Abstract
Background
Targeted temperature management (also known under 'therapeutic hypothermia', 'induced hypothermia'", or 'cooling') has been shown to be beneficial for neurological outcome in patients who have had successful resuscitation from sudden cardiac arrest, but it remains unclear when this intervention should be initiated.
Objectives
To assess the effects of pre‐hospital initiation of cooling on survival and neurological outcome in comparison to in‐hospital initiation of cooling for adults with pre‐hospital cardiac arrest.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL, BIOSIS, and three trials registers from inception to 5 March 2015, and carried out reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria
We searched for randomized controlled trials (RCTs) in adults with out‐of‐hospital cardiac arrest comparing cooling in the pre‐hospital setting to in‐hospital cooling. Our primary outcomes were survival and neurological outcome; our secondary outcomes were adverse events, quality of life, and length of stay in the intensive care unit (ICU) and in the hospital.
Data collection and analysis
We used Cochrane's standard methodological procedures.
Main results
We included seven RCTs (2369 participants randomized) on the induction of pre‐hospital cooling in comparison to in‐hospital cooling. There was considerable methodological heterogeneity and risk of bias mainly due to deficits in the administration of cooling, therefore we refrained from pooling the results for survival and neurological outcome and we presented the results for each study separately. Adverse events were rare: based on four studies with 1713 adults pre‐hospital induction of cooling may increase the risk of cardiac re‐arrests. Risk of bias within the seven individual studies was generally moderate. Overall the quality of the evidence was very low. This was mainly driven by inconsistency and low precision.
Authors' conclusions
Currently, there is no convincing evidence to clearly delineate beneficial or harmful effects of pre‐hospital induction of cooling in comparison to in‐hospital induction of cooling. This conclusion is based on very low quality evidence.
Plain language summary
Should patients experiencing sudden cardiac death be cooled to lower their body temperature prior to or after admission to hospital?
Review question
We reviewed the current available evidence in order to answer the question of whether early cooling in people who receive basic life support for sudden cardiac death influences survival and brain damage compared to cooling that is started after their admission to hospital. Early cooling means the cooling of the person quickly by the ambulance staff, paramedics or doctors, in the field. We included seven studies meeting the Cochrane requirements in this review.
Background
Population
This review deals with people who receive basic life support for sudden cardiac death. Sudden cardiac death means that the heart and subsequently the circulation stops. If these people do not receive early cardiopulmonary resuscitation then their brain cells begin to be irreversibly damaged and subsequently they die. If basic life support is successful, one form of therapy that may help to prevent further cell damage is to cool the body for several hours to 32°C to 36°C. This therapy has been shown to be beneficial in reducing brain damage and is recommended in international guidelines for the treatment of people that have been brought back to life after sudden cardiac death.
Intervention
The optimal timing for the initiation of cooling is unclear. This review compares people who had their cooling therapy started before hospital admission to those who had their cooling therapy started after admission to a hospital.
Outcomes
The effects of the intervention were measured by survival and brain damage, together with side effects, quality of life, and length of hospital stay.
Search date
We completed the review searches in March 2015.
Study characteristics
The seven studies included had a total of 2369 participants and compared the effects of cooling before and after being admitted to the hospital. The mean age of the participants in the studies was between 59 and 68 years with the majority being male. People that were not included in the trials were generally those with trauma, those with a terminal disease, those at the natural end of their life, pregnant women, and those that already had a low body temperature.
Study funding sources
Two out of seven studies were funded by the medical industry, four received funding from the government or non‐profit organizations, and one study did not receive any funding.
Key results
None of the studies found any evidence for a benefit of pre‐hospital cooling versus in‐hospital cooling. However, we discovered that in almost all studies a relevant amount of participants did not receive pre‐hospital cooling or in‐hospital cooling or cooling according to the guidelines at all. The reasons for this were not clearly stated. The question of whether the decision to cool participants may have been influenced by other factors cannot be reliably answered. Proper design and conduct of the included studies was of concern, therefore to avoid making misleading interpretations we did not pool the results of the single studies. We found that in adults that received pre‐hospital cooling the heart was slightly more likely to stop again before they were admitted to the hospital.
Quality of the evidence
Many of the included studies were of limited use because they focused on the practicability and safety of pre‐hospital cooling without specifically emphasising cooling therapy. Other factors that contributed to a downgrading of the quality of the evidence were that the information came from different study populations and from different time points of applying pre‐hospital cooling. In addition, there was risk of bias within the studies. The quality of the individual studies was moderate. In summary, the quality of the evidence to answer our review question was very low.
Summary of findings
Summary of findings for the main comparison. Pre‐hospital cooling compared to in‐hospital cooling for survival, neuroprotection, and adverse events after out‐of‐hospital cardiac arrest.
| Survival, neurological outcome, and adverse events: pre‐hospital cooling compared to in‐hospital cooling after out‐of‐hospital cardiac arrest | ||||||
| Patient or population: out‐of‐hospital cardiac arrest Settings: emergency medicine and intensive care, worldwide Intervention: pre‐hospital cooling Comparison: in‐hospital cooling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| I n‐hospital cooling | Pre‐hospitalcooling | |||||
| Survival and good neurological outcome | Study population | Not pooled | 2369 randomized participants (7 RCTs) (Bernard 2010; Bernard 2012; Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007; Kim 2014) |
⊕⊝⊝⊝ VERY LOW 1,2,3,4,5,6,7 | — | |
| Not pooled | Not pooled | |||||
| Not pooled | Not pooled | |||||
| Adverse events ‐ re‐arrest after randomization | Study population | RR 1.23 (1.02 to 1.48) | 1713 participants with available information
(4 studies) (Castren 2010; Kämäräinen 2009; Kim 2007; Kim 2014) |
⊕⊝⊝⊝ VERY LOW 1,2,3,4, 7 | — | |
| 183 per 1000 | 225 per 1000 (187 to 271) | |||||
| Moderate | ||||||
| 186 per 1000 | 229 per 1000 (190 to 276) | |||||
We downgraded the quality of the evidence to 'very low' for the following reasons:
1Inappropriate application of intervention or control, or both.
2Indirectness in the intervention: two studies evaluated intra‐arrest cooling while all others evaluated post‐arrest cooling (Castren 2010; Debaty 2014).
3Indirectness in the intervention: the rate of application of pre‐hospital cooling varied over all studies; up to 50% of all participants did not receive the full intervention (Bernard 2010; Bernard 2012; Kim 2007; Kim 2014); up to 16% of participants did not receive the intervention at all (Bernard 2010; Bernard 2012; Kim 2007; Kim 2014).
4Indirectness in the comparator: the rate of application of in‐hospital cooling varied over all studies; some studies did not provide information (Castren 2010; Kämäräinen 2009; Kim 2007; Kim 2014); in some only some of the participants received in‐hospital cooling (Kämäräinen 2009; Kim 2007; Kim 2014); temperature curves in some studies indicated that a relevant proportion of participants were not cooled according to the then current guidelines (Bernard 2010; Bernard 2012); in some studies the target temperature was at the upper limit of the then current guidelines (Castren 2010).
5Indirectness in the population: one study included only adults with non‐ventricular fibrillation cardiac arrest (Bernard 2010); another only ventricular fibrillation cardiac arrest (Bernard 2012); the others did not make restrictions.
6Imprecision: three studies were feasibility or pilot studies with sample sizes too small to evaluate clinical outcomes (Castren 2010; Kämäräinen 2009; Kim 2007). Due to the above described reasons we refrained from pooling the estimates, therefore we are left with the lower precision of the individual studies.
7Risk of bias within studies: three studies lacked blinding of outcome assessment, which may substantially bias the assessment of neurological outcome (Castren 2010; Kämäräinen 2009; Kim 2007); insufficient administration and continuation of the intervention and comparator/no information on administration and continuation of the intervention and comparator (see above).
Background
Cooling the body compared with no cooling has been shown to be beneficial for neurological outcome and survival in patients who have had successful resuscitation from sudden cardiac arrest (Arrich 2016), but it remains unclear when this intervention should be initiated.
Description of the condition
The incidence of out‐of‐hospital sudden cardiac arrest is not easily determined. A review of emergency medicine services (EMS)‐treated out‐of‐hospital cardiac arrest in Europe reports an incidence of 41 per 100,000 person‐years (Berdowski 2010), while in North America around 52 per 100,000 person‐years are reported (Nichol 2008). The survival rates of 9% to 11% for all‐rhythm cardiac arrests and 19% to 21% for ventricular fibrillation cardiac arrests seem to have improved in recent decades, but are still disastrously low. When the circulation stops the brain cells are directly damaged through the lack of oxygen and adenosine triphosphate (ATP), but even after the circulation is re‐established continuous damage of the brain cells happens through neuronal necrosis and apoptosis caused by a myriad of pathophysiological mechanisms, microcirculatory failure, and other factors such as pyrexia, hyperglycaemia, and seizures (Holzer 2010; Nolan 2008). After resuscitation and admission to a hospital, post‐resuscitation care aims to reduce the secondary reperfusion injuries caused by the cardiac arrest. This treatment includes early treatment of the cause of the arrest (the majority have a coronary heart disease, thus they will undergo coronary angiography with subsequent percutaneous coronary intervention (PCI) if indicated), cooling, optimization of oxygenation, ventilation, circulation and metabolism, and early seizure detection and treatment following a standardized treatment protocol (Callaway 2015; Soar 2015).
