Abstract
Background
This is an update of the orginal Cochrane review published on the 18th April 2012. Cancer cachexia is a multidimensional syndrome characterised by wasting, loss of weight, loss of appetite, metabolic alterations, fatigue and reduced performance status. A significant number of patients with advanced cancer develop cachexia before death. There is no identified optimum treatment for cancer cachexia. While the exact mechanism of the action of thalidomide is unclear, it is known to have immunomodulatory and anti‐inflammatory properties, which are thought to help reduce the weight loss associated with cachexia. Preliminary studies of thalidomide have demonstrated encouraging results.
Objectives
This review aimed to (1) evaluate the effectiveness of thalidomide, and (2) identify and assess adverse effects from thalidomide for cancer cachexia.
Search methods
For this update we searched CENTRAL, MEDLINE, EMBASE, Web of Science and CINAHL (from ‐‐‐‐‐‐‐‐‐). Reference lists from reviewed articles, trial registers, relevant conference documents and thalidomide manufacturers were also searched.
Selection criteria
This review included randomised controlled trials (RCTs) and non‐RCTs. Participants were adults diagnosed with advanced or incurable cancer and weight loss or a clinical diagnosis of cachexia who were administered thalidomide.
Data collection and analysis
All titles and abstracts retrieved by electronic searching were downloaded to a reference management database. Duplicates were removed and the remaining citations were read by two review authors and checked for eligibility. Studies that were deemed ineligible for inclusion had clear reasons for exclusion documented. Data were extracted independently by two review authors for all eligible studies. While a meta‐analysis was planned for this review, this was not possible due to the small number of studies included and high heterogeneity among them. Thus a narrative synthesis of the findings is presented.
Main results
The literature search revealed a dearth of large, well conducted trials in this area. This has hindered the review authors’ ability to make an informed decision about thalidomide for the management of cancer cachexia. At present, there is insufficient evidence to refute or support the use of thalidomide for the management of cachexia in advanced cancer patients.
Authors' conclusions
The review authors cannot confirm or refute previous literature on the use of thalidomide for patients with advanced cancer who have cachexia and there is inadequate evidence to recommend it for clinical practice. Additional, well conducted, large RCTs are needed to test thalidomide both singularly and in combination with other treatment modalities to ascertain its true benefit, if any, for this population. Furthermore, one study (out of the three reviewed) highlighted that thalidomide was poorly tolerated and its use needs to be explored further in light of the frailty of this population.
Plain language summary
Thalidomide for managing wasting syndrome (cachexia) in advanced cancer
This review aims to assess if thalidomide is an effective treatment for the wasting syndrome (know as cachexia) seen in patients with advanced cancer. However, there was not enough evidence to make an informed decision about the use of thalidomide for patients with advanced cancer who have this wasting syndrome. This means that thalidomide as a treatment for this wasting syndrome remains unproven and its use needs more testing. Additionally, this review highlighted that there may be undesirable side effects of thalidomide when used for this syndrome, which need to be looked at closely to ensure it is suitable for this group of patients.
Background
Description of the condition
Cancer cachexia is a multidimensional syndrome characterised by wasting, loss of weight, loss of appetite, metabolic alterations, fatigue and reduced performance status. Cachexia is a general term meaning a form of ill health in different health conditions. A workshop, held in 1997, highlighted the lack of agreement on a definition of cancer cachexia (Argiles 2010; Dahele 2004). However, recent work has produced an international consensus on a definition for cancer cachexia as "a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. The pathophysiology is characterised by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism" (Fearon 2011, p490). Patients with cancer cachexia typically display profound weight loss from both fat and muscle tissue. Death tends to occur when an affected individual’s weight loss reaches 30% of pre‐morbid levels (Tisdale 2009).
A significant number of patients with advanced cancer develop cachexia before death. At the time of diagnosis, it is estimated that 80% of patients with upper gastrointestinal cancers and 60% of patients with lung cancer already have considerable weight loss (Burch 2000). In general, patients who have solid tumours (excluding breast cancer) have an elevated incidence of cachexia (Bruera 1999). The frequency of weight loss in different tumour types is listed in Table 1 (Laviano 2005). Although this data exists, it is argued that cachexia is so common in progressive end‐stage cancer that its true incidence is difficult to quantify (Ma 1998). Cancer cachexia is, therefore, an important and common clinical problem (Gordon 2005).
1. Incidence of weight loss.
| Tumour type | Incidence of weight loss |
| Pancreas | 85% |
| Gastric | 83% |
| Oesophageal | 79% |
| Head and Neck | 72% |
| Colorectal | 55‐60% |
| Lung | 50‐66% |
| Prostate | 56% |
| Breast | 10‐35% |
| General cancer population | 63% |
The complex metabolic alterations associated with cachexia result in a halting of the accumulation of lean muscle mass that nutrition alone cannot reverse (Tisdale 2002). While the exact pathophysiology of cachexia in advanced cancer remains incomplete, it appears to be mediated through an amalgamation of the pro‐inflammatory cytokine response of the host to tumour presence and the tumour manufacture of specific cytokines and catabolic factors (Gordon 2005). It is postulated that tumours, or host immune cells responding to the tumour, release several pro‐inflammatory cytokines including tumour necrosis factor‐alpha (TNF‐alpha), interleukin‐6 (IL‐6) and interferon‐gamma (IFN‐gamma), which have been implicated in cancer cachexia (Murphy 2009). Furthermore, explicit catabolic factors such as lipid mobilising factor and proteolysis inducing factor, which stimulates muscle breakdown, have also been recognized in patients with cancer who are losing weight (Ramos 2004). Cancer cachexia has multifaceted implications. It is evident that there are extreme biological and metabolic alterations in cancer cachexia that affect physical ability (Bruera 1996). This in turn can negatively affect self esteem (Brown 1999) and quality of life (QoL) (DeBoer 2006) and induce alteration in body image (Bruera 1996). Socially, cancer cachexia can reduce the patient's ability to engage with friends and family at meal times due to the potential conflict over food intake (Higginson 1996). While the impact of cancer cachexia is seen to be holistic for patients, the wasting associated with cancer cachexia is also a significant source of concern and distress for family members (Mearnes 1997).
Description of the intervention
Several treatment options currently exist for cachexia in advanced cancer patients. These include thalidomide, megestrol acetate and eicosapentaenoic acid. Thalidomide is known to have a variety of actions that highlight its usefulness as an anticancer agent (Stroud 2005). It is administered orally, in doses up to 200 mg once daily, generally at bedtime. Thalidomide is associated with severe side effects such as causing developmental malformations in an embryo or fetus, deep venous thrombosis and peripheral neuropathy. Less severe side effects include constipation, vomiting and drowsiness (BNF 2009).
How the intervention might work
While the exact mechanism of the antimalignancy action of thalidomide is unclear, it is known to have multifaceted immunomodulatory and anti‐inflammatory properties, which are thought to help reduce the weight loss associated with cachexia in patients with cancer (Gordon 2005). Thalidomide modifies the cytokine triggers of the wasting response through its potent antiTNF‐alpha effects (Fanelli 2003). This hampers the production of a transcription factor (NkB) and thus limits downstream gene expression, which in turn affects the control of the pro‐inflammatory cytokines, cell growth and regulation (Wilkes 2006). Such direct control of NkB, as opposed to merely cytokine inhibition, may help to clarify why thalidomide appears to be more successful than previously reviewed drugs, such as megestrol acetate (Loprinzi 1993). Studies have highlighted the potential for thalidomide to aid in the attenuation of wasting (Gordon 2005; Khan 2003) and improve the subjective symptoms of cachexia, such as reduced appetite, and sensation of well being (Bruera 1993). Nonetheless, such studies are small and use different doses of thalidomide in different patient populations, thereby providing little indication of a definitive treatment effect in a palliative cancer population.
Why it is important to do this review
Although reviews have examined different treatment modalities for cancer cachexia (Berenstein 2007; Dewey 2007), evidence has not identified an optimum treatment. Preliminary studies of thalidomide for cancer cachexia have demonstrated encouraging results (Bruera 1999; Khan 2003). However, the safety and efficacy of thalidomide for cancer cachexia has not been systematically reviewed.
Objectives
This review will examine thalidomide for managing cancer cachexia, and:
evaluate the effectiveness of thalidomide for cancer cachexia; and
identify and assess any adverse effects from thalidomide when used for cancer cachexia.
Methods
Criteria for considering studies for this review
Types of studies
We will include randomised controlled trials (RCTs) and non‐randomised (quasi‐RCTs, cohort and case control) studies.
Types of participants
Adults diagnosed with advanced or incurable cancer with weight loss or a clinical diagnosis of cachexia.
Although the range of body weight is wide, the individual's range of fluctuation over time is much narrower. It has been demonstrated that the 95% confidence intervals (CI) for change in body weight in healthy adults are approximately (±) 2% in one month, (±) 3.5% in three months, and (±) 5% in six months (Kolter 2000; Rosenbaum 2000). Therefore, any spontaneous weight changes beyond these limits could be described as abnormal. It is stipulated by Inui 2002 that cachexia should be suspected if there is an involuntary weight loss of greater than 5% of pre‐morbid weight observed in a six‐month period. However, there appears to be no agreement on this value as, in reviewing recent studies into cancer cachexia, the inclusion criteria have used patients with weight loss greater than 5% (Bruera 1999; Fearon 2003), above 5% in three months (Mantovani 2008) and above 10% in six months (Gordon 2005). Therefore, minimum limits were set as defined by Kolter 2000, and results were to be stratified by degree of weight loss over time.
Types of interventions
Administration of thalidomide orally (regardless of dosage) for any duration versus placebo or an alternative experimental treatment modality.
Types of outcome measures
Within the literature there is no agreed consensus on the most advantageous measurement, as an outcome, in cachexia trials. Historically, cachexia trials have measured weight gain but since some of this weight gain may be due to water retention or fat mass, body composition is also measured. This enables lean muscle mass to be more accurately measured and thus the impact of potential treatments on cachexia can be more reliably assessed. Additionally, it has been demonstrated that weight loss alone does not identify the full effect of cachexia on physical function for affected individuals (Fearon 2006) and newer mechanism such as ActivPALTM (a lightweight monitor worn by patients which measures physical activity levels) has been tested in a feasibility study focusing on physical activity as an outcome measure in cachexia trials (Maddocks 2010). Further research into this tool will help demonstrate the utility of such devices.
