|
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From 99 patients 4 were excluded due to protocol violations before any data had been collected. "All 95 remaining patients from whom efficacy data were obtained were included in the efficacy analysis". |
| Selective reporting (reporting bias) | Unclear risk | All outcome from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (31 propacetamol, 30 morphine, 34 placebo) |
Varrassi 1999.
| Methods | Randomized, double‐blinded, double‐dummy, active‐controlled Medications administered at time of extubation |
|
| Participants | Type of surgery: elective hysterectomy Propacetamol group Entered/completing: 100/87 Age (mean, SD): 48.4 ± 6.7 Sex (male, %): 0 Ketorolac group Entered/completing: 100/89 Age (mean, SD): 49.8 ± 9.0 Sex (male, %): 0 |
|
| Interventions | Intervention: 2 g propacetamol in 100 ml saline over 15 min Control: 30 mg IV ketorolac |
|
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS and VRS) Patient satisfaction (VRS at 12 h) |
|
| Source of funding | Supported in part by UPSA MEDICA SPA | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, initial pain intensity | |
| Details of preoperative pain | Patients were excluded if they were receiving additional analgesic, antipyretic, or antiinflammatory treatment during the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block (2:2) randomization, each study center receiving 4 case lots |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Propacetamol 2 g or ketorolac 30 mg was administered as an IV infusion (100 ml saline in 15 min). Unclear whether the 2 infusions appeared identical. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24 patients were excluded before blinding and 2 patients, 1 from each treatment arm, were withdrawn from the study for reasons unrelated to treatment. Then 2 were withdrawn due to lack of efficacy. Remaining patients were 87 for propacetamol and 89 for ketorolac group and were all used in the outcome evaluating cumulative morphine consumption. Number of patients used for the outcomes "evaluation of pain intensity" and "percentage of patients rating pain as severe or very severe" differ. |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting |
| Size | Unclear risk | 50 to 199 participants per arm of the study (100 propacetamol, 100 ketorolac) |
Vuilleumier 1998.
| Methods | Randomized, double‐blind, active‐controlled Medications administered at the end of anesthesia |
|
| Participants | Type of surgery: various elective Propacetamol group Entered/completing: 40/38 Age (mean, SD): 39 ± 13 Sex (male, %): 47 Morphine group Entered/completing: 40/39 Age (mean, SD): 39 ± 14 Sex (male, %): 49 |
|
| Interventions | Intervention: 30 mg/kg propacetamol in 150 ml dextrose 5% over 15 min Control: 0.2 mg/kg morphine IV |
|
| Outcomes | Number of patients requiring rescue analgesia (repeat dose of intervention) Pain intensity (VAS) Vigilance (trailmaking test) |
|
| Source of funding | UPSA Laboratories supplied drugs and covered logistical expenses of study | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of anesthesia, type of surgery | |
| Details of preoperative pain | Patients taking opioids were excluded | |
| Notes | French language paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Solutions prepared by third party, but unclear if they appeared identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From the 80 patients 3 were withdrawn. All the remaining 77 patients were included in efficacy analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 morphine) |
Wininger 2010.
| Methods | 2 regimens of IV paracetamol (every 4 h versus every 6 h) versus 2 same regimens of placebo. Participants after abdominal laparoscopy, who present with pain. 24‐h assessment. | |
| Participants | Type of surgery: abdominal laparoscopy Paracetamol 1000 mg group Entered/completing: 92/88 Age (mean, SD): 45.3 ± 12.26 Sex (male, %): 20 Placebo group 100 ml Entered/completing: 43/37 Age (mean, SD): 46.0 ± 11.70 Sex (male, %): 14 Placebo group 65 ml Entered/completing: 67/62 Age (mean, SD): 46.5 ± 13.08 Sex (male, %): 20 |
|
| Interventions | All interventions administered on postoperative day 1 Paracetamol 1000 mg in 100 ml 15‐min IV infusion, every 6 h (total 4 doses over 24 h) Paracetamol 650 mg in 65 ml 15‐min IV infusion, every 4 h (total of 6 doses over 24 h, not included in our analysis) Placebo: 100 ml 15‐min IV infusion, every 6 h (total 4 doses over 24 h) Placebo: 65 ml 15‐min IV infusion, every 4 h (total of 6 doses over 24 h) |
|
| Outcomes | Primary: SPID 0 to 24 h of acetaminophen 1000 mg versus combined placebo Secondary: SPID24 for acetaminophen 650 mg versus placebos (not included in our analysis) TOTPAR24 Pain intensity at baseline, and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 16, 18, 20, 22, and 24 h Adverse events Vital signs CBC, Liver Function Tests |
|
| Source of funding | Cadence Pharmaceuticals | |
| Were treatment groups comparable at baseline? | Yes: age, sex, race, height, weight. No clinically relevant differences observed between the placebo and intravenous acetaminophen groups with regard to type of primary abdominal laparoscopic surgery, additional procedures performed, the duration of surgery, or the time from end of surgery to T0. | |
| Details of preoperative pain | Exclusion: having a chronic pain condition, or use of opioids or tramadol daily for > 7 days immediately before study medication administration | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization by performed by kit randomization, but there was an error (all first 109 participants allocated to either 1000 mg paracetamol or 65 ml placebo group) |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding procedures seem adequate. Active drug and placebo solution, bottles, and labels were identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes seem complete. Dropouts completely described and similar between groups. WOCF imputation used for observations recorded after first request for analgesia. BOCF for observations before first request. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported Protocol published on clinicaltrials.gov. All outcomes from protocol reported as planned. SPID 4 & 6, TOTPAR 4 & 6, time to rescue, rescue requirements all reported despite not being mentioned on clinicaltrials.gov. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (92 paracetamol, 43 placebo, 67 placebo) |
Zhou 2001.
