Abstract
Background
Methadone belongs to a class of analgesics known as opioids, that are considered the cornerstone of therapy for moderate‐to‐severe pain due to life‐threatening illnesses; however, their use in chronic non‐cancer pain (CNCP) is controversial. Methadone has many characteristics that differentiate it from other opioids, which suggests that it may have a different efficacy and safety profile.
Objectives
To assess the analgesic effectiveness and safety of methadone in the treatment of CNCP.
Search methods
We identified both randomized controlled trials (RCTs) and non‐randomized studies of methadone use in chronic pain by searching the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2011, issue 11, MEDLINE (1950 to November 2011), and EMBASE (1980 to November 2011), together with reference lists of retrieved papers and reviews.
Selection criteria
We included RCTs with pain assessment as either the primary or secondary outcome. Quasi‐randomized studies, cohorts and case‐control trials were also considered for inclusion because we suspected that the beneficial and harmful effects of methadone in CNCP may not be adequately addressed in RCTs.
Data collection and analysis
Two review authors independently extracted efficacy and adverse event data and assessed risk of bias.
Main results
We included two RCTs and one non‐randomized study, involving a total of 181 participants. Both RCTs were cross‐over studies, one involving 19 participants with diverse neuropathic pain syndromes, the other involving 76 participants with postherpetic neuralgia. Study phases were 20 days and approximately eight weeks, respectively. The non‐randomized study retrospectively evaluated 86 outpatients over an average of 8.8 ± 6.3 months.
One RCT reported average pain intensity and pain relief, and found statistically significant improvements versus placebo for both outcomes, with 10 mg and 20 mg daily doses of methadone. The second RCT reported differences in pain reduction between methadone and morphine and found morphine to be statistically superior. The non‐randomized study found that in patients initially prescribed methadone it was effective in fewer participants than in those initially prescribed other long‐acting opioids (28% versus 42%, 33% and 50% for morphine, oxycodone and transdermal fentanyl, respectively).
One RCT compared incidences for several individual adverse events, but found a difference between methadone and placebo for only one event, dizziness (P = 0.041).
Authors' conclusions
The three studies provide very limited evidence of the efficacy of methadone for CNCP, and there were too few data for pooled analysis of efficacy or harm, or to have confidence in the results of the individual studies. No conclusions can be made regarding differences in efficacy or safety between methadone and placebo, other opioids, or other treatments.
Plain language summary
Methadone for long‐term non‐cancer pain
The three studies included in our review provide very limited evidence of the effectiveness of methadone for chronic non‐cancer pain. We were unable to combine results statistically, and there were too few participants in each study to be confident in their results. No conclusions can be made regarding differences in effectiveness or side effects between methadone and placebo, other opioids, or other treatments.
Background
Description of the condition
Chronic non‐cancer pain (CNCP) encompasses a wide range of chronic pain conditions. Several definitions of chronic pain exist, including "pain that lasts beyond the term of an injury or painful stimulus" (Gale 2006) and “pain without apparent biological value continuing beyond the normal tissue healing time” (IASP 2003). However, the International Association for the Study of Pain (IASP) task force on Taxonomy does not give a single definition, but rather provides descriptions of chronic pain syndromes.
CNCP includes both neuropathic (e.g. postherpetic neuralgia (PHN), painful diabetic neuropathy (PDN)) and nociceptive syndromes (e.g. arthritides, myofascial pain syndromes, headaches). The prevalence of CNCP in adults is reported to be between 2% and 40% in the developed countries, depending on the type of pain and the population studied (Verhaak 1998). The Pain in Europe report described the prevalence of CNCP as ranging between 11% and 30%, with overall prevalence of 19% in the general adult population in Europe (Breivik 2006). There are modest differences in the prevalence of chronic pain between developed and developing countries (Tsang 2008). Worldwide, back pain, osteoarthritis and headaches are the leading causes of CNCP (Breivik 2006; Tsang 2008). CNCP has a substantial effect on patients’ health‐related quality of life (HRQoL); some studies show a reduction in HRQoL that is comparable to that reported by palliative cancer pain patients (Fredheim 2008). It also causes significant disability, with a recent Australian study indicating an average of 16.4 lost work day equivalents in a 6‐month period in persons with chronic pain (Blyth 2003). The financial burden of chronic pain is also substantial. The annual cost of chronic pain in the United States was estimated at almost 300 billion dollars in 2001 (NAS 2001).
Description of the intervention
Methadone belongs to the class of drugs known as opioids, which are the most effective broad‐spectrum analgesics available. Methadone is used clinically as an analgesic, and for maintenance and detoxification of patients with heroin and other opioid dependencies. Opioids are considered the cornerstone of therapy for moderate‐to‐severe acute pain or pain of similar intensity due to life threatening illnesses, but their long‐term use in non‐cancer pain is controversial. Recent studies and meta‐analyses provided efficacy data on opioids for the treatment of CNCP (Furlan 2006), including chronic neuropathic pain (Eisenberg 2006).
In the United States, the therapeutic use of opioids in general has risen significantly over the last decade, with methadone use increasing by 1177% (Manchikanti 2008). More than 4 million methadone prescriptions were written for pain in the United States in 2009 (CDC 2012). Despite this, the safety and efficacy of the different opioids have yet to be established.
How the intervention might work
Opioids provide analgesia by binding to opioid receptors of the mu and kappa class and blocking the release of neurotransmitters such as substance P. Opioid receptors are expressed both centrally and peripherally (during the inflammatory response in injured tissue). Methadone is a synthetic opioid that shares many of the analgesic and unwanted effects typical of other opioids. However, it has pharmacokinetic and pharmacodynamic properties that distinguish it from other opioids.
Unlike many other opioids, methadone has extensive oral bioavailability. Most opioids have less than 40% oral bioavailability.
Methadone is metabolized in the liver via the cytochrome P‐450 system, and is excreted renally and via the faecal route. Dosage adjustment is not required in renal or hepatic insufficiency, or in haemodialysis. Additionally, methadone does not appear to produce active, potentially toxic metabolites. Methadone has a long, biphasic elimination half‐life. It may take up to 10 days to reach steady‐state serum levels. It is inherently long acting and is significantly less expensive than opioids that are pharmaceutically manipulated into controlled‐release formulations.
N‐methyl‐ D‐aspartate (NMDA) receptor antagonism. Activation of the NMDA receptor by excitatory amino acids, such as glutamate, has been implicated in the development of neuropathic pain and also appears to have a role in the development of opioid tolerance and opioid induced hyperalgesia. While the ability of methadone to block the NMDA receptor has been demonstrated in animal models, it is unclear if this has clinical relevance at normal doses. It is postulated that this property may lend methadone an advantage over other opioids when treating neuropathic pain, with less need for dosage escalations.
Prolongation of the QT interval on electrocardiogram (ECG) at high doses. QT interval is the interval measured from the beginning of the QRS complex to the end of the T wave on ECG, measuring the time between depolarization and repolarization (or recovery) of the heart ventricles. Methadone binds in vitro to the cardiac HERG potassium ion channel (Kv11.1 potassium channel coded by human Ether‐à‐go‐go Related Gene) and has been shown to prolong cardiac depolarization in a dose‐dependent manner. Patients with prolonged QT interval are at risk of developing torsades de pointes, a potentially life‐threatening ventricular tachyarrhythmia. Data indicate that methadone may be responsible for sudden cardiac death, even in concentrations that are considered therapeutic for the majority of patients (Chugh 2008; Krantz 2009). Intravenous methadone is associated with greater QTc interval prolongation than the oral preparation. This risk may also be increased with concurrent use of other QT interval prolonging medications.
These distinct properties suggest that methadone may have a different efficacy and safety profile than other commonly prescribed opioids.
Why it is important to do this review
The increase in prescribing of methadone in recent years has been accompanied by an increase in fatalities associated with its use (Shields 2007). More than 30% of prescription analgesic deaths in the United States involve methadone, even though methadone prescribing accounts for a relatively small share, 2%, of analgesic prescriptions (CDC 2012). In addition to the risk of fatal arrhythmias, patients receiving methadone are thought to be at greater risk of developing respiratory depression than with other opioids, because of methadone’s complex pharmacokinetic properties. Conversely, the potentially beneficial properties of methadone, such as NMDA receptor antagonism, may be responsible for increased effectiveness in certain patients with CNCP; therefore, it is important and timely to conduct this systematic review.
Objectives
To assess the analgesic effectiveness and safety of methadone in the treatment of CNCP.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that investigated the analgesic effects of methadone, with pain assessment as either the primary or secondary outcome. Quasi‐randomized studies comparing methadone to active comparators or placebo, cohorts and case‐control trials with pain assessment as a primary outcome were also considered for inclusion. Full peer‐reviewed journal publication was required; abstracts were not considered for inclusion, unless they were less than three years old. Single case reports and clinical observations were excluded. We excluded studies with fewer than 10 participants to overcome random play of chance on estimation of treatment effect. Studies using racaemic mixtures of methadone or either of the (d‐) or (l‐) methadone isomers were also considered for inclusion.
Rationale for conducting a review that includes non‐randomized studies
We had some concerns that the beneficial and harmful effects of methadone in CNCP may not be studied adequately in RCTs. For example, respiratory depression or clinically significant arrhythmias may be rare enough not to be captured by RCTs. Therefore, in case the goal of the review could not be met by reviewing RCTs only, an attempt to systematically review the non‐randomized studies was undertaken.
Types of participants
Adult participants (aged 18 years and above) having any type of CNCP were included.
Studies with mixed populations, i.e. CNCP and cancer pain were only included if data were presented separately. Studies of methadone for acute or experimental pain were excluded.
Types of interventions
Administration of methadone by oral, rectal, intravenous, sublingual, subcutaneous, transdermal, epidural or intrathecal routes were considered for inclusion. Studies assessing topical administration of methadone on open wounds were also considered. Both single and multiple dose studies were assessed. Placebo or active control comparators were included.
Types of outcome measures
We extracted information about the pain condition, number of participants studied, drug and dosing regimen, study design, duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events from each study and recorded this on a data extraction sheet.
We considered the following participant reported outcomes measurements:
measures of pain relief or reduction of pain intensity (visual analogue scale (VAS), numerical rating scale (NRS), or verbal rating scale (VRS));
time to and number of participants achieving 50% or greater pain relief;
participant’s global impression of clinical change;
pain outcome measures available on pain questionnaires and QoL measurement instruments (brief pain inventory (BPI), the Western Ontario McMaster function pain and stiffness score (WOMAC), McGill pain questionnaire, Medical Outcomes Study Short‐Form 36 (SF‐36)).