Primary outcomes
The primary outcome measures were body composition (dual‐energy X‐ray absorptiometry (DEXA), lean body mass, total body water, mid‐upper arm circumference) including change in weight (kg).
Secondary outcomes
Secondary outcome measures were:
overall survival (OS);
quality of life (QoL);
fatigue and functioning, including ability to carry out normal activities (using psychometrically validated measures such as the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 (http://groups.eortc.be/qol/questionnaires_qlqc30.htm), Multidimensional Fatigue Symptom Inventory‐Short Form (MFSI‐SF) (Stein 2004) and Karnofsky Index (Karnofsky 1949), which allows patients to be classified according to their functional ability and ranges from 0 to 100 with lower scores being indicative of poorer survival);
performance status;
grip strength;
serum levels of pro‐inflammatory cytokines such as IL6 and TNFalpha (pro‐inflammatory cytokines are chemical messengers, produced by the body, that promote systemic inflammation); and
adverse events.
Search methods for identification of studies
Electronic searches
We searched the electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 1), MEDLINE (1950 to week 4 March 2011), EMBASE (1980 to 2011 week 12), Web of Science and CINAHL (from inception to April 2011).
The search strategies are detailed in Appendix 1. No language restrictions were imposed and articles were obtained as necessary.
Searching other resources
We examined the reference lists from retrieved articles for any additional studies. We searched trials registers including Current Controlled Trials (http://www.controlled-trials.com/), National Cancer Institute (http://www.cancer.gov/clinicaltrials) and ClinicalTrials.gov (http://clinicaltrials.gov/). Handsearching included abstracts from relevant conferences, such as the International Cachexia Conference. The primary review author contacted thalidomide manufactures (such as Celgene Corporation) to identify any additional grey literature related to the review.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to a reference management database (Reference Manager). Duplicates were removed and the remaining citations were read by two review authors (JR and MM) and checked for eligibility. The full copy of each article that could not be excluded on the basis of the information presented in the title and abstract was obtained, translated if necessary, and reviewed. Any disagreement was resolved through discussion between the review authors, utilising the wider review team if necessary, until consensus was achieved. If the review authors required any additional information on studies to ascertain eligibility, the primary review author (JR) made direct contact with the study authors. All studies that were deemed ineligible for inclusion had clear reasons for exclusion documented. Non‐randomised studies including quasi‐experimental, cohort and case control studies were considered systematically after all eligible RCTs had been identified.
Data extraction and management
Two review authors (JR and MM) independently extracted data from eligible studies using a standard data extraction form. The extraction form included details on the study type (RCT, non‐randomised trial, cohort, case control), characteristics of participants (inclusion criteria, tumour type, age, stage, co‐morbidity, previous treatment, performance status, weight loss), intervention(s) (dose, formulation, duration of treatment), risk of bias, duration of follow‐up, outcomes and number enrolled in each study arm. Once complete, all information on the data extraction forms was presented in a tabular format. If more than one report of the same study was uncovered, the most recent report was used for data extraction. We expected that studies would have recruited participants with differing cancer sites (for example pancreatic, lung, gastrointestinal), therefore outcomes were extracted for all tumour types. The time points at which outcomes were collected and reported were noted.
Assessment of risk of bias in included studies
Studies which meet the review inclusion criteria were independently assessed for quality by two review authors (JR and MM) and differences resolved by discussion or by appeal to a third review author (MD).
Risk of bias of included RCTs was assessed using the guidelines detailed in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (Higgins 2009), as outlined in Table 2.
2. The Cochrane Collaboration's tool for assessing risk of bias in randomised controlled trials.
| Domain | Description | Review authors’ judgement |
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Was the allocation sequence adequately generated? |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessorsAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Are reports of the study free of suggestion of selective outcome reporting? |
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
Was the study apparently free of other problems that could put it at a high risk of bias? |
Assessment of reporting biases
We planned to develop funnel plots, which provide a visual display with the potential to identify publication bias in studies. The funnel plot would correspond to meta‐analysis of the primary outcome to assess the potential for small study effects such as publication bias. Additionally possible sources of asymmetry in a funnel plot, such as selective outcome reporting, poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity, artefactual and chance, were planned, as outlined by Egger 1997. However, this review has only included three studies and therefore a qualitative assessment of reporting biases was used whereby the review authors have reviewed and summarised the evidence from the studies.
Results
Description of studies
Results of the search
A flow diagram detailing the selection of studies in detailed in Figure 1. The electronic literature searches identified 488 potential articles and 42 were identified from trial registers and grey literature. Following removal of duplicates we (JR and MM) independently examined 445 papers and after initial screening 370 papers were removed for one of the following reasons.
1.
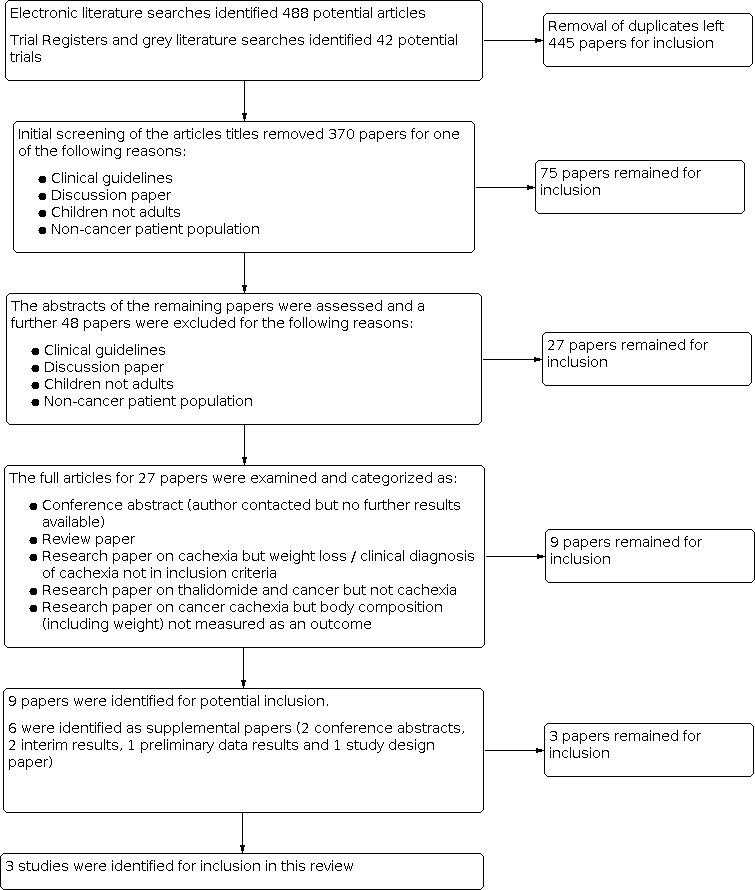
Selection of studies
Clinical guidelines.
Discussion paper.
Children not adults.
Non‐cancer patient population.
We (JR and MM) independently assessed the abstracts of the remaining 75 papers and a further 48 papers were excluded for the reasons above.
We (JR and MM) independently examined 27 full articles and categorized them as:
conference abstract (author contacted but no further results available);
review paper;
research paper on cachexia but weight loss or clinical diagnosis of cachexia not in inclusion criteria;
research paper on thalidomide and cancer but not cachexia;
research paper on cancer cachexia but body composition (including weight) not measured as an outcome.
Nine papers were identified for potential inclusion.
Included studies
Six of the nine articles included were identified as supplemental papers (two conference abstracts, two interim results, one preliminary data results and one study design paper). In total, three studies were identified for inclusion. The included studies were conducted in Italy (Mantovani 2010a), Nottingham, UK (Wilkes 2011) and Portsmouth, UK (Gordon 2005). All articles were written in English. Full details can be found in the Characteristics of included studies table.
Participants
For the three included studies the number of participants recruited were 50 (Gordon 2005), 34 (Wilkes 2011) and 322 (Mantovani 2010a). One study included patients with pancreatic cancer exclusively (Gordon 2005), one recruited oesophageal cancer patients exclusively (Wilkes 2011) and one recruited patients with varied cancer diagnoses (Mantovani 2010a).
Interventions and controls
All included studies randomised participants into groups. In two studies a control group (utilizing a thalidomide placebo) was used (Gordon 2005; Wilkes 2011).
Mantovani 2010a distributed patients across five treatment arms:
arm 1, medroxyprogesterone acetate (500 mg/d) or megestrol acetate (320 mg/d);
arm 2, eicosapentaenoic acid (EPA) enriched (2.2 g/d) nutritional supplement;
arm 3, L‐carnitine (4 g/d);
arm 4, thalidomide (200 mg/d);
arm 5, combination of all four arms.
Thalidomide, 200 mg daily, was taken orally for six weeks in Wilkes 2011 and for 24 weeks in Gordon 2005 and Mantovani 2010a . Additionally, all participants in Mantovani 2010a were given a basic treatment of polyphenols (300 mg/d) plus lipoic acid (300 mg/d) plus carbocysteine (2.7 g/d) plus vitamin E (400 mg/d) plus vitamin A (30,000 IU/d) plus vitamin C (500 mg/d).
All treatments were taken orally.
Outcomes
Primary outcomes
While body composition was measured as the primary outcome in all studies, the mode of measurement or timing of such measurements was not standardized across studies: for example, change in weight in kg at four weeks in Gordon 2005); changes in total body weight (TBW) and lean body mass (LBM) measured using calibrated electronic scales and dual‐energy X‐ray absorptiometry (DEXA), respectively, at six weeks in Wilkes 2011; and LBM (assessed by bioelectrical impedance analysis in all patients, by DEXA in 144 patients, and by CT at lumbar (L) level 3 in 25 patients) in Mantovani 2010a. Mantovani 2010a had two further primary outcomes measured at 16 weeks: resting energy expenditure (REE) (assessed by indirect calorimetry) and fatigue (assessed by the Multidimensional Fatigue Symptom Inventory–Short Form).
Secondary outcomes
Wilkes 2011 included: REE (assessed by indirect calorimetry); triceps skin‐fold thickness; mid‐arm circumference; symptoms of disease progression, performance indices (Karnofsky index); Piper Fatigue Scale questionnaire; and serum level TNF‐alpha and IL‐1beta.
