Editor’s Note
About 30 percent of all people suffering from depression do not respond adequately to available treatments. Our author, a leading researcher in the field of antidepressants, says that the rediscovery of a promising, yet problematic, drug called ketamine is the most significant breakthrough for treating depression in half a century. Will ketamine inspire the next generation of antidepressants?
The World Health Organization reports that depression is the leading cause of disability and loss of work worldwide, affecting more than 300 million people.3 In the United States, the lifetime incidence of major depressive disorder is approximately 17 percent at an estimated cost to the economy of over $200 billion annually.1 This devastating illness is characterized by depressed mood, feelings of worthlessness, inability to experience pleasure, and disruption of eating, sleeping, social, and sexual activities. In severe cases, it can lead to suicide, which has become the third leading cause of death, up 30 percent over the past 10 years, to more than 44,000 in the US alone.2
Despite its prevalence and impact, the available therapeutic medications for depression are limited to one class of drugs, monaminergic agents that increase levels of serotonin and/or norepinephrine. These drugs block the reuptake or metabolism of monoamines and, over time, lead to adaptive changes that underlie their antidepressant actions. This class of antidepressants was discovered in the 1950s, and although there have been refinements to reduce side effects, they have gone largely unchanged in over 60 years. These agents have serious limitations, notably low rates of efficacy (only one in three patients respond to the first prescribed medication), and a delay in therapeutic response of weeks to months.4 Even after trying multiple types of monoaminergic agents, plus supplemental medications, about a third of patients remain nonresponsive and are considered treatment resistant. The time lag and treatment resistance can have fatal consequences for a population at high risk of suicide.
The limitations of currently available agents explain the excitement and hope surrounding the discovery of a new class of antidepressants whose prototype, ketamine, produces rapid (within hours) relief from severe depressive symptoms, even in patients considered treatment resistant.5 Ketamine is an antagonist of the NMDA (N-methyl-D-aspartate) receptor, one type of receptor for glutamate, the major excitatory amino acid in the brain. At high doses, the drug is used as a dissociative anesthetic, producing catalepsy, catatonia, analgesia, and amnesia, that is often used for pediatric and veterinary medicine. A single low dose of ketamine, however, produces rapid antidepressant actions that last for approximately one week in most individuals. The discovery of a new, rapidly acting class of drug with a completely novel mechanism is arguably the biggest breakthrough in the field of depression in over 60 years. However, ketamine also has side effects, notably dissociative and psychotomimetic actions that, while transient, have limited its widespread use.
Nevertheless, the discovery of ketamine provides a much-needed option for treatment resistant depression and has spurred research and drug development efforts to identify new drugs that produce its rapid and efficacious antidepressant actions without its side effects. Let’s explore the discovery and validation of ketamine as a rapid acting antidepressant, its novel mechanism of action, and progress toward the development of like agents.
Drug Development Issues
The monoamine reuptake blocking antidepressants, which include the serotonin selective reuptake inhibitors that are among the most highly prescribed classes of drugs, began with tricyclics antidepressants, an early chemical subclass that blocks the reuptake of serotonin and norepinephrine. Patients given tricyclics reported improvement in depressive symptoms, but only after the drugs had been administered for several weeks. This led to the monoamine hypothesis that deficits in serotonin or norepinephrine in the brain cause depression, although the evidence for this theory is not very strong.
The time lag for the therapeutic action of monoaminergic agents has also been difficult to explain, since these agents rapidly block reuptake and increase synaptic levels of monoamines in a matter of days. This has led to the theory that delayed neuronal adaptation to elevated serotonin is required for an antidepressant response. Among a number of reports on adaptive changes, one key hypothesis attributes therapeutic action to delayed increase in the expression of growth factors, particularly BDNF (brain-derived neurotrophic factor). The elevation of BDNF contributes to the reversal of synaptic deficits caused by chronic stress and depression.
While these drugs provide some therapeutic benefit, their shortcomings have prompted continuous research and development over the past decades. These efforts have focused largely on refinements of monoamine reuptake inhibitors to reduce their side effects, the creation of selective or dual reuptake inhibitors, and the development of agents that target reuptake of dopamine, a monoamine neurotransmitter system involved in motivation and reward. Most of these new monoaminergic agents have the same limitations of the earlier ones, except a somewhat better side effect profile. There have also been efforts to target blockade of neuropeptides, notably corticotrophin releasing factor (CRF), that mediate the endocrine and behavioral responses to stress. These are based on rational design and significant antidepressant-like effects in rodent models, but have not translated into effective treatment options for humans. More generally, this difficulty in translation from experimental animals to patients has been a serious problem for development of novel medications for all psychiatric illnesses and the reason why most major pharmaceutical companies have significantly or completely cut research and development is this area.