Description of the intervention
Cooling the body as the principle of targeted temperature management (formerly 'therapeutic hypothermia' or 'induced hypothermia') may improve survival and reduce the amount of neurological damage after cardiac arrest (Arrich 2016). According to recent guidelines, comatose survivors of out‐of‐hospital ventricular fibrillation cardiac arrest should be cooled with internal or external cooling techniques to a target temperature of 32°C to 36°C (patients with in‐hospital cardiac arrest or other primary rhythms may also benefit) (Callaway 2015; Soar 2015). This targeted temperature should be maintained for at least 24 hours, and after this cooling period the patients should be rewarmed at a rate of 0.25°C to 0.5°C/hour to normothermia). Pre‐hospital application of cooling refers to the reduction of body core temperature for at least 12 hours by the healthcare professionals in the field, either during resuscitation or shortly after resuscitation. Several methods are available to decrease body temperature, including surface cooling methods like ice packs, cold‐air mattresses, water‐circulating cooling pads, and pre‐cooled cooling pads. The core temperature may also be decreased by intravascular cooling catheters (Holzer 2010). Other methods include cooling caps or the application of coolant through the nose, which cools the nasal cavity and subsequently the whole body through evaporation (Castren 2010). The rapid infusion of large volumes of cold intravenous fluid immediately after return of spontaneous circulation was discouraged in the most recent guidelines (Callaway 2015; Soar 2015). However, not all methods that are available after admission to a hospital will be available or practical in the field.
Formally the intervention in this review is the time point of application of cooling. Practically, application of cooling includes significant variation and we expected study protocols and clinical routines to differ substantially. Therefore we pragmatically compared early application in the pre‐hospital phase to later application after hospital admission. However, cooling should be continued after hospital admission. Maintenance cooling and rewarming should be according to the current guidelines on resuscitation at that time and comparable to the control group.
How the intervention might work
Pathophysiologically, brain damage through hypoxia is due to two main mechanisms. The first is direct excitotoxic and ischaemic cell death leading to necrosis and apoptosis, and the second is reperfusion injury, which is also known as 'post‐resuscitation disease or syndrome' (Holzer 2010; Nolan 2008). Cooling the body inhibits numerous pathways of these two mechanisms. In vivo studies comparing different starting points of cooling are scarce, but there are a number of animal studies that have indicated that an earlier start of cooling, even during the resuscitation phase, might lead to an improved neurological outcome (Abella 2008; Janata 2010; Kuboyama 1993; Sterz 1991).
Cooling compared to no cooling has been shown to be beneficial for neurological outcome and survival in adults who have had resuscitation from sudden cardiac arrest (Arrich 2016). This evidence is mainly based on three randomized controlled trials (Bernard 2002; HACA 2002; Hachimi‐Idrissi 2001). In these trials cooling had been initiated as late as four hours after cardiac arrest. However, the question of whether an earlier cooling start may be more beneficial has been raised in many experimental and human studies. It is important to note that 'earlier' refers to different time points of cooling, before resuscitation is started (Janata 2010; Zhao 2008), during resuscitation (Abella 2004; Yannopoulos 2009), immediately after resuscitation (Kuboyama 1993) (as opposed to a 15‐minute delay), and after one hour (as opposed to a four‐hour delay) (Colbourne 1995). All of these studies showed that an earlier initiation of cooling was more beneficial. However, a recent animal study demonstrated that there seemed to be no difference as long as cooling was started less than four hours after cardiac arrest (Che 2011). Also, retrospective human data show conflicting results. Some studies showed a better neurological outcome with an earlier start of cooling or faster cooling (Sendelbach 2012; Wolff 2009), and others did not find that earlier cooling had a beneficial effect (Nielsen 2009). One cohort study even suggested that adults who reached the target temperature earlier also had a poorer prognosis (Haugk 2011). This finding can probably be explained by the fact that patients who are more severely harmed by the cardiac arrest might have compromised thermoregulation and therefore less resistance against induction of cooling (Suffoletto 2009). The dilemma of differentiating a diagnostic sign (an early outcome) from an effective intervention can only be challenged by randomized trials.
Why it is important to do this review
We have conducted this systematic review to explore the uncertainty arising from conflicting results in a number of animal and clinical studies.
Following the animal studies (Abella 2008; Janata 2010; Kuboyama 1993; Sterz 1991), a few case series were published on the potential beneficial effect of an earlier versus a delayed start for cooling, with conflicting results (Nielsen 2009; Wolff 2009).
A few randomized controlled trials have been published that evaluate the feasibility and effectiveness of cooling during resuscitation and shortly after resuscitation versus in‐hospital cooling, which is the standard care in most centres. The results were conflicting, partly due to the small study size of the pilot studies and maybe partly due to methodological shortcomings. Some showed a significant effect in the subgroups (Castren 2010; Kim 2007).
Cooling may improve neurological outcome after resuscitation from sudden cardiac arrest even when its application is delayed. It is important to find out if timing of cooling initiation has an effect on clinical outcomes. If a pre‐hospital start of cooling provides a further improvement of neurological outcome, this effect should not be missed because of methodological shortcomings or small sample sizes of the preceding randomized controlled trials. If there is no further gain with a pre‐hospital cooling start, the extra effort to start cooling in the field could be spared. This would help decision makers and guideline committees in advising healthcare professionals. Currently the guidelines comment that "Early cooling strategies, other than rapid infusion of large volumes of cold IV fluid, and cooling during CPR in the prehospital setting have not been studied adequately" (Callaway 2015; Soar 2015). For the scientific community, a structured evaluation of the available evidence will provide a basis on which to decide whether further studies should be undertaken in this field and will serve as the basis for sample size calculations.
Objectives
To assess the effects of pre‐hospital initiation of cooling on survival and neurological outcome in comparison to in‐hospital initiation of cooling for adults with pre‐hospital cardiac arrest.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized and 'quasi‐randomized' controlled trials. 'Quasi‐randomized' refers to allocation procedures such as alternating days, odd and even days and the like, which we planned to include because of the relative novelty of the intervention and the expected low number of trials available to date. We planned to exclude cross‐over studies given the condition and the nature of the outcomes assessed.
Types of participants
We included studies in adults with out‐of‐hospital cardiac arrest who received targeted temperature management.
Types of interventions
We compared pre‐hospital induction of cooling to in‐hospital induction of cooling.
Pre‐hospital induction of cooling (intervention): cooling during resuscitation or shortly after resuscitation in the out‐of‐hospital setting.
Later induction of cooling (standard therapy; control): in‐hospital cooling.
Types of outcome measures
Primary outcomes
Survival: we investigated short‐term survival (closest to 30 days) and long‐term survival (closest to six months).
Neurological outcome: ideally we expected the outcome to be reported as best neurological outcome during hospital stay and in cerebral performance categories (CPC) (Cummins 1991; Jennett 1975). Good neurological outcome is usually considered if the CPC is 1 or 2. If authors grouped this outcome along with other categories or cut‐offs, or used other instruments for neurological outcome assessment, like the modified Rankin Scale, we accepted this for our meta‐analysis and planned to perform a sensitivity analysis based on the outcome definition.
We considered both survival and neurological outcome as dichotomous data at a given point in time.
Secondary outcomes
Adverse events, as reported by study authors (dichotomous data).
Adverse events related to cooling methods, as reported by study authors (dichotomous data).
Quality of life, as reported by study authors (data as reported).
Length of stay in the intensive care unit (ICU) and in the hospital, as proxies for economic outcomes (continuous data).
Availability of outcomes was not part of the study eligibility criteria. We planned to include studies that met the participant, intervention, and comparison criteria in the review even if they reported no relevant outcomes.
Search methods for identification of studies
Electronic searches
We searched the following databases (from inception to March 2015): the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), CINAHL (Appendix 4), and BIOSIS (Appendix 5), and three trials registers (EudraCT, ClinicalTrials.gov, and the International Clinical Trials Registry Platform).
We did not apply any language restrictions.
We used a search strategy for identifying randomized controlled trials (RCTs) in MEDLINE and EMBASE (Higgins 2011).
Searching other resources
In an attempt to identify further studies we searched the primary clinical trials registers (March 2015) accepted by the International Committee of Medical Journal Editors (seehttp://www.icmje.org/recommendations/browse/publishing‐and‐editorial‐issues/clinical‐trial‐registration.html). We also asked experts in the field whether they were aware of any ongoing, unpublished, or published trials on this subject. We also searched the reference lists of included studies and other reviews on the topic.
Data collection and analysis
Selection of studies
We imported all retrieved results into EndNote (version X7, Thomson Corporation) and eliminated any duplicates. Two authors each (CH + MH and A‐M W + JA) independently scanned titles and abstracts for relevance. For all references that were not excluded by both authors at this stage, we retrieved the full texts and examined them for compliance with the inclusion criteria. We linked multiple reports of the same study. We resolved discrepancies by discussion or by involving a third author as an arbiter (HH).
Data extraction and management
Two authors each (CH + MH and A‐M W + JA) independently extracted all relevant data into a predefined form (Appendix 6). We resolved discrepancies by discussion or by involving a third author as arbiter (HH). We then entered data into the Cochrane software program Review Manager (RevMan 5.3).
Assessment of risk of bias in included studies
To assess the internal validity of the identified trials, we used a domain‐based evaluation, assessing random sequence generation, allocation concealment, blinding of outcome assessment (for neurological outcome and quality of life), incomplete outcome data (primary outcome), selective reporting (neurological outcome), and exclusion of randomized participants from the analysis (primary outcome).
Two authors (as named above) independently extracted and tabulated all relevant information. We resolved discrepancies by discussion or by involving a third author as arbiter.
We assumed that blinding of participants and personnel was not relevant, as participants are unconscious and cooling interventions are almost impossible to blind.
We assessed the domains as low risk of bias, unclear, or high risk of bias.
Measures of treatment effect
The primary measure of treatment effect for the primary outcomes was the risk ratio (relative risk) for surviving and achieving good neurological recovery in participants allocated to pre‐hospital cooling when compared to in‐hospital cooling. The same applied for adverse events. For quality of life and length of hospital stay data, we used mean differences as measures of treatment effect. In the case that several instruments were used for quality of life assessment across studies, we used standardized mean differences instead.
Unit of analysis issues
Cluster‐randomized trials
For cluster‐randomized trials we planned to use estimates that allow for the clustered structure of the data. In the absence of adequate estimates we would have used appropriate approximations.
Studies with multiple treatment groups
In the case of multiple treatments (for example intra‐arrest cooling versus pre‐hospital arrest cooling versus in‐hospital cooling) we planned to combine the groups to create a single pair‐wise comparison, but to avoid overall estimates.
