Abstract
Background
Ovarian cancer is seventh most common cancer in women worldwide. Approximately 1.3% of women will be diagnosed with ovarian cancer at some point during their life time. The majority of tumours arise from surface of the ovary (epithelial). Two thirds of these women will present with advanced disease, requiring aggressive treatment, which includes debulking surgery (removal of as much disease as possible) and chemotherapy. However, most women (75%) with advanced epithelial ovarian cancer (EOC) will relapse following surgery and chemotherapy. Patients who relapse are treated with either platinum or non‐platinum drugs and this is dependent on the platinum‐sensitivity and platinum‐free interval. These drug regimens are generally well‐tolerated although there are potential severe side effects. New treatments that can be used to treat recurrence or prevent disease progression after first‐line or subsequent chemotherapy are important, especially those with a low toxicity profile. Hormones such as luteinising hormone releasing hormone (LHRH) agonists have been used in the treatment of relapsed EOC. Some studies have shown objective remissions, while other studies have shown little or no benefit. Most small studies report a better side‐effect profile for LHRH agonists when compared to standard chemotherapeutic agents used in EOC.
Objectives
To compare the effectiveness and safety of luteinising hormone releasing hormone (LHRH) agonists with chemotherapeutic agents or placebo in relapsed epithelial ovarian cancer (EOC).
Search methods
We searched the Cochrane Gynaecological Cancer Group trials register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase up to January 2016. We also searched registers of clinical trials and abstracts of scientific meetings.
Selection criteria
Randomised controlled trials (RCTs) that compared LHRH agonists with chemotherapeutic agents or placebo in relapsed EOC.
Data collection and analysis
Two review authors independently assessed whether relevant studies met the inclusion criteria, retrieved data and assessed risk of bias.
Main results
Two studies, including 97 women, met our inclusion criteria: one assessed LHRH agonist (leuprorelin) use in relapsed (platinum‐resistant and platinum‐refractory) EOC in comparison with a chemotherapeutic agent (treosulfan) (Du Bois 2002); the other examined LHRH agonist (decapeptyl) versus a placebo (Currie 1994). Since both studies had different control groups, a meta‐analysis was not possible.
There may be little or no difference between treatment with leuprorelin or treosulfan in overall survival (OS) (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.58 to 1.67; verylow‐quality evidence) or progression‐free survival (PFS) at six and 12 months (risk ratio (RR) 0.61, 95% CI 0.22 to 1.68, and RR 0.65, 95% CI 0.12 to 3.66; verylow‐quality evidence), respectively (Du Bois 2002). The duration of follow‐up was 2.5 years and quality of life (QoL) was not reported in this study.
Alopecia and fatigue were probably more common with treosulfan than leuprorelin (alopecia RR 0.32, 95% CI 0.12 to 0.91 (very low‐quality evidence)). There may be little or no difference in other Grade 3/4 side effects: nausea and vomiting (RR 0.65, 95% CI 0.12 to 3.66 (very low‐quality evidence)); neurotoxicity (RR 0.32, 95% CI 0.01 to 7.71 (very low‐quality evidence)) and neutropenia (RR 0.97, 95% 0.06 to 14.97 (very low‐quality evidence)),
The Currie 1994 study, which compared decapeptyl treatment with placebo, reported mean PFS of 16 weeks verus 11.2 weeks, respectively. No relative effects measures or P value at a particular time point were reported. Overall survival (OS) and QoL outcomes were not reported. In addition, adverse events were only mentioned for the decapeptyl group.
Adverse events were incompletely reported (no adverse events in decapeptyl group, but not reported for the placebo group).
Authors' conclusions
Based on this review of two small RCTs, there is not enough evidence to comment on the safety and effectiveness of LHRH agonists in the treatment of platinum‐refractory and platinum‐resistant (relapsed) EOC. Overall, the quality of evidence for all outcomes (including OS, PFS, QoL and adverse events) is very low.
Plain language summary
The use of hormonal treatment in relapsed epithelial ovarian cancer
Background
Epithelial ovarian cancer (EOC) arises from the cells covering the surface of the ovary. The majority of women with this type of cancer present with advanced stage disease at diagnosis. The initial treatment involves surgery (removal of as much disease as possible) followed by chemotherapy. In some cases chemotherapy is given to shrink the cancer before surgery is undertaken. Irrespective of the type of treatment received, cancer will return at some point in some women. Treatment following relapse, usually involves chemotherapy. The choice of chemotherapy depends on the cancer‐free period from the initial chemotherapy (platinum drugs). If relapse occurs after six months from finishing initial treatment with chemotherapy, women are treated with platinum drugs. However if the cancer recurs within six months, women are treated with non‐platinum drugs, since platinum drugs would be unlikely to work again. Eventually, the majority of women develop resistance to any chemotherapeutic drug. Some women also suffer from drug‐related side effects and poor quality of life (QoL) as a result of treatment. Therefore, there is a need for newer drug treatments with fewer side effects. In this context, hormone therapy have been tried. Luteinising hormone releasing hormone (LHRH) agonist are hormones that work by telling the pituitary gland located in the brain to stop producing this hormone and as a result the tumour cells in the ovary, which may be dependent on this hormone, cannot be stimulated. LHRH agonists have been used in relapsed EOC and some studies have shown low toxicity with these hormones.
Review question
We conducted this review to assess whether hormonal therapy (LHRH agonist) was effective and safe compared with chemotherapy or placebo in women with relapsed EOC.
Main findings
We searched electronic databases and other resources for randomised controlled trials (RCTs) comparing LHRH agonists with chemotherapy or placebo in women with relapsed EOC.Two RCTs were identified. Since the comparisons differed, they were reported separately. Available evidence did not show improvement in overall survival and progression‐free survival at six and12 months with hormonal (LHRH) therapy. Also, major side effects (haematological and neurological) did not statistically differ between the two treatment groups, but were incompletely reported. Quality of life data were not reported in either study.
Quality of evidence
Currently, the quality of evidence is very low regarding the effectiveness and safety of LHRH agonists in women who relapse within six months of initial platinum chemotherapy treatment.
Summary of findings
Summary of findings 1. Leuprorelin compared with treosulfan for relapsed epithelial ovarian cancer.
| Leuprorelin compared with treosulfan for relapsed epithelial ovarian cancer | |||||
| Patient or population: Platinum‐resistant and refractory (relapsed) epithelial ovarian cancer Setting: Hospital outpatients Intervention: Leuprorelin Comparison: Treosulfan | |||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | ||
| Without Leuprorelin | With Leuprorelin | Difference | |||
| Progression‐free survival at 6 months № of participants: 73 (1 RCT) | RR 0.61 (0.22 to 1.68) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 22.2% | 13.6% (4.9 to 37.3) | 8.7% fewer (17.3 fewer to 15.1 more) | |||
| Progression‐free survival at 12 months № of participants: 73 (1 RCT) | RR 0.65 (0.12 to 3.66) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 8.3% | 5.4% (1.0 to 30.5) | 2.9% fewer (7.3 fewer to 22.2 more) | |||
| Overall survival follow‐up: median 22 months № of participants: 73 (1 RCT) | HR 0.98 (0.58 to 1.67) | Study population | ⊕⊝⊝⊝ VERY LOW 1 4 5 | ||
| 19.4% | 19.1% (11.8 to 30.3) | 0.3% fewer (7.7 fewer to 10.9 more) | |||
| Skin toxicity (alopecia) № of participants: 73 (1 RCT) | RR 0.32 (0.12 to 0.91) | Study population | ⊕⊝⊝⊝ VERY LOW 2 3 4 | ||
| 33.3% | 10.7% (4.0 to 30.3) | 22.7% fewer (29.3 fewer to 3.0 fewer) | |||
| Gastrointestinal toxicity (nausea and vomiting) № of participants: 73 (1 RCT) | RR 0.65 (0.12 to 3.66) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 8.3% | 5.4% (1.0 to 30.5) | 2.9% fewer (7.3 fewer to 22.2 more) | |||
| Neurotoxicity № of participants: 73 (1 RCT) | RR 0.32 (0.01 to 7.71) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 4 | ||
| 2.8% | 0.9% (0.0 to 21.4) | 1.9% fewer (2.7 fewer to 18.6 more) | |||
| Neutropenia grade 3/4 № of participants: 73 (1 RCT) | not estimable | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 4 | ||
| 2.8% | 0.0% (0.0 to 0.0) | 2.8% fewer (2.8 fewer to 2.8 fewer) | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Imprecision 2 No confidence intervals or HR reported 3 Significant cross‐over between the two treatment groups 4 Trial at overall high risk of bias 5 Treosulfan group had more refractory stage patients compared to the leuprorelin group.
Quality of life data were not reported in this study.