Ketamine and NMDA Receptor Antagonists
The discovery of ketamine as a rapid antidepressant goes back to early attempts to understand the neurobiology of psychosis and schizophrenia. A related compound, phencyclidine (PCP), was first synthesized by Parke-Davis in 1959 and, although it was a potent anesthetic, it produced severe hallucinations upon awakening.
Efforts to circumvent this problem led to the synthesis of ketamine in 1962, which also had potent anesthetic and analgesic properties, and was approved for clinical use by 1970, although it still produced psychotomimetic actions, but less so than PCP. (The psychotomimetic and dissociative effects these drugs produce at low doses6 have led to their abuse; on the street, ketamine and PCP are referred to as “special K” and “angel dust,” respectively.)
These agents block NMDA receptors, a major class of excitatory neurotransmitter receptors in the brain that gate the entry of calcium, an important signaling molecule. When the NMDA receptor is activated by glutamate, the channel opens to allow calcium to flow inside the neuron; in this open state ketamine can also enter and bind inside the channel and block subsequent calcium entry and NMDA receptor function. While these agents were found to produce anesthesia at high doses, at low doses they were found to produce psychotomimetic and dissociative effects in humans.6
The finding that NMDA receptor antagonists produce psychotomimetic effects contributed to the NMDA receptor deficit hypothesis of schizophrenia, and research efforts on the neurobiology of this illness included studies with low doses of ketamine. In addition, evidence was accumulating that linked chronic administration of typical antidepressants to adaptations of NMDA receptor expression and function. Together these findings led John Krystal, Rob Berman, Dennis Charney and colleagues at Yale University to test the antidepressant actions of ketamine. In this small, double blind, placebo-controlled trial, a single dose of ketamine was found to produce a rapid antidepressant response with an average onset of four hours that lasted for at least three days.5 This therapeutic response occurred after approximately one to two hours of transient, psychotomimetic, and dissociative effects. These studies used intravenous administration, since that was the typical route used clinically for anesthesia and received straightforward approval from the Health Insurance Commission.
Surprisingly, this important discovery was not pursued for several years, partly because of negative press about ketamine research conducted in psychiatric patients, particularly those with schizophrenia, and questions about the competency of these patients to give informed consent. It wasn’t until six years later that one of the original investigators, Dennis Charney, together with Carlos Zarate and others at National Institute of Mental Health conducted a second, larger double-blind placebo controlled trial that confirmed the original findings.7 This study described a more detailed time course, which demonstrated significant therapeutic improvements as early as 80 minutes after a single dose of ketamine, and that lasted for at least seven days.
There have now been multiple research trials that confirm and validate the rapid and efficacious actions of ketamine for the treatment of depression. There are still some reservations, partly because it is difficult to account for potential placebo effects since patients receiving ketamine know they have been administered an active compound (due to the drug’s dissociative psychotomimetic effects). Trials are now run with an active comparator, such as midazolam, a short acting benzodiazepine that produces anxiolytic effects, but it is still difficult to completely discount placebo effects because of the potent acute behavioral effects of ketamine.
In addition, recent trials demonstrate that ketamine is effective for suicidal ideation, which is particularly difficult to treat with monoaminergic antidepressants, and represents a second important potential therapeutic indication for the drug. There have also been reports that ketamine has efficacy for post-traumatic stress syndrome (PTSD), another difficult-to-treat illness that responds poorly to currently available medications.
Ketamine Increases Synaptic Connections
Work in my laboratory has focused on the neurobiology of stress and depression, and the mechanisms by which antidepressants produce a therapeutic response. At a meeting where we described our work on typical monoaminergic antidepressants, Charney showed us a video of a severely depressed patient who had failed to respond to a typical antidepressant. The video was incredible in that this chronically depressed patient displayed a dramatic improvement within a few hours after receiving ketamine. In discussions afterwards, Charney suggested that research was sorely needed to understand the neurobiological mechanisms by which ketamine could produce such a rapid and efficacious response, and my laboratory initiated studies to address this question. The hope was that this information could also provide novel targets for the development of new medications.