In addition, we intended to analyse time to and amount of rescue analgesic medications.
The following adverse event outcomes were considered:
number of participants with at least one adverse event;
number of participants with at least one serious adverse event;
number of participants experiencing specific adverse events;
severity of adverse events;
all‐cause withdrawals;
lack of efficacy withdrawals;
adverse event withdrawals.
Search methods for identification of studies
Electronic searches
We searched the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2011, issue 11;
MEDLINE (Ovid) 1950 to November 2011;
EMBASE (Ovid) 1980 to November 2011.
Given the limited literature in this area, a general search strategy was undertaken as seen in the MEDLINE search strategy and this was amended for the other databases (see Appendix 1; Appendix 2; Appendix 3 for details). We applied no language restrictions.
Searching other resources
We sought additional studies from the reference lists of retrieved articles and from reviews.
Data collection and analysis
Selection of studies
Eligibility was initially determined by reading the title and abstracts identified in each search. Full‐length articles of potentially eligible studies were obtained and assessed. Two review authors (SH, EM) independently determined the eligibility of each study for inclusion based on the criteria set in this protocol. Disagreements were resolved by discussion, or if persistent, by a third review author (AL). The studies were not anonymized in any way prior to assessment.
When selecting non‐randomized studies, we intended to include the best available study designs used to answer the review question. Preference was given to quasi‐randomized, case‐control and cohort study designs, since for those types of studies methods for determination of study quality and for controlling confounding factors have been published (Reeves 2011).
Data extraction and management
Data extraction was divided between two review authors (SH, EM) with each extraction being independently duplicated, using a Microsoft Excel data extraction sheet. The data elements abstracted are listed in Appendix 4. Data suitable for pooling were entered into RevMan 5 independently by two review authors (SH, EM) to prevent transcription errors.
The data collection form was modified for extraction of non‐randomized studies. It included the checklist shown in Appendix 5, in order to determine the study design of a non‐randomized study, rather than depending on the study authors’ description of the study design.
Assessment of risk of bias in included studies
Two review authors (EM and SH) independently assessed the risk of bias of all included RCTs in this review. The review authors made critical assessments for each of the following domains: sequence generation (randomization), allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Review author judgment for each domain was entered into a Risk of Bias table, as either “low risk”, “high risk”, or “unclear risk” (indicating either lack of information or uncertainty over the potential for bias).
For non‐randomized studies, we prepared a list of potential confounding factors in Table 1 and evaluated all studies according to this table. We identified the confounding factors that the researchers had considered and those that had been omitted. We assessed the balance between comparator groups at baseline with respect to the main prognostic or confounding factors. We identified what researchers did to control for selection bias, i.e. any design features used for this purpose (e.g. matching or restriction to particular subgroups) and the methods of analysis (e.g. stratification or regression modelling with propensity scores or covariates). Additionally, we assessed quality of case‐control and cohort studies by using the Newcastle‐Ottawa Scale (see Appendix 6; Appendix 7), with a higher number of “stars” indicating a higher quality of study. In studies in which allocation to groups is determined by outcome, as in case‐control studies, the exposure of interest, rather than the outcome, is most susceptible to bias. We intended to assess whether the researchers assessing the exposure were masked to whether the participants had experienced the outcome or not; however, no studies of this design met all inclusion criteria.
1. Confounding factors.
| Did the study restrict participant selection so that all groups had the same value for the confounder? | Did the study demonstrate balance between groups for the confounder? | Did the study match on the confounder? | Did the study adjust for the confounder in statistical analyses to quantify the effect size? | |
| Confounder 1 (Age) | yes/no | yes/no | yes/no | yes/no |
| Confounder 2 (Sex) | yes/no | yes/no | yes/no | yes/no |
| Confounder 3 (Pain diagnosis) | yes/no | yes/no | yes/no | yes/no |
| Confounder 4 (Methadone dose and route of administration) | yes/no | yes/no | yes/no | yes/no |
| Confounder 5 (Duration of methadone treatment) | yes/no | yes/no | yes/no | yes/no |
Confounding factors in each non‐randomized study were assessed according to this table
Measures of treatment effect
Dichotomous data
Discrete events such as preference, numbers of participants reporting at least 50% pain relief, or the number of participants reporting adverse events were used to calculate the absolute risk reduction (ARR, also known as risk difference) using RevMan 5 software. When a statistically significant ARR existed between interventions, we intended to derive the numbers needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH). Dichotomous outcomes are presented in terms of both raw numbers and percentages of participants in each study arm benefiting from therapy or suffering adverse events. We intended to apply a random‐effects model to combine data. We are aware of the possible limitation of using a random‐effects model for meta‐analysis in case of non‐normal distribution of intervention effect data; however, using a fixed‐effect model for this purpose may be less appropriate since we cannot assume to know the direction of the effect.
Continuous data
We intended to undertake meta‐analyses when comparable data were available from continuous outcomes. Comparisons between methadone and active control or placebo groups were to be made separately for total pain relief (TOTPAR), pain intensity post‐intervention, analgesic consumption, and intensity of a specific adverse event, using weighted mean differences (WMDs). We intended to apply a random‐effects model to combine data. Again, there were insufficient data to permit meta‐analysis.
Dealing with missing data
No attempts were made to contact study authors for missing subject data. If subject data were missing, analyses were based on participant populations in which outcomes were reported. Discrepancies between number of participants enrolled and number of participants in whom outcomes were reported are noted in the 'Characteristics of included studies' table. Where studies reported statistics based on intention‐to‐treat (ITT) or modified ITT populations, available case analyses were performed.
Assessment of heterogeneity
We intended to quantify statistical heterogeneity using the I2 statistic, which is a reliable and robust test to quantify heterogeneity, since it does not depend on the number of trials or on the between‐study variance. The I2 statistic measures the extent of inconsistency among studies' results and can be interpreted as the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error. An I2 value of greater than 50% is considered to indicate substantial heterogeneity (Deeks 2008). We also intended to assess heterogeneity by visually studying forest plots. Predetermined subanalyses or sensitivity analyses were not required to explain heterogeneity, due to lack of data.
Assessment of reporting biases
No attempt was made to assess reporting bias. The inclusion of abstracts and searching of the US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) websites was undertaken in an attempt to minimize publication bias.
Data synthesis
We intended to use the random‐effects model by DerSimonian and Laird (Deeks 2008) for meta‐analysis, using RevMan 5.
We did not make any attempt to combine evidence from RCTs and non‐randomized studies.
Subgroup analysis and investigation of heterogeneity
There were insufficient data to allow us to undertake subgroup analysis for:
chronic neuropathic versus nociceptive pain conditions;
specific pain conditions (e.g. postherpetic neuralgia (PHN), diabetic peripheral neuropathy (DPN), low back pain, headache);
older versus younger people; specifically age 18 to 65 versus greater than 65;
studies of less than or equal to 24 hours versus studies of greater than 24 hours;
men versus women.
We were able to undertake subgroup analysis for one study (Morley 2003) in which two methadone dosing regimens were studied ‐ a "low‐dose" phase, where participants received 10 mg daily and a "high‐dose" phase, where participants received 20 mg daily.
Results
Description of studies
Results of the search
The searches yielded 49 potentially relevant studies.
Included studies
Three studies fulfilled the inclusion criteria. Morley 2003 studied two dosing regimens of methadone in a randomized, placebo‐controlled, cross‐over trial in participants with diverse neuropathic pain syndromes. Both regimens were tested over a 20 day phase. Participants received either methadone or placebo on odd days and a rest day on even days, i.e. they received five days of methadone for each phase. The "low‐dose" phase administered 5 mg twice daily, whereas the "high‐dose" phase administered 10 mg twice daily. Raja 2002 compared an opioid (morphine or methadone) with a tricyclic antidepressant (nortriptyline or desipramine) and placebo in a three‐phase randomized double‐blind cross‐over trial, with each phase lasting approximately eight weeks. Participants received methadone only if they were unable to tolerate morphine during the titration phase. Quang‐Cantagrel 2000 in a study described as a "retrospective chart review", evaluated the efficacy and safety of various long‐acting opioids in 86 outpatients followed over a period of an average of 8.8 months. Patients were allowed to switch to (an) alternative opioid(s) if the first opioid prescribed proved ineffective or intolerable. We used Appendix 5 to determine the nature of the study, but were unable to define it precisely; however, it had most of the elements of a case series.
Excluded studies
Forty‐six studies did not meet all inclusion criteria.
Seventeen studies had no comparator group (Altier 2005; Bendiksen 2007; Cruciani 2005; Fredheim 2006; Gagnon 2003; Green 1996; Mironer 1999; Moulin 2005; Peng 2008; Robbins 1996; Robbins 1997; Robbins 2009; Rothrock 1999; Saper 2004; Shir 2001; Urban 1986; Walmsley 2010).
Eight studies had no pain outcome (Arnaert 2006; Fredheim 2007; Krebs 2011; Manchikanti 2005; Manchikanti 2009; Sjøgren 2000; Taylor 2000; Webster 2008).
Eight studies enrolled fewer than 10 participants in the methadone arm (Arner 1988; Bouckoms 1992; Flavell Matts 1964; France 1984; Gallagher 2007; Gardner‐Nix 1996; Morley 1993; Schofferman 1999).
Five studies administered various opioids, and did not present methadone data separately (Byas‐Smith 2005; Haythornthwaite 1998; Naliboff 2011; Robbins 1999; Watson 2010).
Four studies provided no data (Byrne 2001; Fowle 1978; Lockwood 2004; McNulty 2000).
Three manuscripts were case studies (Altier 2001; Berken 1982; Sprenger 2008).
Finally, one study enrolled cancer patients only (Beaver 1967).
Risk of bias in included studies
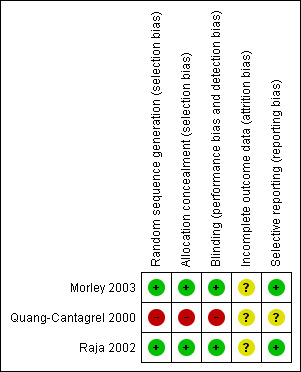
Risk of bias for the two RCTs is discussed under the subheadings below. For the retrospective chart review (Quang‐Cantagrel 2000) we assessed confounding factors (Table 1) and determined that results could potentially be biased based on lack of controlling for the following confounders: age, sex, pain diagnosis, methadone dose and duration of treatment. Additionally, we applied the Newcastle‐Ottawa Scale for assessing the quality of non‐randomized studies in meta‐analysis (Appendix 6 and Appendix 7). Based on this, we assessed its quality using the assessment scale for case control studies (Appendix 6). We assigned scores as follows: Selection 1, Comparability 0, Exposure 2. The findings are presented as Figure 1.