Background
Description of the condition
Ovarian cancer is the seventh most common cancer in women. It is also the fourth most common cause of death due to cancer in women (Denny 2013; Ledermann 2013). The majority of these cancers occur in older and postmenopausal women. A woman's risk of developing ovarian cancer by the age of 65 years ranges from 0.4% in developing countries to 0.6% in developed countries (GLOBOCAN 2012). Epithelial ovarian cancer (EOC) is a disease in which malignant cells arise from the tissues covering the surface of the ovary. The exact cause of EOC remains unknown, but has been found to be associated with nulliparity (women who have not delivered fetuses greater than 24 weeks pregnancy), delayed childbearing, early menarche, late menopause, obesity, and BRCA1 and BRCA2 tumour suppressor gene mutations (inherited faulty genes in the family which increase the risk of both ovarian and breast cancer), and women of Ashkenazi Jewish descent who have a far higher incidence of the BRCA1 and BRCA2 genes (Denny 2013; Feig 2006; Ledermann 2013). Also, recent advances in molecular pathogenesis (the study and diagnosis of disease through examination of molecules within organs, tissues or body fluids) reveals that high‐grade serous ovarian cancer (the most common histological subtype of EOC) appears to develop from intra‐epithelial carcinoma (precancerous cells) in the fallopian tube. This precancerous condition is termed as 'serous tubal intra‐epithelial carcinoma' (STIC) and is often seen in women with BRCA1 and BRCA2 mutations. A bilateral salpingo‐oophorectomy (removal of tubes and ovaries) has shown to reduce the risk of developing EOC (Kurman 2013; Polcher 2015).
In the USA, the five‐year survival rate for ovarian cancer is 45% (Siegel 2011). In the UK, the age‐standardised relative survival rates for ovarian cancer are 72% at one year, falling to 43% at five years or more (Cancer Research UK 2012). Women with advanced EOC may be relatively asymptomatic, or may have only mild, vague symptoms when they present to the clinician. This results in poor survival rates for this group of women, since most women will relapse despite high initial response rates to chemotherapy (Jemal 2008). Other causes of poor survival include late presentation, tumour biology and suboptimal cytoreduction either due to disease distribution or limited surgical ability.
The International Federation of Gynecology and Obstetrics (FIGO) describes ovarian cancer staging as I to IV (see Appendix 1) (Society of Gynaecologic Oncology). Epithelial ovarian cancer spreads trans‐coelomically (across the abdominal cavity) by the spillage of tumour cells. It is also known to spread to pelvic and para‐aortic nodes (lymph nodes situated behind the womb and the upper abdomen) via retroperitoneal lymphatic channels (lymphatic channels situated behind the lining of the abdomen), especially in advanced EOC. It can spread to the liver and outside the peritoneal cavity to the chest and supraclavicular nodes (lymph nodes felt above the collar bone) via lymphatic channels through the diaphragm. Approximately two thirds of women with EOC present with FIGO stage III and IV (Makar 1995; Pettersson 1994; Twombly 2007).
The FIGO surgical stage predicts the five‐year survival rate for women with ovarian cancer. The survival rates are shown in Appendix 2 (Cancer Research UK 2011; Cancer Research UK 2012). Staging is carried out by midline laparotomy (opening the abdomen via a midline vertical cut) and systematic examination of all areas in the abdomen and pelvis. This normally involves obtaining peritoneal washings or ascites for cytology, total abdominal hysterectomy (removal of the womb), bilateral salpingo‐oophorectomy (removal of both ovaries and tubes), omentectomy (removal of the fatty apron covering the bowel), debulking of any visible disease (removal of any visible cancer), random biopsies of the pelvic and abdominal peritoneum and retroperitoneal lymph node assessment (removal of lymph nodes situated within the pelvis and abdomen) (Cancer Research UK; Denny 2013).
A key indicator for survival in women with advanced EOC is the amount of residual disease remaining following primary debulking surgery (Griffiths 1975; Hoskins 1992; Hoskins 1994). The aim of primary debulking surgery is complete cytoreduction (removal of all visible cancer) or residual disease < 1 cm if complete cytoreduction not achievable. A meta‐analysis has demonstrated that patient survival is longer in the absence of residual disease in comparison to the presence of residual disease (whether it is < 1 cm or > 1 cm) (Shih 2010). A systematic review on optimal primary cytoreduction for advanced EOC showed that women with residual disease < 1 cm still do better than women with residual disease > 1 cm (Elattar 2011). To what extent this is due the direct effect of surgical intervention or underlying tumour biology affecting tumour resectability remains a matter of debate.
Women with early stage disease, FIGO stage IC (grade 3) and above, are recommended to have platinum‐based chemotherapy, either after surgery (adjuvant) or increasingly before surgery if disease is widespread (neoadjuvant chemotherapy) (Du Bois 2009; Feig 2006; Vergote 2010). A recent Cochrane review concluded that neoadjuvant chemotherapy (chemotherapy given prior to surgery in order to reduce the bulk of the disease) is a reasonable alternative to primary debulking surgery in patients with bulky advanced EOC that cannot be optimally reduced (Morrison 2012). However, most women (75%) with advanced EOC will relapse following surgery and chemotherapy, despite initial chemosensitivity with platinum‐based regimens. Eventually, sometime after several cycles of chemotherapy, most women who relapse will develop resistance to conventional chemotherapy drugs (Gonzalez‐Martin 2013).
Description of the intervention
Although women with advanced EOC initially respond well to cytoreductive surgery and platinum‐based, first‐line chemotherapy (initial response rate following platinum‐based chemotherapy is around 70%), the majority of women (70% to 80%) will relapse or progress within 12 to 24 months (Colombo 2014; Ledermann 2013; Sourbier 2012). In general, women with relapsed EOC are treated with chemotherapy. However, if complete cytoreduction can be achieved in these women (recurrent disease in single site or few sites with resectable disease), chemotherapy could be held in reserve for when surgery is no longer an option.
The response to chemotherapy and type of chemotherapeutic regimen used for women with relapse depends on the time interval between the initial platinum‐based chemotherapy and recurrence of disease (relapse‐free interval). If relapse occurs after 12 months of completion of initial platinum chemotherapy, the tumour is considered to be platinum‐sensitive, and if relapse occurs within six months after completion of initial chemotherapy, the tumour is considered to be platinum‐resistant. Patients presenting with disease between six to 12 months post completion of initial platinum combination are considered to be partially platinum‐sensitive (Banerjee 2011; Colombo 2014; Friedlander 2011; Pfisterer 2006).
Women with platinum‐sensitive or partially platinum‐sensitive disease are generally treated with carboplatin, alone or in combination with paclitaxel, liposomal doxorubicin or gemcitabine (Ledermann 2013). The Ovarian Cancer Study (OCEANS) assessed the role of bevacizumab (anti‐angiogenic drug) in the treatment of platinum‐sensitive recurrences. In this phase III double‐blind placebo‐controlled trial, chemotherapy (gemcitabine + carboplatin), with or without bevacizumab was used for recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer. The results demonstrated an improved median progression‐free survival (PFS) for patients receiving bevacizumab when compared to those receiving a placebo (Aghajanian 2012).
Women resistant to platinum chemotherapy are treated with non‐platinum drugs; the most commonly‐used drugs are liposomal doxorubicin, gemcitabine, taxanes and topotecan. These patients have low response rates and experience severe side effects. This can negatively influence quality of life (QoL). Eventually women develop resistance to any chemotherapeutic regimen (Ledermann 2013). It is therefore important to balance the benefits and toxicities of prolonged chemotherapy treatment in women with recurrent disease. New treatments that can be used to treat recurrence or prevent disease progression after first‐line chemotherapy or subsequent chemotherapy are important, especially those with a low toxicity profile (few side effects).
Luteinising hormone releasing hormone (LHRH) agonists have been used in both the treatment of advanced EOC (in addition to first‐line, platinum‐based chemotherapy) and also in relapsed disease. Some studies have shown objective remissions, whilst other studies (including phase II studies) have shown little or no benefit (Adelson 1993; Levine 2007; Lind 1992; Medl 1993; Miller 1992; Ron 1995). Most studies have shown a better side‐effect profile for LHRH agonists when compared to standard chemotherapeutic agents used in EOC (relapsed or progressive disease) (Emons 1990; Emons 1994). In addition, LHRH agonists have shown disease stabilisation and improved PFS when used in patients with relapsed EOC, without any major side effects (Lind 1992). Anecdotal reports on LHRH agonists use as consolidation therapy in advanced EOC and recurrent ovarian cancer showed long‐term complete clinical remission and longer (seven to 14 years) overall survival (OS) (Chudecka‐Glaz 2009). Despite several studies showing benefit, to date there has been no systematic review addressing the role of LHRH agonists versus other chemotherapeutic interventions in relapsed EOC.
How the intervention might work
Cramer 1983 hypothesised that elevated gonadotrophin hormonal levels (hormones produced by the pituitary gland) found in postmenopausal women can be a causative factor for developing ovarian cancer. Emons 1990 postulated that EOC can be gonadotrophin‐dependent. Adelson 1993 reported the presence of luteinising hormone (LH), follicle‐stimulating hormone (FSH) and gonadotrophin releasing hormone (GnRH) receptors on ovarian tumours, and manipulation of these hormones resulted in tumour response both in vivo and in vitro. Other studies found that human EOC cell lines express LHRH receptors and proliferation of these cells was decreased by treatment with both agonists and antagonist analogues of LHRH in vitro (Emons 1994; Emons 1996; Emons 2000). It is reported that specific LHRH binding sites can be found in at least 80% of EOC (Emons 1990; Emons 1994; Emons 1996; Emons 2000; Lind 1992), and this could be a target for hormonal therapy with LHRH agonists. In vivo, LHRH agonists cause suppression of LH and FSH levels, which in turn inhibit the growth of EOC (Dowsett 1988; Emons 2000). Hence LHRH analogues could be used in EOC treatment due to their anti‐proliferative effect. A number of phase I and phase II clinical trials have reported that suppression of endogenous LH and FSH secretion by administration of LHRH analogues induces objective remissions of 9% to 12% and disease stabilisation in 15% to 26% of patients with advanced resistant EOC, and with less side effects than chemotherapy (Emons 2000; Paskeviciute 2002).