The initial question was how ketamine, an antagonist of the excitatory neurotransmitter glutamate at the NMDA receptors, which causes transient psychotic and dissociative effects, could produce a subsequent rapid and sustained antidepressant response? Early studies reported that while ketamine blocks NMDA receptors; it produces a paradoxical increase in brain levels of glutamate, the neurotransmitter that excites NMDA receptors. Subsequent studies found that low doses of ketamine selectively block NMDA receptors on tonic firing GABA (gamma-aminobutyric acid) inhibitory neurons because these neurons are tonically firing, which leads to the open channel state that ketamine can access. The reduced activity of the interneurons then results in disinhibition of excitatory neurons, thereby explaining the burst of glutamate. Interestingly, the burst is rapid and transient, and correlates with the time period for the initial psychotic and dissociative effects observed after dosing, when brain levels of ketamine are highest.
But how might this burst of glutamate translate into an antidepressant response? Cellular models of learning and memory demonstrate that stimulation of glutamate neurotransmission and activation of another major class of receptors, referred to as AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, together with NMDA receptors increases the number of synaptic connections on excitatory neurons, which is thought to contribute to long-term memory. We and others in the field reasoned that ketamine, via this glutamate burst, could increase synaptic connections in brain regions known to undergo atrophy and loss of synapses in animals exposed to chronic stress and in depressed patients.8 (Exposing rodents to chronic or traumatic stress, which is known to precipitate or exacerbate depressive-like symptoms, decreases synaptic connections in the hippocampus and prefrontal cortex, two brain regions implicated in depression neurobiology. In addition, brain imaging studies have consistently reported a reduction in the size of the hippocampus and prefrontal cortex in depressed patients, and postmortem studies report a reduction of synapse numbers in the brains of depressed subjects.)
In animal studies in collaboration with George Aghajanian and Rong-Jian Liu at Yale, we tested the effects of ketamine on synapses in excitatory neurons of the prefrontal cortex. The results were astounding: a single dose of ketamine rapidly increased the number and function of these synapses.9 This was determined by recording responses from individual neurons in brain slices from animals previously administered ketamine. In addition, neurons were filled with a dye to label neuronal processes, allowing measurement of the number and size of spines where synapses are found. These studies demonstrated that a single dose of ketamine increased the amplitude and frequency of postsynaptic currents in pyramidal neurons, and that this effect was associated with an increase in the number and size of spine synapses.
Analysis of synaptic proteins that make up the spines demonstrated, further, that this increase in number occurred as early as two hours after a single dose of ketamine, consistent with the time course of its therapeutic actions. We also found that a single dose of ketamine rapidly reversed the synaptic deficits caused by chronic stress exposure, which correlated with reduction in behavioral abnormalities, notably anhedonia (the inability to experience pleasure) and helpless behaviors.10
Subsequent studies of the molecular and cellular mechanisms underlying the actions of ketamine have demonstrated a role for the rapid release of BDNF, and for activation of the mTORC1 signaling pathway known to be important for the synthesis of synaptic proteins required for long-term memory. Questions still remain regarding the initial cellular process triggered by ketamine: notably, is the glutamate burst resulting from ketamine blockade of NMDA receptors on interneurons the key cellular trigger or do the antidepressant actions of ketamine result from blockade of NMDA receptors on excitatory neurons? Studies are ongoing to selectively delete NMDA receptors on the GABA versus glutamate neurons to test these two possibilities, and could provide insight for identifying new targets on these neuronal populations for future drug development.
Novel Classes of Ketamine-Like Agents
The psychotomimetic and dissociative effects of ketamine, and its attendant abuse potential, have stimulated the search for novel agents with the drug’s rapid and efficacious antidepressant actions but without these side effects. An early study demonstrated that an antagonist selective for the GluN2B subunit of the NMDA receptor, CP101,606, also produced an antidepressant response after a single dose.11 Although some mild side effects were observed, this report led to studies of other GluN2B selective antagonists in rodent models, with similar findings. Clinical trials with selective GluN2B agents in depressed patients have reported mixed results, and additional studies are needed to further test this possible target.
There have also been efforts to develop the stereoisomers and metabolites of ketamine as rapid antidepressants, some also with the potential of fewer side effects. The initial ketamine studies discussed above were conducted with a mixture of both stereoisomers [(R,S)-ketamine], but more recent work has examined the actions of each stereoisomer alone. (S)-ketamine has three- to four-fold higher affinity for the NMDA receptor than (R)-ketamine, and a nasal application, referred to as esketamine, is undergoing development by Johnson & Johnson for the treatment of depression and suicide ideation.12 Initial clinical studies have been promising, and the nasal application provides a less expensive, much more accessible mode of administration that can be delivered in a doctor’s office compared to the intravenous route in a hospital setting used in studies of (R,S)-ketamine. Esketamine has received “breakthrough therapy” status from the Food & Drug Administration (FDA), which means that it will be fast tracked for approval, even though it appears to have the same side effects as (R,S)-ketamine.