1.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both RCTs (Morley 2003; Raja 2002) adequately described the methods used to ensure that allocation of participants to treatment groups was concealed.
Blinding
Both studies (Morley 2003; Raja 2002) adequately described the methods used to ensure that participants and interacting investigators were unable to differentiate between the treatment and control tablets/capsules.
Incomplete outcome data
In Morley 2003, 1/19 participants withdrew during the "low‐dose" phase on the first occasion in the methadone arm, 1/18 withdrew between phases, and 6/17 participants withdrew during the "high‐dose" phase ‐ three while taking placebo, three while taking methadone. In Raja 2002, 19/66 participants withdrew while taking an opioid; however, the number withdrawing while taking methadone (26 participants enrolled) was not reported. Seven of 59 participants withdrew while taking a tricyclic antidepressant and 1/56 withdrew while taking placebo.
Morley 2003 performed efficacy analyses only on those participants who completed the study (completer analysis). Although only one participant withdrew during the low dose phase, more than one‐third withdrew during the high dose phase, all because of adverse events. Raja 2002 performed an ITT analysis; however, they did not report how many participants receiving methadone withdrew and for participants not completing the study they used the last three available pain ratings, rather than baseline pain ratings.
Selective reporting
Both RCTs (Morley 2003; Raja 2002) reported the outcomes specified in the methods, although Morley 2003 measured, but did not report the severity of adverse events.
Other potential sources of bias
Treatment group size was an issue, with only 19 participants receiving at least one dose of methadone in one study (Morley 2003) and 26 participants receiving at least one dose in the other (Raja 2002). Studies with small group sizes may overestimate efficacy (Moore 1998; Nüesch 2010). Participants in one study (Morley 2003) received a maximum of five days of methadone in each phase. Evidence from trials in arthritis patients shows that studies lasting less than eight weeks overestimate the effect of treatment (Moore 2010a) ‐ the same may be true in studies of neuropathic‐type pain, given that both are chronic conditions.
Effects of interventions
Morley 2003 measured maximum and average pain intensity and pain relief via a 0 to 100 VAS. Raja 2002 measured pain intensity and pain relief using a 0 to 10 and 0 to 100 NRS, respectively. Quang‐Cantagrel 2000 evaluated efficacy by assessing the number of patients with pain relief of 50% or greater. Raja 2002 also reported number of treatment responders, defined as individuals whose baseline pain rating decreased by 33% during the treatment period; however, they reported a composite of participants receiving each class of intervention (opioid, tricyclic antidepressant, placebo) rather than results for individual drugs, e.g. methadone. We were, therefore, only able to analyse continuous data for pain outcomes, and due to the differences in outcome reporting between studies, we were unable to perform any meta‐analyses.
Morley 2003 investigated the use of methadone in participants with a variety of diagnoses, both central and peripheral neuropathies. Raja 2002 enrolled only participants with PHN. Quang‐Cantagrel 2000 reviewed the cases of patients with a variety of complaints, the most common of which were back pain and neuropathies.
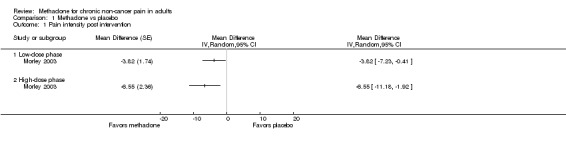
Morley 2003 conducted a completer analysis of 19 participants enrolled in a two phase, cross‐over study. In the first phase, participants received a daily dose of 10 mg and in the second phase 20 mg of oral methadone. The washout period between phases was not specified. All participants had responded poorly to traditional analgesic regimens, with several concurrently receiving other opioids. Participants continued on all current medications during the study. Participants received methadone or placebo on odd numbered days and received no intervention on even numbered days. Efficacy was reported at the end of each phase. The paper supplied individual participant data; therefore, we were able to perform our own statistical analysis of each outcome and compare our results with those results reported by the authors. We (EM, SH) performed a paired t‐test to compare the difference in average pain intensity between methadone and placebo for both the "low‐" and "high‐dose" phases (10 mg and 20 mg daily). Both phases demonstrated that methadone was more effective than placebo in reducing average pain intensity (P = 0.042 and P = 0.020, respectively, Figure 2). Pain relief reported with both doses of methadone also demonstrated statistical superiority versus placebo (P = 0.003 and P < 0.001 for low‐dose and high‐dose phases, respectively, Wilcoxon Signed Rank Test). The results of our analyses of pain intensity and pain relief agreed with those analyses presented in the paper for the high‐dose phase, but differed from those presented for the low‐dose phase, where the authors reported that the differences between methadone and placebo were not statistically significant for either outcome, perhaps reflecting differences in statistical methodology. Three of the 18 participants completing the low‐dose phase had an average pain intensity below 40/100, i.e. mild, while receiving methadone, compared with only one participant receiving placebo. In the high‐dose phase, two participants had average pain intensity below 40/100 whilst receiving methadone, compared with only one while receiving placebo.
2.
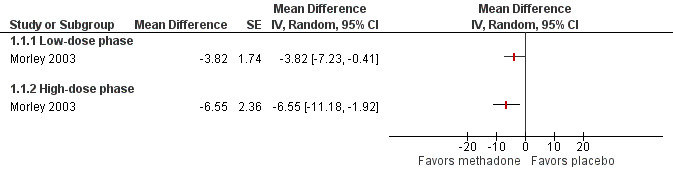
Forest plot of comparison: 1 Methadone vs placebo, outcome: 1.1 Pain intensity post intervention.
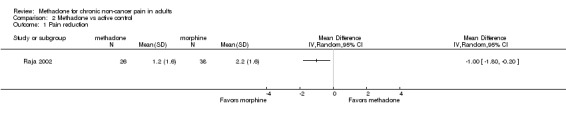
Raja 2002 conducted an ITT analysis of 76 participants enrolled in a three period, cross‐over study. Each treatment period lasted approximately eight weeks and had a titration, maintenance and taper phase. Each period was separated by a one‐week washout. Participants in the opioid phase received morphine as their initial intervention, and received methadone only if they were unable to tolerate 30 mg or less of morphine. Several participants were receiving opioids at the time of enrolment, but were permitted to use only acetaminophen or non‐steroidal anti‐inflammatories (NSAIDs) during the study. The average daily dose of methadone was 15 mg, i.e. similar to the dosing regimen administered by Morley 2003. The change in pain intensity during each treatment period was calculated as the difference between the mean of the last three ratings during the treatment phase, typically made during the end of the maintenance period, and the mean of three baseline ratings obtained prior to the initiation of the drug treatment. Pain relief was calculated in a similar manner. The majority of results were reported as composite data, i.e. data were not reported separately for morphine and methadone. The only outcome for which separate data were available was reduction in pain from baseline to the end of the maintenance period. The authors reported that the reduction in pain was greater with morphine (reduction 2.2/10; 95% CI 2.7 to 1.6, N = 38) than methadone (reduction 1.2/10; 95% CI 1.8 to 0.5, N = 26, P = 0.02) with participants taking an average of 91 mg of morphine daily (Figure 3). During the opioid period, average pain intensity during the maintenance phase was 4.4/10, i.e. moderate pain, but again, results were not reported separately for methadone and morphine.
3.

Forest plot of comparison: 2 Methadone vs active control, outcome: 2.1 Pain reduction.
Quang‐Cantagrel 2000 performed a "retrospective chart review" of 86 outpatients from a pain clinic. Patients received an average of 8.8 months of opioid therapy. Patients were initially assigned one of four long acting opioids (sustained‐release morphine or oxycodone, methadone, or transdermal fentanyl), with choice of opioid based on allergies, cost, and patient preference for delivery route. They received the initial opioid for at least one week, after which rotation to a different opioid was permitted, contingent upon lack of efficacy (pain relief less than 50%) or intolerable side effects (severity greater than 30 on a 0 to 100 scale). Initial doses of methadone were 5 mg to 20 mg four times a day, after which titration in 5 mg increments was allowed for lack of efficacy. On average, the first opioid prescribed was effective for 31 patients, was stopped because of side effects in 25, and was stopped for ineffectiveness in 29. For patients receiving methadone as their initial opioid, 8 of 29 found treatment to be effective versus 14 of 33, 6 of 18 and 3 of 6 for long‐acting formulations of morphine, oxycodone and transdermal fentanyl, respectively. Methadone was stopped because of side effects in 11 patients and because of ineffectiveness in nine. The average dose in those who found it to be effective was 39.0 ± 17.0 mg, with patients receiving therapy for an average of 49.4 weeks. Of the remaining participants, the second opioid prescribed (after the failure of the first) was effective in 16 of 52 patients, the third in 12 of 30, the fourth in 10 of 18, and the fifth in 1 of 7 patients. Twenty‐three participants were switched to methadone from first to final rotations, but details of efficacy and tolerability were not reported. The authors compared the efficacy of the different drugs prescribed by calculating the ratio between the number of participants for which the drug was effective during five different prescriptions and the total number of prescriptions. They found that timed‐release morphine was the most effective, but did not report ratios.
Withdrawals
In Morley 2003, 33 participants were invited to enrol in the study. Fourteen declined to participate; five participants gave no reason, three associated methadone with addiction, two did not want to take any further medication, and four participants gave ‘depression when new therapies fail’, ‘having to declare a methadone script would damage my employment prospects’, ‘not 100% sure I want to be on methadone’ and inability to understand the trial assessments as respective reasons. Thus, 19 participants were enrolled. During the low‐dose phase, one participant withdrew on his first day of receiving methadone because of adverse effects. A further participant did not complete data on rest days, and so was removed from the rest day analysis of Phase 1. The same participant declined to enter the high‐dose phase due to an intercurrent illness. Seventeen participants, therefore, entered the high‐dose phase; six withdrew before completing ‐ three while taking placebo, three while taking methadone ‐ all because of adverse events.