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered on postoperative day 1 when baseline pain reached moderate‐to‐severe intensity |
|
| Participants | Type of surgery: orthopedic Propacetamol group Entered/completing: 60/57 Age (mean, SD): 61.4 ± 12.0 Sex (male, %): 37 Ketorolac group Entered/completing: 28/27 Age (mean, SD): 60.6 ± 11.1 Sex (male, %): 22 Placebo group Entered/completing: 55/52 Age (mean, SD): 60.9 ± 12.4 Sex (male, %): 40 |
|
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 15 mg ketorolac (not included in our analysis) Control: 30 mg ketorolac Placebo: saline |
|
| Outcomes | Time to onset of and number of patients experiencing analgesia (double‐stopwatch method) Pain intensity at rest and with activity (VRS, VAS) and derived summary measures Pain relief (categorical) and derived summary measures Time to, number of patients requesting, and consumption of rescue medication (morphine via PCA) Global evaluation (categorical) |
|
| Source of funding | Supported in part by a grant from UPSA Inc., France, and in part by the White Mountain Institute (a non–for profit public charity) in Los Altos, CA | |
| Were treatment groups comparable at baseline? | Yes: demographic, anesthetic and surgical characteristics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All study medication solutions prepared by a hospital pharmacist who was not involved in the data collection. Double‐dummy technique employed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 172 patients were initially randomized into the study groups, 164 received the study medication and were included in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting. All outcomes from Methods section reported in Results section. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (60 propacetamol, 28 ketorolac, 55 placebo) |
AE = adverse event; ASA = American Society of Anesthesiologists physical status classification system; AUC = area under the curve; BMI: body mass index; BOCF = baseline observation carried forward; BP = blood pressure; DSST = digit symbol substitution test; h = hour; HR = heart rate; ICU = intensive care unit; IM = intramuscular; INR = international normalized ratio; IQR = interquartile range; ITT = intention‐to‐treat; IV = intravenous; LA = local anesthetic; LOCF = last observation carried forward; min = minutes; NRS = numerical rating scale; NS = normal saline; N/V = nausea/vomiting; OR = operating room; PACU = post anesthesia care unit; PCA = patient‐controlled analgesia; PI = pain intensity; PT = prothrombin time; SD = standard deviation; SPID = summed pain intensity difference; TDT = Trieger dot test; THA = total hip arthroplasty; TOTPAR = total pain relief; VAS = visual analog scale; VPS = verbal pain score; VRS = verbal rating scale; WOCF = worst observation carried forward.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alhashemi 2006 | Pain not patient‐reported |
| Alhashemi 2007 | Pain not patient‐reported |
| Alimian 2014 | Paracetamol administered via continuous infusion |
| Anand 2013 | Outcomes assessed over 75 minutes only |
| Ang 1990 | Propacetamol administered intramuscularly |
| Ashrafnejad 2012 | Available as abstract only with no usable data |
| Aydogan 2008 | No pain or analgesic outcome |
| Caliskan 2013 | Preoperative administration of interventions |
| Candiotti 2010 | Not all participants had postoperative pain, efficacy outcomes were assessed at 24 hour intervals, not clear when drugs were administered |
| Cok 2011 | Preemptive administration of interventions |
| Dowling 2014 | Appears that control group did not receive placebo. Pain data only presented up to 1 hour. |
| Elseify 2011 | First dose received after induction of anesthesia, no 4‐ or 6‐hour pain data |
| Fijalkowska 2006 | Compares laparotomy to laparoscopy |
| Fourcade 2005 | No data provided on 4‐ or 6‐hour intervals |
| Garcia 1999 | Participants received propacetamol 30 minutes before operation |
| Gehling 2010 | Paracetamol administered via continuous infusion and no data at 4 or 6 hours |
| Ghaffaripour 2012 | Available as abstract only with no usable data |
| Gousheh 2013 | Interventions administered 10 minutes after induction of anesthesia. Operations were at least 1 hour long; therefore administration > 30 minutes before end of surgery. |
| Granry 1997 | Multiple dose study without data for first dose |
| Grundmann 2006 | Study drug administered more than 30 minutes before the end of surgery |
| Hernandez Palazon 2001 | No data provided for either 4‐ or 6‐hour intervals |
| Irct2012062410102N | All patients initially receive IV paracetamol |
| Ko 2010 | Preoperative administration of interventions |
| Kocum 2013 | Pain not patient‐reported, unclear if interventions within 30 minutes of end of surgery |
| Memis 2010 | Multiple dose study without data for first dose |
| Murat 2005 | Some pain assessments investigator‐reported. Unable to ascertain numbers of participants self reporting pain. |
| NCT01691690 | Pain not patient‐reported |
| NCT01721486 | Pain not patient‐reported |
| Nikoda 2006 | Not randomized |
| Olonisakin 2012 | No data beyond 3 h |
| Pernia 2000 | Received paracetamol in both arms to evaluate efficacy of drug metamizol |
| Rashwan 2013 | No pain data until 8 h post interventions |
| Silvanto 2007 | Study drug administered more than 30 minutes before the end of surgery |
| Topal 2009 | Control group did not receive active control or placebo |
| Toygar 2008 | One control group had intervention administered before induction, and the other control group received no intervention (no placebo was administered) |
| Turner 2014 | Interventions administered 30 minutes before surgical incision |
| Uvarov 2008 | Control group received no intervention |
| Uzun 2010 | Paracetamol plus placebo versus paracetamol plus metamizole, versus no treatment |
| Verchere 2002 | Interventions administered 1 hour before the end of surgery |
| Zeidan 2014 | Dose finding study with outcomes reported at 45 minutes |
| Ziolkowski 2008 | All groups received paracetamol |
IV: intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
Atashkhoyi 2014.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Dawoodi 2014.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Jabalameli 2014.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Majumdar 2014.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Pekmezci 2014.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Rasheed 2007.
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Unable to obtain full article from any source |
Ritchie 2015.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Singla 2015.
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Differences between protocol and review
Differences between the protocol and review were detailed in the previous version of this review (Tzortzopoulou 2011). Briefly, we did not perform certain sensitivity analyses as planned, we included additional subgroup analyses, and we performed a sensitivity analysis using a random‐effects model instead of our original fixed‐effect model. For the 2016 updated review, these changes were incorporated into our planned analysis. We added 'Summary of findings' tables.
Contributions of authors
2016 review
Searched for studies: EM.
Obtained copies of studies: EM, MF.
Selected which studies to include (two plus one arbiter): EM, MF, SH, RS.
Extracted data from studies (two review authors): EM, MF, SH, RS, DC.
Entered data into RevMan: EM, MF
Carried out the analysis: EM, MF.
Interpreted the analysis: EM, MF.
Drafted the final review: EM, MF.
Edited the final review: EM, MF, SH, RS, DC.
2011 review
Drafted the protocol: AT, MSC.
Developed a search strategy: MSC, AT.
Searched for studies (usually two review authors): AT, TF, EM.
Obtained copies of studies: AT, TF, EM.
Selected which studies to include (two plus one arbiter): AT, TF, MSC, EM.
Extracted data from studies (two review authors): AT, TF, EM, MB, RS, TF.
Entered data into RevMan: AT, EM.
Carried out the analysis: EM, AT.
Interpreted the analysis: EM, AT.
Drafted the final review: EM.
Drafted the revised review: EM.
Updated the review: EM, AT.
Sources of support
Internal sources
Saltonstall Fund for Pain Research, USA.
External sources
No sources of support supplied
Declarations of interest
EM: none known.
MF: none known. Prior to initial planning and conception of this review update, the institution at which MF is employed received payment for fee‐for‐service activities from Mallinckrodt Pharmaceuticals, which produces paracetamol/acetaminophen.
SH: none known.
DC: none known.
