Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To evaluate the efficacy and safety of animal‐assisted therapy for people with dementia.
Background
Description of the condition
The term dementia describes a collection of symptoms caused by disorders affecting the brain. Dementia is a chronic and progressive condition characterised by a deterioration in memory, cognitive, social, and daily functional abilities beyond what might be expected from normal ageing. According to a World Health Organization (WHO) report in 2017, five to eight per 100 people worldwide suffer from dementia, with around 50 million people affected globally (WHO 2017a). It is estimated that the number of people with dementia worldwide will increase at a rate of 10 million per year, and that the total population with dementia will reach 82 million by 2030 and 152 million by 2050 (WHO 2017b), with most of the affected population from low‐ and middle‐income countries (WHO 2015). Dementia represents a major cause of disability and dependency among older adults, with the total global societal cost of dementia estimated to be around USD 820 billion, equivalent to 1.1% of the global gross domestic product (GDP) (Prince 2015).
The most common cause of dementia is Alzheimer's disease, which affects 60% to 80% of dementia patients, followed by vascular dementia, mixed dementia, and dementia of Lewy bodies (ALZ 2018). Some types of dementia are reversible, but most are not. People who suffer from dementia experience progressive worsening of symptoms, from occasional forgetfulness and disorientation in place and time, deterioration in self‐care and communication skills, to a total loss of mobility and the ability to recognise family members. Most people with dementia demonstrate behavioural changes characterised by repeated questioning, wandering, and aggressiveness. In the early stages, these changes may not be obvious as the symptoms tend to develop slowly. However, as the disease progresses, the symptoms become more evident as the decline in cognition and functional ability begins to interfere with the person's normal day‐to‐day activities. To date, no treatment has been identified that is clearly and consistently effective in preventing or halting progression of the disease (Chau 2016; Schwarz 2012). The major goals of currently available treatments are symptomatic, targeting challenging behaviour and psychological symptoms of patients, as well as their quality of life and that of their carers (NHS 2015). Animal‐assisted therapy (AAT) is one intervention that has been proposed to improve symptoms and possibly functional abilities in people who suffer from dementia.
Description of the intervention
AAT refers to the use of an animal that is considered suitable to work with human care recipients in the treatment of human physical or psychological disorders, coordinated by a human professional with in‐depth knowledge of the animal(s) involved and who has been formally certified (IAHAIO 2014). AAT is designed to promote improvements in human physical, social, emotional, or cognitive functions, and can be provided in individualised or group settings, with documentation and evaluation of the process and outcomes (AVMA 2018; Lefebvre 2008; Marino 2012). The use of animals in human therapy was first described in 1792 (McCulloch 1986). AAT as a treatment mode was formally introduced in 1969 by Dr Boris Levinson (Levinson 1969), a psychiatrist, who observed the interaction between a dog and a child with autism (Jacobs 2013). AAT for dementia has been documented since the 1990s (Walsh 1995; Behling 2011). Animals used in AAT for dementia include dogs and cats (Filan 2006; Motomura 2004), as well as aquatic animals (Filan 2006).
How the intervention might work
AAT has been reported to help in people with dementia by initiating social interaction in a controlled manner, which may lead to a decreased sense of loneliness and agitation (Banks 2002; LeRoux 2009; Richeson 2003; Sellers 2006). Increased levels of neurochemicals associated with relaxation and bonding have been reported in human recipients of AAT after treatment (Filan 2006). In terms of socio‐emotional aspects, AAT may benefit the care recipients by offering companionship to reduce boredom and the sense of isolation; providing pleasure, relaxation, and a source of motivation (Ohtani 2015); and by addressing unmet physical and emotional needs through joint participation in goal‐related activities (Ebener 2017). In a pilot survey, AAT appeared to be associated with increased muscle strength and range of movement, improved pain management, reduced blood pressure and heart rate, increased responsibility, self‐esteem, and patient independence in nursing home residents (Darrah 1996). In some cohort studies, AAT has been reported to improve nutritional intake (Edwards 2002), reduce depression (Travers 2013), and reduce medication usage in older people with dementia (Lust 2007). It is unclear over what time frame AAT works best in people with dementia, although a study on AAT for institutionalised elderly people showed that it appeared to have different overall effects on the physical, cognitive, and emotional functions of the care recipients in the first six months and thereafter (Kawamura 2007). A Cochrane protocol on the use of AAT in people with serious mental illness uses a cut‐off of six months to define a short‐term outcome assessment period (Downes 2013).
We have constructed a logic framework that delineates the condition, its clinical symptoms and progression, possible or hypothesised consequences, and possible points of intervention where AAT may work, following the guidance by Kneale 2015 and the Cochrane Infectious Diseases Group (CIDG 2016). The logic framework is depicted in a flow diagram (Figure 1).
Figure 1.