Raja 2002 screened 103 participants: 18 declined to participate; four patients' pain decreased; three were excluded due to impaired cognition; and two had a questionable diagnosis. Thus, 76 participants were randomized. Five participants withdrew before receiving therapy (reasons not reported). Twenty‐seven participants withdrew during one of the treatment periods: 19 while receiving opioids, 7 while receiving tricyclic antidepressants and one while receiving placebo. There was a discrepancy in reporting of dropouts between the participant flow diagram and the manuscript text, with the text reporting 20 patients withdrawing during opioid therapy and six while receiving tricyclics. Primary reasons stated for dropping out during the opioid period were side effects (N = 7), other medical problems (N = 6), and concerns of family members (N = 5). Primary reasons stated for dropping out during the tricyclic period were side effects (N = 2), other medical problems (N = 1), and concerns of family members (N = 2). An additional two participants withdrew during the opioid period owing to marked pain reduction and because they wished to use non‐study medications. An additional participant withdrew during tricyclic treatment owing to a significant reduction in pain.
Quang‐Cantagrel 2000 permitted participants to switch to a different opioid based on lack of efficacy or intolerable side effects (see details in 'Effects of interventions' above). Since this was a retrospective review, there was no "study" to withdraw from; however, the authors noted that 16 (19%) of the initial 86 outpatients were unable to find an effective and tolerable opioid after five opioid rotations.
Adverse events
Patient‐reported adverse events in the three included studies were typical of well‐recognized opioid‐induced side effects. Adverse events occurred with placebo in both RCTs. Raja 2002 reported that constipation, nausea, drowsiness, and loss of appetite were more common with opioids than with placebo or tricyclic antidepressants; however, the study authors did not report whether these adverse events occurred while participants were receiving methadone or while receiving morphine.
Morley 2003 listed individual participants reporting adverse events during the 20 day period of both the low‐ and high‐dose methadone phases, but did not report statistical significance of any differences. To compare adverse event rate differences between methadone and placebo we used the McNemar test, as recommended for comparison of dichotomous data in studies with cross‐over design (Elbourne 2002). The test is based on comparing discordant pairs of responses (e.g. adverse event developed under methadone, but not placebo) for each intervention. Our analysis of both phases did not demonstrate a statistically significant difference between methadone and placebo for the incidence of any specific adverse event with the exception of dizziness in the low‐dose phase, which occurred more frequently in those receiving methadone (either on the methadone day or the rest day after methadone) than in those receiving placebo (placebo day or rest day after placebo) (P = 0.041, Table 2). Six of 19 (32%) participants reported dizziness while taking methadone, compared with zero of 19 receiving placebo.
2. Adverse events: Morley 2003, incidence of dizziness, "low‐dose" methadone phase.
| Methadone 10 mg daily | Total | |||
| Dizziness | yes | no | ||
| Placebo | yes | 0 | 0 | 0 |
| no | 6 | 13 | 19 | |
| Total | 6 | 13 | 19 | |
The proportion of observations in the different categories which define the contingency table is significantly different than is expected from random occurrence (P = 0.041, Chi2 = 4.167, with 1 degree of freedom).
Quang‐Cantagrel 2000 noted that nausea/vomiting (40%), sedation (32%) and itching (24%) were the most common adverse events that caused participants to switch to an alternative opioid, but did not report individual adverse events for each opioid. They reported that 52% (N = 15) of those receiving methadone as their initial opioid reported adverse events, with 38% (N = 11) switching to a different opioid because the adverse event was intolerable (versus 24%, 33% and 0% for morphine, oxycodone and transdermal fentanyl, respectively). The authors also noted that addiction occurred in one participant overall, with that participant being prescribed methadone. The participant, who suffered from chronic headaches, had a history of compulsive drug use and alcohol abuse. It was not reported whether the decision to prescribe methadone was based on her substance abuse history.
Withdrawals due to adverse events are detailed above under 'Withdrawals'.
Serious adverse events
No serious adverse events were reported in any included study (Morley 2003; Quang‐Cantagrel 2000; Raja 2002).
Discussion
Summary of main results
We widened our inclusion criteria from not only RCTs, but also to non‐randomized studies in an attempt to increase the amount of available information, in particular that related to safety, as we predicted there would be a lack of data from only RCTs. However, only one non‐randomized study met our inclusion criteria (Quang‐Cantagrel 2000), and only two RCTs were included (Morley 2003; Raja 2002). Forty‐six studies did not meet one or more of our relaxed inclusion criteria, the most frequent reason being due to the lack of a comparator group. Morley 2003 studied a population with mixed neuropathies, Quang‐Cantagrel 2000 reviewed a sample of pain clinic outpatients with diverse chronic pain complaints, while Raja 2002 studied participants with postherpetic neuralgia.
Both RCTs employed a cross‐over design. Whereas Raja 2002 had a one‐week washout between interventions, Morley 2003 designed their study in such a way that participants had only a one‐day washout ("rest day") between receiving methadone and placebo. Given the long half‐life of methadone, the possibility of a carry‐over effect cannot be ruled out.
None of the three included studies presented sufficient data for us to be able to make any firm conclusions regarding the efficacy of methadone as an analgesic. In the case of Morley 2003, continuous VAS data were presented, with no reporting of numbers of participants with clinically significant reductions in pain. Additionally, neither quality of life or functional outcomes were assessed. Raja 2002 assessed many more valid outcomes, but did not present the majority of results in a format that we could analyse, due to the fact that they did not report methadone and morphine data separately. Quang‐Cantagrel 2000 did not assess quality of life or functional outcomes, instead reporting only the number of participants with at least 50% pain relief and those with intolerable side effects, and only reported separate data for each opioid for the first opioid prescribed.
The limited data available to us demonstrated a statistically significant reduction in pain intensity and increase in pain relief when either methadone 10 mg or 20 mg daily were compared with placebo (Morley 2003).
Adverse events were similar to those commonly reported in opioid studies (McNicol 2008). There were no reports of QTc prolongation or respiratory depression, probably because of the low total numbers of participants across the three studies and the relatively low doses of methadone in the Morley 2003 study. Therefore, we were unable to demonstrate any difference between methadone's and other opioids' safety profiles.
Overall completeness and applicability of evidence
Efficacy
One study investigated postherpetic neuralgia (Raja 2002), one a population with mixed neuropathies (Morley 2003) and one a sample of outpatients with diverse complaints, both neuropathic and non‐neuropathic. In the first study (Raja 2002) only 26 participants received methadone. While the second study reported individual participant data, the overall participant numbers for each diagnosis were insufficient to allow us to conduct any subanalyses by diagnosis. The final study did not report data individually, nor did it divide the data based on diagnosis.
All three included studies administered oral methadone. Morley 2003 studied relatively low fixed doses (10 mg to 20 mg daily) over 20 days, whereas Raja 2002 allowed dose titration to a maximum of 80 mg over a period of approximately six weeks, perhaps more closely reflecting clinical practice. Quang‐Cantagrel 2000 allowed participants to titrate dose to efficacy or intolerable side effects, with average doses in those who stopped methadone due to intolerable side effects being statistically significantly lower than doses in participants who found the drug to be effective (39 ± 17 mg) or stopped it due to ineffectiveness. By its nature, this study reflected clinical practice.
Morley 2003 compared methadone with placebo. Raja 2002 compared methadone with placebo, a tricyclic antidepressant, and another opioid (morphine), but only presented data allowing us to compare methadone with morphine. Quang‐Cantagrel 2000 compared methadone with three other first‐line opioids, but did not include a group who did not receive an opioid.
Morley 2003 assessed various pain outcomes, but did not assess functioning or quality of life. Raja 2002 assessed many outcomes in addition to pain reduction, but again, we were only able to use data comparing pain reduction with morphine. Quang‐Cantagrel 2000 assessed only the number of participants who reported pain reduction of at least 50%.
Based on such limited data, we cannot make any recommendations regarding variations in methadone's analgesic efficacy for different types of chronic non‐cancer pain or when administered via different routes. Similarly, we cannot make any conclusions regarding improvements in quality of life or functioning. We have very limited data to make conclusions regarding short‐ versus long‐term administration, low‐ versus high‐dose regimens or efficacy compared to either active or placebo controls. None of the included studies presented data that could aid assessment of potential advantages of methadone regarding its action as an NMDA antagonist and possible related reduction in tolerance, opioid‐induced hyperalgesia or neuropathic pain.
Safety
Morley 2003 compared incidence of various adverse events with methadone versus placebo. Only dizziness was shown to occur statistically more frequently, and only during the low‐dose (10 mg daily) phase. Quang‐Cantagrel 2000 compared both the percentage of participants with any side effect and those that stopped a specific opioid due to side effects between methadone and three other opioids. While methadone demonstrated a higher proportion of participants for both outcomes than the other opioids prescribed, statistical significance was not reported. There were no reports of respiratory depression or QTc prolongation. This may be due to the relative infrequency of these side effects occurrence or because participants were more closely monitored than in regular clinical settings. It is suggested that both side effects occur more frequently in patients receiving methadone than in those receiving other opioids, but there are no data from the current review to support these assumptions (McNicol 2008).
Quality of the evidence
Only two RCTs (Morley 2003; Raja 2002) enrolling a total of 95 participants, and one non‐RCT (Quang‐Cantagrel 2000) reviewing 86 participants, met all inclusion criteria. Neither RCT presented dichotomous data allowing assessment of number of participants with 50% pain relief or better. Instead, both presented continuous outcomes, either pain intensity or pain reduction. Quang‐Cantagrel 2000 did report dichotomous outcomes, but did not have a placebo group for calculation of NNTB versus placebo.
The "Risk of Bias" assessment showed that both RCTs may have risk of bias due to incomplete reporting. Morley 2003 analyzed only those participants completing the study ‐ only 11 of the 19 enrolled completed both phases. Raja 2002 performed an ITT analysis but used last observation carried forward rather than baseline observation carried forward. It is possible, therefore, that both studies may be at risk for overestimating the efficacy of methadone. While not included in our "Risk of Bias" assessments, the short‐term nature of one study (Morley 2003) and the low number of participants receiving methadone in both studies, also produces a high potential for bias. Quang‐Cantagrel 2000 achieved only three stars out of a possible nine on the Newcastle‐Ottawa quality assessment scale and did not report any attempt to control for several potential confounding factors, such as age. By its nature, a retrospective review has a higher risk of bias than an RCT.
Each included study was sufficiently different that it is not possible to assess the consistency of findings. Last, the possibility of publication bias from unpublished negative results cannot be excluded, given that few negative studies would be required to change the positive effects seen in the included studies.
Since the review was completed, additional information has given more cause for concern that there may be significant additional biases in the three included studies, arising from small size or short duration (Moore 2010b), use of completer analyses (Moore 2012), and cross‐over design.