Dealing with missing data
For a negligible amount of missing data (or if they were convincingly missing at random), we analysed only the available data. We did not employ data imputation or data replacement methods.
Assessment of heterogeneity
We evaluated clinical and statistical heterogeneity. We assessed clinical heterogeneity by tabulating and informally inspecting relevant data. We only performed quantitative synthesis if clinical heterogeneity was negligible. We assessed statistical heterogeneity using the I2 statistic (Higgins 2003). We considered statistical heterogeneity to be relevant if the I2 statistic was > 50%. To investigate heterogeneity we performed subgroup analyses and the test for subgroup differences. If possible, we employed meta‐regression to further investigate heterogeneity.
Assessment of reporting biases
We planned to assess the presence of possible reporting bias using funnel plots (plotting the effect against precision) (Egger 1997), and to inspect them visually (Higgins 2011).
Data synthesis
We calculated risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous variables. For continuous variables we planned to calculate mean differences or standardized mean differences depending on the comparability of measurement methods. Generally we used random‐effects models. We used RevMan 5.3 for standard calculations and Stata 11 for meta‐regression.
Subgroup analysis and investigation of heterogeneity
We planned to investigate the following subgroups:
Efficacy of cooling methods in the intervention group and in the control group.
Intra‐arrest versus early post‐arrest cooling in the intervention group.
Duration of cardiac arrest.
Primary cardiac rhythm.
Sensitivity analysis
We planned to perform sensitivity analyses on the effect estimate by omitting studies with an overall 'high risk of bias' to assess the robustness of our estimates against within‐study bias.
If several methods of neurological outcome assessment were used we planned to investigate the robustness of the effect estimates.
Summary of findings
We used the principles of the GRADE system (Guyatt 2008), in order to assess the quality of the body of evidence associated with the specific outcomes of survival and neurological outcome in our review, and we constructed a 'Summary of findings' table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
The systematic search of databases (from inception to March 2015) resulted in 1575 hits (duplicates excluded, see Figure 1). From these, we excluded 1565 papers according to our eligibility criteria, leaving 10 papers to which we added one additional paper found in another review (Callaway 2002). From these remaining 11 papers we excluded three papers after further evaluation and discussion (Belohlavek 2013; Busch 2010; Taccone 2010); see Characteristics of excluded studies. Seven completed studies (Bernard 2010; Bernard 2012; Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007; Kim 2014), and one ongoing study (Nordberg 2013), remained for inclusion in our review.
1.
Study flow diagram.
Included studies
We included seven studies in our review (Bernard 2010; Bernard 2012; Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007; Kim 2014).
The basic points and an assessment of the quality criteria of the included studies can be found in Characteristics of included studies.
Design
All studies were randomized, controlled, parallel‐group trials.
Sample sizes
The sample sizes of the included studies were highly variable, with one small study (Kämäräinen 2009 with 43 participants), five middle‐sized studies (Kim 2007 with 125 participants; Bernard 2012 with 163 participants; Castren 2010 with 200 participants; Bernard 2010 with 234 participants; and Debaty 2014 with 245 participants), and one bigger study (Kim 2014 with 1359 participants). Four studies were aimed primarily at establishing safety and feasibility or differences in inflammation markers and calculated their sample sizes accordingly (Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007).
Setting
All studies included participants with out‐of‐hospital cardiac arrest, hence including ambulance services (Bernard 2010; Bernard 2012; Castren 2010; Debaty 2014; Kim 2007; Kim 2014), or helicopter services and staff (Kämäräinen 2009), or emergency departments of intensive or acute care units in urban areas. All were multicentre studies.
Participants
Inclusion criteria for all studies were heterogenous. Most studies included participants with out‐of‐hospital cardiac arrest due to all causes, regardless of the primary cardiac rhythm (Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007; Kim 2014). One study only included participants with a witnessed collapse (Castren 2010). The two studies by Bernard and colleagues differentiated between participants with ventricular fibrillation (Bernard 2010) and asystole and pulseless electrical activity (PEA) (Bernard 2012) as primary cardiac rhythms but were similar otherwise. In six studies temperature at study inclusion was reported and ranged between 35.2°C to 35.9°C (Bernard 2010; Bernard 2012; Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007) (see also Characteristics of included studies).
Interventions
Pre‐hospital cooling was comparable in five studies (Bernard 2010; Bernard 2012; Kämäräinen 2009; Kim 2007; Kim 2014), using up to 2 L of cold fluids as administered after resuscitation. In contrast, Castren 2010 and Debaty 2014 began with the intervention already during resuscitation, with Castren 2010 using intranasal cooling and Debaty cold fluids and external cooling. However, the actual administration of cold fluid varied considerably in all studies. The pre‐hospital patients' temperature was measured by tympanic probes (Bernard 2010; Bernard 2012; Castren 2010), and oesophageal/nasopharyngeal temperature probes (Kämäräinen 2009; Kim 2007; Kim 2014). In many studies a relevant proportion of participants received no cooling either in the pre‐hospital group (Bernard 2010; Bernard 2012; Kim 2007; Kim 2014), or during the hospital phase (Kämäräinen 2009; Kim 2007; Kim 2014). Most of the studies focused on feasibility and safety in the pre‐hospital phase and there were no reported specific protocols for 'in‐hospital cooling' (Castren 2010; Kämäräinen 2009; Kim 2007; Kim 2014), which was left to the discretion of the treating physicians. These studies also did not provide further information on the initiation of cooling, the methods, cooling rates, cooling durations, and rewarming rates in the hospital phase. Only three out of seven studies provided temperature curves showing that a relevant proportion of the participants did not receive cooling according to guidelines at the time of conduct (Bernard 2010; Bernard 2012; Debaty 2014), which limits the generalizability of the results. In summary we considered methodological heterogeneity to be a relevant limitation in this review.
Outcome
For most of the studies, the primary outcome parameters were feasibility and safety, the differences in patients' temperatures, or differences in serum concentration of inflammation markers on admission to the hospital (Castren 2010; Debaty 2014; Kämäräinen 2009; Kim 2007). All studies additionally provided hospital mortality data and some simple neurological outcome parameters evaluated at discharge like "discharge either to home or to a rehabilitation facility" (Bernard 2010; Bernard 2012), "severe neurologic deficits" (Kim 2007), "full recovery, mildly to moderately impaired, severely impaired, comatose, or dead" (Kim 2014), or the cerebral performance categories (CPC) score (Castren 2010; Debaty 2014; Kämäräinen 2009).
Excluded studies
We excluded three studies (Belohlavek 2013; Busch 2010; Taccone 2010) (see Characteristics of excluded studies). The study by Belohlavek 2013 and colleagues was not considered for inclusion in this review because combinations of treatments were investigated, which makes the estimation of the isolated effect of a single component impossible. The abstracts by Busch 2010 and Taccone 2010 were separate presentations of their centre‐specific patients as part of Castren 2010, with no additional information. We linked these references to the respective study.
Ongoing studies
We found one ongoing study that would meet our eligible criteria (Nordberg 2013). It was started in 2010 and is currently recruiting participants. The estimated completion is December 2016. For further details see Characteristics of ongoing studies.
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
Risk of bias across the individual studies was generally moderate (Figure 2; Figure 3; Characteristics of included studies). This was mainly driven by deficits in blinding the neurological outcome assessment and 'other biases', addressing the inconsistent administration of the intervention and the control. None of the studies was sufficiently designed to show equivalence or non‐inferiority, but failed to prove superiority only. For the feasibility studies this might have been appropriate, however the bigger studies used effectiveness measures like neurological outcome and survival as primary outcomes with a similar design to the feasibility studies. Here the danger of misinterpretation is considerable. Pooling of the results of these studies would have been inappropriate and would have led to an invalid interpretation of the result.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
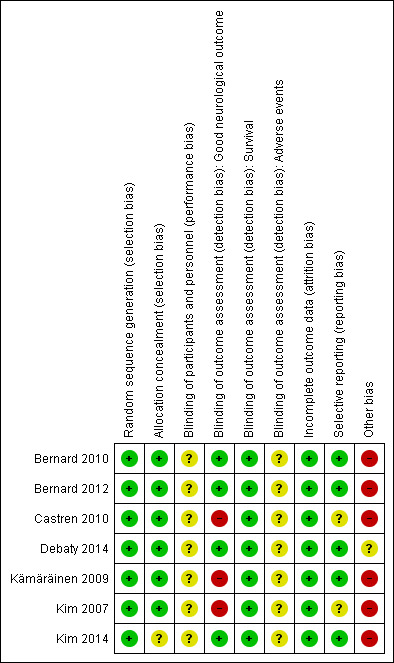
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of bias from inadequate allocation concealment was generally low. Only in one study was allocation concealment not described (Kim 2014).
Blinding
By nature in these trials blinding of staff to treatment allocation (in ambulances, helicopters, and hospitals) would have been difficult and was not done in any of the included studies. Blinding of outcome assessment was reported in four studies (Bernard 2010; Bernard 2012; Debaty 2014; Kim 2014). For all others the outcome assessors were not blinded or not reported to be. Survival may not be sensitive against possible information bias from unblinded outcome assessment. For the assessment of the neurological outcome, however, we classified unblinded assessment and missing information for this item as 'high risk'.
Incomplete outcome data
Three studies had complete outcome data for all randomized participants (Bernard 2010; Bernard 2012; Kim 2007). The remaining four reported some loss to follow‐up but numbers were either too small to have a relevant impact or numbers and reasons for exclusion were balanced between groups. We therefore rated all studies as 'low risk' of bias for incomplete outcome data.
Three studies reported on loss to follow‐up after randomization (Castren 2010; Kämäräinen 2009; Kim 2014) (see Characteristics of included studies). All studies gave reasons for the exclusions, which were either balanced between the study groups or the proportion of missing outcomes compared to the number of observed events was too low to have a clinically relevant impact on the intervention effect estimate.