RS: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abdulla 2012a {published data only}
- Abdulla S, Eckhardt R, Netter U, Abdulla W. Efficacy of three IV non‐opioid‐analgesics on opioid consumption for postoperative pain relief after total thyroidectomy: a randomised, double‐blind trial. Middle East Journal of Anesthesiology 2012;21:543‐52. [PubMed] [Google Scholar]
Abdulla 2012b {published data only}
- Abdulla S, Eckhardt R, Netter U, Abdulla W. A randomized, double‐blind, controlled trial on non‐opioid analgesics and opioid consumption for postoperative pain relief after laparoscopic cholecystectomy. Acta Anaesthesiologica Belgica 2012;63:43‐50. [PubMed] [Google Scholar]
Akarsu 2010 {published data only}
- Akarsu S, Sahin S, Kara C, Akdemir N, Degerli S. A comparison between parenteral paracetamol and diclofenac for acute postoperative pain treatment in patients after caeserean section. Journal of Turkish Society of Obstetrics and Gynecology 2010;7:262‐6. [Google Scholar]
Akil 2014 {published data only}
- Akil A, Api O, Bektas Y, Yilmaz AO, Yalti S, Unal O. Paracetamol vs dexketoprofen for perineal pain relief after episiotomy or perineal tear. Journal of Obstetrics & Gynaecology 2014;34:25‐8. [DOI] [PubMed] [Google Scholar]
Arici 2009 {published data only}
- Arici S, Gurbet A, Turker G, Yavascaoglu B, Sahin S. Preemptive analgesic effects of intravenous paracetamol in total abdominal hysterectomy. Agri Dergisi 2009;21:54‐61. [PubMed] [Google Scholar]
Arslan 2011 {published data only}
- Arslan M, Cicek R, Celep B, lmaz Y, Ustun Kalender H. Comparison of the analgesic effects of intravenous paracetamol and lornoxicam in postoperative pain following thyroidectomies. Agri Dergisi 2011;23:160‐6. [DOI] [PubMed] [Google Scholar]
Arslan 2013 {published data only}
- Arslan M, Celep B, Cicek R, Kalender HU, Yilmaz H. Comparing the efficacy of preemptive intravenous paracetamol on the reducing effect of opioid usage in cholecystectomy. Journal of Research in Medical Sciences 2013;18:172‐7. [PMC free article] [PubMed] [Google Scholar]
Atef 2008 {published data only}
- Atef A, Fawaz AA. Intravenous paracetamol is highly effective in pain treatment after tonsillectomy in adults. European Archives of Oto‐Rhino‐Laryngology 2008;265:351‐5. [17891409] [DOI] [PubMed] [Google Scholar]
Aubrun 2003 {published data only}
- Aubrun F, Kalfon F, Mottet P, Bellanger A, Langeron O, Coriat P, et al. Adjunctive analgesia with intravenous propacetamol does not reduce morphine‐related adverse effects. British Journal of Anaesthesia 2003;90:314‐9. [PUBMED: 12594143] [DOI] [PubMed] [Google Scholar]
Beaussier 2005 {published data only}
- Beaussier M, Weickmans H, Paugam C, Lavazais S, Baechle JP, Goater P, et al. A randomized, double‐blind comparison between parecoxib sodium and propacetamol for parenteral postoperative analgesia after inguinal hernia repair in adult patients. Anesthesia & Analgesia 2005;100:1309‐15. [PUBMED: 15845675] [DOI] [PubMed] [Google Scholar]
Brodner 2011 {published data only}
- Brodner G, Gogarten W, Aken H, Hahnenkamp K, Wempe C, Freise H, et al. Efficacy of intravenous paracetamol compared to dipyrone and parecoxib for postoperative pain management after minor‐to‐intermediate surgery: a randomised, double‐blind trial. European Journal of Anaesthesiology 2011;28:125‐32. [DOI] [PubMed] [Google Scholar]
Cakan 2008 {published data only}
- Cakan T, Inan N, Culhaoglu S, Bakkal K, Basar H. Intravenous paracetamol improves the quality of postoperative analgesia but does not decrease narcotic requirements. Journal of Neurosurgical Anesthesiology 2008;20:169‐73. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen ZF, Jiang H, Li YY. Role of propacetamol in multimodal analgesia after lumbar spine surgery. Journal of Shanghai Jiaotong University (Medical Science) 2011;31:1205‐11. [Google Scholar]
Dejonckheere 2001 {published data only}
- Dejonckheere M, Desjeux L, Deneu S, Ewalenko P. Intravenous tramadol compared to propacetamol for postoperative analgesia following thyroidectomy. Acta Anesthesiologica Belgica 2001;52:29‐33. [PUBMED: 11307656] [PubMed] [Google Scholar]
Delbos 1995 {published data only}
- Delbos A, Boccard E. The morphine‐sparing effect of propacetamol in orthopedic postoperative pain. Journal of Pain & Symptom Management 1995;10:279‐86. [PUBMED: 7541435] [DOI] [PubMed] [Google Scholar]
Eremenko 2008 {published data only}
- Eremenko AA, Kuslieva EV. Analgesic and opioid‐sparing effects of intravenous paracetamol in the early period after aortocoronary bypass surgery. Anesteziologiia i Reanimatologiia 2008;5:11‐4. [PubMed] [Google Scholar]
Faiz 2014 {published data only}
- Faiz HR, Rahimzadeh P, Visnjevac O, Behzadi B, Ghodraty MR, Nader ND. Intravenous acetaminophen is superior to ketamine for postoperative pain after abdominal hysterectomy: Results of a prospective, randomized, double‐blind, multicenter clinical trial. Journal of Pain Research 2014;7:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farkas 1992 {published data only}
- Farkas JC, Larroututou P, Morin JP, Laurian C, Huchet J, Cormier JM, et al. Analgesic efficacy of an injectable acetaminophen versus a dipyrone plus pitofenone plus fenpiverinium association after abdominal aortic repair. Current Therapeutic Research 1992;51:19‐27. [Google Scholar]
Fletcher 1997 {published data only}
- Fletcher D, Negre I, Barbin C, Francois A, Carreres C, Falgueirettes C, et al. Postoperative analgesia with I.V. propacetamol and ketoprofen combination after disc surgery. Canadian Journal of Anaesthesia 1997;44:479‐85. [PUBMED: 9161740] [DOI] [PubMed] [Google Scholar]
Hahn 2003 {published data only}
- Hahn TW, Mogensen T, Lund C, Jacobsen LS, Hjortsoe NC, Rasmussen SN, et al. Analgesic effect of i.v. paracetamol: possible ceiling effect of paracetamol in postoperative pain. Acta Anaesthesiologica Scandinavica 2003;47:138‐45. [PUBMED: 12631041] [DOI] [PubMed] [Google Scholar]
Hans 1993 {published data only}
- Hans P, Brichant JF, Bonhomme V, Triffaux M. Analgesic efficiency of propacetamol hydrochloride after lumbar disc surgery. Acta Anesthesiologica Belgica 1993;44:129‐33. [PUBMED: 8116325] [PubMed] [Google Scholar]
Hiller 2004 {published data only}
- Hiller A, Silvanto M, Savolainen S, Tarkkila P. Propacetamol and diclofenac alone and in combination for analgesia after elective tonsillectomy. Acta Anaesthesiologica Scandinavica 2004;48:1185‐9. [PUBMED: 15352967] [DOI] [PubMed] [Google Scholar]
Hiller 2012 {published data only}
- Hiller A, Helenius I, Nurmi E, Neuvonen PJ, Kaukonen M, Hartikainen T, et al. Acetaminophen improves analgesia but does not reduce opioid requirement after major spine surgery in children and adolescents. Spine 2012;37:E1225‐31. [DOI] [PubMed] [Google Scholar]
Hynes 2006 {published data only}
- Hynes D, McCarroll M, Hiesse‐Provost O. Analgesic efficacy of parenteral paracetamol (propacetamol) and diclofenac in post‐operative orthopaedic pain. Acta Anaesthesiologica Scandinavica 2006;50:374‐81. [PUBMED: 1648474] [DOI] [PubMed] [Google Scholar]
Inal 2006 {published data only}
- Inal MT, Celik NS, Tuncay FS. IV paracetamol infusion is better than IV meperidine infusion for postoperative analgesia after Caesarean section. The Internet Journal of Anesthesiology 2006;15. [Google Scholar]
Jahr 2012 Study 2, 65‐ {published data only}
- Jahr JS, Breitmeyer JB, Pan C, Royal MA, Ang RY. Safety and efficacy of intravenous acetaminophen in the elderly after major orthopedic surgery: subset data analysis from 3, randomized, placebo‐controlled trials. American Journal of Therapeutics 2012;19:66‐75. [DOI] [PubMed] [Google Scholar]