Potential biases in the review process
Our decision to include non‐randomized studies was made to increase the potential for discovering additional useful data, while possibly at the same time increasing the risk of biased conclusions. It could be argued, then, that we should also have included RCTs with a different potential source of bias, i.e. those with less than 10 participants. However, based on recent literature, the risk of invalid findings might be greater with RCTs with less than 10 participants than with non‐RCTs that are otherwise well designed (Moore 1998; Moore 2010b). Regardless, only one non‐randomized study met inclusion criteria.
Agreements and disagreements with other studies or reviews
Despite lack of evidence, there have been dozens of reviews of either opioids as a class, or methadone specifically, for chronic non‐cancer pain. The majority of reviews are either narrative or only contain some elements of a systematic review, such as a structured literature search. Some reviews include only RCTs, with others employing more relaxed criteria. Few reviews report unequivocal support for or against the use of opioids in CNCP.
General opioid reviews
In a Cochrane review of opioids for neuropathic pain, Eisenberg 2006 included the same methadone studies included in our review. A similar, earlier review of opioids for CNCP (Kalso 2004) included only Raja 2002, presumably because of the earlier search date. Both reported similar results as our review, but neither made specific recommendations regarding methadone. A more recent systematic review of opioids for CNCP (Manchikanti 2011) employed more stringent inclusion criteria than our review. Both studies included in our review were not included in Manchikanti 2011 because the follow‐up period in each was less than 12 weeks. The authors concluded that recommendations for opioid use in CNCP must be based on non‐randomized studies. Chou 2009 and colleagues performed a systematic review as part of the production of a clinical guideline for the use of opioids in CNCP. Their recommendations for methadone use are based on epidemiological studies or case series, and highlight the risks of death due to accidental overdosage and the risk of torsade de pointes when taken in high doses, or with concomitant use of drugs that interact with methadone or that themselves prolong QTc interval. None of the studies referenced met our inclusion criteria. The authors make no statement about the benefits of methadone therapy, based on lack of evidence.
Methadone‐specific reviews
Sandoval 2005 conducted a systematic review of oral methadone for CNCP and included not only RCTs but also non‐RCTs including case reports/series. They included Morley 2003, but excluded Raja 2002 because "less than 50% of the participants received methadone in this study; therefore, it does not provide a clear evaluation of the analgesic efficacy of methadone". They also included 13 case reports including 31 participants and seven case series including 495 participants (we excluded such studies). The case reports/series in aggregate reported "meaningful" pain relief in 59% of patients. The review authors noted that this figure "should be interpreted very cautiously, as it seems overrated due to the poor quality of the uncontrolled studies and their tendency to report positive results". The case series/reports also reported adverse events similar to those seen in randomized studies, with nausea and/or vomiting, sedation, constipation and itch occurring in more than 10% of participants. However, in addition, the case series/reports also reported incidences of cardiac arrhythmias, tolerance and addiction (but not respiratory depression) in less than 5% of participants.
Finally, Nicholson 2007 performed a review for The Cochrane Library using similar criteria to ours, but instead looking at methadone for cancer pain. This review also suffered from lack of data due to the small number of studies meeting the inclusion criteria and their design heterogeneity preventing meta‐analysis of outcomes. Therefore, the possibility of extrapolating data from patients with cancer pain to those with non‐cancer pain does not currently exist.
Authors' conclusions
Implications for practice.
There is very limited evidence to judge the effectiveness of methadone in chronic non‐cancer pain. Equally, there is no evidence from RCTs that methadone has a different safety profile to other opioids.
Implications for research.
This review highlights the lack of high quality evidence investigating the use of methadone for chronic non‐cancer pain. In particular, safety issues such as respiratory depression, cardiac arrhythmias and addiction have not been adequately addressed. While well‐designed, long‐duration, randomized, controlled studies would be highly desirable, this review highlights the fact that no RCTs have been conducted since 2003, perhaps reflecting methadone's generic status.
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2019 | Amended | Contact details updated. |
| 2 August 2017 | Review declared as stable | Review superseded. See Published notes. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 1 August 2017 | Amended | Author deceased. See Published notes. |
| 20 May 2015 | Review declared as stable | See Published notes. |
Notes
2015
In May 2015, the authors and editors agreed to re‐assess this review for updating in 2016. The review may be superseded by a new protocol on methadone for neuropathic pain, at which point the original review would be withdrawn. For more information, please contact the Cochrane PaPaS Review Group.
2017
Author Art Lipman sadly passed away in April 2017.
At May 2017, this Cochrane Review has been superseded by a new review on methadone for neuropathic pain. See: McNicol ED, Ferguson MC, Schumann R. Methadone for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No.: CD012499. DOI: 10.1002/14651858.CD012499.pub2.
Acknowledgements
We wish to thank Caroline Struthers, Trials Search Coordinator, PaPaS Review Group for invaluable guidance in developing our various search strategies and for running searches of those databases for which we did not have access.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Pain explode all trees
#2 (pain*):ti,ab,kw
#3 (neuralgi* or myalgi* or neuropath* or arthriti* or osteoarthri* or arthralgi* or sciatica or headache* or migrain*):ti,ab,kw
#4 MeSH descriptor Analgesia explode all trees
#5 (analgesi*):ti,ab,kw
#6 MeSH descriptor Tibial Neuropathy explode all trees
#7 MeSH descriptor Femoral Neuropathy explode all trees
#8 MeSH descriptor Radial Neuropathy explode all trees
#9 MeSH descriptor Alcoholic Neuropathy explode all trees
#10 MeSH descriptor Optic Neuropathy, Ischemic explode all trees
#11 MeSH descriptor Median Neuropathy explode all trees
#12 MeSH descriptor Sciatic Neuropathy explode all trees
#13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12)
#14 MeSH descriptor Methadone explode all trees
#15 (methadon* or d‐methadone or l‐methadone or r‐methadone or s‐methadone or dolophine or phenadone or physeptone or phymet or symoron or metadol or metasedin or methaddict or methadose or methex or pinadone or amidone or biodone)
#16 (#14 OR #15)
#17 (#13 AND #16)
#18 (#17) LIMIT TO CLINICAL TRIALS (CENTRAL)
Appendix 2. MEDLINE search strategy
1. exp PAIN/
2. pain*.mp.
3. (neuralgi* or myalgi* or neuropath* or arthriti* or osteoarthri* or arthralgi* or sciatica or headache* or migrain*).mp.
4. exp ANALGESIA/
5. analgesi*.mp.
6. exp Tibial Neuropathy/ or exp Femoral Neuropathy/ or exp Radial Neuropathy/ or exp Alcoholic Neuropathy/ or exp Optic Neuropathy, Ischemic/ or exp Median Neuropathy/ or exp Sciatic Neuropathy/
7. exp methadone/
8. (methadon* or d‐methadone or l‐methadone or r‐methadone or s‐methadone or dolophine or phenadone or physeptone or phymet or symoron or metadol or metasedin or methaddict or methadose or methex or pinadone or amidone or
biodone).mp.
9. or/1‐6
10. 7 or 8
11. 9 and 10
12. limit 11 to humans
13. limit 12 to (case reports or clinical trial, all or comparative study or meta analysis or "review").
14 randomized controlled trial.pt.
15 controlled clinical trial.pt.
16 randomized.ab.
17 placebo.ab.
18 drug therapy.fs.
19 randomly.ab.
20 trial.ab.
21 groups.ab.
22 or/14‐21
23 (animals not (humans and animals)).sh.
24 22 not 23
25 11 and 24
26 25 not 13
27 13 not 25
28 13 or 25
Appendix 3. EMBASE search strategy
1 exp PAIN/
2 pain*.mp.
3 (neuralgi* or myalgi* or neuropath* or arthriti* or osteoarthri* or arthralgi* or sciatica or headache* or migrain*).mp.
4 exp ANALGESIA/
5 analgesi*.mp.
6 exp tarsal tunnel syndrome/ or exp femoral neuropathy/ or exp radial neuropathy/ or exp ischemic optic neuropathy/ or exp carpal tunnel syndrome/ or exp sciatic neuropathy/
7 exp METHADONE/
8 (methadon* or d‐methadone or l‐methadone or r‐methadone or s‐methadone or dolophine or phenadone or physeptone or phymet or symoron or metadol or metasedin or methaddict or methadose or methex or pinadone or amidone or biodone).mp.
9 or/1‐6
10 7 or 8
11 9 and 10
12 random*.ti,ab.
13 factorial*.ti,ab.
14 (crossover* or cross over* or cross‐over*).ti,ab.
15 placebo*.ti,ab.
16 (doubl* adj blind*).ti,ab.
17 (singl* adj blind*).ti,ab.
18 assign*.ti,ab.
19 allocat*.ti,ab.
20 volunteer*.ti,ab.
21 CROSSOVER PROCEDURE.sh.
22 DOUBLE‐BLIND PROCEDURE.sh.
23 RANDOMIZED CONTROLLED TRIAL.sh.
24 SINGLE BLIND PROCEDURE.sh.
25 or/12‐24
26 ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/
27 HUMAN/
28 26 and 27
29 26 not 28
30 25 not 29
31 11 and 30
Appendix 4. Data extraction form for RCTs
General study data
First author
Year published
Study unique ID number
Title
Journal
Role of funding source mentioned? If Yes, specify
Criteria for including study
Double blinded RCT?
Case series or open‐label prospective study with active comparator or placebo arm?
More than 10 participants?
Study evaluates methadone administered for CNCP?
Adults (age > 18) or mixed population, but separate data on adults presented?
If presented as abstract, < 3 years old?
Pain outcome?
Final decision: if 1 or 2 and 3‐7 replies are "yes" ‐ include. If "no" ‐ exclude and STOP here.
Characteristics of included studies
Risk of bias assessment
Was the allocation sequence adequately generated (randomization)? No‐N Yes‐Y Unclear‐U.
Was the allocation adequately concealed? No‐N Yes‐Y Unclear‐U.
Was knowledge of the allocated intervention adequately prevented during the study (blinding)? No‐N Yes‐Y Unclear‐U.
Were incomplete outcome data adequately addressed? No‐N Yes‐Y Unclear‐U.
Are reports of the study free of suggestion of selective outcome reporting? No‐N Yes‐Y Unclear‐U.
Methodology
Parallel = P, Cross‐over = C.
Single or multiple dose study, or both?
Study duration.
Inclusion criteria.
Exclusion criteria.
Control groups (placebo or active): list drug name, non‐drug intervention, and/or nature of placebo.
Total N randomized (entire study).
Number randomized: methadone/Control groups.