Gordon 2005 included: changes in bone‐free muscle mass, grip strength, quality of life (EORTC QLQ ‐ C30 global health score and EORTC QLQ ‐ C30 physical functioning), survival and bloods for haemoglobin, albumin, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP).
Mantovani 2010a included: appetite, by visual analogue scale (VAS); grip strength, by dynamometer; QoL, by the EORTC QLQ‐C30, EQ‐5D index, and EQ‐5D VAS; serum levels of IL‐6 and TNF‐alpha; Glasgow Prognostic Score (GPS); blood levels of reactive oxygen species and the antioxidant enzyme glutathione peroxidase; total daily physical activity and the associated energy expenditure (SenseWear PRO2 Armband); performance status (PS), Eastern Cooperative Oncology Group Performance Status (ECOG PS) scale; progression‐free survival (PFS), and overall survival (OS).
All studies collected data on adverse events.
Excluded studies
Reasons for exclusion are detailed in the Results of the search section of this review and the Characteristics of excluded studies table.
Risk of bias in included studies
All studies were assessed for risk of bias using the RevMan 5.2 risk of bias tool. This tool specifies criteria for assessing bias as detailed below.
Allocation
Random sequence generation
All studies were randomised and each study provided details of the random sequence generation used. These included randomisation being undertaken in blocks of four using a sequential series (Gordon 2005), randomisation performed by random‐numbers table (Mantovani 2010a), or by a computer generated randomisation protocol (Wilkes 2011). Therefore, all studies were classified as ‘low risk’ of bias for this component. Allocation concealment was clearly discussed and was deemed ‘low risk’ in both the Gordon 2005 and Wilkes 2011 studies. However, it was not mentioned in sufficient detail by Mantovani 2010a and therefore was deemed ‘unclear’ for this study.
Blinding
Blinding of personnel was described by Gordon 2005 and Wilkes 2011. However, insufficient detail was provided by Mantovani 2010a.
Insufficient detail was provided in all studies about the blinding of outcome assessment, so all were deemed ‘unclear’.
Incomplete outcome data
Loss to follow‐up was generally reported across the studies and patient flow details were adequately recorded by both Wilkes 2011 and Mantovani 2010a. It was noted that in Gordon 2005 data on two patients were recorded in the patient flow diagram as being withdrawn from the study, as ‘other’, and no explanation (such as progressive disease) was offered.
Selective reporting
There was no evidence of selective reporting across the three studies. Nonetheless, one study (Wilkes 2011) did not discuss data on skin‐fold thickness while all other primary and secondary measures were reported across all three studies.
Other potential sources of bias
In assessing the methodological quality of studies included in this review, sample size was seen as being problematic for this type of study, which recruited advanced cancer patients. For example, Gordon 2005 and Mantovani 2010a did not achieve the sample size stipulated in their power calculations. Gordon 2005 stated that 25 patients were needed per arm and the study recruited 24 to placebo and 23 to thalidomide; and Mantovani 2010a stated that 95 patients were needed for each arm and the study recruited 87 to 88 in each of the three completed arms. While Wilkes 2011 did achieve the sample size stipulated (17), all but two patients required either a dose reduction or cessation of treatment due to side effects compared to 94% of the placebo group who completed their protocol.
Effects of interventions
The three studies meeting the inclusion criteria involved a total of 416 patients. Two studies tested thalidomide (200 mg/day) versus placebo for different time durations (six weeks and four weeks) (Gordon 2005; Wilkes 2011 respectively). One study tested thalidomide (200 mg daily for four months) versus four other active treatments (Mantovani 2010a).
Patient characteristics
Gordon 2005 randomised 50 patients with inoperable pancreatic cancer, greater than 10% weight loss over the preceding six months, and likely life expectancy of at least six weeks. Mantovani 2010a randomly assigned 332 patients with a histologically confirmed advanced stage tumour at any site, loss of greater than 5% of ideal or pre‐illness body weight in the previous three months, and a life expectancy of four months or more. Wilkes 2011 randomised 34 patients with incurable oesophageal cancer who had a clinical diagnosis of cachexia, life expectancy over eight weeks and who could tolerate a soft diet.
Adjunct antineoplastic treatment
In both Gordon 2005 and Wilkes 2011 potential participants were excluded if they had received any form of treatment for pancreatic cancer in the previous six weeks, or radio‐ or chemotherapy in the previous four weeks respectively. However, in Mantovani 2010a patients could be receiving concomitant antineoplastic chemotherapy or hormone therapy with curative or palliative intent or supportive care.
Interventions
Intervention 1: thalidomide versus placebo
Two of the trials compared thalidomide 200 mg daily to placebo (Gordon 2005; Wilkes 2011). In the Gordon 2005 trial 50 patients were randomised to receive either thalidomide 200 mg daily or an identical placebo for a trial period of 24 weeks. Assessment took place at baseline, four and eight weeks. At eight weeks, there were only 20 patients (8 placebo; 12 treatment) left in the trial and meaningful comparisons were not achievable. Results presented were therefore for four weeks of treatment. Wilkes 2011 randomised 34 patients to receive either thalidomide 200 mg daily or identical placebo for a trial period of six weeks. Assessment took place at baseline and six weeks.
Intervention 2: thalidomide versus alternative active treatment
In the Mantovani 2010a trial, patients were randomised to one of five arms:
arm 1, a progestational agent, that is medroxyprogesterone acetate (MPA) (500 mg/day) or megestrol acetate (MA) (320 mg/day), which were considered equivalent;
arm 2, an oral eicosapentaenoic acid (EPA)‐enriched (2.2 g/day) nutritional supplement, in prescribed dosages of two cartons/day for both ProSure or three cartons/day for Forticare;
arm 3, L‐carnitine (4 g/day);
arm 4, thalidomide (200 mg/day);
arm 5, MPA or MAplus EPA‐enriched nutritional supplement plus L‐carnitine plus thalidomide.
The trial period was four months and endpoints were assessed at baseline and 16 weeks. Additionally, an interim analysis was conducted at the randomisation of the 125th patient and the 204th patient. Analysis showed inferiority of arm 2 (EPA enriched supplement) and arm 1 (medroxyprogesterone) respectively and these arms were withdrawn at that time.
Compliance
Gordon 2005 detailed that compliance was measured by participant self‐reporting and tablet count at each visit, but no details are provided within the article. Wilkes 2011 stated that only 8 out of 17 patients in the thalidomide arm returned for repeated measures at six weeks, two of whom had discontinued treatment but all had taken at least 50% of their trial medication. In the Mantovani 2010a study, they reported that patient compliance was 'very good'; however, there was no offered explanation of how compliance with the medication and dietary regimes was monitored.
Adverse events
A summary of all adverse events is presented in Table 3. While it was anticipated that this review would include tests for difference between the intervention and comparison groups in relation to adverse events, this was not possible due to the high heterogeneity across studies.
3. Adverse events.
| Wilkes 2011 | Gordon 2005 | Mantovani 2010 | |||||||
| Thalidomide | Placebo | Thalidomide | Placebo | Arm 1 |
Arm 2 | Arm 3 | Arm 4 | Arm 5 | |
| Peripheral neuropathy | NR | NR | 2/24 | NR | NR | NR | NR | NR | NR |
| Parasthesia | 1/17 | NR | NR | NR | NR | NR | NR | NR | NR |
| Rash / cutaneous reaction | 5/17 | NR | 2/24 | NR | NR | NR | NR | NR | NR |
|
Daytime somnolence |
3/17 | NR | 4/24 | NR | NR | NR | NR | 2/87 | NR |
| Venous thromboembolism | 1/17 | 1/17 | 1/24 | 2/23 | 1/44 | NR | NR | NR | 1/85 |
| Diarrhea | NR | NR | MIDR | MIDR | 1/44 | 1/25 | 4/88 | NR | 5/85 |
| Constipation | NR | NR | MIDR | MIDR | NR | NR | NR | NR | NR |
| Epigastralgia | NR | NR | NR | NR | NR | 2/25 | NR | NR | 1/85 |
| Headache, nausea and vomiting (resembling raised intracranial pressure) | 1/17 | NR | NR | NR | NR | NR | NR | NR | NR |
| Nausea/vomiting | 1/17 | NR | MIDR | MIDR | NR | NR | NR | NR | NR |
| Neutropenia | 1/17 | NR | NR | NR | NR | NR | NR | NR | NR |
| Pneumonia | 2/17 | NR | NR | NR | NR | NR | NR | NR | NR |
| Deterioration due to disease related symptoms | 3/17 | NR | NR | NR | NR | NR | NR | NR | NR |
| Low blood pressure | 1/17 | NR | NR | NR | NR | NR | NR | NR | NR |
|
Key NR Not reported in paper MIDR Measured (in study) Insufficient Data Reported in paper – author attempted to be contacted 19/01/2012 – email not in use. Arm 1 Medroxyprogesterone acetate (500mgs/d) or megestrol acetate (320 mg/d); Arm 2 Eicosapentaenoic acid (EPA) enriched (2.2g/d) nutritional supplement: Arm 3 L‐Carnitine (4g/d); Arm 4 Thalidomide 200mg/d; Arm 5 Combination of Arms 1, 2, 3 and 4. | |||||||||
In the Wilkes 2011 study nine patients in the thalidomide group experienced recognised side effects of thalidomide. These included skin rashes, hyper‐somnolence, paraesthesia, constipation, headache and neutropenia. Two patients on active treatment developed pneumonia and were withdrawn; a further two patients became too frail to continue and both died within two weeks of withdrawal. One patient elected to discontinue treatment after 11 days due to ongoing emesis; vomiting persisted off treatment. The most common side effects of treatment were cutaneous reactions, experienced by five patients. Three of these cases presented within one week of taking the drug and resolved with drug withdrawal in all but one case, an eczematous reaction with peri‐orbital and limb dermatitis that required an eight‐week course of oral prednisolone. One patient chose to continue the drug after erythematous macules were noticed on his lower limb; however, these resolved on dose reduction for hyper‐somnolence. Two further patients required dose reduction to 100 mg for excessive drowsiness during the day, despite night‐time dosing. One patient experienced severe headaches, nausea and vomiting after three weeks of treatment, resembling raised intracranial pressure; computed tomography did not reveal evidence of metastases and symptoms resolved on drug withdrawal. Neutropenia occurred in one patient after four weeks of treatment (absolute neutrophil count 0.47 × 109 cells/L) and resolved within seven days of stopping thalidomide. One patient developed isolated paraesthesia after two weeks of thalidomide. Confirmed venous thromboembolism occurred in two patients, one from each arm of the study. Bilateral pulmonary emboli where diagnosed on computed tomography pulmonary angiography 15 days after commencing therapy in the patient receiving thalidomide. A deep vein thrombosis was diagnosed seven days after trial completion in the patient allocated to receive placebo.