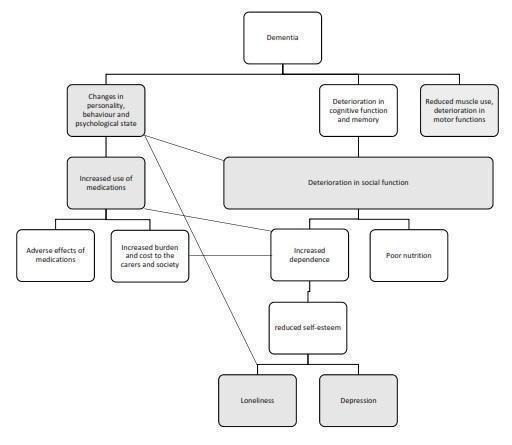
A logic framework model that depicts the progressive clinical manifestations and possible consequences of dementia, as well as possible points where animal‐assisted therapy may act, as shaded in grey
Possible adverse effects of AAT include transmission of zootopic diseases, animal aggression, and compromised animal welfare. A report from Japan found that no zootopic diseases occurred among children with sickle cell disease and healthcare workers in a children's hospital where AAT was regularly used (Yamauchi 2008). To address the issues of animal welfare and aggression, policies and guidelines have been published by established institutions such as the American Veterinary Medical Association (AVMA) (AVMA 2018), and the International Association of Human‐Animal Interaction Organizations (IAHAIO) (IAHAIO 2014). A study that measured the salivary cortisol level of therapy dogs as an indication of their stress level showed no major difference in the animals' salivary cortisol levels between their working days and off days (Glenk 2014). The animal welfare and ethical issues associated with the use of AAT have been studied and commented on (Glenk 2017; Hatch 2007).
Why it is important to do this review
The increasing number of people with dementia worldwide has been accompanied by an increased volume of dementia‐related research, including high‐quality research such as randomised controlled trials (RCTs) on various interventions to alleviate symptoms or to slow progression of the disease. Among the non‐pharmacological interventions studied, RCTs on AAT have been published since the 1990s and include more recently published studies evaluating robotic animals (Sakairi 2004; Tamura 2004; Wada 2008). However, to date, there has been no systematic review of RCTs that has synthesised data specifically on the use of AAT in people with dementia. The closest review is a Cochrane protocol on the use of AAT for people with serious mental illness, and the population does not included people with dementia (Downes 2013). It is important that relevant individual studies on the use of AAT in people with dementia are synthesised in a rigorous manner with regular updates, as we plan to undertake here, to provide a reliable and up‐to‐date guidance on practice, guideline and policy development, and future research.
Objectives
To evaluate the efficacy and safety of animal‐assisted therapy for people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We will include RCTs, cluster‐RCTs (e.g. trials in which randomisation was performed at the level of nursing care home/assisted living facilities or at subunit level within these institutions), and randomised cross‐over studies.
Types of participants
We will include studies that recruit participants with dementia, as defined by the study authors. The dementia may be of any severity.
We will perform sensitivity analyses to assess the impact on the pooled results of different methods used to identify dementia in participants, or of the inclusion of studies in which some participants may not have had dementia (e.g. mixed care home populations) (see Sensitivity analysis).
Setting: we will include studies that enrol participants living in the community or in any type of institution.
Types of interventions
Any form of animal‐assisted therapy, in which a live animal that is considered suitable to serve as companion to human care recipients is introduced with a specific therapeutic aim of improving symptoms and signs of dementia, with or without a concurrent role in providing assistance in daily activities (e.g. the use of guide dogs in facilitating memory training or physical activities as well as helping to retrieve daily items or crossing the road). Ideally, there will be clear documentation of the intervention being coordinated by a human healthcare provider with the appropriate expertise, as stated in the definition of animal‐assisted therapy (see Description of the intervention). However, anticipating that the information may not be available in all potentially eligible studies, we will accept all studies that provide any relevant description of animal involvement in therapeutic activities as mentioned above, with or without documentation on human coordination. The intervention may involve any species of animal, and may be conducted in an individual or group setting.
We will exclude studies that examine animal‐assisted activities alone (e.g. the use of guide dogs only for retrieving daily items or crossing the road), or pet ownership/companion animals, or the use of surrogates such as toys, robotic animals, or animals in digital applications.
We will accept any length and frequency (number of sessions per week) of therapy.
Comparison
Standard care only, or therapy intended to achieve the same goals in physical or mental functions without the involvement of animals, or another form of therapy being compared head‐to‐head with animal‐assisted therapy, such as standardised physical or occupational therapy, or both.
We will also include trials that compare different forms of animal‐assisted therapy, e.g. using different species of animal.
Any concurrent interventions, such as the use of medication and non‐pharmacological treatment as well as lifestyle changes, need to be clearly stated and identical between the two groups.
Types of outcome measures
Among our pre‐defined outcomes, 10 — including all primary outcomes — relate directly to the person with dementia, one to caregivers, and one to the therapy animal.
Primary outcomes
Affect and emotional well‐being, in particular, depression, as measured by suitable scales such as the Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos 1988) or Geriatric Depression Scale (GDS) (Yesavage 1982).
Social functioning, measured by suitable scales such as the Social Functioning in Dementia scale (SF‐DEM); De Jong Gierveld Loneliness Scale (Gierveld 2006); Communication Observation Scale; and Multidimensional Observation Scale for Elderly Subjects (MOSES) withdrawal subscale (Helmes 1987).
Overall behavioural and psychological symptoms of dementia (BPSD), measured with any validated instrument, e.g. the Neuropsychiatric Inventory (NPI) (Cummings 1994).
Agitation and irritability, measured with any validated instrument, e.g. Cohen‐Mansfield Agitation Inventory (CMAI) (Cohen‐Mansfield 1989), MOSES (irritability subscale) (Helmes 1987).