Number completing study: methadone/Control groups.
Total N analyzed (ITT): methadone/Control groups.
Participants
Age methadone group: mean ± SD (if other measure of average and spread, specify).
Age placebo, active control group: mean ± SD (if other measure of average and spread, specify).
Sex: methadone group (n M/F).
Sex: control groups (n M/F).
Chronic pain condition.
Comorbid pathophysiology.
Interventions
Dose of methadone administered.
Number of methadone daily doses.
Total daily dose: methadone.
Duration of methadone administration.
Dose of controls administered.
Number of control daily doses.
Total daily dose: controls.
Duration of control administration.
Route of administration.
Outcome measures evaluated
Pain INTENSITY scale used: categorical, numerical rating scale, VAS?
Categorical scale: specify categories.
Numerical scale: details (0 to 5, 0 to 10, 0 to 100) and anchors.
VAS scale: details (0 to 5, 0 to 10, 0 to 100) and anchors.
Pain RELIEF scale: Number of categories used and details.
Baseline CATEGORICAL pain intensity score methadone: mean ± SD (or specify if other measure of average and spread).
Baseline NUMERICAL pain intensity score methadone: mean ± SD (or specify if other measure of average and spread).
Baseline VAS pain intensity score methadone: mean ± SD (or specify if other measure of average and spread).
Baseline CATEGORICAL pain intensity score CONTROL groups: mean ± SD (or specify if other measure of average and spread).
Baseline NUMERICAL pain intensity score CONTROL groups: mean ± SD (or specify if other measure of average and spread).
Baseline VAS pain intensity score CONTROL groups: mean ± SD (or specify if other measure of average and spread).
Number of participants > 50% pain relief (n/N): methadone.
Number of participants > 50% pain relief (n/N): control groups.
Time to achieve 50% pain relief (mins.): methadone (mean ± SD).
Time to achieve 50% pain relief (mins.): control groups (mean ± SD).
Other pain outcome (e.g. global evaluation, time to onset of analgesia, HRQoL scores). Specify and detail for all groups.
Adverse events
Number of participants reporting ANY adverse event: methadone group (n/N).
Number of participants reporting SPECIFIC adverse events (list each): methadone group (n/N).
If scale used for intensity of specific side effect(s), specify: methadone group.
Number of participants reporting ANY adverse event: control groups (n/N).
Number of participants reporting SPECIFIC adverse events (list each): control groups (n/N).
If scale used for intensity of specific side effect(s), specify: control groups.
Reasons for dropouts: methadone group.
Reasons for dropouts: control groups.
Comments
Appendix 5. List of study design features for non‐randomized studies (studies with allocation to interventions at the individual level)
| RCT | Q‐RCT | NRCT | CBA | PCS | RCS | HCT | NCC | CC | XS | BA | CR/CS | |
| Was there a comparison: | ||||||||||||
| Between two or more groups of participants receiving different interventions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| Within the same group of participants over time? | P | P | N | Y | N | N | N | N | N | N | Y | N |
| Were participants allocated to groups by: | ||||||||||||
| Concealed randomization? | Y | N | N | N | N | N | N | N | N | N | na | na |
| Quasi‐randomization? | N | Y | N | N | N | N | N | N | N | N | na | na |
| By other action of researchers? | N | N | Y | P | N | N | N | N | N | N | na | na |
| Time differences? | N | N | N | N | N | N | Y | N | N | N | na | na |
| Location differences? | N | N | P | P | P | P | P | na | na | na | na | na |
| Treatment decisions? | N | N | N | P | P | P | N | N | N | P | na | na |
| Participants' preferences? | N | N | N | P | P | P | N | N | N | P | na | na |
| On the basis of outcome? | N | N | N | N | N | N | N | Y | Y | P | na | na |
| Some other process? (specify) | ||||||||||||
| Which parts of the study were prospective: | ||||||||||||
| Identification of participants? | Y | Y | Y | P | Y | N | P* | Y | N | N | P | P |
| Assessment of baseline and allocation to intervention? | Y | Y | Y | P | Y | N | P* | Y | N | N | na | na |
| Assessment of outcomes? | Y | Y | Y | P | Y | P | P | Y | N | N | P | P |
| Generation of hypotheses? | Y | Y | Y | Y | Y | Y | Y | Y | P | P | P | na |
| On what variables was comparability between groups assessed: | ||||||||||||
| Potential confounders? | P | P | P | P | P | P | P | P | P | P | N | na |
| Baseline assessment of outcome variables? | P | P | P | Y | P | P | P | N | N | N | N | na |
Y = Yes; P = Possibly; P* = Possible for one group only; N = No; na = not applicable. NB: Note that ‘possibly’ is used in the table to indicate cells where either ‘Y’ or ‘N’ may be the case. It should not be used as a response option when applying the checklist; if uncertain, the response should be ‘can’t tell’
RCT = Randomized controlled trial; Q‐RCT = Quasi‐randomized controlled trial; NRCT = Non‐randomized controlled trial; CBA = Controlled before‐and‐after study; PCS = Prospective cohort study; RCS = Retrospective cohort study; HCT = Historically controlled trial; NCC = Nested case‐control study; CC = Case‐control study; XS = Cross‐sectional study; BA = Before‐and‐after comparison; CR/CS = Case report/Case series.
Appendix 6. Newcastle ‐ Ottawa quality assessment scale case control studies
A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for comparability.
Selection
-
Is the case definition adequate?
yes, with independent validation *
yes, e.g. record linkage or based on self reports
no description
-
Representativeness of the cases
consecutive or obviously representative series of cases *
potential for selection biases or not stated
-
Selection of controls
community controls *
hospital controls
no description
-
Definition of controls
no history of disease (endpoint) *
no description of source
Comparability
-
Comparability of cases and controls on the basis of the design or analysis
study controls for _______________ (Select the most important factor.) *
study controls for any additional factor * (This criteria could be modified to indicate specific control for a second important factor.)
Exposure
-
Ascertainment of exposure
secure record (e.g. surgical records) *
structured interview where blind to case/control status *
interview not blinded to case/control status
written self report or medical record only
no description
-
Same method of ascertainment for cases and controls
yes *
no
-
Non‐response rate
same rate for both groups *
non respondents described
rate different and no designation
Appendix 7. Newcastle ‐ Ottawa quality assessment scale cohort studies
A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for comparability.
Selection
-
Representativeness of the exposed cohort
truly representative of the average _______________ (describe) in the community *
somewhat representative of the average ______________ in the community *
selected group of users e.g. nurses, volunteers
no description of the derivation of the cohort
-
Selection of the non exposed cohort
drawn from the same community as the exposed cohort *
drawn from a different source
no description of the derivation of the non exposed cohort
-
Ascertainment of exposure
secure record (e.g. surgical records) *
structured interview *
written self report
no description
-
Demonstration that outcome of interest was not present at start of study
yes *
no
Comparability
-
Comparability of cohorts on the basis of the design or analysis
study controls for _____________ (select the most important factor) *
study controls for any additional factor * (This criteria could be modified to indicate specific control for a second important factor)
Outcome
-
Assessment of outcome
independent blind assessment *
record linkage *
self report
no description
-
Was follow‐up long enough for outcomes to occur
yes (select an adequate follow up period for outcome of interest) *
no
-
Adequacy of follow up of cohorts
complete follow up ‐ all participants accounted for *
participants lost to follow up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow up, or description provided of those lost) *
follow up rate < ____% (select an adequate %) and no description of those lost
no statement
Data and analyses
Comparison 1. Methadone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity post intervention | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 1.1 Low‐dose phase | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 High‐dose phase | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Methadone vs placebo, Outcome 1 Pain intensity post intervention.
Comparison 2. Methadone vs active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain reduction | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Methadone vs active control, Outcome 1 Pain reduction.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Morley 2003.
| Methods | Randomized, double‐blind, placebo‐controlled cross‐over. Two phases, each 20 days. | |
| Participants | 19 participants (13 men, 6 women) with nonmalignant neuropathic pain lasting > 3 months. | |
| Interventions | Phase I: Methadone oral: 5 mg twice daily alternating with placebo on odd days & rest on even days Phase II: Methadone oral: 10 mg twice daily alternating with placebo on odd days & rest on even days | |
| Outcomes | All outcomes assessed each evening in patient diaries. Maxium and average pain intensity, pain relief (VAS). Adverse effects with severity (mild, moderate, severe). Any additional "prn" medications required |
|
| Notes | For each phase, results of 5 days with active intervention were compared with results of 5 days with placebo. Participants had neuropathic pain that had not been satisfactorily relieved by other interventions or by current or previous drug regimens. Participants were permitted to continue with concurrent medications, some of which were opioids. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eight replications of a Latin square design |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medication containers appeared identical, and medications "were not distinguishable by taste or appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only participants completing study were analyzed. One participant withdrew at end of low‐dose phase due to intercurrent illness. Six participants withdrew during high dose phase due to severe nausea ‐ three while taking placebo, three while taking methadone. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section, although severity of adverse effects not presented. |
Quang‐Cantagrel 2000.
| Methods | "Retrospective chart review" containing mostly elements of a case series. Patients initially assigned to one of four long‐acting opioids: sustained‐release morphine or oxycodone; methadone; or transdermal fentanyl. Patients were switched to (an) alternative opioid(s) if first (or subsequent) opioids were ineffective or intolerable. | |
| Participants | 86 patients (50 women and 36 men) with diverse chronic non‐cancer pain diagnoses. | |
| Interventions | Initial opioid: methadone (starting dose: 5 mg to 20 mg four times a day, titrating up in 5 mg increments), sustained‐release morphine or oxycodone, or transdermal fentanyl. Patients could switch to any one of the other three opioids or immediate‐release oxycodone, levorphanol or hydrocodone. Mean methadone dose from initial opioid = 35.4 mg. | |
| Outcomes | For each rotation, number of patients: with at least 50% pain relief; switching opioids due to intolerable side effects (> 30 on a 0 to 100 scale); switching due to lack of effectiveness (less than 50% pain relief); with any side effect. | |
| Notes | Non‐RCT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised see Table 1 and Appendix 6 for quality assessment and risk of bias |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible due to study design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
Raja 2002.