Mantovani 2010a reported that toxicity was quite negligible and was comparable among treatment arms. All toxicities were assessed by the National Cancer Institute (NCI) common toxicity criteria for adverse events.
Arm 1: one patient had grade 1 to 2 diarrhoea, and one patient had a grade 1 to 2 thromboembolism or deep vein thrombosis.
Arm 2 one patient had grade 1 to 2 diarrhoea, and two patients had grade 1 to 2 epigastralgia.
Arm 3: two patients had grade 1 to 2 and two patients had grade 3 to 4 diarrhoea.
Arm 4: two patients had grade 1 to 2 somnolence.
Arm 5: three patients had grade 1 and two patients had grade 3 to 4 diarrhoea, one patient had grade 1 to 2 epigastralgia, and one patient had grade 1 to 2 thromboembolism or deep vein thrombosis.
Gordon 2005 reported that, overall, thalidomide appeared to be well tolerated. Two patients complained of peripheral neuropathy which resolved on stopping the drug, and two patients developed a rash that necessitated withdrawing from the trial. A further four patients complained of severe daytime somnolence that required a reduction in drug dosage in two patients and cessation of the drug in the other two. In the symptom scales at four weeks, constipation was significantly more common in the thalidomide group compared with placebo (P = 0.04) and insomnia significantly less common (P = 0.023). There was no significant difference between the two groups in any of the other symptom scales (fatigue, pain, nausea and vomiting, dyspnoea, appetite loss, diarrhoea, or financial difficulties). Other side effects were mild and did not differ significantly from placebo. Two patients in the placebo group and one in the thalidomide group developed deep vein thrombosis and were withdrawn from the study.
Withdrawals and dropouts
All three studies reported details of patient withdrawal and dropout. Due to the limited data, analysis was not conducted on the risk of withdrawal and specific adverse events between treatment groups. A narrative of the withdrawals and dropouts is therefore presented below and summarised in Table 4.
4. Patient flow.
| Wilkes 2011 | Gordon 2005 | Mantovani 2010a | |||||||
| Eligibility | |||||||||
| Patients assessed for eligibility | 126 | 64 | 332 | ||||||
| Excluded (ineligible) | 6 | 11 | 0 | ||||||
| Refused participation | 84 | 3 | 0 | ||||||
| Died prior to randomisation | 2 | 0 | 0 | ||||||
| Randomisation | |||||||||
| Total number of patients randomised | 34 | 50 | 332 | ||||||
| Intervention | |||||||||
| Treatment | Placebo | Thalidomide | Placebo | Thalidomide | 1 | 2 | 3 | 4 | 5 |
| Patient numbers | 17 | 17 | 25 | 25 | 44 | 25 | 88 | 87 | 88 |
| Follow up | |||||||||
| Time point 1 | Week 6 | Week 4 | 4 Months | ||||||
| Patients remaining | 16 | 8 | 16 | 17 | 42 | 23 | 85 | 84 | 86 |
| Reasons for loss | 1‐ died |
Drug toxicity Disease progression Elective withdrawal. (numbers not specified) |
1 – wrong diagnosis 4 – disease progression 3‐ died 1 – adverse event |
2 – wrong diagnosis 1 – disease progression 3 – died 2 ‐ adverse event |
Death ‐ 2 patients in arm 1 2 patients in arm 2 2 patients in arm 5 3 patients in arm 3 3 patients in arm 4 |
||||
| Time point 2 | N/A |
Week 8 | N/A |
||||||
| Patients remaining | 8 | 12 | |||||||
| Reasons for loss | 4 ‐ disease progression 1 ‐ died 2 – adverse event 1 ‐ other |
3 – died 1 ‐ adverse event 1 ‐ other |
|||||||
|
Key: 1, a progestational agent, that is, medroxyprogesterone acetate (MPA) (500 mg/day) or megestrol acetate (MA) (320 mg/day); 2, an oral eicosapentaenoic acid(EPA)‐enriched (2.2 g/day) nutritional supplement, in prescribed dosages of two cartons/day for both ProSure or 3 cartons/day for Forticare 3, L‐carnitine (4 g/day); 4, thalidomide (200 mg/day); 5, MPAorMAplus EPA‐enriched nutritional supplement plus L‐carnitine plus thalidomide. | |||||||||
Wilkes 2011 reported that of 126 eligible patients, 84 declined entry into the study, six were excluded due to established neuropathy or frailty and two died prior to randomisation. Thirty‐four patients were randomised to receive thalidomide or placebo. Only eight patients in the thalidomide arm were able to attend for repeat studies at week six, two of whom had discontinued treatment due to toxicity but all had taken at least 50% of their trial medication; a further two patients were able to tolerate treatment but were too unwell to attend for repeat studies. The remaining patients withdrew participation due to drug toxicity, disease progression or elective withdrawal. Sixteen patients who received placebo were able to complete the protocol; one patient on placebo died unexpectedly in his sleep after seven days of participation.
Gordon 2005 assessed 64 patients for eligibility, of these 11 were not eligible and 3 refused consent. The remaining 50 patients were then randomised: 25 placebo and 25 thalidomide group. By week four, there were 16 patients remaining in the placebo group: 1 patient was removed due to wrong diagnosis; 4 patients due to disease progression; 3 died; and 1 due to an adverse event. At the same time point, 17 remained in the thalidomide group: 2 patients were removed due to the wrong diagnosis; 1 due to disease progression; 3 died; and 2 had adverse events. By week eight of the trial only eight patients reminded in the placebo group. Between weeks four and eight, 4 patients were removed due to disease progression; 2 due to adverse events; 1 died and 1 patient was recorded as being removed due to 'other'. At the same time point,in the thalidomide group 12 patients remained. Between week four and eight, 1 patient had an adverse event, 3 died and 1 patient was recorded as being removed due to 'other'.
Mantovani 2010a detailed a CONSORT statement which outlined 332 patients were assessed for eligibility, none were excluded, none refused to participant, and every patient met the inclusion criteria. Patients were randomised into five arms: 44 patients were allocated to intervention arm 1; 25 to intervention arm 2; 88 to intervention arm 3; 87 to intervention arm 4; and 88 to intervention arm 5. Zero patients were lost to follow‐up but patients discontinued their intervention because of death (due to progressive disease): two patients in intervention arms 1, 2 and 5; and three patients in arms 3 and 4.
Overall results
This review focused on specific outcomes measured using validated tools and the results section reflected these pre‐set criteria. Whilst some studies also reported outcomes such as glutathione peroxidase levels, these have not be incorporated into the review. Studies which had varied tumour types and inclusion criteria with differing weight loss (for example 5% versus 10% ) or a clinical diagnosis of cancer cachexia were included in this review as we hoped to have sufficient studies under each of these categories to perform subgroup analysis (as detailed in our protocol). However, analysis across baseline weight loss or cancer diagnosis was not possible due to the small number of studies included in the review (three) and high heterogeneity between them in relation to inclusion criteria, outcome measures being tested at different times and tumour type. Particularly relevant was the high heterogeneity in inclusion criteria, for example Gordon 2005 specified a weight loss greater than 10% over the preceding six months in their inclusion criteria and this was not reflected in either of the other studies. This is synonymous with the ongoing literature, to establish a consensus definition for cachexia (Muscaritoli 2010), but nevertheless highlights the variability in patients recruited into these studies and thus the problems with pooling studies for meta‐analysis. Therefore, a descriptive analysis helped to provide an indication of the possible benefits and harms for the primary and secondary outcomes of interest.
Intervention 1: thalidomide versus placebo
Two studies examined thalidomide versus placebo (Gordon 2005; Wilkes 2011). As these studies looked at differing patient groups (advanced pancreatic cancer or advanced oesophageal cancer respectively) and both measured their primary outcome for the drug effect at differing time (four weeks or six weeks respectively) the data could not be pooled across the two trials.
Intervention 2: thalidomide versus alternative active treatment
In Mantovani 2010a five comparison arms were incorporated into the study design: arm 1, medroxyprogesterone 500 mg/d or megestrol acetate 320 mg/d; arm 2, oral supplementation with eicosapentaenoic acid (EPA); arm 3, L‐carnitine 4 g/d; arm 4, thalidomide 200 mg/d; arm 5, a combination of the above. Due to the early stopping of arms 1 and 2 in this trial, only arms 3, 4 and 5 were included in the analysis.
Primary outcome
Body composition
Measured by DEXA, lean body mass (LBM), total body water (TBW), mid‐upper arm circumference and weight.
Intervention 1: thalidomide versus placebo
Wilkes 2011 reported that over the six‐week study period, TBW and LBM remained unchanged from baseline values in compliant participants from both study groups. Availability of additional mid‐arm muscle circumference data failed to show muscle mass changes at two or four weeks of treatment. Furthermore, the difference observed between the groups was not significant for all body composition endpoints (all P > 0.05).
Gordon 2005 noted that at the primary endpoint of four weeks there was a significant difference in weight change, absolute difference ‐2.59 kg (95% CI ‐4.3 to ‐0.8); P = 0.005. At week eight there was still a significant difference between the two groups, with patients in the treatment group losing 0.06 kg compared with 3.62 kg (absolute difference ‐3.57 kg, 95% CI‐6.8 to ‐0.3; P = 0.034) in the placebo group.