Selective reporting
All expected outcomes were reported. We rated all studies 'low risk' for this item.
Other potential sources of bias
An inappropriate administration of the intervention and control might have resulted in an underestimation of a potential effect. In four studies only between 20% and 50% of all participants received the full intervention of 2000 mL of cold fluids (Bernard 2010; Bernard 2012; Kim 2007; Kim 2014). In the same studies up to 16% of all participants assigned to pre‐hospital cooling did not receive any intervention at all. In all studies the continuation of cooling or the initiation of cooling in the hospital was left to the discretion of the treating physician. In three studies only between 61% and 77% of all participants received cooling in the hospital; no detailed information was provided for these participants (Kämäräinen 2009; Kim 2007; Kim 2014). Further, only three studies provided data on actual patient temperature during treatment (Bernard 2010; Bernard 2012; Debaty 2014). Judging from the graphs in two studies the temperatures of the intervention groups rewarmed shortly after hospital admission with an average temperature of above 34°C in about half of participants in both the intervention and control groups (Bernard 2010; Bernard 2012). This indicates that around 50% of the participants did not receive cooling to the guidelines of that time. One study required a target temperature of 34°C, on the upper limit of the recommended 32°C to 34°C (Castren 2010). For most other studies no further information on the application of in‐hospital cooling or any temperature curves for their participants was provided, so it was not possible to determine if was applied appropriately in these studies (Castren 2010; Kämäräinen 2009; Kim 2007; Kim 2014).
Effects of interventions
See: Table 1
We present the results of seven randomized controlled trials for the primary endpoints of this review separately in Figure 4 and Figure 5.
4.
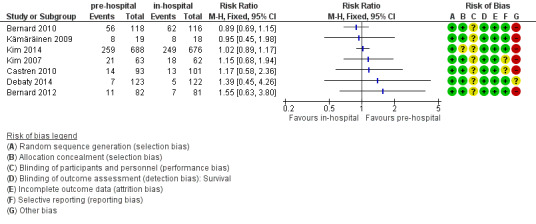
Forest plot of comparison: 2 Survival: pre‐hospital cooling versus in‐hospital cooling, outcome: 2.1 Survival.
5.
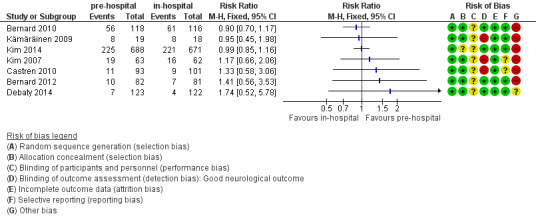
Forest plot of comparison: 1 Neurological outcome: pre‐hospital cooling versus in‐hospital cooling, outcome: 1.1 Good neurological outcome.
Primary outcomes
1. Survival
We investigated short‐term survival (closest to 30 days) and long‐term survival (closest to six months) (see Analysis 1.1). There was considerable methodological heterogeneity and risk of bias, mainly due to deficits in the administration of cooling (see Characteristics of included studies). We have therefore refrained from pooling the results for survival. Insufficient information on six‐month survival was available. Accordingly we did not perform sensitivity or subgroup analyses. Pre‐hospital cooling did not appear to have an effect on survival compared with in‐hospital cooling.
1.1. Analysis.
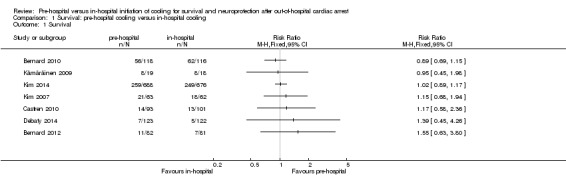
Comparison 1 Survival: pre‐hospital cooling versus in‐hospital cooling, Outcome 1 Survival.
2. Neurological outcome
There was considerable methodological heterogeneity and risk of bias, mainly due to deficits in the administration of cooling (see Characteristics of included studies). We have therefore refrained from pooling the results for neurological outcome (see Analysis 2.1). Accordingly we did not perform sensitivity or subgroup analyses. Pre‐hospital cooling did not appear to have an effect on neurological outcome compared with in‐hospital cooling in any of the included studies.
2.1. Analysis.
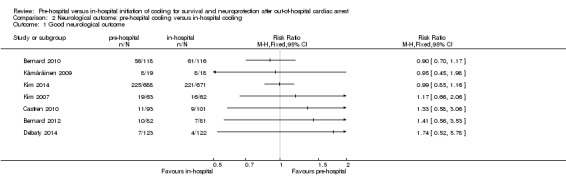
Comparison 2 Neurological outcome: pre‐hospital cooling versus in‐hospital cooling, Outcome 1 Good neurological outcome.
Secondary outcomes
1. Adverse events, as reported by study authors
We pooled the available data on adverse events. Four studies reported on re‐arrests after resuscitation (Castren 2010; Kämäräinen 2009; Kim 2007; Kim 2014). These showed a higher incidence in the pre‐hospital cooling group (risk ratio (RR) 1.23; 95% confidence interval (CI) 1.02 to 1.48; P value = 0.03). There was no effect on the incidence of pulmonary oedema (RR 1.02; 95% CI 0.67 to 1.57; P value = 0.77) (Kämäräinen 2009; Kim 2007; Kim 2014). See Table 2.
1. Adverse events: any.
| Adverse event | Studies | Participants | Statistical method | Effect estimate |
| Pulmonary oedema first evaluation after ROSC | 4 | 1457 | Risk ratio (M‐H, random, 95% CI) | 1.02 (0.67 to 1.57) |
| Pulmonary congestions | 1 | 103 | Risk ratio (M‐H, fixed, 95% CI) | 1.81 (0.17 to 19.40) |
| Cardiomegaly | 1 | 103 | Risk ratio (M‐H, fixed, 95% CI) | 0.53 (0.28 to 0.99) |
| Pleural effusions | 1 | 103 | Risk ratio (M‐H, fixed, 95% CI) | 0.91 (0.19 to 4.29) |
| Re‐arrest after randomization | 4 | 1713 | Risk ratio (M‐H, fixed, 95% CI) | 1.23 (1.02 to 1.48) |
| Acidosis | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 0.21 (0.01 to 4.49) |
| Acute myocardial infarction | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 0.36 (0.01 to 8.90) |
| Bleed | 2 | 271 | Odds ratio (M‐H, fixed, 95% CI) | 0.92 (0.22 to 3.85) |
| Convulsions | 2 | 271 | Odds ratio (M‐H, fixed, 95% CI) | 3.04 (0.78 to 11.81) |
| Lethal/long‐lasting arrhythmia | 2 | 271 | Odds ratio (M‐H, fixed, 95% CI) | 0.57 (0.19 to 1.72) |
| Renal failure | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 0.54 (0.05 to 6.03) |
| Sepsis/multiorgan failure | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 0.36 (0.04 to 3.47) |
| Hyperglycaemia | 1 | 1322 | Odds ratio (M‐H, fixed, 95% CI) | 0.70 (0.55 to 0.89) |
| Hyperthermia | 1 | 77 | Odds ratio (M‐H, fixed, 95% CI) | 1.74 (0.53 to 5.79) |
| Pneumonia | 1 | 77 | Odds ratio (M‐H, fixed, 95% CI) | 2.26 (0.54 to 9.51) |
| Bacteraemia | 1 | 77 | Odds ratio (M‐H, fixed, 95% CI) | 2.70 (0.11 to 68.47) |
| Adverse events any total | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 1.81 (0.86 to 3.82) |
| Adverse events serious total | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 0.51 (0.19 to 1.31) |
CI = confidence interval ROSC = return of spontaneous circulation
2. Adverse events related to cooling methods, as reported by study authors
For device‐related adverse events there was a higher incidence of nasal whitening with the intranasal cooling device used for intra‐arrest cooling (RR 29.30; 95% CI 1.77 to 486.02; P value = 0.02, see Table 3) (Castren 2010). For all other reported adverse events there was no significant difference between pre‐hospital and in‐hospital cooling.
2. Adverse events: device‐related.
| Adverse event | Studies | Participants | Statistical method | Effect estimate |
| Epistaxis | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 7.85 (0.40 to 154.06) |
| Periorbital emphysema | 1 | 194 | Odds ratio (M‐H, fixed, 95% CI) | 3.29 (0.13 to 81.81) |
| Nasal whitening | 1 | 194 | Risk ratio (M‐H, fixed, 95% CI) | 29.30 (1.77 to 486.02) |
CI = confidence interval
3. Quality of life, as reported by study authors
There were no data on quality of life.
4. Length of stay in the intensive care unit (ICU) and in the hospital, as proxies for economic outcomes
For ICU length of stay, Castren 2010 reported no significant difference between the pre‐hospital and in‐hospital cooling group (eight days for the pre‐hospital cooling participants versus 11 days for control participants; no standard deviation (SD) given).
For in‐hospital length of stay, Castren 2010 reported no significant difference between the pre‐hospital and in‐hospital cooling group (24.1 days for the pre‐hospital cooling participants versus 26 days for control participants; no SD given). Kim 2007 reported that the median length of stay was similar for the intervention and control group (12.2 days for the pre‐hospital cooling participants versus 9.9 days for control participants; P value = 0.71). Kim 2014 reported that the median length of stay was similar for the intervention and control groups among those with ventricular fibrillation (9.1 days for the pre‐hospital cooling participants versus 9.4 days for control participants; P value = 0.75) and among those without ventricular fibrillation (11.8 days for the pre‐hospital cooling participants versus 10.5 days for control participants; P value = 0.45).
Overall quality of the evidence
Using the GRADE approach we downgraded the overall quality of the evidence to 'very low', mainly due to a considerable amount of inconsistency, but also due to risk of bias within the studies and low precision (for details see the footnotes of Table 1).
Discussion
Summary of main results
Evidence from seven studies in 2369 people contributing data to the primary outcomes of this review was insufficient to show that pre‐hospital induction of cooling in comparison to in‐hospital cooling improved survival or neurological outcome after out‐of‐hospital cardiac arrest. Studies indicated a slightly increased number of re‐arrests with the application of pre‐hospital hypothermia.