Jahr 2012 Study 2, 65+ {published data only}
- Jahr JS, Breitmeyer JB, Pan C, Royal MA, Ang RY. Safety and efficacy of intravenous acetaminophen in the elderly after major orthopedic surgery: subset data analysis from 3, randomized, placebo‐controlled trials. American Journal of Therapeutics 2012;19:66‐75. [DOI] [PubMed] [Google Scholar]
Jahr 2012 Study 3, 65‐ {published data only}
- Jahr JS, Breitmeyer JB, Pan C, Royal MA, Ang RY. Safety and efficacy of intravenous acetaminophen in the elderly after major orthopedic surgery: subset data analysis from 3, randomized, placebo‐controlled trials. American Journal of Therapeutics 2012;19:66‐75. [DOI] [PubMed] [Google Scholar]
Jahr 2012 Study 3, 65+ {published data only}
- Jahr JS, Breitmeyer JB, Pan C, Royal MA, Ang RY. Safety and efficacy of intravenous acetaminophen in the elderly after major orthopedic surgery: subset data analysis from 3, randomized, placebo‐controlled trials. American Journal of Therapeutics 2012;19:66‐75. [DOI] [PubMed] [Google Scholar]
Jarde 1997 {published data only}
- Jarde O, Boccard E. Parenteral versus oral route increases paracetamol efficacy. Clinical Drug Investigation 1997;14:474‐81. [Google Scholar]
Juhl 2006 {published data only}
- Juhl GI, Norholt SE, Tonnesen E, Hiesse‐Provost O, Jensen TS. Analgesic efficacy and safety of intravenous paracetamol (acetaminophen) administered as a 2 g starting dose following third molar surgery. European Journal of Pain 2006;10:371‐7. [PUBMED: 16085437] [DOI] [PubMed] [Google Scholar]
Kamath 2014 {published data only}
- Kamath V, Lasrado A. Efficacy of intravenous acetaminophen versus intravenous butorphanol as postoperative analgesic in obstetrics and gynaecology: a comparative study. BJOG: An International Journal of Obstetrics and Gynaecology 2014;121:194. [Google Scholar]
Kampe 2006 {published data only}
- Kampe S, Warm M, Landwehr S, Dagtekin O, Haussmann S, Paul M, et al. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Current Medical Research and Opinion 2006;22:1949‐54. [PUBMED: 17022854] [DOI] [PubMed] [Google Scholar]
Kara 2010 {published data only}
- Kara C, Resorlu B, Cicekbilek I, Unsal A. Analgesic efficacy and safety of nonsteroidal anti‐inflammatory drugs after transurethral resection of prostate. International Braz J Urol 2010;36(1):49‐54. [DOI] [PubMed] [Google Scholar]
Karaman 2010 {published data only}
- Karaman Y, Cukurova I, Demirhan E, Gonullu M, Altunbas S. Efficacy of dexketoprofen trometamol for acute postoperative pain relief after ENT surgery: a comparison with paracetamol and metamizole. Nobel Medicus 2010;6:47‐52. [Google Scholar]
Kemppainen 2006 {published data only}
- Kemppainen T, Kokki H, Tuomilehto H, Seppä J, Nuutinen Jl. Acetaminophen is highly effective in pain treatment after endoscopic sinus surgery. Laryngoscope 2006;116:2125‐8. [PUBMED: 17146383] [DOI] [PubMed] [Google Scholar]
Khajavi 2007 {published data only}
- Khajavi MR, Najafi A, PanahKhahi M, Moharari RS. Propacetamol and morphine in postoperative pain therapy after renal transplantation. International Journal of Pharmacology 2007;3:183‐6. [Google Scholar]
Khalili 2013 {published data only}
- Khalili G, Janghorbani M, Saryazdi H, Emaminejad A. Effect of preemptive and preventive acetaminophen on postoperative pain score: a randomized, double‐blind trial of patients undergoing lower extremity surgery. Journal of Clinical Anesthesia 2013;25:188‐92. [DOI] [PubMed] [Google Scholar]
Khan 2007 {published data only}
- Khan ZU, Iqbal J, Saleh H, Deek AM. Intravenous paracetamol is as effective as morphine in knee arthroscopic day surgery procedures. Pakistani Journal of Medical Sciences 2007;26:851‐3. [Google Scholar]
Kilicaslan 2010 {published data only}
- Kilicaslan A, Tuncer S, Yuceaktas A, Uyar M, Reisli R. The effects of intravenous paracetamol on postoperative analgesia and tramadol consumption in cesarean operations. Agri Dergisi 2010;22:7‐12. [PubMed] [Google Scholar]
Koppert 2006 {published data only}
- Koppert W, Frotsch K, Huzurudin H, Boswald W, Griessinger N, Weisbach V, et al. The effects of paracetamol and parecoxib on kidney function in elderly patients undergoing orthopedic surgery. Anesthesia and Analgesia 2006;103:1170‐6. [DOI] [PubMed] [Google Scholar]
Korkmaz 2010 {published data only}
- Korkmaz Dilmen O, Tunali Y, Cakmakkaya OS, Yentur E, Tutuncu AC, Tureci E, et al. Efficacy of intravenous paracetamol, metamizol and lornoxicam on postoperative pain and morphine consumption after lumbar disc surgery. European Journal of Anaesthesiology 2010;27:428‐32. [DOI] [PubMed] [Google Scholar]
Lahtinen 2002 {published data only}
- Lahtinen P, Kokki H, Hendolin H, Hakala T, Hynynen M. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesthesia & Analgesia 2002;95:813‐9. [12351250] [DOI] [PubMed] [Google Scholar]
Landwehr 2005 {published data only}
- Landwehr S, Kiencke P, Giesecke T, Eggert D, Thumann G, Kampe S. A comparison between IV paracetamol and IV metamizol for postoperative analgesia after retinal surgery. Current Medical Research and Opinion 2005;21:1569‐75. [PUBMED: 16238896] [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee SY, Lee WH, Lee EH, Han KC, Ko YK. The effects of paracetamol, ketorolac, and paracetamol plus morphine on pain control after thyroidectomy. Korean Journal of Pain 2010;23:124‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leykin 2008 {published data only}
- Leykin Y, Casati A, Rapotec A, Dalsasso M, Barzan L, Fanelli G, et al. Comparison of parecoxib and proparacetamol in endoscopic nasal surgery patients. Yonsei Medical Journal 2008;49:383‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2003 {published data only}
- Ma EL, Wang XR, Jiang ZM, Cui Y, Wang R, Liu J. A randomized, double blind, and controlled clinical trial of the non‐addictive propacetamol in postoperative analgesia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao [Acta Acadamiae Medicinae Sinicae] 2003;25:329‐32. [PUBMED: 12905750 ] [PubMed] [Google Scholar]
Maghsoudi 2014 {published data only}
- Maghsoudi R, Etemadian M, Lotfi SMT, Movasagi G, Amjadi M. Opioid sparing effects of intravenous paracetamol after PCNL: a randomized double blinded clinical trial. Journal of Endourology 2012;26:A354. [DOI] [PubMed] [Google Scholar]
- Maghsoudi R, Tabatabai M, Radfar MH, Movasagi G, Etemadian M, Shati M, et al. Opioid‐sparing effect of intravenous paracetamol after percutaneous nephrolithotomy: a double‐blind randomized controlled trial. Journal of Endourology 2014;28:23‐7. [DOI] [PubMed] [Google Scholar]
Marty 2005 {published data only}
- Marty J, Benhamou D, Chassard D, Empairaire N, Roche A, Mayaud A, et al. Effects of single‐dose injectable paracetamol versus propacetamol in pain management after minor gynecologic surgery: a multicenter, randomized, double‐blind, active‐controlled, two‐parallel‐group study. Current Therapeutic Research 2005;66:294‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mimoz 2001 {published data only}
- Mimoz O, Incagnoli P, Gillon MC, Kuhlman L, Mirand A, Soilleux H, et al. Analgesic efficacy and safety of nefopam vs. propacetamol following hepatic resection. Anaesthesia 2001;51:520‐5. [DOI] [PubMed] [Google Scholar]
Mitra 2012 {published data only}
- Mitra S, Khandelwal P, Sehgal A. Diclofenac‐tramadol vs. diclofenac‐acetaminophen combinations for pain relief after caesarean section. Acta Anaesthesiologica Scandinavica 2012;56:706‐11. [DOI] [PubMed] [Google Scholar]
Moller 2005a {published data only}