At week four there was a significant difference for change in bone‐free arm muscle area (AMA) between the two groups. Patients in the treatment group had gained an average of 1 cm3 in bone‐free AMA while those in the placebo group lost an average of 4.6 cm3 (absolute difference ‐5.6 cm3, 95% CI ‐8.9 to ‐2.2; P = 0.002). This remained significant at week eight, with patients in the treatment group having lost an average of 0.5 cm3 compared with 8.4 cm3 (absolute difference ‐7.9 cm3, 95% CI ‐14.0 to ‐1.8; P = 0.014) in the placebo group.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a reported that a post hoc analysis showed the superiority of arm 5 over the other arms. LBM (DEXA) arm 5 (combination treatment including thalidomide) versus arm 3 (L‐carnitine) and arm 4 (thalidomide) (P < 0.001). However, there was no significant difference in LBM assessed by bioelectrical impedance analysis (BIA). An analysis of changes from baseline showed that LBM, as assessed by DEXA, significantly increased (P = 0.015) in arm 5 (combination treatment including thalidomide) whereas LBM as assessed by BIA did not change significantly. Additionally, the L3 computed tomography (CT) analysis showed an improvement in the estimated LBM (kg) (P = 0.001) and a trend toward an increase in muscle mass surface area (mm2) in arm 5. There was no significant difference from any primary endpoint in arm 4 (thalidomide) for any of the body composition primary endpoints.
Secondary outcomes
Grip strength
Intervention 1: thalidomide versus placebo
Grip strength was reported by one of the two trials in this section (Gordon 2005). There was no significant difference in grip strength between the two groups at any time point. Additionally, grip strength did not differ significantly from baseline at any time in either group.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a reported within‐group analysis only, which highlighted a trend toward an increase in grip strength in arm 4 (thalidomide) (P = 0.08) but not arm 5 (combination treatment including thalidomide).
Resting energy expenditure (REE)
Intervention 1: thalidomide versus placebo
Only one study in this section reported on REE and data were reported on within‐group analysis only. Wilkes 2011 found that REE did not differ significantly between the study groups at baseline: the median REE was 32.8 (95% CI 28.3 to 36.7) and 33.6 (95% CI 32.0 to 37.3) kcal/kg LBM/day for patients taking placebo and thalidomide respectively. Follow‐up data were available for 13 patients on placebo and 6 patients on thalidomide; the placebo group demonstrated a statistically significant increase from baseline readings from 32.3 to 34.4 kcal/kg LBM/day (P = 0.04). No difference from baseline was observed in the thalidomide group.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a reported REE, which was elevated at enrolment in 85% of patients: arm 5 (combination treatment including thalidomide) versus arm 3 (L‐carnitine) had a significant decrease (P = 0.004), arm 5 (combination treatment including thalidomide) versus arm 4 (thalidomide) had a non‐significant decrease (P = 0.056). In relation to within‐group comparisons REE decreased significantly (P = 0.44) in arm 5 (combination treatment including thalidomide); while it also deceased in arm 4 (thalidomide) this was not significant (P = 0.49).
Fatigue
Intervention 1: thalidomide versus placebo
Only one study in this section measured fatigue (Wilkes 2011). Piper Fatigue Scores did not differ between the groups, either at baseline or on study completion.
Intervention 2: thalidomide versus alternative active treatment
For fatigue, assessed by the Multidimensional Fatigue Symptom Inventory–Short Form, between groups there was an improvement in fatigue between arm 5 (combination treatment including thalidomide) versus arm 3 (L‐carnitine) (P = 0.004) and arm 5 (combination treatment including thalidomide) versus arm 4 (thalidomide) (P = 0.07). Within‐group analysis showed significant improvement in fatigue (P = 0.047) in arm 5 only (combination treatment including thalidomide). However, fatigue increased (from baseline to post‐treatment) in arm 4 (thalidomide) but this was not significant (P = 0.6).
Appetite
Only Mantovani 2010a measured appetite (VAS score). Data were presented for within‐group analysis, which increased significantly (P = 0.0003) in arm 5 only (combination treatment including thalidomide). A smaller increase was seen in arm 4 (thalidomide) (P = 0.3).
Survival
Intervention 1: thalidomide versus placebo
Wilkes 2011 reported that the median time to death for all participants was 109 days from the date of enrolment. Survival was not affected by group allocation, or whether the patient was able to complete the protocol.
Gordon 2005 reported that the median duration of survival from entering the study was 148 days in the thalidomide group (95% CI 67 to 171) compared with 110 days in the placebo group (95% CI 75 to 136), although this was not statistically significant (P = 0.45).
Intervention 2: thalidomide versus alternative active treatment
Survival was not measured by Mantovani 2010a.
Performance status
Intervention 1: thalidomide versus placebo
Performance status was not measured by Gordon 2005, however Wilkes 2011 reported that the Karnofsky score at baseline was 10 points higher (indicative of a better state of health) in the group given placebo, albeit not reaching statistical significance. After treatment, the median score remained unchanged on placebo whereas the thalidomide group had dropped by a further 10 points.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a presented within‐group analysis for GPS and ECOG‐PS scores, which decreased significantly in arm 5 (combination treatment including thalidomide), arm 4 (thalidomide), and arm 3 (L‐carnitine).
Quality of life
Intervention 1: thalidomide versus placebo
Only one study in this section recorded quality of life measurements. Gordon 2005 reported there was no significant difference in global health score or physical functioning between the two groups or from baseline in either group. However, change in physical functioning correlated positively with change in weight (P = 0.001) and there was a trend suggesting change in global health score correlated positively with change in weight, although this was not significant (P = 0.2).
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a reported within group analysis only, which highlighted a trend toward an improvement in EQ‐5D index in arm 5 (combination treatment including thalidomide) with no change in arm 4 (thalidomide). Their data also highlighted a trend towards an increase in EQ‐5D VAS in arm 4 (thalidomide) and a decrease in arm 5 (combination treatment including thalidomide), neither of which were significant (P = 0.7 and P = 0.9 respectively).
Biochemistry
Intervention 1: thalidomide versus placebo
Wilkes 2011 noted that serum concentrations of TNF‐alpha and IL‐1β did not differ significantly at baseline between those individuals given active drug or placebo. Six weeks of treatment did not change serum TNF concentrations in either group, whereas IL‐1β increased significantly (P < 0.05) in patients taking placebo but not in those taking thalidomide. Plasma albumin concentrations fell in both groups over the study period.
Gordon 2005 did not report biochemical markers.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a in their within‐group analysis reported that IL‐6 decreased significantly in arm 5 (combination treatment including thalidomide) (P = 0.02) and arm 4 (thalidomide) (P = 0.03), with a trend toward a decrease in TNF‐alpha in arm 5 only (P = 0.053). Additionally, total energy expenditure (TEE) and activity energy expenditure (AEE) (kcal/day and min/day) increased significantly in arm 5 (P < 0.05).
Discussion
The aim of this systematic review was to evaluate the effectiveness of thalidomide for cancer cachexia and identify and assess any adverse effects. This was done by comprehensive data searching and screening of published and unpublished work related to this topic. Despite undertaking such a thorough search, the literature has revealed a dearth of large, well conducted trials in this area. Indeed a meta‐analysis that was planned for this review was not possible. This has hindered the review authors' ability to make an informed decision about thalidomide for the management of cancer cachexia. At present, there is insufficient evidence to refute or support the use of thalidomide either as a single treatment modality or in combination with other treatments for the management of cachexia in advanced cancer patients.
Summary of main results
Intervention 1: thalidomide versus placebo
Two RCTs with a total of 84 participants were included (Gordon 2005; Wilkes 2011). As a result of the limited data available in the literature on thalidomide versus placebo for managing cancer cachexia, there was insufficient information to determine if thalidomide was better than placebo for the management of cancer cachexia. Most notably though, results from the Gordon 2005 study suggested that thalidomide was well tolerated and this is at odds with results from the Wilkes 2011 study, which highlighted the poor tolerability of thalidomide. Additional work on the tolerability and potential benefits and harms of thalidomide in this population is warranted.
Intervention 2: thalidomide versus alternative active treatment
Mantovani 2010a has conducted the largest (332 patients) randomised controlled trial of patients with advanced cancer who have cachexia. Results from their trial suggest that a multimodal approach (combining thalidomide with additional treatments) to cachexia management may be more effective than single treatment modalities, including thalidomide. However, more research in required to confirm the role of multimodal therapy for cachexia management, particularly as all participants in this study cohort also received supplemental dietary treatment. Additionally, this study only tested one multimodal approach to cachexia management and the usefulness of variations of this also need to be tested to ascertain if there is a most beneficial combination of treatments for this patient cohort.
Overall completeness and applicability of evidence
Treatments for cancer cachexia have generated debate and research activity for a significant amount of time. This review sought to verify if thalidomide was a successful treatment for the management of cachexia in advanced cancer patients. At this time we are unable to draw any conclusions regarding the applicability or otherwise of thalidomide for cancer cachexia because of the general dearth of available evidence.
Quality of the evidence
In assessing the methodological quality of studies included in this review, sample size was seen as being problematic for this type of study, which recruits advanced cancer patients. For example, Gordon 2005 and Mantovani 2010a did not achieve the sample size stipulated in their power calculations (25 and 95 for each arm, respectively). While Wilkes 2011 did achieve the sample size stipulated (17), all but two patients required either a dose reduction or cessation of treatment due to side effects; compared to 94% of the placebo group who completed their protocol. Additionally, the three studies did not provide sufficient information on the blinding of outcome assessors, and Mantovani 2010a also did not provide sufficient information on allocation concealment and blinding of participants and personnel, thereby precluding assessment of bias for these domains.
Potential biases in the review process
While every effort has been made to search for and include in this review all applicable studies in the area of thalidomide for cancer cachexia, there were sources of information which may have been relevant but which could not be included. These include conference abstracts that have not been published as full papers, and authors contacted that did not respond to requests for additional clarification on published research studies. We assessed all the studies included in this review for bias and independently extracted the data.
Agreements and disagreements with other studies or reviews
This is the first systematic review of thalidomide for cancer cachexia. Several papers have sought to summarise the applicability of thalidomide for cancer cachexia but may not have used a systematic approach (Dodson 2011; Murphy 2009). When placed in the context of papers evaluating thalidomide for cancer cachexia, the findings presented in this review confirm that a proven treatment modality for cancer cachexia has yet to be established (Coss 2011). Additionally, this review has highlighted the potential toxicities of thalidomide in an advanced cancer population, which have not been included in previous reviews.
Authors' conclusions
Implications for practice.
Through conducting this systematic review we cannot confirm or refute previous literature on the use of thalidomide for patients with advanced cancer who have cachexia and there is inadequate evidence to recommend it for clinical practice. Furthermore, the results from this systematic review suggest that there may be toxicities associated with thalidomide administration in this patient cohort that need to be explored further in light of the frailty of this population.