However, relevant heterogeneity in terms of methods, interventions, and cohorts, as well as risk of bias in these seven included randomized controlled trials was too large to present a summary estimate of the effect of pre‐hospital cooling on survival and neurological outcome. Overall, pre‐hospital cooling did not appear to have an effect on neurological outcome and survival compared to in‐hospital cooling.
Significant shortcomings in these studies make any inferences about any efficacy outcome difficult. For some studies the focus and primary endpoint was strictly on the pre‐hospital phase so not much attention was given to the continuation of the cooling therapy. As a consequence some participants rewarmed after admission, and a significant number of participants did not receive any continuous cooling therapy.
Nonetheless, the intervention had an effect on procedural outcomes. In all studies the application of cooling in the pre‐hospital phase by the ambulance and helicopter staff resulted in a significantly lower body temperature on admission when compared to the control group.
Overall completeness and applicability of evidence
Unfortunately, the included studies had major heterogeneity in the intervention and control group treatments. The focus of the majority of the studies was put on the pre‐hospital phase; accordingly no common cooling management was in place across all studies. For the pre‐hospital phase cooling in some studies was not applied in the prespecified manner or was omitted. In‐hospital cooling was not required in all feasibility studies and in a relevant number of participants it was not initiated. This does not reflect common practice and adherence to cooling guidelines. Some studies did not document any data on the conduct of in‐hospital cooling preventing an assessment of whether the therapy was applied in an effective way and according to any cooling guidelines at that time. Another main source of heterogeneity stems from the incorporation of intra‐arrest and post‐arrest pre‐hospital cooling, which is evidenced by the large differences in outcome risks in the control groups. Additionally, common practice varies considerably across countries, likewise reflecting the uncertainty of the currently available evidence.
In 2013 one study was published that compared the effectiveness of temperature management at 33°C to temperature management at 36°C (Nielsen 2013). The authors found no difference in neurological outcome and survival. In the context of this review this result challenges the question of whether cooling to 32°C to 34°C is effective in the first place. Currently, there is no clear answer and this has been extensively discussed in the scientific community and by guidelines panels. The most recent guidelines have picked up on the Nielsen 2013 study and stated that temperature control between 32°C and 36°C is recommended, "whether certain subpopulations of cardiac arrest patients may benefit from lower (32–34°C) or higher (36°C) temperatures remains unknown, and further research may help elucidate this" (Callaway 2015; Soar 2015). This may reflect on the fact that in some ways the Nielsen 2013 study differs from previous efficacy studies on hypothermia. Firstly, Nielsen 2013 was a pragmatic study (multicentre, different methods of cooling etc.), which may not be suitable as long as the cooling 'dose', which is mainly target temperature and cooling duration, is not unequivocally characterized in proof of concept studies. Additionally, Nielsen 2013 had a very short duration from collapse to resuscitation (on average one minute) compared to the other trials (around 10 minutes). This is of relevance since there are reports showing that the effect of cooling may depend this no‐flow time (Testori 2012), and the very short times are unrealistic in many countries.
However, if heterogeneity between previous studies and the Nielsen 2013 study were ignored and they were put together, targeted temperature management may still improve neurological outcome by more than 50% (Arrich 2016).
Quality of the evidence
Risk of bias within the seven individual studies was generally moderate (see Figure 2; Figure 3; Characteristics of included studies). An exception was 'blinding of outcome assessment', which was absent in almost half of all studies and may have substantially biased outcomes like neurological state. 'Other sources of bias' (see Characteristics of included studies) formed the other exception and was the reason why we have not pooled the results. Using the GRADE approach we downgraded the quality of the overall evidence to 'very low' (see Table 1). This was mainly driven by a relevant amount of inconsistency due to the inconsistent application of the intervention and control, which significantly reduced confidence in the results and opens up the possibility that future studies with a more rigorous study conduct may have a different result. Adding to the inconsistency were the different study populations and the two different time points of intervention (intra‐arrest and post‐arrest). Imprecision also led to a further downgrading of the evidence as most studies were small, with three out of seven studies being feasibility or pilot studies (Castren 2010; Kämäräinen 2009; Kim 2007). The pre‐hospital participants' temperature was measured by tympanic probes (Bernard 2010; Bernard 2012; Castren 2010), and oesophageal/nasopharyngeal temperature probes (Kämäräinen 2009; Kim 2007; Kim 2014), the former having been shown to be the least reliable method for temperature measurement during the cooling phase (Krizanac 2013).
In the absence of summary estimates for the primary outcome we are left with the low precision of the single study estimates.
Potential biases in the review process
We have strived to find all comparable studies in this field and presented their results separately, together with assessment of their methodology, strengths, and limitations, to give the most comprehensive information on the question of the effectiveness of pre‐hospital cooling. We cannot exclude potential reporting bias due to the limited number of studies available. However, as is known from empirical evidence, reporting bias is usually driven by statistical significance and positive results (Hopewell 2009). It is noteworthy that in our review we only identified non‐significant studies.
Agreements and disagreements with other studies or reviews
We found four systematic reviews, which are at least partly comparable to our review. Two included meta‐analyses, whereas another two abstained from presenting summary estimates.
Cullen and colleagues published a review and meta‐analysis comparing pre‐hospital cooling to a later induction of cooling (Cullen 2011). Up to 2011 they found the same four studies as in our review (Bernard 2010; Castren 2010; Kämäräinen 2009; Kim 2007). They pooled all data and found no difference between the two groups but they were not specific in the description of the outcome and did not present any evaluation of clinical heterogeneity or possible sources of bias in the included studies. They concluded, however, that cooling in the pre‐hospital setting is feasible, but that it is unclear if it is beneficial in the long term, including for improving neurological outcomes.
Diao and colleagues presented a systematic review and meta‐analysis comparing pre‐hospital cooling to in‐hospital cooling or no cooling (Diao 2013). They pooled five studies, which are all included in our review (Bernard 2010; Bernard 2012; Castren 2010; Kämäräinen 2009; Kim 2007). They found that pre‐hospital cooling after cardiac arrest resulted in significantly lower body temperature on hospital admission. No differences were observed in survival to hospital discharge, favourable neurological outcome at hospital discharge, or re‐arrests. The overall quality of the included studies was graded as very low and this coincides with our methodological and clinical heterogeneity assessment.
Scolletta and colleagues summarized studies on intra‐arrest cooling (Scolletta 2012). Among the available randomized controlled trials they only included the study by Castren 2010 and did not otherwise pool data.
Cabanas conducted a systematic review on all available studies (randomized or not) of pre‐hospital cooling in comparison to normothermia or later induction of cooling (Cabanas 2011). Among the studies from our systematic review they only included Kim 2007. They did not pool the data but concluded that cooling can be efficiently induced in the pre‐hospital environment. Further, it was stated that more research would be needed to understand the effectiveness and optimal timing of early cooling.
Authors' conclusions
Implications for practice.
Currently, the overall quality of the available evidence on the effects of pre‐hospital cooling on survival and neuroprotection is very low. There is no convincing evidence to clearly delineate beneficial or harmful effects of pre‐hospital induction of cooling in comparison to in‐hospital cooling. The currently available studies suggest that pre‐hospital cooling induction is feasible to lower body temperature on hospital admission, but it may also increase the risk of cardiac re‐arrest. We do not have sufficient evidence to determine the effects of other determinants (duration, target temperature, etc.) of cooling from this review.
Implications for research.
Previous trials have focused on the feasibility and safety of pre‐hospital cooling, or have not rigorously applied nor reliably controlled either form of targeted temperature management. This resulted in considerable heterogeneity in the pre‐hospital phase but even more in the in‐hospital phase, which made it impossible to pool the outcome data from the single studies. Future trials should tackle these shortcomings, include the full initial 36 hours in the treatment protocols, and evaluate at least neurological outcome and survival at six months. One ongoing trial that compares pre‐hospital intra‐arrest transnasal evaporative cooling with standard targeted temperature management in the hospital, with neurological intact survival as the primary outcome parameter, would fit our inclusion criteria and is currently recruiting participants (Nordberg 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
When we extracted the outcome data from Kim 2014 (in January 2015) we noticed a discrepancy in the presented outcome table. After contacting the authors we were sent a corrected table of the outcome data and accordingly adjusted the events in the control group for the outcome "Good neurologic outcome" from 231 as presented in the paper to 221 in the tables sent to us.
We would like to thank Dr Nicola Petrucci (content editor), Dr Cathal Walsh (statistical editor), Dr Kjetil Sunde, Dr Clifton Callaway, Dr Jasmeet Soar (peer reviewers), and Jane Cracknell (Cochrane Anaesthesia Review Group Managing Editor) for their help and editorial advice during the preparation of the protocol for the systematic review (Arrich 2013).