Why it is important to do this review
In women with stage I or II EOC, around 20% to 25% will have relapse of disease following treatment, while most women (75%) with advanced EOC will relapse (Ushijima 2010). Treatment after relapse is usually palliative and there is a need to find treatments with low toxicity to improve QoL outcomes. There have been observational studies in the literature evaluating the role of LHRH agonists in patients with advanced EOC and some studies report a beneficial effect (Lind 1992; Rzepka‐Gorska 2003) in terms of overall and PFS. Hassan 2005 reported that a combination of goserelin and tamoxifen prolonged survival in women with relapsed and platinum‐resistant disease similar to that seen for single‐agent chemotherapy regimens. Hormonal therapy might be better than conventional chemotherapy due to its relative lack of toxicity, ease of administration and tolerability in the context of compromised bone marrow function, and suitable for women heavily pre‐treated with other chemotherapeutic agents and with platinum‐resistant disease. There have been a few randomised controlled trials (RCTs) addressing this important clinical question (Du Bois 2002; Emons 1996; Jager 1995).
As far as we are aware, there has been no systematic review and meta‐analysis published addressing this clinical question. Due to the lack of consensus in the literature, there was a need to conduct a comprehensive systematic review on this subject.
Objectives
To compare the effectiveness and safety of luteinising hormone releasing hormone (LHRH) agonists with chemotherapeutic agents or placebo in relapsed epithelial ovarian cancer (EOC).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adult women (aged 18 years or older) diagnosed with relapsed (platinum‐resistant and platinum‐refractory) epithelial ovarian cancer (EOC).
Types of interventions
Intervention: Any luteinising hormone releasing hormone (LHRH) agonists (e.g. zoladex or goserelin or triptorelin or leuprorelin or decapeptyl or D‐Trp‐6‐LH‐RH).
Comparison: Chemotherapeutic agents or placebo.
Types of outcome measures
RCTs that reported any one of the following clinical outcomes.
Primary outcomes
Overall survival (OS): survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
Progression‐free survival (PFS): the length of time during and after the treatment during which the cancer does not get worse.
-
Adverse events, classified according to CTCAE 2006, for example:
chemotherapy toxicity;
other side effects not categorised above (e.g. of hormonal treatment).
-
Categories of toxicity, grouped as:
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, infection, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, constipation, diarrhoea, liver);
genitourinary;
skin (stomatitis, mucositis, alopecia, hand foot syndrome, allergy);
neurological (peripheral and central) e.g. neuropathy;
pulmonary.
Quality of life (QoL) measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication by a validated scale.
Grade assessment
We performed Grade assessment using GRADEpro (GRADEproGDT) software to create a 'Summary of findings' table in this review. We downgraded the quality of the evidence for numerous reasons, but the main ones included the small size of the trials (especially Currie 1994 (n = 24), overall high risk of bias, incomplete reporting and potentially selective reporting of outcomes. Each outcome in both trials was downgraded for at least three different reasons so applying the GRADE approach and starting with high‐quality evidence, we justified downgrading a level for each reason and this ultimately reduced the quality of the evidence to very low (See Quality of the evidence and Table 1).
Search methods for identification of studies
We searched for papers in all languages and translations were carried out if necessary.
Electronic searches
The following electronic databases were searched.
The Cochrane Gynaecological Cancer Review Group's Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL), (the Cochrane Library 2016, Issue 1)
MEDLINE (1946 to January 2016)
Embase (1980 to January 2016)
The MEDLINE, Embase and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 3, Appendix 4 and Appendix 5.
All relevant articles found were identified on PubMed using the 'related articles' feature, a further search was carried out for newly‐published articles.
Searching other resources
Unpublished and grey literature
We searched the following sources to check for ongoing trials.
Metaregister (www.controlled-trials.com/rct), Physicians Data Query ( www.nci.nih.gov), www.clinicaltrials.gov; and www.cancer.gov/clinicaltrials.
We did not find any ongoing trials with regards to this topic.
We searched conference proceedings and abstracts through ZETOC (http://zetoc.jisc.ac.uk/) and WorldCat Dissertations.
We conducted a Google search for internet‐based resources and open‐access publications.
Handsearching.
We handsearched the following sources for reports of conferences.
International Gynecological Cancer Society (IGCS)
European Society of Gynaecological Oncology (ESGO)
Society of Gynecologic Oncologists (SGO)
British Gynaecological Cancer Society (BGCS)
Australian Society of Gynaecologic Oncologists (ASGO)
American Society of Clinical Oncology (ASCO)
European Society of Medical Oncology (ESMO)
Clinical Oncological Society of Australia (COSA)
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database (Endnote) and duplicates were removed. Two review authors (RW, SS) independently examined the titles and abstracts of the remaining references to assess the eligibility. Those references that clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (RW, SS). Disagreements were resolved by discussion between the two authors and if necessary by a third review author (AM). Reasons for exclusion were documented
Data extraction and management
Two authors (RW, SS) independently extracted the data onto a data extraction form specifically designed for the review. Differences between review authors were resolved by discussion.
For included studies, data were abstracted as specified in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We extracted the following data.
Author, year of publication and journal citation (including language).
Country.
Setting.
Study design, methodology.
Study population:
total number enrolled;
patient characteristics;
age (aged 18 years or older);
co‐morbidities;
performance status;
type of treatment received for relapsed EOC, including details of dose, duration and combination.
EOC details:
FIGO stage, grade and histology;
recurrent disease;
progressive disease.
Total number in the intervention groups.
Intervention details:
luteinising hormone releasing hormone (LHRH) agonists (any), including details of dosing schedule, route, frequency and duration.
Comparison details:
-
chemotherapeutic agents, including details of dosing schedule, route, frequency and duration
placebo.
Risk of bias in each study (see Assessment of risk of bias in included studies).
Duration of follow‐up.
Outcomes: OS, PFS, response to treatment and adverse events:
for each outcome: outcome definition and unit of measurement (if relevant);
for scales: upper and lower limits, and whether high or low score is good;
results: number of participants allocated to each group;
for each outcome of interest: sample size; missing participants.
Data on outcomes were extracted as follows.
For dichotomous outcomes (e.g. adverse events or deaths, if it was not possible to use a hazard ratio), we therefore, extracted the number of patients in each group who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR).
The HR for overall survival was reported in the Du Bois 2002 trial so this was also included as an outcome and we inputted the log HR and SE of the log HR into RevMan 5 and reported the HR with 95% confidence interval.
Where possible, all data extracted were those relevant to an intention‐to treat analysis, in which participants were analysed in groups to which they were assigned to reduce the bias.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs in accordance with guidelines in the Cochrane Handbook using the Cochrane Collaboration's tool and the criteria specified in Chapter 8 (Higgins 2011). This included assessment of the following.
Selection bias: random sequence generation and allocation concealment.
Performance bias: restricted to blinding of outcome assessors as it is not possible to blind participants and healthcare providers.
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
The proportion of participants whose outcomes are not reported at the end of the trial were recorded; we noted if loss to follow‐up was not reported. For each outcome, the satisfactory level of loss to follow‐up was coded as:
low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment groups;
high risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up were different between treatment groups;
unclear, if loss to follow‐up was not reported.
Reporting bias: selective reporting of outcomes.
Other possible sources of bias.
The 'Risk of bias' tool was applied independently by two review authors (RW, SS) and differences were resolved by discussion or by appeal to a third review author (AM). Results are presented in 'Risk of bias' tables and also as a 'Risk of bias' summary (Figure 1).
1.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We used the following measures of the effect of treatment.
For dichotomous outcomes (e.g. adverse events, or where it was not possible to use a hazard ratio (HR) for time‐to‐event data), we extracted the number of patients in each group who experienced the outcome of interest and the number of patients assessed at the endpoint, in order to estimate a risk ratio (RR).
The HR for overall survival (OS) was reported in the Du Bois 2002 trial so this is included as an outcome.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
This was not relevant as meta‐analyses were not judged to be appropriate due to clinical heterogeneity of the studies.
Assessment of reporting biases
It was not possible to assess small‐study biases such as publication bias due to the inclusion of only two single study analyses in the review.
Data synthesis
The comparisons were different in the two included trials (Du Bois 2002 and Currie 1994) and therefore it was not possible to perform meta‐analysis. It was therefore not relevant to assess heterogeneity between results of trials or conduct any subgroup or sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search strategy identified 577 unique references after removal of duplicates. The abstracts of these were read independently by two review authors. The articles that did not meet the inclusion criteria at this stage were excluded. Five references (two meeting abstracts and three journal articles) were retrieved in full (Figure 2). The full‐text screening excluded two studies for the reasons described in the table of Characteristics of excluded studies; Jager 1993 and Jager 1995 described the results of the same study and were excluded.
2.