- Moller PL, Juhl GI, Payen‐Champenois C, Skoglund LA. Intravenous acetaminophen (paracetamol): comparable analgesic efficacy, but better local safety than its prodrug, propacetamol, for postoperative pain after third molar surgery. Anesthesia & Analgesia 2005;101:90‐6. [DOI] [PubMed] [Google Scholar]
Moller 2005b {published data only}
- Moller PL, Sindet‐Pedersen S, Petersen CT, Juhl GI, Dillenschneider A, Skoglund LA. Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery. British Journal of Anaesthesia 2005;94:642‐8. [DOI] [PubMed] [Google Scholar]
Mowafi 2012 {published data only}
- Mowafi HA, Elmakarim EA, Ismail S, Al‐Mahdy M, El‐Saflan AE, Elsaid AS. Intravenous lornoxicam is more effective than paracetamol as a supplemental analgesic after lower abdominal surgery: a randomized controlled trial. World Journal of Surgery 2012;36:2039‐44. [DOI] [PubMed] [Google Scholar]
Ohnesorge 2009 {published data only}
- Ohnesorge H, Bein B, Hanss R, Francksen H, Mayer L, Scholz J, et al. Paracetamol versus metamizol in the treatment of postoperative pain after breast surgery: a randomized, controlled trial. European Journal of Anaesthesiology 2009;26:648‐53. [DOI] [PubMed] [Google Scholar]
Omar 2011 {published data only}
- Omar AAA, Al Issa KA. Intravenous paracetamol (Perfalgan) for analgesia after cesarean section: a double‐blind randomized controlled study. Rawal Medical Journal 2011;36:1‐12. [Google Scholar]
Oncul 2011 {published data only}
- Oncul AM, Cimen E, Kucukyavuz Z, Cambazoglu M. Postoperative analgesia in orthognathic surgery patients: diclofenac sodium or paracetamol?. British Journal of Oral & Maxillofacial Surgery 2011;49:138‐41. [DOI] [PubMed] [Google Scholar]
Oreskovic 2014 {published data only}
- Oreskovic Z, Bicanic G, Hrabac P, Tripkovic B, Delimar D. Treatment of postoperative pain after total hip arthroplasty: comparison between metamizol and paracetamol as adjunctive to opioid analgesics ‐ Prospective, double‐blind, randomised study. Archives of Orthopaedic & Trauma Surgery 2014;134:631‐6. [DOI] [PubMed] [Google Scholar]
Paech 2014 {published data only}
- Paech MJ, McDonnell NJ, Sinha A, Baber C, Nathan EA. A randomised controlled trial of parecoxib, celecoxib and paracetamol as adjuncts to patient‐controlled epidural analgesia after caesarean delivery. Anaesthesia & Intensive Care 2014;42:15‐22. [DOI] [PubMed] [Google Scholar]
Peduto 1998 {published data only}
- Peduto VA, Ballabio M, Stefanini S. Efficacy of propacetamol in the treatment of postoperative pain. Morphine‐sparing effect in orthopedic surgery. Italian Collaborative Group on Propacetamol. Acta Anaesthesiologica Scandinavica 1998;42:293‐8. [PUBMED: 9542555] [DOI] [PubMed] [Google Scholar]
Salonen 2009 {published data only}
- Salonen A, Silvola J, Kokki H. Does 1 or 2 g paracetamol added to ketoprofen enhance analgesia in adult tonsillectomy patients?. Acta Anaesthesiologica Scandinavica 2009;53:1200‐6. [DOI] [PubMed] [Google Scholar]
Sanyal 2014 {published data only}
- Sanyal P, Pal A, Biswas J, Mukhopadhyay P, Dasgupta S, Das SH. Choice of non‐opioid analgesics for postoperative analgesia in patients undergoing lower abdominal gynecological surgeries. BJOG: An International Journal of Obstetrics and Gynaecology 2014;121:189. [Google Scholar]
Shimia 2014 {published data only}
- Shimia M, Parish M, Abedini N. The effect of intravenous paracetamol on postoperative pain after lumbar discectomy. Asian Spine Journal 2014;8:400‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddik 2001 {published data only}
- Siddik SM, Aouad MT, Jalbout MI, Rizk LB, Kamar GH, Baraka AS. Diclofenac and/or propacetamol for postoperative pain management after cesarean delivery in patients receiving patient controlled analgesia morphine. Regional Anesthesia and Pain Medicine 2001;26:310‐5. [DOI] [PubMed] [Google Scholar]
Sinatra 2005 {published data only}
- Sinatra RS, Jahr JS, Reynolds L, Groudine SB, Royal MA, Breitmeyer JB, et al. Intravenous acetaminophen for pain after major orthopedic surgery: an expanded analysis. Pain Practice 2012;12:357‐65. [DOI] [PubMed] [Google Scholar]
- Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen‐Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology 2005;102:822‐31. [PUBMED: 15791113] [DOI] [PubMed] [Google Scholar]
Tiippana 2008 {published data only}
- Tiippana E, Bachmann M, Kalso E, Pere P. Effect of paracetamol and coxib with or without dexamethasone after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2008;52:673‐80. [DOI] [PubMed] [Google Scholar]
Togrul 2011 {published data only}
- Togrul T, Baysal Yildirim Z, Cengiz M, Cigdem A, San I, Kar M. Comparison of intravenous paracetamol and tramadol for postoperative analgesia in patients with septo‐rhinoplasty. Anestezi Dergisi 2011;19:213‐6. [Google Scholar]
Tunali 2013 {published data only}
- Tunali Y, Akcil EF, Dilmen OK, Tutuncu AC, Koksal GM, Akbas S, et al. Efficacy of intravenous paracetamol and dexketoprofen on postoperative pain and morphine consumption after a lumbar disk surgery. Journal of Neurosurgical Anesthesiology 2013;25:143‐7. [DOI] [PubMed] [Google Scholar]
Tuncel 2012 {published data only}
- Tuncel A, Balci M, Postaci A, Aslan Y, Koseoglu E, Sacan O, et al. Comparison of intraoperative local levobupivacaine infiltraton anaesthesia, intravenous paracetamol and lornoxicam treatments for postoperative pain management in patients underwent transperitoneal laparoscopic renal and adrenal surgery. Journal of Endourology 2012;26:A287. [Google Scholar]
Unal 2013 {published data only}
- Unal C, Cakan T, Baltaci B, Basar H. Comparison of analgesic efficacy of intravenous paracetamol and intravenous dexketoprofen trometamol in multimodal analgesia after hysterectomy. Journal of Research in Medical Sciences 2013;18:897‐903. [PMC free article] [PubMed] [Google Scholar]
Van Aken 2004 {published data only}
- Aken H, Thys L, Veekman L, Buerkle H. Assessing analgesia in single and repeated administrations of propacetamol for postoperative pain: comparison with morphine after dental surgery. Anesthesia & Analgesia 2004;98:159‐65. [PUBMED: 14693612] [DOI] [PubMed] [Google Scholar]
Varrassi 1999 {published data only}
- Varrassi G, Marinangeli F, Agro F, Aloe L, Cillis P, Nicola A, et al. A double‐blinded evaluation of propacetamol versus ketorolac in combination with patient‐controlled analgesia morphine: analgesic efficacy and tolerability after gynecologic surgery. Anesthesia & Analgesia 1999;88:611‐6. [PUBMED: 10072016] [DOI] [PubMed] [Google Scholar]
Vuilleumier 1998 {published data only}
- Vuilleumier PA, Buclin T, Biollaz J. Comparison of propacetamol and morphine in postoperative analgesia. Schweizerische Medizinische Wochenschrift 1998;14:259‐63. [9540151] [PubMed] [Google Scholar]
Wininger 2010 {published data only}
- Wininger SJ, Miller H, Minkowitz HS, Royal MA, Ang RY, Breitmeyer JB, et al. A randomized, double‐blind, placebo‐controlled, multicenter, repeat‐dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clinical Therapeutics 2010;32:2348‐69. [DOI] [PubMed] [Google Scholar]
Zhou 2001 {published data only}
- Zhou TJ, Tang J, White PF. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesthesia & Analgesia 2001;92:1569‐75. [PUBMED: 11375848] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alhashemi 2006 {published data only}
- Alhashemi JA, Daghistani MF. Effects of intraoperative I.V. acetaminophen vs I.M. meperidine on post‐tonsillectomy pain in children. British Journal of Anaesthesia 2006;96:790‐5. [PUBMED: 16613928] [DOI] [PubMed] [Google Scholar]
Alhashemi 2007 {published data only}
- Alhashemi JA, Daghistani MF. Effect of intraoperative intravenous acetaminophen vs. intramuscular meperidine on pain and discharge time after paediatric dental restoration. European Journal of Anaesthesiology 2007;24:128‐33. [PUBMED: 16895621] [DOI] [PubMed] [Google Scholar]