Studies in rodent models have reported that (R)-ketamine also has antidepressant effects but without the behavioral actions that predict (R,S)-ketamine’s side effects (i.e., decreased sensory motor gating behavior, and increased conditioned place preference, a measure of the motivational effects of drugs of abuse ).13 However, there have been no clinical trials to date demonstrating (R)-ketamine’s efficacy. A major ketamine metabolite, (2R,6R)-hydroxynorketamine, is also reported to have antidepressant actions in rodent models without the behavioral side effects of (R,S)-ketamine.14 Moreover, this compound’s antidepressant actions were independent of effects at the NMDA receptor, indicating a potential novel mechanism of action. Efforts are currently underway to test the efficacy of this metabolite in depressed patients.
Another interesting agent under development is GLYX-13, or Rapastinel, which purportedly acts as a glycine-like partial agonist of the NMDA receptor. Activation of the NMDA receptor complex and channel opening requires both glycine and glutamate, which function as co-activators, and Rapastinel acts in a manner similar to glycine to co-activate the NMDA receptor. The drug is a tetrapeptide developed by Joseph Moskal, a professor of biomedical engineering at Northwestern University; his work led to studies demonstrating that Rapastinel produces rapid and long-lasting antidepressant actions in rodent models, but without the behavioral side effect profile of ketamine.15 There have also been preliminary reports on the clinical efficacy of Rapastinel in depressed patients. Together, these results led to a startup company, Naurex, headed by Moskal. Allergan subsequently acquired Naurex and is currently conducting phase III trials of Rapastinel, which like esketamine, has received “breakthrough” status from the FDA.
A Bright Future for Pharmacotherapies
Patients who are unresponsive to typical antidepressant agents have begun seeking out treatment centers that offer ketamine, even though they may find the dissociative and psychotomimetic side effects unpleasant; in fact, many continue receiving treatments on a weekly to monthly basis, even after particularly difficult experiences. Patients often find ketamine treatment via pain clinics that offer the drug for chronic neuropathic pain, because of the lack of availability in hospitals or psychiatry clinics. But that is starting to change. Yale New Haven Hospital, as well as other centers around the country, are now offering ketamine as an outpatient therapy, and insurance companies are beginning to cover these treatments, understanding the importance of treating patients before they must be hospitalized (e.g., those at risk of suicide). However, it is important to remember that ketamine is a drug of abuse and that the potential adverse effects of long-term use have not been studied.
Investigations into ketamine’s novel mechanism of action, including blockade of NMDA receptors, glutamate burst, and rapid induction of synaptic connections that reverses the deficits of stress and depression, have reinvigorated research efforts to identify additional rapid-acting glutamatergic antidepressants with reduced side effects. While further studies are needed to more fully elucidate the neurobiological effects underlying ketamine’s efficacy against depression, the field is poised for major advances in the treatment of this debilitating illness.
Figure 1.
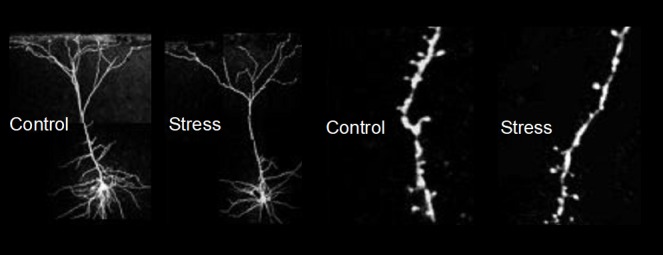
Preclinical studies show evidence of neuronal atrophy and loss in response to stress. Chronic stress, which can lead to depression, decreases synaptic connections in the PFC and hippocampus.
Figure 2.
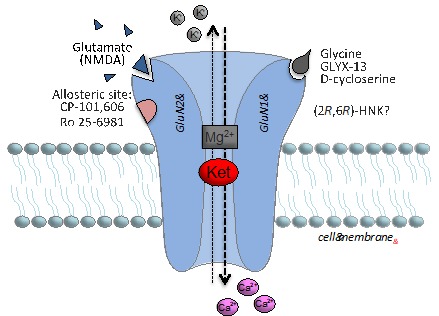
Figure 3.