Acknowledgements
We would like to thank Nicola Petrucci (content editor), Vibeke E Horstmann (statistical editor), Shu Ming Pan, Charles D Deakin, Jasmeet Soar (peer reviewers), and Roy Buffery (consumer referee) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Search strategy: CENTRAL, The Cochrane Library
#1 MeSH descriptor Resuscitation explode all trees #2 MeSH descriptor Cardiopulmonary Resuscitation explode all trees #3 MeSH descriptor Resuscitation Orders explode all trees #4 MeSH descriptor Heart Arrest explode all trees #5 MeSH descriptor Heart Massage explode all trees #6 ((cardio?pulmonary or order*) near2 resuscitation):ti,ab #7 reanimation:ti,ab #8 ((circulatory or circulation or cardiac) near arrest):ti,ab or heart standstill:ti,ab #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Cryotherapy explode all trees #11 MeSH descriptor Hypothermia explode all trees #12 MeSH descriptor Hypothermia, Induced explode all trees #13 ((resuscitative or therapeutic or artificial or induced or extracorporeal) near hypothermia) #14 artificial hibernation or body cooling or refrigeration anesthesia or body temperature:ti,ab or refrigeration:ti,ab #15 (#10 OR #11 OR #12 OR #13 OR #14) #16 (#9 AND #15)
Appendix 2. Search strategy: MEDLINE (OvidSP)
1. Resuscitation/ or Cardiopulmonary Resuscitation/ or Resuscitation Orders/ or Heart Arrest/ or Heart Massage/ or advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab. 2. Cryotherapy/ or Hypothermia/ or Circulatory Arrest, Deep Hypothermia Induced/ or Hypothermia, Induced/ or ((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia).mp. or artificial hibernation.mp. or body cooling.mp. or chilling.mp. or refrigeration anesthesia.mp. or body temperature.ti,ab. or refrigeration.ti,ab. 3. 1 and 2 4. ((randomised controlled trial or controlled clinical trial).pt. or randomised.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy: EMBASE (OvidSP)
1. resuscitation/ or heart arrest/ or heart massage/ or advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab. 2. cryotherapy/ or hypothermia/ or ((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia).mp. or artificial hibernation.mp. or body cooling.mp. or chilling.mp. or refrigeration anesthesia.mp. or body temperature.ti,ab. or refrigeration.ti,ab. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doub* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy: CINAHL (EBSCOhost)
S1 ( (MH "Resuscitation") OR (MH "Resuscitation Orders") OR (MH "Resuscitation, Cardiopulmonary") OR (MH "Heart Arrest") OR (MH "Heart Massage") ) OR AB ( ((cardio?pulmonary or order*) and resuscitation) ) OR AB reanimation OR ( (circulatory or circulation or cardiac) and arrest ) OR heart standstill S2 ( (MH "Cryotherapy") OR (MH "Hypothermia") OR (MH "Hypothermia, Induced") ) OR ( ((resuscitative or therapeutic or artificial or induced or extracorporeal) and hypothermia) ) OR artificial hibernation OR body cooling OR refrigeration anesthesia S3 ( (MH "randomised Controlled Trials") OR (MH "Random Assignment") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") OR (MH "Clinical Trials") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Placebos") ) OR ( random* or controlled clinical trial or placebo ) S4 S1 and S2 and S3
Appendix 5. Search strategy: BIOSIS (OvidSP)
1. advanced cardiac life support.mp. or ((cardio?pulmonary or order*) adj2 resuscitation).ti,ab. or reanimation.ti,ab. or ((circulatory or circulation or cardiac) adj3 arrest).ti,ab. or heart standstill.ti,ab. 2. (((resuscitative or therapeutic or artificial or induced or extracorporeal) adj3 hypothermia) or artificial hibernation or body cooling or chilling or refrigeration anesthesia).mp. or body temperature.ti,ab. or refrigeration.ti,ab. 3. 1 and 2
Appendix 6. Data extraction form
|
Data extraction form Pre‐hospital cooling versus in‐hospital cooling for patients with cardiac arrest | |
| Reviewer: | |
| Date: | |
| Decision: |
|
| Reasons for exclusion: | |
| Study characteristics | Publication type: |
| Language: | |
Randomization:
|
|
| Setting | Multicentre:
|
| Participants | Total number of patients: |
| Mean age: | |
| Percent female: | |
Cause of cardiac arrest
|
|
Primary cardiac rhythm
|
|
| Quality | Allocation concealment A. adequate B. unclear
|
Outcome assessor blind
|
|
Intention‐to‐treat:
|
|
Selective reporting:
|
|
Relevant amount of missing outcome data:
|
|
Baseline characteristics comparable:
|
|
| Intervention | Type of intervention: |
Time point of intervention:
|
|
| Controls: | |
| Duration of cardiac arrest intervention: | |
| Duration of cardiac arrest control: | |
| Target temperature intervention: | |
| Target temperature control: | |
| Cooling rate intervention: | |
| Cooling rate control: | |
| Temperature of patient at admission intervention: | |
| Temperature of patient at admission control: |
| Total duration of cooling intervention: | |
| Total duration of cooling control: | |
| Rewarming rate intervention: | |
| Rewarming rate control: | |
Multiple treatment groups:
|
|
| Outcomes | Types of outcome measures:
|
Time point of assessment of outcome measures:
|
|
| Funding | |
| Notes |
Primary outcomes:
| Type of outcome: | |||
| Intervention | Control | ||
| Events (n) | Total (N) | Events (n) | Total (N) |
| Type of outcome: | |||
| Intervention | Control | ||
| Events (n) | Total (N) | Events (n) | Total (N) |
Secondary outcomes (dichotomous):
| Type of outcome: | |||
| Intervention | Control | ||
| Events (n) | Total (N) | Events (n) | Total (N) |
Secondary outcomes (continuous):
| Type of outcome: | |||||
| Intervention | Control | ||||
| Mean | SD | Total | Mean | SD | Total |
Data and analyses
Comparison 1. Survival: pre‐hospital cooling versus in‐hospital cooling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Neurological outcome: pre‐hospital cooling versus in‐hospital cooling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Good neurological outcome | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bernard 2010.
| Methods | Parallel‐group, randomized trial, October 2005 to November 2007, multicentre study | |
| Participants | Total number of participants 234, mean age 63 years, 15% female, patients' temperature on arrival of resuscitation team: pre‐hospital cooling group 35.9°C, hospital cooling group: 35.8°C Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Funding | Funding: funding for the study was provided by grants from the Australian National Health and Medical Research Council (Drs Bernard, Cameron, Taylor, Cooper, Kelly, and Silvester) and the National Heart Foundation of Australia (Drs Bernard and Smith). | |
| Declarations of interest | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The envelope allocation was computer‐randomized and allocated in blocks of 10 to each intensive care paramedic unit |
| Allocation concealment (selection bias) | Low risk | The treating intensive care paramedics randomized eligible participants by opening an opaque, sealed envelope that indicated treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating ambulance and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | Low risk | Before hospital discharge, conscious participants were evaluated by a rehabilitation physician who was unaware of the study allocation |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants analysed for the primary outcome |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | High risk |
|
Bernard 2012.
| Methods | Parallel‐group, randomized trial, October 2005 to November 2007, multicentre study | |
| Participants | Total number of participants 163, mean age 62 years, 36% female, patients' average temperature on arrival of resuscitation team: pre‐hospital cooling group 35.9°C, hospital cooling group: 35.8°C Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Pre‐hospital cooling: 40 mL/kg and up to 2000 mL ice‐cold lactated Hartmann solution, given after resuscitation, n = 82 Control: in‐hospital cooling, n = 81 |
|
| Outcomes |
|
|
| Funding | Supported by the Australian National Health and Medical Research Committee | |
| Declarations of interest | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐randomized and allocated in blocks of 10 (5 to pre‐hospital cooling and 5 to hospital cooling) to each intensive care paramedic unit rating |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes. The envelope allocation was computer‐randomized and allocated in blocks of 10 to each intensive care paramedic unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating ambulance and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | Low risk | Before hospital discharge, conscious participants were evaluated by a rehabilitation physician who was unaware of the study allocation |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants analysed for primary outcome |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | High risk |
|
Castren 2010.
| Methods | Parallel‐group, randomized trial, November 2008 to June 2009, multicentre study | |
| Participants | Total number of participants 200, mean age: intervention 66, control 64 years, 27% female, patients' average temperature at ROSC: pre‐hospital cooling group 35.5°C, hospital cooling group: 35.8°C Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intra‐arrest cooling with RhinoChill device, continued in‐hospital according to institutional standards Control: in‐hospital cooling according to institutional standards |
|
| Outcomes |
|
|
| Funding | This work was supported by BeneChill, Inc, San Diego, California | |
| Declarations of interest | Dr Barbut is the founder and Chairman of BeneChill. She participated in study design, data analysis, and writing of the manuscript. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization assignments were generated under a randomized permuted‐block design, with block sizes of 8, in a 1:1 allocation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes that contained single randomization assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating ambulance and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | High risk | The discharge assessment may not always have been performed by an individual blinded to the treatment group |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 of 200 participants lost to follow‐up (4 from intervention, 3 from control group), due to misclassification of eligibility criteria and transport to non‐participating hospital. Reasons and numbers are balanced and the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Unclear risk | Presentation of data included only participants with acquired ROSC (after randomization) |
| Other bias | High risk |
|
Debaty 2014.
| Methods | Parallel‐group, randomized trial, February 2009 to August 2012, multicentre study | |
| Participants | Total number of participants 245, mean age 67 years, 29% female, patients' average temperature at randomization: pre‐hospital cooling group 35.2°C, hospital cooling group: 35.4°C (estimated from graph) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intra‐arrest cooling, infusion of up to 2000 mL of ice‐cold 0.9% saline solution at 100 mL/min during cardiac arrest by use of a standard infusion set and a pressure bag inflated to 300 mmHg. Surface cooling was also induced using gel pads. | |
| Outcomes | Types of outcome measures: Primary outcomes
Secondary outcomes
|
|
| Funding | French Society of Emergency Medicine | |
| Declarations of interest | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization assignments were generated under a randomized permuted‐block design, with block sizes of 4, in a 1:1 allocation |
| Allocation concealment (selection bias) | Low risk | Each mobile intensive care unit was given sequentially numbered, sealed envelopes containing single randomization assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Good neurological outcome | Low risk | Neurological outcome at hospital discharge and 30 days was assessed by a physician blinded to the study allocation |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis of clinical outcomes was according to intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | Unclear risk | No information on application of pre‐hospital cold fluids (which proportion of patients received how much) Over 24 hours of cooling the body temperature of the intra‐arrest cooling group seemed to be higher than the body temperature of the hospital cooling group According to the authors the study was not powered to show a clinical difference |
Kim 2007.