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled, cross‐over. Each treatment period lasted approximately 8 weeks and had a titration, maintenance, and taper phase. The treatment periods were separated by a 1‐week drug free, washout period. | |
| Participants | 76 participants (26 received methadone) with postherpetic neuralgia, pain persisting ≥ 3 months after resolution of cutaneous lesions | |
| Interventions | Morphine oral: 15 mg/day to 240 mg/day or methadone oral 5 mg/day to 80 mg/day (means 91 ± 49.3 mg/day and 15 ± 2.0 mg/day, respectively) Nortriptyline or desipramine: 10 mg/day to 160 mg/day (means 89 ± 27.1 mg/day and 63 ± 3.6 mg/day, respectively) Placebo | |
| Outcomes | Primary outcomes: pain intensity, pain relief, cognitive function (symbol substitution task) Secondary outcomes: physical functioning, sleep, mood, side effects, treatment preference Pain intensity (0 to 10 NRS) and pain relief (0 to 100 NRS) values were collected by twice‐weekly telephone interviews during the trial. All other outcome measures were obtained during clinic visits at the end of the drug‐free baseline period and at the end of the maintenance phase for each drug. |
|
| Notes | Study compared opioid (morphine or methadone) vs tricyclic antidepressant (nortriptyline or desipramine) vs placebo. Participants received methadone only if they did not tolerate morphine. Separate data (morphine or methadone) only presented for one outcome: reduction in pain (baseline to end of maintenance period). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was computer generated by the biostatistician" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The pharmacist formulated the study drugs in identical gel capsules to maintain the blinding. All investigators were blinded to the drug treatments during the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis employed. For participants who did not complete a treatment period, the last three available pain ratings were used. Number of participants who did not complete methadone phase not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altier 2001 | Case study |
| Altier 2005 | No comparator group |
| Arnaert 2006 | Patient interview with no pain outcome |
| Arner 1988 | Only one participant received methadone |
| Beaver 1967 | Cancer patients |
| Bendiksen 2007 | No comparator group |
| Berken 1982 | Case study |
| Bouckoms 1992 | Only 7 participants received methadone |
| Byas‐Smith 2005 | Various opioids administered ‐ methadone data not presented separately |
| Byrne 2001 | Letter to editor with no subject data |
| Cruciani 2005 | No comparator group |
| Flavell Matts 1964 | Mixed population ‐ only nine participants in methadone group had chronic non‐cancer pain |
| Fowle 1978 | Letter to the editor with no subject data |
| France 1984 | Only four participants received methadone |
| Fredheim 2006 | No comparator group |
| Fredheim 2007 | No pain outcome |
| Gagnon 2003 | No comparator group |
| Gallagher 2007 | Only three participants received methadone |
| Gardner‐Nix 1996 | Only five participants received methadone |
| Green 1996 | No comparator group |
| Haythornthwaite 1998 | Various opioids administered ‐ methadone data not presented separately |
| Krebs 2011 | No pain outcome |
| Lockwood 2004 | Letter to the editor with no subject data |
| Manchikanti 2005 | No pain outcome |
| Manchikanti 2009 | No pain outcome |
| McNulty 2000 | Data not provided |
| Mironer 1999 | No comparator group |
| Morley 1993 | Only five participants received methadone |
| Moulin 2005 | No comparator group |
| Naliboff 2011 | Various opioids administered ‐ methadone data not presented separately |
| Peng 2008 | No comparator group |
| Robbins 1996 | No comparator group |
| Robbins 1997 | No comparator group |
| Robbins 1999 | Various opioids administered ‐ methadone data not presented separately |
| Robbins 2009 | No comparator group |
| Rothrock 1999 | No comparator group |
| Saper 2004 | No comparator group |
| Schofferman 1999 | Only 8 participants received methadone |
| Shir 2001 | No comparator group |
| Sjøgren 2000 | No pain outcome |
| Sprenger 2008 | Case study |
| Taylor 2000 | No pain outcome |
| Urban 1986 | No comparator group |
| Walmsley 2010 | No comparator group |
| Watson 2010 | Various opioids administered ‐ methadone data not presented separately |
| Webster 2008 | No pain outcome |
Differences between protocol and review
None
Contributions of authors
EM and SH: Conceptualized the research design for this review. Both screened the search results, organized the retrieved studies, screened the retrieved studies for possible inclusion, assessed the risk of bias of the included studies, extracted data from the studies, performed data analysis, entered data into Revman and wrote the full review. SH will be responsible for writing the update of the review.
AL: Co‐designed the review. He acted as mediator when agreement could not be reached between EM and SH regarding possible inclusion of retrieved studies, and assisted in writing the full review.
SH and EM have contributed equally in preparing this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
SH is supported by a grant from the Fulbright Program, United States Department of State, USA.
Financial
-
EM is supported by the Richard Saltonstall Charitable Foundation, USA.
Financial
Declarations of interest
None declared
Deceased
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Morley 2003 {published data only}
- Morley JS, Bridson J, Nash TP, Miles JB, White S, Makin MK. Low‐dose methadone has an analgesic effect in neuropathic pain: a double‐blind randomized controlled crossover trial. Palliative Medicine 2003;17:576‐87. [DOI] [PubMed] [Google Scholar]
Quang‐Cantagrel 2000 {published data only}
- Quang‐Cantagrel ND, Wallace MS, Magnuson SK. Opioid substitution to improve the effectiveness of chronic noncancer pain control: a chart review. Anesthesia and Analgesia 2000;90:933‐7. [DOI] [PubMed] [Google Scholar]
Raja 2002 {published data only}
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo‐controlled trial. Neurology 2002;59:1015‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altier 2001 {published data only}
- Altier N, Dion D, Boulanger A, Choiniere M. Successful use of methadone in the treatment of chronic neuropathic pain arising from burn injuries: a case‐study. Burns 2001;27:771‐5. [DOI] [PubMed] [Google Scholar]
Altier 2005 {published data only}
- Altier N, Dion D, Boulanger A, Choiniere M. Management of chronic neuropathic pain with methadone: a review of 13 cases. Clinical Journal of Pain 2005;21:364‐9. [DOI] [PubMed] [Google Scholar]
Arnaert 2006 {published data only}
- Arnaert AC, Ciccotosto G. Response phases in methadone treatment for chronic nonmalignant pain. Pain Management Nursing 2006;7:23‐30. [DOI] [PubMed] [Google Scholar]
Arner 1988 {published data only}
- Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain 1988;33:11‐23. [DOI] [PubMed] [Google Scholar]
Beaver 1967 {published data only}
- Beaver WT, Wallenstein SL, Houde RW, Rogers A. A clinical comparison of the analgesic effects of methadone and morphine administered intramuscularly, and of orally and parenterally administered methadone. Clinical Pharmacology & Therapeutics 1967;8:415‐26. [DOI] [PubMed] [Google Scholar]
Bendiksen 2007 {published data only}
- Bendiksen A, McGehee E, Handberg G. Methadone in the treatment of chronic non‐malignant pain [Metadon til behandlingaf kroniske ikkemaligne smerter]. Ugeskrift for Laeger 2007;169:1568‐72. [PubMed] [Google Scholar]
Berken 1982 {published data only}
- Berken GH, Stone MM, Stone SK. Methadone: an effective agent in managing intractable pain as a symptom of psychotic anger. Annals of the New York Academy of Sciences 1982;398:83‐6. [DOI] [PubMed] [Google Scholar]
Bouckoms 1992 {published data only}
- Bouckoms AJ, Masand P, Murray GB, Cassem EH, Stern TA, Tesar GE. Chronic nonmalignant pain treated with long‐term oral narcotic analgesics. Annals of Clinical Psychiatry 1992;4:185‐92. [Google Scholar]
Byas‐Smith 2005 {published data only}
- Byas‐Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clinical Journal of Pain 2005;21:345‐52. [DOI] [PubMed] [Google Scholar]
Byrne 2001 {published data only}
- Byrne A. Opioids in chronic non‐malignant pain. Don't forget methadone for chronic pain. BMJ 2001;323:572. [PubMed] [Google Scholar]
Cruciani 2005 {published data only}
- Cruciani RA, Sekine R, Homel P, Lussier D, Yap Y, Suzuki Y, et al. Measurement of QTc in patients receiving chronic methadone therapy. Journal of Pain and Symptom Management 2005;29:385‐91. [DOI] [PubMed] [Google Scholar]
Flavell Matts 1964 {published data only}
- Flavell Matts SG, Swan CH, Wharton BA. Double blind trial of dextromoramide, methadone and pethidine in the treatment of severe pain. Postgraduate Medical Journal 1964;40:103‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowle 1978 {published data only}
- Fowle AS, Letley E. A comparison of diamorphine‐with‐cocaine and methadone. British Journal of Clinical Pharmacology 1978;5:527‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
France 1984 {published data only}
- France RD, Urban BJ, Keefe FJ. Long‐term use of narcotic analgesics in chronic pain. Social Science and Medicine 1984;19:1379‐82. [DOI] [PubMed] [Google Scholar]
Fredheim 2006 {published data only}
- Fredheim OMS, Kaasa S, Dale O, Klepstad P, Landrø NI, Borchgrevink PC. Opioid switching from oral slow release morphine to oral methadone may improve pain control in chronic non‐malignant pain: a nine‐month follow‐up study. Palliative Medicine 2006;20:35‐41. [DOI] [PubMed] [Google Scholar]
Fredheim 2007 {published data only}
- Fredheim OMS, Borchgrevink PC, Klepstad P, Kaasa S, Dale O. Long term methadone for chronic pain: a pilot study of pharmacokinetic aspects. European Journal of Pain 2007;11:599‐604. [DOI] [PubMed] [Google Scholar]
Gagnon 2003 {published data only}
- Gagnon BA, Almahrezi A, Schreier G. Methadone in the treatment of neuropathic pain. Pain Research & Management 2003;8:149‐54. [DOI] [PubMed] [Google Scholar]
Gallagher 2007 {published data only}
- Gallagher RM, Welz‐Bosna M, Gammaitoni A. Assessment of dosing frequency of sustained‐release opioid preparations in patients with chronic nonmalignant pain. Pain Medicine 2007;8:71‐4. [DOI] [PubMed] [Google Scholar]
Gardner‐Nix 1996 {published data only}
- Gardner‐Nix JS. Oral methadone for managing chronic nonmalignant pain. Journal of Pain and Symptom Management 1996;11:321‐8. [DOI] [PubMed] [Google Scholar]