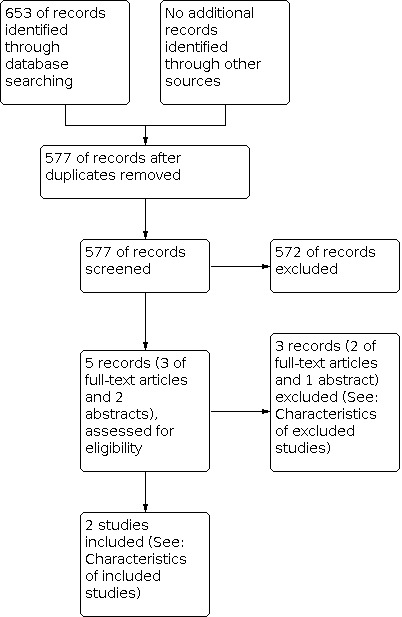
Study flow diagram.
Two randomised controlled trials (RCTs) were identified that met our inclusion criteria; these are described in the table Characteristics of included studies.
Included studies
The two included studies (Du Bois 2002; Currie 1994) included 97 eligible women (73 from Du Bois 2002 and a further 24 from Currie 1994).
Design of studies
Du Bois 2002 examined the effect of luteinising hormone releasing hormone (LHRH) agonist (leuprorelin) versus chemotherapy (treosulfan) in platinum‐refractory or relapsed epithelial ovarian cancer (EOC) within six months of first‐line chemotherapy whereas Currie 1994 assessed the effect of LHRH agonist (decapeptyl) versus placebo in platinum‐resistant EOC. The two studies, Du Bois 2002 and Currie 1994 were conducted in Germany and USA, respectively. Du Bois 2002 was a multi‐centred, prospective and randomised, but unblinded trial, whilst Currie 1994 was a prospective, randomised and placebo‐controlled (type of blinding was unclear) trial. However, the blinding was broken when women progressed, in order to consider cross‐over and treat with LHRH agonists, if on placebo.
Currie 1994 was published as an abstract. We were unable to obtain additional information. This study was included, as it met the inclusion criteria. However, minimal information regarding outcomes was reported without any statistical values (hazard ratios, P values or confidence intervals were not reported).
Patient characteristics
Du Bois 2002 included 73 women. Thirty‐seven women were included in the intervention group (LHRH agonist: leuprorelin) and 36 in the control group (chemotherapy: treosulfan). Both groups were comparable with respect to age, initial FIGO stage and performance status. All relapses occurred within six months. However, the treosulfan group had more refractory stage patients compared to the leuprorelin group.The mean age in the leuprorelin group was 58.8 years (range: 27 to 75) and the mean age in the treosulfan group was 58 years (range: 36 to 75). The initial FIGO stage at diagnosis of most women (94.5%) was stage III and IV, with only few women with stage II. All women had received prior first‐line, platinum‐based chemotherapy.
Currie 1994 included 24 women. Twelve women were included in the intervention arm (D‐TRP‐6‐LHRH: Decapeptyl) and 12 in the comparison arm (placebo). The mean age of participants was 58.4 years. Women had advanced EOC and all had previously received multiple first‐line chemotherapy. However, there was no information regarding the type of prior chemotherapy received. The performance status of women was not reported. Therefore, It is not possible to comment on the comparability of women between the two groups.
Interventions
In the Du Bois 2002 study, 37 women received leuprorelin (LHRH agonist) 3.75 mg injected subcutaneously (SC) or intramuscularly (IM) in the intervention group and 36 women received treosulfan 7 gm/m2 infusion (chemotherapy) in the comparison group. The dose of treosulfan was reduced depending on the toxicity. Both treatments were repeated every four weeks. The mean duration of treatment in the leuprorelin group was 13 weeks and in the treosulfan group 18 weeks. A total of 122 courses in the leuprorelin group and 150 courses in the treosulfan group were evaluated.
In the Currie 1994 study, 12 women received LHRH agonist (D‐TRP‐6‐LHRH: Decapeptyl) in the intervention group and 12 women in the comparison group received placebo. Both treatments were repeated every four weeks. Neither the dose nor route of administration of either of the treatments were reported in this study. Also, the type of follow‐up (clinical or radiological), frequency, and duration of follow‐up were not reported.
Outcomes
In the Du Bois 2002 study, both treatment groups were comparable. Overall survival (OS) and progression‐free survival (PFS) at six and 12 months were reported. Hazard ratio was reported only for OS, but not for PFS. The median OS time was 36 and 30 weeks in the treosulfan and leuprorelin groups, respectively. Adverse events were reported in Du Bois 2002 study. Quality of life (QoL) data were not reported in this study.The duration of follow‐up in this study was 2.5 years. Objective response to treatment was also reported but was not observed in either group.
Currie 1994 was published as a meeting abstract only. The PFS was reported, but there were no statistical comparisons between the two treatment groups. Adverse events were incompletely and selectively reported (no adverse events in decapeptyl group, but not reported for placebo group). Overall survival and QoL data were not reported for either treatment group.We were unable to obtain more detailed information on the two RCTs.
Excluded studies
Three references were excluded after obtaining the full text (see Characteristics of excluded studies).
Jager 1993 (published as abstract) and Jager 1995 (published in peer review journal) reported results of the same study. This was a randomised comparison of triptorelin versus tamoxifen in the treatment of women with progressive ovarian cancer. In this study, hormonal therapy was used in both the intervention (LHRH agonist) and comparison (tamoxifen) groups, hence it did not meet our inclusion criteria.
Emons 1996 was a prospective, randomised, double‐blind trial in which LHRH agonist (triptorelin) was used in combination with first‐line chemotherapy for treatment of advanced EOC following cytoreductive surgery and not in relapsed EOC. Our study criterion was LHRH agonist use in relapsed EOC. Therefore this study was excluded.
Risk of bias in included studies
Both studies were at a high risk of bias, but the Du Bois 2002 trial met three of the six core 'Risk of bias' criteria, compared to Currie 1994, which did not address any (see Figure 1)
Allocation
It was unclear whether an adequate sequence had been generated to assign women to treatment groups and whether the allocation was adequately concealed in the Currie 1994 study. Random assignment of the women to the two treatment groups was performed in the Du Bois 2002 study. Patient groups (leuprorelin and treosulfan) were comparable in the Du Bois 2002 study, but not reported in Currie 1994, making it impossible to tell whether there was an element of allocation bias.
Blinding
Blinding of outcome assessor was not reported in either study (Currie 1994; Du Bois 2002). In the Currie 1994 study, it was unclear whether the blinding was used as it was reported that the blinding was broken in the placebo arm when the disease progressed in these patients and they were subsequently treated with LHRH agonist (decapeptyl). It is unclear whether assessors were unblinded also. While blinding of the outcome assessor is not important for outcomes such as OS, it is vital for PFS and both trials reported this outcome. Therefore the unclear risk of bias in these trials for this item can be deemed as being potentially prone to performance and detection biases.
Incomplete outcome data
All eligible women were assessed at the endpoint for outcomes in the Du Bois 2002 study (low risk of bias), while this unclear in the Currie 1994 abstract (unclear risk of bias).
Selective reporting
Only PFS and incomplete adverse event data were reported in the Currie 1994 study. There was emphasis on one woman with stable disease for 80 weeks in the decapeptyl group (high risk of bias). All outcomes were reported in the Du Bois 2002 study but statistical comparisons were incompletely reported (hazard ratios and confidence intervals were not reported for all outcomes ‐ high risk of bias). QoL data were not reported in either study.
Other potential sources of bias
There was insufficient information to make a judgement on whether any additional risk factor for bias existed (unclear risk of bias).
Effects of interventions
See: Table 1
Summary of outcomes for comparisons of interventions in the included studies are shown in Table 1.
In the case of dichotomous outcomes, we were unable to estimate a risk ratio (RR) for comparisons of treatment, if the data were incompletely reported or if one or both treatment groups experienced no events.
Leuprorelin versus treosulfan
Du Bois 2002 (73 participants) included this comparison and all outcomes below are based on results from this single trial.
Overall survival (OS)
Women received treatment until disease progression or death. The duration of follow‐up was 2.5 years. There may be little or no difference in overall survival (OS) between the two groups (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.58 to 1.67, P value = 0.95) (Analysis 1.1). In this study, there was significant cross‐over between the two treatment groups.
1.1. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 1: Overall survival
Progression‐free survival (PFS)
There may be little or no difference in PFS at six (risk ratio (RR) = 0.61, 95% CI 0.22 to 1.68, P value = 0.34) (Analysis 1.2) and 12 months (RR 0.65, 95% CI 0.12 to 3.66, P value = 0.62) (Analysis 1.3) between the two treatment groups.
1.2. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 2: Progression‐free survival at 6 months
1.3. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 3: Progression‐free survival at 12 months
Adverse events (Minor)
Overall, both treatments (leuprorelin versus treosulfan) were well‐tolerated with only one woman stopping treatment due to toxicity.
Skin toxicity
The study reported that 33% (n = 12) of women had alopecia in the treosulfan group compared with 11% (n = 4) in the leuprorelin group. The women who received leuprorelin probably had a lower risk of alopecia than those who received treosulfan (RR 0.32, 95% CI 0.12 to 0.91, P value = 0.03). (Analysis 1.4)
1.4. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 4: Skin toxicity (alopecia)
Fatigue
Fatigue was reported in 22% (n = 8) of women in the treosulfan group and 2.7% (n =1) in the leuprorelin group (RR 0.12, 95% CI 0.02 to 0.92, P value = 0.04). (Analysis 1.5).