Alimian 2014 {published data only}
- Alimian M, Pournajafian A, Kholdebarin, Ghodraty M, Rokhtabnak F, Yazdkhasti P. Analgesic effects of paracetamol and morphine after elective laparotomy surgeries. Anesthesiology & Pain Medicine 2014;4:e12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 2013 {published data only}
- Anand A, Sprenker CJ, Karlnoski R, Norman J, Miladinovic B, Wilburn B, et al. Intravenous acetaminophen vs. ketorolac for postoperative analgesia after ambulatory parathyroidectomy. Scandinavian Journal of Pain 2013;4:249‐53. [DOI] [PubMed] [Google Scholar]
Ang 1990 {published data only}
- Ang ET, Goldfarb G, Boccard E. Analgesic efficacy of propacetamol hydrochlorate 2 g versus pentazocine 30 mg after orthopedic surgery. European Journal of Pain 1990;11:137‐42. [Google Scholar]
Ashrafnejad 2012 {published data only}
- Ashrafnejad B, Hassani V. Comparison between analgesic effect of morphine and propacetamol after orthopedic surgeries. Pain Practice 2012;12:123. [Google Scholar]
Aydogan 2008 {published data only}
- Aydogan H, Dogru K, Erdem S, Bicer C, Aksu R, Boyaci A. The effect of IV paracetamol on the hemodynamic indices, liver functions and the postoperative analgesia in the patients underwent major orthopaedic surgery. Erciyes Tip Dergisi [Erciyes Medical Journal] 2008;30:71‐7. [Google Scholar]
Caliskan 2013 {published data only}
- Caliskan E, Sener M, Kocum A, Ozyilkan NB, Ezer SS, Aribogan A. The efficacy of intravenous paracetamol versus dipyrone for postoperative analgesia after day‐case lower abdominal surgery in children with spinal anesthesia: a prospective randomized double‐blind placebo‐controlled study. BMC Anesthesiology 2013;13:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Candiotti 2010 {published data only}
- Candiotti KA, Bergese SD, Viscusi ER, Singla SK, Royal MA, Singla NK. Safety of multiple‐dose intravenous acetaminophen in adult inpatients. Pain Medicine 2010;11:1841‐8. [DOI] [PubMed] [Google Scholar]
Cok 2011 {published data only}
- Cok OY, Eker HE, Pelit A, Canturk S, Akin S, Aribogan A, et al. The effect of paracetamol on postoperative nausea and vomiting during the first 24 h after strabismus surgery: a prospective, randomised, double‐blind study. European Journal of Anaesthesiology 2011;28:836‐41. [DOI] [PubMed] [Google Scholar]
Dowling 2014 {published data only}
- Dowling O, Campese C, Macchio F, Aronsohn J, Gold J, Bosshart D, et al. Effect of single dose IV acetaminophen on postoperative/postdischarge nausea and vomiting, pain, and patient satisfaction in outpatients undergoing laparoscopic cholecystectomy. Anesthesia and Analgesia 2014;118:S29. [Google Scholar]
Elseify 2011 {published data only}
- Elseify ZA, El‐Khattab SO, Khattab AM, Atta EM, Aijoub LF. Combined parecoxib and I.V. paracetamol provides additional analgesic effect with better postoperative satisfaction in patients undergoing anterior cruciate ligament reconstruction. Saudi Journal of Anaesthesia 2011;5:45‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fijalkowska 2006 {published data only}
- Fijalkowska A, Trela‐Stachurska K, Rechberger T. Efficacy of intravenous paracetamol for early postoperative analgesia after gynaecological surgery. Anaesthesiology Intensive Therapy 2006;38:66‐8. [Google Scholar]
Fourcade 2005 {published data only}
- Fourcade O, Sanchez P, Kern D, Mazoit JX, Minville V, Samii K. Propacetamol and ketoprofen after thyroidectomy. European Journal of Anaesthesiology 2005;22:373‐7. [PUBMED: 15918387] [DOI] [PubMed] [Google Scholar]
Garcia 1999 {published data only}
- Garcia F, Rodriguez‐Huertas F, Gutierrez M, Bustos A, Sariego M, Garcia‐ Baquero A. Preoperative propacetamol and postoperative pain after laparoscopic surgery for tubal blockage. Revista de la Sociedad Espanola del Dolor 1999;6:83‐7. [Google Scholar]
Gehling 2010 {published data only}
- Gehling M, Arndt C, Eberhart LHJ, Koch T, Kruger T, Wulf H. Postoperative analgesia with parecoxib, acetaminophen, and the combination of both: a randomized, double‐blind, placebo‐controlled trial in patients undergoing thyroid surgery. British Journal of Anaesthesia 2010;104:761‐7. [DOI] [PubMed] [Google Scholar]
Ghaffaripour 2012 {published data only}
- Ghaffaripour JS, Mahmoudi H, Lahsaee S, Jaafari H, Kazemi AP, Mirshamsi M. Comparison of propacetamol and morphine in postoperative pain relief after orthopedic surgery. Pain Practice 2012;12:129. [Google Scholar]
Gousheh 2013 {published data only}
- Gousheh SM, Nesioonpour S, Javaher Foroosh F, Akhondzadeh R, Sahafi SA, Alizadeh Z. Intravenous paracetamol for postoperative analgesia in laparoscopic cholecystectomy. Anesthesiology & Pain Medicine 2013;3:214‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granry 1997 {published data only}
- Granry JC, Rod B, Monrigal JP, Merckx J, Berniere J, Jean N, et al. The analgesic efficacy of an injectable prodrug of acetaminophen in children after orthopaedic surgery. Paediatric Anaesthesia 1997;7:445‐9. [PUBMED: 9365969] [DOI] [PubMed] [Google Scholar]
Grundmann 2006 {published data only}
- Grundmann U, Wornle C, Biedler A, Kreuer S, Wrobel M, Wilhelm W. The efficacy of the non‐opioid analgesics parecoxib, paracetamol and metamizol for postoperative pain relief after lumbar microdiscectomy. Anesthesia & Analgesia 2006;103:217‐22. [PUBMED: 16790656] [DOI] [PubMed] [Google Scholar]
Hernandez Palazon 2001 {published data only}
- Hernandez‐Palazon J, Tortosa JA, Martinez‐Lage JF, Perez‐Flores D. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesthesia & Analgesia 2001;92:1473‐6. [PUBMED: 11375828] [DOI] [PubMed] [Google Scholar]
Irct2012062410102N {published data only}
- IRCT2012062410102N1. A comparison of analgesic effect of intravenous acetaminophen (Apotel) versus oxycodone tablet and oral acetaminophen codeine in rhinoplasty and septoplasty surgery. irct.ir/searchresult.php?keyword=A%20comparison%20of%20analgesic%20effect%20of%20Intravenous%20Acetaminophen%20&id=10102&number=1&field=g&prt=1&total=1&m=1 (accessed 24 March 2015).
Ko 2010 {published data only}
- Ko MJ, Lee JH, Cheong SH, Shin CM, Kim YJ, Choe YK, et al. Comparison of the effects of acetaminophen to ketorolac when added to lidocaine for intravenous regional anesthesia. Korean Journal of Anesthesiology 2010;58:357‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocum 2013 {published data only}
- Kocum AI, Sener M, Caliskan E, Bozdogan N, Micozkadioglu D, Yilmaz I, et al. Intravenous paracetamol and dipyrone for postoperative analgesia after day‐case tonsillectomy in children: a prospective, randomized, double blind, placebo controlled study. Revista Brasileira de Otorrinolaringologia 2013;79:89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Memis 2010 {published data only}
- Memis D, Inal MT, Kavalci G, Sezer A, Sut N. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid‐related adverse effects after major surgery in intensive care unit. Journal of Critical Care 2010;25:458‐62. [DOI] [PubMed] [Google Scholar]
Murat 2005 {published data only}
- Murat I, Baujard C, Foussat C, Guyot E, Petel H, Rod B, et al. Tolerance and analgesic efficacy of a new i.v. paracetamol solution in children after inguinal hernia repair. Paediatric Anaesthesia 2005;15:663‐70. [DOI] [PubMed] [Google Scholar]
NCT01691690 {published data only}
- NCT01691690. Analgesic effect of single dose intravenous acetaminophen in pediatric patients undergoing tonsillectomy. clinicaltrials.gov/ct2/show/NCT01691690 (accessed 24 March 2015).