Health‐related quality of life, using validated condition‐specific quality of life scales.
Adverse effects, including injuries or trauma.
Secondary outcomes
Physical functional, such as activities in daily living (ADL), measured by validated tools such as: the Lawton Physical Self‐Maintenance Scale (PSMS) (Lawton 1969), Alzheimer’s Disease Activities of Daily Living International Scale (ADCS‐ADL) (Galasko 1997); Gottries‐Brane‐Steen‐Skala, ADL subscale (GBS‐ADL) (Brane 2001).
Cognitive functioning in different domains measured by validated scales, e.g. global cognitive function, assessed with Alzheimer’s Disease Assessment scale – Cognitive subscale (ADAS‐cog) (Rosen 1984) or Mini‐Mental State Exam (MMSE) (Folstein 1975), or other global measures of cognition.
Overall dementia severity measured by validated tools such as: Clinical Dementia Rating scale‐Sum of Boxes (CDR‐SOB) (O'Bryant 2008) or Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (CIBIC‐Plus) (Schneider 1997).
Mortality
Rates of institutionalisation
Carer satisfaction and stress.
Animal outcomes: physical, emotional, and other outcomes assessed for the animals involved, including animal injuries or trauma, or other adverse effects.
We will accept all outcomes assessed at variable time‐points throughout the conduct of the study, including short‐term (less than six months) and long‐term (six months or longer) periods. We will note the period of outcome assessment and classify it as short‐ or long‐term, and record this in the ‘Characteristics of included studies' table. If there is substantial heterogeneity in our results, as detailed under the ‘Assessment of heterogeneity' section, we will consider dose of intervention (including session frequency, length of sessions, and duration of intervention) as part of our assessment for possible causes of heterogeneity, and decide whether or not to pool data.
Search methods for identification of studies
We will search ALOIS (www.medicine.ox.ac.uk/alois), which is the Cochrane Dementia and Cognitive Improvement Group’s (CDCIG) Specialized Register.
ALOIS is maintained by the CDCIG Information Specialists and contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. The studies are identified through searching the following.
Major healthcare databases: MEDLINE, Embase, CINAHL, and PsycINFO.
Trial registers: ClinicalTrials.gov (https://www.clinicaltrials.gov/) and the WHO International Clinical Trials Register Platform (ICTRP) (http://apps.who.int/trialsearch/) which covers ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others.
The Cochrane Library's Central Register of Controlled Trials (CENTRAL).
Grey literature sources: ISI Web of Science Core Collection.
All sources will be searched from their inception to the latest issue or edition, and the most up‐to‐date version of the CDCIG Specialized Register used for retrieving potentially relevant studies. We will repeat the search if more than six months have elapsed between the initial search date and final stages of the review, to bring the review up‐to‐date.
To view a list of all sources searched for ALOIS, see the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies run in healthcare bibliographic databases, used for the retrieval of reports of dementia, cognitive improvement, and cognitive enhancement trials, can be viewed on the CDCIG website (http://dementia.cochrane.org/searches).
We will run additional searches in MEDLINE, Embase, PsycINFO, CINAHL, LILACs, ClinicalTrials.gov and the WHO ICTRP to ensure that the searches for this review are as comprehensive and as up‐to‐date as possible. The search strategy for the retrieval of reports of trials from MEDLINE (via the Ovid SP platform) is in Appendix 1.
Additionally, we will search animal‐based journals, including Anthrozoos, Animals, Animal Behaviour, Applied Animal Behaviour Science, the Journal of Animal Science and Technology, and the Journal of Animal Health and Behavioural Science using the term ‘animal‐assisted', ‘animal‐facilitated', ‘pet‐assisted', and ‘pet‐facilitated'.
We will not limit the language of the studies included in our review. For non‐English studies, we will enlist the help of a translator via the Cochrane Task Exchange platform (https://taskexchange.cochrane.org/) to translate the essential information of the studies into English.
Searching other resources
We will try to contact the authors of relevant trials to request details of any additional unpublished or ongoing studies that might meet the inclusion criteria for this review. We will also review the reference lists and citations of retrieved articles to look for additional trials for inclusion.
Data collection and analysis
Selection of studies
We will employ the standard Cochrane methods, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011f).
We will use Covidence to manage the screening and selection process, by importing references retrieved from all databases into the Covidence platform (Covidence 2018). Two review authors (NML and SMWC) will independently screen for potentially eligible studies by inspecting the titles and abstracts to generate a shortlist. Two review authors (NML and SSN) will then independently inspect the abstracts or full texts, or both, of these shortlisted studies further to determine final eligibility, using the predefined inclusion and exclusion criteria. We will resolve any disagreement with the help of a third review author (FS) who will act as an arbiter. We will delineate the study selection process in a PRISMA diagram.
We will include published and unpublished studies available in full‐text article or abstract form, and will contact the authors of unpublished studies and studies available only as abstracts to request additional information not provided in the available reports, including details such as: methods of sequence generation, allocation and blinding, participant withdrawal and prespecified outcomes, and full outcome data. If we find multiple reports of the same study, we will group them under a single study ID, and assign the report with the most amount of relevant information as the primary publication. We will list any studies excluded after full‐text assessment and their reason for exclusion in a ‘Characteristics of excluded studies' table.