Implications for research.
Through conducting this systematic review, the lack of large RCTS is apparent. It is anticipated that the recent consensus on the clinical definition for cancer cachexia will help standardize inclusion criteria for future cachexia trials, as there was a degree of variability in the studies reviewed. Additionally, the majority of trials recruited patients who were extremely frail and at an advanced stage of their cancer trajectory, making if difficult to recruit sufficient numbers into studies and contributing to the high dropout rates. Up to 80% of cancer patients will have cachexia at an advanced stage (Murphy 2009) and it may be worth considering recruiting patients at an earlier juncture to see if potential treatment(s) would help prevent cachexia. It is also interesting to note that combination therapy for cancer cachexia has provided data on novel treatments and may be reflective of the multifaceted nature of this syndrome (Fearon 2008), and indeed its multidimensional impact on patients (Reid 2009a, Reid 2009b). Additional, well conducted, large RCTs are needed to test thalidomide both singularly and in combination with other treatment modalities to ascertain its true benefit, if any, for this population. The number of patients who have this debilitating syndrome underscores the significance of this research direction.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2021 | Review declared as stable | No new studies expected in this topic area. |
History
Protocol first published: Issue 9, 2010 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 23 July 2018 | Amended | No potentially relevant new studies identified after a scoping search (July 2018). The conclusions of this Cochrane Review are therefore still considered up to date. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
Notes
No new studies expected in this topic area.
Acknowledgements
Thanks is afforded to: The HSC R&D division of the Public Health Agency (Northern Ireland) for a Cochrane Fellowship awarded to the first review authors to undertake this systematic review; Jane Hayes, Information Manager, Cochrane Gynaecological Cancer Review Group for assistance with literature searches; Dr Lesley Smith, Principal Lecturer (Research Methods), School of Health & Social Care, Oxford Brookes University for assistance on the protocol and review.
Appendices
Appendix 1. Search strategies
MEDLINE search strategy (1950 to March week 4 2011)
1 exp Neoplasms/ 2 (cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma*).mp. 3 1 or 2 4 Cachexia/ 5 (cachex* or cachectic).mp. 6 (malnourish* or malnutrition or emaciation or weight loss or wasting or wasted).mp. 7 4 or 5 or 6 8 Thalidomide/ 9 thalidomid*.mp. 10 8 or 9 11 3 and 7 and 10 12 (animals not (humans and animals)).sh. 13 11 not 12
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier sh=subject heading
EMBASE search strategy (EMBASE 1980 to 2011 week 12)
1 neoplasm/ 2 (cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma*).mp. 3 1 or 2 4 cachexia/ 5 (cachex* or cachectic).mp. 6 (malnourish* or malnutrition or emaciation or weight loss or wasting or wasted).mp. 7 4 or 5 or 6 8 thalidomide/ 9 thalidomid*.mp. 10 8 or 9 11 3 and 7 and 10
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
CENTRAL (Cochrane Central Register of Controlled Trials) search strategy (2011, Issue 1)
#1 MeSH descriptor Neoplasms explode all trees #2 cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* #3 (#1 OR #2) #4 MeSH descriptor Cachexia explode all trees #5 cachex* or cachectic #6 malnourish* or malnutrition or emaciation or weight loss or wasting or wasted #7 (#4 OR #5 OR #6) #8 MeSH descriptor Thalidomide explode all trees #9 thalidom* #10 (#8 OR #9) #11 (#3 AND #7 AND #10)
CINAHL search strategy (inception to April 2011)
1 neoplasm 2 cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* 3 1 or 2 4 cachexia 5 cachex* or cachectic 6 malnourish* or malnutrition or emaciation or weight loss or wasting or wasted 7 4 or 5 or 6 8 thalidomide 9 thalidomid* 10 8 or 9 11 3 and 7 and 10
Web of Science search strategy (inception to April 2011)
#1 ‐ cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* #2 ‐ cachex* or cachectic #3 ‐ cachexia #4 ‐ malnourish* or malnutrition or emaciation or weight loss or wasting or wasted # 5 ‐ #2 or # 3 or #4 #6 ‐ thalidomide #7 ‐ thalidomid* #8 ‐ #6 or #7 #9 ‐ # 1 and #5 and #8
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gordon 2005.
| Study characteristics | ||
| Methods | Prospective randomised double‐blind, placebo‐controlled trial | |
| Participants | 50 advanced pancreatic cancer patients according to clinical and radiological findings, operative appearance, and/or histological diagnosis; patient deemed inoperable either on the basis of tumour anatomy, inability to survive major surgery, or patient preference; greater than 10% weight loss over the preceding six months; and likely life expectancy of at least six weeks based on clinical judgment. | |
| Interventions | Thalidomide 200mgs for 6 months or placebo | |
| Outcomes | Primary outcome measure was change in weight (Kg) at four weeks (also measured at eight weeks) Secondary outcome measures were change in bone free muscle mass (cm), grip strength, quality of life ‐ Global Health Score (all measured at week 4 and 8) and survival. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was undertaken in blocks of four using a sequential series of sealed envelopes containing a computer generated code” (p541) |
| Allocation concealment (selection bias) | Low risk | “Randomisation envelopes were opened by a third party who dispensed the trial drug in a double blind fashion” (p541) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo used, states double blind (p541) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Participants were subsequently assessed every four weeks for six months with the same measurements and blood tests recorded at each visit. All measurements were undertaken by the same investigator (TJ).” (p541) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram presented to detail patient flow/ attrition etc. Potential bias due to ‘other’ |
| Selective reporting (reporting bias) | Low risk | Results of the primary and 4 secondary outcomes all reported |
| Other bias | Unclear risk | None seen |
Mantovani 2010a.
| Study characteristics | ||
| Methods | Randomised phase III clinical trial | |
| Participants | 332 patients (aged ≥18 years) with a histologically confirmed advanced stage tumor at any site, loss of >5% of ideal or pre‐illness body weight in the previous 3 months with or without abnormal values of pro‐inflammatory cytokines predictive of the onset of clinical cachexia, and a life expectancy ≥4 months, were eligible. Patients could be receiving concomitant antineoplastic chemotherapy or hormone therapy with palliative intent or supportive care. | |
| Interventions | 5 arms: 1‐‐medroxyprogesterone 500 mg/d or megestrol acetate 320 mg/d; 2‐‐oral supplementation with eicosapentaenoic acid (EPA); 3‐‐L‐carnitine 4 g/d; 4‐‐thalidomide 200 mg/d; 5‐‐a combination of the above. Treatment duration: 4 months. | |
| Outcomes | Primary outcomes: Lean body mass, resting energy expenditure and fatigue. These were assessed by bioelectrical impedance analysis / dual‐energy X‐ray absorptiometry, indirect calorimetry and the Multidimensional Fatigue Symptom Inventory–Short Form, respectively. Secondary endpoints: Appetite (visual analogy scale); grip strength (dynamometer) ; quality if life (EORTC QLQ‐C30, EQ‐5Dindex, and EQ‐5DVAS); serum levels of interleukin ‐6 and tumour necrosis factor‐alpha (enzyme‐linked immunosorbent assays); Glasglow Performance Scale, blood levels of reactive oxygen species (FORT test) and antioxidant enzyme glutathione peroxidase (photometer); total daily physical activity and the associated total energy expenditure (SenseWear PRO2 Armband) and performance status (Eastern Cooperative Oncology Group PS scale). All outcomes measured at baseline, 4, 8 and 16 weeks. |
|
| Notes | All patients were given, as basic treatment, polyphenols (300 mg/days) obtained by dietary sources or supplemented with tablets, lipoic acid (300 mg/day), carbocysteine (2.7 g/day), vitamin E (400 mg/day), vitamin A (30,000 IU/day), and vitamin C (500 mg/day). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “patients were randomised to one of five arms” (ERMPS, p. 293)“Random assignment was performed by random‐number tables” (Nutrition, p306) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in sufficient detail to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in sufficient detail to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in sufficient detail to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consort diagram provided (p203) |
| Selective reporting (reporting bias) | Low risk | Data from all primary and secondary outcomes presented |
| Other bias | Unclear risk | None seen |
Wilkes 2011.
| Study characteristics | ||
| Methods | Randomised placebo‐controlled trial | |
| Participants | 34 patients with advanced oesophageal cancer | |
| Interventions | Thalidomide 200mg or placebo for 6 weeks | |
| Outcomes | Primary endpoints were change in total body weight and lean body mass, measured at baseline and 6 weeks using calibrated electronic scales and dual‐energy X‐ray absorptiometry respectively. Secondary outcomes included: Routine biochemistry including tumour necrosis factor‐alpha and interleukin 1‐beta and blood count as well as resting energy expenditure (measured by indirect calorimetry using ventilated hood apparatus); measured at baseline at 6 weeks. Triceps skin‐fold thickness, mid‐arm circumference, symptoms of disease progression, adverse drug reactions, performance indices (Karnofsky index) and Piper Fatigue questionnaire were measured at baseline, weeks 2, 4, and 6. |
|
| Notes | The thalidomide group was significantly heavier in terms of TBW and LBM (P<0.05). Poor tolerability of thalidomide noted in this patient cohort. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Treatment allocation was governed using a computer‐generated randomisation protocol held by the dispensing pharmacy. The protocol was generated by an independent statistician using block randomisation (block of four).“ (p3) |
| Allocation concealment (selection bias) | Unclear risk | “Treatment allocation was disclosed to the investigators after the last participant had completed the protocol” (p3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Treatment allocation was disclosed to the investigators after the last participant had completed the protocol” (p3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Of 126 eligible patients, 84 declined entry into the study, six were excluded due to established neuropathy or frailty and two died prior to randomisation. Thirty‐four patients were randomised to receive thalidomide or placebo. Only eight patients in the thalidomide arm were able to attend for repeat studies at week 6, two of whom had discontinued treatment due to toxicity but all had taken at least 50% of their trial medication; a further two patients were able to tolerate treatment but were too unwell to attend for repeat studies. The remaining patients withdrew participation due to drug toxicity, disease progression or elective withdrawal. Sixteen patients who received placebo were able to complete the protocol; one patient on placebo died unexpectedly in his sleep after 7 days of participation” (p3) |
| Selective reporting (reporting bias) | Unclear risk | Triceps skin‐fold thickness not mentioned specifically in results, all other primary and secondary measures reported. |
| Other bias | Unclear risk | None seen |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boasberg 2000 | Conference proceeding, abstract only published ‐ author contacted 13/03/2009 and full research report not published. |
| Bruera 1999 | Body composition not an outcome. |
| Bruyn 1998 | Review. |
| Calder 2000 | Research paper on thalidomide and cancer but not cachexia. |
| Hackshaw 2008 | Review. |
| Haslett 1998 | Review. |
| Herrera 2001 | Review. |
| Lazenby 2010 | Review. |
| Lee 2009 | Research paper on thalidomide and cancer but not cachexia. |
| Martino 2007 | Research paper on thalidomide and cancer but not cachexia. |
| Ockenga 2006 | Authors' response to journal article, commentary only, not research paper. |
| Pavlakis 2006 | Conference proceeding, abstract only published ‐ author contacted and full research report not published. Insufficient data in abstract. |
| Sharma 2006 | Research paper on thalidomide analogue and cancer. |
| Tassinari 2008 | Body composition not an outcome. |
Characteristics of studies awaiting classification [ordered by study ID]
Sanchetee 2010.