NCT01721486 {published data only}
- NCT01721486. Efficacy of iv vs oral administration of acetaminophen for pain control following tonsillectomy with or without adenoidectomy. clinicaltrials.gov/ct2/show/NCT01721486 (accessed 24 March 2015).
Nikoda 2006 {published data only}
- Nikoda VV, Makarova VV, Maiachkin RB, Bondarenko AV. Clinical aspects of analgesia with intravenous paracetamol in the early postoperative period. Anesteziologiia i Reanimatologiia 2006;6:54‐8. [PubMed] [Google Scholar]
Olonisakin 2012 {published data only}
- Olonisakin RP, Amanor‐Boadu SD, Akinyemi AO. Morphine‐sparing effect of intravenous paracetamol for post operative pain management following gynaecological surgery. African Journal of Medicine & Medical Sciences 2012;31:429‐36. [PubMed] [Google Scholar]
Pernia 2000 {published data only}
- Pernia A, Torres LM, Calderon E. Management of postoperative pain through intravenous patient controlled analgesia (iv PCA) versus propacetamol and metamizol. Revista de la Sociedad Espanola del Dolor 2000;7:354‐60. [Google Scholar]
Rashwan 2013 {published data only}
- Rashwan D, Fathy El‐Rahmawy G. Multimodal analgesia after upper limb orthopedic surgeries: patient controlled intravenous low dose tramadol analgesia with or without intravenous acetaminophen ‐ a comparative study. Egyptian Journal of Anaesthesia 2013;29:231‐4. [Google Scholar]
Silvanto 2007 {published data only}
- Silvanto M, Munsterhjelm E, Savolainen S, Tiainen P, Niemi T, Ylikorkala O, et al. Effect of 3 g of intravenous paracetamol on post‐operative analgesia, platelet function and liver enzymes in patients undergoing tonsillectomy under local anaesthesia. Acta Anaesthesiologica Scandinavica 2007;51:1147‐54. [PUBMED: 17711562] [DOI] [PubMed] [Google Scholar]
Topal 2009 {published data only}
- Topal A, Erol A, Tuncer, S, Tavlan A, Kilicaslan A, Otelcioglu S. The effect of intravenous paracetamol on postoperative analgesia and morphine consumption. Anestezi Dergisi 2009;17:29‐32. [Google Scholar]
Toygar 2008 {published data only}
- Toygar P, Akkaya T, Ozkan D, Ozel O, Uslu E, Gumus H. Does iv paracetamol have preemptive analgesic effect on lumber disc surgeries?. Agri Dergisi 2008;20:14‐9. [PubMed] [Google Scholar]
Turner 2014 {published data only}
- Turner L. The impact of IV acetaminophen on postoperative pain in women undergoing pelvic organ prolapse repair: a double‐blind randomized placebo controlled trial. https://clinicaltrials.gov/show/NCT02155738 2014.
Uvarov 2008 {published data only}
- Uvarov DN, Orlov MM, Levin AV, Sokolov AV, Nedashkovskii EV. Role of paracetamol in a balanced postoperative analgesia scheme after thoracotomy. Anesteziologiia i Reanimatologiia 2008;4:46‐9. [PubMed] [Google Scholar]
Uzun 2010 {published data only}
- Uzun S, Aycan IO, Erden IA, Sahin A, Aypar U. The addition of metamizole to morphine and paracetamol improves early postoperative analgesia and patient satisfaction after lumbar disc surgery. Turkish Neurosurgery 2010;20:341‐7. [DOI] [PubMed] [Google Scholar]
Verchere 2002 {published data only}
- Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. Journal of Neurosurgical Anesthesiology 2002;14:96‐101. [PUBMED: 11907388] [DOI] [PubMed] [Google Scholar]
Zeidan 2014 {published data only}
- Zeidan A, Mazoit JX, Ali Abdullah M, Maaliki H, Ghattas T, Saifan A. Median effective dose (ED50) of paracetamol and morphine for postoperative pain: a study of interaction. British Journal of Anaesthesia 2014;112:118‐23. [DOI] [PubMed] [Google Scholar]
Ziolkowski 2008 {published data only}
- Ziolkowski R, Srebrzynski A, Kaczka K, Butwicka A, Kuzdak K, Pomorski L. Assessment of postoperative analgesia using intravenous paracetamol during first day following thyroid surgery for goiter. Clinical and Experimental Medical Letters 2008;49:41‐6. [Google Scholar]
References to studies awaiting assessment
Atashkhoyi 2014 {published data only}
- Atashkhoyi S, Rasouli S, Fardiazar Z, Ghojazadeh M, Marandi PH. Preventive analgesia with intravenous paracetamol for post‐cesarean section pain control. International Journal of Women's Health and Reproduction Sciences 2014;2:131‐7. [Google Scholar]
Dawoodi 2014 {published data only}
- Dawoodi I, Khety Z. Comparative efficacy and safety study of analgesic effect of fentanyl I.V. and paracetamol I.V. in postoperative patients in multidisciplinary hospital. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5:664‐8. [Google Scholar]
Jabalameli 2014 {published data only}
- Jabalameli M, Goudarzi L. Preventive effects of intravenous paracetamol in post‐partum pain of elective cesarean delivery with spinal anesthesia. Journal of Isfahan Medical School 2014;32:1227‐37. [Google Scholar]
Majumdar 2014 {published data only}
- Majumdar S, Das A, Kundu R, Mukherjee D, Hazra B, Mitra T. Intravenous paracetamol infusion: superior pain management and earlier discharge from hospital in patients undergoing palliative head‐neck cancer surgery. Perspectives in Clinical Research 2014;5:172‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pekmezci 2014 {published data only}
- Pekmezci A, Cesur M, Aksoy M, Ince I, Aksoy AN. The effect of ilioinguinal‐iliohypogastric block with or without intravenous paracetamol for pain relief after caesarean delivery. Acta Medica Mediterranea 2014;30:1183‐8. [Google Scholar]
Rasheed 2007 {published data only}
- Borodiciene J, Gudaityte J, Gerulyte I, Macas A, Suskeviciene I. Randomized controlled study of the acetaminophen effectiveness after adenotonsillectomy or tonsillectomy in children. European Journal of Anaesthesiology 2014;31:173. [Google Scholar]
- Dabir S, Parsa T, Abbasinazari M, Parviz Y. Comparison of a single dose of intravenous paracetamol with single dose of rectal indomethacin for pain management after open septorhinoplasty surgery. La Prensa Medica 2016;102:1. [Google Scholar]
- Rasheed A, Aqil M, Haq A, Usman SK. Comparative study of tramadol & propacetamol for postoperative pain management in patients undergoing nasal surgeries. Medical Forum Monthly 2007;18:21‐5. [Google Scholar]
Ritchie 2015 {published data only}
- Ritchie R, Sloan PA, Reddy A, Chau D, Lukens MF, Nimma S. Intraoperative acetaminophen for pediatric post‐tonsillectomy pain relief: a comparison of 2 surgical techniques. Anesthesia and Analgesia 2015;120:S225. [Google Scholar]
Singla 2015 {published data only}
- Singla NK, Hale ME, Davis JC, Bekker A, Gimbel J, Jahr J, et al. IV acetaminophen: Efficacy of a single dose for postoperative pain after hip arthroplasty: subset data analysis of 2 unpublished randomized clinical trials. American Journal of Therapeutics 2015;22:2‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Agnes 2006
- Agnes RS, Lee YS, Davis P, Ma SW, Badghisi H, Porreca F, et al. Structure‐activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Journal of Medicinal Chemistry 2006;49:2868‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2005
- Anderson BJ, Pons G, Autret‐LecaE, Allegaert K, Boccard E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatric Anaesthesia 2005;15:282‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Apfel 2013
- Apfel CC, Turan F, Souza K, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and metaanalysis. Pain 2013;154:677‐89. [DOI] [PubMed] [Google Scholar]
Apfelbaum 2003
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia & Analgesia 2003;97:534‐40. [DOI] [PubMed] [Google Scholar]
AUREF 2012
- PaPaS Author, Referee Guidance. Cochrane Pain, Palliative and Supportive Care Group. http://papas.cochrane.org/papas‐documents (accessed 5 August 2015).