Green 1996 {published data only}
- Green J, Hickey S, Ansbacher S, Kartsonis P. Methadone use in a small series of selected patients with chronic nonmalignant pain. Pain Digest 1996;6:3‐6. [Google Scholar]
Haythornthwaite 1998 {published data only}
- Haythornthwaite JA, Menefee LA, Quatrano‐Piacentini AL, Pappagallo M. Outcome of chronic opioid therapy for non‐cancer pain. Journal of Pain and Symptom Management 1998;15:185‐94. [DOI] [PubMed] [Google Scholar]
Krebs 2011 {published data only}
- Krebs EEB, Becker WC, Zerzan J, Bair MJ, McCoy K, Hui S. Comparative mortality among Department of Veterans Affairs patients prescribed methadone or long‐acting morphine for chronic pain. Pain 2011;152:1789‐95. [DOI] [PubMed] [Google Scholar]
Lockwood 2004 {published data only}
- Lockwood AH. Daily scheduled opioids for intractable head pain: Long‐term observations of a treatment program. Neurology 2004;63:2459. [DOI] [PubMed] [Google Scholar]
Manchikanti 2005 {published data only}
- Manchikanti L, Manchukonda R, Pampati V, Damron KS. Evaluation of abuse of prescription and illicit drugs in chronic pain patients receiving short‐acting (hydrocodone) or long‐acting (methadone) opioids. Pain Physician 2005;8:257‐61. [PubMed] [Google Scholar]
Manchikanti 2009 {published data only}
- Manchikanti L, Manchikanti KN, Pampati V, Cash KA. Prevalence of side effects of prolonged low or moderate dose opioid therapy with concomitant benzodiazepine and/or antidepressant therapy in chronic non‐cancer pain. Pain Physician 2009;12:259‐67. [PubMed] [Google Scholar]
McNulty 2000 {published data only}
- McNulty JP. Chronic pain: levorphanol, methadone, and the N‐methyl‐D‐aspartate receptor. Journal of Palliative Medicine 2009;12:765‐6. [DOI] [PubMed] [Google Scholar]
Mironer 1999 {published data only}
- Mironer YE, Haasis JC III, Chapple IT, Somerville JJ, Barsanti JM. Successful use of methadone in neuropathic pain:a multicenter study by the National Forum of Independent Pain Clinicians. Pain Digest 1999;9:191‐3. [Google Scholar]
Morley 1993 {published data only}
- Morley JS, Watt JW, Wells JC, Miles JB, Finnegan MJ, Leng G. Methadone in pain uncontrolled by morphine. Lancet 1993;342:1243. [DOI] [PubMed] [Google Scholar]
Moulin 2005 {published data only}
- Moulin DE, Palma D, Watling C, Schulz V. Methadone in the management of intractable neuropathic noncancer pain. Canadian Journal of Neurological Sciences 2005;32:340‐3. [DOI] [PubMed] [Google Scholar]
Naliboff 2011 {published data only}
- Naliboff BDW, Wu SM, Schieffer B, Bolus R, Pham Q, Baria A, et al. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. Journal of Pain 2011;12:288‐96. [DOI] [PubMed] [Google Scholar]
Peng 2008 {published data only}
- Peng PT, Tumber P, Stafford M, Gourlay D, Wong P, Galonski M, et al. Experience of methadone therapy in 100 consecutive chronic pain patients in a multidisciplinary pain center. Pain Medicine 2008;9:786‐94. [DOI] [PubMed] [Google Scholar]
Robbins 1996 {published data only}
- Robbins L. Daily opioids (methadone) for refractory chronic daily headache. Headache Quarterly 1996;7:39‐42. [Google Scholar]
Robbins 1997 {published data only}
- Robbins L. Long‐acting opioids (methadone) for refractory chronic daily headache:Quality of life assessment. Headache Quarterly 1997;8:234‐6. [Google Scholar]
Robbins 1999 {published data only}
- Robbins L. Long‐acting opioids for severe chronic daily headache. Headache Quarterly 1999;10:135‐40. [Google Scholar]
Robbins 2009 {published data only}
- Robbins L. Long‐acting opioids for refractory chronic migraine. Practical Pain Management 2009;July/August:45‐54. [Google Scholar]
Rothrock 1999 {published data only}
- Rothrock JF. Management of chronic daily headache utilizing a uniform treatment pathway. Headache 1999;39:650‐3. [DOI] [PubMed] [Google Scholar]
Saper 2004 {published data only}
- Saper JR, Lake AE III, Hamel RL, Lutz TE, Branca B, Sims DB, et al. Daily scheduled opioids for intractable head pain. Long‐term observations of a treatment program. Neurology 2004;62:1687‐94. [DOI] [PubMed] [Google Scholar]
Schofferman 1999 {published data only}
- Schofferman J. Long‐term opioid analgesic therapy for severe refractory lumbar spine pain. The Clinical Journal of Pain 1999;15:136‐40. [DOI] [PubMed] [Google Scholar]
Shir 2001 {published data only}
- Shir Y, Rosen G, Zeldin A, Davidson EM. Methadone is safe for treating hospitalized patients with severe pain. Canadian Journal of Anaesthesia 2001;48:1109‐13. [DOI] [PubMed] [Google Scholar]
Sjøgren 2000 {published data only}
- Sjøgren P, Thomsen AB, Olsen AK. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long‐term oral opioid therapy. Journal of Pain and Symptom Management 2000;19:100‐8. [DOI] [PubMed] [Google Scholar]
Sprenger 2008 {published data only}
- Sprenger T, Seifert CL, Miederer M, Valet M, Tolle TR. Successful prophylactic treatment of chronic cluster headache with low‐dose levomethadone. Journal of Neurology 2008;255:1832‐3. [DOI] [PubMed] [Google Scholar]
Taylor 2000 {published data only}
- Taylor WF, Finkel AG, Robertson KR, Anderson AC, Toomey TC, Abashian SA, et al. Methadone in the treatment of chronic nonmalignant pain: a 2‐year follow‐up. Pain Medicine 2000;1:254‐9. [DOI] [PubMed] [Google Scholar]
Urban 1986 {published data only}
- Urban BJ, France RD, Steinberger EK, Scott DL, Maltbie AA. Long‐term use of narcotic/antidepressant medication in the management of phantom limb pain. Pain 1986;24:191‐6. [DOI] [PubMed] [Google Scholar]
Walmsley 2010 {published data only}
- Walmsley R, Robson P, Lee MA. Use of methadone for uncontrolled pain: an alternative dosing regimen. Journal of Pain and Symptom Management 2010;40:e3‐4. [DOI] [PubMed] [Google Scholar]
Watson 2010 {published data only}
- Watson CPN, Watt‐Watson J, Chipman M. The long‐term safety and efficacy of opioids: a survey of 84 selected patients with intractable chronic noncancer pain. Pain Research and Management 2010;15:213‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2008 {published data only}
- Webster LR, Choi Y, Desai H, Webster L, Grant BJB. Sleep‐disordered breathing and chronic opioid therapy. Pain Medicine 2008;9:425‐32. [DOI] [PubMed] [Google Scholar]
Additional references
Blyth 2003
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Chronic pain, work performance and litigation. Pain 2003;103:41‐7. [DOI] [PubMed] [Google Scholar]
Breivik 2006
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain 2006;10:287‐333. [DOI] [PubMed] [Google Scholar]
CDC 2012
- Centers for Disease Control and Prevention. Prescription painkiller overdoses: use and abuse of methadone as a painkiller. http://www.cdc.gov/vitalsigns/MethadoneOverdoses/index.html (accessed 16 July 2012).
Chou 2009
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. Journal of Pain 2009;10:113‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chugh 2008
- Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community‐based evaluation of sudden death associated with therapeutic levels of methadone. American Journal of Medicine 2008;121:66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.0. Chichester: John Wiley & Sons, 2008:243‐96. [Google Scholar]
Eisenberg 2006
- Eisenberg E, McNicol E, Carr DB. Opioids for neuropathic pain. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006146] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Fredheim 2008
- Fredheim OM, Kaasa S, Fayers P, Saltnes T, Jordhoy M, Borchgrevink PC. Chronic non‐malignant pain patients report as poor health‐related quality of life as palliative cancer patients. Acta Anaesthesiologica Scandinavica 2008;52:143‐8. [DOI] [PubMed] [Google Scholar]
Furlan 2006
- Furlan AD, Sandoval JA, Mailis‐Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. Canadian Medical Association Journal 2006;174:1589‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gale 2006
- Longe JL, editor. The Gale Encyclopedia of Medicine. 3rd Edition. Thomson Gale, 1996. [Google Scholar]
IASP 2003
- Harstall C, Ospina M. How prevalent is chronic pain?. Pain: Clinical Updates 2003;11(2):1‐4. [Google Scholar]
Kalso 2004
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain 2004;112:372‐80. [DOI] [PubMed] [Google Scholar]
Krantz 2009
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Annals of Internal Medicine 2009;150:378‐95. [DOI] [PubMed] [Google Scholar]
Manchikanti 2008
- Manchikanti L, Singh A. Therapeutic opioids: a ten‐year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008;11:S63‐88. [PubMed] [Google Scholar]
Manchikanti 2011
- Manchikanti LA, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, et al. A systematic review of randomized trials of long‐term opioid management for chronic non‐cancer pain. Pain Physician 2011;14:91‐121. [PubMed] [Google Scholar]
McNicol 2008
- McNicol E. Opioid side effects and their treatment in patients with chronic cancer and noncancer pain. Journal of Pain & Palliative Care Pharmacotherapy 2008;22:270‐81. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;7:209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69:374‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386‐9. [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: Imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153:265‐8. [DOI] [PubMed] [Google Scholar]
NAS 2001
- National Academies of Science and Institute of Medicine. Musculoskeletal disorders and the workplace: Low back pain and upper extremities. National Academies Press, 2001. [PubMed] [Google Scholar]
Nicholson 2007
- Nicholson AB. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003971] [DOI] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Sandoval 2005
- Sandoval JA, Furlan AD, Mailis‐Gagnon A. Oral methadone for chronic noncancer pain: a systematic literature review of reasons for administration, prescription patterns, effectiveness, and side effects. Clinical Journal of Pain 2005;21:503‐12. [DOI] [PubMed] [Google Scholar]
Shields 2007
- Shields LB, Hunsaker Iii JC, Corey TS, Ward MK, Stewart D. Methadone toxicity fatalities: a review of medical examiner cases in a large metropolitan area. Journal of Forensic Sciences 2007;52:1389‐95. [DOI] [PubMed] [Google Scholar]
Tsang 2008
- Tsang A, Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression‐anxiety disorders. Journal of Pain 2008;9:883‐91. [DOI] [PubMed] [Google Scholar]
Verhaak 1998
- Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain 1998;77:231‐9. [DOI] [PubMed] [Google Scholar]