| Methods | Single centre, double‐blind, randomised controlled trial |
| Participants | Fifty patients with gastrointestinal cancer who had lost at least 10% of their body weight |
| Interventions | Thalidomide 100 mg daily, olanzipine 5 mg OD and megestrol acetate 80 mg OD or thalidomide for 24 weeks |
| Outcomes | Weight, arm muscle mass, physical functioning |
| Notes | Author contacted for additional information ‐ no response gained |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN23944748.
| Study name | The Role of Thalidomide in Reversing Cachexia in Patients with Oesophageal Cancer |
| Methods | Double‐blind placebo‐controlled trial |
| Participants | Adults with non‐obstructing and inoperable oesophageal cancer (dysphagia score <3, able to swallow a semi‐solid diet) |
| Interventions | Thalidomide versus placebo |
| Outcomes | Change in lean body mass |
| Starting date | 10/12/2002 |
| Contact information | Derby Hospitals NHS Foundation Trust Derby City Hospital Uttoxeter Road |
| Notes | emailed 18/08/2011 re update (emailed undeliverable) |
ISRCTN51456701.
| Study name | The use of thalidomide as a treatment for cancer cachexia |
| Methods | Randomised controlled trial |
| Participants | 1. Have a histological or cytological diagnosis of upper gastrointestinal (oesophagus, stomach, small bowel, ampulla or pancreas) adenocarcinoma 2. Have no curative options available which are acceptable to the patient 3. Have lost 5% total of pre‐morbid body weight or be actively losing at least 1 kg per month 4. Weight loss may be self‐reported or obtained from previous documentation 5. If a patient is using megesterol acetate (Megace, Megestrol) or eicosapentaenoic acid (Maxepa, Omacor, Prosure) and has been on a stable dose for at least a month but losing weight at the stated rate despite this they may be included. They will be asked to continue on this same dose for the course of the study. 6. Those using corticosteroids, non‐steroidal anti‐inflammatory drugs and other nutritional supplements or complementary therapies will not be restricted, the doses used will be recorded at each clinic visit 7. Have a predicted survival of at least 8 weeks 8. Aged over 18 years at the time to entry into the trial 9. Able to understand the information given and to give written informed consent 10. Able to take oral medications 11. Agree to the conditions of use of thalidomide as enumerated 12. Women who have not had their ovaries or uterus removed or who have been post‐menopausal for at least 2 years, must have a negative urinary pregnancy test and negative pregnancy tests repeated on a monthly basis until 1 month after completion of the trial |
| Interventions | Thalidomide or placebo |
| Outcomes | To evaluate the ability of thalidomide, as compared with placebo, to attenuate loss of weight in patients with incurable upper gastrointestinal carcinomas |
| Starting date | 03/10/2005 |
| Contact information | Gastroenterology Dept Queen Alexandra Hospital Cosham |
| Notes | Analysis ongoing |
NCT01127386.
| Study name | Lenalidomide for Lean Body Mass and Muscle Strength in Inflammatory Cancer Cachexia Syndrome |
| Methods | Randomised double‐blind trail |
| Participants |
|
| Interventions | Lenalidomide versus basic cachexia management |
| Outcomes | Lean body mass and handgrip strength [Time Frame: after 8 weeks treatment] |
| Starting date | May 2010 |
| Contact information | Florian Strasser, MD |
| Notes | Estimated completion date 12/2012 |
Differences between protocol and review
Meta analysis could not be conducted across trials. A qualitative descriptive analysis of trials was therefore reported. The following methodological section will be implemented in a future update if new trials are identified which can be assess through a meta‐analysis.
Measures of treatment effect
We will use the following measures of the effect of treatment.
For continuous outcomes, we will use the mean difference between treatment arms.
For time to event data, we will use the HR, if possible.
For dichotomous outcomes, we will use the risk ratio (RR).
Unit of analysis issues
Detailed below.
Dealing with missing data
We will not impute missing outcome data for the primary outcome. If data are missing we will contact trial authors to request data on the outcomes.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by subgroup analyses. If there is no evidence of heterogeneity, a fixed‐effect model, which assumes a common underlying effect behind every trial, will be used. If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported. If marked heterogeneity is suspected (greater than 75%), estimates will not be combined. All potential causes of such heterogeneity will be explored through subgroup and sensitivity analysis. If there is heterogeneity then a random‐effects model will be used and each trial will be assumed to be measuring a different, true effect. While it is acknowledged that a random‐effects model is more susceptible to publication bias, methods to formally test publication bias will be incorporated into the analysis as outlined below.
Data synthesis
This review will examine different types of studies, including RCTs and non‐randomised studies. Therefore, data analysis will be conducted separately for each study design. If sufficient, clinically similar studies are available, their results will be pooled in meta‐analyses. Adjusted summary statistics will be used if available; otherwise unadjusted results will be used.
For continuous outcomes, the mean differences (MDs) between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardized mean differences (SMDs) will be pooled. For time‐to‐event data, hazard ratios (HRs) will be pooled using the generic inverse variance facility of RevMan 5. For any dichotomous outcomes, the risk ratios (RR) will be calculated for each study and these will then be pooled.
If any trials have multiple treatment groups, the 'shared' comparison group will be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group will be treated as independent comparisons. Random‐effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986). The final discussion will include a narrative synthesis of the findings of each study design.
Subgroup analysis and investigation of heterogeneity
Where appropriate, subgroup analysis will be performed, potentially grouping the trials by type of therapy regime (single versus multimodal therapy), duration of thalidomide administration (short term (two weeks) compared to longer term (four months)), baseline weight loss (5% versus 10%), and cancer diagnosis. Thalidomide tested in combination with other therapy regimes, such as exercise or an alternative drug modality, will be considered providing the treatment effect of thalidomide can be differentiated (that is study testing exercise regime plus thalidomide and exercise regime plus placebo). No post hoc subgroup analysis will be performed.
Sensitivity analysis
Sensitivity analyses will be performed: (i) excluding studies at high risk of bias, and (ii) using unadjusted results.
Contributions of authors
JR is the main author of this review; MM provided editorial support; all authors were involved in the development of the review and CC provided statistical advice.
Sources of support
Internal sources
No sources of support provided
External sources
R&D Office of the DHPSSNI, Cochrane Fellowship, UK
Declarations of interest
None to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Gordon 2005 {published data only}
- Gordon JN, Trebble TM, Ellis RD, Duncan HD, JohnsT, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. GUT 2005;54:540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantovani 2010a {published data only}
- Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, et al. Randomised phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. The Oncologist 2010;15:200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkes 2011 {published data only}
- Wilkes EA, Selby AL, Cole AT, Freeman JG, Rennie MJ, Khan ZH. Poor tolerability of thalidomide in end-stage oesophageal cancer. European Journal of Cancer Care 2011;DOI: 10.1111/j.1365-2354.2011.01255.x. [Epub ahead of print]:1-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boasberg 2000 {published data only}
- Boasberg PD, O'Day SJ, Weisberg M et al. Thalidmode induced cessation of weight loss and improved sleep in advanced cancer patients with cachexia. American Society Clinical Oncology 2000;19:609a, Abstract 2396. [Google Scholar]
Bruera 1999 {published data only}
- Bruera E, Neumann CM, Pituskin E, Calder K, Ball G, Hanson J. Thalidomide in patients with cachexia due to terminal cancer: Preliminary report. Annals of Oncology 1999;10(7):857-9. [DOI] [PubMed] [Google Scholar]
Bruyn 1998 {published data only}
- Bruyn GAW. Thalidomide - Celgene Corporation. Drugs 1998;1(4):490-500. [PubMed] [Google Scholar]
Calder 2000 {published data only}
- Calder K, Bruera E. Thalidomide for night sweats in patients with advanced cancer. Palliative Medicine 2000;12(1):77-8. [DOI] [PubMed] [Google Scholar]
Hackshaw 2008 {published data only}
- Hachshaw A. Small studies: strengths and limitations. European Respiratory Journal 2008;32(5):1141-3. [DOI] [PubMed] [Google Scholar]
Haslett 1998 {published data only}
- Haslett PAJ. Antocytokine approaches to the treatment of anorexia and cachexia. Seminars in Oncology 1998;25(2):53-7. [PubMed] [Google Scholar]
Herrera 2001 {published data only}
- Herrera E, Bruera E. New drugs potentially useful for the cachexia treatment in palliative care. Medicina Paliativa 2001;8(3):144-58. [Google Scholar]
Lazenby 2010 {published data only}
- Lazenby JM, Saif MW. Palliative care from the beginning of treatment for advanced pancreatic cancer. Journal of the Pancreas 2010;11(2):154-7. [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee SM, Rudd R, Woll PJ, Ottensmeier C, Gilligan D, Price A, et al. Randomised double blind placebo controlled trial of thalidomide in combination with gemcitabine and carboplatin in advanced non small cell lung cancer. Journal of Clinical Oncology 2009;27(31):5248-54. [DOI] [PubMed] [Google Scholar]
Martino 2007 {published data only}
- Martino M, Console G, Callea V, Stelitano C, Massara E, Irrera G, et al. Low tolerance and high toxicity of thalidomide as maintenance therapy after double autologous stem cell transplant in multiple myeloma patients. European Journal of Haematology 2007;78(1):35-40. [DOI] [PubMed] [Google Scholar]
Ockenga 2006 {published data only}
- Ockenga J, Valentini L. Thalidomide: an effective anabolic agent in gastrointestinal cancer cachexia. Alimentary Pharmacology and Therapeutics 2006;23(3):446-7. [DOI] [PubMed] [Google Scholar]
Pavlakis 2006 {published data only}
- Pavlakis N, Aslani A, Harvie R, Smith RC, Wheeler H. Malignant mesothelioma and cachexia. description of body composition changes in 27 patients receiving chemotherapy and/or thalidomide. Lung cancer 2006;54:S17. [Google Scholar]
Sharma 2006 {published data only}
- Sharma RA, Stewart WP, Daines CA, Knight RD, O'Byrne KJ, Dalgleish AG. Toxicity profile of the immunomodulatory thalidomide analogue, lenalidomide: Phase I clinical trial of thr5ee dosing schedules in patients with solid malignancies. European Journal of Cancer 2006;42(12):2318-25. [DOI] [PubMed] [Google Scholar]
Tassinari 2008 {published data only}
- Tassinari D, Santelmo C, Tombesi P, Sartori S. Thalidomide in the treatment of cancer cachexia. Journal of Palliative Care 2008;24(3):187-9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Sanchetee 2010 {published data only}
- Sanchetee SC, Sacchetee S. Thalidomide versus thalidomide with olanzapine and megestrol acetate in treatment of cachexia in gastro intestinal cancer: A randomised trial. Support Cancer Care 2010;18 Suppl 3:171. [Google Scholar]
References to ongoing studies
ISRCTN23944748 {published data only}
- The Role of Thalidomide in Reversing Cachexia in Patients with Oesophageal Cancer. Ongoing study. 10/12/2002. Contact author for more information.