Data extraction and management
Two review authors (SMWC and NML) will independently extract and code all data from each included study using Covidence (Covidence 2018). We will collect study characteristics, including study design, setting, country, methods of allocation, participants, interventions, comparators, outcomes, sponsorship details, declaration of interests of the primary investigators and methods used to control possible conflicts of interests, and other information considered relevant according to Chapter 7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We will resolve potential discrepancies through discussion and will involve a third review author if necessary. In case of language ambiguity that remains after translation, we will contact researchers in the field familiar with the language in question, or the study authors for clarification if necessary.
We will also extract the outcome data using an electronic data collection form. For continuous data we will extract the mean value of the outcome measurement in each group at each time point (or, if this is unavailable, the mean change from baseline), the standard deviation (SD) values, and the number of participants used to measure the outcome for each group. For dichotomous outcomes we will extract the number of participants in each outcome group at each time point. We will contact the study authors to obtain important missing data. If the study report only provides the summary effect sizes (e.g. risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data), we will extract those measures as well as the accompanying standard errors (SE) or 95% confidence intervals (CI) to prepare the data for combination via the generic inverse variance method. For studies that provide the outcome data in figures or graphs without accompanying annotation or numerical report, we will attempt to estimate the data from the figures using Plot Digitizer software (Jelicic Kadic 2016; Vucic 2015). Once we collect the data, one review author (SMWC) will transfer the data to Review Manager 5 (RevMan 5) software (RevMan 2014), and a second review author will check the accuracy of the data entry.
Assessment of risk of bias in included studies
Two review authors (NML and FS) will independently assess each included study for risk of bias according to the following six criteria, in accordance with the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other issues (e.g. extreme baseline imbalance).
For cluster‐RCTs and cross‐over trials, we will include additional ‘Risk of bias' domains under ‘other bias', as follows (Higgins 2011e).
Cluster‐RCTs
Was there evidence of further recruitment of participants into the clusters after randomisation (‘recruitment bias')?
Was there clear evidence of baseline imbalance between randomised clusters?
Was there evidence of loss of clusters in addition to the loss of participants after trial commencement?
Was there a unit of analysis error (i.e. failure to adjust for the clustering effect)? (for details please see the ‘Unit of analysis issues' section)
Cross‐over trials
Was the use of cross‐over design appropriate?
Can it be assumed that the trial was not biased from carry‐over effects?
Are unbiased data (e.g. data from both periods of the trial, data with removal of drop‐out from any one period) presented?
We will make a judgement of low, high, or unclear risk of bias, with justifications based on the information obtained from the papers. We will complete a ‘Risk of bias' table for each eligible study and present our overall ‘Risk of bias' assessment using a ‘Risk of bias' graph and ‘Risk of bias' summary. Any disagreement among the review authors will be resolved by discussion to achieve a consensus.
Measures of treatment effect
We will report the pooled outcome estimates for categorical data in relative terms using RRs, and also in absolute terms using risk differences (RDs). For continuous data we will use weighted mean differences (WMDs) with their respective 95% CIs. If pooled analyses are not possible due to reasons such as major discrepancies in study characteristics or outcome reporting, as detailed under the ‘Assessment of heterogeneity' section, we will report the results of the studies individually.
Unit of analysis issues
For cluster‐RCTs (e.g. trials in which the assignment to intervention or control group was made at the level of the institution), we will assess whether adjustment has been made for the effects of clustering in order to account for non‐independence among the participants in a cluster via the use of an appropriate analysis model such as a Generalised Estimating Equation (GEE) model. If the study authors do not state the unit of analysis, we plan to inspect the width of the standard error (SE) or 95% CI of the estimated treatment effects. If we find inappropriately small SEs or narrow 95% CIs, we will ask the study authors to provide information on the unit of analysis.
If no adjustment has been made for the effects of clustering, we will perform adjustment by multiplying the SEs of the final effect estimates by the square root of the ‘design effect', represented by the formula "1 + (M‐1) x ICC", where M is the average cluster size (number of participants per cluster) and ICC is the intracluster correlation. We will determine the average cluster size (M) from each trial by dividing the total number of participants by the total number of clusters. We will use a relatively large assumed ICC of 0.10, which is commonly used and is considered a realistic estimate in general (Campbell 2001). We will combine the adjusted final effect estimates from each trial with their SEs in meta‐analysis using generic inverse‐variance methods, as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
If determination of the unit of analysis is not possible, we will include the studies concerned in a meta‐analysis using the effect estimates reported by the study authors. We will then perform a sensitivity analysis to assess how the overall results are affected by these studies.
For cross‐over studies, our strategy for data analysis depends on the ‘Risk of bias' judgment of the included study. If we consider the included study to have low risk of bias across all three additional domains specific for cross‐over trials, as detailed under the ‘Assessment of risk of bias in included studies' section, we will include data from both phases of the trial, namely, before and after the cross‐over. In such cases, we will attempt to extract paired data from each participant if available. If we judge the trial to have unclear or high risk of bias in any of the additional risk of bias domain, we will only use data from the first phase before cross‐over takes place. If the results are not reported separately for each phase, we will still pool the overall results, but will evaluate the impact of excluding such studies via sensitivity analyses. Additionally, if data from both phases are reported separately but no paired data is extractable, we will also pool the overall results and conduct sensitivity analysis to assess the impact of such studies.