1.5. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 5: Fatigue
Hot Flushes
Hot flushes were similar in both treatment groups (RR 0.97, 95% CI 0.06 to 14.97, P value = 0.98). (Analysis 1.6)
1.6. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 6: Hot flushes
Gastrointestinal toxicity
There may be little or no difference in the risk of nausea and vomiting between women who received leuprorelin and those who received treosulfan (RR 0.65, 95% CI 0.12 to 3.66, P value = 0.62). (Analysis 1.7)
1.7. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 7: Gastrointestinal toxicity (nausea and vomiting)
Neurotoxicity (Grade 3)
There may be little or no difference in the risk of neurotoxicity between women who received leuprorelin and those who received treosulfan (RR 0.32, 95% CI 0.01 to 7.71, P value = 0.49). (Analysis 1.8)
1.8. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 8: Neurotoxicity
Pain (Grade 3)
There may be little or no difference in the risk of grade 3 pain between women who received leuprorelin and those who received treosulfan (RR 0.19, 95% CI 0.01 to 3.92, P value = 0.29). (Analysis 1.9)
1.9. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 9: Grade 3 pain
Haematological toxicity (Grade 3/4)
The incidence of neutropenia grade 3/4 was similar in both treatment groups (RR 0.97, 95% CI 0.06 to 14.97, P value = 0.98) (Analysis 1.10). There may be little or difference in grade 3/4 thrombocytopenia and anaemia (RR 0.24, 95% CI 0.03 to 2.07, and RR.28, 95% CI 0.06 to 1.25) between women who received leuprorelin and those who received treosulfan (Analysis 1.11 and Analysis 1.12).
1.10. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 10: Neutropenia grade 3/4
1.11. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 11: Thrombocytopenia
1.12. Analysis.

Comparison 1: LHRH agonist versus treosulfan for the treatment of relapsed EOC, Outcome 12: Anaemia
Quality of life
Quality of life data were not reported in the Du Bois 2002 study.
Objective response
Objective response to treatment did not differ between the two groups with only few having stable disease in either groups.
LHRH agonist (D‐TRP‐6‐LHRH: Decapeptyl) versus placebo
Only the Currie 1994 trial (24 participants) included this comparison and all outcomes below are based on results from this single trial.
Overall survival (OS)
OS and QoL were not reported in the Currie 1994 trial and PFS was not adequately reported. Twenty‐four patients were enrolled in the study (published as an abstract only). The information regarding blinding was unclear as there was significant cross‐over in the placebo group. All women in the placebo group were treated with LHRH agonist (decapeptyl) when the disease progressed. Mean PFS was 16 weeks and 11.2 weeks in the decapeptyl and the placebo group, respectively.
No adverse events were seen in the decapeptyl group, but were not reported in the placebo group.
We did not apply GRADE assessment (GRADEproGDT) to this trial because the evidence was clearly of very low quality, namely because it is so very small (n = 24), poorly reported and reported in abstract form.
Discussion
Summary of main results
We found two randomised controlled trials (RCTs) that met the inclusion criteria (73 women from Du Bois 2002 and a further 24 from Currie 1994).The data were too sparse and prone to bias to adequately assess the safety and effectiveness of luteinising hormone releasing hormone (LHRH) agonists in the treatment of (platinum‐refractory and platinum‐resistant) relapsed epithelial ovarian cancer (EOC). The comparisons were restricted to individual study data only. In the Du Bois 2002 study, progression‐free survival (PFS) at six and 12 months did not statistically differ between the two treatment groups. Overall survival (OS) was not different between the two treatment groups in the Du Bois 2002 study and it was not reported in the Currie 1994 study.The duration of follow‐up was 2.5 years in the Du Bois 2002 study. Given the poor prognosis of this condition (relapsed EOC), this was considered adequate. The follow‐up period in the Currie 1994 study was not reported. Quality of life (QoL) was not reported in either study and adverse events were incompletely reported in the Currie 1994 study. In the study by Du Bois 2002, minor side effects such as alopecia and fatigue were higher in the treosulfan group than those in the leuprorelin group. However, major side effects of neutropenia, neurotoxicity and grade 3 pain did not statistically differ between the two treatment groups.
Overall completeness and applicability of evidence
Grade assessment of quality of evidence was performed using GRADEproGDT software to create a 'Summary of findings' (SoF) table for this review of two small RCTs. Overall, the quality of evidence for all outcomes was very low in both trials (Currie 1994; Du Bois 2002). The reasons are outlined below and explained in Table 1 and in Effects of interventions.
This review was based on a small number of women (97) in platinum‐refractory and platinum‐resistant (relapsed) EOC. Meta‐analysis was not possible as the study comparisons were different in the two included RCTs. Based on this review, there is not enough evidence from the included studies to support the use of LHRH agonists (leuprorelin) in the treatment of platinum‐refractory or platinum‐resistant EOC.
The current practice in the treatment of women with relapsed EOC depends on platinum sensitivity which in turn is based on a platinum‐free interval from initial chemotherapy. Women with platinum‐sensitive disease are generally treated with carboplatin alone or in combination with paclitaxel, liposomal doxorubicin or gemcitabine. Recently, bevacizumab (anti‐angiogenic drug) has demonstrated an improved median PFS in the treatment of platinum‐sensitive recurrences when compared to those receiving a placebo. Women resistant to platinum chemotherapy are treated with non‐platinum drugs; the most commonly‐used drugs are liposomal doxorubicin, gemcitabine, taxanes and topotecan. It is unlikely that the current practice will change in these patients based on the above findings.
Quality of the evidence
We performed a Grade assessment of quality of evidence as outlined above (see; Table 1 and in Effects of interventions).
Two RCTs were included in this review which assessed 97 women (73 from Du Bois 2002 and a further 24 from Currie 1994). Both studies had different comparisons groups and therefore the data were presented individually and meta‐analysis was not possible. The quality of evidence for OS, PFS and adverse events was very low in both included trials (Currie 1994; Du Bois 2002) (Table 1 and in Effects of interventions).
In the Du Bois 2002 study, LHRH agonist (leuprorelin) was compared with chemotherapeutic agent (treosulfan) whilst in the Currie 1994 study, LHRH agonist (decapeptyl) was compared with placebo. The Currie 1994 study was underpowered and it was also difficult to interpret the data as there was significant cross‐over (all women in the placebo arm received decapeptyl when the disease progressed). Overall survival was not reported and adverse events were reported only for decapeptyl group in the Currie 1994 study. In the Du Bois 2002 study, the power was reported to be adequate. However, there was significant cross‐over between the two treatment groups and there were more patients with refractory stage in the treosulfan group compared to leuprorelin group. This may have had an impact on survival analysis (OS). Major adverse events such as neutropenia, neurotoxicity and grade 3 pain did not statistically differ between the two treatment groups. The PFS at six and 12 months did not differ between treosulfan and leuprorelin group. Quality of life was not reported in either study.
Both studies were at high risk of bias. Currie 1994 did not fulfil any of the core individual 'Risk of bias' items while the study of Du Bois 2002 fulfilled only a few of the core individual 'Risk of bias' items. The follow‐up for all patients was reported in the Du Bois 2002 study and was considered adequate (2.5 years), while in the Currie 1994 study, the type or the duration of follow‐up was not reported. All outcomes were reported in the Du Bois 2002 study, but not in the Currie 1994 study. Hazard ratio, which is the best estimate of statistical analysis to summarise the differences in the two treatment groups, was not reported in the Currie 1994 study and was only reported for OS in the Du Bois 2002 study.
From these data, it is not possible to draw meaningful conclusions about whether LHRH agonists are safe and effective in the treatment of platinum‐refractory and platinum‐resistant (relapsed) EOC. Overall, the quality of the evidence was very low in both included trials (Currie 1994; Du Bois 2002). The quality of the evidence was downgraded for numerous reasons, but the main ones included the small size of the trials (especially Currie 1994 (n = 24)), overall high risk of bias, incomplete reporting and potentially selective reporting of outcomes. There are lots of gaps in the evidence in this area and the evidence base is very limited.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were independently assessed and the data retrieved by two review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce the bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias (studies that did not find the treatments to have been effective may not have been published). We were unable to assess this possibility as we did not find an adequate number of studies that met our inclusion criteria and all the treatment comparisons were restricted to single‐trial analysis.
Agreements and disagreements with other studies or reviews
The comparison between LHRH agonists with chemotherapeutic agent or placebo is difficult, as there are no published systematic reviews in the literature with similar eligibility criteria to this review. Currently there is no convincing evidence to support LHRH agonist use in platinum‐refractory and platinum‐resistant (relapsed) EOC.
Authors' conclusions
Implications for practice.