ISRCTN51456701 {published data only}
- The use of thalidomide as a treatment for cancer cachexia. Ongoing study. 03/10/2005. Contact author for more information.
NCT01127386 {published data only}
- Lenalidomide for Lean Body Mass and Muscle Strength in Inflammatory Cancer Cachexia Syndrome. Ongoing study. May 2010. Contact author for more information.
Additional references
Argiles 2010
- Argiles JM, Anker SD, Evans WJ Morley JE, Fearon KCH, Strasser F, et al. Consensus on cachexia definitions. Journal of the American Medical Directors Association 11;4:229-30. [DOI] [PubMed] [Google Scholar]
Berenstein 2007
- Berenstein G, Ortiz Z. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD004310. [DOI: 10.1002/14651858.CD004310.pub2] [DOI] [PubMed] [Google Scholar]
BNF 2009
- British National Formulary. British National Formulary. Vol. 58. London: British Medical Association, September 2009. [Google Scholar]
Brown 1999
- Brown DJF. The problem of weakness in patients with advanced cancer. International Journal of Palliative Nursing 1999;5(1):6-12. [Google Scholar]
Bruera 1993
- Bruera E, Fainsinger RL. Clinical management of cachexia and anorexia. In: MacDonald N, editors(s). Palliative Medicine: a case based manual. Oxford: Oxford University Press, 1993. [Google Scholar]
Bruera 1996
- Bruera E, Higginson I. Cachexia-anorexia in cancer patients. Oxford University Press: Oxford, 1996. [Google Scholar]
Burch 2000
- Burch PA, Richardson RL, Cha SS, Sargent DJ, Pitot HG, Kaur JS et al. Phase III study of paclitaxel and cisplatin for advanced urothelial cancer. Journal of Urology 2000;164:1538-42. [PubMed] [Google Scholar]
Coss 2011
- Coss CC, Bohl C, Dalton JT. Cancer cachexia therapy. Current Opinion in Clinical Nutrition and Metabolic Care 2011;14(3):268-73. [DOI] [PubMed] [Google Scholar]
Dahele 2004
- Dahele M, Fearon KCH. Research Methodology: Cancer cachexia syndrome. Palliative Medicine 2004;18:409-17. [DOI] [PubMed] [Google Scholar]
DeBoer 2006
- DeBoer M, Marks DL. Adiponectin and insulin resistance associated with endometrial cancer. Endocrinology and Metabolism 2006;2:423-5. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Dewey 2007
- Dewey A, Baughan C, Dean TP, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004597. [DOI: 10.1002/14651858.CD004597.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodson 2011
- Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annual Review of Medicine 2011;18(62):265-79. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Phillips AN. Meta-analysis: principles and procedures. BMJ 1997;315:1533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanelli 2003
- Fanelli M, Sarmiento R, Gattuso D, Carillio G, Capaccetti B, Vacca A, et al. Thalidomide: a new anticancer drug? Expert Opinion on Investigational Drugs 2003;12:1211-25. [DOI] [PubMed] [Google Scholar]
Fearon 2003
- Fearon KCH, Von Meyenfeldt MF, Moses AGW, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia. Gut 2003;52:1479-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fearon 2006
- Fearon FC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. American Journal of Clinical Nutrition 2006;83(6):1345-50. [DOI] [PubMed] [Google Scholar]
Fearon 2008
- Fearon KCH. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. European Journal of Cancer 2008;44:1124-32. [DOI] [PubMed] [Google Scholar]
Fearon 2011
- Fearon K, Strasser F, Bosaeus I, Bruera E, Fainsinger R, Jatoi A, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology 2011;12:489-95. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org 2009.
Higginson 1996
- Higginson I, Winget C. Psychological impact of cancer cachexia on the patient and family. In: Bruera E, Higginson I, editors(s). Cachexia-Anorexia in Cancer Patients. Oxford: Oxford University Press, 1996:172-84. [Google Scholar]
Inui 2002
- Inui A. Cancer anorexia-cachexia syndrome. CA: A Cancer Journal for Clinicians 2002;52:72-91. [DOI] [PubMed] [Google Scholar]
Karnofsky 1949
- Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, editors(s). Evaluation of Chemotherapeutic Agents. New York: Columbia University Press, 1949:199-205. [Google Scholar]
Khan 2003
- Khan ZH, Simpson EJ, Cole AT, Holt M, MacDonald I, Pye D, et al. Oesophageal cancer and cachexia: the effect of short term treatment with thalidomide on weight loss and lean body mass. Alimentary Pharmacology and Therapeutics 2003;17:677-82. [DOI] [PubMed] [Google Scholar]
Kolter 2000
- Kolter DP. Cachexia. Annals of Internal Medicine 2000;133(8):622-34. [DOI] [PubMed] [Google Scholar]
Laviano 2005
- Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy Insight: cancer anorexia–cachexia syndrome—when all you can eat is yourself. Nature 2005;2(3):158-65. [DOI] [PubMed] [Google Scholar]
Loprinzi 1993
- Loprinzi CL, Michalak JC, Schaid DJ, Mailliard JA, Athmann LM, Goldberg RM, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. Journal of Clinical Oncology 1993;22:762-7. [DOI] [PubMed] [Google Scholar]
Ma 1998
- Ma G, Alexander HR. Prevalence and pathophysiology of cancer cachexia. In: Bruera E and Portenoy R, editors(s). Topics in palliative care. Oxford: Oxford University Press, 1998. [Google Scholar]
Maddocks 2010
- Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KCH, Wilcock. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Supportive Care in Cancer 2010;18(12):1539-44. [DOI] [PubMed] [Google Scholar]
Mantovani 2008
- Mantovani G, Maccio A, Madeddu, C, Giulia G, Serpe, R, Massa E, et al. Randomised phase III clinical trial of five different arms of treatment for patients with cancer cachexia: Intrim results. Nutrition 2008;24:305-13. [DOI] [PubMed] [Google Scholar]
Mantovani 2010b
- Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. European Review for Medical and Pharmacological Sciences 2010;14:292-301. [PubMed] [Google Scholar]
Mearnes 1997
- Meares CJ. Primary caregiver perceptions of intake cessation in patients who are terminally ill. Oncology Nursing Forum 1997;24:1751-7. [PubMed] [Google Scholar]
Murphy 2009
- Murphy KT, Lynch GS. Update on emerging drugs for cancer cachexia. Expert Opinion on Emerging Drugs 2009;14:619-32. [DOI] [PubMed] [Google Scholar]
Muscaritoli 2010
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Intreste Group (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clinical Nutrition 2010;29:154-9. [DOI] [PubMed] [Google Scholar]
Ramos 2004
- Ramos EJB, Sukuzi, S Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome. Current Opinion Clinical Nutrition and Metabolic Care 2004;7:427-34. [DOI] [PubMed] [Google Scholar]
Reid 2009a
- Reid J, McKenna H, Fitzsimons D, and McCance T. The experience of cancer cachexia: A qualitative study of advanced cancer patients and their family members. International Journal of Nursing Studies 2009;46(5):606-16. [DOI] [PubMed] [Google Scholar]
Reid 2009b
- Reid J, McKenna H, Fitzsimons D, and McCance T. Fighting over food: Patient and family understanding of cancer cachexia. Oncology Nursing Forum 2009;36(4):439-45. [DOI] [PubMed] [Google Scholar]
Rosenbaum 2000
- Rosenbaum K, Wang J, Pierson R, Kolter D. Time-dependent variation in weight and body composition in healthy adults. Journal of Parenteral and Enteral Nutrition 2000;24:52-5. [DOI] [PubMed] [Google Scholar]
Stein 2004
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain and Symptom Management 2004;27(1):14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroud 2005
- Stroud MA. Thalidomide and cancer cachexia: old problem, new hope? Gut 2005;54:447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tisdale 2002
- Tisdale MJ. Cachexia in cancer patients. Nature Reviews Cancer 2002;2:862-71. [DOI] [PubMed] [Google Scholar]
Tisdale 2009
- Tisdale MJ. Mechanisms of cancer cachexia. Physiological Reviews 2009;89:381-410. [DOI] [PubMed] [Google Scholar]
Wilkes 2006
- Wilkes EA, Freeman JG. Thalidomide: an effective anabolic agent in gastrointestinal cancer cachexia. Alimentary Pharmacology and Therapeutics 2006;23(3):445-6. [DOI] [PubMed] [Google Scholar]