Dealing with missing data
We will follow the recommendations in Chapter 8.13.2 in the Cochrane Handbook for Systematic Reviews of Intervention in assessing the risk of bias from incomplete outcome data (Higgins 2011b).
We will perform our analyses using intention‐to‐treat (ITT) data either with complete case analysis (available outcome data of all participants who completed the study and analysed in the group initially randomised) for all outcomes if this is possible, or with imputation of missing outcome data by the study author if this is the only form of data available. If ITT data is not provided, we will include outcome data of the participants either in a ‘per protocol' or ‘as treated' manner, as provided by the study authors, but will make a corresponding note under the ‘Characteristics of included studies' table.
If sufficient studies are available, we will perform sensitivity analyses to assess how the overall results are affected by the inclusion of studies with a high risk of attrition bias from incomplete outcome data, and studies that do not provide ITT data.
Assessment of heterogeneity
We will use the I2 statistic to quantify the degree of inconsistency in the results (Higgins 2011c), with a cut‐off of 50% and above considered as the level at which the degree of heterogeneity is of sufficient concern to justify an exploration of possible explanations. In such a situation, we will evaluate studies in terms of their clinical and methodological characteristics using the following criteria to determine whether the degree of heterogeneity may be explained by differences in those characteristics, and whether a meta‐analysis is appropriate.
We will assess the following criteria.
Characteristics of the participants (e.g. age, type and severity of dementia).
Settings of the studies (e.g. community or institution).
Interventions (type of animal, dosage (intensity or duration of therapy)).
Risk of bias (as detailed in the ‘Assessment of risk of bias in included studies' section).
If we identify any of the above‐mentioned factors during our exploration that we consider to be a plausible explanation of the observed heterogeneity, we will separate the studies into subgroups according to the factors concerned if there are sufficient studies in each subgroup. In the case of risk of bias, we will conduct sensitivity analyses excluding the studies at higher risk of bias.
Assessment of reporting biases
We will use a funnel plot and Egger's test to screen for publication bias if there are at least 10 studies included in the analysis of the relevant outcomes (Egger 1997) . If publication bias is suggested by significant asymmetry of the funnel plot, we will include a statement in our results with a corresponding note of caution in our discussion, bearing in mind that funnel plot asymmetry does not necessarily equate to the presence of publication bias. If possible, we will compare conference abstracts and available trial protocols of included studies with published data.
Data synthesis
We will perform meta‐analyses if there are at least two studies with broadly similar population, intervention, comparison, and outcome (PICO) measures, using a random‐effects model in RevMan 5 (RevMan 2014). Our primary data analyses will follow the intention‐to‐treat principle; namely, we will analyse all participants in whom relevant outcome data are available in the group originally allocated. We will express our results as RRs, RDs, number needed to treat for an additional patient to benefit (NNTB), number needed to treat to cause an additional harm (NNTH) and MDs with their respective 95% CIs, as detailed in the ‘Measures of treatment effect' section. For cluster‐RCTs, our proposed methods of analysis are detailed in the ‘Unit of analysis issues' section.
If there are substantial differences between the characteristics of the PICO measures that preclude a meta‐analysis, we will summarise the results of the studies narratively.
Subgroup analysis and investigation of heterogeneity
Apart from the assessment of heterogeneity and subgroup analysis as detailed under the ‘Assessment of heterogeneity' section, we will conduct the following subgroup analyses, if data are available.
Type of studies
1. Individually randomised versus cluster‐randomised trials.
Population
2. Setting: community versus institution (such as care home).
3. Stage of dementia, differentiating very mild, mild, moderate, and severe dementia, as defined by validated tools such as the CDR‐SOB (O'Bryant 2008).
4. Type of dementia.
Intervention
5. Individual versus group therapy.
6. The use of different animals, each species forming an individual subgroup.
7. Intensity (‘dosage') of intervention: three or more versus fewer sessions per week.
‘Summary of findings' table
We will develop a ‘Summary of findings' table highlighting the quality of the evidence using the GRADE approach in our major outcomes as listed below (Schünemann 2011). We will use the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the evidence relating to the studies that contributed data to the meta‐analyses for each of these outcomes.
Specifically, for the criterion of study limitation, we will make decision on the overall risk of bias across the pool of relevant studies that contribute to the outcome rated on two levels: i) determining the overall risk of bias of any single study, and ii) determining the risk of bias across the pool of relevant studies (namely, overall study limitation). For i, we will assign the overall risk of bias status of the single study according to the worst risk of bias domain that is relevant to the outcome rated, except the domain of selective outcome reporting. For ii, we will refer to the guideline as detailed in Table 12.2.d of the Cochrane Handbook for Systematic Reviews of Intervention(Schünemann 2011).
If we identify an issue in each of the five GRADE criteria that we consider to pose a serious enough risk to influence the outcome estimate, we will downgrade the quality of evidence by one level, and when we consider the issue to be very serious, we will downgrade the quality of evidence by two levels (Schünemann 2011). Whenever we decide to downgrade the quality of evidence from the default high quality, we will justify our decision and describe the level of downgrading in the footnotes of the table. We will construct the ‘Summary of findings' table using an internet‐based version of GRADEpro software (GRADEpro 2015), according to the methods and recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
We will include the following outcomes in the ‘Summary of findings' table, regardless of the availability of data.
Depression, measured by validated scales such as CSDD or GDS.