Based on this review, there are insufficient data to make a thorough assessment of the effectiveness and safety of luteinising hormone releasing hormone (LHRH) agonists in the treatment of platinum‐refractory and platinum‐resistant (relapsed) epithelial ovarian cancer (EOC). This review is based on two trials, which were incompletely or poorly reported and the conduct and methodology of these trials is also questionable. Overall survival (OS) and progression‐free survival (PFS) did not differ between leuprorelin (LHRH agonist) and treosulfan (chemotherapeutic drug) group. Also, there was no difference in adverse events between the two treatment groups. The data were insufficient and of too low quality to draw any meaningful conclusions regarding comparison of LHRH agonist (decapeptyl) versus placebo in resistant (relapsed) EOC. Quality of life data were not reported in either study.
Currently, the patients who relapse (EOC) are treated with either platinum or non‐platinum drugs and this is dependent on the platinum‐sensitivity and platinum‐free interval. It is unlikely that the current practice will change in these patients based on the above findings.
Implications for research.
Our review indicates that there is a need for well‐conducted randomised controlled trials (RCTs) with adequate power and comparable groups to allow satisfactory comparisons of outcomes or detect meaningful differences in survival outcomes. The RCT by Du Bois 2002, is more than 13 years old, and the comparison is with a chemotherapeutic agent which is not used currently as a standard second‐line chemotherapeutic agent for ovarian cancer. There is no new evidence to justify multi‐centre trials to compare LHRH agonists with placebo or even with standard chemotherapy. Now that there is good evidence to show that the majority of high‐grade serous malignancies are actually of fallopian tube origin, perhaps further scientific research is required in the first instance to determine the effects of LHRH agonists on fallopian tube cells and serous tubal intra‐epithelial carcinoma (STIC) lesions.
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2021 | Review declared as stable | Most recent search 15 February 2021. No potentially relevant new studies identified. The conclusions of this Cochrane Review are therefore still considered up to date for this topic. |
History
Protocol first published: Issue 9, 2014 Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 21 January 2019 | Amended | No potentially relevant new studies identified after a scoping search in January 2019. The conclusions of this Cochrane review are therefore still considered to be up to date for this topic. A further search of the literature will be carried out in 2021, 5 years after the review was first published. |
Acknowledgements
We thank Jo Morrison for clinical expertise, Andrew Bryant for methodological support, Jane Hayes for designing the search strategy and Gail Quinn, Clare Jess and Tracey Bishop for their contribution to the editorial process.
The National Institute for Health Research supported this project via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Ovarian cancer staging
| FIGO classification of ovarian cancer (2014) | |
| Stage | Characteristics |
| I | Growth limited to ovaries |
| IA | Growth limited to one ovary. The ovarian capsule is intact without tumour on the external surface and no malignant cells present in washings or ascites |
| IB | Growth limited to both ovaries. The ovarian capsule is intact without tumour on the external surface and no malignant cells present in washings or ascites |
| IC | Either stage IA or IB but with tumour on the surface of one or both ovaries, or surgical spill, or capsule rupture before surgery or with ascites present containing cancerous cells, or with positive peritoneal washings |
| IC1 | Surgical spill |
| IC2 | Tumour on ovarian surface or capsule rupture before surgery |
| IC3 | Positive peritoneal washings or ascites containing malignant cells |
| II | Growth involving one or both ovaries with spread to other pelvic organs (below the pelvic brim) |
| IIA | Metastasis to uterus or tubes |
| IIB | Metastasis to other pelvic intraperitoneal organs |
| III | Growth involving one or both ovaries with histologically or cytologically confirmed peritoneal implants outside the pelvis and/or positive retroperitoneal lymph nodes |
| IIIA | Positive peritoneal spread confirmed microscopically or positive retroperitoneal lymph nodes |
| IIIA1 | Positive retroperitoneal lymph nodes IIIA (i)‐Metastasis less than 10 mm size IIIA(Iii)‐ Metastasis more than 10 mm size |
| IIIA2 | Microscopic peritoneal metastasis outside the pelvis +/‐ positive retroperitoneal lymph nodes |
| IIIB | Macroscopic peritoneal metastasis < 2 cm size outside the pelvis +/‐ positive retroperitoneal lymph nodes +/‐ involvement of liver and/or splenic capsule |
| IIIC | Macroscopic peritoneal metastasis > 2 cm size outside the pelvis +/‐ positive retroperitoneal lymph nodes +/‐ involvement of liver and/or splenic capsule |
| IV | Distant metastasis excluding peritoneal metastasis |
| IVA | Analysis of pleural effusion showing malignant cells |
| IVB | Parenchymal liver and/or splenic metastasis and metastasis to extra‐abdominal organs including inguinal lymph nodes and other lymph nodes outside the abdominal cavity |
Appendix 2. Five‐year survival rates for ovarian cancer
| Stage | Survival rate (%) |
| Stage I | 92% |
| Stage 2 | 55% |
| Stage 3 | 22% |
| Stage 4 | 6% |
| Overall survival for all stages | 43% |
Appendix 3. MEDLINE (Ovid) search strategy
Medline Ovid 1946 to Jan week 1 2016
1 exp Ovarian Neoplasms/ 2 (Ovar* adj5 (cancer* or tumor* or tumour* or malignant* or neoplasia* or carcinoma* or adenocarcinoma*)).mp. 3 1 or 2 4 exp Gonadotropin‐Releasing Hormone/ 5 LHRH.mp. 6 luteinising hormone releasing hormone*.mp. 7 gonadotropin releasing hormone*.mp. 8 GnRH.mp. 9 (zoladex or goserelin or triptorelin or leuprorelin or decapeptyl or D‐Trp‐6‐LH‐RH).mp. 10 4 or 5 or 6 or 7 or 8 or 9 11 3 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 drug therapy.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 11 and 20 22 exp animals/ not humans.sh. 23 21 not 22
key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
Appendix 4. Embase (Ovid) search strategy
Embase Ovid 1980 to 2016 week 3
1 exp ovary tumor/ 2 (Ovar* adj5 (cancer* or tumor* or tumour* or malignant* or neoplasm* or carcinoma* or adenocarcinoma*)).mp. 3 1 or 2 4 gonadorelin/ 5 LHRH.mp. 6 luteinising hormone releasing hormone*.mp. 7 gonadotropin releasing hormone*.mp. 8 GnRH.mp. 9 (zoladex or goserelin or triptorelin or leuprorelin or decapeptyl or D‐Trp‐6‐LH‐RH).mp. 10 4 or 5 or 6 or 7 or 8 or 9 11 3 and 10 12 crossover procedure/ 13 double‐blind procedure/ 14 randomized controlled trial/ 15 single‐blind procedure/ 16 random*.mp. 17 factorial*.mp. 18 (crossover* or cross over* or cross‐over*).mp. 19 placebo*.mp. 20 (double* adj blind*).mp. 21 (single* adj blind*).mp. 22 assign*.mp. 23 allocat*.mp. 24 volunteer*.mp. 25 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 11 and 25
key:[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 5. CENTRAL search strategy
CENTRAL 2015, Issue 12
#1 MeSH descriptor: [Ovarian Neoplasms] explode all trees #2 ovar* near/5 (cancer* or tumor* or tumour* or malignant* or neoplasm* or carcinoma* or adenocarcinoma*) #3 #1 or #2 #4 MeSH descriptor: [Gonadotropin‐Releasing Hormone] explode all trees #5 LHRH #6 luteinising hormone releasing hormone*. #7 gonadotropin releasing hormone* #8 GnRH #9 (zoladex or goserelin or triptorelin or leuprorelin or decapeptyl or D‐Trp‐6‐LH‐RH) #10 #4 or #5 or #6 or #7 or #8 or #9 #11 #3 and #10
Data and analyses
Comparison 1. LHRH agonist versus treosulfan for the treatment of relapsed EOC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.2 Progression‐free survival at 6 months | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.3 Progression‐free survival at 12 months | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.4 Skin toxicity (alopecia) | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.5 Fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.6 Hot flushes | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.7 Gastrointestinal toxicity (nausea and vomiting) | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.8 Neurotoxicity | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.9 Grade 3 pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.10 Neutropenia grade 3/4 | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.11 Thrombocytopenia | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.12 Anaemia | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Currie 1994.
| Study characteristics | ||
| Methods | RCT, Baltimore, John Hopkins Medical Institution and Sinai Hospital, Maryland. | |
| Participants | Patients with resistant EOC who had prior multiple courses of chemotherapy. The mean age of patients was 58.4 years. There was no information regarding initial FIGO stage or performance status of patients or the type of prior first‐line chemotherapy received by these patients. We are not able to comment on the comparability of patients between the intervention and comparison groups as it is not reported by the authors. | |
| Interventions | Decapeptyl (LHRH agonist) versus placebo. Twelve patients were included in the intervention group (D‐TRP‐6‐LHRH: Decapeptyl) and 12 patients in the comparison group (placebo). Both treatments were repeated every 4 weeks. The dose and duration of treatment was not reported for both groups. | |
| Outcomes | PFS, adverse events (incompletely reported) and response to treatment. Confidence intervals, P values and Hazard ratios were not reported. | |
| Notes | The type of blinding was unclear and it was mentioned that the blinding was broken in the placebo group when the disease progressed in these patients and they were treated with LHRH agonist decapeptyl. OS was not reported and adverse events were incompletely reported. Therefore individual patient data were not presented for these outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | While not an issue for OS, blinding of treatment was broken in the placebo arm when the disease progressed in these patients and they were treated with LHRH agonist decapeptyl. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not documented |
| Selective reporting (reporting bias) | High risk | OS was not reported, only PFS and there was emphasis on one patient experiencing stable disease for 80 weeks in the decapeptyl group |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias may be present |
Du Bois 2002.