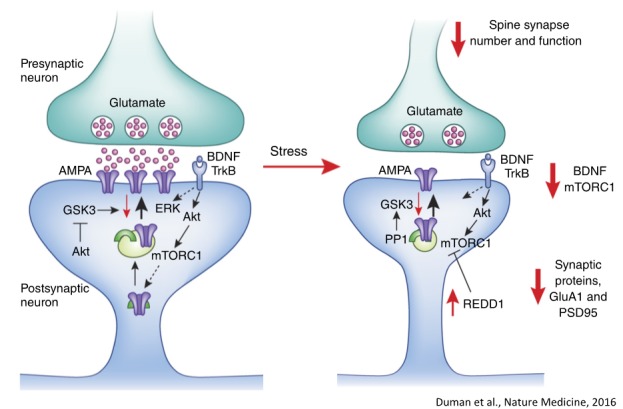
What are the signaling mechanisms underlying synaptic loss caused by stress/MDD: decreased BDNF-mTORC1 signaling? Duman et al, Nature Medicine, 2016.
Figure 4.
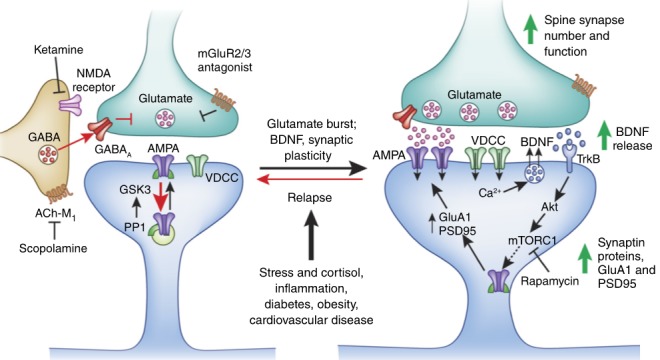
Signaling mechanisms for ketamine and rapid acting antidepressants. Duman et al, Nature Medicine, 2016.
Footnotes
Ronald S. Duman, Ph.D., is Professor of Psychiatry and Neurobiology, director of the Abraham Ribicoff Research Facilities, and the Jameson endowed Professor at Yale University. His work has characterized the molecular and cellular basis of stress, depression, and antidepressant treatments, leading to a neurotrophic and synaptic hypothesis of depression and a framework for the development of novel therapeutic agents. Duman has received the Anna-Monika Prize, Nola Maddox Falcone Prize, Janssen Prize, NIMH MERIT Award, and NARSAD Distinguished Investigator Award, and is a member of the National Academy of Medicine. He has authored over 300 original articles and reviews and has given over 250 invited lectures.
References
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry [Internet] 2005;62(6):617. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. National Violent Death Reporting System [Internet] 2015. Available from: https://www.cdc.gov/violenceprevention/nvdrs/index.html.
- 3.WHO. WHO | Depression [Internet] Who; 2017. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/%0Ahttp://www.who.int/mediacentre/factsheets/fs369/en/%5Cnhttp://www.who.int/mediacentre/factsheets/fs369/en/index.html%5Cnfiles/11/index.html%5Cnfiles/13/index.html%5Cnfiles/16/index.html. [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, Oren Da, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Soc Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Moghaddam B, Krystal JH. Capturing the angel in angel dust: twenty years of translational neuroscience studies of NMDA receptors antagonists in animals and humans. Schizophr Bull. 2012 2012 Sep;38(5):942–9. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry [Internet] 2006;63(8):856. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science [Internet] 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science (80−) [Internet] 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med [Internet] 2016;22(3):238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preskorn S, Macaluso M, Mehra DV, Zammit G, Moskal JR, Burch RM. Randomized Proof of Concept Trial of GLYX-13, an N-Methyl-D-Aspartate Receptor Glycine Site Partial Agonist, in Major Depressive Disorder Nonresponsive to a Previous Antidepressant Agent. J Psychiatr Pract [Internet] 2015;21(2):140–9. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 12.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2016;80(6):424–31. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Shirayama Y, Zhang J, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry [Internet] 2015;5(9):e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature [Internet] 2016;533(7604):481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskal JR, Burgdorf JS, Stanton PK, Kroes RA, Disterhoft JF, Burch RM, et al. The Development of Rapastinel (Formerly GLYX-13); A Rapid Acting and Long Lasting Antidepressant. Curr Neuropharmacol [Internet] 2017;15(1):47–56. doi: 10.2174/1570159X14666160321122703. [DOI] [PMC free article] [PubMed] [Google Scholar]