Social functioning, measured by validated scales such as the SF‐DEM or De Jong Gierveld Loneliness Scale.
Overall BPSD, measured with any validated instrument, e.g. the NPI (Cummings 1994).
Agitation and irritability, measured with any validated instrument, e.g. CMAI (Cohen‐Mansfield 1989) and MOSES (irritability subscale) (Helmes 1987).
Health‐related quality of life, measured using validated condition‐specific quality of life scales.
Activities of daily living measured by suitable scales such as the Lawton PSMS.
Adverse events.
Sensitivity analysis
If a sufficient number of studies are available, we will consider sensitivity analyses for the primary outcomes and secondary outcomes to assess the impact on pooled results of excluding studies based on the following characteristics.
-
High risk of bias:
high risk of selection bias (for either criterion or both criteria of random sequence generation and allocation concealment)
high risk of attrition bias (incomplete outcome data);
studies reporting non‐ITT data only.
-
Participant factors:
studies that do not use recognised criteria to identify dementia (e.g. the Diagnostic and Statistical Manual of Mental Disorders V (DSM V) or previous editions of DSM (APA 2013), the International Classification of Diseases 10 (ICD 10) or previous editions of ICD (WHO 2010), NINDS‐AIREN Criteria for the Diagnosis of Vascular Dementia (Roman 1993), or NINCDS‐ADRDA Alzheimer's criteria (McKhann 2011);
studies which may have included some participants without dementia (e.g. mixed care home populations).
-
Intervention factors:
studies that do not clearly document involvement of an appropriately‐trained human facilitator.
Acknowledgements
Our thanks to Sue Marcus, Managing Editor and Dr Jenny McCleery, Coordinating Editor of Cochrane Dementia along with the editorial team for their support in providing feedback to the draft, and to Candida Fenton, Information Specialist, for developing the search strategy
Appendices
Appendix 1. MEDLINE search strategy
1 exp Dementia/
2 Delirium/
3 Wernicke Encephalopathy/
4 Delirium, Dementia, Amnestic, Cognitive Disorders/
5 dement*.mp.
6 alzheimer*.mp.
7 (lewy* adj2 bod*).mp.
8 (chronic adj2 cerebrovascular).mp.
9 ("organic brain disease" or "organic brain syndrome").mp.
10 "benign senescent forgetfulness".mp.
11 (cerebr* adj2 deteriorat*).mp.
12 (cerebral* adj2 insufficient*).mp.
13 or/1‐12
14 exp Animal Assisted Therapy/
15 exp Animals, Domestic/
16 exp Bonding, Human‐Pet/
17 exp Equine‐Assisted Therapy/
18 Pets/
19 "animal assisted".ti,ab.
20 "animal facilitated".ti,ab.
21 "animal‐assisted".ti,ab.
22 "animal‐facilitated".ti,ab.
23 AAA.ti,ab.
24 AAI.ti,ab.
25 AAT.ti,ab.
26 Animal Human Bond*.ti,ab.
27 animal visit*.ti,ab.
28 Animal‐Human Bond*.ti,ab.
29 aquaria.ti,ab.
30 aquarium*.ti,ab.
31 cat.ti,ab.
32 cats.ti,ab.
33 companion animal*.ti,ab.
34 dog.ti,ab.
35 dogs.ti,ab.
36 equine.ti,ab.
37 Fish tank*.ti,ab.
38 hippotherapy.ti,ab.
39 horse*.ti,ab.
40 horseback riding therap*.ti,ab.
41 human animal teams.ti,ab.
42 human‐animal teams.ti,ab.
43 pet facilitated therap*.ti,ab.
44 Pet Human Bond*.ti,ab.
45 pets.ti,ab.
46 pet‐therap*.ti,ab.
47 (pet adj5 (therap* or visit* or assist* or robot* or resident* or companion*)).ti,ab.
48 recreational horseback riding therapy.ti,ab.
49 resident cat*.ti,ab.
50 dog‐assisted.ti,ab.
51 service animal program*.ti,ab.
52 (therapeutic adj3 animal*).ti,ab.
53 visiting animal*.ti,ab.
54 or/14‐53
55 13 and 54
56 randomized controlled trial.pt.
57 controlled clinical trial.pt.
58 randomized.ab.
59 placebo.ab.
60 drug therapy.fs.
61 randomly.ab.
62 trial.ab.
63 groups.ab.
64 or/56‐63
65 exp animals/ not humans.sh.
66 64 not 65
67 55 and 66
Contributions of authors
NML: designed the protocol, performed co‐ordination with Cochrane, designed the search strategy with the Information Specialist, and wrote the protocol.
SMWC: designed the protocol, designed the search strategy with the Information Specialist, and wrote the protocol.
SSN, FS, SLT, and NC: designed and wrote the protocol.
Sources of support
Internal sources
Taylor's University Flagship Research Grant: Evidence synthesis projects on interventions to improve physical, mental and social well‐being of older adults, Malaysia.
External sources
-
National Institute for Health Research (NIHR), UK.
This protocol was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the National Health Service (NHS), or the Department of Health
Declarations of interest
NML: none known.
SMWC: none known.
SSN: none known.
FS: none known.
SLT: none known.
NC: none known.
New
References
Additional references
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23(3):271‐84. [PUBMED: 3337862] [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. Alzheimer's and dementia: types of Alzheimer's. 2018. https://alz.org/ (accessed 18 January 2018).