Bandolier 2010
- Bandolier. Oxford league table of analgesics in acute pain. http://www.medicine.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/lftab.html (accessed 7 June 2010).
Barbaud 1995
- Barbaud A, Trechot P, Bertrand O, Schmutz JL. Occupational allergy to propacetamol. Lancet 1995;346:902. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107:86‐90. [DOI] [PubMed] [Google Scholar]
Berl 1998
- Berl V, Barbaud A, Lepoittevin JP. Mechanism of allergic contact dermatitis from propacetamol: sensitization to activated N, N‐diethylglycine. Contact Dermatitis 1998;38:185‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Björkman 1994
- Björkman R, Hallman KM, Hedner J, Hedner T, Henning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain 1994;57:259‐64. [DOI] [PubMed] [Google Scholar]
Cadence 2011
- Cadence Pharmaceuticals, Inc. Ofirmev (acetaminophen) injection. Product Monograph 2011.
Campbell 1998
- Campbell WI, Patterson CC. Quantifying meaningful changes in pain. Anaesthesia 1998;53:121‐5. [DOI] [PubMed] [Google Scholar]
Cepeda 2003
- Cepeda MS, Africanoa JM, Poloa R, Alcalaa R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain?. Pain 2003;105:151‐7. [DOI] [PubMed] [Google Scholar]
Chandrasekharan 2002
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99:13926‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandrasekharan 2004
- Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biology 2004;5:241. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith L, McQuay HJ. Seeking a simple measure of analgesia for mega trials: is simple global assessment good enough?. Pain 2001;91:189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The Design of Analgesic Clinical Trials. Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Depre 1992
- Depre M, Hecken A, Verbesselt R, Tjandra‐Maga TB, Gerin M, Schepper PJ. Tolerance and pharmacokinetics of propacetamol, a paracetamol formulation for intravenous use. Fundamental & Clinical Pharmacology 1992;6:259‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004233.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elia 2005
- Elia N, Lysakowski C, Tramer MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase‐2 inhibitors and patient‐controlled analgesia morphine offer advantages over morphine alone? Meta‐analyses of randomized trials. Anesthesiology 2005;103:1296‐304. [DOI] [PubMed] [Google Scholar]
EMC 2010
- The electronic Medicines Compendium (eMC). Perfalgan 10mg/ml solution for infusion. http://www.medicines.org.uk/EMC/medicine/14288/SPC/Perfalgan+10mg+ml++Solution+for+Infusion/ (accessed 7 June 2010).
Flouvat 2004
- Flouvat B, Leneveu A, Fitoussi S, Delhotal‐Landes B, Gendron A. Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 2004;42:50‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gielen 2001
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis 2001;45:273‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.), 2015.
Graham 2005
- Graham GG, Scott KF. Mechanism of action of paracetamol. American Journal of Therapeutics 2005;12:46‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gray 2005
- Gray A, Kehlet H, Bonnet F, Rawal N. Predicting postoperative analgesia outcomes: NNT league tables or procedure‐specific evidence?. British Journal of Anaesthesia 2005;94:710‐4. [DOI] [PubMed] [Google Scholar]
Greco 2003
- Greco A, Ajmone‐Cat MA, Nicolini A, Sciulli MG, Minghetti L. Paracetamol effectively reduces prostaglandin E2 synthesis in brain macrophages by inhibiting enzymatic activity of cyclooxygenase but not phospholipase and prostaglandin E synthase. Journal of Neuroscience Research 2003;71:844‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hama 2010
- Hama AT, Sagen J. Cannabinoid receptor–mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology 2010;58:758‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hyllested 2002
- Hyllested M, Jones S, Pedersen JL, Kehlet H. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. British Journal of Anaesthesia 2002;88:199‐214. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacox 1994
- Jacox AK, Carr DB, Payne R, Berde CB. Management of Cancer Pain. Clinical Practice Guidelines. Rockville: Agency for Health Care Policy and Research, 1994. [AHCPR publication No 94‐0595] [Google Scholar]
Jebaraj 2013
- Jebaraj B, Maitra S, Baidya DK, Khanna P. Intravenous paracetamol reduces postoperative opioid consumption after orthopedic surgery: a systematic review of clinical trials. Pain Research and Treatment 2013;2013:402510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumpulainen 2007
- Kumpulainen E, Kokki H, Halonen T, Heikkinen M, Savolainen J, Laisalmi M. Paracetamol (acetaminophen) penetrates readily into the cerebrospinal fluid of children after intravenous administration. Pediatrics 2007;119:766‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee YS, Kim H, Brahim JS, Rowan J, Lee G, Dionne RA. Acetaminophen selectively suppresses peripheral prostaglandin E2 release and increases COX‐2 gene expression in a clinical model of acute inflammation. Pain 2007;129:279‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mallet 2008
- Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen‐induced analgesia. Pain 2008;139:190‐200. [DOI] [PubMed] [Google Scholar]
Marret 2005
- Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient‐controlled analgesia morphine side effects: meta‐analysis of randomized controlled trials. Anesthesiology 2005;102:1249‐60. [DOI] [PubMed] [Google Scholar]
McNicol 2015
- McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non‐patient controlled opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD003348.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 2005
- Moller PL, Juhl GI, Payen‐Champenois C, Skoglund LA. Intravenous acetaminophen (paracetamol): comparable analgesic efficacy, but better local safety than its prodrug, propacetamol, for postoperative pain after third molar surgery. Anesthesia & Analgesia 2005;101:90‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69:311‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69:127‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
NHS 2010
- NHS Greater Glasgow & Clyde Medicines Information Service. IV Paracetamol – Risk of Hepatic Damage. Available at http://www.ggcprescribing.org.uk/media/uploads/postscript_acute/psacute_01.pdf (accessed 6 May 2016).
Perrott 2004
- Perrott DA, Piira T, Goodenough B, Champion GD. Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever: a meta‐analysis. Archives of Pediatrics & Adolescent Medicine 2004;158:521‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Remy 2005
- Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side‐effects and consumption after major surgery: meta‐analysis of randomized controlled trials. British Journal of Anaesthesia 2005;94:505‐13. [DOI] [PubMed] [Google Scholar]
Remy 2006
- Remy C, Marret E, Bonnet F. State of the art of paracetamol in acute pain therapy. Current Opinion in Anaesthesiology 2006;19:562‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sachs 2005
- Sachs CJ. Oral analgesics for acute nonspecific pain. American Family Physician 2005;71:913‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Subramanyam 2014
- Subramanyam R, Varughese A, Kurth CD, Eckman MH. Cost‐effectiveness of intravenous acetaminophen for pediatric tonsillectomy. Pediatric Anesthesia 2014;24:467‐75. [DOI] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Tzortzopoulou 2011
- Tzortzopoulou A, McNicol ED, Cepeda MS, Francia MBD, Farhat T, Schumann R. Single dose intravenous propacetamol or intravenous paracetamol for postoperative pain. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007126.pub2] [DOI] [PubMed] [Google Scholar]