| Study characteristics | ||
| Methods | RCT, Multicentre trial, Germal Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer, Germany, | |
| Participants | Patients with EOC progressing or experiencing relapse within six months of first‐line platinum‐based chemotherapy. The trial included 73 adult patients. The mean age of patients in the intervention arm (LHRH agonist) was 58.8 years (range:27‐75) and the mean age in the comparison arm (chemotherapy; Treosulfan) was 58 years (range: 36‐75). The initial FIGO stage at diagnosis of most patients (94.5%) was stage III and IV with only few patients with stage II. All patients had received prior first‐line platinum‐based chemotherapy. | |
| Interventions | Leuprorelin versus treosulfan. Thirty‐seven patients received leuprorelin (LHRH agonist) 3.75 mg injected subcutaneously (SC) or intramuscularly (IM) in the intervention group and 36 patients received treosulfan 7 gm/m2 infusion (chemotherapy) in the comparison group. Both treatments were repeated every four weeks. The mean duration of treatment in the leuprorelin group was 13 weeks and in the treosulfan arm 18 weeks. A total of 122 courses in the leuprorelin group and 150 courses in treosulfan group were evaluated. | |
| Outcomes | OS, PFS and adverse effects | |
| Notes | Both groups were comparable. Patients were followed at 3‐monthly intervals until death. Additonal outcomes were reported in this trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not reported in the methods. There was also significant cross‐over between the two treatment groups. In the treosulfan arm, 15 women received third to fifth‐line treatment and three women received leuprorelin. In leuprorelin arm, almost all patients received third‐line chemotherapy. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. While blinding of the outcome assessor is not important for outcomes such as OS it is vital for PFS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up on all patients was reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. In addition other outcomes were reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias may be present |
EOC: epithelial ovarian cancer FIGO: Federation of Gynecology and Obstetrics LHRH: luteinising hormone releasing hormone OS: overall survival PFS: progression‐free survival
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Emons 1996 | In this trial, LHRH agonist (triptorelin) was used in combination with first‐line chemotherapy in patients with advanced EOC following cytoreductive surgery. |
| Jager 1993 | This study was published as abstract. It is the same study as Jager 1995, a randomised trial comparing triptorelin with tamoxifen in the treatment of progressive EOC. A hormonal therapy was compared with another hormonal therapy and did not fit our inclusion criteria. |
| Jager 1995 | This trial was a randomised comparison of triptorelin with tamoxifen for the treatment of progressive EOC. A hormonal therapy was compared with another hormonal therapy and did not fit our inclusion criteria. |
EOC: epithelial ovarian cancer LHRH: luteinising hormone releasing hormone
Differences between protocol and review
Searches and inclusion of studies
We stated in our inclusion criteria that women with relapsed EOC would be included in this review. However, the study of Du Bois 2002 included women who progressed while receiving platinum chemotherapy (platinum‐refractory) and also women who relapsed within six months of platinum chemotherapy (platinum‐resistant) and these were not reported separately. Both were included in this review.
In the protocol we stated:
''If ongoing trials that have not been published are identified through these searches, we will approach the principal investigators and major co‐operative groups active in this area, to ask for relevant data''
However, we did not find an active co‐operative trials group or any relevant ongoing trials, so we did not make these contacts.
Quality of life (QoL)
In the protocol we stated that we would report:
''Quality of life (QoL) measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication by a validated scale''
However, QoL was not reported in either study.
Continous outcome and time‐to‐event data
Time‐to‐event data were incompletely reported and continuous data and adjusted statistics were not reported so the following text was removed from measures of treatment effect sections.
Measures of treatment effect
Time‐to‐event data were incompletely reported and continuous data and adjusted statistics were not reported so the following text was removed from measures of treatment effect sections for this version of the review.
"For time‐to‐event data (survival and disease progression), we will extract the log of the hazard ratio [log (HR)] and its standard error from trial reports. If these are not reported, we will attempt to estimate the log (HR) and its standard error using the methods of Parmar 1998.
For continuous outcomes (e.g. quality of life), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up. We will pool the mean differences between the treatment arms at the end of follow‐up if all trials measure the outcome on the same scale, otherwise standardised mean differences will be pooled if trials measure outcomes on different scales.
If reported, we will extract both unadjusted and adjusted statistics."
Data Synthesis
Since the comparisons differed in the two included RCTs in this review, it was not possible to perform a meta‐analysis. Therefore it was not relevant to assess heterogeneity between results of trials or conduct any subgroup analysis or sensitivity analysis. Continuous data and adjusted statistics were not reported and time‐to‐event data were incompletely reported in the included trials. Therefore, the corresponding sections from the protocol as mentioned below were removed.
"If sufficient, clinically‐similar studies are available we will pool their results in meta‐analyses using Cochrane Review Manager software (Review Manager 2014).
For time‐to‐event data, we will pool hazard ratios (HRs) using the generic inverse variance facility of Review Manager 2014.
For any dichotomous outcomes, we will calculate the risk ratio (RR) for each study and these will then be pooled.
For continuous outcomes, we will pool the mean differences between the treatment arms at the end of follow‐up if all trials measure the outcome on the same scale, otherwise standardised mean differences will be pooled."
"If any trials have multiple treatment groups, we will divide the ‘shared’ comparison group into the number of treatment groups, and comparisons between each treatment group and the split comparison group will be treated as independent comparisons."
"We will use the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986)."
Assessment of heterogeneity
In future updates we will assess heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by subgroup analyses. If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported.
Assessment of reporting biases
We will examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias, if a sufficient number of studies are identified.
Subgroup analysis and investigation of heterogeneity
We will conduct subgroup analysis if possible between different LHRH agonists versus different chemotherapeutic agents. We will consider factors such as age, stage, type of intervention, length of follow‐up and 'Risk of bias' status in the interpretation of any heterogeneity.
Sensitivity analysis
If a sufficient number of trials is included in the review, we will perform sensitivity analyses to examine possible differences in methodological or clinical aspects between the trials e.g. compare trials at low risk of bias versus high risk of bias or trials with different clinical criteria for survival.
Methods
Grade assessment of quality of evidence was performed using GRADEproGDT software to create a 'Summary of findings' table for this review. This was not described in the protocol but has been included in this review (see Table 1 and in Effects of interventions).
Contributions of authors
RW, GL, AM drafted the clinical sections of the review. SS and RW drafted the methodological sections of the review. All authors agreed the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research (NIHR), Department of Health, NHS Cochrane Collaboration Programme, UK
Declarations of interest
Rekha Wuntakal – none known Srividya Seshadri – none known Ana Montes – none known Geoff Lane – none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Currie 1994 {published data only}
- Currie J, Holden T, Sert B, Dudzinski M, Guarino J. A randomised placebo-controlled efficacy and safety study of a sustained release LHRH agonist (Decapeptyl) in patients with advanced ovarian cancer. Gynecologic Oncology 1994;52(1):116-7. [Google Scholar]
Du Bois 2002 {published data only}
- Du Bois BA, Meier W, Luck HJ, Emon G, Moebus V, Schroeder W, et al. Chemotherapy versus hormonal treatment in platinum- and paclitaxel-refractory ovarian cancer: a randomised trial of the German Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer. Annals of Oncology 2002 2002;13(2):251-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Emons 1996 {published data only}
- Emons G, Ortmann O, Teichert HM, Fassl H, Löhrs U, Kullander S, et al. Hormone-releasing hormone agonist triptorelin in combination with cytotoxic chemotherapy in patients with advanced ovarian carcinoma. A prospective double blind randomized trial. Decapeptyl Ovarian Cancer study group. Cancer 1996;78(7):1452-60. [DOI] [PubMed] [Google Scholar]
Jager 1993 {published data only}
- Jager W. A randomized comparison of decapeptyl and tamoxifen as treatment of progressive ovarian cancer. Gynecological Endocrinology 1993;7(Suppl 2):70:(abstr 123). [Google Scholar]
Jager 1995 {published data only}
- Jager W, Sauerbrei W, Beck E, Maassen V, Stumpfe M, Meier W, et al. Comparison of triptorelin and tamoxifen as treatment of progressive ovarian cancer. Anticancer Research 1995;15(6B):2639-42. [PubMed] [Google Scholar]
Additional references
Adelson 1993
- Adelson MD, Reece MT. Effects of gonadotropin-releasing hormone analogues on ovarian epithelial tumours. Clinical Obstetrics and Gynecology 1993;36(3):690-700. [DOI] [PubMed] [Google Scholar]
Aghajanian 2012
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomised, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of Clinical Oncology 2012;30 (17):2039-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2011
- Banerjee S, Bookman M, Gore M. Systemic therapy for ovarian cancer, current treatment, recent advances, and unmet needs. In: Kaye S, Brown R, Gabra H, Gore M, editors(s). Emerging Therapeutic Targets in Ovarian Cancer. London: Springer, 2011:1-34. [Google Scholar]
Cancer Research UK
- Cancer Research UK . Stages of ovarian cancer. www.cancerresearchuk.org/cancer-help/type/ovarian-cancer/treatment/stages-of-ovarian-cancer Accessed 29 June 2014.