- American Veterinary Medical Association. Animal‐Assisted Interventions: guidelines. 2018. AVMA.org (accessed 18 January 2018).
- Banks MR, Banks WA. The effects of animal‐assisted therapy on loneliness in an elderly population in long‐term care facilities. Journal of Gerontology 2002;57A(7):M428‐32. [DOI] [PubMed] [Google Scholar]
- Behling RJ, Haefner J, Stowe M. Animal programs and animal assisted therapy in Illinois long‐term care facilities twenty years later (1990‐2010). Academy of Health Care Management Journal 2011;7(2):109‐17. [Google Scholar]
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [DOI] [PubMed] [Google Scholar]
- Chau SA, Liu CS, Ruthirakuhan M, Lanctôt KL, Herrmann N. Pharmacotherapy of dementia. In: Chiu H, Shulman K editor(s). Mental Health and Illness of the Elderly. Singapore: Springer, 2016. [DOI: 10.1007/978-981-10-0370-7_20-1] [DOI] [Google Scholar]
- Cochrane Infectious Diseases Group. Developing logic models: an introduction to using logic models in CIDG reviews. September 2016. http://cidg.cochrane.org/sites/cidg.cochrane.org/files/public/uploads/cidg_logic_models_sep16.pdf (accessed 1 March 2018).
- Cohen‐Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. Journal of Gerontology 1989;44(3):M77‐84. [PUBMED: 2715584] [DOI] [PubMed] [Google Scholar]
- Veritas Health Innovation. Covidence. Version accessed 1 March 2018. Melbourne, Australia: Veritas Health Innovation, 2018.
- Darrah JP. A pilot survey of animal‐facilitated therapy in Southern California and South Dakota nursing homes. Occupational Therapy International 1996;3(2):105‐21. [DOI: 10.1002/oti.31] [DOI] [Google Scholar]
- Downes MJ, Dean R, Bath‐Hextall FJ. Animal‐assisted therapy for people with serious mental illness. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010818] [DOI] [Google Scholar]
- Ebener J, Oh H. A review of animal‐assisted interventions in long‐term care facilities. Activities, Adaptation & Aging 2017;41(2):107‐28. [Google Scholar]
- Edwards NE, Beck AM. Animal‐assisted therapy and nutrition in Alzheimer’s disease. Western Journal of Nursing Research 2002;24(6):697‐712. [DOI: 10.1177/019394502320555430] [DOI] [PubMed] [Google Scholar]
- Filan SL, Llewellyn‐Jones RH. Animal‐assisted therapy for dementia: a review of the literature. International Psychogeriatrics 2006;18(4):597‐611. [DOI: 10.1017/S1041610206003322] [DOI] [PubMed] [Google Scholar]
- Jong Gierveld J, Tilburg T. A 6‐item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Research on Aging 2006;28(5):582‐98. [Google Scholar]
- Glenk LM, Kothgassner OD, Stetina BU, Palme R, Kepplinger B, Baran H. Salivary cortisol and behavior in therapy dogs during animal‐assisted interventions: A pilot study. Journal of Veterinary Behavior 2014;9(3):98‐106. [DOI: 10.1016/j.jveb.2014.02.005] [DOI] [Google Scholar]
- Glenk LM. Current perspectives on therapy dog welfare in animal‐assisted interventions. Animals 2017;7(2):7. [DOI: 10.3390/ani7020007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Hatch A. The view from all fours: a look at an animal‐assisted activity program from the animals’ perspective. Anthrozoos 2007;20(1):37‐50. [Google Scholar]
- Helmes E, Csapo KG, Short JA. Standardization and validation of the Multidimensional Observation Scale for Elderly Subjects (MOSES). Journal of Gerontology 1987;42(4):395‐405. [PUBMED: 3598087] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 11: Presenting results and "Summary of findings" table. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Deeks J, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Jegatheesan B, Beetz A, Ormerod E, Johnson R, Fine A, Yamazaki K, et al. The IAHAIO definitions for animal‐assisted intervention and guidelines for wellness of animals involved. 2014. http://iahaio.org/wp/wp‐content/uploads/2017/05/iahaio‐white‐paper‐final‐nov‐24‐2014.pdf. 4. San Diego, CA, USA: Elsevier, (accessed 15 January 2018).