| Methods | Parallel‐group, randomized trial, November 2004 to February 2006, multicentre study | |
| Participants | Total number of participants 125, mean age 66 years, 30% female, patients' average temperature at randomization: pre‐hospital cooling group 35.8°C, hospital cooling group: 35.5°C Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Pre‐hospital induction of cooling (32°C to 34°C): up to 2 L of 4°C normal saline after resuscitation Control: standard care with and without hypothermia |
|
| Outcomes | Primary:
Secondary:
|
|
| Funding | This work was supported by a grant from the Medic One Foundation and National Institutes of Health grant HL04346 (F.K.) | |
| Declarations of interest | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...balanced blocks of 4" |
| Allocation concealment (selection bias) | Low risk | Paramedics called the emergency department physician at Harborview Medical Center to verify eligibility and to learn treatment assignment. The emergency room physician opened sequentially numbered envelopes that randomized participants to either receive or not receive cooling. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating ambulance and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | High risk | Study personnel during data collection and analysis could not be entirely unaware of treatment assignment |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants analysed for primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Of participants discharged alive, only severe neurological deficit was reported, but not in detail (neurological scores missing) |
| Other bias | High risk |
|
Kim 2014.
| Methods | Parallel‐group, randomized trial, December 2007 to December 2012, multicentre study | |
| Participants | Total number of participants 1359, mean age VF: 62; non‐VF: 68, 36% female, baseline average temperature of cooling groups not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Pre‐hospital cooling: up to 2L of 4°C saline after resuscitation Control: standard care alone (control) or standard care plus induction of cooling(intervention) |
|
| Outcomes |
|
|
| Funding | National Heart, Lung, and Blood Institute and with additional support from the Medic One Foundation (Seattle, Washington) | |
| Declarations of interest | Dr Nichol reported receiving institutional grant funding from the Asmund S. Laerdal Foundation for Acute Medicine, the National Heart, Lung, and Blood Institute, the National Institutes of Health, Medtronic Foundation, Velomedix Inc, Philips Healthcare Inc, Physio‐Control Inc, HealthSine Technologies Inc, and Zoll Inc; serving on the board of Medic One Foundation; being part of a patent assigned to the University of Washington; and receiving travel reimbursement from the American Heart Association. Dr Hallstrom reported receiving grants, support for travel to meetings, fees for participating in review activities, payment for writing or reviewing a manuscript, and provision for writing assistance, medicines, equipment, or administrative support from the National Heart, Lung, and Blood Institute; and serving as a consultant to Amarin and St Jude Medical for data and safety monitoring board activity on several trials. Dr Rea reported receiving grant support for community‐based resuscitation from Medtronic Foundation. Dr Deem reported receiving institutional grant funding from the National Institutes of Health and Medic One Foundation. No other author reported disclosures. | |
| Notes | Only 77% of all participants with VF received in‐hospital cooling (equally with field cooling or not, 224 in each group). It is unknown how many participants with non‐VF rhythms received in‐hospital cooling (only one hospital cooled participants with non‐VF cardiac arrests) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified by first recorded rhythm (VF or without VF) and destination hospital and by using randomly permuted blocks of concealed size to ensure temporal equality of assignment in each stratum |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating ambulance and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | Low risk | Study personnel who abstracted the medical records for the primary outcome were unaware of the study allocation |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding of survival data considered 'low risk' |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 of 1364 participants withdrawn because in prison; the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk |
|
Kämäräinen 2009.
| Methods | Parallel‐group, randomized trial, May 2005 to December 2008, multicentre study | |
| Participants | Total number of participants 43, mean age intervention: 59, control: 63 years, 2% female, baseline average temperature pre‐hospital cooling group: 35.5°C, hospital cooling group 35.3°C Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Pre‐hospital cooling: a rapid infusion of 4°C Ringer's acetate, 100 mL/min, target nasopharyngeal temperature was set at 33°C or alternatively the maximum volume of cold fluid was 30 mL/kg Control: no fluid cooling/conventional therapy; the use of in‐hospital cooling was left at the discretion of the hospital's physicians |
|
| Outcomes | Primary:
Secondary:
|
|
| Funding | None | |
| Declarations of interest | None | |
| Notes | The authors emphasize that the focus of this study was on the pre‐hospital phase of the participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Low risk | Unmarked, sealed and opaque envelopes opened by helicopter staff upon inclusion |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating helicopter and in‐hospital staff were not blinded |
| Blinding of outcome assessment (detection bias) Good neurological outcome | High risk | Treating personnel and investigators could not be blinded to the treatment |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Lack of blinding considered low risk |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 43 participants, 4 participants in the intervention group and 2 participants in the control group excluded from outcome analysis; 2 in the intervention group died between randomization and administration of interventions; the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | High risk | Only 10 out of 19 participants (52%) in the intervention group and 13 out of 18 (72%) participants in the control group received in‐hospital cooling; no detailed information on the conduct of temperature management in the hospital was given |
CPC = cerebral performance categories CPR = cardiopulmonary resuscitation GCS = Glasgow Coma Scale h = hours N = number of participants OHCA = out‐of‐hospital cardiac arrest PEA = pulseless electric activity ROSC = return of spontaneous circulation VF = ventricular fibrillation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belohlavek 2013 | Mixed intervention/ongoing study |
| Busch 2010 | Substudy to Castren 2010 |
| Taccone 2010 | Substudy to Castren 2010 |
Characteristics of ongoing studies [ordered by study ID]
Nordberg 2013.
| Trial name or title | Pre‐hospital Resuscitation Intra Nasal Cooling Effectiveness Survival Study (PRINCESS) |
| Methods | Randomized, controlled, open‐label, parallel design, phase 2 study |
| Participants | Participants with out‐of‐hospital cardiac arrest Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | June 2010 |
| Contact information | Leif Svensson, MD, PhD, leif.svensson@sodersjukhuset.se Maaret Castrén, MD, PhD, maaret.castren@sodersjukhuset.se |
| Notes | This study is currently recruiting participants |
CPC = cerebral performance categories DNAR = do not attempt resuscitation ROSC = return of spontaneous circulation
Differences between protocol and review
We made the following changes to the protocol (Arrich 2013):
We added one additional author (Alexandra‐Maria Warenits) and slightly changed the order. We did not search the PASCAL database as it was no longer available at our institution.
We changed the title of the published protocol from 'Prehospital versus in‐hospital initiation of mild therapeutic hypothermia for survival and neuroprotection after out‐of‐hospital cardiac arrest' to 'Pre‐hospital versus in‐hospital initiation of cooling for survival and neuroprotection after out‐of‐hospital cardiac arrest' following suggestions in the latest resuscitation guidelines. 'Cooling' seemed the most fitting and simple term especially when the relatively short pre‐hospital period is described. We tried to use the term 'cooling' throughout the manuscript to avoid unnecessary confusion. Occasionally we used 'targeted temperature management' (in the context of the whole cooling period).
For the same reason stated in 2, we changed the wording from 'hypothermia' to 'cooling' or 'targeted temperature management' in the Objectives, Types of participants, and Types of interventions.
We changed the structure of the Objectives in order to comply with Cochrane standards (Methodological Expectations of Cochrane Intervention Reviews).
We have additionally searched three trials registers (EudraCT, ClinicalTrials.gov, and the International Clinical Trials Registry Platform).
As we did not pool the results we did not carry out sensitivity or subgroup analyses.
We did not include cluster‐RCTs and studies with multiple treatment groups. If we include them when we update the review we will handle them as described in the Methods section.
Contributions of authors
Jasmin Arrich: (JA), Christof Havel (CH), Michael Holzer (MH), Alexandra‐Maria Warenits (AMW), Harald Herkner (HH)
Conceiving the review: JA, CH, MH, and HH
Co‐ordinating the review: HH and JA
Undertaking manual searches: JA, HH, MH, AMW, and CH
Screening search results: JA, HH, MH, AMW, and CH
Organizing retrieval of papers: JA
Screening retrieved papers against inclusion criteria: JA, HH, MH, AMW, and CH
Appraising quality of papers:JA, HH, MH, AMW, and CH
Abstracting data from papers: JA, HH, MH, AMW, and CH
Writing to authors of papers for additional information: JA
Providing additional data about papers: JA
Obtaining and screening data on unpublished studies: JA, HH, MH, AMW, and CH
Data management for the review: HH and JA
Entering data into Review Manager (RevMan 5.3): JA and HH
RevMan statistical data: HH and JA
Other statistical analysis not using RevMan: HH
Interpretation of data: JA, HH, MH, AMW, and CH
Statistical inferences: HH and JA
Writing the review: JA, HH, MH, AMW, and CH
Securing funding for the review: not applicable
Performing previous work that was the foundation of the present study: JA, CH, MH, and HH
Guarantor for the review (one author): JA
Person responsible for reading and checking review before submission: JA
Sources of support
Internal sources
Medical University of Vienna, Austria.
External sources
No sources of support supplied
Declarations of interest
Jasmin Arrich has no conflict of interest.
Michael Holzer received travel grants for scientific conferences and honoraria for lectures from Bard Medical, EmCools, Polimed Sp. z o.o. and Zoll Medical Österreich. He also received honoraria for consulting from Zoll Medical Österreich and was responsible for studies where the Department of Emergency Medicine received study grants from Velomedix and Philips.
Michael Holzer and Christof Havel were involved in the design, conduct, and publication of the HACA 2002 trial.