Cancer Research UK 2011
- Cancer Research UK . Ovarian cancer report. www.publications.cancerresearchuk.org/cancerstats/statsovarian 2011.
Cancer Research UK 2012
- Cancer Research UK . Statistics on survival [www.cancerresearchuk.org/cancer-info/cancerstats/types/ovary/survival/]. Cancer Research UK 2012.
Chudecka‐Glaz 2009
- Chudecka-Glaz A, Rzepka-Gorska I. Favorable effects of long-term therapy with gonadoliberin analogues in three patients with advanced and recurrent ovarian cancer. European Journal of Gynaecological Oncology 2009;30(5):589-91. [PubMed] [Google Scholar]
Colombo 2014
- Colombo PE, Fabbro M, Theillet C, Bibeau F, Rouanet P, Ray-Coquard I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Critical Reviews in Oncology/Haematology 2014;89(2):207-16. [DOI] [PubMed] [Google Scholar]
Cramer 1983
- Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. Journal of the National Cancer Institute 1983;71:717-21. [PubMed] [Google Scholar]
CTCAE 2006
- Common terminology criteria for adverse events. ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed 22 September 2014.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. (2001) Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Systematic Reviews in Health Care: Meta-Analysis in Context Published online 2008:285-312. onlinelibrary.wiley.com/advanced/search/results. [10.1002/9780470693926.ch15]
Denny 2013
- Denny L. Staging of ovarian cancer: time to subdivide more? Journal of Gynecologic Oncology 2013;24(4):293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Dowsett 1988
- Dowsett M, Cantwell B, Lal A, Jeffcoate SL, Harris AL. Supression of postmenopausal ovarian steroidogenesis with luteinising hormone-releasing hormone agonist goserelin. Journal of Clinical Endocrinology and Metabolism 1988;66:672. [DOI] [PubMed] [Google Scholar]
Du Bois 2009
- Du Bois A, Reuss A, Oujade-Lauraine E, Harter P, Pfisterer J. The role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer. A combined exploratory analysis of three prospectively randomised phase III multicenter trials by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruooe Ovariaikarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux pour kes Etudes des Cancers de I’Ovaire (GINECO). Cancer 2009;15:1234-44. [DOI] [PubMed] [Google Scholar]
Elattar 2011
- Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD007565. [DOI: 10.1002/14651858.CD007565.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emons 1990
- Emons G, Pahwan GS, Ortmann O, Knuppen R, Oberheuser F, Schulz KD. LHRH-Receptors and LHRH-Agonist treatment in ovarian cancer: an overview. Journal of Steroid Biochemistry and Molecular Biology 1990;37(6):1003-6. [DOI] [PubMed] [Google Scholar]
Emons 1994
- Emons G, Schally AV. The use of luteinising hormone-releasing hormone agonists and antagonists in gynaecological cancers. Human Reproduction 1994;9:1364-79. [DOI] [PubMed] [Google Scholar]
Emons 2000
- Emons G, Schulz KD. Primary and salvage therapy with LHRH analogues in ovarian cancer. Recent Results in Cancer Research 2000;153:83-94. [DOI] [PubMed] [Google Scholar]
Feig 2006
- Feig BW, Berger DH, Fuhrman GM. The M.D. Anderson Surgical Oncology Handbook. 4th edition. New Delhi: Lippincott Williams & Wilkins, 2006. [Google Scholar]
Friedlander 2011
- Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, et al. Clinical trials in recurrent ovarian cancer. International Journal of Gynecological Cancer 2011;21(4):771-5. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. globocan.iarc.fr/Default.aspx.
Gonzalez‐Martin 2013
- Gonzalez-Martin A. Update on randomised trials on recurrent disease. Annals of Oncology 2013;24:42-52. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- Cochrane informatics and knowledge development department. GRADEpro. tech.cochrane.org/gradepro: GRADEproGDT, Accessed 25th May 2015.
Griffiths 1975
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. National Cancer Institute Monographs 1975;42:101-4. [PubMed] [Google Scholar]
Hassan 2005
- Hassan J, Ton N, Mullamitha S, Clamp A, Marshall E, Jayson GC. Phase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancer. British Journal of Cancer 2005;95:647-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane-handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Hoskins 1992
- Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic Oncology 1992;47:159-66. [DOI] [PubMed] [Google Scholar]
Hoskins 1994
- Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American Journal of Obstetrics and Gynecology 1994;170:974-9. [DOI] [PubMed] [Google Scholar]
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: A Cancer Journal for Clinicians 2008;58:71-96. [DOI] [PubMed] [Google Scholar]
Kurman 2013
- Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Annals of Oncology 2013;24(Supp 10):16-21. [DOI] [PubMed] [Google Scholar]
Ledermann 2013
- Ledermann JA, Raja FA, Fotofoulou C, Gonzalez-Martin A, Colombo N, Sessa C on behalf of the ESMO guidelines working group. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO clinical practice guidelines for diagnoses, treatment and follow up. Annals of Oncology 2013;24(6):vi24-vi32. [DOI] [PubMed] [Google Scholar]
Levine 2007
- Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer 2007;110(11):2448-56. [DOI] [PubMed] [Google Scholar]
Lind 1992
- Lind MJ, Cantwell BM, Millward MJ, Robinson A, Proctor M, Simmons D, et al. A phase II trial of goserelin (Zoladex) in relapsed epithelial ovarian cancer. British Journal of Cancer 1992;65(4):621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makar 1995
- Makar AP. Prognostic studies in cancer of the ovary and fallopian tube with emphasis on the CA 125 and c-erbB-2 oncogene. Summary of doctoral thesis. Acta Obstetrica et Gynecologica Scandinavica 1995;74:238-40. [Google Scholar]
Medl 1993
- Medl M, Peters-Engel Ch, Fuchs G, Leodolter S. Triptorelin (D-Trp-6-LHRH) in combination with carboplatin-containing polychemotherapy for advanced ovarian cancer: a pilot study. Anticancer Research 1993;13(6):2373-6. [PubMed] [Google Scholar]
Miller 1992
- Miller DS, Brady MF, Barrett RJ. A phase II trial of leuprolide acetate in patients with advanced epithelial ovarian carcinoma. A Gynecologic Oncology Group Study. American Journal of Clinical Oncology 1992;15(2):125-8. [DOI] [PubMed] [Google Scholar]
Morrison 2012
- Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD005343. [DOI: 10.1002/14651858.CD005343.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Paskeviciute 2002
- Paskeviciute L, Roed H, Engelholm S. No rules without exception: long-term complete remission observed in a study using a LH-RH agonist in platinum-refractory ovarian cancer. Gynecologic Oncology 2002;86:297-301. [DOI] [PubMed] [Google Scholar]
Pettersson 1994
- Pettersson F. Annual Report on the Result of Treatment in Gynecological Cancer. Stockholm, Sweden. FIGO 1994;22:83-102.
Pfisterer 2006
- Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Seminars in Oncology 2006;33:S12-6. [DOI] [PubMed] [Google Scholar]
Polcher 2015
- Pölcher M, Hauptmann S, Fotopoulou C, Schmalfeldt B, Meinhold-Heerlein I, Mustea A, et al. Opportunistic salpingectomies for the prevention of a high-grade serous carcinoma: a statement by the Kommission Ovar of the AGO. Archives of Gynecology and Obstetrics 2015;292(1):231-4. [DOI] [PubMed] [Google Scholar]
Review Manager 2014
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration 2014.
Ron 1995
- Ron IG, Wigler N, Merimsky O, Inbar MJ, Chaitchik S. A phase II trial of D-Trp-6-LHRH (decapeptyl) in pretreated patients with advanced epithelial ovarian cancer. Cancer investigation 1995;13(3):272-5. [DOI] [PubMed] [Google Scholar]
Rzepka‐Gorska 2003
- Rzepka-Gorska I, Chudecka-Glaz A, Kosmider M, Malecha J. GnRH analogues as an adjuvant therapy for ovarian cancer patients. International Journal of Gynaecology and Obstetrics 2000;81:199-205. [DOI] [PubMed] [Google Scholar]
Shih 2010
- Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. Journal of Gynecologic Oncology 2010;21(75):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2011
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: Cancer Journal for Clinicians 2011;61:212-36. [DOI] [PubMed] [Google Scholar]
Society of Gynaecologic Oncology
- Society of Gynaecologic Oncology. New FIGO ovarian cancer staging guidelines, 2014. www.sgo.org/wp-content/uploads/2012/09/FIGO-Ovarian-Cancer-Staging_1.10.14.pdf Accessed 6 September 2014.
Sourbier 2012
- Sourbier C. Ovarian cancer: emerging molecular-targeted therapies. Biologics: Targets and Therapy 2012;6:147-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twombly 2007
- Twombly R. Cancer killer may be silent ‘no more’. Journal of the National Cancer Institute 2007;99(18):1359-61. [DOI] [PubMed] [Google Scholar]
Ushijima 2010
- Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. Journal of Oncology 2010;2010:497429. [DOI: 10.1155/2010/497429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergote 2010
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIc or IV ovarian cancer. New England of Journal of Medicine 2010;363(10):943-53. [DOI] [PubMed] [Google Scholar]