- Jacobs CI. Animal‐assisted therapy and the child‐animal bond: children's well‐being and behavior. Research Papers 2013;427:http://opensiuc.lib.siu.edu/gs_rp/427. [Google Scholar]
- Kawamura N, Niiyama M, Niiyama H. Long‐term evaluation of animal‐assisted therapy for institutionalized elderly people: a preliminary result. Psychogeriatrics 2007;7(1):8‐13. [Google Scholar]
- Kneale D, Thomas J, Harris K. Developing and optimising the use of logic models in systematic reviews: exploring practice and good practice in the use of programme theory in reviews. PLoS One 2015;10(11):e0142187. [DOI: 10.1371/journal.pone.0142187] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9(3):179‐86. [PUBMED: 5349366] [PubMed] [Google Scholar]
- Lefebvre SL, Golab GC, Christensen E, Castrodale L, Aureden K, Bialachowski A, et al. Guidelines for animal‐assisted interventions in health care facilities. American Journal of Infection Control 2008;36(2):78‐85. [DOI: 10.1016/j.ajic.2007.09.005] [DOI] [PubMed] [Google Scholar]
- LeRoux MC, Kemp R. Effects of a companion dog on depression and anxiety levels of elderly residents in a long‐term care facility. Psychogeriatrics 2009;9(1):23‐6. [Google Scholar]
- Levinson BM. Pet‐oriented Child Psychotherapy. 1st Edition. Michigan: Thomas CC, 1969. [Google Scholar]
- Lust E, Ryan‐Haddad A, Coover K, Snell J. Measuring clinical outcomes of animal‐assisted therapy: impact on resident medication usage. Consultant Pharmacist 2007;22(7):580‐5. [DOI: 10.4140/TCP.n.2007.580] [DOI] [PubMed] [Google Scholar]
- Marino L. Construct validity of animal‐assisted therapy and activities: how important is the animal in AAT?. Anthrozoös: a multidisciplinary journal of the interactions of people and animals 2012;25(Suppl 1):s139‐51. [DOI: 10.2752/175303712X13353430377219] [DOI] [Google Scholar]
- McCulloch MJ. Animal‐facilitated therapy. Overview and future direction. National Forum 1986;66(1):19. [Google Scholar]
- Motomura N, Yagi T, Ohyama H. Animal assisted therapy for people with dementia. Psychogeriatrics 2004;4(2):40‐2. [DOI: 10.1111/j.1479-8301.2004.00062.x] [DOI] [Google Scholar]
- National Health Service UK. Dementia guide: is there a cure for dementia? 17 June 2015. NHS.uk (accessed 28 January 2018).
- Ohtani N, Narita S, Yoshihara E, Ohta M, Iwahashi K. Psychological evaluation of Animal‐assisted Intervention (AAI) programs involving visiting dogs and cats for alcohol dependents: a pilot study. Nihon Arukoru Yakubutsu Igakkai zasshi [Japanese Journal of Alcohol Studies & Drug Dependence] 2015;50(6):289‐95. [PUBMED: 26964290] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Yutzu, Prina M. World Alzheimer Report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. www.alz.co.uk/research/world‐report‐2015. London: Alzheimer’s Disease International;, (accessed 15 January 2018).
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Richeson NE. Effects of animal‐assisted therapy on agitated behaviors and social interactions of older adults with dementia. American Journal of Alzheimer's Disease & Other Dementias 2003;18(6):353‐8. [DOI: 10.1177/153331750301800610] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakairi K. Research of robot‐assisted activity for the elderly with senile dementia in a group home. SICE 2004 Annual Conference; 2004 Aug 4‐6. Sapporo, Japan: Institute of Electrical and Electronics Engineers (IEEE), 2005. [Google Scholar]
- Schwarz S, Froelich L, Burns A. Pharmacological treatment of dementia. Current Opinion in Psychiatry 2012;25(6):542‐50. [DOI: 10.1097/YCO.0b013e328358e4f2] [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AJ, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Sellers DM. The evaluation of an animal assisted therapy intervention for elders with dementia in long‐term care. Activities, Adaptation & Aging 2006;30(1):61‐77. [DOI: 10.1300/J016v30n01_04] [DOI] [Google Scholar]
- Tamura T, Yonemitsu S, Itoh A, Oikawa D, Kawakami A, Higashi Y, et al. Is an entertainment robot useful in the care of elderly people with severe dementia?. Journals of Gerontology: Series A 2004;59(1):83‐5. [DOI: 10.1093/gerona/59.1.M83] [DOI] [PubMed] [Google Scholar]
- Travers C, Perkins J, Rand J, Barlett H, Morton J. An evaluation of dog‐assisted therapy for residents of aged care facilities with dementia. Anthrozoös 2013;26(2):213‐25. [DOI: 10.2752/175303713X13636846944169] [DOI] [Google Scholar]
- Wada K, Shibata T, Musha T, Kimura S. Robot therapy for elders affected by dementia. IEEE Engineering in Medicine and Biology Magazine 2008;27(4):53‐60. [DOI: 10.1109/MEMB.2008.919496] [DOI] [Google Scholar]
- Walsh PG, Mertin PG, Verlander DF, Pollard CF. The effects of a ‘pets as therapy’ dog on persons with dementia in a psychiatric ward. Australian Occupational Therapy Journal 1995;42(4):161‐6. [DOI: 10.1111/j.1440-1630.1995.tb01331.x] [DOI] [Google Scholar]
- World Health Organization. The epidemiology and impact of dementia: current state and future trends. 2015. www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf (accessed 15 January 2018). [WHO/MSD/MER/15.3]
- World Health Organization. Dementia. Factsheet updated December 2017. www.who.int/news‐room/fact‐sheets/detail/dementia. Geneva: World Health Organization, (accessed 18 January 2018).
- World Health Organization. Overview of the global situation. Global action plan on the public health response to Dementia 2017‐2025. Geneva: World Health Organization, 2017:2. [Google Scholar]
- Yamauchi T, Pipkin E. Six years experience with animal‐assisted therapy in a children’s hospital: is there patient risk?. American Journal of Infection Control 2008;36(5):E117. [DOI: 10.1016/j.ajic.2008.04.132] [DOI] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1982;17(1):37‐49. [PUBMED: 7183759] [DOI] [PubMed] [Google Scholar]