Alexandra‐Maria Warenits has no conflict of interest
Harald Herkner has no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bernard 2010 {published data only}
- Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, et al. Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out‐of‐hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010;122(7):737‐42. [PUBMED: 20679551] [DOI] [PubMed] [Google Scholar]
Bernard 2012 {published data only}
- Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, et al. Rapid Infusion of Cold Hartmanns Investigators. Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest*. Critical Care Medicine 2012;40(3):747‐53. [PUBMED: 22020244] [DOI] [PubMed] [Google Scholar]
Castren 2010 {published data only}
- Busch HJ, Eichwede F, Födisch M, Taccone FS, Wöbker G, Schwab T, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81(8):943‐9. [PUBMED: 20627524] [DOI] [PubMed] [Google Scholar]
- Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra‐arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre‐ROSC IntraNasal cooling effectiveness). Circulation 2010;122(7):729‐36. [PUBMED: 20679548] [DOI] [PubMed] [Google Scholar]
- Taccone FS, Vincent JL. Trans‐nasal cooling during CPR: a single‐center experience. Critical Care 2010;14(Suppl 1):S107‐8. [DOI: 10.1186/cc8551] [DOI] [Google Scholar]
Debaty 2014 {published data only}
- Debaty G, Maignan M, Savary D, Koch F‐X, Ruckly S, Durand M, et al. Impact of intra‐arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: a randomized controlled trial. Intensive Care Medicine 2014;40(12):1832‐42. [PUBMED: 25348858] [DOI] [PubMed] [Google Scholar]
Kämäräinen 2009 {published data only}
- Kämäräinen A, Virkkunen I, Tenhunen J, Yli‐Hankala A, Silfvast T. Prehospital therapeutic hypothermia for comatose survivors of cardiac arrest: a randomized controlled trial. Acta Anaesthesiologica Scandinavica 2009;53(7):900‐7. [PUBMED: 19496762] [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim F, Olsufka M, Longstreth WT Jr, Maynard C, Carlbom D, Deem S, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out‐of‐hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007;115(24):3064‐70. [PUBMED: 17548731] [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA 2014;311(1):45‐52. [PUBMED: 24240712] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Belohlavek 2013 {published data only}
- Belohlavek J, Franek O, Pokorna M, Danda J, Kandrnal V, Balik M, et al. Hyperinvasive approach to out‐of hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study. JACC. Cardiovascular Interventions 2013;6(2 Suppl 1):S41. [DOI: 10.1016/j.jcin.2013.01.050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busch 2010 {published data only}
- Busch HJ, Eichwede F, Födisch M, Taccone FS, Wöbker G, Schwab T, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81(8):943‐9. [PUBMED: 20627524] [DOI] [PubMed] [Google Scholar]
Taccone 2010 {published data only}
- Taccone FS, Vincent JL. Trans‐nasal cooling during CPR: a single‐center experience. Critical Care 2010;14(1):P319. [DOI: 10.1186/cc8551] [DOI] [Google Scholar]
References to ongoing studies
Nordberg 2013 {published data only}
- Nordberg P, Taccone FS, Castren M, Truhlar A, Desruelles D, Forsberg S, et al. Design of the PRINCESS trial: pre‐hospital resuscitation intra‐nasal cooling effectiveness survival study (PRINCESS). BMC Emergency Medicine 2013;13(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abella 2004
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra‐arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004;109(22):2786‐91. [PUBMED: 15159295] [DOI] [PubMed] [Google Scholar]
Abella 2008
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra‐arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2008;109(22):2786‐91. [PUBMED: 15159295] [DOI] [PubMed] [Google Scholar]
Arrich 2016
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. The Cochrane database of systematic reviews 2016;2:CD004128. [PUBMED: 26878327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berdowski 2010
- Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out‐of‐hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81(11):1479‐87. [PUBMED: 20828914] [DOI] [PubMed] [Google Scholar]
Bernard 2002
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. New England Journal of Medicine 2002;346(8):557‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cabanas 2011
- Cabanas JG, Brice JH, Maio VJ, Myers B, Hinchey PR. Field‐induced therapeutic hypothermia for neuroprotection after out‐of hospital cardiac arrest: a systematic review of the literature. Journal of Emergency Medicine 2011;40(4):400‐9. [PUBMED: 20850254] [DOI] [PubMed] [Google Scholar]
Callaway 2002
- Callaway CW, Tadler SC, Katz LM, Lipinski CL, Brader E. Feasibility of external cranial cooling during out‐of‐hospital cardiac arrest. Resuscitation 2002;52:159‐65. [PUBMED: 11841883] [DOI] [PubMed] [Google Scholar]
Callaway 2015
- Callaway CW, Soar J, Aibiki M, Bottiger BW, Brooks SC, Deakin CD, et al. Part 4: Advanced Life Support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015;132(16 Suppl 1):S84‐S145. [PUBMED: 26472860] [DOI] [PubMed] [Google Scholar]
Che 2011
- Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Critical Care Medicine 2011;39(6):1423‐30. [PUBMED: 21610611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colbourne 1995
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. Journal of Neuroscience 1995;15(11):7250‐60. [PUBMED: 7472479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullen 2011
- Cullen D, Augenstine D, Kaper L, Tinkham S, Utz D. Therapeutic hypothermia initiated in the pre‐hospital setting: a meta‐analysis. Advanced Emergency Nursing Journal 2011;33(4):314‐21. [PUBMED: 22075682] [DOI] [PubMed] [Google Scholar]
Cummins 1991
- Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett PJ, Becker L, et al. Recommended guidelines for uniform reporting of data from out‐of‐hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991;84:960‐75. [PUBMED: 1860248] [DOI] [PubMed] [Google Scholar]
Diao 2013
- Diao M, Huang F, Guan J, Zhang Z, Xiao Y, Shan Y, et al. Prehospital therapeutic hypothermia after cardiac arrest: a systematic review and meta‐analysis of randomized controlled trials. Resuscitation 2013;84(8):1021‐8. [PUBMED: 23454259] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
HACA 2002
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. New England Journal of Medicine 2002;346(8):549‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hachimi‐Idrissi 2001
- Hachimi‐Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51(3):275‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haugk 2011
- Haugk M, Testori C, Sterz F, Uranitsch M, Holzer M, Behringer W, et al. Time to Target Temperature Study Group. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Critical Care (London, England) 2011;15(2):R101. [PUBMED: 21439038] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holzer 2010
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. New England Journal of Medicine 2010;363(13):1256‐64. [PUBMED: 20860507] [DOI] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Louden K, Clarke M, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009; Vol. 1. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed]
Janata 2010
- Janata A, Weihs W, Schratter A, Bayegan K, Holzer M, Frossard M, et al. Cold aortic flush and chest compressions enable good neurologic outcome after 15 mins of ventricular fibrillation in cardiac arrest in pigs. Critical Care Medicine 2010;38(8):1637‐43. [PUBMED: 20543671] [DOI] [PubMed] [Google Scholar]
Jennett 1975
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krizanac 2013
- Krizanac D, Stratil P, Hoerburger D, Testori C, Wallmueller C, Schober A, et al. Femoro‐iliacal artery versus pulmonary artery core temperature measurement during therapeutic hypothermia: an observational study. Resuscitation 2013;84(6):805‐9. [PUBMED: 23200998] [DOI] [PubMed] [Google Scholar]
Kuboyama 1993
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Critical Care Medicine 1993;21(9):1348‐58. [PUBMED: 8370299] [DOI] [PubMed] [Google Scholar]
Nichol 2008
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Resuscitation Outcomes Consortium Investigators. Regional variation in out‐of‐hospital cardiac arrest incidence and outcome. JAMA 2008;300(12):1423‐31. [PUBMED: 18812533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2009
- Nielsen N, Hovdenes J, Nilsson F, Rubertsson S, Stammet P, Sunde K, et al. Hypothermia Network. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiologica Scandinavica 2009;53(7):926‐34. [PUBMED: 19549271] [DOI] [PubMed] [Google Scholar]
Nielsen 2013
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. New England Journal of Medicine 2013;369(23):2197‐206. [PUBMED: 24237006] [DOI] [PubMed] [Google Scholar]
Nolan 2008
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008;79(3):350‐79. [PUBMED: 18963350] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scolletta 2012
- Scolletta S, Taccone FS, Nordberg P, Donadello K, Vincent JL, Castren M. Intra‐arrest hypothermia during cardiac arrest: a systematic review. Critical Care (London, England) 2012;16(2):R41. [PUBMED: 22397519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sendelbach 2012
- Sendelbach S, Hearst MO, Johnson PJ, Unger BT, Mooney MR. Effects of variation in temperature management on cerebral performance category scores in patients who received therapeutic hypothermia post cardiac arrest. Resuscitation 2012;83(7):829‐34. [PUBMED: 22230942] [DOI] [PubMed] [Google Scholar]
Soar 2015
- Soar J, Callaway CW, Aibiki M, Bottiger BW, Brooks SC, Deakin CD, et al. Part 4: Advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015;95:e71‐e120. [PUBMED: 26477429] [DOI] [PubMed] [Google Scholar]
Sterz 1991
- Sterz F, Safar P, Tisherman S, Radovsky A, Kuboyama K, Oku K. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Critical Care Medicine 1991;19(3):379‐89. [PUBMED: 1999100] [DOI] [PubMed] [Google Scholar]
Suffoletto 2009
- Suffoletto B, Peberdy MA, Hoek T, Callaway C. Body temperature changes are associated with outcomes following in‐hospital cardiac arrest and return of spontaneous circulation. Resuscitation 2009;80(12):1365‐70. [PUBMED: 19804929] [DOI] [PubMed] [Google Scholar]
Testori 2012
- Testori C, Sterz F, Holzer M, Losert H, Arrich J, Herkner H, et al. The beneficial effect of mild therapeutic hypothermia depends on the time of complete circulatory standstill in patients with cardiac arrest. Resuscitation 2012;83(5):596‐601. [PUBMED: 22138057] [DOI] [PubMed] [Google Scholar]
Wolff 2009
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. International Journal of Cardiology 2009;133(2):223‐8. [PUBMED: 18353458] [DOI] [PubMed] [Google Scholar]
Yannopoulos 2009
- Yannopoulos D, Zviman M, Castro V, Kolandaivelu A, Ranjan R, Wilson RF, et al. Intra‐cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation 2009;120(14):1426‐35. [PUBMED: 19770397] [DOI] [PubMed] [Google Scholar]
Zhao 2008
- Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, et al. Intra‐arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008;77(2):242‐9. [PUBMED: 18096292] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Arrich 2013
- Arrich J, Havel C, Holzer M, Herkner H. Prehospital versus in‐hospital initiation of mild therapeutic hypothermia for survival and neuroprotection after out‐of‐hospital cardiac arrest. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010570] [DOI] [PMC free article] [PubMed] [Google Scholar]


